Abstract
Background
Implantable methods of contraception offer long‐acting reversible contraception. Their uptake rate in comparison to other contraceptive methods, particularly in developed countries, has historically been low.
Objectives
To assess the contraceptive effectiveness, tolerability and acceptability of subdermal implants in comparison to other reversible contraceptive methods.
Search methods
Literature were identified through database searches, reference lists and individuals/organisations working in the contraceptive field.
Selection criteria
All randomised and controlled trials comparing subdermal implants with other forms of reversible contraceptives and reporting on pre‐determined outcomes in women of reproductive years. Primary outcomes were pregnancy and continuation.
Data collection and analysis
Quality assessment of studies and data extraction were completed independently by two reviewers. A quality checklist was designed to identify general methodological and contraceptive specific factors. Study authors and pharmaceutical companies were contacted to provide additional data. Data were collected on pregnancy rates, continuation, side effects and adverse events.
Main results
All nine identified trials compared different types of contraceptive implant. Eight, involving 1578 women, compared Implanon with Norplant , and one, involving 1198 women, compared Jadelle with Norplant. There was no difference between Implanon and Norplant for contraceptive effectiveness rates or continuation over 4 years. Both were highly effective methods of contraception with no pregnancies occurring in any of the trials during 26,972 and 28,108 women months of follow up respectively. The most common side‐effect with Implanon and Norplant was changes in bleeding pattern. The pattern with Implanon was initially more variable, bleeding with both implants became less frequent with duration of use. After two years use the amenorrhoea rate was significantly higher with Implanon. The trials reported no significant difference in hormonal side‐effects or adverse events. Implanon was significantly quicker to insert and remove than Norplant. There was no difference in contraceptive effectiveness and in continuation rates between Jadelle and Norplant. Jadelle was significantly quicker to remove than Norplant.
Authors' conclusions
Implanon, Norplant and Jadelle are highly effective contraceptive methods. No significant differences were found in contraceptive effectiveness or continuation. The most common side‐effect with all implants was unpredictable vaginal bleeding. Time taken for removal of Implanon and Jadelle was less than that for Norplant. Although this systematic review was unable to provide a definitive answer on relative effectiveness, tolerability and acceptability of contraceptive implants in comparison to other contraceptive methods, it has raised issues around the conduct of contraceptive research.
Plain language summary
This review aimed to assess how effective contraceptive implants were at preventing pregnancy and how acceptable women found them compared to other methods of contraception.
All the trials identified compared different types of contraceptive implant. No trials were found that compared implants to other contraceptive methods. All the implants were highly effective methods of contraception in the selected women. The majority of women using contraceptive implants chose to continue with the method long term, over 80% of women were still using their implant at two years. Women in developed country studies were less likely to continue with these methods when compared to women in developing country studies. The most common reported side ‐effect was of irregular vaginal bleeding. Bleeding with all implants became less frequent with time. Removal was quicker for Implanon and Jadelle than for Norplant. Insertion problems were rare with any of the implants. Problems at removal were uncommon but were significantly more likely to occur in Norplant users than Implanon users.
Background
All the currently available implantable contraceptive methods release progestogen hormones. They offer long acting reversible contraception. Many potential advantages have been cited for contraceptive implants including (IPPF 2000, WHO consultation):
high contraceptive effectiveness;
no need for user compliance, once inserted they are 'forgettable' methods of contraception;
long life‐span;
minimal requirement for medical follow‐up once inserted;
low, stable serum hormone levels minimising metabolic effects;
rapid reversibility upon discontinuation.
The uptake of implantable contraceptives in some countries has, however, been low. A number of reasons have been proposed to explain the low uptake of these contraceptive methods within contraceptive services:
the initial cost of these methods is high. If women continue to use implants as a method of long‐term contraception they may be cost‐effective, but if the discontinuation rates are high soon after starting the method, implants may be a much more expensive option;
insertion and removal of implants requires formal training.;
media publicity surrounding problematic menstrual changes and a few high profile cases of difficult removal with Norplant have affected consumer demand (HMR Ltd 1999, IPPF 1999).
The first available contraceptive implant, Norplant, was registered for use in 1983, and since then several more implants have been developed. They are currently approved for use in more than 60 countries and are being used by over 11 million women worldwide (WHO 2003). This number is rising as the availability of devices that are easier to use increases their popularity. There are currently four implants that are registered for use, several additional systems are under development. This review will consider only those implants that are currently registered for use.
All implants are based on the same principle: the progestogen hormone is released from one or more biologically inert tubes which are placed in the subdermal layer of the upper inner aspect of the woman's non‐dominant arm. An outline of the different implants is given below.
Norplant Progestogen: Levonorgestrel Licensed lifespan: 5 years Reservoir: 6 silicone capsules Registration: In over 60 countries worldwide
Norplant consists of six, sealed silicone capsules which are placed in a fan shaped pattern in the arm. Each Norplant capsule is 34 mm long and 2.4 mm in diameter and contains 36 mg of the progestogen, levonorgestrel. The original version of Norplant, available from 1983 until 1991, was manufactured with 'hard tubing'. Since 1991 Norplant has been manufactured using 'soft tubing' silicone capsules, this version has been shown to have a higher contraceptive effectiveness than the 'hard tubing' version (Sivin 1988, Sivin 1998b). This review will consider only studies that used 'soft tubing' Norplant.
Jadelle Progestogen: Levonorgestrel Licensed lifespan: 5 years Reservoir: 2 silicone rods Registration: In USA, some EU countries
Jadelle consists of two individual sealed silicone rods, each is 2.5 mm in diameter and 4.3 cm in length, each rod contains 75 mg of levonorgestrel. Jadelle differs from a prototype two rod levonorgestrel implant, known as Norplant 2, which was manufactured pre 1990. This prototype was never registered because a supplier ceased manufacture of an elastomer used in the production process (Sivin 1997). Jadelle was developed after a suitable substitute component was identified. Jadelle and Norplant 2 have different contraceptive effectiveness (Sivin, personal communication). This review will consider only studies involving Jadelle.
Implanon Progestogen: Etonogestrel Licensed lifespan: 3 years Reservoir: 1 polymer (ethylvinyl acetate) rod Registration: In over 40 countries worldwide
Implanon is a single rod, 40 mm long, 2 mm in diameter, containing 68 mg of etonogestrel.
Elcometrine Progestogen: Nestorone Licensed lifespan: 6 months Reservoir: 1 silicone capsule Registration: In Brazil
This is the only implant releasing nestorone that is currently registered, others are under development. This implant is likely to be particularly suitable for breastfeeding mothers as this progestogen is inactive when ingested orally, therefore minimising the effect of any hormone transferred to infants via breast milk (WHO 2003).
Objectives
To determine the effectiveness, acceptability and tolerability of subdermal implantable contraceptives. In order to do this the following questions were asked:
1. What is the effectiveness of subdermal implants in comparison to other reversible contraceptive methods? 2. What is the acceptability and tolerability of subdermal implants in comparison to other reversible contraceptive methods (continuation of the method was used as a marker of acceptability and reported side‐effects were used as a marker of tolerability)? 3. How do different types of subdermal implants compare in terms of effectiveness, acceptability and tolerability?
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trial and controlled clinical (i.e. quasi‐randomised) trial comparisons of subdermal implantable contraceptives with other forms of reversible contraceptives.
Types of participants
Women of reproductive years seeking effective contraception. Pregnant women were excluded.
