Abstract
Background
Chronobiological changes have been detected in various physiological functions of patients with breast cancer, suggesting dysregulation in the pineal gland and melatonin secretion. This study aimed to assess and measure serum melatonin levels pre- and postoperatively in patients who had been diagnosed for the first time with breast cancer.
Methods
A sample of first-time breast cancer patients, consisting of 45 women aged 25–65 years, was evaluated and psychometric assessment was completed using the Beck Depression Inventory (BDI), Insomnia Severity Index (White, Weinberg et al) and the Epworth Sleepiness Scale (Cardoso, Spence et al). The Morningness-Eveningness questionnaire (MEQ) was used to assess the chronotype. Serum melatonin levels were measured by radioimmunoassay.
Results
Morning and moderately morning chronotypes were prevalent among the sample (25%, 45.8%, respectively). The finding of a mean BDI score of 13.5±11.2 indicated that depressive symptoms were prevalent among the sample. Despite the finding that a mean of the participants apparently had no symptoms of daytime sleepiness (the mean and standard deviations of the ESS were 7.5±4.4), scores on the ISI (a mean of 16.7±SD 7.3) indicated that insomnia symptoms were prevalent in the sample. Melatonin levels showed an inverse relationship with insomnia severity as measured by the ISI and depression severity, as assessed by the BDI. The postoperative melatonin levels were higher than the preoperative levels. Additionally, the psychometric profile differed among various pathological types of breast cancer according to their hormone receptor profile.
Conclusion
Serum melatonin levels correlated significantly with self-reported sleep quality and psychometric profiles of depression in the present sample of breast cancer patients. The melatonin assay, which is relatively easy to carry out, provided a convenient, objective measure of an important biological correlate of sleep quality and depression. This assay thus represented a confirmatory alternative to the self-report instruments, which may sometimes be unreliable. Future studies should further evaluate the utility of melatonin measures in psychiatric and sleep complaints of breast cancer patients.
Keywords: beck depression, breast cancer, chronotype, circadian, melatonin
Introduction
Cancer is one of the world’s leading fatal diseases. Of the different types of cancer, breast cancer is one of the most common and has a high incidence rate in all countries.16 Global cancer surveys indicate that breast cancer is the second most common type of cancer, with 1.7 million new cases, or 25% of all types of cancer, being reported to occur each year.24 Epidemiological studies have also revealed that, on a worldwide basis, half a million deaths each year are due to metastatic breast cancer.14 A disproportionately greater number of these deaths occur in developing countries, partly because of the higher percentage of affected patients whose cancers were not screened or detected until having reached an advanced stage of pathology.58
Disruption of circadian rhythms has been proven to predispose to medical morbidities, including cardiac diseases, cancer, addiction, diabetes, and obesity.38 Melatonin is the pineal neuro-hormone responsible for controlling the central and peripheral circadian rhythms.59 Among melatonin’s numerous activities is the regulation of the immune system and antitumor defense mechanisms, functions which are inferred to occur most intensely in the nighttime hours, in tandem with the parallel nocturnal surge in serum melatonin levels.61 Recent data have implicated disruption of circadian rhythms and as a causal factor in serious pathologic conditions, including tumorigeneses and progression of cancer.54,59 The first study evaluating the association between melatonin levels and breast cancer risk was investigated by Bartsch and co-workers.6 It was found that the women with advanced breast cancer, when compared with healthy controls, had lower levels of urinary melatonin. Additionally, it was reported that biological assays of women with Estrogen Receptor (ER)-positive breast cancer showed an inverse relationship between ER levels and peak melatonin values.64 Estrogens are involved in several aspects of breast cancer development, including proliferation, metastasis, immune evasion, and mortality.67 Peak melatonin levels have been associated with attenuation of all of the carcinogenic processes in the presence of estrogen.40 Melatonin activity also affects the hypothalamic-pituitary-ovarian (HPO) axis, resulting in lower circulating levels of estrogen and progesterone.51 Experimental interference with circadian clock functioning is associated with higher levels of cancer incidence, faster progression, and shorter survival.9
Current evidence indicates that the nighttime physiological surge of melatonin may serve as a “natural restraint” for tumor initiation, promotion, and progression. The multiple modes of melatonin’s tumor-suppressive effects against breast cancer cells include antiestrogenic (a), antiproliferative (b), anti-metastatic (c), pro-apoptotic (d), antioxidant (e), anti-angiogenic effects (f), and immuno-enhancing (g) effects.40 In a study by46 melatonin was found to decrease the volume of mammary tumors, but did not influence any other tumor characteristics. It was found that melatonin inhibited the growth of breast cancer by interacting with estrogen-responsive pathways, thus behaving as an anti-estrogenic hormone. In a recent study by,27 melatonin was found to reduce sulfatase expression and activity in human breast cancer cells, thus modulating local estrogen biosynthesis. Furthermore, melatonin has been found to play an inhibitory role in the viability and invasiveness of breast cancer mammospheres. Additionally, its potential anti-metastatic role has been shown by its ability to modulate protein expression in the epithelial–mesenchymal transition of breast cancer stem cells.26 Melatonin has also been found to stimulate expression of the pro-apoptotic BAD and BAX genes, and to enhance inhibition of the anti-apoptotic gene BCL-2, thus acting as an adjuvant to chemotherapy.3 Melatonin has been found to regulate angiogenic factors in breast tumor cells, and possesses anti-angiogenic activity, particularly under hypoxic conditions.34,63 Taken together, these findings support the conclusion that melatonin has important anti-tumorigenic functions, and thus may have important therapeutic potential. It has been suggested that an experimental examination of different strategies for manipulating melatonin activity, in particular, of disrupting circadian rhythms, in particular by exposing human subjects to light at night or other forms of sleep disruption could be informative for the development of novel strategies for cancer prevention.50
We hypothesized that the circadian rhythm of melatonin secretion would be disturbed in patients with breast cancer and that such disruption would correlate with the severity of the cancer pathology and the psychometric profile. Previous research has linked a single psychological variable (like Depression & Anxiety) e.g., in the studies by53 or sleep complaint with melatonin secretion in breast cancer patients, e.g.30,43,68 To our knowledge, no previous study has examined the association among multiple psychological variables, sleep profiles, serum melatonin levels, and hormonal status of breast cancer patients (including estrogen, progesterone, and Her/neu receptors). Therefore, this cross-sectional observational study was conducted to assess these multiple effects. The investigations thus examined the psychological and sleep quality profiles of patients with breast cancer and then carried out a correlational analysis of these variables before and after breast cancer surgery with any parallel changes which occurred in serum melatonin levels.
Subject and Methods
Study Design and Sample
Study Population
Forty-five patients with breast cancer and who met inclusion criteria were consecutively recruited from the inpatient ward of Mansoura University Oncology Center. The study included patients aged 20–65 years who had received a first-time diagnosis of breast cancer (non-recurrent patients), and who were candidates for surgical mastectomy. Patients were excluded if prior to admission they had metastasis apart from lymph nodes, or a diagnosis of a primary psychiatric disorder, or if they were taking a psychotropic medication.
