Abstract
Background
Urinary incontinence is common after radical prostatectomy and can also occur in some circumstances after transurethral resection of the prostate (TURP). Conservative management includes pelvic floor muscle training with or without biofeedback, electrical stimulation, extra‐corporeal magnetic innervation (ExMI), compression devices (penile clamps), lifestyle changes, or a combination of methods.
Objectives
To determine the effectiveness of conservative management for urinary incontinence up to 12 months after transurethral, suprapubic, laparoscopic, radical retropubic or perineal prostatectomy, including any single conservative therapy or any combination of conservative therapies.
Search methods
We searched the Cochrane Incontinence Group Specialised Register (5 February 2014), CENTRAL (2014, Issue 1), EMBASE (January 2010 to Week 3 2014), CINAHL (January 1982 to 18 January 2014), ClinicalTrials.gov and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (both searched 29 January 2014), and the reference lists of relevant articles.
Selection criteria
Randomised or quasi‐randomised controlled trials evaluating conservative interventions for urinary continence in men after prostatectomy.
Data collection and analysis
Two or more review authors assessed the methodological quality of the trials and abstracted data. We tried to contact several authors of included studies to obtain extra information.
Main results
Fifty trials met the inclusion criteria, 45 in men after radical prostatectomy, four trials after TURP and one trial after either operation. The trials included 4717 men of whom 2736 had an active conservative intervention. There was considerable variation in the interventions, populations and outcome measures. Data were not available for many of the pre‐stated outcomes. Men's symptoms improved over time irrespective of management.
There was no evidence from eight trials that pelvic floor muscle training with or without biofeedback was better than control for men who had urinary incontinence up to 12 months after radical prostatectomy; the quality of the evidence was judged to be moderate (for example 57% with urinary incontinence in the intervention group versus 62% in the control group, risk ratio (RR) for incontinence after 12 months 0.85, 95% confidence interval (CI) 0.60 to 1.22). One large multi‐centre trial of one‐to‐one therapy showed no difference in any urinary or quality of life outcome measures and had narrow CIs. It seems unlikely that men benefit from one‐to‐one PFMT therapy after TURP. Individual small trials provided data to suggest that electrical stimulation, external magnetic innervation, or combinations of treatments might be beneficial but the evidence was limited.
Amongst trials of conservative treatment for all men after radical prostatectomy, aimed at both treatment and prevention, there was moderate evidence of an overall benefit from pelvic floor muscle training versus control management in terms of reduction of urinary incontinence (for example 10% with urinary incontinence after one year in the intervention groups versus 32% in the control groups, RR for urinary incontinence 0.32, 95% CI 0.20 to 0.51). However, this finding was not supported by other data from pad tests. The findings should be treated with caution because the risk of bias assessment showed methodological limitations.
Men in one trial were more satisfied with one type of external compression device, which had the lowest urine loss, compared to two others or no treatment. The effect of other conservative interventions such as lifestyle changes remained undetermined as no trials involving these interventions were identified.
Authors' conclusions
The value of the various approaches to conservative management of postprostatectomy incontinence after radical prostatectomy remains uncertain. The evidence is conflicting and therefore rigorous, adequately powered randomised controlled trials (RCTs) which abide by the principles and recommendations of the CONSORT statement are still needed to obtain a definitive answer. The trials should be robustly designed to answer specific well constructed research questions and include outcomes which are important from the patient's perspective in decision making and are also relevant to the healthcare professionals. Long‐term incontinence may be managed by an external penile clamp, but there are safety problems.
Plain language summary
Conservative management for men with urinary incontinence after prostate surgery
Background information
The prostate is a male sex gland that surrounds the outlet of the bladder. Two main diseases of the prostate (cancer of the prostate, and benign (non‐cancerous) prostatic enlargement) can be treated by surgery but some men suffer leakage of urine (urinary incontinence) afterwards. Conservative treatments of the leakage such as pelvic floor muscle training with or without biofeedback or anal electrical stimulation are thought to help men control this leakage.
The main findings of the review
The review of trials found that there was conflicting evidence about the benefit of therapists teaching men to contract their pelvic floor muscles for either prevention or treatment of urine leakage after radical prostate surgery for cancer. However, information from one large trial suggested that men do not benefit from seeing a therapist to receive pelvic floor muscle training after transurethral resection (TURP) for benign prostatic enlargement. Overall, there was insufficient evidence to demonstrate a beneficial effect from pelvic floor muscle training.
Of three external compression devices tested, one penile clamp seemed to be better than the others.
Adverse effects
This one penile clamp needed to be used cautiously because of safety risks.
Any limitations of the review
In future updates it may be worth considering two separate reviews, looking separately at 'treatment' and 'prevention' trials. More research that is of better quality is also needed to assess conservative management.
Summary of findings
Summary of findings for the main comparison. Treatment of UI after radical: PFMT ± biofeedback versus no treatment; for postprostatectomy urinary incontinence.
| Treatment of UI after radical: PFMT ±biofeedback versus no treatment; for postprostatectomy urinary incontinence | ||||||
| Patient or population: patients with postprostatectomy urinary incontinence Intervention: treatment of UI after radical: PFMT ± biofeedback versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 623 per 1000 | 529 per 1000 (374 to 760) | RR 0.85 (0.6 to 1.22) | 665 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Urinary Incontinence Score (ICI‐SF) ‐ after first year | The mean urinary incontinence score (ici‐short form) ‐ after first year in the intervention groups was 0.5 lower (1.35 lower to 0.35 higher) | 391 (1 study) | ⊕⊕⊝⊝ low2,3,4 | |||
| Adverse events | See comment | See comment | Not estimable | 138 (1 study) | ⊕⊕⊕⊕ high2,3,5 | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide CI (0.60 to 1.22) 2 Funnel plot could not be used as there are fewer than 10 trials 3 Not applicable (only one trial) 4 95% CI is very wide (‐1.35 to 0.35) 5 Not estimable as the event rate is zero in each arm
Summary of findings 2. Treatment of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy urinary incontinence.
| Treatment of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI Intervention: Treatment of UI after radical: electric or magnetic energy versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 63 per 1000 | 16 per 1000 (6 to 47) | RR 0.26 (0.09 to 0.74) | 413 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Urinary Incontinence Score (ICIQ‐SF UI score) ‐ after 12 months | The mean urinary incontinence score (iciq‐short form ui score) ‐ after 12 months in the intervention groups was 1.4 lower (5.03 lower to 2.23 higher) | 47 (1 study) | ⊕⊕⊝⊝ low2,3,4 | |||
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) ‐ after 12 months | See comment | See comment | Not estimable | 47 (1 study) | ⊕⊕⊝⊝ low2,3,5 | |
| Adverse events | 133 per 1000 | 77 per 1000 (15 to 387) | RR 0.58 (0.11 to 2.9) | 56 (1 study) | ⊕⊕⊝⊝ low2,3,6 | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Random sequence generation and allocation concealment unclear is 1/2 trials taking part in the meta‐analysis 2 Funnel plot could not be used as there are fewer than 10 trials 3 Not applicable. Only one trial 4 95% CI very wide (‐5.03 to 2.23) 5 95% CI very wide (‐2.02 to 1.22) 6 95% CI very wide (0.11 to 2.90)
Summary of findings 3. Treatment of UI after radical: combinations of treatments versus no treatment for postprostatectomy urinary incontinence.
| Treatment of UI after radical: combinations of treatments versus no treatment for postprostatectomy UI | ||||||
| Patient or population: patients with postprostatectomy UI Intervention: Treatment of UI after radical: combinations of treatments versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: combinations of treatments versus no treatment | |||||
| Number of incontinent men with 3 to 6 months | 53 per 1000 | 150 per 1000 (17 to 1000) | RR 2.85 (0.32 to 25.07) | 39 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) after 12 months | Study population | Not estimable | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events ‐ PFMT + anal EStim + BFB | 0 per 1000 | 0 per 1000 (0 to 0) | RR 4.86 (0.24 to 99.39) | 138 (1 study) | ⊕⊕⊝⊝ low2,4,5 | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Random sequence generation and allocation concealment unclear 2 Not applicable, only one trial 3 No explanation was provided 4 Funnel plot cannot be used as there is only one trial 5 95% CI is very wide (0.24 to 99.39)
Summary of findings 4. Treatment of UI after radical: one active treatment versus another active treatment for postprostatectomy urinary incontinence.
| Treatment of UI after radical: one active treatment versus another active treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI Intervention: Treatment of UI after radical: one active treatment versus another active treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men within 6 to 12 months ‐ FES versus ExMI | 83 per 1000 | 167 per 1000 (17 to 1000) | RR 2 (0.21 to 19.23) | 24 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | |
| Quality of Life Score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT | The mean quality of life score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT in the intervention groups was 1.6 lower (2.73 to 0.47 lower) | 24 (1 study) | ⊕⊕⊝⊝ low1,2,5,6 | |||
| Adverse events PFMT + Anal EStim versus PFMT alone | 0 per 1000 | 0 per 1000 (0 to 0) | RR 5 (0.24 to 102.3) | 140 (1 study) | ⊕⊕⊝⊝ low2,5,7 | |
| Economic analysis using QALY | Study population | Not estimable | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Random sequence generation and allocation concealment is unclear 2 Not applicable, only one trial 3 GRADE‐specific outcome was number of incontinent men after 12 months 4 95% CI is very wide (0.21 to 19.23) 5 Funnel plot cannot be used as there was only one trial 6 GRADE‐specific outcome was ICI‐Q‐SF after 12 months 7 95% CI very wide (0.24 to 102.30)
Summary of findings 5. Prevention of UI after radical: PFMT ± biofeedback versus no treatment for postprostatectomy urinary incontinence.
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: All men after radical prostatectomy Intervention: Prevention of UI after radical: PFMT ± biofeedback versus no treatment Comparison: | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men ‐ after 12 months | 321 per 1000 | 103 per 1000 (64 to 164) | RR 0.32 (0.2 to 0.51) | 373 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months | The mean quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months in the intervention groups was 0.69 lower (3.19 lower to 1.81 higher) | 105 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | |||
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment is unclear for Filocamo 2005 which contributes 84.2% weightage 2 Funnel plot cannot be used as there are fewer than 10 trials 3 Sequence generation is unclear in Ribeiro 2008. Allocation concealment is unclear in both the trials taking part in the meta‐analysis 4 95% CI is very wide (‐3.19 to 1.81)
Summary of findings 6. Prevention of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy urinary incontinence.
| Prevention of UI after radical: electric or magnetic energy versus no treatment for UI | ||||||
| Patient or population: All men after radical prostatectomy Intervention: Prevention of UI after radical: electric or magnetic energy versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of life score assessed using (ICIQ‐SF score) ‐ within 6 to 12 months | See comment | See comment | Not estimable | 32 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment is unclear 2 95% CI is very wide (‐2.15 to 5.35) 3 Funnel plot cannot be used as there are fewer than 10 trials
Summary of findings 7. Prevention of UI after radical: combinations of treatments versus no treatment for postprostatectomy urinary incontinence.
| Prevention of UI after radical: combinations of treatments versus no treatment compared to for postprostatectomy UI | ||||||
| Patient or population: All men after radical prostatectomy Intervention: Prevention of UI after radical: combinations of treatments versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: combinations of treatments versus no treatment | ||||||
| Number of incontinent men within 6 to 12 months ‐ PFMT + anal EStim + biofeedback versus no treatment | See comment | See comment | Not estimable | 60 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐ SF UI score) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Sequence generation and allocation concealment are both unclear 2 Funnel plot cannot be used as there are fewer than 10 trials
Summary of findings 8. Prevention of UI after radical: one active treatment versus another active treatment (PFMT pre and post‐operation versus PFMT post‐operation) for postprostatectomy urinary incontinence.
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT pre and post‐operation versus PFMT post‐operation) for UI | ||||||
| Patient or population: All men after radical prostatectomy Intervention: Prevention of UI after radical: one active treatment versus another active treatment Comparison: (PFMT pre and post‐operation versus PFMT post‐operation) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT pre and post‐operation versus PFMT post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | See comment | See comment | Not estimable | 367 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Quality of Life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events | See comment | See comment | Not estimable | 102 (1 study) | ⊕⊕⊕⊕ high3,4,5 | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Sequence generation is unclear 2/3 trials and allocation concealment is unclear in 1/3 trials 2 Due to clinical heterogeneity we decided not to pool the results 3 Not applicable 4 RR is not estimable as there is zero event in both arms of the trial 5 Funnel plot cannot be used as there were fewer than 10 trials
Summary of findings 9. Prevention of UI after radical: one active treatment versus another active treatment (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) for postprostatectomy urinary incontinence.
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) for | ||||||
| Patient or population: All men after radical prostatectomy Intervention: Prevention of UI after radical: one active treatment versus another active treatment Comparison: PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | 71 per 1000 | 100 per 1000 (18 to 555) | RR 1.4 (0.25 to 7.77) | 58 (1 study) | ⊕⊕⊝⊝ low1,2,3 | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) | Study population | Not estimable | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events | See comment | See comment | Not estimable | 68 (1 study) | ⊕⊕⊝⊝ low1,3,4 | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not applicable 2 95% CI very wide (0.25 to 7.77) 3 Funnel plot cannot be used as there were fewer than 10 trials 4 95% CI is very wide (0.80 to 240.77)
Summary of findings 10. Prevention of UI after radical: one active treatment versus another active treatment (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) for postprostatectomy urinary incontinence.
| Prevention of UI after radical: one active treatment versus another active treatment compared to (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) for UI | ||||||
| Patient or population: All men after radical prostatectomy Intervention: Prevention of UI after radical: one active treatment versus another active treatment Comparison: Pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of Life Score assessed using (ICIQ‐SF) within 6 to 12 months | See comment | See comment | Not estimable | 34 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment is unclear 2 Not applicable 3 95% CI very wide (‐3.13 to 4.13) 4 Funnel plot cannot be used as there were fewer than 10 trials
Summary of findings 11. Treatment of UI after TURP: PFMT ± biofeedback versus no treatment for postprostatectomy urinary incontinence.
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: Men with UI after TURP Intervention: Treatment of UI after TURP: PFMT ± biofeedback versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men‐ after 12 months | See comment | See comment | Not estimable | 1609 (1 study) | ⊕⊕⊕⊝ moderate1,2,3 | |
| Quality of life Score assessed using Score (ICIQ‐SF UI score) ‐ after 12 months | See comment | See comment | Not estimable | 397 (1 study) | ⊕⊕⊝⊝ low1,3,4 | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not applicable 2 95% CI is wide (0.91 to 1.23) 3 Funnel plot cannot be used at there are fewer than 10 trials 4 95% CI is very wide (‐0.89 to 0.69) 5 GRADE specific outcome is IIEF score 6 95% CI is very wide (0.86 to 1.72)
Summary of findings 12. Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment for postprostatectomy urinary incontinence.
| Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment for UI | ||||||
| Patient or population: All men after TURP Intervention: Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ within 3 to 6 months | 227 per 1000 | 116 per 1000 (32 to 430) | RR 0.51 (0.14 to 1.89) | 48 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Urinary Incontinence Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) at 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not applicable 2 GRADE specific outcome was number of incontinent men after 12 months 3 95% CI is very wide (0.14 to 1.89) 4 Funnel plot cannot be used as there are fewer than 10 trials
Background
Description of the condition
It is not uncommon for men to have urinary incontinence (UI) after prostatectomy. UI can be divided into three groups of urgency urinary incontinence (UUI), stress urinary incontinence (SUI) and mixed urinary incontinence (MUI). UUI is described by the International Continence Society (ICS) as the complaint of involuntary leakage of urine associated with a sudden desire to void urine (Altman 2013). SUI is defined as the involuntary leakage of urine with concurrent coughing, sneezing or physical exertion, whilst MUI, as the name suggests, is a mixture of the symptoms found in both of these types (Altman 2013). The reported frequency varies depending on the type of surgery and surgical technique (Grise 2001; Peyromaure 2002), the definition and quantification of incontinence (Grise 2001; Peyromaure 2002), the timing of the evaluation relative to the surgery, and who evaluates the presence or absence of incontinence (physician or patient) (Donnellan 1997; McCammon 1999). Furthermore, the costs associated with UI can be substantial. The annual cost to the National Health Service (NHS) in the UK for treating clinically significant storage symptoms in men was estimated to be GBP 303 million (Turner 2004) and the annual direct cost of UI in the US was estimated to be USD 3.8 billion (Wilson 2001).
The prevalence of UI after radical prostatectomy is widely reported, ranging from 2% to 60%, albeit at varying times after operation (Milsom 2009). For example, in one study at three months after radical prostatectomy (Donnellan 1997) 51% were subjectively wet (self‐report) but 36% were wet on pad testing (objective reporting). By 12 months, 20% were subjectively still wet but only 16% were classed as wet using objective criteria.
UI is less common after transurethral resection of the prostate (TURP) for benign prostate disease (Omar 2014) and most cases are due to persistent incontinence pre‐dating the surgery. Early UUI affects up to 30% to 40% of men but late SUI is rare affecting less than 0.5% of men (Rassweiler 2006). This is a less invasive operation than a radical prostatectomy and usually does not involve damage to pelvic nerves. Due to these clinical differences, we have analysed data relating to TURP separately.
After both types of operation the problem tends to improve with time, so that it declines and plateaus within one to two years postoperatively (Hunskaar 2002). However, some men are left with incontinence that persists for years afterwards.
Continence mechanisms
Urinary continence depends on a complex interaction of smooth and striated muscle fibres blended together to form the continence mechanism. Considerable debate has existed in the literature as to whether incontinence after prostatectomy is due to an effect on the detrusor (bladder) muscle or on the sphincter, as commonly these abnormalities coexist (Peyromaure 2002). New detrusor overactivity and intrinsic sphincter deficiency due to sphincteric injury (Ficazzola 1998; Groutz 2000; McGuire 1990) or weakness (Majoros 2006) are cited as the most important causes of persistent incontinence after radical prostatectomy. Debate continues on whether detrusor overactivity is a primary or secondary factor. Whereas some report overactivity as the primary cause of postprostatectomy incontinence (Golubuff 1995; Leach 1995) others argue strongly that even if other factors play a role, intrinsic sphincter deficiency is the primary cause of UI after radical prostatectomy (Aboseif 1996; Chao 1995; Groutz 2000; Gudziak 1996; Kondo 2002; Majoros 2006; Winters 1997).
Risk factors for postprostatectomy UI after radical prostatectomy include pre‐existing abnormalities of detrusor contractility (Leach 1995) and older age (Kondo 2002). This is possibly because in older men there is evidence of rhabdosphincter atrophy and neural degeneration (Burnett 1998; Chao 1995). Other risk factors include previous TURP (Jacobsen 2007); pre‐operative radiotherapy (Kondo 2002; Rainwater 1988); trauma; a spinal cord lesion; new obstruction due to recurrence, bladder neck contracture, or urethral stricture (Litwiller 1997); Parkinson's disease (Kondo 2002); dementia; and medications (Khan 1991). A surgeon's inadequate skill and expertise can determine post‐operative incontinence rates (Eastham 1996). In addition, having surgery in a hospital which performs fewer than 20 radical prostatectomies a year may be a factor (Albertsen 1997).
After TURP, UI is thought most likely to be due to pre‐existing abnormalities of bladder function, such as poor compliance or detrusor overactivity, rather than direct sphincter injury (Abrams 1991), possibly because removal of the prostatic tissue removed some of the protective mechanism for continence.
Description of the intervention
Many of the treatments used in current practice for postprostatectomy UI are 'conservative', which is usually considered as not involving drugs or surgery. Treatments such as biofeedback with surface intra‐anal probes are defined as non‐invasive in this context, as opposed to surgical interventions. Five categories of conservative management are considered in this review, both singly and in combination when appropriate.
1. Pelvic floor muscle training (PFMT)
This involves any method of training the pelvic floor muscles to contract. It includes teaching performance of an accurate voluntary pelvic floor muscle contraction using biofeedback and co‐ordinating and timing the contraction against increases in intra‐abdominal pressure, often called functional PFMT.
Traditionally, biofeedback involves the use of equipment to provide visual or auditory feedback about the pelvic floor muscle function to enable one to train, strengthen and increase endurance and co‐ordination of the pelvic floor muscle contractions. Simple auditory biofeedback can also be provided by the therapist informing the patient when a contraction is felt through digital anal examination during the pelvic floor muscle contraction. Additionally, pelvic floor muscle contraction electromyography (EMG) can be used as a surrogate for biofeedback, as well as for measuring the intra‐rectal pressure.
The theoretical basis of PFMT is that repeated, volitional contractions of selected pelvic floor muscles may improve their strength and efficiency during periods of increased intra‐abdominal pressure and can inhibit detrusor activity. In a systematic review of the literature on female UI, Berghmans and colleagues noted that a pelvic floor muscle contraction may raise the urethra and press it towards the symphysis pubis, prevent urethral descent, and improve structural support of the pelvic organs (Berghmans 1998). They further pointed out that PFMT may result in hypertrophy of the peri‐urethral striated muscles thereby increasing the 'external mechanical pressure' on the urethra.
2. Electrical stimulation (non‐invasive) delivered via surface electrodes
Electrical stimulation (ES) works by activating the motor fibres of the pudendal nerve, which can result in contraction of the pelvic floor muscles or the striated peri‐urethral musculature, supporting the intrinsic part of the urethral sphincter closing mechanism (Berghmans 2013). This may be important in the management of men with SUI by stimulating the intrinsic sphincter, strengthening the pelvic muscles and raising the patient's awareness of these muscles in a similar way to biofeedback. ES can also be helpful in men with detrusor overactivity or UUI because it can stimulate afferent fibres of the pudendal nerve, decreasing the sensation of urgency and inhibiting parasympathetic activity which results in a decrease in involuntary detrusor contractions (Berghmans 2013). Two types of non‐invasive ES are detailed below. The parameters of the ES used in studies vary depending on the type of UI and ES. Parameters include pulse width and duration, current intensity, stimulus frequency, current source, pulse shape, duration of treatment and total number of sessions, and rest to work ratio.
Anal electrical stimulation (ES)
Any type of ES using a non‐invasive surface anal probe designed for the therapy. The intention of ES is to facilitate contraction of the peri‐urethral striated muscle by inserting the probe into the anal canal (Jabs 2001).
Sticky patch electrodes, also called transcutaneous electrical nerve stimulation (TENS)
TENS is a low intensity, sensory nerve stimulation used for detrusor overactivity. It is delivered at various sites using patch electrodes. Sites include the sacral dermatomes, dorsal penile nerve, hamstring and quadriceps muscle, and the posterior tibial or perineal nerves (Berghmans 2013).
3. Lifestyle adjustment
This includes fluid adjustment, healthy diet, avoiding excessive caffeine, physical exercise, weight loss and cessation of smoking.
4. Extra‐corporeal magnetic innervation
This involves the use of a magnetic chair to stimulate contraction of the pelvic floor muscles and sacral nerve roots, without the discomfort of inserting an anal probe (Galloway 2000).
5. External penile compression devices (penile clamps)
These devices use an external clamp to achieve non‐surgical compression of the urethra.
Timing of the intervention
Conservative treatment can be started before or after surgery. In general, when it is delivered to all men (whether before or after) the aim is to prevent the development or persistence of UI. We have therefore distinguished between treatment of all men who do have UI (treatment) as opposed to a mixed population of men some of whom do not have UI (prevention).
How the intervention might work
All of these interventions, apart from lifestyle adjustment and a penile clamp, work by inducing contraction of pelvic muscles to increase their strength and efficiency, whilst improving co‐ordination and bladder control by inhibiting overactive detrusor activity. Repetitive contractions can raise urethral closure pressure at rest and during an increase in intra‐abdominal pressure.
Why it is important to do this review
The uncertainty about the benefit of conservative treatment for men with UI after prostate surgery was confirmed in the initial Cochrane review, first published in 1999 (Moore 1999b) and updated in 2001 (Moore 2001). The review originally only considered post‐operative PFMT, biofeedback and electrical stimulation. In a subsequent update (Hunter 2004) the review was broadened to include trials evaluating lifestyle adjustment, external penile compression devices and extracorporeal magnetic innervation. The most recent update also included trials on men after TURP (Hunter 2007) but still did not provide reliable evidence on the effects of conservative treatment. The current update includes 13 new trials.
Objectives
To determine the effectiveness of conservative management for urinary incontinence (UI) up to 12 months after transurethral or radical retropubic prostatectomy, including any single conservative therapy or any combination of conservative therapies.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised trials of conservative management to prevent or treat UI after TURP or radical prostatectomy were included. Trials were included if they used any single conservative therapy or any combination of conservative therapies. Other forms of clinical trials were excluded. Analysis of trials in men having radical prostatectomy was done separately from those in men having a TURP.
Types of participants
Adult men with UI following prostatectomy.
Types of interventions
PFMT; biofeedback (verbal or machine‐mediated); electrical stimulation (ES) via a surface electrode (e.g. anal probe ES, sticky patch electrode, transcutaneous electrical nerve stimulation (TENS)); extra‐corporeal magnetic innervation (ExMI); lifestyle adjustment; and external penile compression devices. These interventions could be compared with no treatment or with each other, alone or in combination.
The following comparisons were made for treatment or prevention of UI after prostatectomy.
Radical prostatectomy
Treatment (of men with UI after radical prostatectomy)
(1) Treatment of UI after radical prostatectomy: PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(2) Treatment of UI after radical prostatectomy: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, transcutaneous electrical nerve stimulation (TENS), extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(3) Treatment of UI after radical prostatectomy: lifestyle interventions versus no treatment or sham treatment
(4) Treatment of UI after radical prostatectomy: combinations of treatments versus no treatment or sham treatment
(5) Treatment of UI after radical prostatectomy: one treatment versus another active treatment
Prevention (of UI in men after radical prostatectomy)
(6) Prevention of UI after radical prostatectomy: PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(7) Prevention of UI after radical prostatectomy: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(8) Prevention of UI after radical prostatectomy: lifestyle interventions versus no treatment or sham treatment
(9) Prevention of UI after radical prostatectomy: combinations of treatments versus no treatment or sham treatment
(10) Prevention of UI after radical prostatectomy: one treatment versus another active treatment
TURP
Treatment (of men with UI after TURP)
(11) Treatment of UI after TURP: PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(12) Treatment of UI after TURP: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(13) Treatment of UI after TURP: lifestyle interventions versus no treatment or sham treatment
(14) Treatment of UI after TURP: combinations of treatments versus no treatment or sham treatment
(15) Treatment of UI after TURP: one treatment versus another active treatment
Prevention (of UI in men after TURP)
(16) Prevention of UI after TURP: pre or post‐operative PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(17) Prevention of UI after TURP: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, TENS,extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(18) Prevention of UI after TURP: lifestyle interventions versus no treatment or sham treatment
(19) Prevention of UI after TURP: combinations of treatments versus no treatment or sham treatment
(20) Prevention of UI after TURP: one treatment versus another active treatment
Containment of urinary incontinence (UI) from any cause
(21) External penile compression devices (penile clamps) versus no treatment or sham treatment
We have not listed all possible comparisons here. As and when new trials address new comparisons these will be added to the review.
Pharmacological agents will be considered in separate reviews. Verbal or written instructions, as well as sham therapy, were considered as 'no treatment'. The use of the term 'sham therapy' in this review meant any therapy that could not influence the pelvic floor muscles such as placing an ES probe in the anus but not turning it on.
Types of outcome measures
Primary outcomes
Number of men reporting urinary incontinence (UI) after 12 months
Quality of life assessed using the International Consultation on Incontinence Questionnaire Urinary Incontinence Short Form (ICIQ‐UI‐SF) or (ICIQ‐SF)
Number of men reporting adverse effects
Secondary outcomes
1. Participant reported observations
Number of men reporting UI (number not cured, in the short, medium or long term)
Number of men with no improvement in UI (number not cured or improved)
Self‐report of satisfaction with method
Compliance
2. Quantification of symptoms
Standardised pad test (24 hour or 1 hour) measuring grams of urine lost
Frequency of micturitions per 24 hours
Number of pad or clothing changes (pad changes per 24 hours)
Frequency of UI from self‐report or diary (incontinent episodes per 24 hours)
3. Clinician reported urinary outcome measures
Objective or observed leakage
Urodynamic outcome measures
4. Quality of life
Impact of UI e.g. Incontinence Impact Questionnaire (Uebersax 1995)
General health status e.g. Short Form 36 (Ware 1993)
5. Adverse effects
Pain or discomfort
Other adverse outcomes as reported by individual trials and judged to be important
6. Health economics outcomes
Cost of intervention
Resource implications of differences in outcome
Cost effective analysis
7. Other outcomes
Non‐prespecified outcomes judged important when performing the review
The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a; Guyatt 2011b; Guyatt 2013; Guyatt 2013a). This approach divides the quality of evidence into four categories: high, moderate, low and very low. Randomised controlled trials (RCTs) start as high quality evidence and non‐randomised trials begin as low quality evidence. The quality of evidence can be rated down for RCTs and up or down for non‐RCTs depending on predefined characteristics. The factors considered when assessing the quality of evidence included:
limitations in study design and implementation;
indirectness of evidence;
unexplained heterogeneity or inconsistency of results;
imprecision of results;
high probability of publication bias.
Primary and secondary outcomes were classified as critical, important or not important for decision making from the man’s perspective. The GRADE working group strongly advises a maximum of seven outcomes in a systematic review (Guyatt 2011a). The critical outcomes for assessing quality of evidence included in this review were:
number of men reporting UI after 12 months;
quality of life assessed using the ICIQ‐UI‐SF;
number of men reporting adverse effects;
cost effective analysis.
Search methods for identification of studies
We did not impose any language or other limits on the searches. Details of the search methods used for the previous versions of this review can be found in Appendix 1 and Appendix 2.
Electronic searches
This review has drawn on the search strategy developed for the Incontinence Review Group. Relevant trials were identified from the Incontinence Review Group Specialised Register of controlled trials which is described, along with the Group's search strategy, in the Incontinence Group's module in The Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, and handsearching of journals and conference proceedings. The Incontinence Group Specialised Register was searched using the Group's own keyword system; the search terms used were: ({design.cct*} OR {design.rct*}) AND ({topic.urine.incon.postprost*}) (All searches were of the keyword field of Reference Manager 2012). The date of the most recent search of the Specialised Register for this review was 5 February 2014. Most of the trials in the Incontinence Group Specialised Register are also contained in CENTRAL.
Specific searches were also performed for this update of the review.
CENTRAL (OvidSP) (2014, Issue 1) was searched on 26 February 2014.
EMBASE (OvidSP) (January 2010 to Week 3 2014) was searched on 20 January 2014.
CINAHL (EBSCOhost) (January 1982 to 18 January 2014) was searched on 22 January 2014.
ClinicalTrials.gov (via the Cochrane Register of Studies (CRS) interface) and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (both searched on 29 January 2014).
The strategies used to search these databases can be found in Appendix 3.
Searching other resources
Reference lists of relevant articles
The reference lists of relevant articles were searched for other possibly relevant trials.
Contact with investigators in the field
We contacted investigators to ask for other possibly relevant trials, published or unpublished.
Data collection and analysis
Comparisons of the outcomes of the chosen interventions with no treatment, with each other, and in combination were planned a priori for the review update. Data were not available for all planned comparisons. There was considerable diversity in the length of time interventions were carried out for and in the timing of outcome measurements relative to randomisation. The data were therefore reported at three monthly time points.
Selection of studies
The list of abstracts for each update was reviewed independently by two review authors and results compared. The full text articles of references or abstracts identified as potentially relevant by either review author were retrieved and reviewed by both. Reference lists of relevant review articles were reviewed to identify any further trials. References were assessed based on the population, interventions, control management, outcomes and overall study design. Using the full texts of the potentially relevant published studies and abstracts, the same two review authors independently reviewed the studies for relevance and inclusion. Authors were contacted for further data or clarification of methods. Disagreements were resolved through discussion; third party arbitration was not required.
Attempts were made to contact authors of trial reports if clarification was necessary. Studies were excluded from the review if they made comparisons other than those pre‐specified or if data were unavailable. Excluded studies were listed with reasons for their exclusion.
Data extraction and management
Data for the trials were extracted independently by two review authors using a standard form developed for this purpose. The following information was included:
study method and characteristics (design, method of randomisation, inclusion and exclusion criteria, withdrawals and dropouts);
participants (type of surgery, age, timing of randomisation, baseline incontinence or not);
type of intervention, timing (before or after surgery, or both) and duration of therapy, co‐interventions;
control (no treatment or sham therapy or other active treatment);
outcomes (types of outcome measures, reported outcomes, adverse events).
Extracted data were compared by two review authors for completeness and accuracy, and cross‐checked by another review author if necessary. Disagreements were resolved through discussion and review of the trial report. New data were entered using RevMan5 software.
Assessment of risk of bias in included studies
The risk of bias of the trials was assessed using the Cochrane 'risk of bias' tool.
The following methodological parameters were recorded: 1) identification of study as randomised or quasi‐randomised; 2) description of inclusion and exclusion criteria; 3) potential for selection bias (method of sequence generation, adequacy of random allocation concealment) rating; 4) potential for bias around the time of treatment or during outcome assessment (blinding of participants, personnel, outcome assessors); 5) potential for selection bias in the analysis (description of withdrawals, dropouts, participants lost to follow up, analysis based on intention to treat).
Measures of treatment effect
Analyses were based on available data from all included trials that were relevant to the comparisons and outcomes of interest. Meta‐analysis was undertaken where data were available from more than one study assessing the same outcome. A fixed‐effect model was used for calculations of pooled estimates and their 95% confidence intervals (CIs), or a random‐effects model if there was heterogeneity. For categorical outcomes we related the numbers reporting an outcome to the numbers at risk in each group to calculate a risk ratio (RR) with 95% CI. For continuous variables we used means and standard deviations to calculate a mean difference (MD) with 95% CI. If similar outcomes were reported on different scales, we calculated the standardised mean difference (SMD). We reversed the direction of effect if needed to ensure consistency across trials. If data to calculate RRs or MDs were not given, we utilised the most detailed numerical data available to calculate the actual numbers or means and standard deviations (for example test statistics, P values).
Unit of analysis issues
The primary analysis was per man randomised.
Dealing with missing data
Analysis of the data was on an intention‐to‐treat basis to the furthest possible extent. This meant all participants were analysed in the groups to which they were randomised. If this was not the case, we considered whether to exclude the trial. Attempts were made to obtain missing data from the original trialists. However, if this was not possible data were reported as given in the studies, except if there was evidence of differential loss to follow up from the randomised groups. In that case, the use of imputation of missing data was considered. If trials reported sufficient detail to calculate MDs but gave no information on the associated standard deviation (SD), the outcome was assumed to have a SD equal to the highest SD from other trials within the same analysis.
Assessment of heterogeneity
Trials were only combined if they were thought to be clinically similar. We assessed heterogeneity between studies by visual inspection of plots of the data, the Chi2 test for heterogeneity and I2statistic (Higgins 2011). We used the thresholds for interpretation of the I2 statistic in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2003).
Assessment of reporting biases
Due to the difficulty of detecting and correcting for publication bias and other reporting biases, the authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being careful to watch for duplication of data. Funnel plots could not be utilised because there were fewer than 10 trials in each meta‐analysis.
Data synthesis
Included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For dichotomous outcomes, data were summarised (for example number of people for whom an outcome is present or not) and risk ratios (RR) calculated with their 95% CIs. For continuous outcomes, each trial was summarised using the mean value for each group and SD, and combined as mean difference (MD) if the same scale (for example pad test in grams of urine) was used for the outcome measurement in more than one trial. A fixed‐effect model was used to calculate the summary statistic and the 95% CI. Heterogeneity was assessed visually and using the Chi2 test for heterogeneity and the I2 statistic (Higgins 2003). Forest plots were examined and potential sources influencing heterogeneity identified. Possible sources of heterogeneity were explored statistically through subgroup analysis. Where synthesis was deemed not appropriate, a narrative overview was planned.
Trials were combined if interventions were based on similar clinical criteria. To combine trial data, a meta‐analysis was conducted and a fixed‐effect model approach to the analysis was utilised unless there was evidence of heterogeneity across studies, in which case a random‐effects model was used.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis based on cancer stage but there were not enough data.
Sensitivity analysis
We planned to perform sensitivity analysis to investigate the effect of including or excluding trials at high risk of bias, however not enough trials were in the meta‐analysis.
Results
Description of studies
Results of the search
For the current update (2014) of the review 764 possibly relevant articles and abstracts were identified. Sources and numbers of potentially eligible titles were:
Incontinence Review Group Specialised Register (193);
CENTRAL (37);
updated search of EMBASE (354);
CINAHL (23);
ClinicalTrials.gov (125);
WHO ICTRP (32).
Overall 96 reports of 50 studies were included in the qualitative synthesis. Fifty‐nine reports of 27 studies were included in the quantitative synthesis. Four trials are awaiting further information from the authors (Crivellaro 2011;Delmastro 2010; Lilli 2006 NEW; Zhang 2007) and eight trials are ongoing (Burnett 2012; Burnett 2013;Fode 2012 NEW;Goode 2014;Mina 2013; Ng 2011;Terrone 2007; Zopf 2012).
Forty‐one reports of 36 studies were excluded and reasons are given in the 'Characteristics of excluded studies' table. The flow of the literature through the assessment process is shown in the PRISMA study flow chart (Figure 1).
1.
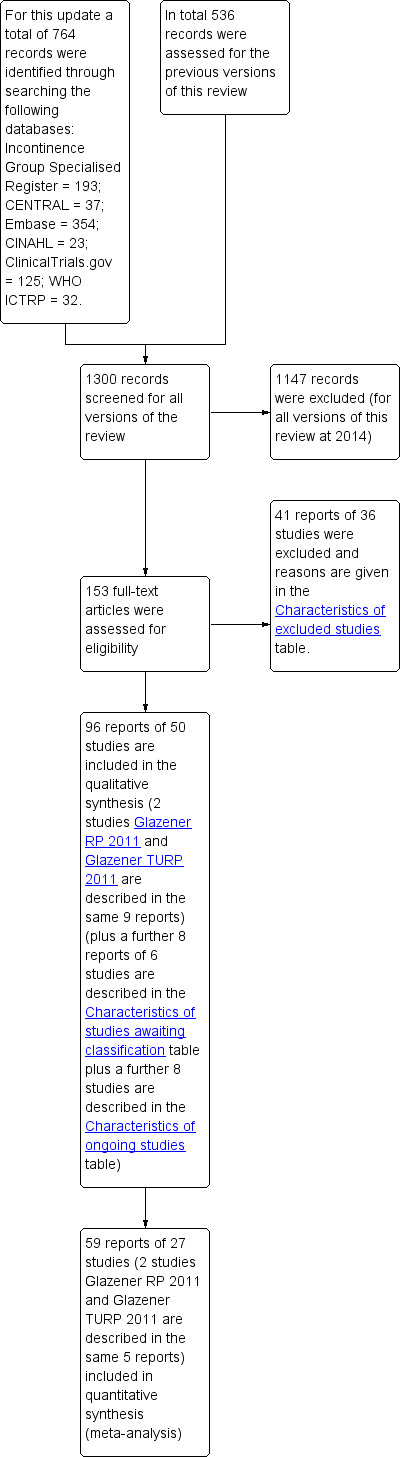
PRISMA study flow diagram.
New included trials
After abstract and full text screening 13 relevant new trials (Ahmed 2012;Dijkstra‐Eshuis 2013; Fader 2013; Fode 2014; Geraerts 2013;Ghanem 2013; Hou 2013; Laurienzo 2013; Marchiori 2010; Martini 2011; Morihiro 2011; Park 2012; Tienforti 2012) were identified. We also identified 12 new reports of the trials which were already identified in the previous update (Campbell 2012). The trialists were contacted for additional information and data.
One previously included trial published as an abstract was updated with data from a full publication (Centemero 2009).
Included studies
Types of populations
The trials included 4717 men, of whom 2736 had an active conservative intervention.
Surgery
Forty‐five trials involved patients undergoing radical prostatectomy (Ahmed 2012;Bales 2000;Burgio 2006;Centemero 2009;Dijkstra‐Eshuis 2013;Dubbelman 2004;Fader 2013;Filocamo 2005;Floratos 2002;Fode 2014;Franke 1998;Geraerts 2013;Ghanem 2013;Glazener RP 2011;Goode 2009;Hoffman 2005;Koo 2009;Laurienzo 2013;Liu 2008;Manassero 2007;Marchiori 2010;Mariotti 2009;Martini 2011;Mathewson‐Chapman 97;Moore 1999;Moore 2004;Moore 2008;Morihiro 2011;Nowak 2007;Opsomer 1994;Overgard 2008;Park 2012;Parekh 2003;Perissinotto 2008;Ribeiro 2008;Robinson 2008;Robinson 2009;Seleme 2008;Tienforti 2012;Tobia 2008;van Kampen 1998;Wille 2003;Yamanishi 2006;Yokoyama 2004;Zhang 2007).
One very small trial included one patient having a TURP while the rest were radical prostatectomy patients (Joseph 2000) but this was included in the radical prostatectomy group for analysis. Also, as all the men in this trial were incontinent for some time after surgery, they may have represented a group with persistent (longer than one to two years) UI. There were many potentially confounding variables in this trial, acknowledged by the author.
Four trials involved patients after TURP (Glazener TURP 2011;Hou 2013;Porru 2001;Tibaek 2007).
The trials involving post‐TURP patients only (Glazener TURP 2011; Hou 2013; Porru 2001; Tibaek 2007) were analysed separately from the trials amongst men having radical prostatectomy.
Continence status of populations
There was variation in continence status, which led to different populations being studied separately: those with persistent UI and those with all men undergoing surgery (many of whom were likely to recover continence spontaneously). The comparisons were therefore structured to reflect this: trials which included only men with post‐operative incontinence were deemed to be trials of treatment, while trials in which all men were treated (irrespective of continence status) were deemed to be trials of prevention.
Twenty‐three treatment trials enrolled only men with post‐operative UI (diagnosis of UI varied with recruitment time) (Dubbelman 2004; Fader 2013; Floratos 2002; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Hoffman 2005; Joseph 2000; Koo 2009; Liu 2008; Manassero 2007; Marchiori 2010; Moore 1999; Moore 2004; Moore 2008; Opsomer 1994; Robinson 2009; Seleme 2008; van Kampen 1998; Yamanishi 2006; Yokoyama 2004; Zhang 2007).
Twenty‐seven prevention trials included all men who underwent surgery, some of whom m.ay have been dry or become dry spontaneously (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Filocamo 2005; Fode 2014; Geraerts 2013; Ghanem 2013; Hou 2013; Laurienzo 2013; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Morihiro 2011; Nowak 2007; Overgard 2008; Park 2012; Parekh 2003; Perissinotto 2008; Porru 2001; Ribeiro 2008; Robinson 2008; Tibaek 2007; Tienforti 2012; Tobia 2008; Wille 2003).
Timing of recruitment
As the populations and the type and timing of interventions varied so greatly among the trials, the decision was made by the authors to also identify the timing of the recruitment to the trials and the timing of the intervention (before or after surgery):
only post‐operative treatment for UI (Ahmed 2012; Dubbelman 2004; Floratos 2002; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Hoffman 2005; Hou 2013; Joseph 2000; Koo 2009; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Moore 1999; Moore 2008; Morihiro 2011; Nowak 2007; Overgard 2008; Park 2012; Ribeiro 2008; Robinson 2009; Seleme 2008; van Kampen 1998; Yokoyama 2004; Zhang 2007) or containment (Fader 2013; Moore 2004); and
pre‐operative recruitment of all men undergoing surgery, which included a pre‐operative intervention with or without a post‐operative intervention (Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Filocamo 2005; Fode 2014; Geraerts 2013; Ghanem 2013; Laurienzo 2013; Martini 2011; Mathewson‐Chapman 97; Parekh 2003; Perissinotto 2008; Porru 2001; Robinson 2008; Tibaek 2007; Tienforti 2012; Tobia 2008; Wille 2003; Yamanishi 2006).
Time of recruitment of participants to the trial relative to the time of their surgery also varied:
pre‐operatively (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Fode 2014; Geraerts 2013; Ghanem 2013; Hou 2013; Laurienzo 2013; Martini 2011; Mathewson‐Chapman 97; Moore 2008; Nowak 2007; Overgard 2008; Parekh 2003; Perissinotto 2008; Robinson 2008; Tibaek 2007; Tienforti 2012; Tobia 2008; Wille 2003);
within days or up to two weeks post‐operatively or after catheter removal (Dubbelman 2004; Filocamo 2005; Floratos 2002; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Hoffman 2005; Koo 2009; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Park 2012; Porru 2001; Ribeiro 2008; Robinson 2009; van Kampen 1998; Yamanishi 2006);
weeks to months after surgery (Goode 2009; Joseph 2000; Moore 1999; Moore 2004; Opsomer 1994; Seleme 2008; Zhang 2007).
