Abstract
Background
Observational data support inverse relationships between exercise or metformin use and disease outcomes in colorectal and breast cancer survivors, although the mechanisms underlying these associations are not well understood.
Methods
In a phase II trial, stage I–III colorectal and breast cancer survivors who completed standard therapy were randomly assigned to structured exercise or metformin or both or neither for 12 weeks. The primary outcome was change in fasting insulin levels; secondary outcomes included changes in other blood-based energetic biomarkers and anthropometric measurements. Analyses used linear mixed models.
Results
In total, 139 patients were randomly assigned; 91 (65%) completed follow-up assessments. Fasting insulin levels statistically significantly decreased in all three intervention arms (−2.47 μU/mL combination arm, −0.08 μU/mL exercise only, −1.16 μU/mL metformin only, + 2.79 μU/mL control arm). Compared with the control arm, all groups experienced statistically significant weight loss between baseline and 12 weeks (−1.8% combination arm, −0.22% exercise only, −1.0% metformin only, +1.55% control). The combination arm also experienced statistically significant improvements in the homeostatic model assessment for insulin resistance (−30.6% combination arm, +61.2% control) and leptin (−42.2% combination arm, −0.8% control), compared with the control arm. The interventions did not change insulin-like growth factor–1 or insulin-like growth factor binding protein–3 measurements as compared with the control arm. Tolerance to metformin limited compliance (approximately 50% of the participants took at least 75% of the planned dosages in both treatment arms).
Conclusions
The combination of exercise and metformin statistically significantly improved insulin and associated metabolic markers, as compared to the control arm, with potential greater effect than either exercise or metformin alone though power limited formal synergy testing. Larger efforts are warranted to determine if such a combined modality intervention can improve outcomes in colorectal and breast cancer survivors.
Dysregulated metabolism is a major hallmark of all solid tumors (1). Mounting evidence demonstrates the importance of energy balance and metabolic factors in cancer development, growth, and recurrence (2–12). Multiple studies have observed associations between high levels of circulating metabolic factors and outcomes (1,2,13–18). Similarly, observational studies have demonstrated that both breast and colorectal cancer survivors who are more physically active either before diagnosis or after diagnosis have improved outcomes (19–24). One hypothesis as to a biological mechanism associating exercise with outcomes relates to insulin and related metabolic factors driving tumor pathogenesis and progression (4,25). Indeed, several studies in colorectal and breast cancer patients suggest exercise statistically significantly lowered insulin and other factors, compared with control groups (26–30).
Metformin is a biguanide derivative approved for type 2 diabetes treatment. Metformin reduces glucose concentration leading to decline in insulin levels and insulin resistance (31), as well as activates the LKB1/AMP-activated protein kinase pathway, decreasing protein synthesis and cell growth (32). Observational studies have demonstrated that diabetics who take metformin are at lower risk than diabetics who take other agents of developing breast and colorectal cancer (33–35), and diabetics who take metformin after diagnosis may have improved response to chemotherapy (36) and lower cancer-related mortality (37–41).
Based on these observations, trials have been conducted or are underway testing the effects of exercise or metformin on circulating metabolic factors, as well as cancer-related outcomes (26,27,29,42–45). Studies to date have largely examined effects as single agents. We conducted a multicenter, randomized phase II trial for colorectal and breast cancer survivors to test the effects of combining an exercise intervention with metformin, compared with either intervention alone or with a control group on insulin, and other metabolic biomarkers, as well as weight, body mass index (BMI), and exercise measures. This trial was supported through the National Cancer Institute’s Transdisciplinary Research in Cancer (TREC) program (46).
Methods
Study Population
The study was an open-label, randomized phase II trial designed to test the effect of exercise, metformin, or both interventions on fasting insulin levels in colorectal and breast cancer survivors. The original protocol was limited to individuals with stage I–III colorectal cancer who had undergone curative-intent surgery and completed adjuvant therapy (if indicated) within 2–24 months before enrollment. Participants had to engage in less than 120 minutes of exercise per week, have Eastern Cooperative Oncology Group performance status 0 or 1, random glucose less than 160 mg/dL or fasting glucose less than 126 mg/dL, no major surgery within 1 month of the start of intervention or planned surgeries within the intervention period, no evidence of metastatic disease, and not be on diabetes pharmacological therapy. The study activated at the Dana-Farber Cancer Institute in June 2011 and at Duke University Medical Center in January 2012. Because of slow accrual, the inclusion criteria were expanded to include patients with stage I–III breast cancer who completed standard treatment (concurrent hormonal therapy and/or trastuzumab were allowed). In addition, the restriction to have completed therapy within 24 months was removed. In October 2013, Duke’s enrollment was discontinued because the site principal investigator changed institutions. Yale Cancer Center was activated in May 2014. Each institution’s institutional review board approved the study, and informed consent was obtained from participants before enrollment. All potential participants’ medical oncologist or surgeon provided medical clearance.
Study Design
Participants were randomly assigned to one of four treatment arms: exercise, metformin, exercise combined with metformin, or wait-list control. Although the initial study design included a 6-month intervention, after 2 patients enrolled, the protocol was amended to a 12-week interventions. The control group was offered consultation with an exercise trainer after the 12-week measurements. Random assignment was performed using a random permuted block design of fixed block sizes with stratification by BMI (less than 30 vs 30 or greater kg/m2), cancer type, and sex.
