Abstract
Background
Nicergoline is an ergot derivative currently in use in over fifty countries for more than three decades, for the treatment of cognitive, affective, and behavioral disorders of older people. It was initially considered as a vasoactive drug and mainly prescribed for cerebrovascular disorders. Recent findings suggest other actions which has provided a rationale for the use of nicergoline for the treatment of various forms of dementia, including Alzheimer's Disease.
Objectives
To determine whether there is evidence of efficacy of nicergoline in the treatment of dementia and other age‐associated forms of cognitive decline,and to assess the safety and tolerability of the drug.
Search methods
1. Electronic databases search. The Cochrane Controlled Trials Register (which contains citations from the MEDLINE, EMBASE, Psych LIT, and hand searches of geriatric, dementia, psychogeriatric journals, and conference abstracts) was searched using the following terms: 'Nicergoline', 'Sermion'. 2. Reference search. The reference lists of all obtained studies was checked. 3. Pharmaceutical company Pharmacia & Upjohn, owners of the rights to produce and market nicergoline in various different countries, was asked to provide data and reports of clinical trials. In case of unavailability of numerical data in published studies, the authors of each paper, were asked for any published or unpublished data.
Selection criteria
‐ All unconfounded, double‐blind, randomized, placebo‐controlled, published and unpublished trials were sought. Non‐randomized trials were excluded. Open trials were considered for inclusion if patients were randomized to the different treatment groups. ‐ All patients diagnosed as having dementia or other cognitive disorder defined according to classification criteria accepted at the time of each study. ‐ Nicergoline given at any dose for more than one day with placebo control.
Type of outcome variables:
1. Cognitive function (as measured by psychometric tests). 2. Clinical impression (such as CIBIC or other clinical global measures of change). 3. Functional performance including dependency. 4. Behavioural disturbance. 5. Safety and acceptability as measured by the incidence of adverse effects (including side‐effects) leading to withdrawal. 6. Death 7. Effect on carer 8. Use of services 9. Quality of life.
Data collection and analysis
A comprehensive search of the international literature and the producing company archives has been performed to identify all possible sources of data for this review. Only those trials fulfilling the inclusion criteria of belonging to either category A or B of allocation concealment, as defined by the Cochrane Organisation, were examined for data extraction by one reviewer. If there was doubt then the other reviewer was consulted. Data availability restricted analyses to 'completers' analyses for the outcome measures. Outcomes able to be assessed included: Behaviour, Cognition, Clinical Judgment, Tolerability, EEG.
Main results
The Sandoz Clinical Assessment Geriatric Scale (SCAG) was the outcome used in the largest number of patients (814 patients). The results from these studies were homogeneous in nature despite including patients observed for periods of time ranging from 2 months to 12 months. There was a difference in favour of the active treatment in reducing the behavioural symptoms described by this scale, ‐5.18 points [‐8.03, ‐2.33]. This scale has a maximum of 133 points. The therapeutic effects of nicergoline seem to be evident by 2 months of treatment and maintained for 6 months. In general other behavioural outcome measures which include the GRS, the IADL, and the MACC and were episodically used in few studies, failed to demonstrate statistically significant results although there was a trend favouring treatment. Cognitive assessment has been performed in a moderate number of patients with the MMSE (261 patients) and the ADAS‐Cog (342 patients). No significant heterogeneity was found for these trials, despite the trials extending over periods of treatment of 3 to 12 months. There was a difference between treatment and control groups on the MMSE favouring nicergoline treatment. At 12 months the effect size was 2.86 [0.98, 4.74] The effect size for the ADAS‐Cog, used exclusively with Alzheimer's disease patients, did not reveal a significant benefit. At 12 months the trend favoured treatment (‐1.64 [‐4.62, 1.34]). The other results from various cognitive measures tended to favour nicergoline but this was based on a small number of cases. The clinical impression of change obtained from a total of 921 patients was homogeneous across the studies, despite reflecting changes over periods of time ranging from 2 to 12 months. The Peto odd ratio for improvement in the subjects treated with nicergoline over these varying time periods was 3.33 [2.50, 4.43]. Tolerability assessed in 1427 patients was homogeneous across all studies and demonstrated a mildly increased risk of adverse events on treatment, OR 1.51[1.10, 2.07].
Authors' conclusions
The clinical studies on nicergoline were carried out with diverse criteria and modalities of evaluation. Despite this, the 14 studies included in this review, have presented generally consistent results. Results of this meta‐analysis provide some evidence of positive effects of nicergoline on cognition and behaviour and these effects are supported by an effect on clinical global impression. There was some evidence that there were increased risk of adverse effects associated with nicergoline. These results were obtained on older patients with mild to moderate cognitive and behavioural impairment of various clinical origins, including chronic cerebrovascular disorders and Alzheimer's dementia. The few studies specifically performed on patients with Alzheimer's disease were performed with too few people to give a definitive answer to the questions concerning the use of nicergoline for this form of dementia. This drug has not been evaluated using current diagnostic categories such as MCI or in association with therapeutic agents of different nature such as cholinesterase or antioxidant drugs.
Plain language summary
Nicergoline may improve cognition and behavioural function of people with mild to moderate dementia
Nicergoline is an ergot derivative which has been registered in over 50 countries and has been used for more than three decades for the treatment of cognitive, affective, and behavioural disorders of older people. During the time it has been in use, the rationale for its clinical use has evolved. Initially regarded as a vasoactive drug, it was mainly prescribed for cerebrovascular disorders. Since then, findings suggesting a more complex pharmacological profile of nicergoline have led to its being considered for the treatment of other forms of dementia, including Alzheimer's Disease. There is some evidence of positive effects of nicergoline on cognition and behaviour and these effects are supported by an effect on clinical global impression.There was some evidence that there was increased risk of adverse effects associated with nicergoline. This drug has not been evaluated using current diagnostic categories such as MCI or in association with therapeutic agents of different nature such as cholinesterase or antioxidant drugs.
Background
Dementia is a clinical condition characterized by global, and usually progressive impairment of cognitive functions. These functions include memory, judgement, reasoning, perception and personality, and impairments lead to difficulties in daily life and to inappropriate behaviour. It is commonest in later life and is most often caused by Alzheimer's disease and inadequate blood supply to the brain, but is also a feature of some other diseases.
Nicergoline, 8‐beta‐(5‐bromonicotinoylhydroxymethyl)‐1,6‐dimethyl‐10alpha‐metoxyergoline, is an ergot derivative which has been registered in over fifty countries and has been used for more than three decades for the treatment of cognitive, affective, and behavioural disorders of older people. During the time it has been in use, the rationale for its clinical use has evolved. Initially regarded as a vasoactive drug, it was mainly prescribed for cerebrovascular disorders. Since then, findings suggesting a more complex pharmacological profile of nicergoline have led to its being considered for the treatment of other forms of dementia, including Alzheimer's Disease. Although cholinergic deficits are the major current targets for pharmacological intervention in Alzheimer's dementia, a wide variety of other neurotransmitter changes can be identified in the disease. Nicergoline has been demonstrated to increase the availability of acetylcholine both through an increased release from cholinergic terminals and a selective inhibition of acetyl cholinesterase. Nicergoline may also enhance noradrenaline and dopamine turnover in some areas of the brain. Nicergoline has a positive effect on the signal transduction system stimulating the phosphoinositide pathway which is specifically impaired in Alzheimer's dementia. Other useful actions of nicergoline in dementia are an increase of phosphoinositide‐PKC translocation which helps in combating beta‐amyloid deposition and in retarding the reduction in nerve‐growth factor (NGF) which may help in preventing the loss of cholinergic neurons.