Types of interventions
In the included trials, there was considerable variation in the type and intensity of interventions. Table 13 gives the exact details of the interventions used in each trial. The duration of the treatment varied from four weeks up to one year. The interventions included:
1. Details of interventions.
| Study ID | Intervention | Control |
| Ahmed 2012 | A: At catheter removal received standard care of verbal and written instructions, instructed by physiotherapist to perform 3 sets of 15‐20 contractions daily, for a duration of 3‐5 seconds with a 6‐10 second rest period, encouraged to perform exercises before functional activities such as sneezing, coughing, or lifting weight, also in the supine position, sitting, squatting and going up and down stairs B: ES, treatment started one week after catheter removal, patients received 15 minutes of twice weekly electrical stimulation for 12 weeks C: PFMT + BFB + ES: Treatment started one week after catheter removal, patients received twice weekly treatment with 15 minutes of electrical stimulation and 15 minutes of biofeedback for 12 weeks, instructed to perform 3 series of 10 rapid contractions, 3 sustained contractions of 5, 7 or 10 seconds and then 10 contractions during prolonged expiration in the supine position All patients were given a logbook to complete daily regarding self‐report of exercises |
|
| Bales 2000 | PFMT + biofeedback 45 minute session with nurse trained in biofeedback. Patients were instructed to perform graded PFMT. Contractions of 5‐10 seconds, 10‐15 repetitions were performed with biofeedback (surface electrodes used to measure muscle strength). Advised to practice the exercises 4 times per day until surgery |
No biofeedback training Written and brief verbal instructions from a nurse on how to perform PFMT (isolate muscle that stops urine flow, practice 4 times per day, 10‐15 repetitions). |
| Burgio 2006 | PFMT + biofeedback Single session of biofeedback (rectal probe to measure intra‐abdominal rectal pressure and external anal sphincter contraction) assisted behavioural training. Feedback and verbal instruction used to teach control of pelvic muscles. Taught to contract sphincter during 2‐10 seconds periods separated by 2‐10 seconds of relaxation, dependent on ability. Written instructions for daily at home practice of 45 PFM exercises daily (3 sessions of 15 exercises each time). Additionally instructed to slow or interrupt voiding once daily. Encouraged to exercise daily preoperatively, then resume when catheter removed post‐operatively |
Usual care of brief verbal instructions post operatively to interrupt the voiding stream plus any instruction from physician. |
| Centemero 2009 | Intervention A: PFMT both pre and post‐operatively. A structured PFMT program 30 and 15 days before surgery, previous physiotherapist evaluation to provide the patients with feedback about the quality of pelvic floor muscle function, PC teste (endurance and contraction quality), breathing coordination, typify muscle contraction as tonic and modify incorrect physical attitudes. This was also repeated after the procedure Intervention B: PFMT post‐operatively only |
|
| Dijkstra‐Eshuis 2013 | 30 mins of guided PFMT + biofeedback weekly for 4 weeks before surgery, received written instructions to: carry out two sets of 30 contractions during abdominal breathing, one breath between each contraction; restart PFMT after catheter removal (7‐10 days after surgery) All men were seen before surgery by a physiotherapist, who explained relevant anatomy, anal visual inspection and digital palpation, biofeedback registration with rectal probe, All patients received PFMT + biofeedback or electrical stimulation, or both, if still incontinent after 6 weeks |
Received written instructions on PFMT after catheter removal (7‐10 days after surgery) |
| Dubbelman 2004 | Nine or less sessions of physiotherapy guided pelvic floor exercises after surgery | Exercise instruction through information folder |
| Filocamo 2005 | Formal instruction (3 treatment sessions plus at home exercises) in PFMT using verbal explanation, palpation and visualization of the base of the penis with a mirror, in different positions and prior to sneezing, coughing or lifting | No formal instruction |
| Floratos 2002 | Initiated after catheter removal, 15 treatment sessions (3 times per week for 30 minutes) of PFMT with EMG (surface) biofeedback in clinic | Instruction with verbal feedback and an information pamphlet with instructions to perform PFMT 50‐100 times daily at home |
| Fode 2014 | Pre‐operative session guided PFMT + instruction on how to use penile vibratory stimulation device. Instructed to stimulate frenulum once daily, 10 seconds of stimulation then 10 second pause, repeated 10 times for 1 week pre‐operatively, instructed to restart stimulation after catheter removal for 6 weeks All men were offered a PDE5 inhibitor after 1 month post‐operatively and also received telephone contact to ensure compliance with treatment |
Preoperative session guided PFMT |
| Franke 1998 | Biofeedback (perineal patch EMG) enhanced PFMT; exercise treatment sessions at 6, 7, 9, 11, and 16 weeks post‐operatively | No treatment. |
| Geraerts 2013 | Intervention A: PFMT + biofeedback 30 mins of guided PFMT + biofeedback weekly for 3 weeks before surgery. Patients were instructed to carry out 60 contractions a day at home; contract their pelvic floor while coughing, and sitting down or getting up from a chair. Patients were also instructed to restart PFMT on day 4 after surgery while catheter was in situ Intervention B: Instructed to start PFMT on the day after catheter removal (e.g. 2‐3 weeks after surgery) All men: Received weekly individual guided exercise programme with digital or EMG biofeedback after surgery. Advice was given on how to contract pelvic floor muscles to prevent leakage during functional activities. When patients carried out the instructed 60 contractions, they were asked to colour in three squares in their diary to assess compliance |
|
| Ghanem 2013 | Pre‐operative PFMT for 2 weeks + postoperative PFMT programme | Postoperative PFMT programme only |
| Goode 2009 | Intervention A: Behavioural therapy with PFMT for 8 weeks Intervention B: Behavioural therapy with biofeedback and electrical stimulation for 8 weeks Behavioural therapy consisted of pelvic floor muscle exercises and bladder control strategies in both groups |
No treatment |
| Hoffman 2005 | Intervention A: perineal EStim plus physiotherapy (PFMT) Intervention B: anal EStim plus physiotherapy (PFMT) |
PFMT alone |
| Hou 2013 | Guided PFMT + biofeedback after catheter removal (2 days post‐operatively), instructed to: contract pelvic muscles for 5 seconds and relax for 10 seconds. After discharge, patients were instructed to carry out 5 mins of each PFE three times daily. Patients also received motivational telephone interviews once weekly | No description |
| Joseph 2000 | Intervention A: Instruction in PFMT including biofeedback with visual feedback as well as verbal to assist in identifying and discriminating muscles Intervention B: Instruction in PFMT, squeezing of finger during digital rectal examination |
|
| Koo 2009 | ExMI, treatment sessions were for 20 minutes twice weekly for 8 weeks | PFMT alone |
| Laurienzo 2013 | A (15): Standard treatment with verbal instructions for PFMT B (17): Pre‐operative guided PFMT, with 10 physiotherapy sessions: contractions of the pelvic floor muscles for 5 seconds in “dorsal decubitus” position for 10 times, in the same position with the waist elevated (10 times), lying down with legs adducted against a plastic ball performed 10 times and standing and flexing the hips to 60̊ (10 times) C (17): Pre‐operative PFMT + ES during 10 physiotherapy sessions, ES was with an anal probe lasting 15 minutes in total, and men also received guided PFMT and followed the same training regime as above Men did not receive treatment post‐operatively |
Instructed to start PFMT at home 15 weeks before surgery. |
| Liu 2008 | Extra‐corporeal magnetic innervation (ExMI), the frequency of the pulse field was 10Hz for 10 minutes, followed by a 3 minute rest and a second treatment of 50 Hz for 20 minutes. This was done twice a week | PFMT alone, instructions given to carry out 20mins x 3 a day. |
| Manassero 2007 | PFMT re‐education program, verbal feedback The training program involved active PFE. verbal feedback of the contraction was used to instruct the patients to correctly and selectively contract their pelvic muscles while relaxing the abdominal muscles. the strength of the pelvic floor muscles was measured by digital anal control using a score of 0 to 5 ( 0 = no contraction, 5 = good contraction against strong resistance) Initially home practice comprised 45 contractions (3 sessions of 15) per day at home, progressively increasing the number until 90 per day. This was taught by two experienced urologists |
No treatment. |
| Marchiori 2010 | Guided PFMT + biofeedback during first session, second session involved 10 sets of pelvic floor electrical stimulation lasting 15 mins each, instructed to: carry out three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) All men received oral and written information on pelvic floor anatomy and on PFME, pelvic floor muscle endurance assessed by digital anal control |
Received oral and written information on pelvic floor anatomy and on PFME, instructed to: perform 30 contractions a day at home for the first month after catheter removal (16 days after surgery) |
| Mariotti 2009 | PFMT plus ES and biofeedback twice a week for 6 weeks ES ‐ a surface electrodes was inserted into the anus and pulsed, the intensity was adequate to induce visual lifting of the levator ani and pubococcygeus muscle, considering the level of comfort to the patient Biofeedback ‐ via surface electrodes both perineal and abdominally |
Instructions to conduct PFMT ‐ verbal and written instructions at catheter removal and follow up visits. |
| Martini 2011 | PFMT: 5 sessions of guided PFMT for 2‐3 weeks pre‐operatively and continued post‐operatively All men underwent clinical examination of pelvic muscles function using digital perineal testing according to “AIPDA score” and evaluation of voiding symptoms |
Postoperative standard care, written instructions for PFMT |
| Mathewson‐Chapman 97 | Pre‐operatively received further instruction and practice with PME protocol Home exercises and biofeedback (anal probe) (Incare 8900); practiced at home 3 times a week, starting with daily 15 PFMT and increasing by 10 every 4 weeks to a maximum of 35 PFMT. | Post‐operatively no further interventions until week 5 when pelvic muscle strength was assessed. |
| Moore 1999 | Intervention A: PFMT alone Intervention B: PFMT plus rectal ES treated by one physiotherapist 30 minutes twice a week for 12 weeks Both included home exercises 3x/day gradually working up to 30 minutes per session lying, standing, sitting; strength, endurance, speed and control with maximum contractions of 5‐10 seconds, 10‐20 second relaxation and 12‐20 repetitions; submaximum contractions at 65‐75% of maximum strength with hold 20‐30 seconds and equal rest time, 8‐10 repetitions; speed was sets of quick repetitive contractions in a 10 second time span; control involved gradual recruitment to maximum contraction in 3 stages with 5 second hold at each stage and a slow release with rest 15‐30 seconds |
oral and written information about PFMT pre and post‐ operatively (standard treatment) |
| Moore 2004 | Each participant had 4 periods (each lasted 1 day) Group A: No device Group B: C3 device Group C: U‐Tex device Group D: Cunningham clamp | |
| Moore 2008 | Maximum 24 weekly, 30‐minute treatment protocol (30 min biofeedback‐assisted PFMT) and home exercise protocol of 2‐3 times a day | Verbal and written information on PFME and weekly telephone contact by a urology nurse |
| Morihiro 2011 | PFMT + sacral surface therapeutic electrical stimulation (ssTES), ssTES 2x a day for 15 minutes each, lasting 1 month after catheter removal (day 5) | PFME only, carried out alone |
| Nowak 2007 | Extra‐corporeal magnetic innervation (EXMI) based pelvic floor device | PFMT alone |
| Opsomer 1994; | PFMT plus biofeedback plus electrical stimulation directed by physiotherapist | PFMT on their own without medical supervision. |
| Overgard 2008; | Instructions on PFMT and physiotherapy, 45 minutes weekly Patients were instructed to perform 3 sets of contractions daily at home, in either a supine, sitting or standing position. Digital anal palpation to teach correct contractions, as well as oral and written instructions DVD of instructions given to those living too far from hospital |
Instructions on PFMT alone. |
| Parekh 2003 | Two treatment sessions preoperatively. Session 1 consisted of PFMT in a hook lying position Session 2 was on an exercise ball. Teaching methods varied and included verbal cues, visualization with an anatomical model, palpation or biofeedback with rectal probe. Post‐operatively, PFMT was reviewed and participants were seen every 3 weeks for 3 months by a physiotherapist Home exercise for 6 months or more for those requiring further physical therapy guidance | No formal education on PFMT pre‐operatively, telephone or face to face follow‐up at least monthly. |
| Park 2012 | Patients performed Kegel exercises together with other types of exercises which included resistance training and pelvic flexibility. The intervention started 3 weeks after surgery and lasted 12 weeks Details of the combined exercise regime: Post‐operative weeks 1‐4 1) Education about postoperative symptoms 2) Performing Kegel exercises, recognizing the parapelvic muscles 3) Pelvic floor flexibility fitness: performing pelvic exercises while sitting on a ball Post‐operative weeks 5‐8 (ball exercises) 1) Performing pelvic exercises while sitting on a ball 2) Performing lower extremity exercises while placing a ball on the wall 3) Lifting a heel on the ball while standing face‐to‐face with the wall 4) Lifting up and down on the ball while spreading and bending legs 5) Performing flank exercises while having a ball in the hand 6) Squeezing the ball with the adductor muscles while lying on a table Post‐operative weeks 9‐12 (elastic band exercises) 1) Lifting the object with an elastic band lateral, anterior, and posterior to the patient’s arms 2) Lifting the legs and then spreading them while attaching an elastic band to the foot |
In the control group, only Kegel exercises were performed |
| Perissinotto 2008 | Early pelvic floor rehabilitation program at home twice dally, Kegel exercises | No formal PFMT |
| Porru 2001 | Initial visit before surgery, digital evaluation of pelvic muscle contraction strength. Verbal instruction, feedback and reinforcement on contraction was given to teach selective contraction of anal sphincter and relaxation of abdominal muscles. Verbal and written instruction given for home PFMT. Weekly digital anal reassessment and grading of pelvic muscle contraction by the therapist. Instructed to practice contractions 45 times per day (3 groups of 15 contractions) | Not specified |
| Ribeiro 2008 | PFMT plus BF weekly for 3 months | PFMT oral instructions only |
| Robinson 2008 | Intervention A: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 33%) plus PFMT for four weeks with biofeedback Intervention B: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 46%) |
|
| Robinson 2009 | Intervention A: routine brief verbal and written PFMT plus one PFMT session and 3 weekly nurse phone calls Intervention B: routine brief verbal and written PFMT plus four BF enhanced PFMT sessions and 4 weekly nurse phone calls |
Routine brief verbal and written PFMT. |
| Seleme 2008 | Verbal instruction and information on PFMT plus information on life style changes. Additional 15 physiotherapy sessions consisting of intensive PFMT with BF and ES | Verbal instruction and information on PFMT plus information on life style changes. |
| Tibaek 2007 | One hour individual session with physiotherapist to teach correct contraction for PFMT, three 1 hour group lessons and home training programme | No pre operative physiotherapy. Information about anatomy and physiology and verbal instructions for 2 to 3 days after TURP in the ward. |
| Tienforti 2012 | PFMT + biofeedback Patients received guided PFMT + biofeedback + information about the anatomy of pelvic floor muscles the day before surgery and after catheter removal. They were also given oral and written instructions on Kegel exercises to be performed at home which involved three sets of contractions daily for 10 mins, contracting their pelvic floor while lying, sitting and standing. The frequency of contractions was recorded in a training diary and visits at monthly intervals after catheter removal involved assisted biofeedback and motivation for 20 min |
No biofeedback training Received standard care, oral and written instructions from urologist on PFMT, Instructed to: start PFMT after catheter removal (e.g. 2‐3 weeks after surgery) |
| Tobia 2008 | PFMT | No PFMT |
| van Kampen 1998 | 1 session of PFMT in hospital before discharge and then saw the physiotherapist for 1‐2 weeks for as long as UI persisted. 90 daily home exercises sitting, standing and lying. 7 men unable to contract PFM or with weak contraction received electrical stimulation by anal probe | No formal PFMT instruction but saw the therapist at 1‐2 weeks and received placebo stimulation and information about aetiology of UI. |
| Wille 2003 | Intervention A: PFMT alone Intervention B: PFMT + ES; PFMT as above plus instructed by dedicated in ES via surface anal electrode and bio‐impulser (biphasic pulse with 1 second bursts, 5 second pulse width, 2 second pulse trains Intervention C: PFMT + ES + biofeedback. As above plus biofeedback (anal probe) 15 minutes twice daily for 3 months All groups: PFMT by physiotherapist, 20‐30 minute sessions for 3 days, instructed to perform exercises twice daily for 3 months plus 3 week rehabilitation program after dischargeRegular interaction with health professional for 6 weeks after surgery, encouraged to performed treatment for 3 months post‐surgery |
|
| Yamanishi 2006 | Oral PFMT plus ES for 15 minutes twice daily Instructed pre‐operatively PFMT by nurses and continued after catheter removal |
Oral PFMT plus sham device. Instructed pre‐operatively PFMT by nurses and continued after catheter removal. |
| Yokoyama 2004 | Intervention A: anal electrode for 15 minutes twice a day for 1 month Intervention B: extra‐corporeal magnetic innervation, neocontrol system, treatment sessions 20 minutes, twice a week for 2 weeks |
PFMT, digital anal teaching of correct contractions, then verbal and written instructions for home practice. |
| Zhang 2007 | PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes. Plus support group, 6 meetings in 3 months with a health psychologist | PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes |
PFMT alone (Centemero 2009; Dubbelman 2004; Filocamo 2005; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Martini 2011; Park 2012; Perissinotto 2008; Porru 2001; Tobia 2008);
PFMT plus biofeedback (Bales 2000; Burgio 2006; Dijkstra‐Eshuis 2013; Floratos 2002; Franke 1998; Geraerts 2013; Hou 2013; Joseph 2000; Manassero 2007; Marchiori 2010; Mathewson‐Chapman 97; Moore 1999; Moore 2008; Overgard 2008; Parekh 2003; Ribeiro 2008; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012);
ESl with PFMT (Ahmed 2012; Hoffman 2005; Laurienzo 2013; Morihiro 2011; Wille 2003; Yamanishi 2006);
ES with PFMT and biofeedback (Ahmed 2012; Goode 2009; Mariotti 2009; Opsomer 1994; Seleme 2008; Wille 2003; Zhang 2007);
extra‐corporeal magnetic innervation (ExMI) with PFMT (Koo 2009; Liu 2008; Nowak 2007);
extra‐corporeal magnetic innervation (ExMI) with PFMT or ES with PFMT (Yokoyama 2004);
penile compression (Fader 2013; Moore 2004);
transcutaneous mechanical nerve stimulation by vibration with PFMT (Fode 2014).
No trials testing lifestyle changes alone were identified.
Types of comparators
There was considerable variation in the types of comparators. Table 13 provides the details of the comparators used in each trial. The comparators included:
no treatment, verbal or written instructions or sham therapy (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dubbelman 2004; Filocamo 2005; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Hou 2013; Laurienzo 2013; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Moore 2004; Moore 2008; Morihiro 2011; Nowak 2007; Opsomer 1994; Overgard 2008; Park 2012; Parekh 2003; Perissinotto 2008; Porru 2001; Ribeiro 2008; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012; Tobia 2008; van Kampen 1998; Wille 2003; Yamanishi 2006; Yokoyama 2004);
active treatment (Ahmed 2012; Dijkstra‐Eshuis 2013; Floratos 2002; Fode 2014; Geraerts 2013; Ghanem 2013; Goode 2009; Hoffman 2005; Joseph 2000; Koo 2009; Laurienzo 2013; Moore 1999; Seleme 2008; Zhang 2007).
Types of outcome measures
There was a lack of consistency in the reporting of outcome measures. In terms of the primary outcomes of interest in this review these included:
number of men with incontinence, for radical surgery (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Filocamo 2005; Floratos 2002; Fode 2014; Franke 1998; Geraerts 2013; Ghanem 2013; Glazener RP 2011; Goode 2009; Manassero 2007; Marchiori 2010; Mariotti 2009; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Morihiro 2011; Opsomer 1994; Overgard 2008; Parekh 2003; Park 2012; Tobia 2008; van Kampen 1998; Yamanishi 2006) and for TURP (Glazener TURP 2011; Porru 2001; Tibaek 2007; Tienforti 2012);
number not cured (Zhang 2007) (assumed to indicate number of incontinent men);
time until continent (Fode 2014; Marchiori 2010; Mariotti 2009);
number of pad changes over 24 hours (Floratos 2002; Koo 2009; Mathewson‐Chapman 97; Ribeiro 2008; Tienforti 2012);
number of men using pads (Glazener RP 2011; Glazener TURP 2011);
number of incontinence episodes per day (Glazener RP 2011; Glazener TURP 2011; Goode 2009; Tienforti 2012);
pad test weights, grams of urine lost in 24 hours (Ahmed 2012; Geraerts 2013; Joseph 2000; Koo 2009; Mariotti 2009; Mathewson‐Chapman 97; Moore 1999; Moore 2008; Overgard 2008; Park 2012; Ribeiro 2008; Yamanishi 2006), 1 hour (Floratos 2002; Geraerts 2013; Hoffman 2005), 20 minutes (Wille 2003);
number with severe incontinence (pad test weight > 150 g) (Centemero 2009);
quality of life (condition‐specific such as incontinence scores): ICIQ‐SF score (Centemero 2009; Glazener RP 2011; Glazener TURP 2011; Park 2012; Ribeiro 2008; Yamanishi 2006), severity of UI (Zhang 2007), I‐QoL (Seleme 2008), ICI‐Q‐SF (Liu 2008), IIQ (Ahmed 2012; Ribeiro 2008), ICIQ‐SF QoL score (Glazener RP 2011; Glazener TURP 2011; Yamanishi 2006), EPIC‐UI (Goode 2009), KHQ (Dijkstra‐Eshuis 2013);
pelvic floor muscle strength (Overgard 2008);
carrying out PFMT or compliance (Glazener RP 2011; Glazener TURP 2011; Goode 2009; Overgard 2008; Zhang 2007).
Excluded studies
In total 36 studies were excluded. The majority of the studies that did not meet the inclusion criteria were excluded because the study design was not appropriate or the intervention was not relevant for the population of interest. See the Excluded studies table for a more detailed description.
Risk of bias in included studies
The assessment criteria of The Cochrane Collaboration assume that the avoidance of bias is best achieved by: a randomised trial with an adequate method of random sequence generation; secure concealment of allocation prior to formal entry; adequate blinding of patients, healthcare providers and outcome assessors; description of reasons and numbers of withdrawals and dropouts; and analysis on an intention‐to‐treat basis. None of the early trials fulfilled all these criteria. However recent trials have fared much better in terms of secure concealment of allocation and blinding but overall this continues to be problematic in many trials (Figure 2; Figure 3).
2.
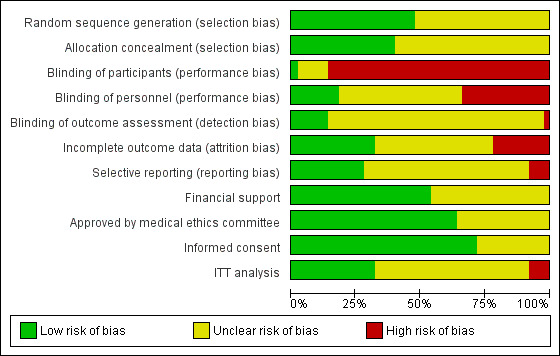
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
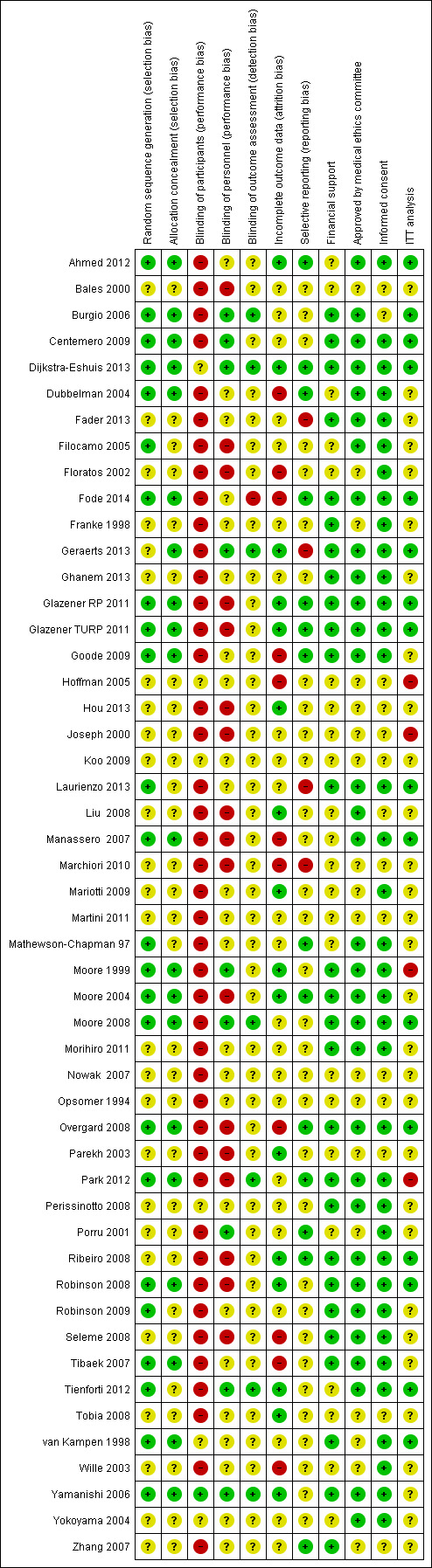
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation
Although all trials were identified as RCTs only 24 trials (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Filocamo 2005; Fode 2014; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Manassero 2007; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012; van Kampen 1998; Yamanishi 2006) described a method of adequate sequence generation (for example computer generated random numbers) and were assessed as low risk of bias. The remainder did not provide enough information to make a judgement and were assessed as unclear.
Allocation concealment
Only 20 trials (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Fode 2014; Geraerts 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Manassero 2007; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Tibaek 2007; van Kampen 1998; Yamanishi 2006) adequately described a technique of allocation concealment (for example sealed envelopes or computerised randomisation) and were assessed as low risk of bias. The remainder did not provide enough information to make a judgement and were assessed as unclear.
Blinding
Blinding was not described in most trials. In complex interventions such as physical therapy it is not possible to blind either the clinicians or the participants from the intervention, however, if blinding did not take place in trials they were judged to be at high risk of bias. This may have an impact on the outcome of interest and was considered while assessing the quality of evidence. Yamanishi 2006 used a sham device for the control group and this was the only trial that was deemed to be at low risk of bias in terms of blinding of participants.
In terms of blinding of personnel:
9 trials (Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Geraerts 2013; Moore 1999; Moore 2008; Porru 2001; Tienforti 2012; Yamanishi 2006) were deemed to be at low risk of bias;
17 trials (Bales 2000; Filocamo 2005; Floratos 2002; Glazener RP 2011; Glazener TURP 2011; Hou 2013; Joseph 2000; Liu 2008; Manassero 2007; Marchiori 2010; Moore 2004; Overgard 2008; Parekh 2003; Park 2012; Ribeiro 2008; Robinson 2008; Seleme 2008) were deemed to be at high risk of bias; and
23 trials (Ahmed 2012; Dubbelman 2004; Fader 2013; Fode 2014; Franke 1998; Ghanem 2013; Goode 2009; Hoffman 2005; Koo 2009; Laurienzo 2013; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Morihiro 2011; Nowak 2007; Opsomer 1994; Perissinotto 2008; Robinson 2009; Tobia 2008; van Kampen 1998; Wille 2003; Yokoyama 2004; Zhang 2007) were at unclear risk.
Burgio 2006; Moore 1999 and Moore 2008 indicated that a single therapist, blinded to control group outcomes, provided all treatment. Dijkstra‐Eshuis 2013 and Geraerts 2013 reported that the post‐operative physiotherapist was blinded to allocation and physical therapy provided by the pre‐operative therapist.
In terms of blinding of outcome assessment:
7 trials (Burgio 2006; Dijkstra‐Eshuis 2013; Geraerts 2013; Moore 2008; Park 2012; Tienforti 2012; Yamanishi 2006) were deemed to be at low risk of bias because they had outcome assessors who were not involved in the provision of the intervention or were not aware of allocation when entering data;
1 trial (Fode 2014) was found to be at high risk of bias; and
42 trials (Ahmed 2012; Bales 2000; Centemero 2009; Dubbelman 2004; Fader 2013; Filocamo 2005; Floratos 2002; Franke 1998; Ghanem 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Hoffman 2005; Hou 2013; Joseph 2000; Koo 2009; Laurienzo 2013; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Morihiro 2011; Nowak 2007; Opsomer 1994; Overgard 2008; Parekh 2003; Perissinotto 2008; Porru 2001; Ribeiro 2008; Robinson 2008; Robinson 2009; Seleme 2008; Tibaek 2007; Tobia 2008; van Kampen 1998; Wille 2003; Yokoyama 2004; Zhang 2007) were at unclear risk because this information was not provided.
Yamanishi 2006 used a sham device for the control group but there was no statement of whether assessors were aware of this or not.
Incomplete outcome data
Several trials gave no description or did not report dropouts (Centemero 2009; Ghanem 2013; Koo 2009; Marchiori 2010; Morihiro 2011; Perissinotto 2008; Ribeiro 2008; Robinson 2009; Seleme 2008; Yamanishi 2006; Yokoyama 2004), or did not have withdrawals or dropouts (Bales 2000; Liu 2008; Moore 2004; Tobia 2008).
All others reported the number of withdrawals or dropouts, although the reasons were not consistently reported and few, except Moore 2008 and Robinson 2008, discussed how this was dealt with in the analysis. In one trial, outcomes beyond eight weeks were not available for the control group because all the men were treated, and data were not available for over a third of the men in the other two intervention groups (Goode 2009). Two trials were thought to be at risk of bias because of differential dropout from the randomised groups (Dubbelman 2004; Manassero 2007). One trial (Marchiori 2010) that was judged to be at high risk of bias reported that the survey questionnaire used for one of their outcomes was completed correctly but returned by fewer than 10% of the men.
Six trials (Fader 2013; Martini 2011; Nowak 2007; Perissinotto 2008; Robinson 2008; Robinson 2009) did not provide any usable data. Three of these trials (Nowak 2007; Perissinotto 2008; Robinson 2009) did not report how many men were randomised to each group.
Selective reporting
There was significant difficulty in assessing selective outcome reporting because the protocols for most of the included trials were not available for assessment or could not be found. For a few of the trials, data were not available for some of the outcomes stated in the methods section.
Other potential sources of bias
Information about funding was available for 27 of the included studies (Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Fader 2013; Fode 2014; Franke 1998; Geraerts 2013; Ghanem 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Moore 1999; Moore 2004; Moore 2008; Morihiro 2011; Overgard 2008; Park 2012; Perissinotto 2008; Ribeiro 2008; Robinson 2008; Robinson 2009; Seleme 2008; Tibaek 2007; van Kampen 1998; Yamanishi 2006; Zhang 2007) and the studies were judged to be at low risk of bias. The rest of the trials were judged to be at unclear risk of bias because there was a lack of information even after contacting the authors.
Thirty‐two trials reported obtaining approval from a medical ethics committee (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Fader 2013; Filocamo 2005; Fode 2014; Geraerts 2013; Ghanem 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Liu 2008; Manassero 2007; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Moore 2008; Morihiro 2011; Overgard 2008; Park 2012; Perissinotto 2008; Ribeiro 2008; Robinson 2008; Robinson 2009; Seleme 2008; Tibaek 2007; Tienforti 2012; Yamanishi 2006; Yokoyama 2004) and were judged to be at low risk of bias. The remaining 18 trials did not report their source of medical ethical approval and were judged to be at unclear risk of bias after no further information was provided by the authors.
Fourteen trials were deemed to be at unclear risk of bias in terms of obtaining informed consent (Bales 2000; Burgio 2006; Hoffman 2005; Hou 2013; Joseph 2000; Koo 2009; Liu 2008; Marchiori 2010; Martini 2011; Nowak 2007; Opsomer 1994; Parekh 2003; Tobia 2008; Zhang 2007). These authors were contacted but no further information on this matter was provided. The other trials did report obtaining informed consent from patients and therefore were deemed to be at low risk of bias for this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12
Radical prostatectomy: treatment of incontinent men after surgery
1. Treatment of UI after radical prostatectomy: post‐operative PFMT with or without biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 1)
Nine trials (Dubbelman 2004; Floratos 2002; Franke 1998; Manassero 2007; Glazener RP 2011; Goode 2009; Moore 1999; Moore 2008; van Kampen 1998) compared PFMT with or without biofeedback to no treatment (sham or verbal instruction) amongst men who had UI after radical prostatectomy. The quality of the evidence is given in Table 1.
Differences between trials
All the men were incontinent at baseline.
In one trial (Manassero 2007) there was evidence of unexplained differential dropout from the control group (13 of 53 men, while there were no dropouts from the 54 in the intervention group). The missing men have therefore been assumed to be dry for the purpose of an intention‐to‐treat analysis. The other trials have been analysed as reported since dropouts (if any) were balanced between the groups.
Sources of heterogeneity
(1) Definition of incontinence varied with each trial:
more than 1 g urine on one hour pad test (Dubbelman 2004);
more than 8 g urine loss on 24 hour pad test (Moore 2008);
more than 2 g urine loss on one hour (van Kampen 1998) or 24 hour pad test (Moore 1999);
men who were not pad free (Franke 1998);
a visual analogue score of 10 = completely incontinent and 0 = completely continent (Manassero 2007); or
no leakage based on bladder diaries (Goode 2009).
(2) The type of PFMT regimens differed between the trials:
four trials (Dubbelman 2004; Goode 2009; Manassero 2007; Moore 1999) evaluated PFMT alone (without biofeedback);
three trials evaluated PFMT with biofeedback, verbal instruction (Manassero 2007; Moore 2008) or ES (van Kampen 1998);
two trials (Floratos 2002; Franke 1998) used PFMT with biofeedback via a perineal patch (surface) EMG.
Formal PFMT post‐operative sessions directed by a therapist ranged from: twice a week for 12 weeks (Moore 1999); three times a week for three weeks (Floratos 2002); in up to nine sessions (Dubbelman 2004); weekly for 24 weeks (Moore 2008); four sessions over eight weeks (Goode 2009); five sessions over 16 weeks (Franke 1998); to as long as the UI persisted (van Kampen 1998). Men received only four therapy sessions in three months in one of these trials (Glazener RP 2011) and men in another trial were seen weekly for up to six months (Moore 2008).
(3) Control interventions differed between the trials and included:
information (verbal or written) about PFMT only (Dubbelman 2004; Floratos 2002; Moore 1999; Moore 2008);
no treatment (Manassero 2007);
sham placebo PFMT and contact with therapist (van Kampen 1998);
monitoring of UI only (e.g. by bladder diary or phone calls) (Franke 1998; Goode 2009).
(4) The participants differed between the trials.
Two trials (Goode 2009; Moore 1999) recruited participants with persistent incontinence (some longer than one year) post‐operatively, and these participants may have differed from those enrolled pre‐operatively (Moore 2008) but still incontinent at four weeks after surgery) or from those recruited within a week or two of catheter removal (Dubbelman 2004;Floratos 2002;Glazener RP 2011; Manassero 2007; van Kampen 1998) or up to six weeks after radical prostatectomy (Franke 1998).
Incontinence in men and incontinence episodes
Because there was evidence of significant statistical heterogeneity between the trials included in this comparison (see below), meta‐analysis was carried out using a random‐effects model, therefore widening the CI. There were no significant differences at any time period in the UI rates, and the CIs were wide (for example RR for UI up to 12 months 0.91, 95% CI 0.73 to 1.14, Analysis 1.1.3; and after 12 months 57% with UI versus 62% in the control group, RR 0.85, 95% CI 0.60 to 1.22, Analysis 1.1.4). Only two trials (Manassero 2007; van Kampen 1998) favoured the treatment and of these, only one (van Kampen 1998) used biofeedback. The estimates from the other trials had CIs that did not rule out clinically important effects. Overall, as one of the pre‐defined GRADE‐specific outcomes, the quality of evidence for the outcome 'number of incontinent men after 12 months' was found to be moderate.
1.1. Analysis.
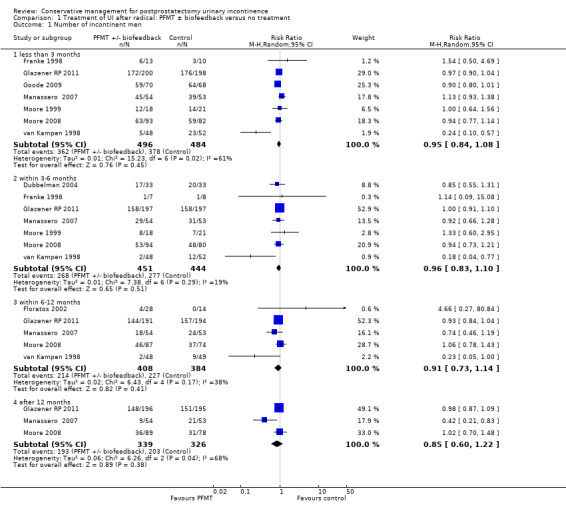
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.
The meta‐analysis was dominated by the Glazener RP 2011 trial, which was a large pragmatic multi‐centre trial conducted in a context where information on PFMT was widely available. This showed no good evidence to support one‐to‐one training by a therapist (for example RR for UI after 12 months 0.98, 95% CI 0.87 to 1.09, Analysis 1.1.4) (Glazener RP 2011). This one large trial had narrow CIs which did not include a clinically significant difference, pre‐specified to be 15%. One other trial (Moore 2008) was in line with the Glazener RP 2011 findings but had wider CIs (RR 1.02, 95% CI 0.70 to 1.48, Analysis 1.1.4) (Moore 2008).
In one large trial (Glazener RP 2011), men did not report differences in UI episodes at any time period, based on urinary diary data (for example after 12 months MD 0.1, 95% CI ‐0.82 to 1.02, Analysis 1.2.4). Alternatively, one trial (Goode 2009) did report a significant difference, however this measurement was obtained at less than 3 months (MD ‐1.14, 95% CI ‐1.46 to ‐0.82, Analysis 1.2.1).
1.2. Analysis.

Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day.
Use of pads
Use of pads could be considered to be a measure of more severe incontinence. There was no statistically significant difference in the number of men using pads in one large trial (40% in intervention group versus 42% in control group after 12 months, RR 0.94, 95% CI 0.72 to 1.22, Analysis 1.3) (Glazener RP 2011). Floratos 2002 used number of pad changes over 24 hours as the outcome measure, with no statistically significant difference in the MD between treatment and control groups at any time period (Analysis 1.4).
1.3. Analysis.
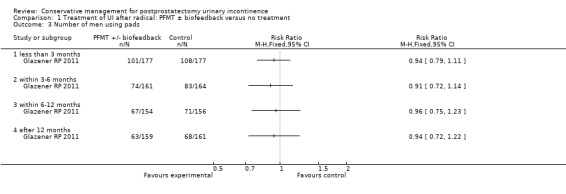
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads.
1.4. Analysis.
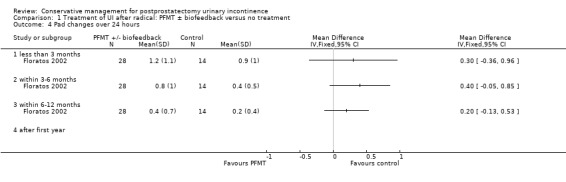
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 Pad changes over 24 hours.
Urinary incontinence score and effect on quality of life
In one large trial (Glazener RP 2011), there was no evidence of a difference in the ICIQ‐SF (a composite score of frequency, amount and effect of UI on quality of life) at any time period after the intervention up to or beyond one year (MD after 12 months ‐0.5, 95% CI ‐1.35 to 0.35, Analysis 1.5) or quality of life as a single score from 0 to 10 (MD ‐0.30, 95% CI ‐0.73 to 0.13, Analysis 1.6), however the quality of evidence for this outcome was found to be low.
1.5. Analysis.
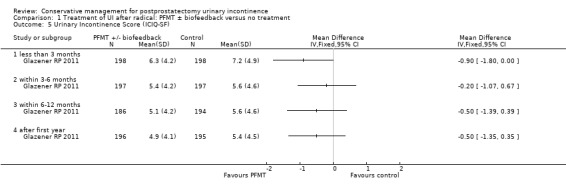
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Urinary Incontinence Score (ICIQ‐SF).
1.6. Analysis.
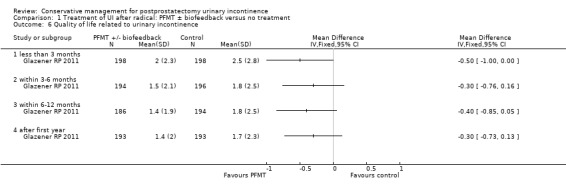
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Quality of life related to urinary incontinence.
Pad tests
Two trials (Moore 1999; Moore 2008) reported 24 hour pad test results and one (Floratos 2002) reported a one hour pad test. Dubbelman 2004 and van Kampen 1998 also measured urine loss on a 24 hour pad test, but did not report SDs and therefore these data could not be included in the meta‐analysis. Amongst the two trials which gave 24 hour pad test data, there were no statistically significant differences between the groups at 3, 6 or 12 months, or after 12 months (Analysis 1.8). Similarly, using a one hour pad test (Floratos 2002), there were no statistically significant differences between the groups up to six months (Analysis 1.9). In the smaller trials (Floratos 2002; Moore 1999; Moore 2008) the SDs were often larger than the means, suggesting highly skewed data.
1.8. Analysis.
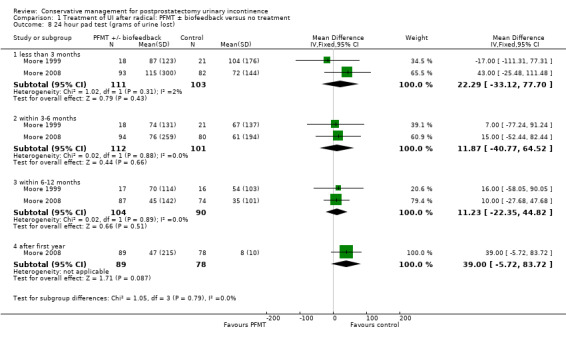
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 24 hour pad test (grams of urine lost).
1.9. Analysis.
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 1 hour pad test (grams of urine lost).
2. Treatment of UI after radical prostatectomy: post‐operative interventions using electric or magnetic energy (for example post‐operative anal ES, perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI) versus no treatment or sham treatment (Comparison 2)
Four trials were identified which addressed this comparison (Marchiori 2010; Moore 1999; Morihiro 2011; Yamanishi 2006). These trials compared anal ES with oral (verbal) PFMT. The control group in Moore's trial received oral information about PFMT only, whereas in Yamanishi's trial the control group also received sham ES. The quality of the evidence is given in Table 2.
Number of incontinent men
In the short term (less than three months), there were fewer incontinent men in the intervention groups in two trials (64% versus 84% in the control groups, RR 0.77, 95% CI 0.60 to 0.98, Analysis 2.1.1) (Moore 1999; Yamanishi 2006) and the quality of the evidence for this outcome was deemed to be moderate. This remained the same at 6 to 12 months (19% versus 53% in the control groups, RR 0.37, 95% CI 0.18 to 0.73, Analysis 2.1.3) and after 12 months (7% versus 33% in the control groups of three trials, RR 0.26, 95% CI 0.09 to 0.74). However, the data were too few to be reliable in the longer term.
2.1. Analysis.
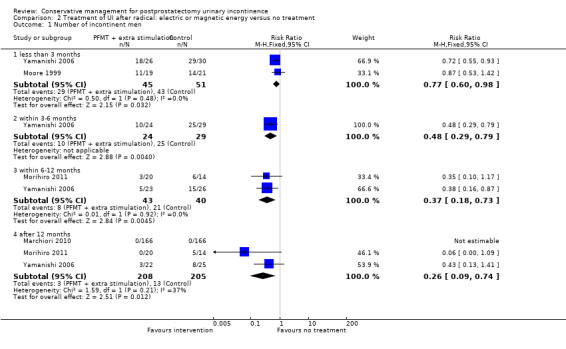
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 Number of incontinent men.
Adverse effects
One small trial (Yamanishi 2006) reported adverse effects, with two men in the active ES group and four men in the group receiving sham treatment reporting anal pain or discomfort. No statistically significant differences were found between the groups (RR 0.58, 95% CI 0.11 to 2.90, Analysis 2.2).
2.2. Analysis.
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 Adverse effects.
Pad test
There were no statistically significant differences between the groups on grams of urine lost (24 hour pad test) at any of the time points (Analysis 2.3). SSs were large, indicating skewed distribution of data, and the CIs were wide with evidence of significant statistical heterogeneity.
2.3. Analysis.
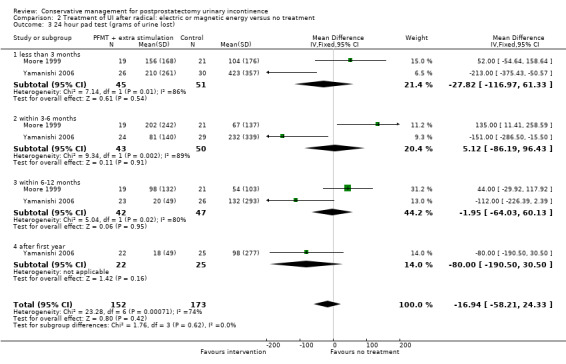
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 3 24 hour pad test (grams of urine lost).
UI score
Men in the intervention group in one trial (Yamanishi 2006) had lower (better) UI scores using a quality of life outcome combined with amount and frequency of urine lost (for example MD ‐3.9, 95% CI ‐7.15 to ‐0.65, Analysis 2.4.3, at one year) though this did not quite reach statistical significance when quality of life was analysed on its own (MD ‐0.40, 95% CI ‐2.02 to 1.22, Analysis 2.5).
2.4. Analysis.
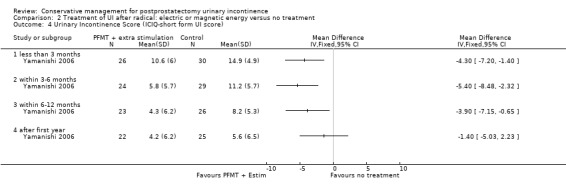
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 4 Urinary Incontinence Score (ICIQ‐short form UI score).
2.5. Analysis.
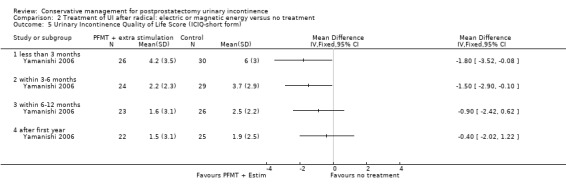
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form).
Time until continence achieved
Men achieved continence on average about 5 months sooner in the intervention group of one trial (MD ‐4.11 months, 95% CI ‐6 to ‐2.23, Analysis 2.6) (Yamanishi 2006).
2.6. Analysis.
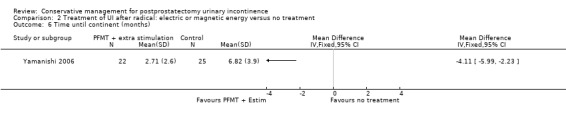
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 6 Time until continent (months).
3. Treatment of UI after radical prostatectomy: post‐operative lifestyle adjustment versus no treatment or sham treatment (Comparison 3)
No trials were identified.
