Abstract
Background
Antidepressants are commonly used in the management of low‐back pain. However, their use is controversial.
Objectives
The aim of this review was to determine whether antidepressants are more effective than placebo for the treatment of non‐specific low‐back pain.
Search methods
Randomised controlled trials were identified from MEDLINE, EMBASE and PsycINFO (to November 2008), the Cochrane Central Register of Controlled Trials 2008, Issue 4, and previous systematic reviews.
Selection criteria
We included randomised controlled trials that compared antidepressant medication and placebo for patients with non‐specific low‐back pain and used at least one clinically relevant outcome measure.
Data collection and analysis
Two blinded review authors independently extracted data and assessed the risk of bias in the trials. Meta‐analyses were used to examine the effect of antidepressants on pain, depression and function, and the effect of antidepressant type on pain. To account for studies that could not be pooled, additional qualitative analyses were performed using the levels of evidence recommended by the Cochrane Back Review Group.
Main results
Ten trials that compared antidepressants with placebo were included in this review. The pooled analyses showed no difference in pain relief (six trials (one trial with two treatment arms and a second trial with 3 treatment arms); standardized mean difference (SMD) ‐0.04 (95% confidence interval (CI) ‐0.25 to 0.17)) or depression (two trials; SMD 0.06 (95% CI ‐0.29 to 0.40)) between antidepressant and placebo treatments. The qualitative analyses found conflicting evidence on the effect of antidepressants on pain intensity in chronic low‐back pain, and no clear evidence that antidepressants reduce depression in chronic low‐back pain patients. Two pooled analyses showed no difference in pain relief between different types of antidepressants and placebo. Our findings were not altered by the sensitivity analyses, which varied the risk of bias allowed for inclusion in the meta‐analyses to allow data from additional trials to be examined.
Authors' conclusions
There is no clear evidence that antidepressants are more effective than placebo in the management of patients with chronic low‐back pain. These findings do not imply that severely depressed patients with back pain should not be treated with antidepressants; furthermore, there is evidence for their use in other forms of chronic pain.
Plain language summary
Antidepressants for non‐specific low‐back pain
Low‐back pain is a common condition affecting up to 80% of adults over their lifetime. In the vast majority of cases, low‐back pain has no identifiable cause and is termed "non‐specific".
Low‐back pain is usually benign and self‐limiting. It generally resolves in six weeks, with or without treatment.
However, up to 30% of individuals who report low‐back pain go on to have recurrent or persistent symptoms. As a result, low‐back pain is one of the most common reasons for medical visits and it results in huge economic losses across developed nations because of reduced productivity, work absence and early retirement.
Antidepressants are a common treatment for low‐back pain. Physicians prescribe them to patients with back pain for three main reasons: to provide pain relief, help with sleep and reduce depression. However, the prescription of antidepressants as a treatment for back pain remains controversial because of conflicting scientific evidence.
This updated review evaluated whether antidepressants are beneficial in the management of non‐specific low‐back pain. We identified ten studies which compared antidepressants to a placebo (an inactive substance that has no treatment value). All patients in these studies had low‐back pain as a primary complaint and some participants also had symptoms of depression.
We looked at the results of individual studies and also combined the results of several studies in larger analyses.
The review could find no convincing evidence that antidepressants relieve back pain or depression more effectively than placebo. Antidepressants did not result in any other apparent benefits in the treatment of back pain.
Antidepressants did cause side‐effects, however, adequate information about these was not provided in the trials.
Patients with significant depression should not avoid antidepressants based on this review, as they continue to play an important role in the treatment of clinical depression. There is also evidence that antidepressants can help patients with other specific types of pain.
The review cautions that existing studies do not provide adequate evidence regarding antidepressants for low‐back pain. There is a need for larger and more sophisticated studies to confirm the conclusions of this review. In the meantime, antidepressants should be regarded as an unproven treatment for non‐specific low‐back pain.
Background
Low‐back pain is a major health problem in developed countries worldwide and a primary cause of medical expense, absenteeism and disability. Although low‐back pain is usually a benign and self‐limiting disease that improves spontaneously over time (Waddell 1987), a wide range of therapeutic interventions are available for the treatment of this condition (Deyo 1987; Spitzer 1987; van Tulder 1997a; Cherkin 2003). Drug therapy is one of the many possible treatments available for symptom relief in patients with low‐back pain. In particular, antidepressant medication has been used in the management of patients with low‐back pain for many decades.
There are three key reasons for using antidepressants in the treatment of low‐back pain. First, chronic low‐back pain patients commonly present with depression, and treatment with antidepressants may elevate mood and increase pain tolerance (Cohen 2001; Micó 2006). The second reason for the use of antidepressants in chronic low‐back pain patients is their supposed analgesic action, which may occur at lower doses than their antidepressant effect (Cohen 2001; Micó 2006). Third, antidepressant drugs (primarily tricyclic antidepressants) are sedating, and it has been suggested that part of their value for managing chronic pain syndromes may be to improve sleep in those who experience insomnia (Deyo 1996). To obtain these specific effects in the management of chronic pain, different classes of antidepressants and treatment doses are used.
However, the use of antidepressants for low‐back pain is controversial. There is no clear message from recent systematic reviews (Salerno 2002; Staiger 2003; Schnitzer 2004) and antidepressants are not recommended in most clinical guidelines (Koes 2001). Nevertheless, two surveys have been conducted in the United States of America, which show that up to 23% of primary care physicians prescribe antidepressants for low‐back pain (DiIorio 2000), and 2%, 7% and 13% of visits for low‐back pain to primary care physicians, neurologists and rheumatologists respectively involve the prescription of antidepressant mediation (Broadhead 1991).
While our previous systematic review concluded that there is no clear evidence that antidepressants are effective in the management of patients with chronic low‐back pain (Urquhart 2008), trials were identified that were not included in the last review, so an updated review of the literature is indicated.
Objectives
The objectives of this systematic review were to: (i) assess whether antidepressants are more effective than placebo for the treatment of non‐specific low‐back pain, (ii) update previous systematic reviews with the latest evidence, and (iii) clarify the conflicting findings between our review and previous systematic reviews.
Methods
Criteria for considering studies for this review
Types of studies
Only trials that were randomised and had a placebo control group were included.
Types of participants
Adult subjects with non‐specific low‐back pain (defined as pain localised below the scapulae and above the cleft of the buttocks) with or without radiation and with or without leg pain were included. Studies that recruited participants with somatic or radicular (neuropathic) pain, or both, were included. We excluded trials that recruited subjects with low‐back pain and specific pathological entities, such as infection, neoplasm, metastasis, osteoporosis, rheumatoid arthritis or fractures. In addition, studies that investigated the effect of antidepressant medication on depression, and selected patients based on a diagnosis of a major depressive disorder, even though some patients also had chronic low‐back pain, were excluded.
Types of interventions
Any type of antidepressant (versus placebo) was included, i.e. tricyclic and heterocyclic antidepressants, selective serotonin reuptake inhibitors, mono‐amine oxidase inhibitors and 'atypical' antidepressants.
Types of outcome measures
Studies were included that used at least one of five primary outcome measures; (a) pain intensity, measured on a visual analog or numerical scale, (b) overall improvement, proportion of patients recovered or improved according to a dichotomised overall assessment of the clinical state by the patient themselves or an assessor, (c) functional status, expressed on a back pain‐specific scale (e.g. Roland Morris and Oswestry Disability Questionnaires) or a more generic scale (e.g. Sickness Impact Profile Scale), (d) return to work, measured as the number of days of sick leave or the proportion of patients returned to work, and (e) depression (or another behavioral outcome). Physiological outcomes (e.g. range of motion, spinal flexibility, degrees of straight leg raising or muscle strength) and generic functional status (e.g. SF‐36, Nottingham Health Profile, Sickness Impact Profile) were considered secondary outcomes.
Search methods for identification of studies
To identify relevant trials for this review, computer‐aided searches of the MEDLINE, EMBASE and PsycINFO databases were performed up to November 2008. The Cochrane Central Register of Controlled Trials (The Cochrane Library 2008, Issue 4) was screened for additional trials, and references of relevant reviews and identified trials were checked. Trials published in English, Dutch, French and German were included, as the authors of the current review were able to read and understand these languages.
We used the search strategy recommended by the Cochrane Back Review Group (Appendix 1: MEDLINE) (van Tulder 2003). Additional search terms for antidepressants included MeSH headings: antidepressive agents (including serotonin uptake inhibitors and adrenergic uptake inhibitors), and free text words: antidepress$, amoxapine, bupropion, citalopram, fluoxetine, fluvoxamine, maprotiline, mianserin, paroxetine, quipazine, ritanserin, sulpiride, trazodone, tryptophan, viloxazine, amitriptyline, clomipramine, desipramine, dothiepin, doxepin, imipramine, iprindole, lofepramine, nortriptyline, opipramol, protriptyline, trimipramine. A similar search strategy was used for EMBASE and PsycINFO.
Data collection and analysis
Study selection
Two review authors independently selected the trials for this systematic review and a consensus method was used to resolve disagreements concerning inclusion of randomised controlled trials. A third review author was consulted if disagreements persisted.
Risk of bias assessment
To assess the risk of bias of the randomised controlled trials, the criteria (and criteria operationalisation) recommended by the Cochrane Back Review Group were used (van Tulder 2003). Eleven items reflecting the internal validity of the trials were used to assess their risk of bias, with each criterion scored as "yes", "no", or "don't know" (Appendix 2). Low risk of bias was defined as fulfilling six or more of the eleven quality criteria (i.e. obtaining a 'yes' score to at least six criteria). A consensus method was used to resolve disagreements. Although all authors were contacted for additional information on their trial, only one author responded to our request (Atkinson 1998).
Data extraction
The studies were blinded with regard to authors, institution, journal and year of publishing by an administrative assistant who was not involved in the review. Data on the characteristics of the study population, type and dose of antidepressant and placebo treatment, and study results were tabulated. A summary of these data are provided in the Characteristics of included studies table.
Clinical Relevance
Two review authors independently assessed the clinical relevance of each study based on the criteria recommended by the Cochrane Back Review Group (van Tulder 2003). These criteria consist of five questions that relate to key factors such as patients, interventions and outcomes, and are scored as "yes", "no", or "don't know" (Appendix 3). The assessment indicates whether sufficient information has been given for the user to determine the relevance of the study's results to their specific patient population.
