Abstract
The aim of this study was to evaluate the effect of growth hormone (GH) deficiency in primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of young mice. Ovaries from six-month-old GH-deficient Ames Dwarf (df/df) and Normal (N/df) mice were used. The number of primordial follicles was higher in df/df mice (p = 0.0026). Also, df/df mice had a lower number of primary (p = 0.023), secondary (p = 0.0052) and tertiary (p = 0.019) follicles. These findings indicate a slower rate of primordial follicle activation in df/df mice. Female df/df mice had decreased γH2AX foci intensity in oocytes of primordial (p = 0.015) and primary (p = 0.0004) follicles compared to N/df mice. Also, df/df mice had reduced γH2AX intensity in granulosa cells of primordial (p = 0.0002) and primary (p < 0.0001) follicles. Overall, this indicate to us that df/df mice accumulate less DNA damage in the ovarian reserve compared to N/df mice. Additionally, macrophage infiltration was also reduced in ovaries of df/df mice compared to N/df mice (p = 0.033). Interestingly, df/df mice had a reduced number of granulosa cells around primordial (p = 0.0024) and primary (p = 0.007) follicles compared to N/df mice. Also, df/df mice had a small diameter of primordial follicle nuclei (p = 0.0093), secondary follicle oocyte (p = 0.046) and tertiary follicle (p = 0.012). This points to the role of granulosa cell proliferation and oocyte growth for primordial follicle activation. The current study points to the role of the GH/IGF-I axis in extending lifespan of reproductive health, along with maintenance of oocyte DNA integrity and reduced ovarian inflammation.
Keywords: Growth hormone, Insulin-like growth factor-1, ovarian reserve, DNA damage, macrophages
1. Introduction
A progressive decline and consequent depletion of the ovarian primordial follicle reserve is the main determinant of the onset of menopause in women (Faddy et al. 1992). Along with the reduction in follicle number, the quality of the remaining oocytes also decreases with age (Faddy et al. 1992; Hirshfield 1994). It is well known that the age-related decline in women’s fertility is paralleled by a decrease in the ovarian reserve and an increase in chromosomally abnormal conceptions (McGowan and McCoy 2013). The underlying cause of the increased risk to pregnancy outcome with age is commonly attributed to the exponential rise in oocyte chromosome mis-segregations, leading to karyotypic imbalances and aneuploidies in the offspring (Woods et al. 2017). Many of these karyotypic abnormalities result in spontaneous abortion in the first trimester and thus contribute to the high frequency of pregnancy loss during this time window (Ashaat and Husseiny 2012). However, advanced maternal age also poses an increased risk of serious complications that manifest in later pregnancy, these include miscarriage, late fetal and perinatal death, stillbirth, preterm and extreme preterm birth, low birth weight, placenta praevia and pre-eclampsia (Abel et al. 2002; Jacobsson et al. 2004).
In mammalian ovaries, the majority of oocytes are meiotically arrested and surrounded by a layer of flattened somatic granulosa cells, a structure known as primordial follicle (Hirshfield 1991; Adhikari and Liu 2009). In order to ensure the proper reproductive lifespan, most of the oocytes are maintained in this quiescent state within primordial follicles (Zhang et al. 2014). Granulosa cells play a fundamental role in initiating the growth of primordial follicles (Zhang et al. 2014). Several molecular networks mediate the interaction between somatic cells and germ cells in controlling the development of dormant mammalian oocytes (Laplante and Sabatini 2012; Zhang et al. 2014). Mammalian target of rapamycin (mTOR), for example, is stimulated by nutrition, oxygen, energy and several growth factors (Laplante and Sabatini 2012). Granulosa cells produced mTOR stimulates Kit which in the oocyte stimulates the phosphoinositide 3-kinase (Pi3k)/protein kinase B (Akt1) pathway and the transcription factor Forkhead Box O3a (FoxO3a) phosphorylation resulting in primordial follicle activation (John et al. 2008; Reddy et al. 2008; Zhang et al. 2014). In mice this pathway is already active after birth and has a role in promoting activation of primordial follicles as early as post-natal day 10 (Zhang et al. 2014). Mathematical models and in vivo evaluation of the primordial reserve suggest a severe reduction in the number of primordial follicles in the reserve from birth to puberty that can play a key role in reproductive longevity of mice (Bristol-Gould et al. 2006).
Ames Dwarf mice (df/df) have a defective Prophet of Pit1 (Prop1) gene, which impairs anterior pituitary gland development, resulting in deficient growth hormone (GH) secretion (Sornson et al. 1996). As a result, df/df mice have very low levels of circulating insulin-like growth factor I (IGF-I), are significantly smaller, have delayed puberty and live around 30 to 65% longer than normal littermates (N/df) (Chandrashekar and Bartke 1993; Brown-Borg et al. 1996). The GH/IGF-I axis also has a central role in ovarian function (Bachelot et al. 2002; Zaczek et al. 2002; Chandrashekar et al. 2004). We have shown that older GH-deficient df/df mice have a larger ovarian primordial follicle reserve than N/df mice and that treatment with exogenous GH activated the primordial follicle reserve in both df/df and N/df mice (Saccon et al. 2017). This indicates that increased longevity is correlated with increased reproductive lifespan. Furthermore, mice overexpressing bovine GH (bGH) had a smaller ovarian reserve than normal mice (Saccon et al. 2017), showing that the GH/IGF-I axis has a central role in ovarian function. Despite the evidences that df/df mice have increase ovarian reserve little is known about the quality of these oocytes.
Several factors can influence oocyte quality during aging. The adverse effects of DNA damage caused by environmental factors on somatic cells have been extensively reported (Saini and Gordenin 2018). Mammalian oocytes enclosed in primordial follicles remain arrested in prophase I of meiosis up to several decades in some species, including humans (Mehlmann 2005; Chiang et al. 2012). This long dormancy period increases the chances of accumulating DNA damage, as shown in female mice and human, both of which, accumulate double strand breaks (DSBs) in primordial follicle oocytes with aging (Titus et al. 2013). Extensive damage occurring throughout meiosis can have serious consequences if an adequate cellular response is not activated, and may result in infertility or development of defective embryos that are unable to result in full-term pregnancy (Kirk and Lyon 1982; Adriaens et al. 2009). In response to DSBs, kinases are known to phosphorylate histone 2Ax (γH2AX) on serine 139 (Burma et al. 2001; Bakkenist and Kastan 2003). Such post-transcriptional modification at the lesion site provides a platform for the ataxia-telangiectasia mutated (ATM)–mediated DNA damage signaling pathway to regulates the repair of DNA DSBs (Collins and Jones 2016). Oocytes are therefore prone to such damage as their lifetime for a single cell is very long and significant damage can accumulate, which may explain the fertility decline as animals become older.
