Abstract
Background
Steroids have been used widely since the early 1970s for the treatment of adult‐onset minimal change disease. The response rates to immunosuppressive agents in adult minimal change disease, especially steroids, are more variable than in children. The optimal agent, dose, and duration of treatment for the first episode of nephrotic syndrome, or for disease relapse(s) has not been determined.
Objectives
To determine the benefits and harms of interventions for the nephrotic syndrome in adults caused by minimal change disease.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, reference articles and abstracts from conference proceedings, without language restriction. Search date: January 2007.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of any intervention for minimal change disease in adults over 18 years with the nephrotic syndrome were included. Studies comparing different routes, frequencies, and duration of immunosuppressive agents were selected. Studies comparing non‐immunosuppressive agents were also assessed.
Data collection and analysis
Two authors independently assessed study quality and extracted data. Statistical analyses were performed using the random effects model and results were expressed as a risk ratio (RR) for dichotomous outcomes, or mean difference (MD) for continuous data with 95% confidence intervals (CI).
Main results
Three RCTs (68 participants) were identified. All treatment comparisons contained only one study. No significant difference was found between prednisone compared with placebo for complete (RR 1.44, CI 0.95 to 2.19) and partial remission (RR 1.00, CI 0.07 to 14.45) of the nephrotic syndrome due to minimal change disease. There was no difference between intravenous methylprednisolone plus oral prednisone compared with oral prednisone alone for complete remission (RR 0.74, CI 0.50 to 1.08). Prednisone, compared with short‐course intravenous methylprednisolone, increased the number of subjects who achieved complete remission (RR 4.95, CI 1.15 to 21.26). The lack of statistical evidence of efficacy associated with prednisone therapy was based on data derived from a single study that compared 'alternate‐day prednisone' to no immunosuppression' with only a small number of participants in each group. No RCTs were identified comparing regimens in adults with a steroid‐dependent or relapsing disease course or comparing treatments comprising alkylating agents, cyclosporine, tacrolimus, levamisole, or mycophenolate mofetil.
Authors' conclusions
Further comparative studies are required to examine the efficacy of immunosuppressive agents for achievement of sustained remission of nephrotic syndrome caused by minimal change disease. Studies are also needed to evaluate treatments for adults with steroid‐dependent or relapsing disease.
Plain language summary
Interventions for minimal change disease in adults with nephrotic syndrome
Nephrotic syndrome is a condition where the kidneys leak protein from the blood into the urine. Minimal change disease is the third most common primary kidney disease in adults with unexplained nephrotic syndrome (10% to 15%). Steroids have been used widely since the early 1970s for the treatment of adult‐onset minimal change disease, however the optimal agent, dose and duration has not been determined. This review identified three small studies (68 participants) comparing: 1) intravenous plus oral steroid treatment versus oral sterids; 2) oral versus short‐course intravenous steroid treatment; and 3) oral steroid treatment versus placebo. Only oral steroid treatment (compared to short‐course intravenous steroid treatment) showed an increase in the number of patients who achieved complete remission. However, the lack of available studies leaves important treatment questions unanswered; what is the optimal dose and duration of steroid treatment in new‐onset adult minimal change disease; how are relapses following steroid‐induced remission prevented and treated; and what are the appropriate treatments for steroid‐dependent or treatment‐resistant minimal change disease?
Background
Nephrotic syndrome is a clinical condition where the glomeruli of the kidney leak protein from the blood into the urine. It is characterised by often severe generalised oedema and hypoproteinaemia and, if untreated, is associated with considerable morbidity. The causes of nephrotic syndrome are either a primary renal process, or a result of injury to the kidney through systemic diseases, most commonly diabetes mellitus. Minimal change disease is the third most common primary kidney disease in adults with unexplained nephrotic syndrome (10% to 15%) (Haas 1995; Korbet 1996), after membranous nephropathy (30% to 40%) and segmental sclerosing glomerular disorders (20% to 30%) (Haas 1997). In fact, minimal change disease may be diagnosed in cases of focal and segmental glomerulosclerosis due to sampling errors in the kidney biopsy process. The incidence of minimal change disease varies depending on the population studied and is reported as 1/1,000,000 in the UK compared with 27/1,000,000 in the USA (Johnson 2003). Adult‐onset minimal change disease is associated with kidney failure in 33% of patients, hypertension in 35%, microscopic haematuria in 47%, and hypercholesterolaemia in 96% (Nakayama 2002). Kidney biopsy is mandatory for a diagnosis of minimal change disease in adults with the nephrotic syndrome.
The kidney biopsy in minimal change disease reveals no, or only minor, changes on light microscopy. The abnormality is the fusion of the foot processes of the podocyte cell which normally forms an impermeable barrier to protein as part of the glomerular membrane that controls the urinary filtrate. While the precise pathogenesis of minimal change disease is yet to be clarified there is increasing evidence that T lymphocytes, and probably other immune cells, are involved in the disease and may produce cytokines that alter the normal glomerular filtration membrane that prevents proteinuria (Grimbert 2003).
The response rates to immunosuppressive agents in adult minimal change disease, especially steroids, are more variable than in children. Uncontrolled studies have suggested that the response rates to steroids in adults is "delayed" by 8 to 16 weeks compared to that demonstrated in children and the reasons for this delay are poorly understood. The development of minimal change disease in those older than 40 years may be characterised by increased rates of kidney impairment and hypertension, although these features may simply reflect age‐related changes (Tse 2003). The most efficacious treatment to achieve sustained remission of proteinuria in adults is less well defined than for children. Relapse of the nephrotic syndrome after remission may occur and refers to the recurrence of an abnormal urine protein excretion rate, with or without oedema. Spontaneous remission is infrequent, although prior to the widespread use of corticosteroids a spontaneous rate of remission was reported in over half of patients in the first two years after diagnosis (Black 1970). Steroids have been used widely since the early 1970s for the treatment of adult‐onset minimal change disease (Nolasco 1986). The response rate to corticosteroids is slower in adults than in children where remission can be achieved in 37% to 50% within four weeks, 51% to 76% within eight weeks, and 76% to 97% within 16 weeks. However, up to 10% of patients may fail to achieve complete remission with corticosteroid therapy alone and require additional immunosuppression. After an initial complete response, up to two‐thirds of adults treated with steroids alone will relapse on one or more occasions, and approximately 25% become frequent relapsers (Nakayama 2002). Many patients develop steroid dependency. Older patients may have different remission rates to corticosteroids (Korbet 1996; Nakayama 2002).
Other agents active against the immune system have been employed to prolong periods of remission or reduce corticosteroid exposure. In adults, alkylating agents (cyclophosphamide and chlorambucil) (Al Khader 1979; Mak 1996) have been tested for the treatment of glomerular diseases. More recently, agents used in transplantation including cyclosporine, (Matsumoto 2004; Meyrier 1991; Woo 2001), tacrolimus (Patel 2005; Schweda 1997), sirolimus (Patel 2005), and mycophenolate mofetil (MMF) (Briggs 1998; Day 2002; Mogyorosi 2002) have all been reported in patients with either steroid‐resistant or frequently relapsing nephrotic syndrome. Adverse effects from immunosuppression may be considerable, depending on the agent used, and include infection, malignancy, peptic ulceration, diabetes mellitus, infertility, kidney failure, bone marrow suppression, hypertrichosis, and alopecia. Other nonspecific supportive treatments to reduce proteinuria and ameliorate the morbidity of the nephrotic syndrome are reported, including angiotensin‐converting enzyme (ACE) inhibitors (Arora 2002; Dilek 1999), non‐steroid anti‐inflammatory drugs (NSAIDs), heparinoids, and hydroxymethylglutaryl coenzyme A (HMG Co‐A) reductase inhibitors (Deighan 2001; Olbricht 1999).
The treatment of adult‐onset minimal change disease appears to have developed without consensus and the most effective second‐line immunosuppressive agent to treat steroid‐resistant nephrotic syndrome or frequently relapsing disease appears uncertain. Initial therapy is most commonly in the form of corticosteroids alone but strong evidence is lacking for the treatment of steroid‐resistant, relapsing or steroid‐dependent subjects. This systematic review examined the existing randomised controlled trials (RCTs) for the benefits and harms of interventions for the nephrotic syndrome caused by minimal change disease in adults to identify areas that require further research. The incidence and nature of treatment related toxicity were emphasised.
Objectives
To evaluate the benefits and harms of different agents, including both immunosuppressive and non‐immunosuppressive agents, in adults with minimal change disease causing the nephrotic syndrome.
To evaluate the efficacy of interventions on 'time‐to‐remission' of nephrotic syndrome, in adults with minimal change disease causing the nephrotic syndrome.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at any intervention for minimal change disease in adults with the nephrotic syndrome were included. Immunosuppressive agents included corticosteroids, cyclophosphamide, chlorambucil, azathioprine, cyclosporine, tacrolimus (FK‐506), sirolimus (target of rapamycin inhibitors (TOR‐I; sirolimus and everolimus), and MMF. Non‐immunosuppressive agents included NSAIDs, ACE inhibitors, angiotensin‐receptor antagonists, heparinoids, parenteral albumin and HMG Co‐A reductase inhibitors. The first period of randomised cross‐over studies were included.
Types of participants
Inclusion criteria
RCTs enrolling adults (> 18 years) with the nephrotic syndrome and biopsy‐proven minimal change disease were included. Nephrotic syndrome was defined as proteinuria >3.0 g/24 h, oedema and hypercholesterolaemia.
Exclusion criteria
Studies enrolling paediatric patients were excluded as these were the subject of previously published reviews (Durkan 2005; Hodson 2006; Hodson 2007). Studies enrolling patients who had any type of segmental sclerosing abnormality on kidney biopsy (focal and segmental glomerulosclerosis (FSGS), collapsing variant of FSGS) were excluded. Any RCT enrolling patients with secondary minimal change nephropathy (e.g. related to drug therapy) were excluded.
