Abstract
Background
Phototherapy is used to treat newborn infants with hyperbilirubinaemia. Fibreoptic phototherapy is a new mode of phototherapy which is reported to lower serum bilirubin (SBR) while minimising disruption of normal infant care.
Objectives
To evaluate the efficacy of fibreoptic phototherapy.
Search methods
The standard search strategy of the Cochrane Collaboration was used including searches of the Cochrane Controlled Trials Register, MEDLINE, EMBASE and discussion with experts in the field.
Selection criteria
Randomised or quasi‐randomised controlled trials evaluating the efficacy of fibreoptic phototherapy in the management of newborn infants with hyperbilirubinaemia.
Data collection and analysis
Thirty‐one studies were identified of which 24 met inclusion criteria. They evaluated the efficacy of fibreoptic phototherapy in a number of different clinical situations and patient populations.
Main results
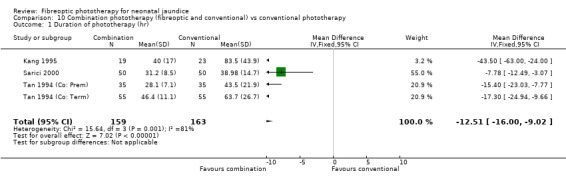
Fibreoptic phototherapy was more effective at lowering SBR than no treatment but less effective than conventional phototherapy (percentage change in SBR after 24 hours of treatment: WMD ‐10.7%, 95%CI ‐18.14, ‐3.26 and WMD 3.59%, 95%CI 1.27, 5.92 respectively). Fibreoptic phototherapy was equally as effective as conventional phototherapy in preterm infants and when two fibreoptic devices were used simultaneously (change in SBR after 24 hours of treatment: WMD 1.7%, 95%CI ‐2.65, 6.05 and change in SBR per day over whole treatment period: WMD 2.82%, 95%CI ‐1.84, 7.48 respectively). A combination of fibreoptic and conventional phototherapy was more effective than conventional phototherapy alone (duration of phototherapy: WMD ‐12.51 hr, 95%CI ‐16.00, ‐9.02, meta‐analysis affected by heterogeneity). No conclusion can be made on the superiority of one fibreoptic device over another as the two studies comparing them (one favouring BiliBlanket, the other finding no difference) did not contain a common outcome measure.
Authors' conclusions
Fibreoptic phototherapy has a place in the management of neonatal hyperbilirubinaemia. It is probably a safe alternative to conventional phototherapy in term infants with physiological jaundice. No trials have been identified which support the widely‐held view that fibreoptic devices interfere less with infant care or impact less on parent‐child bonding.
Plain language summary
Fibreoptic phototherapy for neonatal jaundice
A single fibreoptic phototherapy device is less effective at treating neonatal jaundice than conventional phototherapy, except in preterm infants in whom it is equally effective. Newborn infants often develop jaundice, which is concerning as unconjugated serum bilirubin can damage the developing brain. Since the 1960s, jaundice has been treated with phototherapy, for which the infants have to be naked in a crib with their eyes covered. Fibreoptic phototherapy is a new type of phototherapy in which the light is applied directly to the skin of the infant via optical fibres, enabling the infants to be nursed fully clothed near to their parents. This review has shown that fibreoptic phototherapy is less effective than conventional phototherapy, except in preterm infants in whom it is equally effective.
Background
In the first week of life approximately 50% of newborn infants have clinically detectable jaundice (Maisels 1982). The majority of these infants have no underlying disease and their jaundice results from the increased bilirubin production and decreased excretion which are normally seen in the newborn period (physiological jaundice). In a minority, jaundice indicates a more serious underlying pathology such as haemolysis, septicaemia or metabolic disease (non‐physiological jaundice). Elevated serum bilirubin (SBR) can damage neurones, and for this reason it is measured in a newborn infant with obvious jaundice or signs of underlying disease.
Since the early 1970's neonatal jaundice has been treated with phototherapy. Phototherapy causes photoisomerisation of bilirubin into a water‐soluble form which can be excreted by the kidney. It effectively decreases the SBR in jaundiced newborn infants and decreases the need for exchange blood transfusion (Maisels 1992). To deliver phototherapy the infant is nursed under halogen or fluorescent lamps and the eyes are covered with a mask to prevent retinal damage. The disadvantages of delivering phototherapy in this way are that it can interfere with parent‐child bonding (Fetus & Newborn 1986), and that a displaced eye mask can cause nasal obstruction (Al‐Salihi 1975). However, this method of delivering phototherapy (conventional phototherapy) remains widely used.
In the last 10 years new devices for delivering phototherapy have been developed using optical fibres and a light source. In the first, the infant is nursed on a blanket containing optical fibres that delivers light to the back. In the second a cummerbund‐like band containing optical fibres is wrapped around the infant's trunk. Both types of device have been reported to be effective in reducing serum bilirubin (vs no treatment), and they have the advantage that infants can be nursed close to their parents, without eye protection (Rosenfeld 1990). Because of the easy mode of administration, fibreoptic phototherapy can more easily be delivered at home, with possible economic advantages.
This systematic review includes data from randomised trials in jaundiced newborn infants and evaluates the effects of both fibreoptic phototherapy versus no treatment, and fibreoptic versus conventional phototherapy.
Objectives
The main objective of this review was to evaluate the efficacy of fibreoptic phototherapy in newborn infants with jaundice.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials which evaluated the use of fibreoptic phototherapy in newborn infants with jaundice.
Types of participants
Newborn infants up to 28 days of age with jaundice, or an elevated SBR, who required phototherapy.
Types of interventions
Studies using any type of fibreoptic device to deliver phototherapy were included. Conventional phototherapy was defined as treatment delivered using banks of halogen or fluorescent lamps.
Types of outcome measures
Primary outcome measures:
Rate of change of SBR (micromoles/L/hour)
Duration of phototherapy (hours)
Incidence of kernicterus (%)
Rate of treatment failure (%, defined as use of either exchange blood transfusion, or additional banks of conventional phototherapy lamps)
Incidence of side‐effects including burns and dehydration
Secondary outcome measures:
Parent‐child bonding
Nursing staff satisfaction
Maternal satisfaction
Cost effectiveness
Search methods for identification of studies
The standard search strategy of the Neonatal Review Group, as outlined in the Cochrane Library, was used. The search strategy was applied after 1988, that being the year in which fibreoptic phototherapy devices were first tested in clinical trials. The following sources were searched for eligible reports in any language:
Cochrane Controlled Trials Register (Cochrane Library, Disk Issue 3, 2000)
MEDLINE and EMBASE electronic searches using the terms: "jaundice, jaundice/neonatal, randomized controlled trial, phototherapy, fiber optics, infant/newborn", and the textwords "biliblanket, wallaby, phototherapy, fiber optic, jaundice" (1989 to 2000 inclusive)
Reference lists from the above, and from review articles
Personal communication with primary authors from the above to identify unpublished data
Proceedings of annual meetings of The European Society for Paediatric Research and The Society for Pediatric Research: handsearches of abstracts (1989 to 2000 inclusive)
Data collection and analysis
The standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. The methodological quality of each trial was assessed by both authors, with the second author blinded to trial author and institution. Having decided which trials to include, both authors independently extracted the data and compared results. Disagreements were resolved by consensus. The standard statistical methods of the Cochrane Collaboration were used. For categorical and continuous data the relative risk (RR) and weighted mean difference (WMD) were calculated respectively. 95% confidence intervals were used and a fixed effects model was assumed for the meta‐analysis. Efficacy was compared in the following sub‐groups: preterm infants (less than 37 weeks gestational age), infants with jaundice due to haemolysis, by type of fibreoptic phototherapy device (post hoc) and by conventional phototherapy lamp colour (post hoc).
Results
Description of studies
RESULTS OF SEARCH STRATEGY AND STUDY ELIGIBILITY The search strategy uncovered 31 studies. In four, eligibility was uncertain and further data are awaited from the authors (Amato 1992; de Luca 1991; Ennever 1991; Omenaca 1994). Three studies, in which the allocation procedure was unacceptably susceptible to the introduction of bias, have been excluded (Hysmith 1992; Rosenfeld 1990; Schuman 1992). The remaining 24 randomised or quasi‐randomised studies have been included.
MANUSCRIPTS IN FOREIGN LANGUAGES Four studies written in Italian (Pometta 1997; Romagnoli 1992; Romagnoli 1994 (B); Romagnoli 1994 (W); Romagnoli 1995 (B); Romagnoli 1995 (W)) and one in Portuguese (de Carvalho 1992) have been included. In each case the manuscript was read, and the data collection form completed, by a health‐care researcher fluent in the relevant language working at the institution of the principal reviewer. In four of the five cases the primary author was subsequently contacted to answer further questions.
GESTATIONAL AGE In all included studies the subjects were newborn infants, less than 28 days of age, with clinical or biochemically‐defined jaundice. While most studies enrolled term infants (>=37 weeks gestational age), nine studies included only preterm infants. Some studies included both term and preterm infants and these have been divided and included as separate studies (e.g. Tan 1994 Term, Tan 1994 Pre) if the author provided sufficient data for this to be possible. Data from preterm infants have been analysed in a sub‐group as specified in the protocol.
BIRTHWEIGHT In one study (Ittman 1992), separate birthweight strata were included and a single summary outcome measure including all infants was not provided. In this case the data have been separated and included as different studies (Ittmann 1992 <1250g, Ittmann 1992 >1250g).
HAEMOLYSIS In the majority of the studies, infants were investigated for haemolysis and excluded if it was present, or if there was mother‐infant blood group incompatability likely to cause it. In one study haemolysis was not considered, and in another only Rhesus incompatible infants were excluded. No studies enrolled only infants with haemolysis, and none including haemolysing infants reported separate data for this group, making the planned sub‐group analysis impossible.
DIFFERENT COMPARISON GROUPS Of the 24 included studies, one compared fibreoptic phototherapy with no treatment and 17 compared fibreoptic with conventional phototherapy. Seven studies compared combination treatment (fibreoptic and conventional) with conventional phototherapy and one compared double fibreoptic with conventional phototherapy. Two studies compared different makes of fibreoptic device. These different comparisons address different questions about the efficacy of fibreoptic phototherapy and have therefore been analysed separately. If a study included data comparing combination or double phototherapy with conventional phototherapy, as well as fibreoptic versus conventional, the data have been separated and included as a different study (e.g. Al‐Alaiyan 1996 Co, Tan 1997 Double).
DIFFERENT PHOTOTHERAPY TYPES The studies included various forms of fibreoptic and conventional phototherapy. Two different fibreoptic devices were used (BiliBlanket and Wallaby phototherapy system) and where both were included in one study the data have been divided and included as separate studies (e.g. Romagnoli 1994 B, Romagnoli 1994 W). The manufacturers of these devices specify the irradiance of each device as 35 and 8‐10 microwatts/cm2/nm respectively. Conventional phototherapy was administered with either halogen or fluorescent lamps emitting white light, blue light, or a mixture of the two. In order to allow calculation of meaningful summary effect measures, sub‐group analyses by type of fibreoptic phototherapy device, and by conventional phototherapy lamp colour have been performed. The need for these analyses was not anticipated at the time of writing the protocol for this review and these sub‐groups have been specified post‐hoc.
PRIMARY OUTCOMES The primary outcome measure used to express the efficacy of phototherapy varied widely between studies. Most commonly, some measure of the direct effect of phototherapy on SBR was reported. This was either expressed in "absolute" terms (e.g. SBR before and after treatment, change in SBR with treatment, change in SBR per hour of treatment), or in "relative" terms (percentage change in SBR after 24 or 48 hours of treatment, percentage change in SBR per hour or per day of treatment). The effect of phototherapy on SBR was also expressed indirectly (e.g. duration of phototherapy, use of additional phototherapy, use of exchange transfusion). The incidence of kernicterus was not reported in any of the studies. Maternal migraine, trans‐epidermal water loss and mesenteric blood flow were reported as side‐effects and have been included in the meta‐analysis. The included studies did not share a common primary outcome measure, making a meta‐analysis including all studies impossible without individual patient data.
SECONDARY OUTCOMES No data on the effect of different phototherapy devices on mother‐child bonding were reported in any study. Some studies reported the anecdotal opinions of staff and parents on the different phototherapy devices, but none used validated scales so these data have been omitted. No data on cost‐effectiveness were reported in any study.
ADDITIONAL OUTCOMES A significant rebound in SBR requiring further treatment (defined as an increase in SBR after cessation of phototherapy to a level above that for which phototherapy was initially commenced) was commonly reported. This outcome was not anticipated at the time the protocol was written but is considered by the authors to be an important one and has therefore been included (use of repeat phototherapy for rebound jaundice).
