Abstract
Steroid hormones, such as estrogens, were once thought to be exclusively synthesized in the ovaries and enact transcriptional changes over the course of hours to days. However, estrogens are also locally synthesized within neural circuits, wherein they rapidly (within minutes) modulate a range of behaviors, including spatial cognition and communication. Here, we review the role of brain-derived estrogens (neuroestrogens) as modulators within sensory circuits in songbirds. We first present songbirds as an attractive model to explore how neuroestrogens in auditory cortex modulate vocal communication processing and learning. Further, we examine how estrogens may enhance vocal learning and auditory memory consolidation in sensory cortex via mechanisms similar to those found in the hippocampus of rodents and birds. Finally, we propose future directions for investigation, including: 1) the extent of developmental and hemispheric shifts in aromatase and membrane estrogen receptor expression in auditory circuits; 2) how neuroestrogens may impact inhibitory interneurons to regulate audition and critical period plasticity; and, 3) dendritic spine plasticity as a candidate mechanism mediating estrogen-dependent effects on vocal learning. Together, this perspective of estrogens as neuromodulators in the vertebrate brain has opened new avenues in understanding sensory plasticity, including how hormones can act on communication circuits to influence behaviors in other vocal learning species, such as in language acquisition and speech processing in humans.
Keywords: neurosteroid, sensorimotor, nongenomic, vocal learning, estradiol
Introduction
In nature, animals confront an overwhelming array of sensory cues. Filtering and processing this stream of sensory information is necessary to evaluate potential mates, mediate territory disputes, recognize kin, identify neighbors, and detect predators. Production and perception of air-borne cues manifest across multiple modalities, such as visual displays, tactile/vibrational signals, chemical cues, as well as auditory signals (Ai et al., 2017; Endevelt-Shapira et al., 2018; Mangiamele et al., 2016; Ota et al., 2015; Shamble et al., 2016; Smotherman and Narins, 2000). While most species integrate multimodal information, many rely primarily on acoustic cues for intraspecies communication, i.e., vocal communication.
Vocal communication is widespread among vertebrates. Humans specialize in spoken language. Rodents emit ultrasonic vocalizations (USVs) across many contexts, ranging from mother-pup interactions (Portfors, 2007), courtship and mating (Holy and Guo, 2005), and social play (Knutson et al., 1998). The vast majority of teleosts, such as toadfish and midshipman fish, produce underwater calls (Bass, 2008). But for most acoustically-communicating vertebrates, these vocalizations are innate. Experience-dependent vocal learning is only found in a handful of animals, including songbirds and humans (Petkov and Jarvis, 2012). Thus, hearing serves a unique dual function in songbirds: to both detect and learn their elaborate, species-specific vocal communication signals.
Here, we review the neural circuit modulation of auditory processing in a well-studied songbird species, the Australian zebra finch (Taeniopygia guttata). We suggest that songbirds in general, and zebra finches in particular, offer a unique opportunity to investigate how rapid estrogen signaling in sensory cortex enables both the processing and learning of vocal communication cues across development and in adulthood. Further, we provide suggestions for areas of future research on this topic, and suggest possible clinical implications of this research for understanding human cognition and language.
Neuromodulators that tune neural circuits
For intra-species communication to have adaptive value, an organism must integrate external and internal cues – such as energy reserves, social standing, and reproductive status – and adjust ongoing communication encounters. Such flexibility allows for context (both current and previous) to guide communication for both sender and receiver. In the vocal communication domain, the neural circuits that underlie vocal production as well as hearing must therefore be sensitive to context, by way of neuromodulation.
The recent scientific fascination with neural ‘connectomics’ has produced detailed neural circuit diagrams for a number of organisms. But it has also revealed that a wiring diagram is a useful predictor of behavior only when the dynamic ‘functional connectivity’ of that diagram is taken into account (Bargmann, 2012; Bargmann and Newsome, 2014; Marder et al., 2014). Neuromodulators such as biogenic amines, neurotransmitters, neuropeptides, and even gases like nitric oxide all are produced within neural circuits to exert modulatory effects (Katz and Lillvis, 2014; Nusbaum et al., 2017; Petersen and Hurley, 2017). To momentarily alter the wiring diagram, that is, to shift the functional connectivity of a neural circuit, modulators can influence the efficacy and even the sign (excitation vs. inhibition) of synaptic connections on a minute-by-minute timescale, enabling extraordinary circuit and behavioral flexibility. There is now growing appreciation that steroid hormones can act as neuromodulators via local synthesis and action in neural circuits (Balthazart and Ball, 2006; Kelly and Vitousek, 2017; Remage-Healey, 2014; Rudolph et al., 2016; Woolley, 2007). The emergent perspective that steroids may be genuine neuromodulators of neural circuits and behavior has been useful in guiding the exploration of neuroestrogen synthesis and action in the songbird auditory forebrain, as we describe in detail below.
Estrogens can be rapidly synthesized within sensory circuits to act as neuromodulators
Estrogens were classically thought to be secreted exclusively from the gonads. However, it is now clear that estrogens and other steroid hormones are also synthesized within the brain (Balthazart et al., 2018; London, 2016). Initial evidence for brain-derived estrogens (neuroestrogens) arose from the discovery of brain aromatase expression in multiple vertebrate species. Aromatase, the enzyme necessary for converting precursor androgens into subsequent estrogens, was previously described solely in peripheral tissue. In the 1970s, the first direct evidence for the capacity of central estrogen production showed neural aromatase in humans (Naftolin et al., 1975a; Naftolin et al., 1975b; Naftolin et al., 1971), and across a diverse range of vertebrate taxa, including reptiles, fish, amphibians, and birds (Callard et al., 1978a; Callard et al., 1978b). Follow-up work in songbirds demonstrated that the brain is the primary source of both local and circulating estrogens (Schlinger and Arnold, 1992), which suggested a novel role for central estrogen synthesis to locally target neural circuits.
We now understand that brain-derived estrogens can also rapidly tune neural circuits and impact a diverse range of behaviors. Initial evidence for rapid effects of estrogens on synaptic physiology came from single-neuron recordings in the preoptic area (POA) of female rats, in which 17β-estradiol (estradiol) altered firing rates within seconds (Kelly et al., 1976). Since then, acute effects of estrogens on neuronal activity states and cellular events have been reported for the hypothalamus, hippocampus, striatum, amygdala, brainstem, and more recently auditory cortex (Abraham et al., 2004; Bryant et al., 2005; Chaban et al., 2003; Dufy et al., 1979; Mermelstein et al., 1996; Nabekura et al., 1986; Remage-Healey and Bass, 2004; Remage-Healey et al., 2010a; Vasudevan and Pfaff, 2008; Woolley, 2007). Functionally, estradiol’s impact on circuit physiology is exceptionally diverse in terms of behavioral actions, timing, and species. In mice, aromatization is key to organize the medial amygdala early in life to selectively respond to opposite-sex olfactory cues (Bergan et al., 2014). At a more acute timescale, testosterone rapidly increases visually-guided responses to a female stimulus in male goldfish, likely via rapid aromatization into estrogens (Lord et al., 2009; Mangiamele et al., 2017). Even nociception within the dorsal horn of Japanese quail is rapidly modulated by acute estrogen actions (Evrard and Balthazart, 2004). Therefore, estrogen synthesis in the brain is important for many behaviors, neural circuits, and species, at a range of timescales.
Classically, steroid hormones like estradiol were thought to exclusively target intracellular nuclear receptors and affect transcriptional changes over the course of hours to days. However, estrogen receptors found on dendritic and axonal processes in guinea pig hypothalamic neurons provided evidence for a non-nuclear site for the neural actions of estrogens (Blaustein et al., 1992). Since then, evidence has emerged that estrogens can rapidly influence neuronal activity through membrane-docked estrogen receptors (both ERα and ERβ) that are associated with metabotropic-glutamate receptors (Mermelstein, 2009; Micevych and Mermelstein, 2008). More recently, rapid actions of estrogens have also been found to act through a G-protein coupled estrogen receptor, GPER1 (formerly the orphaned ‘GPR30’) (Barton et al., 2017; Rudolph et al., 2016; Srivastava et al., 2013). The emergent understanding of these many mechanisms for steroid actions were presented in a recent review previewing the current Special Issue (Balthazart et al., 2018). Below, we describe the contribution of recent work in songbirds testing the role of rapid neuroestrogen signaling in shaping sensory processing, and place this work in a broader context.
The songbird auditory circuit as a model to explore rapid estrogen actions on vocal communication processing and learning
Songbirds are a powerful system to explore neuroestrogen actions in sensory circuits across the lifespan. First, the forebrain circuits that guide auditory-dependent behaviors are enriched with estrogen receptors and estrogen synthase (aromatase), especially as compared to rodent sensory cortices (as reviewed in Vahaba and Remage-Healey, 2015). In agreement with high aromatase concentrations, the brain is the primary site of estradiol synthesis in male zebra finches (Schlinger and Arnold, 1992), so much so that circulating estrogen levels persist in castrated males (Adkins-Regan et al., 1990). The abundance of estrogen production and signaling in the songbird auditory forebrain makes it an attractive system to measure and manipulate neuroestrogen signaling and evaluate its effects on audition and learning. Below, we review how neuroestrogens are generated in the songbird brain, and their rapid effects on physiology and related behaviors in both adult and developing songbirds.