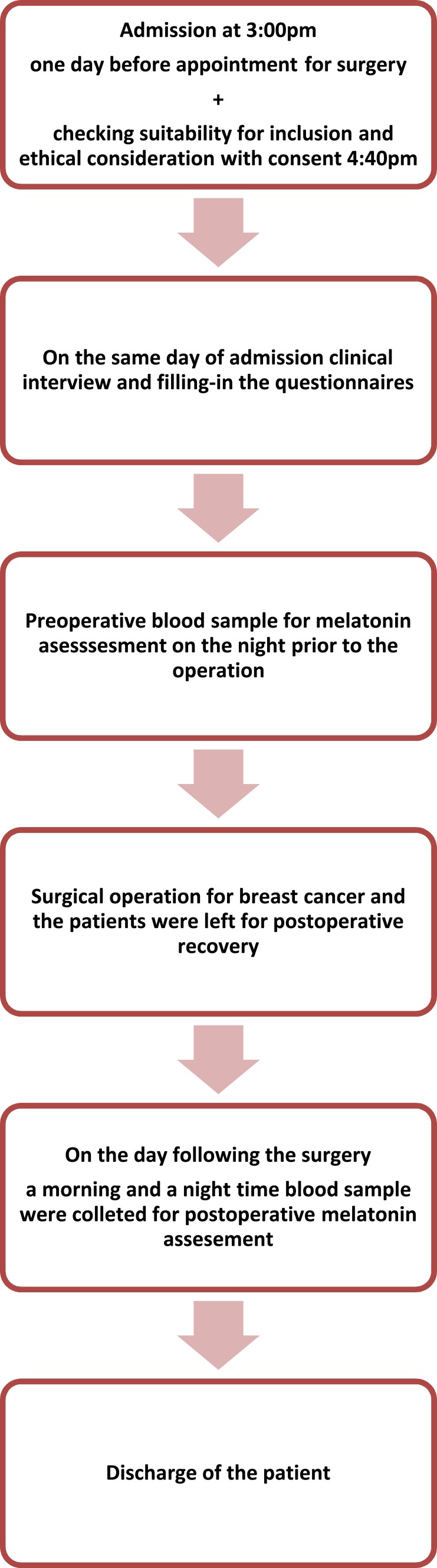
All recruited patients were interviewed by research team members on the day of their admission to the oncology center. Patients were evaluated based on their general health and suitability for participation, and then were asked to sign an informed consent form. The recruitment interview was conducted in a private room, participants were told of the study’s aims and procedure. Pathological and other oncological data were extracted from the patient’s surgical file, especially regarding the patients’ progesterone receptors, estrogen receptors, and HER2/neu receptors. Participants filled in the questionnaires and provided three blood samples for assays of their serum melatonin levels. Three blood samples were collected; the first preoperatively, typically immediately before the surgery (several collections were carried out earlier in the day), and then on two successive occasions, i.e., following the surgery. The blood sample that was collected on the night of the operation (between 9:30 and 10:30 pm) was done under conditions of dim light. An exception was that if patients complained about being in the dimly lit room, they were allowed to change the light intensity according to their preference. The first blood sample was drawn immediately before the scheduled operation time or, in some instances, at around 3 pm the day before the surgery. Two follow up collections were carried out to obtain a rhythm of melatonin secretion over the day and the night before the patient was discharged. Thus, on the day following the operation, a daytime postoperative sample and a nighttime postoperative sample were also withdrawn from most participants. Figure 1 summarizes the steps of the study.
Figure 1.
Stepwise study methodology.
According to a series of studies conducted by Chu et al,16 it was found that daytime samples are reliable for assessing melatonin levels and that a single measurement is sufficient to examine associations between melatonin and risk of cancer in epidemiologic studies.69 In the present study, however, it was decided to take three subsequent samples to cover the daytime, nighttime, preoperative and postoperative levels of the hormone. A graphic display of the serum melatonin levels obtained in the current study is shown in Figure 2.
Figure 2.
(A) Graphic dispersion of serum melatonin levels. (B) 3D histogram representing serum melatonin levels.
Prior to the commencement of the study procedures, the study was approved by the Institutional Review Board (IRB) of Mansoura University (Human IRB Approval.18.06.220). Informed written consent was obtained from all participants prior to the commencement of the study. This study was conducted in accordance with experimental study guidelines recommended in the Declaration of Helsinki.
Data Collection
All questionnaire instruments used in this study were administered in a manner designed to protect the anonymity of respondents and to keep their responses confidential. Data such as anthropometric and sociodemographics (e.g., age, gender, and employment status), and clinical data were also collected. The following instruments were used: The Beck Depression Inventory, Insomnia Severity Index, Epworth Sleepiness Scale, and the Morningness-Eveningness Questionnaire. All instruments were translated and validated in Arabic.
Beck Depression Inventory (BDI)
The presence of depressive symptoms in breast cancer in this study was assessed using the Beck Depression Inventory (BDI) (Beck et al, 1996).70 The BDI is a 21-item self-report multiple-choice inventory that takes about 10 mins to complete. It is a widely used indicator of the severity of depression and has been found to have a sensitivity of 81% and a specificity of 92%. BDI-II items are rated on a 4-point scale ranging from 0 to 3 based on the severity of each item. This self-report scale permits a maximum total score of 63. Scores from 0 to 13 indicate minimal depression; a score in the range of 14–19 indicates mild depression; a score of 20–28 indicates moderate depression; and a score of 29–63 indicates severe depression. In this study, we have used the Arabic validated version.2
Insomnia Severity Index (White, Weinberg et al)
The Insomnia Severity Index (Lissoni, Paolorossi et al) is a brief screening assessment tool designed to evaluate insomnia severity based on DSM V diagnostic criteria for insomnia.7 This self-report instrument measures the patient’s perception of both nocturnal and diurnal symptoms of insomnia over the past 2 weeks. The ISI comprises seven items which assesses the perceived severity of difficulties initiating sleep, staying asleep, early morning awakenings, satisfaction with one’s current sleep pattern, whether there is interference with daily functioning, noticeability of any impairments attributed to the sleep problem, and the degree of personal distress or concern caused by the sleep problem. The seven answers are added up to produce a total score. The score categories and associated insomnia symptoms are: from 0 to 7, no clinically significant insomnia; from 8 to 14, subthreshold insomnia; from 15 to 21, clinical insomnia (moderate severity); scores from 22 to 28 indicate clinical insomnia (severe). In this study, the validated Arabic version of the Index was used.4,62
Epworth Sleepiness Scale (Cardoso, Spence et al)
The Epworth Sleepiness is a brief, self-evaluation instrument intended to measure daytime somnolence.36 The ESS consists of eight questions. Respondents are asked to rate, on a 4-point scale (0–3), their likelihood of dozing off or falling asleep while engaged in eight different activities. Most people engage in the suggested activities at least occasionally, although not necessarily every day. The ESS score (the sum of 8 item scores, 0–3) can range from 0 to 24. The higher the ESS score, the higher that person’s “daytime sleepiness”. A validated Arabic version of the scale was used in this study.1,4
Morningness-Eveningness Questionnaire (MEQ)
The Morningness-Eveningness Questionnaire (MEQ) is a self-rated questionnaire.31 The scale consists of 19-items, the purpose of which is to measure individual differences in morningness and eveningness, defined as the degree to which respondents are active and alert at certain times of day (i.e., morning, in the evening, or in between these times). The abridged version (MEQr) of this scale was later developed and validated by Adan and Almirall (1991).71 The MEQr has been shown to successfully identify human circadian typology in both applied and experimental research.47 The Abridged version contains five items. Three questions establish the preferred morning wake-up time, the evening bedtime, and the hour of the day when personal efficiency is maximal. The two other questions evaluate the extent of tiredness within the first half-hour after morning awakening and determine which circadian type or group the respondent considers himself/herself to be. The MEQr establishes five behavioral chronotypes: definitively morning-type (score, 22–25), moderately morning-type (score, 18–21), neither-type (score, 12–17), moderately evening-type (score, 8–11) and definitively evening-type (score, 4–7). The Arabic version has now been validated in an Arabic-speaking sample.5
Serum Melatonin Measurement
Blood samples were collected at 3 time points and analyzed according to the method described by.45 The sample collection points were as follows: 10 pm (one sample collected preoperatively), on the day immediately preceding the operation; (two samples collected postoperatively) collected on the same night of the operation and 10 am on the morning of the day immediately after the operation). The samples withdrawn thus represented key time points in the 24 hr rhythm of melatonin secretion. The two postoperative samples were taken in order to check the difference between the melatonin levels at both time points as an indicator of circadian variability in the secretion. The 10 pm nighttime postoperative (1.5–2 hrs after sleep onset) sample was withdrawn to assess the level of melatonin secretion after the operation and removal of the cancerous breast tissue. Each participant gave only a single sample at each time point of blood withdrawal. No duplicate samples were collected (only one blood sample was taken at each time point) producing a total of 135 (45×3) collected samples. A histogram summarizing the serum melatonin levels is provided below in Figure 2B.