Data analysis
Given sufficient data were provided, meta‐analyses were performed. The results were tabulated and formally tested for homogeneity. Results were plotted as standardised mean differences (SMD) with corresponding 95% confidence intervals (95% CI). A difference of less than zero indicated a positive effect of antidepressants. Data were pooled using a random‐effects model (in RevMan 5).
Qualitative analyses were also used to summarise the results, as not all studies could be included in the meta‐analyses. The rating system recommended by the Cochrane Back Review Group was used (van Tulder 2003). It consists of four levels of scientific evidence based on the quality and the outcome of the studies: (1) strong evidence : consistent findings among multiple trials with a low risk of bias (2) moderate evidence : consistent findings among multiple trials with a high risk of bias and/or one trial with a low risk of bias (3) limited or conflicting evidence : only one trial with a high risk of bias or inconsistent findings among multiple trials (4) no evidence : no randomised controlled trials
Results
Description of studies
Ten studies were identified that compared antidepressant medication with placebo (Jenkins 1976; Alcoff 1982; Pheasant 1983; Goodkin 1990; Treves 1991; Atkinson 1998; Atkinson 1999; Dickens 2000; Katz 2005; Atkinson 2007). Two studies by Atkinson and colleagues (Atkinson 1999; Atkinson 2007) each included several comparisons, including different types of antidepressants with placebo and two antidepressants with each other. In the current review, we included two comparisons from Atkinson et al (Atkinson 1999) of placebo versus maprotiline (Atkinson 1999a) and paroxetine (Atkinson 1999b) and three comparisons from Atkinson et al (Atkinson 2007) of placebo versus low dose desipramine (<60 ng/mL) (Atkinson 2007a), high dose desipramine (>60 ng/mL) (Atkinson 2007b) and fluoxetine (Atkinson 2007c).
Nine trials included patients with chronic low‐back pain (Alcoff 1982; Pheasant 1983; Goodkin 1990; Treves 1991; Atkinson 1998; Atkinson 1999; Dickens 2000; Katz 2005; Atkinson 2007 ) and one trial did not specify the duration of the low‐back pain of the included patients (Jenkins 1976). All studies included low‐back pain patients with a mix of clinical diagnoses, such as radicular symptoms, herniated disc or spondylolisthesis. Even though all the studies selected patients because they had back pain, one study explicitly selected low back patients with significant depressive symptoms (Dickens 2000). Four studies included a mix of patients who were depressed and patients who were not depressed (i.e. 20% depressed (Alcoff 1982), 40.5% history of depression (Goodkin 1990), 34% moderate or severe depression (Jenkins 1976), 47% depressed (Treves 1991)), three studies excluded patients with major depression (Atkinson 1998; Atkinson 1999; Atkinson 2007), and in two studies it was unclear if patients were depressed or not (Pheasant 1983; Katz 2005).
Several types of antidepressants were investigated: tricyclic antidepressants, serotonin selective reuptake inhibitors, and 'atypical' antidepressants (e.g. aminoketone antidepressants) (Mir 1997; Stahl 1998). Most studies evaluated the effectiveness of tricyclic antidepressants with noradrenergic (maprotiline, nortriptyline, desipramine) (Atkinson 1998; Atkinson 1999; Atkinson 2007) or serotonergic effects (clomipramine) (Treves 1991), or a mix of both (amitriptyline, imipramine) (Jenkins 1976; Alcoff 1982; Pheasant 1983). Three studies evaluated a selective serotonin reuptake inhibitor (paroxetine, fluoxetine) (Atkinson 1999; Dickens 2000; Atkinson 2007) and two studies investigated 'atypical' antidepressants, bupropion (aminoketone antidepressant) (Katz 2005) and trazodone (Goodkin 1990). While three trials used an active placebo (i.e. preparation containing substances that mimic the side effects of antidepressants) (Pheasant 1983; Atkinson 1999; Atkinson 2007), the remaining trials used an inert placebo (Jenkins 1976; Alcoff 1982; Goodkin 1990; Treves 1991; Atkinson 1998; Dickens 2000; Katz 2005; ). In most studies, patients were allowed to continue regular medication (e.g. aspirin, non‐steroidal anti‐inflammatory drugs) (Alcoff 1982; Pheasant 1983; Goodkin 1990; Atkinson 1998; Atkinson 1999; Dickens 2000; Atkinson 2007). Although opioids were usually not allowed, one study reported that analgesics were not prescribed, except on the rare occasions when they became essential, and that the only psychotropic drugs that were used were hypnotics (Jenkins 1976). Two studies reported little or no information on additional medication (Treves 1991; Katz 2005).
Risk of bias in included studies
The results of the risk of bias assessment are presented in Figure 1. Seven trials had a low risk of bias (Jenkins 1976; Goodkin 1990; Atkinson 1998; Atkinson 1999; Dickens 2000; Katz 2005; Atkinson 2007), while only three studies (Alcoff 1982; Pheasant 1983; Treves 1991) were considered to have a high risk of bias (i.e. meeting fewer than six criteria). The most prevalent methodological flaws were associated with inadequate description of compliance and co‐interventions.
1.
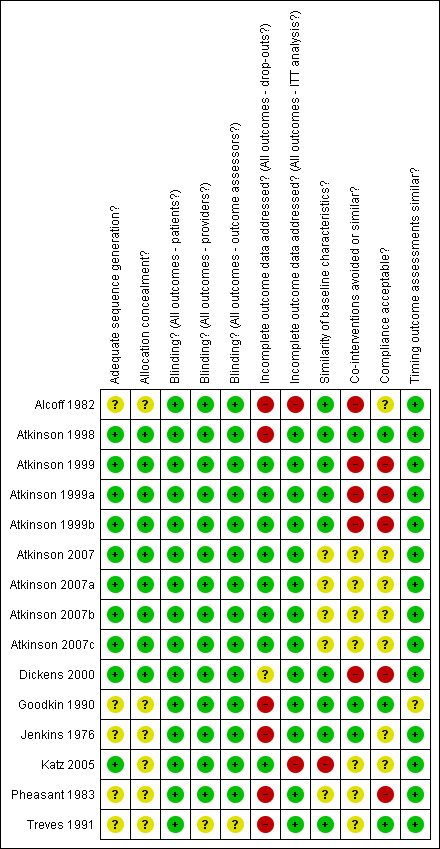
Summary of risks of bias
Clinical relevance
The clinical relevance scores for each trial are presented in Table 1. Five of the 10 trials were found to have moderate to high clinical relevance (a score of three or greater) (Jenkins 1976; Goodkin 1990; Atkinson 1998; Atkinson 1999; Dickens 2000). The other five trials were rated as having low clinical relevance (Alcoff 1982; Pheasant 1983; Treves 1991; Katz 2005; Atkinson 2007), and three of these trials also had a high risk of bias. While most studies provided sufficient data on the patients studied (six trials), interventions/treatment settings used (10 trials) and outcomes measured and reported (six trials), information to determine if there was a clinically important effect (one trial) or whether treatment benefits outweighed the potential harms (no trials) was poorly reported. In summary, these findings indicate that while most studies were found to have moderate to high clinical relevance, the assessment of clinical relevance was limited by a paucity of data.
1. Clinical Relevance.
| Study | Pt description | Interv. description | Outcomes meas/report | Effect size | Benefit vs harm | Score |
| Alcoff 1982 | ? | + | ‐ | ? | ? | 1 |
| Atkinson 1998 | + | + | + | ‐ | ‐ | 3 |
| Atkinson 1999 | + | + | + | + | ‐ | 4 |
| Atkinson 2007 | + | + | ‐ | ‐ | ‐ | 2 |
| Dickens 2000 | + | + | + | ‐ | ‐ | 3 |
| Goodkin 1990 | + | + | + | ‐ | ‐ | 3 |
| Jenkins 1976 | + | + | + | ‐ | ‐ | 3 |
| Katz 2005 | ? | + | + | ‐ | ‐ | 2 |
| Pheasant 1983 | ? | + | ‐ | ‐ | ‐ | 1 |
| Treves 1991 | ? | + | ‐ | ‐ | ? | 1 |
Effects of interventions
Study selection
Our original search strategy identified 1,650 potentially relevant abstracts. Following review of the full text of articles, we identified 20 trials that needed to be considered in detail. Nine studies met our inclusion criteria (Jenkins 1976; Alcoff 1982; Pheasant 1983; Goodkin 1990; Treves 1991; Atkinson 1998; Atkinson 1999; Dickens 2000; Katz 2005). Eleven studies were excluded as they; (i) selected patients with chronic idiopathic pain syndrome (Loldrup 1989), specific low‐back pain (Storch 1982), musculoskeletal pain (Schreiber 2001a), chronic neck and/or back pain (without presenting the data on back pain separately) (Hameroff 1982; Hameroff 1984) or a major depressive disorder (Brannan 2004); (ii) were not randomised (Sternbach 1976; Ward 1986) or placebo‐controlled (Stein 1996; Pirbudak 2003b); or (iii) were published in Turkish (Pirbudak 2003a) (see Characteristics of excluded studies table). Two additional trials were identified from 234 studies when our search was updated in November 2008 (Atkinson 2007; Khoromi 2007). The study by Atkinson et al (Atkinson 2007), with three treatment arms, was included in the current review, while the negative study by Khoromi and colleagues (Khoromi 2007) was excluded from the review as it focused on patients with lumbar radiculopathy. A concurrent search of the WHO‐ICTRP database of registered protocols yielded two studies of interest (Eli Lilly & Co 2006; Eli Lilly & Co 2007).
Primary analyses
Effectiveness of antidepressants versus placebo: Pain intensity
Of the seven studies with a low risk of bias that compared antidepressants with placebo, five trials reported no differences in pain between these treatments (Jenkins 1976; Goodkin 1990; Atkinson 1999b; Dickens 2000; Katz 2005), while three studies with a low risk of bias reported a greater reduction in pain with the use of antidepressants (Atkinson 1998; Atkinson 1999a; Atkinson 2007). Overall, these findings indicate that there is conflicting evidence regarding the effect of antidepressants on pain intensity in patients with chronic low‐back pain.