Objectives
To determine whether there is evidence of efficacy of nicergoline in the treatment of dementia and other age‐associated forms of cognitive decline,and to assess the safety and tolerability of the drug.
Methods
Criteria for considering studies for this review
Types of studies
All unconfounded, double‐blind, randomized, placebo‐controlled, published and unpublished trials were reviewed. Non‐randomized trials were excluded. Open trials were considered for inclusion if patients were randomized to the different treatment groups.
Types of participants
All patients diagnosed as having dementia or other cognitive disorder defined according to classification criteria accepted at the time of each study.
Types of interventions
Nicergoline given at any dose for more than one day with placebo control.
Types of outcome measures
1. Cognitive function (as measured by psychometric tests). 2. Clinical impression (such as CIBIC or other clinical global measures of change). 3. Functional performance including dependency. 4. Behavioural disturbance. 5. Safety and acceptability as measured by the incidence of adverse effects (including side‐effects) leading to withdrawal. 6. Death 7. Effect on carer 8. Use of services 9. Quality of life.
Search methods for identification of studies
1. The trials were identified from a search of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group on 4 May 2001 using the term nicergolin* and sermion. The Specialized Register at that time contained records from the following databases:
CCTR/Central: January 2001 (issue 1); MEDLINE: 1966 to December 2000; EMBASE: 1980 to December 2000; PsycLit: 1887 to December 2000; CINAHL:1982 to December 2000; SIGLE (Grey Literature in Europe): 1980 to December 2000; ISTP (Index to Scientific and Technical Proceedings): to May 2000; INSIDE (BL database of Conference Proceedings and Journals): to June 2000; Aslib Index to Theses (UK and Ireland theses): 1970 to March 2001; Dissertation Abstract (USA): 1861 to March 2001; ADEAR (Alzheimer's Disease Clinical Trials Database): to March 2001; National Research Register (including the MRC Clinical Trials Directory): January 2001 (issue 1) Alzheimers Society Trials Database: to March 2001; Glaxo‐Wellcome Trials Database: to March 2001; Centerwatch Trials Database: to December 2000.
The search strategies used to identify relevant records in MEDLINE, EMBASE, PsycInfo and CINAHL can be found in the Group's module.
2. Reference search. The reference lists of all obtained studies were checked. 3. Pharmaceutical company Pharmacia & Upjohn, owners of the rights to produce and market nicergoline in various different countries, was asked to provide data and reports of clinical trials. In case of unavailability of numerical data in published studies, the authors of each paper, were asked for any published or unpublished data.
Data collection and analysis
A single reviewer (MF) reviewed the list of articles and discarded irrelevant citations based on the title of the publication and its abstract. In the presence of any suggestion that the article could possibly be relevant, it was retrieved for further assessment. The two reviewers independently selected the trials for inclusion in the review from the culled citation list. Disagreements were resolved by discussion.
Results
Description of studies
Eleven studies (one of which, Saletu 1995, comprised a placebo group and two separate groups of patients, with different clinical diagnoses, and thus was included twice in this review) were found compliant with the inclusion criteria and had numerical data available to be extracted for meta‐analysis. Seventy‐six other studies were not included predominantly because they did not include a placebo control group (and thus the studies were confounded) or had no control group at all. Other studies were outside the inclusion criteria since they included patients with diagnoses other than dementia or cognitive decline of older people. All included studies were classified as category A in regard to allocation concealment and were performed in double‐blind, parallel group fashion. Four studies were carried out in single centres, all the others were performed as multicentre. This review is based on studies of which the clinical definitions of patients vary both according to the prevalent diagnostic mode of the time and to the different entities considered for inclusion in the individual studies. The various definitions of patients range from "Senile Cognitive Deterioration" to "Cerebral Metabolic and Nutritional Disturbances", from "Hypertension and Leukoaraiosis" to "Senile Cerebral Insufficiency", from "Chronic Cerebrovascular Disorders" to "Multi Infact Dementia (MID)", and from "Senile Dementia" to "Senile Dementia of Alzheimer's Type (SDAT)". Most of the studies were performed using a total of 60 mg. of nicergoline per day (in some instances 20 mg three times a day, and in others 30 mg twice a day) and all used oral administration. The length of treatment ranged from a minimum of 4 weeks to a maximum of 2 years. The studies were performed in a number of different countries including Italy, USA, Germany, and Austria. Not all the data included in this metanalysis are from published sources; four of the included studies were obtained from the internal archives of Pharmacia & Upjohn.
Risk of bias in included studies
The two reviewers have assessed the methodological quality of each trial with particular emphasis on allocation concealment. Empirical research has shown that lack of adequate allocation concealment is associated with bias (Chalmers 1983, Schulz 1995). Indeed, concealment has been found to be more important in preventing bias than other components of allocation, such as the generation of the allocation sequence.
The trials were ranked using the Cochrane approach:
Category A (adequate) where the report describes allocation of treatment by: (i) some form of centralized randomized scheme, such as having to provide details of an enrolled participant to an office by phone to receive the treatment group allocation; (ii) some form of randomization scheme controlled by a pharmacy; (iii) numbered or coded containers, such as in a pharmaceutical trial in which capsules from identical‐looking numbered bottles are allocated sequentially to enrolled participants; (iv) an on‐site or coded computer system, given that the allocations were in a locked, unreadable file that could be accessed only after inputting the characteristics of an enrolled participant; or (v) if assignment envelopes were used, the report should at least specify that they were sequentially numbered, sealed, opaque envelopes; (vi) other combinations of described elements of the process that provide assurance of adequate concealment.
Category B (intermediate) where the report describes allocation of treatment by: (i) use of a 'list' of 'table' to allocate assignments; (ii) use of 'envelopes' or 'sealed envelopes'; (iii) stating the study as 'randomized' without further detail.
Category C (inadequate) where the report describes allocation of treatment by: (i) alternation; (ii) reference to case record numbers, dates of birth, day of week, or any other such approach; (iii) any allocation procedure that is entirely transparent before assignment, such as an open list of random numbers or assignments. Trials which have taken inadequate measures to conceal allocation have been shown to yield more pronounced estimates of treatment effect than trials which have taken adequate measures. Trials with unclear allocation concealment produce estimates less pronounced than inadequately concealed trials, but more pronounced than adequately concealed trials (Chalmers 1983, ).
INCLUSION CRITERIA Trials were included if they conformed to categories A or B, while those falling into category C were excluded.
DATA EXTRACTION Data were independently extracted by two reviewers and cross‐checked. Any discrepancies were discussed. Data were not sought on every patient for each outcome measure, since single patient data were unavailable for most of the studies. To allow an intention‐to‐treat analysis, data were sought irrespective of compliance, whether or not the patients were subsequently deemed ineligible, or otherwise excluded from treatment or follow‐up. Unfortunately, only completers' analyses could be performed. Data for an 'on‐treatment' or 'completers' analysis were abstracted from publications or internal reports and used for the analysis. In a study where a cross‐over design was used, only data from the first treatment period were included. No titration periods prior to the randomized phase of the study were described in the included studies.
