Abstract
Background
In industrialised countries sterilisation is generally performed by laparoscopy. In settings where the resources for purchase and maintenance of laparoscopic equipment are limited, minilaparotomy may still be the most common approach. The advantages and disadvantages of laparoscopic sterilisation compared to minilaparotomy have not been systematically evaluated. The ideal method would be one which is highly effective, economical, able to be performed on an outpatient basis, allowing rapid resumption of normal activity and producing a minimal or invisible scar. This review considers the methods to enter the abdominal cavity through the abdominal wall, regardless of the technique used for tubal sterilisation.
Objectives
To compare laparoscopic tubal sterilisation to minilaparotomy in terms of operative morbidity and mortality. Trials comparing laparoscopy or minilaparotomy with culdoscopy were also included. Different methods used to interrupt tubal patency and comparison of different forms of anaesthesia will be considered in different reviews.
Search methods
Randomised controlled trials (RCTs) were identified by using the search strategy of the Cochrane Collaboration. Reference lists of identified trials have been searched.
Selection criteria
All randomised controlled trials comparing laparoscopy, minilaparotomy and/or culdoscopy for tubal sterilisation.
Data collection and analysis
Trials were evaluated for methodological quality and appropriateness for inclusion. Data were extracted independently by the reviewers. Results are reported as odds ratio for dichotomous outcomes and weighted mean differences for continuous outcomes.
Main results
Six trials were included in the review. Minilaparotomy vs laparoscopy: There was no difference in major morbidity between the 2 groups. Minor morbidity was significantly less in the laparoscopy group (Peto OR 1.89; 95% CI 1.38, 2.59). Duration of operation was shorter with laparoscopy (WMD 5.34; 95% CI 4.52, 6.16). Minilaparotomy vs culdoscopy: Major morbidity was higher for culdoscopy compared to minilaparotomy (Peto OR 0.14; 95% CI 0.02, 0.98). Duration of operation was shorter with culdoscopy (WMD 4.91; 95% CI 3.82, 6.01). Laparoscopy vs culdoscopy: In the one trial comparing the two interventions there was no significant difference between the groups with regard to major morbidity. Significantly more women suffered from minor morbidities with culdoscopy (Peto OR 0.20; 95% CI 0.05, 0.77).
Authors' conclusions
Major morbidity seems to be a rare outcome for both, laparoscopy and minilaparotomy. Personal preference of the woman and/or of the surgeon can guide the choice of technique. Practical aspects must be taken into account before implementing endoscopic techniques in settings with limited resources. Culdoscopy is not recommended as it carries a higher complication rate.
Plain language summary
Laparoscopy ( "keyhole" surgery ) has fewer complications than other forms of tubal ligation ( tying the tubes for contraception ), but requires more skills and equipment
Tubal ligation or sterilisation ( tying the tubes ) is a common method of fertility regulation. It is usually done by using the following methods: mini‐laparotomy ( through a small cut in the abdomen ), laparoscopy ( "keyhole" surgery ‐ through a tube inserted through the umbilicus ( belly button ) or a very small cut ), or culdoscopy ( using a tube, but through the vagina ). The review found that overall, laparoscopy had fewer complications than mini‐laparotomy, but it requires more sophisticated expensive equipment and greater skills. Culdoscopy has higher rates of complications.
Background
Worldwide, the most commonly used method of fertility regulation is tubal sterilisation (Limpaphayom 1991). Over a hundred million women of childbearing age have been sterilised and it is estimated that more than 100 million women in the developing world alone will seek sterilisation in the next 20 years (WHO 1992). Sterilisation has undergone an evolution similar to many surgical techniques. Initially, surgical sterilisation implied a major intervention requiring an open laparotomy and general anaesthesia, with significant morbidity and mortality. In an effort to simplify the procedure, Steptoe developed the technique of laparoscopic sterilisation, eventually becoming an outpatient procedure with the option of using local anaesthesia (Wheeless 1972).
On a parallel track, laparotomy techniques to perform sterilisation through smaller incisions (minilaparotomy), also with the option of using local anaesthesia, have been developed and are now widely used (Uchida 1975, Osathanondh 1974).
The World Health Organisation's (WHO) Task Force on Female Sterilization stated: "The ideal female sterilization would involve a simple, easily learned, one‐time procedure that could be accomplished under local anaesthesia and involve a tubal occlusion technique that caused minimum damage. The procedure would be safe, have high efficacy, be readily accessible, and be personally and culturally acceptable. The cost for each procedure would be low and there would be minimal costs for the maintenance of equipment". The task force promoted neither laparoscopy or minilaparotomy as the superior technique, though it reported that they both came close to meeting the required criteria listed above according to the data of a large multicentre prospective study [WHO A 1982].
In industrialised countries sterilisation is generally performed by laparoscopy rather than by minilaparotomy, based on the belief that this approach is both safe and effective. In addition, most believe that the laparoscopy scar is aesthetically more acceptable and the period of recuperation is more rapid. In settings where the resources are limited for the purchase and maintenance of the more sophisticated laparoscopic equipment, minilaparotomy may still be the most common approach. In both resource poor and industrialized countries using the technique with the greatest effectiveness and safety, together with the least costs, is extremely important.
The laparoscopic approach uses a long thin needle inserted through the umbilicus into the peritoneal cavity, through which gas (primarily CO2) is introduced. Then, after removal of the needle, a trocar is inserted into the peritoneal cavity. Other approaches to create a pneumoperitoneum are used, including direct trocar insertion and open laparoscopy. Techniques of gasless laparoscopy have been also proposed. Through the trocar sheath, the laparoscope is passed. The actual technique for occluding the fallopian tubes began as unipolar electrocoagulation, which later evolved into bipolar electrocoagulation (electrocautery), diminishing the risks of thermal bowel injuries. In an attempt to simplify the laparoscopic technique, other methods of tubal occlusion were soon introduced, including clips and rings (Wheeless 1992). Minilaparotomy is described as laparotomy through a small (usually less than 5 cm) suprapubic incision. For performing the operation only standard surgical instruments are required. Though both methods are widely used, the advantages and disadvantages of laparoscopic sterilisation compared to mini‐laparotomy have not been systematically evaluated. The ideal method would be one which is highly effective, economical, able to be performed on an outpatient basis, allowing rapid resumption of normal activity, producing a minimal or invisible scar and having a potential for reversibility.
This review considers the methods to enter the abdominal cavity through the abdominal wall, either by minilaparotomy, laparoscopy or culdoscopy regardless of the technique used for tubal sterilisation. Comparison of different techniques for interrupting tubal patency and different types of anaesthetics will be considered in other reviews.
Objectives
To evaluate laparoscopic tubal sterilisation, as compared to mini‐laparotomy in terms of operative morbidity, mortality and failure of surgical approach. Trials comparing laparoscopy or minilaparotomy with culdoscopy were included in the review.
