Abstract
Background
In nephrotic syndrome protein leaks from the blood to the urine through the glomeruli resulting in hypoproteinaemia and generalised oedema. While most children with nephrotic syndrome respond to corticosteroids, 80% experience a relapsing course. Corticosteroids have reduced the mortality rate to around 3%. However corticosteroids have well recognised potentially serious adverse effects such as obesity, poor growth, hypertension, diabetes mellitus, osteoporosis and behavioural disturbances. This is an update of a review first published in 2000 and updated in 2003, 2005 and 2007.
Objectives
The aim of this review was to assess the benefits and harms of different corticosteroid regimens in children with steroid‐sensitive nephrotic syndrome (SSNS). The benefits and harms of therapy were studied in two groups of children 1) children in their initial episode of SSNS, and 2) children who experience a relapsing course of SSNS.
Search methods
We searched the Cochrane Renal Group's Specialised Register to 26 February 2015 through contact with the Trials Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) performed in children (three months to 18 years) in their initial or subsequent episode of SSNS, comparing different durations, total doses or other dose strategies using any corticosteroid agent.
Data collection and analysis
Two authors independently assessed risk of bias and extracted data. Results were expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
Ten new studies were identified so a total of 34 studies (3033 total participants) were included in the 2015 review update. The risk of bias attributes were frequently poorly performed. Low risk of bias was reported in 18 studies for sequence generation, 16 studies for allocation concealment, seven for performance and detection bias, 15 for incomplete reporting and 16 for selective reporting. Three months or more of prednisone significantly reduced the risk of frequently relapsing nephrotic syndrome (FRNS) (6 studies, 582 children: RR 0.68, 95% CI 0.47 to 1.00) and of relapse by 12 to 24 months (8 studies, 741 children: RR 0.80, 95% CI 0.64 to 1.00) compared with two months. Five or six months of prednisone significantly reduced the risk of relapse (7 studies, 763 children: RR 0.62, 95% CI 0.45 to 0.85) but not FRNS (5 studies, 591 children: RR 0.78, 95% CI 0.50 to 1.22) compared with three months. However there was significant heterogeneity in the analyses. Subgroup analysis stratified by risk of bias for allocation concealment showed that the risk for FRNS did not differ significantly between two or three months of prednisone and three to six months among studies at low risk of bias but was significantly reduced in extended duration studies compared with two or three months in studies at high risk or unclear risk of bias. There were no significant differences in the risk of adverse effects between extended duration and two or three months of prednisone. Four studies found that in children with FRNS, daily prednisone during viral infections compared with alternate‐day prednisone or no treatment significantly reduced the rate of relapse.
Authors' conclusions
In this 2015 update the addition of three well‐designed studies has changed the conclusion of this review. Studies of long versus shorter duration of corticosteroids have heterogeneous treatment effects, with the older high risk of bias studies tending to over‐estimate the effect of longer course therapy, compared with more recently published low risk of bias studies. Among studies at low risk of bias, there was no significant difference in the risk for FRNS between prednisone given for two or three months and longer durations or total dose of therapy indicating that there is no benefit of increasing the duration of prednisone beyond two or three months in the initial episode of SSNS.
The risk of relapse in children with FRNS is reduced by the administration of daily prednisone at onset of an upper respiratory tract or viral infection. Three additional studies have increased the evidence supporting this conclusion. This management strategy may be considered for children with FRNS. A paucity of data on prednisone use in relapsing nephrotic syndrome remains. In particular there are no data from RCTs evaluating the efficacy and safety of prolonged courses of low dose alternate‐day prednisone although this management strategy is recommended in current guidelines.
Plain language summary
Corticosteroid therapy for children with nephrotic syndrome
Nephrotic syndrome is a condition where the kidneys leak protein from the blood into the urine. When it is untreated, children can often die from infections. Most children, with nephrotic syndrome, respond to corticosteroid drugs (prednisone, prednisolone) reducing the risk of serious infection. However they usually have repeat episodes, which are often triggered by viral infections. Corticosteroid drugs can have serious side effects.
We looked at evidence from 34 studies enrolling 3033 children. Fourteen of 21 studies, in children with their first episode of nephrotic syndrome, evaluated prednisone for two or three months compared with longer durations. Thirteen studies evaluated different corticosteroid regimens in children with frequently relapsing disease (FRNS). Studies were of variable methodological quality with only about half of the studies at low risk of bias.
Among studies of long versus shorter duration of prednisone, older studies at high or unclear risk of bias tended to over‐estimate the effect of longer course therapy compared with new studies at low risk of bias. Studies at low risk of bias found no significant differences in the risk of relapse or the development of FRNS between prednisone given for three to six months compared with two or three months. Therefore there is no benefit of increasing the duration of prednisone beyond two or three months in the initial episode of SSNS.
Based on four studies in children with frequently relapsing nephrotic syndrome, prednisone given for five to seven days at the onset of a viral infection reduces the risk of relapse.
This review updates information previously published in 2000, 2003, 2005 and 2007. The addition of three new studies evaluating different durations of prednisone in the first episode of nephrotic syndrome has changed the conclusions expressed in previous versions of this review
Summary of findings
Background
Description of the condition
Nephrotic syndrome is a well‐recognised chronic illness in childhood. The characteristic features, including oedema, proteinuria and hypoalbuminaemia, result from alterations of the perm‐selectivity barrier of the glomerular capillary wall. The reported incidence is 2 to 7/100,000 children, with a prevalence of 16/100,000 (Eddy 2003). Recent prospective studies revealed an incidence of 1.15 to 2.1/100,000 children/year (El Bakkali 2011).The incidence of nephrotic syndrome is higher in Asian (McKinney 2001), African‐American (Srivastava 1999) and Arab children (Elzouki 1984). Most children have minimal change disease, in which changes on light microscopy are minor or absent, and respond to corticosteroid agents. Despite the overall incidence of childhood nephrotic syndrome remaining relatively stable over the last three decades, the histological pattern appears to be changing with an increase in the incidence of focal and segmental glomerulosclerosis (FSGS), even after adjustment for biopsy practices (Bonilla‐Felix 1999; Gulati 1999; Srivastava 1999). The histological variant and response to immunosuppressive treatment may be related to ethnicity (Eddy 2003). Steroid‐sensitive nephrotic syndrome (SSNS) is less common in African and African‐American children, and in South Africa 7.2% of 236 African children had SSNS compared with 62% of 286 Indian children (Bhimma 1997). The pathogenesis of SSNS remains unknown but appears to be related to abnormalities in T‐cell and B‐cell regulation. About 80% of children who respond to corticosteroids experience a relapsing course with recurrent episodes of oedema and proteinuria (Koskimies 1982; Tarshish 1997). The complications of nephrotic syndrome are related to effects of the disease itself, and adverse effects related to corticosteroid therapy and corticosteroid sparing agents. Children with nephrotic syndrome, which is resistant to therapy, are at increased risk of bacterial infection, characteristically resulting in peritonitis, cellulitis or septicaemia, of thromboembolic phenomena and of protein calorie malnutrition. Before antibiotics became available, two thirds of children with nephrotic syndrome died (Arneil 1971).The survivors remitted spontaneously after several months. Mortality rates fell to 35% with the introduction of sulphonamides and penicillin (Arneil 1971) and fell further with the use of corticosteroid medications.
Description of the intervention
Corticosteroids have been used to treat childhood nephrotic syndrome since 1950 when large doses of adrenocorticotrophic hormone (ACTH) and cortisone given for two to three weeks were found to induce diuresis with loss of oedema and proteinuria (Arneil 1971). Corticosteroid usage has reduced the mortality rate in childhood nephrotic syndrome to around 3%, with infection remaining the most important cause of death (ISKDC 1984). Of children who present with their first episode of nephrotic syndrome, approximately 80% will achieve remission with corticosteroid therapy (Koskimies 1982). Because of this dramatic before‐after evidence, oral corticosteroids are the first‐line treatment of a child presenting with idiopathic nephrotic syndrome and no randomised controlled prospective studies of corticosteroids compared to placebo were carried out. The achievement of remission with corticosteroid therapy determines long term prognosis for kidney function irrespective of kidney histology (Niaudet 2009). However corticosteroids have known adverse effects. Major complications related to prolonged corticosteroid use in nephrotic syndrome include growth impairment, particularly with steroid therapy administered daily (Hyams 1988), cataracts (Ng 2001) and excessive weight gain or obesity (Rüth 2005).Two recent studies (Mishra 2010; Neuhaus 2010) highlight the impact of psychological and behavioural abnormalities related to corticosteroid therapy. Anxiety, depression, emotional lability, aggressive behaviour and attention problems had already developed with completion of 12 weeks of therapy (Mishra 2010). Neuhaus 2010 demonstrated family background, particularly maternal distress, reduced quality of life and psychosocial adjustment. Adverse effects are particularly prevalent in those children who relapse frequently and thus require multiple courses of corticosteroids.
How the intervention might work
The pharmacology and pharmacodynamics of corticosteroids in SSNS are not fully understood (Mehls 2011). It is widely believed the main effect is through the regulation of nuclear gene expression via the cytosolic glucocorticoid receptor, which activates genes for anti‐inflammatory cytokines and suppresses genes for pro‐inflammatory cytokines. Glucocorticoids are lipid soluble and can easily pass through cell membranes. This process takes several hours. More recently research had identified corticosteroid effects, which are independent of nuclear gene transcription and occur earlier. At high glucocorticoid doses, suppression of T‐cell function occurs. Corticosteroids also act directly to stabilise the podocyte cytoskeleton.
Why it is important to do this review
The original treatment schedules for childhood nephrotic syndrome were developed in an ad hoc manner. The International Study of Kidney Disease in Children (ISKDC) was established in 1966 and determined by consensus a regimen of daily corticosteroids for four weeks followed by corticosteroids given on three consecutive days out of seven for four weeks (Arneil 1971). Since then many physicians have used regimens involving periods of daily followed by alternate‐day or intermittent therapy and several randomised controlled trials (RCTs) have investigated different durations and total corticosteroid therapy doses in an effort to delineate the optimal doses and durations of corticosteroid therapy that are most beneficial and least harmful. These have been evaluated in previous versions of this systematic review. However despite these data there remains no consensus on the most appropriate corticosteroid regimen to achieve and maintain remission with the least adverse effects. Therefore the 2015 update of this review has been undertaken to identify further RCTs, which compare different corticosteroid regimens in the initial episode of SSNS and in relapsing disease.
Objectives
The aim of this review was to assess the benefits and harms of different corticosteroid regimens in children with SSNS. The benefits and harms of therapy were studied in two groups of children:
Children in their initial episode of SSNS
Children who experience a relapsing course of SSNS.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs, in which different doses, dose strategies, routes of administration and durations of treatment with prednisone, prednisolone or other corticosteroid agent are compared in the treatment of SSNS in children, were included.
Types of participants
Inclusion criteria
Children aged three months to 18 years with SSNS (i.e. become oedema free with urine protein ≤ 1+ on dipstick, urinary protein/creatinine ratio ≤ 20 mg/mmol or ≤ 4 mg/m2/h for three consecutive days while receiving corticosteroid therapy). A kidney biopsy diagnosis of minimal change disease was not required for inclusion of the study.
Children with initial episode of SSNS
Children with relapsing SSNS
Exclusion criteria
Children with steroid‐resistant nephrotic syndrome (failure to achieve remission following four weeks or more of prednisone at 60 mg/m2/d) or congenital nephrotic syndrome
Children with other kidney or systemic forms of nephrotic syndrome defined on kidney biopsy, clinical features or serology (e.g. idiopathic membranous glomerulonephritis, mesangiocapillary glomerulonephritis, post‐infectious glomerulonephritis, Henoch‐Schönlein nephritis, systemic lupus erythematosus)
Types of interventions
Prednisone, prednisolone or other corticosteroid medication given orally or intravenously. The following aspects of the corticosteroid regimens were considered.
Shorter duration compared with two months of corticosteroid treatment
Longer durations compared with two or three months of corticosteroid treatment
Comparisons of different doses of corticosteroid medication given for induction of a remission
Comparisons of other regimens of corticosteroid therapy
Different corticosteroid agents (e.g. deflazacort, methylprednisolone) compared with standard agents (e.g. prednisone, prednisolone)
Comparisons of daily, alternate‐day or intermittent administration of corticosteroid medication. Intermittent administration refers to the administration of corticosteroids on three consecutive days of seven days
Single daily dose compared with divided daily doses of corticosteroid medication
Corticosteroid medication given with other agents for the first episode of steroid‐responsive nephrotic syndrome.
Types of outcome measures
Primary outcomes
The numbers of children with and without relapse at six months, 12 months and 24 months after completion of treatment.
The number of children who developed frequently relapsing nephrotic syndrome.
Secondary outcomes
The number of children who required other immunosuppressive therapy because of steroid toxicity
Mean relapse rates/patient
Serious adverse events including reduced growth rates, hypertension, cataracts/glaucoma, psychological disorders, infections, thromboses and osteoporosis
Cumulative corticosteroid dosage
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register up to 26 February 2015 through contact with the Trials Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of nephrology textbooks, review articles and relevant studies and CD‐ROMs and abstract books from nephrology meetings.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Conference proceedings of meetings of the International Pediatric Nephrology Association and European Society for Paediatric Nephrology.
Data collection and analysis
Selection of studies
The initial review was undertaken by four authors. The titles and abstracts were screened by two authors who discarded studies that were not relevant (i.e. studies of lipid lowering agents) although studies and reviews that could have included relevant data or information on studies were retained initially. Three authors independently assessed abstracts, and if necessary the full text, to determine which studies satisfied the characteristics required for inclusion. The 2003, 2005 and 2007 updates were undertaken by three authors (EH, NW, JC).
This 2015 update was undertaken by four authors (DH, EH, NW, JC). Potentially relevant studies were initially determined by two authors from titles and abstracts. Full text articles of potentially eligible articles were reviewed for eligibility by two authors.
Data extraction and management
Data extraction and assessment of risk of bias were performed by two authors using standardised data extraction forms. Studies in languages other than English were translated before data extraction. Where more than one report of a study was identified, data were extracted from all reports. Where there were discrepancies between reports, data from the primary source was used. Study authors were contacted for additional information about studies where possible.
Assessment of risk of bias in included studies
For this update, the following items were assessed during the risk of bias assessment tool (Higgins 2011).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (relapse or no relapse, side effects) the risk ratio (RR) for individual studies were calculated and summary statistics estimated using the random effects model and results compared to those obtained using a fixed effects model. Where continuous scales of measurement were used to assess the effects of treatment (cumulative steroid therapy, relapse rate), these data were analysed as the mean difference (MD) or standardised mean difference (SMD) if different scales had been used. The time to relapse was not included since many children did not experience relapse so the data would be biased.
Unit of analysis issues
Data from cross‐over studies were included in the meta‐analyses if separate data for the first part of the study were available. Otherwise results of cross‐over studies were reported in the text only.
Dealing with missing data
We aimed to analyse available data in meta‐analyses using ITT data. However, where ITT data were not provided, or additional information could not be obtained from authors, available published data were used in the analyses.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test with N‐1 degrees of freedom and an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
The search strategy used aimed to reduce publication bias caused by lack of publication of studies with negative results. Where there were several publications on the same study, all reports were reviewed to ensure that all details of methods and results were included to reduce the risk of selective outcome reporting bias.
Data synthesis
Data were combined using random effects model for dichotomous and continuous data.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to investigate between study differences based on risk of bias, differences between definitions of FRNS and different durations of treatment in the experimental group in studies comparing two months with three or more months of prednisone.
Sensitivity analysis
Where a single study differed considerably from the other studies in the meta‐analysis, this study was temporarily excluded to determine whether its removal altered the results of the meta‐analysis.
Results
Description of studies
Results of the search
Search results are shown in Figure 1.
1.
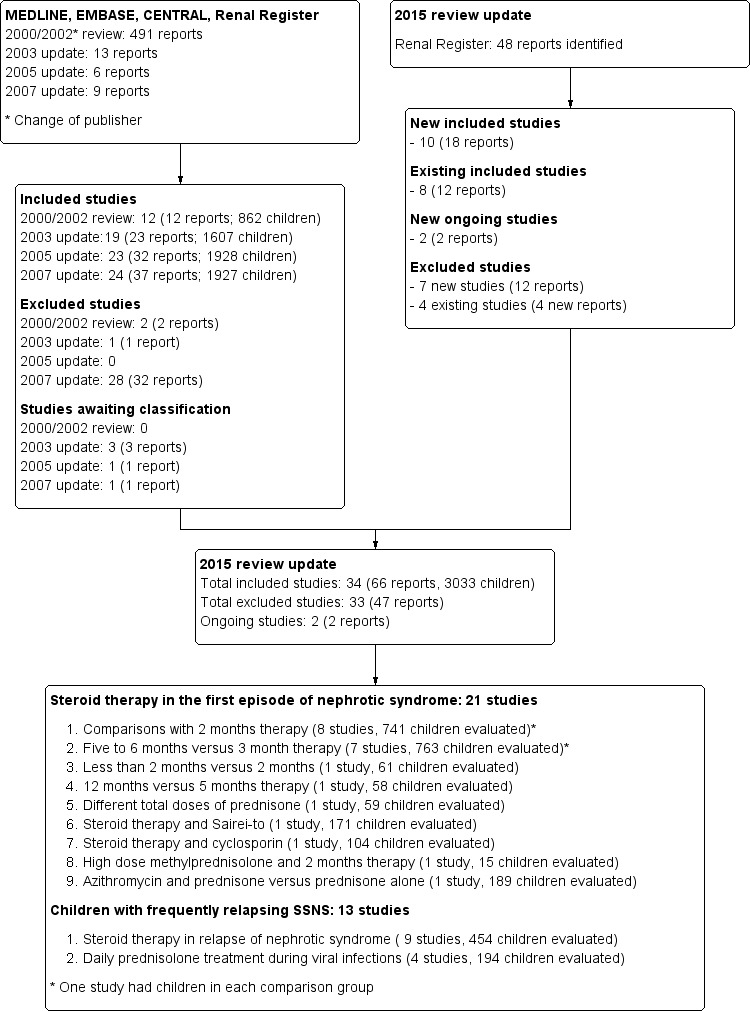
Study flow diagram.
For this latest update our search (to 26 February 2015) identified 48 potentially relevant reports. Of these, nine new studies (16 reports) (Abeyagunawardena 2008; Abeyagunawardena 2014; Gulati 2009; Liern 2008; Mishra 2012; Sinha 2014; Teeninga 2013; Yoshikawa 2014; Zhang 2014) were included. One study awaiting assessment (Mocan 1999) was also included. Our search also identified two studies that are underway; no results were yet available to include in our analysis (PREDNOS Study 2013, PREDNOS 2 Study 2014). We also found 12 reports of nine previously included studies (APN 1988; APN 1993; Bagga 1999; Broyer 1997; Ekka 1997; Hiraoka 2000; Norero 1996; Pecoraro 2003; Yoshikawa 1998). This update includes 34 studies (66 reports) that involved a total of 3033 participants.
