Abstract
Background
Reduced perfusion of organs such as the brain, heart, kidneys and the gastrointestinal tract may lead to acute dysfunction and be associated with permanent injury. Various strategies have been used to provide cardiovascular support to preterm infants including inotropes, corticosteroids and volume expansion.
Objectives
To determine the effect of early volume expansion on morbidity and mortality in very preterm infants. If volume expansion is effective, to determine the type of volume expansion that is most effective.
Search methods
The standard search strategy of the Neonatal Review Group was used. Updated searches were performed of the Cochrane Central Register of Controlled Trials (Issue 3, 2008), MEDLINE (1996 ‐ July 2008), EMBASE (1980 ‐ July 2008), previous reviews including cross references, abstracts and conferences.
Selection criteria
Randomised trials of early volume expansion with normal saline, fresh frozen plasma, albumin, plasma substitutes or blood compared to no treatment or another form of volume expansion in preterm infants ≦ 32 weeks gestation or ≦ 1500 g were included. Volume expansion was defined as at least 10 ml/kg given in the first 72 hours after birth.
Data collection and analysis
Standard methods of the Neonatal Review Group with use of relative risk (RR), risk difference (RD) and weighted mean difference (WMD). The fixed effects model was used for meta‐analysis. Data from individual studies were only eligible for inclusion if a least 80% of infants were reported for that outcome.
Main results
Eight studies were included. Five studies compared volume to no treatment. Most studies enrolled very preterm infants on the basis of gestation or birthweight. Two studies comparing different types of volume expansion enrolled very preterm infants with hypotension. No study enrolled infants on the basis of low blood flow. One study examined the effect of volume expansion on blood flow, but evaluated normotensive very preterm infants.
Four studies comparing volume expansion and no treatment with a total of 940 very preterm infants reported no significant difference in mortality (typical RR 1.11, 95% CI 0.88, 1.40). The large NNNI 1996 study reported no significant difference in severe disability (RR 0.80, 95% CI 0.52, 1.23), cerebral palsy (RR 0.76, 95% CI 0.48, 1.20) and combined death or severe disability (RR 1.00, 95% CI 0.80, 1.24). Although one small study (Beverley 1985) reported reduced P/IVH with volume expansion, this was not supported by any other study. No significant difference was reported in grade 3‐4 P/IVH and combined death or grade 3‐4 P/IVH. One study (NNNI 1996) reported no significant difference in the incidence of hypotension. The finding of decreased necrotising enterocolitis and increased sepsis in infants who received fresh frozen plasma compared to no treatment in one study should be treated with caution. No significant differences in mortality or disability were found in this study. Comparing albumin and saline in hypotensive infants, one study (Lynch 2008) reported a significant increase in mean BP and reduced incidence of treatment failure (persistent hypotension treated with dopamine). The other study (So 1997) and the meta‐analysis of the two studies found no significant difference in treatment failure (typical RR 0.76, 95% CI 0.54, 1.07) or in any other clinical outcome.
Authors' conclusions
There is no evidence from randomised trials to support the routine use of early volume expansion in very preterm infants without cardiovascular compromise. There is insufficient evidence to determine whether infants with cardiovascular compromise benefit from volume expansion. There is insufficient evidence to determine what type of volume expansion should be used in preterm infants (if at all) or to determine the benefit of using early red cell transfusions. The significance of the finding of a significant increase in blood pressure in hypotensive preterm infants in one trial comparing albumin and saline is unclear, but the overall meta‐analyses found no other significant clinical benefit in using albumin compared to saline.
Plain language summary
Early volume expansion for prevention of morbidity and mortality in very preterm infants
Early volume expansion for very preterm babies has not been shown to improve their outcomes but more research is needed. Low blood pressure and blood flow have been linked to brain injury in preterm babies. They can also cause permanent injury to other organs and developmental problems. One way of trying to increase the blood pressure and flow of blood is to increase the amount of fluid in the blood stream (volume expansion). This can be done with products such as albumin (a protein) and salt solutions. The review of evidence from trials found that volume expansion has not been shown to improve outcomes for preterm babies. More research is needed.