Along with DNA damage, inflammation also increases with age and can negatively affect fertility (Bektas et al. 2018). Interleukin 1 deficient female mice have more primordial follicles and increased fertility than control females (Chen et al. 2018). We identified by RNASeq that old df/df mice have more than 150 down-regulated gene ontology terms related to the inflammatory/immune response compared to N/df mice (Schneider et al. 2017), pointing to a role of inflammation in reproductive senescence. Interestingly, transplant of young ovaries to older recipients in mice was associated to a reduced inflammation and increased longevity (Habermehl et al. 2019). Also, it is well known that adipose tissue and blood levels of pro-inflammatory cytokines, interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) are reduced in df/df mice (Berryman et al. 2004; Wang et al. 2006). This reduced inflammation is believed to be one of the main reasons for the increased longevity of df/df mice (Masternak and Bartke 2012). In this context, the presence of inflammatory cells within the ovaries is an important parameter, as macrophages are the most abundant immune cells within the ovary (Turner et al. 2011). A high-fat diet results in increased primordial follicle depletion and increased ovarian macrophage infiltration in mice (Skaznik-Wikiel et al. 2016). Inflammation results in oxidative stress, reducing cellular antioxidant capacity, leading to overproduction of free radicals that react with cell membrane fatty acids and proteins impairing their function permanently (Durackova 2010). In addition, excess free radicals increase DNA damage, a predisposing factor for several age-related disorders (Khansari et al. 2009).
Based on this evidence, the aim of this study was to evaluate the primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of young df/df and N/df mice.
2. Materials and methods:
2.1. Animals
For these experiments, six-month-old female Ames dwarf (df/df, n = 6) and normal littermate control (N/df, n = 6) mice were used. Mice were maintained under temperature (22 ± 2°C) and humidity (40-60%) controlled conditions. All experiments were approved by the Institutional Animal Care and Use Committee from Southern Illinois University School of Medicine, IL, USA.
2.2. Tissue collection and processing
The mice were anesthetized after fasting for 12 h and the pair of ovaries was collected, dissected from surrounding adipose tissue and placed in 10% formalin buffered solution. After that, the ovaries were removed from the formalin solution, dehydrated in alcohol, cleared in xylene and embedded individually in Paraplast Plus (Sigma Chemical Company®, St. Louis, MO, USA). One ovary of the pair was then serially sectioned at 5 μm using a semi-automated rotary microtome (RM2245, Leica Biosystems, San Diego, CA, USA). The sections started at the beginning of the visible area of the ovarian surface until the end of the structure, and every sixth section was selected and placed on a standard histological slide for staining and counting. Intermediate sections were randomly selected for immunofluorescence analysis using slides impregnated with 3% organosilane (Sigma Chemical Company®, St. Louis, MO, USA) in ethanol.
2.3. Follicular classification, counting and measurement
The slides were dried at 56° C for 24 h, stained with hematoxylin-eosin, and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, MO, USA). Images of the ovarian sections were captured with a digital camera coupled to a microscope (Nikon Eclipse E200, Nikon Corporation, Japan) using the 10 and 40 objectives, assisted by the software Moticam 5.0 (Motic, Hong Kong, China). Nucleus, oocytes and follicles diameters were measured using Motic Images Plus 2.0® (Motic, Hong Kong, China). For each mouse 3 random follicles/category (primordial, primary, secondary) were evaluated for diameter measurement (n=6 mice, total of 18/group/category). Only follicles containing an oocyte with clearly visible nucleus were counted in each slide. Follicle classification was based on Myers et al, 2004. Follicles were classified as primordial (oocyte surrounded by a single layer of flattened granulosa cells, Figure 1A), primary (oocyte surrounded by a single layer of cuboidal granulosa cells, Figure 1B), secondary (oocyte surrounded by two or more layers of cuboid granulosa cells without a visible antrum, Figure 1C) and tertiary (follicles with a clearly defined antral space and multiple layers of granulosa cells around the oocyte, Figure 1D) (Myers et al. 2004). To estimate actual follicle quantity the number of follicles in each category was multiplied by six to account for the section sampling and by two to account for the fact that only one ovary of the pair was used for sampling (Saccon et al. 2017).
Figure 1.

Morphological classification of follicles. They were classified according to (Myers et al. 2004) as (A) primordial (oocyte surrounded by a single layer of flattened granulosa cells), (B) primary (oocyte surrounded by a single layer of cuboidal granulosa cells), (C) secondary (oocyte surrounded by two or more layers of cuboid granulosa cells without a visible antrum) and (D) tertiary (follicles with a clearly defined antral space and multiple layers of granulosa cells around the oocyte). Black arrows indicate the follicle.
2.4. Immunofluorescence
For immunofluorescence analysis, the ovarian samples were deparaffinized with xylene and rehydrated with graded alcohols. The primary monoclonal antibodies were obtained from Abeam (Abeam Pic, Cambrigde, UK) and diluted in 1.5% BSA solution. The anti-gamma H2AX (γH2AX) phospo SI39 antibody (abl 1174), to indicate DNA damage (Titus et al. 2013) and anti-CD68 antibody (ab955), to indicate the presence of macrophage (Skaznik-Wikiel et al. 2016) were used at a final dilution of 1:500. The blockage of the endogenous peroxidase activity was achieved with hydrogen peroxide blocking solution, while the antigen recovery was performed in humid heat, during 3 min after the boiling point, in citrate solution at pH 6.0. Non-specific background staining was reduced by covering the tissue sections that received protein block with BSA and goat serum. Thereafter, slides were incubated overnight with the primary antibody in a humid chamber at 4°C. The slides with anti-γH2AX and anti-CD68 antibodies were incubated for 1 hour with secondary Alexa Fluor® 488 (abl50113) and Hoechst (ab228550) for nuclei staining during 15 min. The slides were mounted with a drop of mounting medium under coverslips. The images of the follicles were captured by confocal microscope (Olympus FluoView™ 1000). Fluorescence intensity quantification for γH2AX, measured as pixel intensity in the nuclei area (Anderson et al. 2013), and macrophage counting, measured as number of identified cells/slide, was performed by image analysis software Image J® (Schneider et al. 2012). γH2AX was measured in 3 follicles for each category (primordial, primary, secondary) per mice (n=6 mice; total of 18 follicles/category/group). Macrophage counting was performed as a total number of macrophages per sections of ovary. Each mouse had a total of 4 random sections from the center of the ovary counted for macrophage infiltration. The number of granulosa cells was also counted in 3 follicles/ category/mouse, using the Hoechst (ab228550) for nuclei staining of surrounding granulosa cells (Braw-Tal and Yossefi 1997).