Types of interventions
All immunosuppressive agents were considered and included the following:
Corticosteroid agent versus placebo or no treatment.
Different doses and/or durations and routes of administration of corticosteroid treatments.
Non‐corticosteroid immunosuppressive agent (with or without concomitant corticosteroid treatment) versus corticosteroid agent alone. These non‐corticosteroid agents included azathioprine, cyclophosphamide, chlorambucil, cyclosporine, tacrolimus, sirolimus, levamisole and mycophenolate mofetil.
Comparisons between two different non‐corticosteroid agents (with or without concomitant steroid agent).
Different doses, durations, and routes of the same non‐corticosteroid immunosuppressive agent (with or without concomitant use of corticosteroid agent).
All studies where participants were randomised to a non‐immunosuppressive agent were included. These included:
Non‐immunosuppressive agent versus placebo or no treatment.
Immunosuppressive agent versus non‐immunosuppressive agent.
Comparisons between two different non‐immunosuppressive agents.
Types of outcome measures
Primary outcome measure
Number of patients who achieved complete remission during and following therapy (i.e. oedema free and proteinuria < 1+ (on dipstick), or protein:creatinine < 0.03 g/mmol or complete remission as defined by the investigators).
Secondary outcome measures
Number of patients who achieve partial remission with reduction in proteinuria (i.e. proteinuria < 2+ on dipstick, urine protein:creatinine ratio < 0.3 g/mmol, protein excretion < 3 g/d or partial remission as defined by the triallists).
End of treatment increase in serum albumin ≥ 30 g/L.
End of treatment loss of oedema.
Time to remission (days) of nephrotic syndrome.
End of treatment reduction in total cholesterol to ≤ 5.5 mmol/L.
Doubling of serum creatinine.
End stage kidney disease (GFR ≤ 15 mL/min or requiring renal replacement therapy).
One or more episodes of thrombosis.
All‐cause mortality.
Fatal and non‐fatal cardiovascular events (myocardial infarction, stroke, revascularisation).
Adverse events including major infection requiring parenteral antibiosis or hospitalisation, all‐cause infection, hypertension, malignancy, kidney failure (as defined by the triallists or a rise in the plasma creatinine > 0.03 mmol/L or a rise in the estimated glomerular filtration rate > 25%), diabetes mellitus/impaired glucose tolerance, gonadal failure (sustained amenorrhoea or infertility), bone toxicity (avascular necrosis or fracture), bone marrow toxicity, bladder toxicity (haemorrhagic cystitis), hypertrichosis, gingival hyperplasia, alopecia, peptic ulceration.
The following continuous variables were analysed:
End of treatment mean serum creatinine (mmol/L).
End of treatment mean protein excretion rate (g/24 h) or protein:creatinine ratio (g/mmol).
End of treatment mean serum albumin (g/L).
End of treatment mean serum total cholesterol (mmol/L).
End of treatment mean serum LDL cholesterol/HDL cholesterol/triglycerides (all mmol/L) and HDL:LDL cholesterol ratio.
Protein excretion rate at 6, 12 and 24 months (g/24 h) after treatment.
Duration of complete remission or partial remission (months).
Search methods for identification of studies
Relevant studies were obtained from the following sources without language restriction (Appendix 1 ‐ Electronic search strategies):
The Cochrane Renal Group's specialised register (January 2007) and the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library issue 1, 2007). CENTRAL and the Renal Group's specialised register contain the hand searched results of conference proceedings from general and speciality meetings. This is an ongoing activity across the Cochrane Collaboration and is both retrospective and prospective (Master List 2007).
MEDLINE (1966 to January 2007) using the optimally sensitive strategy developed for the Cochrane Collaboration for the identification of RCTs (Dickersin 1994) with a specific strategy developed with input from the Cochrane Renal Group Trials Search Coordinators.
EMBASE (1980 to January 2007) using a search strategy adapted from that developed for the Cochrane Collaboration for the identification of RCTs (Lefebvre 1996) together with a specific search strategy developed with input from the Cochrane Renal Group Trials Search Coordinators.
Reference lists of nephrology textbooks, review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Included and excluded studies
The review was undertaken independently by three authors (SCP, KN, GFMS). The search strategy was used to obtain titles and abstracts of studies that might be relevant to the review. The titles and abstracts were screened independently by (SCP) and (KN), who discarded studies that were not applicable. Studies and reviews that might include relevant data or information on studies were retained initially. Reviewers (SCP) and (KN) independently assessed retrieved abstracts and, if necessary, the full text of these studies, to determine which studies satisfied the inclusion criteria. Data extraction was carried out independently by the same authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Any further information to clarify study reporting or to request additional information from the original author was be requested by written correspondence and any relevant information obtained in this manner was be included in the review. Disagreements were resolved in consultation with (GFMS).
Study quality
The quality of studies included was assessed independently by (SCP) and (KN) without blinding to authorship or journal using the checklist developed for the Cochrane Renal Group. Discrepancies were resolved by discussion with GFMS. The quality items assessed were allocation concealment, blinding (participants, investigators, outcome assessors and data analysis), intention‐to‐treat analysis and completeness of follow‐up.
Quality checklist
Allocation concealment
Adequate (A): Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study.
Unclear (B): Randomisation stated but no information on method used is available.
Inadequate (C): Method of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group.
Blinding
Blinding of investigators: Yes/no/not stated
Blinding of participants: Yes/no/not stated
Blinding of outcome assessor: Yes/no/not stated
Blinding of data analysis: Yes/no/not stated
The above are considered not blinded if the treatment group can be identified in > 20% of participants because of the side effects of treatment.
Intention‐to‐treat analysis
Yes: Specifically reported by authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment.
Yes: Not stated but confirmed on study assessment.
No: Not reported and lack of intention‐to‐treat analysis confirmed on study assessment. (Patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation).
No: Stated but not confirmed upon study assessment.
Not stated
Completeness of follow‐up
Per cent of participants excluded or lost to follow‐up.
Statistical assessment
For dichotomous outcomes (complete remission, partial remission, doubling of serum creatinine or end‐stage kidney disease, adverse events) results were expressed as a risk ratio (RR) with 95% confidence intervals (CI). Data were pooled using the Der Simonian Laird random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers. Where continuous scales of measurement were used to assess the effects of treatment (proteinuria, kidney outcomes, duration of remission), the mean difference (MD) was used. Heterogeneity was analysed, where applicable, using a chi squared test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003).
Subgroup analysis was planned to explore any possible sources of heterogeneity (e.g. participants, treatments and study quality). Heterogeneity among participants could have been related to; age (dichotomised to between 18‐40 years and greater than 40 years), kidney function, evidence of glomerulosclerosis on biopsy, haematuria, hypertension, response to corticosteroids, characteristics of clinical course (partial remission, frequently relapsing disease, steroid dependence, duration of NS), severity of NS, severity of hyperlipidaemia. Heterogeneity in treatments could have been related to prior agent(s) used and the agent, dose and duration of therapy. Adverse effects were tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used (Table 4). Where possible, the risk difference with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
1. Adverse events.
| Study | Treatment Group | Number of participants | Adverse events |
| Coggins 1985 | Prednisone | 14 | Psychosis (1); avascular necrosis (1) |
| Coggins 1985 | No treatment | 14 | Doubling of serum creatinine (3); renal replacement therapy (1) |
| Imbasciati 1985 | Prednisone | 11 | Thrombosis (1); death (1) |
| Imbasciati 1985 | Methylprednisone and prednisone | 11 | Pulmonary embolism (1) |
| Yeung 1983 | Methylprednisone | 10 | ‐ |
| Yeung 1983 | Prednisone | 8 | Gastroenteric bleed (1) |
Insufficient RCTs were identified to enable examination for publication bias using a funnel plot (Egger 1997).
Results
Description of studies
Figure 1‐ Flow chart of study identification and selection) details the progress through the phases of this systematic review. From the Cochrane Renal Group's specialised register and CENTRAL, 91 citations were received; from MEDLINE 697 citations, and from EMBASE 3057 citations. Of these 3845 potentially eligible publications 3781 were excluded after title and abstract review. The primary reasons for exclusion of citations were a non‐randomised design, not minimal change disease, or a paediatric study population. Full‐text analysis of the remaining 64 publications found three RCTs for inclusion in this review (Coggins 1985; Imbasciati 1985; Yeung 1983). The total number of adult patients randomised in the included studies was 68. No authors responded to our written requests for unpublished study data. Study characteristics are presented in the table Characteristics of included studies.
1.
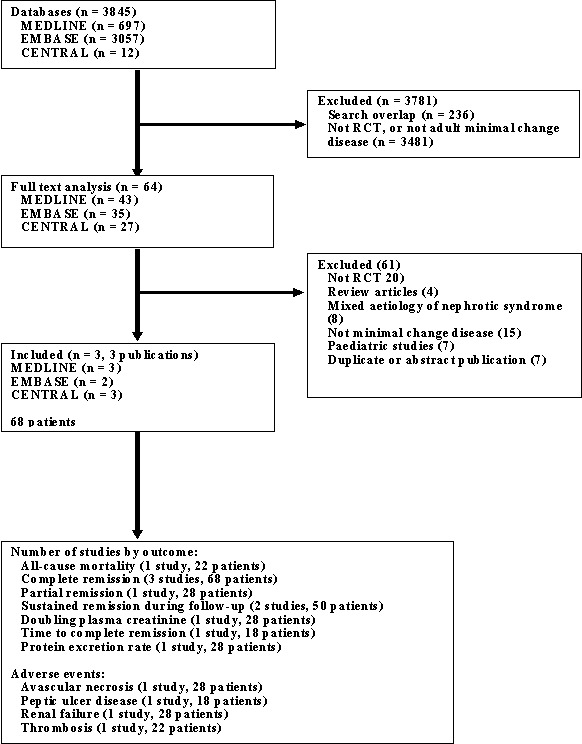
Flow chart of study identification and selection
A number of studies comparing treatment regimens containing cyclosporine, cyclophosphamide and high dose steroids for primary disease, and poorly responsive or relapsing disease were excluded because they were observational, included children, or included heterogenous causes of the nephrotic syndrome. The details of these are presented in the table Characteristics of excluded studies.