For the search results of our previous reviews please see (Hodson 2002; Hodson 2003; Hodson 2005; Hodson 2007).
Included studies
The 34 included studies were divided into groups according to comparisons of corticosteroid regimens. Most studies used prednisone or prednisolone. For ease of reading, the term "prednisone" has been used in the text for both medications.
Prednisone treatment in first episode of nephrotic syndrome
Three months or more versus two months of therapy (741 evaluated children)
Seven studies (APN 1993; Bagga 1999; Jayantha 2002a; Ksiazek 1995; Norero 1996; Satomura 2001; Ueda 1988; Yoshikawa 2014) compared therapies of two months duration with regimens of three months or more. In all of these studies except Satomura 2001, increased duration of treatment resulted in increased total prednisone dose compared with the control group. Satomura 2001 compared three months of treatment with two months weeks using the same total dose of prednisone in each group. In Ksiazek 1995, which compared three different regimens, data from the two month therapy group and the experimental group treated for six months (experimental group 1) were included in the meta‐analysis. Norero 1996 excluded those children who became steroid dependent.
Five to six months versus three months of therapy (763 evaluated children)
Seven studies (Hiraoka 2003; Ksiazek 1995; Mishra 2012, Pecoraro 2003; Sharma 2000; Sinha 2014; Teeninga 2013) compared five or six months of prednisone with three months of therapy. In all studies except Teeninga 2013, increased duration of prednisone resulted in increased total prednisone dose compared with the control group. Teeninga 2013 compared three months with six months therapy, using the same total dose of prednisone in both groups. From Ksiazek 1995, data from the experimental groups treated for three months (experimental group 2) and six months (experimental group 1) were included in this analysis. Pecoraro 2003 had three groups ‐ a control group treated for three months and two experimental groups treated for six months with different total doses of prednisone. Only the control group and treatment group 1 were included in the meta‐analysis.
Less than two months versus two months of therapy (61 evaluated children)
APN 1988 compared less than the two month of prednisone with two months.
12 months versus five months of therapy (58 evaluated children)
Kleinknecht 1982 compared five months of prednisone with one year of therapy; the timing of the follow‐up period in relation to the duration of initial therapy was not stated.
Different total doses of prednisone (59 evaluated children)
Hiraoka 2000 compared different total doses of prednisone given for three months.
Prednisone and Sairei‐to therapy (171 evaluated children)
Yoshikawa 1998 compared two months of prednisone with 4.5 months but both groups received the Chinese herb, Sairei‐to. The assumption was made that the effect of the herb would be the same in both treatment groups. However because this assumption may not be correct, this study was considered separately from other studies comparing different durations of prednisone.
Prednisone and cyclosporin therapy (104 evaluated children)
APN 1999 compared three months of prednisone plus two months of cyclosporin with three months of prednisone only.
High dose oral methylprednisolone therapy (15 evaluated children)
Mocan 1999 compared high dose oral methylprednisolone given over two weeks with six months of prednisone therapy.
Prednisone and azithromycin therapy (211 evaluated children)
Zhang 2014 compared the addition of azithromycin to prednisone with prednisone alone at initiation of therapy in the first episode of nephrotic syndrome.
Relapsing nephrotic syndrome
Daily prednisone treatment during viral infections (194 evaluated children)
Three studies (Abeyagunawardena 2008; Gulati 2009; Mattoo 2000) compared daily with alternate‐day prednisone to prevent relapse during viral infections in children with SSNS receiving alternate‐day prednisone. One study (Abeyagunawardena 2014) compared daily prednisone with placebo to prevent relapse during upper respiratory tract infections in children with SSNS off prednisone.
Relapsing nephrotic syndrome: other interventions (454 evaluated children)
Nine studies investigated relapsing SSNS (APN 1981; Broyer 1997; Ekka 1997; Imbasciati 1985; ISKDC 1984; Jayantha 2002b; Leisti 1978; Li 1994; Liern 2008). Ekka 1997 and Li 1994 compared single daily with three times/day dosage of prednisone. The remaining studies explored different treatment regimens aimed at inducing remission, maintaining remission or both. Jayantha 2002b excluded children with steroid dependent nephrotic syndrome. Imbasciati 1985 and Jayantha 2002b included children with infrequently and frequently relapsing SSNS.
Excluded studies
In total, we excluded 34 studies (66 reports) after full text assessment for this review. Of the 34 excluded studies, five were not RCTs and 29 were RCTs involving non‐corticosteroid interventions in children with SSNS.
Risk of bias in included studies
Risk of bias assessments were performed using Cochrane's risk of bias assessment tool (Appendix 2). Summaries of risk of bias assessments are shown in Figure 2; Figure 3.
2.
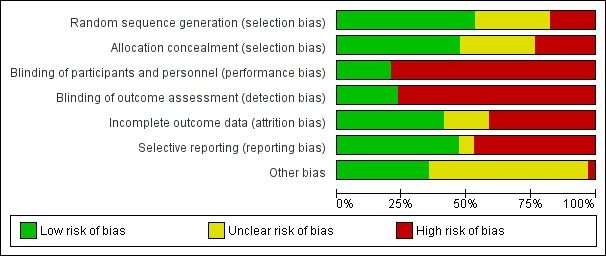
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
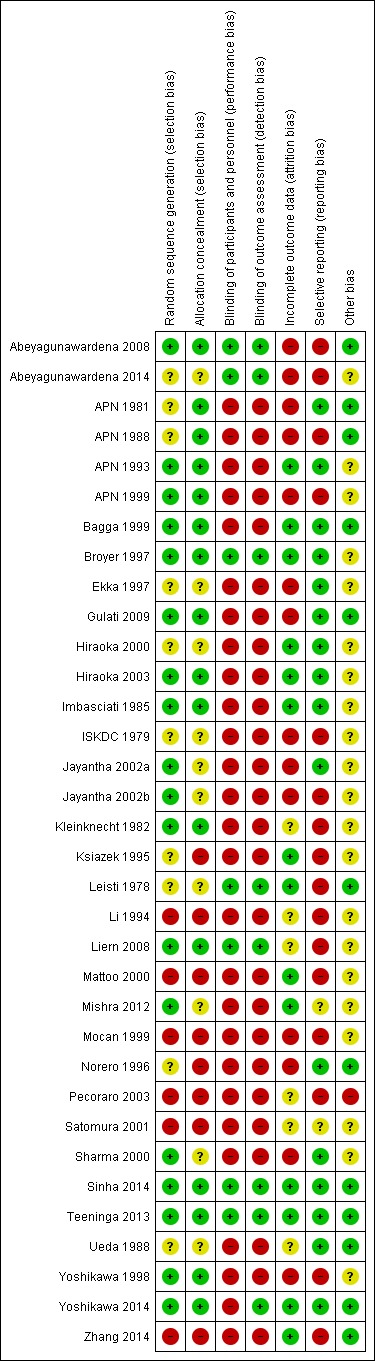
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation was considered to be at low risk of bias in 18 studies (Abeyagunawardena 2008; APN 1993; APN 1999; Bagga 1999; Broyer 1997; Gulati 2009; Hiraoka 2003; Imbasciati 1985; Jayantha 2002a; Jayantha 2002b; Kleinknecht 1982; Liern 2008; Mishra 2012; Sharma 2000; Sinha 2014; Teeninga 2013; Yoshikawa 1998; Yoshikawa 2014), and high risk in six studies (Li 1994; Mattoo 2000; Mocan 1999, Pecoraro 2003; Satomura 2001; Zhang 2014). Sequence generation methods was assessed as unclear in the remaining 10 studies.
Allocation concealment was considered to be at low risk of bias in 16 studies (Abeyagunawardena 2008; APN 1981; APN 1988; APN 1993; APN 1999; Broyer 1997; Gulati 2009; Hiraoka 2003; Imbasciati 1985; Kleinknecht 1982; Liern 2008; Sinha 2014; Teeninga 2013; Yoshikawa 1998; Yoshikawa 2014) and at high risk of bias in eight studies (Ksiazek 1995; Li 1994; Mattoo 2000; Mocan 1999; Norero 1996; Pecoraro 2003; Satomura 2001; Zhang 2014). Ksiazek 1995 stated that parents could influence which treatment group their child was assigned. Allocation concealment methods was assessed as unclear in the remaining 10 studies.
Blinding
Seven studies were considered to be at low risk of performance and detection bias because they were placebo controlled studies (Abeyagunawardena 2008; Abeyagunawardena 2014; Broyer 1997; Leisti 1978; Liern 2008; Sinha 2014; Teeninga 2013). Yoshikawa 2014 was an open‐label study but at low risk of detection bias. The remainder were at high risk of both performance and detection bias. Most studies reported the primary outcome of relapse using the ISKDC definition of relapse (ISKDC 1970).
Incomplete outcome data
We assessed 14 studies to be at low risk of attrition bias because they reported fewer than 10% of participants lost to follow‐up or excluded from analysis (APN 1993; Bagga 1999; Broyer 1997; Hiraoka 2000; Hiraoka 2003; Imbasciati 1985; Ksiazek 1995; Leisti 1978; Mattoo 2000; Mishra 2012; Sinha 2014; Teeninga 2013; Yoshikawa 2014; Zhang 2014). Fourteen studies considered at high risk of attrition bias because more than 10% of participants were lost to follow‐up or excluded from the analysis (Abeyagunawardena 2008; Abeyagunawardena 2014; APN 1981; APN 1988; APN 1999; Ekka 1997; Gulati 2009; ISKDC 1979; Jayantha 2002a; Jayantha 2002b; Mocan 1999; Norero 1996; Sharma 2000; Yoshikawa 1998). The remaining six studies were considered to be unclear risk of attrition bias.
Selective reporting
Studies were deemed to be at risk of reporting bias if outcome data did not include one or more outcomes of frequently relapsing nephrotic syndrome, relapse rate and adverse events. Studies were also considered to be at high risk of bias if data were provided in a format, which could not be entered into the meta‐analyses. Cross‐over studies were considered to be at high risk of bias if data from the first and second parts of the study were not separable. Sixteen studies were at low risk of reporting bias (APN 1981; APN 1993; Bagga 1999; Broyer 1997; Ekka 1997; Gulati 2009; Hiraoka 2000; Hiraoka 2003; Imbasciati 1985; Jayantha 2002a; Norero 1996; Sharma 2000; Sinha 2014; Teeninga 2013; Ueda 1988; Yoshikawa 2014). There were 16 studies at high risk of selective reporting bias (Abeyagunawardena 2008; Abeyagunawardena 2014; APN 1988; APN 1999; ISKDC 1979; Jayantha 2002b; Kleinknecht 1982; Ksiazek 1995; Leisti 1978; Li 1994; Liern 2008; Mattoo 2000; Mocan 1999; Pecoraro 2003; Yoshikawa 1998; Zhang 2014). Mishra 2012 and Satomura 2001 were assessed to be at unclear risk of selective reporting bias
Other potential sources of bias
Twelve studies were considered at low risk of potential bias as they were funded educational or philanthropic organisations (Abeyagunawardena 2008; APN 1981; APN 1988; Bagga 1999; Gulati 2009; Leisti 1978; Norero 1996; Sinha 2014; Teeninga 2013; Ueda 1988; Yoshikawa 2014; Zhang 2014). One study was considered to be at high risk of bias as it was funded by industry and no full‐text publication has been identified 10 years after the first conference abstract (Pecoraro 2003). The remaining 21 studies were deemed unclear of other risk of bias as no information on funding sources was provided.
In Ueda 1988 the calculated total protocol dose (4620 mg/m2) exceeded the dose administered (3132 ± 417 mg/m2) suggesting that the protocol was not adhered to in all patients. In three studies (Jayantha 2002a; Ksiazek 1995; Ueda 1988) the numbers of children in the treatment and control groups differed markedly.
Effects of interventions
Summary of findings for the main comparison. Steroid therapy in first episode of nephrotic syndrome: three months of more versus two months for nephrotic syndrome in children.
| Steroid therapy in first episode of nephrotic syndrome: 3 months of more versus 2 months for nephrotic syndrome in children | ||||||
| Patient or population: patients with nephrotic syndrome in children Settings: Intervention: steroid therapy in first episode of nephrotic syndrome: 3 months of more versus 2 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Steroid therapy in first episode of nephrotic syndrome: 3 months of more compared with 2 months | |||||
| Number with frequent relapses by 12 to 24 months | Study population | RR 0.68 (0.47 to 1) | 582 (6) | ⊕⊕⊝⊝ low1,2 | ||
| 358 per 1000 | 243 per 1000 (168 to 358) | |||||
| Moderate | ||||||
| 359 per 1000 | 244 per 1000 (169 to 359) | |||||
| Number of children relapsing by 12 to 24 months | Study population | RR 0.8 (0.64 to 1) | 741 (8) | ⊕⊕⊝⊝ low1,2 | ||
| 668 per 1000 | 535 per 1000 (428 to 668) | |||||
| Moderate | ||||||
| 647 per 1000 | 518 per 1000 (414 to 647) | |||||
| Number with frequent relapses by 12 to 24 months stratified by risk of bias for allocation concealment: low risk of bias for allocation concealment | Study population | RR 0.92 (0.69 to 1.23) | 362 (3) | ⊕⊕⊕⊕ high | ||
| 359 per 1000 | 330 per 1000 (247 to 441) | |||||
| Moderate | ||||||
| 348 per 1000 | 320 per 1000 (240 to 428) | |||||
| Number with frequent relapses by 12 to 24 months stratified by risk of bias for allocation concealment: unclear or high risk of bias for allocation bias | Study population | RR 0.45 (0.26 to 0.77) | 220 (3) | ⊕⊕⊕⊝ moderate1 | ||
| 357 per 1000 | 161 per 1000 (93 to 275) | |||||
| Moderate | ||||||
| 371 per 1000 | 167 per 1000 (96 to 286) | |||||
| Adverse events: psychological disorders | Study population | RR 2.18 (0.43 to 11.13) | 233 (3) | ⊕⊕⊝⊝ low1,3 | ||
| 18 per 1000 | 40 per 1000 (8 to 202) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse events: hypertension | Study population | RR 1.79 (0.47 to 6.86) | 456 (6) | ⊕⊕⊕⊝ moderate1 | ||
| 60 per 1000 | 107 per 1000 (28 to 410) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse events: eye complications | Study population | RR 0.32 (0.07 to 1.42) | 400 (5) | ⊕⊕⊕⊝ moderate1 | ||
| 43 per 1000 | 14 per 1000 (3 to 62) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Some studies at high or unclear risk of bias 2 Significant heterogeneity between studies 3 Few studies included in analysis
Summary of findings 2. Steroid therapy in first episode of nephrotic syndrome: five or six months versus three months for nephrotic syndrome in children.
| Steroid therapy in first episode of nephrotic syndrome: 5 to 6 months versus 3 months for nephrotic syndrome in children | ||||||
| Patient or population: patients with nephrotic syndrome in children Settings: Intervention: steroid therapy in first episode of nephrotic syndrome: 5 to 6 months versus 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Steroid therapy in first episode of nephrotic syndrome: 5 to 6 months verus 3 months | |||||
| Number with frequent relapses by 12 to 24 months | Study population | RR 0.78 (0.5 to 1.22) | 591 (5) | ⊕⊕⊝⊝ low1 | ||
| 363 per 1000 | 283 per 1000 (182 to 443) | |||||
| Moderate | ||||||
| 393 per 1000 | 307 per 1000 (196 to 479) | |||||
| Number of children relapsing by 12 to 24 months | Study population | RR 0.62 (0.45 to 0.85) | 763 (7) | ⊕⊕⊝⊝ low1,2 | ||
| 694 per 1000 | 430 per 1000 (312 to 590) | |||||
| Moderate | ||||||
| 703 per 1000 | 436 per 1000 (316 to 598) | |||||
| Subgroup analysis by risk of bias for number with frequent relapses: low risk of bias for allocation concealment | Study population | RR 1 (0.74 to 1.34) | 377 (3) | ⊕⊕⊕⊕ high | ||
| 438 per 1000 | 438 per 1000 (324 to 587) | |||||
| Moderate | ||||||
| 441 per 1000 | 441 per 1000 (326 to 591) | |||||
| Subgroup analysis by risk of bias for number with frequent relapses: Unclear or high risk of bias for allocation concealment | Study population | RR 0.36 (0.18 to 0.72) | 214 (2) | ⊕⊕⊕⊝ moderate2 | ||
| 234 per 1000 | 84 per 1000 (42 to 168) | |||||
| Moderate | ||||||
| 185 per 1000 | 67 per 1000 (33 to 133) | |||||
| Adverse events: hypertension | Study population | RR 1.37 (0.91 to 2.05) | 636 (5) | ⊕⊕⊕⊝ moderate2 | ||
| 111 per 1000 | 153 per 1000 (101 to 229) | |||||
| Moderate | ||||||
| 114 per 1000 | 156 per 1000 (104 to 234) | |||||
| Adverse events: eye complications | Study population | RR 0.46 (0.18 to 1.17) | 614 (5 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 36 per 1000 | 17 per 1000 (6 to 42) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse events: cushingoid appearance | Study population | RR 0.92 (0.62 to 1.36) | 646 (5) | ⊕⊕⊕⊝ moderate2 | ||
| 365 per 1000 | 336 per 1000 (226 to 496) | |||||
| Moderate | ||||||
| 386 per 1000 | 355 per 1000 (239 to 525) | |||||
| Adverse events: psychological disorders | Study population | RR 0.38 (0.03 to 4.39) | 389 (3) | ⊕⊕⊝⊝ low2,3 | ||
| 48 per 1000 | 18 per 1000 (1 to 209) | |||||
| Moderate | ||||||
| 46 per 1000 | 17 per 1000 (1 to 202) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Significant heterogeneity between studies
2 Some studies at high or unclear risk of bias
3 Few studies included in analyses
Outcome of children in their first episode of SSNS
Three months or more versus two months therapy
The risk of frequently relapsing nephrotic syndrome (FRNS) was significantly lower with prolonged duration of prednisone compared with two months (Analysis 1.1 (6 studies, 582 children): RR 0.68, 95% CI 0.47 to 1.00; I2 = 36%).
1.1. Analysis.
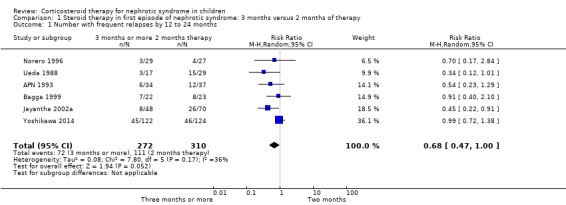
Comparison 1 Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy, Outcome 1 Number with frequent relapses by 12 to 24 months.
The number of children relapsing by 12 to 24 months and the mean relapse rate/patient/year were significantly lower with prolonged duration of prednisone compared with two months (Analysis 1.2 (8 studies, 741 children): RR 0.80, 95% CI 0.64 to 1.00; I2 = 66%); Analysis 1.3 (4 studies, 295 children): MD ‐0.65, 95% CI ‐1.29 to ‐0.00; I2 = 88%). Cumulative prednisone dose did not differ significantly between groups (Analysis 1.4 (3 studies, 245 children): MD 0.71 g/m2, 95% CI ‐0.67 to 2.09; I2 = 60%).