Background
Reduced perfusion of organs such as the brain, heart, kidneys and the gastrointestinal tract may lead to acute dysfunction and be associated with permanent injury. Twenty percent of surviving babies born very premature do so with some degree of neurodevelopmental disability (Lorenz 1998). Low systemic blood pressure (BP) and systemic blood flow (SBF) have both been linked to cerebral injury in preterm infants. Systemic arterial hypotension is associated with peri/intraventricular haemorrhage (P/IVH), ischaemic cerebral lesions and poor long‐term neurodevelopmental outcome (Miall‐Allen 1987; Goldstein 1995; Low 1993). Low upper body blood flow (Kluckow 2000; Osborn 2003; Miletin 2008) and low cerebral blood flow (Meek 1999) on the first day after birth are associated with late P/IVH. In addition, low upper body blood flow on the first day (as measured by flow in the superior vena cava) was associated with a significant increase in mortality (Osborn 2003; Kluckow 2000), necrotising enterocolitis (Osborn 2007) and subsequent neurodevelopmental impairment (Hunt 2004; Osborn 2007) in infants born < 30 weeks' gestation.
Clinical features suggesting reduced perfusion include low BP, reduced cutaneous perfusion and metabolic acidosis. However, systemic arterial pressure has been shown to be poorly correlated with SBF as measured by Doppler ultrasound in preterm infants (Kluckow 1996; Kluckow 2000; Osborn 2004a). In addition, clinical measures of hypovolaemia including systemic hypotension have been found to be poorly correlated to blood volume in premature infants (Barr 1977; Bauer 1993).
Various therapeutic strategies have been used to provide cardiovascular support to preterm infants including inotropes, corticosteroids and volume expansion. Most strategies have targeted low BP using inotropes such as dopamine vs. dobutamine (Greenough 1993; Hentschel 1995; Klarr 1994; Roze 1993), dopamine vs. adrenaline (Pellicer 2005; Valverde 2006), corticosteroids (Gaissmaier 1999), and corticosteroids vs. dopamine (Bourchier 1997). Trials of dopamine vs. dobutamine in very preterm infants with systemic hypotension have not found a difference between these inotropes at preventing mortality and P/IVH. Dopamine was more effective than dobutamine at treating systemic hypotension in very preterm infants (Subhedar 2003). Despite an increase in systemic BP, dopamine was shown to reduce aortic blood flow in one study (Roze 1993). In another study in very preterm infants with low SBF, dobutamine which produced little change in BP but a significantly greater increase in SBF than dopamine, despite dopamine resulting in a significantly greater increase in BP (Osborn 2002). However, there was no significant difference in long‐term outcomes (Osborn 2007; Osborn 2007a). Strategies to correct systemic hypotension and hypovolaemia have also included volume expansion. Observational studies have found increases in cardiac output after albumin infusion in sick preterm infants (Pladys 1997) and a small increase in systemic BP in hypotensive preterm infants (Barr 1977; Bignall 1989). Short‐term increases in SBF have also been reported after saline infusion in preterm infants with low SBF (Osborn 2002). Observational studies have also reported an increase in P/IVH (Goldberg 1980) and chronic lung disease (Van Marter 1990) in preterm infants receiving volume expansion. A systematic review of randomised controlled trials of albumin infusions in critically ill patients including those with hypovolaemia, burns and hypoalbuminaemia found a significantly increased mortality for those patients receiving albumin compared to control (Alderson 2004).