Types of interventions
Subdermal implants versus:
non‐hormonal intrauterine devices (IUDs)
barrier contraceptives
oral contraceptives
injectable contraceptives
progestogen‐releasing intrauterine systems (IUSs)
different subdermal implants (e.g. Norplant vs. Implanon)
Types of outcome measures
Primary outcome measures
Pregnancy due to method failure at 1, 2, 3, 4, 5 years after starting contraceptive method
Continuation of contraceptive method after 1, 2, 3, 4, 5 years of follow up
Secondary outcome measures
Menstrual changes
Hormonal side effects
Adverse clinical events
Study withdrawals/reason for discontinuation
Search methods for identification of studies
The following computerised databases were searched to identify publications, in any language, describing randomised or controlled clinical trials of subdermal implantable contraceptives versus other forms of reversible contraceptive methods.
Cochrane Central Register of Controlled Trials (in The Cochrane Library) Issue 2, 2003
MEDLINE (1966 to June 2003)
EMBASE (1980 to June 2003
POPLINE (June 2003)
Science Citation Index (1981 to June 2003)
PsychLit (1972 to June 2003)
Strategies were devised as follows for the databases searched:
1. The Cochrane Library (searched 26th June 2003)
#1contracepti* near implant* #2exp NORGESTREL/ #3LEVONORGESTREL/ #4norplant* #5uniplant #6keto near desogestrel #7levonorgestrel #8norgestrel #9etonorgestrel #10implanon #11subdermal near implant* #12subcutaneous near implant* #13jadelle #14nestorone #15elcometrine #16normegestrol #17#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17
2. MEDLINE (OvidWeb 1966‐June Week 3 2003) (searched 26th June 2003)
1 (contracepti$ adj implant$).tw. 2 exp NORGESTREL/ 3 LEVONORGESTREL/ 4 norplant$.tw. 5 uniplant.tw. 6 (keto adj desogestrel).tw. 7 levonorgestrel.tw. 8 norgestrel.tw. 9 etonorgestrel.tw. 10 implanon.tw. 11 (subdermal adj implant$).tw. 12 (subcutaneous adj implant$).tw. 13 jadelle.tw. 14 nestorone.tw. 15 elcometrine.tw. 16 normegestrol.tw. 17 or/1‐16 18 randomized controlled trial.pt. 19 controlled clinical trial.pt. 20 RANDOMIZED CONTROLLED TRIALS/ 21 RANDOM ALLOCATION/ 22 DOUBLE‐BLIND METHOD/ 23 SINGLE‐BLIND METHOD/ 24 clinical trial.pt. 25 exp CLINICAL TRIALS/ 26 (clin$ adj25 trial$).tw. 27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 28 PLACEBOS/ 29 (placebo$ or random$).tw. 30 RESEARCH DESIGN/ 31 CROSS‐OVER STUDIES/ 32 (crossover$ or cross‐over$ or cross over$).tw. 33 INTERVENTION‐STUDIES/ 34 multicenter study.pt. 35 (latin‐square$ or latin square$ or factorial$).tw. 36 COMPARATIVE STUDY/ 37 exp EVALUATION STUDIES/ 38 FOLLOW UP STUDIES/ 39 PROSPECTIVE STUDIES/ 40 (control$ or prospective$ or volunteer$).tw. 41 or/18‐40 42 17 and 41 43 (ANIMAL not HUMAN).sh. 44 42 not 43
3. EMBASE (OvidWeb 1980‐2003 week 25) (searched 26th June 2003)
1 (contracepti$ adj implant$).tw. 2 LEVONORGESTREL/ 3 NORGESTREL/ 4 Norplant$.tw. 5 Uniplant.tw. 6 (keto adj desogestrel).tw. 7 levonorgestrel.tw. 8 norgestrel.tw. 9 etonorgestrel.tw. 10 implanon.tw. 11 jadelle.tw. 12 nestorone.tw. 13 elcometrine.tw. 14 normegestrol.tw. 15 (subdermal adj implant$).tw. 16 or/1‐15 17 random$.tw. 18 factorial$.tw. 19 (crossover$ or cross‐over$ or cross over$).tw. 20 placebo$.tw. 21 (doubl$ adj blind$).tw. 22 (singl$ adj blind$).tw. 23 assign$.tw. 24 allocat$.tw. 25 volunteer$.tw. 26 CROSSOVER PROCEDURE/ 27 DOUBLE‐BLIND PROCEDURE/ 28 RANDOMIZED CONTROLLED TRIAL/ 29 SINGLE‐BLIND PROCEDURE/ 30 or/17‐29 31 16 AND 30 32 exp ANIMAL/ or NONHUMAN/ or exp ANIMAL EXPERIMENT/ 33 exp HUMAN/ 34 32 not 33 35 31 not 34
4. POPLINE (via http://db.jhuccp.org/popinform/expert.html updated June 16 2003) (searched 26th June 2003) Contraceptive implant* / levonorgestrel / norgestrel / norplant* / uniplant / keto desogestrel / etonorgestrel / implanon / jadelle / nestorone / elcometrine / normegestrol / subdermal implant*
5. Science Citation Index (Web of Science 1981 ‐ 27 June 2003) (searched 27th June 2003) Contracepti* implant* or norgestrel or levonorgestrel or norplant* or uniplant or keto desogestrel or etonorgestrel or implanon or jadelle or nestorone or elcometrine or normegestrol or subdermal implant*
6. PsycINFO (OvidWeb 1972 ‐ June week 1 2003) (searched 26th June 2003)
1 (contracepti$ adj implant$).tw. 2 CONTRACEPTIVE DEVICES/ 3 Norplant$.tw. 4 Uniplant$.tw. 5 (keto adj desogestrel).tw. 6 levonorgestrel.tw. 7 norgestrel.tw. 8 etonorgestrel.tw. 9 implanon.tw. 10 jadelle.tw. 11 nestorone.tw. 12 elcometrine.tw. 13 normegestrol.tw. 14 (subdermal adj implant$).tw. 15 or/1‐14
In addition:
The reference lists of all identified publications were searched for previously unidentified articles.
The relevant pharmaceutical companies were contacted and asked to release results of any relevant unpublished studies for inclusion.
Individuals and organisations with an interest in contraceptive research were contacted to identify unpublished and ongoing studies relevant to the review.
Data collection and analysis
Study selection The selection of studies for inclusion and their methodological quality were independently assessed and reported by reviewers (JP, RF and FC). Non‐English language publications were assessed by RF and a translator.
Quality assessment The quality of each publication was assessed independently by two of the three reviewers (JP, RF and FC). Standard quality assessment forms were designed, and included general methodological factors, as well as some of contraceptive specific factors recommended by Trussell 1991. The following quality factors were included on the checklist:
method of randomisation described
allocation concealment
blinded assessment of outcomes
groups treated identically other than named intervention
description of women who withdrew or were lost to follow up provided
description of hormonal contraceptive method or pregnancy immediately prior to study enrolment
statistical method (with reference) used to analyse pregnancy and continuation of methods
description of contraceptive failure provided (i.e. user or method failure or both)
active follow up conducted (i.e. analysis of follow up delayed a few months to allow inclusion of undetected pregnancies)
All of the reviewer differences in initial assessment were resolved after discussion and it was not necessary to involve a third party. Data collection and analysis Contraceptive effectiveness and continuation Although single‐decrement life‐table probabilities are the ideal method for the measurement of contraceptive effectiveness and continuation (Trussell 1991), they were not commonly employed in the papers and therefore not used in this analysis. It was, however, usually possible to collect the number of reported pregnancies and the number of women months contributing to follow up. The number of pregnancies per women months of use, akin to the Pearl Index rate was collected at specific follow up points (at one, two, three, four and five years). The proportion of all women still using each implant at the specific time points was calculated. In order to obtain a relative measure of continuation taking account of the time the method was used, the number of women months contributing to follow up and the potential number of women months at the specified time points were collected. Potential women months were calculated by multiplying the number of women recruited onto each of the studies with the total number of months at each of the specified time points (e.g. at one year the number of women recruited into a study was multiplied by 12 months). This method has been described as a way of measuring completeness of follow‐up (Clark 2002).