Different methods used to interrupt tubal patency (excision, occlusion and coagulation) and comparison of different forms of anaesthesia will be considered in different reviews.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials comparing laparoscopy, minilaparotomy or culdoscopy for tubal sterilisation. Trial characteristics have been assessed and trials have been included if they fulfill the following criteria: random allocation to experimental and comparison groups; reasonable measures to ensure allocation concealment; violations of allocated management not sufficient to materially affect outcomes.
Types of participants
Women requesting tubal sterilisation as an interval procedure, independent of other surgical operations. If trials on women requesting postpartum sterilisation will be identified in future they will be analysed separately.
Types of interventions
In this review two endoscopic approaches, laparoscopy and culdoscopy were compared to minilaparotomy for tubal sterilisation irrespective of the technique used for interrupting tubal patency. Laparoscopic sterilisation was defined as any sterilisation using a laparoscope, with or without the use of a camera. Minilaparotomy was defined as any sterilisation through a small incision (less than 5 cm), according to the description by the author of the report. Culdoscopic sterilisation was defined as any sterilisation using an endoscope through an incision in the posterior cul‐de‐sac. The level of expertise of the surgeon (which may have an important impact on the success or failure of the intervention), was included, whenever possible, in the discussion.
The comparison between different tubal occlusion techniques (coagulation, rings, clips, sutures and excision) is evaluated in a different review, as well as the comparison between local, regional and general anaesthesia for surgical sterilisation.
Types of outcome measures
Operative mortality and major morbidity (cardiac arrest, pulmonary embolism, intestinal or vascular injuries requiring additional surgery) Minor morbidity (intestinal or vascular injuries not requiring additional surgery, post operative wound haematoma or infection not requiring hospitalisation, urinary tract infection)
Failure of surgical approach (laparoscopy converted into laparotomy or extension of the mini‐laparotomy incision), failure of anaesthetic approach, duration of operation, hospital stay > 24 hours, complaints (abdominal pain, analgesic use post‐operatively, persistent abdominal pain at follow‐up, other minor complaints at follow‐up) Duration of operation and length of hospital stay Women's satisfaction (as questioned at follow‐up)
Search methods for identification of studies
Randomised controlled trials (RCTs) have been identified by using the search strategy of the Cochrane Collaboration. The Cochrane Controlled Trials Register has been searched (CLIB 2001, Issue 4). Reference lists of identified trials have been searched. An electronic search strategy has been developed, including the following terms: (tubal OR female OR contracep*) AND (sterilis* OR steriliz* OR laparo* OR culdoscopy OR Filshie OR Hulka OR Yoon).
Data collection and analysis
The selection of trials for inclusion in the review was performed by three of the reviewers (RK, MB, DW) after employing the search strategy described previously. A quality score for concealment of allocation has been assigned to each trial, using the following criteria:
(A) adequate concealment of allocation (B) unclear whether concealment of allocation is adequate (C) inadequate concealment of allocation, quasi‐randomisation
Only studies scoring A or B were included in the review.
Data extraction was conducted independently by three co‐reviewers (RK, MB, DW). A form was designed to facilitate the process of data extraction. In case of discrepancies between reviewers in either the decision of inclusion/exclusion of studies or in data extraction, this was resolved by consensus. Whenever possible, the analysis was conducted on an 'intention to treat' basis. Attempts were made to obtain additional information on outcomes of women excluded from the original analysis [WHO A 1982].
Definitions: Major morbidity: any morbidity occurring as a result of the intervention and leading to an additional intervention (e.g. additional surgical procedure, blood transfusion). Minor morbidity: any morbidity occurring as a result of the intervention and which does not lead to major additional interventions. Failure of surgical approach: failure of the surgical approach used to enter the abdomen leading to change of approach (inluding extension of mini‐laparotomy incision). Failure of anaesthetic approach: failure of the anaesthetic approach used leading to change of approach.
In addition to the data on outcomes the following methodological details were extracted from the reports: Details on surgical methods: classification of surgical procedure, type of anaesthesia, setting (country, level of the health care institution, year). Number of randomised women, number of women not included in the study, exclusion after randomisation and losses to follow‐up. Method of randomisation and concealment of allocation
Data on outcomes: Major and minor morbidity, intra‐ and postoperative conditions, death, failure of surgical approach, complaints have been extracted. Data on failure (pregnancy) has not been extracted as it is assumed this would be primarily influenced by the technique used to interrupt tubal patency. Heterogeneity between studies has been explored for each outcome. If significant heterogeneity (p<0.1) between studies was detected, reasons for that were explored, including setting (developing and industrialised countries), year of the study, use of a camera during laparoscopy, single or multiple incisions, selection of women and expertise of the surgeon.
Results expressed as cumulative incidence were combined using the methods available in RevMan.
Results
Description of studies
Four trials of minilaparotomy compared to laparoscopy including a total of 1911 women, one trial of minilaparotomy compared to culdoscopy including 395 women and one trial evaluating minilaparotomy, laparoscopy and culdoscopy in 295 women met the criteria for inclusion in the review. In this meta‐analysis, no statistically significant heterogeneity was detected for any of the outcomes.
Modified Pomeroy technique (ligation and excision) for tubal occlusion was used during culdoscopy and minilaparotomy in all trials but one, where the surgeons used Hulka clips [Letchworth 1980]. Laparoscopic sterilisation was performed by coagulation in 3 trials (cauterisation as described by Wheeless (Wheeless 1992) in 2 trials and in 1 trial electrocoagulation was not further specified ) and either Hulka clips or Pomeroy method in 1 trial. All but two trials mentioned that the physicians performing the sterilisations were experienced surgeons and not trainees [Meyer 1976, Taner 1994]. WHO conducted a multicentre study in 7 developing country and 3 developed country centres. Eight centres compared minilaparotomy with laparoscopy and 2 compared minilaparotomy with culdoscopy. The two comparisons were conducted at different sites with different sample sizes and were therefore in the review included as different trials [WHO A 1982 and WHO B 1982]. The same inclusion criteria were used for both trials and outcomes were reported as major and minor complications, pregnancies, technical problems and women's complaints. The women were discharged usually after 8 hours and follow‐up was scheduled at 1 week and 6 weeks post‐operatively. In the study [WHO A 1982] 1827 women were recruited (912 minilaparotomy and 915 laparoscopy). The post‐randomisation exclusion rate was about 12% (121 women) in the minilaparotomy group and about 10% (96 women) in the laparoscopy group due to protocol violations (mostly because of inclusion of patients with subumbilical scar, which was an exclusion criteria). There were important differences in baseline characteristics mainly due to one centre (Bangkok) where women in the laparoscopy group were older, had more living children and had been married longer. Also, women in the minilaparotomy group were lighter and had a lower ponderal index, mainly due to the contribution of two centres (Bangkok, Havana). These differences were statistically significant for the Bangkok centre. In the three developed country centres (London, Los Angeles, Sydney) all operations were performed under general anaesthesia, whereas in two developing country centres (Bangkok, Seoul) local anaesthesia was used for both procedures. In Havana and Singapore all patients in the laparoscopy group received general aneasthesia and most minilaparotomy procedures were done under spinal/epidural anaesthesia. In Santiago all minilaparotomy cases were performed under spinal, all laparoscopy cases under local anaesthesia. In all centres sedatives for pre‐medication were used.
