Abstract
Background
Despite the increasing use of palliative chemotherapy for advanced colorectal cancer, there remains uncertainty as to the true effectiveness of this intervention. This review was therefore undertaken to assess the available evidence for the benefit of palliative chemotherapy in this disease.
Objectives
To determine the benefits and harms of palliative chemotherapy in patients with locally advanced or metastatic colorectal cancer. A secondary objective was to investigate outcomes for younger and elderly patients.
Search methods
Trials were identified by computerised and hand searches of the literature, scanning references and contacting investigators.
Selection criteria
All randomised controlled trials of palliative chemotherapy compared with supportive care alone in patients with advanced or metastatic colorectal cancer.
Both randomised and non‐randomised studies were considered when searching for data on quality of life, resource use and cost effectiveness of palliative chemotherapy.
Data collection and analysis
Investigators from all eligible studies were asked to supply individual patient data. Meta‐analysis was performed using both published data and individual patient data. Studies were grouped according to whether chemotherapy was administered regionally or systemically.
Main results
13 randomised controlled trials representing a total of 1365 randomised patients met the inclusion criteria. Meta‐analysis of a subset of trials that provided individual patient data demonstrated that palliative chemotherapy was associated with a 35% (95% CI 24% to 44%) reduction in the risk of death. This translates into an absolute improvement in survival of 16% at both 6 months and 12 months and an improvement in median survival of 3.7 months. The overall quality of evidence relating to treatment toxicity, symptom control and quality of life was poor.
Authors' conclusions
Chemotherapy is effective in prolonging time to disease progression and survival in patients with advanced colorectal cancer. The survival benefit may be underestimated by this meta‐analysis, as a proportion of patients in the control arms of some trials received chemotherapy. No age related differences were found in the effectiveness of chemotherapy, but elderly patients were under represented in trials. Treatment toxicity and impact upon quality of life and symptom control have been inadequately assessed in the majority of trials and further research is needed to clarify the palliative benefit of chemotherapy.
Plain language summary
Chemotherapy improves the survival of people with advanced colorectal cancer, but the adverse effects and impact on quality of life are not yet known.
Colorectal (bowel) cancer is common worldwide, but can often be treated effectively with surgery. In some people, however, the cancer returns and sometimes also spread to other parts of the body. When this happens, chemotherapy (anti‐cancer drugs) can be used to try to slow the progression of the cancer. However, chemotherapy also have adverse effects. The review of trials found that chemotherapy improves survival of people with advanced colorectal cancer. However, the effects of treatment upon quality of life are not yet clear and need more study.
Background
Colorectal cancer accounts for approximately 10% of all cancer registrations and is the second most common cancer within the UK (ONS 1997). The primary treatment for colorectal cancer is surgical resection, but over half of all patients will eventually die of metastatic disease (Cunningham 1993). Although the rate of progression of advanced colorectal cancer is variable, patients have a median survival of only 6‐9 months from the diagnosis of metastatic disease, during which time they may develop a wide variety of physical and psychological symptoms that detract from their quality of life and frequently precipitate hospital admission (Seymour 1997).
Chemotherapy for Advanced Colorectal cancer
Palliative chemotherapy is now offered to an increasing proportion of patients with advanced colorectal cancer (Seymour 1997). However, there is no universally accepted standard therapy and the duration of time for which treatment is given varies widely (Wils 1998). A limitation of existing therapies is the frequent development of significant toxicity.
5‐Fluorouracil (5‐FU) either alone or in combination with other agents is widely employed as first line therapy (Wils 1998). When compared with intravenous bolus injection of 5‐FU, administration of 5‐FU by continuous low dose intravenous infusion, or modulation of 5‐FU with agents, such as leucovorin or methotrexate, can enhance the tumour response rate, but not appreciably lengthen survival in patients with advanced colorectal cancer. (Lokich 1989; De Gramont 1997; ACCMAP 1992; ACCMAP 1994). Modulated regimens may, however, increase toxicity, notably myelosuppression, diarrhoea, stomatitis, and alopecia and some are complex to administer requiring frequent hospital admissions or outpatient attendance. Continuous infusion involves less time in hospital but requires a central venous catheter and a portable pump. Administration in this way is less likely to cause myelosuppression than other 5‐FU based regimens, but surgical complications of line insertion, sepsis or thrombosis occur occasionally and palmar‐plantar erythema may occur (Lokich 1989; De Gramont 1997). More recently a number of new agents have been evaluated that appear to be active in the treatment of advanced colorectal cancer, some of which may offer the advantage of reduced toxicity or ease of administration (raltitrexed, irinotecan, oxaliplatin, capecitabine). The optimal duration of chemotherapy for advanced colorectal cancer also remains uncertain. In some trials treatment has been given continuously until disease progression, death or unacceptable toxicity, while in others treatment has been of more limited duration (treatment until best response ± consolidation, or a maximum duration of 6 months in case of complete response, partial response or stable disease) (Wils 1998).
As a consequence of the portal venous drainage system, hepatic metastases occur frequently in patients with colorectal cancer and the liver is often the first site of metastatic disease and may be the only site of spread in as many as 30‐40% of patients with advanced disease (Weiss 1986). Infusional chemotherapy with fluoropyrimidines such as 5‐FU has also been administered via a catheter placed in the hepatic artery or portal vein so as to deliver the highest concentrations of the active agent to the liver. Up to 80% of the drug may be metabolised in the liver so the systemic drug concentration is much lower after hepatic infusion than after intravenous chemotherapy, which may reduce the systemic toxicity. Although the response rates achievable with hepatic infusional chemotherapy are higher than with systemic chemotherapy, extrahepatic relapse and progression is frequent, resulting in an equivalent median patient survival when compared to systemic treatment (MA Group 1996). The therapeutic benefits of hepatic infusional chemotherapy may be outweighed by the requirement for operative placement of the infusion device with its associated inconvenience and risks and the possible side effects of hepatic artery infusion including catheter related complications and the potential for hepato‐biliary toxicity. For these reasons, the use of hepatic infusional chemotherapy is restricted to relatively few experienced centres world wide.
Benefits and risks
As for any treatment, the potential benefits of palliative chemotherapy for advanced colorectal cancer must be balanced against the potential risks of treatment related morbidity and mortality. The aims of treatment are prolongation of survival, symptom control and maintenance or improvement of quality of life (QoL), but chemotherapy can be burdensome for some patients as it is associated with a range of side effects and may cause psychological distress, social isolation, financial difficulties and prolonged hospital stays (Redmond 1998). This balance may also be influenced by the choice of treatment and the expertise of the oncologist and supporting staff in selecting patients and managing side effects (Seymour 1997).
Cost and cost effectiveness
The cost of palliative chemotherapy treatment for advanced colorectal cancer includes not only the costs associated with the administration of chemotherapy, but also the provision of support to manage chemotherapy related complications. If palliative chemotherapy improves symptom control and quality of life this may reduce patient dependency and need for other symptomatic/supportive care measures offsetting the cost of this treatment. On the other hand, if the incidence of chemotherapy related toxicity is high and there is a decrease in quality of life as a result of treatment, then the cost of palliative chemotherapy will become much greater than that of supportive care alone.
Systematic Review
Although there is a great deal of published information reporting the results of palliative chemotherapy for advanced colorectal cancer, there is still uncertainty as to the true effectiveness of this intervention, particularly in elderly people who are often underrepresented in clinical trials. This review has been undertaken to assess the published evidence for the efficacy of palliative chemotherapy for advanced colorectal cancer across all age groups in order to establish whether this treatment is effective.
The best evidence of the effectiveness of healthcare interventions comes from the results of randomised controlled trials (RCTs). To avoid bias and to maximise reliability, systematic reviews are usually restricted to evidence from RCTs. For most of the outcomes that we have evaluated, this approach has been applied in the present review, with quantitative analysis restricted to the results of RCTs.
This systematic review aimed to identify all relevant studies of palliative chemotherapy versus no palliative chemotherapy in patients with advanced colorectal cancer, appraise the currently available evidence and provide a comprehensive summary of that evidence.
Objectives
1. To determine the benefits and harms of palliative chemotherapy in patients with locally advanced or metastatic colorectal cancer.
2. To perform a subgroup analysis in order to investigate outcomes in younger patients and the elderly patient population.
3. To assess the cost effectiveness of palliative chemotherapy for locally advanced/metastatic colorectal cancer.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of palliative chemotherapy compared with supportive care alone were included. Additional interventions were permitted (e.g. hepatic artery ligation) providing that they were common to both arms of the study.
Both randomised and non‐randomised studies were considered when searching for data on quality of life, resource use and cost effectiveness of palliative chemotherapy.
No language restriction was applied when searching for randomised controlled trials. For non‐randomised studies, only those published in English, French, German, Italian or Spanish languages were considered.
Types of participants
Patients with advanced or metastatic colorectal cancer. No restrictions were placed on whether patients had previous surgery, radiotherapy or adjuvant chemotherapy. Both symptomatic and asymptomatic patients were included.
Trials in which patients had previous chemotherapy for advanced / metastatic disease were considered separately.
Types of interventions
Palliative chemotherapy Both single agent and combination chemotherapy regimens. All doses, routes and schedules were included. Patients could be treated in either in‐patient or outpatient settings.
Comparator Supportive care was defined as anything other than chemotherapy to include symptom control by radiotherapy, palliative surgery, pain relief, blood transfusion and social / psychological support. These interventions could be provided with or without input from specialist palliative care services. Studies were not excluded where a proportion of the control group subsequently received chemotherapy.
Types of outcome measures
1. Progression‐free and overall survival
2. Incidence of grade 3 or 4 toxicity during the course of chemotherapy treatment and up to 30 days after the last chemotherapy infusion
3. Quality of life to include measures of pain, fatigue, weight loss, appetite, and independence in activities of daily living
4. Need for hospital admission and nursing support
5. Performance status
6. Tumour response
7. Resource use and cost effectiveness
Patients who had previously received treatment for colorectal cancer were asked to comment upon our protocol and choice of outcomes. Telephone interviews were conducted with four subjects who were over 65 years of age. These subjects had been recruited through Colon Cancer Concern, a UK based charity which supports research and provides information on bowel cancer.
Search methods for identification of studies
The search aimed to provide a comprehensive list of primary studies, both published and unpublished, which may be eligible for inclusion. Multiple sources were used to identify studies and the following electronic databases were searched.
Medline Embase CancerLit Cochrane Controlled Trials Register Cinahl Healthstar Science Citation Index Edina Biosis NHS Economic Evaluation Database Index to scientific and technical proceedings Pascal
Both medical subject headings and free‐text searching were performed in order to improve the sensitivity of searching. The search strategy was piloted and the search terms were modified depending upon the 'hit rate' and 'miss rate' of the identified records. The final search strategy for Medline is given below. This strategy was modified for use with other bibliographic databases. Searching took place between July and October 1998.