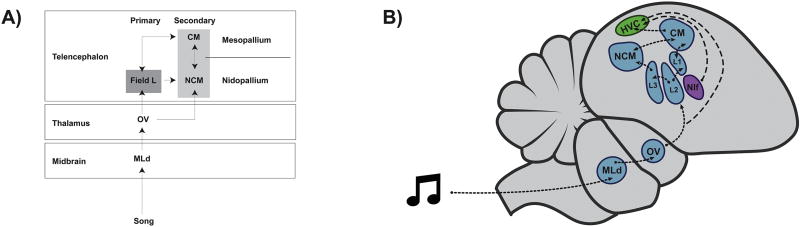
The organization of auditory circuits is relatively conserved across the class Aves. As in other vertebrates, birds perceive acoustic signals beginning at peripheral hair cells in the ear, and these auditory signals reach central cortical regions in the auditory forebrain (reviewed in Jarvis, 2004). As shown in Figure 1, brainstem and midbrain auditory signals are initially relayed from the thalamic nucleus oviodalis (Ov) to the avian auditory telencephalic homologue of primary auditory cortex (Field L complex; Field L2), which sends afferent projections to secondary auditory cortex, including the caudal mesopallium (CM) and the caudomedial nidopallium (NCM) (Jarvis, 2004; Vates et al., 1996; Wang et al., 2010).
Figure 1.
Two schematized songbird auditory circuits. A–B) Auditory stimuli, such as song, arrives from the brainstem and cochlear nuclei (not shown) into the midbrain MLd and into the thalamic OV (ovoidalis). The primary thalamic recipient of auditory information in the cortex is the Field L complex (L1, L2, and L3), which projects to the caudomedial nidopallium (NCM) and caudal mesopallium (CM), which are themselves reciprocally connected. Auditory information reaches HVC (used as a proper name) by way of the nucleus interface (NIf), which itself receives projections from CM (not illustrated for clarity). Not depicted are the forebrain basal ganglia and song motor circuit pathways that are essential for song learning and production. Adapted from Brenowitz and Remage-Healey, 2016 and Vahaba and Remage-Healey, 2017.
Interestingly, while this auditory pathway is conserved across birds (Bonke et al., 1979; Vates et al., 1996; Wang et al., 2010; Wild et al., 1993), a high concentration of aromatase and estrogen receptors in avian forebrain distinguishes vocal learning birds from other species (Metzdorf et al., 1999; Silverin et al., 2000; Yoder and Vicario, 2012), especially within NCM (Caras and Remage-Healey, 2016). Within the auditory forebrain, aromatase is almost exclusively found in NCM, whereas little to no aromatase has been described in Field L or CM of zebra finches (Ikeda et al., 2017; Peterson et al., 2005; Pinaud et al., 2006; Saldanha et al., 2000). Therefore, NCM provides the predominant source of estrogens to the auditory forebrain circuitry in male and female zebra finches. In addition to being regionally concentrated in NCM, aromatase is also co-expressed in specific cell types, namely parvalbumin-positive interneurons (Ikeda et al., 2017). The exceptional capacity for estrogen synthesis in the songbird brain has led to investigations of its functional significance for cognition and sensory processing.
Acutely synthesized estrogens within songbird auditory forebrain rapidly alter physiology. In zebra finches, estrogens are produced through both de novo steroidogenesis and through aromatization of circulating androgens (London et al., 2006; London et al., 2009; Remage-Healey et al., 2010b). Social interactions and song playbacks rapidly increase neuroestrogen production in the NCM of both adult male and female zebra finches (Remage-Healey et al., 2012; Remage-Healey et al., 2008). Functionally, local increases in neuroestrogens within NCM directly enhance auditory function. In anesthetized zebra finches, perfusing estradiol in NCM rapidly increased auditory-evoked firing rates and bursting in NCM, consistent with a neuroestrogen-dependent enhancement of the representation of communication signals (Remage-Healey, 2012; Remage-Healey et al., 2010a). Similarly, acute peripheral estrogen treatment resulted in a stronger song-evoked fMRI BOLD response bilaterally in the auditory lobule (which includes Field L, CMM, and NCM) of male European starlings (De Groof et al., 2017). Interestingly, global suppression of estrogen synthesis in these same animals specifically reduced auditory responsiveness in the left but not right hemisphere auditory lobule (De Groof et al., 2017). In addition to direct effects in NCM, estradiol in NCM also increases stimulus-selectivity and auditory responsiveness in downstream regions, including the key sensorimotor nuclei HVC and NIf (Pawlisch and Remage-Healey, 2015; Remage-Healey and Joshi, 2012). Taken together, estradiol in NCM enhances central auditory processing; however, it remains to be determined whether these enhanced neural representations translate into improved audition as assessed by psychophysic or behavioral measures.
So far, we have limited information about how neuroestrogen signaling in NCM regulates behavior. Inhibiting local estrogen synthesis in the NCM of male zebra finches rapidly suppressed behavioral preferences for the birds’ own song when delivered to the left, but not right hemisphere (Remage-Healey et al., 2010a). Studies using peripheral administration of aromatase inhibitors also support the general idea of estrogen synthesis and auditory function in songbirds (Alward et al., 2016a; Yoder et al., 2012). One intriguing possibly is that in addition to rapidly guiding auditory encoding, local neuroestrogen production in NCM may also facilitate auditory memory consolidation of recent experiences in adults, as we discuss below.
Neuroestrogen provision may help consolidate recent auditory experiences
In addition to facilitating audition, elevated neuroestrogens in NCM may also rapidly enhance the consolidation of recent experiences. While this idea has been explored to a lesser extent in auditory circuits, accumulating evidence indicates that estrogens enhance cognition in another estrogen-sensitive brain region: the hippocampus (HP). Since the 1990s, exogenous estrogens were known to have mnemonic-enhancing properties in spatial memory tests (Luine and Rodriguez, 1994). Early studies by Packard & Teather provided the first behavioral description of memory-enhancement from post-training peripheral and intra-hippocampal presentations of estradiol in rodents (Packard, 1998; Packard and Teather, 1997a, b). This work built on the emerging idea that estrogens mediate ovarian-cycle dependent changes in dendritic spine plasticity in the hippocampus (Woolley and McEwen, 1992), and provided a potential behavioral/functional consequence of this plasticity. Since then, rapid estrogen synthesis and action in hippocampus has become an active area of investigation (see reviews by Choleris, Frick, Luine, and Korol in this same Special Issue), and a more detailed understanding of these mechanisms has emerged.
Generally speaking, estrogens acting in HP enhance spatial memory and object recognition (Galea et al., 2017; Srivastava et al., 2013). In rodents, estradiol’s ability to enhance memory consolidation is limited to a time-sensitive window immediately after learning: subsequent recall is unaffected by estradiol treatments if presented >2 hours after initial training (Fernandez et al., 2008). As such, the relatively acute impact on memory consolidation is thought to be mediated by rapid neuroestrogen signaling (Tuscher et al., 2016b). One puzzle associated with these findings is the limited, indirect evidence for aromatase in the rodent HP (Sato and Woolley, 2016; Tabatadze et al., 2014; Tuscher et al., 2016b; Wu et al., 2009). By contrast, the songbird hippocampus is highly enriched with synaptic and axonal aromatase protein, suggesting it is well positioned to facilitate rapid, non-classical steroid signaling (Ikeda et al., 2017; Peterson et al., 2005; Remage-Healey et al., 2011; Rohmann et al., 2007; Saldanha et al., 2004; Saldanha et al., 2000). In agreement with this, hippocampal estradiol typically facilitates spatial cognition in zebra finches (Bailey et al., 2017; Bailey and Saldanha, 2015; Oberlander et al., 2004; Rensel et al., 2013), and blocking GPER1 in HP completely prevents learning a food caching task (Bailey et al., 2017). With these recent findings in mind, neuroestrogens may play a similar role in sensory learning in songbirds, including the processing and consolidation of recent auditory experiences (Vahaba and Remage-Healey, 2015).
In addition to providing a source of estrogens to the auditory system, NCM is also implicated in auditory learning and recognition memory in adult songbirds (Bolhuis and Gahr, 2006; Chew et al., 1995; Gobes and Bolhuis, 2007; Hahnloser and Kotowicz, 2010; Mello et al., 1995). NCM exhibits a seasonal enlargement during breeding photoperiods in European starlings, who are open-ended song learners (De Groof et al., 2009). In adult zebra finches, NCM is considered a focal region for storing recent auditory representations (Chew et al., 1995; Dong and Clayton, 2008, 2009; Kruse et al., 2000; Smulders and Jarvis, 2013; Soyman and Vicario, 2017; Stripling et al., 2001). A single, brief exposure (40 mins) to song results in a short-term memory for the trained song and subsequent recognition in NCM (Dong and Clayton, 2009). While NCM appears to be required for adult auditory memory consolidation and recognition, the molecular mechanisms supporting this are only recently becoming clearer (Ahmadiantehrani and London, 2017; London and Clayton, 2008) and may involve rapid estrogen signaling.
Neuromodulators act within central auditory circuits to enable post-training memory consolidation. Like estrogens, local noradrenergic modulation of NCM is required for both auditory processing (Ikeda et al., 2015; Lee et al., 2017) and memorization (Velho et al., 2012). Moreover, estradiol levels increase in adult NCM during social and song exposure, which may facilitate changes necessary for auditory memory formation (Remage-Healey et al., 2012; Remage-Healey et al., 2008). Auditory memory consolidation in NCM involves epigenetic modifications (Phan et al., 2017), which is also a route by which estradiol mediates spatial learning in rodents (Zhao et al., 2010). In adult songbirds, inhibiting global estrogen synthesis impairs short-term auditory memorization and recognition in NCM (Yoder and Vicario, 2012). While the specific role for neuroestrogens in sensory learning has yet to be directly tested in adult songbirds, local estradiol in the olfactory bulb of mice improves odor memory consolidation (Dillon et al., 2013), providing an intriguing parallel. In the following section, we consider how a similar mechanism may exist for consolidating sensory (tutor) memories across the song learning critical period in juvenile songbirds.
