Abstract
Objectives
To investigate the efficacy and safety of ixekizumab for up to 52 weeks in two phase 3 studies of patients with active radiographic axial spondyloarthritis (r-axSpA) who were biological disease-modifying antirheumatic drug (bDMARD)-naive (COAST-V) or tumour necrosis factor inhibitor (TNFi)-experienced (COAST-W).
Methods
Adults with active r-axSpA were randomised 1:1:1:1 (n=341) to 80 mg ixekizumab every 2 (IXE Q2W) or 4 weeks (IXE Q4W), placebo (PBO) or 40 mg adalimumab Q2W (ADA) in COAST-V and 1:1:1 (n=316) to IXE Q2W, IXE Q4W or PBO in COAST-W. At week 16, patients receiving ixekizumab continued their assigned treatment; patients receiving PBO or ADA were rerandomised 1:1 to IXE Q2W or IXE Q4W (PBO/IXE, ADA/IXE) through week 52.
Results
In COAST-V, Assessment of SpondyloArthritis international Society 40 (ASAS40) responses rates (intent-to-treat population, non-responder imputation) at weeks 16 and 52 were 48% and 53% (IXE Q4W); 52% and 51% (IXE Q2W); 36% and 51% (ADA/IXE); 19% and 47% (PBO/IXE). Corresponding ASAS40 response rates in COAST-W were 25% and 34% (IXE Q4W); 31% and 31% (IXE Q2W); 14% and 39% (PBO/IXE). Both ixekizumab regimens sustained improvements in disease activity, physical function, objective markers of inflammation, QoL, health status and overall function up to 52 weeks. Safety through 52 weeks of ixekizumab was consistent with safety through 16 weeks.
Conclusion
The significant efficacy demonstrated with ixekizumab at week 16 was sustained for up to 52 weeks in bDMARD-naive and TNFi-experienced patients. bDMARD-naive patients initially treated with ADA demonstrated further numerical improvements after switching to ixekizumab. Safety findings were consistent with the known safety profile of ixekizumab.
Trial registration number
Keywords: ankylosing spondylitis, DMARDs (biologic), spondyloarthritis
Key messages.
What is already known about this subject?
Ixekizumab was superior to placebo at week 16 for treating the signs and symptoms of active radiographic axial spondyloarthritis (r-axSpA) in two phase 3 trials in patients who were biological disease-modifying antirheumatic drug (bDMARD)-naive (COAST-V) or tumour necrosis factor inhibitor (TNFi)-experienced (prior inadequate response or intolerance to one or two TNFi; COAST-W).
What does this study add?
The significant improvements observed with ixekizumab at week 16 were sustained for up to 52 weeks in bDMARD-naive (COAST-V) and TNFi-experienced patients (COAST-W); results were similar between two ixekizumab regimens (80 mg every 2 or every 4 weeks) across endpoints. In COAST-V, bDMARD-naive patients who were initially treated with adalimumab for 16 weeks demonstrated further numerical improvements through week 52 after switching to ixekizumab.
COAST-W is the first phase 3 study of a bDMARD in an exclusively TNFi-experienced patient population and provides robust information on the long-term efficacy and safety of ixekizumab in this population.
Adverse events were consistent with the known safety profile of ixekizumab.
How might this impact on clinical practice or future developments?
The findings from COAST-V and COAST-W suggest that ixekizumab could be a longer-term treatment option for patients with axSpA, regardless of prior experience with TNFi. The additional numeric improvement in adalimumab-treated patients after switching to ixekizumab is of particular interest and deserves further exploration.
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory condition comprising non-radiographic axSpA and radiographic axSpA (r-axSpA). The latter, also known as ankylosing spondylitis (AS), is characterised by inflammatory back pain and radiographic evidence of damage to the sacroiliac joint.1 These manifestations, and peripheral musculoskeletal and extra-articular signs and symptoms, may contribute to limited mobility, progressive disability and decreased quality of life (QoL).2 3 Biological disease-modifying antirheumatic drugs (bDMARDs), including tumour necrosis factor inhibitors (TNFi)4 5 and an interleukin (IL)-17A antagonist,5 are recommended for managing patients with axSpA who do not respond to or tolerate non-steroidal anti-inflammatory drugs (NSAIDs). However, up to 40% of patients fail to achieve satisfactory disease control with TNFi,6 and treatment with TNFi may be contraindicated in other patients.7
The IL-17 signalling pathway plays a key role in the pathogenesis of axSpA.8 9 Ixekizumab, a high-affinity monoclonal antibody that selectively targets IL-17A, is approved for treating active psoriatic arthritis and moderate-to-severe plaque psoriasis and has demonstrated efficacy in two phase 3 trials in patients with r-axSpA who were bDMARD-naive (COAST-V) or TNFi-experienced (prior inadequate response or intolerance to TNFi; COAST-W).10 11 In both studies, ixekizumab resulted in significantly greater improvement versus placebo (PBO) at week 16 for measures of disease activity (including the primary endpoint of Assessment of SpondyloArthritis international Society 40 response (ASAS40)), function, QoL and spinal inflammation.
Here, we evaluated the sustainability of improvements observed at week 16 for treatment of r-axSpA with ixekizumab 80 mg every 4 or 2 weeks (IXE Q4W or IXE Q2W) up to week 52 in COAST-V and COAST-W. We also evaluated the safety of ixekizumab for up to 52 weeks, with a specific focus on overall safety, including events of special interest such as injection site reactions (ISRs) and candidiasis, and extra-articular manifestations such as inflammatory bowel disease (IBD), anterior uveitis (AU) and psoriasis.
Materials and methods
Study design
COAST-V10 and COAST-W11 are phase 3, multicentre, randomised, double-blind, active-controlled (COAST-V only) and PBO-controlled, 52-week trials, followed by an optional 2-year extension study.
All patients provided written informed consent.
Patient and public involvement
Patients were not involved in the design or conduct of the study, development of outcomes or dissemination of study results.
Patients
Patient eligibility criteria have been described previously.10 11 Patients were to be ≥18 years of age, have an established diagnosis of r-axSpA and meet ASAS criteria (with central reading of radiographic sacroiliitis).12 Patients in COAST-W were required to have discontinued one or two TNFi because of intolerance or inadequate response; COAST-V only included bDMARD-naive patients.
Treatment protocol
Study procedures for COAST-V and COAST-W have been described elsewhere.10 11 Patients in COAST-V were randomised 1:1:1:1 to PBO, adalimumab 40 mg (ADA) Q2W, IXE Q2W or IXE Q4W. ADA represents an active reference group; the study was not powered to test equivalence/non-inferiority of the active treatment groups to each other, including ixekizumab versus ADA. Patients in COAST-W were randomised 1:1:1 to PBO, IXE Q2W or IXE Q4W. In both trials, patients assigned ixekizumab were further randomised 1:1 to a 160 mg or 80 mg starting dose.