1.2. Analysis.
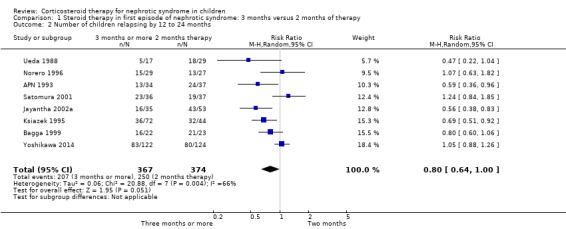
Comparison 1 Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy, Outcome 2 Number of children relapsing by 12 to 24 months.
1.3. Analysis.
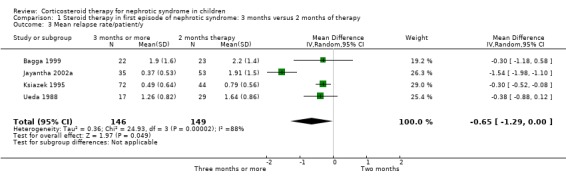
Comparison 1 Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy, Outcome 3 Mean relapse rate/patient/y.
1.4. Analysis.
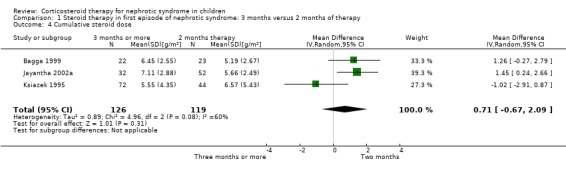
Comparison 1 Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy, Outcome 4 Cumulative steroid dose.
There was medium to high levels of heterogeneity between studies in all analyses.
The heterogeneity was not explained by inclusion/exclusion of patients with steroid‐dependent disease, different durations of prednisone (three months versus more than three months) or different definitions of FRNS (ISKDC definition compared with other definitions) (Analysis 1.5).
1.5. Analysis.
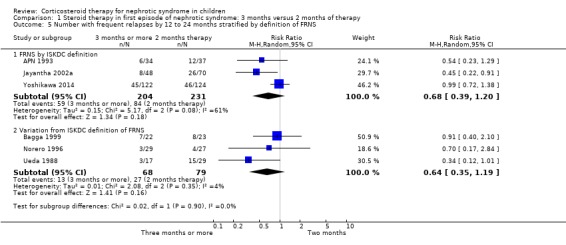
Comparison 1 Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy, Outcome 5 Number with frequent relapses by 12 to 24 months stratified by definition of FRNS.
Subgroup analysis based on risk of bias components (allocation concealment, attrition bias) indicated that there was no significant difference in the risk of FRNS in studies at low risk of bias for allocation concealment (Analysis 1.6.1 (3 studies, 362 children): RR 0.92, 95% CI 0.69 to 1.23; I2 = 0%). In contrast the risk of FRNS was significantly increased in studies at high or unclear risk of bias for allocation concealment (Analysis 1.6.2 (3 studies, 220 children): RR 0.45, 95% CI 0.26 to 0.77; I2 = 0%). The data for attrition bias are identical and are not shown. Thus heterogeneity between studies was explained by differences in risk of bias between studies.
1.6. Analysis.
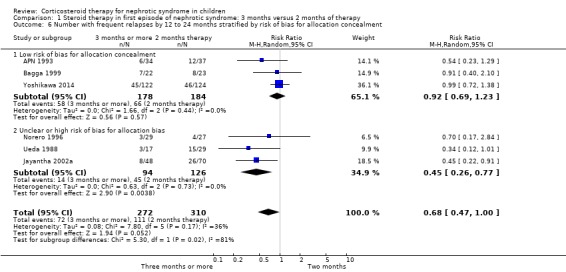
Comparison 1 Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy, Outcome 6 Number with frequent relapses by 12 to 24 months stratified by risk of bias for allocation concealment.
Serious adverse events (growth retardation, hypertension, cataracts/glaucoma, psychological disorders, osteoporosis, infections, features of Cushing's Syndrome) were not significantly different between regimens (Analysis 1.7). In Yoshikawa 2014, results were reported as events not patients and are not included in the meta‐analyses. The authors reported that frequency and severity of adverse events were similar in both groups.
1.7. Analysis.
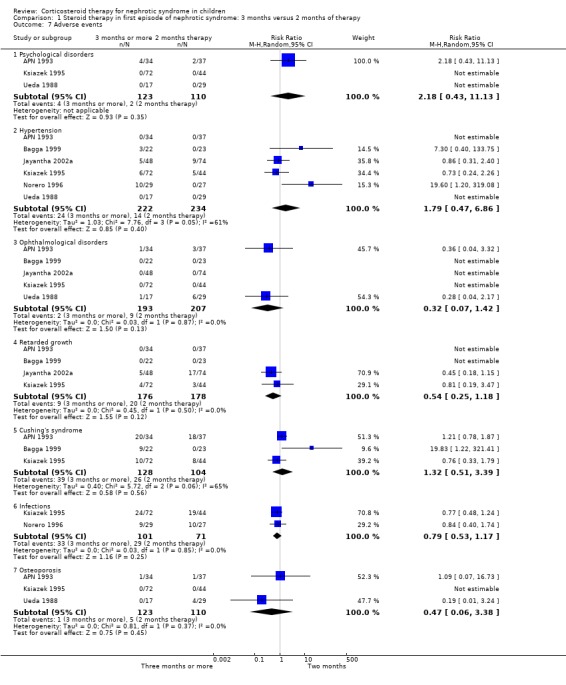
Comparison 1 Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy, Outcome 7 Adverse events.
Five or six months versus three months therapy
There was no significant difference in the risk of FRNS between prednisone treatment for five or six months compared with three months (Analysis 2.1 (5 studies, 591 children): RR 0.78, 95% CI 0.50 to 1.22; I2 = 67%).
2.1. Analysis.
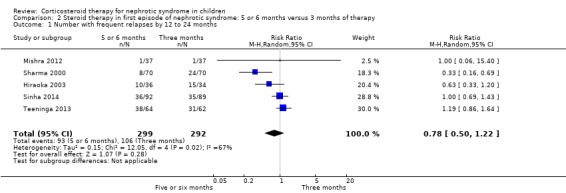
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 1 Number with frequent relapses by 12 to 24 months.
Prednisone given for five to six months significantly reduced the risk of relapse by 12 to 24 months compared with three months (Analysis 2.2 (7 studies, 763 children): RR 0.62, 95% CI 0.45 to 0.85; I2 = 83%) and the mean relapse rate/patient/year was significantly reduced (Analysis 2.3 (3 studies, 460 children): MD ‐0.39, 95% CI ‐0.64 to ‐0.14; I2 = 40%). Cumulative prednisone dose did not differ significantly between groups (Analysis 2.4 (3 studies, 460 children): MD ‐0.47, 95% CI ‐1.67 to 0.73; I2 = 85%).
2.2. Analysis.
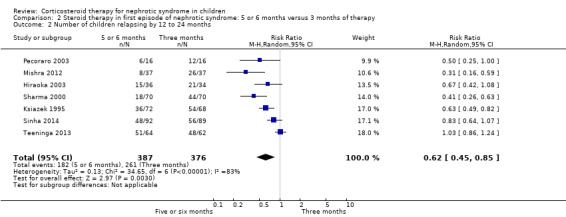
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 2 Number of children relapsing by 12 to 24 months.
2.3. Analysis.
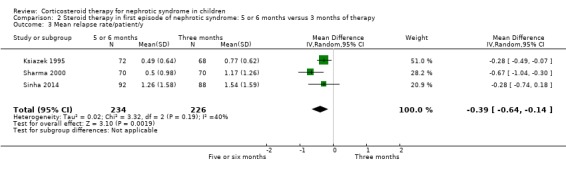
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 3 Mean relapse rate/patient/y.
2.4. Analysis.
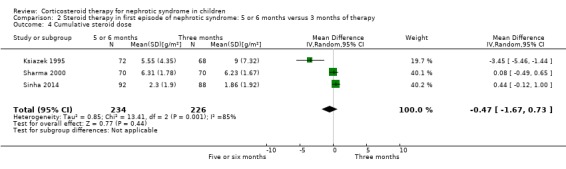
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 4 Cumulative steroid dose.
There was medium to high levels of heterogeneity among studies in all analyses.
The heterogeneity was not explained by inclusion/exclusion of patients with steroid dependent disease or different definitions of FRNS (ISKDC definition compared with other definitions) (Analysis 2.5).
2.5. Analysis.
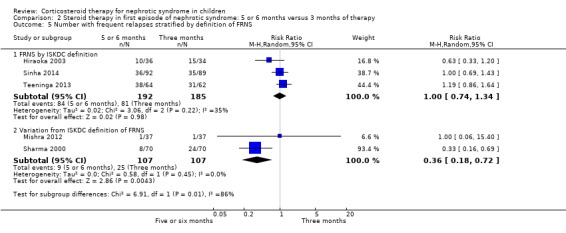
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 5 Number with frequent relapses stratified by definition of FRNS.
Subgroup analysis of the risk for FRNS was performed based on risk of bias for allocation concealment, blinding (performance/detection bias) and attrition. There was no significant difference in the risk of FRNS in studies at low risk of bias for allocation concealment (Analysis 2.6.1 (3 studies, 377 children): RR 1.00, 95% CI 0.74 to 1.34; I2 = 35%). In contrast the risk of FRNS was significantly increased in studies at high or unclear risk of bias for allocation concealment (Analysis 2.6.2 (2 studies, 14 children): RR 0.36, 95% CI 0.18 to 0.72; I2 = 0%). Similarly there was no significant difference in the risk of FRNS in studies at low risk of performance/detection bias or attrition bias (Analysis 2.7.1; Analysis 2.8.1) while there was a significant reduction in the risk of FRNS in studies at unclear or high risk of performance/detection bias or attrition bias (Analysis 2.7.2; Analysis 2.8.2). Thus heterogeneity between studies was explained by differences in risk of bias among studies.
2.6. Analysis.
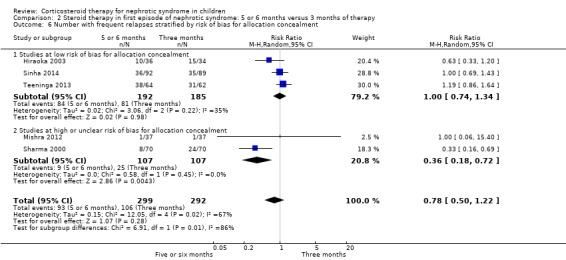
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 6 Number with frequent relapses stratified by risk of bias for allocation concealment.
2.7. Analysis.
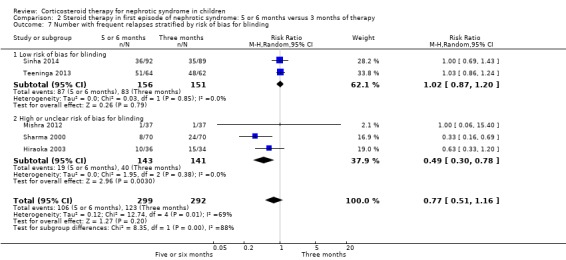
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 7 Number with frequent relapses stratified by risk of bias for blinding.
2.8. Analysis.
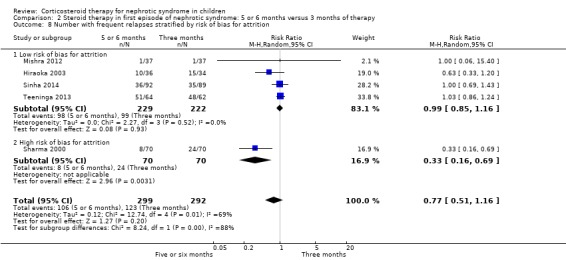
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 8 Number with frequent relapses stratified by risk of bias for attrition.
Adverse effects did not differ significantly between groups (Analysis 2.9).
2.9. Analysis.
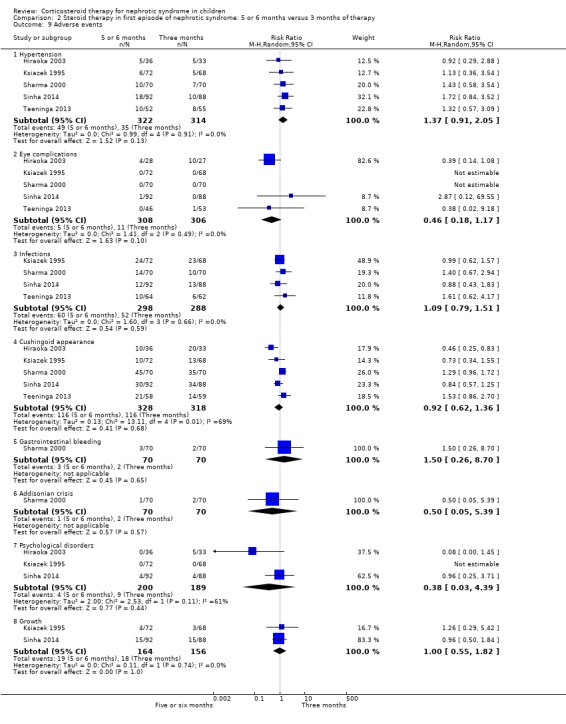
Comparison 2 Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy, Outcome 9 Adverse events.
One month versus two months therapy
APN 1988 showed that prednisone duration less than the two month regimen resulted in a significantly higher relapse rate at six and 12 months (Analysis 3.1 (1 study, 61 children): RR 1.60, 95% CI 1.01 to 2.54; Analysis 3.2 (1 study, 60 children): RR 1.46, 95% CI 1.01 to 2.12). There was no significant differences in the risk for FRNS (Analysis 3.3) and the cumulative prednisone dose (Analysis 3.4).
3.1. Analysis.
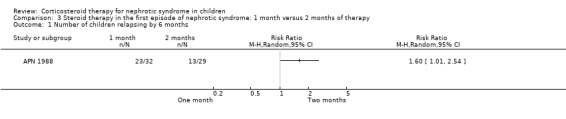
Comparison 3 Steroid therapy in the first episode of nephrotic syndrome: 1 month versus 2 months of therapy, Outcome 1 Number of children relapsing by 6 months.
3.2. Analysis.
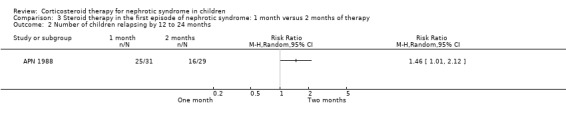
Comparison 3 Steroid therapy in the first episode of nephrotic syndrome: 1 month versus 2 months of therapy, Outcome 2 Number of children relapsing by 12 to 24 months.
3.3. Analysis.
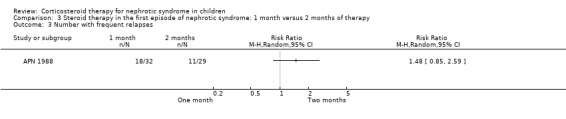
Comparison 3 Steroid therapy in the first episode of nephrotic syndrome: 1 month versus 2 months of therapy, Outcome 3 Number with frequent relapses.
3.4. Analysis.
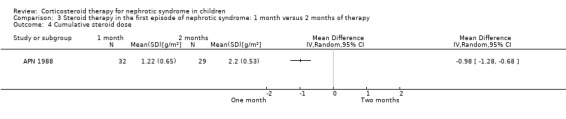
Comparison 3 Steroid therapy in the first episode of nephrotic syndrome: 1 month versus 2 months of therapy, Outcome 4 Cumulative steroid dose.
Five months versus 12 months therapy
Kleinknecht 1982 showed no evidence that the relapse rate was significantly reduced by giving prednisone for one year compared with five months (Analysis 4.1).
4.1. Analysis.
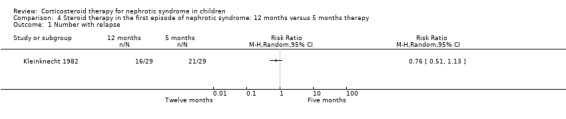
Comparison 4 Steroid therapy in the first episode of nephrotic syndrome: 12 months versus 5 months therapy, Outcome 1 Number with relapse.
Different total doses of prednisone
Hiraoka 2000 used different total prednisone doses in each group with both groups receiving treatment for three months. The number of children relapsing by 12 months was significantly reduced in children treated with the higher dose (Analysis 5.1 (1 study, 59 children): RR 0.63, 95% CI 0.42 to 0.94). However there was no significant difference in the risk for FRNS (Analysis 5.2 (1 study, 60 children): RR 0.69, 95% CI 0.35 to 1.37). Adverse effects did not differ between groups (Analysis 5.3).
5.1. Analysis.

Comparison 5 Steroid therapy in the first episode of nephrotic syndrome: different total doses given over same duration, Outcome 1 Relapse at twelve months.
5.2. Analysis.
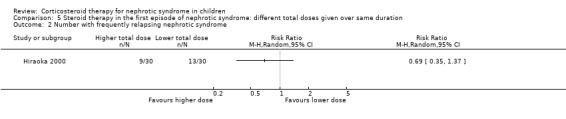
Comparison 5 Steroid therapy in the first episode of nephrotic syndrome: different total doses given over same duration, Outcome 2 Number with frequently relapsing nephrotic syndrome.
5.3. Analysis.
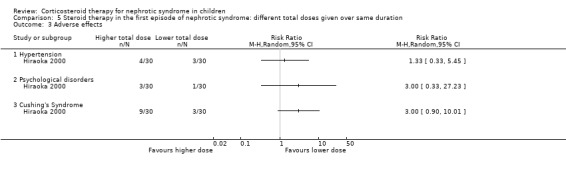
Comparison 5 Steroid therapy in the first episode of nephrotic syndrome: different total doses given over same duration, Outcome 3 Adverse effects.
Two month steroid therapy and Sairei‐to compared with 4.5 months prednisone and Sairei‐to
Yoshikawa 1998 showed no significant difference in relapse rate at two years or in the number of children who relapsed frequently between two months and four and a half months of prednisone when both groups received the Chinese herb, Sairei‐to (Analysis 6.1).
6.1. Analysis.
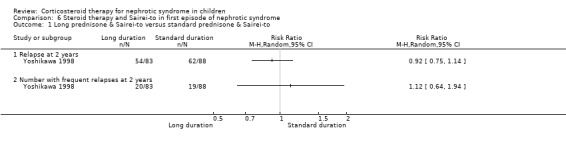
Comparison 6 Steroid therapy and Sairei‐to in first episode of nephrotic syndrome, Outcome 1 Long prednisone & Sairei‐to versus standard prednisone & Sairei‐to.