Evidence that estrogens are involved in auditory processing necessary during developmental song learning
Both male and female developing songbirds form an auditory memory of their tutor’s song that is necessary for accurate vocal (song) learning and imitation. In closed-ended learners, song models are acquired across a critical period early in development classically described as occurring across two phases: 1) tutor song memorization (“sensory phase”), and 2) motor rehearsal (“sensorimotor phase”) (London, 2017). During the sensory phase (tutor song memorization), pre-vocalizing songbirds begin encoding/memorizing their father’s or older sibling’s song beginning around 25 days post-hatch (dph) (Deregnaucourt and Gahr, 2013; Immelmann, 1969; Roper and Zann, 2006), or possibly earlier since some embryonic birds are selectivity responsive to adult conspecific song (Colombelli-Negrel et al., 2012; Spencer and Minderman, 2018). Once a tutor song ‘template’ memory is formed, birds begin to evaluate their burgeoning vocal imitations compared to the tutor memory during the sensorimotor phase. The sensorimotor phase (motor rehearsal) is akin to early infant babbling (Aronov et al., 2008; Doupe and Kuhl, 1999; Lipkind et al., 2013; Prather et al., 2017) and begins with emergent vocalizations, followed by song refinement, and eventual song crystallization that coincides with sexual maturation. In the case of zebra finches, the sensorimotor phase ends with a single highly stereotyped song produced throughout adulthood. While the behavioral study of song learning has intrigued scientists as far back as Aristotle, the neural mechanisms enabling song learning has a relatively more recent history beginning around the 1960s.
Other recent reviews have provided excellent coverage of the role of motor and cortical-basal ganglia pathways in sensorimotor learning (Brainard and Doupe, 2013; Mooney, 2009), and here we restrict our discussion on neuroestrogens and song learning by focusing on tutor memorization during the sensory phase and the contributions of auditory forebrain circuits, namely NCM (Bolhuis and Gahr, 2006; Bolhuis et al., 2010). While other auditory forebrain regions are likely involved in auditory memory acquisition for learned song (e.g. CMM, Jeanne et al., 2011; Terpstra et al., 2006), as well as other auditory-responsive regions (Adret et al., 2012; Mandelblat-Cerf et al., 2014; Piristine et al., 2016; Roberts et al., 2017), these areas are largely devoid of aromatase in cell bodies as well as neurites, compared to the high expression found in NCM (Ikeda et al., 2017; Saldanha et al., 2000), and thus direct roles for neuroestrogens are unlikely.
NCM is considered a primary site required for tutor song memorization and representation (Bolhuis and Gahr, 2006; Bolhuis and Moorman, 2015; Clayton, 2013) but see (Canopoli et al., 2016, 2017). Compared to sensorimotor-aged males, auditory-evoked firing rates and the coding accuracy of single neurons for individual song stimuli in NCM are both elevated in pre-singing, sensory-aged zebra finches that are beginning to form auditory memories of their tutor song (Vahaba et al., 2017). In developing songbirds, tutor song playback evokes higher immediate-early gene expression in NCM than does a novel male’s song (Gobes et al., 2010). Behavioral preferences for tutor song in adults is abolished when NCM is bilaterally lesioned (Gobes and Bolhuis, 2007). Further, like adult songbirds, habituation to specific vocalizations occurs in NCM early in development, suggesting a role in encoding recent/familiar auditory experience (Miller-Sims and Bottjer, 2014; Stripling et al., 2001). Transcript levels for the plasticity-related immediate early gene (IEG) egr-1 (also known as zenk) peak in male NCM during the onset of sensory learning/opening of the song learning critical period (Jin and Clayton, 1997). In agreement with this, blocking plasticity-related MAPK signaling pathway in the auditory lobule (including both NCM and CMM) specifically during developmental tutoring prevents accurate tutor song imitation in adulthood (London and Clayton, 2008). Furthermore, tutoring naïve juvenile songbirds rapidly biases a subpopulation of single NCM neurons towards selectivity for the tutor’s song (Yanagihara and Yazaki-Sugiyama, 2016). These findings together suggest that NCM is required for accurate tutor song encoding, memorization and imitation, yet the molecular mechanisms enabling putative NCM-dependent auditory memory consolidation are less well known (Moorman et al., 2011). Neuronal cell density is adult-like by 20 dph in NCM (Stripling et al., 2001), and auditory responsiveness is markedly enhanced in sensory-aged songbirds compared to sensorimotor-aged males (Vahaba et al., 2017). Therefore, it may be that age-dependent changes in steroid hormones and their cognate receptors across the song learning critical period in development can partially explain NCM’s role in song learning/tutor song memorization.
Steroid hormones can limit song learning critical period plasticity during development, such as androgens which, like estrogens, also exert fast-actions on neural circuits (Bass and Remage-Healey, 2008; Foradori et al., 2008; Kelley and Bass, 2010; Wu et al., 2001). In developing songbirds, administering androgens, such as testosterone (T), to closed-ended learners before adult-like song is achieved leads to premature song and circuit crystallization (Bottjer and Johnson, 1997; Korsia and Bottjer, 1991; Whaling et al., 1995) but see (Templeton et al., 2012). One explanation for this is that testosterone acts to crystallize the song circuit and enable adult-like courtship, binding together the timing of sexual maturation with song maturation. Accordingly, circulating T peaks towards the tail-end of the song learning/sexual maturation period, potentially signifying the ‘closure’ of the critical period for song learning (Marler et al., 1987). In addition to pre-maturely crystallizing plastic song production, androgen implants early in development also lead to parallel premature ‘adult’-like physiology in the song motor pathway (Livingston and Mooney, 2001). According to this model, androgens impede motor variability by blocking vocal exploration, leading to stereotyped/crystallized song during development. In agreement with this interpretation, androgens continue to exert profound influence over song motor circuits in adulthood (Alward et al., 2013, 2017; Alward et al., 2016b; Alward et al., 2014).
Song learning experiments based on circulating levels and peripheral hormone manipulations are confounded by the fact that brain is the main source of circulating estrogens in songbirds (Schlinger and Arnold, 1992). For example, if testosterone acts as a cue to end song learning plasticity once adequate song is achieved, one would expect that peripheral T levels correspond to song learning fidelity. However, peripheral T levels measured at 100 dph in male zebra finches do not correlate with the degree of tutor song imitation (Deregnaucourt et al., 2013). Moreover, circulating T levels do not change in male zebra finches between the sensorimotor phase (50–60 dph) and the closing of the song learning critical period (105 – 130 dph), suggesting peripheral androgen levels are stable across development (Mori and Wada, 2015). Unlike androgens, circulating estradiol levels during the sensory phase of song learning are a more reliable predictor of eventual song similarity in adulthood (Marler et al., 1988). As such, estrogens are a prime candidate for the neuromodulation of tutor song encoding and memorization.
Estrogens are well-positioned to regulate song learning due to the unusual abundance of aromatase and estrogen receptors in vocal learners, as well as the role of estrogen in masculinizing vocal circuits in females. Although NCM is a highly conserved auditory forebrain region across Aves (Wang et al., 2010; Wild et al., 1993), as mentioned above there is a unique abundance of estrogen receptors and aromatase distribution in the avian forebrain of vocal learners, including NCM, compared to innately vocalizing birds (Metzdorf et al., 1999; Silverin et al., 2000; Yoder and Vicario, 2012). It is interesting to note that unlike songbirds, innately vocalizing male ruffed grouses have somatic aromatase protein expression in the Field L complex (Corfield et al., 2013). One role for local estradiol may be to establish song learning neural circuits. Exogeneous estradiol exposure in female zebra finch chicks, who do not normally sing in adulthood, masculinizes the neural song circuit by enlarging song nuclei, and enables male-like vocal learning and production (Gurney and Konishi, 1980). Follow-up studies demonstrated that brain-derived estrogens could account for the masculinization of the song motor pathway in zebra finches (Holloway and Clayton, 2001). Taken together, these studies suggest that neuroestrogens are required for vocal learning (motor) circuits in songbirds. Therefore, estradiol may be important across development for song learning, and perhaps specifically within the sensory phase, given that estradiol enhances auditory processing in adult songbirds.
Peripheral levels of estrogens and membrane estrogen receptors in the auditory cortex peak during the sensory phase of song learning, suggesting local neuroestrogens in NCM may influence tutor song memory consolidation. Sparrows, zebra finches, and canaries all have elevated levels of circulating estradiol exclusively during the sensory phase of song learning, a period critical for encoding and consolidating the model song (Marler et al., 1988; Marler et al., 1987; Pröve, 1983; Weichel et al., 1986), however see (Adkins-Regan et al., 1990). In swamp sparrows, this sensory phase estradiol peak is a reliable predictor for eventual tutor song imitation in adulthood (Marler et al., 1987). Alongside changes in local and global estradiol, GPER1 transcript levels peak at 30 dph in male telencephalon (which includes NCM) and are 5-times higher at that age than in adult males (Acharya and Veney, 2011). As GPER1 is one putative mechanism by which neuroestrogens rapidly enhance auditory processing (Krentzel et al., 2018; Remage-Healey et al., 2013), a coincident peak in circulating estradiol levels and cortical GPER1 expression suggests a role for estradiol in auditory memory consolidation in NCM.
