Abstract
Purpose
The present study was designed to determine the relationships between sarcopenia and diabetic peripheral neuropathy (DPN) in patients with type 2 diabetes mellitus (T2DM) and diabetic foot disease (DFD) respectively.
Patients and Methods
A total of 1104 patients with T2DM and 257 patients with DFD were included in the study, which was designed as a cross-sectional study. Body composition was assessed using dual-energy X-ray-absorptiometry (DXA). The diagnosis of sarcopenia was based on the Baumgartner criteria. DPN was assessed by Neuropathy symptom score (NSS) and Neuropathy disability score (NDS), and the severity of neuropathy was divided into non-neuropathy symptom (NS), Mild NS, Moderate NS and Severe NS according to NSS. Logistic regression analyses were carried out to determine the relations of sarcopenia and DPN in patients with T2DM and NSS in patients with DFD, respectively.
Results
The prevalence of DPN was 80.0% in T2DM patients with sarcopenia and 70.3% in non-sarcopenia patients (P=0.007). Logistic regression analyses showed DPN was one of the independent risk factors for sarcopenia in T2DM patients (OR 1.564 [95% CI: 1.004, 2.435], P=0.048). The prevalence of DPN had no statistical significance in DFD patients with or without sarcopenia. However, the NSS of DFD patients with sarcopenia was higher than that of non-sarcopenia patients. In the multivariate logistic regression analysis, NSS was determined to be associated with sarcopenia in DFD patients (OR 1.387[95% CI: 1.074, 1.789], P=0.012). The appendicular lean mass (ALM) of DFD patients without NS was higher than patients with mild, moderate and severe NS (20.71±2.73 vs 16.57±3.62 vs 17.99±3.54 vs 17.23±3.29 Kg, P=0.028).
Conclusion
DPN is an independent risk factor for sarcopenia in patients with T2DM and NSS is also independently correlated with sarcopenia in patients with DFD, with the latter being more obvious with the aggravation of neurological symptoms in DFD patients.
Keywords: type 2 diabetes mellitus, diabetic peripheral neuropathy, diabetic foot disease, sarcopenia, correlation
Introduction
Having been defined as a geriatric syndrome, sarcopenia is characterized with age-related loss of muscle mass and accompanying decline in physical function.1 Sarcopenia is closely associated with increased risk of adverse events such as falls, fractures, disabilities, physical dysfunction, increased hospitalization, decreased quality of life and death.2–5 In addition to aging, sarcopenia may be related to sports, nutrition, immunity, hormones, central and peripheral nerves, as well as many chronic diseases.6–9 Diabetes is one of the important risk factors for the occurrence of sarcopenia. Studies have shown that people over the age of 60 have a higher risk of developing sarcopenia in type 2 diabetes mellitus (T2DM) than non-diabetic patients. The risk of muscle loss in diabetic patients is three times higher than that in non-diabetic patients.1 The reason why diabetic patients are prone to sarcopenia has not been fully elucidated. But there is evidence that some pathogenesis of sarcopenia is closely related to diabetes, including age, malnutrition, insulin resistance, chronic inflammation, mitochondrial dysfunction, neurovascular complications, poor glycemic control, high blood sugar level and long-term medication.10–15
Diabetic peripheral neuropathy (DPN), one of the common complications of diabetes, has been developed by approximately 30% to 50% of diabetes mellitus patients.16,17 DPN is germane to the occurrence and development of sarcopenia, and its relationship with muscle weakness has been reported in several studies.18–21 Metabolic and microvascular impairments in DPN damage the intraneural capillaries that supply the peripheral nerves and lead to sensory loss, pain, and muscle weakness.22 Compared with diabetes alone, DPN can lead to accelerated muscle mass loss in diabetic patients.23
Remarkably, some clinical studies on the relationship between DPN and sarcopenia as there are in diabetic populations, very few studies have shown the association between two of them in patients with diabetic foot disease (DFD). DFD is a severe chronic diabetic complication, consisting of lesions in the deep tissues associated with neurological disorders and peripheral vascular disease in the lower limbs.24 Nearly 30% of patients with DPN will develop a foot ulcer within 2 years of diagnosis of severe DPN.25 Our research team has found that sarcopenia is one of the independent risk factors for DFD in previous studies,26 but no specific relationship between DPN and sarcopenia in DFD patients has been found in clinical studies. Therefore, the purpose of the present study was to explore the relation between DPN and sarcopenia in two populations of T2DM and DFD.
Materials and Methods
Study Design and Participants
This cross-sectional study included 1104 patients with T2DM admitted to the Department of Endocrinology of the First Affiliated Hospital of Chongqing Medical University from June 2013 to December 2015, and 257 patients with DF treated after the establishment of the Diabetic Foot Working Group in April 2016 with available data were recruited. Inclusion criteria: patients of T2DM with or without DF. Exclusion criteria: duration of DF>3months; individuals with age<20 or>90 years; severe heart failure (New York Heart Association Class II-IV); severe liver impairment (liver enzyme ALT≥3-fold the upper limit of normal range); severe renal dysfunction (estimated glomerular filtration rate [eGFR]<30 mL/min/1.73m2); a history of thyroid or adrenal diseases. The patient consent was written informed consent, and this study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Affiliated Hospital of Chongqing Medical University.
