Alloimmunization to red blood cell (RBC) antigens remains an important and clinically significant problem. About 3% of general, hospital-based patients possess an underlying alloantibody (Tormey et al, 2008), with higher rates observed in chronically transfused groups (Tormey & Hendrickson, 2015). An important goal of transfusion and haematology communities has been to identify characteristics that may predispose an individual to developing RBC alloantibodies. Once identified, such patients could be transfused in ways that avoid provoking an alloimmune response. However, there have been few clues indicating what may constitute a ‘responder’ in the context of RBC transfusion and alloimmunization (Gehrie & Tormey, 2014; Ryder et al, 2014).
We read with great interest the report by Fasano et al (2015) implicating inflammation as a factor that contributes to the alloimmune response among patients with sickle cell disease (SCD). Since that publication, at least one other study has linked inflammation to RBC alloimmunization in SCD (Telen et al, 2015). Given these and past studies examining the effects of inflammation on alloantibody development in general patient groups (Ramsey & Smietana, 1995; Yazer et al, 2009; Papay et al, 2012), we undertook an investigation to examine the relationship between chronic autoimmune illnesses and RBC alloimmunization. In comparison to previous reports, our study is unique in that it was performed at a U.S. Veterans hospital that has an overwhelmingly male patient population lacking hereditary haemoglobin disorders (Tormey et al, 2008). As such, and in comparison to previous investigations, we were able to examine the effects of inflammation on primarily transfusion-associated (rather than pregnancy-induced) alloantibody development in a non-SCD population with diverse autoimmune disorders.
The transfusion and medical records of alloimmunized patients on file at VA Connecticut Healthcare System (West Haven, CT, USA) were retrospectively reviewed to include all subjects who underwent type and screen testing between 1961 and 2015. For alloimmunized patients (n = 220), the following information was collected: gender, race/ethnicity, number and specificity of alloantibodies reactive at 37°C and/or anti-human globulin phase, and the presence of a chronic, autoimmune inflammatory disorder (with the specific diagnosis noted as applicable). In addition, the transfusion and medical records for 250 randomly selected patients undergoing RBC transfusion were similarly reviewed to establish the transfusion rate among individuals with chronic autoimmune disorders. The chi-square test with Yates’ correction for continuity was used for statistical comparisons, with P-values <0·05 considered significant.
Among the 220 alloimmunized patients, 15·9% (35/220) were found to have an underlying chronic autoimmune disorder (Table I). Overall, patients with an autoimmune disorder formed 50 alloantibodies, with anti-K and -D most frequently identified (Fig 1A). These chronically inflamed subjects formed 1·43 antibodies/patient, a rate that was not significantly different than historical data at our facility (577 antibodies among 443 historical controls; 1·30 antibodies/control; P = 0·772; Tormey et al, 2008). Of 250 randomly selected controls undergoing transfusion, 8·4% (21/250) were found to have an underlying chronic autoimmune disorder. The proportion of alloimmunized patients with an autoimmune disorder was significantly higher than the proportion of control/transfused patients with an autoimmune disorder (0·19 vs. 0·09, respectively; P = 0·012, Fig 1B).
Table I.
Characteristics of alloimmunized study patients.
| n | |
|---|---|
| Gender | |
| Male | 30 |
| Female | 5 |
| Race/Ethnicity* | |
| White, non-Hispanic | 32 |
| White, Hispanic | 1 |
| Black, non-Hispanic | 1 |
| Unspecified, non-Hispanic | 1 |
| Types of autoimmune disorders† | |
| Inflammatory bowel disease | 9 |
| Psoriasis/psoriatic arthritis | 9 |
| Rheumatoid arthritis | 7 |
| Polymyalgia rheumatica | 4 |
| Others | 13 |
Race/ethnicity information was extracted from demographic data within the electronic medical record. Patients receiving care at our facility self-identify race and ethnicity at the time of their enrollment. Race/ethnicity data were therefore unconfirmed by the study authors. The majority of alloimmunized patients self-identified as white, non-Hispanic (91·4%, 32/35).
As some patients had >1 autoimmune disorder, the total number of associated disease states was 42. For those patients with inflammatory bowel disease (IBD, n = 9), 5 were diagnosed with ulcerative colitis, 4 with Crohn’s disease, and 1 with IBD not further classifiable. Disorders falling under the ‘Others’ category included: bullous pemphigoid (n = 2), pernicious anaemia (n = 2), ankylosing spondylitis (n = 1), antiphosholipid syndrome (n = 1), celiac disease (n = 1), immune-mediated glomerulonephritis (n = 1), inflammatory/autoimmune idiopathic pulmonary fibrosis (n = 1), sarcoidosis (n = 1), scleroderma (n = 1), Sjogren syndrome (n = 1) and systemic lupus erythematosus (n = 1).
Fig 1.