Interventions
Coggins 1985 (28 participants) compared oral prednisone (125 mg alternate daily) with no treatment, Yeung 1983 (18 participants) compared intravenous bolus methylprednisolone alone with oral prednisone, and Imbasciati 1985 (22 participants) compared intravenous methylprednisolone followed by oral prednisone with oral prednisone alone.
Diagnoses
The three included studies were published over 20 years prior to this review. Yeung 1983 randomised people with a first episode of biopsy‐proven minimal change disease. Imbasciati 1985 enrolled adults with the nephrotic syndrome, persisting for at least two weeks. Patients in this study included those with previous episodes of the nephrotic syndrome who were included only when they had achieved a complete remission with steroids at least one year previously. In Coggins 1985 patients had experienced the nephrotic syndrome for a mean of two months, although it was unclear whether participants were enrolled during their first or subsequent episodes of nephrotic syndrome.
Outcomes
Coggins 1985 reported a pretreatment mean level of proteinuria of 9.8 g/d, whereas the degree of proteinuria prior to randomisation was unclear for Imbasciati 1985 and Yeung 1983. The reporting of biopsy data prior to enrolment was poor and participants with sclerosing lesions were included in the studies. Imbasciati 1985 reported evidence of focal glomerular obsolescence in 2/22 participants. Yeung 1983 reported a single repeat kidney biopsy showing focal and segmental glomerulosclerosis following a poor response to intervention (oral prednisone) despite an entry biopsy showing normal glomeruli. Coggins 1985 did not detail biopsy findings. The age of participants was 30 years in Coggins 1985 and ranged up to 56 years in Imbasciati 1985 and Yeung 1983. Complete remission events were reported in all studies. Relapses and non‐response to treatment were described inconsistently. Imbasciati 1985 and Yeung 1983 detailed one or more episodes of relapse (including time to relapse) and non‐responders to treatment, while Coggins 1985 reported the non‐responders to intervention, however the number of patients who experienced relapse of the nephrotic syndrome during follow up was unclear. This study also reported patients continuing to have 1 g or more proteinuria during months of follow up (Coggins 1985). Follow‐up was up to 840 days (Yeung 1983), 52.5 months (Imbasciati 1985) and 24 months (Coggins 1985).
Cointerventions
Cointerventions included diuretics (Coggins 1985; Imbasciati 1985; Yeung 1983), a low‐salt diet (Imbasciati 1985) and antihypertensive treatment (Imbasciati 1985).
Risk of bias in included studies
The quality of studies was difficult to assess because many details such as methods of allocation concealment, the use of intention‐to‐treat analysis, methods of blinding and the number of patients lost to follow‐up were difficult to ascertain or were not provided. In general, study quality was variable and reporting of study method details was unsatisfactory or incomplete. Of note, all included studies preceded the CONSORT guidelines for reporting of RCTs (Begg 1996).
Allocation concealment
Allocation concealment was unclear in all three studies.
Blinding
Blinding of participants and investigators was not used in Imbasciati 1985 or Yeung 1983, and was unclear in Coggins 1985. Blinding of outcome assessors was not described in any study.
Intention‐to‐treat analysis
Intention‐to‐treat analysis was used in Imbasciati 1985, not stated in Coggins 1985, and not used in Yeung 1983.
Completeness of follow‐up
No participants were lost to follow‐up in Coggins 1985 or Imbasciati 1985, and the loss to follow‐up was not stated in Yeung 1983.
Effects of interventions
The identified treatment comparisons were steroid versus no treatment (comparison 01), intravenous steroid plus oral steroid versus oral steroid alone (comparison 02) and intravenous steroid versus oral steroid (comparison 03). No other treatment modalities were assessed. Each treatment comparison contained a single study, and therefore no meta‐analyses were possible.
Steroid versus placebo or no treatment
Coggins 1985 compared alternate daily oral prednisone 125 mg with no treatment. Treatment was continued for two months. Prednisone did not increase the number of patients who achieved complete remission compared with no treatment (Analysis 1.1: RR 1.44, 95% CI 0.95 to 2.19). No difference was found between treatment groups for the achievement of partial remission (Analysis 1.2: RR 1.00, 95% CI 0.07 to 14.45) or sustained remission (Analysis 1.3: RR 2.50, 95% CI 0.58 to 10.80). The likelihood of resolution of oedema was similar between the two treatment groups (Analysis 1.4: RR 1.21, 95% CI 0.89 to 1.66). The incidence of adverse events (Analysis 1.5) was similar for each treatment group including for avascular necrosis (RR 3.00, 95%CI 0.13 to 67.91), doubling of serum creatinine (RR 0.11, 95% CI 0.01 to 1.89), and renal failure (RR 0.33, 95% CI 0.01 to 7.55). The four patients who experienced doubling of serum creatinine were randomised to no treatment and three achieved subsequent remission with steroid treatment. The number of patients in each group who relapsed during the follow‐up period was unclear.
1.1. Analysis.
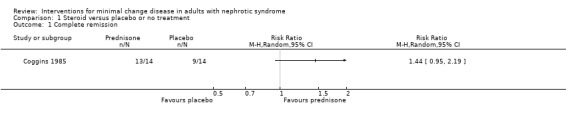
Comparison 1 Steroid versus placebo or no treatment, Outcome 1 Complete remission.
1.2. Analysis.
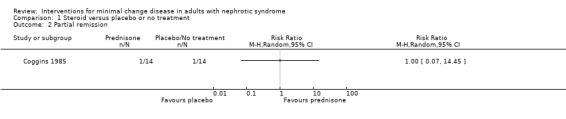
Comparison 1 Steroid versus placebo or no treatment, Outcome 2 Partial remission.
1.3. Analysis.
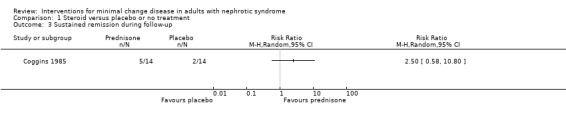
Comparison 1 Steroid versus placebo or no treatment, Outcome 3 Sustained remission during follow‐up.
1.4. Analysis.
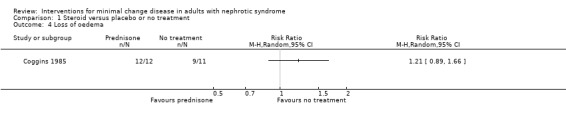
Comparison 1 Steroid versus placebo or no treatment, Outcome 4 Loss of oedema.
1.5. Analysis.
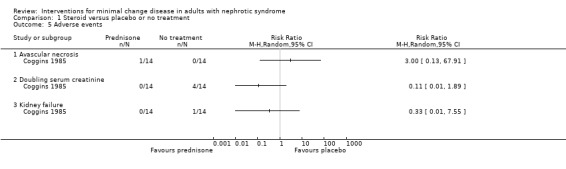
Comparison 1 Steroid versus placebo or no treatment, Outcome 5 Adverse events.
Intravenous and oral steroid versus oral steroid alone
Imbasciati 1985 randomly assigned participants to either methylprednisolone 20 mg/kg/d for three days then oral prednisone 0.5 mg/kg/d for four weeks, 0.5 and 0.25 mg/kg alternate days for four weeks, then 0.5 mg/alternate days for four months, or treatment with oral prednisone 1 mg/kg/d for four weeks, then 1 mg/kg/alternate days for four weeks, then 0.5 mg/kg on alternate days for four months. Relapses during treatment were treated according to the initial treatment allocation. No significant difference was seen following treatment with intravenous and oral steroid compared with steroid alone for complete remission (Analysis 2.1: RR 0.73, 95% CI 0.50 to 1.08), sustained remission during follow up (Analysis 2.2: RR 0.50, 95% CI 0.11 to 2.19), all‐cause mortality Analysis 2.3: RR 0.33, 95% CI 0.02 to 7.39) or adverse events (thrombosis) (Analysis 2.4.1: RR 2.00, 95% CI 0.21 to 18.98). Six patients in the intravenous plus oral steroid group experienced 13 relapses compared with seven patients who developed 11 relapses in the steroid alone group (Analysis 2.5; RR 1.18, 95% CI 0.65 to 2.15).
2.1. Analysis.
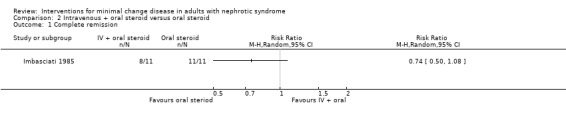
Comparison 2 Intravenous + oral steroid versus oral steroid, Outcome 1 Complete remission.
2.2. Analysis.
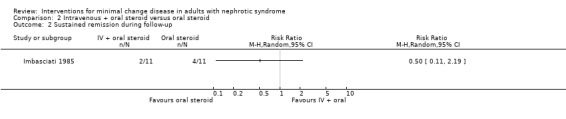
Comparison 2 Intravenous + oral steroid versus oral steroid, Outcome 2 Sustained remission during follow‐up.
2.3. Analysis.
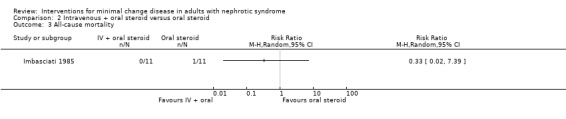
Comparison 2 Intravenous + oral steroid versus oral steroid, Outcome 3 All‐cause mortality.