DATA ANALYSIS For continuous or ordinal variables (such as psychometric test scores, clinical global impression scales, functional and quality of life scales) the main outcomes of interest are the change in score from baseline to the final assessment. If ordinal scale data appeared to be approximately normally distributed, or if the investigators' analysis suggested parametric tests to be appropriate, the outcome measures were treated as continuous data. For binary outcomes such as global impression the endpoint itself is of interest and the Peto method of the 'typical odds ratio' was used. A test for heterogeneity of the treatment effect between the trials was made using a standard chi‐square statistic. If a test of heterogeneity was negative a weighted estimate of the typical treatment effect across trials, the 'typical odds ratio' (i.e. the odds of an unfavourable outcome amongst treatment‐allocated patients to the corresponding odds among controls) was calculated using Peto's log‐rank test adapted for ordinal data (Mulrow 1997). It was planned that if there was evidence of heterogeneity of the treatment effect within a group of trials then only data from a sub‐group of studies showing homogeneity would be pooled. This procedure was not required. The null hypothesis to be tested was that, for any of the above outcomes, nicergoline had no effect compared with placebo.
Effects of interventions
Results are described for the different outcomes comprising different methods of cognitive assessment, behavioural rating scales, global clinical impression and tolerability to the drug which were identified as the relevant measurements used in the included studies and which have varied according to the time, geographical area, type of patients, and goal of each study. Behavioral Rating Scales
Several types of behavioural rating scales were used in the clinical studies with nicergoline. The Sandoz Clinical Assessment Geriatric Scale (SCAG) was the one used with the largest number of patients (in total 814 patients, 405 treated with Nicergoline and 409 with placebo from 7 studies). The results from these diverse studies were homogeneous, even though they included patients with different clinical diagnoses and observed over time periods ranging from 2 months to 12 months. Using the WMD (weighted mean difference) and FE (Fixed Effect) model, the summary effect size was ‐5.80 [95% CI ‐8.18, ‐3.41], p=0.00001 indicating an effect in favour of the active treatment in reducing the behavioural symptoms described by SCAG. This scale has a maximum score of 133 points. The therapeutic effects of nicergoline seem to be evident by 2 months of treatment and maintained for 6 months. In general other behavioural outcome measures which include the GRS, the IADL, and the MACC and were episodically used in few studies, failed to demonstrate statistically significant results although there was a trend favouring treatment.
Cognitive Assessment
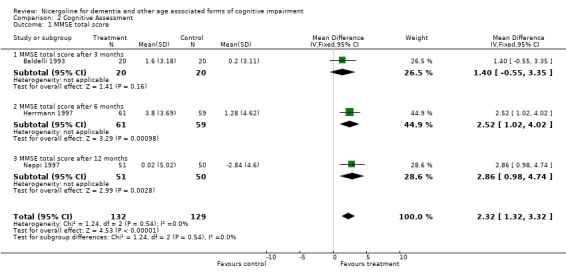
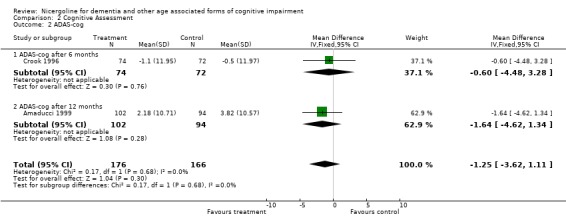
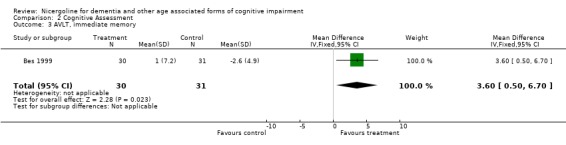
Cognitive assessment had been performed for the largest number of subjects with the MMSE and the ADAS‐Cog, while only few studies used other measures. The total number of patients who underwent an examination with MMSE was 261, with 132 patients in the nicergoline group and 129 in the placebo group from 3 studies. Even though these individual studies were performed over a long period of time with different acceptable diagnostic criteria, and had a different length of observation ranging from 3 months to 12 months, no significant heterogeneity was found. The effect size of the difference between the treatment groups, favouring nicergoline treatment, was 2.32 [95% CI 1.32, 3.32] using the weighted mean difference and fixed effect model . The ADAS‐Cog was used in two studies performed for 6 and 12 months, both restricted to Alzheimer's disease patients (in total 342 patients, 176 in the nicergoline group and 166 in the placebo group). In both studies the differences tended to favour the nicergoline group (the total effect size (weighted mean difference) was ‐1.25 [95% CI ‐3.62, 1.11]) but this difference was not statistically significant. The other results from various cognitive measures tended to favour nicergoline but were based on a small number of cases.(in total 83 patients) One of these studies, performed on a total of 61 patients, provided evidence of a significant effect of nicergoline on a measure of immediate memory when compared with placebo (effect size 3.60 [95% CI 0.50‐6.70], p=0.02). Clinical Global Impression
Clinical impression was evaluated in a total of 6 different studies with 857 patients, 420 in the nicergoline group and 437 in the placebo group. These results were obtained from periods of observation ranging from 2 months to 12 months and were homogeneous across the studies with chi‐square = 7.64 (df = 6, p = 0.27). Using a fixed effects model, the Peto odds ratio for improvement in the subjects treated with nicergoline as opposed to the subjects treated with placebo was 3.33 [95% CI 2.50, 4.43] (p=0.00001).
Tolerability
Tolerability was assessed in 1362 patients, 678 treated with nicergoline and 684 treated with placebo. Safety data were also homogeneous across studies, chi‐square = 14.92 (df = 9). Using a fixed effects model, the Peto Odds Ratio for frequency of adverse effects of this drug compared with placebo was 1.51 [95% CI 1.10, 2.07] indicating a modest increase of adverse effects with nicergoline .
Discussion
This review examined controlled studies of nicergoline starting in 1972 and performed with diverse criteria and modalities of evaluation. Despite these potential sources of variability the data used for this meta‐analysis were homogeneous in nature. The 11 included studies, out of the 124 studies considered for this review, were all double‐blind, placebo controlled clinical studies with extractable numerical data and fulfilled randomization criteria adopted by the Cochrane group. Ten studies are still awaiting an assessment for inclusion, on the assumption that the authors will provide necessary data. At present, the evidence for efficacy of nicergoline is based on studies which have evaluated subjects observed over periods ranging from a minimum of two weeks to a maximum of two years and has been confirmed in different domains such as behaviour, cognition, and clinical judgement. In general, results of this meta‐analysis provide some evidence that there are positive effects of nicergoline on cognition and behaviour, and these effects are confirmed by the relevant effect on clinical global impression. There was some evidence of modest problems in the tolerance for this medication.
The evidence of these results is mostly based on studies performed on older patients with mild to moderate cognitive and behavioural impairment of various clinical origins, including chronic cerebrovascular disorders and Alzheimer's dementia. The few studies specifically performed on patients with Alzheimer's disease, even though consistently indicating a trend in favour of the active treatment, were performed with too few people to give a definite answer to the questions concerning the use of nicergoline for this form of dementia.
Authors' conclusions
Implications for practice.
There is some evidence that nicergoline may have benefits for people with dementia and chronic cognitive disorders. Nicergoline is relatively safe and easy to prescribe and use. The bulk of evidence is obtained from disorders with a cerebrovascular origin, but it is not clear whether the effect is restricted to these conditions. There is little available evidence for a useful role in the treatment of Alzheimer's dementia.
Implications for research.
Since many of the studies done on nicergoline were carried out long ago, the drug has not been evaluated using currently available diagnostic categories or with or against other therapeutic agent such as cholinesterase inibitors or antioxidants.