ADDITIONAL DATA Contact has been made with, and further data requested and received from, 10 of the 19 primary authors (Al‐Alaiyan, Costello, Crawshaw, Dani, Donzelli, Holtrop, Ittman, Maisels, Pezzati and Romagnoli). Efforts continue to make contact with the remaining nine primary authors.
Risk of bias in included studies
In all 24 included studies allocation to intervention or control was at random or quasi‐random. In fourteen studies allocation concealment was adequate, in three unclear, and in seven inadequate (all quasi‐randomised with alternate or sequential allocation). None of the studies stated whether care‐givers were masked to treatment group allocation but in view of the type of intervention such masking is probably impossible. Masking of outcome assessors was not mentioned in any study. A biochemical outcome measure is most appropriate in these studies because it is relatively immune to the introduction of bias. Indirect outcome measures (e.g. duration of phototherapy, use of additional phototherapy, use of exchange transfusion) are particularly susceptible to the introduction of bias. Accordingly the data from indirect outcome measures has only been included in the meta‐analysis when the SBR level at which phototherapy would be ceased, additional phototherapy used and exchange transfusion performed had been stated in the methods section of the study manuscript.
Effects of interventions
FIBREOPTIC PHOTOTHERAPY VERSUS NO TREATMENT (Comparison 1) One study investigated this comparison (Romagnoli 1992). Infants randomised to the fibreoptic group were treated with the Wallaby phototherapy system and infants randomised to control received no treatment. In both groups, if the SBR reached pre‐specified levels conventional phototherapy was added/commenced. Infants in the fibreoptic group were less likely to require conventional phototherapy but this did not reach statistical significance (RR 0.14, 95% CI 0.01, 2.62). However both the percentage change in SBR per hour, and the percentage change in SBR after 24 hours of treatment, were significantly greater in the fibreoptic group (WMD ‐0.44%, 95% CI ‐0.67, ‐0.21 and WMD ‐10.7%, 95% CI ‐18.14, ‐3.26, respectively).
FIBREOPTIC VERSUS CONVENTIONAL PHOTOTHERAPY (Comparison 2) The majority of the studies investigated this comparison. The summary effect measures for duration of phototherapy, percentage change in SBR per hour and percentage change in SBR per day were all affected by heterogeneity. The use of exchange transfusion in the fibreoptic group was increased but did not reach statistical significance (RR 1.62, 95%CI 0.38, 6.93). The use of additional phototherapy in the fibreoptic group was significantly increased (RR 1.68, 95%CI 1.18, 2.38). The percentage change in SBR after 24 and 48 hours of treatment was greater in the conventional phototherapy group (WMD 3.59%, 95%CI 1.27, 5.92 and WMD 10.79%, 95%CI 8.33, 13.26 respectively). The use of repeat phototherapy for rebound jaundice was no different between the groups (RR 2.33, 95%CI 0.92, 5.91). The risk of mothers developing migraine during their infant's treatment with phototherapy was not significantly different between groups (RR 5.59, 95%CI 0.29, 108.39). Trans‐epidermal water loss and mesenteric blood flow velocity after feeding were significantly higher in infants treated with fibreoptic devices (WMD 17.00 mL/m2/hr, 95%CI 7.26, 26.74 and WMD 0.11 m/s, 95%CI 0.10, 0.12 respectively).
FIBREOPTIC VERSUS CONVENTIONAL PHOTOTHERAPY STRATIFIED BY CONVENTIONAL PHOTOTHERAPY LAMP COLOUR (Comparisons 3‐5) Conventional phototherapy was stratified post‐hoc into white light only, blue light only or a mixture of the two for this sub‐group analysis. The wavelength of light used was ascertained from the manuscript or from the author for all studies. Halogen and fluorescent lamps were assumed to be of equivalent efficacy for a given wavelength of light. Infants receiving fibreoptic phototherapy were significantly more likely to require additional phototherapy than those receiving conventional phototherapy with either blue or white lamps (RR 3.08, 95% CI 1.27, 7.48 and RR 1.48, 95% CI 1.01, 2.18 respectively). Similarly, the percentage change in SBR after 48 hours of treatment was greater for infants treated with either white or blue lamps than for fibreoptic phototherapy (WMD 11.72%, 95% CI 8.43, 15.01 and WMD 9.73%, 95% CI 5.92, 13.54 respectively).
FIBREOPTIC VERSUS CONVENTIONAL PHOTOTHERAPY IN PRETERM INFANTS (Comparison 6) Nine studies included only preterm infants, or contained some preterm infants and reported results for them separately. Duration of phototherapy was not significantly different between the groups (WMD 2.00 hours, 95%CI ‐3.52, 7.52) and there was no greater use of additional phototherapy in the fibreoptic group (RR 1.07, 95%CI 0.27, 4.27). Percentage change in SBR per hour and per day was significantly affected by heterogeneity. The percentage change in SBR after 24 hours of treatment was not significantly different between the groups (WMD 1.70%, 95%CI ‐2.65, 6.05), and the use of repeat phototherapy for rebound jaundice was no higher in the fibreoptic group (RR 2.00, 95%CI 0.71, 5.63).
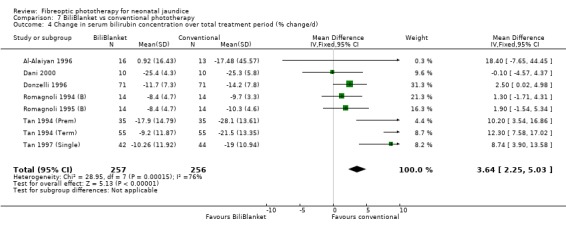
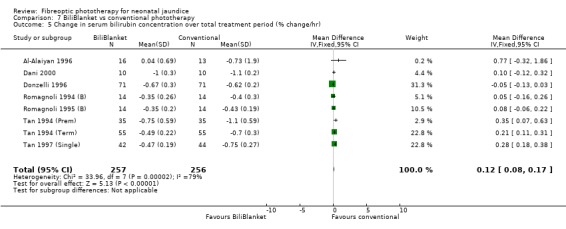
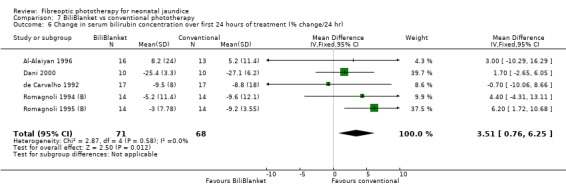
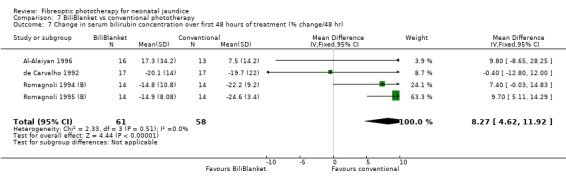
FIBREOPTIC VERSUS CONVENTIONAL PHOTOTHERAPY STRATIFIED BY TYPE OF FIBREOPTIC DEVICE (Comparisons 7‐8) BiliBlanket was compared with conventional phototherapy in 13 studies. The data for duration of phototherapy and percentage change in SBR per hour and per day were affected by heterogeneity. The use of additional phototherapy was significantly increased in the BiliBlanket group (RR 1.57, 95%CI 1.10, 2.24). The use of exchange transfusion and repeat phototherapy for rebound jaundice were not significantly different between the groups (RR 1.62, 95%CI 0.38, 6.93 and RR 1.72, 95%CI 0.70, 4.27 respectively). However the percentage change in SBR after 24 and 48 hours was significantly less in the BiliBlanket group (WMD 3.51%, 95%CI 0.76, 6.25 and WMD 8.27%, 95%CI 4.62, 11.92 respectively).
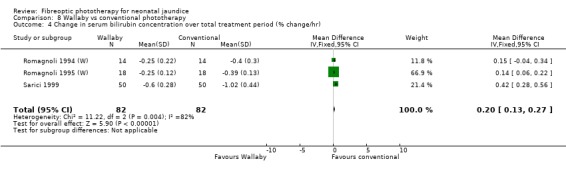
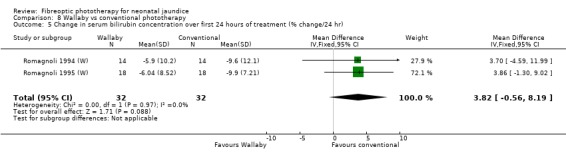
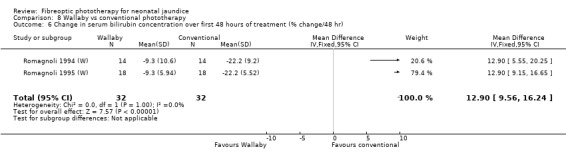
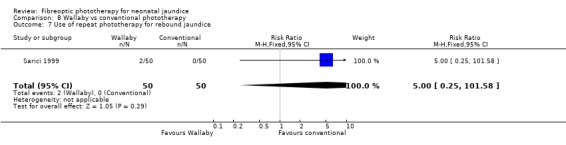
Wallaby phototherapy system was compared with conventional phototherapy system in six studies. The data on percentage change in SBR per hour of treatment were affected by heterogeneity. The use of additional and repeat phototherapy for rebound jaundice was no higher in the Wallaby group (RR 9.00, 95%CI 0.50, 162.90 and RR 5.00, 95%CI 0.25, 101.59 respectively). The duration of phototherapy was significantly longer in the Wallaby group (WMD 22.02 hours, 95%CI 16.55, 27.49). The percentage change in SBR per day and after 48 hours of treatment were both significantly lower in the Wallaby group (WMD 3.37%, 95%CI 1.69, 5.05 and WMD 12.9%, 95%CI 9.56, 16.24 respectively) but percentage change at 24 hours of treatment was no different between the groups (WMD 3.82%, 95%CI ‐0.56, 8.19).
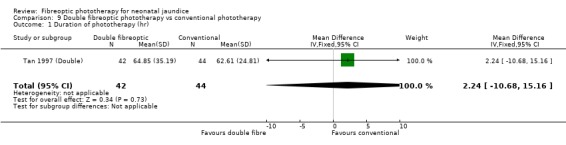
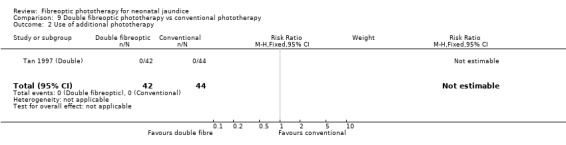
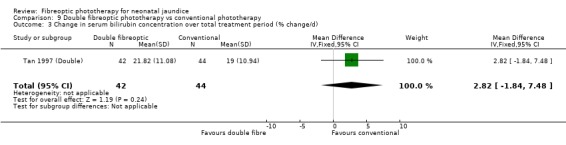
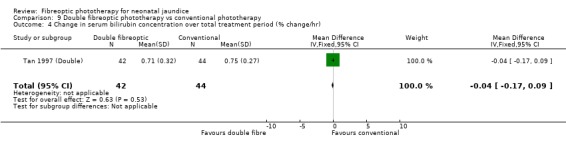
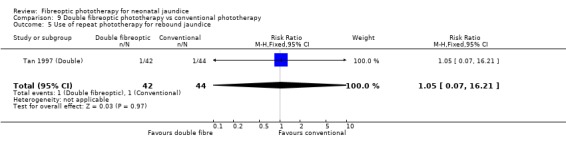
DOUBLE FIBREOPTIC PHOTOTHERAPY VERSUS CONVENTIONAL PHOTOTHERAPY (Comparison 9) In the single study (Tan 1997 (Double)) reporting this intervention, the infants randomised to double fibreoptic treatment were wrapped in two BiliBlankets. There was no difference in duration of treatment or percentage change in SBR per hour or per day between the groups (WMD 2.24 hours, 95%CI ‐10.68, 15.16, WMD ‐0.04%, 95%CI ‐0.17, 0.09 and WMD 2.82%, 95%CI ‐1.84, 7.48 respectively). Infants randomised to the fibreoptic group had no greater use of repeat phototherapy for rebound jaundice (RR 1.05, 95%CI 0.07, 16.22).
