Abstract
Background
Lichen sclerosus is a chronic, inflammatory skin condition that most commonly occurs in adult women, although it may also be seen in men and children. It primarily affects the genital area and around the anus, where it causes persistent itching and soreness. Scarring after inflammation may lead to severe damage by fusion of the vulval lips (labia); narrowing of the vaginal opening; and burying of the clitoris in women and girls, as well as tightening of the foreskin in men and boys, if treatments are not started early. Affected people have an increased risk of genital cancers.
Objectives
To assess the effects of topical interventions for genital lichen sclerosus and adverse effects reported in included trials.
Search methods
We searched the following databases up to 16 September 2011: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE (from 2005), EMBASE (from 2007), LILACS (from 1982), CINAHL (from 1981), British Nursing Index and Archive (from 1985), Science Citation Index Expanded (from 1945), BIOSIS Previews (from 1926), Conference Papers Index (from 1982), and Conference Proceedings Citation Index ‐ Science (from 1990). We also searched ongoing trial registries and scanned the bibliographies of included studies, published reviews, and papers that had cited the included studies.
Selection criteria
Randomised controlled trials (RCTs) of topical interventions in genital lichen sclerosus.
Data collection and analysis
Two authors independently selected trials, extracted data, and assessed the risk of bias. A third author was available for resolving differences of opinion.
Main results
We included 7 RCTs, with a total of 249 participants, covering 6 treatments. Six of these RCTs tested the efficacy of one active intervention against placebo or another active intervention, while the other trial tested three active interventions against placebo.
When compared to placebo in one trial, clobetasol propionate 0.05% was effective in treating genital lichen sclerosus in relation to the following outcomes: 'participant‐rated improvement or remission of symptoms' (risk ratio (RR) 2.85, 95% confidence interval (CI) 1.45 to 5.61) and 'investigator‐rated global degree of improvement' (standardised mean difference (SMD) 5.74, 95% CI 4.26 to 7.23).
When mometasone furoate 0.05% was compared to placebo in another trial, there was a significant improvement in the 'investigator‐rated change in clinical grade of phimosis' (SMD ‐1.04, 95% CI ‐1.77 to ‐0.31).
Both trials found no significant differences in reported adverse drug reactions between the corticosteroid and placebo groups. The data from four trials found no significant benefit for topical testosterone, dihydrotestosterone, and progesterone. When used as maintenance therapy after an initial treatment with topical clobetasol propionate in another trial, topical testosterone worsened the symptoms (P < 0.05), but the placebo did not.
One trial found no differences between pimecrolimus and clobetasol propionate in relieving symptoms through change in pruritus (itching) (SMD ‐0.33, 95% CI ‐0.99 to 0.33) and burning/pain (SMD 0.03, 95% CI ‐0.62 to 0.69). However, pimecrolimus was less effective than clobetasol propionate with regard to the 'investigator‐rated global degree of improvement' (SMD ‐1.64, 95% CI ‐2.40 to ‐0.87). This trial found no significant differences in reported adverse drug reactions between the pimecrolimus and placebo groups.
Authors' conclusions
The current limited evidence demonstrates the efficacy of clobetasol propionate, mometasone furoate, and pimecrolimus in treating genital lichen sclerosus. Further RCTs are needed to determine the optimal potency and regimen of topical corticosteroids, examine other topical interventions, assess the duration of remission or prevention of flares, evaluate the reduction in the risk of genital squamous cell carcinoma or genital intraepithelial neoplasia, and examine the efficacy in improving the quality of the sex lives of people with this condition.
Keywords: Adult; Child; Female; Humans; Male; Anti‐Inflammatory Agents; Anti‐Inflammatory Agents/therapeutic use; Clobetasol; Clobetasol/therapeutic use; Dermatologic Agents; Dermatologic Agents/therapeutic use; Dihydrotestosterone; Dihydrotestosterone/therapeutic use; Genital Diseases, Male; Genital Diseases, Male/drug therapy; Lichen Sclerosus et Atrophicus; Lichen Sclerosus et Atrophicus/drug therapy; Mometasone Furoate; Pregnadienediols; Pregnadienediols/therapeutic use; Randomized Controlled Trials as Topic; Tacrolimus; Tacrolimus/analogs & derivatives; Tacrolimus/therapeutic use; Testosterone Propionate; Testosterone Propionate/therapeutic use; Vulvar Lichen Sclerosus; Vulvar Lichen Sclerosus/drug therapy
Plain language summary
Topical treatments for genital lichen sclerosus
Lichen sclerosus is a chronic skin disease that mostly affects adult women, but also men and children. It mainly occurs in the genital area and around the anus. Affected women and girls frequently report itching, pain, and burning in the involved area. Scarring after inflammation may cause fusion of the vaginal lips, narrowing of the vaginal opening, and burying of the clitoris. Sex is often painful, less pleasurable, or impossible because of the pain. Lichen sclerosus in men and boys may cause tightening of the foreskin, leading to difficulty in passing urine or painful erection. Pain on opening the bowels may also be present, causing constipation, especially in children. Treating this disease is beneficial as the symptoms can be relieved, and further damage to the genital area and around the anus may be prevented. Various topical treatments for lichen sclerosus have been devised. This review aimed to identify which topical treatments are effective and safe.
We included 7 trials, with a total of 249 participants, covering 6 treatments in this review. Topical clobetasol propionate and mometasone furoate were effective in treating genital lichen sclerosus. There was no substantial difference in the efficacy of relieving symptoms (e.g. itching and pain) between pimecrolimus cream and clobetasol propionate, but the former was less effective in improving gross appearance.
More research is needed for a number of reasons: to decide the strength of steroids that should be used, as well as the frequency and length of application to the skin which gives the best results; to examine other skin treatments; to assess the long‐term benefits of topical treatments with regard to relieving symptoms and reducing the risk of developing genital cancers; and to examine the benefits of treatments on the quality of the sex lives of people with this condition.
Background
Definition
Lichen sclerosus is a chronic inflammatory skin condition which causes distressing symptoms and discomfort. It most frequently occurs in women (Brownstein 1973), but also in men and children. Lichen sclerosus may affect any site, but it occurs mainly in the anogenital area (the genital area and around the anus), where it causes itching and pain. Scarring leading to the destruction of the anogenital structures, such as fusion of the vulval lips (labia), narrowing of the vaginal opening, and burying of the clitoris in women and girls, as well as tightening of the foreskin in men and boys, is common (Powell 1999).
Description of the condition
The primary lesions are flat, ivory‐coloured spots, which may merge together into crinkly thin patches. Purpura or ecchymosis (bruising) is common. The Koebner phenomenon (the development of lesions in previously normal skin that has been scratched or damaged) occurs in lichen sclerosus (Wallace 1971). In women and girls, scarring after inflammation may cause fusion of the labia minora (lips), narrowing of the vaginal introitus (vaginal opening), and burying of the clitoris. They commonly report itching, pain, burning, painful or less pleasurable sexual intercourse, and anal or genital bleeding. Constipation causing painful defecation may also be a problem, especially in children.
Lichen sclerosus in men and boys usually occurs on the glans penis and/or foreskin, and it may cause phimosis (narrowing of the opening of the foreskin) in a previously retractable foreskin or adhesions of the foreskin to the glans causing painful erection. Meatal stenosis (narrowing of the urethral opening) may lead to problems passing urine and urinary obstruction. Lichen sclerosus away from the genital area alone has been reported in about 6% of all affected women (Wallace 1971). Lichen sclerosus of the mouth is very unusual and is usually asymptomatic (Meffert 1995; Tremaine 1989).
The diagnosis in most affected people is made clinically, but a confirmatory biopsy is helpful if there is clinical doubt about the diagnosis. It also helps to detect any atypical or malignant changes. A vulval biopsy is not usually performed in children, except in cases that fail to respond to treatments (Neill 2010).
Epidemiology
Lichen sclerosus can occur at any age, but it has two main peaks in incidence: The first occurs before puberty for both girls and boys, and the second peaks are, for women, after the menopause, and, for men, between 30 and 50 years of age. The prevalence is estimated to be between 1:30 and 1:1000 in adults (Leibovitz 2000; Tasker 2003). Women are more commonly affected than men, with the female/male (F/M) ratio varying from 6:1 to 10:1 (Brownstein 1973; Wallace 1971). However, the F/M ratio appears to be the reverse of this in childhood (Kyriakis 2007), as the estimated prevalence is 1:900 in girls aged between 2 and 16 years (Powell 2001) and 1:200 in boys aged 0 to 14 years (Shankar 1999). One explanation for those, seemingly, reverse figures may be that boys with phimosis are seen earlier in life by a physician than girls, who might have little or no subjective symptoms of early lichen sclerosus. Many of the boys with a tightened foreskin will be circumcised and may, therefore, not have active lichen sclerosus in adulthood; whereas, symptoms will increase in women. Lichen sclerosus seems more common in Caucasians, but there are reports in other ethnic groups (Tasker 2003). Extrapolation from the Oxford clinic data suggests that approximately 150 to 200 women per million population present to a clinician each year (Clayton 2006).
Pathogenesis
The cause of lichen sclerosus is unknown, but there is a strong association with autoimmune diseases. Between 21.5% and 34% of those with lichen sclerosus have an associated autoimmune disease: Thyroid disease, alopecia areata, vitiligo, and pernicious anaemia are the most commonly seen (Meyrick 1988). These associations are more common in women and girls (28% to 54%) (Cooper 2008; Marren 1995) than in men and boys (3%) (Azurdia 1999); furthermore, up to 74% of those affected were found to have circulating autoantibodies (Harrington 1981). Recent studies show an increased incidence of autoantibodies to the extracellular matrix protein 1 in lichen sclerosus, which supports the idea of lichen sclerosus being a (humoral) autoimmune disease (Oyama 2003). In addition, there is evidence of both autoantibody and T‐cell reactivity to basement membrane proteins (Baldo 2010; Howard 2004). The high incidence of lichen sclerosus in postmenopausal women suggests a pathogenic role of reduced oestrogen levels; however, a protective effect from oestrogens, e.g. that women before menopause will not develop lichen sclerosus, has not been observed (Powell 1999; Tasker 2003). Genetic factors are implicated, and cases of familial lichen sclerosus have been reported (Sherman 2010).
Immunogenetic studies have demonstrated a significant association with the histocompatibility antigens, HLA class II antigen DQ7 and DRB1*12 (Gao 2005; Marren 1995). An infective aetiology has been postulated, but there are no clear data to show that lichen sclerosus is related to an infectious organism (Funaro 2004).
Impact
Lichen sclerosus has a huge impact on a person's quality of life by interfering with function (particularly sexual functioning) and self image, and the resultant distress and anxiety are immediately apparent. Many affected people feel embarrassed; some have persistent itching and pain (despite successful control of the inflammation), and many are concerned about how the disorder may progress. The lifetime risk of the development of vulval squamous cell carcinoma (SCC) in women with lichen sclerosus is 4% to 5% (Powell 1999; Wallace 1971), whilst the background lifetime risk of vulval SCC in the UK population is 0.3% (CRUK 2010). Also, vulval verrucous carcinoma has been associated with lichen sclerosus (Wang 2010). The mechanism of development of genital malignancies in lichen sclerosus is unclear, but they may involve oncogenic human papillomavirus infection, altered expression of p53 oncogene, chronic inflammation of lichen sclerosus, and oxidative stress (Wang 2010).
Description of the intervention
There is no cure for lichen sclerosus; however, there are good outcomes as a result of treating the disease. These include the relief of subjective symptoms (itching, pain) and prevention of further anatomical changes (due to sclerosis and fusion). Some clinical signs may be reversed, but any scarring that has occurred will remain (Cooper 2004; Renaud‐Vilmer 2004). It is possible that treatment may prevent malignant transformation, but this needs to be evaluated.
At present, potent or very potent topical corticosteroids are generally considered the intervention of choice (e.g. clobetasol propionate 0.05% cream or ointment) when treating lichen sclerosus (Cooper 2004; Dalziel 1991; Dalziel 1993; Funaro 2004). The Guidelines for the management of lichen sclerosus, prepared for dermatologists on behalf of the British Association of Dermatologists, recommend the use of a very potent corticosteroid ointment or cream (Neill 2010), which is also effective in treating lichen sclerosus in children (Fischer 1997; Powell 1999). Intralesional injection of corticosteroids has been shown to be effective and may be an alternative to topical corticosteroids when treating thick, resistant plaques of lichen sclerosus (Mazdisnian 1999). However, the adverse effects of corticosteroid‐induced skin atrophy (skin thinning) and telangiectasia (distended blood capillaries giving a spidery red spot) have to be considered. Whether corticosteroid‐induced immunosuppression increases the risk of vulval malignancy also needs evaluation.