2.5. Statistical analyzes
All statistical analyzes were performed using GraphPad Prism 6 (GraphPad Inc., La Jolla, CA, USA). T-test was performed for comparing the number of follicles. Size of follicles and oocytes, granulosa cell numbers and immunostaining between df/df and N/df mice were evaluated by the GLM procedure in SAS (SAS University Edition, Cary, NC, USA) to correct for multiple measures for the same mice. A p-value lower than 0.05 was considered as significant.
3. Results
The number of primordial follicles was higher in df/df mice (p = 0.0026, Figure 2A). Also, df/df mice had a lower number of primary (p = 0.023, Figure 2B), secondary (p = 0.0052, Figure 2C) and tertiary (p = 0.019, Figure 2D) follicles, suggesting a reduced activation rate of primordial follicles in 6-month-old df/df mice. The number of total follicles was not different between both groups (p > 0.05).
Figure 2.

Comparison between the number of (A) primordial, (B) primary, (C) secondary and (D) tertiary follicles in N/df (n = 6) and df/df (n = 6) mice. Different letters indicate significant differences (p < 0.05). Data presented as media ± SEM.
Female df/df mice had healthier primordial and primary follicles, as indicated by the lower quantity of DSBs, measured by lower fluorescence intensity for γH2AX foci in oocytes from primordial (p = 0.015, Figure 3A) and primary (p = 0.0004, Figure 3B) follicles compared to N/df mice. Also, df/df mice had reduced γH2AX intensity in granulosa cells of primordial (p = 0.0002, Figure 3C) and primary (p < 0.0001, Figure 3D) follicles. Representative images of immunofluorescence of anti-γH2AX are shown in Figure 4. Df/df mice also had ovarian tissue with reduced macrophage infiltration compared to N/df mice (p = 0.033, Figure 5C).
Figure 3.
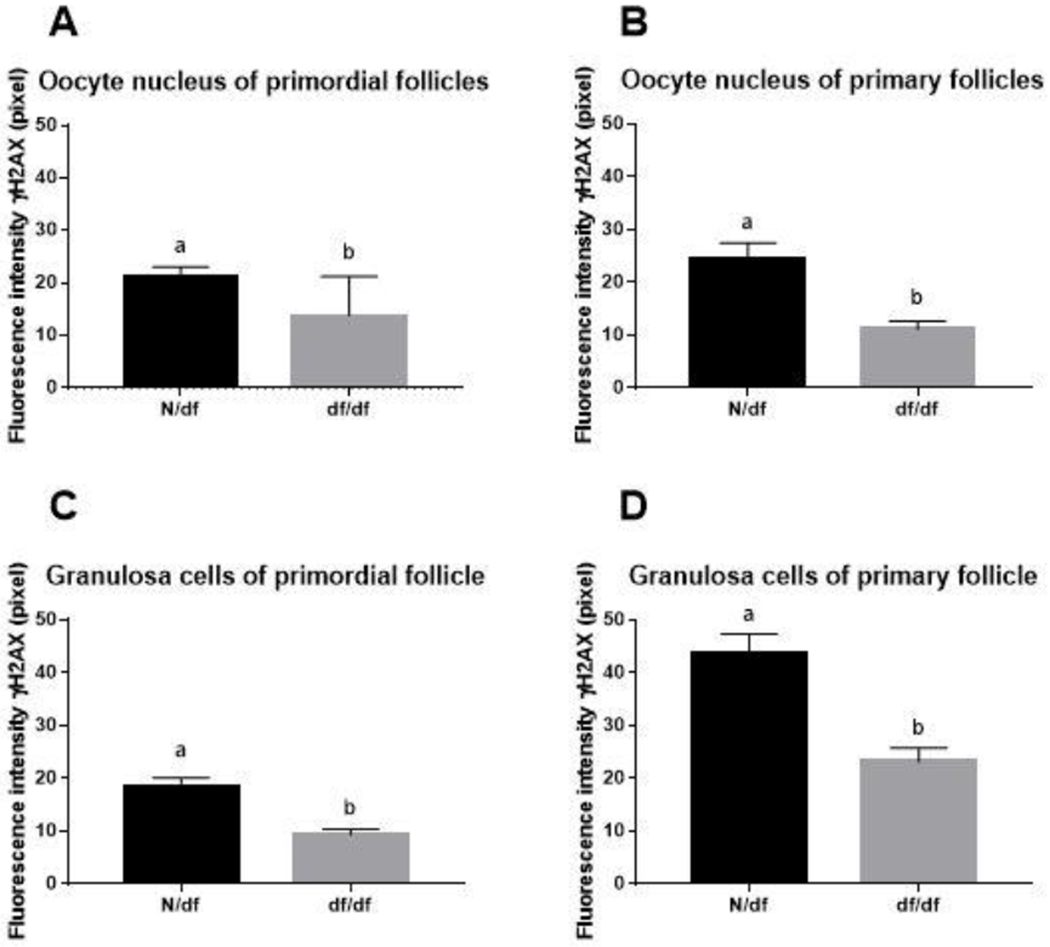
Fluorescence intensity in pixels of γH2AX immunostaining in oocytes nucleus of primordial follicle (n = 18, A), oocyte nucleus of primary follicle (n = 18, B) and granulosa cell nuclei from primordial (n = 18, C) and primary follicles (n = 18, D) of N/df and df/df mice. Different letters indicate significant differences (p < 0.05). Data presented as media ± SEM.
Figure 4.

Representative images of immunofluorescence of anti-γH2AX in primordial and primary follicles of N/df and df/df mice. Blue images represent Hoechst (staining genetic material) and greens represents anti-γH2AX staining γH2AX protein. Merged images are showed as both Hoechst and anti-γH2AX staining combination. Red arrows represent some granulosa cells and yellow arrow represents oocyte nucleus.
Figure 5.
