Abstract
Epitranscriptomic modifications play an important role in RNA function and can impact gene expression. Here, we apply a chemical proteomics approach to investigate readers of N1-methyladenosine (m1A), a poorly characterized modification on mammalian mRNA. We find that YTHDF proteins, known m6A readers, recognize m1A-modified sequences in a methylation-specific manner. We characterize binding of recombinant YTHDF1/2 proteins to m1A-modified oligonucleotides to demonstrate that these interactions can exhibit comparable affinity to m6A-recognition events, and occur in diverse sequence contexts. Further, we demonstrate YTHDF2 interacts specifically with endogenously modified m1A transcripts. Finally, we deplete cellular YTHDF2 to show that the abundance of m1A-modified transcripts is increased in its absence. Similarly, increasing m1A levels through depletion of ALKBH3, an m1A eraser protein, destabilizes known m1A-containing RNAs. Our results shed light on the function of m1A on mRNA, and provide a mechanistic framework to further evaluate the role of m1A in biological processes.
INTRODUCTION
Chemical modifications on macromolecules play an essential role in biological processes. Post-transcriptional modifications of cellular RNA are widespread throughout biology1 and represent an evolutionarily conserved strategy to modulate the properties of this central molecule of life. Investigation into the function of modifications occurring on structured RNAs has revealed how these marks can regulate base pairing, nucleic acid folding, and interactions with associated proteins2–3. In contrast, there still exist major gaps in our understanding of mRNA modification chemistry and the mechanisms by which these modifications affect biological processes.
Transcriptome-wide mapping of N6-methyladenosine (m6A), an abundant modification on eukaryotic mRNA,4–5 has led to the proposal of RNA epigenetics or epitranscriptomics6–7. N6-methyladenosine perturbs RNA structure8 and functions as an epitope for the recruitment of modification-specific reader proteins9–10, resulting in the modulation of RNA stability11, protein translation12, splicing13, and nuclear export14. Recent studies have demonstrated a role for the m6A pathway in diverse biology occurring in normal15–17 and disease18–19 contexts. The effect of m6A on mRNA behavior suggests that other modifications20 may function similarly; however, currently we lack insights into the roles of many modifications.
One such modification is N1-methyladenosine (m1A), which has been found on mRNA through transcriptomic and mass spectrometric studies21–22. Currently, there exists substantial controversy over the sites and frequency of m1A modification on mRNA23–24. While initial estimates based on m1A immunoprecipitation and sequencing suggested that thousands of m1A sites existed22, recent single-nucleotide mapping approaches have arrived at more conservative numbers ranging from ~15–50025–26. The reported single-nucleotide strategies combine immunoprecipitation with m1A-induced mutations or truncations during reverse transcription (RT) to localize modifications at high resolution. Since the propensity of specific RT enzymes to stall or misincorporate opposite an m1A site is not fully understood, the conditions and criteria applied to map m1A are likely responsible for the large discrepancy between studies. In addition, factors such as sequencing depth and RNA preparation could also explain the disparity in called m1A sites. While our manuscript was in review, a new single-nucleotide approach for m1A mapping relying upon an engineered RT enzyme was used to identify 215 m1A sites on mRNA27. This number of sites is within the range of earlier estimates, however, only a fraction of them overlap with prior studies suggesting that further work will be needed in order to generate a definitive m1A map.
Beyond mapping, elucidating m1A-associated proteins is critical towards understanding its regulation and function. Thus far, the demethylases ALKBH1 and ALKBH3 have been characterized as m1A erasers28–29 and TRMT6/61a was shown to install a subset of m1A mRNA sites25–26. Investigation of m1A readers, however, has lagged behind. Recently, Yang and co-workers used affinity proteomics with an m1A-modified oligonucleotide to identify YTH-domain proteins (specifically, YTHDF1–3 and YTHDC1) as m1A readers30. This is an intriguing finding given that YTH-domain proteins are well-established m6A readers. Although the reported m1A-specific interactions were low affinity (Kd ~ 10–100 µM), this study provides insight into how protein-m1A recognition could regulate mRNA behavior and warrants further investigation. Recently, we developed an RNA chemical proteomics approach to profile modification-specific readers and anti-readers10. Our approach relies upon photocrosslinking with synthetic, diazirine-containing oligonucleotides and enables stringent identification of direct interaction partners. Here, we apply our method to identify m1A-specific binding proteins. We find that YTHDF proteins bind specifically to m1A-modified sequences. Interestingly, the related YTH-domain protein YTHDC1 shows no m1A-specific RNA binding. We further investigate the interactions of recombinant YTHDF1/2 using biophysical assays in order to demonstrate that these binding events are high affinity and occur in diverse sequence contexts. Finally, we show that YTHDF2 binds to endogenous m1A-modified transcripts and provide evidence that m1A destabilizes RNA transcripts, thereby demonstrating a role for this mark in post-transcriptional gene regulation.