1 RANDOMIZED‐CONTROLLED‐TRIAL in PT 2 CONTROLLED‐CLINICAL‐TRIAL in PT 3 RANDOMIZED‐CONTROLLED‐TRIALS 4 RANDOM‐ALLOCATION 5 DOUBLE‐BLIND‐METHOD 6 SINGLE‐BLIND‐METHOD 7 #1 or #2 or #3 or #4 or #5 or #6 8 (TG=ANIMAL) not ((TG=HUMAN) and (TG=ANIMAL)) 9 #7 not #8 10 CLINICAL‐TRIAL in PT 11 explode CLINICAL‐TRIALS/ all subheadings 12 (clin* near trial*) in TI 13 (clin* near trial*) in AB 14 (singl* or doubl* or trebl* or tripl*) near (blind* or mask*) 15 (#14 in TI) or (#14 in AB) 16 PLACEBOS 17 placebo* in TI 18 placebo* in AB 19 random* in TI 20 random* in AB 21 RESEARCH‐DESIGN 22 #10 or #11 or #12 or #13 or #15 or #16 or #17 or #18 or #19 or #20 or #21 23 (TG=ANIMAL) not ((TG=HUMAN) and (TG=ANIMAL)) 24 #22 not #23 25 #24 not #9 26 TG=COMPARATIVE‐STUDY 27 explode EVALUATION‐STUDIES/ all subheadings 28 FOLLOW‐UP‐STUDIES 29 PROSPECTIVE‐STUDIES 30 control* or prospectiv* or volunteer* 31 (#30 in TI) or (#30 in AB) 32 #26 or #27 or #28 or #29 or #31 33 (TG=ANIMAL) not ((TG=HUMAN) and (TG=ANIMAL)) 34 #32 not #33 35 #34 not (#9 or #25) 36 explode "COLORECTAL‐NEOPLASMS"/ all subheadings 37 "RECTAL NEOPLASMS"/ all subheadings 38 explode "SIGMOID‐NEOPLASMS"/ all subheadings 39 CARCINOMA* near (COLORECTAL or COLON* or RECT* or INTESTIN* or LARGE BOWEL or BOWEL) 40 NEOPLASIA* near (COLORECTAL or COLON* or RECT* or INTESTIN* or LARGE BOWEL or BOWEL) 41 NEOPLASM* near (COLORECTAL or COLON* or RECT* or INTESTIN* or LARGE BOWEL or BOWEL) 42 ADENOCARCINOMA* near (COLORECTAL or COLON* or RECT* or INTESTIN* or LARGE BOWEL or BOWEL) 43 CANCER* near (COLORECTAL or COLON* or RECT* or INTESTIN* or LARGE BOWEL or BOWEL) 44 #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 45 5FU in TI,AB 46 5‐FLUOROURACIL in TI,AB,NM 47 METHOTREXATE in TI,AB,NM 48 FLUOROURACIL in TI,AB,NM 49 CHEMOTHERAPY in TI,AB 50 explode "Antineoplastic‐Agents"/ all subheadings 51 explode "Antimetabolites" tree: 2/ all subheadings 52 #45 or #46 or #47 or #48 or #49 or #50 or #51 53 "RECTAL‐NEOPLASMS"/ drug‐therapy 54 explode "COLORECTAL‐NEOPLASMS"/ drug‐therapy 55 explode "SIGMOID‐NEOPLASMS"/ drug‐therapy 56 #53 or #54 or #55 57 #52 and #44 58 #57 or #56 59 unresectable 60 inoperable 61 salvage 62 advanced 63 metastatic 64 "PALLIATIVE‐CARE"/ all subheadings 65 (SYMPTOMATIC or SYMPTOM CONTROL or SYMPTOM MANAGEMENT) in TI,AB 66 PALLIAT* in TI,AB 67 SUPPORTIVE in TI,AB 68 #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 69 #68 and #58 70 #69 and #9 71 #69 and #25 72 #69 and #35 73 #70 or #71 or #72 74 explode "COSTS‐AND‐COST‐ANALYSIS"/ all subheadings 75 explode "Economics,‐Hospital"/ all subheadings 76 explode "Economics,‐Medical"/ all subheadings 77 "Economics,‐Nursing"/ all subheadings 78 "Economics,‐Pharmaceutical"/ all subheadings 79 (COST or COSTS or COSTED or COSTLY or COSTING) in TI,AB 80 (ECONOMIC* or PHARMACOECONOMIC* or PRICE* or PRICING*) in TI,AB 81 ECONOMIC EVALUATION* in TI,AB 82 "Quality‐of‐Life"/ all subheadings 83 quality of life in ti, ab 84 QOL in ti,ab 85 well‐being in ti,ab 86 #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 or #85 87 #86 and #69 88 #87 or #73
Handsearching
Conference abstracts were handsearched in order to identify papers which had not been indexed in the above sources. Abstracts were searched from the Proceedings of the American Society of Clinical Oncology (1980‐1998), the Fourth International Conference on Geriatric Oncology 1998, and the First European Conference on the Economics of Cancer 1997.
Other sources of published and unpublished evidence
The following sources were searched for relevant citations and ongoing or recently completed studies: National Research Register Medical Research Council Trials Directory Current Research in Britain UKCCCR Trials Register Center Watch Clinical Trials listing Physician Data Query NCI Cancer Control Intervention Studies NIH Inventory of Clinical Trials and Studies FNCLCC Cancer Clinical Trials Registry (France) The National Clinical Trials Registry: Cancer Trials (Australia) Trial amnesty on Cochrane Library System for information on grey literature in Europe (SIGLE) Index to UK theses DHSS‐data Health CD INAHTA Needs Assessment series
Further studies were obtained by scanning the reference lists and contacting the investigators of all eligible studies. Further information on the search strategy (e.g. dates searched, search terms for each source) can be obtained from the authors upon request.
Data collection and analysis
Assessing trials for eligibility
All studies were assessed against the inclusion criteria above. This was performed independently by two reviewers (one clinical, one non‐clinical) and agreement on eligibility was reached. A standard checklist was used to guide this process. Investigators were contacted for clarification where eligibility could not be determined from the published study.
Appraising validity
Validity was assessed in terms of the generation of allocation schedule (truly random, quasi random, systematic), concealment of treatment allocation from trialists, withdrawal and drop‐out rates, and whether analyses had been performed by intention‐to‐treat. This assessment was based on the Jadad scale (Jadad 1996). Investigators were contacted where this information could not be extracted from the publication.
Data extraction Investigators from all eligible studies were contacted and invited to submit individual patient data from their study, together with details of trial design and conduct. The following data were requested : baseline characteristics including age and tumour site, allocated treatment group, date of randomisation, tumour response, final status with respect to survival and progression, and the date of death or last follow up. Investigators were asked to supply updated data where possible. Aggregate data on quality of life and performance status were also requested in order to supplement published information.
Data were extracted from published papers independently by two reviewers. Data for survival and progression were taken from the text of the publication or estimated from survival curves where necessary.
When a study had generated multiple publications, the most recent (i.e. that with the most mature data) was used for extracting data on outcomes. Earlier publications were used in order to provide information on baseline characteristics or methodology.
Analysis
The risk of death was compared at 6 months, 12 months, 18 months and 24 months using data from published material. The risk of disease progression was compared at 3 months, 6 months, 9 months and 12 months. These time points were specified in advance by the Steering Group for this review. All data were entered into Metaview 3.1. in order to produce a pooled estimate of effect for each of these time points.
Studies were grouped according to whether chemotherapy was administered regionally or systemically, as specified in the review protocol. We had also planned to group studies by high dose vs low dose chemotherapy and protracted intravenous vs bolus intravenous infusion but this was not possible due to the variety of agents and schedules used in the studies. Chi‐square tests for heterogeneity were used to test for gross statistical heterogeneity over all trials and for interaction between the two subsets of regional versus systemic chemotherapy.
Individual patient data for survival and progression were analysed using the Survival Curve and Hazard Ratio Plot (SCHARP) program (developed by the Medical Research Council Clinical Trials Unit and the Instituto Mario Negri, Milan). Survival analyses were stratified by trial and the log‐rank expected number of events and variance were used to calculate the hazard ratios for individual trials and combined across all trials by the fixed‐effects model. All analyses were performed by intention to treat. Studies were grouped according to whether chemotherapy was administered regionally or systemically. The chi‐squared test for interaction was used to test for consistency of effect across these two subsets of trials.
The absolute effects of treatment were at different time points were read from simple (non‐stratified) Kaplan Meier curves. Median survival and time to progression were also estimated from the Kaplan‐Meier curves.
A subgroup analysis was performed using similar stratified methodology in order to determine whether outcomes differ according to age‐group. The following categories were prospectively selected : <= 65 years, 65‐74 years, 75‐84 years, >=85 years. When data was analysed it was evident that there were small numbers of patients in the upper age groups. These categories were collapsed in order to give the analysis greater power. In addition, the categories were altered in order to produce three groups of equal size (<50 years, 50‐64 years, >=65 years). This amendment was performed prior to results being seen. The chi‐squared tests for interaction and trend were used in this sub‐group analysis.
A sensitivity analysis was performed in order to test the effect of removing extraordinary studies which differ in some way from the main body of evidence.
We chose to present results as relative risks as opposed to odds ratios in the Revman software. Odds ratios are a poor estimate of the relative risk when the event rate is high, and may be subject to misinterpretation. However, one limitation of choosing the relative risk summary is that the level of heterogeneity is increased, indicating that it does not represent the 'best fit' for the data.
Data on quality of life were extracted from randomised controlled trials of palliative chemotherapy versus supportive care. Information was tabulated to include study design, comparison groups, quality of life instruments, person assessing quality of life, time points when assessed, and changes in quality of life compared with a pre‐treatment baseline. Other prospective studies of palliative chemotherapy were scanned for additional information, but were not included in the summary of evidence.
Data on chemotherapy toxicity and infusional complications were tabulated, but were not combined quantitatively.
Results
Description of studies
Thirteen randomised controlled trials published between 1983 and 1998 met the inclusion criteria (Allen Mersh, Beretta, Chisholm, Cunningham, Gerard, Glimelius, Hafstrom, Hunt, Nordic, Rougier, Scheithauer, Smyth, Yorkshire). The total number of randomised patients was 1365. Two trials included patients with other digestive tract tumours in addition to colorectal cancer (Beretta, Glimelius). One trial specified an upper age limit of 70 years (Gerard), 7 trials an upper age limit of 75 years (Glimelius, Hafstrom, Hunt, Nordic, Scheithauer, Cunningham, Allen Mersh) and one trial was performed exclusively in patients aged 70 years or over (Beretta).
Five trials assessed the value of regional chemotherapy in which agents were delivered into the portal vein or hepatic artery. There was also some attempt to disrupt hepatic blood flow in three of these trials, by ligation or occlusion of the hepatic artery or injection of degradable starch microspheres. Seven trials assessed the value of intravenous chemotherapy and one trial evaluated an oral agent.
5‐Fluorouracil was alone or in combination with other agents was used in 9 studies. Other trials evaluated the use of floxuridine (2), irinotecan (1) and tauromustine (1). Treatment was administered for either a fixed period (usually 6 months), or until disease progression.
Supportive care included localised radiation therapy, analgesia, corticosteroids, antibiotics, blood transfusion, nutritional support, psychological support and other symptomatic treatment. In many papers the supportive care interventions were poorly described.
One study was conducted exclusively in patients who were asymptomatic at trial entry (Nordic). Other studies appeared to have included both symptomatic and asymptomatic patients, although this is not stated explicitly in many studies. In the largest study (Cunningham), asymptomatic patients represented 29% of the patient population, whereas in the Gerard study (which stratified by symptom status) the proportion was 64%.