Clinical Data Collection
Patient history included data on demographic characteristics, type and duration of diabetes, microvascular and macrovascular comorbidities, and smoking habits. Physical examination included objective evaluation for peripheral neuropathy and peripheral arterial disease (PAD). PAD was defined as an ankle-brachial pressure index (ABI) less than 0.9 with additional investigation by means of duplex ultrasonography or angiography. Laboratory examination: white blood cell, hemoglobin, neutrophil, alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride, total cholesterol, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), glycosylated hemoglobin (HbA1c), serum creatinine, blood uric acid, urinary micro-albumin to creatinine ratio (UACR). Body mass index (BMI) was calculated by dividing weight by the square of height. Plasma glucose levels were measured using a biochemical analyzer (BS-380; Mindray Medical International, Shenzhen, China). HbA1c was measured using borate affinity high-performance liquid chromatography (Trinity Biotech, ultra, Dublin, Ireland). Serum lipids including total cholesterol, triglyceride, HDL-c, and LDL-c were measured enzymatically by an automatic analyzer (Model 7080; Hitachi, Tokyo, Japan) with reagents purchased from Leadman Biochemistry Co. Ltd. (Beijing, China). Serum creatinine, urinary creatinine and albumin were measured with an automatic biochemical analyzer (Modular DDP, Roche). Urinary micro-albuminuria to creatinine ratio (UACR) was calculated. Body composition was measured using DXA Hologic scanner (Hologic Discovery QDR ® Series, Bedford, MA, USA) by a trained technician. All standard procedures followed previous studies. Hologic Whole Body DXA reference database software was used to estimate the regional and whole-body lean tissue.
Diagnostic Criteria
T2DM was diagnosed according to the 1999 WHO Diabetes Diagnostic Criteria. Diagnostic criteria for sarcopenia refer to Baumgartner diagnostic criteria: appendicular lean mass index (ALMI)=appendicular lean mass (ALM=Arm LM+Leg LM)/height2 in kg/m2. The diagnostic criteria of sarcopenia are ALMI<7.01kg/m2 in male and ALMI<5.42kg/m2 in females.27 Chronic diabetic complications such as DPN, diabetic kidney disease, diabetic retinopathy, and DF were evaluated according to the guidelines of the American Diabetes Association (ADA) 2012.28
Assessment and Diagnosis of DPN
Neuropathy symptom score (NSS) and Neuropathy disability score (NDS) were used to assess the degree of neuropathy in patients with DPN. NSS was evaluated by asking patients about their experience of pain or discomfort in the legs. NSS: 3–4 were classified as mild NS, and 5–6 were classified as moderate NS, 7–9 were classified as severe NS. NDS was derived from examination of the ankle reflex, vibration, pin-prick and temperature (cold tuning fork) sensation at the great toe. NDS: 3–5 were classified as mild ND, 6–8 were classified as moderate ND, and 9–12 were classified as severe ND.29
The minimum acceptable criteria for a diagnosis of peripheral neuropathy were moderate signs with or without symptoms, or mild signs with moderate symptoms. Mild signs alone or with mild symptoms were not considered adequate to make a diagnosis of peripheral neuropathy.18 Nerve conduction velocity (NCV) was measured by EMG/Evoked Potentiometer (Type of Keypoint 9033A07, Dantec, Denmark).
Statistical Analysis
SPSS 20.0 statistical software was used for statistical analysis. The normality and variance homogeneity of the data were analyzed by a single sample Kolmogorov–Smirnov test. The continuous variables of the normal distribution were expressed as mean ± standard deviation, and the independent samples test was used for comparison between the two groups. The continuous variables of non-normal distribution such as duration of diabetes, hemoglobin, AST, creatinine, uric acid, triglyceride, HDL-c, LDL-c and HbA1c are represented by median (interquartile range) when they do not obey the normal distribution after data conversion. Cross-group comparisons were performed using two-sample Kolmogorov–Smirnov test. Categorical variables are expressed by frequency and percentage; two group or groups of disordered categorical variables were tested by chi-square test or Fisher exact probability test. Multiple logistic regression model was used to analyze the influencing factors of sarcopenia. All statistical tests were double-tailed, and p<0.05 was considered statistically significant.