RESULTS
Chemoproteomic probes for identifying m1A reader proteins
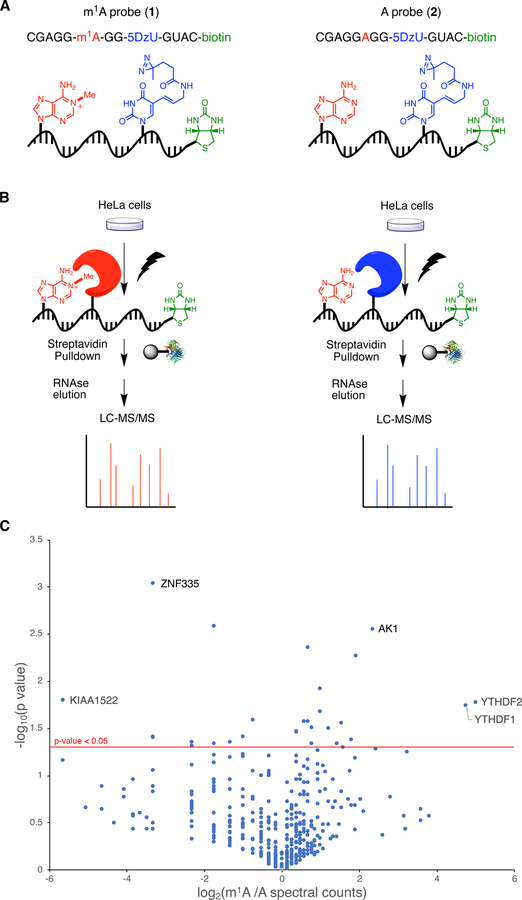
To discover m1A readers, we used our previously reported RNA chemical proteomics method10. First, we designed synthetic RNA probes containing m1A, a photo-activatable diazirine-modified uridine residue (5-DzU), and biotin as an affinity handle for protein enrichment (Figure 1a). We embedded m1A within a purine-rich consensus motif (GAGGA*G) generated from a subset of m1A sites enriched upon knockdown of the m1A eraser ALKBH321. This motif is also similar to one recently identified using an evolved reverse transcriptase enzyme for m1A mapping27. To avoid possible Dimroth rearrangement of m1A to m6A31, we synthesized m1A-containing probe 1 as well as the unmethylated control probe 2 (Figure 1a, Table S1) using ultra-mild phosphoramidite chemistry.
Figure 1.
Chemoproteomic profiling of the m1A interactome. a) Photocrosslinking RNA probes used in this work. b) Schematic for comparative proteomics workflow. c) Volcano plot of protein enrichment from m1A proteomics experiments.
Next, we applied our probes to identify m1A readers in HeLa cells. As described previously10, HeLa cell lysate was incubated with either probe 1 or 2 and exposed to 365 nm UV light (Figure 1b). Cross-linked RNA-protein complexes were next enriched with streptavidin, eluted with mild RNase treatment, and analyzed by LC-MS/MS to identify peptides and quantify abundance by spectral counting. Three independent replicates were performed, and a volcano plot was generated to represent the data (Figure 1c).
Analysis of our proteomics data revealed a number of proteins exhibiting significant (p < 0.05) and probe-specific photocrosslinking behavior. Among these hits, we found proteins from the YTH-domain family32, which are well-established m6A reader proteins. In particular, YTHDF1 and YTHDF2 exhibited 27–32-fold preference for the m1A probe over the unmethylated probe (Figure 1c). The identification of these proteins in both our study and by Wang and co-workers30, using different m1A-containing sequences and different enrichment approaches speaks to the generality of m1A-recognition by YTHDF proteins. In contrast to the earlier report30, YTHDC1, another YTH-domain protein that binds to m6A-modified sequences, was not identified in our data indicating that m1A recognition is likely unique to the YTHDF clade of YTH-domain proteins. In addition, we also found adenylate kinase (AK1), enriched 5-fold against the m1A probe. While AK1 is primarily an enzyme involved in nucleotide metabolism, it has been reported to associate with RNA in vitro33. We did not observe additional m1A specific proteins that met our cutoff (p-value <0.05 and >4-fold enrichment). On the left side of the volcano plot, we identified several putative anti-readers, proteins that bind specifically to unmodified sequences. These included ZNF335 and KIAA1522, enriched 5-fold and 10-fold by the unmethylated probe, respectively (Figure 1c). Our data did not reveal any interactions between unmethylated sequences and Ku70/80 or ILF2/3 as reported previously30, which may reflect that these interactions are sequence specific or indirect. Due to the strong connection between YTHDF proteins and mRNA regulation, we chose to focus on these proteins for further study.