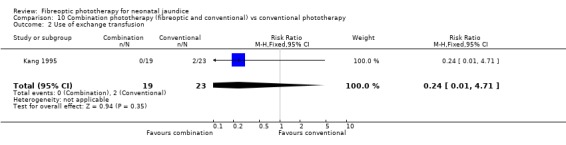
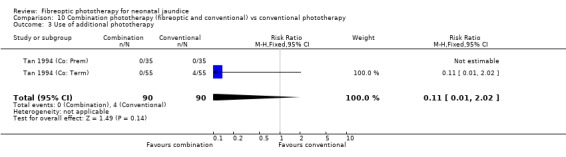
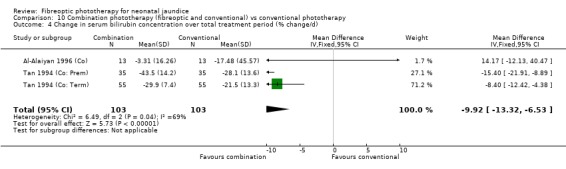
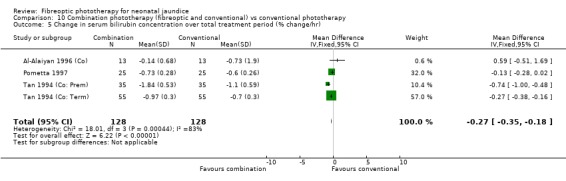
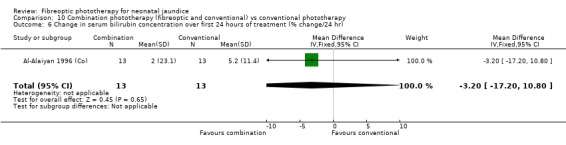
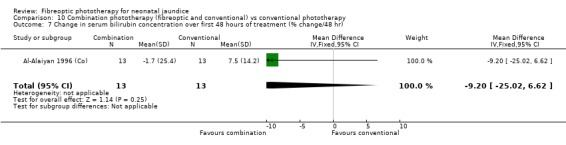
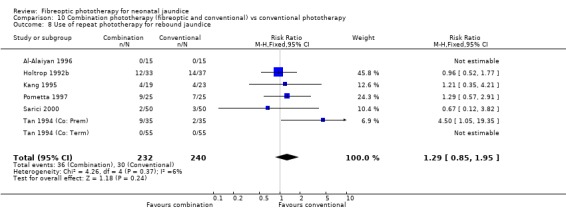
COMBINATION PHOTOTHERAPY (FIBREOPTIC AND CONVENTIONAL) VERSUS CONVENTIONAL PHOTOTHERAPY (Comparison 10) Six studies investigated the use of fibreoptic and conventional devices combined compared to conventional phototherapy. The data for duration of phototherapy and percentage change per hour and per day were affected by heterogeneity. There was a trend to less use of exchange transfusion and additional phototherapy in the combination group but this did not reach statistical significance (RR 0.24, 95%CI 0.01, 4.72 and RR 0.11, 95%CI 0.01, 2.02 respectively). There was also a trend to greater percentage change in SBR at 24 and 48 hours in the combination group (WMD ‐3.2%, 95%CI ‐17.2, 10.8 and WMD ‐9.2%, 95%CI ‐25.02, 6.62 respectively). There was no difference in the use of repeat phototherapy for rebound jaundice between the groups (RR 1.29, 95%CI 0.85, 1.95).
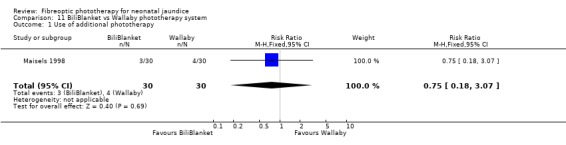
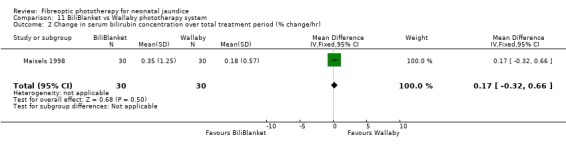
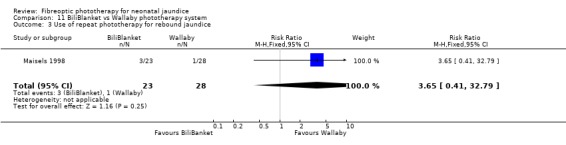
BILIBLANKET VERSUS WALLABY PHOTOTHERAPY SYSTEM (Comparison 11) In the two studies directly comparing BiliBlanket and the Wallaby phototherapy system there are no reported outcomes common to each study, and no additional data have become available which would enable a meta‐analysis to be performed. In the individual studies George et al (George 1994) found a significant difference in the absolute change in SBR favouring BiliBlanket, while Maisels et al (Maisels 1998) found no significant difference in absolute SBR at 12 or 24 hours, or in the use of additional or repeat phototherapy for rebound jaundice. Thus, there is no evidence of a difference in efficacy between the devices.
Discussion
STRENGTHS AND WEAKNESSES OF THE INDEX TRIALS This large systematic review incorporates data from 1753 infants enrolled in 24 trials investigating the efficacy of fibreoptic phototherapy. The methodological quality of the included studies was high with allocation to intervention or control groups being at random or quasi‐random. Allocation concealment was considered adequate in 14, inadequate in seven, and uncertain in three, thus selection bias is unlikely to have been introduced in the majority of studies. No studies reported masking of care‐givers or outcome assessors but as a biochemical outcome was used in the majority of studies, intervention and/or ascertainment biases are unlikely to have been introduced. In a small number of studies some infants were excluded after randomisation so attrition bias may have been introduced.
STRENGTHS AND WEAKNESSES OF THE REVIEW PROCESS The use of the standard search strategy of the Cochrane Collaboration has uncovered a large number of trials, many more than anticipated by either author. The location of a number of separately published abstracts in addition to the main publication, and the fact that contact has been made with the majority of the authors, suggests that the list of included studies is complete. A number of the trials which we detected were published in non‐English journals, suggesting that significant ascertainment bias has been avoided. Agreement between the authors on both methodological quality of the included studies and the extracted data was high, and all disagreements were able to be resolved without reference to a third party.
The included studies reported a variety of outcome measures and unfortunately no single one was common to all studies. This has been partly overcome by some authors kindly re‐analysing their data in different formats. The efficacy of any phototherapy device depends upon the irradiance of the emitted light, its wavelength, and the surface area of the infant's skin onto which it falls. If these had been the only variables in the included comparisons of fibreoptic and conventional phototherapy the meta‐analysis would have been straightforward. However the reviewers have had to consider the following:
The fibreoptic device used differed between studies. The irradiance of the Wallaby phototherapy system and BiliBlanket are different (8‐10 versus 15‐35 microwatts/cm2/nm respectively).
The irradiance setting of the BiliBlanket was not the same in different studies.
Conventional phototherapy varied between studies. Both the lamp colour and lamp type used were not constant.
The infants enrolled in the studies varied widely. Some studies enrolled infants with haemolysis, and phototherapy was instituted at quite different SBR levels.
This variability of intervention has necessitated the inclusion of two sub‐group analyses which were not specified a priori. There is also significant heterogeneity of treatment effect on some outcomes within some comparisons in the meta‐analysis. This may be due to some of the factors listed above.
The following conclusions on the efficacy of fibreoptic phototherapy can be drawn from the available data:
1. The Wallaby phototherapy system is more effective at lowering SBR than no treatment. 2. Fibreoptic phototherapy is less effective at lowering SBR than conventional phototherapy, except in preterm infants and when two BiliBlankets are used simultaneously, in which cases fibreoptic phototherapy is equally effective. The difference in efficacy in term versus preterm infants may reflect the different proportion of the infant's body surface area in contact with the fibreoptic device. 3. The addition of a fibreoptic device to conventional phototherapy (or vice versa) is more effective at lowering SBR than conventional phototherapy alone. 4. There are no data to suggest superiority of one fibreoptic device over another. 5. There is no evidence from randomised controlled trials that the use of fibreoptic phototherapy devices has a more favourable impact on parent‐child bonding or maternal or nurse satisfaction than conventional treatment.
Authors' conclusions
Implications for practice.
There appears to be some place for fibreoptic phototherapy in the management of neonatal hyperbilirubinaemia.
In the term infant with physiological jaundice (by definition at low risk of requiring exchange transfusion), fibreoptic phototherapy may be a reasonable alternative to conventional treatment. Fibreoptic devices are not as effective at lowering SBR and may thus prolong treatment, but this may be of no consequence in a healthy infant with moderate hyperbilirubinaemia and an intact blood brain barrier. Fibreoptic phototherapy does not necessitate the separation of mother from baby, and even though there is no randomised controlled trial data to prove it, there may be some advantage to this.
In the preterm infant, fibreoptic phototherapy is as effective as conventional phototherapy and the two can be used interchangeably.
In the infant with a high SBR who is at risk for an exchange transfusion, the use of double fibreoptic phototherapy, or the addition of conventional phototherapy to a fibreoptic device, will probably lower SBR faster than conventional phototherapy alone. Although this systematic review has not identified any studies specifically investigating infants with haemolysis, the mode of action of phototherapy is the same in this group, so this recommendation is also probably generalisable to the haemolysing infant.
Implications for research.
Further research into the efficacy of fibreoptic phototherapy is required. The use of fibreoptic devices in the treatment of infants with hyperbilirubinaemia due to haemolysis should be investigated in a randomised controlled trial. The possible advantages of fibreoptic over conventional phototherapy (e.g. lack of interference with parent‐child bonding, and improved maternal and nursing staff satisfaction) could be simultaneously evaluated with a validated questionnaire. An economic evaluation of the two forms of phototherapy is also required.
Future investigators should report relative change in bilirubin as a direct outcome and ensure that SBR criteria are stated a priori if indirect outcomes are to be used. Adequate allocation concealment should be ensured and the irradiance of all phototherapy devices should be measured to allow comparison with other studies.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors thank Ms. Deborah Scarcella and Dr. Karin Sitte for their assistance with foreign language translation.
Data and analyses
Comparison 1. Fibreoptic phototherapy vs no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of exchange transfusion | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Use of additional phototherapy | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.62] |
| 3 Change in serum bilirubin concentration over total treatment period (% change/hr) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.67, ‐0.21] |
| 4 Change in serum bilirubin concentration over total treatment period (%change/d) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐10.7 [‐18.14, ‐3.26] |
1.1. Analysis.
Comparison 1 Fibreoptic phototherapy vs no treatment, Outcome 1 Use of exchange transfusion.
1.2. Analysis.
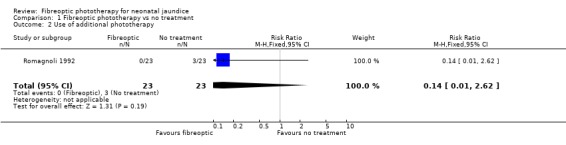
Comparison 1 Fibreoptic phototherapy vs no treatment, Outcome 2 Use of additional phototherapy.
1.3. Analysis.
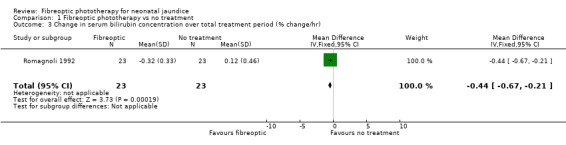
Comparison 1 Fibreoptic phototherapy vs no treatment, Outcome 3 Change in serum bilirubin concentration over total treatment period (% change/hr).
1.4. Analysis.
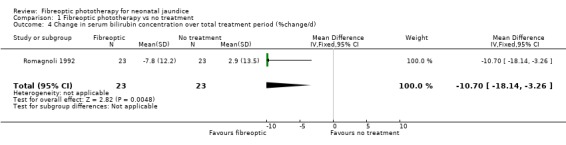
Comparison 1 Fibreoptic phototherapy vs no treatment, Outcome 4 Change in serum bilirubin concentration over total treatment period (%change/d).
Comparison 2. Fibreoptic vs conventional phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 7 | 562 | Mean Difference (IV, Fixed, 95% CI) | 13.62 [10.11, 17.12] |
| 2 Use of exchange transfusion | 4 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.38, 6.93] |
| 3 Use of additional phototherapy | 10 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.18, 2.38] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/d) | 10 | 577 | Mean Difference (IV, Fixed, 95% CI) | 3.53 [2.46, 4.60] |
| 5 Change in serum bilirubin concentration over total treatment period (% change/hr) | 11 | 677 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.11, 0.19] |
| 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 7 | 203 | Mean Difference (IV, Fixed, 95% CI) | 3.59 [1.27, 5.92] |
| 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr) | 6 | 183 | Mean Difference (IV, Fixed, 95% CI) | 10.79 [8.33, 13.26] |
| 8 Use of repeat phototherapy for rebound jaundice | 6 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.92, 5.91] |
| 9 Maternal migraine | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.29, 108.38] |
| 10 Trans‐epidermal water loss (mL/m2/hr) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [7.26, 26.74] |
| 11 Mesenteric blood flow velocity (m/s) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.10, 0.12] |
2.1. Analysis.
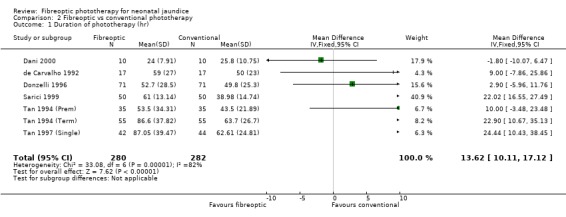
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 1 Duration of phototherapy (hr).