The comparison group were treated differently in each of the trials. Trials conducted by Smyth, Hafstrom and Hunt did not allow cytotoxic agents to be given in the supportive care arm. Other trials allowed chemotherapy to be given under certain conditions (e.g. if supportive care did not achieve palliation or once the patient had become symptomatic). Three trials allowed systemic chemotherapy to be used in the control arm at the outset (Allen Mersh, Rougier, Cunningham).
Individual patient data were obtained from seven of the thirteen studies, representing 866 of 1365 randomised patients (63%). This data had been updated for six of the studies (Hafstrom, Rougier, Cunningham, Nordic, Glimelius, Allen Mersh). Reasons for non‐participation included missing/inaccessible data (Gerard, Scheithauer, Smyth), refusal (Beretta) and being unable to contact investigators due to retirement or death (Chisholm, Yorkshire).
Risk of bias in included studies
All of the included studies were randomised controlled trials. Only three studies specified the method of randomisation in their reports. In two trials the allocation was truly random (computer generated) and in the third trial the allocation was done by minimisation. Further information about treatment allocation was obtained from 7 investigators. In total 9 trials have been categorised as truly random, 1 as quasi‐random (minimisation) and 3 as unclear.
Concealment of allocation was described in four trials. In each of these trials the concealment was 'adequate' as allocation took place centrally at a trials office. This means that those conducting the trial did not have control over allocation and therefore did not have the potential to influence this process.
Further information about allocation concealment was obtained from 9 investigators. In total, concealment was classified as 'adequate' in 10 trials, 'inadequate' in 0 trials and 'unclear' in 3 trials.
In four trials the analysis was not conducted by intention to treat. Patients were excluded after randomisation due to unforeseen ineligibility (Gerard, Yorkshire), protocol violation (Hafstrom) and refusing the assigned treatment or refusing to participate in the study (Scheithauer). These exclusions accounted for a total of 21/231 randomised patients, or roughly 10% for each trial. In a further 2 studies it is not possible to tell whether analysis was performed by intention to treat as these trials have only been published in abstract form (Beretta, Chisholm).
Effects of interventions
SURVIVAL
(I) Meta‐analysis of survival based on published data alone
We analysed the risk of death at different time points as specified in the protocol.
Analyses for 6 months and 12 months are based on published data for 1152 patients from 10 randomised controlled trials. Only 8 trials contributed to the analysis of survival at 18 months and 24 months. It was not possible to extract any data from the Beretta, Glimelius or Hunt studies.
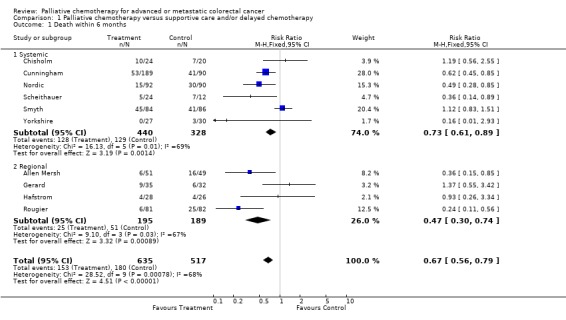
At 6 months, chemotherapy was associated with a significantly reduced risk of death (RR 0.67, 95% CI 0.56 to 0.79). Statistical heterogeneity was apparent in the overall analysis, chi‐square (9) = 28.5, p<0.01. This occurs mainly within the systemic chemotherapy group of trials, rather than reflecting a difference between systemic and regional administration. The Chisholm, Smyth and Gerard studies were not consistent with other studies as they reported a non‐significant trend towards increased mortality in the treatment group.
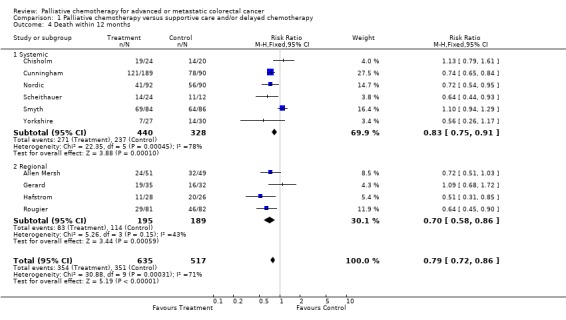
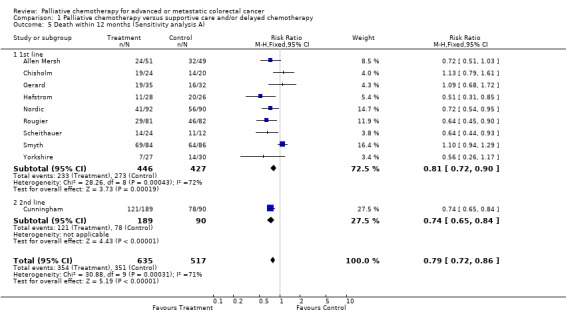
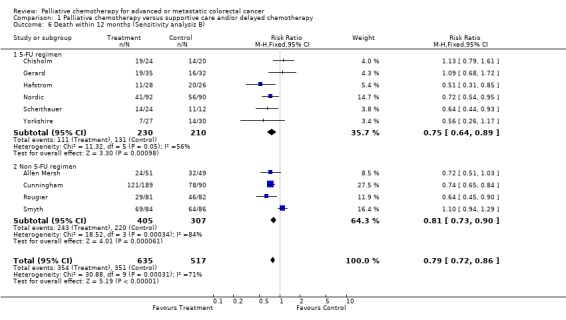
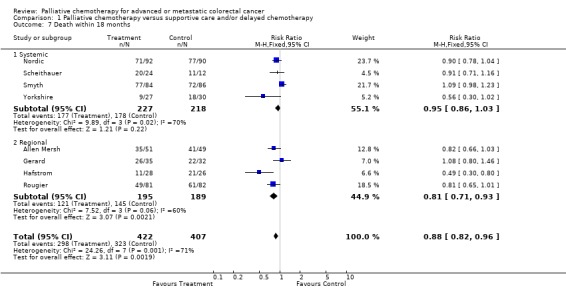
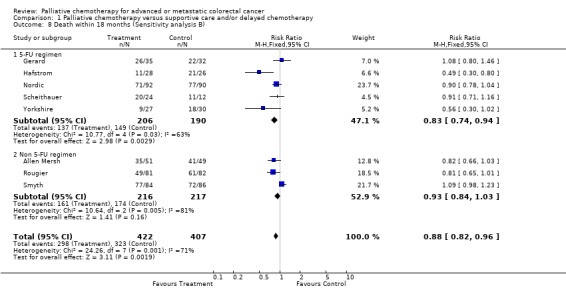
Reduced risk of death was also apparent at 12 months (RR 0.79, 95% CI 0.72 to 0.86) and at 18 months (RR 0.88, 95% CI 0.82 to 0.96). Significant heterogeneity was present in both analyses, indicating that the individual trials were not consistent in their findings. At 24 months, the pooled estimate of effect was non‐significant (RR 0.96, 95% CI 0.90 to 1.01).
The heterogeneity found in these analyses is likely to reflect differences in the patient populations, treatment and control interventions used in the trials.
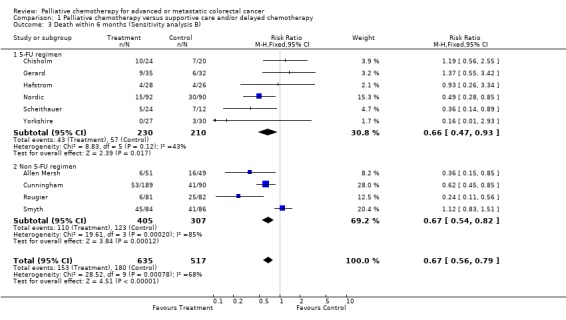
No apparent differences were found between studies which had evaluated fluorouracil and those which had studied other agents (refer to sensitivity analyses).
(II) Meta‐analysis of survival based on individual patient data Data from seven trials on 866 patients and 753 deaths are included in this analysis.
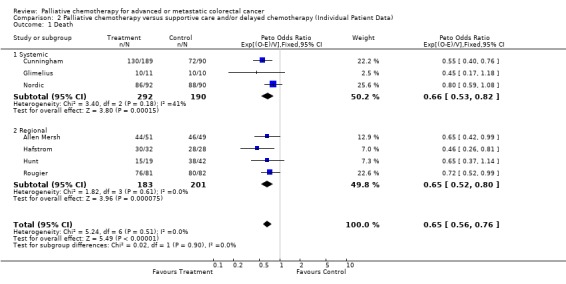
Patients in the treatment group had a significantly reduced risk of death (Hazard ratio 0.65, 95% CI 0.56 to 0.76) in this individual patient data meta‐analysis. This represents a 35% reduction in the risk of death and translates into an absolute benefit of 16% at 6 months, with survival improved from 63% to 79%. At 12 months the absolute benefit is also 16%, representing a survival improvement from 34% to 50%. There was no evidence of gross statistical heterogeneity within subsets of regional and systemic chemotherapy or across all trials, chi‐square (6) = 5.233, p= 0.514 .
Using these data, the number needed to treat (NNT) in order to have one more person alive at both 6 months and 12 months is 6 [95% CI 5 to 10 at 6 months, and 4 to 11 at 12 months].
Median survival is estimated to be 8.0 months in the control group and 11.7 months in the chemotherapy group, a difference of 3.7 months.
(III) Survival according to age group
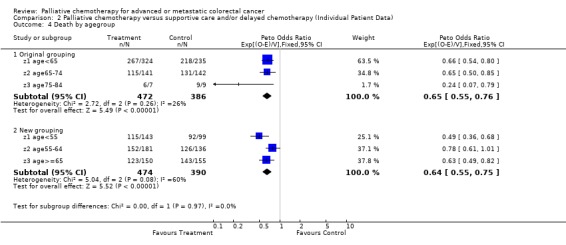
Individual patient data was used for this analysis as described above. There was no evidence of statistical heterogeneity between the age groups using the original or revised age classifications (revised grouping, chi‐squared interaction (2) = 5.039, p=0.08 ). The chi‐squared test for trend was not significant (chi‐squared trend = 0.905, p=0.341), indicating that no relationship was found between age and the effect of treatment upon survival.
It is important to note that very few 'older‐old' and 'oldest‐old' subjects were studied. Only 2.5% of patients were 75 years or older, and no patients in the 85+ age group were included. This compares with an expected colorectal cancer incidence of 31% in patients aged 75‐84 years, and 12% in those aged 85+ years (ONS 1998).
(IV) Stability of survival results
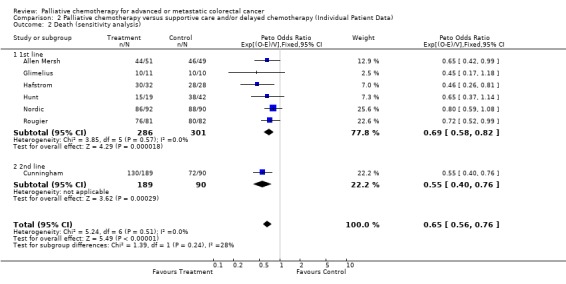
The Cunningham study was considered separately in a sensitivity analysis of survival results. This study was different to the others as it evaluated a second line chemotherapy regimen. This was also the largest study and therefore any differences may have undue influence over the meta‐analysis results. Survival differences between chemotherapy and control groups remained significant when results from this study were excluded (Hazard ratio 0.69, 95% CI 0.58 to 0.82).