A meta‐analysis of six small placebo‐controlled trials was performed (Jenkins 1976; Goodkin 1990; Atkinson 1999; Dickens 2000; Katz 2005; Atkinson 2007), which included studies by Atkinson et al (Atkinson 1999; Atkinson 2007) that had two and three intervention arms respectively. Four trials were excluded from the analysis as two studies did not provide pain intensity data (Pheasant 1983; Treves 1991), one study did not report follow‐up means and standard deviations (SDs) (Atkinson 1998), and one study just provided P values (which does not allow calculation of the component means and SDs) (Alcoff 1982). The Chi2 test value for homogeneity of the standardised mean difference was ‐4.82 (df = 8; P = 0.78), indicating statistical homogeneity among the studies. The pooled analysis of trials (scores of 376 people) failed to show a difference in pain relief between antidepressants and placebo for patients with chronic non‐specific low‐back pain with a standardised mean difference of ‐0.04 (95% CI ‐0.25 to 0.17) (Analysis 1.1).
1.1. Analysis.
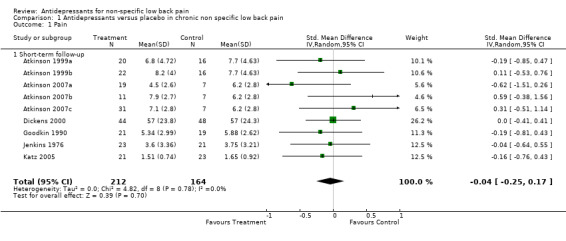
Comparison 1 Antidepressants versus placebo in chronic non specific low back pain, Outcome 1 Pain.
Of the four studies that could not be included in the meta‐analysis, the three studies with a high risk of bias reported no differences in severity of back pain or number of analgesics taken per day (Alcoff 1982) and significantly more symptomatic pain relief with antidepressants (Treves 1991; Atkinson 2007). However, the latter study by Treves and colleagues (Treves 1991) reported on patients who were hospitalised and treated by intravenous infusions of antidepressants. Although there was one study with a low risk of bias that reported a greater reduction in pain with antidepressants (Atkinson 1998), a P value of 0.050 was interpreted as reaching statistical significance and a statistically significant effect was only found in the 'completer's analysis', not for the intention‐to‐treat analysis.
Effectiveness of antidepressants versus placebo: Depression
Six high quality trials included depression as an outcome, which was measured by the Beck Depression Inventory (Jenkins 1976; Goodkin 1990; Atkinson 1998; Atkinson 1999; Katz 2005), Hamilton Depression Scale (Atkinson 1998; Atkinson 1999), and Montgomery Asberg Depression Rating Scale (Dickens 2000). There was considerable variability in the doses of antidepressant used between these trials, with Jenkins et al (Jenkins 1976) trialling 75 mg/day of imipramine and Goodkin and colleagues (Goodkin 1990) using 600 mg/day of trazodone. These studies with a low risk of bias compared antidepressants with placebo and reported no differences in depression (Jenkins 1976; Goodkin 1990; Atkinson 1998; Atkinson 1999; Dickens 2000; Katz 2005). Overall, these results suggest there is no consistent evidence that antidepressants reduce depression in patients with chronic low‐back pain.
Given most studies did not provide data on depression, only two studies (132 people) could be included in a meta‐analysis (Goodkin 1990; Dickens 2000) and this failed to show a difference in reduction of depression between antidepressants and placebo (standardised mean difference of 0.06 (95% CI ‐0.29 to 0.40)) (Analysis 1.2). The one trial with a low risk of bias that included patients with significant depressive symptoms reported conflicting results (Dickens 2000).
1.2. Analysis.
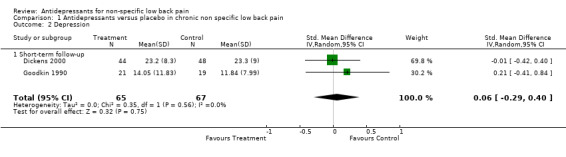
Comparison 1 Antidepressants versus placebo in chronic non specific low back pain, Outcome 2 Depression.
Effectiveness of antidepressants versus placebo: Functional status
Two studies with a low risk of bias included functional status as an outcome measure (Goodkin 1990; Dickens 2000). Neither of these studies found a significant difference in functional status with the use of antidepressants compared to placebo in patients with low‐back pain. The pooled analysis of these two small trials (132 people) failed to show a difference in improvement of functional status between antidepressants and placebo, with a standardised mean difference of ‐0.06 (95% CI ‐0.40 to 0.29) (Analysis 1.3).
1.3. Analysis.
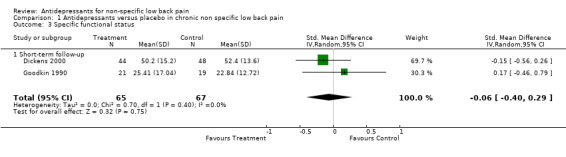
Comparison 1 Antidepressants versus placebo in chronic non specific low back pain, Outcome 3 Specific functional status.
Effectiveness of antidepressant type versus placebo: Pain intensity
Separate pooled analyses were performed to evaluate the effect of antidepressant type on the outcome of pain intensity (Analysis 3.1; Analysis 4.1). The pooled analysis of three trials with a low risk of bias (Jenkins 1976; Atkinson 1999a; Atkinson 2007), including one trial with two intervention arms (Atkinson 2007a; Atkinson 2007b), failed to show a difference in pain relief between tricyclic antidepressants and placebo (standardised mean difference: ‐0.10 (95% CI ‐0.51 to 0.31). Similarly, selective serotonin reuptake inhibitors were found to be no more effective than placebo in the reduction of pain with the pooling of a further three trials with a low risk of bias (Atkinson 1999b; Dickens 2000; Atkinson 2007c) (standardised mean difference: 0.11 (95% CI ‐0.17 to 0.39).
3.1. Analysis.
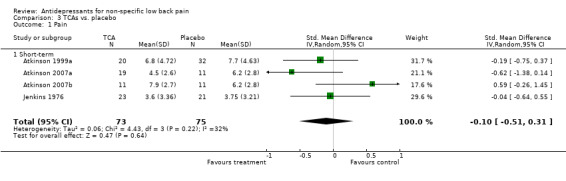
Comparison 3 TCAs vs. placebo, Outcome 1 Pain.
4.1. Analysis.
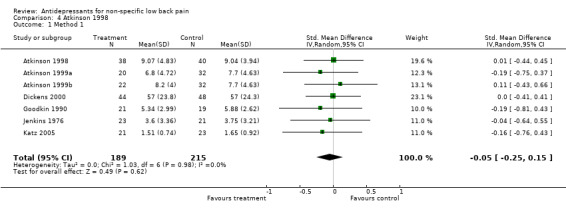
Comparison 4 Atkinson 1998, Outcome 1 Method 1.
Sensitivity analysis
If we assumed that all validity items which were scored 'don't know' met our risk of bias criteria and consequently were scored positively, all studies would have been considered to have a low risk of bias. However, our sensitivity analyses showed that this had no impact on the conclusions of our qualitative analysis. If we had defined low risk of bias as meeting five or more, or seven or more criteria, the conclusions would also have been the same.
We also examined the inclusion of the 'positive' trial by Atkinson et al (Atkinson 1998), which did not report follow‐up means and SDs, in our meta‐analyses. We calculated the follow‐up means for pain intensity and depression from the baseline and change scores, and estimated the follow‐up SDs based on the: (i) baseline SDs, (ii) ratio of the baseline means and SDs, and (iii) ratio of the follow‐up means and SDs of the other RCTs included in the meta‐analyses. The inclusion of Atkinson et al (Atkinson 1998) in the meta‐analyses for both pain and depression would not change our conclusion, that there is no difference in effect between antidepressants and placebo, and demonstrates the robustness of our findings.
Similarly, we examined the addition of three intervention arms from the study by Atkinson et al (Atkinson 2007) in our meta‐analyses. This study did not report statistically significant results for their intention‐to‐treat analysis, only for their 'completer's analysis'. Our meta‐analyses showed that the inclusion of different intervention arms from Atkinson et al (Atkinson 2007) did not change our conclusions for pain and type of antidepressant.
Other analyses
We had planned other subgroup analyses for acute versus chronic low‐back pain, for studies comparing different doses of antidepressants and for long‐term follow‐up versus short‐term follow‐up. However, these subgroup analyses could not be performed because no study explicitly included patients with acute or subacute low‐back pain, administered doses that differed from the guidelines or included a long‐term follow‐up. We also aimed to examine work‐related disability, however no studies investigated this as an outcome.
Discussion
This systematic review identified ten randomised, placebo‐controlled trials. Seven of these trials had a low risk of bias. A qualitative analysis of the trials with a low risk of bias found conflicting evidence regarding the effectiveness of antidepressants in the reduction of pain for chronic low‐back pain patients. However, a meta‐analysis of six trials failed to show a difference in pain relief between antidepressants and placebo. In addition, quantitative analyses that examined the effect of antidepressants and placebo on depression and functional status did not show a difference between treatments, neither did the evaluation of type of antidepressant (versus placebo) on pain relief. This review indicates that there is currently no clear evidence to support the prescription of antidepressants in the treatment of low‐back pain. However, given the paucity of trials with a low risk of bias and large sample sizes, further research is required to confirm the effect of antidepressants on chronic low‐back pain.
Our conclusion, that there is no clear evidence that antidepressants reduce pain, depression or functional status in patients with chronic low‐back pain, may have resulted from differences in patient selection both within and between studies. Although patients were selected because they had low‐back pain, there was variation in the nature and duration of patients' back pain, the percentage of patients with and without depression, and the severity of their depression. Given that a previous Cochrane review has shown that antidepressants are effective in reducing depression in patients with a variety of medical illnesses (e.g. cancer, diabetes, multiple sclerosis) (Gill 2002), future reviews that include large study populations of patients with low‐back pain and severe depression may show antidepressants to be effective. This may also apply to other subgroups of the low‐back pain population.
The frequency and seriousness of adverse effects are also important to consider when evaluating the effectiveness of antidepressants. Although adverse effects, such as dry mouth, constipation, tachycardia, sedation, orthostatic hypotension and tremor, were commonly reported, no serious adverse effects were documented. However, the trials were very small and not designed to evaluate adverse effects. Prospective studies with larger sample sizes are necessary to evaluate the incidence of both minor and major adverse effects.