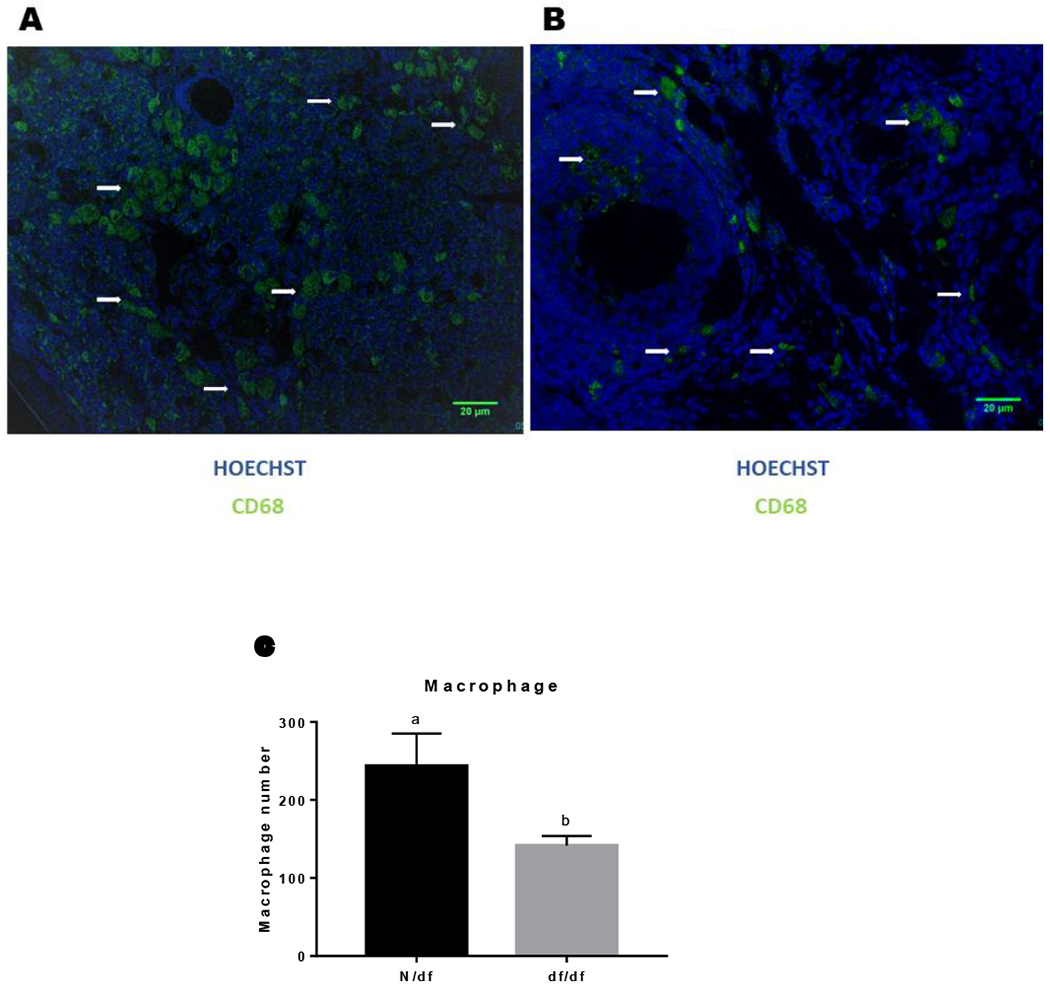
Representative immunofluorescence of anti-CD68 marker of N/df (A) and df/df (B) mice and macrophage number (C) on ovarian section of N/df (n = 18) and df/df (n = 18) mice. White arrows indicate macrophages staining. Different letters indicate significant differences (p < 0.05).
We also evaluated morphological follicle parameters. Female df/df mice had a lower number of granulosa cells in primordial (p = 0.0028, Table 1) and primary (p = 0.021, Table 1) follicles compared to N/df mice. Also, df/df mice had a smaller diameter of primordial follicle nuclei (p = 770.0028, Table 1), secondary follicle oocyte (p = 0.009, Table 1) and tertiary follicle (p = 0.046, Table 1). No difference was found for other parameters such as nuclei diameter of primary, secondary and tertiary follicles, oocyte diameter of primordial, primary and tertiary follicles and follicle diameter of primordial, primary and secondary follicles (p > 0.05).
Table 1.
Number of granulosa cells and diameters of nucleus, oocyte and follicle of primordial, primary, secondary and tertiary follicles of N/df and df/df mice.