In the published data meta‐analysis we were unable to extract survival data from the Beretta, Glimelius and Hunt studies. However, these studies suggest that survival was prolonged in the chemotherapy group. We can therefore conclude that the meta‐analysis results are broadly representative of all known randomised controlled trials in this area.
PROGRESSION FREE SURVIVAL
(I) Meta‐analysis of progression based on published data alone
Only four studies (Gerard, Nordic, Smyth, Yorkshire) provided published data on tumour progression at the time points of 3 months, 6 months, 9 months and 12 months (total 474 patients). Three of these four studies involved the use of Fluorouracil in the treatment regimen.
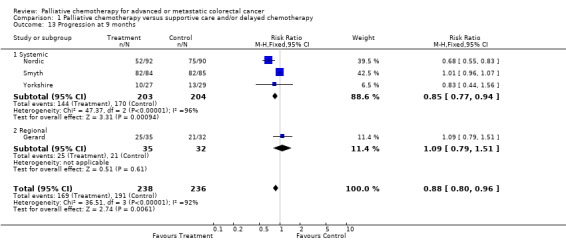
At each of these time points, chemotherapy was associated with a reduced risk of tumour progression. At 3 months the relative risk was estimated to be 0.64 (95% CI 0.54 to 0.76) and at 6 months it was 0.78 (95% CI 0.69 to 0.88). By 12 months the effect was less marked and confidence intervals were wider (RR 0.86, 95% CI 0.77 to 0.96).
At 3 months, 6 months and 9 months there was more variability in results than would be expected by chance (p<0.05). At many of these time points the Gerard study was inconsistent with other studies as it reported a non‐significant trend towards increased progression in the treatment group. This study examined hepatic artery infusion of chemotherapy, and was the only regional chemotherapy trial to report tumour progression at these time points.
(II) Meta‐analysis of progression based on individual patient data
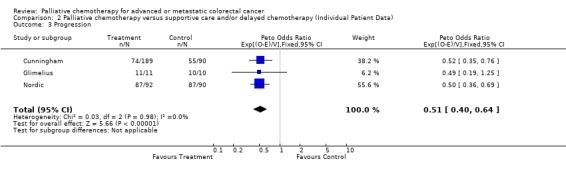
Data from three trials (Cunningham, Glimelius, Nordic) on 482 patients and 324 cases of tumour progression are included in this analysis.
Patients in the treatment group had a significantly reduced risk of progression (Hazard ratio 0.51, 95% CI 0.40 to 0.64) in this individual patient data meta‐analysis. This represents a 49% reduction in the risk of progression and translates into an absolute benefit of 25% at 6 months, with progression‐free survival improved from 36% to 61%. At 12 months the absolute benefit is also 25%, representing a progression‐free survival improvement from 16% to 41%. There was no evidence of gross statistical heterogeneity across the trials, chi‐square (2) = 0.03, p=0.985.
Median progression‐free survival is estimated to be 4.0 months in the control group and 10.0 months in the chemotherapy group, a difference of 6.0 months.
(III) Progression according to age group
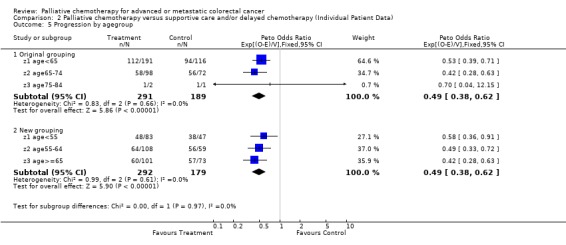
Individual patient data was used for this analysis, as described above. There was no evidence of statistical heterogeneity between the age groups (chi‐squared interaction(2) = 0.99, p=0.61). The chi‐squared test for trend was not significant (chi‐squared trend = 0.99, p=0.32, indicating that no relationship was found between age and the effect of treatment upon progression.
Elderly patients were poorly represented in this dataset, with only 4 patients (0.8%) being over 75 years of age.
TUMOUR RESPONSE
Tumour response was reported in 8 trials. Four trials had used the WHO criteria, one had used the International Union Against Cancer criteria, one assessed percentage hepatic replacement, and two did not specify the method of assessment.
In the treatment arm, overall response ranged from 1.2% to 43% (median 25%). Complete response rates ranged from 0% to 6.3% (median 3.3%), and partial response rates ranged from 1.2% to 37% (median 24%). In the remaining study (Allen Mersh), the percentage hepatic replacement was significantly reduced in the treatment group, with a difference of 16% at 4 months. Patients with immeasurable disease have been excluded from these figures.
TOXICITY / COMPLICATIONS
Data on chemotherapy toxicity were published for 10 out of 13 trials. Only 4 trials graded toxicity according to a standardised validated scale (e.g. World Health Organisation, National Cancer Institute common toxicity criteria). Only one trial reported toxicity for both the treatment and control groups. This comparison is important as events such as nausea, vomiting, anorexia, fatigue and abdominal pain may be related to disease processes rather than being due to chemotherapy.
It has not been possible to combine toxicity results due to differences in chemotherapy regimens and outcome assessment instruments. Studies which have reported significant toxicity (at WHO or NCI grade 3 or 4) are summarised below:
Nordic study : treatment group only, WHO grade 3‐4 leucopenia 2 (2%), thrombocytopenia 2 (2%), renal 2 (2%), stomatitis 1 (1%), nausea / vomiting 1 (1%), diarrhoea 1 (1%), conjunctivitis 1 (1%)
Scheithauer study : treatment group only, WHO grade 3 nausea or vomiting 1 (4%), leucopenia 1 (4%)
Cunningham study : treatment group versus control, NCI grade 3‐4 Any grade 3 or 4 event (79% vs 67%), leucopenia (22% vs 0%), diarrhoea (22% vs 6%), cholinergic symptoms (12% vs 0%), nausea (14% vs 3%), abdominal pain (9% vs 3%), asthenia (15% vs 19%), vomiting (14% vs 8%), cardiovascular symptoms (8% vs 3%).
Where the intervention involved hepatic artery cannulation or ligation, studies have reported complication rates for this procedure and for catheter‐related morbidity. Events such as pump pocket haematoma and infection, cannula dislodgement, laparotomy wound infection, portal thrombosis, biliary fistula, and transient liver failure have been reported, although most reports refer to isolated cases within each series.
None of the trials have reported toxicity or complication rates for different age groups.
QUALITY OF LIFE
Six of the thirteen trials of palliative chemotherapy versus supportive care reported measures which incorporate an assessment of patient well‐being and satisfaction (Smyth, Scheithauer, Allen Mersh, Cunningham, Glimelius, Nordic). Many of the trials included in this analysis were undertaken before validated quality of life instruments specific to patients with cancer were available. Only two studies (Scheithauer, Cunningham) used a cancer‐specific QoL instrument. It is therefore impossible to pool quality of life results for this review.
Many studies did not provide information about who completed the quality of life instrument, and how much assistance was given by carers and health care professionals. It is unclear whether quality of life assessments continued after the cessation of treatment in many studies. In two trials there was no information about the proportion of patients who completed quality of life assessments (Allen Mersh, Glimelius). The statistical analysis of quality of life data may have been inappropriate in some studies, as investigators have not measured the normality of data before applying parametric methods of significance testing.
Results for each of these six trials were incistent. Two trials (Allen Mersh, Scheithauer) found no significant differences between the treatment groups in terms of quality of life. The trial of tauromustine (Smyth) found a more profound decrease in quality of life in the treatment group than in the control group.
In the trial of Irinotecan (Cunningham) the results significantly favoured Irinotecan for the cognitive functioning score, global quality of life score, pain score, dyspnoea, appetite loss and financial impact scores. However, the diarrhoea score was significantly better in the control group. Time to quality of life deterioration was significantly longer in the Irinotecan group.
The trial of 5‐Fluorouracil and leucovorin (Glimelius) included only 21 patients with colorectal cancer out of a total cohort of 61 patients. Quality of life was measured using an ad hoc questionnaire and there was no evidence of validation of this instrument. Quality of life was reported to be more favourable in the treatment group, and authors went on to use these figures in order to derive Quality Adjusted Survival time.
Quality of life during treatment with methotrexate/5‐FU was measured in a subset of 43 patients from the Nordic trial (published separately). Investigators measured pain, presence of symptoms, frequency of troublesome events, tiredness, nausea and vomiting using a non‐validated semi‐structured interview and patient diary. They found that treatment did not negatively influence the well‐being of patients during the period of chemotherapy administration.
CHANGES IN PERFORMANCE STATUS
Change in performance status was measured as a secondary endpoint in three trials, although reporting is sparse in the published papers.
The Nordic and Smyth studies found no significant difference between treatment and control groups during the observation period. The Cunningham study reported that survival without performance status deterioration was significantly longer in the treatment group (p<0.0001) and that a greater proportion of patients with performance status of worse than 0 at baseline had improved their scores (35% vs 11%, p=0.002).
NEED FOR HOSPITAL ADMISSION
Only one study described the need for hospital admission in the treatment and control groups (Cunningham). Admission for adverse events was more common in the treatment group (72% vs 63%) and these patients had a longer duration of stay (median 15 days vs 11 days, range for all patient 1‐168 days).
RESOURCE USE AND COST‐EFFECTIVENESS
Two of the thirteen (13%) trials included in the meta‐analysis produced some evidence of resource consumption and/or of costs per outcome incurred. These were: Glimelius 1995 (a randomised controlled trial with nested cost‐effectiveness analysis) and the Nordic trial.
The usefulness of the economic evidence provided by the Glimelius study is limited by its poor economic methodology and reporting. For example, despite some limited reporting in the first table, resources are not shown separately from unit costs. It is unclear whether the costs (in Swedish Krona, with no pricing year mentioned) are protocol‐driven or not ‐ despite presentation of marginal cost data.
The Nordic trial presents only the number of courses of 5‐FU, methotrexate and leucovorin treatment that were given. No other economic data are available and it is not clear whether these are protocol‐driven resource consumption estimates.
Given the partial and inscrutable nature of the data, these cannot be safely incorporated in a resource consumption model.
Studies not included in the meta‐analysis which provide evidence of resource use
By corresponding with economists active in the field of the economics of colorectal cancer and carrying out searches in parallel with the trial searches, we were able to identify 8 additional reports or abstracts of studies dealing with economic aspects of chemotherapy for advanced colorectal cancer (Delfino 1996, Durand Zalenski 1997, Blijham 1997, Groener 1997, Hale 1997, Ross 1996, Lokich 1996, Kerr 1997). One was subsequently excluded because no new data was presented (Kerr 1997).
Given the relatively abundant nature of studies, we explored whether it would be possible to construct an outline secondary economic model from the available evidence. Usually two methods may be followed to attempt to use data from evaluations outside their original setting (Jefferson 1996) :
'The price adjustment approach': This compares the monetary estimates from different studies after adjustment for price level differences between countries and over time to standardise current values. In order to attempt pooling of cost estimates, economists must be at least certain as to what "secondary" cost estimates represent opportunity costs, charges, or average costs.
'The resource costing approach': this looks at the possibility for deriving data on resource inputs from existing studies, whether designed as economic evaluations or not, and estimating costs and cost‐effectiveness from unit cost data specific to a particular setting.
Both approaches require data on the economics of advanced colorectal cancer of a quantity and quality that at present are not available. Although we identified seven "economic" studies (i.e. which described or analysed resource allocation), four of these were abstracts, thus presenting insufficient data. The remaining four studies present data of different interventions from different settings reflecting likely different cost structures. Some studies (Lokich 1996) were of unclear design with very small denominators.