2.4. Analysis.
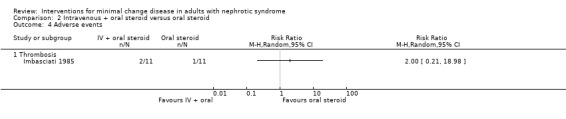
Comparison 2 Intravenous + oral steroid versus oral steroid, Outcome 4 Adverse events.
2.5. Analysis.
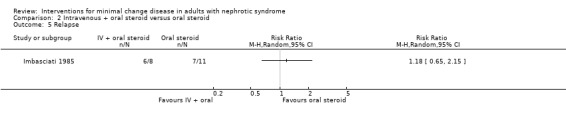
Comparison 2 Intravenous + oral steroid versus oral steroid, Outcome 5 Relapse.
Intravenous steroid versus oral steroid
Yeung 1983 randomised participants to either intravenous methylprednisolone 20 mg/kg/d on three consecutive days or to oral prednisone 1 mg/kg/d given for four to six weeks. Patients in the intravenous methylprednisolone group were administered oral prednisone if they failed to respond to initial treatment within two weeks. Oral prednisone was associated with a significant increase in episodes of complete remission (Analysis 3.1: RR 4.95, 95%CI 1.15 to 21.26). Four of the six participants (all randomised to intravenous methylprednisolone) who did not achieve complete remission within two weeks, and were subsequently administered oral prednisone, experienced complete remission 14 to 31 days after commencing oral prednisone. One of the non‐responders demonstrated FSGS on a follow up biopsy. Time to complete remission (days) was similar between study groups (Analysis 3.2: MD 3.17, 95% CI ‐9.21 to 15.55). No adverse events were reported for adult participants during follow‐up.
3.1. Analysis.
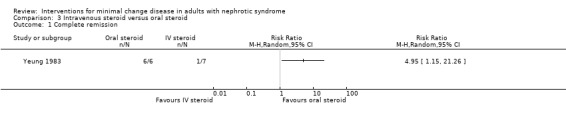
Comparison 3 Intravenous steroid versus oral steroid, Outcome 1 Complete remission.
3.2. Analysis.
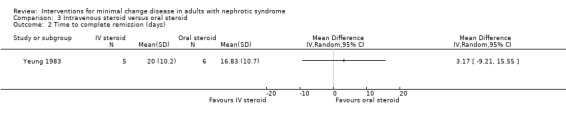
Comparison 3 Intravenous steroid versus oral steroid, Outcome 2 Time to complete remission (days).
Subgroup analyses or assessment for publication bias were not possible. No ongoing studies were identified.
Adverse events
Adverse events for all treatment regimens are detailed in Table 4 ‐ Adverse events.
Discussion
All available RCTs in adults with minimal change disease evaluate treatment of recent onset nephrotic syndrome and compare prednisone with another steroid regime or with no treatment. The review identified three vastly different RCTs performed over two decades, on small numbers of participants without adequate power to detect differences in therapeutic efficacy. This suggests these original studies have little relevance to contemporary treatment of minimal change disease in adults. RCT data for treatment of relapsing disease or steroid‐resistant disease are absent. The likelihood of achieving remission within two months of presentation did not differ between alternate‐day prednisone and no immunosuppression in one study, or between intravenous steroid followed by oral prednisone and oral prednisone alone in another study. The lack of superior efficacy for prednisone over placebo for achievement of remission is surprising and probably explained by the spontaneous remission rate in the small group (N = 14) prescribed no treatment, such that there was no difference in complete remission between groups at the end of the study (Coggins 1985). In children, studies of steroid‐sensitive nephrotic syndrome using either higher doses of steroid or an increased duration of steroid therapy lead to prolonged remission from the nephrotic syndrome (Hodson 2007). This is echoed by the finding in this review of a single RCT in adults where continuous oral prednisone induced complete remission better than short‐course (three day) intravenous steroids, although the small patient numbers could not exclude the possibility that this treatment advantage was due to chance alone.
Adult minimal change disease is steroid‐responsive in approximately 80% of cases (Meyrier 1988) although up to two‐thirds of adults undergo a relapsing course, and steroid‐sensitive forms may become steroid‐dependent. In such cases, repeated or continuous courses of corticosteroid treatment increases the likelihood of glucocorticoid toxicity. In relapsing paediatric nephrotic syndrome, daily prednisone treatment, cyclophosphamide, chlorambucil, levamisole, and cyclosporine all significantly reduce the risk of subsequent relapse (Durkan 2005). No RCT data are available to guide similar treatment for adults, particularly for those who experience relapse during or after initial treatment, for those who frequently relapse, or for those who develop steroid dependency. Agents such as cyclosporine (Matsumoto 2004), tacrolimus (Westhoff 2006) cyclophosphamide, and mycophenolate mofetil (Mogyorosi 2002) are evaluated in uncontrolled studies of adult nephrotic syndrome, and may be associated with significant toxicity, including kidney impairment (Ponticelli 1993; Sharpstone 1969; Tejani 1988; Uldall 1972). The lack of available studies leaves important treatment questions unanswered; what is the optimal dose and duration of steroid treatment in new‐onset adult minimal change disease; how are relapses following steroid‐induced remission prevented and treated; and what are the appropriate treatments for steroid‐dependent or treatment‐resistant minimal change disease?
The absence of evidence to guide management of adults with the nephrotic syndrome due to minimal change disease is in direct contrast to the available data for children with the nephrotic syndrome, summarised in three separate Cochrane systematic reviews (Durkan 2005; Hodson 2006; Hodson 2007). Adult minimal change disease is a rarer condition and this rarity has presented a vital barrier to the conduct of adequately powered studies for this population. The incidence of (all‐cause) nephrotic syndrome in children approaches 2/100,000, probably an order of magnitude more frequent than the condition in adults (Arneil 1961). Moreover, studies in adults with minimal change disease, published in the 1970's, showed an "early and dramatic" decrease in proteinuria following prednisone therapy (Black 1970; Gulati 1973). This profound "before and after" evidence to support the use of steroids in adult minimal change disease lead to the widespread adoption of this empirical approach. No subsequent adequately powered studies to examine the efficacy and toxicity of steroids were conducted. The low incidence of the disease in adults has also prevented adequate RCTs to evaluate treatment for steroid‐dependent or relapsing disease
The combination of causes for nephrotic syndrome together into RCTs has commonly occurred for adults. Frequently adults with minimal change disease have been combined in interventional studies with adults with focal and segmental glomerulosclerosis (Lee 1995; Meyrier 1988; Ponticelli 1993), membranous nephropathy (Black 1970; Mansy 1989), and membrano‐proliferative disease (Gulati 1973). In order to maximise recruitment previous studies have also enrolled both adults and children with minimal change disease into the same study. This approach is problematic as the study populations are necessarily heterogenous and the applicability of such research findings to individuals with adult minimal change disease is limited. This is particularly true where adults are enrolled in studies with children, and where patients with sclerosing glomerular lesions are combined with patients with minimal change disease. The heterogenous response of these different populations to treatment demands that any future studies enrol adults with biopsy‐proven minimal change disease alone.
An international collaborative group to organise multicentre studies of therapy in adult glomerular disease would facilitate the conduct of such studies. The funding of the European Vasculitis Study Group in 1993 is such an example of a collaborative international network coordinated with a central secretariat to ensure appropriate studies of rare diseases are conducted in vasculitis (EUVAS 2006). This group has published a large body of well‐conducted research to guide therapy for vasculitis. For primary glomerulopathies a similar collaborative network could facilitate international studies of treatments that have previously been shown to be efficacious in children and in observational studies in adults. In adults with new‐onset nephrotic syndrome comparisons of different durations and doses of corticosteroids should be considered. Non‐corticosteroid based immunosuppression should be assessed in steroid‐dependent, steroid‐resistant, and relapsing disease, focusing on the duration of remission, kidney outcomes, and treatment‐related toxicity.
Authors' conclusions
Implications for practice.
This review has generated no evidence to support the efficacy of any agent for induction or prolongation of remission for adults with the nephrotic syndrome caused by minimal change disease. The lack of statistical evidence of efficacy associated with prednisone therapy was based on data derived from a single study that compared 'alternate‐day prednisone' to no immunosuppression' with only a small number of participants in each group. The results should be treated with caution due to the small numbers of studies available. No information is available to guide the use of non‐steroid immunosuppression either following the first presentation of nephrotic syndrome or relapsing or steroid‐resistant adult minimal change disease. There are insufficient data to determine whether 'time‐to‐remission' was significantly influenced by any treatments in this review.
Implications for research.
Future adequately powered RCTs are required to compare the benefits and harms of;
Prednisone for the first episode of minimal change disease, comparing different doses and durations of treatment.
Cyclosporine, tacrolimus, or alkylating agents for relapsing disease or steroid‐resistant disease, with a focus on duration on remission, kidney outcomes, and toxicity.
What's new
| Date | Event | Description |
|---|---|---|
| 13 August 2009 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 14 May 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors wish to thank:
Narelle Willis, Review Group Coordinator and Ruth Mitchell, Trials Search Coordinator of the Cochrane Renal Group for their help with this review.
Drs Ponticelli and Goodship for their replies to requests for additional data.