What's new
| Date | Event | Description |
|---|---|---|
| 5 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 24 August 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the full and useful cooperation of the Pharmacia&Upjohn personnel in Milan (Italy) who made available the unpublished data in their archives. We also gratefully acknowledge the contribution of Sara Carfagna, the consumer editor.
Data and analyses
Comparison 1. Behavioral Rating Scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SCAG total scores | 6 | 766 | Mean Difference (IV, Fixed, 95% CI) | ‐5.73 [‐8.17, ‐3.28] |
| 1.1 Scag total score after 2 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐14.14, 7.14] |
| 1.2 SCAG total score after 6 months | 4 | 615 | Mean Difference (IV, Fixed, 95% CI) | ‐5.18 [‐8.03, ‐2.33] |
| 1.3 SCAG total score after 12 months | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐8.19 [‐13.51, ‐2.87] |
| 2 GRS total scores | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐3.50, 1.10] |
| 2.1 GRS total score after 6 months | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐3.50, 1.10] |
| 3 MACC total scores | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [‐2.31, 5.11] |
| 3.1 MACC total scores after 6 months | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [‐2.31, 5.11] |
| 4 IADL total score | 2 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.70, 0.36] |
| 4.1 IADL total score after 3 months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.80, 2.20] |
| 4.2 IADL total score after 12 months | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.87, 0.39] |
1.1. Analysis.
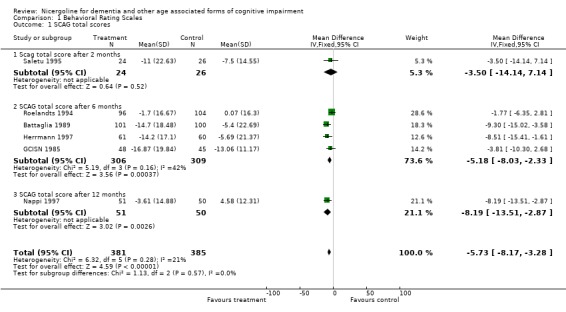
Comparison 1 Behavioral Rating Scales, Outcome 1 SCAG total scores.
1.2. Analysis.
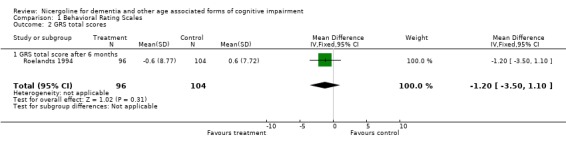
Comparison 1 Behavioral Rating Scales, Outcome 2 GRS total scores.
1.3. Analysis.
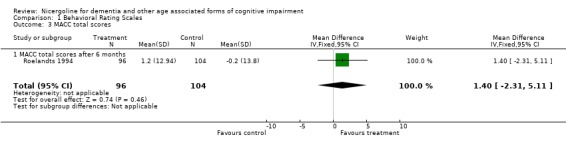
Comparison 1 Behavioral Rating Scales, Outcome 3 MACC total scores.
1.4. Analysis.
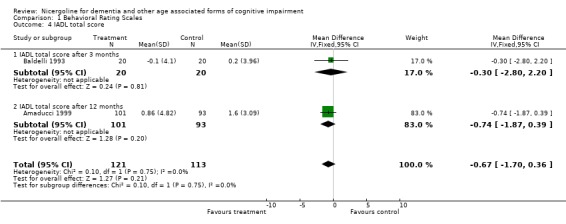
Comparison 1 Behavioral Rating Scales, Outcome 4 IADL total score.
Comparison 2. Cognitive Assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE total score | 3 | 261 | Mean Difference (IV, Fixed, 95% CI) | 2.32 [1.32, 3.32] |
| 1.1 MMSE total score after 3 months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐0.55, 3.35] |
| 1.2 MMSE total score after 6 months | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [1.02, 4.02] |
| 1.3 MMSE total score after 12 months | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | 2.86 [0.98, 4.74] |
| 2 ADAS‐cog | 2 | 342 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐3.62, 1.11] |
| 2.1 ADAS‐cog after 6 months | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.48, 3.28] |
| 2.2 ADAS‐cog after 12 months | 1 | 196 | Mean Difference (IV, Fixed, 95% CI) | ‐1.64 [‐4.62, 1.34] |
| 3 AVLT, immediate memory | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 3.6 [0.50, 6.70] |
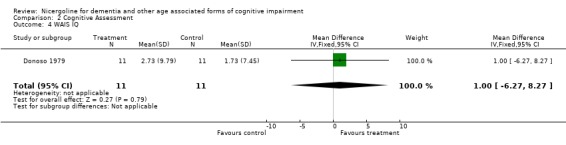
| 4 WAIS IQ | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐6.27, 8.27] |
2.1. Analysis.
Comparison 2 Cognitive Assessment, Outcome 1 MMSE total score.
2.2. Analysis.
Comparison 2 Cognitive Assessment, Outcome 2 ADAS‐cog.
2.3. Analysis.
Comparison 2 Cognitive Assessment, Outcome 3 AVLT, immediate memory.
2.4. Analysis.
Comparison 2 Cognitive Assessment, Outcome 4 WAIS IQ.
Comparison 3. Clinical Evaluation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Global Impression (number showing improvement) | 6 | 809 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.38 [2.48, 4.60] |
| 1.1 CGI after 2 months | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.61 [1.87, 16.83] |
| 1.2 CGI after 6 months | 4 | 658 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.82 [1.99, 3.99] |
| 1.3 CGI after 12 months | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.13 [3.10, 16.42] |
3.1. Analysis.
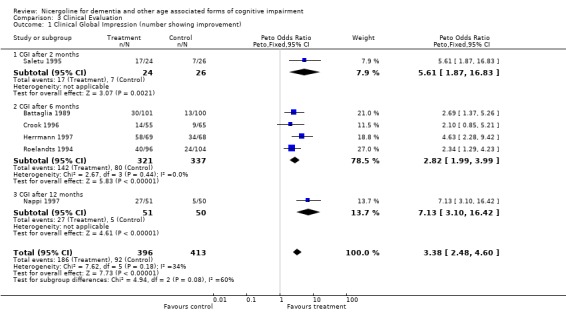
Comparison 3 Clinical Evaluation, Outcome 1 Clinical Global Impression (number showing improvement).
Comparison 4. Tolerability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total number suffering at least one adverse events | 9 | 1314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [1.08, 2.06] |
4.1. Analysis.
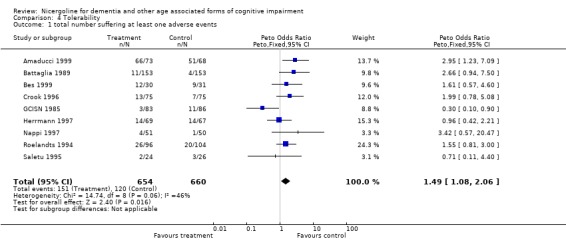
Comparison 4 Tolerability, Outcome 1 total number suffering at least one adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amaducci 1999.
| Methods | multinational, multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | SDAT mild to moderate; 24 patients received nicergoline, 24 placebo; age > 50 yr. | |
| Interventions | Nicergoline or Placebo 30mg. twice a day (os) for 12 months | |
| Outcomes | ADAScog, IADL, CGIC for efficacy; adverse events for safety | |
| Notes | CGIC data were not available. ITT (LOCF) analysis is available | |
Baldelli 1993.
| Methods | single centre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | Senile cognitive deterioration mild to moderate; 20 patients recived nicergoline, 20 placebo; age > 58 yr. | |
| Interventions | Nicergoline or Placebo 20mg. trice a day (os) for 3 months | |
| Outcomes | MMSE, IADL for efficacy | |
| Notes | Patients received a concomitant psychosensory training | |
Battaglia 1989.