4. Treatment of UI after radical prostatectomy: post‐operative combinations of treatments versus no treatment or sham treatment (Comparison 4)
Two trials reported using PFMT with anal ES as well as biofeedback (Goode 2009; Opsomer 1994) versus control management. Goode 2009 compared behavioural therapy comprising biofeedback and ES for eight weeks with a control group. Opsomer 1994 treated incontinent men in the intervention group with two sessions of ES with biofeedback as well as continuing the PFMT taught to both groups at six weeks after radical prostatectomy. The quality of the evidence is given in Table 3.
Number of incontinent men
Goode 2009 reported fewer incontinent men in the intervention group compared with the control group (83% versus 94% in the control group at less than 3 months, RR 0.88, 95% CI 0.78 to 0.99, Analysis 4.1.1). In the other trial (Opsomer 1994), four men in total had incontinence at 3 to 6 months, with 3/20 in the intervention group and 1/19 in the control group, but this was not statistically significant (RR 2.85, 95% CI 0.32 to 25.07, Analysis 4.2). Overall, the quality of evidence for this outcome was very low.
4.1. Analysis.
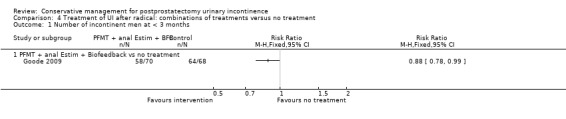
Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men at < 3 months.
4.2. Analysis.
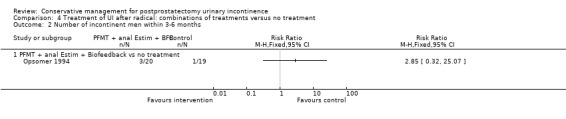
Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 3‐6 months.
Adverse events
There were two adverse events (haemorrhoidal irritation) reported by men receiving ES in one trial (Goode 2009), and the quality of evidence for this outcome was deemed to be of low quality with wide CIs indicating uncertainty (RR 4.86, 95% CI 0.24 to 99.39, Analysis 4.4.1).
4.4. Analysis.
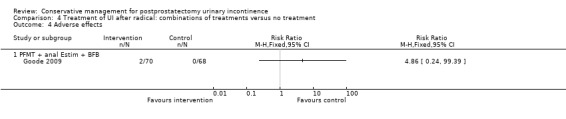
Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 4 Adverse effects.
5. Treatment of UI after radical prostatectomy: post‐operative use of one treatment versus another active treatment (Comparison 5)
Nine trials comparing one active treatment to another were identified (Floratos 2002; Goode 2009; Hoffman 2005; Joseph 2000; Koo 2009; Moore 1999; Seleme 2008; Yokoyama 2004; Zhang 2007).
PFMT plus anal ES (EStim) (Hoffman 2005; Moore 1999).
PFMT plus perineal ES (EStim) (Hoffman 2005).
PFMT plus visual biofeedback (Joseph 2000; Zhang 2007).
PFMT plus visual biofeedback plus support group (Zhang 2007).
PFMT plus oral (verbal) biofeedback (Joseph 2000).
PFMT plus biofeedback plus ES(Estim) (Goode 2009; Seleme 2008).
PFMT alone (Goode 2009; Hoffman 2005; Koo 2009; Moore 1999; Seleme 2008).
Extra‐corporeal Magnetic Innervation (ExMI) (Koo 2009).
The quality of the evidence is given in Table 4.
Number of incontinent men
Four small trials provided data for this outcome (Goode 2009; Moore 1999; Yokoyama 2004; Zhang 2007). The definition of incontinence varied with each trial:
no urine loss recorded in bladder diaries (Goode 2009);
less than 8 g urine loss on 24 hour pad test (Moore 1999);
'urine loss' (Yokoyama 2004); and
use of pad or brief (Zhang 2007).
There was no difference in the incontinence rates in the trials at any time period, but CIs were wide, up to 3 months (RR 0.96, 95% CI 0.83 to 1.12, Analysis 5.1); 3 to 6 months (RR 0.59, 95% CI 0.33 to 1.05, Analysis 5.2); 6 to 12 months (RR 2, 95% CI 0.21 to 18.23, Analysis 5.3) and the quality of evidence was deemed to be of very low quality.
5.1. Analysis.
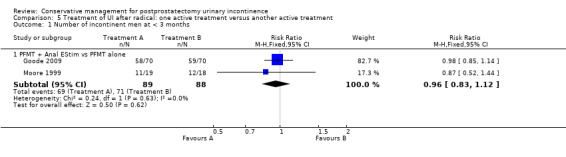
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3 months.
5.2. Analysis.
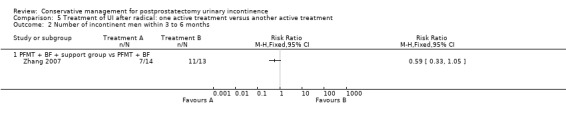
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months.
5.3. Analysis.
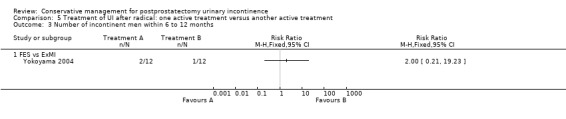
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months.
Pad tests
For the majority of the comparisons there were no statistically significant differences between the groups, SDs were large, indicating skewed distribution of data, and the CIs were wide.
However, men having extra‐corporeal magnetic innervation (ExMI) compared to PFMT alone had less urine loss on the 24 hour pad test at 3 to 6 months in one small trial (Koo 2009) (compared to PFMT alone, MD ‐36 g, 95% CI ‐55 to ‐17, Analysis 5.12.3) and used fewer pads per day (MD ‐0.5, 95% CI ‐0.79 to ‐0.21, Analysis 5.13.1) (Koo 2009).
5.12. Analysis.
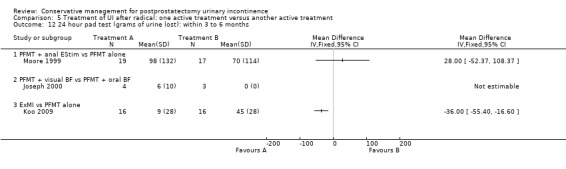
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost): within 3 to 6 months.
5.13. Analysis.
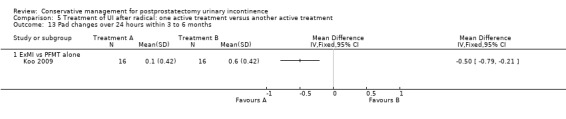
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 13 Pad changes over 24 hours within 3 to 6 months.
Quality of life
In another small trial (Seleme 2008) men receiving PFMT plus biofeedback plus ES reported better quality of life using the Incontinence Quality of life score than those receiving PFMT alone (MD ‐28.63, 95% CI ‐34.60 to ‐22.66, Analysis 5.6.1).
5.6. Analysis.
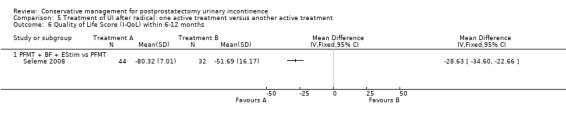
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 6 Quality of Life Score (I‐QoL) within 6‐12 months.
In a third trial (Liu 2008), PFMT supplemented by extra‐corporeal magnetic innervation (ExMI) seemed to be better than PFMT alone in terms of quality of life assessed using the ICIQ‐SF score (MD ‐1.60, 95% CI ‐2.73 to ‐0.47, Analysis 5.7.1) but the quality of the evidence for this outcome was judged to be of low quality.
5.7. Analysis.
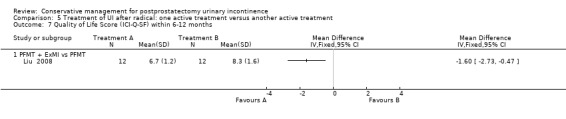
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months.
Adverse events
Two men in one trial (Goode 2009) had an adverse event with ES (haemorrhoidal irritation, RR 5, 95% CI 0.24 to 102.30, Analysis 5.8.1) but the evidence for this outcome was judged to be of low quality.
5.8. Analysis.
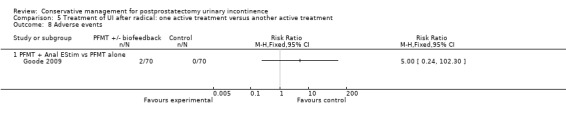
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 8 Adverse events.
Radical prostatectomy: prevention of UI in all men having surgery, intervention before or after prostatectomy or both
6. Prevention of UI after radical prostatectomy: PFMT ± biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 6)
Ten trials addressed this comparison (Bales 2000; Burgio 2006; Filocamo 2005; Laurienzo 2013; Mathewson‐Chapman 97; Overgard 2008; Parekh 2003; Ribeiro 2008; Tienforti 2012; Tobia 2008). The quality of the evidence is given in Table 5.
Differences between trials
The participants were not selected because they were incontinent so included a mixed population of men with and without incontinence after surgery.
Sources of heterogeneity
(1) The type of PFMT regimens differed between the trials:
PFMT plus biofeedback (Bales 2000; Burgio 2006; Laurienzo 2013; Mathewson‐Chapman 97; Parekh 2003; Ribeiro 2008; Tienforti 2012);
PFMT alone (Filocamo 2005; Overgard 2008; Tobia 2008).
Biofeedback was delivered via surface electrodes (Bales 2000) or via a digital or anal probe (Burgio 2006; Mathewson‐Chapman 97; Parekh 2003; Tienforti 2012). In one trial (Ribeiro 2008) the type of biofeedback was not described.
(2) Control interventions differed between the trials and included:
no treatment (Filocamo 2005; Mathewson‐Chapman 97; Parekh 2003; Tobia 2008);
post‐operative verbal or written instruction on PFMT only (Bales 2000; Laurienzo 2013; Overgard 2008; Ribeiro 2008; Tienforti 2012);
usual care with simple instructions to interrupt the stream when voiding (Burgio 2006).
(3) The timing of the interventions relative to surgery also varied:
two trials delivered an intervention before surgery only (Laurienzo 2013; Tobia 2008);
five trials delivered their intervention before and after surgery (Bales 2000; Burgio 2006; Parekh 2003; Mathewson‐Chapman 97; Tienforti 2012);
three trials delivered their intervention after surgery only (Filocamo 2005; Overgard 2008; Ribeiro 2008).
Number of men with UI
Data describing UI were reported by 8 of the 10 trials. While there was no statistically significant difference at 3 months (Analysis 6.1.1), there was evidence from the findings of this systematic review of an overall benefit from PFMT in the number of men with UI within 6 to 12 months (24% versus 52%, RR 0.51, 95% CI 0.35 to 0.75, Analysis 6.1.3) and after 1 year (10% versus 32%, RR 0.32, 95% CI 0.20 to 0.51, Analysis 6.1.4), but the quality of evidence was judged to be moderate. The data were driven mainly by two trials (Filocamo 2005; Overgard 2008), neither of which included biofeedback. One of these trials did not disclose details of allocation concealment (Filocamo 2005) and the other was small (Overgard 2008). The remaining trials showed conflicting results and there was statistically significant heterogeneity, hence the use of a random‐effects model.
6.1. Analysis.
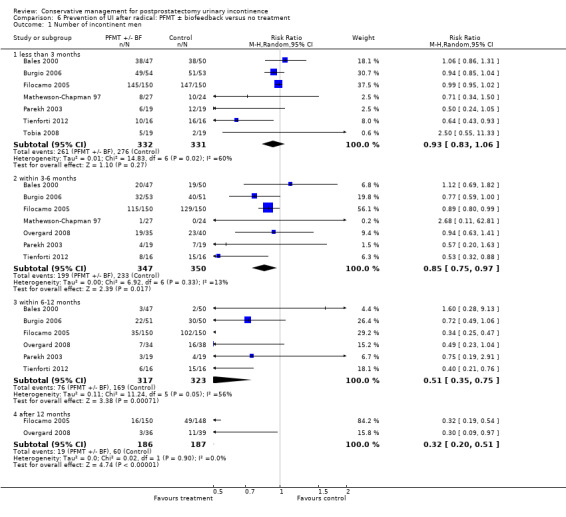
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.
Pad changes and pad tests
In the four trials which reported these outcomes (Filocamo 2005; Mathewson‐Chapman 97; Overgard 2008; Ribeiro 2008) there was statistical heterogeneity. One small trial favoured PFMT (Ribeiro 2008) but using a random‐effects model there was only a significant difference at 6 to 12 months (MD ‐15 g less urine loss on 24 hour pad test with treatment, 95% CI ‐18 to ‐11, Analysis 6.4.3). Men in the intervention group in this trial received PFMT plus biofeedback weekly for three months until they were continent or until three months. The findings from the Filocamo 2005 and Overgard 2008 trials (no significant difference in pad weights) was in contrast to their report of fewer incontinent men with active treatment (RR 0.32, 95% CI0.20 to 0.51, Analysis 6.1.4). However, the SDs were large and the CIs were wide. Laurienzo 2013 did not find a significant difference up to 12 months when using a 1 hour pad test (MD 19.80, 95% CI ‐9.15 to 48.75, Analysis 6.3) and comparing PFMT with no standard treatment.
6.4. Analysis.
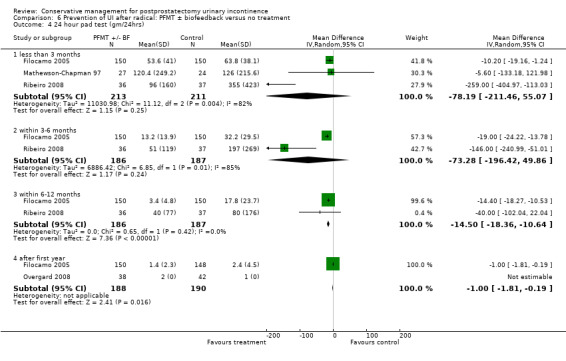
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 24 hour pad test (gm/24hrs).
6.3. Analysis.
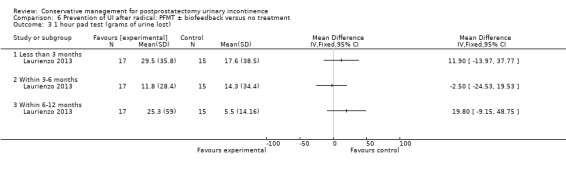
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 1 hour pad test (grams of urine lost).
Mean number of incontinence episodes per day
Tienforti 2012 favoured PFMT at all time points (MD ‐1.43, 95% CI ‐2.35 to ‐0.51, Analysis 6.5) when quantifying episodes of UI in men each day, with men in the intervention group suffering fewer mean numbers of episodes.
6.5. Analysis.
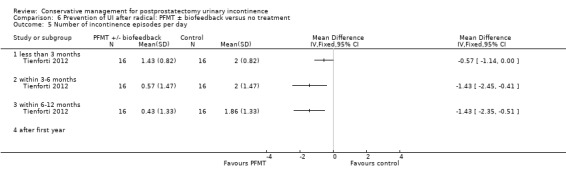
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Number of incontinence episodes per day.
Quality of life
Quality of life was assessed using the ICIQ‐SF by two trials (Laurienzo 2013; Ribeiro 2008) but the quality of the evidence was found to be very low. No significant difference was found within 6 to 12 months (MD ‐0.69, 95% CI ‐3.19 to 1.81, Analysis 6.6).
6.6. Analysis.
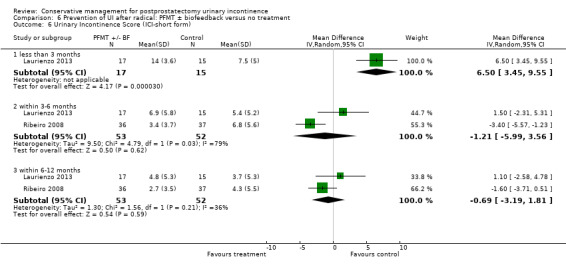
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Urinary Incontinence Score (ICI‐short form).
Ribeiro 2008 also assessed quality of life using the IIQ, favouring the intervention at 3 to 6 months (MD ‐2.70, 95% CI ‐4.88 to ‐0.52, Analysis 6.7).
6.7. Analysis.
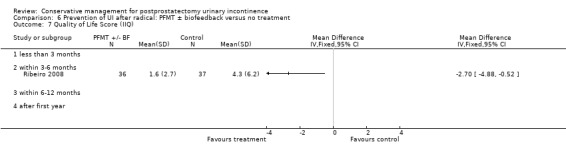
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Quality of Life Score (IIQ).
7. Prevention of UI after radical prostatectomy: electric or magnetic energy (for example anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment (Comparison 7)
One small trial that delivered the intervention pre‐operatively only was identified (Laurienzo 2013). There was no significant difference using a 1 hour pad test at 6 to 12 months (MD ‐1.15, 95% CI ‐9.11 to 6.81, Analysis 7.1) or when assessing quality of life using the ICIQ‐SF (MD 1.60, 95% CI ‐2.15 to 5.35, Analysis 7.2), but the quality of evidence for this outcome was judged to be very low (Table 6).
7.1. Analysis.
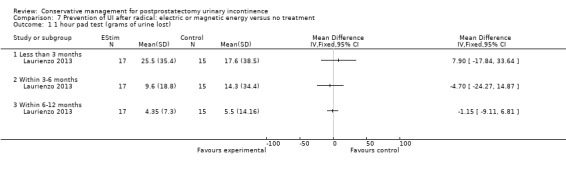
Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 1 hour pad test (grams of urine lost).
7.2. Analysis.
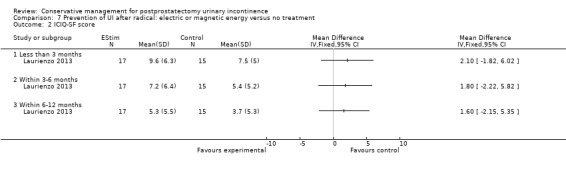
Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 ICIQ‐SF score.
8. Prevention of UI after radical prostatectomy: lifestyle interventions versus no treatment or sham treatment (Comparison 8)
No trials were identified.
9. Prevention of UI after radical prostatectomy: combinations of treatments versus no treatment or sham treatment (Comparison 9)
Only one small trial (Mariotti 2009) looked at this comparison. Men in the intervention group received PFMT plus ES with biofeedback post‐operatively and men in the control group received verbal and written instructions on PFMT. There was a statistical difference with regards to:
number of incontinent men within 6 to 12 months (RR 0.10, 95% CI 0.01 to 0.73, Analysis 9.2);
using a 24 hour pad test (MD ‐24.30, 95% CI ‐45.02 to ‐3.58, Analysis 9.4); and
time until UI was regained (MD ‐1.50, 95% CI ‐2.44 to ‐0.56, Analysis 9.5).
9.2. Analysis.
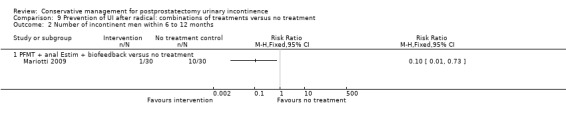
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 6 to 12 months.
9.4. Analysis.
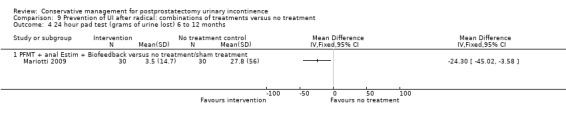
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 4 24 hour pad test (grams of urine lost) 6 to 12 months.
9.5. Analysis.
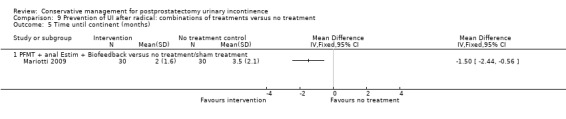
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 5 Time until continent (months).
However, the quality of evidence for the primary outcome (number of incontinent men) was found to be low (Table 7).
Adverse events
Adverse events were not reported.
10. Prevention of UI after radical prostatectomy: one treatment versus another active treatment (Comparison 10)
Eight trials were identified (Ahmed 2012; Centemero 2009; Dijkstra‐Eshuis 2013; Fode 2014; Geraerts 2013Nowak 2007; Park 2012; Wille 2003). Five of these were new in this update (Ahmed 2012; Dijkstra‐Eshuis 2013; Fode 2014; Geraerts 2013; Park 2012) and one was updated with new information (Centemero 2009).
Ahmed 2012 was a three‐armed trial, with patients receiving PFMT plus TENS with biofeedback or TENS only or guided PFMT only.
Centemero 2009 compared PFMT before and after surgery with PFMT delivered after surgery only.
Dijkstra‐Eshuis 2013 compared pre‐operative guided PFMT with biofeedback versus post‐operative written instructions on PFMT; however all men received PFMT plus biofeedback plus ES if they were still incontinent after six weeks.
Fode 2014 compared PFMT + penile vibration before and after surgery with PFMT alone before and after surgery: all men received a phosphodiesterase type 5 (PDE5) inhibitor after the first month.
Geraerts 2013 compared PFMT plus biofeedback versus active PFMT.
Nowak 2007 compared extra‐corporeal magnetic innervation (ExMI) versus PFMT alone but did not provide any useable data.
Park 2012 compared post‐operative PFMT plus general exercise versus post‐operative PFMT alone.
Wille 2003, a three‐arm trial, compared PFMT plus ES versus PFMT plus ES plus anal probe biofeedback versus PFMT alone.
The trials were generally small and few were similar enough to combine in a meta‐analysis. The quality of the evidence is illustrated in Table 8.
Number of incontinent men
This outcome was reported by six trials (Ahmed 2012; Centemero 2009; Dijkstra‐Eshuis 2013; Fode 2014; Ghanem 2013; Park 2012).
In one trial, Centemero 2009 reported fewer incontinent men at less than 3 months and within 3 to 6 months when PFMT was delivered pre and post‐operatively, compared with post‐operatively only, and this correlated with a statistically significant better quality of life score (MD ‐3.70, 95% CI ‐6.00 to ‐1.40, Analysis 10.15.1; MD ‐4.10, 95% CI ‐6.64 to ‐1.56, Analysis 10.16.1). However, when combined with the data from Geraerts 2013, who used the same interventions, there was no statistically significant difference between the interventions at 3 months (RR 0.86, 0.69 to 1.06, Analysis 10.1.1) or 3 to 6 months (RR 0.75, 95% CI 0.54 to 1.04, Analysis 10.2.1). It should be noted that the CIs were very wide.
10.15. Analysis.
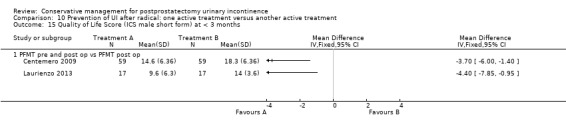
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 15 Quality of Life Score (ICS male short form) at < 3 months.
10.16. Analysis.
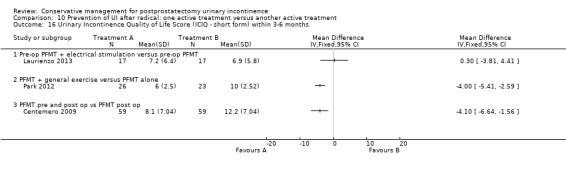
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months.
10.1. Analysis.
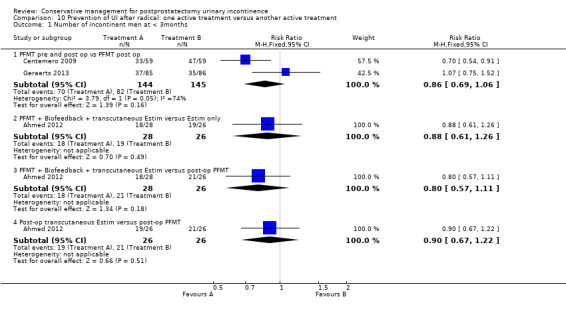
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3months.
10.2. Analysis.
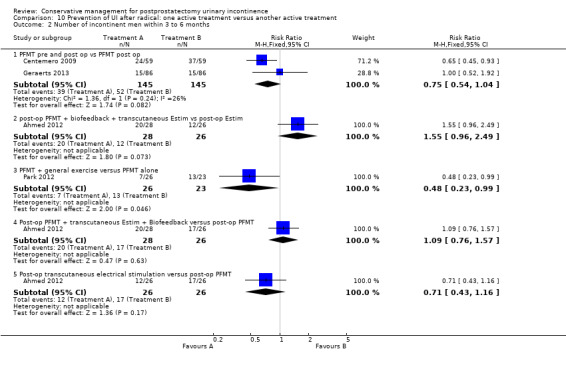
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months.
Ahmed 2012 compared three different treatments (PFMT plus transcutaneous electrical stimulation with biofeedback; TENS only; and guided PFMT only) and found no statistically significant differences between the interventions in terms of number of men with UI (Analysis 10.1, Analysis 10.2; Analysis 10.3) except that at 6 to 12 months PFMT plus ES plus biofeedback proved to be significantly better than PFMT only (RR 0.10, 95% CI 0.01 to 0.76, Analysis 10.3.3).
10.3. Analysis.
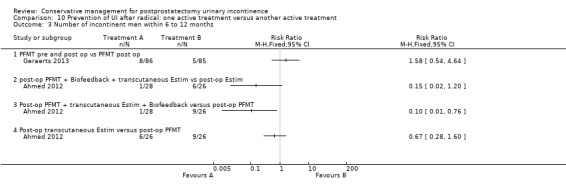
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months.
One small trial (Park 2012) found that general exercise added to PFMT was statistically significantly better than PFMT alone within 3 to 6 months (RR 0.48, 95% CI 0.23 to 0.99, Analysis 10.2.3).
Four trials reported the number of incontinent men after 12 months (Dijkstra‐Eshuis 2013;Fode 2014;Geraerts 2013;Ghanem 2013). The quality of the evidence was moderate (Table 8). Three of these trials, comparing pre and post‐operative PFMT to post‐operative PFMT only, found that more men were incontinent after 12 months when PFMT began before surgery (15.3% versus 10.7% with post‐operative training alone) but this did not reach statistical significance (RR 1.32, 95% CI 0.78 to 2.25, Analysis 10.4.1). The Fode 2014 study was too small to identify a difference between PFMT plus penile vibratory stimulation pre and post‐operatively compared with pre and post‐operative PFMT (Analysis 10.4.2).
10.4. Analysis.
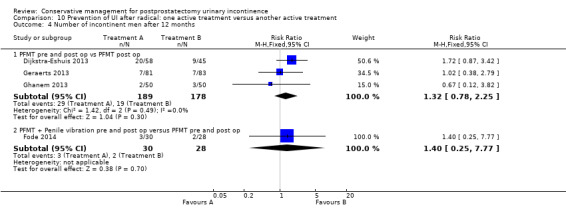
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinent men after 12 months.
Pad tests
In general, the short‐duration pad tests did not distinguish between the various interventions being compared, apart from in one trial. At 6 months (but not at 3 months), Wille 2003 found that PFMT plus anal ES both with and without extra biofeedback were both better than PFMT alone using a 20 minute pad test (MD urine lost ‐3 g, 95% CI ‐6 to ‐0.5 in both comparisons, Analysis 10.8.1 and Analysis 10.8.2), while there was little to choose between the two more intensive interventions (Analysis 10.8.3). However, the trial was small, the SDs large and the CIs wide.
10.8. Analysis.
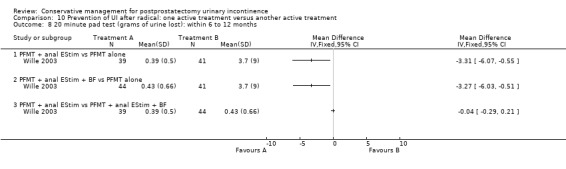
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 8 20 minute pad test (grams of urine lost): within 6 to 12 months.
Using a longer‐duration 24 hour pad test, the groups receiving ES were generally better than those only having PFMT or only having ES (Analysis 10.12; Analysis 10.13; Analysis 10.14) but the interventions were to dissimilar to combine. At three to six months, one small trial (Park 2012) did not find significant benefit when comparing PFMT plus general exercise with PFMT alone (Analysis 10.13.4).
10.12. Analysis.
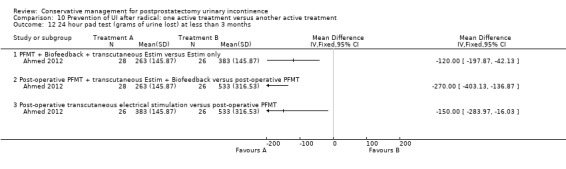
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost) at less than 3 months.
10.13. Analysis.
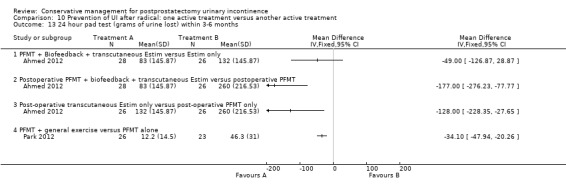
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 13 24 hour pad test (grams of urine lost) within 3‐6 months.
10.14. Analysis.
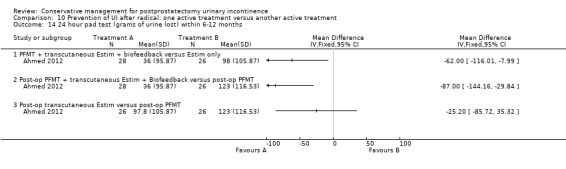
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 14 24 hour pad test (grams of urine lost) within 6‐12 months.
Quality of life
ICIQ‐SF
The ICIQ‐SF score is a mixed measure of both incontinence severity and effect on quality of life. One small trial (Park 2012) found that there was a significant benefit in terms of the ICIQ‐SF and the intervention PFMT plus general exercise versus PFMT alone, but the evidence for this outcome was found to be very low (MD in scores ‐4.00, 95% CI ‐5.41 to ‐2.59, Analysis 10.16.2).
King’s Health Questionnaire
For all domains of the King’s Health Questionnaire, Dijkstra‐Eshuis 2013 did not find a statistically significant difference between PFMT given pre and post‐operatively and PFMT given post‐operatively only (Analysis 10.18).
10.18. Analysis.
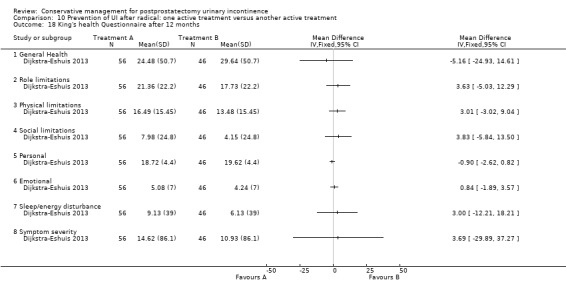
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 18 King's health Questionnaire after 12 months.
SF‐36
In contrast, one trial (Park 2012) found that the intervention PFMT with general exercise was favoured at 3 to 6 months when using the health status measure SF‐36 (MD ‐9.00, 95% CI ‐11.17 to ‐6.83, Analysis 10.19.1) compared with PFMT alone. This may have been more of a measure of an effect of exercise on general health than on incontinence itself.
10.19. Analysis.
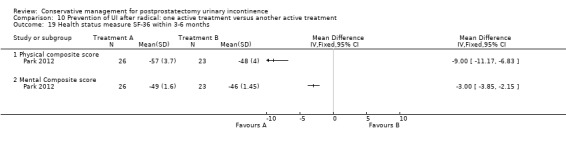
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 19 Health status measure SF‐36 within 3‐6 months.
Adverse events
One trial (Fode 2014) was in the meta‐analysis and the authors stated that 5/30 men reported adverse effects in the intervention group using the group with a penile vibratory stimulation device. The quality of evidence for this outcome was deemed to be low. Adverse effects included:
red spots on the glans penis;
small laceration with some bleeding;
soreness;
frank pain.
TURP: treatment of incontinent men, after surgery
11. Treatment of UI after TURP: PFMT ± biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 11)
One large trial compared PFMT with or without biofeedback to no treatment (sham or verbal instruction) amongst men who had UI after TURP (Glazener TURP 2011). All the men were incontinent at randomisation, six weeks after surgery, and received four one‐to‐one sessions with a trained therapist over a three month period. The quality of the evidence is illustrated in Table 11.
Incontinence in men and incontinence episodes
There were no significant differences at any time period in the incontinence rates (for example RR for incontinence up to 12 months 1.04, 95% CI 0.90 to 1.20, Analysis 11.1.3; and after 12 months, 65% with UI versus 62% in the control group, RR 1.05, 95% CI 0.91 to 1.23, Analysis 11.1.4). The evidence was judged to be moderate.
11.1. Analysis.
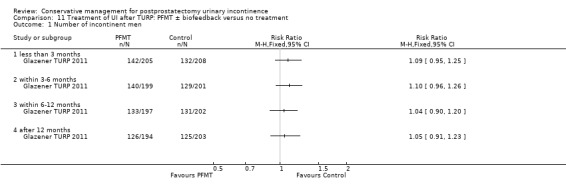
Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.
In one large trial (Glazener TURP 2011) men did not report differences in incontinence episodes at any time period, based on urinary diary data (for example after 12 months MD 0.2, 95% CI ‐0.27 to 0.67, Analysis 11.2).
11.2. Analysis.
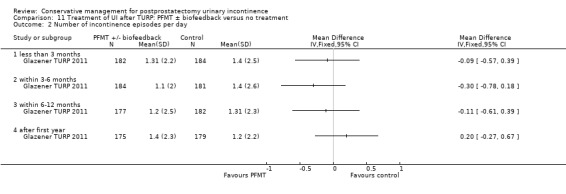
Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day.
Use of pads
Use of pads could be considered to be a measure of more severe incontinence. There was no statistically significant difference in the number of men using pads in one large trial (16% in intervention group versus 18% in control group after 12 months, RR 0.93, 95% CI 0.56 to 1.56, Analysis 11.3) (Glazener TURP 2011).
11.3. Analysis.
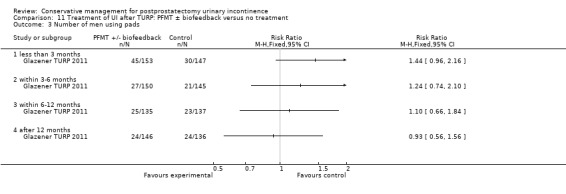
Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads.
Urinary incontinence score and effect on quality of life
In one large trial (Glazener TURP 2011), there was no evidence of a difference in the ICIQ‐SF (a composite score of frequency, amount and effect of UI on quality of life) at any time period after the intervention up to or beyond one year (for example MD after 12 months ‐0.1, 95% CI ‐0.89 to 0.69, Analysis 11.4) or quality of life as a single score from 0 to 10 (MD ‐0.1, 95% CI ‐0.51 to 0.31, Analysis 11.5). The quality of evidence for this outcome was deemed to be low.
11.4. Analysis.
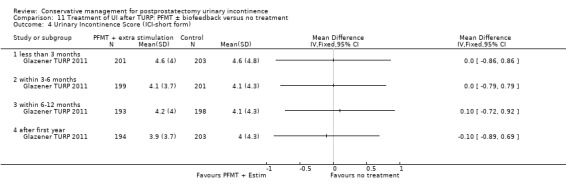
Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 4 Urinary Incontinence Score (ICI‐short form).
11.5. Analysis.
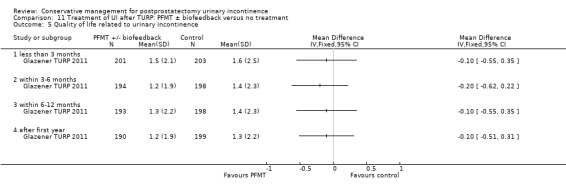
Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 5 Quality of life related to urinary incontinence.
Adverse events
No adverse events were reported.
12. Treatment of UI after TURP: electric or magnetic energy (for example anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment (Comparison 12)
No trials were identified.
13. Treatment of UI after TURP: lifestyle interventions versus no treatment or sham treatment (Comparison 13)
No trials were identified.
14. Treatment of UI after TURP: combinations of treatments versus no treatment or sham treatment (Comparison 14)
No trials were identified.
15. Treatment of UI after TURP: one treatment versus another active treatment (Comparison 15)
No trials were identified.
TURP: prevention of UI in all men having surgery, intervention before or after prostatectomy, or both
16. Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 16)
Three small trials enrolled men before TURP for benign prostatic hyperplasia (Hou 2013; Porru 2001; Tibaek 2007). Men in the intervention groups in both trials received one session with a therapist before surgery to teach them the correct contractions (using verbal biofeedback) and they were expected to practice PFMT afterwards. In the second trial (Tibaek 2007), men also attended three group teaching sessions. The control groups received information only. The quality of the evidence is illustrated in Table 12.
There were no statistically significant differences between the groups in the number of men with incontinence at less than 3 months or 3 to 6 months, but the CIs were wide and the quality of evidence was very low (< 3 months RR 0.60, 95% CI 0.21 to 1.77, Analysis 16.1.1; 3 to 6 months RR 0.51, 95% CI 0.14 to 1.89, Analysis 16.1.2).
16.1. Analysis.
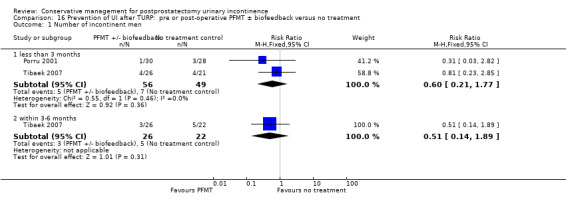
Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.
Quality of life
One trial (Hou 2013) measured quality of life using a health status measure Short‐Form 36 (SF‐36) questionnaire at three to six months. No statistically significant differences were found on any of the domains apart from those associated with mental health (Analysis 16.2).
16.2. Analysis.
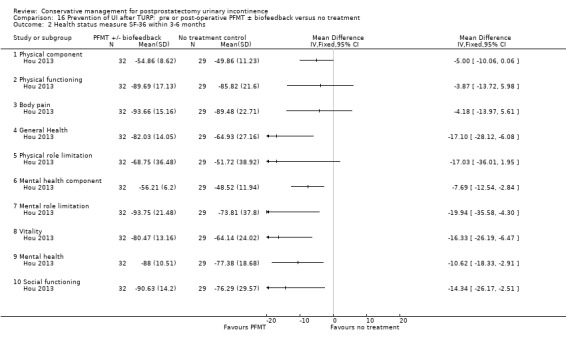
Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 2 Health status measure SF‐36 within 3‐6 months.
17. Prevention of UI after TURP: electric or magnetic energy (for example anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment (Comparison 17)
No trials were identified.
18. Prevention of UI after TURP: lifestyle interventions versus no treatment or sham treatment (Comparison 18)
No trials were identified.
19. Prevention of UI after TURP: combinations of treatments versus no treatment or sham treatment (Comparison 19)
No trials were identified.
20. Prevention of UI after TURP: one treatment versus another active treatment (Comparison 20)
No trials were identified.
Containment of UI (all men with residual UI)
21. External penile compression devices (penile clamps) versus no treatment or sham treatment (Comparison 21)
One trial compared three different penile compression devices (Cunningham clamp, U‐Tex Male Adjustable Tension Band, and C3 penile compression device) with a control period of no device (Moore 2004). A randomised block assignment was used with a multiple period cross‐over design, so that each of the 12 participants had a control period of no device and three periods in which the different devices were used.
All external compression devices reduced the weight of urine lost on a four hour pad test compared to the control period (P < 0.05, Analysis 21.2), but none completely eliminated urine loss. Satisfaction was based on ease of application, comfort and efficacy. The device preferred by the largest number of men (Analysis 21.1) was also that with the lowest urine loss (the Cunningham clamp) (Analysis 21.2).
21.2. Analysis.
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 2 Mean urine loss (grams of urine on pad test).
| Mean urine loss (grams of urine on pad test) | ||||
|---|---|---|---|---|
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | 122.8 gm (SD 130.8) | 53.3 gm (SD 65.7) P<0.05 vs Control (no device) | 32.3 gm (SD 24.3) P<0.05 vs Control (no device) | 17.1 gm (SD 21.3) P<0.05 vs Control (no device) |
21.1. Analysis.
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 1 Number of men satisfied with device.
| Number of men satisfied with device | ||||
|---|---|---|---|---|
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | 0/12 | 0/12 | 2/12 | 10/12 |
Adverse events
The Cunningham clamp was also the device with the greatest reduction in systolic blood flow velocity (P < 0.05 versus control period, Analysis 21.3; Analysis 21.4), raising the possibility of safety issues if applied too tightly. In the trial, men were able to judge when to release the device. The authors recommended that its use should therefore be limited to men who were cognitively intact, aware of bladder filling, had normal genital sensation and intact penile skin, and had sufficient manual dexterity to open and close the device (Moore 2004).
21.3. Analysis.
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 3 Penile Doppler blood flow (mean systolic velocity).
| Penile Doppler blood flow (mean systolic velocity) | ||||
|---|---|---|---|---|
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | N=12 men R: 12.4 (SD 2.8) L: 12.3 (SD 3.0) | N=12 men R: 11.9 (SD 4.4) L: 13.8 (SD 7.3) | N=12 men R: 12.4 (SD 5.5) L: 11.7 (SD 4.7) | N=12 men R: 9.5 (SD 2.3) L: 7.3 (SD 3.0) P<0.05 vs Control (no device) |
21.4. Analysis.
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 4 Penile Doppler blood flow (mean resistence to flow index).
| Penile Doppler blood flow (mean resistence to flow index) | ||||
|---|---|---|---|---|
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | N=12 men R: 0.9 (SD 0.1) L: 0.87 (SD 0.1) | N=12 men R: 0.93 (SD 0.08) L: 0.91 (SD 0.11) | N=12 men R: 0.92 (SD 0.1) L: 0.92 (SD 0.11) | N=12 men R: 0.92 (SD 0.13) L: 0.86 (SD 0.29) |
In another trial with no useable data (Fader 2013), men provided qualitative information which suggested that pads were most highly rated compared with sheath catheters (P = 0.31), clamps (P < 0.01) and the body‐worn urinal (P < 0.001). The clamp was rated as more secure, less leaky and less restrictive on clothing choice than the others (P < 0.05) but was more painful than the rest (P < 0.002).
Discussion
This review incorporates a broad array of possible interventions under the umbrella term of conservative management of postprostatectomy UI. The populations studied included men undergoing prostatectomy for both benign (TURP) and malignant (radical prostatectomy) disease. The interventions were delivered pre‐operatively, post‐operatively or both. In some trials all the men were incontinent at baseline, while at least some were dry in other trials which recruited all men having surgery (these were classed as prevention of incontinence trials). Seven trials (Goode 2009; Joseph 2000; Moore 1999; Moore 2004; Opsomer 1994; Seleme 2008; Zhang 2007) included men who had been incontinent for a considerable time after surgery while the rest recruited men around the time of surgery. More recent trials have focused on the pre‐operative or post‐operative period immediately after catheter removal. It is acknowledged that UI after prostatectomy will resolve over time in many men.
Conservative interventions tend to be resource‐intensive strategies that require people, equipment and clinic space, so administrators will look for evidence of efficacy. Funding has been an issue given the inconclusive nature of the evidence to date. For example, in the United States, the centres for both Medicare and Medicaid services have considered whether to withdraw funding for biofeedback and pelvic floor electrical stimulation (ES) in the treatment of UI of any etiology based on a lack of evidence regarding effectiveness. Through a lobbying effort from service providers and manufacturers, these modalities continued to be covered in the United States (Thompson 2002). However, as controversy about funding is likely to continue, there is a need for continued research in the area to determine which groups of patients are most likely to benefit from conservative interventions.
The findings of this review should continue to be treated with caution. The effectiveness of conservative measures in the longer term or in men with persistent UI remain inconclusive.
Summary of main results
Fifty trials met the inclusion criteria, 45 trials amongst men after radical prostatectomy, four trials after TURP, and one small trial which included one man with benign disease but was classed as a radical prostatectomy trial. There was considerable variation in the interventions, populations and outcome measures. Given this clinical heterogeneity it was decided to differentiate the trials and the comparisons, by type of surgery (TURP or radical prostatectomy) and by whether the intervention was partly preventative (in that not all men were incontinent, for example if all men before or after surgery were recruited, N = 27 trials), for treatment only (when all included men were incontinent at baseline, N = 23 trials) or for containment (external penile compression devices, N = 2 trials). Although the International Prostate Score (IPSS) was used in many of the trials, the authors felt that this questionnaire did not assess UI and therefore was not included in the outcome of quality of life.
Treatment trials for urinary incontinence after radical prostatectomy
Twenty‐one trials investigated the effects of PFMT versus no treatment or a variety of other means of stimulating the pelvic floor muscles. There was considerable clinical and statistical heterogeneity in the populations and the timing and frequency of the interventions, hence a random‐effects model was chosen for most of the comparisons where meta‐analysis was possible. Only two trials (Manassero 2007; van Kampen 1998) showed a statistically significant benefit from active treatment versus no treatment control groups (at 12 months and within 6 to 12 months respectively), and the other trials showed conflicting results. There was differential dropout from the control group in the Manassero 2007 trial (these men were assumed to be dry for analysis purposes). Additionally, men in the experimental group in the van Kampen 1998 trial received one session of PFMT in hospital before discharge and were then seen by a physiotherapist for one to two weeks, whereas those in the Manassero 2007 trial were taught PFMT by two urologists using verbal feedback and instructed to perform contractions at home. Because of the heterogeneity a random‐effects model was used, which led to wider confidence intervals (CIs).
Overall, there was not enough evidence to say whether or not PFMT with or without biofeedback was effective as the CIs were wide (for example number of men with incontinence in the intervention groups 193/339 (57%) versus 203/326 (62%) in the control groups, RR for incontinence after 12 months 0.85, 95% CI 0.60 to 1.22, Analysis 1.1.4).
The meta‐analysis was dominated by the Glazener RP 2011 trial, which was a large pragmatic multi‐centre trial conducted in a context where information on PFMT was widely available. This showed no good evidence to support one‐to‐one training by a therapist (for example RR for UI after 12 months 0.98, 95% CI 0.87 to 1.09, Analysis 1.1.4) (Glazener RP 2011). This one large trial had narrow CIswhich did not include a clinically significant difference, pre‐specified to be 15%. The only other large trial (Moore 2008) was in line with the Glazener RP 2011 findings but with wider CIs (RR 1.02, 95% CI 0.70 to 1.48, Analysis 1.1.4) (Moore 2008) despite a more intensive intervention. While men in the Glazener RP 2011 trial had four therapy sessions over three months, in the Moore 2008 trial men were seen weekly for up to six months. The findings in these two trials concurred despite different intensities of intervention, and the quality of evidence for this GRADE‐specific outcome was moderate. Data from quality of life measures and use of pads and pad tests supported the finding of no differences between intervention and control groups.