Drs Norbert Braun, Tak‐Mao Chan, Richard Glassock and Charles Swainson for their editorial advice during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| EMBASE | 1. exp clinical trial/ 2. comparative study/ 3. drug comparison/ 4. major clinical study/ 5. randomisation/ 6. crossover procedure/ 7. double blind procedure/ 8. single blind procedure/ 9. placebo/ 10. prospective study/ 11. ((clinical or controlled or comparative or placebo or prospective or randomi#ed) adj3 (trial or study)).ti,ab. 12. (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$)).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).ti,ab. 14. (cross?over$ or (cross adj1 over$)).ti,ab. 15. ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).ti,ab. 16. or/1‐10 17. or/11‐15 18. 16 or 17 19. minimal change glomerulonephritis/ 20. Lipoid Nephrosis/ 21. minimal change disease.tw. 22. minimal change glomerulonephritis.tw. 23. minimal change nephr$.tw. 24. nil disease.tw. 25. lipoid nephrosis.tw. 26. idiopathic nephrotic syndrome.tw. 27. or/19‐26 1. exp clinical trial/ 2. comparative study/ 3. drug comparison/ 4. major clinical study/ 5. randomisation/ 6. crossover procedure/ 7. double blind procedure/ 8. single blind procedure/ 9. placebo/ 10. prospective study/ 11. ((clinical or controlled or comparative or placebo or prospective or randomi#ed) adj3 (trial or study)).ti,ab. 12. (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$)).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).ti,ab. 14. (cross?over$ or (cross adj1 over$)).ti,ab. 15. ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).ti,ab. 16. or/1‐10 17. or/11‐15 18. 16 or 17 19. minimal change glomerulonephritis/ 20. Lipoid Nephrosis/ 21. minimal change disease.tw. 22. minimal change glomerulonephritis.tw. 23. minimal change nephr$.tw. 24. nil disease.tw. 25. lipoid nephrosis.tw. 26. idiopathic nephrotic syndrome.tw. 27. or/19‐26 |
| CENTRAL | #1 Nephrosis, Lipoid, this term only in MeSH products #2 lipoid next nephrosis in All Fields #3 minimal next change next disease in All Fields #4 minimal next change next glomerul* in All Fields #5 minimal next change next nephr* in All Fields #6 nil next disease in All Fields #7 idiopathic next nephrotic next syndrome in All Fields #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) |
| MEDLINE | 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized controlled trials/ 4. random allocation/ 5. double blind method/ 6. single blind method/ 7. or/1‐7 8. animals/ not (animals/ and human/) 9. 7 not 8 10. clinical trial.pt. 11. exp clinical trials/ 12. (clinic$ adj25 trial$).ti,ab. 13. cross‐over studies/ 14. (crossover or cross‐over or cross over).tw. 15. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 16. placebos/ 17. placebo$.ti,ab. 18. random$.ti,ab. 19. research design/ 20. or/10‐19 21. 20 not 8 22. 9 or 21 23. Nephrosis Lipoid/ 24. minimal change disease.tw. 25. minimal change glomerulonephritis.tw. 26. minimal change nephro$.tw. 27. nil disease.tw. 28. lipoid nephrosis.tw. 29. idiopathic nephrotic syndrome.tw. 30. or/23‐29 |
Data and analyses
Comparison 1. Steroid versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Sustained remission during follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Loss of oedema | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Avascular necrosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 67.91] | |
| 5.2 Doubling serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.89] | |
| 5.3 Kidney failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.55] |
Comparison 2. Intravenous + oral steroid versus oral steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Sustained remission during follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Thrombosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Intravenous steroid versus oral steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Time to complete remission (days) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Coggins 1985.
| Methods | Country: United States Setting/Design: Collaborative placebo‐controlled RCT Time Frame: NS Randomisation method: NS Blinding ‐ Participants: NS ‐ Investigators: NS ‐ Outcome assessors: NS ‐ Data analyses: NS Intention‐to‐treat: NS Follow‐up period: 77 months Loss to follow‐up: 0% | |
| Participants | INCLUSION CRITERIA
Idiopathic nephrotic syndrome of minimal change disease GROUP 1 (prednisone) Number: 14 Age: 29 years GROUP 2 (no treatment) Number: 14 Age: 32 years |
|
| Interventions | GROUP 1
Prednisone 125 mg PO given in alternate‐day doses for 2 months.
Relapses were re‐treated. GROUP 2 No treatment. If patient reached "stop points" (including doubling of admission creatinine) they were withdrawn from the placebo group and treated with steroids. COINTERVENTIONS: NS |
|
| Outcomes |
|
|
| Notes | Exclusions post‐randomisation but pre‐intervention: NS
Additional data requested from authors: Method of randomisation, allocation concealment COMPLETENESS OF FOLLOW‐UP Eligible/considered for inclusion = NS; Enrolled/randomised = 28; Analysed = 28 ; Percent followed = 100 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Imbasciati 1985.
| Methods | Country: Italy Setting/Design: Multicentre RCT Time Frame: June 1980 to June 1983 Randomisation method: Table of random numbers kept in one centre Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: NS ‐ Data analyses: NS Intention‐to‐treat: Yes Follow‐up period: 12‐24 months Loss to follow‐up: 0% | |
| Participants | INCLUSION CRITERIA
GROUP 1 (Methylprednisone + prednisone) Number: 11 Age: NS GROUP 2 (Prednisone alone) Number: 11 Age: NS |
|
| Interventions | GROUP 1
Methylprednisone 20 mg/kg/d IV for 3 days; then prednisone 0.5 mg/kg/d for 4 weeks PO; then 0.25‐0.5 mg/kg/alternate days PO for 4 weeks; then 0.5 mg/kg/alternate days PO for 4 months. GROUP 2 Prednisone 1 mg/kg/d for 4 weeks; then 1 mg/kg/alternate days for 4 weeks; then 0.5 mg/kg on alternate days 4 months. COINTERVENTIONS Low salt diet, diuretics, anti‐hypertensive changes as needed. |
|
| Outcomes |
|
|
| Notes | Exclusions post‐randomisation but pre‐intervention: 0
Additional data requested from authors: Allocation concealment, age of participants, mean time to remission COMPLETENESS OF FOLLOW‐UP Eligible/considered for inclusion = NS; Enrolled/randomised = 89; Analysed = 89; Percent followed = 100 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yeung 1983.
| Methods | Country: Hong Kong Setting/Design:Single university centre RCT Time Frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: NS ‐ Data analyses: NS Intention‐to‐treat: No Follow‐up period: Up to 750 days Loss to follow‐up: NS | |
| Participants | INCLUSION CRITERIA
Biopsy‐proven minimal change nephrotic syndrome, first episode GROUP 1 (methylprednisolone) Number: 10 (1 F) Age: mean 29.0 y GROUP 2 (prednisone) Number: 8 (3 F) Age: mean 22.4 y |
|
| Interventions | GROUP 1
Methylprednisolone 20 mg/kg/d IV on three consecutive days; then prednisone 1‐2 mg/kg/d 2 weeks after methylprednisolone dose as maintenance if response to methylprednisolone.
If no response within 2 weeks re‐allocated to oral prednisone. GROUP 2 Prednisone 1 mg/kg/d for 4‐6 weeks COINTERVENTIONS Diuretics |
|
| Outcomes |
|
|
| Notes | Exclusions post‐randomisation but pre‐intervention: NS
Additional data requested from authors: Study quality data COMPLETENESS OF FOLLOW‐UP Eligible/considered for inclusion = NS; Enrolled/randomised = 18; Analysed = 18 ; Percent followed = 100 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
MCD ‐ minimal change disease; NS ‐ not stated
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abaigar 1993 | Individuals with NS caused by non‐MCD comparing two levels of dietary protein intake. (Not MCD) |
| Arora 2002 | Case control study of patients with idiopathic nephrotic syndrome (N = 15) receiving either ACE inhibitor or supportive treatment. No patient had MCD. (Not MCD) |
| Bagga 1997 | RCT in children comparing two regimens for prednisone treatment of childhood NS. (Not adult NS) |
| Bagga 1999 | Study in childhood NS. (Not adult NS) |
| Bargman 1999 | Review article of treatment for MCD in adults and children. (Not RCT) |
| Beige 2003 | Abstract of pilot observational study of tacrolimus to treat adults (N = 7) with steroid‐resistant or relapsing NS. Three individuals had MCD. (Not RCT) |
| Black 1970 | Multicentre RCT of oral prednisone compared with no treatment in 125 people (aged 15 years and over), with the NS. Causes of the syndrome were MCD, membranous nephropathy, and proliferative glomerulonephritis. Unable to interpret outcomes in MCD category due to inadequate baseline data. |
| Broyer 1995 | RCT in paediatric NS. (Not adult NS) |
| Choi 2002 | Retrospective analysis of mycophenolate mofetil in biopsy proven glomerular disease complicated by NS and/or kidney failure. (Not RCT) |
| Cole 1994 | Commentary regarding need for collaborative study in childhood NS caused by MCD. (Not RCT) |
| Deighan 2001 | RCT cross‐over design study of adults with proteinuria >3 g/d, biopsy proven glomerulonephritis comparing cerivastatin with fenofibrate. Two out of 12 patients had MCD. (Not MCD) |
| Dilek 1999 | Controlled prospective study comparing enalapril with losartan in NS. Single patient had MCD. (Not MCD) |
| Don 1989 | RCT in 12 individuals with NS comparing reduction in dietary protein intake with enalapril. No patients had MCD. (Not MCD) |
| El‐Reshaid 1995 | Observational study of cyclosporine in refractory adult nephrotic syndrome > 4 months. (Not RCT) |
| Gentile 1993 | RCT comparing vegetarian soy diet with or without fish oil supplements. Not all participants had NS. No patient had biopsy‐proven MCD. (Not NS, not MCD) |