To minimize painful sensations associated with frequent blood samplings, nocturnal blood was drawn through a peripheral intravenous cannula placed in the cubital vein. It was ensured that the nursing staff who collected the blood samples were well trained to minimize technical errors as much as possible. Since melatonin secretion is affected by light intensity,41 a dim light torch (emitting less than 0.25 lux) was used at night during blood sample collections. The nurses used a handheld nocturnal illuminator and avoided exposure of the patients to direct light for the shortest possible duration. The torch illuminator was turned off directly after the sample was successfully withdrawn. The refined melatonin radioimmunoassay (RIA) for serum-based studies, originally described by Manz et al (1989)72 and Hsing, Meyer et al (2010),73 and later modified by Chu, John et al,69 was used.69 The samples were immediately frozen and stored at –70°C until analysis.
Serum melatonin levels were determined using a radioimmunoassay (Melatonin RIA-0355 kit, DRG, Marburg, Germany). The principle of the radioimmunoassay (RIA) method for melatonin measurement is that a known amount of radioactive melatonin (2-I125-iodomelatonin or 3H-melatonin) is mixed with a fixed amount of antibody raised against melatonin. Increasing concentrations of unlabeled melatonin are added to the mixture, which will compete with labeled melatonin causing its displacement from the antibody. Free-labeled melatonin is then separated from the remaining antibody-bound radioactive melatonin and radioactivity is measured. As the concentration of unlabeled melatonin increases in the mixture, competition for the antibodies also increases and bound labeled melatonin decreases. A calibration curve was constructed from the known amounts of labeled and unlabeled melatonin to allow the determination of the unknown melatonin concentrations in the biological samples.18 Thus, in the current study, the amount of 125I-labelled antigen bound to the antibody is inversely proportional to the analyte concentration of the sample. When the system is in equilibrium, the antibody-bound radioactivity is precipitated with a second antibody in the presence of polyethylene glycol. The precipitate was counted in a gamma counter.
Breast Cancer Hormone Receptors
Analysis of breast cancer hormones (estrogen, progesterone, Her/neu) was carried out according to the guidelines recommended by48 Nofech–Mozes, Vella et al48 The tumor was tested based on tissue taken from the surgical excision specimen. Surgeons usually provide the cancerous tissue and part from the surrounding normal breast tissue in the tested specimen. Parrafin sections were used and fixated as soon as possible following removal. Freezing and cryosectioning before fixing the sample were avoided. The fixation period was for 24 hrs. The sections used in this study were freshly cut and none of them were stored for long periods. Better HR detection was found with freshly cut sections compared with slides stored for longer periods of time.23 The slides were oriented, stained for surgical margin assessment, and carefully sliced at 5–10 mm intervals before being placed into formalin. Antibody clone and vendor were then applied together with the Image analysis method.48 The results depend on the percentage of positively stained cells for ER and PR. Interpretation of the stain results was done as follows: 1) Low positive 1–9% for ER or PR, 2) Positive ≥ 10% for ER or PR, 3) Negative < 1% for ER and PR,4.Not interpretable stain results.
Statistical Analysis
Data were transferred from Excel spreadsheets into an SPSS program, version 23 (IBM corp. 2015) to detect the associations and descriptive results. A p-value below 0.05 was considered significant. Categorical data were represented by numbers and frequencies. Correlation coefficients were calculated using the Spearman’s Rho to detect the relationship between melatonin levels and psychometric profiles. Cross-tabulation was used to examine the relationship between the psychometric profiles and cancer pathology. Multivariate logistic regression analysis was carried out to detect which of the variables had the most impact, with serum melatonin levels being used as the dependent variable.
Results
The demographic characteristics of the recruited sample are summarized in Table 1. The mean age was 50.4 ± 10.8 years, indicating that most participants in the sample were menopausal women. Married women were more frequently represented than widowed or divorced ones (50.3%, 33.3%, respectively). The majority of the participants were housewives (79.2%). Sixteen women (33.3%) had a positive family history of breast cancer, 14 women had a positive family history of deaths due to breast cancer, and 34 women had positive medical comorbidities. From Table 1 it can also be noted that the morning and moderately morning chronotypes were predominant among the sample (25% and 45.8%, respectively), followed by neither chronotype, representing only 16.7%.
Table 1.
Summary of Patient Demographics
| Demographic Variable | N | % |
|---|---|---|
| Marital Status | ||
| Married | 25 | 50.3 |
| Divorced | 4 | 8.3 |
| Widow | 16 | 33.3 |
| Occupation | ||
| Housewife | 38 | 79.2 |
| Employed | 10 | 20.8 |
| Family Income | ||
| Low | 24 | 50 |
| Average | 24 | 50 |
| Medical Comorbidity | ||
| Absent | 14 | 29.2 |
| Present | 34 | 70.8 |
| FH of Breast Cancer | ||
| Negative | 32 | 66.7 |
| Positive | 16 | 33.3 |
| FH of Death from Breast Cancer | ||
| None | 36 | 75 |
| Positive | 12 | 25 |
| Chronotype | ||
| Definitely morning | 12 | 25 |
| Moderately morning | 22 | 45.8 |
| Neither | 8 | 16.7 |
| Moderately evening | 4 | 8.3 |
| Definitely evening | 2 | 4.2 |
| Age | ||
| Mean | 50.4 | |
| ±SD | 10.8 | |
| Min | 29 | |
| Max | 65 |
The psychometric profile of the recruited sample is presented in Table 2. It showed a mean BDI score of 13.5±11.2, indicating the prevalence of depressive symptoms in many of the participants. Additionally, the mean of the ESS was found to be 7.5±4.4, indicating that few believed that they suffered from daytime sleepiness. On the other hand, the ISI showed a mean of 16.7±SD 7.3, indicating that insomnia was a prevalent complaint among the studied sample. Regarding the MEQ total score, the mean was 18.25± 4.25. Table 2 summarizes the descriptive data of serum melatonin levels in the study participants. It was noted that the postoperative levels were much higher than the preoperative sample, thus supporting the inference that the sleep quality of many improved following surgery.
Table 2.
Means and Standard Deviations of the Sample Psychometric Tests and Serum Melatonin Levels
| BDI | ESS | MEQ | ISI | Preoperative MEL | Daytime Postoperative MEL | Nighttime Postoperative MEL | |
|---|---|---|---|---|---|---|---|
| Mean | 13.5 | 7.5 | 18.25 | 16.7 | 12.59 | 60 | 79.1 |
| SD | 11.2 | 4.4 | 4.5 | 7.3 | 10.2 | 49.2 | 56.4 |
| Min | 8 | 0 | 5 | 1 | 1.46 | 106 | 8.11 |
| Max | 49 | 16 | 24 | 30 | 35 | 169 | 189 |
| Range | 41 | 16 | 19 | 29 | 33 | 158 | 180 |
Serum melatonin levels were compared to results of the psychometric profile using Spearman’s non-parametric correlation analysis, and the results are shown in Table 3. The finding of significant negative correlations showed that an inverse relationship existed between preoperative serum melatonin levels and the Beck Depression Inventory (preoperative melatonin levels and BDI; r= −0.27, p=0.06), indicating that more depressive symptoms tended to occur with low melatonin levels. Daytime postoperative melatonin significantly correlated with the ESS score (r= −0.9, p=0.001), indicating that when participants tended to experience excessive daytime sleepiness, they also had low melatonin levels, a finding consistent with the established association between increased levels of melatonin and nighttime sleepiness. The nighttime levels were non-significant, but the correlation value showed a strong trend in the expected direction (p=0.07). This finding was expected inasmuch as the ESS is an estimate of daytime sleepiness only. The daytime postoperative melatonin positively correlated with the ISI scores (r=536, p=0.0001), while the nighttime postoperative melatonin negatively correlated with the ISI scores (r=−500, p=0.001), indicating a strong association between lower levels of melatonin secretion at night and reduced sleep quality, as shown by the lower sleep profile scores.