Three small trials provided data and the meta‐analysis suggested that ES was better than control interventions in terms of less incontinence, regaining continence more quickly and better quality of life, at least in the short term up to six months. The quality of evidence was deemed to be moderate, however less information was available for the longer term.
Individual small trials provided data to suggest that extra‐corporeal magnetic innervation (ExMI) or combinations of treatments might be beneficial but the evidence was limited.
Prevention trials for urinary incontinence after radical prostatectomy
Nineteen trials, some of which enrolled men before surgery and others all men as soon as the catheter was removed, included a mixed population of men with and without incontinence after surgery. Again a random‐effects model was chosen to compensate for the considerable clinical and statistical heterogeneity between the trials. Including the information from the quasi‐randomised trial (Filocamo 2005), the chance of incontinence appeared to be lower in the intervention groups in two trials with data after 12 months. The quality of evidence was judged to be moderate (number of men with UI after one year 10.2% versus 32.1% in the control groups, RR 0.32, 95% 0.20 to 0.51, Analysis 6.1.4).
The meta‐analysis of prevention trials included a number of small trials with wide CIs apart from Filocamo 2005, which was out of line with the others. This was the only large trial to favour the intervention group. The worry is that this trial may have been biased due to a lack of reporting on concealment of allocation.
One small trial (Ribeiro 2008) suggested that men were more likely to be carrying out PFMT, at least soon after the intervention (Analysis 6.9), though this was not reflected in significant differences in higher anal squeeze pressures (Analysis 6.8). Another trial of anal ES was too small to be conclusive (Laurienzo 2013). One small trial (Mariotti 2009) reported that adding anal ES and biofeedback to PFMT was beneficial. One further small trial (Wille 2003) found that PFMT plus anal ES with and without extra biofeedback were both better than PFMT alone at six months, but there was little to choose between the two more intensive interventions (Analysis 10.8). Tienforti 2012 found that pre‐operative PFMT was associated with a reduction in number of incontinence episodes per day, but this was a small trial and larger sample sizes would be needed to draw reliable conclusions.
6.9. Analysis.
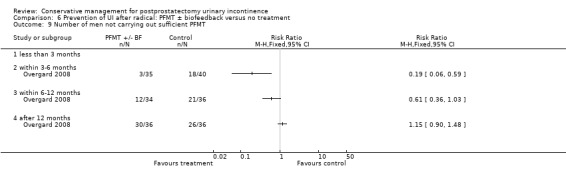
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 Number of men not carrying out sufficient PFMT.
6.8. Analysis.
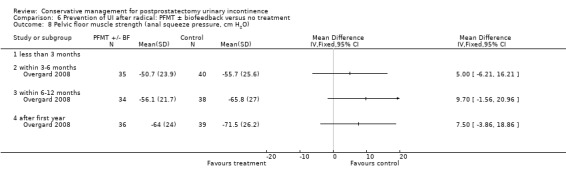
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O).
Nine trials compared one active treatment with another active treatment. Overall there did not seem to be one intervention that proved to be statistically significantly better than another.
Treatment trials for urinary incontinence after TURP
One large trial addressed this comparison (Glazener TURP 2011), comparing four sessions of one‐to‐one therapy with standard management in a context where information about PFMT was widely available. The quality of evidence for the number of incontinent men was moderate but there were no differences between the groups in any of the outcome measures except for performance of PFMT, suggesting that the intervention had changed behaviour but not incontinence or other clinical outcomes.
Prevention trials for urinary incontinence after TURP
Three small trials enrolled men before TURP to receive a minimal PFMT intervention before and after surgery. There were no statistically significant differences in terms of number of incontinent men between the groups but the quality of evidence was deemed to be very low (Analysis 16.1).
Containment of urinary incontinence
One alternative intervention, a clamp fitted to the shaft of the penis, can be used to control unwanted leakage. Men in one trial reported a preference for one type of external compression device compared to two others or no treatment; a Cunningham clamp proved satisfactory to 10 of 12 men with intractable UI (Moore 2004). This may be a viable alternative for some cognitively capable men providing they take into account safety issues such as adequate sensation and the ability to remove the device when it feels too tight or the bladder is full. Another trial which compared pads, sheath catheters, body‐worn urinals and clamps also reported that men found the clamps most effective but painful (Fader 2013).
Men whose incontinence cannot be otherwise controlled can use absorbent pads (Fader 2007; Fader 2008) or a variety of external sheath devices with leg bags. An alternative is an indwelling urinary catheter (Jahn 2007; Moore 2007; Niël‐Weise 2005).
Lifestyle changes
The effect of other conservative interventions such as lifestyle changes remains undetermined as no trials involving these interventions were identified.
Overall completeness and applicability of evidence
Few trials used the primary outcomes of interest, patient reported symptoms and the standardised pad test. Most used a variety of subjective outcomes derived from patient reported symptoms to define continence. There were no trials which examined lifestyle adjustments in alleviating UI after prostatectomy.
Attrition bias may have played a role in the results of some of the included trials and therefore affected the outcome of this review. One of the smaller trials (Franke 1998) lost half of the randomised participants by the end of the data collection period. Although most of those trials that lost participants provided an explanation of these losses, none accounted for the missing data in their primary analyses. The intention‐to‐treat principle mandates, at minimum, that patients stay in the group to which they are randomised (Juni 2001), which the included trials appeared to do. It is also suggested that primary outcomes for all patients randomised to groups should be recorded or estimated if not available. Three of the included trials (Filocamo 2005; Moore 2008; Parekh 2003) reported an analysis using the intention‐to‐treat principle, and one trial (Burgio 2006) used survivor analysis in the original trial analysis. In one trial where there was clear evidence of differential dropout (Manassero 2007), the review authors elected to assume that the men whose data were missing were continent. However, attrition bias may have affected a number of the other trials which did not present relevant data or discuss the issue.
In 21 trials in this review, men who were all incontinent were analysed together. However, in seven of these trials (Goode 2009; Joseph 2000; Moore 1999; Moore 2004; Opsomer 1994; Seleme 2008; Zhang 2007) men had longstanding or persistent incontinence. It is possible that they might respond differently to the interventions compared to men recruited around the time of prostate surgery.
Quality of the evidence
Trial quality and methodological assessment
The quality of the estimated treatment effect of any intervention is determined partly by methodological assessment. Methodological flaws within the included trials of this review were assessed using the reports of the trials and therefore were reliant on the quality of reporting. Data were not available in all the trials for many of the pre‐stated outcomes. CIs tended to be wide except for the more recent large trials, and it was difficult to reliably identify or rule out a useful effect.
All trials claimed to be randomised, but only 24 out of 50 trials provided details of adequate sequence generation (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Filocamo 2005; Fode 2014; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Manassero 2007; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012; van Kampen 1998; Yamanishi 2006). Only 20 of the 50 trials provided details of adequate concealment of randomisation (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Fode 2014; Geraerts 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Manassero 2007; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Tibaek 2007; van Kampen 1998; Yamanishi 2006) and were subsequently judged to be at low risk of selection bias. Additionally, blinding to PFMT was not possible, and blinding of outcome assessment appeared to be absent in many trials as it was not discussed. Therefore, many trials were judged to be at high risk of performance and detection bias.
The quality of the evidence was downgraded for the following.
Study design i.e. there was evidence of methodological flaws in the study design.
Indirectness i.e. a surrogate outcome was selected when a GRADE‐specific outcome was not reported.
Inconsistency, when there was evidence of statistical (either clinical or methodological) heterogeneity.
Imprecision, when the CIwas wide and crossed the line of no effect.
Publication bias. We planned to use funnel plot for publication bias, however, there were fewer than 10 trials in the meta‐analysis and the funnel plot could not be used.
The quality of the evidence for the critical outcomes ranged from moderate to very low, as evident in the summary of findings tables.
Potential biases in the review process
All relevant databases were searched and no language restriction was imposed during the search process, which enabled as many potentially eligible trials as possible to be included. Some reports of trials may not be published and therefore the full extent of the data may not have been obtained. One of the review authors was involved in four of the included trials and another review author was involved in two of the included trials. In order to account for this potential bias in the review process, data extraction and risk of bias assessment were performed by two independent review authors, one of whom was not involved in any of the included trials.
Agreements and disagreements with other studies or reviews
A systematic review conducted by Macdonald et al (MacDonald 2007) was identified which addressed conservative management of post‐prostatectomy urinary incontinence. Macdonald and colleagues included 11 trials (Bales 2000; Burgio 2006; Filocamo 2005; Floratos 2002; Franke 1998; Mathewson‐Chapman 97; Moore 1999; Parekh 2003; van Kampen 1998; Wille 2003; Yokoyama 2004), all of which were included in this review. They did not distinguish between treatment and prevention trials. Macdonald and colleagues' review analysed PFMT and PFMT with biofeedback, focusing on any additional benefit from biofeedback. The authors concluded that the use of guided PFMT was associated with superior patient outcomes compared with no treatment, which differs from the findings of this review. The Macdonald and colleagues review did not include more recent trials because the MEDLINE search only included trials up to 2006. Additionally, the conclusions made in Macdonald's review may have differed because the authors did not utilise the GRADE approach, suggesting the quality of the evidence was not assessed.
Authors' conclusions
Implications for practice.
In keeping with conclusions from earlier versions of this review, at this point there remains no clear support that conservative management of any type is helpful for postprostatectomy UI whether delivered as treatment to men who are incontinent or as prevention to all men undergoing radical prostatectomy. The individual result of one large multi‐centre trial on its own did have narrow confidence intervals which did not include a clinically significant difference (of 15%) in the rate of incontinence between the groups. It seems unlikely that men benefit from one‐to‐one PFMT therapy after TURP.
Some small trials provided data to suggest that electrical stimulation was better than control interventions (in one trial including sham electrical stimulation), or active interventions which did not include electrical stimulation, at least in the short term up to six months. Individual small trials provided data to suggest that extra‐corporeal magnetic innervation (ExMI) or combinations of treatments might be beneficial but the evidence was limited.
The trials suffered from a lack of standardised outcome measures. Definitions of incontinence, measurement of quality of life and types of pad tests (20 minute, 1 hour, 24 hour, number of pads, weight of pads, number of men using pads and so on) varied in almost every trial. The timing for measuring the primary outcome should be at least 1 year.
No trials have tested the effect of lifestyle changes alone. Long‐term UI may be managed by absorbent pads or external penile clamps, but there are safety problems with clamps.
This review did not find sufficient evidence as to whether or not conservative management is effective in treating or preventing postprostatectomy UI.
Implications for research.
Urinary incontinence (UI) after prostatectomy is a distressing problem and, although conclusive evidence does not exist, conservative approaches form part of current management. Well‐designed clinical trials are still needed to clarify the role of these therapies. In addition, men with persistent severe UI could consider surgical treatment for example with an artificial urinary sphincter or male sling. However, these surgical options should also be tested in RCTs as there is currently not enough evidence to support their use (Silva 2011).
As there are known differences in the cause and prevalence of UI between men after TURP and after radical prostatectomy, these groups of men should continue to be studied separately. Prevention trials in all men having surgery should be evaluated separately from treatment trials of men who all have urinary incontinence after surgery.
Most of the trials included in this review used very different protocols, of intervention type, timing and intensity. In order to determine the effects of specific protocols and modalities, large adequately powered trials using common protocols and common standardised outcome measures are needed. Replication studies using similar protocols in different populations would also assist in identifying the populations in which specific conservative management approaches may be effective.
Definitions and measurement of outcomes varied in the included trials. Future trials must attempt to use broadly accepted validated outcome measures, such as those of the International Continence Society (ICS). The primary outcome measure should be the participant's self‐reported UI or its effects on his quality of life. Other objective measures such as the pad test or urinary diaries can be used to determine if continence has been achieved. Researchers must also focus on either the 1 hour or 24 hour pad test, as the results of these two measurements are not equivalent.
Lastly, authors should be encouraged to ensure appropriate measures are taken to avoid the risk of bias from selection, performance, detection and attrition bias, in particular adequate sequence generation and secure concealment of allocation for randomisation, and blinding of outcome measurement, and to report these adequately using the guidelines of the CONSORT statement.
What's new
| Date | Event | Description |
|---|---|---|
| 26 January 2015 | Amended | Incorporated following sentence in the abstract "It seems unlikely that men benefit from one‐to‐one PFMT therapy after TURP." |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 4, 1999
| Date | Event | Description |
|---|---|---|
| 19 January 2015 | New citation required but conclusions have not changed | In this update, the review authors have added 13 new trials (Ahmed 2012; Dijkstra‐Eshuis 2013; Fader 2013; Fode 2014; Geraerts 2013; Ghanem 2013; Hou 2013; Laurienzo 2013; Marchiori 2010; Martini 2011; Morihiro 2011; Park 2012; Tienforti 2012). Risk of bias assessment was performed on all 50 trials in accordance with the current methodology. Overall, 37/50 trials were also included in the previous update (Campbell 2012) and 13/50 trials were identified in this update. Quality of evidence was assessed by adopting the GRADE approach. |
| 19 January 2015 | New search has been performed | In this update, the review authors have added 13 new trials (Ahmed 2012; Dijkstra‐Eshuis 2013; Fader 2013; Fode 2014; Geraerts 2013; Ghanem 2013; Hou 2013; Laurienzo 2013; Marchiori 2010; Martini 2011; Morihiro 2011; Park 2012; Tienforti 2012). Risk of bias assessment was performed on all 50 trials in accordance with the current methodology. Overall, 37/50 trials were also included in the previous update (Campbell 2012) and 13/50 trials were identified in this update. Quality of evidence was assessed by adopting the GRADE approach. |
| 24 August 2011 | New search has been performed | 18 new trials added |
| 24 August 2011 | New citation required and conclusions have changed | In this update, 18 new trials have been added (of which 1 was a previously excluded trial). The total number of trials included is now 37. |
| 16 September 2008 | Amended | Converted to new review format. |
| 21 February 2007 | New citation required and conclusions have changed | Substantive amendment. In this update (Issue 2 2007), 7 trials were added to the review. The total number of studies included was 17. In this update, comparisons were separated on the basis of type of surgery and as well whether the intervention occurred pre‐ or post‐operatively. |
| 25 February 2004 | New citation required and conclusions have changed | Substantive update Issue 2 2004. In this update, five trials were added to the review. One trial previously listed as included was excluded after attempts to contact the author to access data were unsuccessful. The total number of studies included was 10. 7 extra studies were excluded. |
| 23 January 2001 | New citation required and conclusions have changed | Substantive update Issue 2 2001 |
Acknowledgements
Katherine Moore was supported by a postdoctoral fellowship from the Leverhulme Trust, London, England in 1998 thereby allowing the time for the original review (first published 1998). Authors of nine trials (Burgio 2006; Dubbelman 2004; Filocamo 2005; Floratos 2002; Glazener RP 2011; Glazener TURP 2011; Hoffman 2005, Joseph 2000; Wille 2003) generously shared further data so that their trials could be included in this review. We thank Neil Scott for the translation of a German language article (arranged by Sheila Wallace) as well as Matthew Tripp for another. Peter Herbison provided expertise in the area of statistics and assisted in interpreting data provided by authors.
For the 2014 update of the review, the review authors would like to acknowledge Ying Hao for the translation of two Chinese language articles. Authors of five trials (Dijkstra‐Eshuis 2013; Fode 2014; Geraerts 2013; Hou 2013; Park 2012; Tienforti 2012) kindly provided further data. The review authors would also like to acknowledge Katherine Moore for her contribution to the previous versions of the review.
Appendices
Appendix 1. Searches performed for the previous versions of this review up to and including Hunter 2007
Details of the searches performed for previous versions of this review, up to and including 2007 (Hunter 2007) are given below.
Systematic searches of electronic bibliographic databases
MEDLINE (January 1966 to January 2006), EMBASE (January 1988 to January 2006), CINAHL (January 1982 to January 2006), PsycLIT (January 1984 to January 2006), ERIC (January 1984 to January 2006)
The following electronic bibliographic databases were searched (date search was performed: 10 January 2006): MEDLINE ‐ dates searched: January 1966 to January 2006; EMBASE ‐ dates searched: January 1988 to January 2006; PsycLIT ‐ dates searched: January 1984 to January 2006; CINAHL ‐ dates searched: January 1982 to January 2006; ERIC ‐ dates searched: January 1984 to January 2006.
The following search terms were used in each database (no limits were applied to the searches): incontinence, urinary, male, postprostatectomy, stimulation, electrical stimulation, biofeedback, pelvic muscle exercises, Kegel exercises, behavioural, behaviour, behavior, therapy, behaviour modification, therapy, physiotherapy, lifestyle, weight loss, caffeine, smoking, extracorporeal magnetic innervation, external penile compression devices, continence, bladder control, quality of life, randomised (randomized) controlled trial, evaluation, effectiveness, efficacy, outcomes.
Handsearching of conference proceedings
The following conference proceedings were handsearched:
American Urological Association (years searched: 1989‐2005) Supplement to the Journal of Urology, published as a supplement.
Society of Urologic Nurses and Associates (SUNA) (formerly American Urologic Association Allied) these abstracts are not published but are available in the SUNA office. Annual meeting (years searched: 1991 to 2003);1991‐Las Vegas, NV; 1992‐Washington, DC; 1993‐San Antonio, TX; 1994‐San Francisco, CA; 1995‐Las Vegas,NV; 1996‐Orlando, FL, 1997‐New Orleans, LA. Biannual incontinence meeting: 1992‐Tampa, Fla (1st meeting), 1994‐Phoenix, 1996‐Dallas, 1998‐Orlando, 2000‐Nashville, 2004‐Chicago, 2006‐NYC; Understanding urodynamics seminar:1993‐Denver, CO; 1994‐San Antonio, TX; 1995‐Cleveland, OH; 1996‐St Louis, MO.
Wound Ostomy and Continence Nurses (years searched: 1996, 1997,1999 to 2006). Annual meeting: 1996‐ Seattle, WA; 1997‐Nashville, TN; Incontinence meeting (biannual); 1997‐Beverly Hills (1st meeting); 1999‐Austin, TX. (No further Incontinence meetings.)
International Continence Society (years searched: 1980 to 2006). Published proceedings in Neurourology and Urodynamics.
Appendix 2. Searches performed for the previous version of this review (Campbell 2012)
Extra specific searches (additional to the Specialised Register search) were performed for previous version of the review (Campbell 2012). These are detailed below:
CINAHL on EBSCO (January 1982 to 20 November 2009) was searched on 7 December 2009;
EMBASE on Ovid (January 1980 to Week 48 2009) was searched on 3 December 2009.
The search strategies used to search these databases can be found below:
CINAHL on EBSCO (January 1982 to 20 November 2009) was searched on 7 December 2009:
| S38 | S31 and S35 and S37 |
| S37 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S36 |
| S36 | TI ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) or AB ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) |
| S35 | (S32 or S33 or S34) |
| S34 | TI postprostat* OR AB postprostat* |
| S33 | TI post‐prostat* OR AB post‐prostat* |
| S32 | (MH "Prostatectomy") |
| S31 | (S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30) |
| S30 | AB overactive N3 bladder* |
| S29 | TI overactive N3 bladder* |
| S28 | AB urin* N3 leak* |
| S27 | TI urin* N3 leak* |
| S26 | AB incontinen* OR continen* |
| S25 | TI incontinen* OR continen* |
| S24 | (MH "Incontinence") |
| S23 | (MH "Overactive Bladder") |
| S22 | (MH "Urinary Incontinence+") |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | PT Clinical Trial |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
EMBASE on Ovid (January 1980 to Week 48 2009) was searched on 3 December 2009:
| 1 | Randomized Controlled Trial/ |
| 2 | controlled study/ |
| 3 | clinical study/ |
| 4 | major clinical study/ |
| 5 | prospective study/ |
| 6 | meta analysis/ |
| 7 | exp clinical trial/ |
| 8 | randomization/ |
| 9 | crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/ |
| 10 | Placebo/ |
| 11 | latin square design/ |
| 12 | exp comparative study/ |
| 13 | follow up/ |
| 14 | pilot study/ |
| 15 | family study/ or feasibility study/ or pilot study/ or study/ |
| 16 | placebo$.tw. |
| 17 | random$.tw. |
| 18 | (clin$ adj25 trial$).tw. |
| 19 | ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. |
| 20 | factorial.tw. |
| 21 | crossover.tw. |
| 22 | latin square.tw. |
| 23 | (balance$ adj2 block$).tw. |
| 24 | factorial design/ |
| 25 | parallel design/ |
| 26 | triple blind procedure/ |
| 27 | community trial/ |
| 28 | intervention study/ |
| 29 | experimental study/ |
| 30 | prevention study/ |
| 31 | quasi experimental study/ |
| 32 | or/1‐31 |
| 33 | (nonhuman not human).sh. |
| 34 | 32 not 33 |
| 35 | exp urine incontinence/ |
| 36 | incontinence/ |
| 37 | overactive bladder/ |
| 38 | (incontinen$ or continen$).tw. |
| 39 | (urin$ adj2 leak$).tw. |
| 40 | (overactive adj2 bladder$).tw. |
| 41 | 35 or 36 or 37 or 38 or 39 or 40 |
| 42 | prostatectomy/ |
| 43 | post‐prostat$.tw. |
| 44 | postprostat$.tw. |
| 45 | 42 or 43 or 44 |
| 46 | electrostimulation/ or electrostimulation therapy/ |
| 47 | stimulation.mp. |
| 48 | (electric$ adj2 stimulat$).tw. |
| 49 | electrostimulat$.tw. |
| 50 | magnetotherapy/ |
| 51 | exmi.tw. |
| 52 | (magnet$ adj2 (stimulat$ or innervat$)).tw. |
| 53 | feedback system/ |
| 54 | biofeedback.tw. |
| 55 | pelvis floor/ or muscle training/ or pelvic floor muscle training/ or muscle exercise/ or muscle strength/ |
| 56 | (pelvi$ adj5 (exercis$ or train$)).tw. |
| 57 | pfmt.tw. |
| 58 | pfe.tw. |
| 59 | (kegel adj2 exercis$).tw. |
| 60 | behavior therapy/ |
| 61 | (behavio?r$ adj3 (therap$ or train$ or treat$)).tw. |
| 62 | physiotherapy/ |
| 63 | home physiotherapy/ or physiotherapy practice/ |
| 64 | physiotherapist/ or physiotherapist assistant/ |
| 65 | physiotherap$.tw. |
| 66 | (physi$ adj3 (therap$ or treat$)).tw. |
| 67 | lifestyle/ or lifestyle modification/ |
| 68 | (lifestyle$ adj3 (chang$ or modif$)).tw. |
| 69 | (life adj2 style$ adj3 (chang$ or modif$)).tw. |
| 70 | weight reduction/ |
| 71 | (weight adj3 (los$ or reduc$)).tw. |
| 72 | caffeine/ |
| 73 | caffeine.tw. |
| 74 | smoking cessation/ |
| 75 | smoking cessation.tw. |
| 76 | (peni$ adj3 (device$ or clamp$)).tw. |
| 77 | "quality of life"/ |
| 78 | quality of life.tw. |
| 79 | or/46‐78 |
| 80 | 34 and 41 and 45 and 79 |
Appendix 3. Searches performed for the current version of this review
Specific searches were also performed for this update of the review. These are detailed below:
CENTRAL (on OvidSP) (2014, Issue 1) was searched on 26 February 2014;
Embase (on OvidSP) (January 1980 to Week 3 2014) was searched on 20 January 2014;
CINAHL (on EBSCOhost) (January 1982 to 18 January 2014) was searched on 22 January 2014;
ClinicalTrials.gov (via the Cochrane Register of Studies (CRS) interface) and WHO ICTRP (both searched on 29 January 2014)
The search strategies used to search these databases can be found below:
CENTRAL (on OvidSP) (2014, Issue 1) was searched on 26 February 2014
1. exp urinary incontinence/ 2. (incontinen$ or continen$).tw. 3. (urin$ adj2 leak$).tw. 4. or/1‐3 5. prostate/ 6. prostatectomy/ 7. prostatic hyperplasia/ 8. prostatic neoplasms/ 9. prostatitis/ 10. prostatic diseases/ 11. prostat$.tw. 12. post‐prostat$.tw. 13. postprostat$.tw. 14. or/5‐13 15. 4 and 14 16. cochrane incontinence group.gc. 17. 15 not 16
EMBASE (on OvidSP) (January 1947 to Week 3 2014) was searched on 20 January 2014 and limited to entry month January 2010 to Week 3 2014 (using 201$.em.) as the Cochrane Collaboration searches EMBASE centrally and is currently bringing this search up to date.
| 1 | Randomized Controlled Trial/ |
| 2 | controlled study/ |
| 3 | clinical study/ |
| 4 | major clinical study/ |
| 5 | prospective study/ |
| 6 | meta analysis/ |
| 7 | exp clinical trial/ |
| 8 | randomization/ |
| 9 | crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/ |
| 10 | Placebo/ |
| 11 | latin square design/ |
| 12 | exp comparative study/ |
| 13 | follow up/ |
| 14 | pilot study/ |
| 15 | family study/ or feasibility study/ or pilot study/ or study/ |
| 16 | placebo$.tw. |
| 17 | random$.tw. |
| 18 | (clin$ adj25 trial$).tw. |
| 19 | ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. |
| 20 | factorial.tw. |
| 21 | crossover.tw. |
| 22 | latin square.tw. |
| 23 | (balance$ adj2 block$).tw. |
| 24 | factorial design/ |
| 25 | parallel design/ |
| 26 | triple blind procedure/ |
| 27 | community trial/ |
| 28 | intervention study/ |
| 29 | experimental study/ |
| 30 | prevention study/ |
| 31 | quasi experimental study/ |
| 32 | or/1‐31 |
| 33 | (nonhuman not human).sh. |
| 34 | 32 not 33 |
| 35 | exp urine incontinence/ |
| 36 | incontinence/ |
| 37 | overactive bladder/ |
| 38 | (incontinen$ or continen$).tw. |
| 39 | (urin$ adj2 leak$).tw. |
| 40 | (overactive adj2 bladder$).tw. |
| 41 | 35 or 36 or 37 or 38 or 39 or 40 |
| 42 | prostatectomy/ |
| 43 | post‐prostat$.tw. |
| 44 | postprostat$.tw. |
| 45 | 42 or 43 or 44 |
| 46 | electrostimulation/ or electrostimulation therapy/ |
| 47 | stimulation.mp. |
| 48 | (electric$ adj2 stimulat$).tw. |
| 49 | electrostimulat$.tw. |
| 50 | magnetotherapy/ |
| 51 | exmi.tw. |
| 52 | (magnet$ adj2 (stimulat$ or innervat$)).tw. |
| 53 | feedback system/ |
| 54 | biofeedback.tw. |
| 55 | pelvis floor/ or muscle training/ or pelvic floor muscle training/ or muscle exercise/ or muscle strength/ |
| 56 | (pelvi$ adj5 (exercis$ or train$)).tw. |
| 57 | pfmt.tw. |
| 58 | pfe.tw. |
| 59 | (kegel adj2 exercis$).tw. |
| 60 | behavior therapy/ |
| 61 | (behavio?r$ adj3 (therap$ or train$ or treat$)).tw. |
| 62 | physiotherapy/ |
| 63 | home physiotherapy/ or physiotherapy practice/ |
| 64 | physiotherapist/ or physiotherapist assistant/ |
| 65 | physiotherap$.tw. |
| 66 | (physi$ adj3 (therap$ or treat$)).tw. |
| 67 | lifestyle/ or lifestyle modification/ |
| 68 | (lifestyle$ adj3 (chang$ or modif$)).tw. |
| 69 | (life adj2 style$ adj3 (chang$ or modif$)).tw. |
| 70 | weight reduction/ |
| 71 | (weight adj3 (los$ or reduc$)).tw. |
| 72 | caffeine/ |
| 73 | caffeine.tw. |
| 74 | smoking cessation/ |
| 75 | smoking cessation.tw. |
| 76 | (peni$ adj3 (device$ or clamp$)).tw. |
| 77 | "quality of life"/ |
| 78 | quality of life.tw. |
| 79 | or/46‐78 |
| 80 | 34 and 41 and 45 and 79 |
CINAHL (on EBSCOhost) (January 1982 to 18 January 2014) was searched on 22 January 2014
| S38 | S31 and S35 and S37 |
| S37 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S36 |
| S36 | TI ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) or AB ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) |
| S35 | (S32 or S33 or S34) |
| S34 | TI postprostat* OR AB postprostat* |
| S33 | TI post‐prostat* OR AB post‐prostat* |
| S32 | (MH "Prostatectomy") |
| S31 | (S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30) |
| S30 | AB overactive N3 bladder* |
| S29 | TI overactive N3 bladder* |
| S28 | AB urin* N3 leak* |
| S27 | TI urin* N3 leak* |
| S26 | AB incontinen* OR continen* |
| S25 | TI incontinen* OR continen* |
| S24 | (MH "Incontinence") |
| S23 | (MH "Overactive Bladder") |
| S22 | (MH "Urinary Incontinence+") |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | (PT Clinical Trial) OR (PT "randomized controlled trial") |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
ClinicalTrials.gov (via the Cochrane Register of Studies (CRS) interface) (searched on 29 January 2014)
(Continent OR continence OR incontinent OR incontinence OR overactive OR overactivity) AND (prostate OR prostatectomy OR postprostatectomy OR prostatic OR prostatectomies OR prostatic OR prostatectomies OR postprostatectomies)
WHO ICTRP (searched on 29 January 2014)
Simple search with each of these lines searched and assessed separately:
Incontinent AND postprostatectomy
Incontinence AND postprostatectomy
Incontinent AND prostatectomy
Incontinence AND prostatectomy
Data and analyses
Comparison 1. Treatment of UI after radical: PFMT ± biofeedback versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 7 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 1.2 within 3‐6 months | 7 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.10] |
| 1.3 within 6‐12 months | 5 | 792 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.73, 1.14] |
| 1.4 after 12 months | 3 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.22] |
| 2 Number of incontinence episodes per day | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 less than 3 months | 2 | 400 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.39, ‐0.78] |
| 2.2 within 3‐6 months | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.40, 1.00] |
| 2.3 within 6‐12 months | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.33, 0.93] |
| 2.4 after first year | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.82, 1.02] |
| 3 Number of men using pads | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pad changes over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Score (ICIQ‐SF) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of life related to urinary incontinence | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 24 hour pad test (grams of urine lost) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 less than 3 months | 2 | 214 | Mean Difference (IV, Fixed, 95% CI) | 22.29 [‐33.12, 77.70] |
| 8.2 within 3‐6 months | 2 | 213 | Mean Difference (IV, Fixed, 95% CI) | 11.87 [‐40.77, 64.52] |
| 8.3 within 6‐12 months | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | 11.23 [‐22.35, 44.82] |
| 8.4 after first year | 1 | 167 | Mean Difference (IV, Fixed, 95% CI) | 39.0 [‐5.72, 83.72] |
| 9 1 hour pad test (grams of urine lost) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men not carrying out pelvic floor muscle contractions at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.7. Analysis.
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Adverse events.
1.10. Analysis.
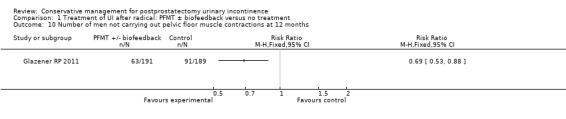
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men not carrying out pelvic floor muscle contractions at 12 months.
Comparison 2. Treatment of UI after radical: electric or magnetic energy versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.98] |
| 1.2 within 3‐6 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.79] |
| 1.3 within 6‐12 months | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.73] |
| 1.4 after 12 months | 3 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.74] |
| 2 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 24 hour pad test (grams of urine lost) | 2 | 325 | Mean Difference (IV, Fixed, 95% CI) | ‐16.94 [‐58.21, 24.33] |
| 3.1 less than 3 months | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐27.82 [‐116.97, 61.33] |
| 3.2 within 3‐6 months | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.12 [‐86.19, 96.43] |
| 3.3 within 6‐12 months | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐64.03, 60.13] |
| 3.4 after first year | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐80.0 [‐190.50, 30.50] |
| 4 Urinary Incontinence Score (ICIQ‐short form UI score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Time until continent (months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Treatment of UI after radical: combinations of treatments versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men at < 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinence episodes per day at > 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT + anal Estim + BFB | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal Estim + BFB | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.3. Analysis.
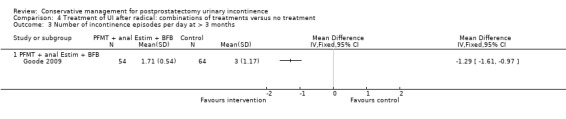
Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 3 Number of incontinence episodes per day at > 3 months.
Comparison 5. Treatment of UI after radical: one active treatment versus another active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men at < 3 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PFMT + Anal EStim vs PFMT alone | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 2 Number of incontinent men within 3 to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinent men within 6 to 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 FES vs ExMI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinence episodes at < 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of Life Score (severity of UI) within 3 to 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT + BF + support group vs PFMT + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of Life Score (I‐QoL) within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 PFMT + BF + EStim vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 PFMT + ExMI vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 PFMT + Anal EStim vs PFMT alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost): at < 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 PFMT + perineal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 PFMT + perineal EStim vs PFMT + anal EStim | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 24 hour pad test (grams of urine lost): at < 3 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 24 hour pad test (grams of urine lost): within 3 to 6 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost): within 3 to 6 months | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Pad changes over 24 hours within 3 to 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Number of men not carrying out sufficient PFMT within 3 to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.4. Analysis.
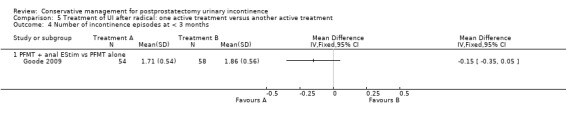
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinence episodes at < 3 months.
5.5. Analysis.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 5 Quality of Life Score (severity of UI) within 3 to 6 months.
5.9. Analysis.
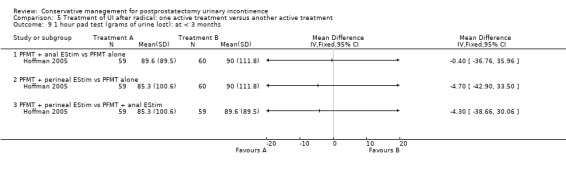
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost): at < 3 months.
5.10. Analysis.
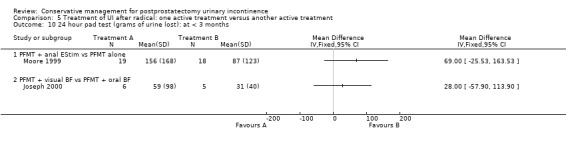
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 10 24 hour pad test (grams of urine lost): at < 3 months.
5.11. Analysis.
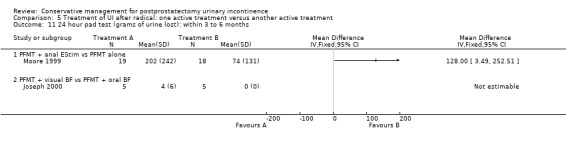
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 11 24 hour pad test (grams of urine lost): within 3 to 6 months.
5.14. Analysis.
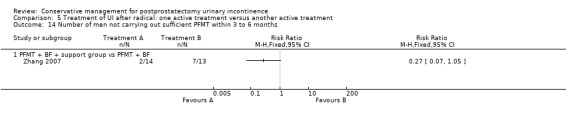
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 14 Number of men not carrying out sufficient PFMT within 3 to 6 months.
Comparison 6. Prevention of UI after radical: PFMT ± biofeedback versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 7 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.06] |
| 1.2 within 3‐6 months | 7 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 1.3 within 6‐12 months | 6 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.35, 0.75] |
| 1.4 after 12 months | 2 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.20, 0.51] |
| 2 Pad changes over 24 hours | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 less than 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6 ‐ 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 1 hour pad test (grams of urine lost) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (gm/24hrs) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 less than 3 months | 3 | 424 | Mean Difference (IV, Random, 95% CI) | ‐78.19 [‐211.46, 55.07] |
| 4.2 within 3‐6 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐73.28 [‐196.42, 49.86] |
| 4.3 within 6‐12 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐14.50 [‐18.36, ‐10.64] |
| 4.4 after first year | 2 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.81, ‐0.19] |
| 5 Number of incontinence episodes per day | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Urinary Incontinence Score (ICI‐short form) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 less than 3 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 6.5 [3.45, 9.55] |
| 6.2 within 3‐6 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐5.99, 3.56] |
| 6.3 within 6‐12 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐3.19, 1.81] |
| 7 Quality of Life Score (IIQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 within 6‐12 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of men not carrying out sufficient PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 less than 3 months | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men having surgery for incontinence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.2. Analysis.
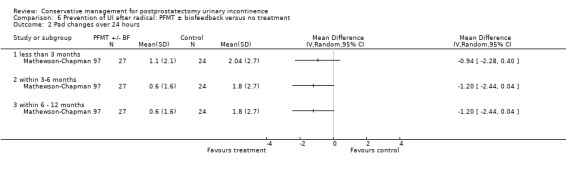
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Pad changes over 24 hours.
6.10. Analysis.
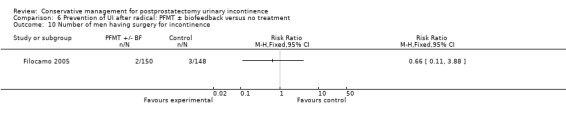
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men having surgery for incontinence.
Comparison 7. Prevention of UI after radical: electric or magnetic energy versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1 hour pad test (grams of urine lost) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ICIQ‐SF score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Prevention of UI after radical: combinations of treatments versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men within 3 to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 6 to 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + anal Estim + biofeedback versus no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 24 hour pad test (grams of urine lost) within 3 to 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (grams of urine lost) 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time until continent (months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
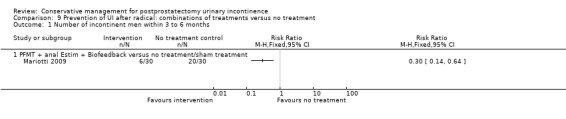
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men within 3 to 6 months.
9.3. Analysis.

Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 3 24 hour pad test (grams of urine lost) within 3 to 6 months.
Comparison 10. Prevention of UI after radical: one active treatment versus another active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men at < 3months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PFMT pre and post op vs PFMT post op | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.06] |
| 1.2 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| 1.3 PFMT + Biofeedback + transcutaneous Estim versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.11] |
| 1.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| 2 Number of incontinent men within 3 to 6 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 PFMT pre and post op vs PFMT post op | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 2.2 post‐op PFMT + biofeedback + transcutaneous Estim vs post‐op Estim | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.96, 2.49] |
| 2.3 PFMT + general exercise versus PFMT alone | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
| 2.4 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.57] |
| 2.5 Post‐op transcutaneous electrical stimulation versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.43, 1.16] |
| 3 Number of incontinent men within 6 to 12 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 post‐op PFMT + Biofeedback + transcutaneous Estim vs post‐op Estim | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinent men after 12 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 PFMT pre and post op vs PFMT post op | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.78, 2.25] |
| 4.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.25, 7.77] |
| 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 20 minute pad test (grams of urine lost): within 3 to 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 20 minute pad test (grams of urine lost): within 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost) at less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Pre‐op PFMT + Estim versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 1 hour pad test (grams of urine lost) within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 1 hour pad test within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost) at less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Post‐operative PFMT + transcutaneous Estim + Biofeedback versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Post‐operative transcutaneous electrical stimulation versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 24 hour pad test (grams of urine lost) within 3‐6 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Postoperative PFMT + biofeedback + transcutaneous Estim versus postoperative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Post‐operative transcutaneous Estim only versus post‐operative PFMT only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 24 hour pad test (grams of urine lost) within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 PFMT + transcutaneous Estim + biofeedback versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Quality of Life Score (ICS male short form) at < 3 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 PFMT pre and post op vs PFMT post op | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 PFMT pre and post op vs PFMT post op | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 King's health Questionnaire after 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Role limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Physical limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Social limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Personal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.6 Emotional | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.7 Sleep/energy disturbance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.8 Symptom severity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Health status measure SF‐36 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Physical composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Mental Composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.5. Analysis.
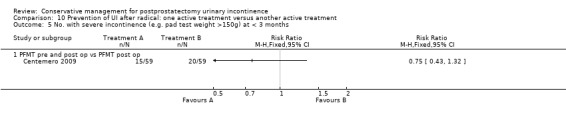
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months.
10.6. Analysis.
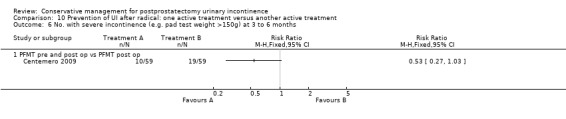
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months.
10.7. Analysis.
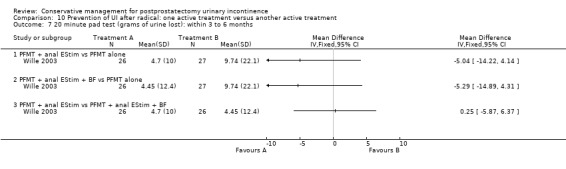
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 7 20 minute pad test (grams of urine lost): within 3 to 6 months.
10.9. Analysis.
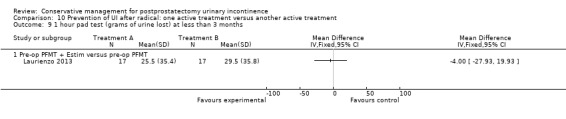
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost) at less than 3 months.
10.10. Analysis.
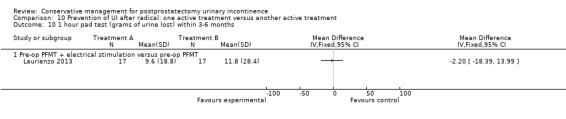
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 10 1 hour pad test (grams of urine lost) within 3‐6 months.
10.11. Analysis.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 11 1 hour pad test within 6‐12 months.
10.17. Analysis.
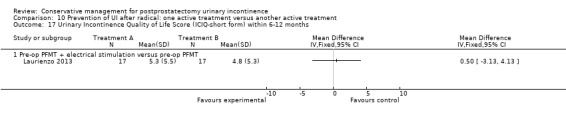
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months.
10.20. Analysis.
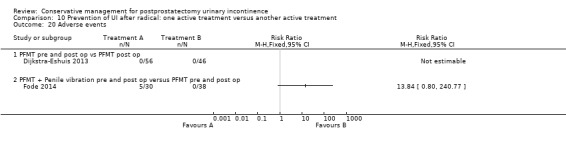
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 20 Adverse events.
Comparison 11. Treatment of UI after TURP: PFMT ± biofeedback versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinence episodes per day | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of men using pads | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Urinary Incontinence Score (ICI‐short form) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life related to urinary incontinence | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of men not carrying out pelvic floor muscle contractions at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
11.6. Analysis.
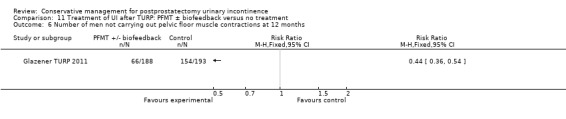
Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 6 Number of men not carrying out pelvic floor muscle contractions at 12 months.