Neuroestrogens in NCM may be important during development for modulating online auditory processing to guide tutor song memory consolidation. In contrast with adults in which song exposure leads to increased neuroestrogen production, juvenile zebra finches have reduced estradiol levels in NCM during social tutoring, and this is followed by a sharp rise one hour post-training (Chao et al., 2015). The functional role of these dynamics during tutoring is unclear. One hypothesis is that acute changes in neuroestrogens within NCM modulates online auditory processing, which may be important for tutor song memory consolidation. Recently, it was revealed that locally delivered estradiol within NCM rapidly transforms auditory encoding in a lateralized, and age-dependent fashion in developing male zebra finches (Vahaba et al., 2017). Therefore, since both adult and developing NCM is left-lateralized for auditory processing and memory consolidation (reviewed above for adults; Chirathivat et al., 2015; Moorman et al., 2012; Moorman et al., 2015), neuroestrogens in NCM may guide tutor song memorization by impacting sensory coding in a hemisphere-specific manner. The extent of interactions between neuroestrogens and established cell-signaling and molecular mechanisms enabling auditory processing and memory consolidation in songbirds remain to be tested (Ahmadiantehrani and London, 2017; London and Clayton, 2008; Moorman et al., 2011). In the sections that follow, we suggest future research directions to elucidate our understanding of how neuroestrogens mediate cognitive and sensory processes (Figure 2).
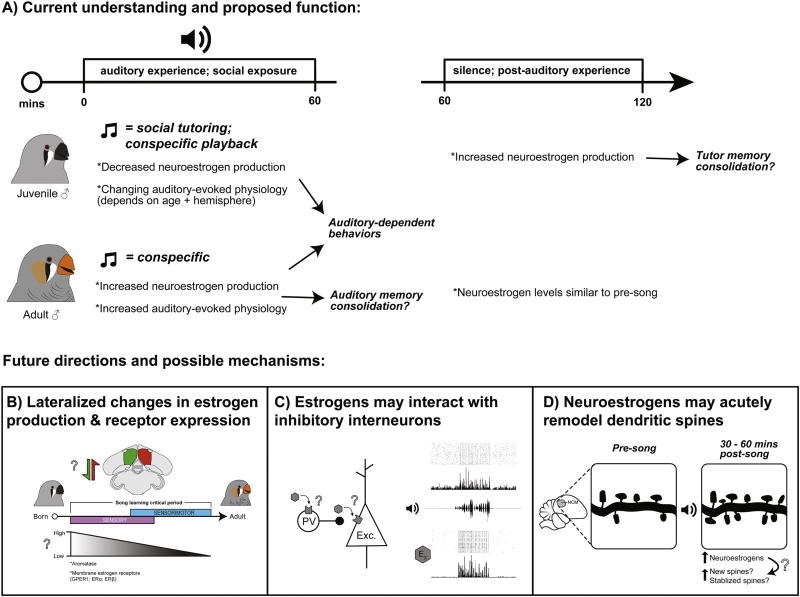
Figure 2.
Current understanding of rapid neuroestrogen signaling in songbird auditory forebrain and proposed future investigations. A) Top: Experimental timeline from previous studies on awake or anesthetized songbirds “Auditory experience” is often conspecific song playback through speakers, whereas social exposure is always a live, adult conspecific (male tutor, or male/female) presentation. Bottom: The physiological effects and temporal fluctuation of neuroestrogen production depends on age. In juvenile male zebra finches, song tutoring leads to an immediate decline in local estrogen content, whereas sixty minutes after the offset of tutoring, there is a rapid elevation in estrogen production that may be important for consolidating tutor song memories. When estrogens are presented in NCM, there are age- and hemisphere-dependent effects on spontaneous and auditory-evoked physiology. In adults, song playbacks and social presentations both elicit immediate elevations in neuroestrogen production, which rapidly enhance auditory responses both physiologically and behaviorally. We propose that the rapid production and actions of neuroestrogens B) change across the critical period for song learning, depending on age and hemisphere; C) impact local inhibitory circuits to modulate auditory signal detection; and D) regulate dendritic spine plasticity necessary for sensory memory consolidation. E2 = 17-beta-estradiol; Exc. = excitatory projection neuron; PV = parvalbumin-expressing interneuron. Portions of this figure adapted from Vahaba and Remage-Healey (2017).
Outlook & future directions
1) How does the expression of aromatase and estrogen receptors change across development?
Presently, much of what is known about estrogen receptor and aromatase expression in the songbird brain is from studies on adult songbirds. Thus, relatively little is known about how the synthesis and action of estrogens may change in the songbird brain across development. Previous work describing songbird aromatase expression in the brain has been limited by age (pre-CP aged subjects, Saldanha et al., 2000), inference of protein expression via mRNA measurement, neuroanatomical spatial resolution and antibody specificity (Palkovits punches: (Balthazart et al., 1990; Schumacher and Balthazart, 1987; Vockel et al., 1990), focusing on non-sensory cortices (Vockel et al., 1988), or limited point-sampling during vocal learning (typically ~45 dph only, Saldanha et al., 1999). While neuroestrogen levels gradually increase within NCM across development, it will be important to verify and expand on this by quantifying bilateral aromatase protein expression, as peripheral hormone changes may also impact these findings (Chao et al., 2015). Further, as neuronal cell density is adult-like by 20 dph in NCM, developmental changes in intrinsic synaptic physiology, and auditory-evoked extracellular activity (Jin and Clayton, 1997; Kudo and Yazaki-Sugiyama, 2017; Vahaba et al., 2017) may in part be explained by changing estrogen production across ontogeny (Chao et al., 2015). Acute estrogen action and/or synthesis may explain developmental changes in auditory properties during development, as GPER1 transcript levels are 5-times higher in sensory-aged male telencephalon compared to adults (Acharya and Veney, 2011). Therefore, as recently suggested by physiological recordings (Vahaba et al., 2017) hemispheric- and age-dependent changes in sensory coding may be accounted for by the expression and/or activity of neuroestrogen-related signaling molecules like aromatase.
2) Do neuroestrogens interact with inhibitory microcircuits to shape developmental song learning?
Sensory circuits primarily consist of interconnected excitatory and inhibitory neurons. Excitatory neurons receive and transmit signals within and across brain regions, whereas local inhibitory interneurons shape the gain, coding, selectivity, and modulation state of local cortical networks (Natan et al., 2017; Pi et al., 2013; Vallentin et al., 2016). Inhibitory neurons therefore shape specific auditory response states, making them primary regulators of processing and plasticity (Blackwell and Geffen, 2017). There is a diverse set of cortical GABAergic interneurons involved in auditory encoding (Tremblay et al., 2016; Wood et al., 2017), including the widely studied parvalbumin (PV) expressing neurons.
Parvalbumin is a protein directly important for calcium buffering and is a reliable marker for a subtype of inhibitory cortical interneurons (reviewed in Aizenberg et al., 2015). In mammalian cortex, parvalbumin-positive neurons are the primary inhibitory cell type, including auditory cortex (Xu et al., 2010). Specifically found within layers 2–6 of mammalian auditory cortex, parvalbumin-positive (PV+) neurons are required for encoding amplitude, frequency tuning, and sensorimotor integration, as well as auditory discrimination and adaption (Aizenberg et al., 2015; Cruikshank et al., 2001; Moore and Wehr, 2013; Natan et al., 2017; Schneider et al., 2014). As such, sharper frequency tuning is associated with recruitment of PV+ cells in auditory cortex (Li et al., 2014).
Tuning by PV+ cells in auditory brain regions may be regulated in part by rapid estrogen synthesis and signaling. In mammals, estrogen receptors are exclusively and highly expressed in PV+ interneurons (≥80%) (Blurton-Jones and Tuszynski, 2002; Higaki et al., 2012). Moreover, peripheral estradiol administration increases PV+ neurons in the arcuate nucleus of adult female rats (Sotonyi et al., 2010), a hypothalamic brain region in which estradiol acts rapidly via membrane-bound estrogen receptors (Roepke et al., 2009). In addition to rapid estrogen actions targeting PV cells, aromatase itself is highly and consistently co-expressed in PV+ neurons within human and nonhuman primate temporal cortex (Azcoitia et al., 2011; Yague et al., 2010; Yague et al., 2006; Yague et al., 2008). Taken together, PV+ neurons are critical for sensory coding in mammalian auditory circuits, and rapid estrogen actions on and synthesis within PV+ cells likely participate in the integration of auditory signals.
In songbirds, inhibitory neurons in NCM likely contribute to auditory learning and processing. Nearly half of all neurons In NCM are GABAergic, which are activated by song presentations (Pinaud et al., 2008; Pinaud et al., 2004), and are necessary for shaping auditory processing, selectivity, and memorization (Pinaud et al., 2008; Yanagihara and Yazaki-Sugiyama, 2016). By rapidly tuning inhibitory neurotransmission necessary for auditory-evoked neural activity, neuroestrogens may modulate auditory physiology in NCM. As with human and nonhuman primate temporal cortex (Yague et al., 2006; Yague et al., 2008), PV and aromatase are co-expressed in neurons within adult songbird auditory brain regions, including NCM (Ikeda et al., 2017). Song learning during development also provides a unique opportunity to explore how estrogens and inhibitory circuits in NCM may regulate critical period plasticity. Like aromatase and estrogen receptors, parvalbumin is uniquely expressed in forebrain song nuclei of avian vocal learners (Hara et al., 2012), and higher activation of PV cells corresponds to ‘better’ visual learning in an avian association cortex-like brain region (Ambalavanar et al., 1999). Songbirds thus offer a powerful model to explore natural mechanisms gating critical period plasticity for learned complex vocal signals in auditory forebrain (London, 2017), as well as testing the role for rapid estrogen actions in PV cells on auditory encoding.