Although variety of designs and poor methodological quality are well known phenomena in economic literature (Gerard 1992, Udvarhelyi 1992, Demicheli 1997, Jefferson 1998), the high number of abstracts indicating studies currently underway or about to be published indicate the future possibility of plentiful data.
Discussion
Discussion of main findings
Few patients have been included in randomised controlled trials comparing chemotherapy with supportive care in three decades of the use chemotherapy for advanced colorectal cancer. Even when all 13 trials that we have identified are combined the total number of randomised patients is only 1365. Considering that there are almost 30,000 new registrations of colorectal cancer and 15,000 deaths annually from this disease in England and Wales alone (ONS 1998, ONS 1997) this number is very small.
As there are no large, high‐quality trials in this area to guide current practice, systematic review and meta‐analysis techniques provide an appropriate way of synthesising the available evidence.
The evaluation of the results of palliative chemotherapy for advanced colorectal cancer presented in this review should be based on the objectives of this treatment. These include the prolongation of survival, control of symptoms and the maintenance or improvement of quality of life.
We have performed meta‐analysis using both published data and individual patient data. The individual patient data analysis is more reliable and informative but was based on only a proportion of the patients (63%). The published data analysis, although more representative of all studies suffers the disadvantages that some patients were excluded or not analysed by intention to treat and there is statistically signficant heterogeneity between these trials. Despite these differences the results of both analyses are broadly similar.
For patients with advanced colorectal cancer palliative chemotherapy was associated with a significant improvement in survival. The results from the individual patient data meta‐analysis show that 6 month survival was increased by 16% from 63% to 79% in patients receiving chemotherapy. A similar benefit was seen at 1 year where survival was increased from 34% to 50%. Median survival was extended by approximately 3.7 months. The time to tumour progression was also prolonged, with the increase in progression free survival estimated to be 6 months. Many of the control subjects ultimately received chemotherapy, and therefore this survival difference may underestimate the true treatment effect. There was no significant variation in the outcomes in trials of hepatic infusional chemotherapy when compared with systemic chemotherapy.
Tumour response (in patients with measurable disease) was recorded in over half of the studies. The rate of overall response was in the region of 25%, indicating that chemotherapy can alter tumour growth. We were unable to obtain sufficient individual patient data in order to analyse response rates by age group. Tumour response is limited as an outcome measure as it may not accurately predict changes in quality of life, symptoms or survival.
The benefits of chemotherapy for metastatic disease might well be reduced in patients who have had prior chemotherapy exposure. This is important as the use of adjuvant chemotherapy for colorectal cancer is increasing. However, the results of the largest single trial (Cunningham), in which all patients had received previous chemotherapy, are consistent with the overall outcome of the meta‐analysis.
The effectiveness of palliative chemotherapy did not appear to vary across different age bands. We were only able to look for age‐related changes within the age‐bands of <=55 years, 55‐65 years and 65+ years, as there were so few patients aged 75 years and older in this analysis. Due to lack of data, we are unable draw any definite conclusions about age‐related trends in chemotherapy effectiveness.
Treatment toxicity could not be pooled as different measurement instruments were used, and a number of different agents, routes and schedules were assessed. Few studies used well recognised, validated instruments in order to classify the severity of events. Information was not available on the distribution of treatment toxicity according to age group, or whether dose‐reductions were routinely prescribed for elderly patients within these trials.
Assessment of quality of life was reported in six trials using six different instruments. The results were inconsistent; two trials found that quality of life was better in patients receiving chemotherapy (Cunningham, Glimelius), one trial found that treatment did not negatively affect well‐being (Nordic), two trials found no significant differences in quality of life between treatment and control groups (Allen Mersh, Scheithauer), and one trial of an agent which appeared ineffective suggested that quality of life was reduced in patients receiving chemotherapy (Smyth). No information was available on quality of life outcomes in older compared to younger patients.
Although some of these studies were carried out before the development of a complete cancer‐specific QoL instrument, the current availability of such instruments makes their use in any future studies a sensible measure, although additional development of such instruments is probably required, especially for their use in older patients.
These studies addressed the minimisation of morbidity and mortality for what is a highly prevalent disease in western society and the third largest cause of cancer death in the UK. All studies were carried out in or focused on identical settings (i.e. tertiary care ‐ oncology units) characterised by high capital and revenue costs. However, we were unable to identify sufficient evidence to enable us to assess the cost effectiveness of palliative chemotherapy.
Limitations of data included in this review
Studies included in this review were heterogeneous in terms of the patient population, interventions and control group considered. Some studies included only patients with metastatic disease confined to the liver, while others included patients with locally advanced disease and widespread metastases. Most trials included both symptomatic and asymptomatic patients, with the exception of the Nordic study, which was conducted solely in asymptomatic patients. Interventions differed considerably across all of these studies. Eight trials examined the effect of systemic chemotherapy, while five examined the effect of hepatic regional chemotherapy. No two studies used the same treatment combination or schedule although nine of the thirteen trials employed a 5‐FU‐based chemotherapy regimen. Chemotherapy was given either for a set period (usually 6 months), or continuously until disease progression. Best supportive care (in terms of the types of interventions and level of expertise) was rarely described in detail within these studies. No common definition was used and therefore the control intervention may have varied considerably between different studies.
The pooled results of these trials therefore represent a generalised estimate of the effectiveness of chemotherapy, rather than being associated with one particular patient population or mode of treatment. It is important to note that a proportion of patients in the control arm of some trials received chemotherapy or other interventions, so these trials may underestimate differences in survival, disease progression, toxicity and QoL. Many trials imposed an upper age restriction of 75 years for the recruitment of subjects (Allen Mersh, Cunningham, Gerard, Glimelius, Hafstrom, Hunt, Nordic, Scheithauer). Given that over 40% of new cases of colorectal cancer occur in patients over 75 years of age (ONS 1998), we can conclude that these trials under represent patients in the older age groups. One trial was conducted solely in patients aged 70 years and over, although only 88 patients with colorectal cancer were included (Beretta) and it is not possible to discern the outcome for this subgroup from the published abstract.
Meta‐analysis was performed using both published data and individual patient data in this review. The published data meta‐analysis is limited by the heterogeneity of the trials. Only seven investigators were able to contribute individual patient data, representing 866/1365 (63%) of randomised patients. Three of the thirteen trials have not been published in full (Chisholm, Beretta, Smyth), two of which suggested negative effects in the chemotherapy group. Although we have attempted to systematically identify all trials, it is possible that further negative trials remain unpublished in this area.
The benefits of chemotherapy must be weighed against treatment toxicity and impact upon quality of life, outcomes that have been inadequately addressed in the majority of trials. Assessment of treatment related toxicity is of fundamental importance in determining the acceptability of chemotherapy in the palliative treatment setting yet many trials reported little or no toxicity data and only a small proportion used validated assessment scales to classify the severity of toxicities. Only one trial reported comparative toxicity data for both the treatment and control groups (Cunningham). This comparison is important as symptoms such as nausea, vomiting, anorexia, fatigue and abdominal pain may be related to the underlying disease process rather than being due to chemotherapy.
Maintenance or improvement in quality of life is one of the most important treatment goals of palliative chemotherapy. The absence of QoL measures in seven of the thirteen RCTs (and the disparate nature of toxicity classification and reporting) is an indictment of the quality of research in this key area. Even when QoL measures were included, the variety of different assessment instruments used over varying periods of time means that it is likely that different aspects of quality of life were assessed in each trial and this makes any direct comparison impossible. Often QoL was measured only during the period of treatment. It would be helpful to know whether changes in QoL occurring as a result of treatment persist once this is completed.
Allocation of treatment was not masked from participants, and therefore expectation of benefit or perceptions of sub‐optimal treatment may have affected quality of life assessments. The 'treatment' effect may be very powerful in patients with incurable cancer whose fear of dying and desire to do something active to overcome the disease may lead to an overestimation of QoL in those receiving chemotherapy.
Conclusions
Relatively few patients have been entered into trials comparing palliative chemotherapy with supportive care alone.
The available evidence suggests that palliative chemotherapy is effective in prolonging survival and time to progression in those selected for entry into these trials. Palliative chemotherapy does not offer long‐term benefits, as life expectancy remains below 12 months in most of those receiving treatment.
One trial compared the use of chemotherapy in asymptomatic patients with delaying chemotherapy until patients became symptomatic and suggested a benefit for earlier chemotherapy. However, most studies did not differentiate between patients with symptomatic and asymptomatic disease at trial entry and it is therefore not possible to determine whether the benefit of chemotherapy is different in these subgroups.
Any benefits of chemotherapy must be offset against treatment toxicity and impact upon quality of life. These outcomes have not been addressed adequately in the majority of randomised trials and it is therefore difficult to evaluate the palliative benefit of chemotherapy.
Elderly patients were poorly represented within these randomised trials. Although we were unable to detect any age‐related differences in the effectiveness of chemotherapy in a subgroup analysis of individual patient data from these trials, there is insufficient evidence on effectiveness, toxicity and effect on symptoms and quality of life in these trials to form a judgement about the benefit of chemotherapy for advanced colorectal cancer in elderly patients.
Authors' conclusions
Implications for practice.
Palliative chemotherapy is currently used in selected patients with advanced or metastatic colorectal cancer within the United Kingdom. Given the incomplete nature of the evidence presented in this review it not possible to make firm recommendations about the use of this treatment, as the balance between benefits, toxicities and cost‐effectiveness of this treatment remains uncertain.
Patients with advanced colorectal cancer who are fit and wish to receive treatment should be encouraged to enter clinical trials.
Implications for research.
§ Further research is needed to examine quality of life and toxicity outcomes for all patients treated with palliative chemotherapy. Investigators should use standardised, validated instruments and continue to assess outcomes beyond the completion of treatment.
§ Further research is also needed to confirm whether it is appropriate to commence chemotherapy treatment in patients with asymptomatic metastatic colorectal cancer.
§ Future trials of chemotherapy for advanced colorectal cancer should not impose upper age limits and consideration should be given to stratification of patients by age at trial entry in order to determine whether there are variations in treatment outcomes in different age groups.
§ Research is required to identify predictors of improved survival and symptom control in order to identify patients who are most likely to benefit from palliative chemotherapy.
§ Further research is required to assess the relative importance of different treatment outcomes from the perspective of patients and their families. The influence of age upon priorities should be explored.
What's new
| Date | Event | Description |
|---|---|---|
| 5 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 16 November 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Collaborators : The trial groups and investigators who contributed individual patient data were invited to comment on the paper ‐ Professor T Allen‐Mersh (Imperial College School of Medicine, London, UK), Professor D Cunningham (Royal Marsden Hospital, Surrey, UK), Professor B Glimelius (Uppsala Hospital, Sweden), Professor L Hafstrom (Sahlgrenska Hospital, Goteburg, Sweden), Dr P Rougier (Hopital Ambroise Pare, Boulogne, France), Professor I Taylor (Institute of Surgical Studies, University College, London, UK)
We would like to thank the Meta‐analysis Group in Cancer (c/o Henri Mondor Hospital, Creteil, France) for providing data for one of the trials, Phil Alderson (UK Cochrane Centre) for guidance when writing the protocol, Jayne Tierney and Lesley Stewart (Meta‐analysis group, MRC Clinical Trials Unit) for help with the individual patient data meta‐analysis, Jon Deeks (Centre for Statistics in Medicine, Oxford) for statistical advice, Andrew Lobb (University of Southampton) for reviewing the search strategy, Jo‐anne Gregory (University of Southampton) for administrative support and Colon Cancer Concern for providing a consumer perspective.