2.2. Analysis.
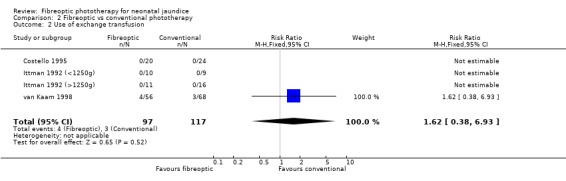
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 2 Use of exchange transfusion.
2.3. Analysis.
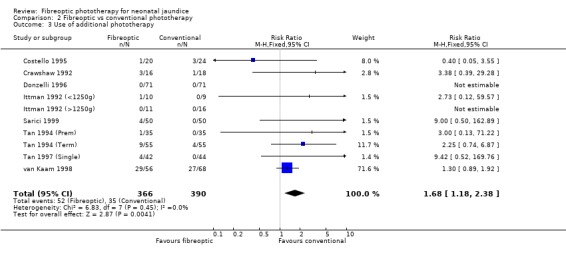
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 3 Use of additional phototherapy.
2.4. Analysis.
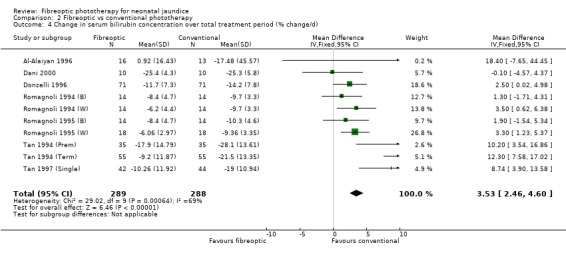
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/d).
2.5. Analysis.
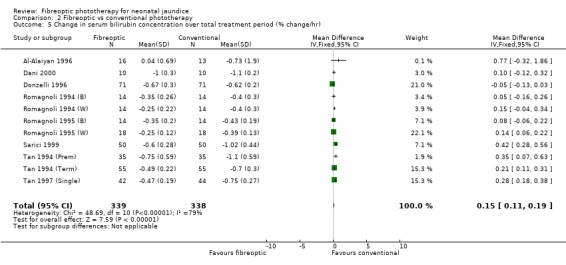
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 5 Change in serum bilirubin concentration over total treatment period (% change/hr).
2.6. Analysis.
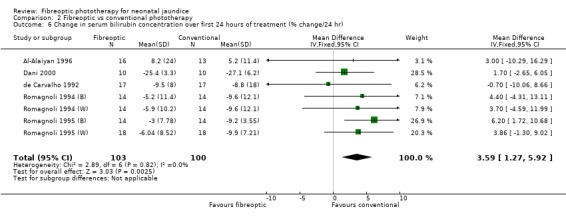
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
2.7. Analysis.
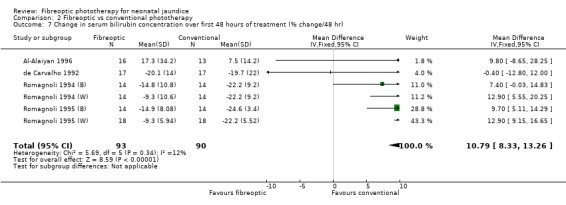
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr).
2.8. Analysis.
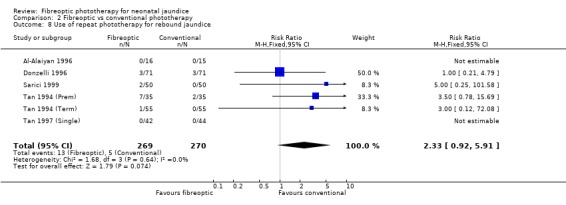
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 8 Use of repeat phototherapy for rebound jaundice.
2.9. Analysis.
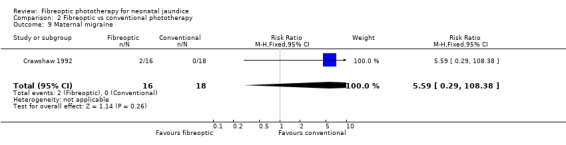
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 9 Maternal migraine.
2.10. Analysis.
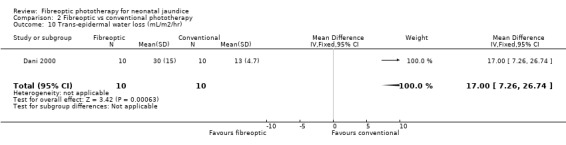
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 10 Trans‐epidermal water loss (mL/m2/hr).
2.11. Analysis.
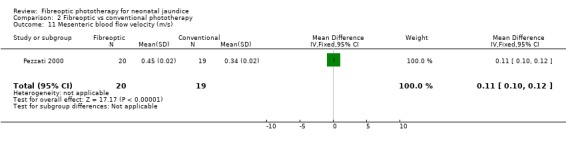
Comparison 2 Fibreoptic vs conventional phototherapy, Outcome 11 Mesenteric blood flow velocity (m/s).
Comparison 3. Fibreoptic vs conventional phototherapy with white light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 4 | 286 | Mean Difference (IV, Fixed, 95% CI) | 9.60 [4.00, 15.20] |
| 2 Use of additional phototherapy | 4 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.27, 7.48] |
| 3 Change in serum bilirubin concentration over total treatment period (% change/d) | 7 | 381 | Mean Difference (IV, Fixed, 95% CI) | 4.14 [2.74, 5.55] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/hr) | 7 | 381 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.14, 0.24] |
| 5 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 4 | 115 | Mean Difference (IV, Fixed, 95% CI) | 2.84 [‐0.19, 5.86] |
| 6 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr) | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | 11.72 [8.43, 15.01] |
| 7 Use of repeat phototherapy for rebound jaundice | 4 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.73, 7.12] |
3.1. Analysis.
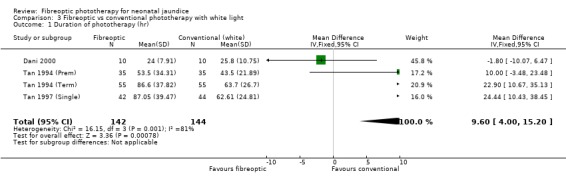
Comparison 3 Fibreoptic vs conventional phototherapy with white light, Outcome 1 Duration of phototherapy (hr).
3.2. Analysis.
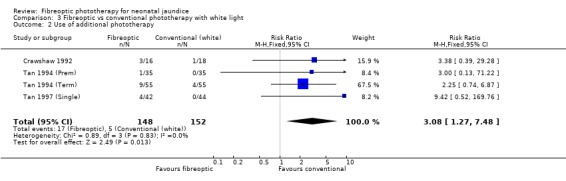
Comparison 3 Fibreoptic vs conventional phototherapy with white light, Outcome 2 Use of additional phototherapy.
3.3. Analysis.
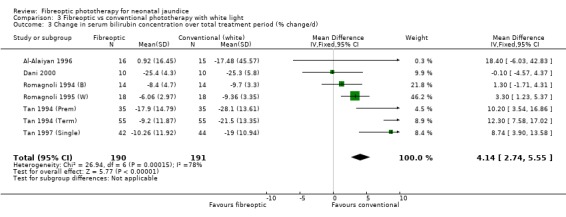
Comparison 3 Fibreoptic vs conventional phototherapy with white light, Outcome 3 Change in serum bilirubin concentration over total treatment period (% change/d).
3.4. Analysis.
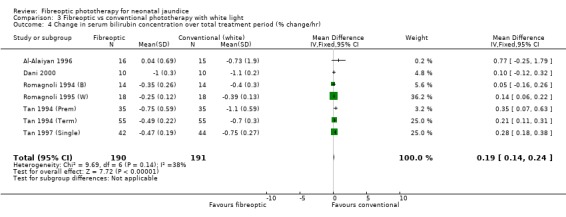
Comparison 3 Fibreoptic vs conventional phototherapy with white light, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/hr).
3.5. Analysis.
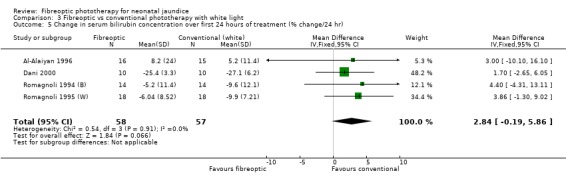
Comparison 3 Fibreoptic vs conventional phototherapy with white light, Outcome 5 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
3.6. Analysis.
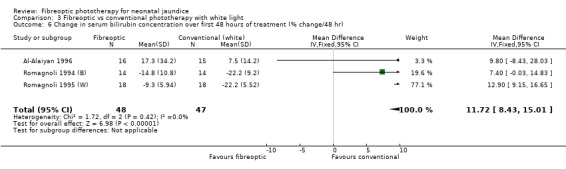
Comparison 3 Fibreoptic vs conventional phototherapy with white light, Outcome 6 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr).
3.7. Analysis.
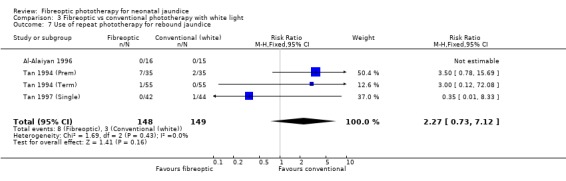
Comparison 3 Fibreoptic vs conventional phototherapy with white light, Outcome 7 Use of repeat phototherapy for rebound jaundice.
Comparison 4. Fibreoptic vs conventional phototherapy with blue light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 2 | 242 | Mean Difference (IV, Fixed, 95% CI) | 18.82 [14.25, 23.38] |
| 2 Use of exchange transfusion | 3 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.38, 6.93] |
| 3 Use of additional phototherapy | 5 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.18] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/d) | 3 | 198 | Mean Difference (IV, Fixed, 95% CI) | 4.95 [3.22, 6.69] |
| 5 Change in serum bilirubin concentration over total treatment period (% change/hr) | 4 | 298 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [0.15, 0.29] |
| 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 5.81 [2.00, 9.63] |
| 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr) | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 9.73 [5.92, 13.54] |
| 8 Use of repeat phototherapy for rebound jaundice | 2 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.36, 4.15] |
4.1. Analysis.
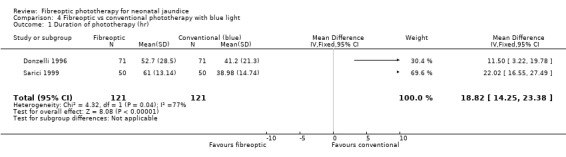
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 1 Duration of phototherapy (hr).
4.2. Analysis.
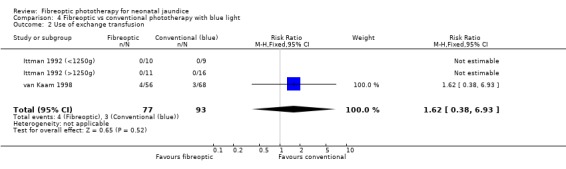
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 2 Use of exchange transfusion.
4.3. Analysis.
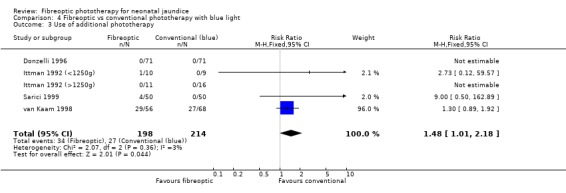
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 3 Use of additional phototherapy.
4.4. Analysis.
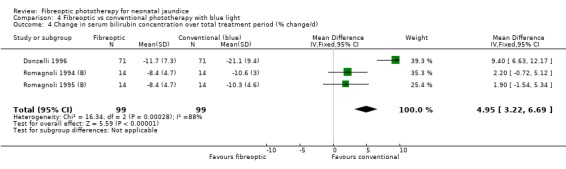
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/d).
4.5. Analysis.
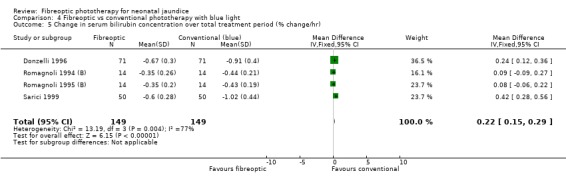
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 5 Change in serum bilirubin concentration over total treatment period (% change/hr).
4.6. Analysis.
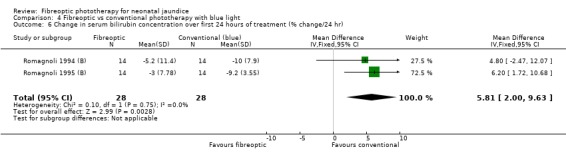
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
4.7. Analysis.
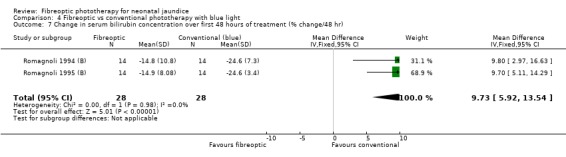
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr).