3) Do neuroestrogens acutely remodel dendritic spines in NCM to facilitate auditory plasticity?
Estrogens enhance cognition via fast-actions on dendritic spines (Luine and Frankfurt, 2012; Srivastava, 2012). Peripheral estrogen treatment improves learning and memory, and rapidly (within 30 – 40 mins) increases hippocampal synaptogenesis and dendritic spine density (Jacome et al., 2016; MacLusky et al., 2005; Phan et al., 2012). Supporting the role of local and fast actions of estradiol mediating synaptic plasticity, estradiol rapidly (after 30 mins) increases dendritic spine densities in cortical neurons via nongenomic mechanisms, (Srivastava et al., 2008), and blocking in vivo estrogen synthesis centrally, within HP, prevents estradiol-dependent circuit plasticity (Vierk et al., 2015). Together, estrogens quickly modify dendritic spine dynamics that are functionally and behaviorally necessary for improved memory raising the prospect of similar mechanisms for auditory memory consolidation in songbirds.
In adult male zebra finches, dendritic spine densities in NCM rapidly double soon after brief (30 mins) exposures to novel song, an effect which is suppressed when endocannabinoid signaling is blocked (Gilbert and Soderstrom, 2013; Holland and Soderstrom, 2017). Intriguingly, acute estrogen treatment rapidly suppresses inhibitory synaptic transmission in rodent HP via an interaction with the cannabinoid receptor type 1 (CB1) (Huang and Woolley, 2012). As NCM is thought to integrate auditory information in adult songbirds by modulating inhibitory activity, rapid estrogen signaling in NCM may help encode and consolidate auditory experience by increasing dendritic spine density.
Developing songbirds may also undergo similar estradiol-dependent spine remodeling for tutor song memorization. In developing zebra finches, experience- and age-dependent changes in dynamic spine stabilization are critical for song learning and HVC circuit development (Roberts et al., 2010). As estradiol rapidly modulates spine dynamics in mammalian neural circuits, post-tutor neuroestrogen elevations in NCM may be important for consolidating recent tutor experience (tutor song) through acute dendritic spine alterations in developing auditory forebrain. Interestingly, both extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) signaling cascades are required in the auditory forebrain of developing male songbirds for tutor memorization and imitation (Ahmadiantehrani and London, 2017; London and Clayton, 2008), which are two intracellular routes of action required for estradiol-induced memory consolidation and related synaptic plasticity modifications in adult rodents (Fortress et al., 2013; Tuscher et al., 2016a).
Does work on neuroestrogens in songbirds and other species have clinical implications for human cognition and communication?
In humans, an association between circulating hormones and hearing has been established most convincingly for women across the menstrual cycle and during pregnancy. There is an abundance of studies showing that hormonal cycles can shift the behavioral threshold to detect sounds, verbal memory (Fernandez et al., 2003; Zimmerman et al., 2011), as well as the otoacoustic emissions detected from women (Al-Mana et al., 2010; Caras, 2013). There is now increasing interest in the role of estrogens in mediating the pathophysiology of auditory dysfunction as well, and the role of hormone-replacement therapy (HRT) in changing auditory function (Frisina and Frisina, 2016). More work is therefore needed in non-human animal models to understand the basic mechanisms of how hormones like neuroestrogens can impact vocal communication processing and memory.
There is also evidence that hormones are important for speech perception and language learning during development in humans. As with songbirds (Marler et al., 1987), elevations in circulating estradiol during development are a positive predictor of future language success in children (Schaadt et al., 2015; Wermke et al., 2014). Children with social and sensory processing difficulties, such as autism, have difficulties with voice processing and recognition, as well as underconnected auditory circuits (Abrams et al., 2013; Gervais et al., 2004). Autism and related speech language disorders may be due in-part to estrogen abnormalities, such as aromatase gene mutations (Anthoni et al., 2012). Therefore, work in animal models such as songbirds will help elucidate how estrogens transform auditory circuits in development, especially as it relates to learned vocal communication.
Conclusions
Studies on songbirds have provided critical progress toward understanding the rapid, nonclassical effects of neuroestrogens on physiological and behavioral endpoints. The current perspectives that: 1) local neuroestrogen production increases in the songbird auditory forebrain during social encounters, and 2) neuroestrogens enhance auditory processing, now direct future studies to address the broader functional significance of this modulation. Songbirds will continue to provide valuable contributions to further reveal how brain-generated estrogens interact with sensory circuits to enable natural vocal communication perception and learning across the lifespan. Going forward, disentangling whether estrogens improve auditory learning due to improved hearing alone, or whether neuroestrogens enhance both hearing and learning via parallel mechanisms, will require new experimental and theoretical approaches. Studies of songbirds should continue to enable such investigations in the future (Vahaba et al., 2017). As songbirds and humans share the rare and remarkable suite of traits for learned vocalizations (Chakraborty and Jarvis, 2015; Prather et al., 2017), understanding the role of neuroestrogens in communication learning and processing may lead to important translational discoveries.
Highlights.
-
-
Neuroestrogens rapidly modulate songbird sensory circuits.
-
-
Songbird vocal learning mechanisms implicate estrogens and cell-signaling pathways.
-
-
The songbird auditory cortex is key for song learning and tutor memory formation.
-
-
Estrogens acting in songbird auditory cortex may enhance memory consolidation.
Acknowledgments
Preparation of this work was supported in part by NIH R01NS082179 and NSF IOS 1354906.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Abrams DA, Lynch CJ, Cheng KM, Phillips J, Supekar K, Ryali S, Uddin LQ, Menon V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110:12060–12065. doi: 10.1073/pnas.1302982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2011;72:1433–1446. doi: 10.1002/dneu.22004. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Adret P, Meliza CD, Margoliash D. Song tutoring in presinging zebra finch juveniles biases a small population of higher-order song-selective neurons toward the tutor song. J Neurophysiol. 2012;108:1977–1987. doi: 10.1152/jn.00905.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadiantehrani S, London SE. Bidirectional manipulation of mTOR signaling disrupts socially mediated vocal learning in juvenile songbirds. Proc Natl Acad Sci U S A. 2017;114:9463–9468. doi: 10.1073/pnas.1701829114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai H, Kai K, Kumaraswamy A, Ikeno H, Wachtler T. Interneurons in the Honeybee Primary Auditory Center Responding to Waggle Dance-Like Vibration Pulses. J Neurosci. 2017;37:10624–10635. doi: 10.1523/JNEUROSCI.0044-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenberg M, Mwilambwe-Tshilobo L, Briguglio JJ, Natan RG, Geffen MN. Bidirectional Regulation of Innate and Learned Behaviors That Rely on Frequency Discrimination by Cortical Inhibitory Neurons. PLoS Biol. 2015;13:e1002308. doi: 10.1371/journal.pbio.1002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM. Alteration in auditory function during the ovarian cycle. Hear Res. 2010;268:114–122. doi: 10.1016/j.heares.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc Natl Acad Sci U S A. 2013;110:19573–19578. doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF. Dissociable Effects on Birdsong of Androgen Signaling in Cortex-Like Brain Regions of Canaries. J Neurosci. 2017;37:8612–8624. doi: 10.1523/JNEUROSCI.3371-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, de Bournonville C, Chan TT, Balthazart J, Cornil CA, Ball GF. Aromatase inhibition rapidly affects in a reversible manner distinct features of birdsong. Scientific reports. 2016a;6:32344. doi: 10.1038/srep32344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Madison FN, Parker SE, Balthazart J, Ball GF. Pleiotropic Control by Testosterone of a Learned Vocal Behavior and Its Underlying Neuroplasticity(1,2,3) eNeuro. 2016b;3 doi: 10.1523/ENEURO.0145-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Mayes WD, Peng K, Stevenson TJ, Balthazart J, Ball GF. Dissociable effects of social context on song and doublecortin immunoreactivity in male canaries. Eur J Neurosci. 2014;40:2941–2947. doi: 10.1111/ejn.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanar R, McCabe BJ, Potter KN, Horn G. Learning-related fos-like immunoreactivity in the chick brain: time-course and co-localization with GABA and parvalbumin. Neuroscience. 1999;93:1515–1524. doi: 10.1016/s0306-4522(99)00217-1. [DOI] [PubMed] [Google Scholar]
- Anthoni H, Sucheston LE, Lewis BA, Tapia-Paez I, Fan X, Zucchelli M, Taipale M, Stein CM, Hokkanen ME, Castren E, Pennington BF, Smith SD, Olson RK, Tomblin JB, Schulte-Korne G, Nothen M, Schumacher J, Muller-Myhsok B, Hoffmann P, Gilger JW, Hynd GW, Nopola-Hemmi J, Leppanen PH, Lyytinen H, Schoumans J, Nordenskjold M, Spencer J, Stanic D, Boon WC, Simpson E, Makela S, Gustafsson JA, Peyrard-Janvid M, Iyengar S, Kere J. The aromatase gene CYP19A1: several genetic and functional lines of evidence supporting a role in reading, speech and language. Behavior genetics. 2012;42:509–527. doi: 10.1007/s10519-012-9532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Makeyeva YV, Paitel ER, Pedersen AL, Hon AT, Gunderson JA, Saldanha CJ. Hippocampal Aromatization Modulates Spatial Memory and Characteristics of the Synaptic Membrane in the Male Zebra Finch. Endocrinology. 2017;158:852–859. doi: 10.1210/en.2016-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Saldanha CJ. The importance of neural aromatization in the acquisition, recall, and integration of song and spatial memories in passerines. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Choleris E, Remage-Healey L. Steroids and the brain: 50years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm Behav. 2018;99:1–8. doi: 10.1016/j.yhbeh.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N. Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: an immunocytochemical study. J Comp Neurol. 1990;301:276–288. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. BioEssays : news and reviews in molecular, cellular and developmental biology. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Newsome WT. The Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) initiative and neurology. JAMA Neurol. 2014;71:675–676. doi: 10.1001/jamaneurol.2014.411. [DOI] [PubMed] [Google Scholar]