Exercise Intervention
The exercise intervention consisted of in-person structured aerobic sessions, administered twice a week, and additional at-home aerobic activity weekly. Exercise training sessions began with a 5-minute warm-up followed by 30–60 minutes of moderate-intensity exercise, followed by a 5-minute cool down and 5–10 minutes of static stretching. Participants gradually increased exercise duration and intensity over the 12-week intervention, under the guidance of the trainer. The weekly aim was for a 10–20% increase in total exercise duration until participants reached the goal of 220 minutes of moderate-intensity exercise per week. Trainers used heart rate monitors during supervised sessions so that patients learned to recognize moderate-intensity exertion.
Metformin Intervention
Metformin treatment initiated at 850 mg daily for 2 weeks, increasing to 850 mg twice daily in participants tolerating initial dosing. Participants with poor tolerance continued at 850 mg daily for an additional week and then adjusted to twice a day on discussion with investigators. If dose escalation was not tolerated, participants remained on 850 mg daily for the 12-week intervention.
Measurements and Outcomes
Demographic data, disease and treatment information, and baseline physical activity information were collected via interview and/or review of medical records. Participants underwent a series of anthropometric measurements at study enrollment and at completion of the 12-week study period by study staff who were blinded to group assignment.
Patients completed a 7-day physical activity recall interview at baseline and 12 weeks, measuring duration and intensity of exercise performed, as well as time spent sleeping and engaging in other sedentary activities (47–49). Participants underwent the 6-Minute Walk Test at baseline and 12 weeks, a validated measure of functional capacity evaluating the distance an individual can walk over a flat, indoor surface in 6 minutes (50).
Fasting (greater than 12 hours) blood was drawn at baseline and 12 weeks. Insulin resistance was calculated by the homeostatic model assessment (HOMA), with the following formula: HOMA = [insulin (μU/mL) x glucose (mg/dL)]/405. Insulin and leptin were measured using a radioimmunoassay method. Insulin-like growth factor–1 (IGF 1) was assessed using an enzyme-linked immunosorbent assays. All assays were carried out by laboratory personnel blinded to participant assignment. Each sample was assayed in duplicate for each analyte. The correlations between replicates exceeded 0.95. The mean intrabatch coefficients of variation calculated from the quality-control samples were 2.5%, 3.0%, 3.1%, 2.8%, and 2.3% for glucose, insulin-like growth factor binding protein–3 (IGFBP 3), IGF 1, insulin, and leptin, respectively.
Sample-Size Justification and Statistical Analysis
Baseline characteristics are presented as median and interquartile range (IQR) or frequency and percent age. All available outcome data were analyzed in an intention-to-treat analysis with a mixed model that was adjusted for baseline level, BMI, sex, cancer type, and study site. The main effect of each intervention, which is the difference in the least squares means from baseline to 12 weeks, was presented with the standard error. One-sided P was also performed to test if the changes in treatment arms were greater than in the control group, or if the change in combined arm was greater than exercise only or metformin only. To have an overall significance level of 5%, the Holm method was used to split alpha for multiple comparison testing (51). The trial was powered for the primary endpoint of change in insulin levels for a sample size of 200 participants. We assumed a between-subject SD of 4 μU /mL in all four arms (27). As such, the study had 94% power to detect a difference of 3.0 μU /mL between the control and the combination arms with a significance level of 0.0167, 87% power to detect a difference of 2.5 μU /mL between control and supervised exercise with a significance level of 0.025, and 34% power to detect a difference of 1 μU/mL between control and metformin alone with alpha of 0.05, using one-sided two-sample Student t tests. Because of slow accrual and end in grant funding, we ultimately enrolled 139 patients. Post hoc statistical assumptions, the accrued cohort led to 83% power to detect a difference of 3.0 μU /mL between control and combination arms with a statistical significance level of 0.0167, 73% power to detect a difference of 2.5 μU /mL between control and supervised exercise with a significance level of 0.025, and 27% power to detect a difference of 1 μU/mL between control and metformin alone with alpha of 0.05, using one-sided two-sample Student t tests. We tested synergistic effects of the two treatments by including a three-way interaction term for time (baseline/12 weeks) × metformin × exercise in the mixed models.
Results
Cohort Characteristics
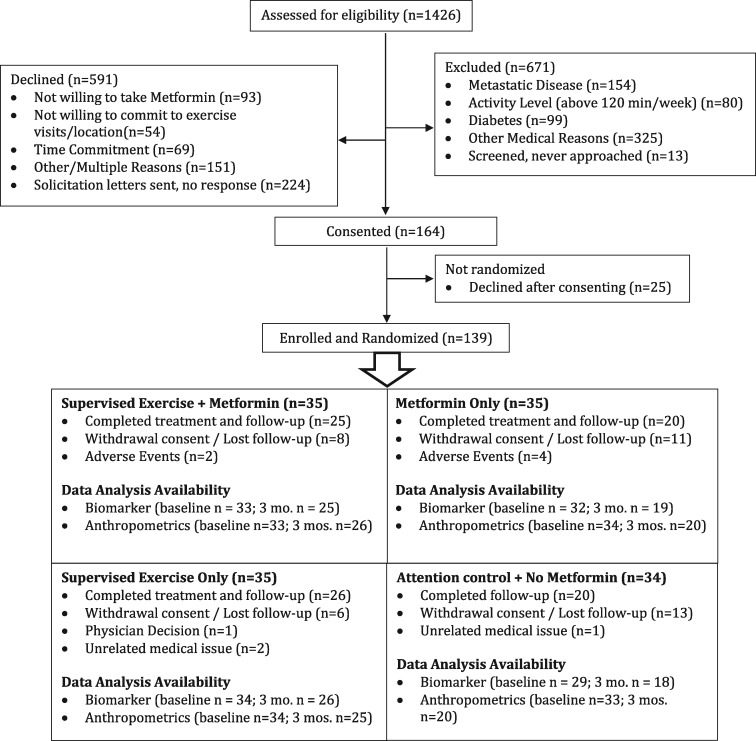
A total of 139 participants were randomly assigned between September 2011 and December 2015 (Figure 1). Ninety-one participants (65%) completed study requirements, including assigned intervention through 12 weeks and follow-up measurements. Reasons for study discontinuation included adverse events (primarily metformin toxicity), time commitment, withdrawal of consent, loss to follow-up, physician decision, or unrelated medical issues. Attrition was greater in the metformin arm (43%) and usual care arm (41%) than in either arm with an exercise component (29% in the combination arm and 26% in the exercise-only arm). Table 1 represents baseline characteristics by treatment arm.