4.8. Analysis.
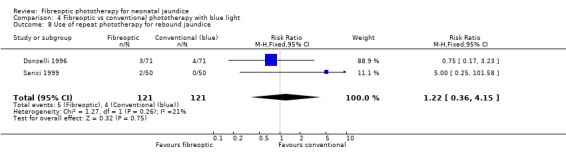
Comparison 4 Fibreoptic vs conventional phototherapy with blue light, Outcome 8 Use of repeat phototherapy for rebound jaundice.
Comparison 5. Fibreoptic vs conventional phototherapy with a combination of white and blue light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐5.96, 11.76] |
| 2 Use of exchange transfusion | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Use of additional phototherapy | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.05, 3.55] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/d) | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | 3.85 [2.05, 5.65] |
| 5 Change in serum bilirubin concentration over total treatment period (% change/hr) | 2 | 178 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.00, 0.13] |
| 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐0.83, 7.42] |
| 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr) | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 15.93 [12.49, 19.38] |
| 8 Use of repeat phototherapy for rebound jaundice | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.79] |
5.1. Analysis.
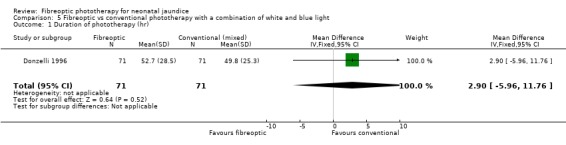
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 1 Duration of phototherapy (hr).
5.2. Analysis.
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 2 Use of exchange transfusion.
5.3. Analysis.
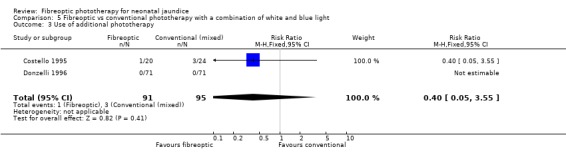
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 3 Use of additional phototherapy.
5.4. Analysis.
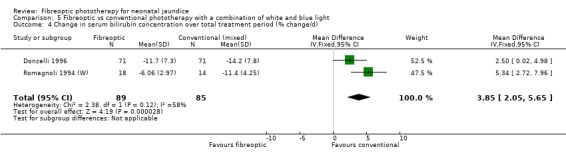
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/d).
5.5. Analysis.
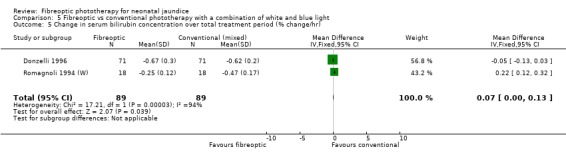
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 5 Change in serum bilirubin concentration over total treatment period (% change/hr).
5.6. Analysis.
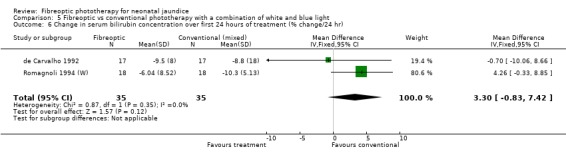
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
5.7. Analysis.
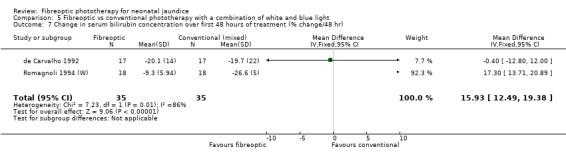
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr).
5.8. Analysis.
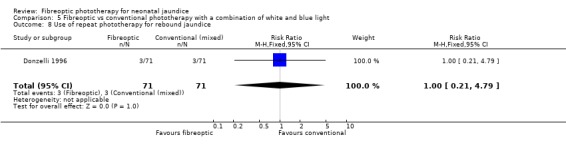
Comparison 5 Fibreoptic vs conventional phototherapy with a combination of white and blue light, Outcome 8 Use of repeat phototherapy for rebound jaundice.
Comparison 6. Fibreoptic vs conventional phototherapy in preterm infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 3 | 232 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐3.52, 7.52] |
| 2 Use of exchange transfusion | 4 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Use of additional phototherapy | 6 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.27, 4.27] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/d) | 3 | 232 | Mean Difference (IV, Fixed, 95% CI) | 2.69 [0.62, 4.75] |
| 5 Change in serum bilirubin concentration over total treatment period (% change/hr) | 3 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.08, 0.07] |
| 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐2.65, 6.05] |
| 7 Use of repeat phototherapy for rebound jaundice | 3 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.71, 5.63] |
6.1. Analysis.
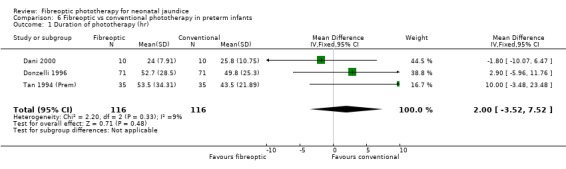
Comparison 6 Fibreoptic vs conventional phototherapy in preterm infants, Outcome 1 Duration of phototherapy (hr).
6.2. Analysis.
Comparison 6 Fibreoptic vs conventional phototherapy in preterm infants, Outcome 2 Use of exchange transfusion.
6.3. Analysis.
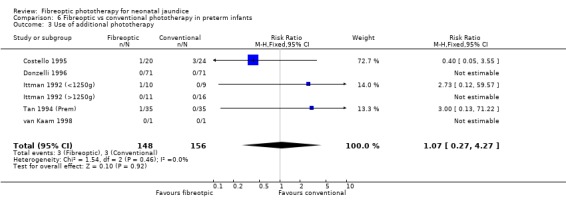
Comparison 6 Fibreoptic vs conventional phototherapy in preterm infants, Outcome 3 Use of additional phototherapy.
6.4. Analysis.
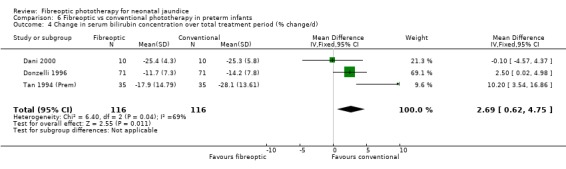
Comparison 6 Fibreoptic vs conventional phototherapy in preterm infants, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/d).
6.5. Analysis.
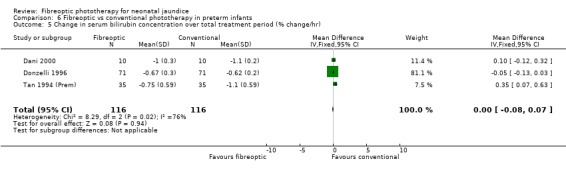
Comparison 6 Fibreoptic vs conventional phototherapy in preterm infants, Outcome 5 Change in serum bilirubin concentration over total treatment period (% change/hr).
6.6. Analysis.
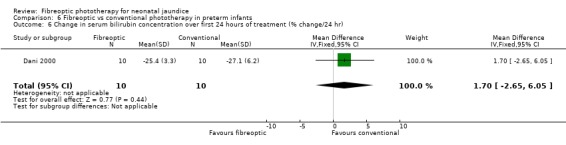
Comparison 6 Fibreoptic vs conventional phototherapy in preterm infants, Outcome 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
6.7. Analysis.
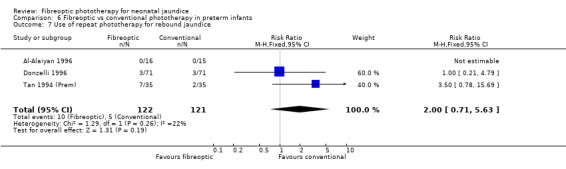
Comparison 6 Fibreoptic vs conventional phototherapy in preterm infants, Outcome 7 Use of repeat phototherapy for rebound jaundice.
Comparison 7. BiliBlanket vs conventional phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 6 | 462 | Mean Difference (IV, Fixed, 95% CI) | 7.79 [3.23, 12.34] |
| 2 Use of exchange transfusion | 4 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.38, 6.93] |
| 3 Use of additional phototherapy | 9 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.10, 2.24] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/d) | 8 | 513 | Mean Difference (IV, Fixed, 95% CI) | 3.64 [2.25, 5.03] |
| 5 Change in serum bilirubin concentration over total treatment period (% change/hr) | 8 | 513 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.08, 0.17] |
| 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 5 | 139 | Mean Difference (IV, Fixed, 95% CI) | 3.51 [0.76, 6.25] |
| 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr) | 4 | 119 | Mean Difference (IV, Fixed, 95% CI) | 8.27 [4.62, 11.92] |
| 8 Use of repeat phototherapy for rebound jaundice | 5 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.70, 4.27] |
7.1. Analysis.
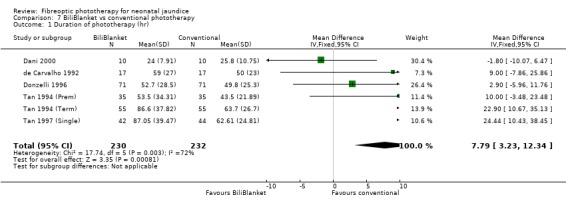
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 1 Duration of phototherapy (hr).
7.2. Analysis.
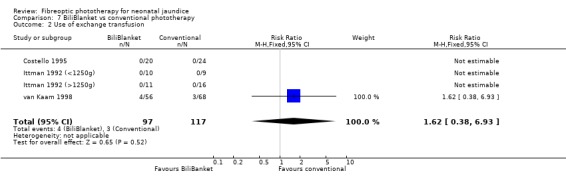
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 2 Use of exchange transfusion.
7.3. Analysis.
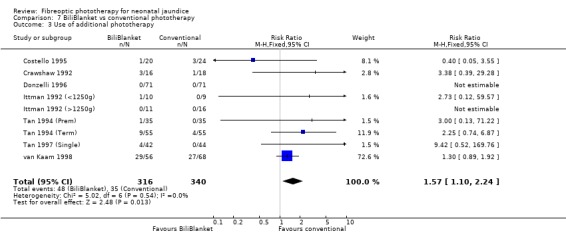
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 3 Use of additional phototherapy.
7.4. Analysis.
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/d).
7.5. Analysis.
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 5 Change in serum bilirubin concentration over total treatment period (% change/hr).
7.6. Analysis.
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
7.7. Analysis.
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr).
7.8. Analysis.
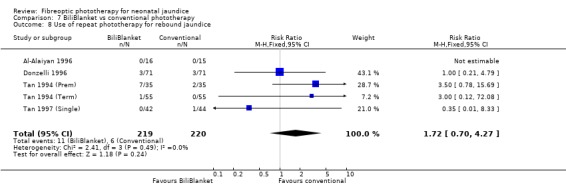
Comparison 7 BiliBlanket vs conventional phototherapy, Outcome 8 Use of repeat phototherapy for rebound jaundice.
Comparison 8. Wallaby vs conventional phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 22.02 [16.55, 27.49] |
| 2 Use of additional phototherapy | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.50, 162.89] |
| 3 Change in serum bilirubin concentration over total treatment period (% change/d) | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 3.37 [1.69, 5.05] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/hr) | 3 | 164 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.13, 0.27] |
| 5 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 3.82 [‐0.56, 8.19] |
| 6 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr) | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 12.90 [9.56, 16.24] |
| 7 Use of repeat phototherapy for rebound jaundice | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.58] |
8.1. Analysis.
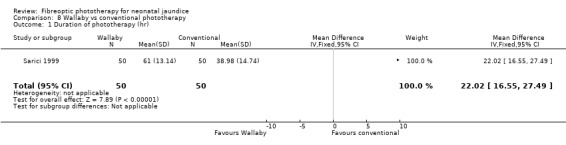
Comparison 8 Wallaby vs conventional phototherapy, Outcome 1 Duration of phototherapy (hr).
8.2. Analysis.
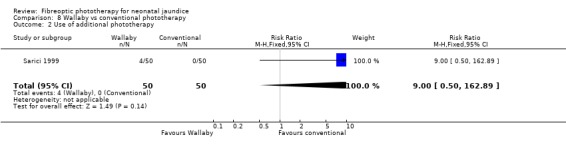
Comparison 8 Wallaby vs conventional phototherapy, Outcome 2 Use of additional phototherapy.
8.3. Analysis.
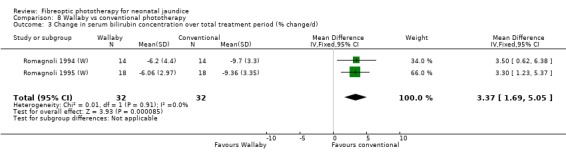
Comparison 8 Wallaby vs conventional phototherapy, Outcome 3 Change in serum bilirubin concentration over total treatment period (% change/d).