In the study [WHO B 1982] 400 women were randomised (200 minilaparotomy and 200 culdoscopy) and 5 women were excluded after randomisation because of protocol violation (4 minilaparotomy, 1 culdoscopy). All operations were performed under local anaesthesia. It is somehow not clear if this trial was conducted only in one centre (Manila).
In the trial conducted by Letchworth [Letchworth 1980] 200 women were randomised to either minilaparotomy or laparoscopy (Steptoe technique). Three women (1 minilaparotomy, 2 laparoscopy) were excluded after randomisation due to protocol violation. Main outcome measures were duration of operation and hospitalisation, post operative pain and analgesia use. The women were usually discharged the next day after the operation and contacted 14 days later for a follow‐up questioning. No baseline data comparing the two groups were reported.
In the trial of Meyer [Meyer 1976] 60 women were randomised, 30 to the minilaparotomy group and 30 to the laparoscopy group. Main outcome measures were serious complications and duration of operation. All operations were performed on an outpatient basis and women were discharged after 6 hours. All but four of the laparoscopic procedures were performed under local anaesthesia. In the minilaparotomy group, after 3 unsuccessful attempts using local anaesthesia all operations were performed under general anaesthesia. Codeine was prescribed routinely for women undergoing minilaparotomy.
In the study of Sitompul [Sitompul 1984] an equal number of women were randomly allocated to three groups (100 for minilaparotomy, laparoscopy and culdoscopy), 5 women were excluded after randomisation (3 minilaparotomy, 2 laparoscopy). All women had terminated their last pregnancy at least 6 weeks prior to sterilisation.
In the trial of Taner [Taner 1994], 24 women were randomised to minilaparotomy and 20 to laparoscopy. Four women in the laparoscopy group and 2 women in the minilaparotomy group underwent 1st trimester termination of pregnancy at the same time.
Surgical incision for minilaparotomy was described in 3 studies as transverse suprapubic incision [Letchworth 1980, Meyer 1976, Sitompul 1984]. Laparoscopy was performed by using 3 trocar technique in one study [Taner 1994] and an one hole incision in two studies [Meyer 1976, Sitompul 1984]. No data on the type and amount of gas insufflated during laparoscopy were reported.
Risk of bias in included studies
Three trials [ WHO A 1982, WHO B 1982, Meyer 1976] received an A allocation concealment score, based on adequate concealment prior to randomisation. The three remaining trials received a score of ´B´ for unclear methods of randomisation and of concealment of allocation.
The two WHO trials [WHO A 1982 and WHO B 1982] used random allocation by envelope system generated centrally by WHO. No further information could be obtained from the trialists as the system used could not be retrieved. However, the significant baseline differences in centres in Bangkok and Havana suggest that aversion of randomisation may have taken place. Meyer [Meyer 1976] used sealed, opaque envelopes randomly drawn with no consecutive numbering. In the Letchworth [Letchworth 1980] trial the allocation took place on the day of surgery when patients were randomly selected for either minilaparotomy or laparoscopy and all patients were operated on by one of the authors. Three patients were excluded after randomisation because the operation has been performed by a surgeon other than the authors. Sitompul [Sitompul 1984] and Taner [Taner 1994] only mentioned random allocation to two groups. Due to the type of interventions evaluated blinding after randomisation was not possible and is therefore not considered for the evaluation of the methodological quality for this review.
Effects of interventions
There were no cases of operative mortality in the two trials reporting this outcome [WHO A 1982 and WHO B 1982]. Minilaparotomy vs laparoscopy: There was no difference in major morbidity between the two groups. There were statistically significant fewer cases in the laparoscopy group having total minor morbidity (Peto OR 1.89; 95% CI 1.38,2.59) and minor vascular injuries (Peto OR 2.06; 95% CI 1.18, 3.59). These results are mainly based on one multicentre trial [WHO A 1982] where one centre reported excess minor bleeding during minilaparotomy. If data from that centre were excluded no difference between the groups remained. Wound infection or haematoma (Peto OR 2.40; 95% CI 1.47,3.92) were reported only in the multicentre trial [WHO A 1982] and were significantly less in the laparoscopy group (Peto OR 2.40; 95% CI 1.47,3.92), but again this was mainly due to one centre and by excluding this centre's data there was no difference between the groups. Failure of anaesthetic approach occurred more often in the minilaparotomy group, but this was based on the results of one trial with a small sample size. Duration of operation was about 5 minutes shorter in the laparoscopy group (WMD 5.39; 95% CI 4.55, 6.22). Postoperative abdominal pain (Peto OR 4.19; 95% CI 3.13, 5.61), analgesic use (Peto OR 3.33; 95% CI 1.89, 5.88) and minor complaints at 4‐6 weeks follow‐up (Peto OR 1.96; 95% CI 1.08, 3.57) were significantly increased in the minilaparotomy group.
Minilaparotomy vs culdoscopy: Women undergoing culdoscopy had more major morbidity than women for whom minilaparotomy was performed (Peto OR 0.14; 95% CI 0.02, 0.98) in the only trial included in this review. Minor morbidity: Wound infection or haematoma occurred significantly more often in the minilaparotomy group in the one trial reporting this outcome (Peto OR 7.66; 95% CI 1.32, 44.62). Duration of operation was significantly shorter in women undergoing culdoscopy (about 5 minutes)(WMD 4.91; 95% CI 3.82, 6.01). There was a trend for women in the culdoscopy group to have a change in surgical approach (change from culdoscopy to laparoscopy/laparotomy). Significantly less women in the culdoscopy group reported postoperative abdominal pain (Peto OR 2.03; 95% CI 1.16, 3.55).
Laparoscopy vs culdoscopy: In the one trial comparing the two interventions [Sitompul 1984] there were no significant differences between the groups with regard to major morbidity (1 woman in the laparoscopy group received blood transfusion and 1 woman in the culdoscopy group developed a pelvic abscess which resulted in hysterectomy). Significantly more women suffered minor morbidities in the culdoscopy group compared to the laparoscopy group (Peto OR 0.20; 95% CI 0.05, 0.77). No data on surgical failures were reported. There was no difference between groups in duration of operation but significantly more women in the culdoscopy group were hospitalised for more than 24 hours (Peto OR 0.20; 95% CI 0.05, 0.77). In one trial [Sitompul 1984] one pregnancy occurred in the culdoscopy group during the four years of follow‐up.