Table 3.
Spearman Correlative Statistics Between Serum Melatonin Levels and Psychometric Tests
| Melatonin Level | BDI | ESS | MEQ | ISI |
|---|---|---|---|---|
| Preoperative Melatonin | ||||
| r | 0.27 | 157 | 163 | 0.2 |
| p | 0.06* | 0.31 | 0.003** | 0.16 |
| Daytime Postoperative Melatonin | ||||
| r | 6.53 | 0.9 | 185 | 536 |
| p | 0.7 | 0.001** | 0.06* | 0.0001** |
| Nighttime Postoperative Melatonin | ||||
| r | 25 | 0.6 | 116 | 500 |
| p | 0.38 | 0.07* | 0.4 | 0.001** |
Notes: *Trend toward significance. **Significant correlation.
Table 4 shows comparisons among pathological subtypes of breast cancer and psychometric profile scores. It can be observed that patients with positive indices for cancer in estrogen receptors tended to have higher scores for depression on the BDI, as well as higher insomnia complaint scores on the ISI (p=0.0001 and 0.004, respectively). Positive indices for cancer in progesterone receptors were associated with elevated scores for depression on the BDI (p=0.0001) but did not show associations with insomnia complaints. Detection of cancer in HER2/neuroreceptors only affected the MEQ scores and chronotype distribution, with p=0.03. Table 5 shows comparison between menopausal and premenopausal subjects, statistically significant differences were found between the two groups on depression and insomnia scores (p=0.007, p=0.05, respectively) .
Table 4.
Psychometric Properties of Different Breast Cancer Receptors
| Estrogen Receptors N (%) | Progesterone Receptors N% | HER2/neu Receptors N% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Weak | Mild +ve | Moderately+ve | Strong +ve | p | Negative | Weak | Moderate +ve | Strong +ve | p | Absent | TypeI | Type IV | p | |
| BDI | |||||||||||||||
| Minimal | 0 | 0 | 0 | 0 | 2(4.1) | 0.0001 | 2(4.1) | 0 | 0 | 0 | 0.0001 | 2(4.1) | 0 | 0 | 0.07 |
| Mild | 2(4.1) | 2(4.1) | 0 | 2(4.1) | 0 | 2(4.1) | 0 | 2(4.1) | 2(4.1) | 6(12.5) | 0 | 0 | |||
| Moderate | 2(4.1) | 4(8.3) | 4(8.3) | 2(4.1) | 0 | 4(8.3) | 4(8.3) | 4(8.3) | 0 | 6(12.5) | 4(8.3) | 2(4.1) | |||
| Severe | 10 | 4(8.3) | 12 | 2(4.1) | 0 | 8(16.6) | 2(4.1) | 16 | 2(4.1) | 14(29.1) | 8(16.6) | 6(12.5) | |||
| ISI | |||||||||||||||
| No Insomnia | 4(8.3) | 0 | 0 | 0 | 2(4.1) | 0.004 | 4(8.3) | 0 | 2(4.1) | 0 | 0.19 | 4(8.3) | 2(4.1) | 0 | 0.09 |
| Subthreshold | 4(8.3) | 2(4.1) | 6(12.5) | 0 | 0 | 4(8.3) | 2(4.1) | 6(12.5) | 0 | 6(12.5) | 4(8.3) | 2(4.1) | |||
| Moderate Clinical Insomnia | 2(4.1) | 6(12.5) | 4(8.3) | 2(4.1) | 4(8.3) | 2(4.1) | 2(4.1) | 8(16.6) | 6(12.5) | 8(16.6) | 2(4.1) | 8(16.6) | |||
| Severe Clinical Insomnia | 4(8.3) | 2(4.1) | 2(4.1) | 2(4.1) | 2(4.1) | 6(12.5) | 2(4.1) | 2(4.1) | 2(4.1) | 10(20.8) | 2(4.1) | 0 | |||
| ESS | |||||||||||||||
| No Eds | 12 | 6(12.5) | 8(16.6) | 4(8.3) | 6(12.5) | 0.45 | 12(25) | 6(12.5) | 12(25) | 6(12.5) | 0.46 | 24(50) | 6(12.5) | 6(12.5) | 0.05 |
| Positive Eds | 2(4.1) | 4(8.3) | 4(8.3) | 2(4.1) | 0 | 4(8.3) | 2(4.1) | 6(12.5) | 0 | 4(8.3) | 4(8.3) | 4(8.3) | |||
| MEQ | |||||||||||||||
| Definitely Morning | 4(8.3) | 2(4.1) | 6(12.5) | 0 | 6(12.5) | 0.05 | 4(8.3) | 2(4.1) | 6(12.5) | 6(12.5) | 0.57 | 8(16.6) | 2(4.1) | 2(4.1) | 0.03 |
| Moderately Morning | 6(12.5) | 6(12.5) | 6(12.5) | 2(4.1) | 2(4.1) | 6(12.5) | 8(16.6) | 6(12.5) | 2(4.1) | 12(25) | 6(12.5) | 4(8.3) | |||
| Neither | 4(8.3) | 0 | 2(4.1) | 2(4.1) | 0 | 4(8.3) | 2(4.1) | 2(4.1) | 0 | 6(12.5) | 2(4.1) | 0 | |||
| Moderately Evening | 0 | 2(4.1) | 2(4.1) | 0 | 0 | 2(4.1) | 0 | 2(4.1) | 0 | 2(4.1) | 0 | 2(4.1) | |||
| Definitely Evening | 0 | 2(4.1) | 0 | 0 | 0 | 0 | 0 | 2(4.1) | 0 | 0 | 2(4.1) | 0 | |||
Notes: Shows comparisons among pathological subtypes of breast cancer and psychometric profile scores. It can be observed that patients with positive indices for cancer in estrogen receptors tended to have higher scores for depression on the BDI, as well as higher insomnia complaint scores on the ISI (p=0.0001 and 0.004, respectively). Positive indices for cancer in progesterone receptors were associated with elevated scores for depression on the BDI (p=0.0001) but did not show associations with insomnia complaints. Detection of cancer in HER2/neuroreceptors only affected the MEQ scores and chronotype distribution, with p=0.03. Bold values indicate statistical significance.
Table 5.
Comparison Between Menopausal and Premenopausal Subjects
| Variable | Menopausal N(%) |
Premenopausal N(%) |
X2 | p |
|---|---|---|---|---|
| BDI Depression Degree | ||||
| Minimal | 2(4.2) | 0 | 12 | 0.007 |
| Mild | 4(8.3) | 2(4.2) | ||
| Moderate | 4(8.3) | 8(16.7) | ||
| Severe | 24(50) | 4(8.3) | ||
| ISI Insomnia Severity | ||||
| No insomnia | 4(8.3) | 2(4.2) | 7.6 | 0.05 |
| Subthreshold | 12(25) | 0 | ||
| Moderate | 12(25) | 2(4.2) | ||
| Severe | 2(4.2) | 2(4.2) | ||
| ESS | ||||
| Not sleepy | 24(50) | 12(25) | 1.2 | 0.4 |
| Sleepy | 10(20.8) | 2(4.2) | ||
| Chronotype | 1.8 | 0.76 | ||
| Definitely morning | 8(16.7) | 4(8.3) | ||
| Moderately morning | 16(33.3) | 6(12.5) | ||
| Neither | 6(12.5) | 2(4.2) | ||
| Moderately evening | 2(4.2) | 2(4.2) | ||
| Definitely evening | 2(4.2) | 0 |
Notes: Shows comparison between menopausal and premenopausal subjects, statistically significant differences were found between the two groups on depression and insomnia scores (p=0.007, p=0.05, respectively). Bold values indicate statistical significance.