We performed both qualitative and quantitative analyses, and concluded that the results are robust and sufficiently conclusive. Our qualitative analyses included trials that had a lower risk of bias. Although the trials had small study populations which may have resulted in insufficient statistical power to detect clinically relevant differences in effects, statistical pooling was included in our analysis to overcome this problem and the meta‐analyses consistently showed very small effect sizes of less than 0.2 (Cohen 1988). Therefore, it is unlikely that an increase in sample size would have led to a major shift in the magnitude of the estimated effect or our conclusion that antidepressants are ineffective in low‐back pain. Given a meta‐analysis could only be performed on a subset of available trials, it is possible that statistical pooling may have also biased the results. However, we showed the robustness of our findings through our sensitivity analyses, suggesting that this was not the case.
Three other systematic reviews have recently investigated the efficacy of antidepressants for chronic low‐back pain (Salerno 2002; Staiger 2003; Schnitzer 2004). While there is agreement between some of the conclusions of these reviews, and between these reviews and the current review, some of their findings are conflicting. Two reviews reported antidepressants to be more effective than placebo in reducing pain severity for chronic low‐back pain (Salerno 2002; Schnitzer 2004), while our review found no consistent evidence to support this conclusion, and a fourth review concluded that their use was dependent on the type of antidepressant prescribed, with tricyclic antidepressants shown to be effective in reducing pain (unlike selective serotonin reuptake inhibitors) (Staiger 2003). In addition, only three of the four reviews (including the current review) concluded that there is insufficient evidence to support the use of antidepressants to improve functional status in chronic low‐back pain patients (Salerno 2002; Staiger 2003). Furthermore, although two reviews did not specifically report on the effect of antidepressants on depression, a third review reported antidepressants to be effective in reducing depression in chronic low‐back pain (Schnitzer 2004), while our review found no clear evidence for their use in patients with low‐back pain and depression.
These conflicting conclusions may have resulted from methodological differences between reviews. Based on Jadad's decision algorithm for analysing and interpreting discordant systematic reviews (Jadad 1997), the reviews were found to primarily differ in the selection criteria used and the trials that were included. Given we identified studies published up until November 2008 and included non‐English trials, our review was the only one to include the study of Katz et al (Katz 2005), Treves et al (Treves 1991) and Atkinson et al (Atkinson 2007). Unlike Schnitzer et al (Schnitzer 2004), we excluded a trial by Schreiber et al (Schreiber 2001a) that did not analyse data for low‐back pain patients separately, and in contrast to Salerno et al (Salerno 2002), we excluded a study by Ward et al (Ward 1986) that did not specify randomisation of subjects to groups.
Given that three of the reviews performed a meta‐analysis, the differences in conclusions can also be explained by the trials selected for statistical pooling. Salerno et al (Salerno 2002) included all ten studies in their meta‐analysis, while our review was limited to six trials, and Staiger et al (Staiger 2003) performed two analyses, with seven trials in the analysis of tricyclic antidepressants and five in the analysis of selective serotonin reuptake inhibitors. Schnitzer et al (Schnitzer 2004) did not perform a meta‐analysis. Given Salerno et al (Salerno 2002) included the study by Ward et al (Ward 1986) (which made no mention of randomisation), which showed the largest difference in effect, it is very likely that this caused an overestimation of the treatment effect. We did not include the study of Alcoff et al (Alcoff 1982) as only P ‐ values were reported, the study of Pheasant et al (Pheasant 1983) as it did not provide data on pain intensity, and the study of Atkinson et al (Atkinson 1998) because only means and standard deviations of the baseline and change scores were reported and standard deviations for the follow‐up scores could not be calculated because correlation data for the baseline and change scores were not provided. However, even when we included the studies by Atkinson et al (Atkinson 1998; Atkinson 2007) in our sensitivity analysis, our conclusions remained unchanged. Salerno et al (Salerno 2002) did not report how they imputed data for the studies of Alcoff et al (Alcoff 1982) and Pheasant et al (Pheasant 1983). We were unable to find recent contact information for Alcoff and Pheasant, nor clarify the methodology used by Salerno and colleagues. Similarly, the review by Staiger et al (Staiger 2003) excluded the trial of Jenkins et al (Jenkins 1976) from their meta‐analysis on the basis that there were insufficient data. However, we were able to calculate standardised mean differences from the post treatment means and standard deviations for both of these and thereby include these in our meta‐analysis. Our review reflects the findings of Jadad and colleagues (Jadad 1997), which showed that a meta‐analysis with a more comprehensive set of trials is less likely to generate positive results (i.e. a statistically significant favourable effect).
The current review is the most up‐to‐date, high quality meta‐analysis on antidepressants and has included all available randomised controlled trials. We found no clear evidence to support the clinicians' prescription of antidepressants in reducing pain and depression for patients with chronic low‐back pain. These findings do not imply that severely depressed patients with back pain shouldn't be treated with antidepressants; furthermore, there is evidence for the use of tricyclic antidepressants in other forms of chronic pain, such as neuropathic pain (Saarton 2005; Sindrup 2005) and fibromyalgia (O'Malley 2000). Given this review was limited by a paucity of trials, small study populations and variation in study quality and patients recruited, further research is required to confirm the effect of antidepressants on low‐back pain.
Authors' conclusions
Implications for practice.
We found no clear evidence to support the clinicians' prescription of antidepressants in reducing pain and depression for patients with chronic low‐back pain. These findings do not imply that severely depressed patients with back pain should not be treated with antidepressants; furthermore, there is evidence for the use of tricyclic antidepressants in other forms of chronic pain, such as neuropathic pain (Saarton 2005; Sindrup 2005) and fibromyalgia (O'Malley 2000).
Implications for research.
To confirm the effect of antidepressants on low‐back pain, more studies with large, homogenous study populations, that meet high methodological standards and involve long‐term follow‐up are required. Future research should also focus on the (cost‐) effectiveness of antidepressants for the management of patients with chronic low‐back pain.
What's new
| Date | Event | Description |
|---|---|---|
| 8 September 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 19 February 2009 | New search has been performed | The literature search was updated on 11th November 2008. No new studies were identified. However, two studies that were classified in the first review as 'waiting for assessment' were examined. While the study by Atkinson (2007) has been included in the review, the negative trial by Khoromi (2007) has been excluded. The addition of the study by Atkinson (2007) has not changed the conclusions of the original review. |
| 19 June 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank Mrs Vera Veldman for blinding the studies and Ms Rachel Couban, Trials Search Co‐ordinator, Cochrane Back Review Group, for updating the literature search.
Appendices
Appendix 1. MEDLINE search strategy
01 randomised controlled trial.pt. 02 controlled clinical trial.pt. 03 Randomised Controlled Trials/ 04 Random Allocation/ 05 Double‐Blind Method/ 06 Single‐Blind Method/ 07 or/1‐6 08 Animals/ not Human/ 09 7 not 8 10 clinical trial.pt. 11 exp Clinical Trials/ 12 (clin$ adj25 trial$).tw. 13 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 14 Placebos/ 15 placebo$.tw. 16 random$.tw. 17 Research Design/ 18 (latin adj square).tw. 19 or/10‐18 20 19 not 18 21 20 not 9 22 Comparative Study/ 23 exp Evaluation Studies/ 24 Follow‐Up Studies/ 25 Prospective Studies/ 26 (control$ or prospective$ or Volunteer$).tw. 27 Cross‐Over Studies/ 28 or/22‐27 29 28 not 8 30 29 not (9 or 21) 31 9 or 21 or 30 32 dorsalgia.ti,ab. 33 exp Back Pain/ 34 backache.ti,ab. 35 (lumbar adj pain).ti,ab. 36 coccyx.ti,ab. 37 coccydynia.ti,ab. 38 sciatica.ti,ab. 39 sciatica/ 40 spondylosis.ti,ab. 41 lumbago.ti,ab. 42 or/32‐41 43 neck muscles.sh. 44 exp Neck/ 45 whiplash injuries.sh. 46 neck.ti,ab. 47 or/44‐46 48 exp Spine/ 49 discitis.ti,ab. 50 exp Spinal Diseases/ 51 (disc adj degeneration).ti,ab. 52 (disc adj prolapse).ti,ab. 53 (disc adj herniation).ti,ab. 54 spinal fusion.sh. 55 spinal neoplasms.sh. 56 (facet adj joints).ti,ab. 57 intervertebral disk.sh. 58 postlaminectomy.ti,ab. 59 arachnoiditis.ti,ab. 60 (failed adj back).ti,ab. 61 or/48‐60 62 Oswestry.tw. 63 Roland‐Morris.tw. 64 or/62‐63 65 42 or 47 or 61 or 64 66 31 and 65
Appendix 2. Criteria for the Risk of Bias Assessment
Was the method of randomisation adequate? A random (unpredictable) assignment sequence. Examples of adequate methods are computer‐generated random numbers table and use of sealed opaque envelopes. Methods of allocation using date of birth, date of admission, hospital numbers, or alternation should not be regarded as appropriate. Was the treatment allocation concealed? Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. Was the patient blinded to the intervention? The review author determines if enough information about the blinding is given in order to score a "yes." Was the care provider blinded to the intervention? The review author determines if enough information about the blinding is given in order to score a "yes." Was the outcome assessor blinded to the intervention? The review author determines if enough information about the blinding is given in order to score a "yes."
Was the drop‐out rate described and acceptable? The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop‐outs does not exceed 20% for immediate and short‐term follow‐ups, 30% for intermediate and long‐term follow‐ups and does not lead to substantial bias a "yes" is scored.
Did the analysis include an intention‐to‐treat analysis? All randomized patients are reported/analyzed in the group to which they were allocated by randomization for the most important moments of effect measurement (minus missing values), irrespective of noncompliance and co‐interventions.
Were the groups similar at baseline regarding the most important prognostic indicators? In order to receive a "yes," groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of patients with neurological symptoms, and value of main outcome measure(s). Were co‐interventions avoided or similar? Co‐interventions should either be avoided in the trial design or be similar between the index and control groups. Was the compliance acceptable in all groups? The review author determines if the compliance to the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). Was the timing of the outcome assessment in all groups similar? Timing of outcome assessment should be identical for all intervention groups and for all important outcome assessments.