Data and analyses
Comparison 1. Palliative chemotherapy versus supportive care and/or delayed chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death within 6 months | 10 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.79] |
| 1.1 Systemic | 6 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.89] |
| 1.2 Regional | 4 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.30, 0.74] |
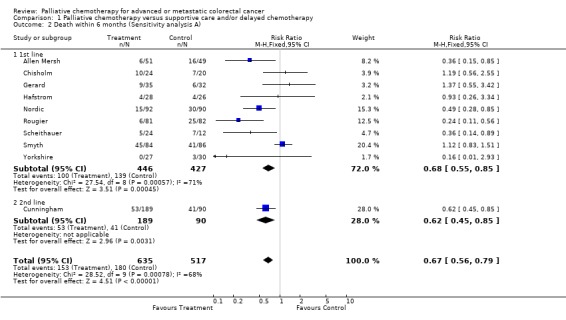
| 2 Death within 6 months (Sensitivity analysis A) | 10 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.79] |
| 2.1 1st line | 9 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.55, 0.85] |
| 2.2 2nd line | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.85] |
| 3 Death within 6 months (Sensitivity analysis B) | 10 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.79] |
| 3.1 5‐FU regimen | 6 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.47, 0.93] |
| 3.2 Non 5‐FU regimen | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.82] |
| 4 Death within 12 months | 10 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.72, 0.86] |
| 4.1 Systemic | 6 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.91] |
| 4.2 Regional | 4 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.58, 0.86] |
| 5 Death within 12 months (Sensitivity analysis A) | 10 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.72, 0.86] |
| 5.1 1st line | 9 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.90] |
| 5.2 2nd line | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.65, 0.84] |
| 6 Death within 12 months (Sensitivity analysis B) | 10 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.72, 0.86] |
| 6.1 5‐FU regimen | 6 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.89] |
| 6.2 Non 5‐FU regimen | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.73, 0.90] |
| 7 Death within 18 months | 8 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.82, 0.96] |
| 7.1 Systemic | 4 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.03] |
| 7.2 Regional | 4 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.93] |
| 8 Death within 18 months (Sensitivity analysis B) | 8 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.82, 0.96] |
| 8.1 5‐FU regimen | 5 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.94] |
| 8.2 Non 5‐FU regimen | 3 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 9 Death within 24 months | 8 | 837 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.01] |
| 9.1 Systemic | 4 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 9.2 Regional | 4 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 10 Death within 24 months (Sensitivity analysis B) | 8 | 837 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.01] |
| 10.1 5‐FU regimen | 5 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.01] |
| 10.2 Non 5‐FU regimen | 3 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.05] |
| 11 Progression at 3 months | 4 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.54, 0.76] |
| 11.1 Systemic | 3 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.78] |
| 11.2 Regional | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.14, 1.19] |
| 12 Progression at 6 months | 4 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.69, 0.88] |
| 12.1 Systemic | 3 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.66, 0.84] |
| 12.2 Regional | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.74, 1.72] |
| 13 Progression at 9 months | 4 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.96] |
| 13.1 Systemic | 3 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.94] |
| 13.2 Regional | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
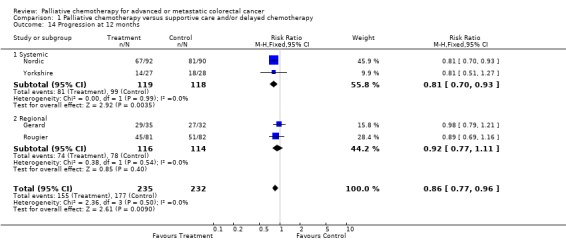
| 14 Progression at 12 months | 4 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.96] |
| 14.1 Systemic | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.93] |
| 14.2 Regional | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.77, 1.11] |
1.1. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 1 Death within 6 months.
1.2. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 2 Death within 6 months (Sensitivity analysis A).
1.3. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 3 Death within 6 months (Sensitivity analysis B).
1.4. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 4 Death within 12 months.
1.5. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 5 Death within 12 months (Sensitivity analysis A).
1.6. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 6 Death within 12 months (Sensitivity analysis B).
1.7. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 7 Death within 18 months.
1.8. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 8 Death within 18 months (Sensitivity analysis B).
1.9. Analysis.
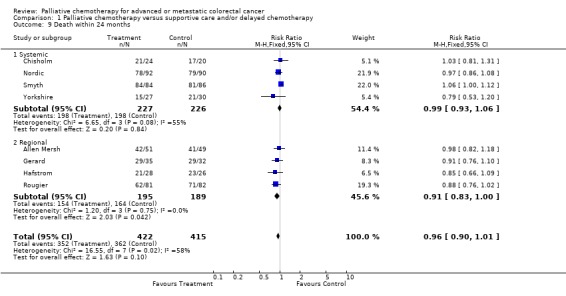
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 9 Death within 24 months.
1.10. Analysis.
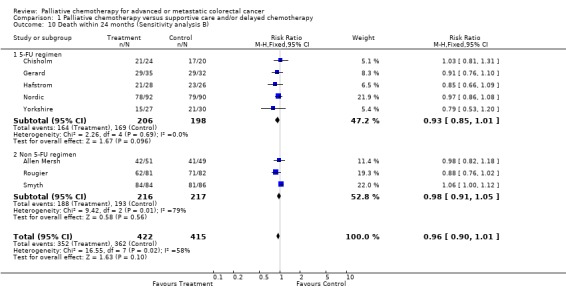
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 10 Death within 24 months (Sensitivity analysis B).
1.11. Analysis.
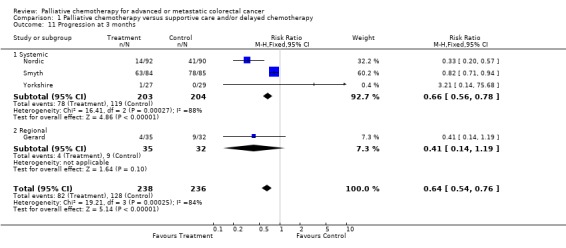
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 11 Progression at 3 months.
1.12. Analysis.
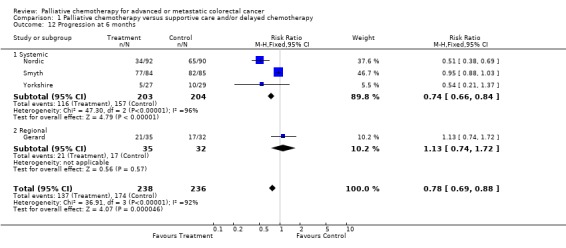
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 12 Progression at 6 months.
1.13. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 13 Progression at 9 months.
1.14. Analysis.
Comparison 1 Palliative chemotherapy versus supportive care and/or delayed chemotherapy, Outcome 14 Progression at 12 months.
Comparison 2. Palliative chemotherapy versus supportive care and/or delayed chemotherapy (Individual Patient Data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 7 | 866 | Peto Odds Ratio (95% CI) | 0.65 [0.56, 0.76] |
| 1.1 Systemic | 3 | 482 | Peto Odds Ratio (95% CI) | 0.66 [0.53, 0.82] |
| 1.2 Regional | 4 | 384 | Peto Odds Ratio (95% CI) | 0.65 [0.52, 0.80] |
| 2 Death (sensitivity analysis) | 7 | 866 | Peto Odds Ratio (95% CI) | 0.65 [0.56, 0.76] |
| 2.1 1st line | 6 | 587 | Peto Odds Ratio (95% CI) | 0.69 [0.58, 0.82] |
| 2.2 2nd line | 1 | 279 | Peto Odds Ratio (95% CI) | 0.55 [0.40, 0.76] |
| 3 Progression | 3 | 482 | Peto Odds Ratio (95% CI) | 0.51 [0.40, 0.64] |
| 4 Death by agegroup | 6 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 4.1 Original grouping | 3 | 858 | Peto Odds Ratio (95% CI) | 0.65 [0.55, 0.76] |
| 4.2 New grouping | 3 | 864 | Peto Odds Ratio (95% CI) | 0.64 [0.55, 0.75] |
| 5 Progression by agegroup | 6 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 5.1 Original grouping | 3 | 480 | Peto Odds Ratio (95% CI) | 0.49 [0.38, 0.62] |
| 5.2 New grouping | 3 | 471 | Peto Odds Ratio (95% CI) | 0.49 [0.38, 0.62] |
2.1. Analysis.
Comparison 2 Palliative chemotherapy versus supportive care and/or delayed chemotherapy (Individual Patient Data), Outcome 1 Death.
2.2. Analysis.
Comparison 2 Palliative chemotherapy versus supportive care and/or delayed chemotherapy (Individual Patient Data), Outcome 2 Death (sensitivity analysis).
2.3. Analysis.
Comparison 2 Palliative chemotherapy versus supportive care and/or delayed chemotherapy (Individual Patient Data), Outcome 3 Progression.
2.4. Analysis.
Comparison 2 Palliative chemotherapy versus supportive care and/or delayed chemotherapy (Individual Patient Data), Outcome 4 Death by agegroup.
2.5. Analysis.
Comparison 2 Palliative chemotherapy versus supportive care and/or delayed chemotherapy (Individual Patient Data), Outcome 5 Progression by agegroup.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen Mersh.
| Methods | Single centre randomised controlled trial. Generation of allocation schedule by minimisation. |
|
| Participants | 100 patients with synchronous or metachronous colorectal liver metastases, excluding those with extensive metastases (>60% liver replacement), less than 4 discrete resectable metastases, ascites, raised bilibubin, evidence of disease outside the liver or a history of systemic chemotherapy. Age < 75 years | |
| Interventions | Hepatic artery infusion Floxuridine (0.2 mg/kg body weight). 14‐day continuous infusion followed by 14‐day rest, and cycle repeated. Treatment continued until progression or toxicity. vs Conventional palliation : resection of primary tumour if diagnosed synchronously. Analgesia, corticosteroids, and systemic chemotherapy permitted. Hepatic artery infusion was not allowed. | |
| Outcomes | Overall survival, Quality of life, Toxicity | |
| Notes | Paper suggests that most of the control group (39/49) did not receive systemic chemotherapy in addition to conventional palliation. Analysis was by intention to treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Beretta.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule unclear. Part of a larger study of all gastrointestinal cancers (n=163). Analysis stratified by primary tumour site |
|
| Participants | 88 patients with advanced metastatic colorectal cancer. All patients were 70 years or older. | |
| Interventions | 5‐Fluorouracil (500mg/m2 iv infusion) with racemic folinic acid (300mg/m2 iv bolus). Given weekly for 6 months or until progression vs Standard treatment (no further details provided) | |
| Outcomes | Tumour response Overall survival Toxicity | |
| Notes | Only published as an abstract. Cannot tell whether analysis was by intention to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chisholm.