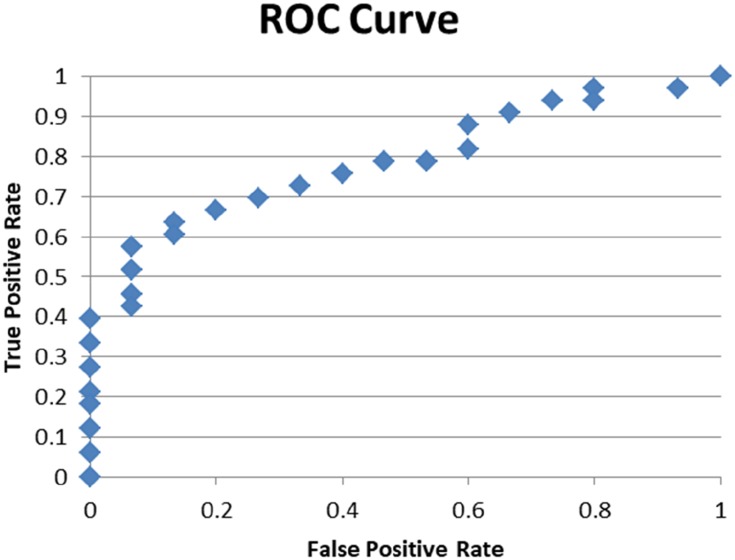
A multiple logistic regression was carried out to determine which of the variables had the strongest association with melatonin levels. It was found that the preoperative melatonin levels appeared to be most strongly influenced by the status of estrogen receptors and the BDI scores (p= 0.032 and 0.05) respectively. Daytime postoperative melatonin levels were strongly affected by the participant’s chronotype (p=0.004), while nighttime postoperative melatonin levels were most affected by the Her/neu receptor status and the chronotype (p=0.04 and 0.025, respectively). These results are summarized in Tables 6–8. Additionally, the ROC curve for each serum melatonin level can be seen in Figures 3–5.
Table 7.
Multivariate logistic regression for study variables impacting postoperative daytime serum melatonin
| Variable | Coefficient | p | R Square | F | P |
|---|---|---|---|---|---|
| Estrogen receptors | 0.26 | 0.8 | 0.3 | 3.4 | 0.008 |
| Progesterone receptors | 0.21 | 0.11 | |||
| Her/neu receptors | 0.019 | 0.15 | |||
| Depression severity | 0.14 | 0.9 | |||
| Insomnia | −0.1 | 0.4 | |||
| Sleepiness | −0.1 | 0.4 | |||
| Chronotype | 0.48 | 0.004 |
Note: Bold value indicate statistical significance.
Figure 4.
ROC curve for regression analysis considering postoperative (night) melatonin as dependent variable.
Table 6.
Multivariate logistic regression for study variables impacting the preoperative serum melatonin
| Variable | Coefficient | p | R Square | F | P |
|---|---|---|---|---|---|
| Estrogen receptors | 0.3 | 0.032 | 0.132 | 1.8 | 0.1 |
| Progesterone receptors | 0.1 | 0.95 | |||
| Her/neu receptors | −0.06 | 0.39 | |||
| Depression severity | 0.34 | −0.05 | |||
| Insomnia | 0.06 | 0.7 | |||
| Sleepiness | 0.02 | 0.9 | |||
| Chronotype | 0.27 | 0.12 |
Note: Bold value indicate statistical significance.
Table 8.
Multivariate logistic regression for study variables impacting postoperative night time serum melatonin
| Variable | Coefficient | p | R square | F | p |
|---|---|---|---|---|---|
| Estrogen receptors | 0.7 | 0.8 | 0.22 | 2.6 | 0.026 |
| Progesterone receptors | 0.32 | 0.3 | |||
| Her/neu receptors | 0.04 | 0.04 | |||
| Depression severity | 0.06 | 0.8 | |||
| Insomnia | −0.015 | 0.7 | |||
| Sleepiness | −0.014 | 0.9 | |||
| Chronotype | 0.37 | 0.025 |
Note: Bold value indicate statistical significance.
Figure 3.
ROC curve for regression analysis considering preoperative melatonin as dependent variable.
Figure 5.
ROC curve for regression analysis considering postoperative (daytime) melatonin as dependent variable.
Discussion
Breast cancer is one of the most frequently occurring cancers among women, and one of the leading causes of death among women aged 40 to 55 years. In Egypt, breast cancer represents the second most commonly diagnosed type of cancer, being found in around 35% of all cancer patients, according to a study conducted by Ibrahim et al.32 Women with breast cancer are prone to various psychological problems, including depression. Additionally, they show changes in their sleep patterns. Some evidence has suggested that the elevated levels of psychological symptoms are driven by increases in hormone receptor-positive breast cancer, whereas psychological symptom severity tended to be much less in patients with hormone receptor-negative breast cancers.19 To our knowledge, no previous study has examined the association of breast cancer receptors and psychometric scales, including insomnia. In the current study, we found a noticeable rise in serum melatonin levels after surgical mastectomy. The mean levels that were found preoperatively increased unexpectedly after removal of the breast tissue from 12.59 pg to 60 pg for daytime samples and to 79.1 pg for the nighttime postoperative sample. This difference was expected since melatonin levels normally increase at night. However, the association between melatonin levels and breast surgery remains complex. Lissoni et al42 found that melatonin was not affected by mastectomy in 13/24 of their studied patients, whereas it was enhanced in 5 women and decreased in 6 cases. Thus, providing evidence that radical mastectomy influences melatonin secretions.42 Our own results, although lacking a non-surgery comparative group, were similar to those of Lissoni et al,42 in as much as they showed that the effect of breast cancer surgery on melatonin secretion is complicated, with the directionality of the change being mixed. Most studies have conflicting results about the direction of circadian rhythm disturbances after surgical operations. Surgical operations and anesthesia have been found to disturb the circadian rhythm secretin of melatonin in different and inconsistent ways.37 The clinical significance of our own findings and that of others thus remains to be established. Stress is a moderator variable. Depression tends to be correlated with anxiety, both lead to disturbed melatonin secretion. According to29, after surgery, melatonin secretion has shown to be acutely disturbed with a delay of secretion and reduced amplitude. Various lines of evidence show that depressed patients exhibit disturbances in both the amplitude and the shape of the melatonin secretion rhythm,29 with some studies showing a low nocturnal melatonin secretion60 and others showing an increase in secretion. Further elucidating the non-uniform directionality of melatonin levels in relation to breast cancer. Most previous studies on the effects of circadian rhythm sleep disorders and exogenous melatonin have focussed on the resultant behavior of breast cancer patients. Circadian disruption however also has an important hormonal and tumorigenic effect. Melatonin has been found to reduce the expression of estradiol-induced genes and thus decreasing mammary tumor viability.10 It could also exert its anti-tumorigenic effects on hormone-dependent mammary tumors by down-regulating the sulfatase pathway of the tumor tissue. Srinivasan et al59 found a correlation between tumor size and the nocturnal amplitude of melatonin secretion. Peak nocturnal amplitude of melatonin was reduced in 50% of the patients with primary breast cancer and was inversely correlated with tumor size.59
Overall, the present study found an association between melatonin levels and the psychometric profile of breast cancer patients. We found a significant inverse association between melatonin and insomnia severity and depression severity, as measured by the BDI. Various studies have documented the changes in sleep patterns which occur in breast cancer patients.13,74,75 Chen et al (2014) reported that, even after exogenous melatonin administration, no improvement in the depressive symptoms of breast cancer patients.15 Lower levels of daytime melatonin were also associated with a lesser degree of daytime sleepiness. Among women with breast cancer, the prevalence of depression might range from 1.5% to 50%, depending on the sample, and particularly the definition of depression and method of assessment.15 This is in line with the findings of the current investigation. Furthermore, the melatonin levels correlated with BDI-measured levels of depression. Previous studies examining the association between sleep and breast cancer have yielded conflicting results, with some finding a positive association and some others not confirming a significant association between sleep or melatonin levels and breast cancer overall.11,51,56,67
The majority of the recruited sample consisted of menopausal and premenopausal women. Studies have shown that insomnia is a frequent complaint in menopausal women.12,55 These hormonal changes seem to affect sleep directly. A decline in the levels of these hormones in menopausal and postmenopausal women and the complex interaction among these hormones can significantly contribute to sleep problems.35,50 The sleep problems occurring in later phases of cancer are characterized by comorbidities such as depression, deviations in breathing, or fibromyalgia, and progressively by age-associated processes. Insomnia is a frequent complaint among breast cancer patients.49 In the current study, the elevated mean ISI scores indicated that the study sample had a noticeable insomnia problem. In a study by Fang et al, it was found that insomnia patients have a higher risk of developing cancers.22 However, another study found that the presence of insomnia symptoms did not predispose nor associate with breast cancer. This finding, however, might have been due to suppression of the immune system indirectly through an insomnia-associated reduction in melatonin levels.57 The ISI scores correlated with the postoperative melatonin levels, indicating that the cancerous tissue might have played a role in the complaint; these findings are to some extent similar to that of Jablonska et al,33 who stated that there was a higher expression of melatonin receptors inside the cancerous breast tissue.33 This would suggest that