Appendix 3. Questions used to assess Clinical Relevance
Based on information provided in the study report, the review authors should answer these questions with 'yes', 'no', 'unsure' 1. Are the patients described in detail so that you can decide whether they are comparable to those that you see in your practice? 2. Are the interventions and treatment settings described well enough so that you can provide the same for your patients? 3. Were all clinically relevant outcomes measured and reported? 4. Is the size of the effect clinically important? 5. Are the likely treatment benefits worth the potential harms?
Data and analyses
Comparison 1. Antidepressants versus placebo in chronic non specific low back pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 9 | 376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 1.1 Short‐term follow‐up | 9 | 376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 2 Depression | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.29, 0.40] |
| 2.1 Short‐term follow‐up | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.29, 0.40] |
| 3 Specific functional status | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.40, 0.29] |
| 3.1 Short‐term follow‐up | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.40, 0.29] |
Comparison 2. SSRIs vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | 199 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.17, 0.39] |
| 1.1 Short‐term | 3 | 199 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.17, 0.39] |
2.1. Analysis.
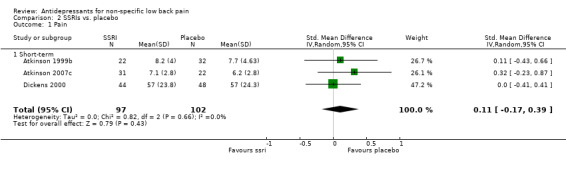
Comparison 2 SSRIs vs. placebo, Outcome 1 Pain.
Comparison 3. TCAs vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 4 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.51, 0.31] |
| 1.1 Short‐term | 4 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.51, 0.31] |
Comparison 4. Atkinson 1998.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Method 1 | 7 | 404 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.25, 0.15] |
| 2 Method 2 | 7 | 404 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.25, 0.15] |
| 3 Method 3 | 7 | 404 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.25, 0.15] |
| 4 Method 1 Depression | 3 | 210 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.33, 0.21] |
| 5 Method 2 Depression | 3 | 210 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.42, 0.22] |
| 6 Method 3 Depression | 3 | 210 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.62, 0.31] |
4.2. Analysis.
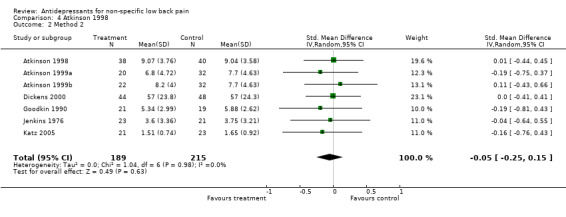
Comparison 4 Atkinson 1998, Outcome 2 Method 2.
4.3. Analysis.
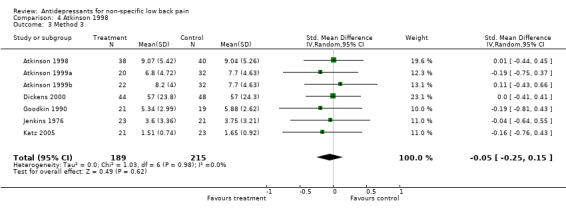
Comparison 4 Atkinson 1998, Outcome 3 Method 3.
4.4. Analysis.
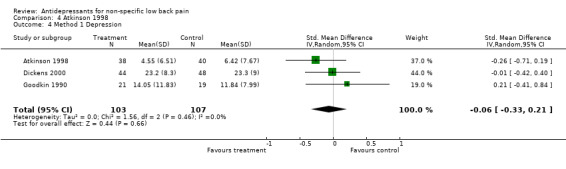
Comparison 4 Atkinson 1998, Outcome 4 Method 1 Depression.
4.5. Analysis.
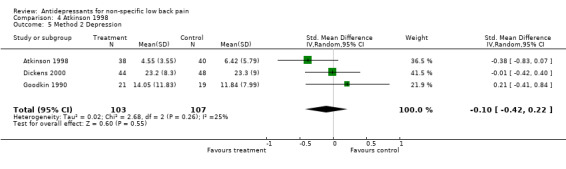
Comparison 4 Atkinson 1998, Outcome 5 Method 2 Depression.
4.6. Analysis.
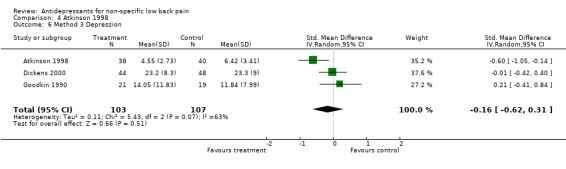
Comparison 4 Atkinson 1998, Outcome 6 Method 3 Depression.
Comparison 5. Atkinson 2007.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Atkinson 2007abc | 9 | 376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 2 Atkinson 2007ab | 8 | 346 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.28, 0.15] |
| 3 Atkinson 2007a | 7 | 335 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.35, 0.09] |
5.1. Analysis.
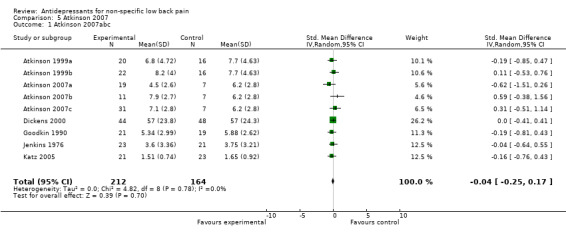
Comparison 5 Atkinson 2007, Outcome 1 Atkinson 2007abc.
5.2. Analysis.
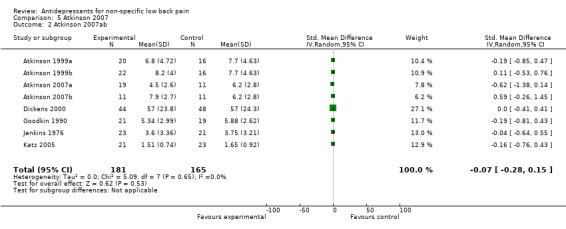
Comparison 5 Atkinson 2007, Outcome 2 Atkinson 2007ab.
5.3. Analysis.
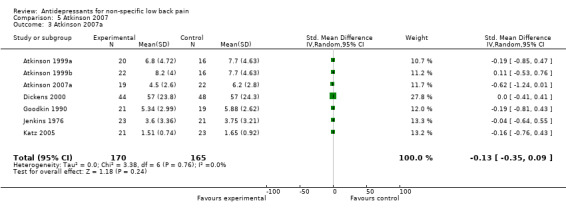
Comparison 5 Atkinson 2007, Outcome 3 Atkinson 2007a.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alcoff 1982.
| Methods | RCT, double blind, placebo controlled. Randomization procedure not described. | |
| Participants | 50 patients randomised, 41 completers, 48 in analysis. Inclusion: low‐back pain for at least 6 weeks if it was a first episode, or two or more prior episodes lasting at least 2 weeks with a current episode of a minimum of 2 weeks. Only 10 of the 50 patients were judged clinically depressed. | |
| Interventions | Antidepressant (i) imipramine 75 mg pills, one pill / day for the first three days, than two pills/day. Duration of the study was 8 weeks (N = 28). Reference treatment (ii) placebo (N = 22). |
|
| Outcomes | No differences in pain and depression after 8 weeks. (i) had a statistically significant effect over (ii) in no. of days with at least some restriction of normal activity, limitation of work or restriction of recreational activities. | |
| Notes | Data not presented; only P ‐ values. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomization procedure not described |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | High risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | High risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | High risk | |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | |
Atkinson 1998.
| Methods | RCT, double blind, placebo controlled. Randomization using a random number table held by a research pharmacist not involved in other aspects of the trial. | |
| Participants | 78 patients (all men) randomised. Inclusion: age 21 to 65 years, low‐back pain on a daily basis for 6 months. Exclusion: mood disorder, major depression, history of psychoactive substance use disorder within the preceding 12 months. | |
| Interventions | Antidepressant (i) nortriptyline, in a dose escalation schedule of 25 mg/d for three days, then 50 mg/d for four days, then 75 mg/d for three days, and then 100 mg/d for four days (depending upon side‐effects) for eight weeks (N = 28). Reference treatment (ii) placebo was administered in identical capsules as (i) and also in a single dose at 21.00 hrs for eight weeks (N = 29). |
|
| Outcomes | Difference (95%CI) in mean change scores for pain intensity (0‐20 point scale) 1.68 (‐0.001 ; 3.36) and for general functional status (SIP) 1.80 (‐.04 ; 3.66) in favour of (i). Health related quality of life, mood, depression and anxiety no differences. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using a random number table held by a research pharmacist not involved in other aspects of the trial. |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | High risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Low risk | |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
Atkinson 1999.
| Methods | RCT, double blind, placebo controlled. Randomization using a random number table held by a research pharmacist not involved in other aspects of the trial. | |
| Participants | 103 chronic low‐back pain patients (65 men, 38 women; age 21 to 65 years). Recruitment through clinics and local advertisements. Exclusion: current mood disorder or major depression and history of a psychoactive substance use disorder within the preceding 12 months. |
|
| Interventions | Antidepressant (i) maprotiline, 50 mg 3 days, 100 mg 3 days, 150 mg thereafter. 8 weeks. (N = 33; Completer's analysis = 20). (Atkinson 1999a) Antidepressant (ii) paroxetine, 10 mg 3 days, 20 mg 3 days, 30 mg thereafter. 8 weeks. (N = 34; Completer's analysis = 22). (Atkinson 1999b) Reference treatment (ii) active placebo (diphenhydramine hydrochloride), 12.5 mg 3 days, 25 mg 3 days, 37.5 mg thereafter. 8 weeks. (N = 36; Completer's analysis = 32). All administered at 9 PM. |
|
| Outcomes | In 'completers' analysis mean (SD) reduction in pain intensity scores (0 to 20 scale) after 8 weeks (i) 5.41 (4.99), (ii) 2.83 (3.31). (i) statistically significant effect over (ii). No differences in depression. Intention to treat analysis showed similar results. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using a random number table held by a research pharmacist not involved in other aspects of the trial. |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | High risk | |
| Compliance acceptable? | High risk | |
| Timing outcome assessments similar? | Low risk | |
Atkinson 1999a.