Low serum levels of dihydrotestosterone, free testosterone, and androstenedione were found in women with untreated vulval lichen sclerosus (Friedrich 1984). Based on a hormone‐deficient theory of lichen sclerosus, topical hormones (e.g. androgens) have been used widely in the past in women with lichen sclerosus. A few studies found topical testosterone propionate effective in treating vulval lichen sclerosus (Friedrich 1971; Skierlo 1987). However, androgen‐associated side‐effects are common, for example, hirsutism and clitoral hypertrophy (Bornstein 1998). A study reported topical retinoids are effective, but skin irritation may limit their use (Virgili 1995). Topical immunomodulators have pharmacological effects similar to topical corticosteroids, but they do not have the side‐effects of skin atrophy and telangiectasia (Hengge 2006). A pilot study of topical ciclosporin did not find beneficial effect in genital lichen sclerosus (Carli 1992). Other topical immunomodulators, e.g. tacrolimus and pimecrolimus, have better skin penetration than topical ciclosporin and seem more promising in treating genital lichen sclerosus (Bohm 2003; Boms 2004; Hengge 2006). However, a burning sensation may be experienced with their use, and the long‐term safety profile of these newer immunomodulators needs to be evaluated.
Photodynamic therapy employs light irradiation of the skin pretreated with a photosensitiser, such as 5‐aminolevulinic acid, and generates highly reactive oxidants (e.g. superoxide or hydroxyl radicals). The photodynamic effect might cause a light‐induced cytotoxic effect (Hillemanns 1999). Irradiation of a photosensitiser, psoralen applied to the skin by ultraviolet A known as PUVA (Psoralen‐ultraviolet A), may have clinical efficacy in treating skin conditions through an immunomodulatory mechanism or via an inhibitory effect on DNA synthesis. In addition, UVA irradiation induces collagenase expression and activity in the skin, possibly through generation of singlet oxygen (Reichrath 2002). Anecdotal reports have found photodynamic therapy and topical PUVA effective for treating genital lichen sclerosus (Hillemanns 1999; Reichrath 2002); however, the potential increased risk of skin cancers associated with PUVA may limit its use (Patel 2009).
Emollients increase the water content of the skin and improve the skin barrier function, protecting it from attack by noxious substances (Loden 1999). Daily use of emollients accelerates normalisation of the damaged skin (Held 2001) and is effective in improving mild subclinical inflammation in experiments (Kikuchi 2003). Therefore, emollients have been used as a maintenance therapy for vulval lichen sclerosus (Simonart 2008).
Why it is important to do this review
Although there is some evidence to support the use of the topical interventions for lichen sclerosus listed below (under Types of interventions), as there had been no systematic review of the literature, we conducted this Cochrane systematic review to find out if the currently recommended treatment regimens are based on evidence. Our aim with this review was to determine the appropriate interventions, possible risks, and side‐effects, as well as assess the level and quality of the currently available evidence, in order to identify areas of uncertainty or gaps in knowledge that require further research.
Objectives
To assess the efficacy of topical interventions for genital lichen sclerosus and adverse effects reported in included trials.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) to evaluate the efficacy of topical interventions for genital lichen sclerosus. Cross‐over trials and within‐patient studies were also included.
Types of participants
We included anyone who had been diagnosed with genital lichen sclerosus by a medical practitioner. The diagnosis of lichen sclerosus was ideally made upon clinical and histological criteria; however, we also accepted clinical diagnosis alone if the diagnosis of lichen sclerosus was made by an experienced investigator (dermatologist, urologist, or gynaecologist).
Types of interventions
We searched for RCTs on the following topical interventions:
topical corticosteroids (potency defined by the British National Formulary (BNF 2010)) (see Table 1);
topical androgens;
topical oestrogen;
topical progesterone;
topical retinoids;
topical immunomodulators (tacrolimus, pimecrolimus, ciclosporin);
topical oxatomide;
photodynamic therapy;
topical PUVA therapy;
cryotherapy;
emollients;
ultrasound; and
other topical interventions.
1. Potency of topical corticosteroids (as defined by the British National Formulary) .
| Potency | Topical corticosteroids |
| Mild | Hydrocortisone 0.1% to 2.5% Fluocinolone acetonide 0.00625% |
| Moderate | Alclometasone dipropionate 0.05% Betamethasone valerate 0.025% Clobetasone butyrate 0.05% Fludroxycortide (flurandrenolone) 0.0125% Fluocortolone 0.25% |
| Potent | Betamethasone dipropionate 0.05% to 0.064% Betamethasone valerate 0.1% to 0.12% Diflucortolone valerate 0.1% Fluocinolone acetonide 0.025% Fluocinonide 0.05% Fluticasone propionate 0.005% to 0.05% Hydrocortisone butyrate 0.1% Mometasone furoate 0.1% Triamcinolone acetonide 0.1% |
| Very potent | Clobetasol propionate 0.05% Diflucortolone valerate 0.3% |
Types of outcome measures
Primary outcomes
1) Participant‐rated improvement or remission of symptoms (in terms of quality of life, pain, itching, and less pain with intercourse).
2) Investigator‐rated global degree of improvement (in terms of pallor, purpura, hyperkeratosis, ulceration, erosion, erythema, sclerosis, and scarring).
When we assessed these primary outcomes, we set a time point of measurement at three to six months into therapy.
3) Adverse drug reactions severe enough to require withdrawal of treatment, including severe skin irritation or infection.
Secondary outcomes
1) Adverse drug reactions that were not severe enough to require cessation of treatment, such as mild skin irritation, atrophy, or telangiectasia.
2) Duration of remission or prevention of subsequent flares, or both.
3) Development of genital squamous cell carcinoma (SCC) or genital intraepithelial neoplasia.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 16 September 2011:
the Cochrane Skin Group Specialised Register using the following search terms: (lichen and (sclerosus or atrophi* or albus or scleureux or sclero‐atrophi* or vulva*)) or (kraurosis and (vulva* or penis)) or (balanitis and (xerotica or obliteran* or sclerotica)) or (vulva* and dystroph*) or (white and spot and disease*);
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library using the search strategy in Appendix 1;
MEDLINE (from 2005) using the search strategy in Appendix 2;
EMBASE (from 2007) using the search strategy in Appendix 3;
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the search strategy in Appendix 4;
CINAHL (Cumulative Index to Nursing and Allied Health Literature, from 1981) using the search strategy in Appendix 5;
BNIA (British Nursing Index and Archive, from 1985) using the search strategy in Appendix 6; and
SCI‐EXPANDED (Science Citation Index Expanded, from 1945) using the search strategy in Appendix 7.
The UK and US Cochrane Centres (CCs) have an ongoing project to systematically search MEDLINE and EMBASE for reports of trials, which are then included in the Cochrane Central Register of Controlled Trials. Searching has currently been completed in MEDLINE to 2004 and in EMBASE to 2006. Further searching has been undertaken for this review by the Cochrane Skin Group to cover the years that have not been searched by the UK and US CCs.
A final prepublication search for this review was undertaken on 16 September 2011. No further completed studies were identified, but two relevant ongoing RCTs were found and added to the references. They will be incorporated into the next update of the review.
Ongoing Trials Registers
On 20 September 2011 we searched for reports of trials in the following ongoing trials databases using the search strategy in Appendix 8:
The metaRegister of controlled trials (www.controlled‐trials.com).
The US National Institutes of Health ongoing trials register (www.clinicaltrials.gov).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The Ongoing Skin Trials Register (www.nottingham.ac.uk/ongoingskintrials).
Searching other resources
Grey literature
On 16 September 2011 we searched BIOSIS Previews (from 1926) for relevant studies using the search strategy in Appendix 7.
Conference proceedings
On 16 September 2011 we searched the Conference Papers Index (from 1982) and Conference Proceedings Citation Index ‐ Science (CPCI‐S) (from 1990) for relevant studies using the search strategies in Appendix 9 and Appendix 7, respectively.
References lists
We scanned the bibliographies of the included studies and published reviews for relevant references. We used SCI‐EXPANDED to identify further papers that cited the included studies, and we scanned them for relevant studies.
Unpublished literature
We contacted the original researchers of the most recent studies to ask if they knew of any other relevant trials, but they did not respond to these queries.
Language
We did not impose any language restrictions.
Adverse Effects
We did not run separate searches for adverse effects, but we extracted relevant data from the included studies.
Data collection and analysis
Selection of studies
Two authors (CC, GK) independently checked titles and abstracts identified from the searches. The authors were not blinded to the names of original researchers, journals, or institutions. If it was clear from the abstract that the study did not refer to a RCT on genital lichen sclerosus (LS), it was excluded. The same two authors independently assessed the full‐text version of each remaining study to determine whether it met the pre‐defined selection criteria. Disagreement was resolved by discussion. We listed the studies that were excluded, after reading the full text, and reasons for exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
Two authors (CC, GK) independently extracted the data using a specialised data extraction form. Discrepancies were resolved by discussion with another author (FW). One author (CC) entered the data into Review Manager (RevMan).
Assessment of risk of bias in included studies
We evaluated the following components since there is some evidence that these are associated with biased estimates of treatment effect (Higgins 2011): (a) adequacy of random sequence generation; (b) allocation concealment ‐ it was considered "adequate" if the assignment could not be foreseen; (c) blinding ‐ adequacy of prevention of knowledge of the allocated interventions; (d) incomplete outcome data ‐ whether this was adequately addressed; and (e) whether the study was free of selective reporting (if the trial study protocol was available, were all prespecified outcomes reported? If the trial study protocol was unavailable, were all primary outcomes of our interest (global degree of improvement rated by participants or researchers and adverse drug reactions that were severe enough to require withdrawal of treatment) reported?).
In addition we reported other potential threats to validity: (f) the degree of certainty that the participants had LS; (g) the baseline assessment of the participants for age, duration of disease, location involved, and severity of LS; (h) drug identity, source, dose, duration of treatments, and adequacy of instructions; (i) whether the outcome measures were described and their assessment was standardised; (j) whether previous treatments for LS were discontinued; (k) whether concomitant treatments for LS were permitted or standardised; and (l) the use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication.
Measures of treatment effect
We expressed the results as risk ratios (RR) and 95% confidence intervals (CI) for dichotomous outcomes, and standardised mean difference (SMD) and 95% CI for ordinal outcomes. We also expressed the dichotomous results as number needed to treat (NNT) and number needed to treat to harm (NNH), where appropriate, for a range of plausible control event rates.
Participant‐rated global degree of improvement was the primary outcome measure when available. If unavailable, the investigator‐rated global degree of improvement was used. Both measures were reported where both were available. We did not combine the two measures.
Unit of analysis issues
Where there were multiple intervention groups within a trial, we made pair‐wise comparisons of an intervention versus no treatment, placebo, and another intervention. We analysed internally‐controlled trials using appropriate techniques for paired designs, and these studies were not be pooled with studies of other designs.
Dealing with missing data
We contacted the original researchers of studies less than 15 years old for missing data. If participant dropout led to missing data, we had planned to conduct an intention‐to‐treat analysis. For dichotomous outcomes we would have regarded participants with missing outcome data as treatment failures and included them in the analysis. For continuous outcomes we would have carried forward the last recorded value for participants with missing outcome data. Where high levels of missing data were seen within the analyses, we would have conducted sensitivity analyses to assess the robustness of the results from the approach described above, by comparing the results with those which had excluded the missing data from the analyses. However, we did not carry out these planned analyses because the majority of the included studies did not report the level of participant dropout.
Assessment of heterogeneity
We assessed statistical heterogeneity using the I² statistic. We assessed clinical heterogeneity arising from the study design (e.g. parallel or cross‐over studies), interventions, participants, and outcome measures. If the I² statistic was less than 80% with reasonable clinical homogeneity, we applied meta‐analysis techniques as appropriate.
Assessment of reporting biases
We planned to test publication bias by using a funnel plot if adequate data for a topical intervention were available. However, the limited number of trials meant it was not possible to do this test.
Data synthesis
For studies on a similar type of intervention (e.g. topical testosterone) we applied meta‐analysis using a random‐effects model to calculate a weighted treatment effect across trials when the I² statistic was 80% or less with reasonable clinical homogeneity. Where it was inappropriate or impossible to perform a meta‐analysis, we summarised the data for each trial.
Subgroup analysis and investigation of heterogeneity
We discussed similarities and differences in the study design, interventions, participants, and outcome measures of the included RCTs. We planned to perform subgroup analyses accordingly. With regard to the participants, we planned to conduct subgroup analyses of the following:
genital LS in adult women;
genital LS in adult men;
genital LS in female children; and
genital LS in male children.
But the number of included trials was too few to conduct these planned subgroup analyses.
Sensitivity analysis
A sensitivity analysis to examine the effects of excluding poor quality studies was planned but not conducted, because of the limited number of trials for each intervention.
Other
A consumer (FB) was involved throughout the review process to help improve the relevance and readability of the final review.
Results
Description of studies
Results of the search
Our search identified 312 citations, of which 18 were considered potentially eligible (Bracco 1993; Cattaneo 1992; Cattaneo 1996; Chari 1994; Diakomanolis 2002; Friedrich 1971; Goldstein 2011; Kiss 2001; Li 2004; NCT00757874; Origoni 1996; Paslin 1991; Paslin 1996; Sideri 1994; Skierlo 1987; Sotiriou 2008; Zarcone 1996; Zhu 2006).
Included studies
This review included 7 studies (8 publications), with a total of 249 participants covering 6 treatments: We included 7 studies that met our inclusion criteria (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001; Paslin 1991; Paslin 1996; Sideri 1994). One paper (Cattaneo 1992) was a duplicate publication of part of another study (Bracco 1993); so we, therefore, combined the data reported by the two papers. The details of the included studies are described in the 'Characteristics of included studies' tables.