Patients completing week 16 entered a dose double-blind extended treatment period (ETP; weeks 16 to 52). During this period, patients originally randomised to PBO or ADA (COAST-V only) were rerandomised 1:1 to IXE Q2W or IXE Q4W (160 mg starting dose for patients switching from PBO, 80 mg starting dose for patients switching from ADA). Patients originally randomised to IXE Q2W or IXE Q4W continued these regimens.
Assessments
Efficacy
Efficacy assessments were made at weeks 20, 24, 28, 32, 36, 44 and 52 in the ETP, except where specified below.
Categorical efficacy endpoints assessed included the proportion of patients achieving ASAS40,13 ASAS20, ASAS partial remission, Ankylosing Spondylitis Disease Activity Score (ASDAS)14 low disease activity (score <2.1), ASDAS inactive disease (score <1.3), ASDAS clinically important improvement (≥1.1 change from baseline), ASDAS major improvement (≥2.0 change from baseline or reached a minimal ASDAS score of 0.6361) and ≥50% improvement in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50).15 Continuous endpoints included changes from baseline in ASDAS, BASDAI, Bath Ankylosing Spondylitis Functional Index (BASFI),16 Medical Outcomes Study Short Form 36 (SF-36) health survey Physical Component Score (PCS),17 ASAS Health Index (ASAS HI),18 19 Spondyloarthritis Research Consortium of Canada (SPARCC) MRI20 of the spine and sacroiliac joint (the latter in COAST-V only) and serum C reactive protein (CRP) concentrations. After week 16, SF-36 PCS and ASAS HI assessments were performed at weeks 36 and 52; MRI assessments were performed at week 16 in both studies and at week 52 in COAST-V only. MRI from baseline, week 16 and week 52 were read in a single campaign. Concomitant NSAID use and ASAS-NSAID21 scores were assessed.
Safety
Safety assessments included the evaluation of adverse events (AEs; per the Medical Dictionary for Regulatory Activities) and treatment-emergent antidrug antibodies (TE-ADAs).10 11 Cerebrocardiovascular events and suspected IBD were adjudicated by an independent clinical event committee. All IBD events were adjudicated by an external committee following EPIMAD criteria.22
At every visit, patients were evaluated for any symptoms of AU; AU events were confirmed by an ophthalmologist. Psoriasis and IBD were not proactively evaluated, but new onset or flares were recorded as AEs.
Statistical analysis
Efficacy analyses through 52 weeks were performed on the intent-to-treat population (ITT; IXE Q4W, IXE Q2W), which included all patients initially randomised to ixekizumab, and the ETP population, which included all patients who received ≥1 dose of ixekizumab during the ETP. Considering the consistent performance of the IXE Q4W and IXE Q2W regimens, data for patients in the ETP who were initially randomised to PBO or ADA were analysed as single groups (PBO/IXE or ADA/IXE), regardless of which ixekizumab dose they received during the ETP. Safety analyses were performed on the ETP population and on the all ixekizumab exposure safety population (IXE Q4W, IXE Q2W), which included all patients who received ≥1 dose of ixekizumab at any time during the 52-week study period.
No between-treatment group comparisons were made for ETP data. For primary analyses of the ITT and ETP populations, the most conservative approach was followed, where missing data were imputed using non-responder imputation (NRI) for categorical variables and modified baseline observation carried forward (mBOCF, a more stringent method of analysis than last observation carried forward) for continuous variables. For secondary analyses of ITT data, missing data were imputed using modified NRI for categorical variables and multiple imputation for continuous variables. ITT data were also analysed as observed. SF-36 PCS data are reported as t-scores, based on 2009 US general population norms.
Statistical analyses were performed using SAS V.9.2 or higher (SAS Institute).
Results
Patients
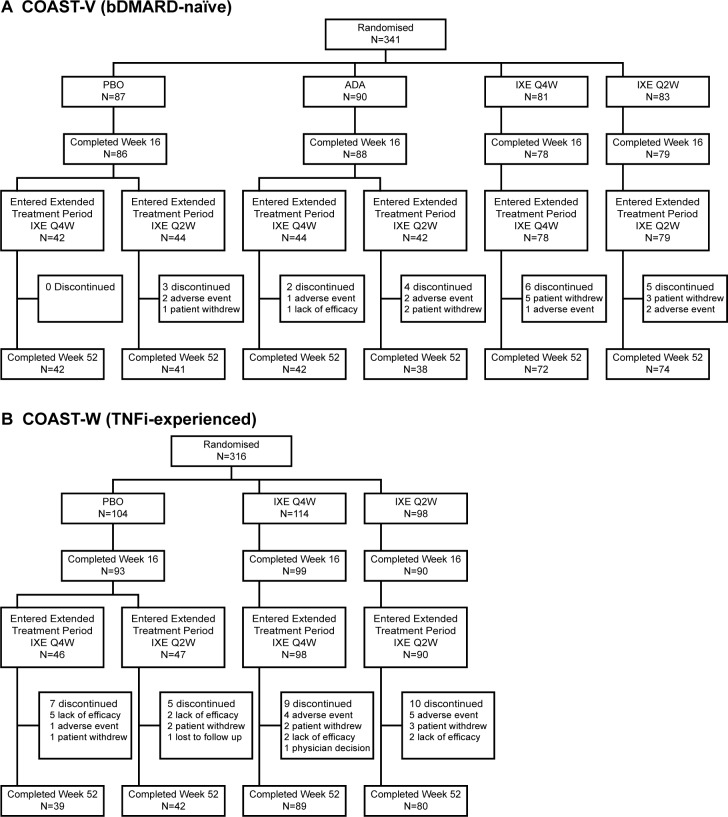
The majority of patients in COAST-V (309/329; 93.9%) and COAST-W (250/281; 89.0%) who entered the ETP completed week 52 (figure 1). Of the patients initially randomised to ixekizumab, 146/164 (89.0%) in COAST-V and 169/212 (79.7%) in COAST-W completed week 52. The most common reason for discontinuation was patient withdrawal (n=11; 3.3%) in COAST-V and lack of efficacy (n=11; 3.9%) in COAST-W.
Figure 1.
Patient disposition: (A) COAST-V; (B) COAST-W. ADA, adalimumab; bDMARD, biological disease-modifying antirheumatic drug; extended treatment period, dose double-blind extended treatment period; IXE Q4W, ixekizumab 80 mg every 4 weeks; IXE Q2W, ixekizumab 80 mg every 2 weeks; PBO, placebo; TNFi, tumour necrosis factor inhibitor.