Results
Study Population Characteristics of T2DM and DFD Patients
Clinical characteristics of patients with T2DM are presented in Table 1. A total of 1104 patients were included in this study, including 204 with sarcopenia and 900 without sarcopenia. The mean age of the participants was 64.35±9.32 years, of which 57.4% were male. Sarcopenia was diagnosed in 16.5% of males and 16.4% of females. As can be seen from Table 1, compared with subjects without sarcopenia, patients exhibited higher percentage of DPN (Figure 1A) and DF in T2DM patients with sarcopenia. The age and levels of HDL-c were also higher than that of subjects without sarcopenia. Sarcopenia patients exhibited lower levels of BMI, waist circumference, hypertension, hemoglobin, ALT, AST, uric acid, triglyceride, HDL-c and left motor peroneal nerve conduction velocity compared to subjects without sarcopenia.
Table 1.
Characteristics of Patients with Type 2 Diabetes with or Without Sarcopenia
| Non-Sarcopenia | Sarcopenia | P value | |
|---|---|---|---|
| Male/Female (n) | 515/385 | 117/87 | 0.973 |
| Age (year) | 64.10±9.35 | 66.88±10.16 | <0.001 |
| Duration of Diabetes (year) | 9(4, 14) | 9(3, 14) | 0.559 |
| BMI (Kg/m2) | 25.62±2.84 | 21.00±2.19 | <0.001 |
| Waist Circumference (cm) | 96.72±8.37 | 86.64±8.05 | <0.001 |
| Smoking (%) | 80.0 | 79.4 | 0.844 |
| Hypertension (%) | 64.4 | 54.4 | 0.008 |
| Coronary heart disease (%) | 16.2 | 14.1 | 0.485 |
| Cerebrovascular disease (%) | 8.8 | 13.1 | 0.065 |
| White Blood Cell (*109/L) | 6.53±1.78 | 6.56±2.47 | 0.377 |
| Hemoglobin (g/L) | 135(121, 147) | 133(116, 144) | 0.007 |
| Neutrophil (*109/L) | 4.24±1.66 | 4.48±2.37 | 0.694 |
| ALT (U/L) | 26.00±22.39 | 21.57±13.97 | 0.009 |
| AST (U/L) | 19(15, 25) | 17(14, 22) | 0.004 |
| Creatinine (μmol/L) | 72.5(60, 87) | 68(56, 86) | 0.150 |
| Uric Acid (μmol/L) | 311(258, 367) | 272(222, 318) | <0.001 |
| UACR (mg/g) | 10.3(3.8, 40.0) | 13.4(4.8, 65.0) | 0.682 |
| Triglyceride (mmol/L) | 1.39(0.99, 2.13) | 1.14(0.8, 1.76) | 0.002 |
| Total Cholesterol (mmol/L) | 4.22±1.13 | 4.25±1.12 | 0.890 |
| HDL-cholesterol (mmol/L) | 1.07(0.9, 1.28) | 1.17(0.98, 1.35) | 0.004 |
| LDL-cholesterol (mmol/L) | 2.5(1.89, 3.20) | 2.53(1.96, 3.31) | 0.941 |
| HbA1c (%) | 7.9(6.8, 9.8) | 8.4(7, 10.7) | 0.069 |
| Right motor tibial NCV (m/s) | 42.78±4.56 | 40.99±5.72 | 0.136 |
| Left motor tibial NCV (m/s) | 43.56±4.41 | 41.71±4.38 | 0.084 |
| Right motor peroneal NCV (m/s) | 44.72±4.93 | 43.34±4.63 | 0.255 |
| Left motor peroneal NCV (m/s) | 45.80±5.32 | 42.46±5.35 | 0.011 |
| Chronic Diabetic Complications | |||
| Diabetic Peripheral Neuropathy (%) | 70.3 | 80 | 0.007 |
| Diabetic Retinopathy (%) | 28.3 | 32.8 | 0.206 |
| Diabetic Kidney Disease (%) | 33.3 | 29.4 | 0.281 |
| Diabetic Foot Ulcer (%) | 8.6 | 20.6 | <0.001 |
Notes: Data are presented as mean ± SD, median (interquartile range), or %.
Abbreviations: BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UACR, urinary micro-albumin to creatinine ratio; HDL-cholesterol, high-density lipoprotein cholesterol; LDL-cholesterol, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; NCV, nerve conduction velocity.