As it was not possible to calculate rate ratio and follow‐up time ratios in RevMan, Microsoft Excel was used to calculate a summary effect size of pregnancies per women months. The rates of the experimental and control events were compared. This method gave a relative measure of 'treatment' effect, that is how much more or less likely implant users were to experience pregnancy in comparison to users of other contraceptive methods or different implants. In terms of continuation, this method provided a crude measure of how well the contraceptives were tolerated. The log rate ratios and the log follow‐up ratios, and their variances were calculated for each study (Hasselblad 1995). It was then possible to combine studies using the inverse weighted average of the log rate or follow‐up ratios. Events and follow‐up were only combined if they were measured over the same time period (i.e. one years, two years and so on) because of their variability over time. For the purpose of data synthesis, in situations where there were no pregnancies in one arm of the trial a continuity correction was implemented by adding a half to each cell.
Menstrual changes, hormonal side‐effects and adverse events Menstrual change outcomes were only collected if investigators had stipulated that they had been measured over 90 day intervals as recommended by Rodriguez 1976 and Belsey 1986. Standard WHO definitions were used to describe vaginal bleeding patterns (Belsey 1986). Amenorrhoea was no bleeding or spotting (B‐S) throughout the reference period (RP). Infrequent bleeding was less than three B‐S episodes starting within a RP excluding amenorrhoea; frequent bleeding was more than five B‐S episodes starting within a RP; and prolonged bleeding was at least one B‐S episode lasting greater than 14 days starting within a RP. The number of women who experienced an event and total number of women at each 90 day interval were collected to calculate odds ratios for menstrual change outcomes. Data on hormonal side effects and adverse events were to be collected at yearly time intervals for the calculation of odds ratios. RevMan was used to obtain a summary effect for the odds ratios. These were compared at specific time points e.g. after one year of use.
A description of the demographic characteristics of the study participants was collected so that a decision could be made whether it was appropriate to combine the data. The degree of heterogeneity was investigated and reported. A fixed effects approach was used for the meta‐analysis (DerSimonian 1986).
Results
Description of studies
Nine subdermal implant trials met the inclusion criteria (See Table of Included Studies for further information including the total number of women and duration for each study). None of these trials compared implants to other reversible methods of contraception, they all compared different types of contraceptive implants.
Eight trials compared Implanon versus Norplant . The data from seven of these eight trials (Organon 34508, Organon 34509, Organon 34510, Organon 34511, Organon 34512, Organon 34514, Organon 34520) had already been combined and published as a series of five meta‐analyses (Croxatto 1998, Mascarenhas 1998a, Huber 1998, Affandi 1998, Urbancsek 1998). The seven trials from these meta‐analyses are referred to by the trial numbers allocated by Organon who supplied the individual trial data from these trials for this review. One trial compared Jadelle versus Norplant (Sivin 1998).
Three of the nine trials were conducted in developing or transitional countries (Organon 34510, Organon 34520, Zheng 1999), four in developed countries (Organon 34508, Organon 34509, Organon 34511, Organon 34512); and the remaining two were multicentre trials conducted in both developing and developed countries (Organon 34514, Sivin 1998). However, when the number of women who were recruited is looked at, the gap between studies that were conducted in developing countries compared to those conducted in developed countries becomes even wider (1219 women recruited versus 278 women recruited, respectively). The international multicentre trials, undertaken in both developed and developing countries, recruited 1279 women. It was not possible to ascertain whether the women were from predominantly developing or developed countries.
The age of recruited women, across all studies, ranged from 18‐40 years.
Information was collected from the papers on factors, other than the use of the contraceptive methods under investigation, that could potentially effect the fecundity of the study participants. It was only stated in one of the trials that all of the recruited women had had a previous birth or pregnancy, thus ensuring the proven fertility of the population under investigation (Zheng 1999). It was stated for one of the trials that the women were not breast feeding (Zheng 1999). One study stated that women were excluded if they had used hormonal injections in the six months prior to the study starting, or oral contraceptives in the month prior to commencement (Zheng 1999). Women were reported to have regular menses at recruitment in eight of the trials (Organon 34508, Organon 34509, Organon 34510, Organon 34511, Organon 34512, Organon 34514, Organon 34520, Zheng 1999). Information was collected on additional factors that could potentially affect the study results. The extent of contraceptive counselling received prior to starting the method was not reported in any of the trials. None of the trials reported whether the health worker inserting the subdermal implants had received specialist training. Eight of the trials reported whether the timing of the insertion in relation to the menstrual cycle was documented (Organon 34508, Organon 34509, Organon 34510, Organon 34511, Organon 34512, Organon 34514, Organon 34520, Zheng 1999).
Risk of bias in included studies
In only one of the nine trials was both the method of randomisation and allocation concealment described by the authors (Sivin 1998). In this trial randomisation was done in blocks of 50 and women were allocated their method in sealed envelopes. In all other trials randomisation was computer generated but there was no mention of allocation concealment. The investigators were not blind at follow up assessments to allocated contraceptive methods in any of the trials.
The authors clearly stated the intervention groups were treated identically in all nine of the trials (Organon 34508, Organon 34509, Organon 34510, Organon 34511, Organon 34512, Organon 34514, Organon 34520, Sivin 1998, Zheng 1999). None of the included studies provided any information on women who withdrew or who were lost to follow‐up, so it was not possible to determine whether or not this group was similar to the women who remained in the study.
In the Organon studies data Pearl Indices were used to provide summary effect sizes for pregnancy and discontinuation, the remaining study used life table analysis to report rates for these outcomes (Sivin 1998).
One of the nine included studies provided a description of the contraceptive methods women were using prior to enrolment (Sivin 1998). Active follow‐up analysis, to complete the assessment of pregnancy status at the time the trial concluded, was conducted in one study (Sivin 1998). Details of the methodological quality for each trial are provided in the table 'Characteristics of Included Studies'.
Effects of interventions
Implanon versus Norplant It was possible to combine eight studies in the meta‐analysis comparing contraceptive effectiveness, continuation and bleeding patterns (Organon 34508, Organon 34509, Organon 34510, Organon 34511, Organon 34512, Organon 34514, Organon 34520, Zheng 1999).
Contraceptive effectiveness There was no difference in contraceptive effectiveness between the two implants. There were no pregnancies in either the Implanon or Norplant groups after 26,972 and 28,108 women months of follow up, respectively. We could not assess the effect of weight on contraceptive effectiveness as it was not possible to extract individual patient weight data. Women with a BMI above 29 were excluded from most of the Organon trials.
Continuation There was no significant difference in the continuation of methods at 1 (Table 2), 2 (Table 3), 3 (Table 4) or 4 years (Table 5) (these were measured as the total number of women months contributed at each time interval compared with the total possible number of women months if all women had continued using the implants). The proportion of women followed up and still using Implanon and Norplant, respectively, were 91.6% and 92.4% at Year 1, 82.5% and 81.4% at Year 2, 67.4% and 72.5% at Year 3, and 17.1% and 16.9% at Year 4. The summary follow‐up time ratios for continuation of Implanon versus Norplant at 1, 2, 3 and 4 years were 0.98 (95% confidence intervals 0.96 to 1.01), 1.00 (95% CI 0.98 to 1.02), 0.99 (95% CI 0.97 to 1.01) and 1.04 (95% CI 1.00 to 1.09) respectively. There were however marked differences in the proportion of women continuing contraceptive methods depending on geographical area, the overall proportion being higher in the studies conducted in developing (90.6% of women continuing to use Implanon and 91.4% Norplant at two years) as compared to developed countries (55.4% or Implanon and 47.5% for Norplant at 2 years).