Design
All of the included studies were RCTs, with two being cross‐over RCTs (Paslin 1991; Paslin 1996).
Sample sizes
The number of participants in the included studies ranged from 5 (Paslin 1991; Paslin 1996) to 79 (Bracco 1993). The two cross‐over trials only included 5 women each (Paslin 1991; Paslin 1996). Four other trials included 16 to 20 participants in each arm (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001). The other trial included 30 and 28 women in the treatment and placebo groups, respectively (Sideri 1994).
Setting
The setting of all of the included studies was either a single hospital or a specialist clinic. All of the studies were conducted in either Europe or the USA.
Participants
The participants were adult women in the majority of the studies (Bracco 1993; Cattaneo 1996; Goldstein 2011; Paslin 1991; Paslin 1996; Sideri 1994). Only one study included boys as participants (Kiss 2001).
Interventions
We only found RCTs on topical clobetasol, mometasone, testosterone, dihydrotestosterone, progesterone, and pimecrolimus. One study (Cattaneo 1996) investigated the effectiveness of topical testosterone propionate as maintenance therapy. All of the interventions were single therapy. The comparator was placebo in five studies (Bracco 1993; Cattaneo 1996; Kiss 2001; Paslin 1991; Sideri 1994). The placebo was a vehicle (e.g. the petrolatum base of the ointment or cream without the active ingredient) and not truly inert. One of the trials compared three topical interventions (clobetasol propionate, testosterone, and progesterone) against placebo (Bracco 1993). The other two studies used an active control: One compared testosterone and dihydrotestosterone (Paslin 1996), while the other compared pimecrolimus and clobetasol propionate (Goldstein 2011).
Outcomes
All of the studies included participant‐rated change in symptoms and investigator‐rated improvement of gross appearance. We originally planned a time point of outcome measurement at three to six months into therapy, but in one included trial outcomes were measured five weeks into therapy (Kiss 2001), while in another trial, measurement was made one year into therapy (Sideri 1994). We included the two trials, and the validity of this review did not appear to be affected (see Differences between protocol and review).
Two studies did not report adverse drug reactions (Paslin 1991; Paslin 1996), while one study did not fully report adverse drug reactions (Bracco 1993).
Funding source
One study was supported by a pharmaceutical company (Goldstein 2011), while the other six studies did not report any funding source (Bracco 1993; Cattaneo 1996; Kiss 2001; Paslin 1991; Paslin 1996; Sideri 1994).
Excluded studies
Of the 18 potentially eligible studies, we excluded 9 when we had looked at them in detail. The reasons for exclusion are listed in the 'Characteristics of excluded studies' tables.
Ongoing studies
We identified three ongoing trials; the available data are in the 'Characteristics of ongoing studies' tables.
Risk of bias in included studies
Many of the elements assessed in the risk of bias analysis were lacking in most of the studies (see 'Characteristics of included studies' tables). Our judgements about each methodological quality item for each included study are summarised in Figure 1, and our judgements about each methodological quality item presented as percentages across all of the included studies are summarised in Figure 2.
1.
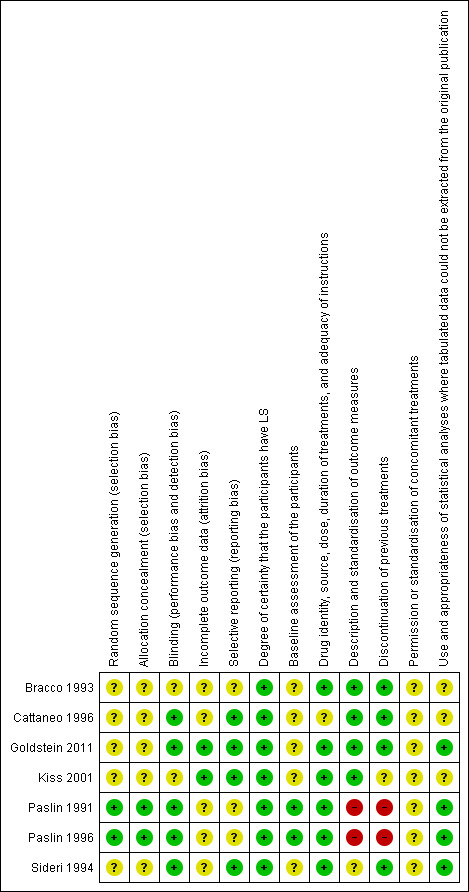
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
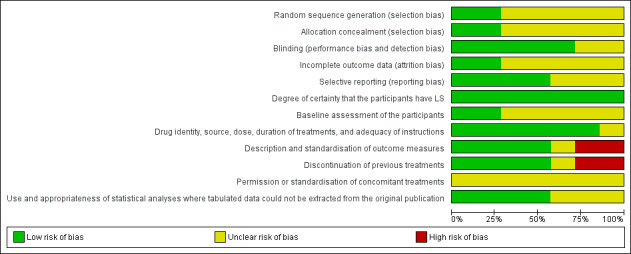
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Random sequence generation
Two studies used an adequate method of randomisation by coin tossing (Paslin 1991; Paslin 1996). All of the other six studies did not describe the process of randomisation (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001; NCT00757874; Sideri 1994).
Allocation concealment
Allocation could not be foreseen in the two studies using coin tossing for randomisation (Paslin 1991; Paslin 1996), as, in both of these studies, allocation was done by the office technician after the participants were enrolled.
It is unclear if allocation was concealed in the other six studies (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001; NCT00757874; Sideri 1994).
Blinding
In four studies (Goldstein 2011; Paslin 1991; Paslin 1996; Sideri 1994), both the investigators and participants were blinded. In another study (Cattaneo 1996) the investigator was blinded to the allocated treatments.
Incomplete outcome data
Only two studies reported withdrawal and dropout (Goldstein 2011; Kiss 2001).
Selective reporting
Four studies reported all of the three prespecified primary outcomes (Cattaneo 1996; Goldstein 2011; Kiss 2001; Sideri 1994). One trial did not fully report or fully describe adverse drug reactions (Bracco 1993), and two other trials did not report adverse drug reactions at all (Paslin 1991; Paslin 1996).
Other potential sources of bias
Certainty of diagnosis of LS
The diagnosis was confirmed by biopsy in all of the included studies (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001; Paslin 1991; Paslin 1996; Sideri 1994).
Baseline assessment of participants
Baseline assessment of the participants was not performed in five studies (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001; Sideri 1994). The participants in the two cross‐over trials were all middle‐ to old‐aged women (Paslin 1991; Paslin 1996).
Drug identity, source, dose, duration of treatments, and adequacy of instructions
The intervention was standardised in all of the included studies (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001; Paslin 1991; Paslin 1996; Sideri 1994).
Description and standardisation of outcome measures
Various scoring systems of symptoms and gross appearance were used, but they were only described in four studies (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001). One study used a scoring system to assess clinical and symptomatic status, but it did not provide relevant details (Sideri 1994). Another trial only standardised one outcome measure, vulval itching, by using a scoring system, but it did not report the rating results (Paslin 1996). Another trial by the same author did not describe the standardisation of outcome measures (Paslin 1991).
Discontinuation of previous treatments
Previous treatments were discontinued in five studies (Bracco 1993; Cattaneo 1996; Goldstein 2011; Paslin 1991; Paslin 1996). In one study, all of the participants received an initial two‐week course of topical antibiotic/corticosteroid cream before allocation (Sideri 1994). Another study did not report if previous treatments were discontinued (Kiss 2001).
Permission or standardisation of concomitant treatments
None of the studies provided relevant descriptions of concomitant treatments (Bracco 1993; Cattaneo 1996; Goldstein 2011; Kiss 2001; Paslin 1991; Paslin 1996; Sideri 1994).
Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publications
Two studies used appropriate statistical analyses (Goldstein 2011; Sideri 1994). Three studies did not use appropriate non‐parametric statistical methods (e.g. Wilcoxon signed rank test) for ranked outcome data (Bracco 1993; Cattaneo 1996; Kiss 2001). No statistical analyses were appropriate for the very small sample used in the two cross‐over studies (Paslin 1991; Paslin 1996).
Effects of interventions
We have addressed our prespecified outcomes, in relation to the interventions listed above, under Types of interventions. Our prespecified primary outcomes included the following:
1) Participant‐rated improvement of symptoms or remission of symptoms (in terms of quality of life, pain, itching, and less pain with intercourse).
2) Investigator‐rated global degree of improvement (in terms of pallor, purpura, hyperkeratosis, ulceration, erosion, erythema, sclerosis, and scarring).
3) Adverse drug reactions severe enough to require withdrawal of treatment, including severe skin irritation or infection.
All of the included trials, but one (Kiss 2001), reported participant‐rated improvement in symptoms. Only one trial specifically assessed pain with intercourse (Paslin 1996).
All seven included studies reported investigator‐rated improvement of gross appearance.
Five trials (Cattaneo 1996; Goldstein 2011; Kiss 2001; Sideri 1994; Bracco 1993) reported adverse drug reactions severe enough to require withdrawal of treatment, but the study by Bracco 1993 omitted reporting relevant data regarding progesterone. Two trials (Paslin 1991; Paslin 1996) did not report this primary outcome.
Our prespecified secondary outcomes included the following:
1) Adverse drug reactions that were not severe enough to require cessation of treatment, such as mild skin irritation, atrophy, or telangiectasia.
2) Duration of remission or prevention of subsequent flares, or both.
3) Development of genital squamous cell carcinoma (SCC) or genital intraepithelial neoplasia.
Four trials (Cattaneo 1996; Goldstein 2011; Kiss 2001; Bracco 1993) reported adverse drug reactions not severe enough to require withdrawal of treatment. The study by Bracco 1993 addressed this outcome after applying clobetasol propionate and testosterone, but it did not report adverse drug reactions to progesterone. Two of the included studies did not report this outcome at all (Paslin 1991; Paslin 1996).
None of the included studies reported the other two secondary outcomes.
Topical corticosteroids
The efficacy of only two topical corticosteroids, clobetasol propionate 0.05% (very potent) and mometasone furoate 0.05% ointment (potent), were assessed in two of the included studies (Bracco 1993; Kiss 2001).
Clobetasol propionate vs placebo
In the study by Bracco 1993, the efficacy of topical clobetasol propionate was compared to placebo after three months' application. Clobetasol propionate was significantly better than placebo in relation to the following outcomes: 'participant‐rated improvement or remission of symptoms' (RR 2.85, 95% CI 1.45 to 5.61) (see Analysis 1.1) and 'investigator‐rated global degree of improvement' (SMD 5.74, 95% CI 4.26 to 7.23) (see Analysis 1.2). The study found no events of adverse drug reactions (e.g. predisposition to infection, worsening of skin atrophy, and contact dermatitis) in either the clobetasol propionate or placebo group.
1.1. Analysis.
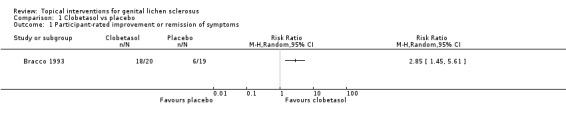
Comparison 1 Clobetasol vs placebo, Outcome 1 Participant‐rated improvement or remission of symptoms.
1.2. Analysis.
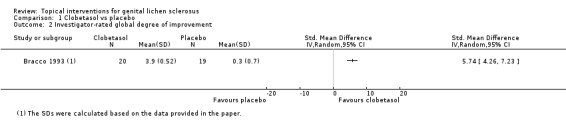
Comparison 1 Clobetasol vs placebo, Outcome 2 Investigator‐rated global degree of improvement.
Mometasone furoate vs placebo
In the study by Kiss 2001, the efficacy of topical mometasone furoate was compared to placebo after five weeks' application. With regard to the outcome 'investigator‐rated change in clinical score of phimosis from baseline', the mean clinical grade of phimosis improved in the mometasone furoate group, but worsened in the placebo group (SMD ‐1.04, 95% CI ‐1.77 to ‐0.31) (see Analysis 2.1). No local or systemic adverse drug reactions occurred in either group.
2.1. Analysis.
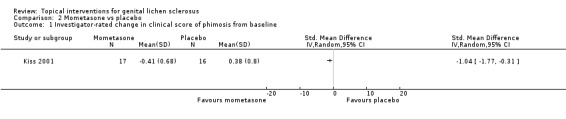
Comparison 2 Mometasone vs placebo, Outcome 1 Investigator‐rated change in clinical score of phimosis from baseline.
Topical androgens
The efficacy of two androgens, testosterone propionate (2% cream) and dihydrotestosterone (2% cream), were studied in five of the included studies (Bracco 1993; Cattaneo 1996; Paslin 1991; Paslin 1996; Sideri 1994).
Testosterone vs placebo
The study period of the Bracco 1993 and Sideri 1994 studies was three months and one year, respectively. In the studies by Bracco 1993 and Sideri 1994, there was no significant difference in the efficacy of testosterone compared to placebo in relation to the outcome 'participant‐rated improvement or remission of symptoms' when the 2 studies were combined (RR 1.21, 95% CI 0.56 to 2.64) (see Analysis 3.1). The outcome 'investigator‐rated improvement of gross appearance' was only reported by Bracco 1993, but there was no significant difference between the 2 groups (SMD 0.42, 95% CI ‐0.21 to 1.06) (see Analysis 3.2).