| Methods | multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | Dementia mild to moderate; 158 patients received nicergoline, 157 placebo; age > 55 yr. | |
| Interventions | Nicergoline or Placebo 20mg. twice a day (os) for 6 months | |
| Outcomes | SCAG for efficacy; HR and blood pressure for safety | |
| Notes | Only completers' analysis is available for 101nicergoline and 100 placebo cases (unpublished report from Pharmacia‐Upjohn) | |
Bes 1999.
| Methods | multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | Hypertension & leukoaraiosis; 36 patients received nicergoline, 36 placebo; age > 60 yr. | |
| Interventions | Nicergoline or Placebo 30mg. twice a day (os) for 24 months | |
| Outcomes | Rey Auditory Verbal Learning Test | |
| Notes | ITT (LOCF) analysis is available | |
Crook 1996.
| Methods | multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | SDAT mild to moderate; 74 patients received nicergoline, 75 placebo; age > 50 yr. | |
| Interventions | Nicergoline or Placebo 30mg. twice a day (os) for 6 months | |
| Outcomes | ADAScog, CGIC for efficacy; adverse events for safety | |
| Notes | ITT (LOCF) analysis is available | |
Donoso 1979.
| Methods | single centre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | Senile cognitive deterioration with no signs of dementia; 11 patients received nicergoline, 11 placebo; age > 65 yr. | |
| Interventions | Nicergoline or Placebo 10mg. trice a day (os) for 6 weeks | |
| Outcomes | WAIS IQ | |
| Notes | Completers' analysis | |
GCISN 1985.
| Methods | multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | Senile cerebral insufficiency mild to moderate; 82 patients received nicergoline, 80 placebo; age > 55 yr. | |
| Interventions | Nicergoline or Placebo 20mg. twice a day (os) for 6 months | |
| Outcomes | SCAG for efficacy; adverse events for safety | |
| Notes | It describes also a crossover study but with no data available for inclusion in this review | |
Herrmann 1997.
| Methods | multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | MID; 70 patients received nicergoline, 69 placebo; age 55‐85 yr. | |
| Interventions | Nicergoline or Placebo 30mg. twice a day (os) for 6 months | |
| Outcomes | SCAG, MMSE, CGI for efficacy; adverse events for safety | |
| Notes | ||
Nappi 1997.
| Methods | multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | SDAT or MID mild to moderate; 51patients received nicergoline, 50 placebo; age 60‐80 yr. | |
| Interventions | Nicergoline or Placebo 30mg. twice a day (os) for 12 months | |
| Outcomes | SCAG, MMSE, CGI for efficacy; adverse events for safety | |
| Notes | ||
Roelandts 1994.
| Methods | multinational, multicentre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | Senile dementia mild to moderate; 82 patients received nicergoline, 80 placebo; age > 55. | |
| Interventions | Nicergoline or Placebo 30mg. twice a day (os) for 6 months | |
| Outcomes | SCAG, GRS, MACC for efficacy; adverse events for safety | |
| Notes | ||
Saletu 1995.
| Methods | single centre, double blind, randomized, placebo controlled, parallel groups | |
| Participants | MID mild to moderate; 24 patients received nicergoline, 26 placebo; age > 60 yr. | |
| Interventions | Nicergoline or Placebo 30mg. twice a day (os) for 8 weeks | |
| Outcomes | SCAG, MMSE, CGI, EEG/ERP for efficacy; side effects for safety | |
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ardia 1985 | cross‐over study with no data divided for each arm |
| Battaglia 1990 | controlled study vs another active drug |
| Battaglia 1995b | preliminary data of another included study |
| Bente 1979 | controlled study vs dihydroergotoxin‐mesylate |
| Bernini 1977 | not a controlled study |
| Blaha 1979 | clinical study with geriatric patients with transitory cognitive and behavioural deficits |
| Brambilla 1978 | controlled study between three different dosage of nicergoline |
| Cascone 1978 | data not available in numerical form |
| Crook 1997a | not clinical study |
| Crook 1997b | preliminary results of another included study |
| Dauverchain 1979 | not controlled study. Chronic cerebrovascular insufficiency |
| Efimov 1991 | not controlled clinical study, Diabetic patients |
| Fabbrini 1995 | not controlled study |
| Gara 1993 | controlled study vs pentoxifylline in cerebral atherosclerosis patients |
| Guardamagna 1972 | not controlled study |
| Iliff 1979 | not controlled study. Blood flow in patients with cerebrovascular disease |
| Kaemmerer 1980 | not controlled study |
| Kobayashi 1992 | not controlled study |
| Kugler 1984 | not controlled study |
| Kugler 1985 | controlled study vs dihydroergotamine mesilate in patients with cerebral insufficiency |
| Kugler 1990 | clinical study on normal subjects taking barbiturates |
| Ladurner 1991 | controlled clinical study vs co‐dergocrine mesilate in patients with multinfarct dementia |
| Maiolo 1972 | not controlled study |
| Man'kovskii 1989a | controlled study vs prostacyclin in brain hypoxia |
| Man'kovskii 1989b | not controlled study |
| Marolda 1978 | controlled study vs eburnamonine |
| Michelangeli 1975 | clinical study performed with chronic cerebrovascular patients included without a formal assessment of cognitive deficits but on the basis only of clinical signs of vascular pathology |
| Miletto 1976 | not controlled study |
| Moglia no year | a review of different studies |
| Montanini 1973 | not controlled study |
| NCSG 1990 | controlled study vs ergoloid mesylates |
| Nico 1981 | controlled study vs papaveroline on cerebral ischaemic vasculopathy |
| no author 1989 | data presented in another included study |
| Ohtomo 1986a | controlled study vs Ca‐hopantenate |
| Ohtomo 1986b | controlled study vs Hydergine |
| Ohtomo 1986c | not controlled study |
| Prencipe 1974 | not controlled study |
| Saletu 1979a | multiple treatments controlled study run in a cross‐over fashion with each patient taking all different treatments at different periods of the observation |
| Saletu 1980 | review with no data in numerical format |
| Saletu 1987 | controlled study vs a different dosage of nicergoline |
| Saletu 1996a | same data of another included study |
| Saletu 1996b | same data of another included study |
| Saletu 1997 | same data of another included study |
| Samso 1979 | not controlled neurophysiological study on patients with chronic cerebrovascular disorders |
| Schneider 1994 | controlled study vs antagonic‐stress |
| Shtok 1985 | controlled study vs dihydroergotoxin on cerebral haemodynamics of patients with acute and chronic cerebrovascular diseases |
| Toni 1973 | not controlled study |
| Vesel'skii 1991 | not placebo controlled clinical study |
| Vesel'skii 1992 | not placebo controlled clinical study |
| Winblad 2000 | not a clinical study, a review |
Contributions of authors
‐MF produced a culled reference list for approval of LF. MF obtained the unpublished references and extracted the data from all references. MF wrote the first draft of the review. ‐LF: checked the analyses and revised the protocol and review. ‐MF: all correspondence
‐CDCIG contact editor: Peter Whitehouse
Declarations of interest
In past years one of the authors (MF) has worked as consultant for Farmitalia (later incorporated into Pharmacia) in the development of some clinical studies with nicergoline. None of those studies was of interest for this review owing to the type of patients considered or the research protocol used.