YTHDF1/2 bind preferentially to m1A-modified transcripts
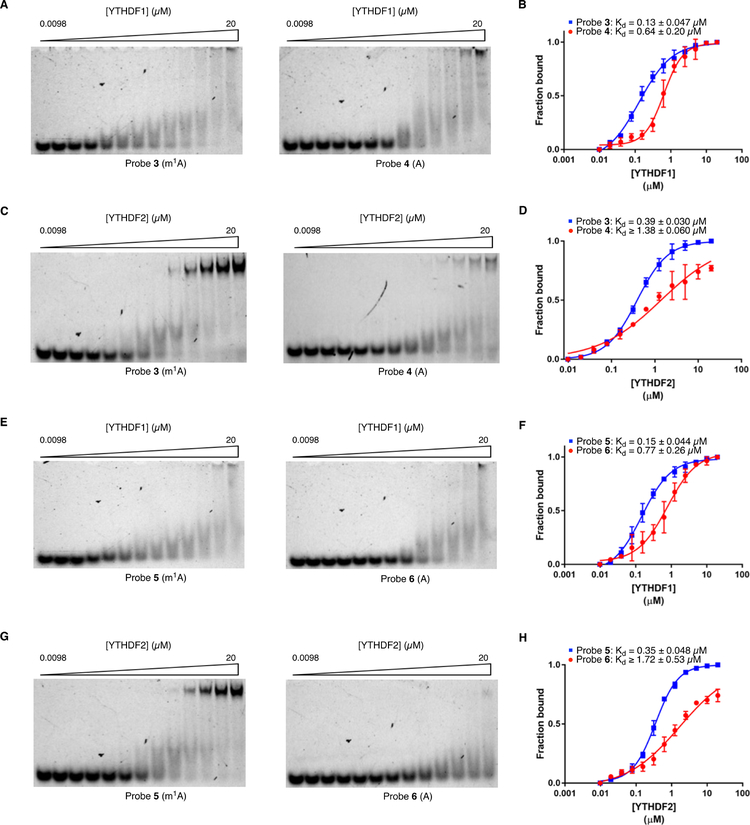
To further investigate our findings, we biochemically characterized the interaction between m1A-containing RNA and YTHDF1/2 proteins. For in vitro binding assays, we synthesized a new set of RNA oligonucleotides, 3 and 4 (Table S1), with identical sequence to probes 1 and 2 except lacking the 5-DzU photocrosslinker and containing 3’ fluorescein instead of biotin. We also generated recombinant YTH domains from YTHDF1 and YTHDF2, which have been shown to interact specifically with m6A-modified RNA oligonucleotides34,35. Next, we performed electrophoretic mobility shift assay (EMSA) to measure binding of each protein to the m1A-containing and corresponding unmethylated RNA oligonucleotides. We observed tight-binding of YTHDF1 and YTHDF2 to the m1A-modified oligonucleotide (Figure 2a–2d, Kd = 0.13 +/− 0.047 μM for YTHDF1:probe 3; Kd = 0.39 +/− 0.030 μM for YTHDF2:probe 3). These values are similar to those reported for YTHDF1/2 binding to m6A-modified sequences 34–35. In contrast, both proteins interacted more weakly with the corresponding unmethylated oligonucleotides, as characterized by a ~5-fold higher Kd for the YTHDF1:probe 4 complex and a >3.5-fold higher Kd for YTHDF2:probe 4 binding (saturation binding was not reached at the highest protein concentration tested) (Figure 2a–2d). Further, we confirmed the binding preference of YTHDF1 and YTHDF2 for the m1A-containing sequence by microscale thermophoresis (MST) assay, which showed similar trends to those measured by EMSA (Figure S1 and Figure 2a–2d).
Figure 2.
Characterization of YTHDF1/2-m1A binding. a) EMSA for YTHDF1 and probe 3/4. b) Quantification of YTHDF1-probe 3/4 binding. c) EMSA for YTHDF2 and probes 3 /4. d) Quantification of YTHDF2-probe 3/4 binding. e) EMSA images YTHDF1 and probes 5/6. f) Quantification of YTHDF1-probe 5/6 binding. g) EMSA for YTHDF2 and probes 5/6. h) Quantification of YTHDF2-probe 5/6 binding.