| Parameter | N/df | df/df | p-Value |
|---|---|---|---|
| Granulosa cells number (SEM) | (n=18) | (n=18) | |
| Primordial Follicles | 8.0 (±0.4) | 5.3 (±0.5) | 0.0028 |
| Primary Follicles | 17.9 (±2.7) | 12.3 (±0.7) | 0.021 |
| Diameters (μm) (SEM) | |||
| Primordial Follicle | |||
| Nuclei diameter (μm) | 3.1 (±0.2) | 2.6 (±0.1) | 0.009 |
| Oocyte diameter (μm) | 6.3 (±0.4) | 5.6 (±0.3) | 0.31 |
| Follicle diameter (μm) | 9.5 (±1.0) | 8.0 (±0.3) | 0.17 |
| Primary Follicle | |||
| Nuclei diameter (μm) | 3.5 (±0.2) | 3.3 (±0.2) | 0.61 |
| Oocyte diameter (μm) | 9.3 (±0.9) | 9.5 (±0.8) | 0.88 |
| Follicle diameter (μm) | 16.3 (±1.4) | 14.9 (±1.2) | 0.47 |
| Secondary Follicle | |||
| Nuclei diameter (μm) | 6.6 (±0.6) | 5.9 (±0.3) | 0.29 |
| Oocyte diameter (μm) | 25.2 (±1.3) | 21.8 (±1.0) | 0.046 |
| Follicle diameter (μm) | 53.5 (±3.5) | 54.0 (±5.0) | 0.93 |
| Tertiary Follicle | |||
| Nuclei diameter (μm) | 5.56 (±0.4) | 5.2 (±0.8) | 0.65 |
| Oocyte diameter (μm) | 30.6 (±1.0) | 29.8 (±1.3) | 0.43 |
| Follicle diameter (μm) | 180.6 (±8.0) | 147.0 (±9.7) | 0.012 |
4. Discussion
The current study points to the role of the GH/IGF-I axis in keeping the primordial follicle reserve in the ovary, along with maintenance of oocyte DNA integrity and reduced ovarian macrophage infiltration. GH-deficient df/df mice are known for being smaller-sized and living longer and healthier life than normal littermate controls (Chandrashekar and Bartke 1993; Brown-Borg et al. 1996). Our present study confirms that these mice have a larger ovarian primordial follicle reserve than N/df already at six months of age. Dwarf mice had almost four times more primordial follicles than N/df mice. This increased primordial ovarian reserve can increase the ovarian lifespan. A previous study from our group demonstrated that df/df mice had a larger ovarian reserve than N/df mice at 18-months-old, with twice as many primordial follicles (Saccon et al. 2017). Also, evaluating 12-month-old df/df mice, we observed three times more primordial follicles (Schneider et al. 2017). This suggests that the difference in the ovarian primordial reserve between df/df and N counterparts decreased with aging, as the reserve becomes smaller. Additionally, we observed that df/df mice had a lower number of primary, secondary and tertiary follicles, which further supports the notion that df/df mice are activating a lower number of follicles, explaining the origins of its prolonged reserve. This means in the absence of GH less primordial follicles are activated to primary follicles and are kept in the reserve for longer periods, therefore, extending the ovarian lifespan. Young female GH receptor gene knockout (GHRKO) mice, which also have lower serum IGF-I and increased longevity (Bartke 1966), also have an increased number of primordial follicles (Slot et al. 2006), and still have ovarian activity at older ages, when age matched normal mice have completely exhausted the ovarian follicular reserve (Sluczanowska-Glabowska et al. 2012). These GHRKO females were able to successfully deliver pups when normal littermates already exhausted reproductive function (Selesniemi et al. 2008; Sluczanowska-Glabowska et al. 2012). Increasing the number of primordial follicles in the ovary can improve the reproductive lifespan. This was demonstrated in calorie restricted mice, which had a increased primordial follicle reserve and delivered pups much later in life when control mice stopped reproducing (Selesniemi et al. 2008).
Activation of the Pi3k/Akt1 signaling pathway and the phosphorylation of downstream effector FoxO3a is an essential step for the activation of the primordial follicle and its irreversible growth (Castrillon et al. 2003; John et al. 2007). Hyperphosphorylation of FoxO3a results in its nuclear exclusion, culminating in the global activation of primordial follicles and premature ovarian failure (Castrillon et al. 2003). Therefore, the presence of FoxO3a in its non-phosphorylated form is crucial to maintaining the primordial follicles in their quiescent state (John et al. 2008). Primordial follicles begin to grow irreversibly when oocyte FoxO3a is phosphorylated by stimulus from the surrounding granulosa cells (John et al. 2007; John et al. 2008; Zhang et al. 2014). We previously demonstrated a higher level of non-phosphorylated FoxO3a in primordial follicles oocytes of 18-month-old df/df mice (Saccon et al. 2017). In addition, GH treatment reduced the FoxO3a protein level, while it increased its phosphorylated form in primordial follicles oocytes (Saccon et al. 2017). Another study observed that pFoxO3a protein levels in primordial/primary follicles were lower in 12-month-old df/df compared to N/df mice, despite no difference in total FoxO3a protein levels (Schneider et al. 2014), which suggests an age-dependent FoxO3a regulation in the ovary. Besides our findings, FOXO3a is a central transcription factor that mediates multiple physiological and pathological processes by inducing transcription of target genes involved in apoptosis (Chen et al. 2018), proliferation (McClelland Descalzo et al. 2016), cell cycle progression (McGowan and McCoy 2013), survival (Joseph et al. 2016) and DNA damage (Fluteau et al. 2015). It also respond to several cellular stresses such as UV irradiation (Wang et al. 2012) and oxidative stress (Lim et al. 2017; Wang et al. 2017). Besides, FOXO3a is strongly associated with human longevity (Willcox et al. 2008). Therefore, this previous evidence shows us that GH/IGF-I-dependent activation of the FoxO3a pathways could be one of the main mechanisms involved in increased reproductive longevity in df/df mice and it is effects can be in 6-month-old df/df mice.
DNA damage in oocytes and granulosa cell can be caused by various endogenous or exogenous stresses, including oxidative stress, telomere erosion, oncogenic mutations, genotoxic stress, and metabolic stress (Lopez-Otin et al. 2013). DNA damage is a challenge that all somatic and germline cells are exposed during their lifetime (Jackson and Bartek 2009). Cells interpret DSBs as a serious threat to their integrity, activating DNA damage checkpoints (DDC) and reacting with a DNA damage response resulting in cell cycle arrest as a downstream effect, allowing activation of repair mechanisms (Bartek and Lukas 2007). DSBs trigger a DDC response by activating two major kinases, i.e. ATM and ataxia telangiectasia and Rad3-related (ATR) (Reinhardt and Yaffe 2009; Smith et al. 2010). Taking advantage of the cell cycle arrest, the cell can then repair the damaged DNA. At the DNA damage site, ATM gives rise to the phosphorylated form of the H2AX histone, which acts as a catalyst for the recruitment of the necessary checkpoint and repair factors (Burgoyne et al. 2007). Snell dwarf mice, endocrinologically identical to df/df mice, have increased cellular DNA repair capacity and upregulation of several DNA repair-related genes compared to normal littermates (Podlutsky et al. 2017). Our data, therefore, prompts several considerations on the relevance of DNA damage in df/df mice oocytes. Our study showed that df/df mice have a higher number of primordial follicles; therefore, more oocytes are kept longer in the ovary, considering also that these mice live 30-70% longer than N/df mice. Additionally, we observed that oocytes from primordial and primary follicles of df/df mice had fewer DSBs than those of N/df mice, indicating that df/df primordial and primary oocytes have a better quality. This is interesting, as it demonstrates that GH-deficiency not only preserves the ovarian primordial reserve for a longer period, but also oocytes accumulate less DNA damage. The impairment of DNA DSBs repair is associated with accelerated loss of ovarian follicular reserve and accumulation of DSBs in human and mouse oocytes (Titus et al. 2013). Other have also observed in monkeys that DSBs increase with age in granulosa cells (Zhang et al. 2015), also suggesting this damage is not confined to the oocyte as we observed. This suggests, that besides Fox03a activation, reduced DSBs in primordial follicle oocytes can be a mechanism of increased ovarian reserve in df/df mice. Moreover, the expression of Breast Cancer 1 protein (BRCA1) and other key genes in the ATM-pathway decline with age in human oocytes, associated with an increase in γH2AX foci (Titus et al. 2013), suggesting a relationship between DNA DSB repair and ovarian aging. The same decline in repair mechanisms was observed for monkey granulosa cells (Zhang et al. 2015). We previously observed a three-fold reduction in BRCA1 gene expression with aging in N mice, although its expression did not change with aging in df/df mice (Schneider et al. 2017). Therefore, it is suggested that maintenance of high levels of DNA protecting factors could be a key element for preserving the ovarian reserve for a longer period. The agreement between mouse and human data pinpoints impaired DNA DSBs repair as a universal mechanism of oocyte aging in mammals. However, further studies on DSB repair in oocytes of df/df mice are needed to establish a mechanistic link.