Edited (no change to conclusions)
References
References to studies included in this review
Amaducci 1999 {unpublished data only}
- Amaducci L, Maurer K, Winblad B, Dom R, Bullock R. Efficacy and safety of nicergoline, 30mg tablets b.i.d. for twelve months, in the treatment of probable Alzheimer's disease. A multicentre, multinational, double‐blind, randomized, placebo‐controlled, parallel group clinical trial. Unpublished report 1999.
Baldelli 1993 {published and unpublished data}
- Baldelli MV, Pirani A, Toschi A, Ballotti A. Association of nicergoline and psychosensory training treatment in a group of elderly patients with cognitive impairment. Congress abstract and unpublished report 1993.
Battaglia 1989 {published data only}
- Battaglia A, Bruni G, Ardia A, Sacchetti G, & the Italian Nicergoline Study Group. Nicergoline in Mild to Moderate Dementia: a multicenter, double‐blind, placebo‐controlled study. Journal of the American Geriatrics Society 1989;37(4):295‐302. [DOI] [PubMed] [Google Scholar]
Bes 1999 {published data only}
- Bes A, Orgogozo JM, Poncet M, Rancurel G, Weber M, Bertholom N, Calvez R, Stehle B. A 24‐month, double‐blind, placebo‐controlled multicentre pilot study of the efficacy and safety of nicergoline 60 mg per day in elderly hypertensive patients with leukoaraiosis. European Journal of Neurology 1999;6:313‐322. [DOI] [PubMed] [Google Scholar]
Crook 1996 {unpublished data only}
- Crook TH, Lakin DM. A double‐blind, randomized, placebo‐controlled trial of 30mg b.i.d. nicergoline administered for six months to patients with probable Alzheimer's Disease. Unpublished report from Pharmacia & Upjohn 1996.
Donoso 1979 {published data only}
- Donoso A, Santander M, Sarce H. Effect of nicergoline on intellectual performance [Efecto de la nicergolina en el deterioro psicorganico senil]. Revista Medica de Chile 1979;107:817‐819. [PubMed] [Google Scholar]
GCISN 1985 {published data only}
- Gruppo Coperativo Italiano per lo Studio della Nicergolina. The activity of nicergoline in senile cerebral deterioration: controlled clinical studies vs placebo. Giornale di Gerontologia 1985;Suppl. 62:27‐43. [Google Scholar]
Herrmann 1997 {published data only}
- Herrmann WM, Stephan K, Gaede K, Apeceche M. A multicenter randomized double‐blind study on the efficacy and safety of nicergoline in patients with multi‐infarct dementia. Dementia and Geriatric Cognitive Disorders 1997;8:9‐17. [DOI] [PubMed] [Google Scholar]
Nappi 1997 {published data only}
- Nappi G, Bono G, Merlo P, Borromei A, Caltagirone C, Lomeo C, Martucci N, Fabbrini G, Annoni K, Battaglia A. Long‐term nicergoline treatment of mild to moderate senile dementia. Clinical Drug Investigation 1997;13:308‐316. [DOI] [PubMed] [Google Scholar]
Roelandts 1994 {unpublished data only}
- Roelandts F, Moglia A, Binder H, Jordy C. Multicenter, multinational, double‐blind, placebo‐controlled study of the efficacy and tolerability of nicergoline in patients suffering from mild to moderate senile dementia. Unpublished internal report 1994.
Saletu 1995 {published data only}
- Saletu B, Paulus E, Linzmeyer L, Anderer P, Semlitsch HV, Grunberger J, Wicke L, Neuhold A, Podreka I. Nicergoline in senile dementia of Alzheimer type and multi‐infarct dementia: a double‐blind, placebo‐controlled, clinical and EEG/ERP mapping study. Psychopharmacology 1995;117(4):385‐395. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ardia 1985 {unpublished data only}
- Ardia A, Dubini A, Jori MC, Groppi W. Multicentric, double‐blind, crossover study of nicergoline vs placebo in mild to moderate senile dementia. Unpublished internal report 1985.
Battaglia 1990 {published data only}
- Battaglia A, Bruni G, Sacchetti G, Pamparana F. A double‐blind randomized study of two ergot derivatives in mild to moderate dementia. Current Therapeutic Research 1990;48:597‐612. [Google Scholar]
Battaglia 1995b {published data only}
- Battaglia A, Annoni K, Pamparana F, Paolis C, Bonura M, Stekke W, and Nicergolina Dementia Long Term Study Italian Group. Nicergoline in the long term tratment of mild or moderate senile dementia: a multicenter double‐blind, randomized, placebo‐controlled trial. 8th European College of Neuropsychopharmacology. Venice, 1995.
Bente 1979 {published data only}
- Bente D, Glatthaar G, Ulrich G, Lewinsky M. Quantitative EEG examinations on the vigilance stabilizing effect of nicergoline / Results of a double blind study with gerontopsychiatric patients. Arzneimittel Forschung 1979;29:1804‐1808. [PubMed] [Google Scholar]
Bernini 1977 {published data only}
- Bernini FP, Muras I, Maglione F, Smaltino F. Action of nicergoline on the cerebral circulatory insufficiency due to arteriosclerosis. Clinical and angioserographic evaluation. Farmaco 1977;32:32‐46. [PubMed] [Google Scholar]
Blaha 1979 {published data only}
- Blaha L, Burkard G, Lehrl S, Kapinas K. Psychopathometric double blind study with nicergoline versus placebo in geriatric patients with slight transit syndromes. Arzneimittel Forschung 1979;29:1295‐1301. [PubMed] [Google Scholar]
Brambilla 1978 {published data only}
- Brambilla G, Malisardi PA, Forni G. Double blind comparison of 3 dosages of nicergoline in senile dementia. Atti dell'Accademia Medica Lombarda 1978;33:37‐47. [Google Scholar]
Cascone 1978 {published data only}
- Cascone A, Liverta C, Pollini C. Controlled study with nicergoline and placebo in cerebral and peripheral vascular insufficiency in the aged. Minerva Cardioangiologica 1978;26:95‐100. [PubMed] [Google Scholar]
Crook 1997a {published data only}
- Crook TH. Nicergoline: parallel evolution of clinical trial methodology and drug development in dementias. Dementia and Geriatric Cognitive Disorders 1997;8(Suppl 1):22‐26. [DOI] [PubMed] [Google Scholar]
Crook 1997b {published data only}
- Crook TH. Nicergoline in the treatment of probable Alzheimer's disease: preliminary results of a double‐blind, randomized, placebo‐controlled study. Journal of the Neurological Sciences 1997;150(Suppl):18. [Google Scholar]
Dauverchain 1979 {published data only}
- Dauverchain J. The role of nicergoline in the symptomatic treatment of arterial hypertension and chronic cerebrovascular insufficiency. A study on 359 observations. Arzneimittel Forschung 1979;29:1308‐1310. [PubMed] [Google Scholar]
Efimov 1991 {published data only}
- Efimov AS, Man'kovskii BN. The effect of sermion on brain function in diabetics. Vrachebnoe Delo 1991;11:73‐76. [PubMed] [Google Scholar]
Fabbrini 1995 {published data only}
- Fabbrini G, Martucci N, Battaglia A, Pamparana F, Annoni K. Nicergoline in the treatment of dementia: the effects on cerebral blood flow measured by SPECT. 8th European College of Neuropsychopharmacology Congress. Venice, 1995.