- Barton M, Filardo EJ, Lolait SJ, Thomas P, Maggiolini M, Prossnitz ER. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. The Journal of steroid biochemistry and molecular biology. 2017 doi: 10.1016/j.jsbmb.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH. Steroid-dependent plasticity of vocal motor systems: novel insights from teleost fish. Brain Res Rev. 2008;57:299–308. doi: 10.1016/j.brainresrev.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. eLife. 2014;3:e02743. doi: 10.7554/eLife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JM, Geffen MN. Progress and challenges for understanding the function of cortical microcircuits in auditory processing. Nat Commun. 2017;8:2165. doi: 10.1038/s41467-017-01755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol. 2002;452:276–287. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nature reviews. Neuroscience. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Moorman S. Birdsong memory and the brain: in search of the template. Neurosci Biobehav Rev. 2015;50:41–55. doi: 10.1016/j.neubiorev.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nature reviews. Neuroscience. 2010;11:747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Bonke BA, Bonke D, Scheich H. Connectivity of the auditory forebrain nuclei in the guinea fowl (Numida meleagris) Cell Tissue Res. 1979;200:101–121. doi: 10.1007/BF00236891. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Translating birdsong: songbirds as a model for basic and applied medical research. Annual review of neuroscience. 2013;36:489–517. doi: 10.1146/annurev-neuro-060909-152826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, Bosch MA, Ronnekleiv OK, Dorsa DM. 17-Beta estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience. 2005;133:343–352. doi: 10.1016/j.neuroscience.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ. Conversion of androgen to estrogen and other steroids in the vertebrate brain. Am. Zool. 1978a;18:511–523. [Google Scholar]
- Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978b;103:2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- Canopoli A, Zai A, Hahnloser R. Lesions of a higher auditory brain area during a sensorimotor period do not impair birdsong learning. Matters. 2016 doi: 10.19185/matters.201603000018. [DOI] [Google Scholar]
- Canopoli A, Zai A, Hahnloser R. Bilateral neurotoxic lesions in NCM before tutoring onset do not prevent successful tutor song learning. Matters. 2017 doi: 10.19185/matters.201612000007. [DOI] [Google Scholar]
- Caras ML. Estrogenic modulation of auditory processing: a vertebrate comparison. Front Neuroendocrinol. 2013;34:285–299. doi: 10.1016/j.yfrne.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras ML, Remage-Healey L. Modulation of Peripheral and Central Auditory Processing by Estrogens in Birds. In: Bass AH, Sisneros JA, Popper AN, Fay RR, editors. Hearing and Hormones. Springer International Publishing; Cham: 2016. pp. 77–99. [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chakraborty M, Jarvis ED. Brain evolution by brain pathway duplication. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2015.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Paon A, Remage-Healey L. Dynamic variation in forebrain estradiol levels during song learning. Dev Neurobiol. 2015;75:271–286. doi: 10.1002/dneu.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci U S A. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirathivat N, Raja SC, Gobes SM. Hemispheric dominance underlying the neural substrate for learned vocalizations develops with experience. Sci Rep. 2015;5:11359. doi: 10.1038/srep11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. The genomics of memory and learning in songbirds. Annual review of genomics and human genetics. 2013;14:45–65. doi: 10.1146/annurev-genom-090711-163809. [DOI] [PubMed] [Google Scholar]
- Colombelli-Negrel D, Hauber ME, Robertson J, Sulloway FJ, Hoi H, Griggio M, Kleindorfer S. Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Current biology : CB. 2012;22:2155–2160. doi: 10.1016/j.cub.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Corfield JR, Harada N, Iwaniuk AN. Aromatase expression in the brain of the ruffed grouse (Bonasa umbellus) and comparisons with other galliform birds (Aves, Galliformes) J Chem Neuroanat. 2013;47:15–27. doi: 10.1016/j.jchemneu.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Killackey HP, Metherate R. Parvalbumin and calbindin are differentially distributed within primary and secondary subregions of the mouse auditory forebrain. Neuroscience. 2001;105:553–569. doi: 10.1016/s0306-4522(01)00226-3. [DOI] [PubMed] [Google Scholar]
- De Groof G, Balthazart J, Cornil CA, Van der Linden A. Topography and Lateralized Effect of Acute Aromatase Inhibition on Auditory Processing in a Seasonal Songbird. J Neurosci. 2017;37:4243–4254. doi: 10.1523/JNEUROSCI.1961-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groof G, Verhoye M, Poirier C, Leemans A, Eens M, Darras VM, Van der Linden A. Structural changes between seasons in the songbird auditory forebrain. J Neurosci. 2009;29:13557–13565. doi: 10.1523/JNEUROSCI.1788-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregnaucourt S, Gahr M. Horizontal transmission of the father's song in the zebra finch (Taeniopygia guttata) Biology letters. 2013;9:20130247. doi: 10.1098/rsbl.2013.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregnaucourt S, Poirier C, Kant AV, Linden AV, Gahr M. Comparisons of different methods to train a young zebra finch (Taeniopygia guttata) to learn a song. Journal of physiology, Paris. 2013;107:210–218. doi: 10.1016/j.jphysparis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Dillon TS, Fox LC, Han C, Linster C. 17 beta-Estradiol Enhances Memory Duration in the Main Olfactory Bulb in CD-1 Mice. Behavioral Neuroscience. 2013;127:923–931. doi: 10.1037/a0034839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Clayton DF. Partial dissociation of molecular and behavioral measures of song habituation in adult zebra finches. Genes, brain, and behavior. 2008;7:802–809. doi: 10.1111/j.1601-183X.2008.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Clayton DF. Habituation in songbirds. Neurobiol Learn Mem. 2009;92:183–188. doi: 10.1016/j.nlm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annual review of neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Dufy B, Vincent JD, Fleury H, Du Pasquier P, Gourdji D, Tixier-Vidal A. Dopamine inhibition of action potentials in a prolactin secreting cell line is modulated by oestrogen. Nature. 1979;282:855–857. doi: 10.1038/282855a0. [DOI] [PubMed] [Google Scholar]
- Endevelt-Shapira Y, Perl O, Ravia A, Amir D, Eisen A, Bezalel V, Rozenkrantz L, Mishor E, Pinchover L, Soroka T, Honigstein D, Sobel N. Altered responses to social chemosignals in autism spectrum disorder. Nat Neurosci. 2018;21:111–119. doi: 10.1038/s41593-017-0024-x. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Weis S, Stoffel-Wagner B, Tendolkar I, Reuber M, Beyenburg S, Klaver P, Fell J, de Greiff A, Ruhlmann J, Reul J, Elger CE. Menstrual cycle-dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci. 2003;23:3790–3795. doi: 10.1523/JNEUROSCI.23-09-03790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD, Frisina DR. Hormone Replacement Therapy and Its Effects on Human Hearing. In: Bass AH, Sisneros JA, Popper AN, Fay RR, editors. Hearing and Hormones. Springer International Publishing; Cham: 2016. pp. 191–209. [Google Scholar]
- Galea LAM, Frick KM, Hampson E, Sohrabji F, Choleris E. Why estrogens matter for behavior and brain health. Neurosci Biobehav Rev. 2017;76:363–379. doi: 10.1016/j.neubiorev.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, Barthelemy C, Brunelle F, Samson Y, Zilbovicius M. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Gilbert MT, Soderstrom K. Novel song-stimulated dendritic spine formation and Arc/Arg3.1 expression in zebra finch auditory telencephalon are disrupted by cannabinoid agonism. Brain Res. 2013;1541:9–21. doi: 10.1016/j.brainres.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobes SM, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Current biology : CB. 2007;17:789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Gobes SM, Zandbergen MA, Bolhuis JJ. Memory in the making: localized brain activation related to song learning in young songbirds. Proc Biol Sci. 2010;277:3343–3351. doi: 10.1098/rspb.2010.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Konishi M. Hormone-Induced Sexual-Differentiation of Brain and Behavior in Zebra Finches. Science. 1980;208:1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Kotowicz A. Auditory representations and memory in birdsong learning. Curr Opin Neurobiol. 2010;20:332–339. doi: 10.1016/j.conb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Hara E, Rivas MV, Ward JM, Okanoya K, Jarvis ED. Convergent differential regulation of parvalbumin in the brains of vocal learners. PLoS One. 2012;7:e29457. doi: 10.1371/journal.pone.0029457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki S, Takumi K, Itoh M, Watanabe G, Taya K, Shimizu K, Hayashi M, Oishi T. Response of ERbeta and aromatase expression in the monkey hippocampal formation to ovariectomy and menopause. Neurosci Res. 2012;72:148–154. doi: 10.1016/j.neures.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Holland TL, Soderstrom K. Chronic CB1 cannabinoid receptor antagonism persistently increases dendritic spine densities in brain regions important to zebra finch vocal learning and production in an antidepressant-sensitive manner. Brain Research. 2017;1672:1–9. doi: 10.1016/j.brainres.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda MZ, Jeon SD, Cowell RA, Remage-Healey L. Norepinephrine Modulates Coding of Complex Vocalizations in the Songbird Auditory Cortex Independent of Local Neuroestrogen Synthesis. J Neurosci. 2015;35:9356–9368. doi: 10.1523/JNEUROSCI.4445-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda MZ, Krentzel AA, Oliver TJ, Scarpa GB, Remage-Healey L. Clustered organization and region-specific identities of estrogen-producing neurons in the forebrain of Zebra Finches (Taeniopygia guttata) J Comp Neurol. 2017;525:3636–3652. doi: 10.1002/cne.24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches. In: Thorpe WH, editor. Bird Vocalizations. 1969. [Google Scholar]
- Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN. Gonadal Hormones Rapidly Enhance Spatial Memory and Increase Hippocampal Spine Density in Male Rats. Endocrinology. 2016;157:1357–1362. doi: 10.1210/en.2015-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne JM, Thompson JV, Sharpee TO, Gentner TQ. Emergence of learned categorical representations within an auditory forebrain circuit. J Neurosci. 2011;31:2595–2606. doi: 10.1523/JNEUROSCI.3930-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron. 1997;19:1049–1059. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- Katz PS, Lillvis JL. Reconciling the deep homology of neuromodulation with the evolution of behavior. Curr Opin Neurobiol. 2014;29:39–47. doi: 10.1016/j.conb.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Bass AH. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Curr Opin Neurobiol. 2010;20:748–753. doi: 10.1016/j.conb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Vitousek MN. Dynamic modulation of sociality and aggression: an examination of plasticity within endocrine and neuroendocrine systems. Philos Trans R Soc Lond B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Korsia S, Bottjer SW. Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. J Neurosci. 1991;11:2362–2371. doi: 10.1523/JNEUROSCI.11-08-02362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Macedo-Lima M, Ikeda MZ, Remage-Healey L. A membrane g-protein coupled estrogen receptor is necessary but not sufficient for sex-differences in zebra finch auditory coding. Endocrinology. 2018:en.2017-03102–en.02017-03102. doi: 10.1210/en.2017-03102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Minimal experience required for immediate-early gene induction in zebra finch neostriatum. Neurobiol Learn Mem. 2000;74:179–184. doi: 10.1006/nlme.2000.3968. [DOI] [PubMed] [Google Scholar]
- Kudo T, Yazaki-Sugiyama Y. Early auditory experience modifies neuronal firing properties in zebra finch auditory cortex. Society for Neuroscience; Washington, D.C., USA: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Pawlisch BA, Macedo-Lima M, Remage-Healey L. Norepinephrine enhances song responsiveness and encoding in the auditory forebrain of male zebra finches. J Neurophysiol. 2017 doi: 10.1152/jn.00251.2017. jn 00251 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Ji XY, Liang F, Li YT, Xiao Z, Tao HW, Zhang LI. A feedforward inhibitory circuit mediates lateral refinement of sensory representation in upper layer 2/3 of mouse primary auditory cortex. J Neurosci. 2014;34:13670–13683. doi: 10.1523/JNEUROSCI.1516-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind D, Marcus GF, Bemis DK, Sasahara K, Jacoby N, Takahasi M, Suzuki K, Feher O, Ravbar P, Okanoya K, Tchernichovski O. Stepwise acquisition of vocal combinatorial capacity in songbirds and human infants. Nature. 2013;498:104–108. doi: 10.1038/nature12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston FS, Mooney R. Androgens and isolation from adult tutors differentially affect the development of songbird neurons critical to vocal plasticity. J Neurophysiol. 2001;85:34–42. doi: 10.1152/jn.2001.85.1.34. [DOI] [PubMed] [Google Scholar]
- London SE. Influences of non-canonical neurosteroid signaling on developing neural circuits. Current Opinion in Neurobiology. 2016;40:103–110. doi: 10.1016/j.conb.2016.06.018. [DOI] [PubMed] [Google Scholar]
- London SE. Developmental song learning as a model to understand neural mechanisms that limit and promote the ability to learn. Behavioural Processes. 2017 doi: 10.1016/j.beproc.2017.11.008. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord LD, Bond J, Thompson RR. Rapid steroid influences on visually guided sexual behavior in male goldfish. Horm Behav. 2009;56:519–526. doi: 10.1016/j.yhbeh.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17 alpha and 17 beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Las L, Denisenko N, Fee MS. A role for descending auditory cortical projections in songbird vocal learning. eLife. 2014;3 doi: 10.7554/eLife.02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiamele LA, Fuxjager MJ, Schuppe ER, Taylor RS, Hödl W, Preininger D. Increased androgenic sensitivity in the hind limb muscular system marks the evolution of a derived gestural display. Proceedings of the National Academy of Sciences. 2016;113:5664–5669. doi: 10.1073/pnas.1603329113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiamele LA, Gomez JR, Curtis NJ, Thompson RR. GPER/GPR30, a membrane estrogen receptor, is expressed in the brain and retina of a social fish (Carassius auratus) and colocalizes with isotocin. J Comp Neurol. 2017;525:252–270. doi: 10.1002/cne.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, O'Leary T, Shruti S. Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annual review of neuroscience. 2014;37:329–346. doi: 10.1146/annurev-neuro-071013-013958. [DOI] [PubMed] [Google Scholar]
- Marler P, Peters S, Ball GF, Dufty AM, Jr, Wingfield JC. The role of sex steroids in the acquisition and production of birdsong. Nature. 1988;336:770–772. doi: 10.1038/336770a0. [DOI] [PubMed] [Google Scholar]
- Marler P, Peters S, Wingfield J. Correlations between song acquisition, song production, and plasma levels of testosterone and estradiol in sparrows. J Neurobiol. 1987;18:531–548. doi: 10.1002/neu.480180605. [DOI] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene's response to that song in zebra finch telencephalon. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG. Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. J Neuroendocrinol. 2009;21:257–262. doi: 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Sims VC, Bottjer SW. Development of neural responsivity to vocal sounds in higher level auditory cortex of songbirds. J Neurophysiol. 2014;112:81–94. doi: 10.1152/jn.00484.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci. 2013;33:13713–13723. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman S, Gobes SM, Kuijpers M, Kerkhofs A, Zandbergen MA, Bolhuis JJ. Human-like brain hemispheric dominance in birdsong learning. Proc Natl Acad Sci U S A. 2012;109:12782–12787. doi: 10.1073/pnas.1207207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman S, Gobes SM, van de Kamp FC, Zandbergen MA, Bolhuis JJ. Learning-related brain hemispheric dominance in sleeping songbirds. Sci Rep. 2015;5:9041. doi: 10.1038/srep09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman S, Mello CV, Bolhuis JJ. From songs to synapses: molecular mechanisms of birdsong memory. Molecular mechanisms of auditory learning in songbirds involve immediate early genes, including zenk and arc, the ERK/MAPK pathway and synapsins. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:377–385. doi: 10.1002/bies.201000150. [DOI] [PubMed] [Google Scholar]
- Mori C, Wada K. Audition-independent vocal crystallization associated with intrinsic developmental gene expression dynamics. J Neurosci. 2015;35:878–889. doi: 10.1523/JNEUROSCI.1804-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233:226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Petro Z, Kuhn M. The formation and metabolism of estrogens in brain tissues. Advances in the biosciences. 1975a;15:105–121. [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent progress in hormone research. 1975b;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab. 1971;33:368–370. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- Natan RG, Rao W, Geffen MN. Cortical Interneurons Differentially Shape Frequency Tuning following Adaptation. Cell Rep. 2017;21:878–890. doi: 10.1016/j.celrep.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Marder E. Functional consequences of neuropeptide and small-molecule co-transmission. Nature reviews. Neuroscience. 2017;18:389–403. doi: 10.1038/nrn.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm Behav. 2004;45:250–258. doi: 10.1016/j.yhbeh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ota N, Gahr M, Soma M. Tap dancing birds: the multimodal mutual courtship display of males and females in a socially monogamous songbird. Sci Rep. 2015;5:16614. doi: 10.1038/srep16614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997a;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997b;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Pawlisch BA, Remage-Healey L. Neuroestrogen signaling in the songbird auditory cortex propagates into a sensorimotor network via an 'interface' nucleus. Neuroscience. 2015;284:522–535. doi: 10.1016/j.neuroscience.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CL, Hurley LM. Putting it in Context: Linking Auditory Processing with Social Behavior Circuits in the Vertebrate Brain. Integr Comp Biol. 2017;57:865–877. doi: 10.1093/icb/icx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, Jarvis ED. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Frontiers in evolutionary neuroscience. 2012;4:12. doi: 10.3389/fnevo.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. Low Doses of 17 beta-Estradiol Rapidly Improve Learning and Increase Hippocampal Dendritic Spines. Neuropsychopharmacology. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Gergues MM, Mahidadia S, Jimenez-Castillo J, Vicario DS, Bieszczad KM. HDAC3 Inhibitor RGFP966 Modulates Neuronal Memory for Vocal Communication Signals in a Songbird Model. Frontiers in Systems Neuroscience. 2017;11:65. doi: 10.3389/fnsys.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J Neurobiol. 2006;66:182–195. doi: 10.1002/neu.20211. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Terleph TA, Tremere LA, Phan ML, Dagostin AA, Leao RM, Mello CV, Vicario DS. Inhibitory network interactions shape the auditory processing of natural communication signals in the songbird auditory forebrain. J Neurophysiol. 2008;100:441–455. doi: 10.1152/jn.01239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Veiho TAF, Jeong JK, Tremere LA, Leao RM, von Gersdorff H, Mello CV. GABAergic neurons participate in the brain's response to birdsong auditory stimulation. Eur J Neurosci. 2004;20:1318–1330. doi: 10.1111/j.1460-9568.2004.03585.x. [DOI] [PubMed] [Google Scholar]