1. Year 1 Continuation Implanon v Norplant (women months [wm] / potential wm [pwm]).
| Study | Implanon wm | Implanon pwm | Norplant wm | Norplant pwm | Follow‐up time ratio | 95% CI | |
| 34508 | 179.8 | 192 | 166.7 | 192 | 1.08 | 4800 | 0.87‐1.33 |
| 34509 | 434.5 | 516 | 457 | 516 | 0.95 | 0.83‐1.08 | |
| 34510 | 711.7 | 720 | 719.9 | 720 | 0.99 | 0.89‐1.10 | |
| 34511 | 473.9 | 480 | 451.3 | 480 | 1.05 | 0.92‐1.19 | |
| 34512 | 407.2 | 480 | 437.1 | 480 | 0.93 | 0.81‐1.07 | |
| 34514 | 341.7 | 492 | 345.2 | 480 | 0.97 | 0.83‐1.12 | |
| 34520 | 5302.2 | 5388 | 5348.7 | 5400 | 0.99 | 0.96‐1.03 | |
| Zheng | 1173.6 | 1200 | 1096.8 | 1200 | 0.93 | 0.86‐1.01 | |
| Meta‐analysis | 0.98 | 0.96‐1.01 |
2. Year 2 Continuation Implanon v Norplant (women months [wm] / potential wm [pwm]).
| Study | Implanon wm | Implanon pwm | Norplant wm | Norplant pwm | Follow‐up ratio | 95% CI |
| 34508 | 297.0 | 384 | 285.6 | 384 | 1.04 | 0.88‐1.22 |
| 34509 | 712.3 | 1032 | 761.5 | 1032 | 0.94 | 0.84‐1.04 |
| 34510 | 1309.2 | 1440 | 1432.3 | 1440 | 0.91 | 0.85‐0.99 |
| 34511 | 924.2 | 960 | 842.4 | 960 | 1.10 | 1.0‐1.20 |
| 34512 | 667.1 | 960 | 737.2 | 960 | 0.90 | 0.81‐1.00 |
| 34514 | 643.3 | 984 | 633.2 | 960 | 0.99 | 0.89‐1.11 |
| 34520 | 10461.9 | 10776 | 10555.7 | 10800 | 0.99 | 0.97‐1.02 |
| Zheng | 2028 | 2400 | 1812 | 2400 | 1.12 | 1.05‐1.19 |
| Meta‐analysis | 1.0 | 0.98‐1.02 |
3. Year 3Continuation Implanon v Norplant (women months[wm] / potential wm [pwm]).
| Sudy | Implanon wm | Implanon pwm | Norplant wm | Norplant pwm | Follow‐up ratio | 95% CI |
| 34508 | 381.1 | 576 | 322.6 | 576 | 1.18 | 1.02‐1.37 |
| 34510 | 1529.5 | 2160 | 1587.7 | 2160 | 0.96 | 0.90‐1.03 |
| 34520 | 14839.4 | 16164 | 15095 | 16200 | 0.99 | 0.96‐1.01 |
| Zheng | 2750.4 | 3600 | 2644.8 | 3600 | 1.04 | 0.99‐1.10 |
| Meta‐analysis | 0.99 | 0.97‐1.01 | ||||
4. Year 4 Continuation Implanon v Norplant (women months [wm] / potential wm [pwm]).
| Study | Implanon wm | Implanon pwm | Norplant wm | Norplant pwm | Follow‐up ratio | 95% CI |
| Zheng | 4099.2 | 21552 | 3949.2 | 21600 | 1.04 | 1.00‐1.09 |
| Meta‐analysis | 1.04 | 1.00‐1.09 | ||||
Menstrual changes Menstrual changes were common with both Implanon and Norplant (see Figure 1, Figure 2, Figure 3 and Figure 4). These figures show the percentage of women with a given bleeding pattern at each reference period. These were calculated as the number of women across all the studies with each bleeding pattern in a particular reference period as a percentage of the total number of women across all the studies in that reference period. Only one trial reported data beyond three years use (Zheng 1999), hence the bleeding data for reference periods 12 ‐ 16 in the figures represents data from this single trial. Bleeding patterns were analysed at the end of the first shifted reference period and at the end of the 4th (equating to one years use) and 8th (equating to two years use) reference periods (see tables in comparisons and data section). In the first shifted reference interval infrequent and prolonged bleeding was more likely amongst Implanon users compared to Norplant users (odds ratios 1.30 [95% CI 1.04 to 1.63], and 1.49 [95% CI 1.09 to 2.03] respectively). No significant difference between the implants was observed for amenorrhoea (this was heterogeneous, p=0.01) and frequent bleeding. At the end of the 4th and 8th reference periods Implanon users were significantly more likely to report amenorrhoea (odds ratio 1.87 [95% CI 1.45 to 2.42] for reference period 4 and 2.14 [95% CI 1.63 to 2.81] for reference period 8). No significant differences between Implanon and Norplant users were observed for infrequent, frequent and prolonged (heterogeneous, p=0.004) bleeding. Amenorrhoea increased with duration of use with both implants (Figure 1) up to three years use, data beyond three years was limited. It was not possible to extract individual trial data for discontinuation due to vaginal bleeding. Affandi 1998 reports no significant differences between the two implants. There were, however, marked differences in the discontinuation of method because of menstrual disturbance depending on geographical area (Affandi 1998). Women in Europe were more likely to discontinue implants because of menstrual disturbance (30.2% discontinuing Implanon and 22.5% discontinuing Norplant at two years) compared to women in South Asia (0.9% discontinuing Implanon and 1.4% discontinuing Norplant at two years). Frequent irregular bleeding was the least acceptable bleeding pattern, constituting roughly 50% of the bleeding related discontinuations. Amenorrhoea, in contrast, was rarely a reason for discontinuation (Affandi 1998).
1.
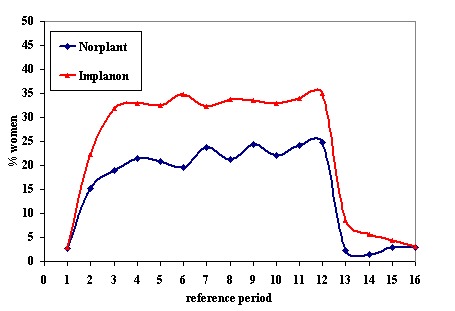
% of women, across all included trials, with amenorrhoea at each reference period
2.
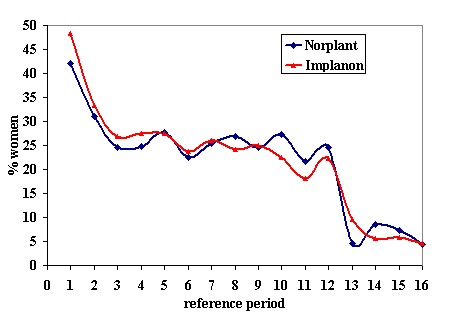
% of women, across all included trials, with infrequent bleeding at each reference period
3.
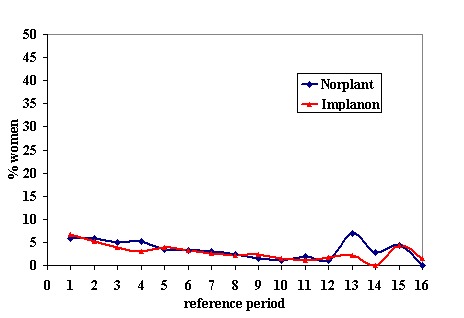
% of women, across all included trials, with frequent bleeding at each reference period
4.