removal of cancer produces a drop-in hormone levels and action. Another study, however, reported that the surgical removal of breast cancer tissue was not associated with changes in melatonin levels, and that only the circadian rhythm was affected.20 In another study, Kubatka and associates the investigators found however that beyond a certain increase in tumor size, further increases were associated with decreases in melatonin secretion.40 The peak of melatonin declined with increasing tumor size. These findings further confirm that pineal melatonin secretion is transiently reduced in primary breast cancer and that as melatonin levels continue to decline, ISI total scores tend to increase, suggesting that melatonin has a role in sleep and mood complaints in breast cancer patients. Similar results have been reported in other studies, demonstrating that increases in serum melatonin concentrations to within the normal nocturnal range, whether this occurs naturally or as the result of exogenous administration, may have beneficial effects on sleep quality. Slightly higher exogenous doses may produce hypnotic effects, including a decrease in objective and self-estimated sleep-onset latency, an increase in sleep duration, and sleepiness upon waking.21,56 In a study by Toffol and colleagues, postmenopausal women were found to have lower nighttime serum melatonin levels. The duration of melatonin secretion tended to be shorter in postmenopausal women. The mean melatonin concentrations and exposure levels did not correlate with follicle-stimulating hormone level, estradiol level, body mass index, BDI score, State-Trait Anxiety Inventory score, BNSQ insomnia score, BNSQ sleepiness score, nor the subjective sleep score.65 These results, which differ from those that were observed in the present study, underscore the need for further research regarding the correlation between melatonin and the psychometric profile of breast cancer patients. Additionally, subjects with estrogen-positive receptors had a higher degree of insomnia severity. Some studies reported that insomnia is associated with suppression of melatonin levels that in turn increases estrogen production and subsequently leads to increases in the number of estrogen receptors.8,17 On the other hand, ISI scores did not show any association with the status of progesterone and HER/neu receptors. These findings are in line with the findings of Vaughn et al who reported that, among breast cancer cases, sleep disturbances were higher for women with ER−/PR− tumors when compared to women with ER+/PR+ tumors, indicating that sleep problems might be associated with a more aggressive type of cancer.66 Also, in a study by Khawaja et al, patients with HER2 positive breast cancer reported getting, on average, less nightly sleep. Additionally, no statistically significant association was found between sleep duration and stage at diagnosis, nor with ER or HER2 receptor status.39 Hence, further research with larger sample sizes are needed to confirm these findings.
The most abundant chronotype in our recruited breast cancer patient was the moderately morning chronotype, followed by the definitely morning type, the neither type. The least abundant were the moderately evening and definitely evening chronotypes. This distribution of chronotypes is different from that reported by Hansen and Lassen who found that subjects with an evening chronotype or a neither chronotype had a greater risk of breast cancer compared to those with morning chronotype.28 This difference can be explained by the cultural background of the women recruited in the current research study, most of whom were married housewives, and who had to take care of their children and house duties, obligations which usually occurred during the daytime, with evening and nighttime periods being reserved for rest and sleep. The same distribution of chronotypes was also observed in a study conducted by Ramin et al, in which participants reported being neither morning nor evening chronotypes had a 27% increased risk of breast cancer when compared to definite morning types.52 None of the other chronotypes were significantly associated with breast cancer risk. A statistically significant difference was found between the chronotypes in relation to the breast cancer pathology, especially the estrogen and the HER/neu receptors status, indicating that the chronotype might play a role in the aggressiveness of breast cancer. These observations should be carefully interpreted however due to the small sample size. In the current study, scores on the MEQ correlated with serum melatonin levels, where the morning chronotype subjects tended to have high MEQ scores; among these subjects, less melatonin was secreted during daytime and correspondingly more was secreted during the nighttime hours. These results are in harmony with Madokoro et al, who found that a higher MES scale score correlated with melatonin ratio.44 Another study measured the nocturnal melatonin levels in blood hourly and assessed the circadian type of each participant; it was found that circadian type was strongly related to the melatonin acrophase and that morning types experienced a more rapid decline in melatonin levels after the peak relative to evening types.25
This study presented unique findings related to associating the screening of the psychometric profile with pathological features of breast cancer and serum melatonin levels. Also, most of the studied women were housewives; thus, the findings can be totally attributed to their illness and not related to shiftwork status that has been abundantly discussed as a major risk for melatonin level dysregulation in breast cancer patients. The present study showed that a strong association existed between melatonin secretions and the sleep profile of BC patients. Additionally, it was found that the pathological type of BC was linked to the severity of psychological symptoms and sleep complaints. A limitation of the study was the small sample size, which prevented firm conclusions regarding premenopausal and postmenopausal women. Additionally, it was not possible to confirm whether this psychometric profile obtained in this study can be considered a reliable risk factor for early screening and detection of cases. This study nevertheless provides some clinical clues for practicing physicians who offer health services to BC patients; based upon the results obtained it is suggested that screening for psychological symptoms during and after treatment of BC is a must. In patients with complaints of insomnia and depressive symptoms, the administration of melatonin might be useful for reducing such symptoms. Detection of melatonin receptor levels in the cancerous tissue might help in selecting patients who are targets of chronotherapies and those who might need adjustment of the rhythm of melatonin secretion either behaviorally or pharmacologically.
The current work has some limitations that need to be addressed. The restriction of recruitment to only patients with a first-time cancer diagnosis made it difficult to obtain a larger sample. For this reason, it was not possible to assess the types or level of melatonin receptors in the cancerous breast tissue. Additionally, more samples of melatonin were needed to detect the actual circadian rhythm of the hormone. A 24-h plasma melatonin profile would have provided accurate measures of circadian phase, duration, and amplitude of melatonin secretion. Comparing the obtained results with a result of a control group who did not have surgery or with those who did not have breast cancer would have been of greater value to the current work.
Despite these limitations, the study findings showed that melatonin secretion is dysregulated in subjects with breast cancer and that removal of the cancerous tissue by surgical procedures helped to some extent in restoring melatonin to normal nocturnal levels. The study further showed that the pathological subtype of cancer was linked to the severity of depressive and insomnia symptoms, and the presence of a tendency towards daytime sleepiness. Further work and cohort studies are needed to determine whether these processes could be diagnostically predictive for the development of breast cancer. If this is confirmed then screening for psychological difficulties, particularly depressive symptoms, or disrupted sleep could be useful as a routine checkup for women at different age groups during their regular health visits.
Acknowledgments
Authors appreciate the efforts of Dr John Zaki (Lecturer of Engineering-Faculty of Engineering at Mansoura University) for his help in conducting the logistic regression analysis of this study. We also appreciate the time and effort given by the participants of the study and the Nursing staff at Mansoura University Oncology center for their help in withdrawing the blood samples and taking care of the included patients during and after the study.