3.1. Analysis.
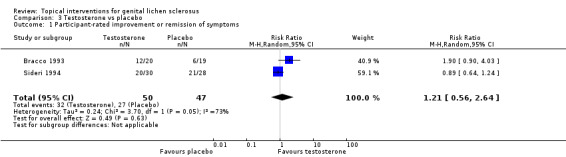
Comparison 3 Testosterone vs placebo, Outcome 1 Participant‐rated improvement or remission of symptoms.
3.2. Analysis.
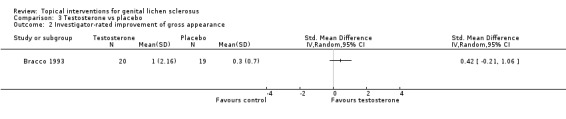
Comparison 3 Testosterone vs placebo, Outcome 2 Investigator‐rated improvement of gross appearance.
No significant difference in severe adverse drug reactions was found between the testosterone and placebo groups when the 2 studies were combined (RR 5.19, 95% CI 0.62 to 43.19) (see Analysis 3.3).
3.3. Analysis.
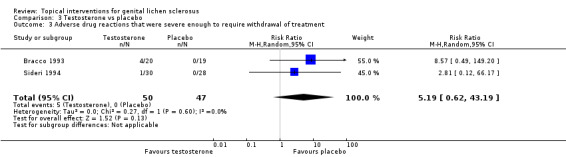
Comparison 3 Testosterone vs placebo, Outcome 3 Adverse drug reactions that were severe enough to require withdrawal of treatment.
Dihydrotestosterone vs placebo
A very small cross‐over trial (Paslin 1991) randomised five participants to receive either dihydrotestosterone or placebo for three months, before switching to the other for three months. The trial lacked a wash‐out period, and a carry‐over effect appeared in two out of three women who used dihydrotestosterone before cross‐over. We, therefore, used only the data from the first period before cross‐over for analysis. No participants showed an improvement in their symptoms after either preparation. No significant difference in 'investigator‐rated improvement of gross appearance' was found between dihydrotestosterone and placebo (RR 5.25, 95% CI 0.41 to 67.73) (see Analysis 4.1).
4.1. Analysis.
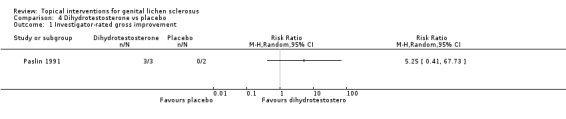
Comparison 4 Dihydrotestosterone vs placebo, Outcome 1 Investigator‐rated gross improvement.
Testosterone vs clobetasol propionate
The study by Bracco 1993 found that after 3 months' application, testosterone was significantly less effective than clobetasol propionate with regard to the following outcomes: 'participant‐rated improvement or remission of symptoms' (RR 0.67, 95% CI 0.45 to 0.98) (see Analysis 5.1) and 'investigator‐rated global degree of improvement' (SMD ‐1.81, 95% CI ‐2.56 to ‐1.06) (see Analysis 5.2).
5.1. Analysis.
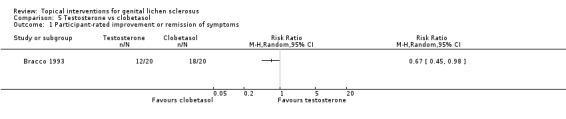
Comparison 5 Testosterone vs clobetasol, Outcome 1 Participant‐rated improvement or remission of symptoms.
5.2. Analysis.
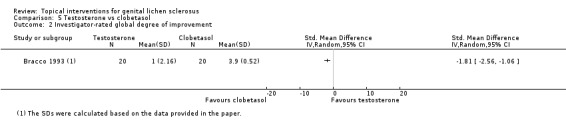
Comparison 5 Testosterone vs clobetasol, Outcome 2 Investigator‐rated global degree of improvement.
No significant differences in adverse drug reactions were found between the testosterone and clobetasol propionate groups with regard to our primary outcome 'adverse drug reactions that were severe enough to require withdrawal of treatment' (RR 3.00, 95% CI 0.13 to 69.52) (see Analysis 5.3) or our secondary outcome 'adverse drug reactions that were not severe enough to require cessation of treatment' (RR 7.00, 95% CI 0.38 to 127.32) (see Analysis 5.4).
5.3. Analysis.
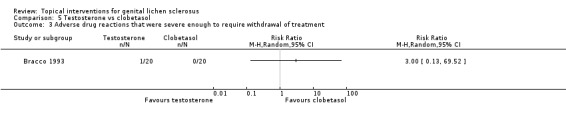
Comparison 5 Testosterone vs clobetasol, Outcome 3 Adverse drug reactions that were severe enough to require withdrawal of treatment.
5.4. Analysis.
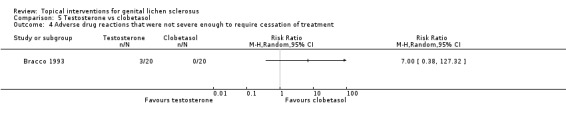
Comparison 5 Testosterone vs clobetasol, Outcome 4 Adverse drug reactions that were not severe enough to require cessation of treatment.
Testosterone vs dihydrotestosterone
A very small cross‐over trial (Paslin 1996) randomised five participants to receive either testosterone or dihydrotestosterone for three months, before switching to the other for three months. The trial lacked a wash‐out period, and we, thus, used only the data from the first period before cross‐over for analysis. The trial did not find significant differences in efficacy between the 2 androgens in relation to the outcomes 'participant‐rated remission of itching' (RR 0.25, 95% CI 0.01 to 4.23) (see Analysis 6.1) and 'investigator‐rated gross improvement' (RR 1.00, 95% CI 0.53 to 1.87) (see Analysis 6.2). Of the two women who were sexually active and received testosterone before cross‐over, one did not have dyspareunia after treatment. Both of the women who received dihydrotestosterone before cross‐over were not sexually active. Therefore, we could not compare the effects of the two androgens on dyspareunia.
6.1. Analysis.
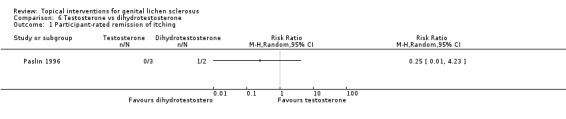
Comparison 6 Testosterone vs dihydrotestosterone, Outcome 1 Participant‐rated remission of itching.
6.2. Analysis.
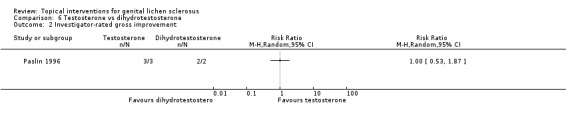
Comparison 6 Testosterone vs dihydrotestosterone, Outcome 2 Investigator‐rated gross improvement.
Testosterone vs placebo as maintenance therapy
The study by Cattaneo 1996 investigated whether topical testosterone could control the symptoms and signs of vulval LS after an initial 24‐week treatment with 0.05% clobetasol propionate cream. The study found that testosterone when used as maintenance therapy for 24 weeks worsened the symptoms (P < 0.05), while the vehicle‐based placebo caused no change in symptoms or gross appearance (see Analysis 7.1 and Analysis 7.2). The Mann‐Whitney U test was used to compare the scores before and after the interventions. However, the Wilcoxon signed rank test should be used to compare the paired data. No adverse drug reaction events 'severe enough to require withdrawal of treatment' occurred in either of the two groups. There was no significant difference in adverse drug reactions 'severe enough to require withdrawal of treatment' in either the testosterone or the placebo groups (RR 9.00, 95% CI 0.52 to 154.56) (see Analysis 7.3).
7.1. Analysis.
Comparison 7 Testosterone vs placebo as maintenance therapy, Outcome 1 Participant‐rated global degree of improvement.
| Participant‐rated global degree of improvement | ||
|---|---|---|
| Study | Testosterone | Placebo |
| Cattaneo 1996 | The symptom score significantly worsened from 6 to 23 (p < 0.05). | No significant change in the symptom score. |
7.2. Analysis.
Comparison 7 Testosterone vs placebo as maintenance therapy, Outcome 2 Investigator‐rated global degree of improvement.
| Investigator‐rated global degree of improvement | ||
|---|---|---|
| Study | Testosterone | Placebo |
| Cattaneo 1996 | No significant change in the score of gross appearance. | No significant change in the score of gross appearance. |
7.3. Analysis.
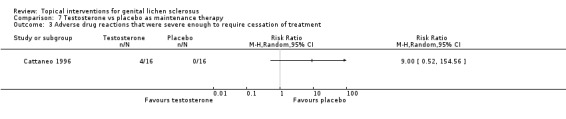
Comparison 7 Testosterone vs placebo as maintenance therapy, Outcome 3 Adverse drug reactions that were severe enough to require cessation of treatment.
Topical progesterone
In the study by Bracco 1993, topical application of progesterone (2% cream) for 3 months was not significantly better than placebo for either 'participant‐rated improvement or remission of symptoms' (RR 1.58, 95% CI 0.72 to 3.50) (see Analysis 8.1) or for 'investigator‐rated global degree of improvement' (SMD 0.34, 95% CI ‐0.29 to 0.97) (see Analysis 8.2).
8.1. Analysis.
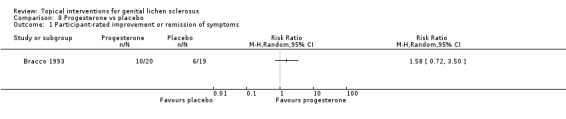
Comparison 8 Progesterone vs placebo, Outcome 1 Participant‐rated improvement or remission of symptoms.
8.2. Analysis.
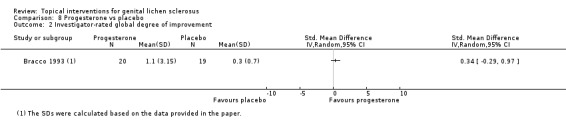
Comparison 8 Progesterone vs placebo, Outcome 2 Investigator‐rated global degree of improvement.
Topical immunomodulators (tacrolimus, pimecrolimus, ciclosporin)
One study tested the efficacy and safety of pimecrolimus (1% cream) against clobetasol propionate (0.05% cream) after 12 weeks' application (Goldstein 2011). Both were effective in relieving pruritus and burning/pain, and there were no significant differences between pimecrolimus and clobetasol propionate in relieving pruritus and burning/pain: change in pruritus (SMD ‐0.33, 95% CI ‐0.99 to 0.33) (see Analysis 9.1), change in burning/pain (SMD 0.03, 95% CI ‐0.62 to 0.69) (see Analysis 9.2). Investigator Global Assessment showed both preparations were effective. However, pimecrolimus was less effective than clobetasol propionate in relation to the 'investigator‐rated global degree of improvement' (SMD ‐1.64, 95% CI ‐2.40 to ‐0.87) (see Analysis 9.3). No adverse drug reactions occurred in either the pimecrolimus or clobetasol propionate group.
9.1. Analysis.
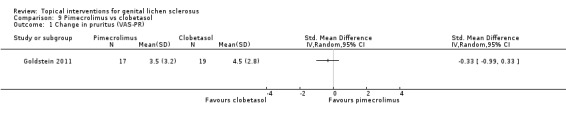
Comparison 9 Pimecrolimus vs clobetasol, Outcome 1 Change in pruritus (VAS‐PR).
9.2. Analysis.
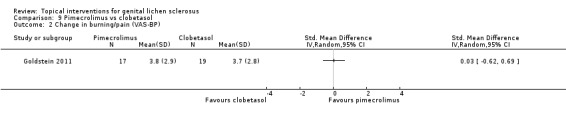
Comparison 9 Pimecrolimus vs clobetasol, Outcome 2 Change in burning/pain (VAS‐BP).
9.3. Analysis.
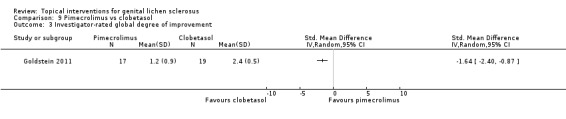
Comparison 9 Pimecrolimus vs clobetasol, Outcome 3 Investigator‐rated global degree of improvement.
Discussion
Summary of main results
The very potent topical steroid, clobetasol propionate 0.05%, was found to be significantly more effective than placebo in treating genital lichen sclerosus in relation to the outcomes 'participant‐rated improvement or remission of symptoms' (RR 2.85, 95% CI 1.45 to 5.61) and 'investigator‐rated global degree of improvement' (SMD 5.74, 95% CI 4.26 to 7.23) (Bracco 1993). When the potent topical steroid, mometasone furoate 0.05%, was compared to placebo in treating penile lichen sclerosus, there was a significant improvement in the 'investigator rated change in clinical grade of phimosis' (SMD ‐1.04, 95% CI ‐1.77 to ‐0.31) (Kiss 2001).