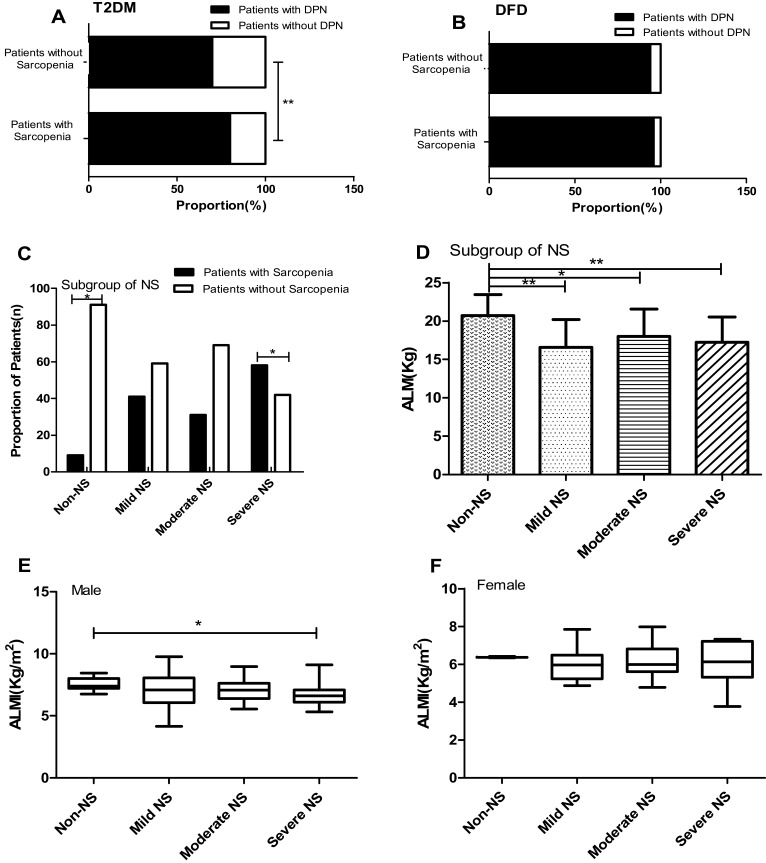
Figure 1.
(A) The proportion of DPN in patients of T2DM with or without sarcopenia. (B) The proportion of DPN in patients of DFD with or without sarcopenia. (C) The proportion of patients with sarcopenia and without sarcopenia in four NS of different degrees in patients with DFD. (D) The appendicular lean mass in four NS of different degrees in patients with DFD. (E) The ALMI in four NS of different degrees in male patients with DFD. (F) The ALMI in four NS of different degrees in female patients with DFD. *P < 0.05; **P < 0.01.
Abbreviations: ALM, appendicular lean mass; ALMI, appendicular lean mass index; NS, neuropathy symptom.
Clinical characteristics of patients with DFD are shown in Table 3. A total of 257 patients were included, including 91 patients with sarcopenia, and 166 patients without sarcopenia. The mean age of the participants was 66.42 ± 11.07 years, of which 64.6% were male. Sarcopenia was diagnosed in 48.8% of males and 23.1% of females. Compared with non-sarcopenia patients, DFD patients with sarcopenia had higher level of age, duration of DFD. The proportion of male, smoking and coronary heart disease were significantly higher in patients with sarcopenia (Table 3). The proportion of DPN was not statistically different between subjects with and without sarcopenia in DFD patients (Figure 1B). Importantly, patients with sarcopenia had larger NSS than DFD patients without sarcopenia.
Table 3.
Characteristics of Patients with Diabetic Foot Disease with or Without Sarcopenia
| Non-Sarcopenia | Sarcopenia | P value | |
|---|---|---|---|
| Gender (male/female) | 85/81 | 70/21 | <0.001 |
| Age (year) | 64.90±10.62 | 68.74±11.39 | 0.006 |
| Duration of Diabetes (year) | 10(5,18) | 9(4,15) | 0.215 |
| Duration of DFD (year) | 1.0(0.3,3.0) | 2.0(0.7,4.0) | 0.044 |
| BMI (Kg/m2) | 24.87±2.85 | 21.51±2.30 | <0.001 |
| History of foot ulcer (%) | 27.1 | 23.5 | 0.522 |
| Smoking (%) | 43.9 | 65.7 | 0.001 |
| Hypertension (%) | 59.4 | 57.8 | 0.810 |
| Coronary heart disease (%) | 15.5 | 29.4 | 0.007 |
| Cerebrovascular disease (%) | 9.7 | 12.7 | 0.440 |
| White Blood Cell (*109/L) | 8.22±3.62 | 9.12±4.19 | 0.113 |
| Hemoglobin (g/L) | 119.03±18.89 | 115.73±23.24 | 0.214 |
| Neutrophil (*109/L) | 71.83±9.49 | 73.37±10.45 | 0.224 |
| ALT (U/L) | 21.11±14.68 | 18.72±15.61 | 0.102 |
| AST (U/L) | 17(13, 27) | 17(13, 23) | 0.427 |
| Creatinine (μmol/L) | 77(58, 97) | 81(66, 114) | 0.078 |
| Uric Acid (μmol/L) | 285.52±105.27 | 287.31±111.99 | 0.897 |
| UACR (mg/g) | 427.0(11.6, 225.5) | 124.7(507.1, 947.8) | 0.010 |
| Triglyceride (mmol/L) | 1.43±1.52 | 1.39±0.68 | 0.397 |
| Total Cholesterol (mmol/L) | 3.73±1.06 | 3.82±1.14 | 0.577 |
| HDL-cholesterol (mmol/L) | 1.03(0.84, 1.22) | 1.00(0.79, 1.17) | 0.235 |
| LDL-cholesterol (mmol/L) | 2.14(1.64, 2.75) | 2.09(1.82, 2.83) | 0.479 |
| HbA1c (%) | 9.1(7.4, 11.7) | 9.9(7.5, 12.0) | 0.200 |
| NSS (point) | 4.67±2.12 | 5.44±1.62 | 0.032 |
| NDS (point) | 8.30±2.51 | 8.10±2.19 | 0.647 |
| Right motor tibial NCV (m/s) | 38.47±4.70 | 38.62±6.78 | 0.908 |
| Left motor tibial NCV (m/s) | 38.88±5.22 | 37.29±3.36 | 0.152 |
| Right motor peroneal NCV (m/s) | 40.42±8.02 | 37.99±7.15 | 0.208 |
| Left motor peroneal NCV (m/s) | 41.32±5.81 | 38.14±6.11 | 0.033 |
| Chronic Diabetic Complications | |||
| Diabetic Peripheral Neuropathy (%) | 94.2 | 96.1 | 0.500 |
| Peripheral arterial disease (%) | 14.8 | 33.0 | 0.001 |
| Diabetic Retinopathy (%) | 47.1 | 42.3 | 0.458 |
| Diabetic Kidney Disease (%) | 48.4 | 55.0 | 0.302 |
Notes: Data are presented as mean ± SD, median (interquartile range), or %.