Comparison 16. Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of incontinent men | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.21, 1.77] |
| 1.2 within 3‐6 months | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.14, 1.89] |
| 2 Health status measure SF‐36 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Physical component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Body pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Physical role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Mental health component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mental role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 21. Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of men satisfied with device | Other data | No numeric data | ||
| 2 Mean urine loss (grams of urine on pad test) | Other data | No numeric data | ||
| 3 Penile Doppler blood flow (mean systolic velocity) | Other data | No numeric data | ||
| 4 Penile Doppler blood flow (mean resistence to flow index) | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2012.
| Methods | RCT | |
| Participants |
Time of recruitment: Pre‐operatively Population: 95 men after radical prostatectomy (whole population, with or without UI) Included: men who underwent RP for clinically localized prostate cancer. Patients were not taking anticholinergic drugs or any drug that may affect continence for the duration of the study Excluded: previous urethral, bladder or prostate surgery, prior urinary or faecal incontinence, neurogenic and psychiatric disorders, pre‐operative urinary tract complications, radiotherapy Age (mean, SD): A 57.2 (3.25); B 58.8 (5.4); C 56.3 (6.8) Dropouts: 10 A: 4 (2 received radiotherapy, 2 had post‐operative complications); B: 4 (2 received radiotherapy, 2 refused follow up); C: 2 (2 received radiotherapy) No differential dropout Baseline characteristics: Comparable at baseline |
|
| Interventions |
Time of intervention: Post‐operative treatment A (26): PFMT alone. At catheter removal men received standard care of verbal and written instructions, active instructions from physiotherapist to perform 3 sets of 15 to 20 contractions daily, for a duration of 3 to 5 seconds with a 6 to 10 second rest period, encouraged to perform exercises before functional activities such as sneezing, coughing, or lifting weight, also in the supine position, sitting, squatting and going up and down stairs. B (26): ES: treatment started one week after catheter removal, patients received 15 minutes of twice weekly electrical stimulation for 12 weeks C (28): PFMT + BFB + ES: treatment started one week after catheter removal, patients received twice weekly treatment with 15 minutes of electrical stimulation and 15 minutes of biofeedback for 12 weeks, instructed to perform 3 series of 10 rapid contractions, 3 sustained contractions of 5, 7 or 10 seconds and then 10 contractions during prolonged expiration in the supine position All patients were given a logbook to complete daily regarding self‐report of exercises Duration of treatment: 12 weeks Follow up: 6, 12 and 24 weeks |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (defined as some pads required and weight gain of the pad > 1 g during the test) Baseline: A 22/26; B 22/26; C 23/28 2 months: A 21/26; B 19/26; C 18/28 3 months: A 17/26; B 12/26; C 20/28 6 months: A 9/26; B 6/26; C 1/28 Other outcomes Leakage weight in grams on 24 hour pad test (mean (SD) N) Baseline: A 791 (380.3) 26; B 790 (399.46) 26; C 785 (311.98) 28 2 months: A 533 (316.53) 26; B 383 (145.87) 26; C 263 (145.87) 28 3 months : A 260 (216.53) 26; B 132 (145.87) 26; C 83 (145.87) 28 6 months : A 123 (116.53) 26; B 98 (105.87) 26; C 36 (95.87) 28 Quality of life (Higher score = worse) Mean scores of IIQ‐7 (mean (SD) N) 2 months: 40 (23) 26; B 36 (25) 26; C 26 (25) 28 3 months: 32 (26) 26; B 29 (28) 26; C 20 (24) 28 6 months: 25 (26) 26; B 23 (24) 26; C 15 (25) 28 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using “a computer‐generated random‐number list” |
| Allocation concealment (selection bias) | Low risk | “sealed envelopes” |
| Blinding of participants (performance bias) | High risk | Blinding to treatment not possible |
| Blinding of personnel (performance bias) | Unclear risk | “One experienced physiotherapist delivered all therapy” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (2 received radiotherapy, 2 had post‐operative complications); B: 4 (2 received radiotherapy, 2 refused follow‐up); C: 2 (2 received radiotherapy). No differential dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk. |
| Approved by medical ethics committee | Low risk | “At the time of this study, there was no Human Research Ethics Committee established in the faculty, but the study was approved by the postgraduate affairs and departmental committee” |
| Informed consent | Low risk | “All patients signed an informed consent form” |
| ITT analysis | Low risk | Assumed from flow diagram of patients |
Bales 2000.
| Methods | Randomised: yes Method of allocation: not stated Blinding: Outcome assessment nurse not involved in intervention Dropouts: None mentioned | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 100 consecutive patients with stage T1c to T2c prostate cancer undergoing radical retropubic prostatectomy by a single surgeon randomised into 2 groups |
|
| Interventions | Pre‐operative intervention Group A (50) intervention: 2 to 4 weeks prior to surgery, participants underwent a 45 minute session with nurse trained in biofeedback. Patients were instructed to perform graded PFMT. Contractions of 5 to 10 seconds, 10 to 15 repetitions were performed with biofeedback (surface electrodes used to measure muscle strength). Advised to practice the exercises 4 times per day until surgery Group B (50) control: no biofeedback training. Written and brief verbal instructions from a nurse on how to perform PFMT (isolate muscle that stops urine flow, practice 4 times per day, 10 to 15 repetitions) Both groups: Encouraged to perform PME 4 times per day after catheter removal 2 weeks post‐operatively Length of follow‐up: 6 months |
|
| Outcomes | Main outcome: time to return of continence measured by number of pads used Continence definition: use of 1 pad or less per day Data collection: at 1, 2, 3, 4, and 6 months post‐operatively There was no significant difference in incontinence between the groups |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment nurse not involved in intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three patients dropped out of the biofeedback arm of the study because they never completed their biofeedback session |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | No description |
| Approved by medical ethics committee | Unclear risk | Not reported |
| Informed consent | Unclear risk | Not reported |
| ITT analysis | Unclear risk | Not specified |
Burgio 2006.
| Methods | Randomised: yes Method of allocation: stratified by age and tumour differentiation, then randomised using computer generated random numbers, block size of 4 to ensure equity of number in each group Blinding: intervention providers and bladder diary scorers were blinded Dropouts: 6 participants in the intervention group, and 7 in the control were excluded after randomisation as surgery was cancelled. At 6 months, 6 in the intervention and 4 in the control were lost to follow‐up | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 125 volunteer patients randomised, 13 excluded after randomisation Analysis on N = 112 men aged 53 to 68 years who underwent radical prostatectomy for prostate cancer. To be eligible, the men had to be ambulatory, continent and identified at least 1 week prior to their surgery |
|
| Interventions | Pre‐operative intervention Group A (57) intervention: single session of biofeedback (rectal probe to measure intra‐abdominal rectal pressure and external anal sphincter contraction) assisted behavioural training. Feedback and verbal instruction used to teach control of pelvic muscles. Taught to contract sphincter during 2 to 10 seconds periods separated by 2 to 10 seconds of relaxation, dependent on ability. Written instructions for daily at home practice of 45 PFM exercises daily (3 sessions of 15 exercises each time). Additionally instructed to slow or interrupt voiding once daily. Encouraged to exercise daily preoperatively, then resume when catheter removed post‐operatively Group B (55) control: usual care of brief verbal instructions post operatively to interrupt the voiding stream plus any instruction from physician Length of follow‐up: 6 months |
|
| Outcomes | Main outcome:
Continual or episodic urine loss using bladder diaries, incontinent pads or other products
Secondary outcomes:
Impact of incontinence and quality of life pre‐operatively and at follow‐up contacts by IIQ, SCL‐90‐R and SF‐36 Continence definition: 3 consecutive weekly 1 day diaries showing no leakage or a 7 day diary showing no leakage Data collection: 1 day bladder diaries mailed in each week. Questionaire on bladder control, lifestyle and 7 day bladder diary at 6 weeks, 3 months and 6 months post‐surgery Time to continence was significantly reduced in the intervention group. The intervention group had a significantly smaller proportion of those with severe or continual leakage at 6 months, and stress type urine loss. No differences on quality of life, return to work or activities between the groups |
|
| Notes | Analysis by "intention to treat". Additional data supplied to KFH by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by age and tumour differentiation, then randomised using computer generated random numbers, block size of 4 to ensure equity of number in each group |
| Allocation concealment (selection bias) | Low risk | Computer allocated. "The randomization schedule was implemented by the research nurse, so that interventionists would be blinded to the next group assignment." |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Intervention providers and bladder diary scorers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Bladder diary scorers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 and 4 lost to follow‐up at 6 months; 6 and 7 excluded after randomisation as surgery cancelled |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "Supported by Grant RO1 DK50283 from the National Institute for Diabetes and Digestive and Kidney Diseases, National Institutes of Health" "The funding organization did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; or the preparation, review or approval of the manuscript." |
| Approved by medical ethics committee | Low risk | "This study was reviewed and approved by the University and VA Medical center Institutional Review Boards for Human use" |
| Informed consent | Unclear risk | "All participants provided informed consent" |
| ITT analysis | Low risk | "intention to treat". Patient flow diagram |
Centemero 2009.
| Methods | Randomised: yes Method of allocation: 100 consecutive patients Blinding: no |
|
| Participants | Number of men 100 Recruitment: pre‐operative Included: all men undergoing radical prostatectomy Excluded: impaired mental status, BMI.27, diabetes mellitus, neurological‐rheumatic‐immune disease, neck‐urethral surgery, prior catheterisation, post‐operative catheterisation time longer than 6 days. Aged: 48‐68 years |
|
| Interventions | Group A (50) intervention: PFMT both pre and post‐operatively. A structured PFMT program 30 and 15 days before surgery, previous physiotherapist evaluation to provide the patients with feedback about the quality of pelvic floor muscle function, PC test (endurance and contraction quality), breathing co‐ordination, typify muscle contraction as tonic and modify incorrect physical attitudes. This was also repeated after the procedure Group B (50) intervention: PFMT post‐operatively only (no details as to whether this is the same as the treatment pre‐operatively above) Duration of treatment: not stated Length of follow‐up: at one and three months |
|
| Outcomes | UI at 1 month: A 33/59; B 47/59 3 month: A 24/59; B 37/59 24 hour pad test, number of subjects with pad test weight of > 150 g 1 month: A 15/59; B 20/59 3 month: A 10/59; B 19/59 Quality of life measured by the ICS male sf questionnaire, mean score 1 month: A 14.6 (6.36) 59; B 18.3 (6.36) 59 3month: A 8.1 (7.04) 59; B 12.2 (6.36) 59 Satisfaction scale (PGI‐I) used only for Group A and 75% reported extreme satisfaction for pre‐operative PFMT |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Individuals were randomised by a computer‐generated list that was centrally maintained". "Permuted block randomisation was used, with a block size of every 10 consecutively enrolled participants" |
| Allocation concealment (selection bias) | Low risk | "Individuals were randomised by a computer‐generated list that was centrally maintained". "Permuted block randomisation was used, with a block size of every 10 consecutively enrolled participants" |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | Low risk | "The surgeon who performed the procedures was blinded to randomisation allocation throughout the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Only the statistician and the data monitoring committee saw unblinded data" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description. It appears that there were was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | "The study was approved by the university institutional review board" |
| Informed consent | Low risk | Patients were "provided written informed consent" |
| ITT analysis | Low risk | Assumed from patient flow chart |
Dijkstra‐Eshuis 2013.
| Methods | RCT | |
| Participants |
Time of recruitment: pre‐operative Population: men having a laparoscopic radical prostatectomy (whole population, with or without UI) Included: patients with prostate cancer, undergoing laparoscopic radical prostatectomy Excluded: neurological disorders, a medical history with invasive perineal and/or rectal surgery, preoperatively existing stress urinary incontinence, radiation, ≥ 75 years Age (mean, SD): A 63.7 (5.3); B 63.7 (5.3) Dropouts: 9 from A (1 unable to understand Dutch, 3 post‐operative radiotherapy, 1 oesophageal cancer, 3 discontinued intervention at own request, 1 excluded due to poor compliance) 8 from B (2 post operative radiotherapy, 1 pelvic lymph node dissection, 1 died of cause unrelated to prostate cancer, 5 discontinued intervention at own request, 1 prolonged catheter) Not differential dropout Baseline characteristics: comparable at baseline |
|
| Interventions |
Time of intervention: pre‐operative (+ postoperative treatment for all men) A (56): 30 mins of guided PFMT + biofeedback weekly for 4 weeks before surgery, received written instructions to: carry out two sets of 30 contractions during abdominal breathing, one breath between each contraction; restart PFMT after catheter removal (7 to 10 days after surgery) B (46): received written instructions on PFMT after catheter removal (7 to 10 days after surgery) All men were seen before surgery by a physiotherapist, who explained relevant anatomy, anal visual inspection and digital palpation, biofeedback registration with rectal probe All patients received PFMT + biofeedback and/or electrical stimulation if still incontinent after 6 weeks Duration of treatment Follow up: 6 weeks, 3 months, 6 months, 9 months and 12 months after surgery |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (leakage on 24 hour pad test) 12 months: A 20/58; B 9/45 Other outcomes Number of continent men after 1 year (no leakage at all on 24 hour pad test) 12 months: 38/58; B 36/45 Adverse effects: A 0/56; B 0/46 Quality of life King’s Health Questionnaire (KHQ) (mean (SD) N): General health 12 months: A 24.48 (50.7) 56; B 29.64 (50.7) 46 Role limitations 12 months: A 21.36 (22.2) 56; B 17.73 (22.2) 46 Physical limitations 12 months: A 16.49 (15.45) 56; B 13.48 (15.45) 46 Social limitations 12 months: A 7.98 (24.8) 56; B 4.15 (24.8) 46 Personal 12 months: A 18.72 (4.4) 56; B 19.62 (4.4) 46 Emotional 12 months: A 5.08 (7.0) 56; B 4.24 (7.0) 46 Sleep or energy disturbance: A 9.13 (39.0) 56; B 6.13 (39.0) 46 Symptom severity: A 14.62 (86.1) 56; B 10.93 (86.1) 46 |
|
| Notes | Trial was stopped early because interim analysis found no benefit for group A Additional information supplied by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer‐generated random numbers with block randomization and variable block size” |
| Allocation concealment (selection bias) | Low risk | "central computer system" |
| Blinding of participants (performance bias) | Unclear risk | “participants were also blinded until their first visit to the pelvic floor physiotherapist” |
| Blinding of personnel (performance bias) | Low risk | “The pelvic floor physiotherapists were blinded to randomization” (to pre‐operative randomisation) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 from A (1 unable to understand Dutch, 3 post‐operative radiotherapy, 1 oesophageal cancer, 3 discontinued intervention at own request, 1 excluded due to poor compliance) 8 from B (2 post‐operative radiotherapy, 1 pelvic lymph node dissection, 1 died of cause unrelated to prostate cancer, 5 discontinued intervention at own request, 1 prolonged catheter á demeure). Not differential dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | “Medical ethical approval was obtained from the Medical Ethics committee of our university hospital” |
| Informed consent | Low risk | “Informed consent was obtained” |
| ITT analysis | Low risk | Assumed from flow diagram |
Dubbelman 2004.
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy (≥ 1 g urine loss on 1 hour pad test), one week after catheter removal Excluded: pre‐operative UI N = 66 men completing the trial, 33 in intervention group, 33 in control All participants had a radical retropubic prostatectomy and lived within 75 km of hospital Age range 61 to 67 years |
|
| Interventions | Post‐operative intervention A (35) intervention: 9 or less sessions of physiotherapy guided pelvic floor exercises after surgery plus information folder B (44) control: exercise instruction through information folder only Length of follow‐up: 6.5 months Dropouts: A 1, B 2 due to stricture; + A 1, B 3 refused further measurements; + B 5 withdrew consent or 1 did not understand |
|
| Outcomes | Continence definition: incontinence defined as loss of at least 1 gram of urine on 1 hour pad test and 4 grams on the 24 hour pad test Main outcome: urinary incontinence on both 1 hour (> 1 g) and 24 hour (> 4 g) pad tests Secondary outcome: urodynamic study (urethral pressure profilometry) Data collection: 1 and 26 weeks after catheter removal Number of wet men at 6 months: A: 17/33, B: 20/33 No significance difference in continence rates between the groups |
|
| Notes | Sample size required 96 men in each arm Other data presented as median (IQR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator to achieve 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, sequentially numbered, opened by trial nurse after result of pad test was known |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | "The physiotherapist who guided men in the PGPFME group was unaware of the outcome data of both treatment groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The data for outcome assessment (e.g. pad‐tests, voiding diaries) were collected and entered in a data base by a trial nurse who was not involved in the treatment or intervention" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 dropped out (of which 2 from intervention group) |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods reported |
| Financial support | Unclear risk | No description |
| Approved by medical ethics committee | Low risk | "approved by our institutional review board" |
| Informed consent | Low risk | "informed consent" |
| ITT analysis | Unclear risk | "the concept of an intent to treat analysis was not applied". Authors also state, "Participants were analysed in the group to which they were allocated at randomization" |
Fader 2013.
| Methods | RCT Cross‐over design | |
| Participants |
Time of recruitment: post‐operative Population: 74 men with incontinence after prostate surgery Included: men who were experiencing incontinence more than a year after prostate cancer treatment and using absorbent pads Excluded: no description Age (mean, SD): no description Dropouts: no information Baseline characteristics: no information |
|
| Interventions |
Time of intervention: post‐operative A: penile compression device (clamp) B: sheath drainage system (sheath) C: body‐worn urinals (BWU) D: pads alone All men tested each device for three weeks and asked to state which device was preferred Duration of treatment: 3 weeks with each device Follow‐up: 3 months |
|
| Outcomes |
Primary outcome (number of men with UI) Not reported Other outcomes Overall opinion: patient satisfaction questionnaire related to device performance Asked to state which device they preferred: Pads were most highly rated compared with sheaths (P = 0.31), clamps (P < 0.01) and BWUs (P < 0.001) The clamp was rated as more secure, less leaky and less restrictive of clothing choice than the others (P < 0.05) but was more painful than the rest (P<0.002) Three months later asked which products they were actually using and for what activities and circumstances: 30/56 using a combination of devices and pads Quality of life EORTC QLC C30 IIQ‐7 ICIQ‐UI King's Health Questionnaire |
|
| Notes | Awaiting further information from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “random order” cross‐over design |
| Allocation concealment (selection bias) | Unclear risk | “random order” |
| Blinding of participants (performance bias) | High risk | Blinding was not possible for participants |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | High risk | Quality of life outcome not reported |
| Financial support | Low risk | Prostate Cancer UK |
| Approved by medical ethics committee | Low risk | Southampton and South West Hampshire Research Ethics Committee (REC) |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
Filocamo 2005.
| Methods | Randomised: yes
Method of allocation: block randomisation, block size of 4 for 2 groups (A and B) with only one permutation code (ABBA) Blinding: not described Dropouts: at 12 months, 2 participants dropped out of the control group Intention to treat: yes |
|
| Participants | Recruitment: post‐operative Included: all men undergoing RRP N = 300 consecutive men post RRP, randomised after catheter removal to 2 groups Intervention group: N = 150 Control group: N = 150 |
|
| Interventions | Post‐operative intervention Group A (150) intervention: formal instruction (3 treatment sessions plus at home exercises) in PFMT using verbal explanation, palpation and visualization of the base of the penis with a mirror, in different positions and prior to sneezing, coughing or lifting Group B (150) control: no formal instruction Length of follow‐up: 12 months |
|
| Outcomes | Main outcome: urine loss on 1 hour and 24 hour pad tests plus number of pads used daily Continence definition: 0 to 1 pads per day Data collection: 1, 3, 6, and 12 months Wet (leakage or use of pads) 1 month: A 145/150, B 147/150 3 months: A 115/150, B 129/150 6 months: A 35/150, B 102/150 12 months: A 16/150, B 49/148 Surgical implantation of artificial urinary sphincter: A 2/150, B 3/148 |
|
| Notes | 74% of the intervention group achieved continence at 3 months compared to only 30% of the control (a significant difference favouring intervention) Differences between the groups declined between 6 to 12 months, with most participants achieving continence in 1 year |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, block size of 4 for 2 groups (A and B) with only one permutation code (ABBA) |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding of pad test or data entry from questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 dropped out of control group but none from intervention |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Low risk | "Approved by the Ethics Committee of our Institution" |
| Informed consent | Low risk | "All patients signed an informed consent form" |
| ITT analysis | Unclear risk | Not specified |
Floratos 2002.
| Methods | Randomised: yes
Method of allocation: randomised 2:1 to intervention: control groups
Blinding: not mentioned
Dropouts: 1 participant randomised to intervention unable to follow intervention protocol (unable to attend clinic, provided with control invention) Intention to treat: yes |
|
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy one week after catheter removal N = 42 consecutive patients |
|
| Interventions | Post‐operative intervention Group A (28) intervention: initiated after catheter removal. Intervention group received 15 treatment sessions (3 times per week for 30 minutes) of PFMT with EMG (surface) biofeedback in clinic Group B (14) control: instruction with verbal feedback and an information pamphlet with instructions to perform PME 50 to 100 times daily at home Length of follow‐up: 6 months |
|
| Outcomes | Main outcome: incontinence episodes measured by 1 hour pad test and continence questionnaire (pads used, number of incontinence episodes) Continence definition: incontinence defined as a urine loss of > 1 g on the 1 hour pad test; 2 or more pads/day a not deemed a "socially acceptable continence rate" Data collection: baseline, 1, 2, 3 and 6 months Level of incontinence in both groups declined over the 6 months of the study. Control group had less urine loss and appeared to regain continence sooner, but the difference was not significant |
|
| Notes | Additional data supplied to KFH by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Randomised 2:1 to intervention: control groups |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 dropped out of intervention group but followed control intervention ‐ unclear if analysed as control |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Patients were informed about the aims and perspectives of the study. Eligible patients consented" |
| ITT analysis | Unclear risk | "Analysed using the intention‐to‐treat approach". Authors also state "One of the patients initially randomized to group A could not follow the programme but performed PMEs under verbal instruction" |
Fode 2014.
| Methods | RCT | |
| Participants |
Time of recruitment: pre‐operative Population: 83 men undergoing nerve sparing radical prostatectomy (whole population, with or without UI) Included: sexually active men with an IIEF score of at least 18 without aids, continent pre‐operatively Excluded: condition that may prevent patient being able to have post‐operative treatment with PDE5‐inhibitor Age (mean, range): A 62 (46‐73); B 65 (49 to 76) Dropouts: 12 from group A (3 excluded because underwent non‐nerve sparing surgery, 2 withdrew consent, 1 lost a partner, 6 non‐compliance), 3 from group B (2 excluded because underwent non‐nerve sparing surgery, 1 withdrew consent). Differential dropout Baseline characteristics: comparable except Group A significantly more LUTS pre‐operatively |
|
| Interventions |
Time of intervention: pre‐operative + post‐operative A (30): pre‐operative session guided PFMT + instruction on how to use penile vibratory stimulation device, instructed to stimulate frenulum once daily, 10 seconds of stimulation then 10 second pause, repeated 10 times for 1 week pre‐operatively, Instructed to restart stimulation after catheter removal for 6 weeks B (38): pre‐operative session guided PFMT All men were offered a PDE5 inhibitor after 1 month post‐operatively and also received telephone contact to ensure compliance with treatment Duration of treatment: 6 weeks Follow up: 3, 6 and 12 months post‐operatively |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (men reporting use of more than one pad daily) 3 months: A 14/42; B 15/41 6 months: A 7/42; B 3/41 12 months: A 3/30; B 2/38 (dropout figures added to 3 and 6 months) Other outcomes Continence rate (patients reporting use of up to one pad daily for security reasons only) 3 months: A 65.5%; B 62.9%, P = 0.83 6 months : A 83.3%; B 91.9%, P = 0.28 12 months : A 90%; B 94.7%, P = 0.46 Median (range) pad use 3 months: A 1 (0 to 6); B 5 (0 to ‐34), P = 0.09 6 months: A 0 (0 to 3); B 1/3 (0 to 6), P = 0.14 12 months: A 0 (0 to 2); B 0 (0 to 3), P = 0.56 Median (range) IIEF‐5 3 months : A 5 (0 to 25); B 5 (0‐25), P = 0.25 6 months : A 10.5 (0 to 25); B 5 (0‐25), P = 0.08 12 months : 18 (0 to 25); B 7.5 (0‐25), P = 0.09 IIEF ≥ 18, n/N (%) 3 months: 5/30 (17); B 4/38 (11), P = 0.46 6 months: 13/30 (43); B 9/38 (24), P = 0.09 12 months: 16/30 (53); B 12/38 (32), P = 0.07 Adverse effects: A: 5/30 reported side effects as a result of penile vibratory stimulation (1 red spots on glans penis, 1 small laceration + some bleeding, 2 complained of soreness, 1 frank pain post‐operatively) B: 0/38 Quality of life Median (range) DAN‐PSS post‐operatively 3 months: A 1 (0 to 34); B 5 (0‐34), P = 0.74 6 months: A 2 (0 to 41); B 1 (0‐48), P = 0.74 12 months: A 3 (0 to 36); B 0.5 (0‐21), P = 0.13 |
|
| Notes | Further information provided by authors PDE5 (phosphodiesterase yype 5) inhibitor is used for erectile dysfunction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized prospective trial" and “randomized by a draw” |
| Allocation concealment (selection bias) | Low risk | Used opaque sealed envelopes |
| Blinding of participants (performance bias) | High risk | “It was not possible to create a believable sham device, which could maintain blinding of the study subjects” |
| Blinding of personnel (performance bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 from group A (3 excluded because underwent non‐nerve sparing surgery, 2 withdrew consent, 1 lost a partner, 6 non‐compliance), 3 from group B (2 excluded because underwent non‐nerve sparing surgery, 1 withdrew consent). Differential dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Low risk | “This study was funded by unrestricted grants from the Velux Foundation and Grosserer L.F. Foghts Foundation” |
| Approved by medical ethics committee | Low risk | “The study was approved by the Danish ethical counsel and the Danish Data protection Agency” |
| Informed consent | Low risk | Assumed as they acquired ethical approval |
| ITT analysis | Low risk | Assumed from patient flow diagram |
Franke 1998.
| Methods | Randomised: yes Method of allocation: not stated Blinding: none Dropouts: 2 with gravitational incontinence consistent with intrinsic sphincter deficiency Intention to treat: not clear | |
| Participants | Recruitment: post‐operative Included: men incontinence post‐radical prostatectomy at 6 weeks post surgery N = 30 men: 6 weeks post‐radical prostatectomy with post‐void residual of < 50 ml; no previous TURP, no urinary tract infection, no neurological conditions |
|
| Interventions | Post‐operative intervention. Group A (13): intervention, biofeedback (perineal patch EMG) enhanced PFMT; exercise treatment sessions at 6, 7, 9, 11, and 16 weeks post‐operatively Group B (10): control, completed bladder diary but did not have any other intervention Length of follow‐up: 12 months |
|
| Outcomes | Main outcome: urine loss measured by voiding diary, 48 hour pad test (reported as mean grams of urine lost in 24 hours), and incontinence questionnaire Continence definition: not clear. Participants described as "completely dry" or with "significant incontinence" Data collection: 6, 12 and 24 weeks There were no significant differences between treatment or control groups on any of the outcome measures at any of the measurement intervals |
|
| Notes | Numbers in the groups unclear as 5 withdrew from the study after initial randomisation. Not clear how many were in each group prior to follow‐up at 6 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Blinding not possible. Therefore judged to be at high risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Five men withdrew after initial randomisation. Dropouts from 25 left at 6 weeks appears to be 10 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Informed consent was obtained" |
| ITT analysis | Unclear risk | Not specified |
Geraerts 2013.
| Methods | RCT | |
| Participants |
Time of recruitment: pre‐operative Population: men having a radical prostatectomy (whole population, with or without UI) Included: men planning to undergo open radical prostatectomy (ORP) or robot‐assisted laparoscopic radical prostatectomy (RARP) Willing to accept ambulatory visits once a week until total continence was achieved; willing to perform measurements pre‐operatively and at 1 month, 3 months, 6 months and 12 months after surgery Excluded: cognitive problems; non‐Dutch speaking; simultaneous other surgery; transport problems; lack of time; psychosocial/other medical problems; refused participation; insisted on preoperative PFMT; not approachable; not enough time between diagnosis and date of planned surgery Age (mean, SD): A 62 (5.90); B 62 (6.33) Dropouts: 6 from A; (1 died, 1 cerebrovascular accident, 3 transport problems, 1 refused further participation) 4 from B: (2 transport problems, 2 refused further participation). Not differential dropout Baseline characteristics: Comparable at baseline |
|
| Interventions |
Time of intervention: pre‐operative A (85): 30 mins of guided PFMT + biofeedback weekly for 3 weeks before surgery instructed to: carry out 60 contractions a day at home; contract their pelvic floor while coughing, and sitting down or getting up from a chair; restart PFMT on day 4 after surgery while catheter was in situ B (85): instructed to start PFMT on the day after catheter removal (e.g. 2 to 3 weeks after surgery) All men performed an individual guided exercise programme with digital or EMG biofeedback postoperatively weekly, delivered by a therapist (blinded to group allocation) different from the pre‐operative Group A therapist. This included advice on using PF muscles to prevent leakage during functional activities Duration of treatment: as long as any degree of UI persisted Follow up: 1 month, 3 months, 6 months and 12 months after surgery |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (1 hour pad test defined as ≤ 1 g) 1 month: A 37/85; B 35/86, P = 0.758 3 months: A 15/86; B 15/86, P = 1.000 6 months: A 8/86; B 5/85, P = 0.566 12 months: A 7/81; B 7/83, P = 1.000 Other outcomes Cumulative incidence of number of continent men 1 month: A 44/85; B 44/85 3 months: A 67/85; B 71/85 6 months: A 80/85; B 80/85 12 months: A 83/85; B 81/85 Point prevalence of continence, 1 hour pad test, defined as 0 g 1 month: A 42/85; B 41/86 3 months: A 63/86; B 61/86 6 months: A 76/86; B 73/85 12 months: A 68/81; B 73/83 Point prevalence of continence, VAS scale, defined as ≤ 1/10 1 month: A 35/89; B 38/88 3 months: A 64/88; B 52/87 6 months: A 73/88; B 65/86 12 months: A 72/84; B 62/84 Urine loss on 24 hour pad test in grams (mean (SD) N): 1 month: A 90 (?) 85; B 85 (?) 85 3 months: A 17 (?) 85; B 13 (?) 85 6 months: A 12 (?) 85; B 3 (?) 85 12 months: A 2 (?) 85; B 3 (?) 85 Quality of life International prostate Symptom Score (IPSS), King’s Health Questionnaire (KHQ): data not given Only one aspect of the King’s Health Questionnaire, incontinence impact, favoured A at 3 (P = 0.008) and 6 months (P = 0.024) after surgery |
|
| Notes | Some men had pre‐operative incontinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence of randomisation was carried out using a “computer program” and was “determined by the patients’ presence at the outpatient urology clinic”. It is unclear what influence the patients’ presence had on randomisation |
| Allocation concealment (selection bias) | Low risk | “Allocation to the treatment groups was concealed”. Method not reported |
| Blinding of participants (performance bias) | High risk | Blinding was not possible for participants |
| Blinding of personnel (performance bias) | Low risk | Post‐operative treatment was delivered by a therapist who was blinded to group allocation and treatment delivered by the pre‐operative Group A therapist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “One blinded and well‐trained assessor performed the measurements” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: Group A: 6 (1 died, 1 cerebrovascular accident, 3 transport problems, 1 refused further participation); Group B: 4 (2 transport problems, 2 refused further participation) |
| Selective reporting (reporting bias) | High risk | Results not reported for quality of life outcomes |
| Financial support | Low risk | Unconditional funding from the “Agency for innovation by Science and Technology (Applied Biomedical Research): governmental grant” |
| Approved by medical ethics committee | Low risk | “Ethical approval from the commission on medical ethics of the University Hospitals Leuven” |
| Informed consent | Low risk | Patients “signed written informed consent” |
| ITT analysis | Low risk | “Data were analyzed according to the intention‐to‐treat principle” |
Ghanem 2013.
| Methods | RCT | |
| Participants |
Time of recruitment: pre‐operative Population: 100 men undergoing a radical prostatectomy (whole population, with or without UI) Included: men undergoing RP for clinically localized prostate cancer. Excluded: patients who had previous pelvic organ surgeries, patients with central or peripheral neurologic diseases Age (mean, SD): not reported Dropouts: not reported Baseline characteristics: not reported |
|
| Interventions |
Time of intervention: pre‐operative (post‐operative treatment for all men) A (50): pre‐operative PFMT for 2 weeks + post‐operative PFMT programme B (50): post‐operative PFMT programme only Duration of treatment Follow‐up: 3.5, 4.5, 12, 13 and 13.5 months |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (defined as using > 1 pad on pad test) 12 months: A 2/50; B 3/50 13 months: A 2/50; B2/50 Other outcomes Quality of life ICS male SF questionnaire, results not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were divided randomly” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to treatment not possible |
| Blinding of personnel (performance bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | “Faculty of Physical Therapy Ethical committee, Cairo University” |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
Glazener RP 2011.
| Methods | RCT | |
| Participants | Recruitment: post‐operative Included: men with persistent urinary incontinence at 6 weeks after radical prostatectomy Excluded: radiotherapy planned; unable to comply with study or intervention; previous formal PFMT Age (mean, SD): A 62.4 (5.8); B 62.3 (5.6) |
|
| Interventions | A (205): one‐to‐one therapy sessions including PFMT and BT if OAB or urgency symptoms + PFMT and lifestyle leaflet Duration of treatment: 4 sessions in 3 months starting 6 weeks after surgery B (206): control group with standard care + lifestyle leaflet only, no individual PFMT instruction or sessions |
|
| Outcomes | UI defined as positive response to ICIQ‐SF questionnaire UI at 3 months: A 172/200, B 176/198 UI at 6 months: A 158/197, B 158/197 UI at 9 months: A 144/191, B 157/194 UI at 12 months: A 148/196, B 151/195 Severe UI at 12 months: A 74/196, B 78/195 UI episodes at 12 months from diaries (mean (SD N): A 3 (3.8) 105, B 2.9 (3) 106 ICI‐Q score at 12 months (mean (SD N): A 4.9 (4.1) 196, B 5.4 (4.5) 195 QoL due to UI at 12 months (mean (SD N): A 1.4 (2) 193, B 1.7 (2.3) 193 Use of pads at 12 months: A 63/159, B 68/161 Men not doing PFMT at 12 months: A 63/191, B 91/189 Erectile dysfunction (no erection): A 105/189, B 105/190 QALYs virtually identical Cost: NHS intervention cost was GBP 181 higher in intervention group (95% CI 107 to 255) Other outcomes: use of other protection, catheters, sheath catheters, urinary frequency, nocturia, faecal incontinence, urgency, constipation, EQ5D, SF‐12 |
|
| Notes | Low dropout rates ICI‐Q score: 0 = no UI, no effect on QoL; 21 = maximum amount, frequency and effect on QoL QoL due to UI measured using ICIQ‐SF: 0 = no effect, 10 = maximum effect Compliance with therapy high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, minimised on centre, age and pre‐existing urinary incontinence |
| Allocation concealment (selection bias) | Low risk | Remote computer allocation |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible for men |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible for therapists |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes from questionnaires completed by men, data entry clerks blinded to group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential dropout from the groups |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods were reported |
| Financial support | Low risk | "The trial was funded by the National Institute of Health Research Health Technology Assessment (NIHR HTA) Programme (project number 03‐14‐03) and will be published in full in Health Technology Assessment. HSRU, HERU, and NMAHP RU are funded by the Chief Scientist Office of the Scottish Government Health Directorates" |
| Approved by medical ethics committee | Low risk | "Our trials were approved by the Multicentre Research Ethics Committee, Edinburgh, Scotland and overseen by an independent trial steering committee and a separate independent data monitoring committee" |
| Informed consent | Low risk | "All men gave signed informed consent" |
| ITT analysis | Low risk | "We used intention‐to‐treat analysis" |
Glazener TURP 2011.
| Methods | RCT | |
| Participants | Recruitment: post‐operative Included: men with persistent urinary incontinence at 6 weeks after transurethral resection of the prostate (TURP) Excluded: radiotherapy planned; channel TURP for palliation for prostate cancer; unable to comply with study or intervention; previous formal PFMT Age (mean, SD): A 68.2 (7.7); B 67.9 (8.1) |
|
| Interventions | A (220): one‐to‐one therapy sessions including PFMT and BT if OAB or urgency symptoms + PFMT and lifestyle leaflet Duration of treatment: 4 sessions in 3 months starting 6 weeks after surgery B (222): control group with standard care + lifestyle leaflet only, no individual PFMT instruction or sessions |
|
| Outcomes | UI defined as positive response to ICIQ‐short form questionnaire UI at 3 months: A 142/205, B 132/208 UI at 6 months: A 140/199, B 129/201 UI at 9 months: A 133/197, B 131/202 UI at 12 months: A 126/194, B 125/203 Severe UI at 12 months: A 48/194, B 49/203 UI episodes at 12 months from diaries (mean (SD N): A 1.4 (2.3) 175, B 1.2 (2.2) 179 ICI‐Q score at 12 months (mean (SD N): A 3.9 (3.7) 194, B 4 (4.3) 203 QoL due to UI at 12 months (mean (SD N): A 1.2 (1.9) 190, B 1.3 (2.2) 199 Use of pads at 12 months: A 24/146, B 24/136 Men not doing PFMT at 12 months: A 66/188, B 154/193 Erectile dysfunction (no erection): A 52/177, B 43/178 QALYs virtually identical Cost: NHS intervention cost was GBP 209 higher in intervention group (95% CI 147 to 271) Other outcomes: use of other protection, catheters, sheath catheters, urinary frequency, nocturia, faecal incontinence, urgency, constipation, EQ5D, SF‐12 |
|
| Notes | Low dropout rates ICI‐Q score: 0= no UI, no effect on QoL; 21 = maximum amount, frequency and effect on QoL QoL due to UI measured using ICIQ‐SF: 0 = no effect, 10 = maximum effect Compliance with therapy high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, minimised on centre, age and pre‐existing urinary incontinence |
| Allocation concealment (selection bias) | Low risk | Remote computer allocation |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible for men |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible for therapists |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes from questionnaires completed by men, data entry clerks blinded to group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential dropout from the groups |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods were reported |
| Financial support | Low risk | "The trial was funded by the National Institute of Health Research Health Technology Assessment (NIHR HTA) Programme (project number 03‐14‐03) and will be published in full in Health Technology Assessment. HSRU, HERU, and NMAHP RU are funded by the Chief Scientist Office of the Scottish Government Health Directorates" |
| Approved by medical ethics committee | Low risk | "Our trials were approved by the Multicentre Research Ethics Committee, Edinburgh, Scotland and overseen by an independent trial steering committee and a separate independent data monitoring committee" |
| Informed consent | Low risk | "All men gave signed informed consent" |
| ITT analysis | Low risk | "We used intention‐to‐treat analysis" |
Goode 2009.
| Methods | Randomised controlled trial | |
| Participants | Recruitment: post‐operative Included: men incontinent 1 to 16 years after radical prostatectomy (mean years since operation: A 5.1, B 3.9, C 5.1) N = 208 (prior to dropout). Analysis of 172 men at 8 weeks Age between 51 to 84 years % of men with prior PFMT instruction: A 36%, B 56%, C 47% % of men using antimuscarinics: A 16%, B 20%, C 28% % of men with urgency UI: A 1%, B 3%, C 2% % of men with stress UI: A 44%, B 47%, C 44% % of men with mixed UI: A 54%, B 50%, C 54% |
|
| Interventions | A (70): behavioural therapy with PFMT alone for 8 weeks B (70): behavioural therapy with biofeedback and electrical stimulation for 8 weeks C (68): control, no treatment for 8 weeks, then offered choice of intervention A or B Behavioural therapy consisted of pelvic floor muscle exercises and bladder control strategies in both groups Dropouts: A 19 at 6 months, 23 at 12 months; B 22 at 6 months, 36 at 12 months; C 3 at 8 weeks Length of follow‐up: 12 months for groups A and B C transferred to treatment at 8 weeks so no further follow up possible |
|
| Outcomes | Frequency of UI, mean accidents in a week Number of continent men at 8 weeks: A 11/70, B 12/70, C 4/68 Incontinence episodes per day at 8 weeks (mean, SD, N): A 1.86 (0.56) 58; B 1.71 (0.54) 54; C: 3 (1.17) 64 Change in quality of life at 8 weeks using EPIC UI subscale (bigger change is better, mean, SD, N): A 13.1 (15.5) 58; B 12.3 (14.6) 54; C 2.9 (12.4) 64 Adverse events: A 0/70, B 2/70 (haemorrhoidal irritation), C 0/68 Patient's Global Perceptions of Improvement (much better): A 90%, B 91%, C 10% Completely satisfied with treatment progress: A 47%, B 47%, C not reported Compliance with PFMT and bladder control strategies at 8 weeks: A 100%, B 93% Compliance at 6 months: A 82%, B 84% Compliance at 12 months: A 91%, B 81% |
|
| Notes | Some baseline differences between groups, did not quite reach statistical significance High dropout rates No data available for control group after eight weeks as all received treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by site, type and frequency of UI, generated by computer programme |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, opened sequentially |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | Data entry staff blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes from questionnaires completed by men, data entry staff blinded to group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis and reported tables on 172 men |
| Selective reporting (reporting bias) | Low risk | Results of outcomes reported |
| Financial support | Low risk | National Institutes of Health ‐ National Institute of Diabetes and Digestive and Kidney Diseases, grant R01 DK60044‐01A2 |
| Approved by medical ethics committee | Low risk | Approved by "University of Alabama at Birmingham Institutional Review Board" |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
Hoffman 2005.
| Methods | Randomised: yes Method of allocation: computerised randomisation Blinding: unclear Dropouts: 1 participant from each intervention group had dropped out by discharge; 15 dropouts from the perineal group, 31 from the anal group and 5 from the control group dropped out by 3 months Intention to treat: no | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy in an inpatient rehabilitation program N= 180 men (prior to dropouts). Randomly assigned to 3 groups (60 in each group) |
|
| Interventions | Post‐operative intervention
Group A (60) intervention: perineal ES plus physiotherapy (PFMT) Group B (60) intervention: anal ES plus physiotherapy (PFMT) Group C (60) control: PFMT alone. Length of follow‐up: 3 months |
|
| Outcomes | Main outcome: urine loss measure on 1 hour pad test Secondary outcomes: quality of life (QLQ‐C30) Continence definition: self‐reports of incontinence Data collection: admission and discharge from the rehabilitation program and at 3 months after discharge All groups improved on continence and quality of life. Use of ES was only of additional value in a compliant subgroup. Perineal ES was better accepted than anal |
|
| Notes | Additional data supplied to KFH by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not specified |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 22 out of 60 in anal ES group, 4 out of 60 in perineal ES group. No reasons for dropouts given |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | High risk | No intention‐to‐treat analysis; insufficient information on methods of statistical analysis; interventions unclear and insufficiently specified |
Hou 2013.
| Methods | RCT | |
| Participants |
Time of recruitment: pre‐operative Population: 66 men who underwent TURP (whole population, with or without UI) Included: patients with benign prostatic hyperplasia and underwent TURP, aged 60 to 90 years, remarkable lower urinary tract symptoms (LUTS) with poor response to medication, ambulatory, able to communicate verbally Excluded: indwelling catheter‐dependent postdischarge, neurogenic bladder, dementia or disability affecting verbal communication Age (mean, SD): A 69.67 (6.09); B 71.41 (6.67) Dropouts: 5 (2 catheter still in situ after discharge from hospital, 3 lost to follow‐up). Not differential dropout Baseline characteristics: comparable at baseline |
|
| Interventions |
Time of intervention: post‐operative treatment A (32): guided PFMT + EMG biofeedback after catheter removal (2 days postoperatively), instructed to: contract pelvic muscles for 5 seconds and relax for 10 seconds. After discharge, patients were instructed to carry out 5 mins of each PFE three times daily. Patients also received motivational telephone interviews once weekly B (29): no description Duration of treatment: 12 weeks Follow up: 1 week, 1 month, 2 months and 3 months |
|
| Outcomes |
Primary outcome (number of men with UI) Not reported Other outcomes Quality of life SF‐36 scores (mean (SD) N) Physical component 3 months: A 54.86 (8.62) 32; B 49.86 (11.23) 29 Physical functioning 3 months: A 89.69 (17.13) 32; B 85.82 (21.60) 29 Body pain 3 months: A 93.66 (15.16) 32; B 89.48 (22.71) 29 General health 3 months: A 82.03 (14.05) 32; B 64.93 (27.16) 29 Physical role limitation 3 months: A 68.75 (36.48) 32; B 51.72 (38.92) 29 Mental health component 3 months: A 56.21 (6.20) 32; B 48.52 (11.94) 29 Mental role limitation 3 months: A 93.75 (21.48) 32; B 73.81 (37.80) 29 Vitality 3 months: A 80.47 (13.16) 32; B 64.14 (24.02) 29 Mental health 3 months: A 88.00 (10.51) 32; B 77.38 (18.68) 29 Social functioning 3 months: A 90.63 (14.20) 32; B 76.29 (29.57) 29 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly classified" |
| Allocation concealment (selection bias) | Unclear risk | "randomly classified" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention was not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (2 catheter still in situ after discharge from hospital, 3 lost to follow‐up). Not differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Joseph 2000.
| Methods | Randomisation: yes Method of allocation: not described Blinding: none Dropouts: 3 did not return to clinic for all appointments, one had other health problems Intention to treat: no | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy or post‐TURP. UI of at least 6 months duration N = 11 patients at least 6 months post‐surgery (4 radical retropubic, 6 radical peritoneal, 1 TURP) |
|
| Interventions | Post‐operative intervention Group A (6): intervention: Instruction in PFMT including biofeedback with visual feedback as well as verbal to assist in identifying and discriminating muscles Group B (5): comparator: Instruction in PFMT, squeezing of finger during digital rectal examination Both: weekly visit for a total of 4 clinic visits Length of follow‐up: 12 months |
|
| Outcomes | Main outcome: urine loss measure by standardised pad test, bladder diary, subjective estimation of degree of incontinence Secondary outcomes: leak point pressure measured by video‐urodynamics, Joseph Continence Assessment Tool Continence definition: subjective evaluation by participants Data collection: baseline, 3, 6, and 12 months No differences between the groups. Improvement seen in all patients at 12 months |
|
| Notes | Data not published in article. Raw data supplied to review author (KFH) who calculated means and standard deviations. These were reviewed by a second review author (KNM) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Reported as "Randomised". No additional information provided |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk.\ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | High risk | No |
Koo 2009.