Improvement in gross appearance after topical application of either testosterone or dihydrotestosterone was found, according to the investigators, in a very small cross‐over trial without placebo control on 5 women (3 women used topical testosterone, and 2 women used topical dihydrotestosterone first) (Paslin 1996). However, no improvement in subjective symptoms was observed. Furthermore, two other studies did not find significant efficacy of testosterone in either symptoms or gross appearance (Bracco 1993; Sideri 1994). When used as maintenance therapy following initial corticosteroid therapy, topical testosterone worsened symptoms (P < 0.05), but the vehicle‐based placebo did not (Cattaneo 1996). After considering all of the data, we concluded that there is no evidence to support the use of topical androgens in treating genital lichen sclerosus. And the observed gross changes, e.g. clitoral enlargement (Paslin 1996), were, most likely, a result of their virilising effect (e.g. development of male sex characteristics in women, such as swelling of the clitoris). There is also no evidence to support the use of another topical sex hormone, progesterone, in treating genital lichen sclerosus (Bracco 1993).
The current evidence found no differences between topical pimecrolimus and topical clobetasol propionate in reducing pruritus (SMD ‐0.33, 95% CI ‐0.99 to 0.33) and burning/pain (SMD 0.03, 95% CI ‐0.62 to 0.69). However, clobetasol propionate was only applied once daily in this trial. Thus, the comparable efficacy of pimecrolimus might have been overestimated. On the other hand, pimecrolimus was less effective than clobetasol propionate in relation to the outcome 'investigator‐rated global degree of improvement' (SMD ‐1.64, 95% CI ‐2.40 to ‐0.87). Furthermore, on histopathological examinations, clobetasol propionate was superior to pimecrolimus in improving inflammation (P = 0.015). However, this was not a prespecified clinical outcome of interest for our review (Goldstein 2011).
Overall completeness and applicability of evidence
All but one study enrolled adult women with vulval lichen sclerosus as participants (Bracco 1993; Cattaneo 1996; Goldstein 2011; Paslin 1991; Paslin 1996; Sideri 1994). Only one study enrolled boys with penile lichen sclerosus as participants (Kiss 2001). This limitation compromises the external validity of the current evidence.
The efficacy of only two different topical corticosteroids, clobetasol propionate and mometasone furoate, has been demonstrated in RCTs (Bracco 1993; Kiss 2001). The concentration of mometasone furoate used in the trial was 0.05% (Kiss 2001), which was half the usual concentration of 0.1%. It is likely that other potent or moderate topical corticosteroids are effective in treating lichen sclerosus, but relevant RCTs are unavailable.
The regimen of clobetasol propionate varied among the trials. In 1 trial (Bracco 1993), clobetasol propionate 0.05% was applied twice daily for 1 month then once daily for 2 months. In another trial comparing pimecrolimus and clobetasol propionate, clobetasol propionate was applied once daily (Goldstein 2011).
We did not find RCTs comparing the efficacy of different regimens of topical clobetasol propionate in treating genital lichen sclerosus. Two previous RCTs of halcinonide (another very potent topical corticosteroid) on extragenital skin found that once‐daily application of halcinonide 0.1% cream was as effective as thrice‐daily application of the same cream in treating psoriasis and atopic dermatitis (Fredriksson 1980; Sudilovsky 1981). However, thrice‐daily application had a more rapid onset of action (Fredriksson 1980) and was superior to once‐daily application, especially for severe psoriasis (Sudilovsky 1981). In an uncontrolled before‐and‐after study on topical clobetasol propionate 0.05% cream for treating vulval lichen sclerosus in 15 women (Dalziel 1991), the cream was applied twice daily for 12 weeks and had remarkable efficacy on symptoms and clinical appearance in 13 women who completed the study. Randomised controlled trials comparing the efficacy of various regimens of topical clobetasol propionate should be conducted.
One trial found pimecrolimus effective in treating genital lichen sclerosus (Goldstein 2011); however, it is only licensed as a second‐line therapy for atopic dermatitis, and it is not indicated for use in children younger than two years of age (Novartis 2010). We identified an ongoing trial on tacrolimus (NCT00757874), but the author did not provide us with any data, despite repeated requests, so we are unable to include this study in the current review.
The study period of the included studies ranged from five weeks to one year. None of them were able to assess the long‐term effects on development of genital squamous cell carcinoma or genital intraepithelial neoplasia. This question may only be answered by trials with very long follow‐up periods.
The scarring of lichen sclerosus may cause fusion of the labia, narrowing of the vaginal introitus, and burying of the clitoris. Thus, women may have dyspareunia (painful sexual intercourse) or less pleasurable sex, and the quality of sex life should be a clinical outcome. However, only a very small study ever specifically examined this outcome (Paslin 1996).
Quality of the evidence
The sample size of all seven studies was small; the total number of participants was 249. The small sample size may lead to insufficient statistical power to detect significant differences in outcomes. However, most of the efficacy estimates were quite precise except for the two small cross‐over trials (Paslin 1991; Paslin 1996).
Most of the studies were published before 1996, when the Consolidated Standards of Reporting Trials (CONSORT) Statement was proposed. Therefore, most studies did not provide a full description of the methods of random sequence generation, allocation concealment, blinding, withdrawal or dropout, and permission or standardisation of concomitant treatments (Bracco 1993; Cattaneo 1996; Paslin 1991; Paslin 1996; Sideri 1994). Although two studies were published after 1996 (Goldstein 2011; Kiss 2001), they did not report the methods of random sequence generation, allocation concealment, and permission or standardisation of concomitant treatments.
A drawback of the included studies was the use of inappropriate statistical methods (Figure 1). Three studies used parametric methods like the Student's t‐test to compare the scores before and after interventions (Bracco 1993; Cattaneo 1996; Kiss 2001), but non‐parametric methods, such as the Wilcoxon signed rank test, should be used in such circumstances. However, it is unclear whether the study results would change substantially if the trialists had used non‐parametric tests. The Cattaneo 1996 trial compared the scores before and after topical testosterone and placebo, respectively. However, it did not make a direct comparison of the efficacy of the two tested interventions. Two studies did not report numerical data of the change in symptoms and gross appearance, nor use statistical analysis to assess the efficacy of interventions (Paslin 1991; Paslin 1996).
We did not use histopathological change as an outcome measure in this review, because it is not a clinical outcome relevant to the well‐being of affected people. Six studies included histopathological changes as an outcome (Bracco 1993; Cattaneo 1996; Goldstein 2011; Paslin 1991; Paslin 1996; Sideri 1994). In most of these studies, the histopathological changes were in line with the clinical outcomes. The exception was one study comparing topical testosterone and placebo in which the symptoms were reported to improve in both groups, but gross appearance or histological findings did not change (Sideri 1994).
Potential biases in the review process
All but one of the included studies were published more than a decade ago. Therefore, some authors did not keep the original data and could not provide missing data for our analyses. For example, the authors of one study could not find the original data and provide us with the standard deviation (SD) of the mean change in gross appearance (Bracco 1993). We, thus, calculated the data based on the information reported in the paper, which might reduce the precision of our effect estimates.
Agreements and disagreements with other studies or reviews
A guideline (Neill 2010) and a review (Pugliese 2007) on the management of lichen sclerosus were published prior to our review. The guideline searched MEDLINE and EMBASE from 2002 to 2009 (Neill 2010), and the review searched PubMed, MEDLINE, and other electronic databases between 1950 and 2006 (Pugliese 2007). However, neither of them were systematic reviews because of a lack of critical appraisal (e.g. assessment of 'Risk of bias') of the included studies.
The British Association of Dermatologists' 2010 guidelines for the management of lichen sclerosus (Neill 2010) were in line with our findings in this review that very potent topical corticosteroids are the mainstay of treatments for genital lichen sclerosus. The guidelines also stated that there was a lack of evidence for the use of topical testosterone or other hormonal treatments. They did not recommend topical calcineurin inhibitors as first‐line treatment because of case reports of the development of squamous cell carcinoma following the use of these drugs and lack of relevant long‐term safety data.
The review published in 2007 (Pugliese 2007) also agreed with our review that very potent topical corticosteroids are the primary topical treatments for genital lichen sclerosus, but it had no data on topical calcineurin inhibitors.
Authors' conclusions
Implications for practice.
The current evidence indicates that topical clobetasol propionate 0.05% is effective in treating genital lichen sclerosus. Topical mometasone furoate has been shown to be effective in boys with penile lichen sclerosus. It is unclear whether other topical corticosteroids are effective. The current evidence found no significant difference between topical pimecrolimus and clobetasol propionate in the efficacy of relieving symptoms, but the former is less effective than the latter in improving gross appearance and reducing inflammation.
Implications for research.
The current evidence is limited, and further studies are required to fill in some gaps in knowledge.
Firstly, we need RCTs determining the potency and regimen (e.g. frequency and duration of application) of topical corticosteroids that have adequate therapeutic efficacy but with the least desirable adverse effects (e.g. infections and skin thinning).
Secondly, we only found that a limited number of topical interventions (e.g. topical corticosteroids), sex hormones, and calcineurin inhibitors have been tested. Randomised controlled trials testing other interventions (see Types of interventions) are awaited.
Thirdly, one of our secondary outcomes, 'duration of remission or prevention of subsequent flares', should be included in future RCTs, although this means that long follow‐up periods are required.
Fourthly, it remains unknown whether effective treatments can reduce the risk of development of genital squamous cell carcinoma (SCC) or genital intraepithelial neoplasia from lichen sclerosus. Randomised controlled trials of adequate length to answer this question should be conducted. Given the 5% risk of SCC development in women with genital lichen sclerosus, a sample size of at least 984 treated and 984 untreated participants is needed to determine if a treatment can halve this risk, based on a significance level of 0.05 and power of 0.8.
Last, but not least, the quality of the sex lives of people with this condition should be examined in future trials.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2015 | Amended | Author information (affiliation) updated. |
Acknowledgements
The Cochrane Skin Group editorial base would like to thank the following people who commented on this update: our Key Editor Michael Bigby, our Statistical Editor Jo Leonardi‐Bee, our Methodological Editor Sarah Garner, Hazel Bell and Sallie Neill who were the clinical referees, and Jack Tweed who was the consumer referee.
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1(lichen and (sclerosus or atrophi* or albus or scleureux or sclero‐atrophi* or vulva*)) #2(balanitis and (xerotica or obliteran* or sclerotica)) #3(vulva* and dystroph*) or (white and spot and disease*) #4MeSH descriptor Lichen Sclerosus et Atrophicus explode all trees #5MeSH descriptor Vulvar Lichen Sclerosus explode all trees #6MeSH descriptor Balanitis explode all trees #7(#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8(SR‐SKIN) #9(#7 AND NOT #8)
Appendix 2. MEDLINE (OVID) search strategy
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. (animals not (human and animals)).sh. 10. 8 not 9 11. lichen sclerosus.mp. or exp Lichen Sclerosus et Atrophicus/ 12. (kraurosis vulvae or kraurosis vulva).mp. or exp Vulvar Lichen Sclerosus/ 13. lichen albus.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 14. exp Balanitis Xerotica Obliterans/ or exp Balanitis/ or balanitis.mp. 15. kraurosis penis.mp. 16. balanitis sclerotica obliterans.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 17. balanitis sclerotica.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 18. white spot disease.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 19. sclero‐atrophic lichen.mp. 20. lichen scleureux.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 21. lichen sclero‐atrophique.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 22. vulval dystrophy.mp. 23. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 24. 23 and 10
Appendix 3. EMBASE (OVID) search strategy
1. random$.mp. 2. factorial$.mp. 3. (crossover$ or cross‐over$).mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. (assign$ or allocat$).mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. lichen sclerosus.mp. or exp Lichen Sclerosus et Atrophicus/ 15. (kraurosis vulvae or kraurosis vulva).mp. or exp Vulvar Lichen Sclerosus/ 16. lichen albus.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 17. exp Balanitis Xerotica Obliterans/ or exp Balanitis/ or balanitis.mp. 18. kraurosis penis.mp. 19. balanitis sclerotica obliterans.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 20. balanitis sclerotica.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 21. white spot disease.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 22. sclero‐atrophic lichen.mp. 23. lichen scleureux.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 24. lichen sclero‐atrophique.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 25. vulval dystrophy.mp. 26. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. 13 and 26
Appendix 4. LILACS search strategy
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Words] and (lichen or liquen) or balanitis or (krausosis or craurosis) or vulva$ [Words]
Appendix 5. CINAHL search strategy
(PT Clinical trial OR PT Drugs OR AB randomi?ed OR AB placebo OR AB randomly OR TI trial) AND (lichen sclerosus OR kraurosis vulvae OR kraurosis vulva OR vulvar lichen sclerosus OR lichen albus OR balanitis xerotica obliterans OR balanitis OR kraurosis penis OR balanitis sclerotica obliterans OR balanitis sclerotica OR white spot disease OR sclero‐atrophic lichen OR lichen scleureux OR lichen sclero‐atrophique OR vulval dystrophy)
Appendix 6. BNIA search strategy
1. randomi#ed controlled trial.mp. 2. controlled clinical trial.mp. 3. randomi#ed.ab. 4. placebo.ab. 5. clinical trial.mp. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. lichen sclerosus.mp.
10. (kraurosis vulvae or kraurosis vulva).mp.
11. lichen albus.mp.
12. balanitis.mp.
13. kraurosis penis.mp.
14. balanitis sclerotica obliterans.mp.