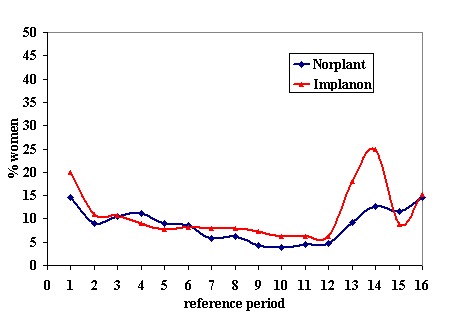
% of women, across all included trials, with prolonged bleeding at each reference period
Hormonal side effects It was not possible to extract individual trial data for meta‐analysis of side effects other than bleeding irregularities or for reasons for discontinuation. Urbancsek 1998 had already combined data from seven trials (Organon 34508, Organon 34509, Organon 34510, Organon 34511, Organon 34512, Organon 34514, Organon 34520). He found no significant difference in the occurrence of drug related adverse events (61% versus 69% for Implanon and Norplant respectively, p=0.17). The most common hormonal side effects were acne (incidence 18.5% versus 21.2% for Implanon and Norplant respectively), headaches (16.8% versus 20.1%), breast pain (9.8% versus 11.4%) and increase in body weight (6.5% versus 7.1%). There was no significant difference in the percentage of women discontinuing Implanon or Norplant due to these adverse events (6.0% versus 7.6%).
Procedure times It was not possible to extract individual trial data on procedure times for meta‐analysis. Data from seven trials had already been combined by Mascarenhas 1998a (Organon 34508, Organon 34509, Organon 34510, Organon 34511, Organon 34512, Organon 34514, Organon 34520). Procedure times were available for 670 women having Implanon inserted and 665 women having Norplant inserted. It was not possible to extract these data from the other trial (Zheng 1999). The mean time for Implanon insertions was 1.1 minutes (SD 0.9, range 0.03 to 5 minutes) and for Norplant insertions was 4.3 minutes (SD 2.1, 0.83 to 18 minutes). Removal times were available for 633 Implanon users and 137 Norplant users. The mean time for Implanon was 2.6 minutes (SD 2.0, range 0.2 to 20 minutes) and for Norplant was 10.2 minutes (SD 8.2, range 1.3 to 50 minutes). Problems at insertion were rare, being reported in two (0.3%) of the Implanon insertions (one case of bleeding and one case where the rod followed the cannula out of the skin) and none of the Norplant insertions. Norplant users were significantly more likely to experience problems at removal than Implanon users (0.2% vs. 4.8%; p<0.001), although the number of problematic removals was small (one complication out of 644 removals for Implanon and 7 out of 145 removals for Norplant). The most common problem with Norplant removals was broken capsules. Zheng 1999 also found the mean insertion and removal times to be significantly less for Implanon than Norplant: insertion 0.61 versus 3.90 mins p<0.001, removal 2.18 versus 11.25 mins p<0.001.
Jadelle versus Norplant One study was identified that compared Jadelle with Norplant (Sivin 1998). There was no significant difference in pregnancy rate (0.13 and 0.09 per 100 women years for Jadelle and Norplant respectively), cumulative continuation rates (55.1 and 53.0 per hundred users of Jadelle and Norplant respectively at five years) or cumulative discontinuation rates for medical or menstrual problems (15 and 16.4 per 100 respectively for Jadelle versus 12 and 19.2 per hundred for Norplant). The mean time taken to remove Jadelle (4.84 minutes SE 0.22) was significantly less than that taken to remove Norplant (9.59 minutes SE 0.44) (p<0.0001).
Subdermal implants versus other reversible contraceptives No RCTs were identified that compared subdermal implants with either IUDS, oral contraceptives, barrier methods or injectable contraceptives.
Discussion
All of the studies included in the review were comparisons of different types of implants rather than studies comparing implants with other types of contraceptive method. Therefore, it was not possible to determine the relative effectiveness or acceptability of subdermal implants when compared to other forms of contraception. While the comparisons of different types of implants may provide useful information to policy makers and providers of family planning services, they are not necessarily informative to the contraceptive user who wishes to decide between different contraceptive methods.
The studies included demonstrated that subdermal implants were very effective methods for preventing unwanted pregnancy, with only two pregnancies in 4377 women years of follow up in Norplant users, three in 2307 women years of follow up with Jadelle and none in 2068 women years of follow up in those using Implanon. Giving pregnancy rates of 0.05, 0.13 and 0 per 100 women years of use for Norplant, Jadelle and Implanon respectively. These pregnancy rates were not significantly different.
There was no difference in continuation or rates of hormonal side effects for Implanon, Norplant or Jadelle. Continuation was much higher in developing countries than in developed. This might reflect access to alternative contraceptive methods as well as differences in culture and expectations. The most common side effect , with all the implants, was of irregular vaginal bleeding. Although the pattern of bleeding varied slightly between Implanon and Norplant, there was no significant difference in discontinuation rates due to vaginal bleeding between the two implants. However, the numbers involved, especially in the European studies where discontinuation appeared to be higher with Implanon, were small. Amenorrhoea increased with duration of use up to 3 years use, however, this may reflect the fact that women with unacceptable bleeding dropped out of the study. The apparent fall in amenorrhoea after the 12th reference period needs to be interpreted with caution as only one trial reported data beyond three years use, and in the 13th reference period data from only 6 women for Norplant and 2 for Implanon were included. Non‐bleeding adverse effects were similar for all the implant types, the most common were acne, breast tenderness, headaches and weight gain.
Eight of the nine trials in this review were sponsored by the manufacturers of Implanon, Organon. In July 2004 a press release was issued explaining that incorrect data had been recorded in the some of study reports of the trials conducted in Indonesia (Rekers 2004). This may have affected trials Organon 34510, Organon 34514 and Organon 34520 included in this review. In order to assess what impact this had on our findings we removed these trials from meta‐analysis. Our sensitivity analysis found that this had no significant effect on the findings for the effectiveness or continuation. However, there were some changes noted for menstrual outcomes reported. Without knowing what the discrepancies were, in terms of the data recorded, it is difficult to reach conclusions on the findings and quality of these studies.
It is presumed that pre‐treatment counselling, to ensure that women are informed about the potential side effects of a contraceptive method, has a positive effect on continuation, but, we could find little unbiased published evidence to support this assumption.
There was very little information on failed insertion or failed removal of implants.
Women who agree to be part of a contraceptive RCT are not likely to be representative of the general population of female contraceptive users. They are more likely to be motivated and able to commit to continued follow up. Importantly, women who are prepared to be randomised are not likely to be representative; user choice of contraceptive method is related to effectiveness.
Only one of the trials described allocation concealment. Allocation concealment is always feasible, even if unblinding happens immediately afterwards. Schulz and colleagues (Schulz 2002) demonstrated that inadequate or unclear allocation concealment exaggerated treatment effect by up to 40%. Unfortunately, it was not possible to investigate what effect allocation concealment , as well as other quality factors, had on the findings because of the small number of eligible studies. The fact that in most studies the investigators were not blind to the methods of contraceptive at follow up visits for assessment of outcomes would not affect the number of pregnancies reported. However, reporting of hormonal side effects and menstrual disturbance, and even continuation, could be affected by either the investigator or the contraceptive user knowing the method.
It was interesting to note that none of the studies in the review provided any information on the characteristics of those women who withdrew or were lost to follow up. This may provide insight into the acceptability and tolerability of a contraceptive method as women who are dissatisfied with a method are more likely to drop out of a study and bias the results of a method's effectiveness.
Although this systematic review was unable to provide a definitive answer on the relative effectiveness, tolerability and acceptability of contraceptive implants in comparison to other contraceptive methods, it has raised issues around the conduct of contraceptive research; namely about study quality, the interpretation of results, the usefulness of the information to contraceptive users and the difficulties in trying to synthesize contraceptive data.