Figure 1.
Consolidated Standards of Reporting Trials diagram.
Table 1.
Baseline characteristics*
| All | Metformin + exercise (N = 35) | Exercise only (N = 35) | Metformin only (N = 35) | Control (N = 34) |
|---|---|---|---|---|
| Tumor location, No. (%) | ||||
| Breast cancer | 22 (62.9) | 21 (60.0) | 22 (62.9) | 22 (64.7) |
| Colorectal cancer | 13 (37.1) | 14 (40.0) | 13 (37.1) | 12 (35.3) |
| Female, No. (%) | 29 (82.9) | 29 (82.9) | 29 (82.9) | 29 (85.3) |
| Study site, No. (%) | ||||
| Dana-Farber | 28 (80.0) | 22 (62.9) | 24 (68.6) | 25 (73.5) |
| Duke | 4 (11.4) | 4 (11.4) | 5 (14.3) | 6 (17.6) |
| Yale | 3 (8.6) | 9 (25.7) | 6 (17.1) | 3 (8.8) |
| Age, median (IQR), y | 53.4 (47.9–58.5) | 58.4 (49.3–64.6) | 54.7 (49.5–60.9) | 56.1 (48.1–65.9) |
| Years from diagnosis to registration, median (IQR) | 2.0 (1.1–3.8) | 1.8 (1.0–4.8) | 2.5 (1.3–4.9) | 1.4 (1.0–2.8) |
| Cancer stage, No. (%) | ||||
| I | 14 (40.0) | 11 (31.4) | 14 (40.0) | 12 (35.3) |
| II | 8 (22.9) | 11 (31.4) | 9 (25.7) | 12 (35.3) |
| III | 13 (37.1) | 12 (34.3) | 12 (34.3) | 9 (26.5) |
| Unknown | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (2.9) |
| Prior chemotherapy, No. (%) | 25 (71.4) | 22 (62.9) | 23 (65.7) | 22 (64.7) |
| Prior radiation, No. (%) | 17 (48.6) | 16 (45.7) | 14 (40.0) | 16 (47.1) |
| Weight, median (IQR), kg | 78.1 (68.9–92.5) | 82.2 (68.8–96.8) | 81.1 (73.0–99.4) | 75.3 (68.3–91.5) |
| BMI, median (IQR) | 27.7 (25.5–34.2) | 28.5 (26.4–32.8) | 29.0 (26.4–36.2) | 28.4 (25.5–31.9) |
| Waist-to-hip ratio, median (IQR) | 0.82 (0.76–0.90) | 0.84 (0.78–0.91) | 0.84 (0.78–0.91) | 0.85 (0.80–0.91) |
| Waist, median (IQR), cm | 94.5 (80.8–100.2) | 94.6 (85.3–101.0) | 97.5 (84.5–102.5) | 90.6 (80.9–107.5) |
| Hip, median (IQR), cm | 107.0 (101.0–120.2) | 110.3 (103.8–118.0) | 110.4 (100.3–120.0) | 110.5 (100.5–118.0) |
| Exercise, minutes/week, median (IQR) | 30 (0–87) | 21 (0–90) | 45 (0–70) | 42 (0–100) |
| Physical activity, MET hours/week, median (IQboR) | 2.0 (0.0–5.3) | 1.2 (0.0–5.8) | 3.4 (0.0–4.7) | 2.7 (0.0–6.7) |
| Walking distance in 6 min, median(IQR), ft | 1495 (1439–1745) | 1572.0 (1410–1737) | 1551 (1495–1736) | 1556 (1476–1706) |
| Insulin, median (IQR), μU/L | 8.2 (6.2–10.8) | 8.2 (6.2–11.8) | 10.8 (5.2–14.7) | 7.5 (6.0–13.5) |
| Glucose, median (IQR), mg/dL | 86.0 (80.3–93.4) | 87.2 (82.0–97.9) | 89.8 (80.8–104.5) | 87.4 (79.2–100.8) |
| HOMA, median (IQR) | 1.5 (1.3–2.3) | 1.8 (1.3–2.8) | 2.0 (1.1–3.7) | 1.7 (1.1–3.1) |
| Leptin, median (IQR), ng/mL | 21.8 (14.3-30.9) | 24.1 (16.7–38.2) | 25.7 (10.8–43.0) | 30.3 (22.2–39.0) |
| IGF-I, median (IQR), ng/mL | 100.9 (87.9–123.8) | 111.4 (73.0–139.7) | 104.1 (82.7–142.4) | 88.7 (81.6–109.2) |
| IGFBP 3, median (IQR), ng/mL | 4426 (3845–5148) | 4282 (3521–4921) | 4210 (3644–4874) | 4250 (3846–4940) |
*HOMA = homeostatic model assessment for insulin resistance; IGF = insulin-like growth factor; IGFBP = insulin-like growth factor binding protein; IQR = intraquartile range; MET = metabolic equivalent task; ng = nanograms; μU = microunits.