15. balanitis sclerotica.mp.
16. white spot disease.mp.
17. sclero‐atrophic lichen.mp.
18. lichen scleureux.mp.
19. lichen sclero‐atrophique.mp.
20. vulval dystrophy.mp.
21. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22. 8 and 21
Appendix 7. SCI‐EXPANDED, BIOSIS Previews, and CPCI‐S search strategy
#1 TS=(randomi*ed controlled trial)
#2 TS=(controlled clinical trial)
#3 TS=(randomi*ed)
#4 TS=(placebo)
#5 TS=(clinical trial)
#6 TS=(randomly)
#7 TI=(trial)
#8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#9 TS=(lichen sclerosus)
#10 TS=(kraurosis vulvae OR kraurosis vulva)
#11 TS=(lichen albus)
#12 TS=(balanitis)
#13 TS=(kraurosis penis)
#14 TS=(balanitis sclerotica obliterans)
#15 TS=(balanitis sclerotica)
#16 TS=(white spot disease)
#17 TS=(sclero‐atrophic lichen)
#18 TS=(lichen scleureux)
#19 TS=(lichen sclero‐atrophique)
#20 TS=(vulval dystrophy)
#21 #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9
#22 #8 AND #21
Appendix 8. Trials registers search strategy
lichen sclerosus OR kraurosis vulvae OR kraurosis vulva OR lichen albus OR balanitis xerotica obliterans OR balanitis OR kraurosis penis OR balanitis sclerotica obliterans OR balanitis sclerotica OR white spot disease OR sclero‐atrophic lichen OR lichen scleureux OR lichen sclero‐atrophique OR vulval dystrophy
Appendix 9. Conference Papers Index search strategy
(KW=(randomi*ed controlled trial OR controlled clinical trial OR randomi*ed OR placebo OR clinical trial OR randomly) OR (TI=trial)) AND (KW=lichen sclerosus OR kraurosis vulvae OR kraurosis vulva OR lichen albus OR balanitis xerotica obliterans OR balanitis OR kraurosis penis OR balanitis sclerotica obliterans OR balanitis sclerotica OR white spot disease OR sclero‐atrophic lichen OR lichen scleureux OR lichen sclero‐atrophique OR vulval dystrophy)
Data and analyses
Comparison 1. Clobetasol vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐rated improvement or remission of symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Investigator‐rated global degree of improvement | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Mometasone vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐rated change in clinical score of phimosis from baseline | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Testosterone vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐rated improvement or remission of symptoms | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.56, 2.64] |
| 2 Investigator‐rated improvement of gross appearance | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Adverse drug reactions that were severe enough to require withdrawal of treatment | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 5.19 [0.62, 43.19] |
Comparison 4. Dihydrotestosterone vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐rated gross improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Testosterone vs clobetasol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐rated improvement or remission of symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Investigator‐rated global degree of improvement | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Adverse drug reactions that were severe enough to require withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse drug reactions that were not severe enough to require cessation of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Testosterone vs dihydrotestosterone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐rated remission of itching | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Investigator‐rated gross improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Testosterone vs placebo as maintenance therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐rated global degree of improvement | Other data | No numeric data | ||
| 2 Investigator‐rated global degree of improvement | Other data | No numeric data | ||
| 3 Adverse drug reactions that were severe enough to require cessation of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Progesterone vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐rated improvement or remission of symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Investigator‐rated global degree of improvement | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Pimecrolimus vs clobetasol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in pruritus (VAS‐PR) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Change in burning/pain (VAS‐BP) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Investigator‐rated global degree of improvement | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bracco 1993.
| Methods | This is a randomised controlled trial. | |
| Participants |
|
|
| Interventions | 4 topical drugs including the following: A: testosterone (2%); B: progesterone (2%); C: clobetasol propionate (0.05%); and D: a cream‐based preparation. All topical drugs were applied twice daily for 3 months, except clobetasol propionate which was applied twice daily for 1 month then once daily for 2 months. |
|
| Outcomes |
Outcomes of the trial 1) Symptoms (itching, burning, pain, and dyspareunia) 2) Gross appearance (hyperkeratosis, purpura, thickness of plaques, atrophy, and erosions) 3) Histological features (epidermal atrophy, oedema, intensity of inflammatory infiltrate, and fibrosis) All were classified according to a 0‐ to 3‐point scoring system. |
|
| Notes | Setting: university hospital Country: Italy Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no description of the process of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | There was no description of the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | There was no description of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of withdrawal or dropout. |
| Selective reporting (reporting bias) | Unclear risk | Symptoms and gross appearance were assessed, but adverse drug reactions were not fully described. |
| Degree of certainty that the participants have LS | Low risk | LS was proven by biopsy. |
| Baseline assessment of the participants | Unclear risk | No comparisons of baseline characteristics between the 2 groups were made. |
| Drug identity, source, dose, duration of treatments, and adequacy of instructions | Low risk | The treatments were standardised. |
| Description and standardisation of outcome measures | Low risk | A 0‐to‐3 point scoring system of symptoms was used which included gross appearance and histological features. |
| Discontinuation of previous treatments | Low risk | There was no use of a topical corticosteroid for at least 3 months preceding the trial. |
| Permission or standardisation of concomitant treatments | Unclear risk | There was no relevant description. |
| Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication | Unclear risk | The only outcome we could not extract tabulated data for was 'investigator‐rated global degree of improvement'. The trialist used Student's t‐test to compare the scores before and after treatments, but Wilcoxon signed rank test should be used. |
Cattaneo 1996.
| Methods | This was a randomised controlled trial. | |
| Participants |
|
|
| Interventions | A: testosterone propionate 2% B: placebo (petrolatum vehicle alone) These were taken once daily as maintenance therapy for 24 weeks. |
|
| Outcomes |
Outcomes of the trial 1) Symptoms (itching, burning, soreness, and dryness) 2) Gross aspects (hyperkeratosis, atrophy, and sclerosis) 3) Histological features (epidermal atrophy, oedema, inflammatory infiltrate, and fibrosis) All were evaluated using a 0‐ to 3‐point scoring system. |
|
| Notes | Setting: university hospital Country: Italy Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no description of the process of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | There was no description of the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The investigators were blinded to the treatment used by each participant. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of withdrawal or dropout. |
| Selective reporting (reporting bias) | Low risk | Symptoms, gross aspects, and adverse drug reactions were reported. |
| Degree of certainty that the participants have LS | Low risk | LS was proven by biopsy. |
| Baseline assessment of the participants | Unclear risk | This was not performed. |
| Drug identity, source, dose, duration of treatments, and adequacy of instructions | Unclear risk | The treatments were standardised. |
| Description and standardisation of outcome measures | Low risk | A 0‐to‐3 point scoring system of symptoms was used which included gross aspects and histological features. |
| Discontinuation of previous treatments | Low risk | All of the women discontinued previous treatments. |
| Permission or standardisation of concomitant treatments | Unclear risk | There was no relevant description. |
| Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication | Unclear risk | The Mann‐Whitney U test was used to compare the scores before and after treatments, but the Wilcoxon signed rank test should be used. |
Goldstein 2011.
| Methods | This was a randomised controlled trial. | |
| Participants |
|
|
| Interventions | A: pimecrolimus cream 1% twice daily B: alternate clobetasol cream 0.05% (in the evening) and vehicle cream (in the morning) These were taken for 12 weeks. |
|
| Outcomes |
Primary outcomes of the trial 1) Histopathological change in inflammation (0 to 4 scale) Secondary outcomes of the trial 1) Change from baseline in pruritus (VAS‐PR) and burning/pain (VAS‐BP) rated by participants using 0 to 10 point visual analogue scale questionnaires 2) Investigator's Global Assessment of the severity of disease (0 to 3 scale), clinical evaluation of lichenification (0 to 3 scale), and clinical valuation of ulceration/fissuring (0 to 3 scale) |
|
| Notes | Setting: specialist clinic (Center for Vulvovaginal Disorders) Country: US Funding source: Novartis Pharmaceuticals Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no description of the process of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | There was no description of the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The investigators and participants were blinded to the treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women were excluded (in 1 LS was not confirmed, and in the other the biopsy slide was lost). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the prespecified outcomes have been reported. |
| Degree of certainty that the participants have LS | Low risk | LS was proven by biopsy. |
| Baseline assessment of the participants | Unclear risk | This was not performed. |
| Drug identity, source, dose, duration of treatments, and adequacy of instructions | Low risk | The treatments were standardised. |
| Description and standardisation of outcome measures | Low risk | The outcome measures were described and standardised. |
| Discontinuation of previous treatments | Low risk | There was no use of systemic immunosuppressants or topical therapy within 4 weeks prior to participation in the trial. |
| Permission or standardisation of concomitant treatments | Unclear risk | There was no relevant description. |
| Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication | Low risk | The trialists used correct statistical tests in their paper and provided us with further detailed data for analysis at our request. |
Kiss 2001.
| Methods | This was a randomised controlled trial. | |
| Participants |
|
|
| Interventions | A: mometasone furoate 0.05% ointment B: placebo once daily These were taken for 5 weeks. |
|
| Outcomes |
Outcomes of the trial 1) Clinical score of phimosis |
|
| Notes | Setting: children's hospital Country: Hungary Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no description of the process of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | There was no description of the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | There was no description of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 boys were lost to follow up ‐ 3 in whom clinically suspected penile LS was not confirmed by pathological examination. There was no significant difference in the withdrawal rate between the treatment and control groups. |
| Selective reporting (reporting bias) | Low risk | A change in the clinical score of phimosis and adverse drug reactions was reported. |
| Degree of certainty that the participants have LS | Low risk | All penile LS cases underwent circumcision, and those that could not be proven by biopsy were excluded. |
| Baseline assessment of the participants | Unclear risk | This was not performed. |
| Drug identity, source, dose, duration of treatments, and adequacy of instructions | Low risk | The treatments were standardised. |
| Description and standardisation of outcome measures | Low risk | Phimosis was graded 1 to 4. |
| Discontinuation of previous treatments | Unclear risk | There was no relevant description. |
| Permission or standardisation of concomitant treatments | Unclear risk | There was no relevant description. |
| Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication | Unclear risk | Student's t‐test was used to compare the scores before and after treatments, but Wilcoxon signed rank test should have been used. |
Paslin 1991.
| Methods | This was a randomised cross‐over trial. | |
| Participants |
|
|
| Interventions | A: dihydrotestosterone 2% B: placebo (white petrolatum vehicle) These were taken twice daily for 3 months, then treatment was reversed for another 3 months. |
|
| Outcomes |
Outcomes of the trial 1) Subjective symptoms 2) Objective gross improvement (documented by photographs) 3) Histopathological findings |
|
| Notes | Setting: private practice Country: US Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on coin tossing. |
| Allocation concealment (selection bias) | Low risk | Allocation could not be foreseen since randomisation was based on coin tossing by the office technician after the participants were enrolled. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither the investigator nor the participants knew which medicine was being applied. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of withdrawal or dropout. |
| Selective reporting (reporting bias) | Unclear risk | Symptoms and objective gross improvement were assessed, but adverse drug reactions were not reported. |
| Degree of certainty that the participants have LS | Low risk | LS was proven by biopsy. |
| Baseline assessment of the participants | Low risk | The participant was her own control since the study was a cross‐over trial. All of the participants were middle‐ to old‐aged women. |
| Drug identity, source, dose, duration of treatments, and adequacy of instructions | Low risk | The treatments were standardised. |
| Description and standardisation of outcome measures | High risk | No grading system was used to assess the outcomes. |
| Discontinuation of previous treatments | High risk | All forms of treatment were withheld for at least 1 month before enrolment. The trial lacked a wash‐out period, and a carry‐over effect appeared in 2 out of 3 women who used dihydrotestosterone in the first 3‐month segment of the trial. |
| Permission or standardisation of concomitant treatments | Unclear risk | There was no relevant description. |
| Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication | Low risk | We extracted tabulated data from the first period before cross‐over. |
Paslin 1996.
| Methods | This was a randomised cross‐over trial. | |
| Participants |
|
|
| Interventions | A: dihydrotestosterone 2% B: testosterone propionate 2% These were taken twice daily for 3 months, then treatment was reversed for another 3 months. |
|
| Outcomes |
Outcomes of the trial 1) Vulval itching (0 to 4 scale) 2) Dyspareunia (presence or absence) 3) Gross appearance (photographic improvement) 4) Histopathological findings (formation of elastic fibres) |
|
| Notes | Setting: university hospital Country: US Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on coin tossing. |
| Allocation concealment (selection bias) | Low risk | Allocation could not be foreseen since randomisation was based on coin tossing by the office technician after the participants were enrolled. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither the investigator nor the participants knew which androgen was being applied. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of withdrawal or dropout. |
| Selective reporting (reporting bias) | Unclear risk | Itching, dyspareunia, and objective gross improvement were assessed, but pain and adverse drug reactions were not reported. |
| Degree of certainty that the participants have LS | Low risk | LS was proven by biopsy. |
| Baseline assessment of the participants | Low risk | The participant was her own control since the study was a cross‐over trial. All of the participants were middle‐ to old‐aged women. |
| Drug identity, source, dose, duration of treatments, and adequacy of instructions | Low risk | The treatments were standardised. |
| Description and standardisation of outcome measures | High risk | Only 1 outcome measure, vulval itching, was standardised at planning, but no rating was reported. No grading system was used for other outcome measures. |
| Discontinuation of previous treatments | High risk | For at least 1 month before enrolment, the participants were allowed to use pramozine hydrochloride gel, cold compresses, and/or plain white petrolatum, but no androgens or corticosteroids were applied. However, the trial lacked a wash‐out period. |
| Permission or standardisation of concomitant treatments | Unclear risk | There was no relevant description. |
| Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication | Low risk | We extracted tabulated data from the first period before cross‐over. |
Sideri 1994.