| Groggel 1989 | RCT comparing gemfibrozil with no treatment in adult NS (N = 11). No participant had MCD. (Not MCD) |
| Gulati 1973 | Patients between 13 and 56 years with NS and biopsy‐proven glomerular disease. Randomised study comparing prednisolone with placebo. Fourteen of 42 participants with MCD. (Not MCD) |
| Ishikawa 1982 | Not MCD. |
| Koike 2002 | Observational study. (Not RCT) |
| Kumar 2004 | RCT comparing ramipril with verapamil in adults with idiopathic NS not showing reduction in proteinuria despite 12 weeks of oral prednisone 2 mg/kg on alternate days. No patient had biopsy‐proven MCD. (Not MCD) |
| Lee 1995 | Observational study of the efficacy and tolerability of cyclosporine in 30 patients with adult NS. Rate of relapse after withdrawal was assessed after treatment. (Not RCT) |
| Leisti 1978 | Paediatric study. (Not adult NS) |
| Mansy 1989 | RCT cross‐over study of 3 levels of protein intake in patients with NS. Two of 12 patients had biopsy proven MCD. (Not MCD) |
| Martins 1994 | Observational single‐arm study. Open prospective 2‐year study of lovastatin in biopsy‐proven primary NS. (Not RCT) |
| Matl 1997 | Quasi‐RCT of 30 patients with chronic glomerular disease proven by biopsy and protein excretion rate higher than 3 g/d randomised to either cyclosporine (5 mg/kg/d) or cyclophosphamide (1.5 mg/kg/d) for 6 months or until remission. Combined aetiology for NS. (Non MCD) |
| Matsubara 2002 | Correspondence describing 2 case reports of combined therapy with camostat mesilate and glycyrrhin for steroid‐dependent NS. (Not RCT) |
| Matsumoto 2004 | Prospective non‐randomised study of methylprednisolone + cyclosporine versus cyclosporine versus prednisone in 36 adults with biopsy‐proven MCD and NS. (Not RCT) |
| Matzkies 1999 | Observational study of fluvastatin (N = 10) in adults with all‐cause NS. (Not RCT) |
| Meyrier 1988 | Observational study of cyclosporine treatment in 56 participants over 14 years of age with NS, MCD in 23. (Not RCT) |
| Meyrier 1991 | Two single arm observational studies in patients with NS (either cyclosporine monotherapy or cyclosporine and prednisone). (Not RCT) |
| Meyrier 1994 | Longitudinal follow up study of cyclosporine in 36 adults with steroid‐dependent or steroid‐resistant idiopathic NS. (Not RCT) |
| Meyrier 1996 | Review article of the utility of cyclosporine treatment for NS in adults. (Not RCT) |
| Mocan 1997 | Paediatric participants. (Not adult NS) |
| Naigui 1997 | RCT for cyclosporine versus prednisone and cyclophosphamide versus prednisone in nephrotic syndrome. Undefined numbers of patients had NS caused by MCD, focal and segmental glomerulosclerosis, membranous nephropathy and membranoproliferative glomerulonephritis. (Not MCD) |
| Ni 2003 | Unclear whether RCT. Comparison of leflunomide and prednisone with cyclophosphamide and prednisone in 41 patients with NS (MCD=12). (Not RCT, not MCD) |
| Niaudet 1994 | Review article. (Not RCT) |
| Olbricht 1999 | RCT of simvastatin versus placebo in 56 patients with NS. No patient had MCD. (Not MCD) |
| Pecoraro 2003 | Paediatric study. (Not adult NS) |
| Piccoli 1993 | RCT comparing deflazacort and prednisone for adults with NS. No patients with MCD were enrolled. (Non‐MCD) |
| Pirisi 1998 | Observational study of immunosuppression in NS (No patient with MCD was enrolled). (Not MCD, not RCT) |
| Ponticelli 1993 | Multicentre RCT of 73 patients (adults and children) comparing cyclophosphamide with cyclosporine in steroid dependent or frequently relapsing idiopathic NS. Included people with a kidney biopsy showing focal and segmental glomerulosclerosis. |
| Ponticelli 1993A | RCT of cyclosporine in steroid‐resistant idiopathic NS. Included people with either MCD or focal segmental glomerulosclerosis. |
| Rabelink 1988 | RCT of simvastatin versus cholestyramine in NS. No patient had MCD. (Not MCD) |
| Reichert 1999 | Pharmacokinetic observational study of prednisone in all‐cause NS. No patient had MCD. (Not RCT) |
| Sharpstone 1969 | RCT of 8‐week courses of prednisone with or without azathioprine. Patients entering study with MCD were not randomised. (Not RCT) |
| Shibasaki 2004 | Comparative study of mizoribine versus standard care in steroid resistant NS. 28/175 had NS caused by MCD or focal and segmental glomerulosclerosis (combine histological category). (Not MCD) |
| Spitalewitz 1993 | Prospective double‐blind placebo‐controlled study of pravastatin versus placebo in patients with NS. No patient had MCD. (Not MCD) |
| Sural 2001 | RCT of levamisole versus steroid and cyclophosphamide in childhood NS. (Not adult NS) |
| Tejani 1988 | Children (1‐18 years) with NS > 1 year randomised to high‐dose prednisone versus cyclosporine and low‐dose prednisone. (Not adult NS) |
| Thomas 1993 | Patients with sub‐nephrotic range proteinuria, steroid‐responsive MCD was an exclusion criteria. (Not MCD, Not NS) |
| Toto 2000 | RCT of adults with NS from any glomerular cause (not described in detail) dichotomised into those with hypercholesterolaemia or mixed dyslipidaemia. (Not MCD) |
| Uldall 1972 | Observational study of cyclophosphamide in 10 individuals with NS due to MCD. (Not RCT) |
| Wyszynska 1988 | Single arm study in children. (Not RCT) |
| Ye 1993 | Cause of NS not defined. RCT comparing short duration prednisone (< 8 weeks) versus longer duration (> 8 weeks). (Not MCD) |
| Yoshikawa 1995 | Observational study in children. (Not RCT) |
MCD ‐ minimal change disease; NS ‐ nephrotic syndrome
Contributions of authors
Writing of protocol and review ‐ SCP, KN, GFMS Screening of titles and abstracts ‐ SCP, KN Assessment for inclusion ‐ SCP, KN Quality assessment ‐ SCP, KN Data extraction ‐ SCP, KN Data entry into RevMan ‐ SCP, KN Data analysis ‐ SCP, KN Disagreement resolution ‐ SCP, KN, GFMS
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Coggins 1985 {published data only}
- Coggins CH. Adult minimal change nephropathy: experience of the collaborative study of glomerular disease. Transactions of the American Clinical & Climatological Asoociation 1985;97:18‐26. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Imbasciati 1985 {published data only}
- Imbasciati E, Gusmano R, Edefonti A, Zucchelli P, Pozzi C, Grassi C, et al. Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. British Medical Journal Clinical Research Ed 1985;291(6505):1305‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeung 1983 {published data only}
- Yeung CK, Wong KL, Ng WL. Intravenous methylprednisolone pulse therapy in minimal change nephrotic syndrome. Australian & New Zealand Journal of Medicine 1983;13(4):349‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abaigar 1993 {published data only}
- Abaigar LP, Torres G, Vazquez A, Toro R, Santos J, Carrasco ML, et al. Influence of protein contents of the diet and angiotensin converting inhibitors in the adult nephrotic syndrome. Randomized and crossed, prospective study. Nefrologia 1993;13(Suppl 5):87‐93. [EMBASE: 1994008853] [Google Scholar]
Arora 2002 {published data only}
- Arora A, Ahlawat RS, Arora S, Mandel AK. Randomised controlled study of enalapril in steroid resistant nephrotic syndrome. Indian Journal of Nephrology 2002;12(3):http://www.ijnephrol.com/july_sep02/articleq13.html. [CENTRAL: CN‐00460300] [Google Scholar]
Bagga 1997 {published data only}
- Bagga A, Ekka BK, Srivastava RN. Single versus divided dose prednisolone therapy for relapses of nephrotic syndrome (NS) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A113. [CENTRAL: CN‐00261391] [DOI] [PubMed] [Google Scholar]
Bagga 1999 {published data only}
- Bagga A, Hari P, Srivastava RN. Long (lp) versus standard (sp) initial prednisolone treatment for nephrotic syndrome (ns) [abstract]. Nephrology, Urology, Transplantation Society of SAARC, Sri Lanka. 1999:158. [CENTRAL: CN‐00460325]
- Bagga A, Hari P, Srivastava RN. Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatric Nephrology 1999;13(9):824‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bargman 1999 {published data only}
- Bargman JM. Management of minimal lesion glomerulonephritis: Evidence‐based recommendations. Kidney International ‐ Supplement 1999;55:S3‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beige 2003 {published data only}
- Beige J, Moosmayer I, Liefeldt L, Neumayer HH, Zidek W, Peters H. Effective and safe treatment of primary nephrotic syndrome with tacrolimus (FK 506) [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):65. [CENTRAL: CN‐00444378] [Google Scholar]
Black 1970 {published data only}
- Black DA, Rose G, Brewer DB. Controlled trial of prednisone in adult patients with the nephrotic syndrome. British Medical Journal 1970;3(5720):421‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Broyer 1995 {published data only}
- Broyer M, Terzi F, Gagnadoux MF, Guest G, Niaudet P. A randomised double blind study of deflazacort (d) versus prednisone (p) in the treatment of idiopathic nephrotic syndrome (ins) [abstract]. Journal of the American Society of Nephrology 1995;6(3):414. [CENTRAL: CN‐00483349] [Google Scholar]
Choi 2002 {published data only}
- Choi MJ, Eustace JA, Gimenez LF, Atta MG, Scheel PJ, Sothinathan R, et al. Mycophenolate mofetil treatment for primary glomerular diseases. Kidney International 2002;61(3):1098‐114. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cole 1994 {published data only}
- Cole B. Cyclosporine for nephrotic syndrome‐‐isn't it time for a collaborative trial?. Journal of the American Society of Nephrology 1994;5(4):1047‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deighan 2001 {published data only}
- Deighan CJ, Caslake MJ, McConnell M, Boulton‐Jones JM, Packard CJ. Comparative effects of cerivastatin and fenofibrate on the atherogenic lipoprotein phenotype in proteinuric renal disease. Journal of the American Society of Nephrology 2001;12(2):341‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dilek 1999 {published data only}