Abbreviations: DFD, diabetic foot disease; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UACR, urinary albumin-to-creatinine ratio; HDL-cholesterol, high-density lipoprotein cholesterol; LDL-cholesterol, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; NSS, Neuropathy symptom score; NDS, Neuropathy disability score; NCV, nerve conduction velocity.
Risk Factors of Sarcopenia in Logistic Regression in Patients with T2DM and DFD
Univariate and multivariable-adjusted models were used for evaluating the relationship between potential risk factors and sarcopenia in patients with T2DM. The continuous variables age was transformed into grade variables according to the intervals as below: age (30≤age<60 years, 60≤age<70 years, 70≤age<80 years, 80≤age<90 years). In separate univariate logistic regression models, age, hemoglobin, triglyceride, DFD and DPN were significant risk increasing factors, and DPN (OR 1.678 [95% CI 1.148,2.480], P =0.008) was a significant risk factor (Table 2). Hypertension, uric acid and ALT were protective factors for sarcopenia. After adjusting for age (Model 1), the relationship between DPN and sarcopenia remains the same (OR 1.507[95% CI 1.016,2.236], P=0.042). In the multivariate-adjusted model analysis, DPN was an independent risk factor for sarcopenia (OR 1.564[95% CI 1.004, 2.435], P=0.048) after adjusting for age, hypertension, hemoglobin, uric acid, triglyceride, ALT and DFD (Model 2).
Table 2.
Univariate and Multivariable Analysis for Logistic Regression of Sarcopenia in T2DM Patients
| Univariate* | Multivariate₤ (Model 1†) | Multivariate₤ (Model 2‡) | ||||
|---|---|---|---|---|---|---|
| OR (95% Cl) | P value | OR (95% Cl) | P value | OR (95% Cl) | P value | |
| Age (30≤Age<60) | Referent category | – | – | Referent category | – | |
| 60≤Age<70 | 1.279(0.856,0.911) | 0.231 | – | – | 1.299(0.812,2.078) | 0.276 |
| 70≤Age<80 | 1.779(1.155,2.740) | 0.009 | – | – | 1.745 (1.037, 2.938) | 0.036 |
| 80≤Age<90 | 3.243(1.818,5.786) | <0.001 | – | – | 3.591 (1.798, 7.171) | <0.001 |
| Hypertension | 0.661(0.486,0.899) | 0.008 | 0.516(0.367,0.726) | <0.001 | 0.618 (0.424, 0.901) | 0.012 |
| ALT | 0.983(0.971,0.995) | 0.006 | 0.984(0.971,0.997) | 0.016 | 0.985 (0.972, 0.999) | 0.037 |
| Uric Acid | 0.995(0.993,0.997) | <0.001 | 0.995(0.993,0.997) | <0.001 | 0.995 (0.993, 0.997) | <0.001 |
| Diabetic foot Disease | 2.771(1.835,4.184) | <0.001 | 2.511(1.641,3.843) | <0.001 | 2.138 (1.330, 3.438) | 0.002 |
| DPN | 1.687(1.148,2.480) | 0.008 | 1.507(1.016,2.236) | 0.042 | 1.564 (1.004, 2.435) | 0.048 |
Notes: *Univariate: Univariate logistic regression analyses for the association between potential risk factors and sarcopenia. ₤Multivariate: Multivariable logistic regression analyses to test whether DPN is independently associated with sarcopenia. In the multivariable logistic regression analyses, DPN was used as the dependent. †Model 1: Adjusted for age; ‡Model 2: Model 1 + adjusted for hypertension, hemoglobin, uric acid, triglyceride, ALT and DFD.