Discussion
This systematic review does not report on efficacy (pregnancy). It is more likely that the technique performed to interrupt tubal patency influences this outcome and is therefore considered in another review. We think that safety issues, hospital stay and costs are the important factors in deciding to choose one method over the other. The results of this systematic review must be interpreted in the light that all of the results are based on a limited number of participants or on one trial only. The trials included in the review have inadequate sample sizes to detect differences in rare outcomes, such as mortality and major morbidity. The results of minilaparotomy versus laparoscopy were dominated by one multicentre trial [WHO A 1982]. Management for the comparison groups, with regard to anaesthesia and post operative care, was mainly according to the centres´ local routine. Using epidural anaesthesia for minilaparotomy as compared to general anaesthesia for laparoscopy might have led to the higher number of immediate pain reported from one centre. Also, one centre used prophylactic antibiotics for all women in the minilaparotomy group and was the only centre that reported less incisional complications in that group as compared to the laparoscopy group. With regard to minor vascular injuries and wound haematoma/infection occurring significantly more often in the minilaparotomy group, again the weight lies on two centres. The four trials included had a follow up period of 4‐6 weeks and only one presented data after a follow‐up of 4 years [Sitompul 1984]. Therefore, no long‐term assessment of complications can be made. The small trend of failure of anaesthetic approach occurred more frequently in the minilaparotomy group. Although this is based on the results of one small trial only, the results may reflect a greater difficulty in obtaining adequate analgesia with minilaparotomy. Culdoscopy seems to be associated with more complications than either minilaparotomy or laparoscopy without any obvious advantages except for less immediate postoperative pain. With pooling data from approximately 1000 women in each group the review is underpowered in its ability to detect differences in operative mortality. Life threatening events or death were not observed in a cohort of 3500 women undergoing interval laparoscopic sterilizations (Destefano 1983). In this study, less than 2 % of women undergoing laparoscopy experienced intra or postoperative complications. Another limitation of the review is the relatively short follow‐up (maximum 1 year) of most included studies. Possible long‐term consequences may differ between laparoscopy and minilaparotomy. With laparoscopy, the likelihood of diagnosing incidental pathologies such as endometriosis and uterine fibroids may be higher and hence lead to higher incidence of subsequent gynaecological interventions. Women who underwent sterilisation were 4 times more likely to have a hysterectomy than women whose husbands had vasectomy (Hillis 1998). Unintended laparotomy for attempted laparoscopy for tubal sterilisation was significantly increased in women with previous abdominal or pelvic surgery (Franks 1987) and was found to be the most frequent complication during interval laparoscopic sterilisation (Destefano 1983). These findings may be important in view of counseling women regarding the procedure and the associated risks involved. Laparoscopy was found to have statistically significant shorter duration of operation. However, the 5 minutes reduction in operating time might not be of great clinical importance. Pregnancy was not included as an outcome in this review because the efficacy of the procedure is related to the tubal occlusion technique rather than the abdominal entry method. Although certain tubal occlusion techniques may be used more frequently with laparoscopy and vice versa, the actual abdominal entry technique should not determine the efficacy of the procedure. Considering factors discussed above the review's main objective was to identify major and minor operative and postoperative complications, costs and hospital stay. Data on women's satisfaction with the procedures were not available from the trials retrieved. Overall, culdoscopy seems to be associated with poorer results and without obvious advantages and therefore should not be recommended. Regarding minilaparotomy and laparoscopy, the decision‐making should be a trade‐off between advantages and disadvantages of each procedure. The experience of the surgeon is important especially with laparoscopy. The purchase and maintenance of laparoscopy equipment and the training required may be limiting factors in centres with limited resources. However, laparoscopy seems to be associated with fewer instances of minor operative morbidity and has a further advantage of minimal or no scarring and less postoperative discomfort.
Authors' conclusions
Implications for practice.
Major morbidity seems to be a rare outcome for laparoscopy and minilaparotomy. The decision which method to chose should be a multifactorial one, depending on the setting, the surgeons experience and the woman's preference. Laparoscopy is a preferred method in many developed country settings. Culdoscopy is not recommended by various international organisations (IPPF 1999, WFHAAVSC 1988) as it has been associated with high rates of complications, which is in agreement with the limited data from randomised controlled trials.
Implications for research.
Data on long term outcomes are available form cohort studies, rather than randomised controlled trials. Minilaparotomy and laparoscopy are safe procedures with short hospital stay. Further comparative trials are not considered to be high priority for research.
What's new
| Date | Event | Description |
|---|---|---|
| 16 February 2009 | Amended | text edited |
| 2 September 2008 | New search has been performed | no new trials were identified |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 15 April 2008 | Amended | Converted to new review format. |
| 26 May 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Herbert B Peterson for his contribution to this review.
Data and analyses
Comparison 1. Minilaparotomy vs laparoscopy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative mortality | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major morbidity (total) | 4 | 2062 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.80 [0.78, 4.17] |
| 3 Major morbidity (details) | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 bowel injury, requiring additional surgery | 2 | 1807 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.06] |
| 3.2 bladder injury, requiring additional surgery | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [0.48, 122.70] |
| 3.3 vascular injury, requiring transfusion or additional surgery | 2 | 1805 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.06, 16.36] |
| 3.4 other operative morbidity, requiring additional surgery | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [0.48, 122.70] |
| 3.5 cardiac arrest | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [0.15, 386.03] |
| 3.6 pulmonary embolism | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 PID requiring hospitalisation | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.26, 2.82] |
| 3.8 re‐hospitalisation | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [0.48, 122.70] |
| 3.9 other anaesthetic morbidity | 2 | 1807 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [0.15, 386.03] |
| 4 Minor morbidity (total) | 5 | 2106 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.89 [1.38, 2.59] |
| 5 Minor morbidity (details) | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 bowel injury with no additional surgery | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.75] |
| 5.2 bladder injury with no additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 vascular injury, not requiring transfusion or additional surgery | 2 | 1670 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.06 [1.18, 3.59] |
| 5.4 other minor intraabdominal injuries | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.45 [0.55, 10.81] |
| 5.5 PID, no hospitalisation | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.18, 3.43] |
| 5.6 urinary tract infection | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.35, 1.81] |
| 5.7 wound infection or haematoma | 2 | 1654 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [1.42, 3.75] |
| 5.8 post‐op temperature > 38°C | 2 | 392 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.26 [0.64, 7.93] |
| 6 Failure of surgical approach | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.16, 1.42] |
| 7 Failure of anaesthetic approach | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.93 [0.79, 79.26] |
| 8 Duration of operation | 3 | 436 | Mean Difference (IV, Fixed, 95% CI) | 5.34 [4.52, 6.16] |
| 9 Hospital stay > 24 hours | 4 | 496 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 23.97 [8.71, 65.92] |
| 10 Complaints | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 abdominal pain post‐op (<24h) | 2 | 1805 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.19 [3.13, 5.61] |
| 10.2 analgesic use post‐op | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.33 [1.89, 5.88] |
| 10.3 persistent pain post‐op | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.81, 1.47] |
| 10.4 women`s satisfaction | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 other minor complaints at follow‐up | 2 | 1756 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [1.08, 3.57] |
1.1. Analysis.
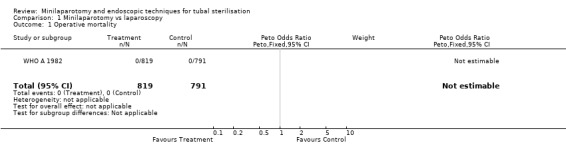
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 1 Operative mortality.