8.4. Analysis.
Comparison 8 Wallaby vs conventional phototherapy, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/hr).
8.5. Analysis.
Comparison 8 Wallaby vs conventional phototherapy, Outcome 5 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
8.6. Analysis.
Comparison 8 Wallaby vs conventional phototherapy, Outcome 6 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr).
8.7. Analysis.
Comparison 8 Wallaby vs conventional phototherapy, Outcome 7 Use of repeat phototherapy for rebound jaundice.
Comparison 9. Double fibreoptic phototherapy vs conventional phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 2.24 [‐10.68, 15.16] |
| 2 Use of additional phototherapy | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in serum bilirubin concentration over total treatment period (% change/d) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 2.82 [‐1.84, 7.48] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/hr) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
| 5 Use of repeat phototherapy for rebound jaundice | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.21] |
9.1. Analysis.
Comparison 9 Double fibreoptic phototherapy vs conventional phototherapy, Outcome 1 Duration of phototherapy (hr).
9.2. Analysis.
Comparison 9 Double fibreoptic phototherapy vs conventional phototherapy, Outcome 2 Use of additional phototherapy.
9.3. Analysis.
Comparison 9 Double fibreoptic phototherapy vs conventional phototherapy, Outcome 3 Change in serum bilirubin concentration over total treatment period (% change/d).
9.4. Analysis.
Comparison 9 Double fibreoptic phototherapy vs conventional phototherapy, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/hr).
9.5. Analysis.
Comparison 9 Double fibreoptic phototherapy vs conventional phototherapy, Outcome 5 Use of repeat phototherapy for rebound jaundice.
Comparison 10. Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of phototherapy (hr) | 4 | 322 | Mean Difference (IV, Fixed, 95% CI) | ‐12.51 [‐14.00, ‐9.02] |
| 2 Use of exchange transfusion | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.71] |
| 3 Use of additional phototherapy | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.02] |
| 4 Change in serum bilirubin concentration over total treatment period (% change/d) | 3 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐9.92 [‐13.32, ‐6.53] |
| 5 Change in serum bilirubin concentration over total treatment period (% change/hr) | 4 | 256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.35, ‐0.18] |
| 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐17.20, 10.80] |
| 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐9.2 [‐25.02, 6.62] |
| 8 Use of repeat phototherapy for rebound jaundice | 7 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.85, 1.95] |
10.1. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 1 Duration of phototherapy (hr).
10.2. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 2 Use of exchange transfusion.
10.3. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 3 Use of additional phototherapy.
10.4. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 4 Change in serum bilirubin concentration over total treatment period (% change/d).
10.5. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 5 Change in serum bilirubin concentration over total treatment period (% change/hr).
10.6. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 6 Change in serum bilirubin concentration over first 24 hours of treatment (% change/24 hr).
10.7. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 7 Change in serum bilirubin concentration over first 48 hours of treatment (% change/48 hr).
10.8. Analysis.
Comparison 10 Combination phototherapy (fibreoptic and conventional) vs conventional phototherapy, Outcome 8 Use of repeat phototherapy for rebound jaundice.
Comparison 11. BiliBlanket vs Wallaby phototherapy system.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of additional phototherapy | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.07] |
| 2 Change in serum bilirubin concentration over total treatment period (% change/hr) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.32, 0.66] |
| 3 Use of repeat phototherapy for rebound jaundice | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.65 [0.41, 32.79] |
11.1. Analysis.
Comparison 11 BiliBlanket vs Wallaby phototherapy system, Outcome 1 Use of additional phototherapy.
11.2. Analysis.
Comparison 11 BiliBlanket vs Wallaby phototherapy system, Outcome 2 Change in serum bilirubin concentration over total treatment period (% change/hr).
11.3. Analysis.
Comparison 11 BiliBlanket vs Wallaby phototherapy system, Outcome 3 Use of repeat phototherapy for rebound jaundice.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Alaiyan 1996.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term and premature infants, >36 weeks GA, >24 hours of age. Qualifying SBR 170‐300 (direct SBR <25). Haemolysis excluded. Fibreoptic n=15, conventional n=15. | |
| Interventions | Fibreoptic: BiliBlanket at 22.34 microwatts/cm2/nm (measured). Conventional: Air‐Shields (white) at 11.6 microwatts/cm2/nm (measured). | |
| Outcomes | Duration of phototherapy (no SBR level specified for cessation of phototherapy therefore not included). Absolute SBR at beginning and end of treatment. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Al‐Alaiyan 1996 (Co).
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term and premature infants, >36 weeks GA, >24 hours of age. Qualifying SBR 170‐300 (direct SBR <25). Haemolysis excluded. Combination n=15, conventional n=15. | |
| Interventions | Combination: BiliBlanket at 22.34 microwatts/cm2/nm (measured) below infant, Air‐Shields (white) at 11.6 microwatts/cm2/nm (measured) above. Conventional: Air‐Shields (white) at 11.6 microwatts/cm2/nm. | |
| Outcomes | Duration of phototherapy (no SBR level specified for cessation of phototherapy therefore not included). Absolute SBR at beginning and end of treatment. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Costello 1995.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: no Blinding of outcome measurement: can't tell | |
| Participants | Premature infants 27‐36 weeks GA. No investigation for haemolysis. Qualifying SBR 125‐300. Birthweight significantly lower in fibreoptic group. Fibreoptic n=20, conventional n=24. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (set). Conventional: white and blue lamps at 8 microwatts/cm2/nm. | |
| Outcomes | Duration of phototherapy (no SBR level specified for cessation of phototherapy therefore not included). Use of exchange transfusion. Use of additional phototherapy. | |
| Notes | Data complete (no SBR data available) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Crawshaw 1992.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: no Blinding of outcome measurement: can't tell | |
| Participants | Term infants >37 weeks GA. Haemolysis excluded. Initiation of phototherapy determined by physician. Fibreoptic n=16, conventional n=18. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (set). Conventional: white lamps. | |
| Outcomes | Duration of phototherapy. Use of additional phototherapy. Absolute SBR at 12, 24, 36 & 48 hours. % change in SBR at 0‐12, 12‐24, 24‐36 & 36‐48 hrs. | |
| Notes | Unpublished trial. Written data supplied by author August 2000. Data complete (no SBR data available) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dani 2000.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants 31‐36 weeks GA, >3 days of age. Infants with haemolysis and congenital malformations excluded. Qualifying SBR >220. Fibreoptic n=10, conventional n=10. | |
| Interventions | Fibreoptic: BiliBlanket. Conventional: Drager Photo‐Therapie 800 (white). | |
| Outcomes | Duration of phototherapy. Absolute change in SBR per hour over treatment period. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
de Carvalho 1992.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants, >2500g, >24 hours of age. Infant with haemolytic disease, sepsis, respiratory distress, direct SBR >2% or unable to be breast fed during study period excluded. Qualifying SBR >205. Fibreoptic n=17, conventional n=17. | |
| Interventions | Fibreoptic: BiliBlanket at 42.6 microwatts/cm2/nm (measured). Conventional: 5 Daylight and 2 Blue "Interelectric Biliblue" F20T12" BBY (General Electric) at 9.8 microwatts/cm2/nm (measured). | |
| Outcomes | % reduction in SBR at 8, 16, 24, 32, 40 and 48 hours. Duration of phototherapy. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Donzelli 1996.
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants <37 weeks GA. Haemolysis excluded. Qualifying SBR >205.2. Fibreoptic n=71, conventional (white) n=71, conventional (blue) n=71. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (measured). Conventional (white): Sylvania Daylight (1 bank with 8 white tubes) at 9 microwatts/cm2/nm (measured). Conventional (blue): Philips Special Blue (1 bank with 8 blue tubes) at 27 microwatts/cm2/nm (measured). | |
| Outcomes | % decline in SBR/day over treatment period. % decline in SBR/hr over treatment period. Duration of phototherapy. | |
| Notes | Further data awaited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Gale 1990.
| Methods | Blinding of randomisation: can't tell Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants >37 weeks GA. Haemolysis excluded. Qualifying SBR >200 (or lower if rapidly rising). Fibreoptic n=20, conventional n=22. | |
| Interventions | Fibreoptic: Wallaby at 7 microwatts/cm2/nm (measured). Conventional: Air Shields (white and blue) at 7 microwatts/cm2/nm (measured). | |
| Outcomes | Absolute change in SBR at baseline, 8, 16, 24, 32, 40 & 48 hours | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
George 1994.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants, 48‐96 hours old, feeding well with normal clinical examination. Qualifying SBR 171.0‐307.8. Haemolysis excluded. BiliBlanket n=26, Wallaby n=27. | |
| Interventions | BiliBlanket: 35 microwatts/cm2/nm (set). Wallaby. | |
| Outcomes | Absolute SBR at 24 hours after initiation of treatment. | |
| Notes | Author unable to be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Holtrop 1992a.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: no Blinding of outcome measurement: can't tell | |
| Participants | Term and premature infants >2500g, >24 hours of age. Haemolysis due to ABO incompatability permitted. Initiation of phototherapy determined by physician. Fibreoptic n=14, conventional n=12. | |
| Interventions | Fibreoptic: Wallaby at 8.2 microwatts/cm2/nm (measured). Conventional: Olympic Bili‐lite (1 bank with 4 white and 4 blue tubes) at 9.2 microwatts/cm2/nm (measured). | |
| Outcomes | Absolute SBR at baseline, 6, 18, 30 & 42 hours. Absolute decline in SBR/hr in first 18 hours. | |
| Notes | Further data awaited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Holtrop 1992b.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants, <2500g, weight appropriate for GA, >24 hours of age. Infants with Rhesus incompatibility and congenital anomalies excluded. Qualifying SBR 85‐256. Combination n=33, conventional n=37. | |
| Interventions | Combination: Wallaby at 8.2 microwatts/cm2/nm (measured) with either Olympic Bili‐lite (1 bank with 4 white and 4 blue tubes) at 9.2 microwatts/cm2/nm or 6 Air Shields halogen lamps at 7 microwatts/cm2/nm. Conventional: Olympic Bili‐lite (1 bank with 8 tubes) at 9.2 microwatts/cm2 or 6 Air Shields halogen lamps at 7 microwatts/cm2/nm. | |
| Outcomes | Absolute SBR at baseline, 6 and 18 hours. Absolute decline in SBR after 18 hours of treatment. % decline in SBR after 18 hours of treatment. | |
| Notes | Further data awaited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ittman 1992 (<1250g).
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants <1250g. Haemolysis permitted. Initiation of phototherapy determined by physician. Fibreoptic n=10, conventional n=9. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (set). Conventional: Halogen spotlight (blue). | |
| Outcomes | Duration of phototherapy. Use of exchange transfusion. Use of additional phototherapy. | |
| Notes | Further data awaited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ittman 1992 (>1250g).
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants 1250‐2500g. Haemolysis permitted. Initiation of phototherapy determined by physician. Fibreoptic n=11, conventional n=16. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (set). Conventional: Halogen spotlight (blue). | |
| Outcomes | Duration of phototherapy. Use of exchange transfusion. Use of additional phototherapy. | |
| Notes | Further data awaited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kang 1995.
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants <37 weeks GA, <2000g, <1 week of age. Haemolysis excluded. Qualifying SBR 120‐222. Combination n=19, conventional n=23. | |
| Interventions | Combination: BiliBlanket at 33‐35 microwatts/cm2/nm (measured) and 3 or 4 white/special blue lamps at 7‐9 microwatts/cm2/nm (measured). Conventional: 3 or 4 white/special blue lamps at 7‐9 microwatts/cm2/nm (measured). | |
| Outcomes | Duration of phototherapy. Use of exchange transfusion. Absolute change in SBR at 8, 16 and 24 hours. | |
| Notes | Author unable to be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Maisels 1998.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants, <2500g. Infants with haemolysis, extensive bruising or cephalohaematomata excluded. Qualifying SBR as per nursery protocol. BiliBlanket n=30, Wallaby n=30. | |
| Interventions | BiliBlanket: 19.9 microwatts/cm2/nm (measured). Wallaby: 18.2 microwatts/cm2/nm (measured). | |
| Outcomes | Absolute SBR at 0, 12 and 24 hours. Use of additional phototherapy. Use of repeat phototherapy. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pezzati 2000.