- Dilek K, Yavus M, Usta M, Ersoy A, Ilcol YO, Gullulu M, et al. The comparison of antiproteinuric effects of angiotensin converting enzyme inhibitor enalapril and angiotensin ii receptor antagonist losartan in nephrotic syndrome cases [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A88. [CENTRAL: CN‐00483748] [Google Scholar]
Don 1989 {published data only}
- Don BR, Wada L, Kaysen GA, Schambelan M. Effect of dietary protein restriction and angiotensin converting enzyme inhibition on protein metabolism in the nephrotic syndrome. Kidney International ‐ Supplement 1989;27:S163‐7. [MEDLINE: ] [PubMed] [Google Scholar]
El‐Reshaid 1995 {published data only}
- El‐Reshaid K, Amer E, Madda JP, Kapoor M. Long‐term cyclosporin A treatment in adults with refractory nephrotic syndrome. Renal Failure 1995;17(6):695‐703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gentile 1993 {published data only}
- Gentile MG, Fellin G, Cofano F, Delle FA, Manna G, Ciceri R, et al. Treatment of proteinuric patients with a vegetarian soy diet and fish oil. Clinical Nephrology 1993;40(6):315‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Groggel 1989 {published data only}
- Groggel GC, Cheung AK, Ellis‐Benigni K, Wilson DE. Treatment of nephrotic hyperlipoproteinemia with gemfibrozil. Kidney International 1989;36(2):266‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gulati 1973 {published data only}
- Gulati PD, Bazaz MG, Vaishnava H. A double‐blind trial of "alternate‐day" steroid therapy in adult nephrotics ‐ a preliminary report. Journal of the Association of Physicians of India 1970;18(12):963‐73. [MEDLINE: ] [PubMed] [Google Scholar]
- Gulati PD, Malik GB, Vaishnava H. Alternate‐day steroid therapy in adult nephrotics. Journal of Medicine 1973;4(5):266‐75. [MEDLINE: ] [PubMed] [Google Scholar]
Ishikawa 1982 {published data only}
- Ishikawa H, Honjo A, Hayashi M, Hamaguchi T, Morita T, Shindo T, et al. Effects of dipyridamole on proteinuria in chronic glomerulonephritis and the nephrotic syndrome. Arzneimittel‐Forschung 1982;32(3):301‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Ishikawa H, Honjo A, Hayashi M, Hamaguchi T, Morita T, Tesutani T, et al. The therapeutical effect of dipyridamole on chronic glomerulonephritis and nephrotic syndrome in large dose administration‐‐the study in a double blind group comparison method. Nippon Jinzo Gakkai Shi. Japanese Journal of Nephrology 1979;21(2):135‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Koike 2002 {published data only}
- Koike M, Honda K, Tsukada M, Itabashi M, Suzuki K, Uchida K, et al. Low‐dose cyclosporin therapy combined with prednisolone for relapsing minimal change nephrotic syndrome in adults. Nippon Jinzo Gakkai Shi. Japanese Journal of Nephrology 2002;44(5):447‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Kumar 2004 {published data only}
- Kumar NS, Mishra RN, Singh AK, Prakash J. Effect of Ace inhibitor and calcium channel blocker on proteinuria in subjects with steroid resistant idiopathic nephrotic syndrome [abstract]. Indian Journal of Nephrology 2001;11(3):110‐1. [CENTRAL: CN‐00461120] [Google Scholar]
- Kumar NS, Singh AK, Mishra SN, Prakash J. Comparative study of angiotensin converting enzyme inhibitor and calcium channel blocker in the treatment of steroid‐resistant idiopathic nephrotic syndrome. Journal of the Association of Physicians of India 2004;52:454‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Lee 1995 {published data only}
- Lee HY, Kim HS, Kang CM, Kim SG, Kim MJ. The efficacy of cyclosporine A in adult nephrotic syndrome with minimal change disease and focal‐segmental glomerulosclerosis: a multicenter study in Korea. Clinical Nephrology 1995;43(6):375‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Leisti 1978 {published data only}
- Leisti S, Koskimies O, Perheentupa J, Vilska J, Hallman N. Idiopathic nephrotic syndrome: prevention of early relapse. British Medical Journal 1978; Vol. 1, issue 6117:892. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Mansy 1989 {published data only}
- Mansy H, Goodship TH, Tapson JS, Hartley GH, Keavey P, Wilkinson R. Effect of a high protein diet in patients with the nephrotic syndrome. Clinical Science 1989;77(4):445‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martins 1994 {published data only}
- Martins PM, Nogueira AC, Reimao PJ, Correia AM, Vicente O, Rodrigues MC, et al. Long‐term effect of lovastatin on lipoprotein profile in patients with primary nephrotic syndrome. Clinical Nephrology 1994;41(5):277‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Matl 1997 {published data only}
- Matl I, Matousovic K, Herout V, Konecny K, Kovac A, Rychlik I, et al. A controlled clinical trial of Consupren versus cyclophosphamide in chronic glomerulonephritis. Casopis Lekaru Ceskych 1997;136(4):120‐123. [MEDLINE: ] [PubMed] [Google Scholar]
- Matl I, Matousovic K, Herout V, Konency K, Kovac A, Rychlik I, et al. A controlled clinical study of Consupren versus Cyclophosphamide in chronic glomerulonephritis. I. Efficacy of therapy. Casopis Lekaru Ceskych 1997;136(4):120‐3. [EMBASE: 1997068194] [PubMed] [Google Scholar]
Matsubara 2002 {published data only}
- Matsubara M, Nogae S, Ito S. Novel trial for the treatment of steroid dependency in minimal change disease: Combined therapy of camostat mesilate and glycyrrhizin. Nephron 2002;90(3):357‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsumoto 2004 {published data only}
- Matsumoto H, Nakao T, Okada T, Nagaoka Y, Takeguchi F, Tomaru R, et al. Favorable outcome of low‐dose cyclosporine after pulse methylprednisolone in Japanese adult minimal‐change nephrotic syndrome. Internal Medicine 2004;43(8):668‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matzkies 1999 {published data only}
- Matzkies FH, Bahner U, Teschner M, Hohage H, Heidland A, Schaefer RM. Efficiency of 1‐year treatment with fluvastatin in hyperlipidemic patients with nephrotic syndrome. American Journal of Nephrology 1999;19(4):492‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meyrier 1988 {published data only}
- Meyrier A, Condamin MC, Simon P. Treatment with cyclosporine of adult idiopathic nephrotic syndrome resistant to corticosteroid and other immunosuppressants. Transplantation Proceedings 1988;20(3 Suppl 4):259‐61. [EMBASE: 1988158714] [PubMed] [Google Scholar]
Meyrier 1991 {published data only}
- Meyrier A, Condamin MC, Broneer D. Treatment of adult idiopathic nephrotic syndrome with cyclosporin A: minimal‐change disease and focal‐segmental glomerulosclerosis. Collaborative Group of the French Society of Nephrology. Clinical Nephrology 1991;35(1):S37‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Meyrier 1994 {published data only}
- Meyrier A, Noel L‐H, Auriche P, Callard P. Long‐term renal tolerance of cyclosporin A treatment in adult idiopathic nephrotic syndrome. Collaborative Group of the Societe de Nephrologie. Kidney International 1994;45(5):1446‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meyrier 1996 {published data only}
- Meyrier A. Use of cyclosporine A in the treatment of refractory nephrotic syndrome in adults. Renal Failure 1996;18(5):775‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mocan 1997 {published data only}
- Mocan H, Mocan MZ. The effect of high dose methylprednisolone therapy in patients with minimal change nephrotic syndrome [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A74. [CENTRAL: CN‐00261380] [Google Scholar]
Naigui 1997 {published data only}
- Naigui X, Jian X, Jing H. A clinical study on low dose cyclosporin a with diltiazem in the treatment of primary nephrotic syndrome [abstract]. Nephrology 1997;3(Suppl 1):S125. [CENTRAL: CN‐00461383] [Google Scholar]
Ni 2003 {published data only}
- Ni ZH, Qian JQ, Lin AW, Mu S, Zhu ML, Fang W. A controlled, prospective study of efficacy of leflunomide in patients with nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):524A. [CENTRAL: CN‐00520368] [Google Scholar]
Niaudet 1994 {published data only}
- Niaudet P, Habib R. Cyclosporine in the treatment of idiopathic nephrosis. Journal of the American Society of Nephrology 1994;5(4):1049‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olbricht 1999 {published data only}
- Olbricht CJ, Wanner C, Thiery J, Basten A. Simvastatin in nephrotic syndrome. Simvastatin in Nephrotic Syndrome Study Group. Kidney International ‐ Supplement 1999;71:S113‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pecoraro 2003 {published data only}
- Pecoraro C, Caropreso MR, Passaro G, Fereto AV, Malgieri G. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):63. [CENTRAL: CN‐00447140] [Google Scholar]
Piccoli 1993 {published data only}
- Piccoli A, Gastaldon F, Pillon L, Mussap M, Faggian D, Plebani M, et al. Bioequivalence of deflazacort and prednisone in the treatment of idiopathic nephrotic syndrome. A pilot study. Current Therapeutic Research ‐ Clinical and Experimental 1993;54(5):588‐97. [EMBASE: 1993345953] [Google Scholar]
Pirisi 1998 {published data only}
- Pirisi M, Faedda R, Satta A, Bartoli E. Immunosuppressive treatment for idiopathic syndrome with corticosteroids and cyclophosphamide. Factors associated with a favorable outcome. Clinical Drug Investigation 1998;16(3):211‐8. [EMBASE: 1998351604] [DOI] [PubMed] [Google Scholar]
Ponticelli 1993 {published data only}
- Ponticelli C. A multicenter controlled prospective trial with cyclosporine vs cyclophosphamide in frequent relapsers and steroid dependent patients with idiopathic nephrotic syndrome. Journal of Nephrology 1989;2(2):147‐51. [EMBASE: 1991014828] [Google Scholar]