Gara 1993 {published data only}
- Gara II. The effect of pentoxifylline and nicergoline on the systemic and cerebral hemodynamics and on the blood rheological properties in patients with an ischemic stroke and atherosclerotic lesions of the major cerebral arteries. Zhurnal Nevropatologii i Psikhiatrii Imeni S.S. Korsakova 1993;93:28‐32. [PubMed] [Google Scholar]
Guardamagna 1972 {published data only}
- Guardamagna C, Negri S. Clinical efficacy of nicergoline in cerebrovascular diseases. Statistical study. Minerva Cardioangiologica 1972;20:636‐641. [PubMed] [Google Scholar]
Iliff 1979 {published data only}
- Iliff LD, Du Boulay GH, Marshall J, Ross Russell RW, Symon L. Effects of nicergoline on cerebral blood flow. Arzneimittel Forschung 1979;29:1277‐1278. [PubMed] [Google Scholar]
Kaemmerer 1980 {published data only}
- Kaemmerer E. Effectiveness of nicergoline in the treatment of chronic cerebrovascular insufficiency. Nervenarzt 1980;51:368‐372. [PubMed] [Google Scholar]
Kobayashi 1992 {published data only}
- Kobayashi H, Handa Y, Kawano H, Kubota T. Effects of nicergoline (Sermion) in patients with cerebrovascular disease. Progress in Medicine 1992;12:2439‐2446. [Google Scholar]
Kugler 1984 {published data only}
- Kugler J, Heidrich H. Nicergoline and cerebral performance insufficiency. Observations in 1 year treatment controls. Fortschr Med 1984;102:1091‐1096. [PubMed] [Google Scholar]
Kugler 1985 {published data only}
- Kugler JE, Meurer‐Krull BC. Electroencephalography and psychometric measurements during the treatment of cerebral insufficiency with nicergoline and dihydroergotamine mesylate. Arzneimittel Forschung 1985;35:1865‐1870. [PubMed] [Google Scholar]
Kugler 1990 {published data only}
- Kugler J, Groll S, Upmeyer HJ, Schmidt A. Nicergoline in the treatment of organic brain syndrome in old age. Zeitschrift fur Gerontopsychologie und Psychiatrie 1990;3:277‐284. [Google Scholar]
Ladurner 1991 {published data only}
- Ladurner G, Erhart P, Erhart C, Scheiber V. Therapy of organic brain syndrome with nicergoline given once a day. [Article in German]. Wiener Klinische Wochenschrift 1991;103:8‐14. [PubMed] [Google Scholar]
Maiolo 1972 {published data only}
- Maiolo AT, Bianchi Porro G, Galli C, Sessa M. Effects of nicergoline on hemodynamics and metabolism of the brain in primary arterial hypertension and in arteriosclerosis. Clinica Terapeutica 1972;62:239‐252. [PubMed] [Google Scholar]
Man'kovskii 1989a {published data only}
- Man'kovskii NB, Mints AIa, Karaban' IN. Initial dyscirculatory encephalopathy in middle‐aged and elderly patients. Zh Nevropatol Psikhiatr Im S S Korsakova 1989;89:16‐20. [PubMed] [Google Scholar]
Man'kovskii 1989b {published data only}
- Man'kowskii NB, Mints AIa, Bachinskaia NIu, Kochubei NV. Sermion treatment of middle‐aged and elderly patients with initial atherosclerotic encephalopathy. Vrachebnoe Delo 1989;4:60‐63. [PubMed] [Google Scholar]
Marolda 1978 {published data only}
- Marolda M, Fragassi N, Buscaino GA. Clinical evaluation of (‐)eburnamonine in comparison with nicergoline in patients suffering from chronic brain ischemia.. European Neurology 1978;17 Suppl 1:159‐166. [DOI] [PubMed] [Google Scholar]
Michelangeli 1975 {published data only}
- Michelangeli J, Sevilla M, Lavagna J, Darcourt G. Action of nicergoline (Sermion) in chronic vascular disease in old age. Annee Medicale de Psychologie (Paris) 1975;1:499‐510. [PubMed] [Google Scholar]
Miletto 1976 {published data only}
- Miletto D, Julou M. Electroencephalographic study during long‐term use of nicergoline. Ann Med Psychol (Paris) 1976;134:824‐831. [PubMed] [Google Scholar]
Moglia no year {unpublished data only}
- Moglia A, Arrigo A, Battaglia A, Sacchetti G. Psychopharmacology of senile dementia: placebo controlled clinical studies with nicergoline. unobtainable. unobtainable; Vol. unobtainable:117.
Montanini 1973 {published data only}
- Montanini R, Gasco P, Manfredini G. Clinical and electroencephalographic studies of the effect of nicergoline in cerebrovascular diseases. Acta Neurologica 1973;28:133‐149. [PubMed] [Google Scholar]
NCSG 1990 {published data only}
- Nicergoline Cooperative Study Group. A double‐blind randomized study of two ergot derivatives in mild to moderate dementia. Current Therapeutic Research 1990;48:597‐612. [Google Scholar]
Nico 1981 {published data only}
- Nico F, Guercini F, Biagi A, Fanfoni S. Cerebral ischemic vasculopathy: clinical and thermographic study in patients treated with papaveroline. Clinica Terapeutica 1981;98:195‐200. [PubMed] [Google Scholar]
no author 1989 {published data only}
- No author. Nicergolin in the treatment of geriatric patients with chronic cerebral insufficiency. Folha Medica 1989;98:409‐414. [Google Scholar]
Ohtomo 1986a {published data only}
- Ohtomo E, Hirai S, Kato N, Araki G, Kuzuya F, Utsumi S, Handa H, Daraj K, Fujishima M. Clinical evaluation of TA‐079 (nicergoline) in the treatment of cerebrovascular disorders. Clinical Evaluation 1986;14:575‐602. [Google Scholar]
Ohtomo 1986b {published data only}
- Otomo E, Hirai S, Hasegawa K, Kato N, Araki G, Kuzuya F, Utsumi S, Handa H, Sarai K, Fujishima M. Clinical evaluation of TA‐079 (Nicergoline) in the treatment of cerebrovascular disorders. Folia Pharmacologica Japonica 1986;87:73‐106. [Google Scholar]
Ohtomo 1986c {published data only}
- Ohtomo E, Hirai S, Hasegawa H, Kato N, Araki G, Kuzuya F, Utsumi S, Handa H, Sarai K, Fujishima M. Clinical evaluation of TA‐079 (Nicergoline) in long‐term treatment of cerebrovascular disorders. Folia Pharmacologica Japonica 1986;87:451‐466. [Google Scholar]
Prencipe 1974 {published data only}
- Prencipe M, Cecconi V, Pisarri F. Preliminary study on the effect of a nicergoline composition (F.I. 6714) on cerebral blood flow. Farmaco 1974;29:278‐284. [PubMed] [Google Scholar]
Saletu 1979a {published data only}
- Saletu B, Grunberger J, Linzmayer L. Determination of the encephalotropic, psychotropic and pharmacodynamic properties of nicergoline by means of quantitative pharmaco‐ electroencephalography and psychometric analysis. Arzneimittel Forschung 1979;29:1251‐1261. [PubMed] [Google Scholar]
Saletu 1980 {published data only}
- Saletu B, Grunberger J. Antihypoxidotic and nootropic drugs: proof of their encephalotropic and pharmacodynamic properties by quantitative EEG investigations. Progress in Neuropsychopharmacology 1980;4:469‐489. [DOI] [PubMed] [Google Scholar]
Saletu 1987 {published data only}
- Saletu B, Hochmayer I, Grunberger J, Bohmer F, Paroubek J, Wicke L, Neuhold A. Therapy of multi‐infarct dementia with nicergoline: double‐blind, clinical, psychometric and EEG imaging studies with 2 dosage schedules. Wien Med Wochenschr 1987;137:513‐524. [PubMed] [Google Scholar]
Saletu 1996a {published data only}
- Saletu B, Paulus E, Linzmayer L, Anderer P, Semlitsch HV, Grunberger J, Wicke L, Neuhold A, Podreka I. Nicergoline in senile dementia of Alzheimer type and multi‐infarct dementia: a double‐blind, placebo‐controlled, clinical and EEG/ERP mapping study. Folha Medica 1996;113:103‐114. [DOI] [PubMed] [Google Scholar]
Saletu 1996b {published data only}
- Saletu B. On the relations between symptomatology and brain function in dementias. Proceedings of the Fourth International Nice/Springfield Symposium on Advances in Alzheimer Therapy. 1996.