| Methods | As per Atkinson 1999 | |
| Participants | ||
| Interventions | Maprotiline 50 mg 3 days, 100 mg 3 days, 150 mg thereafter. (N = 33; Completer's analysis = 20) versus active placebo (diphenhydramine hydrochloride), 12.5 mg 3 days, 25 mg 3 days, 37.5 mg thereafter. (N = 36; Completer's analysis = 32). 8 weeks. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | |
| Allocation concealment? | Low risk | |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | High risk | |
| Compliance acceptable? | High risk | |
| Timing outcome assessments similar? | Low risk | |
Atkinson 1999b.
| Methods | As per Atkinson 1999. | |
| Participants | ||
| Interventions | Paroxetine, 10 mg 3 days, 20 mg 3 days, 30 mg thereafter (N = 34; Completer's analysis = 22) versus active placebo (diphenhydramine hydrochloride), 12.5 mg 3 days, 25 mg 3 days, 37.5 mg thereafter. (N = 36; Completer's analysis = 32). 8 weeks. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | |
| Allocation concealment? | Low risk | |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | High risk | |
| Compliance acceptable? | High risk | |
| Timing outcome assessments similar? | Low risk | |
Atkinson 2007.
| Methods | Randomised, double blind, controlled‐concentration trial with active placebo. Randomization using a computerised random number generator held by a pharmacist who was not involved in other aspects of the trial. | |
| Participants | 121 chronic low back pain patients (74 men, 47 women; mean (SD) age: 46.4 (10.2) years). Recruitment through primary care clinics, local advertisements and word of mouth. Inclusion: aged 21 to 65 years, low back pain on a daily basis for the previous 6 months or longer, English speaking and literate, not a candidate for surgery and extensive metabolizer phenotype. Exclusion: major co‐existing medical illness, another co‐existing pain problem, significant co‐existing musculoskeletal disorder, history of substance abuse, major depression or dysthymia, history of bipolar disorder or psychosis, dementia, known allergy to study drugs, and use of psychoactive agents that would need to be continued during the study. |
|
| Interventions | Antidepressant treatment: (i) Desipramine (50, 110 and 150 ng/mL, which was later analysed as < and > 60ng/mL) (N=52; Completer's analysis: < 60 ng/mL = 19; > 60 ng/mL = 11) (Atkinson 2007a, b) 12 weeks. (ii) Fluoxetine (100, 200 and 400 ng/mL) (N = 43; Completer's analysis: 31 (Atkinson 2007c)). 12 weeks. Reference treatment (iii) active placebo (benztropine mesylate). 0.5mg daily. (N = 26; Completer's analysis: 22). 12 weeks. All administered at 9 PM. |
|
| Outcomes | In 'completers' analysis, pain intensity means (SE) were significantly lower for the low concentration desipramine group (4.5 (0.6)) than for placebo (6.2 (0.6)) and other treatment groups (7.1(0.5)) after 12 weeks. This was also the case for the Roland Morris Disability and Physician Clinical Global scores. However, intention‐to‐treat analyses demonstrated no significant differences. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using a computerised random number generator. |
| Allocation concealment? | Low risk | Performed by a pharmacist who was not involved in other aspects of the trial. |
| Blinding? All outcomes ‐ patients? | Low risk | Patients blinded ‐ re administration of medication (colour of pills etc) and all received venipuncture. Evidence provided to suggest successful blinding. |
| Blinding? All outcomes ‐ providers? | Low risk | Drug concentrations were monitored and adjusted by an off‐site study research pharmacologist who alone had access to the results. A blinded study physician also completed a safety and side‐effect rating. |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | Outcome measures administered by blinded research assistants in sessions separate to the study physician session. Evidence to suggest successful blinding. |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | Number and reasons for drop‐outs provided. Approx 30% drop‐out for intermediate to longer‐term follow‐up. |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | Intention to treat analysis performed. |
| Similarity of baseline characteristics? | Unclear risk | Stated there was no difference but no data were provided, particularly related to baseline outcome measures. |
| Co‐interventions avoided or similar? | Unclear risk | Opioids ceased for trial but not non‐opioids. Urine toxicology screens used to monitor the use of other drugs but results not reported. |
| Compliance acceptable? | Unclear risk | Only 69% retention rate. Limited detail regarding compliance. |
| Timing outcome assessments similar? | Low risk | |
Atkinson 2007a.
| Methods | As per Atkinson 2007 | |
| Participants | ||
| Interventions | Desipramine (50, 110 and 150 ng/mL, which was later analysed as < and > 60ng/mL) (N=52; Completer's analysis: < 60 ng/mL = 19) (Atkinson 2007a) versus active placebo (benztropine mesylate). 0.5mg daily. (N = 26; Completer's analysis: 22). 12 weeks. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | |
| Allocation concealment? | Low risk | |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Unclear risk | Stated there was no difference but no data were provided, particularly related to baseline outcome measures. |
| Co‐interventions avoided or similar? | Unclear risk | Opioids ceased for trial but not non‐opioids. Urine toxicology screens used to monitor the use of other drugs but results not reported. |
| Compliance acceptable? | Unclear risk | Only 69% retention rate. Limited detail regarding compliance. |
| Timing outcome assessments similar? | Low risk | |
Atkinson 2007b.
| Methods | As per Atkinson 2007 | |
| Participants | ||
| Interventions | Desipramine (50, 110 and 150 ng/mL, which was later analysed as < and > 60ng/mL) (N=52; Completer's analysis: > 60 ng/mL = 11) (Atkinson 2007b) 12 weeks versus active placebo (benztropine mesylate). 0.5mg daily. (N = 26; Completer's analysis: 22). 12 weeks. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | |
| Allocation concealment? | Low risk | |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Unclear risk | Stated there was no difference but no data were provided, particularly related to baseline outcome measures. |
| Co‐interventions avoided or similar? | Unclear risk | Opioids ceased for trial but not non‐opioids. Urine toxicology screens used to monitor the use of other drugs but results not reported. |
| Compliance acceptable? | Unclear risk | Only 69% retention rate. Limited detail regarding compliance. |
| Timing outcome assessments similar? | Low risk | |
Atkinson 2007c.
| Methods | As per Atkinson 2007 | |
| Participants | ||
| Interventions | Fluoxetine (100, 200 and 400 ng/mL) (N = 43; Completer's analysis: 31) (Atkinson 2007c) 12 weeks versus active placebo (benztropine mesylate). 0.5mg daily. (N = 26; Completer's analysis: 22). 12 weeks. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | As per Atkinson 2007 |
| Allocation concealment? | Low risk | |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Unclear risk | Stated there was no difference but no data were provided, particularly related to baseline outcome measures. |
| Co‐interventions avoided or similar? | Unclear risk | Opioids ceased for trial but not non‐opioids. Urine toxicology screens used to monitor the use of other drugs but results not reported. |
| Compliance acceptable? | Unclear risk | Only 69% retention rate. Limited detail regarding compliance. |
| Timing outcome assessments similar? | Low risk | |
Dickens 2000.
| Methods | RCT, double blind, placebo controlled. Randomization using a computer‐generated list. Sequentially numbered treatment packs were distributed by the pharmacy. | |
| Participants | 98 patients (42 men, 56 women) randomised. 61 completers. Recruitment in outpatient rheumatology clinic. Inclusion: chronic low back pain (> 6 months), significant depressive symptoms (MADRS > 15), significant disability. Exclusion: any other significant disorder, specific low back pain, recent surgery. | |
| Interventions | First week placebo.
Antidepressant (i) paroxetine 20 mg once a day, 56 days. (N = 44). Reference treatment (ii) placebo. (N = 48). |
|
| Outcomes | There were no significant difference. Mean (SD) scores at baseline and 8 weeks for: 1. Pain (VAS): (i) 55.1 (22.8), 57 (23.8), (ii) 56.1 (21.4), 57 (24.3) 2. Functional status (Oswestry): (i) 54.2 (13.7), 50.2 (15.2), (ii) 54.7 (10.3), 52.4 (13.6) 3. Depression (MADRS): (i) 28.4 (5.3), 23.2 (8.3), (ii) 26.2 (5.8), 23.3 (9.0). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using a computer‐generated list. Sequentially numbered treatment packs were distributed by the pharmacy. |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Unclear risk | Unclear from text |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | High risk | |
| Compliance acceptable? | High risk | |
| Timing outcome assessments similar? | Low risk | |
Goodkin 1990.
| Methods | RCT, double blind, placebo controlled. Randomization procedure not described. | |
| Participants | 59 patients were screened, 44 were enrolled and 42 randomised. Mean age 53.6 (± 12.9) years and average history of back pain 20.3 years. A minimum of one year continuous low back pain or two prior episodes of at least 2 weeks with a current episode of at least 2 weeks. 17 patients had a history of depression. Exclusion: Patients with major psychiatric disorder | |
| Interventions | Antidepressant (i) trazodone: 50 mg tablets, one tablet once a day for three days and then increased by one tablet every 3 days to a maximum of 4 tablets 3 times a day (600 mg/day). 6 weeks (N = 22). Reference treatment (ii) placebo (N = 20). |
|
| Outcomes | There were no significant differences. Mean (SD) scores at baseline and after 6 weeks: 1. Pain (VAS): (i) 6.45 (1.70), 5.34 (2.99), (ii) 6.51 (1.49), 5.88 (2.62) 2. Functional status (SIP physical): (i) 26.69 (15.62), 25.41 (17.04), (ii) 27.65 (10.53), 22.84 (12.72) 3. Depression (Beck): (i) 16.27 (10.39), 14.05 (11.83), (ii) 15.20 (7.01), 11.84 (7.99) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomization procedure not described. |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | High risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Low risk | |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Unclear risk | Unclear from text. |
Jenkins 1976.
| Methods | RCT, placebo‐controlled. Randomization of the treatments with the use of four stratification factors: 1. History of fracture 2. History of low back pain 3. Presence of structural abnormality 4. Type of onset | |
| Participants | 59 patients were randomised, 44 completers. 41 men and 3 women, aged 18‐49 years. Type and duration of low back pain unknown. No major depression. | |
| Interventions | Antidepressant (i) imipramine (tofranil) 25 mg three times per day for 4 weeks (N = 23). Reference treatment (ii) placebo (N = 21). |
|
| Outcomes | No significant differences after 4 weeks in pain and depression. | |
| Notes | Data only for subgroups with and without previous history of back pain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomization of the treatments with the use of four stratification factors: 1. History of fracture 2. History of low back pain 3. Presence of structural abnormality 4. Type of onset |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | High risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Low risk | |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | Low risk | |
Katz 2005.