Abbreviations: OR, odds ratio; 95% CI, 95% confidential interval; ALT, alanine aminotransferase; DPN, diabetic peripheral neuropathy.
As shown in Table 4, univariate and multivariable-adjusted models were used for evaluating the relationship between potential risk factors and sarcopenia in patients with DFD. Univariate analysis revealed that the following variables were significantly associated with mortality: age (OR 1.033[95% CI 1.009,1.057], P=0.007), gender (Male) (OR 3.176[95% CI 1.788,5.644], P<0.001), NSS (OR 1.242[95% CI 1.013,1.521], P=0.037). After adjusting for age (Model 1), the relationship between NSS and sarcopenia remains the same (OR 1.507 [95% CI 1.011,1.526], P=0.039). Gender, age, duration of DFD, smoking, coronary heart disease, UACR and peripheral arterial disease were adjusted in model 2, NSS was an independent risk factor for sarcopenia (OR 1.387[95% CI 1.074, 1.789], P=0.012) in DFD patients.
Table 4.
Univariate and Multivariable Analysis for Logistic Regression of Sarcopenia in Patients with Diabetic Foot Disease
| Univariate* | Multivariate₤ (Model 1†) | Multivariate₤ (Model 2‡) | ||||
|---|---|---|---|---|---|---|
| OR (95% Cl) | P value | OR (95% Cl) | P value | OR (95% Cl) | P value | |
| Age | 1.033(1.009,1.057) | 0.007 | – | – | 1.053 (1.009,1.098) | 0.018 |
| Gender (Male) | 3.176(1.788,5.644) | <0.001 | 3.548(1.502,8.379) | 0.004 | 3.227(1.234,8.436) | 0.017 |
| Duration of DFD | 1.006(0.975,1.037) | 0.715 | 1.036(0.982,1.092) | 0.193 | 1.074(0.999, 1.155) | 0.052 |
| NSS | 1.242(1.013,1.521) | 0.037 | 1.243(1.011,1.526) | 0.039 | 1.387(1.074, 1.789) | 0.012 |
Notes: *Univariate: Univariate logistic regression analyses for the association between potential risk factors and sarcopenia. ₤Multivariate: Multivariable logistic regression analyses to test whether NSS is independently associated with sarcopenia. In the multivariable logistic regression analyses, NSS was used as the dependent. †Model 1: Adjusted for age; ‡Model 2: Model 1+ adjusted for gender, duration of DFD, smoking, coronary heart disease, UACR and peripheral arterial disease.
Abbreviations: OR, odds ratio; 95% CI, 95% confidential interval; DFD, diabetic foot disease; NSS, neuropathy symptom score.
The Relationship Between Varying Degrees of Neuropathy and Sarcopenia in Patients with DFD
Patients with diabetic foot disease were divided into non-NS, Mild NS, Moderate NS and Severe NS according to NSS. There were significant differences in the proportion of sarcopenia among the four groups (2% vs 18% vs 50% vs 30%, P = 0.015). Among patients without NS, the proportion of non-sarcopenia was higher than that of sarcopenia (90.9% vs 9.1%, P < 0.05), while in patients with severe NS, the proportion of sarcopenia was higher than that of non-sarcopenia (58.4% vs 41.7%, P < 0.05) (Figure 1C). Further study found that the ALM in patients without NS was higher than that of in patients with mild, moderate and severe NS (20.71±2.73 vs 16.57±3.62 vs 17.99±3.54 vs 17.23±3.29, P=0.028) (Figure 1D). In addition, ALMI in patients without NS in males with DFD was higher than in patients with severe NS (7.59±0.51 vs 6.66±0.92, P=0.016) (Figure 1E). According to the NSS of female DFD patients, they were divided into 4 groups, with no statistically significant difference in ALMI (P=0.387) (Figure 1F).
Discussion
In the present study, we investigated the relationship between DPN and sarcopenia in patients with T2DM and DPN. The prevalence of DPN ranges from 13% to 68% in diabetes populations.30 Prolonged DPN is known to result in significant skeletal muscle deficits in this patient population, including neurogenic muscle atrophy, loss of muscle strength, power, and endurance.21,31 Among the various mechanisms of DPN leading to the decline of muscle mass and strength, one of the important factors is acceleration of DPN on axonal loss.23,32,33 The loss of motor units results in muscle weakness, atrophy, and intramuscular fatty infiltration.34,35 As expected, DPN is associated with sarcopenia in patients with T2DM in this present study, which agrees with data from previous reports of the correlation between DPN and sarcopenia in diabetic patients.36 Our study shows that diabetic patients with sarcopenia have a significantly higher prevalence of DPN than non-sarcopenia patients. In addition, this present study suggested that DPN was an independent risk factor for sarcopenia adjusted by multiple influencing factors.