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Healthy, premature infants <37 weeks GA. Infants with congenital malformations, birth asphyxia, respiratory distress, renal or gastrointestinal pathologies, patent ductus arteriosus, hypo‐ or hypertension, anaemia and polycythaemia excluded. Haemolysis permitted. Qualifying SBR >171. Fibreoptic n=20, conventional n=19. | |
| Interventions | Fibreoptic: BiliBlanket. Conventional: Drager blue light. | |
| Outcomes | Duration of phototherapy. Use of exchange transfusion. Absolute SBR at baseline, 24 hrs and at end of phototherapy. | |
| Notes | Further data awaited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Pometta 1997.
| Methods | Blinding of randomisation: can't tell Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants, >2500g, >48 hours old with physiological jaundice. Infants with haemolysis, blood group incompatability, sepsis, cephalhaematomata, asphyxia excluded. Infants whose mothers took medication during pregnancy excluded. Combination n=25, conventional n=25. | |
| Interventions | Combination: Wallaby II at 8 microwatts/cm2/nm (measured) and 6 Air Shields blue lights at 24 microwatts/cm2/nm (measured). Conventional: 6 Air Shields blue lights at 24 microwatts/cm2/nm (measured). | |
| Outcomes | Duration of phototherapy. Use of additional phototherapy. Absolute SBR at 4 and 16 hrs and at end of phototherapy. Absolute change in SBR at 4 and 16 hours, and at end of phototherapy. Absolute change in SBR/hr over whole period of treatment. % change in bilirubin at 4 and 16 hours, and over whole period of treatment. % change in SBR/hr over whole period of treatment. | |
| Notes | Author unable to be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Romagnoli 1992.
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants. Infants with birth asphyxia, hypoglycaemia, gastrointerstinal pathology or haemolysis excluded. Infants of mothers with diabetes or who received corticosteroids or barbiturates excluded. Qualifying SBR >205 up to 72 hours, >256 after 72 hours. Fibreoptic n=23, no treatment n=23. | |
| Interventions | Fibreoptic: Wallaby at 8‐10 microwatts/cm2/nm (set). | |
| Outcomes | Absolute change in SBR at 12 and 24 hour. % change in SBR at 12 and 24 hours. Use of exchange transfusion. Use of additional phototherapy. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Romagnoli 1994 (B).
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants. Infants with haemolysis, dehydration or malformations excluded. Infants of mothers with diabetes or who received corticosteroids or barbiturates excluded. Qualifying SBR >239. Fibreoptic (BiliBlanket) n=14, conventional (white) n=14, conventional (blue) n=14, conventional (white and blue) n=14. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (set). Conventional (white): True Light Duro‐Test 20 TH 12 TXC at 4‐6 microwatts/cm2/nm (set). Convnetional (white and blue): 4 True Light Duro‐Test 20 TH 12 TXC and 4 Philips TL 20 W/03T at 8‐12 microwatts/cm2/nm (set). Conventional (blue): 8 Philips TL 20 W/52 at 12‐14 microwatts/cm2/nm (set). | |
| Outcomes | % reduction in SBR at 24, 48 and 72 hours. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Romagnoli 1994 (W).
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants. Infants with haemolysis, dehydration or malformations excluded. Infants of mothers with diabetes or who received corticosteroids or barbiturates excluded. Qualifying SBR >239. Fibreoptic (Wallaby) n=14, conventional (white) n=14, conventional (blue) n=14, conventional (white and blue) n=14. | |
| Interventions | Fibreoptic: Wallaby at 8‐10 microwatts/cm2/nm (set). Convnetional (white): True Light Duro‐Test 20 TH 12 TXC at 4‐6 microwatts/cm2/nm (set). Conventional (white and blue): 4 True Light Duro‐Test 20 TH 12 TXC and 4 Philips TL 20 W/03T at 8‐12 microwatts/cm2/nm (set). Convnetional (blue): 8 Philips TL 20 W/52 at 12‐14 microwatts/cm2/nm (set). | |
| Outcomes | % reduction in SBR at 24, 48 and 72 hours. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Romagnoli 1995 (B).
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants. Infants with birth asphyxia, gastrointerstinal pathology or haemolysis excluded. Infants of mothers with diabetes or who received corticosteroids or barbiturates excluded. Qualifying SBR >239. Fibreoptic (BiliBlanket) n=14, conventional (blue) n=14. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (set). Convnetional: Blue light (8 Philips TL 20 W/52) at 81 microwatts/cm2/nm (measured). | |
| Outcomes | Absolute SBR at 24, 48 and 72 hours. % reduction in SBR at 24, 48 and 72 hours. Use of exchange transfusion. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Romagnoli 1995 (W).
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants. Infants with birth asphyxia, gastrointerstinal pathology or haemolysis excluded. Infants of mothers with diabetes or who received corticosteroids or barbiturates excluded. Qualifying SBR >239. Fibreoptic (Wallaby) n=18, conventional (white) n=18, conventional (white and blue) n=18. | |
| Interventions | Fibreoptic: Wallaby at 8‐10 microwatts/cm2/nm (measured). Conventional (white and blue): 4 True Light Duro‐Test 20 TH 12 TXC and 4 Philips TL 20 W/03T at 44 microwatts/ cm2/nm. Convnetional (white): True Light Duro‐Test 20 TH 12 TXC at 6.5 microwatts/cm2/nm (measured). | |
| Outcomes | Absolute SBR at 24, 48 and 72 hours. % reduction in SBR at 24, 48 and 72 hours. Use of exchange transfusion. | |
| Notes | Data complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sarici 1999.
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: no Blinding of outcome measurement: can't tell | |
| Participants | Term infants, >2500g. Infants with haemolysis, contained haemorrhage, infection, congenital malformations excluded. Qualifying SBR >220 up to 72 hours, and >255 after 72 hours. Fibreoptic n=50, conventional n=50. | |
| Interventions | Fibreoptic: Wallaby at 9.2 microwatts per cm2/nm (measured). Conventional: Ohio at 18.4 microwatts/cm2/nm (measured, 5 special blue lamps). | |
| Outcomes | Duration of phototherapy. Absolute change in SBR/hr over treatment period. % change in SBR/hr over treatment period. Failure of treatment. | |
| Notes | Author unable to be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Sarici 2000.
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: no Blinding of outcome measurement: can't tell | |
| Participants | Term infants, >2500g. Infants with haemolysis, contained haemorrhage, infection, congenital malformations or elevated direct SBR excluded. Qualifying SBR >255. Combination n=50, conventional n=50. | |
| Interventions | Combination: Wallaby at 9.8 microwatts/cm2/nm (measured) and Ohio 5 special blue lamps at 18.7 microwatts/cm2/nm (measured). Conventional: Ohio 5 special blue lamps at 18.4 microwatts/cm2/nm (measured). | |
| Outcomes | Duration of phototherapy. Absolute change in SBR/hr over treatment period. % change in SBR/hr over treatment period. Failure of treatment. | |
| Notes | Author unable to be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Tan 1994 (Co: Prem).
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants. Haemolysis excluded. Qualifying SBR >222 up to 48 hours and >255 after 48 hours. Combination n=35, conventional n=35. | |
| Interventions | Combination: BiliBlanket at 19.01 microwatts/cm2/nm (measured) and Phillips Daylight (1 bank with 7 white tubes) at 6.73 microwatts/cm2/nm (measured). Conventional: Phillips Daylight (1 bank with 7 tubes) at 6.73 microwatts/cm2/nm (measured). | |
| Outcomes | % decline in SBR/day over treatment period. % decline in SBR/hr over treatment period. Duration of phototherapy. Use of additional phototherapy. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Tan 1994 (Co: Term).
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Healthy term infants. Haemolysis excluded. Qualifying SBR >222 up to 48 hours and >255 after 48 hours. Combination n=55, conventional n=55. | |
| Interventions | Combination: BiliBlanket at 19.01 microwatts/cm2/nm (measured) and Phillips Daylight (1 bank with 7 white tubes) at 6.73 microwatts/cm2/nm (measured). Conventional: Phillips Daylight (1 bank with 7 tubes) at 6.73 microwatts/cm2/nm (measured). | |
| Outcomes | % decline in SBR/day over treatment period. % decline in SBR/hr over treatment period. Duration of phototherapy. Use of additional phototherapy. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Tan 1994 (Prem).
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Premature infants. Haemolysis excluded. Qualifying SBR >222 up to 48 hours and >255 after 48 hours. Fibreoptic n=35, conventional n=35. | |
| Interventions | Fibreoptic: BiliBlanket at 19.01 microwatts/cm2/nm (measured). Conventional: Phillips Daylight (1 bank with 7 white tubes) at 6.73 microwatts/cm2/nm (measured). | |
| Outcomes | % decline in SBR/day over treatment period. % decline in SBR/hr over treatment period. Duration of phototherapy. Use of additional phototherapy. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Tan 1994 (Term).
| Methods | Blinding of randomisation: no Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Healthy term infants. Haemolysis excluded. Qualifying SBR >222 up to 48 hours and >255 after 48 hours. Fibreoptic n=55, conventional n=55. | |
| Interventions | Fibreoptic: BiliBlanket at 19.01 microwatts/cm2/nm (measured). Convnetional: Phillips Daylight (1 bank with 7 white tubes) at 6.73 microwatts/cm2/nm (measured). | |
| Outcomes | % decline in SBR/day over treatment period. % decline in SBR/hr over treatment period. Duration of phototherapy. Use of additional phototherapy. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Tan 1997 (Double).
| Methods | Blinding of randomisation: can't tell Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants >37 weeks GA. Haemolysis excluded. Qualifying SBR >222 up to 48 hours and >255 after 48 hours. Double fibreoptic n=42, conventional n=44. | |
| Interventions | Double: Two BiliBlankets at 19.01 microwatts/cm2/nm (measured). Convnetional: Phillips Daylight (1 bank of 7 white tubes) at 6.73 microwatts/cm2/nm (measured). | |
| Outcomes | % decline in SBR/day over treatment period. % decline in SBR/hr over treatment period. Duration of phototherapy. Use of additional phototherapy. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tan 1997 (Single).
| Methods | Blinding of randomisation: can't tell Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Term infants >37 weeks GA. Haemolysis excluded. Qualifying SBR >222 up to 48 hours and >255 after 48 hours. Fibreoptic n=42, conventional n=44. | |
| Interventions | Fibreoptic: BiliBlanket at 19.01 microwatts/cm2/nm (measured). Convnetional: Phillips Daylight (1 bank of 7 white tubes) at 6.73 microwatts/cm2/nm (measured). | |
| Outcomes | % decline in SBR/day over treatment period. % decline in SBR/hr over treatment period. Duration of phototherapy. Use of additional phototherapy. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
van Kaam 1998.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: no Blinding of outcome measurement: can't tell | |
| Participants | Premature infants, <2000g. Haemolysis excluded. Qualifying SBR determined by age and birthweight from graphs. Fibreoptic n=56, conventional n=68. | |
| Interventions | Fibreoptic: BiliBlanket at 35 microwatts/cm2/nm (measured). Convnetional: White light (4 Phillips TLK 40 W/03) at 16 microwatts/cm2/nm (measured). | |
| Outcomes | Duration of phototherapy. Use of exchange transfusion. Use of additional phototherapy. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Woodall 1992.
| Methods | Blinding of randomisation: yes Blinding of intervention: can't tell but probably impossible Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Healthy term infants >37 weeks GA, 48‐96 hours of age. Haemolysis excluded. Qualifying SBR 171‐308. Fibreoptic n=7, conventional n=8. | |
| Interventions | Fibreoptic: Wallaby at 8 microwatts/cm2/nm (measured). Convnetional: Aequitron 7100(1 bank of daylight and blue lamps) at 8 microwatts/cm2/nm (measured). | |
| Outcomes | Duration of phototherapy. Absolute change in SBR/hr over treatment period. | |
| Notes | Further data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GA = Gestational age in weeks BiliBlanket = Phototherapy device (Ohmeda) Wallaby = Phototherapy device (Wallaby Phototherapy Systems) SBR = Unconjugated serum bilirubin in micromoles/L SA = Survival analysis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hysmith 1992 | Patients allocated to intervention or control according to physician preference. |
| Rosenfeld 1990 | Patients allocated to intervention or control according to physician preference. |
| Schuman 1992 | Patients allocated to intervention or control according to physician preference and equipment availability. |
Characteristics of studies awaiting assessment [ordered by study ID]
Amato 1992.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
de Luca 1991.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Ennever 1991.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Omenaca 1994.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Contributions of authors
Dr J Mills is the primary author of the review. He specified its objectives and decided on the types of studies, participants, interventions and outcomes to be included. He was responsible for carrying out the search, assessing study methodology, extracting the data and writing the results.
Dr D Tudehope assisted in writing the protocol. He independently assessed study methodology, extracted data and collaborated with Dr Mills in writing the results.