Demographics and baseline clinical characteristics for the ETP populations were similar between treatment groups within each study (online supplementary table S1) and similar to those of the ITT populations.10 11 Baseline and historical peripheral/extra-articular manifestations of axSpA are summarised in online supplementary table S2.
annrheumdis-2019-216118supp001.pdf (17.5KB, pdf)
annrheumdis-2019-216118supp002.pdf (24.6KB, pdf)
Efficacy
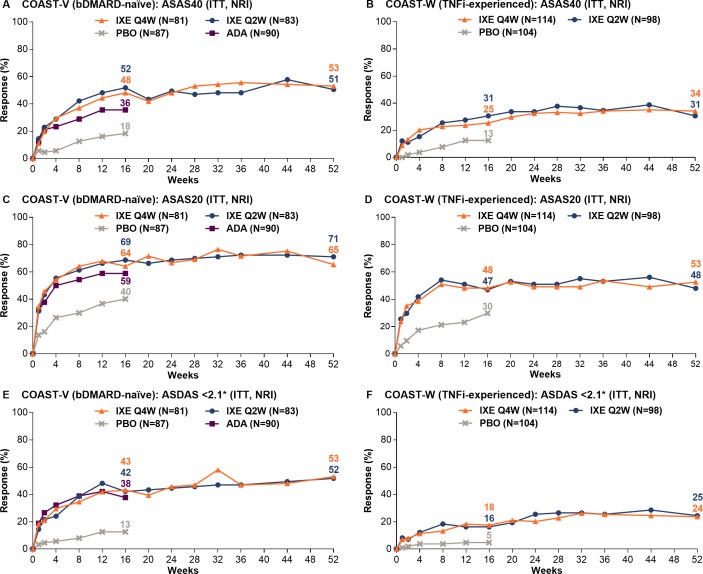
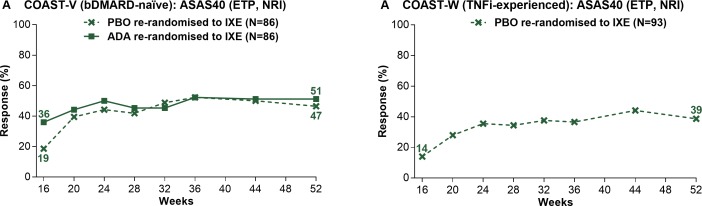
Among patients continuously treated with ixekizumab, week 16 ASAS40 response rates were sustained for up to 52 weeks (figure 2, table 1). Week 52 ASAS40 response rates were 53.1% (IXE Q4W) and 50.6% (IXE Q2W) in COAST-V and 34.2% (IXE Q4W) and 30.6% (IXE Q2W) in COAST-W. Patients randomised to PBO and rerandomised to ixekizumab at week 16 (PBO/IXE) showed rapid improvement in ASAS40 response rates after switching to ixekizumab (figure 3, table 2); week 52 response rates (46.5% in COAST-V, 38.7% in COAST-W) were numerically similar to those in patients initially randomised to ixekizumab. In COAST-V, patients randomised to ADA showed further numerical improvements in ASAS40 response rates (36.0% at week 16, 51.2% at week 52) after switching to ixekizumab (figure 3A, table 2); week 52 response rates were numerically similar to those in patients initially randomised to ixekizumab. Among ADA/IXE patients who were ASAS40 non-responders at week 16, but ASAS40 responders at week 52, 47.4% were ASAS20 non-responders and 52.6% were ASAS20 responders at week 16.
Figure 2.
Proportion of patients achieving ASAS40, ASAS20 and ASDAS <2.1 responses through 52 weeks in COAST-V (A, C, E) and COAST-W (B, D, F). ITT population. Missing data were imputed using NRI. ADA represents an active reference group; the study was not powered to test equivalence or non-inferiority of the active treatment groups to each other, including ixekizumab versus ADA. *ASDAS <2.1 indicates low disease activity. ADA, adalimumab; ASAS, Assessment of SpondyloArthritis international Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; bDMARD, biological disease-modifying antirheumatic drug; ITT, intent to treat; IXE Q4W, ixekizumab 80 mg every 4 weeks; IXE Q2W, ixekizumab 80 mg every 2 weeks; NRI, non-responder imputation; PBO, placebo; TNFi, tumour necrosis factor inhibitor.
Table 1.
Weeks 16* and 52 efficacy endpoints for patients treated continuously with ixekizumab: COAST-V and COAST-W (ITT population: NRI, modified baseline observation carried forward)
| COAST-V (bDMARD-naive) | COAST-W (TNFi-experienced) | |||||||
| IXE Q4W (n=81) |
IXE Q2W (n=83) |
IXE Q4W (n=114) |
IXE Q2W (n=98) |
|||||
| Patients achieving response, n (%) | ||||||||
| NRI | Week 16 | Week 52 | Week 16 | Week 52 | Week 16 | Week 52 | Week 16 | Week 52 |
| ASAS40 | 39 (48.1) | 43 (53.1) | 43 (51.8) | 42 (50.6) | 29 (25.4) | 39 (34.2) | 30 (30.6) | 30 (30.6) |
| ASAS20 | 52 (64.2) | 53 (65.4) | 57 (68.7) | 59 (71.1) | 55 (48.2) | 60 (52.6) | 46 (46.9) | 47 (48.0) |
| ASAS partial remission | 12 (14.8) | 22 (27.2) | 12 (14.5) | 20 (24.1) | 7 (6.1) | 13 (11.4) | 5 (5.1) | 8 (8.2) |
| ASDAS clinically important improvement | 50 (61.7) | 51 (63.0) | 50 (60.2) | 51 (61.4) | 51 (44.7) | 53 (46.5) | 48 (49.0) | 44 (44.9) |
| ASDAS major improvement | 24 (29.6) | 30 (37.0) | 19 (22.9) | 29 (34.9) | 18 (15.8) | 27 (23.7) | 21 (21.4) | 26 (26.5) |
| ASDAS <2.1 (low disease activity) |
35 (43.2) | 43 (53.1) | 35 (42.2) | 43 (51.8) | 20 (17.5) | 27 (23.7) | 16 (16.3) | 24 (24.5) |
| ASDAS <1.3 (inactive disease) |
13 (16.0) | 18 (22.2) | 9 (10.8) | 16 (19.3) | 4 (3.5) | 10 (8.8) | 5 (5.1) | 4 (4.1) |
| BASDAI50 | 34 (42.0) | 40 (49.4) | 36 (43.4) | 37 (44.6) | 25 (21.9) | 31 (27.2) | 23 (23.5) | 27 (27.6) |
| Mean change from baseline (SD) | ||||||||
| mBOCF† | Week 16 | Week 52 | Week 16 | Week 52 | Week 16 | Week 52 | Week 16 | Week 52 |
| ASDAS | −1.4 (1.2) | −1.6 (1.1) | −1.4 (1.0) | −1.6 (1.0) | −1.1 (1.0) | −1.2 (1.1) | −1.2 (1.1) | −1.3 (1.2) |
| BASDAI | −3.0 (2.4) | −3.3 (2.5) | −2.7 (2.1) | −3.1 (2.3) | −2.1 (2.0) | −2.4 (2.4) | −2.1 (2.3) | −2.4 (2.3) |
| BASFI | −2.4 (2.3) | −2.8 (2.5) | −2.5 (2.2) | −2.8 (2.4) | −1.6 (2.1) | −2.1 (2.5) | −1.9 (2.3) | −2.1 (2.3) |
| SF-36 PCS‡ | 7.6 (8.4) | 8.3 (9.5) | 7.8 (7.0) | 8.1 (7.5) | 6.3 (7.5) | 6.5 (8.5) | 6.0 (7.7) | 7.1 (7.6) |
| ASAS Health Index | −2.3 (3.3) | −2.7 (3.3) | −2.8 (3.2) | −3.3 (3.6) | −2.0 (3.1) | −2.3 (3.7) | −1.8 (3.9) | −2.5 (3.5) |
| SPARCC MRI spine score§ | −8.9 (16.2) | −8.8 (17.3) | −8.7 (16.5) | −8.5 (15.9) | −3.2 (8.3) | NA | −5.1 (11.9) | NA |
| SPARCC MRI sacroiliac joint score¶ | −3.4 (7.6) | −3.3 (8.7) | −4.1 (7.3) | −4.2 (7.5) | NA | NA | NA | NA |
| CRP, mg/L | −6.8 (16.7) | −9.2 (12.4) | −8.4 (15.7) | −9.6 (14.5) | −11.5 (30.1) | −10.4 (31.1) | −10.3 (19.3) | −10.0 (18.5) |
*Except for ASAS partial remission (both studies), ASDAS clinically important improvement (both studies), ASDAS major improvement (both studies), ASDAS <1.3 (COAST-W) and BASDAI50 (COAST-W), all week 16 data have been previously reported.10 11
†For patients who discontinued study drug because of an adverse event, the baseline observation was carried forward to the corresponding time point for evaluation. For patients discontinuing study drug for any other reason, the last non-missing observation before discontinuation was carried forward to the corresponding time point for evaluation.