| Methods | Single centre randomised controlled trial. Generation of allocation schedule unclear. |
|
| Participants | 46 patients with locally advanced or disseminated colorectal cancer | |
| Interventions | 5‐Fluorouracil (500‐750mg), methotrexate (25‐50mg) and cyclophosphamide (100‐200mg) i.v. every 2 weeks + Prednisolone (5mg b.d. p.o.) vs Supportive treatment only | |
| Outcomes | Survival, Toxicity | |
| Notes | Only published as an abstract. Cannot tell whether analysis was by intention to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cunningham.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule by computer generation (truly random). Ratio of irinotecan to supportive care 2:1. |
|
| Participants | 279 patients with metastatic colorectal cancer, which had progressed within 6 months of treatment with fluorouracil. Age 18‐75 years, WHO performance status 0‐2, had 1 adjuvant and no more than 2 palliative fluorouracil‐based regimes. Excluded if had previous treatment with topoisomerase I inhibitors, bulky disease, metastases in central nervous system, or unresolved bowel obstruction or diarrhoea. Specified levels of neutrophils, platelets, bilirubin, liver transaminases and creatinine required for inclusion | |
| Interventions | Irinotecan 350mg/m2 over 90min intravenous infusion every 3 weeks + supportive care vs Supportive care alone : antibiotics, analgesics, transfusions, corticosteroids, psychotherapy, and other symptomatic therapy except irinotecan / other topoisomerase I inhibitors. Localised radiation therapy was allowed provided that the dose delivered was in the palliative range according to institutional standards | |
| Outcomes | Overall survival, Disease progression, Performance status, Body weight, Tumour‐related symptoms, Quality of life. | |
| Notes | In the supportive care group 28 (31%) received chemotherapy, mainly with fluorouracil regimens. Analysis was by intention to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gerard.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule truly random (personal communication with investigator). Stratified according to measurability of lesion and presence / absence of symptoms |
|
| Participants | 74 patients with unresectable colorectal liver metastases. Primary colorectal adenocarcinoma had been resected during previous surgical operation. Age < 70 years, Karnofsky performance score >= 60. | |
| Interventions | Hepatic artery ligation with portal infusion 5‐Fluorouracil 600mg/m2/day for at least 10 days (max. 20 days) . Treatment repeated every 6 weeks until progression or severe side effects vs Hepatic artery ligation alone | |
| Outcomes | Survival, Progression, Tumour response, Complications, Toxicity | |
| Notes | Paper does not report whether patients in the control group received subsequent systemic chemotherapy. Analysis not by intention to treat as 7 patients withdrawn from analysis after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Glimelius.
| Methods | Single centre randomised controlled trial. Generation of allocation schedule truly random. |
|
| Participants | 21 patients with surgically non‐curable colorectal cancer. Age <= 75 years. Excluded if Karnofsky performance status <50, previous chemotherapy, other primary tumours or exceeded specified levels of serum creatinine and bilirubin. | |
| Interventions | Primary chemotherapy : 5‐Fluorouracil (500 mg/m2 ) and leucovorin (60 mg/m2 ) on days 1&2, every 2nd week. Treatment continued until tumour progression, either objectively or subjectively, as long as toxicity was low. Treatment could be discontinued after 4 months if stable disease and it was believed that that this would provide better palliation. If a patient had no tumour‐related symptoms, the planned number of treatments was 10 courses. vs Best supportive care including psychosocial support and attempts to relieve any symptoms. Chemotherapy was allowed in this group if the supportive measure did not achieve palliation, or if the patient requested chemotherapy. | |
| Outcomes | Survival, Tumour response, Toxicity, Quality of Life, Costs | |
| Notes | These patients are taken from a randomised controlled trial of 61 patients with advanced gastrointestinal cancer (stratified according to primary disease). In the entire group, 15/28 patients who were randomised to supportive care ultimately received chemotherapy. Analysis appears to be intention to treat (not explicitly stated) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hafstrom.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule truly random. Concealment of allocation adequate (personal communication with investigator) |
|
| Participants | 60 patients with non‐resectable liver metastases and no extrahepatic cancer. Age<= 75 years. | |
| Interventions | Temporary hepatic artery occlusion with intraportal infusion of 5‐Fluorouracil (1000mg/m2/day) for 5 days plus allopurinol 300mg p.o. for 10 days. Repeated every 6 weeks until progression. After 2 years, interval between treatments was prolonged. vs Control : no regional or systemic treatment | |
| Outcomes | Overall survival, Tumour response, Complications | |
| Notes | 57 of 60 randomised patients were treated at one centre. 6 patients were excluded after randomisation because of major protocol violations (not intention to treat) 5/32 in treatment group and 3/28 in control group had subsequent systemic chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hunt.
| Methods | Single centre randomised controlled trial. Generation of allocation schedule truly random. |
|
| Participants | 61 patients with unresectable liver metastases and no evidence of extrahepatic disease. Karnofsky score >60. Age 18‐75 years. Fit enough to undergo laparotomy. Patients excluded if extensive disease (jaundice, ascites, percentage liver replacement >75%) | |
| Interventions | Hepatic artery injection of 5‐Fluorouracil (500mg) plus Degradable starch microspheres (300‐900mg). First course consisted of 4 injections on consecutive days. Each subsequent course consisted of 2 injections on consecutive days, repeated every 28 days. vs Hepatic artery embolisation vs No active therapeutic intervention. Symptomatic treatment provided where necessary | |
| Outcomes | Overall survival, Extrahepatic disease, Toxicity, Complications | |
| Notes | Analysis was by intention to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Nordic.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule truly random (personal communication with investigator). |
|
| Participants | 183 patients with noncurable, asymptomatic colorectal cancer who have received no previous cytostatic therapy. Age <= 75 years. Normal kidney function, no icterus, no evidence of ascites or pleural effusion | |
| Interventions | Methotrexate (250mg/m2 infusion over 2 hours) + 5‐Fluorouracil (500mg/m2 iv bolus x 2) + Leucovorin rescue (15mg IM followed by seven oral doses of 15mg). Treatment repeated every 14 days for 8 courses, every 3 weeks for 2 courses and every 4 weeks for 2 courses. Therapy continued for 12 courses (6 months) unless tumour progression or severe adverse effects vs Primary expectancy. Chemotherapy was allowed when patients became symptomatic. | |
| Outcomes | Survival, Symptom‐free survival, Progression, Tumour response, Toxicity Quality of life was studied in a subset at one centre | |
| Notes | 51/90 patients in supportive care group received chemotherapy when became symptomatic. Analysis by intention to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rougier.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule truly random. |
|
| Participants | 166 patients with unresectable hepatic metastases from primary colorectal cancer. Primary tumour had been resected. Hepatic metastases unresectable. No evidence of extrahepatic disease or ascites. WHO perfomance status 0‐2. Less than 75% hepatic involvement with tumour. Specified bilirubin level for inclusion. | |
| Interventions | Hepatic artery infusion of Floxuridine (0.3mg/kg/day) for 14 days every 4 weeks vs Observation or systemic bolus 5‐FU infusion (500mg/m2/day) for 5 days every 4 weeks | |
| Outcomes | Survival, Progression, Tumour response, Complications, Toxicity | |
| Notes | 24/82 in treatment group and 41/82 in control group had systemic chemotherapy Analysis by intention to treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Scheithauer.
| Methods | Single centre randomised controlled trial. Generation of allocation schedule by computer generated random number list (adequate). Ratio of chemotherapy to supportive care 2:1. |
|
| Participants | 40 previously untreated patients with inoperable, locally recurrent or metastatic colorectal cancer. Age < 75 yrs, life expectancy > 2 months, ECOG performance status <=3. Specified levels of haematological, renal and hepatic function for inclusion. | |
| Interventions | Leucovorin bolus i.v. (200mg/m2/day) plus 5‐Fluorouracil bolus i.v. (550mg/m2/day) plus Cisplatin infusion (20mg/m2/day). All drugs given on 4 consecutive days at 4 week intervals. Continued for total of 6 months or until evidence of disease progression vs Supportive care : analgesia, nutritional support, blood transfusion, and psychosocial support as required. | |
| Outcomes | Survival, Quality of life, Toxicity | |
| Notes | 2/12 evaluable patients in supportive care group received chemotherapy. Analysis not by intention to treat as 4 patients were withdrawn after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Smyth.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule truly random. |
|
| Participants | 190 patients with recurrent or metastatic colorectal cancer not amenable to curative surgery and/or radiotherapy. WHO performance status 0‐2, life expectancy at least 3 months. Specified levels of haematological and hepatic function required. Excluded if previous chemotherapy or radiotherapy to lesions. Patients with concomitant malignant disease were excluded. Excluded if active uncontrolled infection or poor medical risks because of non‐malignant systemic disease. | |
| Interventions | Tauromustine (130mg/m2 p.o. every 5 weeks). Continued until evidence of disease progression
vs
No chemotherapy Radiotherapy was permitted for palliative treatment. Other cytotoxic agents were not permitted. |
|
| Outcomes | Survival, Progression, Tumour response, Quality of life, Performance status, Toxicity | |
| Notes | Only published as a short communication. Additional information supplied by Kabi Pharmacia. Analysis was by intention to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yorkshire.
| Methods | Multicentre randomised controlled trial. Generation of allocation schedule unclear. |
|
| Participants | 57 patients with minimal residual malignant disease after palliative resection for colorectal cancer | |
| Interventions | Methyl CCNU (130mg/m2 i.v. day 1) plus 5‐Fluorouracil (300mg/m2/day i.v. days 1‐5). Courses repeated every 8 weeks for 2 years, unless signs of disease progression vs Symptomatic treatment only | |
| Outcomes | Survival, Progression, Toxicity | |
| Notes | Paper does not report whether patients in the control group received subsequent systemic chemotherapy. Analysis not by intention to treat as some patients were withdrawn from analysis after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
z1 age<55.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
z1 age<65.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
z2 age55‐64.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
z2 age65‐74.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
z3 age75‐84.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
z3 age>=65.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
z4 age >=85.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Contributions of authors
Review Group : Dr Peter Simmonds (CRC Wessex Medical Oncology Unit, Southampton), Miss Lesley Best (CRC Wessex Medical Oncology Unit, Southampton), Dr Chris Baughan (Royal South Hants Hospital, Southampton), Dr Roger Buchanan (Royal South Hants Hospital, Southampton), Dr Carol Davis (Countess Mountbatten House, Southampton), Professor Ian Fentiman (ICRF Clinical Oncology Unit, London), Dr Steve George (Health Care Research Unit, Southampton), Dr Margot Gosney (Geriatric Medicine, Liverpool), Mr John Northover (St Mark's Hospital, Middlesex), Dr Chris Williams (Institute of Health Sciences, Oxford).