1.2. Analysis.
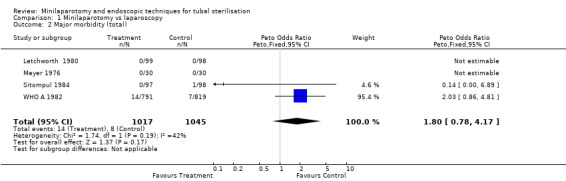
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 2 Major morbidity (total).
1.3. Analysis.
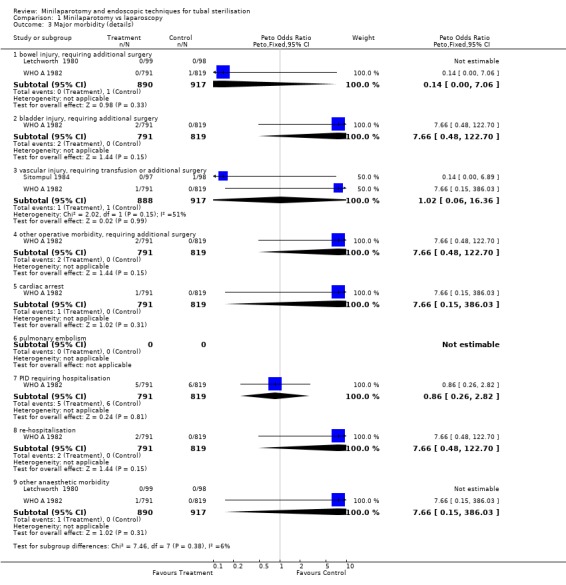
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 3 Major morbidity (details).
1.4. Analysis.
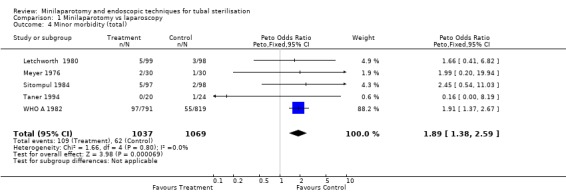
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 4 Minor morbidity (total).
1.5. Analysis.
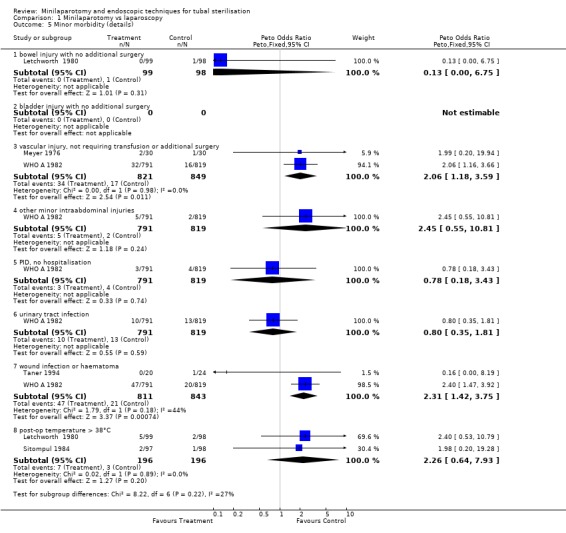
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 5 Minor morbidity (details).
1.6. Analysis.
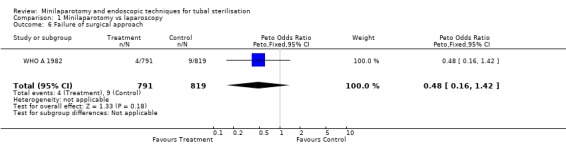
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 6 Failure of surgical approach.
1.7. Analysis.
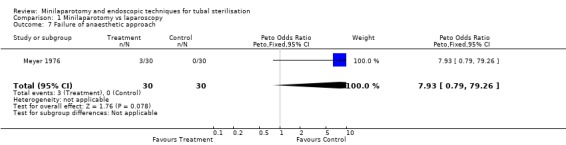
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 7 Failure of anaesthetic approach.
1.8. Analysis.
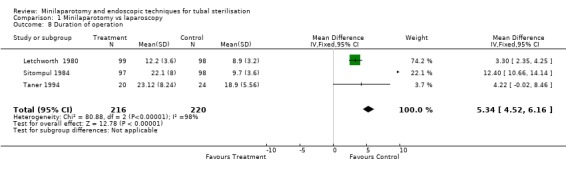
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 8 Duration of operation.
1.9. Analysis.
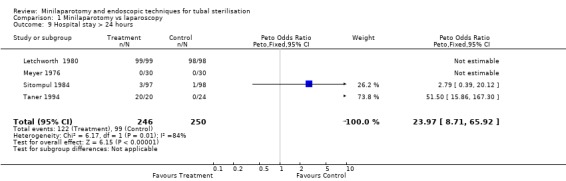
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 9 Hospital stay > 24 hours.
1.10. Analysis.
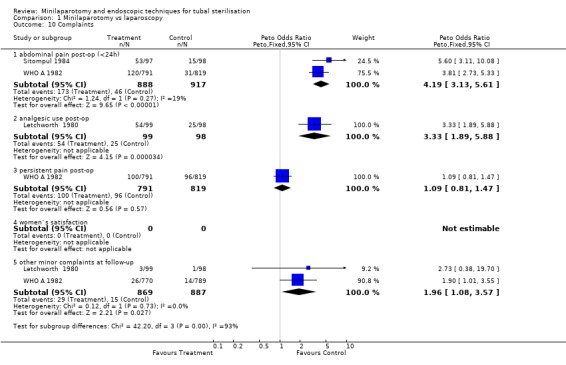
Comparison 1 Minilaparotomy vs laparoscopy, Outcome 10 Complaints.