Compliance With Interventions and Toxicities
Participants randomly assigned to exercise and metformin increased exercise by 167 minutes/week from baseline, and participants in the exercise-only group increased by 140 minutes/week, both statistically significantly greater than increases in the control and metformin-alone groups (30 and 27 minutes/week, respectively; P < .0001 for both exercise arms, compared with control and with metformin only, Table 2).
Table 2.
Change in exercise behaviors and fitness by treatment arm
| Measurement | Metformin + exercise (arm 1) absolute change, minutes (SE) | P arm 1 vs 4* | Exercise only (arm 2) absolute change (SE) | P arm 2 vs 4* | Metformin only (arm 3) absolute change (SE) | P arm 3 vs 4* | Control (arm 4) absolute change (SE) | P arm 1 vs 2† | P arm 1 vs 3† |
|---|---|---|---|---|---|---|---|---|---|
| Exercise, min/wk | 166.6 (16.4) | < .0001 | 140.3 (16.4) | < .0001 | 26.9 (17.7) | 0.69 | 30.2 (19.4) | .07 | < .0001 |
| Physical activity, MET h/wk | 13.6 (1.26) | < .0001 | 11.3 (1.26) | < .0001 | 2.40 (1.36) | 0.65 | 2.76 (1.49) | .04 | < .0001 |
| Walking distance in 6 min, ft | 105.4 (23.6) | .02 | 81.9 (24.5) | .09 | −2.02 (26.1) | 0.92 | 39.9 (28.2) | .20 | < .0001 |
One-sided P tests if the decrease in treatment arms is greater than in the control arm. Analyses by mixed modeling. MET = metabolic equivalent task.
One-sided P tests if the decrease in the combined arm is greater than in the exercise-only arm or metformin-only arm.
Metformin adherence was assessed through self-report, with confirmation by pill count, and exercise compliance by completion of required sessions (Table 3). Adherence to the metformin intervention was moderate, with approximately 50% of participants taking at least 75% of planned dosages in both treatment arms.
Table 3.
Compliance* with assigned treatments
| % completed | Exercise in combined arm N = 35 | Exercise in exercise only N = 35 | Metformin in combined arm N = 35 | Metformin in metformin only N = 35 |
|---|---|---|---|---|
| 90–100 | 5 (14.3) | 7 (20.0) | 14 (41.2) | 10 (28.6) |
| 75–89 | 15 (42.9) | 10 (28.6) | 7 (20.0) | 8 (22.9) |
| 50–74 | 8 (22.9) | 9 (25.7) | 6 (17.1) | 2 (5.7) |
| 10–49 | 2 (5.7) | 5 (14.3) | 2 (5.7) | 6 (17.1) |
| 0–9, missing | 5 (14.3) | 4 (11.4) | 6 (17.1) | 9 (25.7) |
Compliance rate: required exercise sessions 24; required metformin intake is based on drug log, or 154 pills if no records.
No clinically meaningful exercise intervention–associated complications were reported. Toxicities for metformin were as anticipated (Table 4). Gastrointestinal toxicities were most prominent, with 40% of patients experiencing any grade diarrhea in the combination arm and 23% in the metformin-only arm. Although grade 3 toxicities were uncommon, 50% of patients who dropped out of the combination arm and 60% who dropped out of metformin-only arm experienced at least grade 1 toxicity related to metformin.
Table 4.
Toxicity for metformin, highest grade for each patient
| Metformin + exercise, No. (%) |
Metformin only, No. (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity | Any grade | Grade 1 | Grade 2 | Grade 3 | Any grade | Grade 1 | Grade 2 | Grade 3 |
| Diarrhea | 14 (40) | 8 (22.9) | 2 (5.7) | 2 (5.7) | 8 (22.9) | 6 (17.1) | 1 (2.9) | 1 (2.9) |
| Nausea | 5 (14.3) | 4 (11.4) | 0 | 1 (2.9) | 9 (25.7) | 6 (17.1) | 3 (8.6) | 0 |
| Abdominal Pain | 6 (17.1) | 5 (14.3) | 1 (2.9) | 0 | 6 (17.1) | 4 (11.4) | 1 (2.9) | 1 (2.9) |
| Vomiting | 3 (8.6) | 2 (5.7) | 0 | 1 (2.9) | 7 (20.0) | 3 (8.6) | 4 (11.4) | 0 |
| Bloating | 3 (8.6) | 1 (2.9) | 2 (5.7) | 0 | 3 (8.6) | 1 (2.9) | 2 (5.7) | 0 |
| Flatulence | 4 (11.4) | 3 (8.6) | 1 (2.9) | 0 | 2 (5.7) | 1 (2.9) | 1 (2.9) | 0 |
| Fatigue | 2 (5.8) | 1 (2.9) | 1 (2.9) | 0 | 2 (5.7) | 1 (2.9) | 1 (2.9) | 0 |
Compliance and toxicities with exercise and metformin (alone or in combination) did not differ by cancer type. There were no statistically significant differences in baseline characteristics for those who completed intervention and measurements per protocol and those who did not.
Effects on Insulin and Metabolic Biomarkers
Table 5 lists all hormonal measurements. Whereas the control group demonstrated an increase in insulin (2.79 μU/mL), all three intervention arms showed a statistically significant decrease compared with the control arm, with greatest decrease in the combination arm (−2.47 μU/mL). Insulin also decreased by −0.08 μU/mL in the exercise-only arm and -1.16 μU/mL in the metformin-only arm. The decrease in fasting insulin was statistically significantly greater in the combination arm vs the exercise-only arm (P = .03), but not the metformin-only arm (P = .11). There was no evidence that metformin and exercise had synergistic effects on biomarkers; the three-way interaction term for time × metformin × exercise was not statistically significant in mixed models (P = .49), albeit power was limited for this test. There was no interaction between insulin change and on going hormonal therapy usage (P = .13).