- Ponticelli C. Ciclosporin in the treatment of idiopathic nephrotic syndrome [abstract]. 10th Asian Colloquium in Nephrology; 1994 Dec 2‐6; Karachi (Pakistan). 1994:116. [CENTRAL: CN‐00461528]
- Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, et al. Cyclosporin versus cyclophosphamide for patients with steroid‐dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrology Dialysis Transplantation 1993;8(12):1326‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Ponticelli 1993A {published data only}
- Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, et al. A randomized trial of cyclosporine in steroid resistant idiopathic nephrotic syndrome. Kidney International 1993;43(6):1377‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rabelink 1988 {published data only}
- Rabelink AJ, Hene RJ, Erkelens DW, Joles JA, Koomans HA. Effects of simvastatin and cholestyramine on lipoprotein profile in hyperlipidaemia of nephrotic syndrome. Lancet 1988;2(8624):1335‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reichert 1999 {published data only}
- Reichert LJ, Koene RA, Wetzels JF. Acute haemodynamic and proteinuric effects of prednisolone in patients with a nephrotic syndrome. Nephrology Dialysis Transplantation 1999;14(1):91‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharpstone 1969 {published data only}
- Sharpstone P, Ogg CS, Cameron JS. Nephrotic syndrome due to primary renal disease in adults: II. A controlled trial of prednisone and azathioprine. British Medical Journal 1969;2(5656):535‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shibasaki 2004 {published data only}
- Shibasaki T, Koyama A, Hishida A, Muso E, Osawa G, Yamabe H, et al. A randomized open‐label comparative study of conventional therapy versus mizoribine onlay therapy in patients with steroid‐resistant nephrotic syndrome (postmarketing survey). Clinical & Experimental Nephrology 2004;8(2):117‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spitalewitz 1993 {published data only}
- Spitalewitz S, Porush JG, Cattran D, Wright N. Treatment of hyperlipidemia in the nephrotic syndrome: the effects of pravastatin therapy. American Journal of Kidney Diseases 1993;22(1):143‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sural 2001 {published data only}
- Sural S, Pahari DK, Mitra K, Bhattacharya S, Mondal S, Taraphder A. Efficacy of levamisole compared to cyclophosphamide and steroid in frequently in frequently relapsing (FR) minimal change nephrotic syndrome (MCNS) [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):126A. [CENTRAL: CN‐00447910] [Google Scholar]
Tejani 1988 {published data only}
- Tejani A, Gonzalez R, Rajpoot D, Sharma R, Pomrantz A. A randomized trial of cyclosporine with low‐dose prednisone compared with high‐dose prednisone in nephrotic syndrome. Transplantation Proceedings 1988;20(3 Suppl 4):262‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Thomas 1993 {published data only}
- Thomas ME, Harris KP, Ramaswamy C, Hattersley JM, Wheeler DC, Varghese Z, et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney International 1993;44(5):1124‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toto 2000 {published data only}
- Toto RD, Grundy SM, Vega GL. Pravastatin treatment of very low density, intermediate density and low density lipoproteins in hypercholesterolemia and combined hyperlipidemia secondary to the nephrotic syndrome. American Journal of Nephrology 2000;20(1):12‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uldall 1972 {published data only}
- Uldall PR, Feest TG, Morley AR, Tomlinson BE, Kerr DN. Cyclophosphamide therapy in adults with minimal change nephrotic syndrome. Lancet 1972;1(7763):1250‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wyszynska 1988 {published data only}
- Wyszynska T, Ksiasek J, Uszycka‐Karcz M, Kobierska‐Szczepanska A, Morawska Z, Zoch‐Zwierz W. Evaluation of prednisolone pulse therapy in steroid‐resistant nephrotic syndrome. A multicenter collaborative study. Contributions to Nephrology 1988;67:229‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ye 1993 {published data only}
- Ye R, Zhang D. Contrasting efficacy of two different treatment programs in adult frequently relapsing nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts). [CENTRAL: CN‐00486532] [Google Scholar]
Yoshikawa 1995 {published data only}
- Yoshikawa N, Tanaka R, Kitano Y, Nakamura H, Ito H. Long‐term cyclosporin in steroid‐dependent nephrotic syndrome. Contributions to Nephrology 1995;114:19‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Al Khader 1979
- Al‐Khader AA, Lien JW, Aber GM. Cyclophosphamide alone in the treatment of adult patients with minimal change glomerulonephritis. Clinical Nephrology 1979;11(1):26‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Arneil 1961
- Arneil GC. 164 children with nephrosis. Lancet 1961;II:1103‐10. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Briggs 1998
- Briggs WA, Choi MJ, Scheel PJ Jr. Successful mycophenolate mofetil treatment of glomerular disease. American Journal of Kidney Diseases 1998;31(2):213‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Day 2002
- Day CJ, Cockwell P, Lipkin GW, Savage CO, Howie AJ, Adu D. Mycophenolate mofetil in the treatment of resistant idiopathic nephrotic syndrome. Nephrology Dialysis Transplantation 2002;17(11):2011‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durkan 2005
- Durkan A, Hodson EM, Willis NS, Craig JC. Non‐corticosteroid treatment for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD002290. DOI: 10.1002/14651858.CD002290.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EUVAS 2006
- The European Vasculitis Study Group. http://www.vasculitis.org (accessed November 2007).
Grimbert 2003
- Grimbert P, Audard V, Remy P, Lang P, Sahali D. Recent approaches to the pathogenesis of minimal‐change nephrotic syndrome. Nephrology Dialysis Transplantation 2003;18(2):245‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haas 1995
- Haas M, Spargo BH, Coventry S. Increasing incidence of focal‐segmental glomerulosclerosis among adult nephropathies. American Journal of Kidney Diseases 1995;26(5):740‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haas 1997
- Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976‐1979 and 1995‐1997. American Journal of Kidney Diseases 1997;30(5):621‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodson 2006
- Hodson EM, Habashy D, Craig JC. Interventions for idiopathic steroid‐resistant nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2006, Issue 2. [Art. No.: CD003594. DOI: 10.1002/14651858.CD003594.pub3] [DOI] [PubMed] [Google Scholar]
Hodson 2007
- Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2007, Issue 4. [Art. No.: CD001533. DOI: 10.1002/14651858.CD001533.pub4] [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson RJ, Feehally J (editors). Comprehensive clinical nephrology. 2nd Edition. Edinburgh: Mosby, Elsevier Limited, 2003. [Google Scholar]
Korbet 1996
- Korbet SM, Genchi RM, Borok RZ, Schwartz MM. The racial prevalence of glomerular lesions in nephrotic adults. American Journal of Kidney Diseases 1996;27(5):647‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium; 1996 Oct 20‐24; Adelaide (Australia). 1996.
Mak 1996
- Mak SK, Short CD, Mallick NP. Long‐term outcome of adult‐onset minimal‐change nephropathy. Nephrology Dialysis Transplantation 1996;11(11):2192‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Master List 2007
- United States Cochrane Center. Master list of journals being searched. http://apps1.jhsph.edu/cochrane/masterlist.asp (accessed May 2007).
Mogyorosi 2002
- Mogyorosi A, Lippman HR, Feldman GM. Successful treatment of steroid‐resistant minimal change disease with mycophenolate mofetil. American Journal of Nephrology 2002;22(5‐6):569‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakayama 2002
- Nakayama M, Katafuchi R, Yanase T, Ikeda K, Tanaka H, Fuijimi S. Steroid responsiveness and frequency of relapse in adult‐onset minimal change nephrotic syndrome. American Journal of Kidney Diseases 2002;39(3):503‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nolasco 1986
- Nolasco F, Cameron JS, Heywood EF, Hicks J, Ogg C, Williams DG. Adult‐onset minimal change nephrotic syndrome: a long‐term follow‐up. Kidney International 1986;29(6):1215‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patel 2005
- Patel P, Pal S, Ashley C, Sweny P, Burns A. Combination therapy with sirolimus (rapamycin) and tacrolimus (FK‐506) in treatment of refractory minimal change nephropathy, a clinical case report. Nephrology Dialysis Transplantation 2005;20(5):985‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schweda 1997
- Schweda F, Liebl R, Riegger GA, Kramer BK. Tacrolimus treatment for steroid‐ and cyclosporin‐resistant minimal‐change nephrotic syndrome. Nephrology Dialysis Transplantation 1997;12(11):2433‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tse 2003
- Tse KC, Lam MF, Yip PS, Li FK, Choy BY, Lai KN, et al. Idiopathic minimal change nephrotic syndrome in older adults: steroid responsiveness and pattern of relapses. Nephrology Dialysis Transplantation 2003;18(7):1316‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Westhoff 2006
- Westhoff TH, Schmidt S, Zidek W, Beige J, Giet M. Tacrolimus in steroid‐resistant and steroid‐dependent nephrotic syndrome. Clinical Nephrology 2006;65(6):393‐400. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woo 2001
- Woo KT, Chew ST, Vathsala A, Chiang GS. Case reports of low dose cyclosporine. A therapy in adult minimal change nephrotic syndrome. Annals of the Academy of Medicine, Singapore 2001;30(4):430‐5. [MEDLINE: ] [PubMed] [Google Scholar]