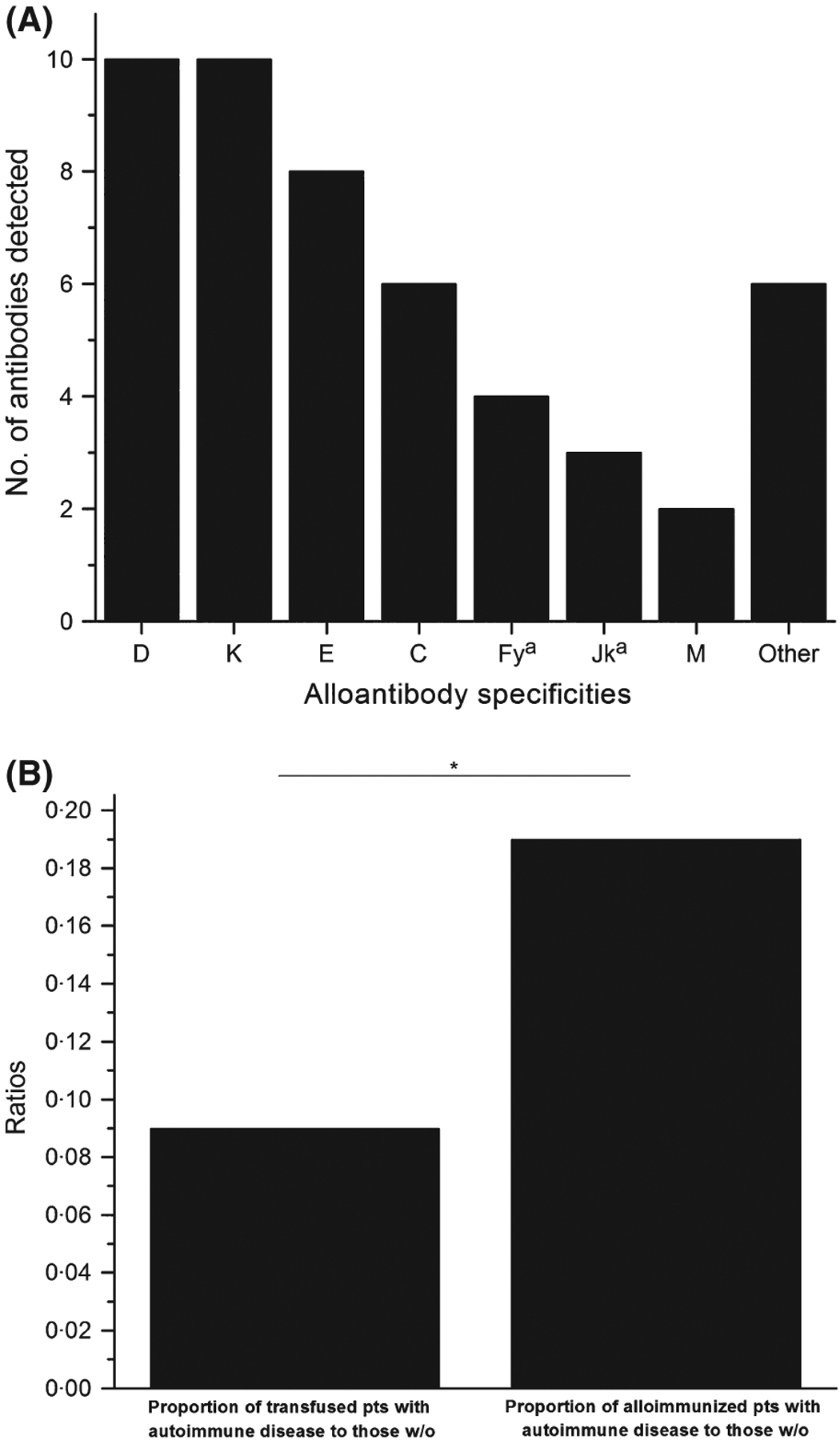
(A) The number and specificity of alloantibodies detected among patients with autoimmune disease. Because some patients made >1 alloantibody, the total number of antibodies detected was 50 (1·4 antibodies/alloimmunized patient). Alloantibodies falling under the ‘Others’ category included: anti-c, - S, -P1, -Lea, -Kna, and -McCa (all n = 1). One unspecified alloantibody reactive at 37°C and anti-human globulin phase was also detected in this cohort (detected in the patient with anti-P1), but this alloantibody is not reflected in the figure above as the definitive specificity could not be identified during the retrospective review. Only 4·0% of alloantibodies (2/50; anti-Kna and -McCa) were considered uncommon and both of these antibodies were detected in the same patient. The determination of uncommon antibody specificities was made by comparing our results to those generated from large studies of general, hospital-based alloimmunized patients (Ramsey & Smietana, 1995; Tormey et al, 2008; Gehrie & Tormey, 2014). (B) The proportion of transfused patients with a chronic, autoimmune disease versus the proportion of alloimmunized patients with a chronic, autoimmune disease. Of randomly selected patients who underwent transfusion, 8·4% (21/250) had an underlying chronic autoimmune disorder. By comparison, 15·9% (35/220) of alloimmunized patients were noted to have an autoimmune disease. The ratio of alloimmunized patients with an autoimmune disease to those without (0·19) was significantly different than the ratio of transfused patients with an autoimmune disease to those without (0·09; P = 0 012; the asterisk (*) indicates a significant difference between the proportions of compared patients). pts = patients; w/o = without.