GH-deficient df/df mice exhibited reduced ovarian macrophage infiltration associated with reduced ovarian aging. The long-living df/df, GHRKO and calorie restricted mice have all been extensively characterized as having a reduced pro-inflammatory profile, which may represent one of the major mechanisms promoting increased insulin sensitivity and extended longevity in these mice (Masternak and Bartke 2012). Macrophages located in the ovaries, by secreting growth factors and/or cytokines, may play a synergistic role in stimulating cellular proliferation and follicle growth (Fukumatsu et al. 1992). Indeed, co-culture of rat granulosa cells with peritoneal macrophages results in proliferation of the granulosa cells (Fukumatsu et al. 1992). Some of the macrophage- derived factors that are known to impact follicular growth are hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), tumor necrosis factor (TNF) α and β, and IGF-I (Stirling et al. 1991; Spaczynski et al. 1999; Nakayama et al. 2003). Macrophages are present in atretic and well as healthy follicles (Turner et al. 2011). Ablation of ovarian macrophages disrupts vascular integrity indicating its pivotal role in ovarian function (Turner et al. 2011). On the other hand, prolonged exposure (both short-term and long-term) to a high-fat diet in young adult female mice reduced primordial follicle numbers, compromised fertility, produced higher systemic proinflammatory cytokine levels, and increased ovarian macrophage infiltration in the stroma, independent of obesity (Skaznik-Wikiel et al. 2016). Pro-inflammatory Interleukin 1 deficient female mice have more primordial follicles and increased fertility than control females (Chen et al. 2018). Also, LPS exposure in mice reduced the primordial follicle pool mediated by TLR4 (McClelland Descalzo et al. 2016), further indicating the role of inflammation on ovarian reserve depletion. Our ovarian transcriptome study showed that the top 150 down-regulated terms between old df/df and old N mice were related to the inflammatory/immune response (Schneider et al. 2017). It was observed that macrophage chemotaxis, macrophage activation and macrophage differentiation were also among the top down-regulated biological processes in old df/df compared to old N mice. Therefore, these findings point to an association between the depletion of the ovarian reserve and an increased ovarian pro-inflammatory status, which were both attenuated in the ovaries of aging df/df mice. Osteopetrotic mice have severely reduced numbers of mature macrophages due to a natural mutation in the CSF- 1 gene (Cohen et al. 2002). These mice have reduced numbers of ovarian macrophages, which may be either a cause or effect of the decreased follicle growth. Caloric restriction also reduced the numbers of macrophages surrounding preovulatory follicles in rats (Duggal et al. 2002). Taken together, this evidence provides an interesting link between ovarian longevity and macrophage population, although more functional studies are necessary.
In addition to reduced activation of primordial follicle numbers in df/df mice, the microenvironment within follicles and ovaries also could be influenced by GH/IGF-I deficiency. Particularly, pre-antral follicles granulosa cells are intimately connected with oocytes, and both play critical roles in oocyte growth and follicular development (Eppig et al. 1997; Su et al. 2009). Oocyte growth is accompanied by the active accumulation of mRNA, proteins, and lipids, and by modifications of chromatin configuration and DNA methylation status, and these molecular reactions require enough energy (Munakata et al. 2016). The number of granulosa cells in primordial follicles is associated with the energy sufficiency of oocytes (Munakata et al. 2016), which can affect oocyte and follicle growth. A complete set of molecular networks that mediate the interaction between granulosa cells and oocytes in controlling the development of dormant mammalian oocytes had been described. The model proposed by Zhang et al. (2014) has two key steps. The first step is the mTOR complex 1 (mTORC1) signaling in granulosa cells that acts as the key decision-making process regarding whether or not a primordial follicle will be activated (Zhang et al. 2014). The second step involves the tightly regulated communication channel from the granulosa cells to the oocytes via KITL-KIT to trigger the awakening of the oocyte through FoxO3a phosphorylation (Zhang et al. 2014). It is proposed that these processes ensure the progressive activation of a limited number of primordial follicles throughout the reproductive lifespan. Thus, it is likely that follicular activation is initiated by molecular and cellular changes in the granulosa cells that are followed by awakening of the dormant oocytes. Our study shows that df/df mice had fewer granulosa cells surrounding oocytes than N/df mice in both primordial and primary follicles, supporting the reduced activation of primordial follicle observed for df/df mice. Also, df/df mice had a smaller oocyte nuclei diameter for primordial follicles and oocyte diameter for secondary follicles. Growth of oocyte nuclei is associated with increased gene expression activity (Moore and Lintern-Moore 1974), indicating that the oocyte is awakening the machinery necessary for its growth and differentiation. We previously observed that oocytes from primordial and primary follicles of bGH mice had increased nuclei and oocyte diameter (Saccon et al. 2017), suggesting the opposite effect associated to increased primordial follicle activation. Overall this may be an indication that GH deficiency is associated with decreased granulosa cell proliferation, which leads to reduced primordial oocyte activation, resulting in a smaller nucleus.
5. Conclusion
In conclusion, the present study indicates a reduced primordial reserve in young long-living df/df mice in comparison to N/df mice. Oocytes from the primordial and primary follicles of df/df mice presented fewer DSBs than N/df mice, indicating that GH-deficiency not only increases the primordial reserve, but also the oocytes accumulate less DNA damage. Additionally, df/df mouse ovaries presented less macrophage infiltration. It is suggested that reduced DSBs along with a reduced number of granulosa cells surrounding the primordial oocyte are involved in the preservation of the ovarian reserve. Overall, these important observations confirm, that delayed aging phenotype in these unique long-living df/df mice are associated to beneficial characteristics of longevity in the female reproductive organs.