Comparison 2. Minilaparotomy versus culdoscopy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative mortality | 1 | 395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major morbidity (total) | 2 | 592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.02, 0.98] |
| 3 Major morbidity (details) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 bowel injury, requiring additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 bladder injury, requiring additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 vascular injury, requiring transfusion or additional surgery | 2 | 592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.19] |
| 3.4 other operative morbidity, requiring additional surgery | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.03] |
| 3.5 cardiac arrest | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 pulmonary embolism | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 other anaesthetic morbidity | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 PID requiring hospitalisation | 1 | 395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.92] |
| 3.9 re‐hospitalisation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Minor morbidity (total) | 2 | 592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.39, 2.22] |
| 5 Minor morbidity (details) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 bowel injury with no additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 bladder injury with no additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 vascular injury, not requiring transfusion or additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 other minor intraabdominal injuries | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 PID, no hospitalisation | 1 | 395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.92] |
| 5.6 urinary tract infection | 1 | 395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.54 [0.47, 121.01] |
| 5.7 wound infection or haematoma | 1 | 395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [1.32, 44.61] |
| 5.8 post‐op temperature >38°C | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [0.21, 19.68] |
| 6 Failure of surgical approach | 1 | 395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 1.32] |
| 7 Duration of operation | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | 4.91 [3.82, 6.01] |
| 8 Hospital stay >24 hours | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.12, 1.33] |
| 9 Complaints | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 abdominal pain post‐op (<24h) | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [1.16, 3.55] |
| 9.2 analgesic use post‐op | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 persistent pain post‐op | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 women`s satisfaction | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 other minor complaints at follow‐up | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
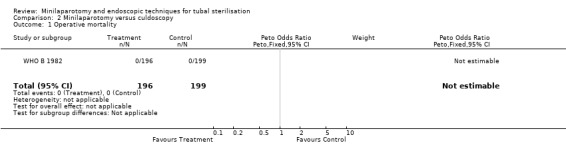
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 1 Operative mortality.
2.2. Analysis.
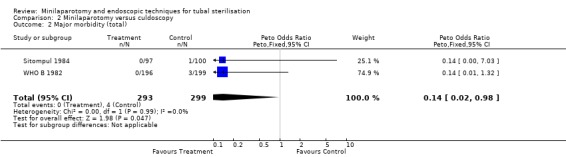
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 2 Major morbidity (total).
2.3. Analysis.
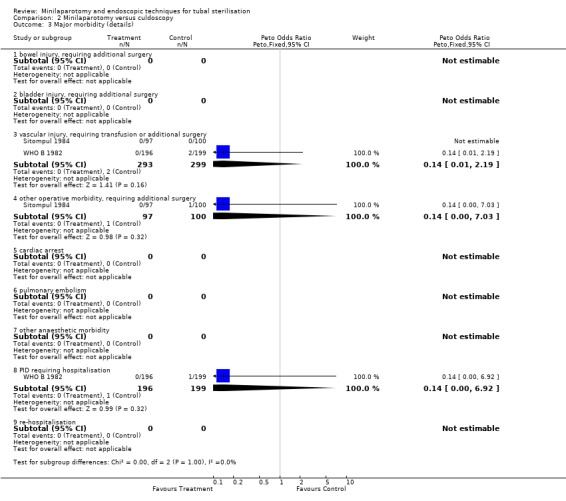
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 3 Major morbidity (details).
2.4. Analysis.
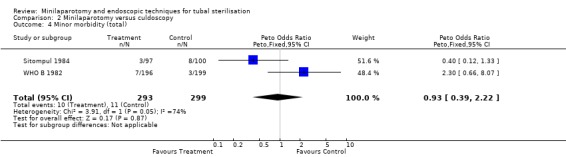
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 4 Minor morbidity (total).
2.5. Analysis.
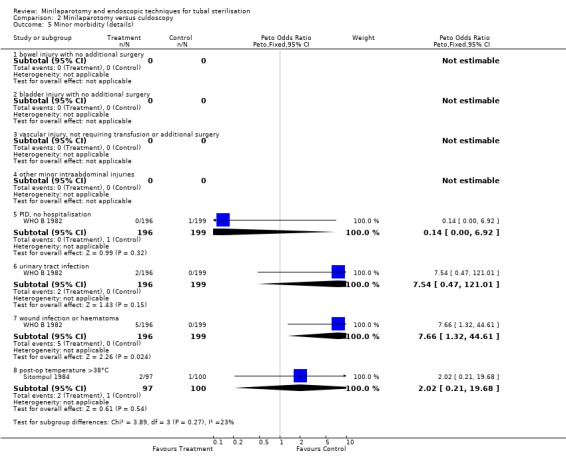
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 5 Minor morbidity (details).
2.6. Analysis.
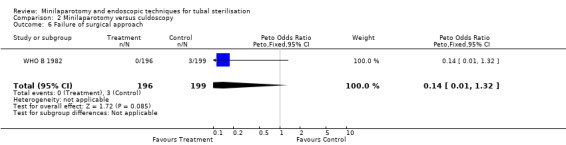
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 6 Failure of surgical approach.
2.7. Analysis.
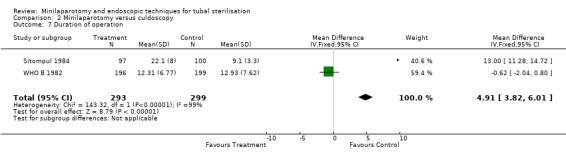
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 7 Duration of operation.
2.8. Analysis.
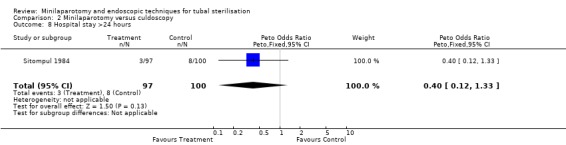
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 8 Hospital stay >24 hours.
2.9. Analysis.
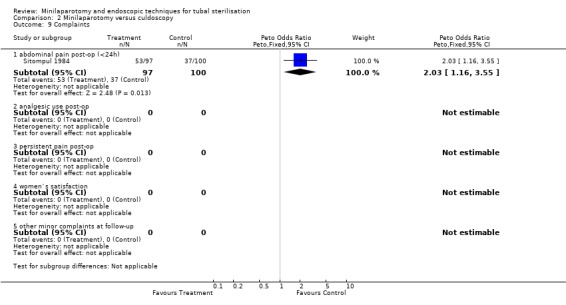
Comparison 2 Minilaparotomy versus culdoscopy, Outcome 9 Complaints.