| Methods | This was a randomised controlled trial. | |
| Participants |
|
|
| Interventions | A: testosterone propionate 2% B: placebo (petrolatum ointment) These were taken for 1 year. |
|
| Outcomes |
Outcomes of the trial 1) Symptoms 2) Gross appearance 3) Histological changes |
|
| Notes | Setting: university hospital Country: Italy Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no description of the process of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | There was no description of the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither the investigator nor the participants knew which medicine was being applied. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of withdrawal or dropout. |
| Selective reporting (reporting bias) | Low risk | Improvement in symptoms, gross appearance, and adverse drug reactions were reported. |
| Degree of certainty that the participants have LS | Low risk | LS was proven by biopsy. |
| Baseline assessment of the participants | Unclear risk | This was not performed. |
| Drug identity, source, dose, duration of treatments, and adequacy of instructions | Low risk | The treatments were standardised. |
| Description and standardisation of outcome measures | Unclear risk | Although a scoring system was used to assess clinical and symptomatic status, no relevant details were provided. |
| Discontinuation of previous treatments | Low risk | All participants received an initial 2‐week course of topical antibiotic‐corticosteroid cream before allocation, then they were randomised to the testosterone and placebo groups. |
| Permission or standardisation of concomitant treatments | Unclear risk | There was no relevant description. |
| Use and appropriateness of statistical analyses where tabulated data could not be extracted from the original publication | Low risk | We extracted tabulated data from the trial. |
VAS= visual analogue scale PR=pruritus BP= burning/pain
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chari 1994 | This was a case series. |
| Diakomanolis 2002 | Randomisation was not mentioned (this was a retrospective cohort study). |
| Friedrich 1971 | Randomisation was not mentioned (this was a controlled study). |
| Li 2004 | This was a phase 2 study, not a RCT. |
| Origoni 1996 | Randomisation was not mentioned (this was a controlled study). |
| Skierlo 1987 | This was a case series. |
| Sotiriou 2008 | This was a case series. |
| Zarcone 1996 | Randomisation was not mentioned (this was a retrospective cohort study). |
| Zhu 2006 | This was not a RCT according to the Chinese content of the paper. |
Characteristics of ongoing studies [ordered by study ID]
NCT00757874.
| Trial name or title | A Double Blind Phase II Study Comparing Safety and Efficacy of Tacrolimus Versus Topical Clobetasol Propionate in the Treatment of Vulvar Lichen Sclerosus |
| Methods | This is a RCT. |
| Participants |
Inclusion criteria of the trial
|
| Interventions | A: tacrolimus cream B: clobetasol propionate These were taken for 3 months. |
| Outcomes |
Primary outcomes of the trial
1) Efficacy assessed by medical examinations and reporting of symptoms Secondary outcomes of the trial 1) Presence and severity of side‐effects |
| Starting date | April 2006 |
| Contact information | Dr Deana Funaro (rouleaufunaro@videotron.ca) |
| Notes | ‐ |
NCT01126255.
| Trial name or title | Efficacy of a Topic Therapy With Progesterone Compared to the Conventional Therapy With Clobetasol Propionate in Patients With Vulvar Lichen Sclerosus. A Double Blind, Randomized Phase II Pilot Study |
| Methods | This is a RCT. |
| Participants |
Inclusion criteria of the trial
|
| Interventions | A: clobetasol propionate 0.05% B: progesterone 8% These topical applications were taken once daily for 12 weeks. |
| Outcomes |
Primary outcomes of the trial 1) Score of the characteristics of LS based on vulvar efflorescences Secondary outcomes of the trial 1) Patient‐reported symptom 2) Quality of life 3) Adverse events |
| Starting date | June 2011 |
| Contact information | Dr Andreas Guenthert (andreas.guenthert@insel.ch) Professor Michel Mueller (michel.mueller@insel.ch) |
| Notes | ‐ |
NCT01400022.
| Trial name or title | A Randomized Clinical Study Comparing Topical 0.05% Clobetasol Propianate in Vaseline With UVA‐1 Phototherapy in the Treatment of Vulvar Lichen Sclerosus |
| Methods | This is a RCT. |
| Participants |
Inclusion criteria of the trial
|
| Interventions | A: UVA1‐phototherapy 4 times per week B: clobetasol propionate (0.05%) in white Vaseline applied thinly once daily over a period of 3 months |
| Outcomes |
Primary outcomes of the trial 1) Clinical improvement during UVA1/cortisone treatment Secondary outcomes of the trial 1) Subjective patient score 2) Influence on quality of life 3) Colorimetry 4) Ultrasound to determine the severity of the sclerosis 5) Immunological, RT‐PCR, and histological parameters in skin biopsies |
| Starting date | August 2010 |
| Contact information | Dr Sarah Terras (s.terras@klinikum‐bochum.de) |
| Notes | ‐ |
Differences between protocol and review
After discussion with our consumer author (FB), we made amendments in the Background to stress that lichen sclerosus can affect the quality of the sex lives of people with this condition, including painful sex and less pleasurable sex. This issue is under‐investigated, and in the Implications for research we encourage researchers to examine this aspect in future trials.
We revised the Description of the intervention to describe what the treatments are, how they work, and their side‐effects.
We have updated some references in the Background text.
In the protocol, we prespecified the first primary outcome as 'participant‐rated global degree of improvement'. However, the global scale may not pick up the various effects of interventions on different symptoms (itching, pain, pain in sex, etc). One trial (Bracco 1993) did not report the degree of improvement; instead, they reported the number of participants who had improvement or remission of symptoms. We, therefore, revised the outcome as 'participant‐rated improvement or remission of symptoms' and reported effects on various symptoms, respectively, if the trialists provided relevant data.
In the protocol, we planned a time point of outcome measurement at three to six months into therapy. In one trial (Kiss 2001), outcome measurement was made at five weeks into therapy, which was earlier than we had planned to make the measurement, so although the Kiss trial was too short according to our review protocol, we made the decision to include the trial in our review. In this trial, a significant difference in the clinical score of phimosis was detected (Kiss 2001). Therefore, inclusion of the trial conducted shorter than originally planned did not appear to affect the validity of our review (i.e. if this trial had not found a significant therapeutic benefit from mometasone furoate in the short study period, we may have been led to say that this intervention was not effective). However, the fact is that even over the shorter study period it did have a beneficial effect, so inclusion of this trial has not led to a false negative conclusion regarding mometasone furoate.
Contributions of authors
Link with the editorial base and co‐ordinating contributions from co‐authors ‐ CC Drafting the protocol ‐ CC, MB, GK, FW, FL, and FB Dialogue with the Trials Search Co‐ordinator ‐ CC Identifying relevant titles and abstracts from the searches ‐ CC and GK Obtaining copies of the trials ‐ CC Selecting the trials ‐ CC and GK Extracting the data ‐ CC and GK Entering the data into RevMan ‐ CC Carrying out the analysis ‐ CC and GK Interpreting the data ‐ CC, GK, and FW Drafting the final review ‐ CC with contribution from all Updating the review ‐ CC
Sources of support
Internal sources
Vrije Universiteit Medisch Centrum, Netherlands.
St John's Institute of Dermatology, UK.
External sources
-
British Society for the Study of Vulval Disease, UK.
Funding
-
Chang Gung Memorial Hospital‐Chiayi (CMRPG690041), Taiwan.
Funding
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bracco 1993 {published data only}
- Bracco GL, Carli P, Sonni L, Maestrini G, Marco A, Taddei GL, et al. Clinical and histologic effects of topical treatments of vulval lichen sclerosus. A critical evaluation. Journal of Reproductive Medicine 1993;38(1):37‐40. [PubMed] [Google Scholar]
- Cattaneo A, Marco A, Sonni L, Bracco GL, Carli P, Taddei GL. Clobetasol vs. testosterone in the treatment of lichen sclerosus of the vulvar region [Clobetasolo vs testosterone nel trattamento del lichen scleroso della regione vulvare]. Minerva Ginecologica 1992;44(11):567‐71. [PubMed] [Google Scholar]
Cattaneo 1996 {published data only}
- Cattaneo A, Carli P, Marco A, Sonni L, Bracco G, Magnis A, et al. Testosterone maintenance therapy. Effects on vulvar lichen sclerosus treated with clobetasol propionate. Journal of Reproductive Medicine 1996;41(2):99‐102. [PubMed] [Google Scholar]
Goldstein 2011 {published and unpublished data}
- Goldstein AT, Creasey A, Pfau R, Phillips D, Burrows LJ. A double‐blind, randomized controlled trial of clobetasol versus pimecrolimus in patients with vulvar lichen sclerosus. Journal of the American Academy of Dermatology 2011;64(6):e99‐e104. [DOI] [PubMed] [Google Scholar]
Kiss 2001 {published data only}
- Kiss A, Csontai A, Pirot L, Nyirady P, Merksz M, Kiraly L. The response of balanitis xerotica obliterans to local steroid application compared with placebo in children. Journal of Urology 2001;165(1):219‐20. [DOI] [PubMed] [Google Scholar]
Paslin 1991 {published data only}
- Paslin D. Treatment of lichen sclerosus with topical dihydrotestosterone. Obstetrics & Gynecology 1991;78(6):1046‐9. [PubMed] [Google Scholar]
Paslin 1996 {published data only}
- Paslin D. Androgens in the topical treatment of lichen sclerosus. International Journal of Dermatology 1996;35(4):298‐301. [DOI] [PubMed] [Google Scholar]
Sideri 1994 {published data only}
- Sideri M, Origoni M, Spinaci L, Ferrari A. Topical testosterone in the treatment of vulvar lichen sclerosus. International Journal of Gynaecology & Obstetrics 1994;46(1):53‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chari 1994 {published data only}
- Chari SP, Conolly P, Shafi MI, Luesley DM. The treatment of lichen sclerosus et atrophicus and squamous cell hyperplasia with graduated topical steroids. Journal of Obstetrics & Gynaecology 1994;14(3):172‐4. [Google Scholar]
Diakomanolis 2002 {published data only}
- Diakomanolis ES, Haidopoulos D, Syndos M, Rodolakis A, Stefanidis K, Chatzipapas J, et al. Vulvar lichen sclerosus in postmenopausal women: a comparative study for treating advanced disease with clobetasol propionate 0.05%. European Journal of Gynaecological Oncology 2002;23(6):519‐22. [PubMed] [Google Scholar]
Friedrich 1971 {published data only}
- Friedrich EG Jr. Topical testosterone for benign vulvar dystrophy. Obstetrics & Gynecology 1971;37(5):677‐86. [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li C, Bian D, Chen W, Zhao C, Yin N, Wang Z. Focused ultrasound therapy of vulvar dystrophies: a feasibility study. Obstetrics & Gynecology 2004;104(5 Pt 1):915‐21. [DOI] [PubMed] [Google Scholar]
Origoni 1996 {published data only}
- Origoni M, Ferrari D, Rossi M, Gandini F, Sideri M, Ferrari A. Topical oxatomide: an alternative approach for the treatment of vulvar lichen sclerosus. International Journal of Gynaecology & Obstetrics 1996;55(3):259‐64. [DOI] [PubMed] [Google Scholar]
Skierlo 1987 {published data only}
- Skierlo P, Heise H. Testosterone propionate ointment‐‐a therapeutic trial in lichen sclerosus et atrophicus [Testosteronpropionat‐Salbe‐‐ein Therapieversuch beim Lichen sclerosus et atrophicus.]. Hautarzt 1987;38(5):295‐7. [PubMed] [Google Scholar]
Sotiriou 2008 {published data only}
- Sotiriou E, Panagiotidou D, Ioannidis D. An open trial of 5‐aminolevulinic acid photodynamic therapy for vulvar lichen sclerosus. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2008;141(2):187‐8. [DOI] [PubMed] [Google Scholar]
Zarcone 1996 {published data only}
- Zarcone R, Vicinanza G, Bellini P, Cardone A. Drug treatment in vulvar lichen sclerosus [Il trattamento medico nel lichen scleroso vulvare]. Minerva Ginecologica 1996;48(10):441‐4. [PubMed] [Google Scholar]
Zhu 2006 {published data only}
- Zhu JH, Feng LH, Zheng H, Han SM, Cong YF, Liu YR. Evaluation of curative effects of focused ultrasound treatment for nonneoplastic epithelial disorders of vulva. Journal of Jilin University Medicine Edition 2006;32(4):714‐6. [Google Scholar]
References to ongoing studies
NCT00757874 {unpublished data only}
- Funaro D. Tacrolimus versus clobetasol propionate in the treatment of vulvar lichen sclerosus. clinicaltrials.gov/ct2/show/NCT00757874 (accessed 16 March 2010).