Highlights:
Growth hormone deficient Ames Dwarf mice have delayed ovarian aging
Decreased oocyte DNA damage on Ames Dwarf mice points to a better reproductive health
Reduced ovarian macrophage infiltration was observed in Ames dwarf mice
Reduced number of granulosa cells was associated to reduced primordial follicle activation
6. Acknowledgments
This work was supported by CAPES, CNPq, FAPERGS and NIH/NIA grants R15 AG059190, R03 AG059846 and R56 AG061414 (MMM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Abel EL, Kruger M and Burd L. 2002. Effects of maternal and paternal age on Caucasian and Native American preterm births and birth weights. Am J Perinatol. 19, 49–54. [DOI] [PubMed] [Google Scholar]
- Adhikari D and Liu K. 2009. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 30, 438–464. [DOI] [PubMed] [Google Scholar]
- Adriaens I, Smitz J and Jacquet P. 2009. The current knowledge on radiosensitivity of ovarian follicle development stages. Hum Reprod Update. 15, 359–377. [DOI] [PubMed] [Google Scholar]
- Anderson D, Andrais B, Mirzayans R, Siegbahn E, Fallone B and Warkentin B. 2013. Comparison of two methods for measuring γ-H2AX nuclear fluorescence as a marker of DNA damage in cultured human cells: applications for microbeam radiation therapy. Journal of Instrumentation. 8, C06008. [Google Scholar]
- Ashaat N and Husseiny A. 2012. Correlation between missed abortion and insertional translocation involving chromosomes 1 and 7. Iran J Reprod Med. 10, 15–22. [PMC free article] [PubMed] [Google Scholar]
- Bachelot A, Monget P, Imbert-Bollore P, Coshigano K, Kopchick JJ, Kelly PA and Binart N. 2002. Growth hormone is required for ovarian follicular growth. Endocrinology. 143, 4104–4112. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ and Kastan MB. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 421, 499–506. [DOI] [PubMed] [Google Scholar]
- Bartek J and Lukas J. 2007. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 19, 238–245. [DOI] [PubMed] [Google Scholar]
- Bartke A 1966. Reproduction of female dwarf mice treated with prolactin. J Reprod Fertil. 11, 203–206. [DOI] [PubMed] [Google Scholar]
- Bektas A, Schurman SH, Sen R and Ferrucci L. 2018. Aging, inflammation and the environment. Exp Gerontol. 105, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman DE, List EO, Coschigano KT, Behar K, Kim JK and Kopchick JJ. 2004. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 14, 309–318. [DOI] [PubMed] [Google Scholar]
- Braw-Tal R and Yossefi S. 1997. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil. 109, 165–171. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD and Woodruff TK. 2006. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 298, 149–154. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ and Bartke A. 1996. Dwarf mice and the ageing process. Nature. 384, 33. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK and Turner JM. 2007. The management of DNA doublestrand breaks in mitotic G2, and in mammalian meiosis viewed from a mitotic G2 perspective. Bioessays. 29, 974–986. [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A and Chen DJ. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 276, 42462–42467. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW and DePinho RA. 2003. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 301, 215–218. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V and Bartke A. 1993. Effects of age and endogenously secreted human growth hormone on the regulation of gonadotropin secretion in female and male transgenic mice expressing the human growth hormone gene. Endocrinology. 132, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Zaczek D and Bartke A. 2004. The consequences of altered somatotropic system on reproduction. Biol Reprod. 71, 17–27. [DOI] [PubMed] [Google Scholar]
- Chen YF, Pandey S, Day CH, Jiang AZ, Ho TJ, Chen RJ, Padma VV, Kuo WW and Huang CY. 2018. Synergistic effect of HIF-1alpha and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J Cell Physiol. 233, 3660–3671. [DOI] [PubMed] [Google Scholar]
- Chiang T, Schultz RM and Lampson MA. 2012. Meiotic origins of maternal age-related aneuploidy. Biol Reprod. 86, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PE, Zhu L, Nishimura K and Pollard JW. 2002. Colony-stimulating factor 1 regulation of neuroendocrine pathways that control gonadal function in mice. Endocrinology. 143, 1413–1422. [DOI] [PubMed] [Google Scholar]
- Collins JK and Jones KT. 2016. DNA damage responses in mammalian oocytes. Reproduction. 152, R15–22. [DOI] [PubMed] [Google Scholar]
- Duggal PS, Ryan NK, Van der Hoek KH, Ritter LJ, Armstrong DT, Magoffin DA and Norman RJ. 2002. Effects of leptin administration and feed restriction on thecal leucocytes in the preovulatory rat ovary and the effects of leptin on meiotic maturation, granulosa cell proliferation, steroid hormone and PGE2 release in cultured rat ovarian follicles. Reproduction. 123, 891–898. [PubMed] [Google Scholar]
- Durackova Z 2010. Some current insights into oxidative stress. Physiol Res. 59, 459–469. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Chesnel F, Hirao Y, O’Brien MJ, Pendola FL, Watanabe S and Wigglesworth K. 1997. Oocyte control of granulosa cell development: how and why. Hum Reprod. 12, 127–132. [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ and Nelson JF. 1992. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 7, 1342–1346. [DOI] [PubMed] [Google Scholar]
- Fluteau A, Ince PG, Minett T, Matthews FE, Brayne C, Garwood CJ, Ratcliffe LE, Morgan S, Heath PR, Shaw PJ, Wharton SB and Simpson JE. 2015. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci Lett. 609, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumatsu Y, Katabuchi H, Naito M, Takeya M, Takahashi K and Okamura H. 1992. Effect of macrophages on proliferation of granulosa cells in the ovary in rats. J Reprod Fertil. 96, 241–249. [DOI] [PubMed] [Google Scholar]
- Habermehl TL, Parkinson KC, Hubbard GB, Ikeno Y, Engelmeyer JI, Schumacher B and Mason JB. 2019. Extension of longevity and reduction of inflammation is ovarian-dependent, but germ cell-independent in post-reproductive female mice. Geroscience. 41, 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN 1991. Development of follicles in the mammalian ovary. Int Rev Cytol. 124, 43–101. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN 1994. Relationship between the supply of primordial follicles and the onset of follicular growth in rats. Biol Reprod. 50, 421–428. [DOI] [PubMed] [Google Scholar]
- Jackson SP and Bartek J. 2009. The DNA-damage response in human biology and disease. Nature. 461, 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson B, Ladfors L and Milsom I. 2004. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 104, 727–733. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ and Castrillon DH. 2008. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 321, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD and Castrillon DH. 2007. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 133, 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Ametepe ES, Haribabu N, Agbayani G, Krishnan L, Blais A and Sad S. 2016. Inhibition of ROS and upregulation of inflammatory cytokines by FoxO3a promotes survival against Salmonella typhimurium. Nat Commun. 7, 12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari N, Shakiba Y and Mahmoudi M. 2009. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 3, 73–80. [DOI] [PubMed] [Google Scholar]