| Methods | RCT, double blind, placebo controlled, crossover trial. Randomization using a computer‐generated list of random numbers. | |
| Participants | 54 patients were randomised. 40 completers. Low back pain for greater than 3 months. Depression status unclear. | |
| Interventions | Antidepressant (i) bupropion SR 150 mg tablets once daily for 3 days, 150 mg twice daily until the end of the 6th week (doses 8 hrs apart) and 150 mg once daily for the seventh week (N = 21). Reference treatment (ii) placebo, dosage as per treatment group (N = 23). |
|
| Outcomes | No significant differences in daily and weekly pain intensity ratings for the first treatment period. However, patients satisfaction with bupropion SR at the end of the second treatment period was greater. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using a computer‐generated list of random numbers. |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | High risk | |
| Similarity of baseline characteristics? | High risk | |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | Low risk | |
Pheasant 1983.
| Methods | RCT, double blind, placebo controlled, crossover trial. Randomization procedure not described. | |
| Participants | 32 patients screened and 16 patients randomised (4 men and 12 women; mean age 47.2 years). Mean duration of symptoms 9.9 years (range 1 to 37 years). All patients suffered from depression. 9 patients completed the trial. | |
| Interventions | Antidepressant (i) amitriptyline 50mg tablets 1 to 3 tablets a day (at once), depending on side‐effects for 6 weeks (N = 6). Reference treatment (ii) atropine (placebo) 0.2 mg tablets (N = 10). |
|
| Outcomes | Analgesics use in (i) during 6 weeks 4.7 per week vs. 8.7 per week in (ii). No data on pain intensity, overall improvement, functional status, return to work or depression. | |
| Notes | Low compliance rate: only 7 out of 16 included in analysis. Data poorly presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomization procedure not described. |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Low risk | |
| Blinding? All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | High risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Unclear risk | unclear from text |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text. |
| Compliance acceptable? | High risk | |
| Timing outcome assessments similar? | Low risk | |
Treves 1991.
| Methods | RCT, double blind, placebo controlled. Randomization procedure not described. | |
| Participants | 68 patients (33 men, 35 women; age 19 to 76 years) with acute or chronic refractory low back pain with or without sciatica. 47% with clinical depression. | |
| Interventions | Antidepressant (i) clomipramine injection IV, progressive doses for 8 days in the morning (placebo in the evening), maximum dosage (75mg) reached at 3rd day, maintained next 7 days (N = 16). Antidepressant (ii) clomipramine, injection IV, in the evening (placebo in the morning), similar to (i) (N = 25). Reference treatment: placebo (iii) in the morning and placebo in the evening, for 10 days (N = 27). | |
| Outcomes | Improvement after 10 days: (i) 16/25 (64%), (ii) 20/27 (74%), (iii) 10/16 (62.5%). (ii) statistically significantly better than (i) and (iii). | |
| Notes | Data on pain intensity not presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomization procedure not described. |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes ‐ patients? | Low risk | |
| Blinding? All outcomes ‐ providers? | Unclear risk | Unclear from text |
| Blinding? All outcomes ‐ outcome assessors? | Unclear risk | Unclear from text |
| Incomplete outcome data addressed? All outcomes ‐ drop‐outs? | High risk | |
| Incomplete outcome data addressed? All outcomes ‐ ITT analysis? | Low risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
mg = milligrams hrs = hours vs = versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brannan 2004 | This is a randomised, double‐blind placebo‐controlled study that recruited patients based on a diagnosis of major depressive disorder (as defined by the DSM‐IV*). |
| Goldstein 2004 | This study recruited patients based on a diagnosis of DSM‐IV* major depressive disorder that was confirmed by means of the Mini‐International Neuropsychiatric Interview. |
| Hameroff 1982 | This paper reported on the same trial as another paper by Hameroff which was published in 1984. |
| Hameroff 1984 | This study recruited patients with chronic neck and/or low back pain. |
| Khoromi 2007 | This study specifically recruited subjects with lumbar radiculopathy, not because they had back pain. It also had fatal flaws, reporting a drop out rate of > 45% and performing analyses on very small numbers of subjects (5‐9 per group). |
| Loldrup 1989 | This is a study on pain in general. The number of patients with low back pain was very low (< 5 for each group). |
| Pirbudak 2003a | This is a randomised trial which compared the effectiveness of epidural corticosteroid injection and amitriptyline/ epidural corticosteroid injection for chronic low back pain with radiculopathy. This study was not placebo controlled. |
| Pirbudak 2003b | This is a randomised trial which examined the effectiveness of epidural corticosteroid injection/amitriptyline and epidural corticosteroid injection/placebo for acute low back pain with lumbar disc herniation. This study was published in Turkish. |
| Schreiber 2001 | This is a randomised single‐blind trial of patients with musculoskeletal pain in which the data on whiplash associated cervical pain and low back pain were not analysed separately. |
| Stein 1996 | This randomised double‐blind trial compared antidepressants (amitriptyline) and analgesics (acetaminophen). |
| Sternbach 1976 | This is a non‐randomized, single‐blind trial in patients with chronic pain. |
| Storch 1982 | This is a non‐randomized trial including patients with specific low back pain (radicular symptoms / nerve root compression) and 23 of the 58 patients received surgery during the intervention period. |
| Ward 1986 | This is a non‐randomised double blind trial of two types of antidepressants (desipramine vs doxepin) in patients with chronic low back pain and depression. |
* Diagnostic and Statistical Manual Of Mental Disorders (4th edition)
Characteristics of ongoing studies [ordered by study ID]
Eli Lilly & Co 2006.
| Trial name or title | Protocol F1J‐MC‐HMEO: Duloxetine Versus Placebo in the Treatment of Chronic Low Back Pain |
| Methods | |
| Participants | Patients with chronic low‐back pain (n=408) |
| Interventions | Duloxetine |
| Outcomes | Not stated |
| Starting date | December 2006 |
| Contact information | Not stated |
| Notes | Main ID #: NCT00408876 |
Eli Lilly & Co 2007.
| Trial name or title | Protocol F1J‐MC‐HMEN: Effect of Duloxetine 60 mg to 120 mg Once Daily in Patients With Chronic Low Back Pain |
| Methods | |
| Participants | Patients with chronic low‐back pain (n=230) |
| Interventions | Duloxetine |
| Outcomes | Not stated |
| Starting date | January 2007 |
| Contact information | Not stated |
| Notes | Main ID #: NCT00424593 |
Contributions of authors
Van Tulder conducted the original literature search, data extraction and quality assessment. Urquhart, Hoving and van Tulder updated this review and prepared the manuscript in 2006/7 and 2008/9. Roland and Assendelft provided clinical interpretation of the results and assisted with writing of the review.
Sources of support
Internal sources
Department of Epidemiology and Preventive Medicine, Monash University, Australia.
Coronel Institute of Occupational Health and Research Centre for Insurance Medicine, Universiteit van Amsterdam, Netherlands.
Department of Public Health and Primary Care, Leiden University Medical Centre, Netherlands.
National Primary Care Research and Development Centre, University of Manchester, UK.
EMGO Institute, VU University Medical Centre, Netherlands.
Department of Health Economics and Health Technology Assessment, VU University, Netherlands.
External sources
National Health and Medical Research Council (NHMRC) Clinical Research Fellowship (284402), Australia.
Declarations of interest
One author (Maurits van Tulder) is co‐ordinating editor of the Cochrane Back Review Group. Editors are required to conduct at least one Cochrane review. This requirement ensures that editors are aware of the processes and commitment needed to conduct reviews. This involvement does not seem to be a source of conflict of interest in the Cochrane Back Review Group. Any editor who is a review author is excluded from editorial decisions on the review in which they are contributors.