We next investigated the generality of m1A recognition by YTHDF1/2. We synthesized fluorescein-labeled oligonucleotides 5 and 6 (Table S1), which contain m1A/A within an entirely different sequence context (UUUUA*AA) from the A/G-rich motif interrogated above. This motif was also identified as a cellular substrate of ALKBH321. We measured the affinity of YTHDF1 and YTHDF2 for probes 5 and 6 by EMSA and observed tight binding for the m1A-containing probe 5 (Figure 2e–2h, Kd = 0.15 +/− 0.044 μM for YTHDF1:probe 5; Kd = 0.35 +/− 0.048 μM for YTHDF2:probe 5), similar to the measured affinity of YTHDF1/2 for the A/G-rich probe 3 sequence. For both YTH-domain proteins, we observed a >5-fold reduction in affinity for the interaction with the corresponding unmethylated oligo 6 (Fig. 2e–2h) with binding failing to reach saturation with YTHDF2 (Figure 2h). Taken together, our results demonstrate that YTHDF1 and YTHDF2, canonical m6A-reader proteins, can also bind to diverse m1A-modified sequences in a methylation-specific fashion.
YTHDC1 does not recognize m1A-modified sequences
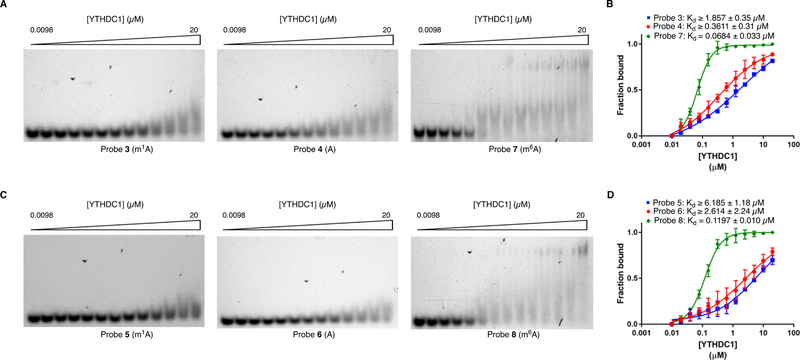
Interestingly, YTHDC1, another YTH-domain containing m6A reader36, was not enriched by m1A in our proteomics data. To explore this, we measured the affinity of recombinant YTHDC1 for oligos 3/4 and 5/6 using EMSA. In both sequence contexts, we observed a slight preference for YTHDC1 binding to the unmethylated sequence, although the affinities were modest and binding curves did not reach saturation (Figure 3a–3d). In contrast, YTHDC1 exhibited high-affinity binding to probes 7 and 8, analogous sequences containing a single m6A residue (Figure 3a–3d and Table S1, Kd = 0.068 +/− 0.033 μM for YTHDC1:probe 7; Kd = 0.12 +/− 0.010 μM for YTHDC1:probe 8), with 27–60-fold lower Kd than what was measured for binding to the corresponding m1A-modified sequences. As a related comparison, we also measured the affinity of YTHDF1/2 for the m6A-modified probes 7 and 8. While binding to these sequences was tighter than to the analogous m1A sequences (probes 3 and 5) (Figure S2), the difference in affinity was much less drastic than for YTHDC1. Moreover, as these sequences do not represent canonical m6A-modified motifs, they may not exist in this form in the cell. The large difference in binding of YTHDC1 to the m6A- and m1A-modified sequences also strongly suggests that our synthetic m1A-containing oligonucleotides have not undergone Dimroth rearrangement, a base-catalyzed chemical transformation that would convert m1A to m6A 31. We further confirmed that the extent of Dimroth rearrangement in our m1A probes was minimal using LC-MS/MS (Figure S3) Altogether, our binding assay results demonstrate that among YTH-domain protein, m1A recognition is specific to YTHDF1/2.
Figure 3.
Characterization of YTHDC1-m1A/m6A binding. a) EMSA for YTHDC1 and probes 3, 4, or 7. b) Quantification of YTHDC1-probe 3/4/7 binding. c) EMSA for YTHDC1 and probes 5, 6, or 8. d) Quantification of YTHDC1-probe 5/6/8 binding.