Table 5.
Effect of exercise and metformin on biomarkers and anthropometric measures (difference in least square means and standard error)
| Measurement | Metformin + exercise (arm 1) absolute change (SE) (% change [SE]) | P arm 1 vs 4* | Exercise only (arm 2) absolute change (SE) (% change [SE]) | P arm 2 vs 4* | Metformin only (arm 3) absolute change (SE) (% change [SE]) | P arm 3 vs 4* | Control (arm 4) absolute change (SE) (% change [SE]) | P arm 2 vs 1† | P arm 3 vs 1† |
|---|---|---|---|---|---|---|---|---|---|
| Blood markers changes | |||||||||
| No. | 33 | 34 | 32 | 29 | |||||
| Fasting insulin, μU/L | −2.47 (1.07) | < .0001‡ | −0.08 (1.06) | .01‡ | −1.16 (1.18) | .003‡ | 2.79 (1.27) | .03‡ | .11 |
| −32.3% (3.2) | −1.2% (0.2) | −12.9% (1.9) | 32.7% (4.7) | ||||||
| Glucose, mg/dL | −0.09 (2.11) | .007‡ | 2.93 (2.08) | .17 | −4.11 (2.32) | .0004‡ | 4.92 (2.44) | .04 | .88 |
| −0.1% (0) | 3.3 % (0.1) | −4.6% (0.2) | 5.4% (0.3) | ||||||
| HOMA IR | −0.50 (0.38) | .0002 | 0.01 (0.38) | .007‡ | −0.41 (0.42) | .0009 ‡ | 1.16 (0.45) | .09 | .38 |
| −30.6% (3.0) | 0.4 % (1.0) | −22.1% (3.8) | 61.2% (11) | ||||||
| Leptin, ng/mL | −5.09 (1.21) | .0002‡ | −0.54 (1.19) | .33 | −2.56 (1.33) | .07 | −0.20 (1.40) | .0002‡ | .02‡ |
| −42.2% (11.7) | −4.0 (1.0) | −15.2 (3.5) | −0.8% (0.1) | ||||||
| IGF 1 ng/mL | −1.29 (2.98) | 0.72 | 8.22 (2.94) | 1.00 | −2.66 (3.28) | .63 | −3.05 (3.46) | .0008‡ | .59 |
| −1.2% (0.1) | 8.9% (0.6) | −2.4% (0.2) | −3.1% (0.2) | ||||||
| IGFBP 3, ng/mL | −178.8 (82.0) | .75 | 53.4 (80.7) | .05 | 25.9 (90.2) | .11 | −96.9 (94.9) | .99 | .98 |
| −4.3% (0.2) | 1.4% (0.11) | 0.61% (0.03) | −2.3% (0.12) | ||||||
| Anthropometric changes | |||||||||
| Weight, kg | −1.37 (0.30) | < .0001 | −0.17 (0.31) | < .0001 | −0.81 (0.33) | < .0001 | 1.24 (0.35) | .0002 | .05 |
| −1.8 % (0.1) | −0.22% (0.01) | −1.0% (0.1) | 1.55% (0.1) | ||||||
| BMI, kg/m2 | −0.50 (0.11) | < .0001 | −0.07 (0.11) | < .0001 | −0.29 (0.12) | < .0001 | 0.43 (0.12) | .0001 | .05 |
| −1.74% (0.1) | −0.24% (0.01) | −1.0% (0.1) | 1.43% (0.1) | ||||||
| Waist-to-hip ratio | −0.007 (0.01) | .01 | 0.006 (0.01) | .21 | 0.012 (0.01) | .38 | 0.016 (0.01) | .06 | .02 |
| −0.78% (0.02) | 0.74% (0.02) | 1.45% (0.03) | 1.91% (0.04) | ||||||
| Waist, cm | −1.31 (0.90) | .0004 | −1.51 (0.91) | .0005 | 1.32 (0.98) | .22 | 2.40 (1.04) | .51 | .004 |
| −1.42% (0.1) | −1.60% (0.04) | 1.44% (0.1) | 2.53% (0.1) | ||||||
One-sided P tests if the decrease in treatment arms is greater than in the control arm. Mixed model adjusted for baseline biomarker or anthropometric value, BMI (30 or greater or not), sex (female, male), cancer (breast or colorectal), study site. BMI = body mass index; HOMA IR = homeostatic model assessment for insulin resistance; IGF-1 = insulin-like growth factor–1; IGFBP-3 = insulin-like growth factor binding protein–3 Negative least square means indicate a decrease at 3 months compared to baseline value.
One-sided P tests if the decrease in the combined arm is greater than in the exercise-only arm or metformin-only arm.
Indicates statistically significant differences based on Holm’s method.
The HOMA for insulin resistance was statistically significantly improved for each intervention arm compared with the control arm, with a greater decrease in the combination arm. The combination arm also experienced a statistically significant decrease in leptin level vs the control arm (−5.09 vs −0.02 nanograms [ng]/mL, P = .0002). This change was also statistically significantly greater than either single intervention arm. There were no statistically significant differences in changes IGF 1 or in IGFBP3 between any of the intervention arms and the control arm, though there was a statistically significant difference in change in IGF between the combination arm and the exercise-only arm. The change in IGFBP 3 for the combination arm was unexpected; we tested for one or two outliers, skewing the data, but 68% of patients in this arm either had stable or decreased IGFBP 3 levels, so the results were not driven by a limited number of patients and may be due to chance or an interactive effect that was unexpected.