NCT01126255 {unpublished data only}
- Guenthert A. Progesterone vs. clobetasol propionate in vulvar lichen sclerosus. http://clinicaltrials.gov/show/NCT01126255 (accessed 20 September 2011).
NCT01400022 {unpublished data only}
- Kreuter A. Topical 0.05% clobetasol propianate in vaseline versus UVA‐1 phototherapy in vulvar lichen sclerosus. http://clinicaltrials.gov/show/NCT01400022 (accessed 20 September 2011).
Additional references
Azurdia 1999
- Azurdia RM, Luzzi GA, Byren I, Welsh K, Wojnarowska F, Marren P, et al. Lichen sclerosus in adult men: A study of the HLA associations and susceptibility to autoimmune disease. British Journal of Dermatology 1999;140(1):79‐83. [DOI] [PubMed] [Google Scholar]
Baldo 2010
BNF 2010
- Martin J, editor. British National Formulary. London: BMJ Group and RPS Publishing, 2010. [Google Scholar]
Bohm 2003
- Bohm M, Frieling U, Luger A, Bonsmann G. Successful treatment of anogenital lichen sclerosus with topical tacrolimus. Archives of Dermatology 2003;139(7):922‐4. [DOI] [PubMed] [Google Scholar]
Boms 2004
- Boms S, Gambichler T, Freitag M, Altmeyer P, Kreuter A. Pimecrolimus 1% cream for anogenital lichen sclerosus in childhood. BMC Dermatology 2004;4(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bornstein 1998
- Bornstein J, Heifetz S, Kellner Y, Stolar Z, Abramovici H. Clobetasol dipropionate 0.05% versus testosterone propionate 2% topical application for severe vulvar lichen sclerosus. American Journal of Obstetrics & Gynecology 1998;178(1 Pt 1):80‐4. [DOI] [PubMed] [Google Scholar]
Brownstein 1973
- Brownstein MH. Lichen sclerosus et atrophicus [Comment]. Archives of Dermatology 1973;108(3):433. [Google Scholar]
Carli 1992
- Carli P, Cattaneo A, Taddei G, Giannotti B. Topical cyclosporine in the treatment of vulvar lichen sclerosus: clinical, histologic, and immunohistochemical findings. Archives of Dermatology 1992;128(11):1548‐9. [DOI] [PubMed] [Google Scholar]
Cattaneo 1992
- Cattaneo A, Marco A, Sonni L, Bracco GL, Carli P, Taddei GL. Clobetasol vs. testosterone in the treatment of lichen sclerosus of the vulvar region [Clobetasolo vs testosterone nel trattamento del lichen scleroso della regione vulvare]. Minerva Ginecologica 1992;44(11):567‐71. [PubMed] [Google Scholar]
Clayton 2006
- Clayton R, Stewart E, Wojnarowska F. Rising demand for the services of a dedicated dermatological vulval clinic without changes in disease profile. Proceedings of the 15th Congress of the European Academy of Dermatology and Venereology. 2006.
Cooper 2004
- Cooper S, Gao XH, Powell JJ, Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis?. Archives of Dermatology 2004;140(6):702‐6. [DOI] [PubMed] [Google Scholar]
Cooper 2008
- Cooper SM, Ali I, Baldo M, Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: a case‐control study. Archives of Dermatology 2008;144(11):1432‐5. [DOI] [PubMed] [Google Scholar]
CRUK 2010
- Cancer Research UK. UK Vulva Cancer incidence statistics. http://info.cancerresearchuk.org/cancerstats/ (accessed 24th March 2010).
Dalziel 1991
- Dalziel KL, Millard P, Wojnarowska F. The treatment of lichen sclerosus with a very potent corticosteroid (clobetasol propionate 0.05%) cream. British Journal of Dermatology 1991;124(5):461‐4. [DOI] [PubMed] [Google Scholar]
Dalziel 1993
- Dalziel KL, Wojnarowska F. Long‐term control of vulval lichen sclerosus after treatment with a potent topical steroid cream. Journal of Reproductive Medicine 1993;38(1):25‐7. [PubMed] [Google Scholar]
Fischer 1997
- Fischer G, Rogers M. Treatment of childhood vulvar lichen sclerosus with potent topical corticosteroid. Pediatric Dermatology 1997;14(3):235‐8. [DOI] [PubMed] [Google Scholar]
Fredriksson 1980
- Fredriksson T, Lassus A, Bleeker J. Treatment of psoriasis and atopic dermatitis with halcinonide cream applied once and three times daily. British Journal of Dermatology 1980;102(5):575‐7. [DOI] [PubMed] [Google Scholar]
Friedrich 1984
- Friedrich EG Jr, Kalra PS. Serum levels of sex hormones in vulvar lichen sclerosus, and the effect of topical testosterone. The New England Journal of Medicine 1984;310(8):488‐91. [DOI] [PubMed] [Google Scholar]
Funaro 2004
- Funaro D. Lichen sclerosus: a review and practical approach. Dermatologic Therapy 2004;17(1):28‐37. [DOI] [PubMed] [Google Scholar]
Gao 2005
- Gao XH, Barnardo MC, Winsey S, Ahmad T, Cook J, Agudelo JD, et al. The association between HLA DR, DQ antigens, and vulval lichen sclerosus in the UK: HLA DRB112 and its associated DRB112/DQB10301/04/09/010 haplotype confers susceptibility to vulval lichen sclerosus, and HLA DRB10301/04 and its associated DRB10301/04/DQB10201/02/03 haplotype protects from vulval lichen sclerosus. Journal of Investigative Dermatology 2005;125(5):895‐9. [DOI] [PubMed] [Google Scholar]
Harrington 1981
- Harrington CI, Dunsmore IR. An investigation into the incidence of autoimmune disorders in patients with lichen sclerosus and atrophicus. British Journal of Dermatology 1981;104(5):563‐6. [DOI] [PubMed] [Google Scholar]
Held 2001
- Held E, Lund H, Agner T. Effect of different moisturizers on SLS‐irritated human skin. Contact Dermatitis 2001;44(4):229‐34. [DOI] [PubMed] [Google Scholar]
Hengge 2006
- Hengge UR, Krause W, Hofmann H, Stadler R, Gross G, Meurer M, et al. Multicentre, phase II trial on the safety and efficacy of topical tacrolimus ointment for the treatment of lichen sclerosus. British Journal of Dermatology 2006;155(5):1021‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Hillemanns 1999
- Hillemanns P, Untch M, Prove F, Baumgartner R, Hillemanns M, Korell M. Photodynamic therapy of vulvar lichen sclerosus with 5‐aminolevulinic acid. Obstetrics & Gynecology 1999;93(1):71‐4. [DOI] [PubMed] [Google Scholar]
Howard 2004
- Howard A, Dean D, Cooper S, Kirtshig G, Wojnarowska F. Circulating basement membrane zone antibodies are found in lichen sclerosus of the vulva. Australasian Journal of Dermatology 2004;45(1):12‐5. [DOI] [PubMed] [Google Scholar]
Kikuchi 2003
- Kikuchi K, Kobayashi H, Hirao T, Ito A, Takahashi H, Tagami H. Improvement of mild inflammatory changes of the facial skin induced by winter environment with daily applications of a moisturizing cream. A half‐side test of biophysical skin parameters, cytokine expression pattern and the formation of cornified envelope. Dermatology 2003;207(3):269‐75. [DOI] [PubMed] [Google Scholar]
Kyriakis 2007
- Kyriakis KP, Emmanuelides S, Terzoudi S, Palamaras I, Damoulaki E, Evangelou G. Gender and age prevalence distributions of morphea en plaque and anogenital lichen sclerosus. Journal of the European Academy of Dermatology & Venereology 2007;21(6):825‐6. [DOI] [PubMed] [Google Scholar]
Leibovitz 2000
- Leibovitz A, Kaplun VV, Saposhnicov N, Habot B. Vulvovaginal examinations in elderly nursing home women residents. Archives of Gerontology and Geriatrics 2000;31(1):1‐4. [DOI] [PubMed] [Google Scholar]
Loden 1999
- Loden M, Andersson AC, Lindberg M. Improvement in skin barrier function in women with atopic dermatitis after treatment with a moisturizing cream (Canoderm). British Journal of Dermatology 1999;140(2):264‐7. [DOI] [PubMed] [Google Scholar]
Marren 1995
- Marren P, Yell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. British Journal of Dermatology 1995;132(2):197‐203. [DOI] [PubMed] [Google Scholar]
Mazdisnian 1999
- Mazdisnian F, Degregorio F, Mazdisnian F, Palmieri A. Intralesional injection of triamcinolone in the treatment of lichen sclerosus. Journal of Reproductive Medicine 1999;44(4):332‐4. [PubMed] [Google Scholar]
Meffert 1995
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. Journal of the American Academy of Dermatology 1995;32(3):393‐416. [DOI] [PubMed] [Google Scholar]
Meyrick 1988
- Meyrick Thomas RH, Ridley CM, McGibbon DH, Black MM. Lichen sclerosus et atrophicus and autoimmunity‐‐a study of 350 women. British Journal of Dermatology 1988;118(1):41‐6. [DOI] [PubMed] [Google Scholar]
Neill 2010
- Neill SM, Lewis FM, Tatnall FM, Cox NH. British Association of Dermatologists' guidelines for the management of lichen sclerosus 2010. British Journal of Dermatology 2010;163(4):672‐82. [DOI] [PubMed] [Google Scholar]
Novartis 2010
- Novartis. Elidel prescribing information. http://www.pharma.us.novartis.com/product/pi/pdf/elidel.pdf (accessed 15th August 2011) 2010.
Oyama 2003
- Oyama N, Chan I, Neill SM, Hamada T, South AP, Wessagowit V, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet 2003;362(9378):118‐23. [DOI] [PubMed] [Google Scholar]
Patel 2009
- Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. Journal of the American Academy of Dermatology 2009;60(6):1001‐17. [DOI] [PubMed] [Google Scholar]
Powell 1999
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet 1999;353(9166):1777‐83. [DOI] [PubMed] [Google Scholar]
Powell 2001
- Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. Journal of the American Academy of Dermatology 2001;44(5):803‐6. [DOI] [PubMed] [Google Scholar]
Pugliese 2007
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of literature and current recommendations for management. Journal of Urology 2007;178(6):2268‐76. [DOI] [PubMed] [Google Scholar]
Reichrath 2002
- Reichrath J, Reinhold U, Tilgen W. Treatment of genito‐anal lesions in inflammatory skin diseases with PUVA cream photochemotherapy: an open pilot study in 12 patients. Dermatology 2002;205(3):245‐8. [DOI] [PubMed] [Google Scholar]
Renaud‐Vilmer 2004
- Renaud‐Vilmer C, Cavelier‐Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus: effect of long‐term topical application of a potent steroid on the course of the disease. Archives of Dermatology 2004;140(6):709‐12. [DOI] [PubMed] [Google Scholar]
Shankar 1999
- Shankar KR, Rickwood, AMK. incidence of phimosis in boys. BJU International 1999;84(1):101‐2. [DOI] [PubMed] [Google Scholar]
Sherman 2010
- Sherman V, McPherson T, Baldo M, Salim A, Gao XH, Wojnarowska F. The high rate of familial lichen sclerosus suggests a genetic contribution: an observational cohort study. Journal of the European Academy of Dermatology & Venereology 2010;24(9):1031‐4. [DOI] [PubMed] [Google Scholar]
Simonart 2008
- Simonart T, Lahaye M, Simonart JM. Vulvar lichen sclerosus: effect of maintenance treatment with a moisturizer on the course of the disease. Menopause 2008;15(1):74‐7. [DOI] [PubMed] [Google Scholar]
Sudilovsky 1981
- Sudilovsky A, Muir JG, Bocobo FC. A comparison of single and multiple applications of halcinonide cream. International Journal of Dermatology 1981;20(9):609‐13. [DOI] [PubMed] [Google Scholar]
Tasker 2003
- Tasker GL, Wojnarowska F. Lichen sclerosus. Clinical & Expermental Dermtology 2003;28(2):128‐33. [DOI] [PubMed] [Google Scholar]
Tremaine 1989
- Tremaine RD, Miller RA. Lichen sclerosus. International Journal of Dermatology 1989;28(1):10‐6. [DOI] [PubMed] [Google Scholar]
Virgili 1995
- Virgili A, Corazza M, Bianchi A, Mollica G, Califano A. Open study of topical 0.025% tretinoin in the treatment of vulvar lichen sclerosus. One year of therapy. Journal of Reproductive Medicine 1995;40(9):614‐8. [PubMed] [Google Scholar]
Wallace 1971
- Wallace HJ. Lichen sclerosus et atrophicus. Transactions of the St John's Hospital Dermatological Society 1971;57(1):9‐30. [PubMed] [Google Scholar]
Wang 2010
- Wang SH, Chi CC, Wong YW, Salim A, Manek S, Wojnarowska F. Genital verrucous carcinoma is associated with lichen sclerosus: a retrospective study and review of the literature. Journal of the European Academy of Dermatology and Venereology 2010;24(7):815‐9. [DOI] [PubMed] [Google Scholar]