YTHDF2 promotes the degradation of m1A-containing transcripts in cells
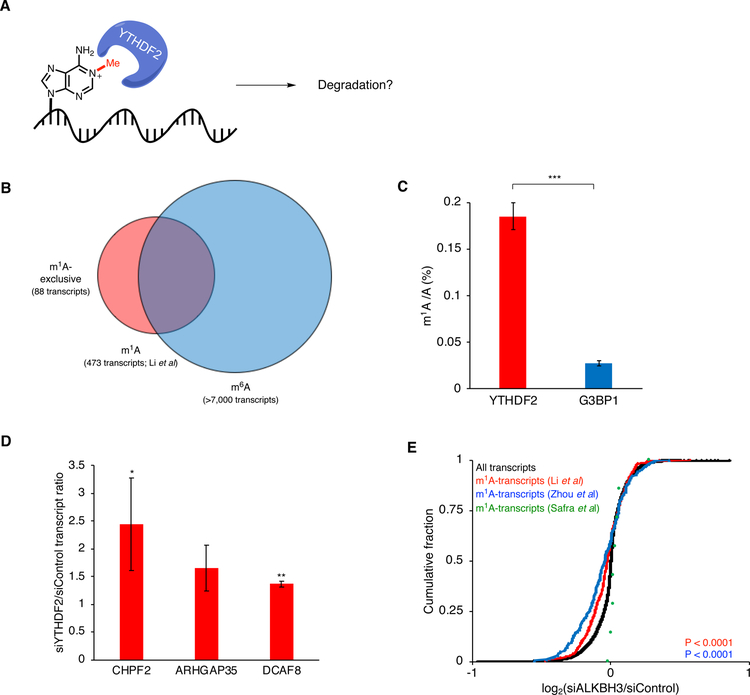
After characterizing binding between YTHDF2 and m1A- RNA in vitro, we next investigated the biological effect of YTHDF2-m1A-binding in human cells. YTHDF2 has been shown to promote the destabilization of m6A-modified RNA by direct binding of these transcripts through its C-terminal YTH-domain and recruitment to processing bodies (sites of mRNA decay) via its P/Q/N-rich N-terminal domain11. Moreover, the simple recruitment of YTHDF2 to mRNA transcripts (independent of m6A modification) is sufficient to facilitate their degradation37. Therefore, we hypothesized that m1A-directed YTHDF2 recruitment could also lead to degradation of m1A-modified transcripts (Figure 4a and 4b). To test this, we first needed to establish that YTHDF2 binds to endogenous m1A-modified RNAs. Towards this end, we generated a stable cell line containing 3xFLAG-tagged YTHDF2 (Fig. S7) and performed RNA immunoprecipitation coupled with LC-MS/MS (RIP-LC-MS/MS). In brief, YTHDF2-RNA complexes were immunoprecipitated from cells and RNA nucleosides were nalyzed using QQQ-LC-MS. We quantified the levels of m1A/A and found a ratio of 0.19%, well above reported values for total polyadenylated RNA21 (Figure 4c and Figure S5). As a control, we performed the same experiment with G3BP1, an abundant RNA binding protein with no known affinity for m1A and found the m1A/A levels to be ~7-fold lower (Figure 4c), consistent with a preference of YTHDF2 for binding to m1A-modified transcripts.
Figure 4.
YTHDF2-m1A regulation in living cells. a) Model for YTHDF2-m1A-mediated regulation of transcript stability. b) Venn diagram to classify transcripts as m1A-exclusive, m6A-exclusive, and both m1A- and m6A-modified. c) LC-MS/MS quantification of m1A levels in RNA enriched by immunoprecipitation of 3xFLAG-tagged YTHDF2 or G3BP1. d) Relative abundance of exclusive m1A-modified transcripts upon YTHDF2 knockdown. e) Cumulative distribution of transcript abundance upon ALKBH3 knockdown (*, p < 0.05; **, p < 0.005; ***, p < 0.0005).
We next investigated regulation of annotated m1A-modified transcripts by YTDHF2. For this purpose, we employed several published datasets including transcriptome-wide abundance data upon YTHDF2 knockdown11 and the three reported single-nucleotide m1A maps25–27. Among m1A-modified transcripts found in all 3 maps, only PRUNE and BRD2 were identified in the YTHDF2 RNAseq dataset and both transcripts increase in abundance upon YTHDF2 knockdown (Figure S8). Analysis of m1A-modified transcripts shared between the Zhou et al.27 and Li et al.26 maps for which YTHDF2 knockdown data exists identified 8 transcripts (ARHGDIA, ACTB, ILF3, PRPF8, HNRNPU, BRD2, PRUNE, and MAVS) – of these 7 were upregulated upon YTHDF2 knockdown (Figure S8). Further, we performed analysis of candidate m1A transcripts upon YTHDF2 siRNA knockdown using RT-qPCR. We focused on transcripts containing m1A and lacking m6A (Figure 4b) to avoid the possibility that the observed effects would be due to the presence of m6A, as well as those that were annotated YTHDF2 substrates based upon PAR-CLIP analysis11. We picked three transcripts (CHPF2, ARHGAP35, and DCAF8) to characterize by RT-qPCR. Consistent with the previous study11, upon siRNA knockdown of YTHDF2 (Figure S4), we observed a significant 1.5–2-fold increase (relative to cells transfected with a scrambled siRNA oligo) in transcript abundance for CHPF2 and DCAF8, indicating a role of YTHDF2 in their destabilization (Figure 4d).