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative Included: men with UI after radical prostatectomy Randomised: N = 32 |
|
| Interventions | A (16) intervention: extra‐corporeal magnetic innervation (ExMI), treatment sessions were for 20 minutes twice weekly for 8 weeks B (16) control: PFMT alone. Duration of treatment not specified Length of follow‐up: six months |
|
| Outcomes | 24 hour pad test, g of urine Baseline: A 655, B 646 1 month: A 147, B 187 2 months: A 33, B 81, P = 0.001 3 months: A 9 (SD 28), B 45 (28), P = 0.001 6 months: Less than 10 g in both groups Number of pads used daily Baseline: A 4.2, B 4.1 I month: A 1.5, B 1.8 2months: A 0.6, B 0.9, P = 0.033 3 months: A 0.1 (0.42), B 0.6 (0.42), P = 0.002 6 months: A 0, B 0.1 Quality of life measured by I‐QoL |
|
| Notes | Awaiting further translation ‐ information from abstract only SDs calculated using P values |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description, Chinese language |
| Allocation concealment (selection bias) | Unclear risk | "Randomly assigned" |
| Blinding of participants (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported, Chinese language |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Laurienzo 2013.
| Methods | RCT | |
| Participants |
Time of recruitment: pre‐operative Population: men having a radical prostatectomy (whole population, with or without UI) Included: patients with prostate cancer (stage T2) and candidates for RPP who were referred for treatment Excluded: radiotherapy (previous or after RPP), previous transurethral resection, pre‐existing neurological disease, urinary fistula after RPP, prolonged indwelling urethral catheterization (more than 15 days), clinical situations that rendered the patient unsuitable for surgical procedure, failure to attend all PFMR or electrical stimulation sessions, loss of follow‐up and desistance Age (mean, SD): A 64 (8); B 62 (7); C 60 (8) Dropouts: 9 (2 failed to attend all sessions, 2 desistance, 1 adjuvant radiotherapy, 1 postoperative urethral stenosis, 1 urinary fistula, 1 unsuitable for surgery due to cardiovascular risk, 1 inadequate follow up) Unclear from which group Baseline characteristics: Comparable at baseline |
|
| Interventions |
Time of intervention: pre‐operative only A (15): standard treatment with verbal instructions for PFMT B (17): pre‐operative guided PFMT, with 10 physiotherapy sessions: contractions of the pelvic floor muscles for 5 seconds in “dorsal decubitus” position for 10 times, in the same position with the waist elevated (10 times), lying down with legs adducted against a plastic ball performed 10 times and standing and flexing the hips to 60̊ (10 times) C (17): pre‐operative PFMT + electrical stimulation during 10 physiotherapy sessions, electrical stimulation was with an anal probe lasting 15 minutes in total, and men also received guided PFMT and followed the same training regime as above Men did not receive PFMT post‐operatively Duration of treatment: 10 pre‐operative sessions Follow up: 1, 3 and 6 months |
|
| Outcomes |
Primary outcome (number of men with UI) Not reported Other outcomes 1 hour pad test score (mean (SD) N) 1 month: A 17.6 (38.5) 15; B 29.5 (35.8) 17; C 25.5 (35.4) 17 3 months:14.3 (34.4) 15; B 11.8 (28.4) 17; C 9.6 (18.8) 17 6 months: A 5.5 (14.16) 15; B 25.3 (59) 17; 4.35 (7.3) 17 Quality of life ICIQ‐SF score (mean (SD) N) 1 month: A 7.5 (5) 15; B 14 (3.6) 17; C 9.6 (6.3) 17 3 months: A 5.4 (5.2) 15; B 6.9 (5.8) 17; C 7.2 (6.4) 17 6 months: A 3.7 (5.3) 15; B 4.8 (5.3) 17; C 5.3 (5.5) 17 SF‐36 Results not reported: “There were no differences between groups on the various domains of the SF‐36 (p > 0.05)” |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomized (computer generated list using Randomizer, v4)” |
| Allocation concealment (selection bias) | Unclear risk | “The patients were randomized (computer generated list using Randomizer, v4)” |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | “PFMR was performed in the preoperative period by the same physiotherapist.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 (2 failed to attend all sessions, 2 desistance, 1 adjuvant radiotherapy, 1 post‐operative urethral stenosis, 1 urinary fistula, 1 unsuitable for surgery due to cardiovascular risk, 1 inadequate follow‐up). Unclear from which group |
| Selective reporting (reporting bias) | High risk | Results of SF‐36 not reported |
| Financial support | Low risk | “Sao Paulo State Foundation for Research Support – FAPESP (number 08/54585‐1)” |
| Approved by medical ethics committee | Low risk | “After approval by the ethical committee and internal review board, 58 consecutive males were included in this analysis” |
| Informed consent | Low risk | “All subjects received and signed an informed consent form” |
| ITT analysis | Low risk | Data presented for all men randomised and not excluded. No differential dropout apparent |
Liu 2008.
| Methods | Randomised controlled clinical trial | |
| Participants | Recruitment: post‐operative Included: men with UI after radical prostatectomy Randomised: N = 24 |
|
| Interventions | Group A (12) intervention: extra‐corporeal magnetic innervation (ExMI), the frequency of the pulse field was 10 Hz for 10 minutes, followed by a 3 minute rest and a second treatment of 50 Hz for 20 minutes. This was done twice a week Group B (12) control: PFMT alone, instructions given to carry out 20 mins x 3 a day Duration of treatment: six weeks Length of follow up: 1, 3 and 6 months |
|
| Outcomes | Main outcome measures: quality of life scale and the ICI‐Q‐SF 1 month: both scores were decreased with no significant differences between the groups At 3 and 6 months: both scores decreased with group A having a significantly lower (better) score than group B (P < 0.05) |
|
| Notes | Information from abstract, awaiting translation of paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "randomly assigned". No additional information provided |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 24 patients included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Low risk | Hospital board, local military university hospital |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Manassero 2007.
| Methods | Randomised: prospective randomised controlled trial Method of allocation: computer generated random numbers Blinding: blinded outcome assessors, not instructors Dropouts: 12 excluded as the couldn't attend regularly for PFMT; 33 continent after surgery and were not randomised; 13 lost to follow‐up in the control group (5 social reasons and 8 non‐responders) Intention to treat: no |
|
| Participants | Recruitment: post‐operative Included: men incontinent (UI > 2g/24 hour pad test), post‐radical prostatectomy who were able to attend hospital Excluded: those with a history of preoperative incontinence, significant perioperative complications, rectal lesion, infection, psychiatric neurological disorders, inability to contract PF muscles or weak contraction with increased detrusor activity Mean age: A 66.8 (6.3 years), B 67.9 (5.5 years) |
|
| Interventions | Group A (54) intervention: PFMT re‐education program, verbal feedback The training program involved active PFE. Verbal feedback of the contraction was used to instruct the patients to correctly and selectively contract their pelvic muscles while relaxing the abdominal muscles. The strength of the pelvic floor muscles was measured by digital anal control using a score of 0 to 5 ( 0 = no contraction, 5 = good contraction against strong resistance) Initially home practice comprised 45 contractions (3 sessions of 15) per day at home, progressively increasing the number until 90 per day. This was taught by two experienced urologists Group B (53) control: no treatment Duration of treatment: up to a year or until incontinence ceased Length of follow‐up: 1, 3, 6 and 12 months |
|
| Outcomes | UI at ‐ 1 month: A 83.3% (45/54), B 97.5% (39/40), P = 0.04 3 months: A 53.7% (29/54), B 77.5% (31/40), P = 0.03 6 months: A 33.3% (18/54), B 60% (24/40), P = 0.01 12 months: A 16.6% (9/54), B 52.5% (21/40), P < 0.01 Subjective assessment of continence using VAS: P = 0.01 at 12 months Quality of lIfe (single question): P = 0.03 at 12 months |
|
| Notes | ITT analysis used for data entry, assuming that all 13 men who dropped out of the control group were dry, because of differential dropout of 13 men from B versus none from A with no explanation for difference between groups If unable to contract anal sphincter or strength 2 or less, not randomised. These men were given ES treatment at home with anal probe |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Stratified on volume of urine lost on pad test |
| Blinding of participants (performance bias) | High risk | Blinding of intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinidng of intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential dropout of 13 from control group, ITT analysis used for data entry by review authors |
| Selective reporting (reporting bias) | Unclear risk | Outcomes in methods reported |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Low risk | "The study was approved by the Medical Centre Institutional Review Board" |
| Informed consent | Low risk | "All men provided informed consent" |
| ITT analysis | Low risk | Assumed from patient flow chart |
Marchiori 2010.
| Methods | RCT | |
| Participants |
Time of recruitment: post‐operative Population: men with incontinence after retropubic radical prostatectomy, open or laparoscopic Included: moderate to severe incontinence at 30 days after catheter removal Excluded: lack of cooperation, pre‐operative incontinence, early recovery of continence Age (mean): A 67; B 66.5 Dropouts: “Survey questionnaire were correctly filled in and returned by fewer than 10% of the patients” Baseline characteristics: comparable at baseline |
|
| Interventions |
Time of intervention: post‐operative treatment A (166): one‐to‐one guided PFMT + biofeedback during first session, second session involved 10 sets of pelvic floor electrical stimulation lasting 15 mins each, instructed to: carry out three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) B (166): received oral and written information on pelvic floor anatomy and on PFME, instructed to: perform three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) and continue for duration of All men received oral and written information on pelvic floor anatomy and on PFME, pelvic floor muscle endurance assessed by digital anal control + PFMT consisting of 3 sets of 30 contractions daily for the first month after catheter removal Duration of treatment Follow up: 3 months, 6 months and 12 months |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (defined as 0 or 2 minipads daily) 3 months: A 36/166; B 81/166 6.5 months: A 1/166; B 28/166 12 months: A 0/166; B 0/166 Other outcomes Median time of continence recovery, days: A 44 ± 2, B 76 ± 4, P ≤ 0.01 Quality of life ICIQ‐male: Results not reported RAND 36‐Item Health Survey questionnaire: results not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Prospectively randomized” Sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | "Prospectively randomized" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported for primary outcome |
| Selective reporting (reporting bias) | High risk | No reporting of primary outcome |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Mariotti 2009.
| Methods | Randomised: yes | |
| Participants | Randomised post‐operatively Included: radical prostatectomy, all men after catheter removal Age: Group A mean 61.86 years, Group B, 61.43 years |
|
| Interventions | Intervention post‐operative Group A (30) intervention: PFMT plus ES and biofeedback twice a week for 6 weeks ES ‐ a surface electrode was inserted into the anus and pulsed, the intensity was adequate to induce visual lifting of the levator ani and pubococcygeus muscle, considering the level of comfort to the patient Biofeedback ‐ via surface electrodes both perineal and abdominally Group B (30) control: instructions to conduct PFMT ‐ verbal and written instructions at catheter removal and follow‐up visits Duration of treatment: 6 weeks Length of follow up: 3 and 6 months |
|
| Outcomes | 24 hour pad test: g/24hrs, mean (SD) 3 months: A 16.67 (30.55), B 136.67 (152.62), P = 0.000 6 months: A 3.47 (14.67), B 27.83 (55.98), P = 0.0004 ICS‐male questionnaire, number of men incontinent, n/N 3 months: A 6/30, B 20/30 6 months: A 1/30, B 10/30 Time to regain continence: A 8 (6.49) weeks, B 13.88 (8.32) weeks, P = 0.003 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Consecutive patients |
| Allocation concealment (selection bias) | Unclear risk | Quote ‐ "Randomized fashion" |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "All patients signed an informed consent before randomization" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Martini 2011.
| Methods | RCT (abstract only) | |
| Participants |
Time of recruitment: pre‐operative Population: 70 consecutive men undergoing a laparoscopic radical prostatectomy (whole population, with or without UI) Included: men undergoing RP for clinically localized prostate cancer T1 to T3 Excluded: history of incontinence or overactive bladder, central or peripheral neurologic disease and cognitive impairment Age (mean, SD): not reported Dropouts: 5 lost to follow up, unclear from which group Baseline characteristics: not reported |
|
| Interventions |
Time of intervention: pre‐operative (post‐operative treatment for all men) A (24): PFMT: 5 sessions of guided PFMT for 2 to 3 weeks pre‐operatively and continued post‐operatively B (25): post‐operative standard care, written instructions for PFMT All men underwent clinical examination of pelvic muscles function using digital perineal testing according to “AIPDA score” and evaluation of voiding symptoms Duration of treatment: Follow up: 1, 3 and 6 months |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (need to wear a pad) No useable data Other outcomes 24 hour pad test Pad use Bladder diary Quality of life Instrument unspecified |
|
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomised” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to treatment not possible |
| Blinding of personnel (performance bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “Five patients lost at follow up”. Not clear why there were dropouts or from which group |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Mathewson‐Chapman 97.
| Methods | Randomised: yes, block procedure Method of allocation: not reported Blinding: none Dropouts: 2, not accounted for Intention to treat: not clear | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 53 men Randomised pre‐operatively |
|
| Interventions | Pre and post‐operative intervention Group A (27) intervention: pre‐operatively received further instruction and practice with PME protocol home exercises and biofeedback (anal probe) (Incare 8900); practiced at home 3 times a week, starting with daily 15 PFMT and increasing by 10 every 4 weeks to a maximum of 35 PFMT Group B (24) control: post‐operatively no further interventions until week 5 when pelvic muscle strength was assessed Both: pre‐operatively, both groups received 30 minutes' prostate education programme and baseline 'perineal muscle evaluation' (not defined); as well all were taught to contract the perineal muscle and hold for a few seconds prior to standing, lifting or coughing and limit the amount of tea, chocolate, alcohol and over‐the‐counter medications Length of follow‐up: 12 weeks |
|
| Outcomes | Main outcome: urine loss measured by 24 hour pad test, frequency of micturitions (self‐recorded bladder diary), number of pads used; days to achieve continence from baseline Secondary outcomes: perineal muscle strength (method not described) Continence definition: self‐report of return of continence Data collection: 3 day bladder diaries at weeks 2, 5, 9 and 12. 24 hour pad test at weeks 5 and 12 |
|
| Notes | Inclusion of other modalities such as caffeine limitation and using perineal muscles during any event which increased abdominal stress may have masked any treatment benefit Extra information obtained from thesis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block procedure |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Unclear risk | Not reported |
| Approved by medical ethics committee | Low risk | "Permission to conduct this study was obtained from the Univerisity of Florida Health Center Institutional Review Board (IRB)." |
| Informed consent | Low risk | "The informed consent was explained to each subject, and his signature was obtained to confirm consent to participate in the study" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Moore 1999.
| Methods | Randomised: yes
Method of allocation: sealed envelopes Blinding: physiotherapist blinded to results of control group Dropouts: 5 |
|
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy. Median duration of UI 8 weeks post‐surgery, range 4 to 200 weeks N = 63 men (53 completed study) Randomised to 3 groups |
|
| Interventions | Post‐operative intervention Intervention Group A (18) intervention: PFMT alone Group B (19) intervention: PFMT plus rectal electrical stimulation treated by one physiotherapist 30 minutes twice a week for 12 weeks Intervention groups also did home exercises 3 times/day gradually working up to 30 minutes per session lying, standing, sitting; strength, endurance, speed and control with maximum contractions of 5 to 10 seconds, 10 to 20 second relaxation and 12 to 20 repetitions; submaximum contractions at 65% to 75% of maximum strength with hold 20 to 30 seconds and equal rest time, 8 to 10 repetitions; speed was sets of quick repetitive contractions in a 10 second time span; control involved gradual recruitment to maximum contraction in 3 stages with 5 second hold at each stage and a slow release with rest 15 to 30 seconds Group C (21) control: oral and written information about PFMT pre and post‐operatively (standard treatment) Length of follow‐up: 24 weeks |
|
| Outcomes | Main outcome: urine loss measured by 24 hour pad test Secondary outcomes: quality of life measures (Incontinence Impact Questionnaire, European Organization for the research and treatment of Cancer‐EORTC QLQ C‐30, version 2), physical symptom inventory (adapted from Herr 1994) Continence definition: ≤ 2 g urine/24 hours Data collection: baseline, 12, 16, 24 weeks after baseline |
|
| Notes | Intervention perhaps administered too early ‐ all subjects improved at the same rate; wide range of severity of urinary incontinence at study entry and size of SD of pad test results also may have resulted in Type II error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned using a computer‐generated random‐number list placed in sealed envelopes at the end of the assessment visit, with patient and researcher opening the sealed envelope" |
| Allocation concealment (selection bias) | Low risk | "Participants were assigned using a computer‐generated random‐number list placed in sealed envelopes at the end of the assessment visit, with patient and researcher opening the sealed envelope" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Physiotherapist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (3 from group B, 2 from group A), 3 bladder neck contractures, 1 rectal pain when performing exercises, 1 vacation for 4 months). No differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "Funding for the research project was received from the Oncology Nurses' Society, Canadian Nurses' Foundation, Caritas Health, Alberta Physiotherapy Association, Edna Minton Foundation, and the University of Alberta" |
| Approved by medical ethics committee | Low risk | "approved by the University of Alberta and Caritas Health Group ethics review boards" |
| Informed consent | Low risk | "All patients signed informed consent" |
| ITT analysis | High risk | Intention‐to‐treat‐analysis not performed |
Moore 2004.
| Methods | Randomised: yes (order of product testing: in threes to treatment block of 4 periods (1 no device, 3 with devices)
Block, multiple period cross‐over design using Latin square configuration
Method of allocation: sealed envelopes. Blinding: research assistant not involved in study chose envelope; but research assistant and participants could not be blinded to intervention Dropouts: none Intention to treat: not discussed |
|
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy who required continuous pad protection for stress incontinence Inclusion criteria: normal perineal and penile sensation, intact penile skin, sufficient manual dexterity Exclusion criteria: overactive bladder, neurological disorders affecting sensation or circulation, cognitive impairment. N = 12 men |
|
| Interventions | Post‐operative intervention Each participant had 4 periods (each lasted 1 day) Group A: no device Group B: C3 device Group C: U‐Tex device Group D: Cunningham clamp |
|
| Outcomes | Main outcome: 4 hour pad test Secondary outcomes: resistive index, cavernosal flow None of the devices completely eliminated urine loss when applied at a comfortable pressure. Each device showed improvement in terms of urine lost, with Cunningham clamp having the lowest mean loss Cunningham clamp significantly lowered flow, but ranked positively by participants |
|
| Notes | Unable to blind participants and research assistant to intervention Sample size calculation given and required size achieved | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomized list of device assignments was prepared by one of the investigators" Block, multiple period crossover design using Latin square configuration |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, research assistant not involved in study chose envelope |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "A research assistant not directly involved with recruitment or data collection entered the data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Financial support | Low risk | "This study was supported by the University of Alberta Internal Allocations Fund and Department of Radiology, University of Alberta Hospital." |
| Approved by medical ethics committee | Low risk | "The Institutional review Board at the University of Alberta approved the study" |
| Informed consent | Low risk | "the study was explained and informed consent obtained" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Moore 2008.
| Methods | Randomised: yes Method of allocation: computer generated list of numbers; group allocation placed in sealed opaque envelopes; opened by subject after initial post‐operation instruction session with therapist Blinding: data entry by clerk blinded to group; therapist blinded to outcome of non‐intervention group; pads weighed by third party Dropouts: control = 7; treatment = 12 | |
| Participants | Recruitment: post‐operative (but approached before surgery) Included: men incontinent after radical prostatectomy (> 8 grams urine lost on 24 hour pad test) at 4 weeks post‐surgery N = 217 men from 3 centres with early stage prostate cancer Inclusion criteria: English speaking, living within 1 hour drive of research centre |
|
| Interventions | Post‐operative intervention Group A (106) intervention: maximum 24 weekly, 30 minute treatment protocol (30 min biofeedback‐assisted PFMT) and home exercise protocol of 2 to 3 times a day Group B (99) control: verbal and written information on PFME and weekly telephone contact by a urology nurse Both: at 4 weeks post‐surgery, both groups received standardised verbal and written instruction about PFMT and recovery after radical prostatectomy by one dedicated physiotherapist or registered nurse at each site Length of follow‐up: 12 months |
|
| Outcomes | Main outcome: grams of urine loss on 24 hour pad test (> 8 g defined as incontinence) Definition of continence: < 8 g of urine loss on 24 hour pad test; subjective continence defined as yes or no Secondary outcome: IPSS, IIQ‐7 (Incontinence Impact Questionnaire), voiding diary, and subjective continence All measures obtained at baseline (pre‐operatively) and at 4, 8, 12, 28 weeks and 1 year post‐operatively 24 hour pad test, mean (SD) N 12 weeks: A 115 (300) 93, B 72 (144) 82 16 weeks: A 76 (259) 94, B 61 (194) 80 28 weeks: A 45 (142) 87, B 35 (101) 74 12 months: A 47 (215) 89, B 8 (10) 78 Dry at 8 weeks: A 20/101 (20%), B 20/88 (23%) Dry at 12 weeks: A 30/93 (32%), B 23/82 (28%) Dry at 16 weeks: A 41/94 (44%), B 32/80 (40%) Dry at 28 weeks: A 41/87 (47%). B 37/74 (50%) Dry at 12 months: A 53/89 60%, B 47/78 60% (< 8 g on pad test) No significant differences between groups on continence or on symptom and quality of life measures or diary at any time point post‐operatively Cost: A: CAD 400; B 240 Adverse events: none in either group The majority of men reported a low impact of incontinence as per the IIQ‐7 and fewer LUTS at 12 months than at baseline on the IPSS. The majority were very satisfied with treatment and support from the continence nurse |
|
| Notes | Groups comparable at pre‐operation baseline on PSA, Gleason score, IPPS, IIQ, pad test and voiding diary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers, random blocked allocation to groups |
| Allocation concealment (selection bias) | Low risk | Group allocation placed in sealed opaque envelopes; opened by participant after initial post‐operation instruction session with therapist |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Therapist blinded to outcome of non‐intervention group; pads weighed by third party |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data entry by clerk blinded to group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: control = 7; treatment = 12; no differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | "Funded by the Alberta Heritage Foundation for Medical Research, the Northern Alberta Urology Foundation, and Pfizer Corporation (unrestricted)" |
| Approved by medical ethics committee | Low risk | "Healthcare ethics approval was obtained at all sites" |
| Informed consent | Low risk | "After the consent form was signed, baseline data were collected" |
| ITT analysis | Low risk | Patient flow chart give details of patient dropouts and withdrawals |
Morihiro 2011.
| Methods | RCT abstract only | |
| Participants |
Time of recruitment: not reported Population: men having laparoscopic radical prostatectomy Included: patients who underwent laparoscopic radical prostatectomy performed by a single surgeon Excluded: not reported Age (mean, SD): not reported Dropouts: not reported Baseline characteristics: comparable at baseline |
|
| Interventions |
Time of intervention: post‐operative treatment for all men A (20): PFMT + sacral surface therapeutic ES (ssTES), ssTES 2 times a day for 15 minutes each, lasting 1 month after catheter removal (day 5) B (14): PFMT only, carried out alone Duration of treatment: 1 month Follow‐up: 1 month, 3 months, 6 months and 12 months post‐operatively |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (defined as requirement for a pad to keep clothing dry) 6 months: A 3/20; B 6/14 12 months: A 0/20; B 5/14 Other outcomes Recovery rate of urinary continence (defined as no requirement for a pad to keep clothing dry) 6 months: A 17/20, B 8/14 12 months: A 20/20, B 9/14, P = 0.007 Quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not enough information. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | “ethics committee of Kitasato university of medicine” |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | No description. Therefore judged to be unclear risk |
Nowak 2007.
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: men undergoing radical prostatectomy Aged: 59 to 72 years |
|
| Interventions | Group A intervention: extra‐corporeal magnetic innervation (ExMI) based pelvic floor device Group B control: PFMT alone Treatment initiated one week after catheter removal Duration of treatment: 10 weeks Length of follow‐up: 12 months |
|
| Outcomes | On first day following catheter removal 16.8% of patients were continent Subsequent follow‐up data unclear if N = 105 or 88 subjects. Group numbers not stated UI at ‐ 4 weeks: A 49%, B 56% 3 months: A 36%, B 50% 6 months; A 18%, B 32% Twenty minute pad test at 12 months, significantly better in Group A at 12 months, P =0.004 QoL score and urinary symptom inventory also carried out, numbers not given |
|
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One patient withdrew from Group A for non‐medical reasons |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | No description. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | No description. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | No description. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Opsomer 1994.
| Methods | Randomised: yes Method of allocation: method not described Blinding: none Dropouts: 4 Intention to treat: not specified | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy 6 weeks after six week after surgery N = 43 (39 completed study) |
|
| Interventions | Post‐operative intervention Group A (21) intervention: PFMT plus biofeedback plus ES directed by physiotherapist Group B (22) control: PFME on their own without medical supervision Length of follow‐up: 12 weeks |
|
| Outcomes | Main outcome: urine loss measured by pad test No statistical difference between groups as to recovery of continence |
|
| Notes | Abstract only ‐ unable to contact author for further data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | No description. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | No description. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | No description. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Overgard 2008.
| Methods | Randomised: yes | |
| Participants | Recruitment: Pre‐operative Included: radical prostatectomy, all men Age: Group A 48 to 68 years, Group B 49 to 72 years |
|
| Interventions | Intervention: post operative Group A (38) intervention: instructions on PFMT and physiotherapy, 45 minutes weekly. Patients were instructed to perform 3 sets of contractions daily at home, in either a supine, sitting or standing position. Digital anal palpation to teach correct contractions, as well as oral and written instructions DVD of instructions given to those living too far from hospital Group B (42) control: instructions on PFMT alone Duration of treatment: up to 1 year Length of follow‐up: 3, 6 and 12 months |
|
| Outcomes | Self‐reported continence (not using pads) 3 months: A 16/35 (46%), B 17/40 (43%), P = 0.73 6 months: A 27/34 (79%), B 22/38 (58%), P = 0.061 12 months: A 33/36 (92%), B 28/39 (72%), P = 0.028 24 hour pad test: g/24hrs, mean (range) 3 months: A 17 (0‐282), B 7 (0‐46), P = 0.53 6 months: A 9 (0‐203), B 2 (0‐12), P = 0.73 12 months: A 2 (0‐55), B 1 (0‐14), P = 0.95 PFM strength (anal squeeze pressure, cm H2O), mean (SD) 3 months: A 50.7 (23.9), B 55.7 (25.6), P = 0.398 6 months: A 56.1 (21.7), B 65.8 (27.0), P = 0.117 12 months: A 64.0 (24.0), B 71.5 (26.2), P = 0.237. |
|
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Norwegian University performed the computerised randomisation procedure immediately after pre‐operative test |
| Allocation concealment (selection bias) | Low risk | Norwegian University performed the computerised randomisation procedure immediately after pre‐operative test. Urologist no prior knowledge of randomisation procedure |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop out rate was 6% Four lost to follow up in physiotherapy group, one lost in instructions only group |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Low risk | "The work was funded by The Norwegian Fund for Postgraduate Training in Physiotherapy and The Norwegian Cancer Society" |
| Approved by medical ethics committee | Low risk | "The study was approved by the Regional Committee for Medical and Health Research Ethics" |
| Informed consent | Low risk | "Eighty‐five men provided written informed consent" |
| ITT analysis | Low risk | Assumed from patient flow chart |
Parekh 2003.
| Methods | Randomised: yes Method of allocation: not described Blinding: none Dropouts: 1 from each of the control and treatment groups. Reasons not described Intention to treat: yes, dropouts categorised as incontinent | |
| Participants | Recruitment: pre‐operative Included: all men scheduled for radical prostatectomy N = 38 patients with localized carcinoma of the prostate |
|
| Interventions | Pre and post‐operative interventions Group A (19) intervention: 2 treatment sessions pre‐operatively. Session 1 consisted of PFMT in a hook lying position Session 2 was on an exercise ball. Teaching methods varied and included verbal cues, visualization with an anatomical model, palpation or biofeedback with rectal probe. Post‐operatively, PFMT was reviewed and participants were seen every 3 weeks for 3 months by a physiotherapist Home exercise for 6 months or more for those requiring further physical therapy guidance Group B (19) control: no formal education on PFMT pre‐operatively, telephone or face to face follow‐up at least monthly Length of follow‐up: 12 months |
|
| Outcomes | Main outcome: urine loss measured by number of pads used daily Continence definition: 0 pads or 1 precautionary pad used Data collection: UI questionnaires at 6, 12, 16, 20, 28, and 52 weeks Greater number of the intervention group gained continence earlier than the control group at 3 months (only point of statistical difference). Minimal long‐term effect as continence rates the same at 1 year |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were enrolled in prospective, randomized fashion into a treatment or a control group" |
| Allocation concealment (selection bias) | Unclear risk | "Randomly assigned" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout from each arm. Categorised as incontinent |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Park 2012.
| Methods | RCT | |
| Participants |
Time of recruitment: post‐operative Population: 121 men who underwent radical prostatectomy (whole population, with or without UI) Included: elderly male patients aged ≥ 65 years, clinically localized prostate cancer (cT1 to T2), Eastern Cooperative Oncology Group performance status of 0 or 1, and written informed consent Excluded: adjuvant or neoadjuvant therapy, severe postoperative complications, a history of intrapelvic surgery, diseases that can affect voiding function, and limitations for exercise intervention, such as patients with serious cardiovascular events or spinal or articular disease Age (mean, SD): A 69.1 (5.7); B 69.4 (7.2) Dropouts: A: 7 (1 orthopaedic surgery for a pre‐existing ankle problem, 1 transurethral surgery for urethral stricture, 4 non‐compliance with follow‐up due to a long distance from the centre to the home or personal affairs, 1 new employment after surgery) B: 8 (1 ophthalmologic surgery for a cataract, 2 adjuvant radiotherapy, 4 non‐compliance with follow‐up due to a long distance from the center to the home or personal affairs, 1 new employment after surgery) Not differential dropout Baseline characteristics: comparable at baseline |
|
| Interventions |
Time of intervention: post‐operative treatment for all men A (26): patients performed Kegel exercises twice weekly, together with other types of exercises which included resistance training and pelvic flexibility. The intervention started 3 weeks after surgery and lasted 12 weeks B (23): ‘In the control group, only kegel exercises were performed’ Duration of treatment: 15 weeks Follow‐up: 1 week before surgery, 3 weeks and 15 weeks after surgery |
|
| Outcomes |
Primary outcome (number of men with UI) Cumulative number of incontinent men [defined as > 1 g on 24 hour pad test) 15 weeks: A 7/26; B 13/23 Other outcomes Cumulative number of continent men [defined as < 1 g on 24 hour pad test) 15 weeks: A 19/26; B 10/23, P = 0.035 Urine loss in grams using 24 hour pad test (mean (SD) N) 1 week before surgery: A 0 (NR) 26; B 0 (NR) 23 3 weeks post‐operatively: A 60 (NR) 26; B 83 (NR) 23 15 weeks: A 12 (NR) 26; B 46 (NR) 23 Quality of life ICIQ score (mean (SD) N) 1 week before surgery: A 4 (NR) 26; B 3 (NR) 23 3 weeks post‐operatively: A 10 (NR) 26; B 10 (NR) 23 15 weeks: A 6 (NR) 26; B 10 (NR) 23 SF‐36 physical composite score (mean (SD) N) 1 week before surgery: A 57 (NR) 26; B 54 (NR) 23 3 weeks post‐operatively: A 45 (NR) 26; B 44 (NR) 23 15 weeks: A 57 (NR) 26; B 48 (NR) 23 SF‐36 mental composite score (mean (SD) N) 1 week before surgery: A 45 (NR) 26; B 44.6 (NR) 23 3 weeks post‐operatively: A 44 (NR) 26; B 43 (NR) 23 15 weeks: A 49 (NR) 26; B 46 (NR) 23 Beck Depression Inventory (mean (SD) N) 1 week before surgery: A 9 (NR) 26; B 7.4 (NR) 23 3 weeks post‐operatively: A 8 (NR) 26; B 9 (NR) 23 15 weeks: A 6 (NR) 26; B 9 (NR) 23 NR = Not reported |
|
| Notes | Details of the combined exercise regime Post‐operative weeks 1 to 4 1) Education about post‐operative symptoms 2) Performing Kegel exercises, recognizing the parapelvic muscles 3) Pelvic floor flexibility fitness: performing pelvic exercises while sitting on a ball Post‐operative weeks 5 to 8 (ball exercises) 1) Performing pelvic exercises while sitting on a ball 2) Performing lower extremity exercises while placing a ball on the wall 3) Lifting a heel on the ball while standing face‐to‐face with the wall 4) Lifting up and down on the ball while spreading and bending legs 5) Performing flank exercises while having a ball in the hand 6) Squeezing the ball with the adductor muscles while lying on a table Post‐operative weeks 9‐12 (elastic band exercises) 1) Lifting the object with an elastic band lateral, anterior, and posterior to the patient’s arms 2) Lifting the legs and then spreading them while attaching an elastic band to the foot Further information provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A random number generator was used to determine the randomization allocation in a 1:1 ratio” |
| Allocation concealment (selection bias) | Low risk | “Sealed envelope, sequentially numbered, and opened by the trial nurse” |
| Blinding of participants (performance bias) | High risk | Blinding of participants was not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent assessor performed serial measurements |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A: 7 (1 orthopaedic surgery for a pre‐existing ankle problem, 1 transurethral surgery for urethral stricture, 4 non‐compliance with follow‐up due to a long distance from the centre to the home or personal affairs, 1 new employment after surgery) B: 8 (1 ophthalmologic surgery for a cataract, 2 adjuvant radiotherapy, 4 non‐compliance with follow up due to a long distance from the center to the home or personal affairs, 1 new employment after surgery) No differential dropout |
| Selective reporting (reporting bias) | Low risk | Results of outcomes reported |
| Financial support | Low risk | Unconditional funding from the “Medical Research Institute, Pusan National University Hospital, Busan, Korea.” |
| Approved by medical ethics committee | Low risk | “Our institutional review board approved this prospective, randomized, controlled trial” |
| Informed consent | Low risk | Patients signed “written informed consent” |
| ITT analysis | High risk | No |
Perissinotto 2008.
| Methods | Randomised: yes. Method of allocation: consecutive patients |
|
| Participants | Pre‐operative randomisation Included: all men undergoing radical prostatectomy Pre‐operative intervention Age: not given |
|
| Interventions | Group A (N not given) intervention: early pelvic floor rehabilitation program at home twice dally, Kegel exercises Group B (N not given) control: no formal PFMT Duration of treatment: for six months or until continence was achieved Length of follow‐up: at 3 and 6 months |
|
| Outcomes | PFM strength: P = 0.002 Quality of life using ICIQ‐SF not significant 24 hour pad test not significant |
|
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Consecutive patients. No additional information provided. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Randomised controlled trial. No additional information provided. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "FAPESP" (Sao Paulo Research Foundation) |
| Approved by medical ethics committee | Low risk | "COMITE DE ESTICA E PESQUISA ‐ UNICAMP" |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
Porru 2001.
| Methods | Randomised: yes Method of allocation: not described Blinding: report stated that urologist performing digital evaluation of pelvic floor muscle contraction was blinded to the study group Dropouts: intervention 2, control 1. Reason reported was non‐attendance at all clinic appointments Intention to treat: none | |
| Participants | Recruitment: pre‐operative Included: all men undergoing TURP N = 58 men (55 completed study) with benign prostatic hypertrophy randomised to 2 groups |
|
| Interventions | Pre and post‐operative intervention Group A (30) intervention: initial visit before surgery, digital evaluation of pelvic muscle contraction strength. Verbal instruction, feedback and reinforcement on contraction was given to teach selective contraction of anal sphincter and relaxation of abdominal muscles. Verbal and written instruction given for home PFMT. Weekly digital anal reassessment and grading of pelvic muscle contraction by the therapist. Instructed to practice contractions 45 times per day (3 groups of 15 contractions) Group B (28) control: not specified Both A and B: voiding diaries initiated after catheter removal Length of follow‐up: 4 weeks. Data collection at catheter removal and weekly for 4 weeks |
|
| Outcomes | Main outcome: urine loss (incontinence episodes) measured by 48 hour bladder diaries completed weekly Secondary outcomes Muscle contraction strength by digital evaluation Scale 0 to 4 (0 = none, 4 = strong) Pressure flow: urine flowmetry pre‐operatively and 1 month post‐operatively Symptoms: AUA (American Urological Association) symptom score preoperatively and 30 days after surgery Quality of life: ICS male questionnaire Significant increase in muscle strength in intervention group by week 4 Both groups showed improvement in symptom score and quality of life post‐operatively, no significant difference between groups Significantly better satisfaction with life in intervention group A compared to control B at 4 weeks Significant difference in voiding intervals between the groups at weeks 2 and 3, but not week 4 No difference in uroflowmetry Significantly less incontinence in the intervention group A at weeks 1, 2 and 3. No difference at week 4 Concluded that PFMT quickens the return to normal voiding post‐TURP |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | B ‐ 'randomised' |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Urologist performing digital evaluation of pelvic floor muscle contraction was blinded to the study group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "One urologist, who was blinded to the study group of the patients, performed only the digital evaluation of the pelvic floor muscle contraction and established and reported the grading during all the visits" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts due to non‐attendance at all clinic appointments (A 2, B 1) |
| Selective reporting (reporting bias) | Low risk | Results reported for outcomes stated in methods |
| Financial support | Unclear risk | No description. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | No description. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Informed consent was given by all patients" |
| ITT analysis | Unclear risk | Not specified |
Ribeiro 2008.
| Methods | Randomised: yes | |
| Participants | Post‐operative intervention Included: radical prostatectomy, all men after catheter removal Age: 51 to 76 years |
|
| Interventions | Group A (36) intervention: PFMT plus BF weekly for 3 months Group B (37) control: PFMT oral instructions only Duration of treatment: weekly until continent or to a maximum of 3 months Length of follow‐up: 3 months after treatment finished |
|
| Outcomes | UI severity (24 hour pad test weights) 1 month (N, mean, SD): A 96 g (160) 36, B 355 (423) 37, P = 0.007 3 months: A 51 (119), 36, B 197 (269) 37 6 months: A 40 (77), 36, B 80 (176) 37 ICI‐SF score: 3 months:A 3.4 (3.7), 36, B 6.8 (5.6) 37, P = 0.022 6 months: A 2.7 (3.5), 36, B 4.3 (5.5) 37, P = 0.339 PFM Strength, A versus B: 1 month, P = 0.006; 3 months P < 0.001; 6 months P = 0.799 Quality of life (IIQ): 3 months: A 1.6 (2.7), 36, B 4.3 (6.2) 37 |
|
| Notes | Groups comparable at baseline before operation on age, BMI, voiding symptoms and PFMT strength | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised controlled trial" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19: A:10 (2 refused further follow‐up, 7 post‐operative complications, 1 radiotherapy); B: 9 (6 refused further follow‐up, 2 post‐operative complications, 1 radiotherapy). No differential dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Low risk | Grant FAPESP 2003/07656‐7 (Sao Paulo Research Foundation) |
| Approved by medical ethics committee | Low risk | "institutional review board approval" |
| Informed consent | Low risk | "All patients signed an informed consent before randomization" |
| ITT analysis | Low risk | Assumed from patient flow chart |
Robinson 2008.
| Methods | Randomisation: yes | |
| Participants | Recruitment: pre‐operatively Included: all men undergoing radical prostatectomy Groups comparable at baseline Age range 39 to 74 years Pre‐operative UI 9% |
|
| Interventions | Group A (62) intervention: brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 33%) plus PFMT for four weeks with biofeedback Group B (64) control: brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 46%) |
|
| Outcomes | No urinary outcomes provided No between group differences in intensity and distress of lower urinary tract symptoms nor in impact on health‐related quality of life |
|
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | The co‐project director who supervised the intervention was responsible for recruitment, but did not have access to the randomisation list The co‐project director who supervised data collection was responsible for concealment of the randomisation list and allocation to the next available assignment on the list to participants sequentially as they enrolled |
| Blinding of participants (performance bias) | High risk | Participants were advised by the research assistant of their group assignment |
| Blinding of personnel (performance bias) | High risk | Questionnaires were filled in by research assistants either in person or by telephone interview |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant difference between groups in the number of participants who either withdrew prematurely or were dropped from the study. Questionnaires with > 20% data missing were excluded from analysis. In remainder mean substitution was inputted for missing data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "This study was supported by the American Cancer Society (TPRB‐98‐118‐01‐PBP) and a Rutgers College of Nursing Faculty Research Development Award" |
| Approved by medical ethics committee | Low risk | "Recruitment was initiated in January 1998 after approval of the parent study was obtained from the institutional review boards of both medical centres and the university" |
| Informed consent | Low risk | "Written informed consent was obtained by a research assistant" |
| ITT analysis | Low risk | "Data analysis was by intention‐to‐treat" |
Robinson 2009.
| Methods | Randomisation: randomly assigned via sealed envelopes | |
| Participants | Number of men 54 but no numbers in groups Recuitment: post‐operatively Included: radical prostatectomy, all with UI who were 50 + years, English speaking and were within a 50 mile radius of treatment centre Age: mean 59.5 (6.3) years |
|
| Interventions | Group A intervention: routine brief verbal and written PFMT plus one PFMT session and 3 weekly nurse phone calls Group B intervention: routine brief verbal and written PFMT plus four BF enhanced PFMT sessions and 4 weekly nurse phone calls Group C control: routine brief verbal and written PFMT Duration of treatment: 3 months Length of follow‐up: 9 months |
|
| Outcomes | Urine stream interruption test (PFM strength) Mishell Uncertainty in Illness Scale Broome Pelvic Muscle self‐Efficacy Scale UI frequency (3 day bladder diary) 24 hour pad test (volume of urine lost) Male Urogenital Distress Inventory (UI distress) Male Urinary Symptom Impact Questionnaire (QoL) |
|
| Notes | No useable data in abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "NIH/NINR" |
| Approved by medical ethics committee | Low risk | "Yes" |
| Informed consent | Low risk | "Following informed consent" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
Seleme 2008.
| Methods | Randomisation: yes, single blind Method of allocation: using coloured cards |
|
| Participants | Post‐operative intervention Included: men with UI eight weeks after radical prostatectomy Exclusion: previous radiotherapy, anterior transurethral resection, diabetes mellitus and urethral obstruction after surgery Age: median 63.7 years, range 46 to 83 years |
|
| Interventions | A (44) intervention: verbal instruction and information on PFMT plus information on life style changes. Additional 15 physiotherapy sessions consisting of intensive PFMT with BF and ES B (32) control: verbal instruction and information on PFMT plus information on life style changes Duration of treatment: no description Length of follow‐up: 6 months |
|
| Outcomes | Incontinence Quality of life (I‐QoL, higher score better), mean (SD) Directly after treatment: A 44.23 (14.61), B 37.53 (9.94) At 6 months: A 80.32 (7.01), B 51.69 (16.17), P = 0.001 At 6 months for Group A (44) intervention only: 1 hour pad test: mean urine loss before treatment 54.2 g and after treatment 8.8 g (P > 0.001) VAS severity of UI: before treatment 9.3, after treatment 1.3 (P > 0.001) |
|
| Notes | Unexplained disparity between numbers in randomised groups No results for Group B control for pad test or VAS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Coloured cards |
| Allocation concealment (selection bias) | Unclear risk | Method of selection unknown |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information for Group B control for both the one hour pad test and the VAS severity of UI |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "None" |
| Approved by medical ethics committee | Low risk | "Medical Ethical Committee of Nossa Senhora das Gracas Hospital in Curitiba, Brazil" |
| Informed consent | Low risk | "after signing informed consent" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Tibaek 2007.
| Methods | Randomisation: yes, mathematical table, grouped in blocks of 10 Method of allocation: sealed envelopes by independent third party Blinding: Slingle blind. Independent physiotherapist undertook initial assessment and 4 week outcome assessment Dropouts: 9 before intervention (4, training too time consuming; 1, didn't have TURP; 4, operated elsewhere) Setting: Hospital, Denmark |
|
| Participants | Pre‐operative intervention Included: TURP, all men Exclusion: prostate cancer, previous lower urinary tract surgery, neurological disease Age: A 70 (58 to 77) years, B 68 (52 to 79) years |
|
| Interventions | Group A (26) intervention: 1 hour individual session with physiotherapist to teach correct contraction for PFMT, three 1 hour group lessons and home training programme Group B (23) control: no pre‐operative physiotherapy. Information about anatomy and physiology and verbal instructions for 2 to 3 days after TURP in the ward Duration of treatment: 4 weeks after surgery Length of follow‐up: 2 and 4 weeks and 3 months after operation |
|
| Outcomes | Compliance: A 24/26 attended all 4 training sessions Use of urinary pads per 24 hours, at 4 weeks: A 4/26, B 4/21. At 3 months: A 3/26, B 5/22 UI (pad test weight g/24hrs): 4 weeks (N, Median, range): A 26, 12 (0 to 374), B 23, 4 (0 to 56), P = 0.755 Danish Prostatic Symptom Scale: 3 months (N, median, range): A 26, 3 (0 to 24), B 23, 4.5 (0 to 51), P = 0.754 Also data on muscle function, muscle strength, static endurance and dynamic endurance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mathematical table, grouped in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes by independent third party |
| Blinding of participants (performance bias) | High risk | Not possible to blind to intervention |
| Blinding of personnel (performance bias) | Unclear risk | Independent physiotherapist undertook initial assessment and 4 week outcome assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Nine dropped out before intervention |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | "This study was approved by the ethical committee in Copenhagen County and followed the Declaration of Helsinki" |
| Informed consent | Low risk | "Informed consent was obtained from the patients" |
| ITT analysis | Unclear risk | Not specified |
Tienforti 2012.
| Methods | RCT | |
| Participants |
Time of recruitment: pre‐operative Population: men undergoing radical prostatectomy (whole population, with or without UI) Included: men who underwent open retropubic radical prostatectomy for clinically localized prostate cancer (cT1a to cT2b), able to regularly attend an ambulatory schedule Excluded: prior diseases with a possible impact on urinary continence, preoperative radiotherapy and any medical condition that could limit participation in the training programme Age (mean, range): A 67 (60 to 74); B 64 (52 to 74) Dropouts: 1 from A (intolerance to procedure using rectal probe), 1 from B (surgical complication) Not differential dropout Baseline characteristics: comparable at baseline |
|
| Interventions |
Time of intervention: pre‐operative A (16): on the day before RP + the day after catheter removal, patients received guided PFMT + biofeedback + information about the anatomy of pelvic floor muscles and wrong execution was corrected, also given oral and written instructions on Kegel exercises to be performed at home, instructed to: perform three sets daily for 10 mins, each contraction lasting 5 seconds with 5 seconds of relaxation, contract their pelvic floor while lying, sitting and standing, frequency recorded in training diary, After RP visits at monthly intervals after catheter removal involving assisted biofeedback and motivation for 20 min B (16): after catheter removal, men received standard care, oral and written instructions from urologist on PFMT, Instructed to: start PFMT (e.g. 2 to 3 weeks after surgery), control visits at 3 + 6 months after catheter removal All men were given oral and written instructions post‐operatively to perform PFMT at home, 3 sets daily of 10 min each Duration of treatment: monthly visits as long as patient required pads, including safety pads Follow up: at least 6 months after catheter removal |
|
| Outcomes |
Primary outcome (number of men with UI) Number of incontinent men (defined as ICIQ‐UI > 0) 1 month: A 10/16; B 16/16, P = 0.02 3 months: A 8/16; B 15/16, P = 0.01 6 months: A 6/16; B 15/16, P = 0.002 Other outcomes Number of continent men (efined as ICIQ‐UI = 0) 1 month: A 6/16; B 0/16, P = 0.02 3 months: A 8/16; B 1/16, P = 0.01 6 months : A 10/16; B 1/16, P = 0.002 Mean number of incontinence episodes per week/24 hours (mean (SD) N) 1 month: A 1.43 (0.82) 16; B 14 (0.82) 16, P = N.S 3 months: A 0.57 (1.47) 16; B 2 (1.47) 16, P = 0.01 6 months: A 0.43 (1.33) 16; B 1.86 (1.33) 16, P = 0.005 Mean number of pads used per week/24 hours (mean (SD) N) 1 month: A 0.46 (0.67) 16; B 0.94 (0.67) 16 P = NS 3 months: A 0.23 (0.63) 16; B 0.91 (0.63) 16 P = 0.005 6 months: A 0.2 (0.57) 16; B 0.66 (0.57) 16 P = 0.03 Quality of life Mean ICIQ‐OAB score (mean (SD) N) 1 month: A 11.5 (3.6) 16; B 14 (3.6) 16, P = NS 3 months: A 11 (0.92) 16; B 11.7 (0.92) 16, P = 0.04 6 months: A 9 (4.1) 16; B 13 (4.1) 16, P = 0.01 Mean UCLA‐PCI score (mean (SD) N) 1 month: A 330 (?) 16; B 260 (?) 16, P = NS 3 months: A 400 (500) 16; B 270 (338) 16, P = 0.006 6 months: A 430 (487) 26; B 275 (311) 16, P = 0.003 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Generated by computer and was stratified with a 1:1 allocation” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants (performance bias) | High risk | “Participants were unblinded to treatment assignment” |
| Blinding of personnel (performance bias) | Low risk | “Surgeons and person scoring the evaluation questionnaires were blinded throughout the duration of the study” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “nurse scoring the evaluation questionnaires was blinded” to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 from A (intolerance to procedure) 1 from B (surgical complication). Not differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported |
| Approved by medical ethics committee | Low risk | “Work was carried out in accordance with the ethical standards of the appropriate institutional committee on human experimentation and with the last revision of the Helsinki Declaration |
| Informed consent | Low risk | “All eligible patients gave informed signed consent” |
| ITT analysis | Low risk | Assumed from patient flow diagram |
Tobia 2008.