Test for heterogeneity of the treatment effects between the two cancers types were not statistically significant for any of the biomarkers. In exploratory analyses, we did not detect any statistically significant interactions between compliance with therapy and change in biomarker levels.
There were greater effects of the interventions, particularly exercise with metformin, on insulin, glucose, HOMA, and BMI for participants with greater baseline values for each marker/measure. (Pinteraction< 0.05; Supplementary Table 1, available online.)
Changes in Anthropometrics
Baseline weight, BMI, waist-to-hip ratio, and waist circumference were similar across all four arms (Table 1). All interventions led to statistically significant improvements in weight and BMI, compared with the control group (P < .0001, Table 5). The combination arm also statistically significantly improved waist-to-hip ratio compared with the control group (0.78% decrease vs 1.9% increase, P = .01). In exploratory analyses, there was a marginally statistically significant interaction between intervention compliance and change in weight (P = .08) and BMI (P = .05) for participants in the combination and metformin-only groups. Participants who had 75% or greater intervention compliance had a trend toward larger changes in weight and BMI, as compared with participants who had less than 75% compliance.
Discussion
In a cohort of 139 physically inactive, breast and colorectal cancer survivors, we found that exercise and metformin had a favorable effect on fasting insulin and other metabolic biomarkers implicated in prognosis in breast and colorectal cancer. Participants randomly assigned to any of the three intervention arms experienced a statistically significant decrease in fasting insulin, as compared to control participants. The effects of exercise and metformin on metabolic markers were suggestive of an additive effect, as compared to the effect of either intervention alone, though power limited demonstration of additivity or synergy.
A number of studies, primarily in breast cancer, have demonstrated the effects of exercise or metformin on metabolic markers (26,27,29,42–45,52). Recent meta-analyses reported statistically significant reductions of fasting insulin and non–statistically significant reductions in insulin resistance, adiponectin, and C-reactive protein (CRP) with exercise (53) and statistically significant reductions in fasting insulin and glucose, CRP, HOMA, BMI, and leptin with metformin (54) in breast cancer patients. The National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) MA.32 trial demonstrated that 6 months of metformin led to an 11.1% decrease in insulin (P = .002) and a 3% decrease in weight (P < .001) relative to controls (52).
There are a few studies that have looked at the effect of combining or comparing the effects of different types of interventions that target metabolic markers in cancer populations. One recent report by Patterson et al investigated the impact of metformin or a weight loss intervention, alone or in combination, on metabolic, inflammatory and sex steroid biomarkers in 333 postmenopausal breast cancer survivors with BMI greater than 25 kg/m2 (55). Weight loss and metformin both led to reductions in fasting insulin as compared to controls. Reduction in insulin was numerically greater in patients who received both interventions than either intervention alone. Notably, metformin led to a median 5.3% weight loss, compared to 5.5% in the weight loss groups (and 2.7% in the control group), making it more challenging to separate the effects of the drug vs weight loss. The CHOICE Study assigned 370 breast cancer survivors with a BMI of 25–35 kg/m2 to a calorie-restricted low-carbohydrate diet, a calorie-restricted low-fat diet, or to a usual diet control (56). The study demonstrated statistically significant reductions in fasting glucose and other metabolic markers in both diet groups and found the magnitude of change was directly dependent on amount of weight lost.
Such comparative studies will be essential in determining which types of energy-balance interventions may be the most effective in subgroups of patients defined by cancer types and host characteristics. Cancer treatments have become increasingly specialized, focusing on individual targets within cancer cells and genetic factors. Observational evidence increasingly shows associations between host factors—physical activity, dietary elements, adiposity, and use of drugs like metformin—and cancer outcomes, but these types of data make it difficult to determine which types of interventions will benefit which patients. Randomized trials that compare the effects of different energy-balance interventions, alone and in combination, on biomarkers linked to cancer recurrence and mortality will not only provide mechanistic insight into the biological pathways through which energy-balance factors affect cancer outcomes, but also demonstrate the most effective means to use energy-balance strategies to improve prognosis in cancer patients.
A number of limitations of our trial must be acknowledged. Our study was slow to accrue participants, especially those with colorectal cancer, ultimately preventing us from meeting our target enrollment goal and limiting power for many of our analyses. Other studies have demonstrated slow accrual of colorectal cancer patients to energy-balance intervention studies. The reasons for this are not entirely clear but may be related to degree of symptom burden after completion of systemic therapy in this population. We also had a higher-than-anticipated rate of attrition, with 35% of participants not completing the 12-week intervention and follow-up period. Adherence to the exercise intervention was good, with exercise participants increasing weekly minutes of moderate or vigorous activity by 140–167 minutes/week, vs 30 minutes in controls (P < .001). Adherence to the metformin intervention was more problematic, with only 51% of patients taking at least 75% of prescribed doses. The dosage was similar to that used in other cancer studies, including NCIC CTG MA.32 in breast cancer (43) and a study in advanced pancreatic cancer with standard chemotherapy (which dosed up to 1000 mg twice a day) (57). Notably, Patterson et al also reported moderate rates of noncompliance with metformin, with only 65.9% of participants taking greater than 80% of prescribed pills (55). These compliance rates are lower than have been reported in the diabetes literature (58) and may reflect the relative inexperience of oncologists in managing the gastrointestinal toxicity of metformin or differences in the patient populations including motivation to continue treatment. It is possible that larger differences in biomarkers would have been seen with better adherence to metformin.