Three month steroid therapy and cyclosporin compared with 3 months prednisone
APN 1999 showed that the addition of cyclosporin to 12 weeks of prednisone therapy reduced the risk for relapse at six months (Analysis 7.1 (1 study, 104 children): RR 0.33, 95% CI 0.33 to 0.83) and at 12 months (Analysis 7.2 (1 study, 104 children): RR 0.72, 95% CI 0.46 to 1.13). The numbers needing cytotoxic therapy were not significantly different between groups (Analysis 7.3 (1 study, 104 children): RR 0.47, 95% CI 0.18 to 1.23). The median time of cumulative sustained remission after completing initial therapy was 22.8 months (95% CI 11.6 to 34) in the cyclosporin/prednisone group and 12.5 months (95% CI 5.9 to 19.1) in the prednisone only group. There was a delay in the time to relapse at six and 12 months but there were no differences at 18 and 24 months. The mean relapse rates/patient at six and 12 months of 0.12 and 0.63 in the cyclosporin/prednisone group were significantly lower than those in the prednisone‐only group (0.57 and 1.03) but there was no difference at 18 and 24 months after therapy. Serum creatinine did not differ at the end of follow‐up (Analysis 7.4). Temporary hirsutism and gum hypertrophy were seen in 60% and 9% of children given cyclosporin. Psychological disturbances occurred in 27% of cyclosporin‐treated patients compared with 14% of patients treated with prednisone alone. Mean systolic and diastolic blood pressures were increased by 10 mmHg and 8 mmHg respectively during cyclosporin therapy.
7.1. Analysis.
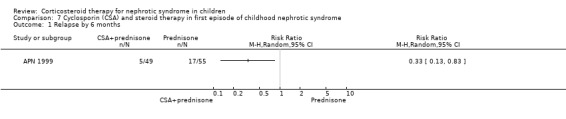
Comparison 7 Cyclosporin (CSA) and steroid therapy in first episode of childhood nephrotic syndrome, Outcome 1 Relapse by 6 months.
7.2. Analysis.
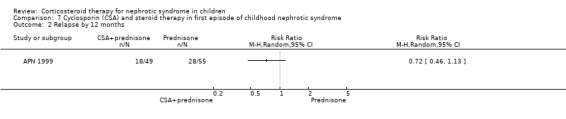
Comparison 7 Cyclosporin (CSA) and steroid therapy in first episode of childhood nephrotic syndrome, Outcome 2 Relapse by 12 months.
7.3. Analysis.
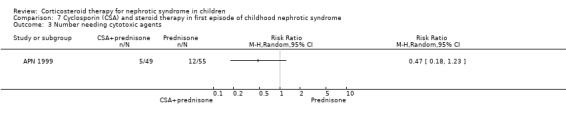
Comparison 7 Cyclosporin (CSA) and steroid therapy in first episode of childhood nephrotic syndrome, Outcome 3 Number needing cytotoxic agents.
7.4. Analysis.
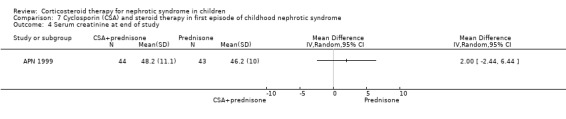
Comparison 7 Cyclosporin (CSA) and steroid therapy in first episode of childhood nephrotic syndrome, Outcome 4 Serum creatinine at end of study.
High dose oral methylprednisolone
Mocan 1999 showed no significant difference in the time to relapse and the relapse rate at one year in patients receiving high dose oral methylprednisolone given over two weeks versus six months of prednisone therapy (Analysis 8.1 (1 study, 15 children): MD ‐8.10, 95% CI ‐30.51 to 14.31); Analysis 8.2 (1 study, 15 children): MD 0.00, 95% CI ‐0.27 to 0.27). However the mean time to remission was significantly shorter in the methylprednisolone group (Analysis 8.3 (1 study, 15 children): MD ‐7.70, 95% CI ‐13.24 to ‐2.16).
8.1. Analysis.
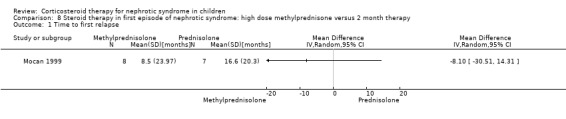
Comparison 8 Steroid therapy in first episode of nephrotic syndrome: high dose methylprednisone versus 2 month therapy, Outcome 1 Time to first relapse.
8.2. Analysis.
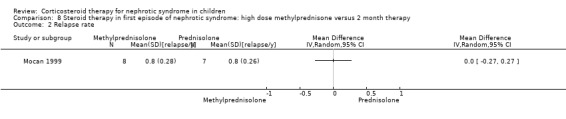
Comparison 8 Steroid therapy in first episode of nephrotic syndrome: high dose methylprednisone versus 2 month therapy, Outcome 2 Relapse rate.
8.3. Analysis.
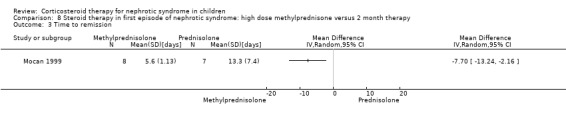
Comparison 8 Steroid therapy in first episode of nephrotic syndrome: high dose methylprednisone versus 2 month therapy, Outcome 3 Time to remission.
Azithromycin and prednisone versus prednisone alone
Zhang 2014 found no significant difference in the risk of relapse by six months between prednisone with azithromycin and prednisone alone (Analysis 9.1).
9.1. Analysis.
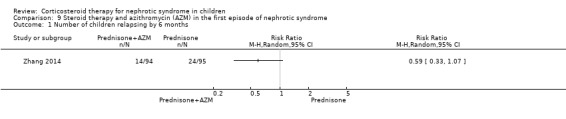
Comparison 9 Steroid therapy and azithromycin (AZM) in the first episode of nephrotic syndrome, Outcome 1 Number of children relapsing by 6 months.
Outcome of children with frequently relapsing SSNS
Daily prednisone treatment during viral infections
Abeyagunawardena 2008 demonstrated in a cross‐over study that daily prednisone administered during an infection significantly reduced the risk of relapse compared with continuing alternate‐day prednisone (Analysis 10.1 (1 study, 40 children): RR 0.49, 95% CI 0.18 to 1.30; first part of study).
10.1. Analysis.
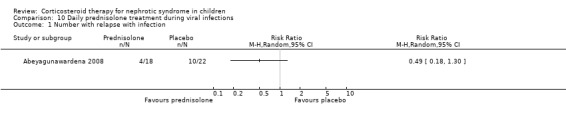
Comparison 10 Daily prednisolone treatment during viral infections, Outcome 1 Number with relapse with infection.
Gulati 2009 reported a significant reduction in infection related relapses per patient year (Analysis 10.2.1 (1 study, 95 children): MD ‐0.70, 95% CI ‐0.87 to ‐0.53) and in the total number of relapses/patient‐year (Analysis 10.2.2 (1 study, 95 children): MD ‐0.90, 95% CI ‐1.08 to ‐0.72) in children receiving daily prednisone during infections compared with alternate daily prednisone.
10.2. Analysis.
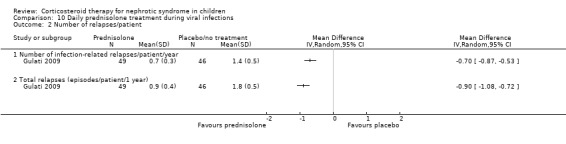
Comparison 10 Daily prednisolone treatment during viral infections, Outcome 2 Number of relapses/patient.
Mattoo 2000 showed a significant reduction in the total relapse episodes/patient at two years (Analysis 10.3 (1 study, 36 participants): MD ‐3.30, 95% CI ‐4.03 to ‐2.57) in children receiving daily prednisone during infections compared with alternate‐day prednisone.
10.3. Analysis.
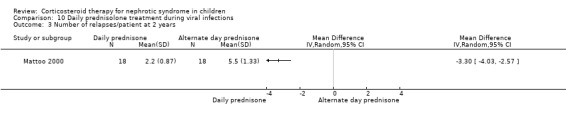
Comparison 10 Daily prednisolone treatment during viral infections, Outcome 3 Number of relapses/patient at 2 years.
Abeyagunawardena 2014 found in a cross‐over study that children with SSNS off prednisone for at least three months had fewer relapses when administered prednisone for five days at the onset of upper respiratory tract infection (URTI) compared with placebo (11 relapses in 113 URTI versus 25 relapses in 101 URTI; P = 0.014). 65.5% of children in the prednisone group had no relapses compared with 40.6% in the placebo group.
Other comparisons of prednisone usage
Nine studies included children with relapsing SSNS (APN 1981; Broyer 1997; Ekka 1997; Imbasciati 1985; ISKDC 1979; Jayantha 2002b; Leisti 1978; Li 1994; Liern 2008) (Table 15).
1. Outcomes of studies in children with relapsing nephrotic syndrome.
| Study ID | Relapse on therapy (RR (95% CI)) | Relapse by 9 months (RR (95% CI)) | Relapse by 12 months (RR (95% CI)) | Mean relapse rate MD (95% CI)) | Mean time to remission (MD (95% CI) | Steroid therapy |
| APN 1981 | 0.60 (0.36 to 1.02) | 1.20 (0.93 to 1.55) | ‐0.20 (‐0.65 to 0.25) | Alternate‐day versus intermittent | ||
| Broyer 1997 | 0.44 (0.25 to 0.78) | ‐1.90 (‐2.77 to ‐1.03) | Deflazacort versus prednisone | |||
| Ekka 1997 | 1.07 (0.77 to 1.50) | Daily versus divided dose prednisone | ||||
| Imbasciati 1985 | 1.06 (0.75 to 1.52) | IV and oral versus oral prednisone | ||||
| ISKDC 1979 | 0.20 (0.05 to 0.82) | 1.00 (0.89 to 1.12) | 0.54 (‐0.50 to 1.58) | Daily versus intermittent prednisone | ||
| Jayantha 2002b | 0.43 (0.29 to 0.65) | ‐1.78 (‐2.30 to ‐1.26) | 7 months prednisone versus standard ISKDC regimen for relapse | |||
| Leisti 1978 | Cortisol versus placebo in FRNS (cross‐over): 5/13 (38%) on cortisol relapsed versus 12/13 (92%) on placebo | |||||
| Li 1994 | 0.04 days (‐0.98 to 1.06) | Daily versus divided dose prednisone | ||||
| Liern 2008 | Time to relapse (cross‐over): deflazacort 105 ± 4.19 days versus methylprednisolone 85 ± 3.8 days |
FRNS ‐ frequently relapsing nephrotic syndrome; ISKDC ‐ International Study of Kidney Disease in Children
Alternate‐day therapy (APN 1981) was more effective than intermittent therapy in maintaining remission in frequently relapsing children during six months of therapy (Analysis 11.1.1: RR 0.60, 95% CI 0.36 to 1.02) but there was no difference by 12 months (Analysis 11.2.1: RR 1.20, 95% CI 0.93 to 1.55).
11.1. Analysis.
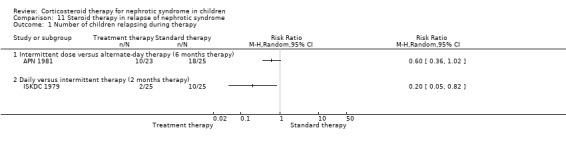
Comparison 11 Steroid therapy in relapse of nephrotic syndrome, Outcome 1 Number of children relapsing during therapy.
11.2. Analysis.
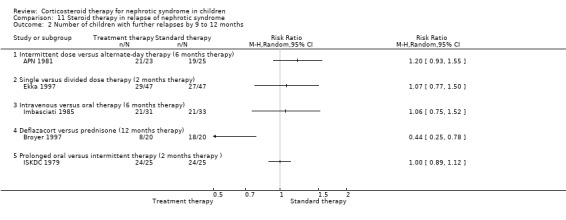
Comparison 11 Steroid therapy in relapse of nephrotic syndrome, Outcome 2 Number of children with further relapses by 9 to 12 months.
Single daily dosing (Ekka 1997) was as effective as multiple daily dosing in maintaining remission in children who relapsed frequently (Analysis 11.2.1: RR 1.07, 95% CI 0.77 to 1.50) with no significant difference in the mean relapse rate/patient (Analysis 11.3.1 (94 children): MD ‐0.20, 95% CI ‐0.64 to 0.24). The time to remission did not differ between single and multiple daily dosing patient groups (Analysis 11.6 (2 studies, 138 children): MD 0.04 days, 95% CI ‐0.98 to 1.06; I2 = 0%). Serious side effects including hypertension were less common in the single daily dose patients compared with divided dose patients (Analysis 11.7 (2 studies, 138 children): RR 0.41; 95% CI 0.18 to 0.91; I2 = 0%). In one study, cushingoid features and obesity were less common in the single daily dose group (Li 1994).
11.3. Analysis.
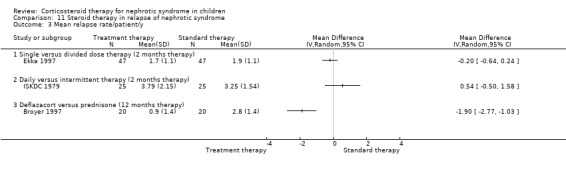
Comparison 11 Steroid therapy in relapse of nephrotic syndrome, Outcome 3 Mean relapse rate/patient/y.
11.6. Analysis.
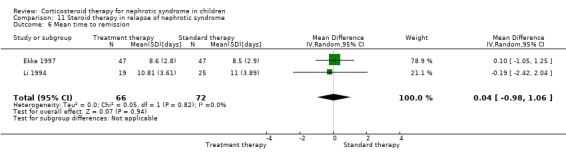
Comparison 11 Steroid therapy in relapse of nephrotic syndrome, Outcome 6 Mean time to remission.
11.7. Analysis.
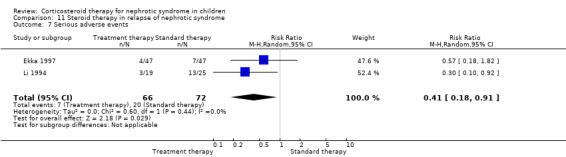
Comparison 11 Steroid therapy in relapse of nephrotic syndrome, Outcome 7 Serious adverse events.
Deflazacort (Broyer 1997) significantly reduced the number of children who relapsed during therapy (Analysis 11.2.4 (40 children): RR 0.44, 95% CI 0.25 to 0.78) and reduced the relapse rate among those who relapsed (Analysis 11.3.3 (40 children): MD ‐1.90, 95% CI ‐2.77 to ‐1.03) without significant differences in side effects.
The mean time to relapse in a cross‐over study (Liern 2008), comparing alternate‐day methylprednisolone with an equivalent dose of deflazacort after the first relapse, was longer in deflazacort treated patients (105 ± 4.19 days) compared with those treated with methylprednisolone (85 ± 3.8 days). There was no differences in the mean time to remission.
Children (ISKDC 1979) relapsed significantly less frequently during treatment on daily prednisone compared with intermittent therapy (Analysis 11.1.2 (50 children): RR 0.20, 95% CI 0.05 to 0.82) but the numbers with relapse (Analysis 11.2.5 (50 children): RR 1.00, 95% CI 0.89 to 1.12) and the mean relapse rate/patient did not differ by nine months after treatment (Analysis 11.3.2 (50 children): MD 0.54, 95% CI ‐0.50 to 1.58). During treatment the mean time to relapse was significantly longer in children treated with daily prednisone (Analysis 11.4.2 (50 children): MD 1.79, 95% CI 0.90 to 2.68).
11.4. Analysis.
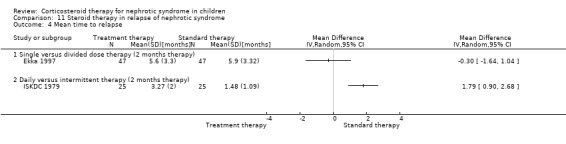
Comparison 11 Steroid therapy in relapse of nephrotic syndrome, Outcome 4 Mean time to relapse.
Remission rate at one year was not significantly different between children who received intravenous methylprednisolone during induction and those who received oral prednisone only (Imbasciati 1985) (Analysis 11.2.3 (64 children): RR 1.06, 95% CI 0.75 to 1.53) but the total dose of oral prednisone administered was higher in the control group than in the group receiving intravenous prednisone.
A cross‐over study (Leisti 1978) showed that fewer children with post‐prednisone adrenocortical suppression relapsed during a six month period if they received partial cortisol substitution with 5 mg of cortisol during remission in comparison with placebo. The data for the patients were combined for each treatment period so the data for the first comparison could not be displayed in a meta‐analysis. After three months treatment, 5/13 children (38%) receiving cortisol had relapsed compared with 12/13 receiving placebo (92%) (Chi2 = 4.0, P = 0.05), and at six months 9/13 children receiving cortisol had relapsed compared with 12/13 receiving placebo.
Significantly fewer children treated with prednisone for seven months relapsed by six months (Analysis 12.1.1 (90 children): RR 0.04, 95% CI 0.01 to 0.25), 12 months (Analysis 12.1,2 (76 children): RR 0.43 95% CI 0.29 to 0.65), two years (Analysis 12.1.3 (64 children): RR 0.60, 95% CI 0.45 to 0.80) and three years (Analysis 12.1.4 (53 children) RR 0.71, 95% CI 0.56 to 0.90) compared with standard duration therapy (Jayantha 2002b). The relapse rate/patient/year excluding patients who became steroid dependent was reduced at one (Analysis 12.2.1 (72 children): MD‐1.78, 95% CI ‐2.30 to ‐1.26), two (Analysis 12.2.2 (56 children): MD ‐1.79, 95% CI ‐2.39 to ‐1.19) and three years (Analysis 12.2.3 (41 children): MD‐1.74, 95% CI ‐2.39 to ‐1.09) after treatment and the number of children who developed steroid dependence or relapsed frequently by one year was reduced (Analysis 12.3 (72 children): RR 0.43, 95% CI 0.19 to 0.95) in the long duration group compared with standard duration. Cumulative steroid dose excluding patients who became steroid dependent during the study was higher at one year (Analysis 12.4.1 (72 children): MD 0.59 g/kg, 95% CI 0.02 to 1.16) in the long treatment group but did not differ at two (Analysis 12.4.2 (56 children): MD ‐0.32 g/kg, 95% CI ‐1.52 to 0.88) and three years (Analysis 12.4.3: MD ‐1.13 g//kg, 95% CI ‐3.08 to ‐0.82). Hypertension was more common in the long duration group but the difference was not statistically significant (Analysis 12.5.1 (72 children): RR 2.40, 95% CI 0.86 to 6.73); the number of children with growth failure did not differ between groups (Analysis 12.5.2 (72 children): RR 1.24, 95% CI 0.62 to 2.50).
12.1. Analysis.
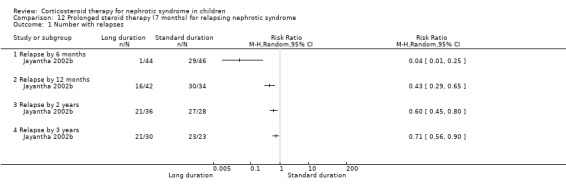
Comparison 12 Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 1 Number with relapses.