‡SF-36 PCS data are reported as t-scores, based on 2009 US general population norms.
§Observed data only (not assessed after week 16 in COAST-W). COAST-V: week 16, n=78 (IXE Q4W) and n=74 (IXE Q2W); week 52, n=72 (IXE Q4W) and n=68 (IXE Q2W). COAST-W: week 16, n=49 (IXE Q4W) and n=45 (IXE Q2W).
¶Observed data only (not assessed in COAST-W). COAST-V: week 16, n=78 (IXE Q4W) and n=75 (IXE Q2W); week 52, n=72 (IXE Q4W) and n=69 (IXE Q2W).
ASAS, Assessment of SpondyloArthritis international Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARD, biological disease-modifying antirheumatic drug; CRP, C reactive protein; ITT, intent to treat; IXE Q2W, ixekizumab 80 mg every 2 weeks; IXE Q4W, ixekizumab 80 mg every 4 weeks; mBOCF, modified baseline observation carried forward; NA, not applicable; NRI, non-responder imputation; SF-36 PCS, Medical Outcomes Study 36-item Short-Form Health Survey Physical Component Score; SPARCC, Spondyloarthritis Research Consortium of Canada; TNFi, tumour necrosis factor inhibitor.
Figure 3.
Proportion of patients initially randomised to PBO or ADA achieving ASAS40 responses on treatment with ixekizumab from week 16 through week 52 in COAST-V (A) and COAST-W (B). ETP population. Missing data were imputed using NRI. ADA, adalimumab; ASAS, Assessment of SpondyloArthritis international Society; bDMARD, biological disease-modifying antirheumatic drug; ETP, dose double-blind extended treatment period; IXE, ixekizumab; NRI, non-responder imputation; PBO, placebo; TNFi, tumour necrosis factor inhibitor.
Table 2.
Week 16* and 52 efficacy endpoints for PBO and ADA patients rerandomised to ixekizumab at week 16: COAST-V and COAST-W (ETP population: NRI, modified baseline observation carried forward)
| COAST-V (bDMARD-naive) | COAST-W (TNFi-experienced) | |||||
| PBO/IXE (n=86) |
ADA/IXE (n=86) |
PBO/IXE (n=93) |
||||
| Patients achieving response, n (%) | ||||||
| NRI | Week 16 | Week 52 | Week 16 | Week 52 | Week 16 | Week 52 |
| ASAS40 | 16 (18.6) | 40 (46.5) | 31 (36.0) | 44 (51.2) | 13 (14.0) | 36 (38.7) |
| ASAS20 | 35 (40.7) | 58 (67.4) | 52 (60.5) | 58 (67.4) | 31 (33.3) | 50 (53.8) |
| ASAS partial remission | 7 (8.1) | 16 (18.6) | 13 (15.1) | 18 (20.9) | 1 (1.1) | 9 (9.7) |
| ASDAS clinically important improvement | 20 (23.3) | 55 (64.0) | 48 (55.8) | 55 (64.0) | 18 (19.4) | 49 (52.7) |
| ASDAS major improvement | 4 (4.7) | 27 (31.4) | 21 (24.4) | 28 (32.6) | 4 (4.3) | 25 (26.9) |
| ASDAS<2.1 (low disease activity) | 11 (12.8) | 35 (40.7) | 33 (38.4) | 41 (47.7) | 5 (5.4) | 27 (29.0) |
| ASDAS<1.3 (inactive disease) | 2 (2.3) | 14 (16.3) | 14 (16.3) | 15 (17.4) | 1 (1.1) | 6 (6.5) |
| BASDAI50 | 15 (17.4) | 40 (46.5) | 28 (32.6) | 39 (45.3) | 10 (10.8) | 35 (37.6) |
| Mean change from baseline (SD) | ||||||
| mBOCF† | Week 16 | Week 52 | Week 16 | Week 52 | Week 16 | Week 52 |
| ASDAS | −0.6 (0.8) | −1.6 (1.0) | −1.3 (1.2) | −1.5 (1.1) | −0.2 (1.1) | −1.4 (1.3) |
| BASDAI | −1.5 (1.7) | −2.9 (2.1) | −2.4 (2.3) | −3.0 (2.3) | −1.0 (2.1) | −2.7 (2.6) |
| BASFI | −1.3 (1.8) | −2.4 (2.2) | −2.2 (2.2) | −2.7 (2.3) | −0.7 (2.1) | −2.2 (2.7) |
| SF-36 PCS‡ | 4.2 (6.3) | 7.7 (8.0) | 6.6 (7.2) | 7.7 (8.0) | 1.0 (7.2) | 6.2 (8.7) |
| ASAS Health Index | −1.4 (2.5) | −2.5 (3.3) | −2.4 (3.1) | −2.9 (3.6) | −0.9 (3.2) | −2.4 (3.6) |
| SPARCC MRI spine score§ | −1.1 (5.9) | −8.5 (14.6) | −12.6 (21.4) | −13.9 (21.2) | NA | NA |
| SPARCC MRI sacroiliac joint score¶ | 0.76 (5.4) | −2.7 (6.2) | −2.8 (8.4) | −3.0 (9.0) | NA | NA |
| CRP, mg/L | −1.0 (22.9) | −11.2 (22.3) | −8.4 (17.3) | −9.4 (17.0) | 6.8 (29.9) | −9.7 (25.8 |
*Except for ASAS partial remission (both studies), ASDAS clinically important improvement (both studies), ASDAS major improvement (both studies), ASDAS <1.3 (COAST-W) and BASDAI50 (COAST-W), all week 16 data have been previously reported.10 11
† For patients who discontinued study drug because of an adverse event, the baseline observation was carried forward to the corresponding timepoint for evaluation. For patients discontinuing study drug for any other reason, the last non-missing observation before discontinuation was carried forward to the corresponding time point for evaluation.