Finally, we took advantage of the known role of ALKBH3 as an m1A eraser enzyme21 to probe the effect of m1A on transcript stability. We depleted ALKBH3 (which should concomitantly increase m1A levels) in HeLa cells using siRNA-mediated knockdown (Figure S6) and analyzed RNA transcript abundance compared to a scrambled siRNA control. We interpreted our results in the context of the Zhou et al.27, Li et al.26, and Safra et al.25 m1A maps. For the first two maps, which identify between 200–500 sites on mRNA, we found a statistically significant decrease in transcript abundance upon ALKBH3 knockdown (Figure 4e), suggesting that m1A destabilizes RNA transcripts, likely through a YTHDF2-dependent mechanism. We did not observe an overall decrease in abundance for the handful of transcripts from the Safra et al. study25, perhaps because the m1A levels on these transcripts do not significantly increase upon ALKBH3 knockdown.
DISCUSSION
In this manuscript, we use chemical proteomics to profile the m1A interactome in HeLa cells. Our results demonstrate that YTHDF proteins recognize m1A-modified RNA in diverse sequence contexts. Further, we show that m1A-modified transcripts in cells exhibit lower stability, likely through YTHDF2-mediated mRNA decay, as has been demonstrated for m6A-modified mRNAs11.
YTH-domain proteins are established readers of m6A-modified mRNA transcripts32, but their interactions with other methylated bases remain largely unexplored. Wang and co-workers30 recently reported direct binding between YTH-domain proteins including YTHDF1, YTHDF2, and YTHDC1 and an m1A-modified RNA oligonucleotide. Our study supports and expands upon their findings with regards to YTHDF1/2, however, we were unable to observe m1A-specific binding by YTHDC1. The observed discrepancy may be due to sequence context effects or specific assay conditions. Notably, Wang and co-workers found that YTHDC1 binds considerably less tightly to their m1A-modified oligonucleotide than YTHDF1/2, which is in line with our observations. How YTHDF proteins can bind to both m6A and m1A residues is still unclear. In YTHDF2, the m6A methyl group is accommodated in a hydrophobic cavity lined with aromatic Trp residues 34, therefore we speculate that the positively charged m1A may engage in cation-π interactions with these amino acid side chains. While YTHDC1 also possesses a similar aromatic cage 36, studies examining the substrate specificity of YTHDC1 in comparison to YTHDF1/2 have found that YTHDC1 has a stronger preference for specific nucleotides surrounding the m6A site 38–39, which may preclude the recognition of an alternate substrate conformation required for accommodation of the m1A residue. Interestingly, we also find substantial affinity between YTHDF1/2 and unmethylated sequences, suggesting that YTHDF1/2 may have a broad role in modulating mRNA translation and metabolism in both a methylation dependent and independent fashion. The ability of YTH-domain proteins to bind diversely modified transcripts will likely depend on the relative concentrations of these molecules in cells, as well as additional mechanisms governing RNA and protein localization. Further, while our biochemical studies provide insight into preferences for different marks, they likely do not fully reflect the native interactions, which may exhibit additional selectivity for adenosine methylation. Additional work will be necessary to elucidate the principles of substrate recognition by these proteins in their native context.
We provide evidence indicating that m1A destabilizes mRNA transcripts. Combined with our biochemical analysis of YTHDF2-m1A binding, this suggests that YTHDF2 recognizes and facilitates the degradation of m1A-modified RNA, likely in a manner similar to its role in m6A RNA turnover. We cannot exclude the possibility that other mechanisms exist for the clearance of m1A-modified transcripts, however these mechanisms remain as yet unknown.
Mapping m1A sites on eukaryotic mRNA has presented a considerable challenge to the field24. While there is still a lack of clarity on the exact number and frequency of m1A modification sites in mammalian cells, we are encouraged that among the transcripts found by multiple studies, several exhibit YTHDF2-dependent abundance. Just as well, our biochemical finding that YTDHF proteins can recognize diverse m1A-modified sequence motifs suggests that this is a general phenomenon. While more robust approaches to m1A sequencing will be required in order to definitively characterize the m1A epitranscriptome, our work demonstrates the functional potential of m1A on mRNA through the recruitment of YTHDF reader proteins, providing support for the regulatory role of this modification in mRNA biology.
METHODS
General Experimental Procedures.
A complete description of methods is given in the Supporting Information.
Supplementary Material
ACKNOWLEDGEMENTS
We thank T. Srikumar and the Princeton University Mass Spectrometry and Proteomics Core Facility for performing proteomics analysis. We thank W. Wang and R. Leach at the Princeton University Genomics Core Facility for performing Illumina sequencing and associated data analysis. We thank J. Dragon for experimental assistance. This research was supported by the NIH (R01 GM132189 to R. E. K.), the Sidney Kimmel Foundation (Sidney Kimmel Scholar Award to R. E. K.), the Damon Runyon Cancer Research Foundation (DFS #21–16 to R. E. K.), and the Alfred P. Sloan Foundation (Sloan Foundation Research Fellowship to R. E. K.). K. W. S. was supported by a generous gift from the Edward C. Taylor 3rd Year Graduate Fellowship in Chemistry. All authors thank Princeton University for financial support.