12.2. Analysis.
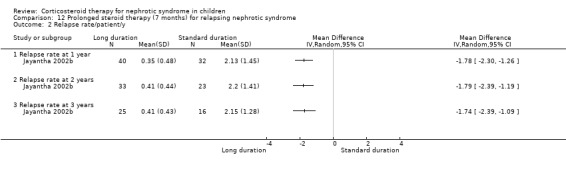
Comparison 12 Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 2 Relapse rate/patient/y.
12.3. Analysis.
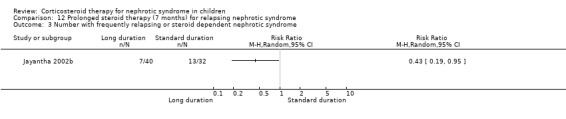
Comparison 12 Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 3 Number with frequently relapsing or steroid dependent nephrotic syndrome.
12.4. Analysis.
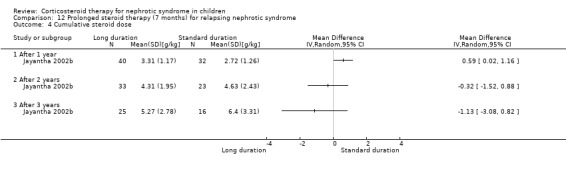
Comparison 12 Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 4 Cumulative steroid dose.
12.5. Analysis.
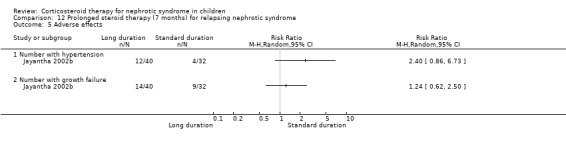
Comparison 12 Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 5 Adverse effects.
Discussion
Summary of main results
We added 10 studies to this 2015 update to bring the total number of included studies to 34 enrolling 3033 children. Analysis of data from three studies has led to the new conclusion that in studies at low risk of bias, there is no significant difference in the risk for FRNS between two and three months of prednisone and more than three months of prednisone in the initial episode of SSNS. Data from three other studies increased the evidence base to support administering prednisone or increasing the dose during URTI to reduce the risk of relapse in children with FRNS.
Prednisone in the first episode of SSNS
Our initial review in 2000 demonstrated that prednisone administered for three months or more significantly reduced the risk of relapse by 12 to 24 months and of FRNS compared with two months in the initial episode of SSNS. Increasing the duration of prednisone up to seven months increased the benefit obtained. It was unclear whether the increase in benefit was related to increased duration or increased dose though indirect analyses suggested that duration was more important than dose. However it was also noted that some included studies in the analyses were at unclear or high risk of bias. In the 2003 and 2005 updates, additional studies were identified which reported that six months of prednisone significantly reduced the risk of relapse compared with three months. In clinical practice, paediatric nephrologists tended to use increasing durations of prednisone in the initial episode of nephrotic syndrome though considerable variation existed between physicians reflecting in part the poor quality of the evidence from randomised studies (MacHardy 2009; Samuel 2013).
This 2015 update included three large, well designed studies (Sinha 2014; Teeninga 2013; Yoshikawa 2014) comparing different durations of prednisone. In Teeninga 2013 children received the same total dose of prednisone administered over three or six months, however children included in Sinha 2014 and Yoshikawa 2014 received a higher total dose of prednisone in the six months treatment group compared with the shorter treatment group. Inclusion of these studies in meta‐analyses showed that the risk of relapse by 12 to 24 months and of FRNS continued to favour extended duration of prednisone except for the analysis of FRNS in the comparison of five to six months with three months of prednisone. However there was considerable heterogeneity between studies with I2 results varying between 36% and 83%. Subgroup analysis stratified for the risk of bias domains showed that studies at low risk of bias for allocation concealment, attrition bias and performance/detection bias found no significant differences in the risk of relapse by 12 to 24 months or FRNS between two to three months of prednisone and three to seven months. Studies at high risk of bias on the other hand showed significant benefits of increasing treatment duration. Therefore studies of long versus shorter duration corticosteroids have heterogeneous treatment effects, with the older high risk of bias studies tending to over‐estimate the effect of longer course therapy, compared with more recently published low risk of bias studies. Among studies at low risk of bias, there was no significant difference in the risk for FRNS between prednisone given for two to three months and longer durations or total dose of therapy indicating that there is no benefit of increasing the duration of prednisone beyond two to three months in the initial episode of SSNS. A study comparing two months with four months of prednisone is currently underway in Europe (PREDNOS Study 2013).
Prednisone in relapsing SSNS
Daily prednisone during viral infections compared with alternate‐day prednisone therapy reduced the rate of relapse. Three additional larger studies of improved methodological quality have increased the power of this analysis so that this management may be considered for children with frequently relapsing SSNS, who are already receiving alternate‐day prednisone. Preliminary data from a fourth study suggests that daily prednisone during URTI reduces the risk of relapse in children not receiving prednisone. A further study addressing this question is currently underway in Europe (PREDNOS 2 Study 2014).
Overall completeness and applicability of evidence
This review includes studies evaluating corticosteroid therapy and nephrotic syndrome, with the majority of studies focusing on therapy for the initial presentation of nephrotic syndrome. Three additional well designed studies involving 562 children has led to the change in conclusions for this review so that the optimum duration of prednisone is suggested to be two or three months of prednisone in the initial episode of SSNS rather than longer durations as argued in previous versions of this review.
There remain few data on the treatment of relapsing nephrotic syndrome with prednisone. In particular there are no studies addressing the use of long term alternate‐day corticosteroid therapy to maintain remission in children with frequently relapsing nephrotic syndrome although this management is widely recommended in guidelines (KDIGO 2012).
Adverse effects of medications were either not reported or there was limited reporting in studies. Among 16 studies evaluating increased duration or dose in the initial episode of SSNS, hypertension, ophthalmological disorders and Cushing's syndrome were reported in 12, 11 and eight studies respectively. Prednisone therapy is known to be associated with significant behavioural and psychological adverse effects (Mishra 2010; Neuhaus 2010). However only seven of these studies reported this outcome and no studies reported data on quality of life for the child or their family. Adverse effects of medications were reported in more detail in the three well designed studies published in 2013 and 2014 (Sinha 2014; Teeninga 2013; Yoshikawa 2014).
The studies included the major ethnic groups, but there are few separable data for African‐American or African children. These groups of children, who are known to have a higher incidence of initial and late steroid‐resistant nephrotic syndrome (Kim 2005; Gipson 2011), may show different responses in studies of increased dose or duration of prednisone.
Quality of the evidence
Of the 34 studies included, only 18 (53%) and 16 studies (47%) revealed adequate random sequence generation and allocation concealment respectively. In part this may be due to suboptimal reporting of these parameters in earlier studies. However some studies published recently failed to provide adequate information on these parameters. Blinding of participants, investigators and outcome assessors was only reported in seven (21%) studies. Studies without blinding were considered at high risk of bias because knowledge of treatment groups could influence both patient management and reporting of remission and relapse by urinalyses. Both attrition bias (incomplete reporting of outcome data) and reporting bias (selective outcome reporting) were at low risk of bias in fewer than 50% of studies. Studies with inadequate allocation concealment can exaggerate the efficacy of the experimental treatment by 30% to 40% (Schulz 1995) and meta‐analyses of low quality studies may overestimate the benefit of therapy (Moher 1998). Addition of recently published well designed studies resulted in significant heterogeneity in the primary efficacy outcomes (relapse, FRNS) in studies assessing the duration of prednisone for the initial episode of SSNS. Subgroup analyses of these studies demonstrated that older studies at higher risk of bias overestimated the benefit of increased duration of prednisone while newer studies at low risk of bias found no significant benefit of prolonging prednisone beyond two to three months in the initial episode of nephrotic syndrome resulting in changed conclusions for this review.
In summary of finding tables (Table 1; Table 2) for comparisons of two months with three months or more, and of three months with five or six months in the first episode of SSNS, the overall quality of studies was considered low for efficacy outcomes because of a high risk of bias in some studies and heterogeneity between studies. When studies with the outcome of FRNS were separated into subgroups according to risk of bias for allocation concealment, the quality of the evidence was considered high for studies at low risk of allocation concealment and moderate for studies at high or uncertain risk of bias of allocation concealment. The quality of studies for the adverse effects was considered moderate or low because of inclusion of some poor quality studies and few included studies.
Only 15/34 studies were included in the summary of findings tables and all compared treatment regimens in the first episode of nephrotic syndrome. The remaining studies were single studies or data could not be included in the meta‐analyses.
Potential biases in the review process
A detailed search using the Cochrane Renal Group's Specialised Register was completed in February 2015. The Renal Register contains conference abstracts as well as published studies and there is no language restriction. This minimised the risk that eligible studies were omitted, although more recently published eligible studies and eligible studies in some congress proceedings not searched could have been missed. There were 10 (29%) included studies that were only available in abstract form with limited information on study methods and outcomes. Failure to include these studies could result in overestimation of treatment effect since it is known that negative studies are less likely to be published or may be published later than positive studies (Hopewell 2007). Alternately, some authors have argued that inclusion of these studies could result in overestimation of treatment effect through selective outcome reporting and incomplete reporting of the number of patients completing follow‐up (Egger 2001).
Many studies were small and had incomplete information on study methods and results and further results particularly of older studies could not be obtained despite contacting authors. Of the 34 included studies 19 were published in 2000 or earlier ‐ before the CONSORT checklist first published in 1996 would be likely to influence study methodology and reporting (Moher 2001).
This was an extensive review; each step was completed independently by at least two authors thus minimising risks of errors in determining study eligibility, data extraction and risk of bias assessment and data synthesis.
Agreements and disagreements with other studies or reviews
New studies at low risk of bias included in this review indicate that there is no significant benefit of treating children for more than two to three months in the initial episode of SSNS. The KDIGO 2012 and other country based guidelines (Gipson 2009; Haute Autorité de Santé 2008; IPNG‐IAP 2008) recommend treatment with three months or more of prednisone for the initial episode of SSNS.
In support of the KDIGO guidelines (KDIGO 2012), this review identified four studies showing that increasing prednisone administration from alternate‐day to daily or giving prednisone to children not on prednisone at the onset of an intercurrent viral infection reduces the risk of relapse.
This review did not identify any RCTs evaluating the use of prolonged courses of alternate‐day prednisone to reduce the risk for relapse in children with frequently relapsing nephrotic syndrome although guidelines (Gipson 2009; Haute Autorité de Santé 2008; IPNG‐IAP 2008; KDIGO 2012) have recommended this practice.
The listed guidelines and narrative reviews (Greenbaum 2012) emphasise the use of non‐corticosteroid immunosuppressive medications in children with frequently relapsing or steroid dependent disease. These medications are the subject of another Cochrane systematic review (Pravitsitthikul 2013).
Authors' conclusions
Implications for practice.
Prolongation of prednisolone therapy beyond two to three months in the initial episode of SSNS does not reduce the risk of relapse in studies at low risk of bias whether the same total dose of prednisone is used for short and long durations or whether the total dose of prednisone is increased with longer durations of treatment. The results of a further well designed study evaluating different durations and therefore total doses of prednisone are awaited (PREDNOS Study 2013).
Daily prednisone therapy during an upper respiratory infection or other infection reduces the risk of relapse compared with continuing alternate‐day prednisone or no prednisone.
During daily therapy, prednisone is as effective when administered as a single daily dose compared with divided doses.
Implications for research.
We now know that administering prednisone over six months rather than two or three months does not reduce the risk of relapse or of developing frequently relapsing disease. However all studies evaluating the duration of prednisolone have used similar daily and alternate daily doses of prednisolone based on the empirical regimens established by ISDKC and Arbeitsgemeinschaft für Pädiatrische Nephrologie in the 1970s and 1980s so we still do not know whether the same results could be obtained with lower total doses of prednisolone.
Adverse events including hypertension, ophthalmological disorders and behavioural or psychological effects are poorly reported. Recently published studies have provided additional information on adverse effects. The results of the PREDNOS Study 2013 comparing eight weeks with 16 weeks of prednisone will include a detailed assessment of the behavioural effects of prednisone.
Further RCTs are required to evaluate different durations of alternate‐day prednisone regimens in children with frequently relapsing SSNS as prolonged alternate‐day prednisone is recommended in current guidelines although there are no RCT data to support this recommendation.
Four RCTs from emerging countries have shown that daily prednisone administered during an intercurrent infection reduces the risk of relapse. A further well designed RCT is currently assessing this intervention in European children, where the pattern of intercurrent infections may be different (PREDNOS 2 Study 2014).
There is some evidence that children with SSNS suffer post‐prednisone adrenal insufficiency and that this state may predispose to relapse. The efficacy of cortisol substitution in such children should be examined in a further RCT.
Further studies of deflazacort in comparison with prednisone with larger numbers of patients and longer follow‐up periods are required in children with frequently relapsing nephrotic syndrome to confirm its efficacy. Studies including the use of ACTH in comparison with prednisone may also be of benefit.
What's new
| Date | Event | Description |
|---|---|---|
| 16 September 2015 | Amended | Minor amendment to forest plot description 2.8.2 ‐ changed from 'Low risk...' to "High risk..." |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 11 March 2015 | New citation required and conclusions have changed | 10 new studies included |
| 11 March 2015 | New search has been performed | New studies identified |
| 13 May 2009 | Amended | Contact details updated. |
| 23 September 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to Dr John F Knight who contributed to the design, quality assessment, data collection, entry, analysis and interpretation, and writing of early versions of this review (Hodson 2000; Hodson 2005).
The authors would like to thank Professor A Bagga, Professor A Abeyagunawardena, Professor PF Hoyer, Professor UK Jayantha, Dr C Kleinknecht, Professor M Liern, Professor TE Mattoo, Professor O Mishra, Professor RK Sharma and Professor N Yoshikawa for the information that they provided about their studies. The authors wish to thank Professors Barratt, Brodehl, Broyer and Ponticelli for responding to our requests for information about unpublished studies.
We would like to thank the referees for their editorial advice during the preparation of this review and its updates.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Steroid therapy in first episode of nephrotic syndrome: 3 months versus 2 months of therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with frequent relapses by 12 to 24 months | 6 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.47, 1.00] |
| 2 Number of children relapsing by 12 to 24 months | 8 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 1.00] |
| 3 Mean relapse rate/patient/y | 4 | 295 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.29, ‐0.00] |
| 4 Cumulative steroid dose | 3 | 245 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐0.67, 2.09] |
| 5 Number with frequent relapses by 12 to 24 months stratified by definition of FRNS | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 FRNS by ISKDC definition | 3 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.39, 1.20] |
| 5.2 Variation from ISKDC definition of FRNS | 3 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.35, 1.19] |
| 6 Number with frequent relapses by 12 to 24 months stratified by risk of bias for allocation concealment | 6 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.47, 1.00] |
| 6.1 Low risk of bias for allocation concealment | 3 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.23] |
| 6.2 Unclear or high risk of bias for allocation bias | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 7 Adverse events | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Psychological disorders | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.43, 11.13] |
| 7.2 Hypertension | 6 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.47, 6.86] |
| 7.3 Ophthalmological disorders | 5 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.07, 1.42] |
| 7.4 Retarded growth | 4 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.25, 1.18] |
| 7.5 Cushing's syndrome | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.51, 3.39] |
| 7.6 Infections | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.17] |
| 7.7 Osteoporosis | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.38] |
Comparison 2. Steroid therapy in first episode of nephrotic syndrome: 5 or 6 months versus 3 months of therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with frequent relapses by 12 to 24 months | 5 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.50, 1.22] |
| 2 Number of children relapsing by 12 to 24 months | 7 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 3 Mean relapse rate/patient/y | 3 | 460 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.64, ‐0.14] |
| 4 Cumulative steroid dose | 3 | 460 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.67, 0.73] |
| 5 Number with frequent relapses stratified by definition of FRNS | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 FRNS by ISKDC definition | 3 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.34] |
| 5.2 Variation from ISKDC definition of FRNS | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.18, 0.72] |
| 6 Number with frequent relapses stratified by risk of bias for allocation concealment | 5 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.50, 1.22] |
| 6.1 Studies at low risk of bias for allocation concealment | 3 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.34] |
| 6.2 Studies at high or unclear risk of bias for allocation concealment | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.18, 0.72] |
| 7 Number with frequent relapses stratified by risk of bias for blinding | 5 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.16] |
| 7.1 Low risk of bias for blinding | 2 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.20] |
| 7.2 High or unclear risk of bias for blinding | 3 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.78] |
| 8 Number with frequent relapses stratified by risk of bias for attrition | 5 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.16] |
| 8.1 Low risk of bias for attrition | 4 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.85, 1.16] |
| 8.2 High risk of bias for attrition | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.16, 0.69] |
| 9 Adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Hypertension | 5 | 636 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.91, 2.05] |
| 9.2 Eye complications | 5 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.17] |
| 9.3 Infections | 4 | 586 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.79, 1.51] |
| 9.4 Cushingoid appearance | 5 | 646 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.62, 1.36] |
| 9.5 Gastrointestinal bleeding | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.26, 8.70] |
| 9.6 Addisonian crisis | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.39] |
| 9.7 Psychological disorders | 3 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.03, 4.39] |
| 9.8 Growth | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.55, 1.82] |
Comparison 3. Steroid therapy in the first episode of nephrotic syndrome: 1 month versus 2 months of therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of children relapsing by 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Number of children relapsing by 12 to 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number with frequent relapses | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Cumulative steroid dose | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Steroid therapy in the first episode of nephrotic syndrome: 12 months versus 5 months therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Steroid therapy in the first episode of nephrotic syndrome: different total doses given over same duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse at twelve months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Number with frequently relapsing nephrotic syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Psychological disorders | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Cushing's Syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Steroid therapy and Sairei‐to in first episode of nephrotic syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long prednisone & Sairei‐to versus standard prednisone & Sairei‐to | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Relapse at 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Number with frequent relapses at 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Cyclosporin (CSA) and steroid therapy in first episode of childhood nephrotic syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse by 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Relapse by 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number needing cytotoxic agents | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Serum creatinine at end of study | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Steroid therapy in first episode of nephrotic syndrome: high dose methylprednisone versus 2 month therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first relapse | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Relapse rate | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Time to remission | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Steroid therapy and azithromycin (AZM) in the first episode of nephrotic syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of children relapsing by 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Daily prednisolone treatment during viral infections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with relapse with infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Number of relapses/patient | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Number of infection‐related relapses/patient/year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Total relapses (episodes/patient/1 year) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of relapses/patient at 2 years | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 11. Steroid therapy in relapse of nephrotic syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of children relapsing during therapy | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Intermittent dose versus alternate‐day therapy (6 months therapy) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Daily versus intermittent therapy (2 months therapy) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of children with further relapses by 9 to 12 months | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Intermittent dose versus alternate‐day therapy (6 months therapy) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Single versus divided dose therapy (2 months therapy) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Intravenous versus oral therapy (6 months therapy) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Deflazacort versus prednisone (12 months therapy) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Prolonged oral versus intermittent therapy (2 months therapy ) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean relapse rate/patient/y | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Single versus divided dose therapy (2 months therapy) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Daily versus intermittent therapy (2 months therapy) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Deflazacort versus prednisone (12 months therapy) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean time to relapse | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Single versus divided dose therapy (2 months therapy) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Daily versus intermittent therapy (2 months therapy) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Cumulative steroid dose | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Mean time to remission | 2 | 138 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.98, 1.06] |
| 7 Serious adverse events | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.91] |
11.5. Analysis.
Comparison 11 Steroid therapy in relapse of nephrotic syndrome, Outcome 5 Cumulative steroid dose.