In conclusion, in one of the first trials evaluating the effects of two different energy-balance interventions, independently and in combination, on metabolic biomarkers in breast and colorectal cancer survivors, our study demonstrates that both exercise and metformin statistically significantly lowered levels of fasting insulin and led to improvements in other metabolic biomarkers. Changes from combination of the two interventions suggested larger reduction in biomarkers than either intervention alone, despite modest compliance to the prescribed metformin dosage. These findings require validation in future studies, with efforts to improve compliance particularly for metformin, but they could help inform future management of patients after cancer diagnosis.
Funding
This work was supported by the Transdisciplinary Research on Energetics and Cancer mechanism from the National Cancer Institute; grant funding U54 CA155626. LWJ is supported by AKTIV Against Cancer, The KavliTrust, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). JAM is supported by the Douglas Gray Woodruff Chair Fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, and the George Stone Family Foundation. JAL is supported by the Susan G. Komen Foundation.
Notes
Affiliations of authors: Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA (JAM, SZ, NC, JCB, AS, SMT, EW, KN, TA, JAL); Yale School of Public Health, Yale University, New Haven, CT (MLI, BC, MH, TS); Memorial Sloan Kettering Cancer Center, New York, NY (LWJ); Weil Cornell Medical Center, New York, NY (LWJ); Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA (JCB); Oncology Department, McGill University and Segal Cancer Centre, Jewish General Hospital, Montreal, QC, Canada (MP); Yale School of Medicine, Yale University, New Haven, CT (CSF); Duke University School of Medicine, Durham, NC (PSD); Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA (FH).
JAM received institutional research funding from Boston Biomedical and Biothera, and has served as an advisor/consultant to Ignyta and Cota. SMT receives institutional research funding from Novartis, Genentech, Eli Lilly, Pfizer, Merck, Exelixis, Eisai, Bristol-Myers Squibb, AstraZeneca, Cyclacel, Immunomedics, Odenate, and Nektar; and has served as an advisor/consultant to Novartis, Eli Lilly, Pfizer, Merck, AstraZeneca, Eisai, Puma, Genentech, Immunomedics, Nektar, Tesaro, and Nanostring. C.S.F. holds ownership interest (including patents) in Cytomx Therapeutics andEntrinsic Health, and is an advisory board member/unpaid consultant forAgios, Bain Capital, Bayer, Celgene, Dicerna, Five Prime Therapeutics, GileadSciences, Eli Lilly, Entrinsic Health, Genentech, KEW, Merck, MerrimackPharma, Pfizer, Sanofi, Taiho, Unum Therapeutics, and CytomX Therapeutics. K.N. is an employee/paid consultant forSeattle Genetics, Bayer, Lilly, Genentech, and Tarrex, and reports receivingcommercial research grants from Pharmavite, LLC, Genentech, Gilead,Tarrex, Trovagene, and Celgene. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
ClinicalTrials.gov Identifier: NCT01340300.
Supplementary Material
References
- 1. Hirschey MD, DeBerardinis RJ, Diehl AME, et al. Dysregulated metabolism contributes to oncogenesis. Semin Cancer Biol. 2015;35(suppl):S129–S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaaks R, Lukanova A.. Energy balance and cancer: The role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. [DOI] [PubMed] [Google Scholar]
- 3. Slattery ML, Potter J, Caan B, et al. Energy balance and colon cancer—beyond physical activity. Cancer Res. 1997;57(1):75–80. [PubMed] [Google Scholar]
- 4. Bowers LW, Rossi EL, O'Flanagan CH, et al. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol (Lausanne). 2015;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doerstling SS, O'Flanagan CH, Hursting SD.. Obesity and cancer metabolism: a perspective on interacting tumor-intrinsic and extrinsic factors. Front Oncol. 2017;7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hursting SD, Berger NA.. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hursting SD, Digiovanni J, Dannenberg AJ, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila). 2012;5(11):1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown JC, Meyerhardt JA.. Obesity and energy balance in GI cancer. J Clin Oncol. 2016;34(35):4217–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J, Jeon JY, Meyerhardt JA.. Diet and lifestyle in survivors of colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1244–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ligibel J. Lifestyle factors in cancer survivorship. J Clin Oncol. 2012;30(30):3697–3704. [DOI] [PubMed] [Google Scholar]
- 12. Meyerhardt JA, Ma J, Courneya KS.. Energetics in colorectal and prostate cancer. J Clin Oncol. 2010;28(26):4066–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolpin BM, Meyerhardt JA, Chan AT, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27(2):176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deming SL, Ren Z, Wen W, et al. Genetic variation in IGF1, IGF-1R, IGFALS, and IGFBP3 in breast cancer survival among Chinese women: a report from the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2007;104(3):309–319. [DOI] [PubMed] [Google Scholar]
- 15. Gu L, Shigemasa K, Ohama K.. Increased expression of IGF II mRNA-binding protein 1 mRNA is associated with an advanced clinical stage and poor prognosis in patients with ovarian cancer. Int J Oncol. 2004;24(3):671–678. [PubMed] [Google Scholar]
- 16. Haffner MC, Petridou B, Peyrat JP, et al. Favorable prognostic value of SOCS2 and IGF-I in breast cancer. BMC Cancer. 2007;7(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li L, Liu S, Liu L, et al. Impact of phosphorylated insulin-like growth factor-1 receptor on the outcome of breast cancer patients and the prognostic value of its alteration during neoadjuvant chemotherapy. Exp Ther Med. 2018;16(4):2949–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulligan AM, O’Malley FP, Ennis M, et al. Insulin receptor is an independent predictor of a favorable outcome in early stage breast cancer. Breast Cancer Res Treat. 2007;106(1):39–47. [DOI] [PubMed] [Google Scholar]