Comparison 3. Laparoscopy vs culdoscopy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major morbidity (total) | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.06, 16.43] |
| 2 Major morbidity (details) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 bowel injury, requiring additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 bladder injury, requiring additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 vascular injury, requiring transfusion or additional surgery | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.54 [0.15, 380.14] |
| 2.4 other operative morbidity requiring additional surgery | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.96] |
| 2.5 cardiac arrest | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 pulmonary embolism | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 other anaesthetic morbidity | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 PID requiring hospitalisation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 re‐hospitalisation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Minor morbidity (total) | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.05, 0.77] |
| 4 Minor morbidity (details) | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.06, 16.43] |
| 4.1 bowel injury with no additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 bladder injury with no additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 vascular injury, not requiring transfusion or additional surgery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 other minor intraabdominal injuries | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 PID, no hospitalisation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 urinary tract infection | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 wound infection or haematoma | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 post‐op temperature >38°C | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.06, 16.43] |
| 5 Duration of operation | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.36, 1.56] |
| 6 Hospital stay >24 hours | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.05, 0.77] |
| 7 Complaints | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 abdominal pain post‐op (<24h) | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.17, 0.62] |
| 7.2 analgesic use post‐op | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 persistent pain post‐op | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 women`s satisfaction | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 other minor complaints at follow‐up | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
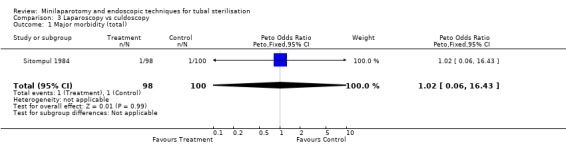
Comparison 3 Laparoscopy vs culdoscopy, Outcome 1 Major morbidity (total).
3.2. Analysis.
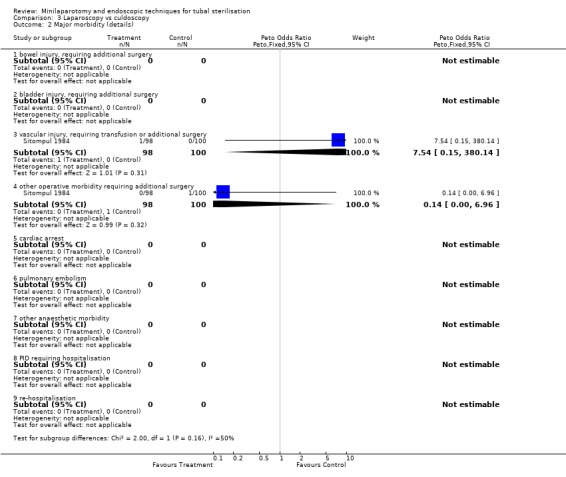
Comparison 3 Laparoscopy vs culdoscopy, Outcome 2 Major morbidity (details).
3.3. Analysis.
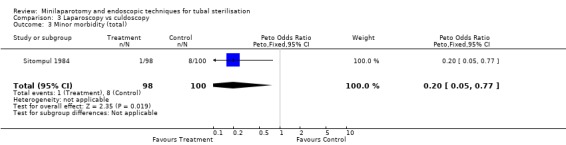
Comparison 3 Laparoscopy vs culdoscopy, Outcome 3 Minor morbidity (total).
3.4. Analysis.
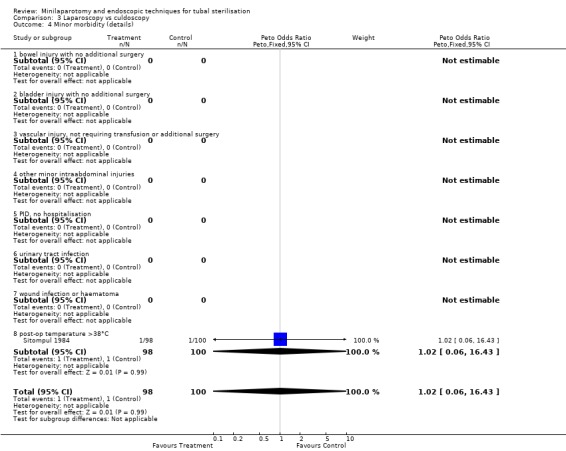
Comparison 3 Laparoscopy vs culdoscopy, Outcome 4 Minor morbidity (details).
3.5. Analysis.
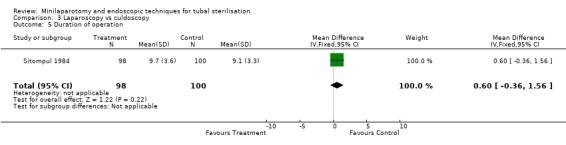
Comparison 3 Laparoscopy vs culdoscopy, Outcome 5 Duration of operation.
3.6. Analysis.
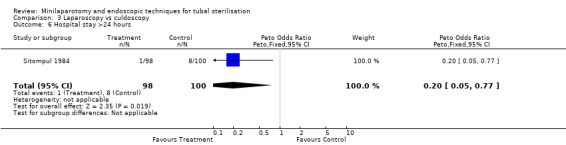
Comparison 3 Laparoscopy vs culdoscopy, Outcome 6 Hospital stay >24 hours.
3.7. Analysis.
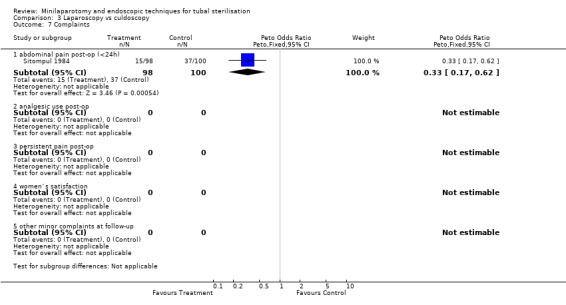
Comparison 3 Laparoscopy vs culdoscopy, Outcome 7 Complaints.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Letchworth 1980.
| Methods | Prospective randomly selected | |
| Participants | 200 women in Southampton/UK requesting sterilisation. Women with previous pelvic surgery were excluded from the study. | |
| Interventions | Minilaparotomy versus laparoscopy using modified Hulka Clemens clip. | |
| Outcomes | Difficulties at surgery, duration of operation and hospital stay, analgesia use, post operative pain | |
| Notes | All operations were performed by experienced surgeons (authors only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Meyer 1976.
| Methods | Randomisation by sealed, opaque envelopes | |
| Participants | 60 women at the Johns Hopkins Gynecologic Clinic, USA requesting tubal sterilisation | |
| Interventions | Minilaparotomy and Pomeroy tubal ligation versus one incision laparoscopy and 3 burn modification of Wheeless and Thompson. | |
| Outcomes | Major morbidity, operation times, | |
| Notes | Minilaparotomy: after 3 unsuccessful attempts with local anaesthesia among the first 6 women, all patients received general anesthesia. Codeine was prescribed routinely for these patients shortly after study begin Laparoscoopy: all but 4 patients received local anesthesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sitompul 1984.