‡ SF-36PCS data are reported as t-scores, based on 2009 US general population norms.
§ Observed data only (not assessed after week 16 in COAST-W). COAST-V: week 16, n=81 (PBO/IXE) and n=80 (ADA/IXE); week 52, n=76 (PBO/IXE) and n=76 (ADA/IXE). COAST-W: week 16, n=49 (IXE Q4W) and n=45 (IXE Q2W).
¶ Observed data only (not assessed in COAST-W). COAST-V: week 16, n=81 (PBO/IXE) and n=80 (ADA/IXE); week 52, n=76 (PBO/IXE) and n=76 (ADA/IXE).
ADA, adalimumab; ASAS, Assessment of SpondyloArthritis international Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARD, biological disease-modifying antirheumatic drug; CRP, C reactive protein; ETP, dose double-blind extended treatment period; mBOCF, modified baseline observation carried forward; NA, not applicable; NRI, non-responder imputation;PBO, placebo; SF-36 PCS, Medical Outcomes Study 36-item Short-Form Health Survey Physical Component Score; IXE Q2W, ixekizumab 80 mg every 2 weeks; IXE Q4W, ixekizumab 80 mg every 4 weeks; TNFi, tumour necrosis factor inhibitor.
Consistent with the ASAS40 findings, week 16 improvements in ASAS20 response rates were sustained for up to 52 weeks among patients continuously treated with ixekizumab in both studies (figure 2C and D). Week 16 improvements in other measures of disease activity were sustained for up to 52 weeks, including changes from baseline in ASDAS and BASDAI, and achievement of ASAS partial remission, ASDAS improvement categories (low disease activity (figure 2), inactive disease, clinically important improvement and major improvement) and BASDAI50 (table 1). Improvements in patient function at week 16 (change from baseline in BASFI) were sustained for up to 52 weeks in patients continuously treated with ixekizumab, as were improvements in measures of QoL (change from baseline in SF-36 PCS) and health functioning (change from baseline in ASAS HI) (table 1). Week 16 improvements in spinal MRI and objective inflammation were sustained for up to 52 weeks per SPARCC spine and sacroiliac joint scores (assessed beyond week 16 in COAST-V only) and changes from baseline in CRP (table 1).
In COAST-V, patients randomised to ADA showed further numerical improvements in most efficacy endpoints on switching to ixekizumab (table 2).
Results from secondary analyses (online supplementary table S3 and S4) were consistent with the primary analyses (NRI and mBOCF).
annrheumdis-2019-216118supp003.pdf (19KB, pdf)
annrheumdis-2019-216118supp004.pdf (17.6KB, pdf)
Concomitant NSAID and ASAS-NSAID findings are summarised in online supplementary table S5.
annrheumdis-2019-216118supp005.pdf (13.2KB, pdf)
Safety
ETP population (weeks 16 to 52)
Overall, 201 (61.1%) patients in COAST-V and 179 (63.7%) patients in COAST-W reported treatment-emergent adverse events (TEAEs) during the ETP (table 3). Most TEAEs were of mild or moderate severity. Eight (2.4%) patients in COAST-V and 10 (3.6%) patients in COAST-W discontinued treatment because of an AE. The most common TEAEs were nasopharyngitis, ISRs and upper respiratory tract infection. Serious adverse events (SAEs) occurred in 18 (5.5%) patients in COAST-V and 9 (3.2%) patients in COAST-W; the frequency of SAEs was similar between ixekizumab regimens. The only SAE reported by more than one patient was bradycardia (n=2 patients; neither SAE was considered related to treatment). There were no deaths during the ETP in either study.
Table 3.
Safety summary: COAST-V and COAST-W (ETP population (weeks 16 to 52) and all ixekizumab exposure safety population (weeks 0 to 52))
| COAST-V (bDMARD-naive) ETP population (weeks 16–52) |
COAST-W (TNFi-experienced) ETP population (weeks 16–52) |
COAST-V+COAST W All ixekizumab exposure safety population (weeks 0–52) |
|||||||
| PBO/ IXE (n=86) n (%) |
ADA/ IXE (n=86) n (%) |
IXE Q4W/ IXE Q4W (n=78) n (%) |
IXE Q2W/ IXE Q2W (n=79) n (%) |
PBO/ IXE (n=93) n (%) |
IXE Q4W/ IXE Q4W (n=98) n (%) |
IXE Q2W/ IXE Q2W (n=90) n (%) |
Total IXE Q4W (n=327) n (%) (IR*) |
Total IXE Q2W (n=314) n (%) (IR*) |
|
| Exposure, patient-years | 58.5 | 51.7 | 51.9 | 53.2 | 59.6 | 64.1 | 58.2 | 259.4 | 250.8 |
| Any TEAE | 57 (66.3) | 50 (58.1) | 50 (64.1) | 44 (55.7) | 52 (55.9) | 69 (70.4) | 58 (64.4) | 234 (71.6) (90.2) | 217 (69.1) (86.5) |
| Mild | 31 (36.0) | 32 (37.2) | 34 (43.6) | 28 (35.4) | 21 (22.6) | 30 (30.6) | 24 (26.7) | 115 (35.2) (44.3) | 97 (30.9) (38.7) |
| Moderate | 22 (25.6) | 15 (17.4) | 13 (16.7) | 13 (16.5) | 23 (24.7) | 33 (33.7) | 30 (33.3) | 101 (30.9) (38.9) | 98 (31.2) (39.1) |
| Severe | 4 (4.7) | 3 (3.5) | 3 (3.8) | 3 (3.8) | 8 (8.6) | 6 (6.1) | 4 (4.4) | 18 (5.5) (6.9) | 22 (7.0) (8.8) |
| Discontinuation due to AE | 2 (2.3) | 3 (3.5) | 1 (1.3) | 2 (2.5) | 1 (1.1) | 4 (4.1) | 5 (5.6) | 17 (5.2) (6.6) | 17 (5.4) (6.8) |
| SAEs | 4 (4.7) | 7 (8.1) | 4 (5.1) | 3 (3.8) | 6 (6.5) | 2 (2.0) | 1 (1.1) | 17 (5.2) (6.6) | 19 (6.1) (7.6) |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.3) (0.4) |
| Most common TEAEs† | |||||||||
| Nasopharyngitis | 17 (19.8) | 7 (8.1) | 8 (10.3) | 7 (8.9) | 3 (3.2) | 3 (3.1) | 4 (4.4) | 37 (11.3) (14.3) | 25 (8.0) (10.0) |
| Injection site reaction | 8 (9.3) | 8 (9.3) | 3 (3.8) | 6 (7.6) | 3 (3.2) | 2 (2.0) | 5 (5.6) | 13 (4.0) (5.0) | 30 (9.6) (12.0) |
| Upper respiratory tract infection | 4 (4.7) | 4 (4.7) | 4 (5.1) | 8 (10.1) | 5 (5.4) | 4 (4.1) | 8 (8.9) | 29 (8.9) (11.2) | 27 (8.6) (10.8) |
| AEs of special interest | |||||||||
| Grade 3 or 4 neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.3) (0.4) | 0 |
| Infections | 34 (39.5) | 19 (22.1) | 25 (32.1) | 25 (31.6) | 32 (34.4) | 29 (29.6) | 33 (36.7) | 134 (41.0) (51.7) | 118 (37.6) (47.1) |
| Serious infections | 1 (1.2) | 1 (1.2) | 0 | 1 (1.3) | 2 (2.2) | 0 | 1 (1.1) | 3 (0.9) (1.2) | 7 (2.2) (2.8) |
| Candida infection | 2 (2.3) | 0 | 0 | 0 | 0 | 2 (2.0) | 0 | 4 (1.2) (1.5) | 1 (0.3) (0.4) |