Funding
Nevin FW Zaki, Yomna M Sabri, Omar Farouk, Amany Abdelfatah and John Zaki funded the chemicals and lab kits needed from their own personal resources.
Disclosure
Mr Seithikurippu R Pandi-Perumal reports non-financial support from Somnogen Canada Inc. and personal fees from Springer, during the conduct of the study, although these financial statements had no role in relation to the current research. The authors report no other conflicts of interest.
References
- 1.Ahmed AE, Fatani A, Al-Harbi A, et al. Validation of the Arabic version of the epworth sleepiness scale. J Epidemiol Glob Health. 2014;4(4):297–302. doi: 10.1016/j.jegh.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alansari BM. Internal consistency of an Arabic adaptation of the beck depression inventory-II with college students in eighteen Arab countries. Social Behav Personality. 2006;34(4):425–430. doi: 10.2224/sbp.2006.34.4.425 [DOI] [Google Scholar]
- 3.Alonso-Gonzalez C, Menendez-Menendez J, Gonzalez-Gonzalez A, Gonzalez A, Cos S, Martinez-Campa C. Melatonin enhances the apoptotic effects and modulates the changes in gene expression induced by docetaxel in MCF7 human breast cancer cells. Int J Oncol. 2018;52(2):560–570. doi: 10.3892/ijo.2017.4213 [DOI] [PubMed] [Google Scholar]
- 4.Assad T. Sleep Disorders,Diagnosis and Management. Cairo-Egypt: eTrac; 2009. [Google Scholar]
- 5.BaHammam AS, Almistehi W, Albatli A, AlShaya S. Distribution of chronotypes in a large sample of young adult Saudis. Ann Saudi Med. 2011;31(2):183–186. doi: 10.4103/0256-4947.78207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartsch C, Bartsch H, Bellmann O, Lippert TH. Depression of serum melatonin in patients with primary breast cancer is not due to an increased peripheral metabolism. Cancer. 1991;67(6):1681–1684. doi: 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 7.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 8.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257–264. doi: 10.1016/j.smrv.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 9.Bojková B, Kajo K, Kisková T, et al. Metformin and melatonin inhibit DMBA-induced mammary tumorigenesis in rats fed a high-fat diet. Anticancer Drugs. 2018;29(2):128–135. [DOI] [PubMed] [Google Scholar]
- 10.Bondy S, Campbell A. Mechanisms underlying tumor suppressive properties of melatonin. Int J Mol Sci. 2018;19(8):2205. doi: 10.3390/ijms19082205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown SB, Hankinson SE, Eliassen AH, et al. Urinary melatonin concentration and the risk of breast cancer in nurses’ health study II. Am J Epidemiol. 2015;181(3):155–162. doi: 10.1093/aje/kwu261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brzezinski A. “Melatonin replacement therapy” for postmenopausal women: is it justified?”. Menopause. 1998;5(1):60–64. [PubMed] [Google Scholar]
- 13.Budhrani PH, Lengacher CA, Kip K, Tofthagen C, Jim H. An integrative review of subjective and objective measures of sleep disturbances in breast cancer survivors. Clin J Oncol Nurs. 2015;19(2):185–91. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso F, Spence D, Mertz S, et al. Global analysis of advanced/metastatic breast cancer: decade report (2005–2015). Breast. 2018;39:131–138. doi: 10.1016/j.breast.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 15.Chen WY, Giobbie-Hurder A, Gantman K, et al. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 2014;145(2):381–388. doi: 10.1007/s10549-014-2944-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407 [DOI] [PubMed] [Google Scholar]
- 18.de Almeida EA, Di Mascio P, Harumi T, et al. Measurement of melatonin in body fluids: standards, protocols and procedures. Childs Nerv Syst. 2011;27(6):879–891. doi: 10.1007/s00381-010-1278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 20.Dogliotti L, Berruti A, Buniva T, et al. Melatonin and human cancer. J Steroid Biochem Mol Biol. 1990;37(6):983–987. doi: 10.1016/0960-0760(90)90454-S [DOI] [PubMed] [Google Scholar]
- 21.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91(5):1824–1828. doi: 10.1073/pnas.91.5.1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang H-F, Miao N-F, Chen C-D, Sithole T, Chung M-H. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J Cancer. 2015;6(11):1140. doi: 10.7150/jca.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Prev Biomarkers. 2004;13(4):667–672. [PubMed] [Google Scholar]
- 24.Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(S3):43–46. doi: 10.7314/APJCP.2016.17.S3.43 [DOI] [PubMed] [Google Scholar]
- 25.Gibertini M, Graham C, Cook MR. Self-report of circadian type reflects the phase of the melatonin rhythm. Biol Psychol. 1999;50(1):19–33. doi: 10.1016/S0301-0511(98)00049-0 [DOI] [PubMed] [Google Scholar]
- 26.Goncalves Ndo N, Colombo J, Lopes JR, et al. Effect of melatonin in epithelial mesenchymal transition markers and invasive properties of breast cancer stem cells of canine and human cell lines. PLoS One. 2016;11(3):e0150407. doi: 10.1371/journal.pone.0150407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez A, Alvarez-Garcia V, Martinez-Campa C, et al. In vivo inhibition of the estrogen sulfatase enzyme and growth of DMBA-induced mammary tumors by melatonin. Curr Cancer Drug Targets. 2010;10(3):279–286. doi: 10.2174/156800910791190201 [DOI] [PubMed] [Google Scholar]
- 28.Hansen J, Lassen CF. Nested case–control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69(8):551–556. doi: 10.1136/oemed-2011-100240 [DOI] [PubMed] [Google Scholar]
- 29.Hansen MV, Madsen MT, Hageman I, et al. The effect of MELatOnin on depression, anxietY, cognitive function and sleep disturbances in patients with breast cancer. The MELODY trial: protocol for a randomised, placebo-controlled, double-blinded trial. BMJ Open. 2012;2(1):e000647. doi: 10.1136/bmjopen-2011-000647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho S-Y, Rohan KJ, Parent J, Tager FA, McKinley PS. A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J Pain Symptom Manage. 2015;49(4):707–715. doi: 10.1016/j.jpainsymman.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 32.Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971. doi: 10.1155/2014/437971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jablonska K, Pula B, Zemla A, et al. Expression of melatonin receptor MT 1 in cells of human invasive ductal breast carcinoma. J Pineal Res. 2013;54(3):334–345. doi: 10.1111/jpi.12032 [DOI] [PubMed] [Google Scholar]
- 34.Jardim-Perassi BV, Lourenco MR, Doho GM, et al. Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anticancer Agents Med Chem. 2016;16(3):347–358. doi: 10.2174/1871520615666150511094201 [DOI] [PubMed] [Google Scholar]
- 35.Jehan S, Jean-Louis G, Zizi F, et al. Sleep, melatonin, and the menopausal transition: what are the links? Sleep Sci. 2017;10(1):11. doi: 10.5935/1984-0063.20170003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 37.Kärkelä J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand. 2002;46(1):30–36. doi: 10.1034/j.1399-6576.2002.460106.x [DOI] [PubMed] [Google Scholar]
- 38.Kelleher FC, Rao A, Maguire A. Circadian molecular clocks and cancer. Cancer Lett. 2014;342(1):9–18. doi: 10.1016/j.canlet.2013.09.040 [DOI] [PubMed] [Google Scholar]
- 39.Khawaja A, Rao S, Li L, Thompson CL. Sleep duration and breast cancer phenotype. J Cancer Epidemiol. 2013;2013:467927. doi: 10.1155/2013/467927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubatka P, Zubor P, Busselberg D, et al. Melatonin and breast cancer: evidences from preclinical and human studies. Crit Rev Oncol Hematol. 2018;122:133–143. doi: 10.1016/j.critrevonc.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 41.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267–1269. doi: 10.1126/science.7434030 [DOI] [PubMed] [Google Scholar]