Authors' conclusions
Implications for practice.
This systematic review found that subdermal implants were very effective methods of contraception, with few reported pregnancies over the course of the included studies in the selected women. No one subdermal implant was found to be any more or less effective in preventing unwanted pregnancy than another. Implanon and Jadelle were quicker to remove than Norplant.
Menstrual disturbances were common, these menstrual side effects should be explained to women so that they can make an informed choice as to whether or not implants are the most appropriate method of contraception for them.
Implications for research.
1. Standardisation of methods and measurements employed in contraceptive research This systematic reviews highlights the problems which arise because of inconsistent methods used to measure and report contraceptive effectiveness. We were not able to assess what impact these factors had on pooled data. Standardised methods need to be encouraged, from the recruitment to analysis stages. These problems do not just impact on individuals conducting systematic reviews. They affect how health care practitioners, policy makers, contraceptive users, researchers and the media interpret the contraceptive literature, whether it comes from articles in peer reviewed journals or from contraceptive information leaflets.
Guidance has been provided by Trussell 1991on the methodological issues which need to be considered when undertaking as well as interpreting contraceptive efficacy and effectiveness research. We would advocate that Trussell's recommendations are considered when contraceptive research is undertaken. We would also advocate that the CONSORT statement should be followed when reporting trials Moher 2001.
2. Designing studies to measure relative effectiveness of contraceptive methods While RCTs provide the best level of evidence and these comparisons of different types of subdermal implant may provide useful information to policy makers and providers of family planning services, they are not necessarily informative to the contraceptive user who wants to decide which of the various different contraceptive methods to use (e.g. implants versus the IUD). Non‐randomised studies were not included in this review. This type of study design may be the most feasible way to compare subdermal implantable contraceptives with a broader spectrum of methods. However, they would need to be designed in such a way to minimise bias.
3. Consumer involvement in the development of contraceptive research It is vital that contraceptive research is able to answer the queries and concerns of contraceptive users. Rates of pregnancy and continuation may not accurately reflect the acceptability of a method. Not all women experiencing problematic side effects will discontinue the method. Their decision to discontinue may reflect many other factors as is illustrated by the marked differences in the discontinuation of implants in developing as compared to developed countries. If lay contraceptive users are involved in research development, attention can be directed to answering consumer related questions.
What's new
| Date | Event | Description |
|---|---|---|
| 7 March 2012 | Amended | Additional tables linked to the text |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 15 April 2008 | Amended | Converted to new review format. |
| 22 April 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
This review was developed from work that was originally funded by the National Health Service Health Technology Assessment Programme. The following individuals assisted in the Health Technology Assessment: Ms Diana Mansour, Dr David Hughes, Professor John Guillebaud, Ms Caroline Summerbell, Dr Stuart Logan and Ms Tina Proctor.
The following individuals have assisted in trying to locate unpublished data and provided general advice: Dr Irvin Sivin (The Population Council), Dr Patrick Rowe (World Health Organization), Dr Catherine d'Arcangues (World Health Organization), Dr Régine Sitruk‐Ware (Laboratoire Théramex), Ms Toni Belfield (FPA) and Dr Iain Chalmers (The UK Cochrane Centre).
The following pharmaceutical companies have co‐operated with this work: Organon Laboratories Ltd.
We would like to acknowledge Dr Yu Yi (Chinese) and Mr Patrick Austin (Swedish) for their help with the translation of papers and Anne Eisenga who developed the search strategy.
Dr. Julian Higgins and Dr Andrew Copas have provided assistance with the methodological aspects of conducting the meta‐analyses. We would also like to acknowledge the support and encouragement received from the Cochrane Fertility Regulation Group and the UK Cochrane Centre, in particular the help and advice received from Frances Fairman.
Data and analyses
Comparison 1. Implanon vs Norplant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amenorrhoea at end of 1st shifted ref period | 8 | 1463 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.58] |
| 2 Amenorrhoea at end of 4th ref period | 8 | 1360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.45, 2.42] |
| 3 Amenorrhoea at end of ref period 8 | 8 | 1228 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.63, 2.81] |
| 4 Infrequent bleeding at end 1st shifted ref period | 8 | 1482 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.63] |
| 5 Infrequent bleeding at end of 4th ref period | 8 | 1363 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.92, 1.49] |
| 6 Infrequent bleeding at end of 8th ref period | 8 | 1228 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.09] |
| 7 Frequent bleeding at end of 1st shifted ref period | 8 | 1482 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.80, 1.79] |
| 8 Frequent bleeding at end 4th ref period | 8 | 1363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.11] |
| 9 Frequent bleeding at end 8th ref period | 8 | 1228 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.53, 2.15] |
| 10 Prolonged bleeding at end 1st shifted ref period | 8 | 1482 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.09, 2.03] |
| 11 Prolonged bleeding at end 4th ref period | 8 | 1363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.10] |
| 12 Prolonged bleeding at end 8th ref period | 8 | 1167 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.70] |
1.1. Analysis.
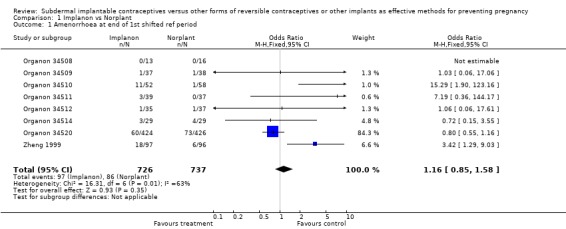
Comparison 1 Implanon vs Norplant, Outcome 1 Amenorrhoea at end of 1st shifted ref period.
1.2. Analysis.
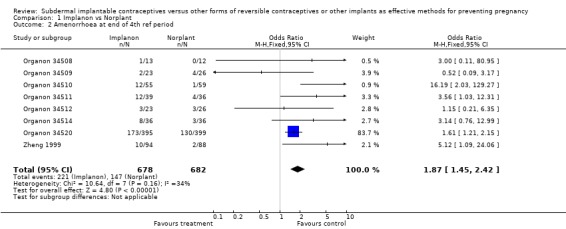
Comparison 1 Implanon vs Norplant, Outcome 2 Amenorrhoea at end of 4th ref period.
1.3. Analysis.
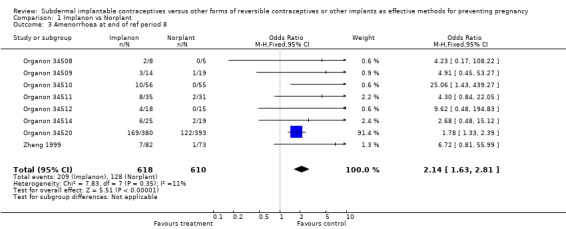
Comparison 1 Implanon vs Norplant, Outcome 3 Amenorrhoea at end of ref period 8.
1.4. Analysis.
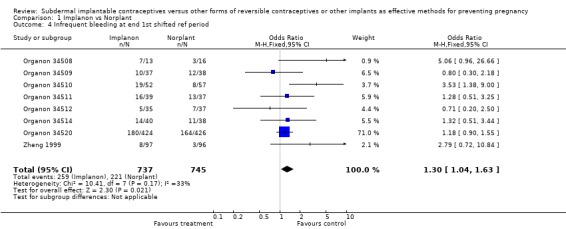
Comparison 1 Implanon vs Norplant, Outcome 4 Infrequent bleeding at end 1st shifted ref period.