Comparison 12. Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with relapses | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Relapse by 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Relapse by 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Relapse by 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Relapse by 3 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Relapse rate/patient/y | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Relapse rate at 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Relapse rate at 2 years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Relapse rate at 3 years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with frequently relapsing or steroid dependent nephrotic syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Cumulative steroid dose | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 After 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 After 2 years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 After 3 years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Number with hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Number with growth failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abeyagunawardena 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Randomised at onset of URTI to receive one of the interventions. At next URTI received alternate therapy |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated, sealed envelopes, sequential patients |
| Allocation concealment (selection bias) | Low risk | Randomly allocated, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators and parents blinded to contents of containers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators and parents blinded to contents of containers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/48 excluded from study (17%) for need for additional immunosuppression (4), no second viral infection (3), number without further relapses (1) |
| Selective reporting (reporting bias) | High risk | Not all the review's pre‐specified outcomes were recorded; no mention of adverse events |
| Other bias | Low risk | The study appears to be free of other source of bias |
Abeyagunawardena 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered to control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo administered to control group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/48 (31%) did not complete both parts of the 2 year cross‐over study |
| Selective reporting (reporting bias) | High risk | No report of adverse effects; cross‐over study and no separate results available for first part of the study so results could not be included in meta‐analyses |
| Other bias | Unclear risk | Abstract only; no information provided |
APN 1981.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (alternate)
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes provided to each centre. "One opened when patient qualified to enter the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding no mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/64 withdrawn: steroid toxicity (8); incorrect treatment or uncooperative parents (6); late non‐response (1); one patient unaccounted for in the text |
| Selective reporting (reporting bias) | Low risk | Recorded the review's pre‐specified outcomes (number with relapse, frequency of relapses, adverse events) |
| Other bias | Low risk | Supported by grants from the VW Foundation |
APN 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (4weeks)
Treatment group 2 (8 weeks)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation to permit judgement |
| Allocation concealment (selection bias) | Low risk | "Central random allocation" reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "77 patients were initially recruited into the trial, but 16 had to be removed at an early stage due to steroid resistance (8), or early deviations from the treatment protocol
(8)" "34 patients completed the study for the full 2 years. Data for the other 27 patients were included for the period that they remained in the study protocol. Of the 27, 5 patients of the short‐course group and 4 from the standard group were removed when they required other immunosuppressive agents; 2 patients from each group left the country during the course of the study; 7 children from the short‐course group, and 3 from the standard group, were lost to follow‐up due to failure of continuous parental cooperation; and late treatment faults were observed in 3 cases after short‐course treatment, and in 1 patient after standard therapy. The full course was completed by 15 patients receiving the short course and by 19 receiving standard treatment." |
| Selective reporting (reporting bias) | High risk | Did not report all the review's pre‐specified outcomes. No report on number of FRNS |
| Other bias | Low risk | Supported by grants from the VW Foundation |
APN 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (3 months)
Treatment group 2 (2 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central random allocation |
| Allocation concealment (selection bias) | Low risk | Central random allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.7% excluded for protocol violation. This proportion of missing outcomes are not sufficient to impact results |
| Selective reporting (reporting bias) | Low risk | Reported the review's pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to assess |
APN 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Co‐ordinating centre, centrally allocated |
| Allocation concealment (selection bias) | Low risk | Centrally allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 152 randomised, 25 withdrew consent (127 entered study) CSA/prednisone group: 13/62 (17%) excluded (SRNS (7), Infection (3), thrombosis (2), protocol deviation (1)) Prednisone group: 10/65 (15%) excluded (infection (1), SRNS (7), protocol deviation (1), unstated (1)) |
| Selective reporting (reporting bias) | High risk | Not all of review's pre‐specified outcomes have been reported. No report on number of frequently relapsing nephrotic syndrome (FRNS). Adverse events and outcomes in reported in percentages |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Bagga 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (4 months)
Treatment group 2 (2 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author that sequence generation was random |
| Allocation concealment (selection bias) | Low risk | Information from author that allocation occurred after child had entered study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal/lost to follow‐up: 6/51; steroid resistance (4); poor compliance (2) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Low risk | Research grant from the All India Institute of Medical Sciences, New Delhi, India. The study appears to be free of other source of bias |
Broyer 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Blocks of 10 packages containing equal numbers of each intervention in order determined by random code" |
| Allocation concealment (selection bias) | Low risk | "Block randomisation and sealed packages, lots of 10" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured. "Medication in identical bottles and identical tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured. "Blinded until end of study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal/lost to follow‐up: 2/40 (loss to follow‐up (1); protocol treatment deviation (1)) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported (cannot report on SDNS, as all remained on steroids as per protocol). |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Ekka 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (single dose)
Treatment group 2 (divided dose)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised " ‐ insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal/lost to follow‐up: 12/106; did not report for follow‐up (11); steroid resistant (1) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Gulati 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. "randomised by stratified randomisation" on basis of therapy with or without levamisole |
| Allocation concealment (selection bias) | Low risk | "allocation was concealed with opaque sealed envelopes opened at inclusion" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/100 (11%) patients excluded or lost to follow‐up; lost to follow‐up (5), discontinued treatment (6) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Low risk | Funded by the Indian Council of Medical Research. CTRI/2008/091/000245 |
Hiraoka 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (high dose)
Treatment group 2 (standard)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated " ‐ insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Withdrawal/lost to follow‐up: 8/68 excluded for steroid resistance |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Hiraoka 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (long duration)
Treatment group 2 (standard duration)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated" ‐ sealed envelopes |
| Allocation concealment (selection bias) | Low risk | "Simple randomisation using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal/ lost to follow‐up: 3/73; steroid resistance (3) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Imbasciati 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned from a table with random numbers" |
| Allocation concealment (selection bias) | Low risk | Central randomisation centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. All 89 randomised patients followed for 12‐24 months |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
ISKDC 1979.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated " ‐ insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/64 (15.6%) not included in analysis because of protocol violation |
| Selective reporting (reporting bias) | High risk | Not all of the review's pre‐specified primary outcomes have been reported. Adverse events not reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Jayantha 2002a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (7 months)
Control group (ISKDC)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation table" ‐ notes received from author |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal/loss to follow‐up: 46/135 (34%) lost to follow‐up at 2 years |
| Selective reporting (reporting bias) | Low risk | Reported on all of review's pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Jayantha 2002b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (7 months)
Treatment group 2 (2 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random allocation table". Information from author |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24% lost to follow‐up at 1 year (23/95) |
| Selective reporting (reporting bias) | High risk | Not all the review's pre‐specified outcomes have been reported. No report on adverse effects |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Kleinknecht 1982.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (13 months)
Treatment group 2 (6 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Sealed closed number envelopes in series of ten". Information obtained from author |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported. No data on adverse effects |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Ksiazek 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (6 months)
Treatment group 2 (3 months)
Treatment group 3 (2 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", insufficient information about sequence generation to permit judgement |
| Allocation concealment (selection bias) | High risk | "Parents had an influence on assignment, favouring Protocol C" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed for two years |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported. No data on numbers with FRNS |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Leisti 1978.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment
Group 1
Group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allotted". No other information |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and personnel blinded. Tablets were of identical taste and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants and personnel blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported. No data on adverse events |
| Other bias | Low risk | Sigrid Juselius Foundation financial support, Medica OY, Helsenki drug preparations |
Li 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (single dose)
Treatment group 2 (divided dose)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated by alternation |
| Allocation concealment (selection bias) | High risk | Patients allocated by alternation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data to permit judgement |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported. No data on frequent relapses |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Liern 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer generated table (information received from author) |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind to patients and medical caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind to patients and medical caregivers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all patients completed both arms of the study |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported. No data on relapse, frequent relapses and minimal data on adverse effects |
| Other bias | Unclear risk | No information provided |
Mattoo 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Data received from authors, alternate patients allocated to groups |
| Allocation concealment (selection bias) | High risk | "alternate patients allocated to groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Each patient was followed for a period of two years |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported. No data on adverse events. Only steroid dependent patients included. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Mishra 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (prolonged treatment)
Treatment group 2 (standard treatment)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | "randomly allocated" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/80, 6.3% lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Did not reported on all of review's pre‐specified outcomes The number of patients with at least one relapse is unclear |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Mocan 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (high dose group)
Treatment group 2 (standard therapy)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Children arbitrarily randomised into two groups" |
| Allocation concealment (selection bias) | High risk | "Children arbitrarily randomised into two groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/21 excluded; 4/21 (21%) lost to follow‐up and this could influence results; 2/21 SRNS |
| Selective reporting (reporting bias) | High risk | Reported on adverse events, relapse rate but not number with FRNS |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Norero 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (3 months)
Treatment group 2 (2 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | High risk | Patients allocated by odd or even numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number excluded or lost to follow‐up: 56/96 completed follow‐up. Of 40 excluded patients, 19 had SRNS. Remaining 21 excluded inappropriately: SDNS (5); deviation from protocol (3); duration of follow‐up insufficient (11); loss to follow‐up (2) |
| Selective reporting (reporting bias) | Low risk | Reported on all of review's pre‐specified outcomes |
| Other bias | Low risk | Grant No 1940506 from FONDECYT (National Scientific and Technology Foundation) |
Pecoraro 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Information from authors suggests "alternation" was used |
| Allocation concealment (selection bias) | High risk | 'Alternation" was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Said that all patients completed follow‐up but unclear whether any patients had been excluded |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported. No data on frequent relapses |
| Other bias | High risk | Educational grant from Fresenius |
Satomura 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (high dose)
Treatment group 2 (low dose)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients assigned "alternately" |
| Allocation concealment (selection bias) | High risk | "Alternation" used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data to permit judgement |
| Other bias | Unclear risk | Insufficient data to permit judgement |
Sharma 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (6 months)
Treatment group 2 (3 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'table of random numbers". Randomisation at 12 weeks after the beginning of initial therapy. Information provided by authors |
| Allocation concealment (selection bias) | Unclear risk | 'table of random numbers' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/156 excluded (10.3%); 160 consecutive patients, 4 refused consent. Of 156 entered, 10 were non‐compliant and 6 lost to follow‐up and their results were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All the reviews pre‐specified outcomes have been reported |
| Other bias | Unclear risk | Non information provided |
Sinha 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (3 months)
Treatment group 2 (6 months)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. Randomly assigned 1:1 in permuted blocks of four |
| Allocation concealment (selection bias) | Low risk | "Procedures for randomisation and packing and distribution were conducted at this centre by individuals, who were not involved in trial implementation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "External pharmacy manufactured identical‐appearing sugar coated tablets of prednisolone and placebo, packaged in matching blister packs of 10 tablets each" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators, patients and outcome assessors were blinded to randomisation schedule. Masking was maintained during data analysis, following which the randomisation code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/181 (3%) excluded (SRNS 1, loss to follow‐up 5) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Funded by Indian Council of Medical Research |
Teeninga 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (3 months)
Treatment group 2 (6 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central pharmacy with a computer generated random number table |
| Allocation concealment (selection bias) | Low risk | Central pharmacy, controlled allocation concealment with a computer generated random number table. Provided prepackaged medications, with fixed and blinded dose. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, health care providers, data collectors and researchers were blinded to group allocation. Identical tasteless capsules containing prednisolone or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, health care providers, data collectors and researchers were blinded to group allocation. Randomisation code broken September 2011. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients with consent and not SRNS were included and followed up (13 withdrew consent, 11 steroid resistant). |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Low risk | No disclosures. Trial registered Netherlands Trial Registry number 255. Funded by Dutch kidney Foundation Grant C03 and by Vrienden van het Sophia Foundation |
Ueda 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (prolonged)
Treatment group 2 (standard)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'allocated randomly', insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned, randomisation stated but no information on method used available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether any patients, who were randomised, were not included in analysis; complete 1 year follow‐up |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes of the review have been reported |
| Other bias | Low risk | Supported by a grant from the Ministry of Health and Welfare in Japan |
Yoshikawa 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (prolonged)
Treatment group 2 (standard)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomly assigned, concealed envelopes' |
| Allocation concealment (selection bias) | Low risk | 'randomly assigned, concealed envelopes' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25/196 (13%) did not complete study |
| Selective reporting (reporting bias) | High risk | Not all the reviews, pre‐specified outcomes were reported. No reports of adverse effects of steroids |
| Other bias | Unclear risk | Insufficient data to permit judgment |
Yoshikawa 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (6 months)
Treatment group 2 (2 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence in 1:1 ratio, stratified for age (1 to 10 years or 11 to 15 years), sex and institution |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned....at the Japan Clinical Research Support Unit" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label, patients, guardians, treating physicians and individuals were data were not blinded to treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Apart from trial statistician and data monitoring committee, all treating physicians and other investigators remained blinded to the trial results until follow up was completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded 9/255 (3%): early relapses after remission (5), 3 no follow‐up data available (3), withdrew consent before allocated study medication (1) |
| Selective reporting (reporting bias) | Low risk | All studies pre‐specified outcomes mentioned |
| Other bias | Low risk | Grant from the Ministry of Health, Labour and Welfare, Japan |
Zhang 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomly categorized into intervention and control groups using a random number table of odd or even numbers" |
| Allocation concealment (selection bias) | High risk | Allocation sequence could be predicted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and lack of blinding could influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/208 (excludes 4 patients with SRNS); 10.1% lost to follow‐up or excluded (15 lost to follow‐up, 6 excluded for hypocomplementaemia) |
| Selective reporting (reporting bias) | High risk | Not all reviews prespecified outcomes are mentioned ‐ no adverse events mentioned |
| Other bias | Low risk | Tiajin Bureau of Health Sciences and Technology Fund. Project number 09KZ34 |
APN ‐ Arbetsgemeinschaft für Pädiatrische Nephrologie; BMI ‐ body mass index; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; CPA ‐ cyclophosphamide; CSA ‐ cyclosporin; eGFR ‐ estimated glomerular filtration rate; FRNS ‐ frequently relapsing steroid‐sensitive nephrotic syndrome; INS ‐ idiopathic nephrotic syndrome; IQR ‐ interquartile range; ISKDC ‐ International Study of Kidney Disease in Children; SCr ‐ serum creatinine; SDNS ‐ steroid‐dependent nephrotic syndrome; SSNS ‐ steroid‐sensitive nephrotic syndrome; SRNS ‐ steroid‐resistant nephrotic syndrome; URTI ‐ upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alatas 1978 | RCT; non‐corticosteroid interventions (chlorambucil) |
| Anonymous 1968 | Not RCT |
| APN 1982 | RCT, non‐corticosteroid interventions (cyclophosphamide/chlorambucil) |
| Arun 2009 | RCT; non‐corticosteroid interventions (zinc) |
| Baluarte 1978 | RCT; non‐corticosteroid interventions (chlorambucil) |
| BAPN 1991 | RCT; non‐corticosteroid interventions (levamisole) |
| Barratt 1970 | RCT; non‐corticosteroid interventions (cyclophosphamide) |
| Barratt 1973 | RCT; non‐corticosteroid interventions (cyclophosphamide) |
| Barratt 1977 | RCT; non‐corticosteroid interventions (azathioprine) |
| Cerkauskiene 2005 | RCT; non‐corticosteroid interventions (fusidic acid) |
| Chiu 1973 | RCT; non‐corticosteroid interventions (cyclophosphamide) |
| Dayal 1994 | RCT; non‐corticosteroid interventions (levamisole) |
| Donia 2005 | RCT; non‐corticosteroid interventions (cyclophosphamide/levamisole) |
| Edefonti 1988 | RCT; non‐corticosteroid interventions (cyclosporin/cyclophosphamide) |
| Grupe 1976 | RCT; non‐corticosteroid interventions (chlorambucil) |
| Hu 2006 | Probably RCT; non‐corticosteroid interventions (herbal medicines) |
| Idczak‐Nowicka 1996 | Not RCT |
| ISKDC 1970 | RCT; non‐corticosteroid interventions (azathioprine) |
| ISKDC 1974 | RCT; non‐corticosteroid interventions (cyclophosphamide) |
| Liu 1995 | Non‐corticosteroid interventions (Chinese medicines). Not sure whether it is RCT |
| Martinelli 2004 | Non‐corticosteroid interventions (cyclophosphamide) in children with FSGS. Not sure whether it is RCT |
| McCrory 1973 | RCT; non‐corticosteroid interventions (cyclophosphamide) |
| Niaudet 1992 | RCT; non‐corticosteroid interventions (cyclosporin/chlorambucil) |
| Prasad 2004 | RCT; non‐corticosteroid interventions (cyclophosphamide) |
| Rashid 1996 | RCT; non‐corticosteroid interventions (levamisole) |
| Sharipov 2007 | Not RCT |
| Ueda 1990 | RCT; non‐corticosteroid interventions (cyclophosphamide) |
| Wang 2005 | Possibly RCT; non‐corticosteroid interventions |
| Weiss 1993 | RCT; non‐corticosteroid interventions (levamisole) |
| Wingen 1990 | Not RCT |
| Yamashita 1971 | Not RCT |
| Yang 2001 | Non‐corticosteroid interventions (herbal). Unclear whether RCT |
| Yoshioka 2000 | RCT; non‐corticosteroid interventions (mizoribine) |
FSGS ‐ focal segmental glomerulonephritis; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
PREDNOS 2 Study 2014.
| Trial name or title | Short course daily prednisolone therapy at the time of upper respiratory infection in children with relapsing steroid sensitive nephrotic syndrome: The PREDNOS 2 study |
| Methods | Parallel RCT |
| Participants | Subjects aged over 1 year and less than 19 years with relapsing SSNS who have had two or more relapses in the preceding 12 months |
| Interventions | Standard course therapy: weeks 1 to 4 prednisolone 60 mg/m2/d (max 80 mg), weeks 5 to 8, prednisolone 40 mg/m2 on alternate days for 28 days Extended course therapy: weeks 1 to 4 60mg/m2/d, weeks 5 to 16 prednisolone 60 mg/m2 on alternate days tapering by 10 mg/m2 every 2 weeks |
| Outcomes | The primary end point will be the incidence of URTI‐related relapse following the first URTI during the 12 month follow‐up period |
| Starting date | |
| Contact information | Nicholas Webb. nicholas.webb@cmft.nhs.uk |
| Notes |
PREDNOS Study 2013.
| Trial name or title | Long‐term tapering versus standard prednisolone (steroid) therapy for the treatment of the initial episode of nephrotic syndrome: national multicentre randomised double blind trial |
| Methods | Parallel RCT |
| Participants | Children presenting with first episode of SSNS, over 1 year and less than 1 year |
| Interventions | Treatment group: children will receive daily prednisolone for 6 days at the onset of URTI at the onset of each URTI during 12 months Control group: children will continue their current treatment (with placebo to maintain double blind) at the onset of each URTI during 12 months |
| Outcomes | Time to first relapse. To determine whether an extended course of prednisolone reduces the relapse rate, reduces the proportion of children who develop frequently relapsing or steroid dependent disease, reduces the requirement for a second or third line agent, is associated with an increased incidence of steroid related adverse events including behavioural problems, is more effective than standard course therapy |
| Starting date | |
| Contact information | nicholas.webb@cmft.nhs.uk |
| Notes |
SSNS ‐ steroid‐sensitive nephrotic syndrome; URTI ‐ upper respiratory tract infection
Differences between protocol and review
Risk of bias assessment tool has replaced the Quality assessment checklist list used in the previous versions of this review.