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: all men, radical prostatectomy Age: 45 to 75 years |
|
| Interventions | Group A (19) intervention: PFMT Group B (19) control: no PFMT length of follow‐up: 2, 4 and 8 weeks |
|
| Outcomes | Dry at 2 weeks: A 9/19, B 9/19 Dry at 4 weeks: A 9/19, B 9/19 Dry at 8 weeks: A 15/19, B 17/19, P = 0.374 No significant differences for age (P = 0.674), PSA (P = 0.208), Gleason score pre (P = 0.762) and post‐operation (P = 0.824) |
|
| Notes | Awaiting translation for more information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | No description, Spanish language |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Spanish language |
| Financial support | Unclear risk | Unable to be determined |
| Approved by medical ethics committee | Unclear risk | Unable to be determined |
| Informed consent | Unclear risk | Unable to be determined |
| ITT analysis | Unclear risk | Unable to be determined |
van Kampen 1998.
| Methods | Randomised: yes
Method of allocation: stratified randomisation with sealed envelopes. Stratified by grams of urine loss (< 50 , > 50, < 250, > 250 g) Blinding: yes (outcome assessor not involved with the study) Dropouts: 5 Intention to treat: yes |
|
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy 15 days after surgery after catheter removal N = 102 eligible, 98 completed |
|
| Interventions | Post‐operative intervention Group A (50) intervention: 1 session of PFMT in hospital before discharge and then saw the physiotherapist for 1 to 2 weeks for as long as UI persisted; 90 daily home exercises sitting, standing and lying; 7 men unable to contract PFM or with weak contraction received electrical stimulation by anal probe Group B (52) control: No formal PFMT instruction but saw the therapist at 1 to 2 weeks and received placebo stimulation and information about aetiology of UI Both A and B: received bladder training to increase bladder capacity Length of follow‐up: 12 months |
|
| Outcomes | Main outcome: urine loss measured by 24 and 1 hour pad tests; 24 hour pad test done daily until continence achieved; 1 hour pad test when loss of < 2 g of urine to confirm continence Secondary outcomes: Subjective UI by visual analogue scale Fluid Volume Chart Quality of Life ‐ questionnaire designed for study Continence definition: Numbers cured defined as < 2 g urine loss on 24 and 1 hour pad tests Data collection: subject assessment of continence preoperatively (during screening), and at 1, 6 and 12 months. Daily weighing of pads by participants (24 hour pad test) |
|
| Notes | Pragmatic study; policy of management left to clinical judgment as to which protocols to add to PFMT regime. 63 of the eligible subjects were unable to participate because of geographical reasons; demographics and post‐operative variables did not differ from the 102 subjects who were in the treatment groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by grams of urine loss (< 50 , > 50, < 250, > 250 g) |
| Allocation concealment (selection bias) | Low risk | A ‐ stratified randomisation with sealed envelopes |
| Blinding of participants (performance bias) | Unclear risk | The control group "received placebo electrotherapy that could not affect the pelvic‐floor muscle function." |
| Blinding of personnel (performance bias) | Unclear risk | "The patients in both groups were treated by the same therapist (MVK) until they became continent, within a period of 1 year" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor not involved with the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: 5 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "supported by a grant from the Fund of Scientific Research, Flanders, Belgium" |
| Approved by medical ethics committee | Unclear risk | Not specified |
| Informed consent | Low risk | "All patients included in the study gave written informed consent" |
| ITT analysis | Low risk | "The groups were analysed on an intention‐to‐treat basis" |
Wille 2003.
| Methods | Randomised: yes Method of allocation: not described Blinding: not mentioned Dropouts: numbers participating at 3 and 12 months identified (for pad test, N = 116 at baseline, 79 at 3 months and 124 at 12 months), reason for dropouts not described | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 139 randomised (number in each group at various data collection points varied) |
|
| Interventions | Post‐operative intervention Group A (47): PFMT alone Group B (46): PFMT + ES; PFMT as above plus instructed by dedicated in ES via surface anal electrode and bio‐impulser (biphasic pulse with 1 second bursts, 5 second pulse width, 2 second pulse trains Group C (46): PFMT + ES + biofeedback. As above plus biofeedback (anal probe) 15 minutes twice daily for 3 months All groups A and B and C: PFMT by physiotherapist, 20‐30 minute sessions for 3 days, instructed to perform exercises twice daily for 3 months plus 3 week rehabilitation program after discharge. Regular interaction with health professional for 6 weeks after surgery, encouraged to performed treatment for 3 months post‐surgery Length of follow‐up: 12 months |
|
| Outcomes | Main outcome: urine loss measure by continence questionnaire and 20 minute provocative pad test Continence definition: reported use of 0 to 1 pads on questionnaire (subjective) or loss of less than 1 gram of urine on pad test Data collection: baseline (after catheter removal), 3 months and 12 months post‐operatively Willingness to undergo surgery again: A 73%, B 83%, C 73% Quality of life EORCT QLQ‐C30: scores for physical; role; emotional; social; and global quality of life were not significantly different between the groups at 3 or 12 months (no SDs provided) No significant differences in continence rates between the three groups at baseline, 3 months or 12 months (objective) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective randomized trial" Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | "Prospective randomized trial" Method of sequence generation not specified |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Results at baseline after catheter removal, at 3 and 12 months postoperatively were available for 139, 120 and 128 (questionnaires at three different time points) and 116, 79 and 124 (pad test at three different time points) patients, respectively". However, no information about individual groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Informed consent was obtained" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Yamanishi 2006.
| Methods | Randomised: yes. Blinding: double blind Dropouts: 1 due to pain in the intervention group |
|
| Participants | Randomisation: postoperative Included: radical prostatectomy, all with severe post‐operative UI of > 100 g after catheter removal Age: mean 65.7 (7.0) years Pre‐operative intervention |
|
| Interventions | All patients instructed pre‐operatively PFMT by nurses and continued after catheter removal A (26) intervention: oral PFMT plus electrical stimulation for 15 minutes twice daily (50 Hz square waves, 300 µsec pulse duration, maximum output 70 mA (5 sec on, 5 sec off duty cycle) B (30) control: oral PFMT plus sham stimulation (output 3 mA, 2 sec on, 13 sec off duty cycle) Duration of treatment: until continent or 12 months Length of follow‐up: 1, 3, 6 and 12 months after treatment Dropouts: A 4/26, B 5/30 (including 2+4 with adverse effects) |
|
| Outcomes | Number of incontinent men 1 month: A 18/26, B 29/30 3 months: A 10/24, B 25/29 6 months: A 5/23, B 15/26 12 months: A 3/22, B 8/25 24 hour pad test weights (mean ml, SD, N) 1 month: A 210 (261) 26, B 423 (357) 30 3 months: A 81 (140) 24, B 232 (339) 29 6 months: A 20 (49) 23, B 132 (293) 26 12 months: A 18 (49) 22, B 98 (277) 25 Time until continent in months (mean, SD, N): A 2.71 (2.6) 22, B 6.82 (3.9) 25, P = 0.0006 ICIQ‐SF (mean score SD N; 0 to 21, higher = worse) 1 month: A 10.6 (6) 26, B 14.9 (4.9) 30 3 months: A 5.8 (5.7) 24, B 11.2 (5.7) 29 6 months: A 4.3 (6.2) 23, B 8.2 (5.3) 26 12 months: A 4.2 (6.2) 22, B 5.6 (6.5) 25 ICIQ‐QoL score (mean score SD N; 0 to 21; 0 to 10, higher = worse) 1 month: A 4.2 (3.5) 26, B 6 (3) 30 3 months: A 2.2 (2.3) 24, B 3.7 (2.9) 29 6 months: A 1.6 (3.1) 23, B 2.5 (2.2) 26 12 months: A 1.5 (3.1) 22, B 1.9 (2.5) 25 Adverse effects: A 2/26, B 4/30 (discomfort or anal pain) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer |
| Allocation concealment (selection bias) | Low risk | "None of the patients, doctors or medical staff knew which type of stimulation had been assigned until the key code was opened" |
| Blinding of participants (performance bias) | Low risk | Men were blinded to the intervention (sham, low energy stimulation in control group |
| Blinding of personnel (performance bias) | Low risk | Blinding of doctors, nurses and medical staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of assessors, medical staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is reported that "In the active ES group 2 patients discontinued after 2 and 3 months, respectively, due to urethral stricture at the bladder neck. In the sham group 1 patient discontinued treatment at 7 months because of an increase in prostate specific antigen and he then underwent radiation therapy". However, there is no evidence that dropout was related to trial interventions |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "None" |
| Approved by medical ethics committee | Low risk | "Local ethical committee approval" was obtained |
| Informed consent | Low risk | "written informed consent from each subject was obtained" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Yokoyama 2004.
| Methods | Randomised: yes Method of allocation: not stated Blinding: not mentioned Dropouts: it appears that there are no dropouts but this is not specifically mentioned |
|
| Participants | Recruitment: post‐operative Included: 36 men with urinary incontinence, >100g on 24hour pad test, one day after catheter removal Mean age: Group A 67.2 years, Group B 68.2 years, Group C 66.2 years |
|
| Interventions | A (12) intervention: anal electrode for 15 minutes twice a day for 1 month B (12) intervention: extra‐corporeal magnetic innervation, neocontrol system, treatment sessions 20 minutes, twice a week for 2 weeks C (12) control: PFMT, digital anal teaching of correct contractions, then verbal and written instructions for home practice Length of follow‐up: 2, 3, 4, 5 and 6 months |
|
| Outcomes | 24 hour pad test weight (grams) 3 months: A 34 g, B 7.3 g, C 50 g. 6 months: for all groups less than 10 g Quality of life measured by I‐QOL: improvement in all groups over time, no statistically significant difference between the groups Remaining UI at 6 months: A 2/12, B 1/12, C 2/12 |
|
| Notes | Adverse effects: None in any of the groups, no discomfort or irritation from anal probe | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned |
| Blinding of participants (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers not given |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported |
| Approved by medical ethics committee | Low risk | "The local ethics committee approved the protocol procedure" |
| Informed consent | Low risk | "Each patient provided written informed consent" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
Zhang 2007.
| Methods | Randomised: yes Method of allocation: not stated Blinding: none Dropouts: two did not complete the control follow‐up assessment because they believed the control group was not helpful |
|
| Participants | 58 men approached, 33 consented, 3 dropouts Recruitment: post‐operative Included: all incontinent men 6 months after radical prostatectomy |
|
| Interventions | Group A (14) intervention: PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes. Plus support group, 6 meetings in 3 months with a health psychologist Group B (15) control: PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes |
|
| Outcomes | Length of follow‐up: 3 months Frequency of PFMT: 4 to 7 times per week A 12/14, B 6/13, P = 0.077 Use of pad or brief: A 7/14 (50%), B 11/13 (85%), P = 0.057 Not able to control urge to urinate and prevent leakage: A 4/14, B 8/13, P = 0.085 Nocturia per week (mean): A 13, B 15.08, P = 0.484 VAS for severity of UI: A 3.21, B 4.65, P = 0.057 (t = ‐1.902) QoL measured by Illness Intrusiveness Questionnaires (IIRS): A 10.96, B 17.27, P = 0.037 Mann Whitney U = 48.5 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Group therapy (unable to blind to intervention) |
| Blinding of personnel (performance bias) | Unclear risk | A research assistant, who was a doctoral candidate in medical anthropology, collected data under supervision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two dropouts in the control group |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Financial support | Low risk | "This study was supported by an American Cancer Society pilot research grant and the Frances Payne Bolton School of Nursing at Case Western Reserve University" |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | "33 patients consented to participate" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
ExMI = extra‐corporeal magnetic innervation; g = gram(s); PFMT = pelvic floor muscle training; TURP = transurethral resection of the prostate; UI = urinary incontinence
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bennett 1997 | Insufficient information to assess study for inclusion. Abstract only, no data included. Attempts to contact the author for data unsuccessful |
| Bocker 2002 | Data from study that included male postprostatectomy and female post‐polio patients. Translation obtained as reported in German. Data from the two groups were not separated and therefore not in a usable form |
| Burkert 2011 | Not measuring incontinence |
| Ceresoli 2002 | Insufficient information to assess study for inclusion. Attempts to contact the author for data unsuccessful |
| Chang 1998 | Data from study which involved post‐TURP patients. Two groups, treatment and control. Not randomly assigned to groups, first 25 consecutively assigned to control, next 25 to intervention |
| Cornel 2005 | Descriptive study. No control group |
| Cornu 2011 | RCT. PFMT + Duloxetine (drug) versus PFMT + placebo |
| Crevenna 2003 | Descriptive pilot study. No control group |
| Dieperink 2013 | Intervention after radiotherapy only |
| Eren 2013 | RCT, 58 men after RP. Pharmacological intervention: Duloxetine + PFMT versus PFMT alone |
| Filocamo 2007 | RCT, 112 men after RP. Pharmacological intervention: Duloxetine + PFMT versus PFMT alone |
| Griebling 1999 | Insufficient information to assess study for inclusion. Data reported in paper presentation and in later published report did not contain sufficient detail of analysis to include in tables of comparison. Attempts to contact authors not successful in providing further data |
| Hotston 2006 | Pharmacological intervention |
| Ip 2004 | Education intervention (refrigerator magnet) not an intervention included in review |
| Kahihara 2006 | A comparative study. Early versus delayed PFMT no randomisation |
| Lin 2012 | Measuring erectile dysfunction only |
| McGlynn 2004 | Descriptive study of change in education delivery approach. No control group |
| Mishel 2002 | Data not separated for men who received radiotherapy and those who underwent prostate surgery |
| Nehra 2001 | Insufficient information to assess study for inclusion. Abstract only. Attempts to contact authors for further data unsuccessful. Possibly ongoing trial but no further data available |
| Ottenbacher 2013 | RCT but of written information about diet and general exercise |
| Pemberton 2006 | Comparative study of different types of urinary sheath |
| Prota 2012 | Measuring erectile dysfunction, no useable data |
| Pulker 2002 | Descriptive study. No control group |
| Ribeiro 2013 | Not prostatectomy |
| Ricci 2004 NEWa | Measuring "sensory urgency" only, not incontinence |
| Robinson 2012 | Measuring the validity of a specific test, no useable data |
| Salinas Casado 1991 | Descriptive study. No control group. Article in Spanish with English abstract |
| Salinas Casado 1996 | Descriptive study. No control group. Article in Spanish with English abstract |
| Seki 2005 | Descriptive study. No control group |
| Shen 2012 NEWa | Not looking at incontinence. Translation obtained as reported in Chinese |
| Traeger 2013 | Data for men who received radiotherapy not separated from those who underwent prostate surgery |
| Yang 2010 NEWa | Not randomised. Translation obtained as reported in Chinese |
| Yao 2012 | Not RCT. Physiotherapist‐guided PFMT versus control (retrospective analysis) |
| Zahariou 2009 | Not randomised |
| Zermann 1999 | Descriptive study. No control group |
| Zhang 2009 | Data for men who received radiotherapy not separated from those who underwent surgery |
Estim = Electrical stimulation
ExMI = Extra‐corporeal magnetic innervation
TURP = Transurethral resection of the prostate
Characteristics of studies awaiting assessment [ordered by study ID]
Crivellaro 2011.
| Methods | Not enough information |
| Participants | 73 men after retropubic radical prostatectomy |
| Interventions | Ultrasound‐guided biofeedback versus biofeedback using verbal instructions and digital biofeedback |
| Outcomes | |
| Notes | Authors to be contacted regarding whether assignment was randomised |
Delmastro 2010.
| Methods | Open label RCT |
| Participants | Men scheduled for radical prostatectomy |
| Interventions | Preoperative intensive PFMT with or without proprioceptive training |
| Outcomes | Anal examination to assess pelvic floor muscle function; subjective and objective voiding and incontinence parameters; four tests of pelvic floor muscle function; PGI‐I; ICIQ‐male score |
| Notes | Further information needed from authors |
Lilli 2006 NEW.
| Methods | Unclear if randomised, further information from authors required |
| Participants |
Time of recruitment: pre‐operative Population: Men having a radical prostatectomy (whole population, with or without UI) Included: Men who were candidates for retropubic radical prostatectomy Excluded: Acquired or congenital disability, cardiovascular diseases requiring the administration of drugs that interfere with voiding, e.g. diuretics and/or alphalytics, problems relating to vesico‐sphincteral innervations, episodes of unstable or transitory continence during their lifetime, any type of neuropathy, other cancers, and psychoaffective disturbances such as depression or insomnia Age (mean, SD): A 68 (?); B 68 (?) Dropouts: Not reported Baseline characteristics: Comparable at baseline |
| Interventions |
Time of intervention: Pre‐operative A (45): 20 mins of PFMT + biofeedback daily for 15 weeks before surgery, instructed to: carry out exercises during increased abdominal pressure (coughing, extending the abdomen, raising the head and keeping it raised), instructed how to carry out rapid, brief contractions without increasing abdominal activity and how to perform slow contractions B (45): Instructed to start PFMT at home 15 weeks before surgery After surgery and removal of catheter, all men were instructed to carry out four series of PF contractions at home on a daily basis for six months Duration of treatment: 6 months Follow up: 1 month, 3 months and 6 months after surgery |
| Outcomes |
Primary outcome (number of men with UI): Number of incontinent men (defined as use of pads): Pre‐operative: A 0/45; B 0/45 1 month: A 42/45; B 42/45 3 months: A 30/45; B 33/45 6 months: A 13/45; B 15/45 Other outcomes: Number of continent men (defined as completely dry without the use of pads): Pre‐operative: A 45/45; B 45/45 1 month: A 3/45; B 3/45 3 months: A 15/45; B 12/45 6 months: A 32/45; B 30/45 |
| Notes | Unclear if randomised |
Simeit 2010 NEW.
| Methods | RCT |
| Participants | Men with urinary incontinence after radical prostatectomy |
| Interventions | A: PFMT B: PFMT with additional 'BBS trainer' |
| Outcomes | Quality of life (EORTC questionnaire), impact of incontinence |
| Notes | Awaiting German translation |
Zellner 2011 NEW.
| Methods | RCT |
| Participants | Men after radical prostatectomy |
| Interventions | A: PFMT + biofeedback + electrical stimulation B: Whole body vibration C: Guided PFMT |
| Outcomes | International Prostate Symptom Score (IPSS), the enclosed question about quality of life (IPSS‐QL), pad test, pelvic floor strength, maximum uroflow, micturition volume, serum testosterone and blood glucose |
| Notes | Awaiting German translation |
Zhang 2013.
| Methods | RCT |
| Participants | 127 Men with incontinence after “cancer treatment” Radical prostatectomy? |
| Interventions | A: Biofeedback + PFMT + 6 biweekly sessions of problem‐solving therapy delivered through a support group, B: Biofeedback + PFMT + 6 biweekly sessions of Problem‐solving therapy delivered through telephone contact, C (?): Standard care |
| Outcomes | Number of incontinent men [need for wearing a pad] |
| Notes | Further information required about participants + no useable data |
Characteristics of ongoing studies [ordered by study ID]
Burnett 2012.
| Trial name or title | Health Interventions in Men Undergoing Radical Prostatectomy‐ A Randomized Controlled Clinical Trial |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | Intensive fitness intervention |
| Outcomes | Expanded Prostate Cancer Index (EPIC)‐26, RAND‐12 Questionnaire, body weight change, body mass index (BMI) change, blood pressure (BP) change, International Index of Erectile Function (IIEF), Quality of Erection Questionnaire (QEQ) |
| Starting date | December 2012 |
| Contact information | Arthur L Burnett, Johns Hopkins Hospital, Baltimore, United States |
| Notes |
Burnett 2013.
| Trial name or title | Study of Non‐Invasive Viberect Penile Vibratory Stimulation Regimen to Enhance Recovery of Erectile Function/Rigidity and Urinary Control/Continence After Nerve Sparing Radical Prostatectomy (RP) for Clinically Localized Prostate Cancer |
| Methods | RCT |
| Participants | Men with Urinary Incontinence |
| Interventions | Post‐operative use of Viberect device 3 days after catheter removal, daily usage for 7‐10 minutes versus no treatment |
| Outcomes | Recovery of erectile function following radical prostatectomy, IIEF, recovery of continence, EPIC urinary and and sexual domain, AUA, EHS EDITS and TSS questionnaires |
| Starting date | April 2013 |
| Contact information | Arthur L. Burnett, M.D., Johns Hopkins University |
| Notes |
Fode 2012 NEW.
| Trial name or title | Mechanical Nerve Stimulation in the Treatment of Post Prostatectomy Incontinence |
| Methods | RCT |
| Participants | Men with urinary incontinence more than 1 year after radical prostatectomy |
| Interventions | Medical vibrator used daily for 6 weeks |
| Outcomes | 24 hour pad test, Micturition diary, Validated symptom score ICI‐Q, IPSS |
| Starting date | June 2012 |
| Contact information | Copenhagen University Hospital at Herlev |
| Notes |
Goode 2014.
| Trial name or title | Perioperative Post‐Prostatectomy Incontinence Home Telehealth Program (ProsTel) |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | Guided PFMT using a telehealth device (home messaging unit) |
| Outcomes | Time to continence using ICIQ‐SF, EPIC‐UI, HRQOL, IIQ‐SF, IPSS, patient satisfaction questionnaire, Estimated Percent improvement, Global perception of Improvement |
| Starting date | January 2012 |
| Contact information | Department of Veterans Affairs |
| Notes |
Mina 2013.
| Trial name or title | A Multicentre, Pilot Randomized Controlled Trial to Examine the Effects of Prehabilitation on Functional Outcomes After Radical Prostatectomy |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | Behavioral: Prehabilitation (PREHAB) |
| Outcomes | Adherence to Prehabilitation Program, Recruitment, Contamination, Study Retention, Physical Fitness, Quality of Life, Psychosocial Wellbeing, Physical Activity, Treatment Complications, Post‐operative length of stay |
| Starting date | November 2013 |
| Contact information | Daniel Santa Mina, University Health Network, Toronto, Ontario, Canada |
| Notes |
Ng 2011.
| Trial name or title | Trial study of the efficacy of intensive preoperative pelvic floor muscle training to decrease post‐prostatectomy urinary incontinence |
| Methods | RCT |
| Participants | Men with urinary incontinence after radical prostatectomy |
| Interventions | Preoperative guided PFMT 3 weeks before surgery versus PFMT on the day of admission for surgery |
| Outcomes | Pad test, IIQ‐7, SF 12 |
| Starting date | February 2011 |
| Contact information | Sau‐loi NG, Queen Mary Hospital, Hong Kong |
| Notes |
Terrone 2007.
| Trial name or title | Prevention of Urinary Incontinence After Prostatectomy |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | BioFeedback; Functional Electrical Stimulation; Pelvic Floor Muscle training exercises |
| Outcomes | 24‐hour PAD test: Complete continence |
| Starting date | February 2007 |
| Contact information | Carlo Cisari, Azienda Ospedaliero Universitaria Maggiore della Carita |
| Notes |
Zopf 2012.
| Trial name or title | Implementation and scientific evaluation of rehabilitative sports groups for prostate cancer patients: study protocol of the ProRehab Study |
| Methods | "Patient preference RCT"? |
| Participants | Men after radical prostatectomy |
| Interventions | exercise intervention ‐ rehabilitative sports group |
| Outcomes | "quality of life using EORTC‐QLQ‐C30/PR 25, incontinence using pad test and erectile dysfunction using IIEF" |
| Starting date | |
| Contact information | German Sport University Cologne |
| Notes |
Estim = Electrical stimulation
ExMI = Extra‐corporeal magnetic innervation
Differences between protocol and review
Trials were reclassified as 'treatment' or 'prevention' trials in a previous version of this review, and hence trials amongst men having radical prostatectomy or TURP were analysed separately. The trials of containment (penile clamps) were analysed separately from those of PFMT and its variations.
In the current update, the GRADE method was used to assess quality of evidence.
Contributions of authors
For the updates in 2004 and 2006, the original lead review author (KNM) and an additional review author (KFH) independently undertook the quality assessment, data extraction and collation. KFH took the lead in updating the text and completed the data entry, which were then checked and commented upon by the other review authors.
For the earlier versions, two of the original review authors undertook the quality assessment of the trials and the data extraction independently. This information was then collated and checked by the original lead review author (KNM) for agreement and, in the few instances where this did not occur, consensus was reached after checking with the other review authors. For the 2004 and 2006 updates, KFH updated the text and entered the data. These were checked by the other review authors, whose additional comments and edits were then incorporated.
For the update in 2012, CG and SC undertook quality assessment and data abstraction for the 18 new included trials, revised the previous data as appropriate, analysed the data and wrote the review text assisted by JC. All review authors contributed to writing or editing the text of the review.
For this update in 2014, CA, MO and CG undertook abstract and full text screening. CA and CG performed data abstraction, cross‐checked by MO. CA, MO and CG performed risk of bias assessment of trials. Quality of evidence was assessed by CA and MO. Previous data were updated, if necessary, and previously included trials were re‐assessed with the additional risk of bias domains. CA took the lead in drafting the manuscript of the review. All review authors contributed to the analysis of data and made comments and suggestions on the manuscript, which were incorporated in the review.
Sources of support
Internal sources
University of Alberta, Edmonton, Alberta, Canada.
External sources
-
National Institute for Health Research (NIHR), UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group.
Chief Scientist Office, Scottish Executive Health Department, UK.
Declarations of interest
Coral A Anderson: none known
Muhammad Imran Omar: none known
Susan E Campbell: none known
Kathleen F Hunter has been a speaker at the Jewish General Hospital (Montreal, Canada) and is currently a local co‐investigator on a multi‐national drug trial funded by Astelles
June D Cody: none known
Cathryn MA Glazener was the Chief Investigator on one of the included trials, MAPS (Glazener RP 2011; Glazener TURP 2011)
Edited (no change to conclusions)
References
References to studies included in this review
Ahmed 2012 {published data only}
- Ahmed MT, Mohammed AH, Amansour A. Effect of pelvic floor electrical stimulation and biofeedback on the recovery of urinary continence after radical prostatectomy. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2012;58(3):170‐6. [sr‐incont47262] [Google Scholar]
- Omar M, Mohammed AH. Effect of pelvic floor exercises, electrical stimulation and biofeedback on urinary incontinence after radical prostatectomy: single blind randomized clinical trial. ANZCTR (http://www.anzctr.org.au/ACTRN12610000975099.aspx) 2010.
Bales 2000 {published data only}
- Bales G, Gerber G, Minor T, Mhoon D, McFarland J, Hyung L, et al. Effect of preoperative biofeedback/pelvic floor training on continence in men undergoing radical prostatectomy. Urology 2000;56:627‐30. [SRINCONT11831] [DOI] [PubMed] [Google Scholar]
Burgio 2006 {published and unpublished data}
- Burgio KL, Goode P, Urban D. Umlauf M, Locher J, Bueschen A, Redden D. Preoperative biofeedback‐assisted behavioural training to reduce post‐prostatectomy incontinence: A randomized controlled trial. Journal of Urology 2006;175(1):196‐201. [SRINCONT21637] [DOI] [PubMed] [Google Scholar]
- Burgio KL, Goode P, Urban D. Umlauf M, Locher J, Bueschen A, Redden D. Preoperative biofeedback‐assisted behavioural training to reduce post‐prostatectomy incontinence: A randomized controlled trial [abstract]. Neurourology and Urodynamics 2005;24(5/6):477. [SRINCONT20970] [DOI] [PubMed] [Google Scholar]
Centemero 2009 {published data only}
- Centemero A, Rigatti L, Andrea L, Gallina A, Lughezzani G, Montorsi F, et al. Effectiveness of pre‐operative pelvic floor muscle training for post‐prostatectomy early incontinence recovery (Abstract number 1642). Journal of Urology 2009;181(4 (Suppl 1)):591. [SR‐INCONT34592] [Google Scholar]
- Centemero A, Rigatti L, Giraudo D, Lazzeri M, Lughezzani G, Zugna D, et al. Preoperative pelvic floor muscle exercise for early continence after radical prostatectomy: a randomised controlled study. European Urology 2010;57(6):1039‐43. [SR‐INCONT40316] [DOI] [PubMed] [Google Scholar]
Dijkstra‐Eshuis 2013 {published data only}
- Dijkstra‐Eshuis J, Splinter R, Zonneveld W, Putter H, Bos T, Bevers R, et al. Effect of preoperative pelvic floor muscle therapy with biofeedback versus standard care on stress urinary incontinence and quality of life in men undergoing laparoscopic radical prostatectomy (Abstract number 137). Neurourology and Urodynamics 2013;32(6):712‐3. [sr‐incont49210] [DOI] [PubMed] [Google Scholar]
- Dijkstra‐Eshuis J, Bos TW, Splinter R, Bevers RF, Zonneveld WC, Putter H, et al. Effect of preoperative pelvic floor muscle therapy with biofeedback versus standard care on stress urinary incontinence and quality of life in men undergoing laparoscopic radical prostatectomy: A randomised control trial [epub ahead of print published online 19 November 2013]. Neurourology and Urodynamics 2013. [sr‐incont49547] [DOI] [PubMed]
- Voorham‐Van Der Zalm P, Dijkstra‐Eshuis J, Splinter R, Putter H, Bevers RFM, Pelger RCM. Effect of preoperative pelvic floor muscle therapy versus standard care on stress urinary incontinence in men undergoing radical laparoscopic prostatectomy (Abstract number 1365). Journal of Urology 2013;189(4 Suppl 1):e558‐9. [sr‐incont50002] [Google Scholar]
- Voorham‐Van Der Zalm PJ, Stoetman AM, Putter H, Bevers RFM, Pelger RCM. Effect of preoperative pelvic floor physiotherapy versus standard care on incontinence in men undergoing radical laparoscopic prostatectomy: an ongoing study (Abstract number 590). Proceedings of the Joint Meeting of the International Continence Society (ICS) and the International Urogynecological Association, 2010 Aug 23‐27, Toronto, Canada. 2010. [SR‐INCONT40184]
Dubbelman 2004 {published and unpublished data}
- Dubbelman Y, Groen J, Wildhagen M, Rikken B, Bosch R. The recovery of urinary continence after radical retropubic prostatectomy: a randomized trial comparing the effect of physiotherapist‐guided pelvic floor muscle exercises with guidance by an instruction folder only. BJU International 2010;106(4):515‐22. [SR‐INCONT40056] [DOI] [PubMed] [Google Scholar]
- Dubbelman Y, Groen J, Wildhagen M, Rikken B, Bosch R. UPDATE Quantification of changes in detrusor function and pressure‐flow parameters after radical prostatectomy: relation to postoperative continence status and the impact of intensity of pelvic floor muscle exercises. Neurourology and Urodynamics 2012;31(5):637‐41. [srincont45959] [DOI] [PubMed] [Google Scholar]
- Dubbelman YD, Groen J, Bosch R. Post prostatectomy incontinence: Significance of the pre‐operative urethral pressure profile and the role of physiotherapy [abstract]. Neurourology and Urodynamics 2004;23(5‐6):471. [SRINCONT19009] [Google Scholar]
- Dubbelman YD, Groen J, Wildhagen MF, Rikken B, Bosch JL. UPDATE Urodynamic quantification of decrease in sphincter function after radical prostatectomy: relation to postoperative continence status and the effect of intensive pelvic floor muscle exercises. Neurourology and Urodynamics 2012;31(5):646‐51. [srincont45958] [DOI] [PubMed] [Google Scholar]
Fader 2013 {published data only}
- Fader M, Macaulay M, Broadbridge J, Cottenden A, Birch B, Moore KN. A trial of devices for the management of urinary incontinence following treatment for prostate cancer (Abstract number 264). Neurourology and Urodynamics 2013;32(6):891‐2. [sr‐incont49194] [Google Scholar]
Filocamo 2005 {published and unpublished data}
- Popolo G, Filocamo MT, Li Marzi V, Cecconi F, Marzocco M, Tosto A, et al. Effectiveness of early pelvic floor rehabilitation treatment for post‐prostatectomy incontinence (Abstract number 610). Proceedings of the International Continence Society (ICS), 35th Annual Meeting, 2005 Aug 28‐Sep 2, Montreal, Canada 2005. [SR‐INCONT21063] [DOI] [PubMed]
- Filocamo MT, Li Marzi V, Popolo G, Cecconi F, Marzocco M, Tosto A, et al. Effectiveness of early pelvic floor rehabilitation treatment for post‐prostatectomy incontinence. European Urology 2005;48(5):734‐8. [SRINCONT21252] [DOI] [PubMed] [Google Scholar]
Floratos 2002 {published data only}
- Foratos DL, Sonke GS, Rapidou CA, Alivizatos GJ, Deliveliotis C, Constantinides CA, et al. Biofeedback versus verbal feedback as learning tools for pelvic muscle exercises in the early management of urinary incontinence after radical prostatectomy. British Journal of Urology International 2002;89(7):714‐9. [SRINCONT15333] [DOI] [PubMed] [Google Scholar]
Fode 2014 {published data only}
- Fode M, Borre M, Ohl DA, Lichtbach J, Sonksen J. Penile vibratory stimulation in the recovery of urinary continence and erectile function after nerve‐sparing radical prostatectomy: a randomized, controlled trial [epub ahead of print] [first published online 22 Jan 2014]. BJU International 2014. [NCT01067261; sr‐incont50324] [DOI] [PMC free article] [PubMed]
- Sonksen JR. Transcutaneous Mechanical Nerve Stimulation (TMNS) by Vibration in the Preservation and Restoration of Urinary Continence and Erectile Function and in the Treatment of Erectile Dysfunction and Urinary Incontinence in Conjunction With Nerve Sparing Radical Prostatectomy. http://clinicaltrials.gov/show/NCT01067261 2010. [NCT01067261; sr‐incont47825]
Franke 1998 {published data only}
- Franke JJ, Gilbert WB, Grier J, Koch MO, Shyr Y, Smith JA Jr. Early post‐prostatectomy pelvic floor biofeedback. Journal of Urology 2000;163(1):191‐3. [SRINCONT9180] [PubMed] [Google Scholar]
- Franke JJ, Grier J, Koch MO, Smith JA. Biofeedback‐enhanced pelvic floor exercises in the early post prostatectomy period. Journal of Urology 1998;159 Suppl 5:37. [SRINCONT5734] [Google Scholar]
Geraerts 2013 {published data only}
- Geraerts I, Poppel H, Devoogdt N, Joniau S, Cleynenbreugel B, Groef A, et al. Influence of preoperative and postoperative pelvic floor muscle training (PFMT) compared with postoperative PFMT on urinary incontinence after radical prostatectomy: a randomized controlled trial. European Urology 2013;64(5):766‐72. [NTR1953; sr‐incont49488] [DOI] [PubMed] [Google Scholar]
Ghanem 2013 {published data only}
- Ghanem A, Khallaf M, Assem A, Hassan A. Does preoperative pelvic floor muscle exercise improve post prostatectomy urinary incontinence? (Abstract number 695). Proceedings of the 43rd Annual Meeting of the International Continence Society (ICS), 2013 Aug 26‐30, Barcelona, Spain 2013. [sr‐incont49728]
Glazener RP 2011 {published data only}
- Dorey G, Glazener C, Buckley B, Cochran C, Moore K. Developing a pelvic floor muscle training regimen for use in a trial intervention. Physiotherapy 2009;95(3):199‐209. [SR‐INCONT32096] [DOI] [PubMed] [Google Scholar]
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. A randomized controlled trial of conservative treatment (pelvic floor muscle training and bladder training) for urinary incontinence in men after prostate surgery (MAPS) (Abstract number 200). Neurourology and Urodynamics 2010;29(6):1093‐4. [SR‐INCONT40156] [Google Scholar]
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Conservative treatment for urinary incontinence in Men After Prostate Surgery (MAPS): two parallel randomised controlled trials. Health Technology Assessment (Winchester, England) 2011;15(24):1‐290. [SR‐INCONT41754] [DOI] [PubMed] [Google Scholar]
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Urinary incontinence in men after formal one‐to‐one pelvic‐floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet 2011;378(9788):328‐37. [SR‐INCONT41752] [DOI] [PubMed] [Google Scholar]
- Glazener CMA. MAPS (Men After Prostate Surgery): Conservative Treatment for Men With Urinary Incontinence After Prostate Surgery; Multicentre Randomised Controlled Trial of Pelvic Floor Muscle Training and Biofeedback [MAPS]. http://clinicaltrials.gov/show/NCT00632138 2005. [NCT00632138; sr‐incont47844]
- Hagen S, Glazener C, Cochran C, Campbell L. Continence care for men having prostate surgery: advice and care before and after radical prostatectomy and transurethral resection of prostate (Abstract number 96). Neurourology and Urodynamics 2009;28(7):690‐1. [SRINCONT34583] [Google Scholar]
- Update Buckley B, Lapitan MCM, Glazener C. The effect of urinary incontinence on health utility and health related quality of life in men following prostate surgery (Abstract number 542). Proceedings of the 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland 2011. [ISRCTN87696430; MAPS; srincont47758]
- Update Buckley BS, Lapitan MC, Glazener CM, MAPS Trial Group. The effect of urinary incontinence on health utility and health‐related quality of life in men following prostate surgery. Neurourology and Urodynamics 2012;31(4):465‐9. [ISRCTN87696430; MAPS; NCT00632138; srincont44658] [DOI] [PubMed] [Google Scholar]
- Update Glazener C, Boachie C, Hagen S, Kilonzo M, Cochran C, Buckley B, et al. Clinical outcomes two years after a randomised controlled trial of pelvic floor muscle training after radical prostatectomy or TURP: men after prostate surgery trial (MAPS) (Abstract number 254). Neurourology and Urodynamics 2011;30(6):1150‐1. [MAPS; srincont:42203] [Google Scholar]
Glazener TURP 2011 {published data only}
- Dorey G, Glazener C, Buckley B, Cochran C, Moore K. Developing a pelvic floor muscle training regimen for use in a trial intervention. Physiotherapy 2009;95(3):199‐209. [DOI] [PubMed] [Google Scholar]
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. A randomized controlled trial of conservative treatment (pelvic floor muscle training and bladder training) for urinary incontinence in men after prostate surgery (MAPS) (Abstract number 200). Neurourology and Urodynamics 2010;29(6):1093‐4. [Google Scholar]
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Conservative treatment for urinary incontinence in Men After Prostate Surgery (MAPS): two parallel randomised controlled trials. Health Technology Assessment (Winchester, England) 2011;15(24):1‐290. [DOI] [PubMed] [Google Scholar]
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Urinary incontinence in men after formal one‐to‐one pelvic‐floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet 2011;378(9788):328‐37. [DOI] [PubMed] [Google Scholar]
- Hagen S, Glazener C, Cochran C, Campbell L. Continence care for men having prostate surgery: advice and care before and after radical prostatectomy and transurethral resection of prostate (Abstract number 96). Neurourology and Urodynamics 2009;28(7):690‐1. [Google Scholar]
- Update Buckley B, Lapitan MCM, Glazener C. The effect of urinary incontinence on health utility and health related quality of life in men following prostate surgery (Abstract number 542). Proceedings of the 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland 2011. [ISRCTN87696430; MAPS; srincont47758]
- Update Buckley BS, Lapitan MC, Glazener CM, MAPS Trial Group. The effect of urinary incontinence on health utility and health‐related quality of life in men following prostate surgery. Neurourology and Urodynamics 2012;31(4):465‐9. [ISRCTN87696430; MAPS; NCT00632138; srincont44658] [DOI] [PubMed] [Google Scholar]
- Update Glazener C, Boachie C, Hagen S, Kilonzo M, Cochran C, Buckley B, et al. Clinical outcomes two years after a randomised controlled trial of pelvic floor muscle training after radical prostatectomy or TURP: men after prostate surgery trial (MAPS) (Abstract number 254). Neurourology and Urodynamics 2011;30(6):1150‐1. [MAPS; srincont:42203] [Google Scholar]
Goode 2009 {published data only}
- Goode P, Burgio K, Johnson T, Roth D, Clay O, Burkhardt J, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent post‐prostatectomy incontinence ‐ a randomized controlled trial (Abstract number 87). Neurourology and Urodynamics 2009;28(7):681‐2. [SR‐INCONT34582] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode PS. Conservative Treatment of Postprostatectomy Incontinence. http://clinicaltrials.gov/show/NCT00212264 2003. [NCT00212264; sr‐incont47864]
- Goode PS, Burgio KL, Johnson TM, Clay OJ, Roth DL, Markland AD, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA 2011;305(2):151‐9. [SR‐INCONT40806] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 2005 {published and unpublished data}
- Hoffmann W, Liedke S, Dombo O, Otto U. Electrical stimulation to treat postoperative incontinence: Therapeutic benefit in regard to quality of life [Die Electrostimulation in der Therapie der posopertiven Harninkontinenz. Therapeutischer Nutzen unter Berucksichtigung der Lebensqualitat]. Urologe 2004;44:33‐40. [SRINCONT20402] [DOI] [PubMed] [Google Scholar]
Hou 2013 {published data only}
- Hou CP, Chen TY, Chang CC, Lin YH, Chang PL, Chen CL, et al. Use of the SF‐36 quality of life scale to assess the effect of pelvic floor muscle exercise on aging males who received transurethral prostate surgery. Clinical Interventions In Aging 2013;8:667‐73. [sr‐incont48064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2000 {published and unpublished data}
- Joseph AC, Chang MK. Comparison of behavior therapy methods for urinary incontinence following prostate surgery: a pilot study. Urologic Nursing 2000;20(3):203‐4. [SRINCONT15334] [PubMed] [Google Scholar]
Koo 2009 {published data only}
- Kim YH, Hwang EG, Shin JH, Kim YW, Lim JS, Na YG, Song KH, Sul CK. Effect of extracorporeal magnetic innervation (ExMI) pelvic floor therapy for urinary incontinence after radical prostatectomy. Urology 2009;74 Suppl 4A:S227. [SR‐INCONT42741] [Google Scholar]
- Koo D, So SM, Lim JS. Effect of Extracorporeal Magnetic Innervation (ExMI) pelvic floor therapy on urinary incontinence after radical prostatectomy [Korean]. Korean Journal of Urology 2009;50(1):23‐7. [SR‐INCONT34595] [Google Scholar]
Laurienzo 2013 {published data only}
- Laurienzo CE, Sacomani CA, Rodrigues TR, Zequi Sde C, Guimaraes GC, Lopes A. Results of preoperative electrical stimulation of pelvic floor muscles in the continence status following radical retropubic prostatectomy. International Brazilian Journal of Urology 2013;39(2):182‐8. [sr‐incont48139] [DOI] [PubMed] [Google Scholar]
Liu 2008 {published data only}
- Liu F. [Extracorporeal magnetic innervation in the treatment of urinary incontinence after radical prostatectomy]. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(17):3289‐92. [SRINCONT34489] [Google Scholar]
Manassero 2007 {published data only}
- Manassero F, Traversi C, Ales V, Pistolesi D, Panicucci E, Valent F, et al. Contribution of early intensive prolonged pelvic floor exercises on urinary continence recovery after bladder neck‐sparing radical prostatectomy: results of a prospective controlled randomized trial. Neurourology and Urodynamics 2007;26(7):985‐9. [SRINCONT23915] [DOI] [PubMed] [Google Scholar]
Marchiori 2010 {published data only}
- Marchiori D, Bertaccini A, Manferrari F, Ferri C, Martorana G. Pelvic floor rehabilitation for continence recovery after radical prostatectomy: role of a personal training re‐educational program. Anticancer Research 2010;30(2):553‐6. [PubMed] [Google Scholar]
Mariotti 2009 {published data only}
- Mariotti G, Sciarra A, Gentilucci A, Salciccia S, Alfarone A, Pierro GD, et al. Early recovery of urinary continence after radical prostatectomy using early pelvic floor electrical stimulation and biofeedback associated treatment. Journal of Urology 2009;181(4):1788‐93. [SRINCONT31079] [DOI] [PubMed] [Google Scholar]
- Sciarra A, Salciccia S, Gentilucci A, Alfarone A, Pierro GB, Mariotti G, et al. Early recovery of urinary continence after radical prostatectomy using early pelvic floor electric stimulation and biofeedback associated treatment. Journal of Urology 2009;181(4 Suppl 1):680. [SRINCONT42739] [DOI] [PubMed] [Google Scholar]
- Sciarra A, Salciccia S, Gentilucci A, Alfarone A, Mariotti G, Cattarino S, et al. Early recovery of urinary continence after radical prostatectomy using early pelvic floor electric stimulation and biofeedback associated treatment (Abstract number 412). European Urology Supplements 2009;8(4):223. [SR‐INCONT42738] [Google Scholar]
Martini 2011 {published data only}
- Martini M, Bernardini S, Blanc E, Piretta K, Tappero R. Relationship between integrity of pelvic floor function and recovery of continence after laparoscopic prostatectomy and effects of preventive pelvic floor training in males with pelvic floor weakness (Abstract number 14). Neurourology and Urodynamics 2011;30 Suppl 1:11‐2. [sr‐incont47398] [Google Scholar]
Mathewson‐Chapman 97 {published data only}
- Mathewson‐Chapman M. The effect of pelvic muscle exercises with biofeedback for urinary incontinence post‐prostatectomy. [Unpublished PhD dissertation]. Gainsville: University of Florida, 1995. [SRINCONT11221] [Google Scholar]
- Mathewson‐Chapman M. Pelvic muscle exercise/biofeedback for urinary incontinence after prostatectomy: an education program. Journal of Cancer Education 1997;12(4):218‐23. [SRINCONT5381] [DOI] [PubMed] [Google Scholar]
Moore 1999 {published and unpublished data}
- Moore KN. The effect of urinary incontinence on the quality of life following radical prostatectomy [Unpublished PhD Thesis]. Edmonton: University of Alberta, 1997. [SRINCONT8006] [Google Scholar]
- Moore KN, Griffiths DJ, Hughton A. A randomised controlled trial of pelvic muscle exercises or pelvic muscle exercises plus electrical stimulation for post radical prostatectomy urinary incontinence [abstract]. Neurourology and Urodynamics 1998;17:424‐6. [SRINCONT5677] [Google Scholar]
- Moore KN, Griffiths DJ, Hughton A. Urinary incontinence after post radical prostatectomy: A randomised controlled trial comparing pelvic muscle exercises with pelvic muscle exercises plus electrical stimulation. British Journal of Urology International 1999;83:57‐65. [SRINCONT5730] [DOI] [PubMed] [Google Scholar]
Moore 2004 {published data only}
- Moore KN, Schieman S, Ackerman T, Drus H, Metcalfe JB, Voaklander DC. Assessing the comfort, safety and patient satisfaction with three commonly used penile compression devices. Urology 2004;63(1):150‐4. [SRINCONT19156] [DOI] [PubMed] [Google Scholar]
Moore 2008 {published and unpublished data}
- Moore KN. The effectiveness of biofeedback assisted pelvic floor muscle exercises in the treatment of incontinence post radical prostatectomy. [Personal Communication] (as supplied 11 May 2006). Data on file.