| Injection site reactions | 15 (17.4) | 13 (15.1) | 5 (6.4) | 9 (11.4) | 8 (8.6) | 3 (3.1) | 7 (7.8) | 30 (9.2) (11.6) | 54 (17.2) (21.5) |
| Allergic reactions/ hypersensitivities | 4 (4.7) | 4 (4.7) | 4 (5.1) | 2 (2.5) | 2 (2.2) | 6 (6.1) | 4 (4.4) | 20 (6.1) (7.7) | 20 (6.4) (8.0) |
| Potential anaphylaxis | 0 | 1 (1.2) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.3) (0.4) |
| Hepatic | 6 (7.0) | 1 (1.2) | 3 (3.8) | 4 (5.1) | 4 (4.3) | 2 (2.0) | 2 (2.2) | 16 (4.9) (6.2) | 13 (4.1) (5.2) |
| Cerebrocardiovascular events‡, adjudicated | 1 (1.2) | 0 | 0 | 0 | 1 (1.1) | 1 (1.0) | 0 | 3 (0.9) (1.2) | 3 (1.0) (1.2) |
| MACE | 0 | 0 | 0 | 0 | 1 (1.1) | 0 | 0 | 0 | 1 (0.3) (0.4) |
| Malignancies | 0 | 1 (1.2) | 0 | 0 | 0 | 0 | 0 | 2 (0.6) (0.8) | 0 |
| Anterior uveitis | 2 (2.3) | 2 (2.3) | 1 (1.3) | 1 (1.3) | 2 (2.2) | 4 (4.1) | 5 (5.6) | 9 (2.8) (3.5) | 11 (3.5) (4.4) |
| Depression | 0 | 0 | 0 | 0 | 0 | 1 (1.0) | 1 (1.1) | 1 (0.3) (0.4) | 2 (0.6) (0.8) |
| Crohn’s disease | 1 (1.2) | 1 (1.2) | 0 | 0 | 0 | 0 | 0 | 2 (0.6) (0.8) | 2 (0.6) (0.8) |
| Ulcerative colitis | 1 (1.2) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.6) (0.8) | 0 |
| IBD not otherwise specified | 0 | 0 | 1 (1.3) | 0 | 0 | 0 | 0 | 2 (0.6) (0.8) | 0 |
| Psoriasis | 0 | 0 | 0 | 0 | 0 | 3 (3.1) | 1 (1.1) | 4 (1.2) (1.5) | 1 (0.3) (0.4) |
*IR calculated per 100 patient-years.
†Defined as events reported by ≥5% of all patients in either of the two studies in the ETP population.
‡Cerebrocardiovascular events included death, cardiac ischaemic events including myocardial infarction and hospitalisation for unstable angina, hospitalisation for heart failure, serious arrhythmia, resuscitated sudden death, cardiogenic shock, coronary revascularisation procedure, stroke/transient ischaemic attack, peripheral revascularisation procedure and peripheral arterial event and hospitalisation for hypertension.
ADA, adalimumab; AE, adverse event; bDMARD, biological disease-modifying antirheumatic drug; ETP, dose double-blind extended treatment period; IBD, inflammatory bowel disease; IR, incidence rate; IXE, IXE Q4W and IXE Q2W combined; MACE, major adverse cerebrocardiovascular events; PBO, placebo; IXE Q2W, ixekizumab 80 mg every 2 weeks; IXE Q4W, ixekizumab 80 mg every 4 weeks; SAE, serious adverse event; TEAE, treatment-emergent adverse event; TNFi, tumour necrosis factor inhibitor.
Malignancy (bladder cancer) was reported by one patient (ADA/IXE) in COAST-V; the event was rated severe and led to study discontinuation. Depression was reported by two patients in COAST-W (both continued treatment); there were no events of suicide or attempted suicide in the ETP (one event of suicide occurred during the placebo-controlled period in a patient (IXE Q2W) with a history of depression).11 There were no events of grade 3/4 neutropenia in either study.
Cerebrocardiovascular events were reported by one patient in COAST-V and two patients in COAST-W. One patient (PBO/IXE) in COAST-W reported a major adverse cerebrocardiovascular event of acute myocardial infarction; the event was severe, resolved and did not lead to study nor treatment discontinuation. Allergic reactions/hypersensitivities were reported by 14 (4.3%) patients in COAST-V and 12 (4.3%) patients in COAST-W.
Infections were reported by 103 (31.3%) patients in COAST-V and 94 (33.5%) patients in COAST-W; most were mild or moderate in severity. Serious infections were reported by three patients (cellulitis, pneumonia and tonsillitis; all n=1 patient) in COAST-V and three patients (gastroenteritis, pneumonia and sinusitis; all n=1 patient) in COAST-W; one of these patients discontinued the study. Candida infection was reported by two patients (oesophageal candidiasis and fungal oesophagitis; both n=1 patient) in COAST-V and two patients (oesophageal candidiasis and oral candidiasis; both n=1 patient) in COAST-W (all were mild or moderate in severity); one of these patients discontinued the study. Three Candida infection events resolved and the other was resolving at the time of patient discontinuation. ISRs were reported by 42 (12.8%) patients in COAST-V and 18 (6.4%) patients in COAST-W. Most were mild or moderate in severity; two were severe. One patient discontinued study drug due to an ISR.
AU was reported by 17 patients, 6 (1.8%) in COAST-V and 11 (3.9%) in COAST-W; none were SAEs and 14 had a history of AU. One patient in COAST-W (IXE Q4W/IXE Q4W) discontinued the study because of AU.
In COAST-V, two patients (with no prior diagnosis) reported Crohn’s disease and two patients with a prior diagnosis of ulcerative colitis reported a flare (online supplementary table S6). All events were mild or moderate in severity; one patient discontinued treatment. All events, except one, were adjudicated as ‘probable’; one event of Crohn’s disease (ADA/IXE) was adjudicated as ‘definitive’. There were no events of Crohn’s disease or ulcerative colitis in COAST-W.
annrheumdis-2019-216118supp006.pdf (24.4KB, pdf)
All ixekizumab exposure safety population (weeks 0 to 52)
During the 52-week study periods of COAST-V and COAST-W (n=641), the pooled exposure-adjusted incidence rate per 100 patient years (EAIR) of serious infections was 2.0 among patients treated with ixekizumab (table 3). Pooled EAIRs of Candida infection and grade 3/4 neutropenia were 1.0 and 0.2, respectively. Corresponding EAIRs for Crohn’s disease, ulcerative colitis and IBD not otherwise specified (NOS) were 0.8, 0.4 and 0.4, respectively (total IBD EAIR: 1.6). The EAIR for AU was 3.9; 15/20 (75%) patients had a history of AU and 14/20 (70%) patients were from COAST-W. The pooled EAIR for psoriasis was 1.0. One patient had a major adverse cerebrocardiovascular event (acute myocardial infarction) and two malignancies were reported (acute promyelocytic leukaemia and bladder cancer).