Contributions of authors
Deidre Hahn: Study selection, quality appraisal, data extraction, data analysis, writing review, updating review.
Elisabeth Hodson: Study selection, quality appraisal, data extraction, data analysis, writing review, updating review.
Narelle Willis: Literature search, obtaining articles, organising translation, data extraction, data analysis, data display, updating review.
Jonathan Craig: Data analysis, writing review, updating review.
Sources of support
Internal sources
No sources of support supplied
External sources
Australian Kidney Foundation, Australia.
National Health and Medical Research Council, Australia.
Commonwealth Department of Health and Aging, Australia.
Declarations of interest
Deirdre Hahn: none known
Elisabeth Hodson: none known
Narelle Willis: none known
Jonathan Craig: none known
Edited (no change to conclusions)
References
References to studies included in this review
Abeyagunawardena 2008 {published data only}
- Abeyagunawardena AS, Trompeter RS. Increasing the dose of prednisolone during viral infections reduces the risk of relapse in nephrotic syndrome: a randomised controlled trial. Archives of Disease in Childhood 2008;93(3):226‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abeyagunawardena 2014 {published and unpublished data}
- Abeyagunawardena A, Thalgahagoda S, Illangasekera Y, Trompeter R. A short course of prednisolone during an upper respiratory tract infection reduces the risk of relapse in childhood nephrotic syndrome [abstract]. Pediatric Nephrology 2014;29(9):1689. [EMBASE: 71662409] [DOI] [PubMed] [Google Scholar]
- Abeyagunawardena AS, Thalgahagoda S, Illangasekera Y, Trompeter RS. Short course of daily prednisolone during upper respiratory tract infection reduces the risk of relapse in childhood nephrotic syndrome [abstract]. Archives of Disease in Childhood 2014;99:A155‐6. [EMBASE: 71566356] [Google Scholar]
APN 1981 {published data only}
- Anonymous. Alternate‐day prednisone is more effective than intermittent prednisone in frequently relapsing nephrotic syndrome. A report of "Arbeitsgemeinschaft fur Padiatrische Nephrologie". European Journal of Pediatrics 1981;135(3):229‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Anonymous. Alternate‐day versus intermittent prednisone in frequently relapsing nephrotic syndrome. A report of "Arbetsgemeinschaft fur Padiatrische Nephrologie". Lancet 1979;1(8113):401‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Brodehl J, Krohn HP. Steroid trial in frequently relapsing nephrotic syndrome in children. Glomerulonephritis. International Conference on pathogenesis, pathology and treatment. New York: Wiley, 1977:210‐5.
APN 1988 {published data only}
- Anonymous. Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Lancet 1988;1(8582):380‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Brodehl J, Krohn HP, Ehrich JH. The treatment of minimal change nephrotic syndrome (lipoid nephrosis): cooperative studies of the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Klinische Padiatrie 1982;194(3):162‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ehrich JH. Initial treatment of idiopathic nephrotic syndrome: short vs standard prednisone [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:59. [CENTRAL: CN‐00644361]
- Ehrich JH for the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Short initial prednisone therapy versus standard prednisone therapy in the steroid responsive nephrotic syndrome [abstract]. Pediatric Nephrology 1987;1(1):C28. [CENTRAL: CN‐00445202] [Google Scholar]
APN 1993 {published data only}
- Ehrich JH, Arbeitsgemeinschaft fur Padiatrische Nephrologie. Minimal change nephrotic syndrome: long prednisone therapy vs standard prednisone therapy [abstract]. Nephrology Dialysis Transplantation 1991;6(10):771. [CENTRAL: CN‐00260613] [Google Scholar]
- Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. European Journal of Pediatrics 1993;152(4):357‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
APN 1999 {published data only}
- Arbeitsgemeinschaft fur Padiatrische Nephrologie. Results of the nephrotic syndrome study VIII of the APN: new standard treatment versus new standard treatment plus 8 weeks cyclosporin A [abstract]. Pediatric Nephrology 1999;13:C26. [CENTRAL: CN‐00636143] [Google Scholar]
- Hoyer PF. Results of the nephrotic syndrome study VIII of the APN: new standard treatment versus new standard treatment plus 8 weeks cyclosporin A [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):104A. [CENTRAL: CN‐00550522] [Google Scholar]
- Hoyer PF for the Arbeitsgemeinschaft fur Padiatrische Nephrologie. The initial treatment of idiopathic nephrotic syndrome with prednisone and cyclosporin A: preliminary results of a therapeutic trial [abstract]. Pediatric Nephrology 1995;9(6):C91. [CENTRAL: CN‐00401335] [Google Scholar]
- Hoyer PF, Brodehl J. Initial treatment of idiopathic nephrotic syndrome in children: prednisone versus prednisone plus cyclosporine A: a prospective, randomized trial. Journal of the American Society of Nephrology 2006;17(4):1151–7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bagga 1999 {published and unpublished data}
- Bagga A, Hari P, Srivastava RN. Long (LP) versus standard (SP) initial prednisolone treatment for idiopathic nephrotic syndrome (NS) [abstract no: P225]. Pediatric Nephrology 1998;12:C155. [CENTRAL: CN‐00583944] [Google Scholar]
- Bagga A, Hari P, Srivastava RN. Long (LP) versus standard (SP) initial prednisolone treatment for nephrotic syndrome (NS) [abstract]. 3rd Congress. Nephrology Urology Transplantation Society (NUTS) of SAARC; 1999 Feb 18 ‐ 21; Colombo, Sri Lanka. 1999:158. [CENTRAL: CN‐00460325]
- Bagga A, Hari P, Srivastava RN. Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatric Nephrology 1999;13(9):824‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Broyer 1997 {published data only}
- Broyer M, Terzi F, Gagnadoux MF, Guest G, Niaudet P. A randomized double blind study of deflazacort (D) versus prednisone (P) in the treatment of idiopathic nephrotic syndrome (INS) [abstract]. Journal of the American Society of Nephrology 1995;6(3):414. [CENTRAL: CN‐00483349] [Google Scholar]
- Broyer M, Terzi F, Lehnert A, Gagnadoux MF, Guest G, Niaudet P. A controlled study of deflazacort in the treatment of idiopathic nephrotic syndrome. Pediatric Nephrology 1997;11(4):418‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekka 1997 {published data only}
- Bagga A, Ekka BK, Srivastava RN. Single versus divided dose prednisolone therapy for relapses of nephrotic syndrome (NS) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A113. [CENTRAL: CN‐00261391] [DOI] [PubMed] [Google Scholar]
- Ekka BK, Bagga A, Srivastava RN. Single‐ versus divided‐dose prednisolone therapy for relapses of nephrotic syndrome. Pediatric Nephrology 1997;11(5):597‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gulati 2009 {published data only}
- Gulati A, Math A, Sreeniwas V, Hari P, Bagga A. Administration of daily corticosteroids prevents infection associated relapses in frequently relapsing nephrotic syndrome [abstract no: SU714]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
- Gulati A, Sinha A, Sreenivas V, Math A, Hari P, Bagga A. Daily corticosteroids reduce infection‐associated relapses in frequently relapsing nephrotic syndrome: a randomized controlled trial. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(1):63‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiraoka 2000 {published data only}
- Hiraoka M, Sudo M, West Japanese Cooperative Study of Kidney Disease in Children. Low versus standard dosage of prednisolone for initial treatment of idiopathic nephrotic syndrome in children [abstract no: A0445]. Journal of the American Society of Nephrology 1996;7(9):1335. [Google Scholar]
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. The West Japan Cooperative Study of Kidney Disease in Children. Kidney International 2000;58(3):1247‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from intensive initial prednisolone therapy for nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):103A. [CENTRAL: CN‐00550605] [Google Scholar]
Hiraoka 2003 {published data only}
- Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M, Takeda N, Haruki S, et al. A randomized study of two long‐course prednisolone regimens for nephrotic syndrome in children. American Journal of Kidney Diseases 2003;41(6):1155‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Imbasciati 1985 {published data only}
- Imbasciati E, Gusmano R, Edefonti A, Zucchelli P, Pozzi C, Grassi C, et al. Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. British Medical Journal Clinical Research Ed 1985;291(6505):1305‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISKDC 1979 {published data only}
- Anonymous. Nephrotic syndrome in children: a randomized trial comparing two prednisone regimens in steroid‐responsive patients who relapse early. Report of the International Study of Kidney Disease in Children. Journal of Pediatrics 1979;95(2):239‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Jayantha 2002a {published and unpublished data}
- Jayantha UK. Comparison of ISKDC regime with a 7 months steroid regime in the first attack of nephrotic syndrome [abstract]. Pediatric Nephrology 2004;19:C81. [CENTRAL: CN‐00583710] [Google Scholar]
- Jayantha UK. Comparison of ISKDC regime with a six month steroid regime in the treatment of steroid sensitive nephrotic syndrome. Unpublished results 2002.
- Jayantha UK. Comparison of ISKDC regime with a six month steroid regime in the treatment of steroid sensitive nephrotic syndrome [abstract no: FP2B]. 7th Asian Congress of Pediatric Nephrology; 2000 Nov 1‐4; Singapore. 2000:28. [CENTRAL: CN‐00583708]
Jayantha 2002b {published and unpublished data}
- Jayantha UK. Comparison of ISKDC regime with 6 month regime in patients with relapsing nephrotic syndrome. Unpublished results 2002.
- Jayantha UK. Prolong versus standard steroid therapy for children with relapsing course of nephrotic syndrome [abstract no: P026]. Pediatric Nephrology 2004;19(9):C99. [MEDLINE: ] [Google Scholar]
Kleinknecht 1982 {published and unpublished data}
- Kleinknecht C, Broyer M, Parchoux B, Loriat C, Nivet H, Palcoux JB, et al. Comparison of short and long treatment at onset of steroid sensitive nephrosis (SSN). Preliminary results of a multicenter controlled trial for the French Society of Pediatric Nephrology [abstract]. International Journal of Pediatric Nephrology 1982;3(1):45. [CENTRAL: CN‐00550480] [Google Scholar]
Ksiazek 1995 {published data only}
- Ksiazek J, Wyszynska T. Short versus long initial prednisone treatment in steroid‐sensitive nephrotic syndrome in children. Acta Paediatrica 1995;84(8):889‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leisti 1978 {published data only}
- Leisti S, Koskimies O, Perheentupa J, Vilska J, Hallman N. Idiopathic nephrotic syndrome: prevention of early relapse. British Medical Journal 1978;1(6117):892. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 1994 {published data only}
- Li X, Li Z, Cheng Z. Treatment of children with simple nephrotic syndrom using prednison once per day. Acta Academiae Medicinae Hubei 1994;15(4):386‐8. [EMBASE: 1995005617] [Google Scholar]
Liern 2008 {published data only}
- Liern M, Dieguez S, Canepa C. Recovery of total immunoglobulin and immunoglobulin subclasses in nephrotic syndrome: deflazacort vs methylprednisone [Recuperacion de la inmnoglobulina total y sus subclases en el sindrome nefrotico: deflazacort vs metilprednisona]. Nefrologia 2008;28(5):563. [MEDLINE: ] [PubMed] [Google Scholar]
Mattoo 2000 {published data only}
- Mattoo TK, Mahmoud MA. Increased maintenance corticosteroids during upper respiratory infection decrease the risk of relapse in nephrotic syndrome. Nephron 2000;85(4):343‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mishra 2012 {published data only}
- Mishra OP, Thakur N, Mishra RN, Prasad R. Prolonged versus standard prednisolone therapy for initial episode of idiopathic nephrotic syndrome. Journal of Nephrology 2012;25(3):394‐400. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mocan 1999 {published data only}
- Mocan H, Erduran E, Karaguzel G. High dose methylprednisolone therapy in nephrotic syndrome. Indian Journal of Pediatrics 1999;66(2):171‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mocan H, Mocan MZ, Erduran E, Karaguzel G, Aslan Y. The effect of high dose methylprednisolone therapy in patients with minimal change nephrotic syndrome [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A74. [CENTRAL: CN‐00261380] [Google Scholar]
Norero 1996 {published data only}
- Norero C, Delucchi A, Lagos E, Rosati P. Initial therapy of primary nephrotic syndrome in children: evaluation in a period of 18 months of two prednisone treatment schedules. Chilean Co‐operative Group of Study of Nephrotic Syndrome in Children [Cuadro inicial del sindrome nefrosico primario del nino: evaluacion a 18 meses de dos esquemas de tratamiento con prednisona. Groupo Cooperativo Chileno de Estudio del Sindrome Nefrosico del Nino]. Revista Medica de Chile 1996;124(5):567‐72. [MEDLINE: ] [PubMed] [Google Scholar]
- Norero C, Delucchi A, Lagos E, Rosati P. Long term evaluation of two prednisone treatments in initial idiopathic nephrotic syndrome (INS) in children. Preliminary findings [abstract]. Pediatric Nephrology 1995;9(6):C88. [CENTRAL: CN‐00550669] [Google Scholar]
Pecoraro 2003 {published data only}
- Pecoraro C, Caropreso MR, Malgieri G, Ferretti A, Nuzzi F. Therapy of first episode steroid responsive nephrotic syndrome (FESRNS): a randomised controlled trial [abstract no: MP087]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v230. [CENTRAL: CN‐00644161] [Google Scholar]
- Pecoraro C, Caropreso MR, Malgieri G, Ferretti AVS, Raddi G, Piscitelli A, et al. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial [abstract no: OFC41]. Pediatric Nephrology 2004;19(9):C72. [CENTRAL: CN‐00644160] [Google Scholar]
- Pecoraro C, Caropreso MR, Passaro G, Ferretti AVS, Malgieri G. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):63. [CENTRAL: CN‐00447140] [Google Scholar]
Satomura 2001 {published data only}
- Satomura K, Yamaoka K, Shima M, Tanaka Y, Ashino N, Nakagawa K, et al. Standard vs low initial dose of prednisolone therapy for first episodes of nephrotic syndrome in children [abstract]. Pediatric Nephrology 2001;16(8):C117. [CENTRAL: CN‐00447593] [Google Scholar]
Sharma 2000 {unpublished data only}
- Gulati S, Ahmed M, Sharma RK, Gupta A, Pokhariyal S. Comparison of abrupt withdrawal versus slow tapering regimen of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A87. [CENTRAL: CN‐00445595] [Google Scholar]
- Sharma RK, Ahmed M, Gulati S, Gupta A, Pokhariyal S. Comparison of abrupt withdrawal versus slow tapering regimen of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome. Unpublished results 2002.