- 42.Lissoni P, Paolorossi F, Barni S, et al. Correlation between changes in prolactin and melatonin serum levels after radical mastectomy. Tumori J. 1987;73(3):263–267. doi: 10.1177/030089168707300309 [DOI] [PubMed] [Google Scholar]
- 43.Maass SW, Roorda C, Berendsen AJ, Verhaak PF, de Bock GH. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: a systematic review. Maturitas. 2015;82(1):100–108. doi: 10.1016/j.maturitas.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 44.MADOKORO S, NAKAGAWA H, MISAKI K, IHARA H, ITO T, ISAKI K. Nocturnal melatonin profiles before and one year after beginning shift‐work. Psychiatry Clin Neurosci. 1997;51(1):17–22. doi: 10.1111/j.1440-1819.1997.tb02360.x [DOI] [PubMed] [Google Scholar]
- 45.Middleton B. Measurement of melatonin and 6-sulphatoxymelatonin In: Hormone Assays in Biological Fluids. Humana Press; 2013:171–199. [DOI] [PubMed] [Google Scholar]
- 46.Mockova K, Mnichova M, Kubatka P, Bojkova B, Ahlers I, Ahlersova E. Mammary carcinogenesis induced in Wistar: hanrats by the combination of ionizing radiation and dimethylbenz(a)anthracene: prevention with melatonin. Neoplasma. 2000;47(4):227–229. [PubMed] [Google Scholar]
- 47.Natale V, Esposito MJ, Martoni M, Fabbri M. Validity of the reduced version of the Morningness–eveningness Questionnaire. Sleep Biol Rhythms. 2006;4(1):72–74. doi: 10.1111/sbr.2006.4.issue-1 [DOI] [Google Scholar]
- 48.Nofech–Mozes S, Vella E, Dhesy–Thind S, Hanna W. Guideline on hormone receptor testing in breast cancer. Evidence Based Ser. 2011;22. [DOI] [PubMed] [Google Scholar]
- 49.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: university of rochester cancer center–community clinical oncology program. J Clin Oncol. 2010;28(2):292. doi: 10.1200/JCO.2009.22.5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandi‐Perumal SR, Srinivasan V, Maestroni G, Cardinali D, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS J. 2006;273(13):2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x [DOI] [PubMed] [Google Scholar]
- 51.Pizzorno JE. Textbook of Natural Medicine. Elsevier Health Sciences; 2013. [Google Scholar]
- 52.Ramin C, Devore EE, Pierre-Paul J, Duffy JF, Hankinson SE, Schernhammer ES. Chronotype and breast cancer risk in a cohort of US nurses. Chronobiol Int. 2013;30(9):1181–1186. doi: 10.3109/07420528.2013.809359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeves KW, Okereke OI, Qian J, Tamimi RM, Eliassen AH, Hankinson SE. Depression, antidepressant use, and breast cancer risk in pre-and postmenopausal women: A prospective cohort study. Cancer Epidemiol Prev Biomarkers. 2018;27(3):306–314. doi: 10.1158/1055-9965.EPI-17-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reszka E, Przybek M, Muurlink O, Peplonska B. Circadian gene variants and breast cancer. Cancer Lett. 2017;390:137–145. doi: 10.1016/j.canlet.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 55.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. doi: 10.1002/pon.860 [DOI] [PubMed] [Google Scholar]
- 56.Schernhammer ES, Hankinson SE. Urinary melatonin levels and postmenopausal breast cancer risk in the nurses’ health study cohort. Cancer Epidemiol Biomarkers Prev. 2009;18(1):74–79. doi: 10.1158/1055-9965.EPI-08-0637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen A, Opdahl S, Strand LB, Vatten LJ, Laugsand LE, Janszky I. Insomnia and the risk of breast cancer: the HUNT study. Psychosom Med. 2017;79(4):461–468. doi: 10.1097/PSY.0000000000000417 [DOI] [PubMed] [Google Scholar]
- 58.Smith RA, DeSantis CE. Breast cancer epidemiology. Breast Imaging. 2018. [Google Scholar]
- 59.Srinivasan V, Pandi-Perumal SR, Brzezinski A, Bhatnagar KP, Cardinali DP. Melatonin, immune function and cancer. Recent Pat Endocr Metab Immune Drug Discov. 2011;5(2):109–123. doi: 10.2174/187221411799015408 [DOI] [PubMed] [Google Scholar]
- 60.Srinivasan V, Pandi-Perumal SR, Trakht I, et al. Pathophysiology of depression: role of sleep and the melatonergic system. Psychiatry Res. 2009;165(3):201–214. doi: 10.1016/j.psychres.2007.11.020 [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther. 2008;7(3):189–203. doi: 10.1177/1534735408322846 [DOI] [PubMed] [Google Scholar]
- 62.Suleiman KH, Yates BC. Translating the insomnia severity index into Arabic. J Nurs Scholarsh. 2011;43(1):49–53. doi: 10.1111/jnu.2011.43.issue-1 [DOI] [PubMed] [Google Scholar]
- 63.Talib WH, Saleh S. Propionibacterium acnes augments antitumor, anti-angiogenesis and immunomodulatory effects of melatonin on breast cancer implanted in mice. PLoS One. 2015;10(4):e0124384. doi: 10.1371/journal.pone.0124384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamarkin L, Danforth D, Lichter A, et al. Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science. 1982;216(4549):1003–1005. doi: 10.1126/science.7079745 [DOI] [PubMed] [Google Scholar]
- 65.Toffol E, Kalleinen N, Haukka J, Vakkuri O, Partonen T, Polo-Kantola P. Melatonin in perimenopausal and postmenopausal women: associations with mood, sleep, climacteric symptoms, and quality of life. Menopause. 2014;21(5):493–500. doi: 10.1097/GME.0b013e3182a6c8f3 [DOI] [PubMed] [Google Scholar]
- 66.Vaughn CB, Freudenheim JL, Nie J, et al. Sleep and breast cancer in the Western New York Exposures and Breast Cancer (WEB) study. J Clin Sleep Med. 2018;14(1):81–86. doi: 10.5664/jcsm.6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viswanathan AN, Schernhammer ES. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281(1):1–7. doi: 10.1016/j.canlet.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whisenant M, Wong B, Mitchell SA, Beck SL, Mooney K. Trajectories of depressed mood and anxiety during chemotherapy for breast cancer. Cancer Nurs. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chu LW, John EM, Yang B, et al. Measuring serum melatonin in postmenopausal women: implications for epidemiologic studies and breast cancer studies. PLoS One. 2018;13(4):e0195666. doi: 10.1371/journal.pone.0195666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Beck depression inventory-II. San Antonio.1996;78(2):490-498. doi: 10.1207/s15327752jpa6703_13 [DOI] [Google Scholar]
- 71.Adan, A., & Almirall, H. Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Personality and Individual differences. 1991;12(3):241-253 [Google Scholar]
- 72.Manz, B., Seidel, A., Alexander, H., Vollrath, L., Wagner, B., Zimmermann, G., ... & Pollow, K. Development and validation of a radioimmunoassay for serum melatonin. Clinical Chemistry and Laboratory Medicine. 1989;27(10):797-802. doi: 10.1515/cclm.1989.27.10.797 [DOI] [PubMed] [Google Scholar]
- 73.Hsing, A. W., Meyer, T. E., Niwa, S., Quraishi, S. M., & Chu, L. W. Measuring serum melatonin in epidemiologic studies. Cancer Epidemiology and Prevention Biomarkers. 2010;19(4):932-937. doi: 10.1158/1055-9965.EPI-10-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White AJ, Weinberg CR, Park YM, et al. Sleep characteristics, light at night and breast cancer risk in a prospective cohort. Int J Cancer. 2017;141(11):2204–2214. doi: 10.1002/ijc.30920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao Q, Signorello LB, Brinton LA, Cohen SS, Blot WJ, Matthews CE. Sleep duration and breast cancer risk among black and white women. Sleep Med. 2016;20:25–29. doi: 10.1016/j.sleep.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]