Edited (no change to conclusions)
References
References to studies included in this review
Alcoff 1982 {published data only}
- Alcoff J, Jones E, Rust P, Newman R. Controlled trial of imipramine for chronic low back pain. J Fam Pract 1982;14:841‐6. [PubMed] [Google Scholar]
Atkinson 1998 {published data only}
- Atkinson HJ, Slater MA, Williams RA, Zisook S, Patterson TL, Grant I, et al. A placebo‐controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain 1998;76:287‐96. [DOI] [PubMed] [Google Scholar]
Atkinson 1999 {published data only}
- Atkinson JH, Slater MA, Wahlgren DR, Williams RA, Zisook S, Pruitt SD, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999;83:137‐45. [DOI] [PubMed] [Google Scholar]
Atkinson 1999a {published data only}
- Atkinson JH, Slater MA, Wahlgren DR, Williams RA, Zisook S, Pruitt SD, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999;83:137‐45. [DOI] [PubMed] [Google Scholar]
Atkinson 1999b {published data only}
- Atkinson JH, Slater MA, Wahlgren DR, Williams RA, Zisook S, Pruitt SD, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999;83:137‐45. [DOI] [PubMed] [Google Scholar]
Atkinson 2007 {published data only}
- Atkinson JH, Slater MA, Capparelli EV, Wallace MS, Zisook S, Abramson I, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain. Journal of Clinical Psychopharmacology 2007;27(2):135‐42. [DOI] [PubMed] [Google Scholar]
Atkinson 2007a {published data only}
- Atkinson JH, Slater MA, Capparelli EV, Wallace MS, Zisook S, Abramson I, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain. Journal of Clinical Psychopharmacology 2007;27(2):135‐42. [DOI] [PubMed] [Google Scholar]
Atkinson 2007b {published data only}
- Atkinson JH, Slater MA, Capparelli EV, Wallace MS, Zisook S, Abramson I, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain. Journal of Clinical Psychopharmacology 2007;27(2):135‐42. [DOI] [PubMed] [Google Scholar]
Atkinson 2007c {published data only}
- Atkinson JH, Slater MA, Capparelli EV, Wallace MS, Zisook S, Abramson I, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain. Journal of Clinical Psychopharmacology 2007;27(2):135‐42. [DOI] [PubMed] [Google Scholar]
Dickens 2000 {published data only}
- Dickens C, Jayson M, Sutton C, Creed F. The relationship between pain and depression in a trial using paroxetine in sufferers of chronic low back pain. Psychosomatics 2000;41:490‐9. [DOI] [PubMed] [Google Scholar]
Goodkin 1990 {published data only}
- Goodkin K, Gullion CM, Agras WS. A randomized, double‐blind, placebo‐controlled trial of trazodone hydrochloride in chronic low back pain syndrome. J Clin Psychopharmacol 1990;10:269‐78. [PubMed] [Google Scholar]
Jenkins 1976 {published data only}
- Jenkins DG, Ebbutt AF, Evans CD. Tofranil in the treatment of low back pain. J Int Med Res 1976;4:28‐40. [PubMed] [Google Scholar]
Katz 2005 {published data only}
- Katz J, Pennella‐Vaughan J, Hetzel RD, Kanazi GE, Dworkin RH. A randomised, placebo‐controlled trial of bupropion sustained release in chronic low back pain. J Pain 2005;6(10):656‐61. [DOI] [PubMed] [Google Scholar]
Pheasant 1983 {published data only}
- Pheasant H, Bursk A, Goldfarb J, Azen SP, Weiss JN, Borelli L. Amitriptyline and chronic low‐back pain. A randomized double‐blind crossover study. Spine 1983;8:552‐7. [DOI] [PubMed] [Google Scholar]
Treves 1991 {published data only}
- Treves R, Montane De La Roque P, Dumond JJ, Bertin P, Arnaud M, Desproges‐Gotteron R. Prospective study of the analgesic action of clomipramine versus placebo in refractory low back pain and sciatica (68 cases). Rev Rhum 1991;58:549‐52. [PubMed] [Google Scholar]
References to studies excluded from this review
Brannan 2004 {published data only}
- Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60mg once‐daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res 2005;39(1):43‐53. [DOI] [PubMed] [Google Scholar]
Goldstein 2004 {published data only}
- Goldstein DJ, Lu Y, Detke MJ, Hudson J, Iyengar S, Demitrack MA. Effects of duloxetine on painful physical symptoms associated with depression. Psychosomatics 2004;45(1):17‐28. [DOI] [PubMed] [Google Scholar]
Hameroff 1982 {published data only}
- Hameroff SR, Cork RC, Scherer K, Crago BR, Neuman C, Womble JR, et al. Doxepin effects on chronic pain , depression and plasma opioids. J Clin Psychiatry 1982;43:22‐7. [PubMed] [Google Scholar]
Hameroff 1984 {published data only}
- Hameroff SR, Weiss JL, Lerman JC, Cork RC, Watts KS, Crago BR, et al. Doxepin's effects on chronic pain and depression: a controlled study. Journal of Clinical Psychiatry 1984;45(3 Pt 2):47‐53. [PubMed] [Google Scholar]
Khoromi 2007 {published data only}
- Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain 2007;130:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loldrup 1989 {published data only}
- Loldrup D, Langemark M, Hansen HJ, Olesen J, Bech P. Clomipramine and mianserin in chronic idiopathic pain syndrome. A placebo controlled study. Psychopharmacology 1989;99:1‐7. [DOI] [PubMed] [Google Scholar]
Pirbudak 2003a {published data only}
- Pirbudak L, Karakurum G, Oner U, Gulec A, Karadalsi H. Epidural corticosteroid injection and amitriptyline for the treatment of chronic low back pain associated with radiculopathy. The Pain Clinic 2003;15(3):247‐53. [Google Scholar]
Pirbudak 2003b {published data only}
- Pirbudak L, Karakurum G, Satana T, Karadasli H, Topalhan M, Oner U, et al. Epidural steroid injection and amitriptyline in the management of acute low back pain originating from lumbar disc herniation. Clin Res 2003;14(2):83‐93. [Google Scholar]
Schreiber 2001 {published data only}
- Schreiber S, Vinokur S, Shavelzon V, Pick CG, Zahavi E, Shir Y. A randomised trial of fluoxetine versus amytriptyline in musculoskeletal pain. Israel Jounal of Psychiatry and Related Sciences 2001;38(2):88‐94. [PubMed] [Google Scholar]
Stein 1996 {published data only}
- Stein D, Peri T, Edelstein E, Elizur A, Floman Y. The efficacy of amitriptyline and acetaminophen in the management of acute low back pain. Psychosomatics 1996;37:63‐70. [DOI] [PubMed] [Google Scholar]
Sternbach 1976 {published data only}
- Sternbach A, Janowski DS, Huey LY, Segal DS. Effects of altering brain Serotonin activity in human chronic pain. In: JJ Bonica, D Albe‐Fessard editor(s). Advances in Pain Research and Therapy. Vol. 1, New York: Raven Press Inc., 1976:601‐6. [Google Scholar]
Storch 1982 {published data only}
- Storch H, Steck P. Antidepressive medication in the treatment of low back pain: a controlled trial [Begeleitende thymoleptische Therapie im Rahmen einer kontrollierten Studie mit Maprotilin (Ludiomil) bei der Behandlung von Kreuzschmerzen]. Nervenarzt 1982;53:445‐50. [PubMed] [Google Scholar]
Ward 1986 {published data only}
- Ward NG. Tricyclic antidepressants for chronic low‐back pain. Mechanisms of action and predictors of response. Spine 1986;11(7):661‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Eli Lilly & Co 2006 {published data only}
- Eli Lilly, Company. Protocol F1J‐MC‐HMEO: Duloxetine Versus Placebo in the Treatment of Chronic Low Back Pain. ClinicalTrials.gov 2006. [NCT00408876]
Eli Lilly & Co 2007 {published data only}
- Eli Lilly, Company. Protocol F1J‐MC‐HMEN: Effect of Duloxetine 60 mg to 120 mg Once Daily in Patients With Chronic Low Back Pain. ClinicalTrials.gov 2007. [NCT00424593 http://clinicaltrials.gov/show/NCT00424593 http://clinicaltrials.gov/show/NCT00424593]
Additional references
Broadhead 1991
- Broadhead WE, Larson DB, Yarnall KS, Blazer DG, Tse CK. Tricyclic antidepressant prescribing for nonpsychiatric disorders. An analysis based on data from the 1985 National Ambulatory Medical Care Survey. J Fam Pract 1991;33(1):24‐32. [PubMed] [Google Scholar]
Cherkin 2003
- Cherkin DC, Sherman KJ, Deyo RA, Shekelle PG. A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain. Ann Intern Med 2003;138(11):898‐906. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. New Jersey: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Cohen 2001
- Cohen SP, Abdi S. New development in the use of antidepressants for the management of pain. Curr Opin Anaesthesiol 2001;14(5):505‐11. [DOI] [PubMed] [Google Scholar]
Deyo 1987
- Deyo RA, Tsui‐Wu YJ. Descriptive epidemiology of low‐back pain and its related medical care in the United States. Spine 1987;12:264‐8. [DOI] [PubMed] [Google Scholar]
Deyo 1996
- Deyo RA. Drug therapy for back pain. Which drugs help which patients?. Spine 1996;21:2840‐50. [DOI] [PubMed] [Google Scholar]
DiIorio 2000
- Iorio D, Henley E, Doughty A. A survey of primary care physician practice patterns and adherence to acute low back problem guidelines. Arch Fam Med 2000;9:1015‐21. [DOI] [PubMed] [Google Scholar]
Gill 2002
- Gill D, Hatcher S. Antidepressants for depression in medical illness. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001312.pub2] [DOI] [PubMed] [Google Scholar]
Jadad 1997
- Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. Can Med Assoc J 1997;156(10):1411‐6. [PMC free article] [PubMed] [Google Scholar]
Koes 2001
- Koes BW, Tulder MW, Ostelo R, Burton KA, Waddell G. Clinical guidelines for the management of low back pain in primary care: an international comparison. Spine 2001;26:2504‐13. [DOI] [PubMed] [Google Scholar]
Micó 2006
- Micó JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci 2006;27(7):348‐54. [DOI] [PubMed] [Google Scholar]
Mir 1997
- Mir S, Taylor D. The adverse effects of antidepressants. Curr Opin Psychiatry 1997;10:88‐94. [Google Scholar]
O'Malley 2000
- O'Malley PG, Balden E, Tomkins G, Santoro J, Kroenke K, Jackson JL. Treatment of fibromyalgia with antidepressants: a meta‐analysis. J Gen Intern Med 2000;15 (9):659‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Saarton 2005
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005454.pub2] [DOI] [PubMed] [Google Scholar]
Salerno 2002
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta‐analysis. Arch Intern Med 2002;162:19‐24. [DOI] [PubMed] [Google Scholar]
Schnitzer 2004
- Schnitzer TJ, Ferraro A, Hunsche E, Kong SX. A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manage 2004;28(1):72‐95. [DOI] [PubMed] [Google Scholar]
Schreiber 2001a
- Schreiber S, Vinokur S, Shavelzon V, Pick CG, Zahavi E, Shir Y. A randomised trial of fluoxetine versus amytriptyline in musculoskeletal pain. Is Jn Psychiatry Relat Sci 2001;38(2):88‐94. [PubMed] [Google Scholar]
Sindrup 2005
- Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96 (6):399‐409. [DOI] [PubMed] [Google Scholar]
Spitzer 1987
- Spitzer WO, LeBlancd FE, Depuis M (Eds). Scientific approach to the assessment and management of activity‐related spinal disorders. Spine 1987;7(Suppl.):1‐59. [PubMed] [Google Scholar]
Stahl 1998
- Stahl SM. Basic psychopharmacology of antidepressants: Antidepressants have seven distinct mechanisms of action. J Clin Psychiatry 1998;59(Suppl. 4):5‐14. [PubMed] [Google Scholar]
Staiger 2003
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine 2003;28(22):2540‐5. [DOI] [PubMed] [Google Scholar]
van Tulder 1997a
- Tulder MW, Koes BW, Bouter LM. Conservative treatment of acute and chronic non‐specific low back pain: a systematic review of randomised controlled trials of the most common interventions. Spine 1997;22:2128‐56. [DOI] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Waddell 1987
- Waddell G. A new clinical model for the treatment of low back pain. Spine 1987;12:632‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Urquhart 2008
- Urquhart D, Hoving JL, Assendelft WJJ, Roland M, Tulder MW. Antidepressants for non‐specific low back pain. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]