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information including supplemental figures, experimental methods, oligonucleotide characterization, mass spectrometry proteomics data, and RNA sequencing is available free of charge on the ACS Publications website.
REFERENCES
- 1.Boccaletto P; Machnicka MA; Purta E; Piatkowski P; Baginski B; Wirecki TK; de Crecy-Lagard V; Ross R; Limbach PA; Kotter A; Helm M; Bujnicki JM, MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 2018, 46, D303–D307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Yacoubi B; Bailly M; de Crecy-Lagard V, Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet 2012, 46, 69–95. [DOI] [PubMed] [Google Scholar]
- 3.Pan T, Modifications and functional genomics of human transfer RNA. Cell Res 2018, 28, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominissini D; Moshitch-Moshkovitz S; Schwartz S; Salmon-Divon M; Ungar L; Osenberg S; Cesarkas K; Jacob-Hirsch J; Amariglio N; Kupiec M; Sorek R; Rechavi G, Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [DOI] [PubMed] [Google Scholar]
- 5.Meyer KD; Saletore Y; Zumbo P; Elemento O; Mason CE; Jaffrey SR, Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He C, Grand challenge commentary: RNA epigenetics? Nat Chem Biol 2010, 6, 863–865. [DOI] [PubMed] [Google Scholar]
- 7.Saletore Y; Meyer K; Korlach J; Vilfan ID; Jaffrey S; Mason CE, The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol 2012, 13, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu N; Dai Q; Zheng G; He C; Parisien M; Pan T, N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015, 518, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edupuganti RR; Geiger S; Lindeboom RGH; Shi H; Hsu PJ; Lu Z; Wang SY; Baltissen MPA; Jansen P; Rossa M; Muller M; Stunnenberg HG; He C; Carell T; Vermeulen M, N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 2017, 24, 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arguello AE; DeLiberto AN; Kleiner RE, RNA Chemical Proteomics Reveals the N(6)-Methyladenosine (m(6)A)-Regulated Protein-RNA Interactome. J Am Chem Soc 2017, 139, 17249–17252. [DOI] [PubMed] [Google Scholar]
- 11.Wang X; Lu Z; Gomez A; Hon GC; Yue Y; Han D; Fu Y; Parisien M; Dai Q; Jia G; Ren B; Pan T; He C, N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X; Zhao BS; Roundtree IA; Lu Z; Han D; Ma H; Weng X; Chen K; Shi H; He C, N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao W; Adhikari S; Dahal U; Chen YS; Hao YJ; Sun BF; Sun HY; Li A; Ping XL; Lai WY; Wang X; Ma HL; Huang CM; Yang Y; Huang N; Jiang GB; Wang HL; Zhou Q; Wang XJ; Zhao YL; Yang YG, Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Molecular cell 2016, 61, 507–519. [DOI] [PubMed] [Google Scholar]
- 14.Roundtree IA; Luo GZ; Zhang Z; Wang X; Zhou T; Cui Y; Sha J; Huang X; Guerrero L; Xie P; He E; Shen B; He C, YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife 2017, 6, e31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao BS; Wang X; Beadell AV; Lu Z; Shi H; Kuuspalu A; Ho RK; He C, m(6)A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 2017, 542, 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Y; Laurent B; Hsu CH; Nachtergaele S; Lu Z; Sheng W; Xu C; Chen H; Ouyang J; Wang S; Ling D; Hsu PH; Zou L; Jambhekar A; He C; Shi Y, RNA m(6)A methylation regulates the ultraviolet-induced DNA damage response. Nature 2017, 543, 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler R; Gillis E; Lasman L; Safra M; Geula S; Soyris C; Nachshon A; Tai-Schmiedel J; Friedman N; Le-Trilling VTK; Trilling M; Mandelboim M; Hanna JH; Schwartz S; Stern-Ginossar N, m(6)A modification controls the innate immune response to infection by targeting type I interferons. Nat Immunol 2019, 20, 173–182. [DOI] [PubMed] [Google Scholar]
- 18.Vu LP; Pickering BF; Cheng Y; Zaccara S; Nguyen D; Minuesa G; Chou T; Chow A; Saletore Y; MacKay M; Schulman J; Famulare C; Patel M; Klimek VM; Garrett-Bakelman FE; Melnick A; Carroll M; Mason CE; Jaffrey SR; Kharas MG, The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med 2017, 23, 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han D; Liu J; Chen C; Dong L; Liu Y; Chang R; Huang X; Liu Y; Wang J; Dougherty U; Bissonnette MB; Shen B; Weichselbaum RR; Xu MM; He C, Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 2019, 566, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roundtree IA; Evans ME; Pan T; He C, Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X; Xiong X; Wang K; Wang L; Shu X; Ma S; Yi C, Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol 2016, 12, 311–316. [DOI] [PubMed] [Google Scholar]