- 19. Je Y, Jeon JY, Giovannucci EL, et al. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905–1913. [DOI] [PubMed] [Google Scholar]
- 20. Jeon JY, Meyerhardt JA.. Exercise after cancer diagnosis: time to get moving. Oncology (Williston Park). 2013;27(6):585–586, 588. [PubMed] [Google Scholar]
- 21. Furmaniak AC, Menig M, Markes MH.. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ibrahim EM, Al-Homaidh A.. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753–765. [DOI] [PubMed] [Google Scholar]
- 23. Lahart IM, Metsios GS, Nevill AM, et al. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1:CD011292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedenreich CM, Neilson HK, Farris MS, et al. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22(19):4766–4775. [DOI] [PubMed] [Google Scholar]
- 25. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11):3109S–3120S. [DOI] [PubMed] [Google Scholar]
- 26. Lee DH, Kim JY, Lee MK, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21(9):2537–2545. [DOI] [PubMed] [Google Scholar]
- 27. Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26(6):907–912. [DOI] [PubMed] [Google Scholar]
- 28. Friedenreich CM, Neilson HK, Woolcott CG, et al. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr Relat Cancer. 2011;18(3):357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Selhub J, Rosenberg IH.. Excessive folic acid intake and relation to adverse health outcome. Biochimie. 2016;126:71–78. [DOI] [PubMed] [Google Scholar]
- 31. Bailey CJ, Turner RC.. Metformin. N Engl J Med. 1996;334(9):574–579. [DOI] [PubMed] [Google Scholar]
- 32. Vallianou NG, Evangelopoulos A, Kazazis C.. Metformin and cancer. Rev Diabet Stud. 2013;10(4):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sehdev A, Shih YC, Vekhter B, et al. Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer. 2015;121(7):1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh S, Singh H, Singh PP, et al. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2258–2268. [DOI] [PubMed] [Google Scholar]
- 35. Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010;3(11):1451–1461. [DOI] [PubMed] [Google Scholar]
- 36. Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meng F, Song L, Wang W.. Metformin improves overall survival of colorectal cancer patients with diabetes: a meta-analysis. J Diabetes Res. 2017;2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paulus JK, Williams CD, Cossor FI, et al. Metformin, diabetes, and survival among U.S. veterans with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(10):1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thent ZC, Zaidun NH, Azmi MF, et al. Is metformin a therapeutic paradigm for colorectal cancer: insight into the molecular pathway? Curr Drug Targets. 2017;18(6):734–750. [DOI] [PubMed] [Google Scholar]
- 40. El-Benhawy SA, El-Sheredy HG.. Metformin and survival in diabetic patients with breast cancer. J Egypt Public Health Assoc. 2014;89(3):148–153. [DOI] [PubMed] [Google Scholar]
- 41. Kim HJ, Kwon H, Lee JW, et al. Metformin increases survival in hormone receptor-positive, HER2-positive breast cancer patients with diabetes. Breast Cancer Res. 2015;17(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dowling RJ, Niraula S, Chang MC, et al. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015;17(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goodwin PJ, Stambolic V, Lemieux J, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat. 2011;126(1):215–220. [DOI] [PubMed] [Google Scholar]
- 44. Courneya KS, Booth CM, Gill S, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15(6):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Courneya KS, Vardy JL, O'Callaghan CJ, et al. Effects of a structured exercise program on physical activity and fitness in colon cancer survivors: one year feasibility results from the CHALLENGE Trial. Cancer Epidemiol Biomarkers Prev. 2016;25(6):969–977. [DOI] [PubMed] [Google Scholar]
- 46. Schmitz KH, Gehlert S, Patterson RE, et al. TREC to WHERE? Transdisciplinary research on energetics and cancer. Clin Cancer Res. 2016;22(7):1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dishman RK, Steinhardt M.. Reliability and concurrent validity for a 7-d re-call of physical activity in college students. Med Sci Sports Exerc. 1988;20(1):14–25. [DOI] [PubMed] [Google Scholar]
- 48. Jacobs DR Jr, Ainsworth BE, Hartman TJ, et al. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. [DOI] [PubMed] [Google Scholar]
- 49. Taylor CB, Coffey T, Berra K, et al. Seven-day activity and self-report compared to a direct measure of physical activity. Am J Epidemiol. 1984;120(6):818–824. [DOI] [PubMed] [Google Scholar]
- 50. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;1979(6):65–70. [Google Scholar]
- 52. Goodwin P J, Parulekar W R, Gelmon K A et al. . Effect of Metformin vs Placebo on and Metabolic Factors in NCIC CTG MA.32. JNCI Journal of the National Cancer Institute. 2015;107(3):djv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kang DW, Lee J, Suh SH, et al. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2017;26(3):355–365. [DOI] [PubMed] [Google Scholar]
- 54. Rahmani J, Manzari N, Thompson J, et al. The effect of metformin on biomarkers associated with breast cancer outcomes: a systematic review, meta-analysis, and dose-response of randomized clinical trials. Clin Transl Oncol. 2019;10.1007/s12094-019-02108-9. [DOI] [PubMed] [Google Scholar]
- 55. Patterson RE, Marinac CR, Sears DD, et al. The effects of metformin and weight loss on biomarkers associated with breast cancer outcomes. J Natl Cancer Inst. 2018;110(11):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thompson HJ, Sedlacek SM, Playdon MC, et al. Weight loss interventions for breast cancer survivors: impact of dietary pattern. PLoS One. 2015;10(5):e0127366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kordes S, Pollak MN, Zwinderman AH, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(7):839–847. [DOI] [PubMed] [Google Scholar]
- 58. Rozenfeld Y, Hunt JS, Plauschinat C, et al. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care. 2008;14(2):71–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.