- Piristine HC, Choetso T, Gobes SM. A sensorimotor area in the songbird brain is required for production of vocalizations in the song learning period of development. Dev Neurobiol. 2016;76:1213–1225. doi: 10.1002/dneu.22384. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Prather JF, Okanoya K, Bolhuis JJ. Brains for birds and babies: Neural parallels between birdsong and speech acquisition. Neurosci Biobehav Rev. 2017;81:225–237. doi: 10.1016/j.neubiorev.2016.12.035. [DOI] [PubMed] [Google Scholar]
- Pröve E. Hormonal Correlates of Behavioural Development in Male Zebra Finches. In: Balthazart J, Pröve E, Gilles R, editors. Hormones and behaviour in higher vertebrates. Springer-Verlag; Berlin; New York: 1983. [Google Scholar]
- Remage-Healey L. Brain estrogen signaling effects acute modulation of acoustic communication behaviors: A working hypothesis. BioEssays : news and reviews in molecular, cellular and developmental biology. 2012;34:1009–1016. doi: 10.1002/bies.201200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L. Frank Beach Award Winner: Steroids as Neuromodulators of Brain Circuits and Behavior. Hormones and Behavior. 2014 doi: 10.1016/j.yhbeh.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010a;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. J Neuroendocrinol. 2013;25:1024–1031. doi: 10.1111/jne.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat. 2010b;39:72–81. doi: 10.1016/j.jchemneu.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, Schlinger BA. Estradiol synthesis and action at the synapse: evidence for "synaptocrine" signaling. Frontiers in endocrinology. 2011;2:28. doi: 10.3389/fendo.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Salwiczek L, Roth J, Schlinger BA. Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learn Mem. 2013;100:41–47. doi: 10.1016/j.nlm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Hisey E, Tanaka M, Kearney MG, Chattree G, Yang CF, Shah NM, Mooney R. Identification of a motor-to-auditory pathway important for vocal learning. Nat Neurosci. 2017;20:978–986. doi: 10.1038/nn.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Bosch MA, Ronnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21:263–270. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- Roper A, Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology. 2006;112:458–470. [Google Scholar]
- Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville JR, Remage-Healey L, Sinchak K, Micevych PE. Actions of Steroids: New Neurotransmitters. J Neurosci. 2016;36:11449–11458. doi: 10.1523/JNEUROSCI.2473-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Clayton NS, Schlinger BA. Androgen metabolism in the juvenile oscine forebrain: a cross-species analysis at neural sites implicated in memory function. J Neurobiol. 1999;40:397–406. doi: 10.1002/(sici)1097-4695(19990905)40:3<397::aid-neu11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J Comp Neurol. 2004;469:522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sato SM, Woolley CS. Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. eLife. 2016;5 doi: 10.7554/eLife.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaadt G, Hesse V, Friederici AD. Sex hormones in early infancy seem to predict aspects of later language development. Brain and language. 2015;141:70–76. doi: 10.1016/j.bandl.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci U S A. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone-metabolizing enzymes in the Japanese quail. Brain Res. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- Shamble PS, Menda G, Golden JR, Nitzany EI, Walden K, Beatus T, Elias DO, Cohen I, Miles RN, Hoy RR. Airborne Acoustic Perception by a Jumping Spider. Current biology : CB. 2016;26:2913–2920. doi: 10.1016/j.cub.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen Comp Endocrinol. 2000;117:34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- Smotherman MS, Narins PM. Hair cells, hearing and hopping: a field guide to hair cell physiology in the frog. Journal of Experimental Biology. 2000;203:2237–2246. doi: 10.1242/jeb.203.15.2237. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Jarvis ED. Different mechanisms are responsible for dishabituation of electrophysiological auditory responses to a change in acoustic identity than to a change in stimulus location. Neurobiol Learn Mem. 2013;106:163–176. doi: 10.1016/j.nlm.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotonyi P, Gao Q, Bechmann I, Horvath TL. Estrogen promotes parvalbumin expression in arcuate nucleus POMC neurons. Reprod Sci. 2010;17:1077–1080. doi: 10.1177/1933719110379651. [DOI] [PubMed] [Google Scholar]
- Soyman E, Vicario DS. Principles of auditory processing differ between sensory and premotor structures of the songbird forebrain. J Neurophysiol. 2017;117:1266–1280. doi: 10.1152/jn.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KA, Minderman J. Advances in the Study of Behavior. Academic Press; 2018. Developmental Programming via Activation of the Hypothalamic–Pituitary–Adrenal Axis: A New Role for Acoustic Stimuli in Shaping Behavior? [Google Scholar]
- Srivastava DP. Two-step wiring plasticity--a mechanism for estrogen-induced rewiring of cortical circuits. The Journal of steroid biochemistry and molecular biology. 2012;131:17–23. doi: 10.1016/j.jsbmb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci U S A. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacological reviews. 2013;65:1318–1350. doi: 10.1124/pr.111.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J Neurobiol. 2001;48:163–180. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Sato SM, Woolley CS. Quantitative Analysis of Long-Form Aromatase mRNA in the Male and Female Rat Brain. Plos One. 2014;9 doi: 10.1371/journal.pone.0100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton CN, Burt JM, Campbell SE, Lent K, Brenowitz EA, Beecher MD. Immediate and long-term effects of testosterone on song plasticity and learning in juvenile song sparrows. Behavioural Processes. 2012;90:254–260. doi: 10.1016/j.beproc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Riebel K, van der Burg JM, den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. J Comp Neurol. 2006;494:784–791. doi: 10.1002/cne.20831. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM. Estradiol-Mediated Spine Changes in the Dorsal Hippocampus and Medial Prefrontal Cortex of Ovariectomized Female Mice Depend on ERK and mTOR Activation in the Dorsal Hippocampus. J Neurosci. 2016a;36:1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav. 2016b;83:60–67. doi: 10.1016/j.yhbeh.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahaba DM, Macedo-Lima M, Remage-Healey L. Sensory Coding and Sensitivity to Local Estrogens Shift during Critical Period Milestones in the Auditory Cortex of Male Songbirds. eNeuro. 2017;4 doi: 10.1523/ENEURO.0317-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahaba DM, Remage-Healey L. Brain estrogen production and the encoding of recent experience. Current Opinion in Behavioral Sciences. 2015;6:148–153. doi: 10.1016/j.cobeha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallentin D, Kosche G, Lipkind D, Long MA. Inhibition protects acquired song segments during vocal learning in zebra finches. Science. 2016;351:267–271. doi: 10.1126/science.aad3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Velho TA, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLoS One. 2012;7:e36276. doi: 10.1371/journal.pone.0036276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Bayer J, Freitag S, Muhia M, Kutsche K, Wolbers T, Kneussel M, Sommer T, Rune GM. Structure-function-behavior relationship in estrogen-induced synaptic plasticity. Horm Behav. 2015;74:139–148. doi: 10.1016/j.yhbeh.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Changes in the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches during the post-hatching period. Brain Res. 1988;463:330–340. doi: 10.1016/0006-8993(88)90406-4. [DOI] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990;511:291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci U S A. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichel K, Schwager G, Heid P, Güttinger HR, Pesch A. Sex Differences in Plasma Steroid Concentrations and Singing Behaviour during Ontogeny in Canaries (Serinus canaria) Ethology. 1986;73:281–294. [Google Scholar]
- Wermke K, Hain J, Oehler K, Wermke P, Hesse V. Sex hormone influence on human infants' sound characteristics: melody in spontaneous crying. Biology letters. 2014;10:20140095. doi: 10.1098/rsbl.2014.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaling CS, Nelson DA, Marler P. Testosterone-induced shortening of the storage phase of song development in birds interferes with vocal learning. Dev Psychobiol. 1995;28:367–376. doi: 10.1002/dev.420280703. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory forebrain in the pigeon (Columba livia) J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- Wood KC, Blackwell JM, Geffen MN. Cortical inhibitory interneurons control sensory processing. Curr Opin Neurobiol. 2017;46:200–207. doi: 10.1016/j.conb.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annual review of pharmacology and toxicology. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KH, Tobias ML, Kelley DB. Estrogen and laryngeal synaptic strength in Xenopus laevis: opposite effects of acute and chronic exposure. Neuroendocrinology. 2001;74:22–32. doi: 10.1159/000054667. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda SI, Harada N, Shah NM. Estrogen Masculinizes Neural Pathways and Sex-Specific Behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138:389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–127. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Yazaki-Sugiyama Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat Commun. 2016;7:11946. doi: 10.1038/ncomms11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Lu K, Vicario DS. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport. 2012;23:922–926. doi: 10.1097/WNR.0b013e3283588b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Vicario DS. To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behav Neurosci. 2012;126:17–28. doi: 10.1037/a0026673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci U S A. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Lipton RB, Santoro N, McConnell DS, Derby CA, Katz MJ, Baigi K, Saunders-Pullman R. Endogenous estradiol is associated with verbal memory in nondemented older men. Brain Cogn. 2011;76:158–165. doi: 10.1016/j.bandc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]