Sources of support
Internal sources
Murdoch Childrens Research Institute, Australia.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Al‐Alaiyan 1996 {published and unpublished data}
- Al‐Alaiyan S. Fiberoptic, conventional and combination phototherapy for treatment of nonhemolytic hyperbilirubinemia in neonates. Ann Saudi Med 1996;16:633‐6. [DOI] [PubMed] [Google Scholar]
Al‐Alaiyan 1996 (Co) {published and unpublished data}
- Al‐Alaiyan S. Fiberoptic, conventional and combination phototherapy for treatment of nonhemolytic hyperbilirubinemia in neonates. Ann Saudi Med 1996;16:633‐6. (Same study as Al‐Alaiyan 1996). [DOI] [PubMed] [Google Scholar]
Costello 1995 {published and unpublished data}
- Costello SA, Nyikal J, Yu VYH, McCloud P. BiliBlanket phototherapy system versus conventional phototherapy: A randomized controlled trial in preterm infants. J Paediatr Child Hlth 1995;31:11‐3. [DOI] [PubMed] [Google Scholar]
Crawshaw 1992 {unpublished data only}
- Crawshaw A. Bili‐Blanket versus conventional phototherapy: a prospective trial. Unpublished trial.
Dani 2000 {published and unpublished data}
de Carvalho 1992 {published and unpublished data}
- Carvalho M, Lins M, Goldani M, Lopes J, Ennever J. Comparison between conventional and fibreoptic phototherapy [Comparacao entre fototerapia convencional e de fibra otica]. Jornal de Pediatria 1992;68:289‐92. [Google Scholar]
Donzelli 1996 {published and unpublished data}
- *Donzelli GP, Moroni M, Rapisardi G, Agati G, Fusi F. Fibreoptic phototherapy in the management of jaundice in low birthweight infants. Acta Paediatr 1996;85:366‐70. [DOI] [PubMed] [Google Scholar]
- Donzelli GP, Moroni M, Paparo M, Cardellini L, Vecchi C. Phototherapy for neonatal jaundice: a comparative study of fiber optic light and fluorescent lamps. Pediatr Res 1992;32:625A. [Google Scholar]
Gale 1990 {published data only}
- Gale R, Dranitzki Z, Dollberg S, Stevenson DK. A randomized, controlled application of the Wallaby phototherapy system compared with standard phototherapy. J Perinatol 1990;10:239‐42. [PubMed] [Google Scholar]
George 1994 {published data only}
- George P, Lynch M. Ohmeda Bililanket vs Wallaby Phototherapy System for the reduction of bilirubin levels in the home‐care setting. Clin Pediatr 1994;33:178‐80. [DOI] [PubMed] [Google Scholar]
Holtrop 1992a {published data only}
- *Holtrop PC, Madison K, Maisels J. A clinical trial of fiberoptic phototherapy vs conventional phototherapy. Amer J Dis Child 1992;146:235‐7. [DOI] [PubMed] [Google Scholar]
- Holtrop PC, Madison K, Maisels MJ. A randomized trial of fiberoptic phototherapy (FP) vs conventional phototherapy (CP). Pediatr Res 1990;27:209A. [DOI] [PubMed] [Google Scholar]
Holtrop 1992b {published data only}
- *Holtrop PC, Ruedisueli K, Maisels MJ. Double versus single phototherapy in low birthweight newborns. Pediatrics 1992;90:674‐7. [PubMed] [Google Scholar]
- Holtrop PC, Ruedisueli K, Regan R, Maisels MJ. Double versus single phototherapy in low birthweight infants. Pediatr Res 1991;29:218A. [PubMed] [Google Scholar]
Ittman 1992 (<1250g) {published and unpublished data}
- Ittman PI, Schumacher RE. Blue light special: randomized trial of fiberoptic phototherapy in preterm infants. Pediatr Res 1992;31:205A. [Google Scholar]
Ittman 1992 (>1250g) {published and unpublished data}
- Ittman PI, Schumacher RE. Blue light special: randomized trial of fiberoptic phototherapy in preterm infants. Pediatr Res 1992;31:205A. (Same study as Ittman 1992 (<1250g)). [Google Scholar]
Kang 1995 {published data only}
- *Kang JH, Shankaran S. Double phototherapy with high irradiance compared with single phototherapy in neonates with hyperbilirubinemia. Am J Perinatol 1995;12:178‐80. [DOI] [PubMed] [Google Scholar]
- Kang JH, Shankaran S. Double phototherapy with high irradiance compared with standard phototherapy. Pediatr Res 1992;31:207A. [DOI] [PubMed] [Google Scholar]
Maisels 1998 {published and unpublished data}
- Maisels MJ, Kring EA, Klarr J. Comparison of the efficacy of two fiberoptic phototherapy blankets. Pediatr Res 1998;43:183A. [Google Scholar]
Pezzati 2000 {published and unpublished data}
- Pezzati M, Biagiotti R, Vangi V, Lombardi E, Wiechmann L, Rubaltelli F. Changes in mesenteric blood flow response to feeding: conventional versus fiber‐optic phototherapy. Pediatrics 2000;105:350‐3. [DOI] [PubMed] [Google Scholar]
Pometta 1997 {published data only}
- Pometta P, Rodono A, Distefano G, Amato M. Double phototherapy with Wallaby optic fibers versus conventional phototherapy. Case reports [Fototerapia doppia con fibre ottiche Wallaby versus fototerapia convenzionale. Contributo casistico]. Pediatr Med Chir 1997;19:187‐91. [PubMed] [Google Scholar]
Romagnoli 1992 {published and unpublished data}
- Romagnoli C, Frezza S, Carolis MP, Zecca E, Papacci E, Tortorolo G. A new device for phototherapy of neonatal jaundice [Un nuovo sistema di fototerapia nel trattamento dell'itero neonatale]. Minerva Pediatr 1992;44:551‐4. [PubMed] [Google Scholar]
Romagnoli 1994 (B) {published and unpublished data}
- Romagnoli C, Frezza S, Greco F, Vento G, Papacci P, Carolis MP, Zecca E, Menonna A, Tortorolo G. Phototherapic treatment of the hyperbilirubinemia of the full‐term neonate: Fiberoptic or conventional systems? [Il trattamento fototerapico dell'iperbilirubinemia del neonato a termine: Fibre ottiche o sistemi tradizionali?]. Aggiornamento Pediatrico 1994;45:61‐7. [Google Scholar]
Romagnoli 1994 (W) {published and unpublished data}
- Romagnoli C, Frezza S, Greco F, Vento G, Papacci P, Carolis MP, Zecca E, Menonna A, Tortorolo G. Phototherapic treatment of the hyperbilirubinemia of the full‐term neonate: Fiberoptic or conventional systems? [Il trattamento fototerapico dell'iperbilirubinemia del neonato a termine: Fibre ottiche o sistemi tradizionali?]. Aggiornamento Pediatrico 1994;45:61‐7. (Same study as Romagnoli 1994 (B)). [Google Scholar]
Romagnoli 1995 (B) {published and unpublished data}
- Romagnoli C, Frezza S, Menonna NM, Carolis MP, Gallini F, Rizzo V, Tortorolo G. Fiberoptic phototherapy or conventional phototherapy in the treatment of neonatal hyperbilirubinemia [Fototerapia a fibre ottiche o tradizionale nel trattamento dell'iperbilirubinemia neonatale]. Riv Ital Pediatr 1995;21:198‐205. [Google Scholar]
Romagnoli 1995 (W) {published and unpublished data}
- Romagnoli C, Frezza S, Menonna NM, Carolis MP, Gallini F, Rizzo V, Tortorolo G. Fiberoptic phototherapy or conventional phototherapy in the treatment of neonatal hyperbilirubinemia [Fototerapia a fibre ottiche o tradizionale nel trattamento dell'iperbilirubinemia neonatale]. Riv Ital Pediatr 1995;21:198‐205. (Same study as Romagnoli 1995 (B)). [Google Scholar]
Sarici 1999 {published data only}
- Sarici SU, Alpay F, Unay B, Ozcan O, Gokcay E. Comparison of the efficacy of conventional special blue light phototherapy and fiberoptic phototherapy in the management of neonatal hyperbilirubinaemia. Acta Paediatr 1999;88:1249‐53. [DOI] [PubMed] [Google Scholar]
Sarici 2000 {published data only}
- Sarici SU. Alpay F, Unay B, Ozcan O, Gokcay E. Double versus single phototherapy in term newborn infants with significant hyperbilirubinemia. J Trop Pediatr 2000;46:36‐9. [DOI] [PubMed] [Google Scholar]
Tan 1994 (Co: Prem) {published data only}
- Tan KL. Comparison of the efficacy of fiberoptic and conventional phototherapy for neonatal hyperbilirubinemia. J Pediatr 1994;125:607‐12. [DOI] [PubMed] [Google Scholar]
Tan 1994 (Co: Term) {published data only}
- Tan KL. Comparison of the efficacy of fiberoptic and conventional phototherapy for neonatal hyperbilirubinemia. J Pediatr 1994;125:607‐12. (Same study as Tan 1994 (Co: Prem)). [DOI] [PubMed] [Google Scholar]
Tan 1994 (Prem) {published data only}
- Tan KL. Comparison of the efficacy of fiberoptic and conventional phototherapy for neonatal hyperbilirubinemia. J Pediatr 1994;125:607‐12. (Same study as Tan 1994 (Co: Prem)). [DOI] [PubMed] [Google Scholar]
Tan 1994 (Term) {published data only}
- Tan KL. Comparison of the efficacy of fibreoptic and conventional phototherapy for neonatal hyperbilirubinaemia. J Pediatr 1994;125:607‐12. (Same study as Tan 1994 (Co: Prem)). [DOI] [PubMed] [Google Scholar]
Tan 1997 (Double) {published data only}
- Tan KL. Efficacy of bidirectional fiber‐optic phototherapy for neonatal hyperbilirubinemia. Pediatr Res 1997;99:E13. [DOI] [PubMed] [Google Scholar]
Tan 1997 (Single) {published data only}
- Tan KL. Efficacy of bidirectional fiber‐optic phototherapy for neonatal hyperbilirubinemia. Pediatr Res 1997;99:E13. (Same study as Tan 1997 (Double)). [DOI] [PubMed] [Google Scholar]
van Kaam 1998 {published data only}
- Kaam AHLC, Beek RHT, Vergunst‐van Keulen JG, Heijden J, Lutz‐Dettinger N, Hop W, Sauer PJJ. Fibre optic versus conventional phototherapy for hyperbilirubinaemia in preterm infants. Eur J Pediatr 1998;157:132‐7. [DOI] [PubMed] [Google Scholar]
Woodall 1992 {published data only}
- Woodall D, Karas JG. A new light on jaundice. Clin Pediatr 1992;31:353‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hysmith 1992 {published data only}
- Hysmith T, Hysmith S, Farmer D. A comparison of fiberoptic vs overhead fluorescent bank methods of phototherapy for the home‐care‐appropriate preterm infant. J Perinatol 1992;XII:91. [Google Scholar]
Rosenfeld 1990 {published data only}
- Rosenfeld W, Twist P, Concepcion L. A new device for phototherapy treatment of jaundiced infants. J Perinatol 1990;10:243‐8. [PubMed] [Google Scholar]
Schuman 1992 {published data only}
- Schuman AJ, Karush G. Fiberoptic vs conventional home phototherapy for neonatal hyperbilirubinemia. Clin Pediatr 1992;31:345‐52. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Amato 1992 {published data only}
- Amato M, Feller CH, Huppi P. Conventional versus fiberoptic phototherapy for treatment of neonatal hyperbilirubinemia. Dev Physiopat Clin 1992;3:61. [Google Scholar]
de Luca 1991 {published data only}
- Luca G, Mocerino P, Vetrella A. A new phototherapy: the optical fibers phototherapy. Proceedings of the Fourth Neonatology Congress of North Italy; 1991 Nov 21‐23; Venice. 1991.
Ennever 1991 {published data only}
- Ennever JF, Carvalho M, Lopes JM, Gerdes JS, Polin RA. Bright light is the right light: multicenter trial of a novel phototherapy device. Pediatr Res 1991;29:213A. [Google Scholar]
Omenaca 1994 {published data only}
- Omenaca F, Paredes C, Peszek I, Lain D. A multi‐center, open label, controlled, randomized, clinical trial using fiberoptic light for treatment of jaundice in neonates. Proceedings of the 39th Annual Japanese Society for Premature and Newborn Medicine; 1994 Oct 28‐29; Tokyo. 1994.
Additional references
Al‐Salihi 1975
- Al‐Salihi FL, Curran YP. Airway obstruction by displaced eye mask during phototherapy. Am J Dis Child 1975;129:1362. [DOI] [PubMed] [Google Scholar]
Fetus & Newborn 1986
- Fetus and Newborn Committee, Canadian Pediatric Society. Use of phototherapy for neonatal hyperbilirubinaemia. Can Med Assoc J 1986;134:1237‐1245. [PMC free article] [PubMed] [Google Scholar]
Maisels 1982
- Maisels MJ. Jaundice in the newborn. Pediatr Rev 1982;3:305‐319. [Google Scholar]
Maisels 1992
- Maisels MJ. Neonatal jaundice. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:507‐19. [Google Scholar]