Patients with autoimmune diseases comprised a large percentage of total alloimmunized subjects and, as a group, appeared to be immunized at higher rates than they are transfused at our facility. These findings, which are also consistent with results from murine models (Hendrickson et al, 2006), suggest that inflammation is a risk factor for alloantibody development in general patient populations. Moreover, history of an autoimmune disease may serve as a marker of a potential antibody ‘responder’ in the context of RBC transfusion. The fact that our inflamed, alloimmunized cohort was primarily white/non-Hispanic decreases the likelihood that recipient Rh variant status contributed to the observed high alloimmunization rate (Tormey & Hendrickson, 2015).
Notably, whilst the chronically inflamed patients in our study were highly alloimmunized, rates of multiple antibodies per patient were not significantly different than controls, nor did we find a higher incidence of ‘uncommon’ antibodies [both of which have been reported previously by Ramsey and Smietana (1995)]. A major difference between our study and that of Ramsey and Smietana (1995) is that their findings were exclusively in women, while our results were in a predominantly male group. This may indicate that the effects of inflammation on pregnancy-associated alloimmunization are somewhat different to those associated with transfusion, or alternatively, that there are gender differences in the degree of responsiveness to blood group antigens in the setting of inflammation.
Our study is not without limitations. Perhaps foremost among these is identifying what constitutes an ‘inflammatory disorder.’ We elected to include patients with chronic diseases associated with well-defined autoantibodies and/or long-standing autoimmune conditions that are treated with immunosuppression. Such a strategy allowed capture of a diverse population of patients with autoimmune diseases. It is also important to note that we were not capable of objectively assessing the degree of inflammation at the time of alloantibody development. In order to minimize this shortcoming, we examined patients with chronic autoimmune disorders under the rationale that there should be persistent, on-going inflammation in such a study group. We also did not collect data on immunosuppressive therapies; it is plausible that such medications may mitigate RBC alloantibody formation. Although we utilized randomly selected controls to establish a transfusion rate of patients with chronic autoimmune disorders, the potential impact of past transfusion burden on alloimmunization is another important variable to consider and was not a factor we could assess in this study. Finally, this investigation was retrospective and patient numbers were limited. Therefore, a larger, prospective study would be helpful to confirm the link between chronic inflammation and RBC alloimmunization.
In summary, our study of a primarily male, non-SCD, transfused cohort is in agreement with recent reports in humans and animal models indicating inflammation as a possible risk factor for RBC alloimmunization. Precautionary interventions (e.g., phenotypic matching for K, E, and C antigens, which represented ~50% of alloantibodies in this study) may be useful for patients with inflammatory disorders. Future investigations exploring the mechanistic links between inflammation and RBC alloimmunization are also warranted.
Footnotes
Conflicts of interest
The authors (ABR, JEH, CAT) report no relevant financial conflicts of interest.
References
- Fasano RM, Booth GS, Miles M, Du L, Koyama T, Meier ER & Luban NL (2015) Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. British Journal of Haematology, 168, 291–300. [DOI] [PubMed] [Google Scholar]
- Gehrie EA & Tormey CA (2014) The influence of clinical and biological factors on transfusion-associated non-ABO antigen alloimmunization: responders, hyper-responders, and non-responders. Transfusion Medicine and Hemotherapy, 41, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD & Zimring JC (2006) Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion, 46, 1526–1536. [DOI] [PubMed] [Google Scholar]
- Papay P, Hackner K, Vogelsang H, Novacek G, Primas C, Reinisch W, Eser A, Mikulits A, Mayr WR & Körmöczi GF (2012) High risk of transfusion-induced alloimmunization of patients with inflammatory bowel disease. American Journal of Medicine, 125, 717.e1–717.e8. [DOI] [PubMed] [Google Scholar]
- Ramsey G & Smietana SJ (1995) Multiple or uncommon red blood cell alloantibodies in women: association with autoimmune disease. Transfusion, 35, 582–586. [DOI] [PubMed] [Google Scholar]
- Ryder AB, Zimring JC & Hendrickson JE (2014) Factors influencing RBC alloimmunization: lessons learned from murine models. Transfusion Medicine and Hemotherapy, 41, 406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telen MJ, Afenyi-Annan A, Garrett ME, Combs MR, Orringer EP & Ashley-Koch AE (2015) Alloimmunization in sickle cell disease: changing antibody specificities and association with chronic pain and decreased survival. Transfusion, 55, 1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormey CA & Hendrickson JE (2015) Routine non-ABO blood group antigen genotyping in sickle cell disease: the new frontier in pretransfusion testing? Transfusion, 55, 1374–1377. [DOI] [PubMed] [Google Scholar]
- Tormey CA, Fisk J & Stack G (2008) Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion, 48, 2069–2076. [DOI] [PubMed] [Google Scholar]
- Yazer MH, Triulzi DJ, Shaz B, Kraus T & Zimring JC (2009) Does a febrile reaction to platelets predispose recipient to red blood cell alloimmunization? Transfusion, 49, 1070–1075. [DOI] [PubMed] [Google Scholar]