- 22.Dominissini D; Nachtergaele S; Moshitch-Moshkovitz S; Peer E; Kol N; Ben-Haim MS; Dai Q; Di Segni A; Salmon-Divon M; Clark WC; Zheng G; Pan T; Solomon O; Eyal E; Hershkovitz V; Han D; Dore LC; Amariglio N; Rechavi G; He C, The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature 2016, 530, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz S, m(1)A within cytoplasmic mRNAs at single nucleotide resolution: a reconciled transcriptome-wide map. RNA 2018, 24, 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grozhik AV; Jaffrey SR, Epitranscriptomics: Shrinking maps of RNA modifications. Nature 2017, 551, 174–176. [DOI] [PubMed] [Google Scholar]
- 25.Safra M; Sas-Chen A; Nir R; Winkler R; Nachshon A; Bar-Yaacov D; Erlacher M; Rossmanith W; Stern-Ginossar N; Schwartz S, The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 2017, 551, 251–255. [DOI] [PubMed] [Google Scholar]
- 26.Li X; Xiong X; Zhang M; Wang K; Chen Y; Zhou J; Mao Y; Lv J; Yi D; Chen XW; Wang C; Qian SB; Yi C, Base-Resolution Mapping Reveals Distinct m(1)A Methylome in Nuclear- and Mitochondrial-Encoded Transcripts. Molecular Cell 2017, 68, 993–1005.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou H; Rauch S; Dai Q; Cui X; Zhang Z; Nachtergaele S; Sepich C; He C; Dickinson BC, Evolution of a reverse transcriptase to map N(1)-methyladenosine in human messenger RNA. Nat Methods 2019, 16, 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aas PA; Otterlei M; Falnes PO; Vagbo CB; Skorpen F; Akbari M; Sundheim O; Bjoras M; Slupphaug G; Seeberg E; Krokan HE, Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 2003, 421, 859–863. [DOI] [PubMed] [Google Scholar]
- 29.Liu F; Clark W; Luo G; Wang X; Fu Y; Wei J; Wang X; Hao Z; Dai Q; Zheng G; Ma H; Han D; Evans M; Klungland A; Pan T; He C, ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell 2016, 167, 816–828.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X; Wang T; Gonzalez G; Wang Y, Identification of YTH Domain-Containing Proteins as the Readers for N1-Methyladenosine in RNA. Anal Chem 2018, 90, 6380–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macon JB; Wolfenden R, 1-Methyladenosine. Dimroth rearrangement and reversible reduction. Biochemistry 1968, 7, 3453–3458. [DOI] [PubMed] [Google Scholar]
- 32.Liao S; Sun H; Xu C, YTH Domain: A Family of N(6)-methyladenosine (m(6)A) Readers. Genomics Proteomics Bioinformatics 2018, 16, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlattner U; Wagner E; Greppin H; Bonzon M, Binding of adenylate kinase to RNA. Biochem Biophys Res Commun 1995, 217, 509–514. [DOI] [PubMed] [Google Scholar]
- 34.Li F; Zhao D; Wu J; Shi Y, Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res 2014, 24, 1490–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu T; Roundtree IA; Wang P; Wang X; Wang L; Sun C; Tian Y; Li J; He C; Xu Y, Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res 2014, 24, 1493–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C; Wang X; Liu K; Roundtree IA; Tempel W; Li Y; Lu Z; He C; Min J, Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 2014, 10, 927–929. [DOI] [PubMed] [Google Scholar]
- 37.Rauch S; He C; Dickinson BC, Targeted m(6)A Reader Proteins To Study Epitranscriptomic Regulation of Single RNAs. J Am Chem Soc 2018, 140, 11974–11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arguello AE; Leach RW; Kleiner RE, In Vitro Selection with a Site-Specifically Modified RNA Library Reveals the Binding Preferences of N(6)-Methyladenosine Reader Proteins. Biochemistry 2019, 58, 3386–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu C; Liu K; Ahmed H; Loppnau P; Schapira M; Min J, Structural Basis for the Discriminative Recognition of N6-Methyladenosine RNA by the Human YT521-B Homology Domain Family of Proteins. J Biol Chem 2015, 290, 24902–24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.