The important effect of DPN on skeletal muscle is the accelerated loss of muscle mass, compared to diabetes without Complications.23 Reduction of lower limb muscles can reduce the activity of DPN patients, leading to gait changes, increased risk of falling and impaired balance. This may give rise to fracture, poor wound healing, chronic infection, and even amputation.2,3,5 To the best of our knowledge, there are very few studies showing the association between DPN and sarcopenia in patients with diabetic foot disease. In our study, the proportion of DPN had no statistical difference in DFD patients with or without sarcopenia. However, this does not mean that DPN is not associated with sarcopenia in DFD patients, as the majority of the entire DFD population has neuropathy. This probably resulted in no statistically significant difference in the proportion of DPN in DFD patients with or without sarcopenia. Our research team found in previous clinical studies that skeletal muscle loss is an independent risk factor for DFD. It was observed that NSS was higher in DFD patients with sarcopenia. The severities of DPN were classified into non-neuropathy, mild, moderate and severe according to NSS in order to explore the detailed relationship between DPN and sarcopenia in patients with DFD.
Our study shows that very few patients with no neuropathy symptoms suffer from sarcopenia in patients with DFD, whereas the proportion of patients with severe NS is significantly higher (Figure 1C). This may indicate to some extent that NS may be detrimental to skeletal muscle mass and may become more pronounced as NS severity increases. Similarly, appendicular lean mass of patients without NS was higher than in patients with NS, regardless of severity. In accordance with other studies, DPN patients had a 57% decline in dorsiflexor muscle volume (4.5% per year), a 61% decline in plantar flexion volume (5% per year) and a 29% loss in foot muscle volume (3% per year) over a 12-year period.18 Skeletal muscle atrophy may be related to increased advanced glycation end products and receptors or reduced insulin signaling in skeletal muscle.37,38 Interestingly, in our study, the ALMI of DFD patients without NS was higher than patients with severe NS but that seems to occur only in male patients. This may be due to the fact that the proportion of males in our cohort with DFD is significantly higher than that of females, but may also indicate that there might be a sexual dimorphism in the susceptibility to muscle loss associated with DPN.
In addition to affecting the skeletal muscle mass of diabetic patients, DPN also affects the strength of skeletal muscle. Studies by Andreassen et al suggested that muscle strength losses of lower limb are more severe in patients with DPN than in simple diabetes.18 The muscle strength of the leg muscles is reduced by 3% to 6% per year according to the severity of neuropathy.18,21 Importantly, muscle strength loss in the leg muscles is associated with neuropathy scores.39,40 Unfortunately, we were unable to assess the correlation between neuropathy and muscle strength due to the lack of grip strength measurements in our subjects.
There are some limitations in this study. This study aimed at investigating the association of sarcopenia and DPN, therefore, subjects with type 2 diabetes were recruited. Due to a high risk of sarcopenia in patients with type 2 diabetes, the detection signal bias might be caused. In addition, subjects were selected among hospitalized patients in a single center, which might lead to an admission rate bias. These biases should be considered when looking at the effect size of DPN on sarcopenia. Only NSS was used to explore the relationship between neuropathy and sarcopenia may not be convincing enough in DFD patients. We do need to collect larger sample size data to confirm a more specific relationship between DPN and sarcopenia. As a retrospective and cross-sectional study, our objective was to investigate whether DPN was independently associated with sarcopenia. We could not identify any causal relationship between sarcopenia and DPN, and prospective or interventional studies in the future are needed to unravel this question.
Conclusion
In conclusion, it is shown that DPN is an independent risk factor for sarcopenia in patients with T2DM and NSS was also independently correlated with sarcopenia in patients with DFD. Moreover, with the aggravation of neurological symptoms, the correlation between NSS and sarcopenia became more obvious. Although a causal relationship between sarcopenia and DPN remains to be established, the current study expands the current knowledge on the relationship between sarcopenia and DPN, and highlights DPN might be an important risk factor for sarcopenia in T2DM patients, especially in patients with DFD.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
No potential conflicts of interest relevant to this article were reported.