1.5. Analysis.
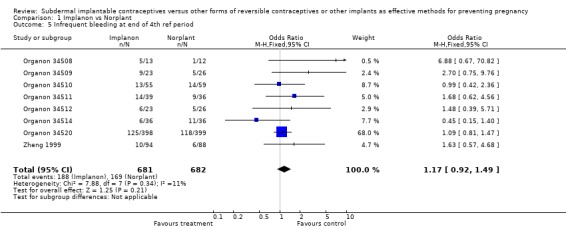
Comparison 1 Implanon vs Norplant, Outcome 5 Infrequent bleeding at end of 4th ref period.
1.6. Analysis.
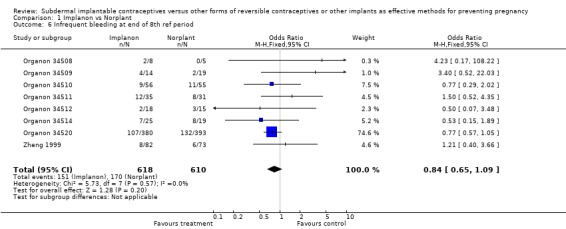
Comparison 1 Implanon vs Norplant, Outcome 6 Infrequent bleeding at end of 8th ref period.
1.7. Analysis.
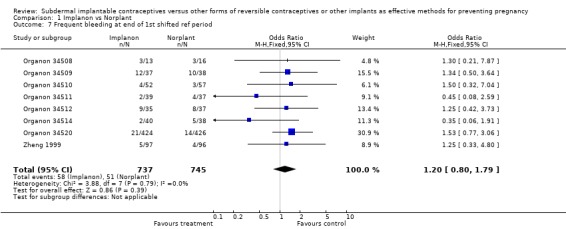
Comparison 1 Implanon vs Norplant, Outcome 7 Frequent bleeding at end of 1st shifted ref period.
1.8. Analysis.
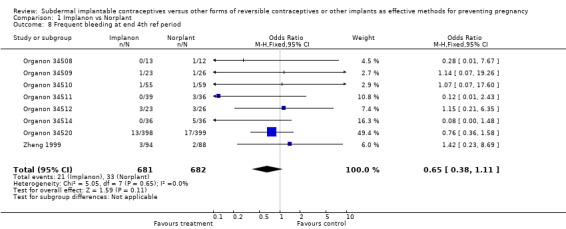
Comparison 1 Implanon vs Norplant, Outcome 8 Frequent bleeding at end 4th ref period.
1.9. Analysis.
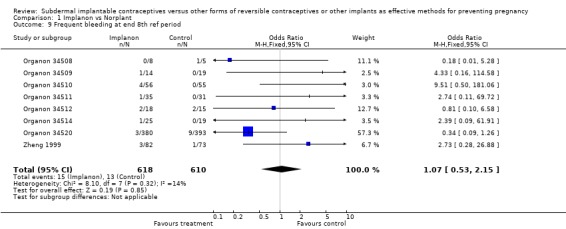
Comparison 1 Implanon vs Norplant, Outcome 9 Frequent bleeding at end 8th ref period.
1.10. Analysis.
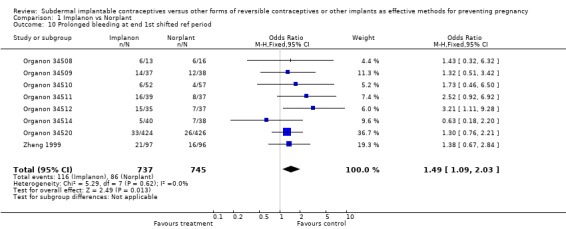
Comparison 1 Implanon vs Norplant, Outcome 10 Prolonged bleeding at end 1st shifted ref period.
1.11. Analysis.
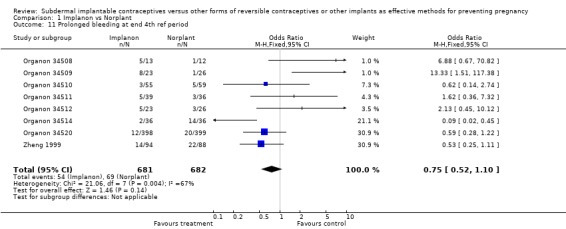
Comparison 1 Implanon vs Norplant, Outcome 11 Prolonged bleeding at end 4th ref period.
1.12. Analysis.
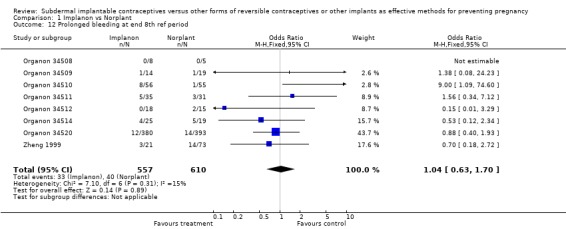
Comparison 1 Implanon vs Norplant, Outcome 12 Prolonged bleeding at end 8th ref period.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Organon 34508.
| Methods | Setting: Finland and Sweden Randomised 3 years follow up | |
| Participants | 32 women randomised, 18‐40 years Regular menses | |
| Interventions | Implanon [n=16] vs. Norlant [n=16] | |
| Outcomes | Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals | |
| Notes | Quality assessment: Groups treated identically Computer generated randomisation User/method failure reported: Not applicable as no pregnancies No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Organon 34509.
| Methods | Setting: Finland and Sweden Randomised 2 years follow up | |
| Participants | 86 women randomised, 18‐40 years Regular menses | |
| Interventions | Implanon [n=43] vs. Norlant [n=43] | |
| Outcomes | Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals | |
| Notes | Quality assessment: Groups treated identically Computer generated randomisation User/method failure reported: Not applicable as no pregnancies No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Organon 34510.
| Methods | Setting: Indonesia and Thailand Randomised 3 year follow up | |
| Participants | 120 women randomised, 18‐40 years Regular menses | |
| Interventions | Implanon [n=60] vs. Norlant [n=60] | |
| Outcomes | Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals | |
| Notes | Quality assessment: Groups treated identically Computer generated randomisation User/method failure reported: Not applicable as no pregnancies No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Organon 34511.
| Methods | Setting Singapore Randomised 2 year follow up | |
| Participants | 80 women randomised 18‐40 years Regular menses | |
| Interventions | Implanon [n=40] vs. Norlant [n=40] | |
| Outcomes | Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals | |
| Notes | Quality assessment: Groups treated identically Computer generated randomisation User/method failure reported: Not applicable as no pregnancies No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Organon 34512.
| Methods | Setting: Finland Randomised 2 year follow up | |
| Participants | 80 women randomised, 18‐40 years Regular menses | |
| Interventions | Implanon [n=40] vs. Norlant [n=40] | |
| Outcomes | Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals | |
| Notes | Quality assessment: Groups treated identically Computer generated randomisation User/method failure reported: Not applicable as no pregnancies No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Organon 34514.
| Methods | Setting: Indonesia and UK Randomised 2 year follow up | |
| Participants | 81 women randomised 18‐40 years Regular menses | |
| Interventions | Implanon [n=41] vs. Norlant [n=40] | |
| Outcomes | Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals | |
| Notes | Quality assessment: Groups treated identically Computer generated randomisation User/method failure reported: Not applicable as no pregnancies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Organon 34520.
| Methods | Setting: Indonesia Randomised 3 year follow up | |
| Participants | 899 women randomised 18‐40 years Regular menses | |
| Interventions | Implanon [n=449] vs. Norlant [n=450] | |
| Outcomes | Pregnancy Continuation rates Menstrual disturbance Hormonal side effects Adverse events Insertions and removals | |
| Notes | Quality assessment: Groups treated identically Computer generated randomisation User/method failure reported: Not applicable as no pregnancies No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Sivin 1998.