Fewer ISRs were reported with IXE Q4W (9.2%) versus IXE Q2W (17.2%). The number of patients reporting an ISR decreased over time. Specifically, 6.4%, 3.8% and 3.4% of patients on IXE Q4W and 14.3%, 8.6% and 5.2% of patients on IXE Q2W reported an ISR from weeks 0–12, weeks 12–24 and weeks 24–36, respectively. Few patients (IXE Q4W ≤1%; IXE Q2W approximately 3%) reported an ISR beyond week 36.
Treatment-emergent antidrug antibodies
TE-ADAs were detected during the 52-week study period in 23 (6.9%) patients in COAST-V and 27 (8.9%) patients in COAST-W. Most TE-ADA-positive patients had low titres (COAST-V, 18 (78%); COAST-W, 23 (85%)) and four patients (COAST-V, 1 (0.3%); COAST-W, 3 (1.0%)) were neutralising antibody positive. There were no associations between TE-ADA positivity and ASAS40 response, ISRs or allergic reaction/hypersensitivity events.
Discussion
In COAST-V and COAST-W, the significant improvements observed at week 1610 11 were sustained for up to 52 weeks with ixekizumab treatment as measured by ASAS40 responses and other efficacy outcomes assessing disease activity, function, objective inflammation, QoL, health status and overall functioning. The results for IXE Q4W and IXE Q2W were similar across endpoints. ASAS40 response rates in patients rerandomised from PBO rapidly increased to levels consistent with those seen with continuous ixekizumab treatment. Patients rerandomised from ADA to ixekizumab at week 16 achieved numerically greater response rates for ASAS40 and other efficacy outcomes at week 52 than at week 16. Collectively, the data from COAST-V and COAST-W demonstrate that ixekizumab is an effective treatment in patients with active r-axSpA who are bDMARD-naive or TNFi-experienced.
In general, treatment responses were numerically smaller in TNFi-experienced (COAST-W) versus bDMARD-naive (COAST-V) patients, reflecting a more difficult to treat population with prior treatment failure and more long-standing disease.
Currently approved biological therapies for axSpA include several TNFi and one IL-17A antagonist. Although only head-to-head trials can fully assess the relative efficacy and safety of different treatments, the week 52 ASAS40 findings reported herein are consistent with those reported for TNFi in patients who were bDMARD-naive23–26 and for secukinumab in subgroups of patients who were bDMARD-naive or had previously failed TNFi treatment.27
The safety profile of ixekizumab during the ETP (week 16 to 52) in both COAST-V and COAST-W is consistent with that observed during weeks 0 to 16.10 11 Discontinuation due to AEs was <4% in both studies, whereas <6% of patients reported SAEs. Most infections and ISRs were mild or moderate in severity and did not result in study discontinuation. ISRs were more frequent with IXE Q2W than IXE Q4W. Furthermore, ISRs were most frequently reported during the first 4 weeks of treatment and decreased in frequency over time. During the 52-week study period, pooled EAIRs for Crohn’s disease, ulcerative colitis, IBD NOS, Candida infection and grade 3/4 neutropenia were ≤1 event/100 patient-years among patients treated with ixekizumab. Among patients who reported IBD events, most had a prior diagnosis of IBD or a gastrointestinal history potentially indicative of IBD. Fewer IBD events were reported with IXE Q2W versus IXE Q4W, and there was no apparent relationship between the length of ixekizumab exposure and IBD. Previous reports have indicated that the EAIR for AU in patients with AS ranges from 2.6 to 3.5 for patients treated with TNFi.28 The EAIR of AU reported herein is at the upper limit of this range, primarily driven by patients from the TNF-experienced population. All but one patient were HLA-B27 positive, with the majority having a history of AU.
An important strength of these analyses is use of the most conservative methods of missing data imputation (NRI and mBOCF) for the primary analyses. Furthermore, as COAST-V and COAST-W exclusively enrolled bDMARD-naive and TNFi-experienced patients, respectively, both studies were fully powered for analyses in these populations. Notably, patients in COAST-W had very active disease (baseline ASDAS >4) and more than 30% had failed two prior TNFi. Another strength is the use of objective measures of inflammation, including MRI at week 52 (COAST-V only); to date, ixekizumab is the only IL-17A antagonist for which short-term and long-term MRI clinical trial data are available. The ETP results are limited by the lack of any placebo or active control comparators.
In conclusion, ixekizumab provided sustained and clinically meaningful improvement in the signs and symptoms of active r-axSpA for up to 52 weeks in COAST-V and COAST-W, with a high rate of completion. The safety findings were consistent with the known safety profile of ixekizumab. These findings suggest that ixekizumab could be a treatment option for axSpA in patients who are bDMARD-naive or who have had a prior inadequate response or intolerance to TNFi.
Acknowledgments
These results were presented in part at the European Congress of Rheumatology 2019, Madrid, Spain (12–15 June). Medical writing assistance was provided by Luke Carey, PhD, CMPP of ProScribe – Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3). The authors would like to thank all study participants and Emily Seem, Eli Lilly and Company, for assistance with statistical analyses.
Footnotes
Handling editor: Josef S Smolen
Contributors: All authors participated in the interpretation of study results, and in the drafting, critical revision and approval of the final version of the manuscript. MD, JC-CW, JS, FVdB, WPM, AD, DvdH, XL, FZ and HC contributed to study conception and/or design. JC-CW, RL, WPM, AD, DvdH, FZ, XL and HC contributed to the acquisition of study results. MD, RL, JS, XL, FZ and HC contributed to the analysis of study results.
Funding: The studies described in this manuscript were sponsored by Eli Lilly and Company, which was involved in the study design, data collection, data analysis and preparation of the manuscript.