Saletu 1997 {published data only}
- Saletu B, Anderer P, Semlitsch HV. Relations between symptomatology and brain function in dementias: double‐ blind, placebo‐controlled, clinical and EEG/ERP mapping studies with nicergoline. Dementia and Geriatric Cognitive Disorders 1997;8(Suppl 1):12‐21. [DOI] [PubMed] [Google Scholar]
Samso 1979 {published data only}
- Samso Dies JM, Villa Bado J, Villalobos J, Iriarte JL, Ordeix R. Neurophysiological study of 36 patients with cerebral vascular processes treated with nicergoline. Arzneimittel Forschung 1979;29:1301‐1307. [PubMed] [Google Scholar]
Schneider 1994 {published data only}
- Schneider F, Popa R, Mihalas G, Stefaniga P, Mihalas IG, Maties R, Mateescu R. Superiority of antagonic‐stress composition versus nicergoline in gerontopsychiatry. Annals of New York Academy of Sciences 1994;30:332‐342. [DOI] [PubMed] [Google Scholar]
Shtok 1985 {published data only}
- Shtok VN, Varlamova IV, Fedorova NV, Timurbaator. Clinico‐rheographic study of the cerebrovascular effect of alpha‐adrenergic blockers in vascular diseases of the brain. Zh Nevropatol Psikhiatr Im S S Korsakova 1985;85:1333‐1338. [PubMed] [Google Scholar]
Toni 1973 {published data only}
- Toni E. Therapeutic activity of nicergoline in cerebrovascular syndromes. Minerva Medica 1973;64:4466‐4473. [PubMed] [Google Scholar]
Vesel'skii 1991 {published data only}
- Vesel'skii ISh, Sanik AV. The correction of microcirculatory disorders in patients with circulatory encephalopathy. Vrachebnoe Delo 1991;7:85‐87. [PubMed] [Google Scholar]
Vesel'skii 1992 {published data only}
- Vesel'skii ISh, Gritsai NN. The correction of lipid peroxidation metabolism, the hemostatic system and cerebral hemodynamics in patients with the initial manifestations of cerebral circulatory insufficiency. Lik Sprava 1992;7:50‐52. [PubMed] [Google Scholar]
Winblad 2000 {published data only}
- Winblad B, Carfagna N, Bonura L, Rossini B, M, Wong EHF, Battaglia A. Nicergoline in dementia: a review of its pharmacological properties and therapeutical potential. CNS Drugs 2000;14:267‐287. [Google Scholar]
References to studies awaiting assessment
Arrigo 1982 {published data only}
- Arrigo A, Moglia A, Borsotti L. A double‐blind, placebo‐controlled, crossover trial with nicergoline in patients with senile dementia. International Journal of Clinical Pharmacological Research 1982;2(suppl 1):33‐41. [Google Scholar]
Blaha 1983 {published data only}
- Blaha L. Monitoring therapy in the treatment of cerebrovascular insufficiency. Fortschr Med 1983;101:1187‐1190. [PubMed] [Google Scholar]
Gessner 1985 {published data only}
- Gessner B, Voelp A, Klasser M. Study of the long‐term action of a Ginkgo biloba extract on vigilance and mental performance as determined by means of quantitative pharmaco‐EEG and psychometric measurements. Arzneimittel Forschung 1985;35:1459‐1465. [PubMed] [Google Scholar]
Grel 1975 {published data only}
- Grel P, Normand F. Comparative double‐blind study versus placebo of nicergoline in cerebral circulatory insufficiency. Psychologie Medicale 1975;7:1789‐1793. [Google Scholar]
Karyofilis 1980 {published data only}
- Karyofilis A. Therapy of demonstrated cerebral ischemia with nicergoline. Ther Ggw 1980;119:1478‐1485. [PubMed] [Google Scholar]
Lebedeva 1990 {published data only}
- Lebedeva NV, Khrapova EV. Use of sermion, Cavinton and trental in patients with cerebrovascular disorders. Sov Med 1990;1:60‐63. [PubMed] [Google Scholar]
Moglia 1991 {unpublished data only}
- Moglia A, Bergonzoli S, Merlo P, Battaglia A, Valzelli S. Demented patients of low or moderate grade treated with nicergoline: preliminary data of a double blind placebo controlled study. Pan European Society of Neurology, 2nd Congress 1991.
Moglia 1993 {published data only}
- Moglia A, Murri L, Battistini N, Battaglia A, Novellini R, Annoni K, Pamparana F. Nicergoline in mild to moderate SDAT and MID: clinical and EEG brain mapping study. 13th International Congress of Electroencephalography & Clinical Neurophysiology 1993.
Nobuhara 1993 {published data only}
- Nobuhara K. A quantitative pharmaco‐EEG study on psychotropic properties of cerebral metabolic enhancers: comparison between young and elderly healthy volunteers. Seishin Shinkeigaku Zasshi 1993;95:392‐416. [PubMed] [Google Scholar]
Saletu 1990 {published data only}
- Saletu B, Grunberger J, Linzmayer L, Anderer P. Brain protection of nicergoline against hypoxia: EEG brain mapping and psychometry. Journal of Neural Transmission, Parkinson's Disease, Dementia Section 1990;2:305‐325. [DOI] [PubMed] [Google Scholar]
Wiedenhammer 1991 {published data only}
- Wiedenhammer W, Groll S, Ladurner G, Erhart P, Erhart C, Scheiber V. Therapy of organic brain syndrome with nicergoline given once a day. Zeitschrift Fuer Gerontopsychologie und Psychiatrie 1991;103:1‐14. [Google Scholar]
Zappoli 1987 {published data only}
- Zappoli R, Arnetoli G, Paganini M, Versari A, Battaglia A, Grignani A, Sacchetti G. Contingent negative variation and reaction time in patients with presenile idiopathic cognitive decline and presenile Alzheimer‐type dementia. Preliminary report on long‐term nicergoline treatment. Neuropsychobiology 1987;18:149‐154. [DOI] [PubMed] [Google Scholar]
Additional references
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H, Junior. Bias in Treatment Assignment in Controlled Clinical Trials.. N Eng J Med 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
Mc Arthur 1997
- Arthur RA, Carfagna N, Banfi L, Cavanus S, Cervini MA, Fariello R. Effects of nicergoline on age‐related decrements in radial maze performance and acetylcholine levels. Brain Research Bulletin 1997;43:305‐311. [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD (eds). Cochrane Collaboration Handbook [updated 9 December 1996]. Available in The Cochrane Library [database on disk and CDROM].. The Cochrane Collaboration; Issue 1, updated quarterly. Oxford: Update Software, 1997. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials.. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]