All members were involved in the study design, data extraction, interpretation of the results and commented on early and final drafts of the report. PS and LB performed all searches, determined inclusion, analysed the data and wrote the report. TJ provided an economic commentary.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS National Cancer Research & Development Programme, UK.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Allen Mersh {published and unpublished data}
- Allen Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic‐artery floxuridine infusion for colorectal liver metastases. Lancet 1994;344(8932):1255‐60. [DOI] [PubMed] [Google Scholar]
- Earlam S, Glover C, Davies M, Fordy C, Allen Mersh TG. Effect of regional and systemic fluorinated pyrimidine chemotherapy on quality of life in colorectal liver metastasis patients. J‐Clin‐Oncol 1997;15(5):2022‐9. [DOI] [PubMed] [Google Scholar]
Beretta {published data only}
- Beretta G, Bollina R, Cozzi C, Morabito A. Should we consider the weekly chemotherapy with fluorouracil + racemic folic acid a standard treatment for advanced / metastatic carcinoma of the digestive tract in elderly patients?. Proceedings ASCO 1997;16:A920. [Google Scholar]
- Beretta G, Bollina R, Labianca R, et al. A controlled trial of supportive care (SC) versus supportive care plus 5‐fluorouracil/folinic acid for advanced metastatic gastrointestinal carcinomas in elderly patients. Proceedings ASCO 1994;113:A669. [Google Scholar]
Chisholm {published data only}
- Chishom E, Malhotra A, Giles GR. Quadruple chemotherapy for advanced colorectal cancer. Clinical Oncology 1983;9(2):185‐186. [Google Scholar]
Cunningham {published and unpublished data}
- Cunningham D, Pyrhonen S, Janes RD, Punt CJA, Hickish T, Heikkila T, Johannesen T, Starkhammer H, Topham CA, Ong E, Herait P, Jacques C. A phase III multicenter randomized study of CPT‐11 versus supportive care alone in patients with 5FU‐resistant metastatic colorectal cancer. Proceedings ASCO 1998. [Google Scholar]
- Cunningham, D, Pyrhonen, S, James, R. D, Punt, C. J. A, Hickish, T. F, Heikkila, R, Johannesen, T. B, Starkhammer, H, Topham, C. A, Awad, L, Jacques, C, Herait. P. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352(9138):1413‐8. [DOI] [PubMed] [Google Scholar]
Gerard {published data only}
- Gerard A, Buyse M, Pector JC, Bleiberg H, Arnaud J P, Willems G, Delvaux G, Lise M, Nitti D, Depadt G, et al. Hepatic artery ligation with and without portal infusion of 5‐FU. A randomized study in patients with unresectable liver metastases from colorectal carcinoma. The E.O.R.T.C. Gastrointestinal Cancer Cooperative Group (G.I. Group).. Eur‐J‐Surg‐Oncol 1991 Jun;17(3):289‐94. [PubMed] [Google Scholar]
Glimelius {published and unpublished data}
- Glimelius B, Hoffman K, Graf W, Haglund U, Nyren O, Pahlman L, Sjoden P O. Cost‐effectiveness of palliative chemotherapy in advanced gastrointestinal cancer. Ann‐Oncol 1995 Mar;6(3):267‐74. [DOI] [PubMed] [Google Scholar]
Hafstrom {published and unpublished data}
- Hafstrom L, Engras B, Holmberg SB, Gustavsson B, Jonsson P, Linder P, Naredi P, Tidebrant. Treatment of liver metastases from colorectal cancer with hepatic artery occlusion, intraportal 5‐fluouracil infusion, and oral allopurinol. Cancer 1994;74(10):2749‐2756. [DOI] [PubMed] [Google Scholar]
Hunt {published and unpublished data}
- Hunt TM, Flowerdew A D, Birch S J, Williams J D, Mullee M A, Taylor I. Prospective randomized controlled trial of hepatic arterial embolization or infusion chemotherapy with 5‐fluorouracil and degradable starch microspheres for colorectal liver metastases. Br‐J‐Surg 1990 Jul;77(7):779‐82. [DOI] [PubMed] [Google Scholar]
Nordic {published and unpublished data}
- Glimelius B, Graf W, Hoffman K, Pahlman L, Sjoden PO, Wennberg A. General condition of asymptomatic patients with advanced colorectal cancer receiving palliative chemotherapy. A longitudinal study. Acta‐Oncol 1992;31(6):645‐51. [DOI] [PubMed] [Google Scholar]
- Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. J‐Clin‐Oncol 1992 Jun;10(6):904‐11. [DOI] [PubMed] [Google Scholar]
Rougier {published and unpublished data}
- Rougier P, Hay J M, Ollivier J M, Escat J, Laplanche A, Elias D, Lasser P, Huguier M. A controlled multicenter trial of intrahepatic chemotherapy (IHC) vs standard palliative treatment for colorectal liver metastases. Proceesings ASCO 1990;9:A403. [Google Scholar]
- Rougier P, Laplanche A, Huguier M, Hay J M, Ollivier J M, Escat J, Salmon R, Julien M, Roullet Audy J C, Gallot D. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long‐term results of a prospective randomized trial. J‐Clin‐Oncol 1992;10(7):1112‐8. [DOI] [PubMed] [Google Scholar]
Scheithauer {published data only}
- Scheithauer W, Depisch D, Schiessel R, Rosen H, Sebesta C, Ludwig H. Influence of chemotherapy on survival and quality of life (QOL) in patients with metastatic colorectal cancer (CC): a randomized study. Proceedings ASCO 1990;9:a1176. [Google Scholar]
- Scheithauer W, Rosen H, Kornek G V, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993;306(6880):752‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smyth {published and unpublished data}
- Smyth JF, Hardcastle J D, Denton G, Alderson D, Grace R H, Mansi J L, Yosef H M, Nordle O, Lauri H, Wahlby S. Two phase III trials of tauromustine (TCNU) in advanced colorectal cancer. Ann‐Oncol 1995 Nov;6(9):948‐9. [DOI] [PubMed] [Google Scholar]
Yorkshire {published data only}
- Yorkshire Gastrointestinal Tumour Group. Chemotherapy after palliative resection of colorectal cancer. Br‐J‐Surg 1984 Apr;71(4):283‐6. [DOI] [PubMed] [Google Scholar]
z1 age<55 {published data only}
z1 age<65 {published data only}
z2 age55‐64 {published data only}
z2 age65‐74 {published data only}
z3 age75‐84 {published data only}
z3 age>=65 {published data only}
z4 age >=85 {published data only}
Additional references
ACCMAP 1992
- Advanced Colorectal Cancer Meta‐analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer : evidence in terms of response rate. J Clin Oncol 1992;10(6):896‐903. [DOI] [PubMed] [Google Scholar]
ACCMAP 1994
- Advanced Colorectal Cancer Meta‐analysis Project. Meta‐analysis of randomized trials testing the biochemical modulation of 5‐fluorouracil by methotrexate in metastatic colorectal cancer. J Clin Oncol 1994;12:960‐9. [DOI] [PubMed] [Google Scholar]
Blijham 1997
- Blijham G, Schmitt C, Aussage P, HenryLaunois B, Jolain B, Herait P. Cost‐effectiveness of Irinotecan (CPT‐11) and best estimated chemotherapy regimen in patients with metastatic colorectal cancer after failure of 5fluorouracil (5FU) containing regimen: Results based on a phase III trial. Eur‐J‐Cancer 1997;33(S9):OP4. [Google Scholar]
Cunningham 1993
- Cunningham D, Findlay M. The chemotherapy of colon cancer can no longer be ignored. Eur J Cancer 1993;29A(15):2077‐9. [DOI] [PubMed] [Google Scholar]
De Gramont 1997
- Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low‐dose leucovorin and fluorouracil bolus with bimonthly high‐dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J‐Clin‐Oncol 1997;15(2):808‐15. [DOI] [PubMed] [Google Scholar]
Delfino 1996
- Delfino C, Bosch B, Caccia G. Cost effectiveness of palliative care for advanced colorectal carcinoma. Proceedings ASCO 1996;A 942. [Google Scholar]
Gerard 1992
- Gerard K. Cost‐utility in practice: a policy maker's guide to the state of the art. Health Policy 1992;21:249‐79. [DOI] [PubMed] [Google Scholar]
Groener 1997
- Groener M G, Ineveld B M, Byttebier G. Evaluation of relative costs of the cytostatic agents Tomudex and 5‐Fluorouracil plus Leucovorin as treatments for advanced colorectal cancer. Eur‐J‐Cancer 1997;33(S9):OP11. [Google Scholar]
Hale 1997
- Hale J, Cohen D, Maughan T. Economics of the MRC Colorectal Working Party CR06 Trial. Eur J Cancer 1997;33(S9):OP13. [Google Scholar]
Jadad 1996
- Jadad AR, Moore R A, Carroll D, Jenkinson C, Reynolds D J, Gavaghan D J, McQuay H J. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control‐Clin‐Trials 1996 Feb;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jefferson 1996
- Jefferson TO, Demicheli V, Mugford M. Elementary Economic Evaluation in Health Care. Blackwell BMJ Books May 2000:BMJ Books 1996. [Google Scholar]
Jefferson 1998
- Jefferson TO, Demicheli M. Methodological quality of economic modelling studies: a case study with hepatitis B vaccines. Pharmacoeconomics 1998;14:251‐7. [DOI] [PubMed] [Google Scholar]
Kerr 1997
- Kerr D J, O'Connor K M. The costs of managing advanced colorectal cancer: a broad perspective. Anticancer‐Drugs 1997;Suppl 2:S23‐6. [DOI] [PubMed] [Google Scholar]
Lokich 1989
- Lokich JJ, Ahlgren JD, Gullo JJ, Philips JA, Fryer JG. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: a Mid‐Atlantic Oncology Program Study. J Clin Oncol 1989;7(4):425‐32. [DOI] [PubMed] [Google Scholar]
Lokich 1996
- Lokich J J, Moore C L, Anderson N R. Comparison of costs for infusion versus bolus chemotherapy administration: Analysis of five standard chemotherapy regimens in three common tumors .1. Model projections for cost based on charges. Cancer 1996;78(2):294‐299. [DOI] [PubMed] [Google Scholar]
MA Group 1996
- Meta‐analysis Group in Cancer. Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. Journal of National Cancer Institute 1996;252‐8. [DOI] [PubMed] [Google Scholar]
ONS 1997
- Office for National Statistics. Mortality Statistics: cause, England and Wales 1997. London: HMSO.
ONS 1998
- Office for National Statistics. Cancer Statistics Registrations : England and Wales, 1992. London. HMSO, 1998.
Redmond 1998
- Redmond K. Assessing patients' needs and preferences in the management of advanced colorectal cancer. Br J Cancer 1998;77(Suppl. 2):5‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 1996
- Ross P, Heron J, Cunningham D. Cost of treating advanced colorectal cancer: a retrospective comparison of treatment regimens. Eur‐J‐Cancer 1996;32A Suppl 5:S13‐7. [DOI] [PubMed] [Google Scholar]
Seymour 1997
- Seymour MT, Stenning SP, Cassidy J. Attitudes and practice in the management of metastatic colorectal cancer in Britain. Colorectal Cancer Working Party of the UK Medical Research Council. Clin‐Oncol‐R‐Coll‐Radiol 1997;9(4):248‐51. [DOI] [PubMed] [Google Scholar]
Udvarhelyi 1992
- Udvarhelyi S, Colditz GA, Rai A, Epstein AM. Cost‐effectiveness and cost‐benefit analyses in the medical literature. Are methods being used correctly?. Annals of Internal Medicine 1992;116:238‐44. [DOI] [PubMed] [Google Scholar]
Weiss 1986
- Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder Mea. Hematogenous metastatic patterns in colonic carcinoma : an analysis of 1541 necropsies. J Pathol 1986;150:195‐203. [DOI] [PubMed] [Google Scholar]
Wils 1998
- Wils J, Sahmoud T, Sobrero A, et al. Evaluation of clinical efficacy of new medical treatments in advanced colorectal cancer. Results of a workshop organized by the EORTC GITCCG. Tumori 1998;84(3):335‐47. [DOI] [PubMed] [Google Scholar]