- Sharma RK, Ahmed M, Gupta A, Gulati S, Sharma AP. Comparison of abrupt withdrawal versus slow tapering regimens of prednisolone therapy in management of first episode of steroid responsive childhood idiopathic nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):97A. [CENTRAL: CN‐00550434] [Google Scholar]
Sinha 2014 {published and unpublished data}
- Bagga A, Sinha A, Saha A, Kumar M, Afzal K, Mehta A, et al. Randomized double blind, placebo controlled trial to compare the efficacy of 3‐months versus 6‐months therapy with prednisolone for the first episode of idiopathic nephrotic syndrome (CTRI/2010/091/001095) [abstract]. Pediatric Nephrology 2012;27(9):1707. [EMBASE: 71386357] [Google Scholar]
- Sinha A, Bagga A, Sharma S, Saha A, Kumar M, Afzal K, et al. Randomized, double blind, placebo controlled trial to compare the efficacy of 3‐months versus 6‐months therapy with prednisolone for the first episode of idiopathic nephritic syndrome [abstract]. Pediatric Nephrology 2013;28(8):1361‐2. [EMBASE: 71126981] [Google Scholar]
- Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, et al. Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid‐ sensitive nephrotic syndrome. Kidney International 2014;87(1):217‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teeninga 2013 {published data only}
- Teeninga N, Kist‐Van Holthe JE, Ruswuk N, Mos NI, Hop WC, Wetzels JF, et al. Effect of extended prednisolone treatment from three to six months, with equal cumulative doses, on childhood nephrotic syndrome: a nation‐wide randomised controlled trial [abstract]. Pediatric Nephrology 2012;27(9):1637‐8. [EMBASE: 71386208] [Google Scholar]
- Teeninga N, Kist‐van Holthe JE, Rijswijk N, Mos NI, Hop WC, Wetzels JF, et al. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. Journal of the American Society of Nephrology 2013;24(1):149‐59. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeninga N, Kist‐van Holthe JE, Akker EL, Kersten MC, Boersma E, Krabbe HG, et al. Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney International 2014;85(6):1444‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ueda 1988 {published data only}
- Ueda N, Chihara M, Kawaguchi S, Niimomi Y, Nonada T, Matsumoto J, et al. Intermittent versus long‐term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. Journal of Pediatrics 1988;112(1):122‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoshikawa 1998 {published data only}
- Takekoshi Y. Treatment of idiopathic nephrotic syndrome [abstract]. Pediatric Nephrology 1996;10(1):C3. [CENTRAL: CN‐00402800] [Google Scholar]
- Yoshikawa N, Ito H, Takehoshi Y, Honda M, Awazu M, Iijima K, et al. Standard versus long‐term prednisolone with Sairei‐to for initial therapy in childhood steroid‐responsive nephrotic syndrome: a prospective controlled study. Nippon Jinzon Gakkai Shi. Japanese Journal of Nephrology 1998;40(8):587‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Yoshikawa 2014 {published and unpublished data}
- Yoshikawa N, Nakanishi K, Oba MS, Sako M, Ohashi Y, Iijima K. Increased duration and dose of prednisolone (PSL) treatment does not reduce relapses in childhood nephrotic syndrome [abstract no: SA‐PO1080]. Journal of the American Society of Nephrology 2014;24(Abstracts):3B. [Google Scholar]
- Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R, Ota E, et al. A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six‐month treatment. Kidney International 2014;87(1):225‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang B, Liu T, Wang W, Zhang X, Fan S, Liu Z, et al. A prospective randomly controlled clinical trial on azithromycin therapy for induction treatment of children with nephrotic syndrome. European Journal of Pediatrics 2014;173(4):509‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alatas 1978 {published data only}
- Alatas H, Wirya IG, Tambunan T, Himawan S. Controlled trial of chlorambucil in frequently relapsing nephrotic syndrome in children (a preliminary report). Journal of the Medical Association of Thailand 1978;61 Suppl 1:222‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Anonymous 1968 {published data only}
- Anonymous. Nephrotic syndrome in children [Die nefrotiese sindroom by kinders]. South African Medical Journal. Suid‐Afrikaanse Tydskrif Vir Geneeskunde 1968;42(2):21‐2. [MEDLINE: ] [PubMed] [Google Scholar]
APN 1982 {published data only}
- Anonymous. Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. New England Journal of Medicine 1982;306(8):451‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brodehl J, Krohn HP, Ehrich JH. The treatment of minimal change nephrotic syndrome (lipoid nephrosis): cooperative studies of the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Klinische Padiatrie 1982;194(3):162‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arun 2009 {published data only}
- Arun S, Bagga A, Bhatnagar S, Hari P, Menon S, Saini S. Efficacy of Zinc(Zn) in reducing relapses in steroid sensitive nephrotic syndrome (SSNS) double blind, randomized controlled trial (RCT) (CRG030600044) [abstract no: 184OP]. Pediatric Nephrology 2007;22(9):1481. [CENTRAL: CN‐00724915] [DOI] [PubMed] [Google Scholar]
- Arun S, Bhatnagar S, Menon S, Saini S, Hari P, Bagga A. Efficacy of zinc supplements in reducing relapses in steroid‐sensitive nephrotic syndrome. Pediatric Nephrology 2009;24(8):1583‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baluarte 1978 {published data only}
- Baluarte HJ, Hiner L, Gruskin AB. Chlorambucil dosage in frequently relapsing nephrotic syndrome: a controlled clinical trial. Journal of Pediatrics 1978;92(2):295‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
BAPN 1991 {published data only}
- Anonymous. Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood. British Association for Paediatric Nephrology. Lancet 1991;337(8757):1555‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Barratt 1970 {published data only}
- Barratt TM, Soothill JF. A controlled trial of cyclophosphamide in steroid sensitive relapsing nephrotic syndrome of childhood [abstract]. Nephron 1971;8:95. [CENTRAL: CN‐00253521] [DOI] [PubMed] [Google Scholar]
- Barratt TM, Soothill JF. Controlled trial of cyclophosphamide in steroid‐sensitive relapsing nephrotic syndrome of childhood. Lancet 1970;2(7671):479‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barratt 1973 {published data only}
- Barratt TM, Cameron JS, Chantler C, Ogg CS, Soothill JF. Comparative trial of 2 weeks and 8 weeks cyclophosphamide in steroid‐sensitive relapsing nephrotic syndrome of childhood. Archives of Disease in Childhood 1973;48(4):286‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barratt 1977 {published data only}
- Barratt TM, Cameron JS, Chantler C, Counahan R, Ogg CS, Soothill JF. Controlled trial of azathioprine in treatment of steroid‐responsive nephrotic syndrome of childhood. Archives of Disease in Childhood 1977;52(6):462‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerkauskiene 2005 {published data only}
- Cerkauskiene R, Kaltenis P. Comparative study of prednisolone alone and prednisolone plus fusidic acid in the treatment of children with steroid‐responsive nephrotic syndrome. Medicina (Kaunas, Lithuania) 2005;41 Suppl 1:26‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Chiu 1973 {published data only}
- Chiu J, McLaine PN, Drummond KN. A controlled prospective study of cyclophosphamide in relapsing, corticosteroid‐responsive, minimal‐lesion nephrotic syndrome in childhood. Journal of Pediatrics 1973;82(4):607‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dayal 1994 {published data only}
- Dayal U, Dayal AK, Shastry JC, Raghupathy P. Use of levamisole in maintaining remission in steroid‐sensitive nephrotic syndrome in children [erratum appears in Nephron 1994;67(4):507]. Nephron 1994;66(4):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donia 2005 {published data only}
- Donia A, Ammar H, Moustafa F, Sobh M. Long‐term efficacy of two unconventional adjunctive therapies in minimal change nephrotic children [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:37. [CENTRAL: CN‐00509161]
- Donia AF, Ammar HM, Agroudy A, Moustafa F, Sobh MA. Long‐term results of two unconventional agents in steroid‐dependent nephrotic children. Pediatric Nephrology 2005;20(10):1420‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edefonti 1988 {published data only}
- Edefonti A, Ghio L, Bettinelli A, Paterlini G, Giani M, Nebbia G, et al. Unconjugated hyperbilirubinemia due to ciclosporin administration in children with nephrotic syndrome. Contributions to Nephrology 1988;67:121‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Edefonti A, Ghio L, Bettinelli G, Paterlini M, Giani G Nebbia A, et al. Unconjugated hyperbilirubinemia due to cyclosporin A (CYA) administration in children with nephrotic syndrome [abstract]. Pedatric Nephrology 1987;1(1):C8. [CENTRAL: CN‐00465774] [DOI] [PubMed] [Google Scholar]
- Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, et al. Cyclosporine (CSA) vs cyclophosphamide (CYC) for children with frequently relapsing/steroid dependent nephrotic syndrome (FR/SDNS): long term study [abstract]. Journal of the American Society of Nephrology 1992;3(3):310. [Google Scholar]
- Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, et al. Cyclosporine (CSA) vs cyclophosphamide (CYC) for children with frequently relapsing/steroid dependent nephrotic syndrome: long term study [abstract]. 9th Congress of the International Pediatric Nephrology Association; 1992 Aug 30‐Sep 4; Jerusalem (Israel). 1992:C70. [CENTRAL: CN‐00483820]
- Ponticelli C. A multicenter controlled prospective trial with cyclosporine vs cyclophosphamide in frequent relapsers and steroid dependent patients with idiopathic nephrotic syndrome. Journal of Nephrology 1989;2(2):147‐51. [EMBASE: 1991014828] [Google Scholar]
- Ponticelli C. Ciclosporin in the treatment of idiopathic nephrotic syndrome [abstract]. 10th Asian Colloquium in Nephrology; 1994 Dec 2‐6; Karachi, Pakistan. 1994:116.
- Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, et al. Cyclosporin versus cyclophosphamide for patients with steroid‐dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrology Dialysis Transplantation 1993;8(12):1326‐32. [MEDLINE: ] [PubMed] [Google Scholar]
- Ponticelli C, Rivolta E. Ciclosporin in minimal‐change glomerulopathy and in focal segmental glomerular sclerosis. American Journal of Nephrology 1990;10(Suppl 1):105‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grupe 1976 {published data only}
- Grupe WE, Makker SP, Ingelfinger JR. Chlorambucil treatment of frequently relapsing nephrotic syndrome. New England Journal of Medicine 1976;295(14):746‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hu 2006 {published data only}
- Hu GH, Dang XQ, Wang JH. Clinical effect of shenbing mistura combined with glucocorticoid on recurrent nephrotic syndrome in children and levels of interleukin‐6 and tumor necrosis factor‐alpha in blood and urine. Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional & Western Medicine] 2006;26(10):892‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Idczak‐Nowicka 1996 {published data only}
- Idczak‐Nowicka E, Ksiazek J, Krynski J, Wyszynska T. Verification of indications for kidney biopsy in children with steroid‐dependent nephrotic syndrome [Weryfikacja wskazan do biopsji nerki u dzieci ze sterydozaleznym zespolem nerczycowym]. Pediatria Polska 1996;71(8):679‐83. [MEDLINE: ] [PubMed] [Google Scholar]
ISKDC 1970 {published data only}
- Abramowicz M, Barnett HL, Edelmann CM Jr, Griefer I, Kobayashi O, Arneil GC, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970;1(7654):959‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Arneil GC. International trial of azathioprine in nephrotic syndrome in childhood. Nephron 1971;8:95. [CENTRAL: CN‐00253520] [Google Scholar]
ISKDC 1974 {published data only}
- Anonymous. Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Report of the International study of Kidney Disease in Children. Lancet 1974;2(7878):423‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Liu 1995 {published data only}
- Liu XY. Therapeutic effect of chai‐ling‐tang (sairei‐to) on the steroid‐dependent nephrotic syndrome in children. American Journal of Chinese Medicine 1995;23(3‐4):255‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martinelli 2004 {published data only}
- Martinelli R, Pereira LJ, Silva OM, Okumura AS, Rocha H. Cyclophosphamide in the treatment of focal segmental glomerulosclerosis. Brazilian Journal of Medical & Biological Research 2004;37(9):1365‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCrory 1973 {published data only}
- McCrory WW, Shibuya M, Lu WH, Lewy JE. Therapeutic and toxic effects observed with different dosage programs of cyclophosphamide in treatment of steroid‐responsive but frequently relapsing nephrotic syndrome. Journal of Pediatrics 1973;82(4):614‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niaudet 1992 {published data only}
- Niaudet P. Comparison of cyclosporin and chlorambucil in the treatment of steroid‐dependent idiopathic nephrotic syndrome: A multicentre randomized controlled trial. The French Society of Paediatric Nephrology. Pediatric Nephrology 1992;6(1):1‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prasad 2004 {published data only}
- Prasad N, Gulati S, Sharma RK, Singh U, Ahmed M. Pulse cyclophosphamide therapy in steroid‐dependent nephrotic syndrome. Pediatric Nephrology 2004;19(5):494‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rashid 1996 {published data only}
- Rashid HU, Ahmed S, Fatima N, Khanam A. Levamisole in the treatment of steroid dependent or frequent relapsing nephrotic syndrome in children. Bangladesh Renal Journal 1996;15(1):6‐8. [EMBASE: 1996266847] [Google Scholar]
Sharipov 2007 {published data only}
- Sharipov AM, Khamzaeva KA. Current aspects of transformation of morphological changes in children with nephrotic syndrome depending on the treatment regimen. Urologiia (Moscow, Russia) 2007, (2):68‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Ueda 1990 {published data only}
- Ueda N, Kuno K, Ito S. Eight and 12 week courses of cyclophosphamide in nephrotic syndrome. Archives of Disease in Childhood 1990;65(10):1147‐59. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang YP, Liu AM, Dai YW, Yang C, Tang HF. The treatment of relapsing primary nephrotic syndrome in children. Journal of Zhejiang University Science. B 2005;6(7):682‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 1993 {published data only}
- Weiss R. Results of a randomized, double‐blind, placebo controlled, multi‐center clinical trial of levamisole for the treatment of children with frequently relapsing or steroid dependent nephrotic syndrome. Personal communication 2005.
- Weiss R, NY‐NJ‐Phila‐Pediatric Nephrology Study Group. Randomized, double‐blind, placebo (P) controlled trial of levamisole (L) for children (CH) with frequently relapsing/steroid dependant (FR/SD) nephrotic syndrome (NS) [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):289. [CENTRAL: CN‐00520404] [Google Scholar]
Wingen 1990 {published data only}
- Wingen AM, Muller‐Wiefel DE, Scharer K. Comparison of different regimens of prednisone therapy in frequently relapsing nephrotic syndrome. Acta Paediatrica Scandinavica 1990;79(3):305‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamashita 1971 {published data only}
- Yamashita F, Funatsu T, Nagayama K, Arihiro H, Anan S. Evaluation of alternate‐day steroid therapy for nephrotic syndrome in childhood by cross‐over study. Kurume Medical Journal 1971;18(3):153‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2001 {published data only}
- Yang RY, Xu LF, Wang Q. The effect of Xuezhikang on lipid metabolism in patients with nephrotic syndrome. Hubei Yikedaxue Xuebao 2001;22(1):55‐7. [Google Scholar]
Yoshioka 2000 {published data only}
- Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney International 2000;58(1):317‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. Placebo‐controlled trial of mizoribine (MZR) in children with frequently relapsing nephrotic syndrome (FRNS). The Pediatric Mizoribine Study Group [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):163A. [Google Scholar]
References to ongoing studies
PREDNOS 2 Study 2014 {published data only}
- Webb NJ, Frew E, Brettell EA, Milford DV, Bockenhauer D, Saleem MA, et al. Short course daily prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid‐sensitive nephrotic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials 2014;15:147. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PREDNOS Study 2013 {published data only}
- Webb N, Trompeter R, Cummins C, Wheatley K, Frew E. Long‐term tapering versus standard prednisolone (steroid) therapy for the treatment of the initial episode of childhood nephrotic syndrome: national multicentre randomised double‐blind trial. www.controlled‐trials.com/ISRCTN16645249 (accessed 26 February 2015).
Additional references
Arneil 1971
- Arneil GC. The nephrotic syndrome. Pediatric Clinics of North America 1971;18(2):547‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bhimma 1997
- Bhimma R, Coovadia HM, Adhikari M. Nephrotic syndrome in South African children: changing perspectives over 20 years. Pediatric Nephrology 1997;11(4):429‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonilla‐Felix 1999
- Bonilla‐Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney International 1999;55(5):1885‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eddy 2003
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet 2003;362(9384):629‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Dickersin K, Davey Smith G. Problems and limitations in conducting systematic reviews. In: Egger M, Davey Smith G, Altman D editor(s). Systematic Reviews in Health care: Meta‐analysis in context. Second Edition. London: BMJ Publishing Group, 2001. [Google Scholar]
El Bakkali 2011
- Bakkali L, Rodrigues Pereira R, Kiuk DJ, Ket JC, Wijk JA. Nephrotic syndrome in the Netherlands: a population‐based cohort study and a review of the literature. Pediatric Nephrology 2011;26(8):1241‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elzouki 1984
- Elzouki AY, Amin F, Jaiswal OP. Primary nephrotic syndrome in Arab children. Archives of Disease in Childhood 1984;59(3):253‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gipson 2009
- Gipson DS, Massengill SF, Yoa L, Nagaraj S, Smoyer WE, Mahan JD, et al. Management of childhood onset nephrotic syndrome. Pediatrics 2009;124(2):747‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gipson 2011
- Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, et al. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney International 2011;79(6):678‐85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenbaum 2012
- Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome‐‐current and future therapies. Nature Reviews Nephrology 2012;8(8):445‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gulati 1999
- Gulati A, Sharma AP, Sharma RK, Gupta A. Changing trends of histopathology in childhood nephrotic syndrome. American Journal of Kidney Diseases 1999;34(4):159‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haute Autorité de Santé 2008
- Haute Autorité de Santé. [Syndrome néphrotique idiopathique de l'enfant. Protocole national de diagnostic et de soinspour une maladie rare. 2008; 1‐22]. www.has‐sante.fr/portail/upload/docs/application/pdf/2008‐06/pnds_sni_enfant.pdf (accessed 26 February 2015).
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopewell 2007
- Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta‐analysis of randomized trials of health care interventions. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000010.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyams 1988
- Hyams JS, Carey DE. Corticosteroids and growth. Journal of Pediatrics 1988;113(2):249‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IPNG‐IAP 2008
- Indian Pediatric Nephrology Group, Indian Academy of Pediatrics, Bagga A, Ali U, Banerjee S, Kanitkar M, Phadke KD, et al. Management of steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatrics 2008;45(3):203‐14. [MEDLINE: ] [PubMed] [Google Scholar]
ISKDC 1984
- Anonymous. Minimal change nephrotic syndrome in children: deaths during the first 5‐15 years' observation. Report of the International Study of Kidney Disease in Children. Pediatrics 1984;73(4):497‐501. [MEDLINE: ] [PubMed] [Google Scholar]
KDIGO 2012
- Lombel RM, Gipson DS, Hodson EM, Kidney Disease: Improving Global Outcomes. Treatment of steroid‐sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatric Nephrology 2013;28(3):415‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim JS, Bellew CA, Silverstein DM, Aviles DH, Boineau FG, Vehaskari VM. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney International 2005;68(3):1275‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koskimies 1982
- Koskimies O, Vilska J, Rapola J, Hallman N. Long‐term outcome of primary nephrotic syndrome. Archives of Disease in Childhood 1982;57(7):544‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacHardy 2009
- MacHardy N, Miles PV, Massengill SF, Smoyer WE, Mahan JD, Greenbaum L, et al. Management patterns of childhood‐onset nephrotic syndrome. Pediatric Nephrology 2009;24(11):2193‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McKinney 2001
- McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatric Nephrology 2001;16(12):1040‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehls 2011
- Mehls O, Hoyer PF. Dosing of glucocorticosteroids in nephrotic syndrome. Pediatric Nephrology 2011;26(12):2095‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mishra 2010
- Mishra OM, Basu B, Upadhyay SK, Prasad R, Schaefer F. Behavioural abnormalities in children with nephrotic syndrome. Nephrology Dialysis Transplantation 2010;25(8):2537‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Jones A, Lepage L, CONSORT Group (Consolidated Standards for Reporting of Trials). Use of the CONSORT statement and quality of reports of randomized trials: a comparative before‐and‐after evaluation. JAMA 2001;285(15):1992‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neuhaus 2010
- Neuhaus TJ, Langlois V, Licht C. Behavioural abnormalities in children with nephrotic syndrome‐‐an underappreciated complication of a standard treatment?. Nephrology Dialysis Transplantation 2010;25(8):2397‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ng 2001
- Ng JS, Wong W, Law RW, Hui J, Wong EN, Lam DS. Ocular complications of paediatric patients with nephrotic syndrome. Clinical & Experimental Ophthalmology 2001;29(4):239‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niaudet 2009
- Niaudet P. Long‐term outcome of children with steroid‐sensitive idiopathic nephrotic syndrome. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(10):1457‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pravitsitthikul 2013
- Pravitsitthikul N, Willis NS, Hodson EM, Craig JC. Non‐corticosteroid immunosuppressive medications for steroid‐sensitive nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD002290.pub4] [DOI] [PubMed] [Google Scholar]
Rüth 2005
- Rüth EM, Kemper M, Leumann EP, Laube GF, Neuhaus TJ. Children with steroid sensitive nephrotic syndrome come of age: long‐term outcome. Journal of Pediatrics 2005;147(2):202‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Samuel 2013
- Samuel S, Morgan CJ, Bitzan N, Mammen C, Dart AB, Manns BJ, et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatric Nephrology 2013;29(12):2289‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Srivastava 1999
- Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatric Nephrology 1999;13(1):13‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tarshish 1997
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. Journal of the American Society of Nephrology 1997;8(5):769‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hodson 2000
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy in nephrotic syndrome: a meta‐analysis of randomised controlled trials. Archives of Disease in Childhood 2000;83(1):45‐51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodson 2002
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001533] [DOI] [Google Scholar]
Hodson 2003
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD001533.pub2] [DOI] [PubMed] [Google Scholar]
Hodson 2005
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001533.pub3] [DOI] [PubMed] [Google Scholar]
Hodson 2007
- Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001533.pub4] [DOI] [PubMed] [Google Scholar]