Competing interests: MD has served as a consultant and received research grants from AbbVie, Eli Lilly and Company, Pfizer and UCB Pharma. JC-CW has served as a consultant and/or speaker and/or has received research grants from Abbott, Bristol-Myers Squibb, Celgene, Chugai, Eisai, Eli Lilly and Company, Janssen, Novartis, Pfizer, Sanofi-Aventis, TSH Taiwan and UCB Pharma. RL has served as a consultant and/or advisor and/or has received research grants from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Galapagos, Merck, Novartis, Pfizer and UCB Pharma. RL is the director of Rheumatology Consultancy BV, a company that was indirectly contracted by Eli Lilly and Company to perform read services for the COAST program. JS has served as a consultant and/or speaker for AbbVie, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, Novartis, Pfizer, Roche and UCB Pharma. XB has served as a consultant and/or has received research grants from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, MSD, Novartis, Pfizer, Roche and UCB Pharma. FVdB has served as a consultant and/or speaker and/or has received research grants from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Merck, Novartis, Pfizer, Sanofi and UCB Pharma. WPM has served as a consultant and/or received honoraria and/or research/educational grants from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galapagos, Janssen, Novartis, Pfizer and UCB Pharma, and is Chief Medical Officer of CARE Arthritis Limited. JE has served as a consultant and/or received research grants from AbbVie, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Novartis, Pfizer, Takeda and UCB Pharma. JAW has been a consultant and/or received research grants from AbbVie, Amgen, Celgene, Eli Lilly and Company, Novartis, Pfizer and UCB Pharma. AD has been a consultant and/or received research support from AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Glaxo Smith & Klein, Janssen, Novartis, Pfizer and UCB Pharma. DvdH has been a consultant for AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly and Company, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB Pharma and is a Director of Imaging Rheumatology BV. TT has been a consultant and speaker for AbbVie, Astellas, Bristol-Myers Squibb, Eisai, Eli Lilly and Company, Janssen, Mitsubishi Tanabe, Novartis, Pfizer and Takeda. XL, FZ, CCB, GG and HC are current employees and shareholders of Eli Lilly and Company. LSG has been a consultant and/or received research grants/support from AbbVie, Amgen, Eli Lilly and Company, Galapagos, Janssen, Novartis, Pfizer and UCB Pharma.
Patient consent for publication: Not required.
Ethics approval: Trial protocols were approved by ethics review boards at each study site. Trials were performed in accordance with the ethical principles of the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Lilly provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and the European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
References
- 1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. 10.1016/S0140-6736(16)31591-4 [DOI] [PubMed] [Google Scholar]
- 2. Gao X, Wendling D, Botteman MF, et al. . Clinical and economic burden of extra-articular manifestations in ankylosing spondylitis patients treated with anti-tumor necrosis factor agents. J Med Econ 2012;15:1054–63. 10.3111/13696998.2012.692341 [DOI] [PubMed] [Google Scholar]
- 3. Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl 2006;78:4–11. [PubMed] [Google Scholar]
- 4. Ward MM, Deodhar A, Akl EA, et al. . American College of Rheumatology/Spondylitis association of America/Spondyloarthritis research and treatment network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res 2016;68:151–66. 10.1002/acr.22708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Heijde D, Ramiro S, Landewé R, et al. . 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. 10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 6. Sepriano A, Regel A, van der Heijde D, et al. . Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open 2017;3:e000396 10.1136/rmdopen-2016-000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D’Angelo S, Carriero A, Gilio M, et al. . Safety of treatment options for spondyloarthritis: a narrative review. Expert Opin Drug Saf 2018;17:475–86. 10.1080/14740338.2018.1448785 [DOI] [PubMed] [Google Scholar]
- 8. Kenna TJ, Davidson SI, Duan R, et al. . Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum 2012;64:1420–9. 10.1002/art.33507 [DOI] [PubMed] [Google Scholar]
- 9. Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum 2009;60:1647–56. 10.1002/art.24568 [DOI] [PubMed] [Google Scholar]
- 10. van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. . Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018;392:2441–51. 10.1016/S0140-6736(18)31946-9 [DOI] [PubMed] [Google Scholar]
- 11. Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. . Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results rrom a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol 2019;71:599–611. 10.1002/art.40753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rudwaleit M, van der Heijde D, Landewé R, et al. . The development of Assessment of Spondyloarthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 13. Sieper J, Rudwaleit M, Baraliakos X, et al. . The Assessment of Spondyloarthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68 Suppl 2:ii1–44. 10.1136/ard.2008.104018 [DOI] [PubMed] [Google Scholar]
- 14. Machado PMMC, Landewé RBM, van der Heijde DM. Endorsement of definitions of disease activity states and improvement scores for the Ankylosing Spondylitis Disease Activity Score: results from OMERACT 10. J Rheumatol 2011;38:1502–6. 10.3899/jrheum.110279 [DOI] [PubMed] [Google Scholar]
- 15. Garrett S, Jenkinson T, Kennedy LG, et al. . A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 16. Calin A, Garrett S, Whitelock H, et al. . A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 17. Ware JE, Snow KK, Kosinski M, et al. . Sf-36 health survey: manual and interpretation guide. 2nd ed Lincoln, RI: QualityMetric, 2000. [Google Scholar]
- 18. Kiltz U, van der Heijde D, Boonen A, et al. . The ASAS Health Index (ASAS HI) - a new tool to assess the health status of patients with spondyloarthritis. Clin Exp Rheumatol 2014;32:S-105-8. [PubMed] [Google Scholar]
- 19. Kiltz U, van der Heijde D, Boonen A, et al. . Development of a health index in patients with ankylosing spondylitis (ASAS HI): final result of a global initiative based on the ICF guided by ASAS. Ann Rheum Dis 2015;74:830–5. 10.1136/annrheumdis-2013-203967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maksymowych WP, Inman RD, Salonen D, et al. . Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. 10.1002/art.21337 [DOI] [PubMed] [Google Scholar]
- 21. Dougados M, Simon P, Braun J, et al. . ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. 10.1136/ard.2010.133488 [DOI] [PubMed] [Google Scholar]
- 22. Gower-Rousseau C, Salomez JL, Dupas JL, et al. . Incidence of inflammatory bowel disease in northern France (1988-1990). Gut 1994;35:1433–8. 10.1136/gut.35.10.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Heijde D, Schiff MH, Sieper J, et al. . Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis 2009;68:922–9. 10.1136/ard.2007.087270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis JC, van der Heijde DM, Braun J, et al. . Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis 2005;64:1557–62. 10.1136/ard.2004.035105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braun J, Deodhar A, Inman RD, et al. . Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis 2012;71:661–7. 10.1136/ard.2011.154799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sieper J, Landewé R, Rudwaleit M, et al. . Effect of certolizumab pegol over ninety-six weeks in patients with axial spondyloarthritis: results from a phase III randomized trial. Arthritis Rheumatol 2015;67:668–77. 10.1002/art.38973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sieper J, Deodhar A, Marzo-Ortega H, et al. . Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 study. Ann Rheum Dis 2017;76:571–92. 10.1136/annrheumdis-2016-210023 [DOI] [PubMed] [Google Scholar]
- 28. Deodhar A, Miceli-Richard C, Baraliakos X, et al. . Low incidence of both new-onset and flares of uveitis in secukinumab-treated patients with ankylosing spondylitis: clinical trial and post-marketing safety analysis. Ann Rheum Dis 2018;77(Suppl 2):999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2019-216118supp001.pdf (17.5KB, pdf)
annrheumdis-2019-216118supp002.pdf (24.6KB, pdf)
annrheumdis-2019-216118supp003.pdf (19KB, pdf)
annrheumdis-2019-216118supp004.pdf (17.6KB, pdf)
annrheumdis-2019-216118supp005.pdf (13.2KB, pdf)
annrheumdis-2019-216118supp006.pdf (24.4KB, pdf)