References
- 1.Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396. doi: 10.2337/dc07-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horlings CGC, van Engelen BGM, Allum JHJ, Bloem BR. A weak balance: the contribution of muscle weakness to postural instability and falls. Nat Clin Pract Neurol. 2008;4:504–515. doi: 10.1038/ncpneuro0886 [DOI] [PubMed] [Google Scholar]
- 4.Yoowannakul S, Tangvoraphonkchai K, Vongsanim S, Mohamed A, Davenport A. Differences in the prevalence of sarcopenia in haemodialysis patients: the effects of gender and ethnicity. J Hum Nutr Diet. 2018;31:689–696. doi: 10.1111/jhn.2018.31.issue-5 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV. Diabetes mellitus: does it affect bone? Calcif Tissue Int. 2003;73:515–519. doi: 10.1007/s00223-003-0023-7 [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7. doi: 10.1097/MCO.0b013e328333c1c1 [DOI] [PubMed] [Google Scholar]
- 7.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 9.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi: 10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leenders M, LB V, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14:585–592. doi: 10.1016/j.jamda.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Lee JSW, Auyeung TW, Leung J, Kwok T, Leung PC, Woo J. Physical frailty in older adults is associated with metabolic and atherosclerotic risk factors and cognitive impairment independent of muscle mass. J Nutr Health Aging. 2011;15:857–862. doi: 10.1007/s12603-011-0134-1 [DOI] [PubMed] [Google Scholar]
- 12.Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414. doi: 10.1515/hsz-2012-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38:82–90. doi: 10.2337/dc14-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–1818. doi: 10.2337/db05-1183 [DOI] [PubMed] [Google Scholar]
- 15.Cetrone M, Mele A, Tricarico D. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr Diabetes Rev. 2014;10:231–237. doi: 10.2174/1573399810666140918121022 [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA, Weigelt JA, Sun X, Johannes RS, Derby KG, Tabak YP. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695–1700. doi: 10.2337/dc11-0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28 Suppl 1:8–14. doi: 10.1002/dmrr.2239 [DOI] [PubMed] [Google Scholar]
- 18.Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles-a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia. 2009;52:1182–1191. doi: 10.1007/s00125-009-1320-0 [DOI] [PubMed] [Google Scholar]
- 19.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes. 2004;53:1543–1548. doi: 10.2337/diabetes.53.6.1543 [DOI] [PubMed] [Google Scholar]
- 20.Allen MD, Choi IH, Kimpinski K, Doherty TJ, Rice CL. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve. 2013;48:298–300. doi: 10.1002/mus.23792 [DOI] [PubMed] [Google Scholar]
- 21.Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes. 2006;55:806–812. doi: 10.2337/diabetes.55.03.06.db05-1237 [DOI] [PubMed] [Google Scholar]
- 22.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956 [DOI] [PubMed] [Google Scholar]
- 23.Ramj N, Toth C, Kennedy J, Zochodne DW. Does diabetes mellitus target motor neurons? Neurobiol Dis. 2007;26:301–311. doi: 10.1016/j.nbd.2006.11.016 [DOI] [PubMed] [Google Scholar]
- 24.Apelqvist J. Diagnostics and treatment of the diabetic foot. Endocrine. 2012;41:384–397. doi: 10.1007/s12020-012-9619-x [DOI] [PubMed] [Google Scholar]
- 25.Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–2947. doi: 10.2337/db12-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng QF, Hu JB, Yang P, et al. Sarcopenia is independently associated with diabetic foot disease. Sci Rep. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes A. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35 Suppl 1:S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–154. doi: 10.1007/BF00400697 [DOI] [PubMed] [Google Scholar]
- 30.van Dieren S, Beulens JWJ, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17:S3–S8. doi: 10.1097/01.hjr.0000368191.86614.5a [DOI] [PubMed] [Google Scholar]
- 31.Andersen H, Stalberg E, Gjerstad MD, Jakobsen J. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle Nerve. 1998;21:1647–1654. doi: [DOI] [PubMed] [Google Scholar]
- 32.Allen MD, Kimpinski K, Doherty TJ, Rice CL. Length dependent loss of motor axons and altered motor unit properties in human diabetic polyneuropathy. Clin Neurophysiol. 2014;125:836–843. doi: 10.1016/j.clinph.2013.09.037 [DOI] [PubMed] [Google Scholar]
- 33.Hansen S, Ballantyne JP. Axonal dysfunction in the neuropathy of diabetes mellitus: a quantitative electrophysiological study. J Neurol Neurosurg Psychiatry. 1977;40:555–564. doi: 10.1136/jnnp.40.6.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen MD, Major B, Kimpinski K, Doherty TJ, Rice CL. Skeletal muscle morphology and contractile function in relation to muscle denervation in diabetic neuropathy. J Appl Physiol (1985). 2014;116:545–552. doi: 10.1152/japplphysiol.01139.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344. doi: 10.2522/ptj.20080079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasemin O, Seydahmet A, Ozcan K. Relationship between diabetic neuropathy and sarcopenia. Prim Care Diabetes. 2019;13:521–528. doi: 10.1016/j.pcd.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 37.Chiu CY, Yang RS, Sheu ML, et al. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J Pathol. 2016;238:470–482. doi: 10.1002/path.4674 [DOI] [PubMed] [Google Scholar]
- 38.O’Neill ED, Wilding JPH, Kahn CR, et al. Absence of insulin signalling in skeletal muscle is associated with reduced muscle mass and function: evidence for decreased protein synthesis and not increased degradation. Age. 2010;32:209–222. doi: 10.1007/s11357-009-9125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care. 2004;27:2382–2385. doi: 10.2337/diacare.27.10.2382 [DOI] [PubMed] [Google Scholar]
- 40.Andersen H, Poulsen PL, Mogensen CE, Jakobsen J. Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes. 1996;45:440–445. doi: 10.2337/diab.45.4.440 [DOI] [PubMed] [Google Scholar]