| Methods | Not specified Random allocation into equal groups | |
| Participants | 300 women requesting sterilisation at the University Hospital in Medan, Indonesia. | |
| Interventions | 1)Minilaparotomy with modified Pomeroy technique 2) Laparoscopy with 1 hole incision and cauterisation as described by Wheeless 3) Culdoscopy with modified Pomeroy method all under local anaesthesia and 10mg Valium intravenous | |
| Outcomes | Complaints during operation, operation times, hospitalisation, post‐op complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Taner 1994.
| Methods | Random selection | |
| Participants | 44 women requesting sterilisation. | |
| Interventions | Minilaparotomy vs laparoscopy with 3 puncture method) and Pomeroy method for sterilisation. General anaesthesia for all women. | |
| Outcomes | Duration of operation, length of hospital stay, length of excised tube, minor morbidity, failure rate | |
| Notes | No inclusion/exclusion criteria were reported. Discussion refers mostly to other studies done in that field. Company providing equipment for laparoscopy mentioned, possible conflict of interest not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
WHO A 1982.
| Methods | Multicenter, randomised study. Random allocation by envelopes centrally generated by WHO. | |
| Participants | Healthy women with at least one child and eligible for both interventions. Exclusion criteria were pelvic pathologies, history of previous PID or peritonitis, scar below the umbilicus or any condition which would increase the risk of any surgical procedure. Conducted in Bangkok, Havana, London, Los Angeles, Santiago, Seoul, Singapore, Sydney | |
| Interventions | Minilaparotomy and modified Pomeroy method versus laparoscopy and electrocoagulation for tubal sterilisation | |
| Outcomes | Major: excessive bleeding requiring transfusion or additional surgery, injury to other organs requiring additional surgery, PID requiring hospitalisation, incision‐related problems requiring re‐hospitalization or additional operation Minor: bloodloss <50 ml, PID, injuries, incision‐ all not requiring hospitalisation or additional surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
WHO B 1982.
| Methods | Randomly allocated by envelope system generated by WHO. | |
| Participants | 400 healthy women with at least 1 leaving child and fulfilling the national eligibility criteria; conducted in Manila | |
| Interventions | Minilaparotomy versus culdoscopy, using modified Pomeroy method. | |
| Outcomes | Major and minor complications as defined by the authors | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Sherman 1984 | This is a retrospective study. |
| Tiras 2000 | comparion between two types of laparoscopy. |
Contributions of authors
RK, MB and DW wrote the protocol, conducted the literature search, critically appraised the studies and did the data extraction. RK wrote the manuscript, GdC and AC critically commented on the review.
Sources of support
Internal sources
University Hospital of Geneva, Department of Obstetrics and Gynaecology, Switzerland.
Geneva Foundation for Medical Education and Research, Switzerland.
External sources
No sources of support supplied
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Letchworth 1980 {published data only}
- Letchworth AT, Kane JL, Noble AD. Laparoscopy or laparotomy for sterilization of women. Obstet Gynecol 1980;56(1):119‐121. [PubMed] [Google Scholar]
Meyer 1976 {published data only}
- Meyer JH, King TM. Advances in Female Sterilisation Techniques. Hagerstown, Maryland, 1976. journal 1976;1:Advances in Female Sterilisation Techniques. Hagerstown, Maryland, 1976. Harper & Row, Publishers. [Google Scholar]
Sitompul 1984 {published data only}
- Sitompul H, Lun KC, Lumbanraja M, Kaban RM, Albar E, Simanjuntak P, Hanafiah MJ. Comparison of three types of tubal sterilisation: the Medan experience. Contraception 1984;29(1):55‐63. [DOI] [PubMed] [Google Scholar]
Taner 1994 {published data only}
- Taner CE, Aban M, Yilmaz N, Senturk N, Toy E. Pomeroy tubal ligation by laparoscopy and minilaparotomy. Adv Contracep 1994;10:151‐155. [DOI] [PubMed] [Google Scholar]
WHO A 1982 {published data only}
- World Health Organization, Task Force on Female Sterilization, Special programme of Research, Development and Research Training in Human Reproduction. Minilaparotomy or laparoscopy for sterilization. Am J Obstet Gynecol 1982;143:645‐652. [PubMed] [Google Scholar]
WHO B 1982 {published data only}
- World Health Organization. Task Force on Female Sterilization, Special Programme of Research, Development and Research Training in Human Reproduction. Randomized comparative study of culdoscopy and minilaparotomy for surgical contraception in women. Contraception 1982;26(6):587‐593. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Sherman 1984 {published data only}
- Sherman PA, Burigo JA. Comparison of laparoscopic Falope‐ring and minilaparotomy sterilization. Obstetrics & Gynecology 1984;63:71‐74. [PubMed] [Google Scholar]
Tiras 2000 {published data only}
Additional references
Destefano 1983
- Destefano F, Greenspan JR, Dicker RC, Peterson HB, et al. Complications of interval laparoscopic tubal sterilisation. journal 1983;61:153‐158. [PubMed] [Google Scholar]
Franks 1987
- Franks AL, Kendrick JS, Peterson HB. Unintended laparotomy associated with laparoscopic tubal sterilization. American Journal of Obstetrics and Gynecology 1987;157:1102‐1105. [DOI] [PubMed] [Google Scholar]
Hillis 1998
- Hillis SD, Marchbanks PA, Taylor LR, Peterson HB. Higher hysterectomy risk for sterilized than nonsterilized women: findings from the US Collaborative Review of Sterilization. The US Collaborative Review of Sterilization Working Group. Obstetrics Gynecology 1998;91:241‐246. [DOI] [PubMed] [Google Scholar]
Limpaphayom 1991
- Limpaphayom K. Sterilization. Curr Opin Obstet Gynecol 1991;3:501‐509. [PubMed] [Google Scholar]
Osathanondh 1974
- Osathanondh V. Suprapubic mini‐laparotomy, uterine elevation technique: simple, inexpensive and out‐patient procedure for interval female sterilization. Contraception 1974;10:251‐262. [DOI] [PubMed] [Google Scholar]
Uchida 1975
- Uchida H. Uchida tubal sterilization. Am J Obstet Gynecol 1975;121:153‐158. [DOI] [PubMed] [Google Scholar]
Wheeless 1972
- Wheeless CR. Outpatient laparoscope sterilization under local anesthesia. Obstet Gynecol 1972;39:767‐770. [PubMed] [Google Scholar]
Wheeless 1992
- Wheeless CR Jr. Tubal sterilization. In: Te Linde's Operative gynecology. [Tubal sterilization]. In: Thompson JD, Rock JA editor(s). Book. Vol. 1, Philadelphia: JB Lippincott, 1992:343‐59. [Google Scholar]
WHO 1992
- World Health Organization. Female sterilization: a guide to provision of services. journal 1992;1:a guide to provision of services. WHO, Geneva, 1992. [Google Scholar]