| Methods | Setting: International multicentre 1198 women randomised Follow up: 5 years | |
| Participants | 18‐40 years Variable parity (<2 births on average) | |
| Interventions | Jadelle [n=600] vs. Norplant [n=598] | |
| Outcomes | Pregnancy Continuation Reasons for discontinuation LNG serum levels | |
| Notes | Quality asessement: Groups treated identically Randomisation by blocks of 50 ‐ sealed envelopes Description of prior contraceptive method / pregnancy provided Measurement: Groups treated identically Method of analysis: Life tables (single decrement rates) User / method failure reported: Not applicable Active follow up conducted No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Low risk | A ‐ Adequate |
Zheng 1999.
| Methods | Setting: China multicentre Randomised Follow up: 2 yrs with optional extension to 4 yrs | |
| Participants | 200 women randomised 20‐35 yrs, regular menses, proven fertility, no recent hormonal contraception or pregnancy | |
| Interventions | Implanon [n=100] vs. Norlant [n=100] | |
| Outcomes | Pregnancy, continuation rates, bleeding patterns | |
| Notes | Quality assessment: Groups treated identically No info on women lost to follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Contributions of authors
RF had the idea to write the review and did the data extractions. Jo Power joined in later and together they finished the review.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Organon 34508 {published and unpublished data}
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
- Makarainen L, Beek A, Tuornivaara L, Asplund B, Bennick H. Ovarian function during the use of a single contraceptive implant: Implanon compared with Norplant. Fertility and Sterility 1998;69(4):714‐21. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
- Organon. Unpublished. Data on file.
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
Organon 34509 {published and unpublished data}
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
- Egberg N, Beek A, Gunnervik C, Hulkko S, Hirvonen E, Larsson‐Cohn U, Bennick H. Effects on the hemostatic system and liver function in relation to Implanon and Norplant. Contraception 1998;58:93‐8. [DOI] [PubMed] [Google Scholar]
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
- Organon. Unpublished. Data on file.
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
Organon 34510 {published and unpublished data}
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
- Organon. Unpublished. Data on file.
- Suherman S, Affandi B, Korver T. The effects of Implanon on lipid metabolism in comparison with Norplant. Contraception 1999;60:281‐7. [DOI] [PubMed] [Google Scholar]
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
Organon 34511 {published and unpublished data}
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
- Biswas A, Viegas O, Bennick C, Korver T, Ratnam S. Effect of Implanon use on selected parameters of thyroid and adrenal function. Contraception 2000;62:247‐51. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
- Organon. Unpublished. Data on file.
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
Organon 34512 {published and unpublished data}
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
- Organon. Unpublished. Data on file.
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
Organon 34514 {published and unpublished data}
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L, Beek A, Bennick H, Newton J. A 2‐year comparative study of endometrial histology and cervical cytology of contraceptive implant users in Birmingham, UK. Human Reproduction 1998;13(11):3057‐60. [DOI] [PubMed] [Google Scholar]
- Organon. Unpublished. Data on file.
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
Organon 34520 {published and unpublished data}
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
- Affandi B, Hoesni H, Barus R, Amran R, Iskander F, Noerpramono N, et al. A multcentred phase III comparative study between single‐implant containing 3‐ketodesogestrel (Implanon) and implants containing levonorgestrel (Norplant) I. Efficacy, acceptability and safety (three‐year results).. Med J Indonesia 1999; Vol. 8:49‐55.
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
- Organon. Unpublished. Data on file.
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
Sivin 1998 {published data only}
- Sivin I, Campodonico I, Kiriwat O, Holma P, Diaz S, Wan L, et al. The performance of levonorgestrel rod and Norplant (TM) cantraceptive implants: A 5 year randomised study. Human Reproduction 1998;13(12):3371‐8. [CN‐00251885] [DOI] [PubMed] [Google Scholar]
- Sivin I, Lahteenmaki P, Ranta S, Darney P, Klaisle C, Wan L, et al. Levonorgestrel concentrations during use of levonorgestrel rod (LNG ROD) implants. Contraception 1997(b);55:81‐5. [DOI] [PubMed] [Google Scholar]
- Sivin I, Viegas O, Campodonico I, et al. Clinical performance of a new two‐rod levonorgestrel contraceptive implant: a three‐year randomized study with Norplant implants as controls. Contraception 1997(a);55:73‐80. [DOI] [PubMed] [Google Scholar]
Zheng 1999 {published data only}
- Zheng SR, Zheng HM, Qian SZ, Kaper RF. A randomised multicenter study comparing the efficacy and bleeding pattern of a single‐rod (Implanan(TM)) and a six‐capsule (Norplant(TM)) hormonal contraceptive implant. Contraception 1999;60(1):1‐8. [1999375035] [DOI] [PubMed] [Google Scholar]
Additional references
Affandi 1998
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58(6S):99S‐107S. [DOI] [PubMed] [Google Scholar]
Belsey 1986
- Belsey EM, Machin D, D'Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. Contraception 1986;34:253‐60. [DOI] [PubMed] [Google Scholar]
Clark 2002
- Clark TG, Altman DG, Stavola BL. Quantification of the completeness of follow‐up.. The Lancet 2002;359:1309‐10. [DOI] [PubMed] [Google Scholar]
Croxatto 1998
- Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. Contraception 1998;58(6S):91S‐98S. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- Dersimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Hasselblad 1995
- Hasselblad V, McCrory DC. Meta‐analytic tools for medical decision making: a practical guide. Med Decis Making 1995;15(1):81‐86. [DOI] [PubMed] [Google Scholar]
HMR Ltd 1999
- Hoechst Marion Roussell Ltd. Litigation against Norplant Collapses. Press release 9 February 1999.
Huber 1998
- Huber J. Pharmacokinetics of Implanon. Contraception 1998;58(6S):85S‐90S. [DOI] [PubMed] [Google Scholar]
IPPF 1999
- Boostra H, Duran V, Weaver K. Norplant and the boom or bust phenomenom. IPPF Medical Bulletin October 1999;33(5):3‐4. [Google Scholar]
IPPF 2000
- Croxatto H. Progestagen implants. IPPF Medical Bulletin February 2000;34(1):1‐4. [ISSN 0019‐0357] [Google Scholar]
Mascarenhas 1998a
- Mascarenhas L. Insertion and removal of Implanon. Contraception 1998;58(6S):79S‐84S. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Sculz KF, Altman DG. The CONSORT statement. Lancet 2001;357 (9263):1191‐4. [PubMed] [Google Scholar]
Rekers 2004
Rodriguez 1976
- Rodriguez G, Faundes‐Latham A, Atkinson L. An approach to the analyses of menstrual patterns in the critical evaluation of contraceptives. Studies in Family Planning 1976;7:42‐5. [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF Grimes DA. Allocation concealment in randomised trials. Lancet 2002;359(9306):614‐618. [DOI] [PubMed] [Google Scholar]
Sivin 1988
- Sivin I. International experience with Norplant and Norplant‐2 contraceptives. Studies in Family Planning 1988;19:81‐94. [PubMed] [Google Scholar]
Sivin 1998b
- Sivin I, Mishell D.R. Jr, Darney P, et al. Levonorgestrel capsule implants in the United States. Obstet. Gynecol. 1998;92:337‐44. [DOI] [PubMed] [Google Scholar]
Trussell 1991
- Trussell J. Methodological pitfalls in the analysis of contraceptive failure. Statistics in Medicine 1991;10:201‐20. [DOI] [PubMed] [Google Scholar]
Urbancsek 1998
- Urbancsek J. An integrated analysis of nonmenstrual adverse events with Implanon. Contraception 1998;58(6S):109S‐114S. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organisation. Contraceptive implants come of age. www.who.int/reproductive‐health/hrp/progress/61/news61.htm (accessed 10 July 2003).