- Kirk M and Lyon MF. 1982. Induction of congenital anomalies in offspring of female mice exposed to varying doses of X-rays. Mutat Res. 106, 73–83. [DOI] [PubMed] [Google Scholar]
- Laplante M and Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell. 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M and Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell. 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SW, Jin L, Luo K, Jin J, Shin YJ, Hong SY and Yang CW. 2017. Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 8, e2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M and Kroemer G. 2013. The hallmarks of aging. Cell. 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM and Bartke A. 2012. Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis. 2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland Descalzo DL, Satoorian TS, Walker LM, Sparks NR, Pulyanina PY and Zur Nieden NI. 2016. Glucose-Induced Oxidative Stress Reduces Proliferation in Embryonic Stem Cells via FOXO3A/beta-Catenin-Dependent Transcription of p21(cip1). Stem Cell Reports. 7, 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan SE and McCoy DM. 2013. Platelet-derived growth factor-A regulates lung fibroblast S-phase entry through p27(kip1) and FoxO3a. Respir Res. 14, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM 2005. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 130, 791–799. [DOI] [PubMed] [Google Scholar]
- Moore GP and Lintern-Moore S. 1974. A correlation between growth and RNA synthesis in the mouse oocyte. J Reprod Fertil. 39, 163–166. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Ichinose T, Ogawa K, Itami N, Tasaki H, Shirasuna K, Kuwayama T and Iwata H. 2016. Relationship between the number of cells surrounding oocytes and energy states of oocytes. Theriogenology. 86, 1789–1798.e1781. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ and Kerr JB. 2004. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 127, 569–580. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Manabe N, Inoue N, Matsui T and Miyamoto H. 2003. Changes in the expression of tumor necrosis factor (TNF) alpha, TNFalpha receptor (TNFR) 2, and TNFR-associated factor 2 in granulosa cells during atresia in pig ovaries. Biol Reprod. 68, 530–535. [DOI] [PubMed] [Google Scholar]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A and Ungvari Z. 2017. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 39, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I and Liu K. 2008. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 319, 611–613. [DOI] [PubMed] [Google Scholar]
- Reinhardt HC and Yaffe MB. 2009. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol. 21, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccon TD, Moreira F, Cruz LA, Mondadori RG, Fang Y, Barros CC, Spinel L, Bartke A, Masternak MM and Schneider A. 2017. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol Cell Endocrinol. 455, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini N and Gordenin DA. 2018. Somatic mutation load and spectra: A record of DNA damage and repair in healthy human cells. Environ Mol Mutagen. 59, 672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Matkovich SJ, Saccon T, Victoria B, Spinel L, Lavasani M, Bartke A, Golusinski P and Masternak MM. 2017. Ovarian transcriptome associated with reproductive senescence in the long-living Ames dwarf mice. Mol Cell Endocrinol. 439, 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Zhi X, Moreira F, Lucia T Jr., Mondadori RG and Masternak MM. 2014. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 7, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS and Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ and Tilly JL. 2008. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 7, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaznik-Wikiel ME, Swindle DC, Allshouse AA, Polotsky AJ and McManaman JL. 2016. High-Fat Diet Causes Subfertility and Compromised Ovarian Function Independent of Obesity in Mice. Biol Reprod. 94, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N and Teerds KJ. 2006. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 131, 525–532. [DOI] [PubMed] [Google Scholar]
- Sluczanowska-Glabowska S, Laszczynska M, Piotrowska K, Glabowski W, Kopchick JJ, Bartke A, Kucia M and Ratajczak MZ. 2012. Morphology of ovaries in laron dwarf mice, with low circulating plasma levels of insulin-like growth factor-1 (IGF-1), and in bovine GH-transgenic mice, with high circulating plasma levels of IGF-1. J Ovarian Res. 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Tho LM, Xu N and Gillespie DA. 2010. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 108, 73–112. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG and Rosenfeld MG. 1996. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 384, 327–333. [DOI] [PubMed] [Google Scholar]
- Spaczynski RZ, Arici A and Duleba AJ. 1999. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 61, 993–998. [DOI] [PubMed] [Google Scholar]
- Stirling D, Waterman MR and Simpson ER. 1991. Expression of mRNA encoding basic fibroblast growth factor (bFGF) in bovine corpora lutea and cultured luteal cells. J Reprod Fertil. 91, 1–8. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K and Eppig JJ. 2009. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 27, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatone C and Amicarelli F. 2013. The aging ovary--the poor granulosa cells. Fertil Steril. 99, 12–17. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S and Oktay K. 2013. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 5, 172ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EC, Hughes J, Wilson H, Clay M, Mylonas KJ, Kipari T, Duncan WC and Fraser HM. 2011. Conditional ablation of macrophages disrupts ovarian vasculature. Reproduction. 141, 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen WR and Xing D. 2012. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol. 227, 1168–1178. [DOI] [PubMed] [Google Scholar]
- Wang X, Meng L, Zhao L, Wang Z, Liu H, Liu G and Guan G. 2017. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res Clin Pract. 126, 172–181. [DOI] [PubMed] [Google Scholar]
- Wang Z, Al-Regaiey KA, Masternak MM and Bartke A. 2006. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 61, 323–331. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B and Curb JD. 2008. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 105, 13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L, Perez-Garcia V, Kieckbusch J, Wang X, DeMayo F, Colucci F and Hemberger M. 2017. Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat Commun. 8, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek D, Hammond J, Suen L, Wandji S, Service D, Bartke A, Chandrashekar V, Coschigano K and Kopchick J. 2002. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biol Reprod. 67, 1115–1124. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, Wu X, Keefe DL and Liu L. 2015. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet. 32, 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, Bosbach B, Brannstrom M and Liu K. 2014. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 24, 2501–2508. [DOI] [PubMed] [Google Scholar]