- Moore KN, Valiquette L, Chetner MP, Byrniak S, Herbison GP. Return to continence after radical retropubic prostatectomy: A randomized trial of verbal and written instructions versus therapist‐directed pelvic floor muscle therapy. Urology 2008;72(6):1280‐6. [SR‐INCONT26907] [DOI] [PubMed] [Google Scholar]
Morihiro 2011 {published data only}
- Morihiro N, Masatsugu I, Shinji K, Kenichi T, Kazumasa M, Shiro B. Effectiveness of sacral surface therapeutic electrical stimulation (SSTES) on early recovery of urinary incontinence after laparoscopic radical prostatectomy: a prospective study (Abstract number 64). Neurourology and Urodynamics 2011;30(6):889‐90. [SR‐INCONT42173] [Google Scholar]
Nowak 2007 {published data only}
- Nowak M, Jordan M, Haberl S, Herwig R, Kuehhas F, Brausi M, et al. Prospective study of extracorporeal magnetic innervation pelvic floor therapy (EXMI) versus standard pelvic floor training following radical prostatectomy: Impact on timing and magnitude of recovery of continence (Abstract number 482). European Urology Supplements 2007;6(2):143. [SRINCONT34792] [Google Scholar]
Opsomer 1994 {published data only}
- Opsomer RJ, Castille Y, Abi Aad AS, Cangh PJ. Urinary incontinence after radical prostatectomy: Is professional pelvic floor training necessary?. Neurourology and Urodynamics 1994;13(4):382‐4. [SRINCONT2687] [Google Scholar]
Overgard 2008 {published data only}
- Morkved S. Urinary Incontinence After Radical Prostatectomy. ‐ Effect of Pelvic Floor Muscle Training. A Randomised Controlled Trial. http://clinicaltrials.gov/show/NCT00239824 2005. [NCT00239824; sr‐incont47830]
- Morkved S, Overgard M, Lydersen S, Angelsen A. Does pelvic floor muscle training with follow up instructions by a physiotherapist reduce urinary incontinence after radical prostatectomy? ‐ A randomised controlled trial (Abstract number 15). Neurourology and Urodynamics 2008;27(7):587‐8. [SR‐INCONT31841] [Google Scholar]
- Overgard M, Angelsen A, Lydersen S, Morkved S. Does physiotherapist‐guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy?: A randomised controlled trial. European Urology 2008;54:438‐48. [SR‐INCONT26906] [DOI] [PubMed] [Google Scholar]
- Update Angelsen A, Nilssen S, Overgard M, Lydersen S, Morkved S. Does physiotherapist‐guided pelvic floor muscle training increase the quality of life in patients after radical prostatectomy? A randomized clinical study (Abstract number 733). Proceedings of the 42nd Annual Meeting of the International Continence (ICS), 2012 Oct 15 to 19, Beijing, China 2012. [NCT00239824; srincont47938] [DOI] [PubMed]
- Update Nilssen SR, Morkved S, Overgard M, Lydersen S, Angelsen A. Does physiotherapist‐guided pelvic floor muscle training increase the quality of life in patients after radical prostatectomy? A randomized clinical study. Scandinavian Journal of Urology and Nephrology 2012;46(6):397‐404. [NCT00239824; SR‐INCONT46190] [DOI] [PubMed] [Google Scholar]
Parekh 2003 {published data only}
- Parekh AR, Feng Mi, Kirages D, Bremner H, Kaswick J, Aboseif S. The role of pelvic floor exercises on post‐prostatectomy incontinence. Journal of Urology 2003;170:130‐3. [SRINCONT16004] [DOI] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park S‐W, Park C‐S, Kim T‐N, Lee W, Nam J‐K. The effects of a 12‐week's combined exercise intervention on physical function and mental health after radical prostatectomy in elderly patients with prostate cancer: a prospective, randomised controlled study (Abstract number 1309). Journal of Urology 2011;185(4S):e524. [SR‐INCONT42511] [Google Scholar]
- Park SW, Kim TN, Nam JK, Ha HK, Shin DG, Lee W, et al. Recovery of overall exercise ability, quality of life, and continence after 12‐week combined exercise intervention in elderly patients who underwent radical prostatectomy: a randomized controlled study. Urology 2012;80(2):299‐306. [SR‐INCONT45132] [DOI] [PubMed] [Google Scholar]
Perissinotto 2008 {published data only}
- Perissinotto MCR, D'Ancona CAL, Campos RM, Lucio AC, Silva JrW. Physiotherapeutic for treatment of post radical prostatectomy urinary incontinence (Abstract number 265). Proceedings of the International Continence Society (ICS), 38th Annual Meeting, 2008 Oct 20‐24, Cairo, Egypt. 2008. [SRINCONT31843]
Porru 2001 {published data only}
- Porru D, Campus G, Caria A, Madeddu, Cucchi A, Rovereto B, et al. Impact of early pelvic floor rehabilitation after transurethral resection of the prostate. Neurourology and Urodynamics 2001;20:53‐9. [SRINCONT11849] [DOI] [PubMed] [Google Scholar]
Ribeiro 2008 {published data only}
- Ribeiro LH, Prota C, Gomes CM, Bessa J Jr, Boldarine MP, Dall'Oglio MF, et al. Long‐term effect of early postoperative pelvic floor biofeedback on continence in men undergoing radical prostatectomy: a prospective, randomized, controlled trial. Journal of Urology 2010;184(3):1034‐9. [SR‐INCONT40040] [DOI] [PubMed] [Google Scholar]
- Ribeiro LS, Prota C, Gomes CM, Dall'Oglio MF, Bruschini H, Srougi M. Effect of early postoperative pelvic‐floor biofeedback on continence in men undergoing radical prostatectomy: A randomized, controlled trial (Abstract number 1412). Journal of Urology 2008;179(4 Suppl S):483. [SRINCONT26921] [DOI] [PubMed] [Google Scholar]
- Ribiero LS, Prota C, Gomes CM, Boldarine MP, Nakano E, Dall'Oglio M, et al. Early pelvic‐floor biofeedback training promotes long‐term improvement of urinary continence after radical prostatectomy (Abstract number 1882) [.]. Journal of Urology 2009;181(4 Suppl):680. [SR‐INCONT42736] [Google Scholar]
Robinson 2008 {published data only}
- Robinson JP, Bradway CW, Nuamah I, Pickett M, McCorkle R. Systematic pelvic floor training for lower urinary tract symptoms post‐prostatectomy: a randomized clinical trial. Internatonal Journal of Urological Nursing 2008;2(1):3‐13. [SRINCONT34593] [Google Scholar]
Robinson 2009 {published data only}
- Robinson J, Weiss R, Avi‐Itzhak T, McCorkle R. Pilot‐testing of a theory‐based pelvic floor training intervention for radical prostatectomy patients (Abstract number 88). Neurourology and Urodynamics 2009;28(7):682‐3. [SRINCONT34587] [Google Scholar]
Seleme 2008 {published data only}
- Seleme M, Ribeiro V, Moreno A, Berghmans B, Bendhack M. Efficacy of physiotherapy after radical prostatectomy (Abstract number 256). Proceedings of the International Continence Society (ICS), 38th Annual Meeting, 2008 Oct 20‐24, Cairo, Egypt. 2008. [SRINCONT31842]
Tibaek 2007 {published data only}
- Tibaek S, Klarskov P, Hansen BL, Thomsen H, Andresen H, Jensen CS, et al. Pelvic floor muscle training before transurethral resection of the prostate: A randomized, controlled, blinded study. Scandinavian Journal of Urology and Nephrology 2007;41(4):329‐34. [SRINCONT23879] [DOI] [PubMed] [Google Scholar]
- Tibaek S, Klarskov P, Lund Hanzen B, Thomsen H, Andresen H, Schmidt Jensen C, et al. Pelvic floor muscle training before transurethral resection of the prostate (Abstract number 31).. Neurourology and Urodynamics 2006;25(6):546‐7. [SR‐INCONT26614] [Google Scholar]
Tienforti 2012 {published data only}
- Sacco E, Tienforti D, D'Addessi A, Racioppi M, Gulino G, Pinto F, et al. Efficacy of a supervised, affordable program of perioperative pelvic floor muscle training in improving the recovery of continence after radical prostatectomy: a randomized controlled trial (Abstract number 138). Neurourology and Urodynamics 2011;30(6):995‐7. [SR‐INCONT42188] [DOI] [PubMed] [Google Scholar]
- Sacco E, Tienforti D, Marangi F, D'Addessi A, Racioppi M, Gulino G, et al. Efficacy of a supervised low‐intensity regimen of perioperative pelvic floor muscle training in reducing postprostatectomy urinary incontinence: A randomized controlled trial (Abstract number 286). European Urology Supplements 2012;11(1):e286. [sr‐incont47271] [Google Scholar]
- Tienforti D, Sacco E, Marangi F, D'Addessi A, Racioppi M, Gulino G, et al. Efficacy of an assisted low‐intensity programme of perioperative pelvic floor muscle training in improving the recovery of continence after radical prostatectomy: a randomized controlled trial. BJU International 2012;110(7):1004‐10. [SR‐INCONT45339] [DOI] [PubMed] [Google Scholar]
Tobia 2008 {published data only}
- Tobia I, Gonzalez MS, Martinez P, Tejerizo JC, Gueglio G, Damia O, et al. [Randomized study on urinary continence after radical prostatectomy with previous kinesic perineal physiotherapy]. [Spanish]. Archivos Espanoles de Urologia 2008;61(7):793‐8. [SRINCONT27688] [DOI] [PubMed] [Google Scholar]
van Kampen 1998 {unpublished data only}
- Kampen M, Weerdt W, Poppel H, Baert L. Urinary incontinence after radical prostatectomy can be treated by pelvic floor re‐education and predicted by measuring urine loss at catheter withdrawal: a controlled study. Proceedings of the International Continence Society (ICS), 29th Annual Meeting; 1999 Aug 22‐26; Denver, Colorado. 1999:246‐7. [SR‐INCONT9919]
- Kampen M, Weerdt W, Poppel H, Ridder D, Feys H, Baert L. Effect of pelvic floor re‐education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet 2000;355(9198):98‐102. [SRINCONT9012] [DOI] [PubMed] [Google Scholar]
- Kampen M, Weerdt W, Poppel H, Ridder D, Feys H, Baert, L. The efficacy of physiotherapy on the degree and the duration of incontinence after radical prostatectomy: a randomised controlled study [abstract]. Neurourology and Urodynamics 1998;17(4):49‐50. [SRINCONT5681] [Google Scholar]
- Kampen M, Weerdt W, Poppel H, Ridder D, Feys H, Baert L. The efficacy of physiotherapy on the degree and the duration of incontinence after radical prostatectomy: a randomised controlled study. [Unpublished doctoral thesis]. Belgium: University of Leuven, 1998. [SRINCONT5732] [Google Scholar]
Wille 2003 {published and unpublished data}
- Wille S, Sobottka A, Heidenreich A, Hofmann R. Pelvic floor exercises, electrical stimulation and biofeedback after radical prostatectomy: Results of a prospective randomized trial. Journal of Urology 2003;170(2 Part 1):490‐3. [SRINCONT16931] [DOI] [PubMed] [Google Scholar]
- Wille S, Sobottka A, Olbert P, Heidenreich A, Hofmann R. Impact of electrical stimulation or biofeedback on quality of life after radical prostatectomy: Results of a prospective randomized trial (Abstract number 167). Journal of Urology 2004;171(4 Suppl):44. [SRINCONT27408] [DOI] [PubMed] [Google Scholar]
Yamanishi 2006 {published data only}
- Yamanishi K, Mizuno T, Sakakibara R, Uchiyama T, Ito Y, Yamamoto T, et al. A randomized, placebo‐controlled, double‐blind study of electrical stimulation with pelvic floor muscle training for the treatment of urinary incontinence after radical prostatectomy (Abstract number 30). Neurourology and Urodynamics 2006;25(6):545‐6. [SRINCONT26613] [Google Scholar]
- Yamanishi T, Mizuno T, Watanabe M, Honda M, Yoshida K‐I. Randomized, placebo controlled study of electrical stimulation with pelvic floor muscle training for severe urinary incontinence after radical prostatectomy. Journal of Urology 2010;184(5):2007‐12. [SR‐INCONT40344] [DOI] [PubMed] [Google Scholar]
Yokoyama 2004 {published data only}
- Yokoyama T, Nishiguchi J, Watanabe T, Nose H, Nozaki K, Fujita O, Inoue M, et al. Comparative study of effects of extracorporeal magnetic innervation versus electrical stimulation for urinary incontinence after radical prostatectomy. Urology 2004;63(2):264‐7. [SRINCONT17116] [DOI] [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang AY, Galanek J, Strauss GJ, Siminoff LA. What it would take for men to attend and benefit from support groups after prostatectomy for prostate cancer: a problem‐solving approach. Journal of Psychosocial Oncology 2008;26(3):97‐112. [SRINCONT29254] [DOI] [PubMed] [Google Scholar]
- Zhang AY, Strauss GJ, Siminoff LA. Effects of combined pelvic floor muscle exercise and a support group on urinary incontinence and quality of life of postprostatectomy patients. Oncology Nursing Forum Online 2007;34(1):47‐53. [SRINCONT31506] [DOI] [PubMed] [Google Scholar]
- Zhang AY, Strauss GJ, Siminoff LA. Intervention of urinary incontinence and quality of life outcome in prostate cancer patients. Journal of Psychosocial Oncology 2006;24(2):17‐30. [SRINCONT22147] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bennett 1997 {published data only}
- Bennett JK, Foote JE, Green BG, Killorin EW, Martin SH. Effectiveness of biofeedback/electrostimulation in the treatment of post prostatectomy urinary incontinence [abstract]. Proceedings of the Urodynamics Society Annual Meeting. 1997.
Bocker 2002 {published data only}
- Bocker B, Smolenski UC. [Physikalische therapie der beckenbodeninsuffizienz‐ methodenvergleich]. Journal fur Urologie und Urogynakologie 2002;2:20‐7. [Google Scholar]
Burkert 2011 {published data only}
- Burkert S, Scholz U, Gralla O, Roigas J, Knoll N. Dyadic planning of health‐behavior change after prostatectomy: a randomized‐controlled planning intervention. Social Science & Medicine 2011;73(5):783‐92. [srincont46861] [DOI] [PubMed] [Google Scholar]
Ceresoli 2002 {unpublished data only}
- Ceresoli A, Kartalas Goumas J, Colombo F, Barbetti E, Dell'Aglio F, Bonacina P, et al. Daily transcutaneous electrical nerve stimulation (DTENS) after radical perineal prostatectomy: A free cost effective biofeedback technique in the treatment of post operative urinary incontinence (abstract). Proceedings of the 32nd Annual Meeting of the International Continence Society; 2002 Aug 28‐30; Heidelberg, Germany. 2002.
Chang 1998 {published data only}
- Chang PL, Tsai LH, Huang ST, Hsieh ML, Tsui KH. The early effect of pelvic floor muscle exercise after transurethral prostatectomy. Journal of Urology 1998;160(2):402‐5. [PubMed] [Google Scholar]
Cornel 2005 {published data only}
- Cornel EB, Wit R, Witjes JA. Evaluation of early pelvic floor physiotherapy on the duration and degree of urinary incontinence after radical retropubic prostatectomy in a non‐teaching hospital. World Journal of Urology 2005;23:353‐5. [DOI] [PubMed] [Google Scholar]
Cornu 2011 {published data only}
- Cornu JN, Merlet B, Ciofu C, Mouly S, Peyrat L, Sebe P, et al. Duloxetine for mild to moderate postprostatectomy incontinence: preliminary results of a randomised, placebo‐controlled trial. European Urology 2011;59(1):148‐54. [SR‐INCONT41370] [DOI] [PubMed] [Google Scholar]
Crevenna 2003 {published data only}
- Crevenna R, Zoch C, Kellani M, Quittan M, Fialka‐Moser V. Implementation of a physical rehabilitation group for post‐prostatectomy urinary incontinence patients and its effects on quality of life. Physikalische Medicin Rehabilitationsmedizin Kurotmedizin 2003;13(6):339‐44. [Google Scholar]
Dieperink 2013 {published data only}
- Dieperink KB, Johansen C, Hansen S, Wagner L, Andersen KK, Minet LR, et al. The effects of multidisciplinary rehabilitation: RePCa‐a randomised study among primary prostate cancer patients. British Journal of Cancer 2013;109(12):3005‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eren 2013 {published data only}
- Alan C, Ersay AR, Eren AE, Altintas R, Kocoglu H, Basturk G. Efficacy of early duloksetin therapy in urinary incontinence occurred after radical prostatectomy (Abstract number 704). Proceedings of the 43rd Annual Meeting of the International Continence Society (ICS), 2013 Aug 26‐30, Barcelona, Spain 2013. [sr‐incont49727]
- Eren AE, Alan C, Bastruk G. Efficacy of early duloxetine therapy in urinary incontinence occurred after radical prostatectomy (Abstract number 25226). BJU International 2013;112 Suppl S1:18‐9. [sr‐incont49943] [Google Scholar]
- Eren AE, Ersay AR, Alan C, Basturk G. Efficacy of early Duloksetin therapy in urinary incontinence occurred after radical prostatectomy (Abstract number S90). European Urology Supplements 2013;12(4):e1198. [sr‐incont49916] [Google Scholar]
Filocamo 2007 {published data only}
- Popolo G, Filocamo M, Li Marzi V, Cecconi F, Marzocco M, Nicita G. Stress incontinence after radical prostatectomy: a randomized controlled trial on combination of duloxetine and rehabilitation versus rehabilitation alone (Abstract number 29). Neurourology and Urodynamics 2006;25(6):544‐5. [SR‐INCONT26612] [Google Scholar]
- Filocamo MT, Li Marzi V, Popolo G, Cecconi F, Villari D, Marzocco M, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. European Urology 2007;51(6):1559‐64. [23000] [DOI] [PubMed] [Google Scholar]
Griebling 1999 {published data only}
- Griebling TL, Kreder KJ, Sueppel CA, See WA. Timing of biofeedback and pelvic floor muscle exercise training for men undergoing radical prostatectomy (Abstract number 322). Proceedings of the 29th Annual Meeting of the International Continence Society; 1999 Aug 22‐26; Denver, Colorado. 1999:401. [SR‐INCONT9928]
- Sueppel C, Kreder K, See W. Improved continence outcomes with preoperative pelvic floor muscle strengthening exercises. Urologic Nursing 2001;21:201‐10. [PubMed] [Google Scholar]
Hotston 2006 {published data only}
- Hotston MR, Hurley K, Patel S, Persad R. The use of duloxetine for stress incontinence after prostatectomy. BJU International 2006;98(4):916‐7. [26028] [DOI] [PubMed] [Google Scholar]
Ip 2004 {published data only}
- Ip V. Evaluation of a patient education tool to reduce the incidence of incontinence post‐prostate surgery. Urologic Nursing 2004;24(5):401‐7. [PubMed] [Google Scholar]
Kahihara 2006 {published data only}
- Kahihara CT, Ferreira U, Pedro RN, Matheus WE, Netto NR. [Early versus delayed physiotherapy in the treatment of post‐prostatectomy male urinary incontinence]. Archivos Espanoles de Urologia 2006;59(8):773‐8. [DOI] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin YH, Yu TJ, Lin VC, Wang HP, Lu K. Effects of early pelvic‐floor muscle exercise for sexual dysfunction in radical prostatectomy recipients. Cancer Nursing 2012;35(2):106‐14. [SR‐INCONT46858] [DOI] [PubMed] [Google Scholar]
McGlynn 2004 {published data only}
- McGlynn B, Al‐Saffar N, Begg H, Gurun M, Hollins G, McPhee S, et al. Management of urinary incontinence following radical prostatectomy. Urologic Nursing 2004;24(6):475‐82. [PubMed] [Google Scholar]
Mishel 2002 {published data only}
- Mishel MH, Belyea M, Germino BB, Stewart JL, Bailey DE Jr, Robertson C, et al. Helping patients with localized prostate carcinoma manage uncertainty and treatment side effects: nurse‐delivered psychoeducational intervention over the telephone. Cancer 2002;94(6):1854‐66. [sr‐incont15336] [DOI] [PubMed] [Google Scholar]
Nehra 2001 {published data only}
- Nehra A, Rovner E, Wein A, Lange P, Ellis W, Keane R, et al. Interim analysis of a multi‐center study of extracorporeal magnetic innervation (ExMI) for the treatment of urinary incontinence following radical prostatectomy (Abstract). Proceedings of the 31st Annual Meeting of the International Continence Society; 2001 Sept 18‐21; Seoul, Korea. 2001.
Ottenbacher 2013 {published data only}
- Ottenbacher A, Sloane R, Snyder DC, Kraus W, Sprod L, Demark‐Wahnefried W. Cancer‐specific concerns and physical activity among recently diagnosed breast and prostate cancer survivors. Integrative Cancer Therapies 2013;12(3):206‐12. [sr‐incont50477] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pemberton 2006 {published data only}
- Pemberton P, Brooks A, Eriksen CM, Frost S, Graham S, Greenman L, et al. A comparative study of two types of urinary sheath. Nursing Times 2006;102(7):36‐41. [PubMed] [Google Scholar]
Prota 2012 {published data only}
- Prota C, Gomes CM, Ribeiro LH, Bessa J Jr, Nakano E, Dall'Oglio M, et al. Early postoperative pelvic‐floor biofeedback improves erectile function in men undergoing radical prostatectomy: a prospective, randomized, controlled trial. International Journal of Impotence Research 2012;24(5):174‐8. [SR‐INCONT45891] [DOI] [PubMed] [Google Scholar]
Pulker 2002 {published data only}
- Pulker E. Long term results of physiotherapy in men with urinary stress incontinence after radical prostatectomy (Abstract). Proceedings of the 32nd Annual Meeting of the International Continence Society; 2002 Aug 28‐30; Heidelburg, Germany. 2002.
Ribeiro 2013 {published data only}
- Ribeiro AM, Oliveira HF. Pelvic Floor Muscles Training in Urinary Disorders in Men With Prostate Cancer Undergoing Radiotherapy. http://clinicaltrials.gov/show/NCT01762956 2013. [NCT01762956; sr‐incont47831]
Ricci 2004 NEWa {published data only}
- Ricci L, Minardi D, Romoli M, Galosi AB, Muzzonigro G. Acupuncture reflexotherapy in the treatment of sensory urgency that persists after transurethral resection of the prostate: a preliminary report. Neurourology and Urodynamics 2004;23(1):58‐62. [SR‐INCONT16645] [DOI] [PubMed] [Google Scholar]
Robinson 2012 {published data only}
- Robinson JP, Burrell SA, Avi‐Itzhak T, McCorkle R. Validity testing of the stopwatch urine stream interruption test in radical prostatectomy patients. Journal of Wound, Ostomy & Continence Nursing 2012;39(5):545‐51. [SR‐INCONT45900] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salinas Casado 1991 {published data only}
- Salinas Casado J, Varela E, Prieto Chaparro L, Virseda Rodriguez M, Salomon S, Guerrero A, et al. Results of perineal electric stimulation in stress urinary incontinence [Resultatos de la estimulacion electrica perineal en la incontinencia urinaria de esfuerzo]. Archivos Espanoles di Urologia 1991;44(4):437‐40. [PubMed] [Google Scholar]
Salinas Casado 1996 {published data only}
- Salinas Casado J, Virseda Chamorro M, Salomon Mohamed S, Bravo de Rueda C, Aristizabal JM, Resel Estevez L. Results of electrical stimulation in the treatment of post‐prostatectomy urinary incontinence. Actas Urologicas Espanolas 1996;20(6):544‐50. [PubMed] [Google Scholar]
Seki 2005 {published data only}
- Seki N, Kai N, Takano N, Naito S, Kawajiri M, Kira J, Tobimatsu S. Efficacy of high‐frequency magnetic stimulation of the sacral root in patients with stress urinary incontinence following radical prostatectomy [abstract]. 35th Annual Meeting of International Continence Society; 2005 Aug 29 ‐ Sep 2; Montreal, Canada.
Shen 2012 NEWa {published data only}
- Shen YZ, Lin X, Lin Q. [Overactive bladder after transurethral resection of prostate treated with electroacupuncture therapy and tolterodine]. Zhongguo Zhen Jiu = Chinese acupuncture & moxibustion 2012;32(5):404‐8. [SR‐INCONT47454] [PubMed] [Google Scholar]
Traeger 2013 {published data only}
- Traeger L, Penedo FJ, Benedict C, Dahn JR, Lechner SC, Schneiderman N, et al. Identifying how and for whom cognitive‐behavioral stress management improves emotional well‐being among recent prostate cancer survivors. Psycho‐Oncology 2013;22(2):250‐9. [sr‐incont48709] [DOI] [PubMed] [Google Scholar]
Yang 2010 NEWa {published data only}
- Yang BS, Ye DW, Yao XD, Peng JY, Zhang SL, Dai B, et al. [The study of electrical acupuncture stimulation therapy combined with pelvic floor muscle therapy for postprostatectomy incontinence]. [Chinese]. Chung‐Hua Wai Ko Tsa Chih [Chinese Journal of Surgery] 2010;48(17):1325‐7. [SR‐INCONT42595] [PubMed] [Google Scholar]
Yao 2012 {published data only}
- Yao J, Hirschhorn AD, Mungovan SF, Patel MI. Preoperative pelvic floor physiotherapy improves continence following radical prostatectomy (Abstract number 182). Neurourology and Urodynamics 2012;31(6):964‐5. [sr‐incont50072] [Google Scholar]
Zahariou 2009 {published data only}
- Zahariou A, Kyriakides A. Intensive pelvic floor muscle training after radical prostatectomy improves continence outcomes (Abstract number S40). European Urology Supplements 2009;8(8):620. [sr‐incont49168] [Google Scholar]
Zermann 1999 {published and unpublished data}
- Zermann DH, Ishgooka M, Wunderlich H, Wilhelm S, Schubert J. Post‐prostatectomy incontinence and pelvic floor dysfunction (abstract). Proceedings of the 29th Annual Meeting of the International Continence Society; 1999 Aug 22‐26; Denver, Colorado. 1999.
- Zermann DH, Ishigooka M, Wunderlich H, Reichelt O, Schubert J. A study of pelvic floor function pre‐and post radical prostatectomy using clinical neurourological investigations, urodynamics and electromyography. European Urology 2000;37:72‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang A, O'Neill J. Improving Continence and Quality of Life in Prostate Cancer Patients (StayDry). http://clinicaltrials.gov/show/NCT01365182 2009. [NCT01365182; StayDry; sr‐incont47806]
References to studies awaiting assessment
Crivellaro 2011 {published data only}
- Abbinante M, Crivellaro S, Palazzetti A, Tosco L, Frea B. Efficacy of ultrasound‐guided pelvic muscle training (Abstract number 45). Neurourology and Urodynamics 2012;31 Suppl 1:S35. [sr‐incont47301] [Google Scholar]
- Crivellaro S, Abbinante M, Palazzetti A, Tosco L, Frea B. Efficacy of ultrasound‐guided pelvic muscle training (Abstract number 285). European Urology Supplements 2012;11(1):e285a. [sr‐incont47272] [Google Scholar]
- Crivellaro S, Lorenzo T, Abbinante M, Martinez G, Palazzetti A, Frea B. Efficacy of pelvic training under transrectal ultrasonography (Abstract number 16). Neurourology and Urodynamics 2011;30 Suppl 1:13‐4. [sr‐incont47397] [Google Scholar]
Delmastro 2010 {published data only}
- Delmastro F, Marchisio C, Lamberti G, Giraudo D. Urinary incontinence after radical prostatectomy: a randomized controlled trial comparing preoperative intensive pelvic muscle exercises with or without proprioceptive training (Abstract). Neurourology and Urodynamics 2010;29(2 Suppl):62‐3. [SR‐INCONT39878] [Google Scholar]
Lilli 2006 NEW {published data only}
- Lilli P, Mercuriali M, Fiori M, Hanitzsch H, Gunelli R, Bercovich E. Impact of preoperative biofeedback on incontinence in cancer patients undergoing radical prostatectomy. Archivio Italiano di Urologia e Andrologia 2006;78(3):92‐6. [SR‐INCONT25089] [PubMed] [Google Scholar]
Simeit 2010 NEW {published data only}
- Simeit R, Deck R, Drechsler T, Fiedrich M, Schonrock‐Nabulsi P. [Quality of life and impact of incontinence in male patients with prostate carcinoma after radical retropubic prostatectomy]. [German]. Rehabilitation 2010;49(3):180‐9. [SR‐INCONT40661] [DOI] [PubMed] [Google Scholar]
Zellner 2011 NEW {published data only}
- Zellner M. [Incontinence after radical prostatectomy and cystectomy: are combined training with mechanical devices and whole body vibration effective?]. [German]. Urologe (Ausg.A) 2011;50(4):433‐44. [SR‐INCONT41808] [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang A. The problem‐solving therapy and urinary incontinence in prostate cancer patients (Abstract number 14‐5). Psycho‐Oncology 2013;22 Suppl 2:33‐4. [NCT01365182; sr‐incont49941] [Google Scholar]
References to ongoing studies
Burnett 2012 {published data only}
- NCT01748110, Burnett AL, Segal RL, Cheskin LJ, Blitz JU. Health Interventions in Men Undergoing Radical Prostatectomy ‐ A Randomized Controlled Clinical Trial. http://clinicaltrials.gov/show/NCT01748110 2012.
Burnett 2013 {published data only}
- Burnett AL, Tajkarimi K. Study of non‐invasive Viberect© penile vibratory stimulation regimen to enhance recovery of erectile function/rigidity and urinary control/continence after nerve sparing radical prostatectomy (RP) for clinically localized prostate cancer. http://clinicaltrials.gov/show/NCT01718704 2013. [NCT01718704; sr‐incont49360]
Fode 2012 NEW {published data only}
- Fode M. Mechanical nerve stimulation in the treatment of post prostatectomy incontinence. http://clinicaltrials.gov/show/NCT01540656 2012. [NCT01540656; sr‐incont49358]
Goode 2014 {published data only}
- Goode PS. Perioperative Post‐Prostatectomy Incontinence Home Telehealth Program (ProsTel). http://clinicaltrials.gov/show/NCT01960998 2014. [NCT01960998; ProsTel; sr‐incont49364]
Mina 2013 {published data only}
- NCT02036684, Mina DS, Matthew AG, Carli F. A Multicentre, Pilot Randomized Controlled Trial to Examine the Effects of Prehabilitation on Functional Outcomes After Radical Prostatectomy. http://clinicaltrials.gov/show/NCT02036684 2013.
Ng 2011 {published data only}
- Ng S‐I. A Randomized Controlled Trial Study of the Efficacy of Intensive Preoperative Pelvic Floor Muscle Training to Decrease Post‐prostatectomy Urinary Incontinence. http://clinicaltrials.gov/show/NCT01338584 2011. [NCT01338584; sr‐incont47833]
Terrone 2007 {published data only}
- Terrone C, Cisari C. Prevention of Urinary Incontinence After Prostatectomy. http://clinicaltrials.gov/show/NCT00982098 2007. [NCT00982098; sr‐incont47873]
Zopf 2012 {published data only}
- Zopf EM, Braun M, Machtens S, Zumbe J, Bloch W, Baumann FT. Implementation and scientific evaluation of rehabilitative sports groups for prostate cancer patients: study protocol of the ProRehab Study. BMC Cancer 2012;12:312. [DRKS00004184; SR‐INCONT45125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aboseif 1996
- Aboseif SR, O'Connell HE, Usui A, McGuire EJ. Collagen injection for intrinsic sphincteric deficiency in men. Journal of Urology 1996;155:10‐3. [PubMed] [Google Scholar]
Abrams 1991
- Abrams PH. Investigation of post prostatectomy problems. Urology 1991;15:209‐12. [DOI] [PubMed] [Google Scholar]
Albertsen 1997
- Albertsen PC, Lu‐Yao GL, Warren J. Risk of complication and readmission associated with radical prostatectomy in community practice: A Medicare claims analysis. Journal of Urology 1997;157 Suppl 4:93. [Google Scholar]
Altman 2013
- Altman D, Cartwright R, Lapitan MC, Nelson R, Sillen U, Tikkinen K. Epidemiology of Urinary Incontinence (UI) and other Lower Urinary tract Symptoms (LUTS), Pelvic organ Prolapse (POP) and Anal Incontinence (AI). In: Abrams P, Cardozo L, Khoury S, Wein A editor(s). Incontinence: 5th International Consultation on Incontinence. Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence; 2012 Feb 23‐25; Paris. Belgium: International Consultation on Urological Diseases, 2013:27‐9. [Google Scholar]
Berghmans 1998
- Berghmans LC, Hendriks HJ, Bo K, Hay‐Smith EJ, Bie RA, Waalwijk van Doorn ES. Conservative treatment of stress urinary incontinence in women: a systematic review of randomized clinical trials. British Journal of Urology 1998;82:181‐91. [DOI] [PubMed] [Google Scholar]
Berghmans 2013
- Berghmans B, Hendriks E, Bernards A, Bie R, Omar MI. Electrical stimulation with non‐implanted electrodes for urinary incontinence in men. Cochrane Database of Systematic Reviews 13, Issue 6. [DOI: 10.1002/14651858.CD001202.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burnett 1998
- Burnett AL, Mostwin JL. In situ anatomical study of the male urethral sphincteric complex: relevance to continence preservation following major pelvic surgery. Journal of Urology 1998;160(4):1301‐6. [PubMed] [Google Scholar]
Chao 1995
- Chao R, Mayo ME. Incontinence after radical prostatectomy: Detrusor or sphincter causes?. Journal of Urology 1995;154:16‐8. [DOI] [PubMed] [Google Scholar]
Donnellan 1997
- Donnellan SM, Duncan HJ, MacGregor RJ, Russell JM. Prospective assessment of incontinence after radical retropubic prostatectomy: objective and subjective analysis. Urology 1997;49(2):225‐30. [DOI] [PubMed] [Google Scholar]
Eastham 1996
- Eastham JA, Kattan MW, Rogers E, Goad JR, Ohori M, Boone TB, et al. Risk factors for urinary incontinence after radical prostatectomy. Journal of Urology 1996;156(5):1707‐13. [PubMed] [Google Scholar]
Fader 2007
- Fader M, Cottenden Alan M, Getliffe K. Absorbent products for light urinary incontinence in women. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD001406.pub2; CD001406] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fader 2008
- Fader M, Cottenden Alan M, Getliffe K. Absorbent products for moderate‐heavy urinary and/or faecal incontinence in women and men. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007408; CD007408] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ficazzola 1998
- Ficazzola MA, Nitti VW. The etiology of post‐radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. Journal of Urology 1998;160:1317‐20. [PubMed] [Google Scholar]
Galloway 2000
- Galloway N, El‐Galley R, Sand P, Appell R, Russell H, Carlan S. Update on extracorporeal magnetic innervation (ExMI) therapy for stress urinary incontinence. Urology 2000;56(6A):82‐6. [DOI] [PubMed] [Google Scholar]
Golubuff 1995
- Golubuff ET, Chang DT, Olsson CA, Kaplan SA. Urodynamics and the etiology of post‐prostatectomy urinary incontinence: The initial Columbia experience. Journal of Urology 1995;153:1034‐7. [PubMed] [Google Scholar]
Grise 2001
- Grise P, Thurman S. Urinary incontinence following treatment of localized prostate cancer. Cancer Control 2001;8(6):532‐9. [DOI] [PubMed] [Google Scholar]
Groutz 2000
- Groutz A, Blaivais JG, Chaikin DC, Weiss JP, Verhaaren M. The pathophysiology of post‐radical prostatectomy incontinence: a clinical and video urodynamic study. Journal of Urology 2000;163(6):1767‐70. [PubMed] [Google Scholar]
Gudziak 1996
- Gudziak MR, McGuire EJ, Gormley EA. Urodynamic assessment of urethral sphincter function in post‐prostatectomy incontinence. Journal of Urology 1996;156:1131‐4. [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing Summary of Findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester, UK: John Wiley & Sons, Ltd.
Hunskaar 2002
- Hunskaar S, Burgio K, Diokno AC, Herzog AR, Hjalmas K, Lapitan MC. Epidemiology and natural history of urinary incontinence. Incontinence: 2nd International Consultation on Incontinence. Plymouth, UK: Health Publications, 2002:165‐200.
Jabs 2001
- Jabs CF, Stanton SL. Urge incontinence and detrusor instability. International Urogynecology Journal and Pelvic Floor Dysfunction 2001;12(1):58‐8. [DOI] [PubMed] [Google Scholar]
Jacobsen 2007
- Jacobsen NE, Moore KN, Estey E, Voaklander D. Open versus laparoscopic radical prostatectomy: a prospective comparison of postoperative urinary incontinence rates. Journal of Urology 2007;177(2):615‐9. [DOI] [PubMed] [Google Scholar]
Jahn 2007
- Jahn P, Preuss M, Kernig A, Langer G, Seifert‐Huehmer A. Types of indwelling urinary catheters for long‐term bladder drainage in adults. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004997.pub2; CD004997] [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Assessing the quality of randomized controlled trials. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in context. 2nd Edition. London: BMJ Books, 2001:87‐108. [Google Scholar]
Khan 1991
- Khan Z, Mieza M, Starer P, Singh VK. Post‐prostatectomy incontinence. A urodynamic and fluoroscopic point of view. Urology 1991;38(5):483‐8. [DOI] [PubMed] [Google Scholar]
Kondo 2002
- Kondo A, Lin TL, Nordling J, Siroky M, Tammela T. Conservative management in men. Incontinence: 2nd International Consultation on Incontinence. Plymouth, UK: Health Publications, 2002:553‐68.
Leach 1995
- Leach GE. Post‐prostatectomy incontinence: the importance of bladder dysfunction. Journal of Urology 1995;153(3 Pt 2):1038. [DOI] [PubMed] [Google Scholar]
Litwiller 1997
- Litwiller SE, McIntire DD, Schnitzer B, Roehrborn CG. Risk factors for incontinence following radical prostatectomy in a community practice setting [abstract]. Urodynamics Society Meeting. New Orleans, April 1997.
MacDonald 2007
- MacDonald R, Fink HA, Huckabay C, Monga M, Wilt TJ. Pelvic floor muscle training to improve urinary incontinence after radical prostatectomy: a systematic review of effectiveness. BJU International 2007;100:76–81. [DOI] [PubMed] [Google Scholar]
Majoros 2006
- Majoros A, Bach D, Keszthelyi A, Hamvas A, Romics I. Urinary incontinence and voiding dysfunction after radical retropubic prostatectomy (prospective urodynamic study). Neurourology and Urodynamics 2006;25:2‐7. [DOI] [PubMed] [Google Scholar]
McCammon 1999
- McCammon KA, Kolm P, Main B, Schellhammer PF. Comparative quality of life analysis after radical prostatectomy or external beam radiation for localized prostate cancer. Urology 1999;54(3):509‐16. [DOI] [PubMed] [Google Scholar]
McGuire 1990
- McGuire EJ. Editorial comment: Pathophysiology of urinary incontinence after radical prostatectomy. Journal of Urology 1990;143:978. [DOI] [PubMed] [Google Scholar]
Milsom 2009
- Milsom I, Altman D, Lapitan MC, Nelson R, Sillen U, Thom D. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP). Incontinence: 4th International Consultation on Incontinence. Paris, France: Health Publication Ltd, 2009:35‐112. [Google Scholar]
Moore 2007
- Moore KN, Fader M, Getliffe K. Long‐term bladder management by intermittent catheterisation in adults and children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006008.pub2; CD006008] [DOI] [PubMed] [Google Scholar]
Niël‐Weise 2005
- Niël‐Weise BS, Broek PJ. Urinary catheter policies for long‐term bladder drainage. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004201.pub2; CD004201] [DOI] [PubMed] [Google Scholar]
Omar 2014
- Omar MI, Lam T, Alexander C, Graham J, Mamoulakis C, Imamura M, et al. Systematic review and meta‐analysis of the clinical effectiveness of bipolar compared with monopolar transurethral resection of the prostate (TURP). BJU International 2014;113:24–35. [DOI: 10.1111/bju.12281] [DOI] [PubMed] [Google Scholar]
Peyromaure 2002
- Peyromaure M, Ravery V, Boccon‐Gibod I. The management of stress urinary incontinence after radical prostatectomy. British Journal of Urology International 2002;90(2):155‐61. [DOI] [PubMed] [Google Scholar]
Rainwater 1988
- Rainwater LM, Zincke H. Radical prostatectomy after radiation for cancer of the prostate: feasibility and prognosis. Journal of Urology 1988;140(6):1455‐9. [DOI] [PubMed] [Google Scholar]
Rassweiler 2006
- Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of Transurethral Resection of the Prostate (TURP) – Incidence, management, and prevention. European Association of Urology 2006;50:969‐80. [DOI] [PubMed] [Google Scholar]
Reference Manager 2012
- Reference Manager Professional Edition Version 12. New York: Thomson Reuters 2012.
Silva 2011
- Silva LA, Andriolo RB, Atallah ÁN, Silva EMK. Surgery for stress urinary incontinence due to presumed sphincter deficiency after prostate surgery. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD008306.pub2] [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson DL. The national coverage decision for reimbursement for biofeedback and pelvic floor electrical stimulation for treatment for urinary incontinence. Journal of Wound, Ostomy & Continence Nursing 2002;29:11‐9. [DOI] [PubMed] [Google Scholar]
Turner 2004
- Turner DA, Shaw C, McGrother CW, Dallosso HM, Cooper NJ, MRC Incontinence Team. The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. BJU International 2004;93(9):1246‐52. [DOI] [PubMed] [Google Scholar]
Uebersax 1995
- Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. The Continence Program for Women Research Group. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourology and Urodynamics 1995;14:131‐9. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE. Measuring patients' views: the optimum outcome measure. BMJ 1993;306(6890):1429‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2001
- Wilson L, Brown J, Shin GP, Luc K, Subak L. Annual direct cost of urinary incontinence. Obstetrics and Gynecology 2001;98(3):398‐406. [DOI] [PubMed] [Google Scholar]
Winters 1997
- Winters JC, Rackley RR, Fralick RA, Simich SC, Appell RA. Urodynamic findings in post‐prostatectomy incontinence [Abstract 398]. Journal of Urology 1997;157 Suppl 4:102. [Google Scholar]
References to other published versions of this review
Campbell 2012
- Campbell SE, Glazener CMA, Hunter KF, Cody JD, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD001843.pub4] [DOI] [PubMed] [Google Scholar]
Hunter 2004
- Hunter KF, Moore KN, Cody DJ, Glazener CMA. Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD001843.pub2] [DOI] [PubMed] [Google Scholar]
Hunter 2007
- Hunter KF, Moore KN, Glazener CMA. Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD001843.pub3] [DOI] [PubMed] [Google Scholar]
Moore 1999b
- Moore KN, Cody DJ, Glazener CM. Conservative management for post prostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001843] [DOI] [PubMed] [Google Scholar]
Moore 2001
- Moore KN, Cody DJ, Glazener CM. Conservative management for post prostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001843] [DOI] [PubMed] [Google Scholar]