The question addressed by this review is what is the evidence from randomised controlled trials for the use of early volume expansion to prevent mortality and morbidity in very preterm infants. In view of the difficulties of identifying infants with poor perfusion and hypovolaemia, subgroup analysis was planned according to method of diagnosis of poor perfusion (unselected preterm infants, preterm infants with clinical indicators of poor perfusion [low BP, reduced cutaneous perfusion and metabolic acidosis] and infants with ultrasound Doppler detected low blood flow). As there is evidence to suggest that late intraventricular haemorrhage is associated with systemic hypotension and low SBF in the first day of life (Miall‐Allen 1987; Goldstein 1995; Kluckow 2000; Osborn 2003), subgroup analysis was performed with the hypothesis that trials that used early volume expansion were more likely to prevent intraventricular haemorrhage. As different volume expanders have different effects, subgroup analysis was performed according to type of volume expansion used. This is an update of a previous Cochrane Review (Osborn 2001; Osborn 2004).
Objectives
To determine the effect of early volume expansion on morbidity and mortality in very preterm infants. Subgroup analysis was planned according to method of diagnosis of poor perfusion (unselected preterm infants, preterm infants with clinical indicators of poor perfusion and infants with ultrasound Doppler detected low blood flow), postnatal age of treatment and type of volume expansion used. In trials among such infants which compare two or more different types of infusate for volume expansion, to determine which type of volume expansion is more effective.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials that compare volume expansion with control (no treatment), and randomised controlled trials comparing two or more types of infusate for volume expansion. Trials with adequate randomisation and ≧ 80% follow‐up of participants were eligible for inclusion.
Types of participants
Very preterm infants born ≦ 32 weeks' gestation or ≦ 1500 g and enrolled and treated in the first 72 hours after birth. Trials were eligible if they enrolled either unselected preterm infants, preterm infants with clinically suspected poor perfusion (eg. low BP, poor cutaneous perfusion, or metabolic acidosis), or preterm infants with low blood flow (eg. determined by Doppler ultrasound). Low BP may be defined as BP either less than a specified percentile of a standard chart, a mean BP ≦ 30 mmHg in any preterm infant or a mean BP ≦ 1 mmHg per week of gestation.
Types of interventions
Volume expansion including normal saline, fresh frozen plasma, albumin, plasma substitute or blood. Volume expansion was defined as at least 10ml/kg additional volume above maintenance requirements given over less than 6 hours.
Types of outcome measures
Primary outcome measures included any of the following:
Neonatal mortality and mortality to discharge
Peri/intraventricular haemorrhage (any or severe grades)
Periventricular leucomalacia
Neurodevelopmental disability (either neurological abnormality including cerebral palsy, developmental delay or sensory impairment)
Secondary outcome measures included any of the following;
Use of inotropes (in first 72 hours)
Failure to correct low SBF (eg. Doppler ultrasound after volume expansion)
Failure to correct systemic hypotension (enrolment criteria used in trial)
Patent ductus arteriosus (PDA)
Renal impairment (creatinine ≧ 120 micromol/l, oliguria ≦ 0.5ml/kg/hour)
Airleak
Chronic lung disease (CLD) (at 28 postnatal days or near term postmenstrual age)
Proven necrotising enterocolitis
Retinopathy of prematurity (any stage and severe)
Planned subgroup analyses included the following identified subcategories:
Including only trials where volume expansion was given a) in the first day after birth, and b) in the first 12 hours after birth
According to type of volume expansion used (normal saline, fresh frozen plasma, albumin, plasma substitute or blood)
Comparing different types of volume expansion to determine which type volume expansion is most effective
According to whether trials enrolled :
Unselected preterm infants
Preterm infants with clinical indicators of poor perfusion (low BP, poor cutaneous perfusion or metabolic acidosis)
Preterm infants with ultrasound Doppler diagnosed low blood flow
All primary and secondary outcomes were included in subgroup analysis where available.
Search methods for identification of studies
An updated search was performed of Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 3, 2008), MEDLINE (1996 ‐ July 2008), EMBASE (1980 ‐ July 2008), previous reviews including cross references (all articles referenced), abstracts and conferences (Perinatal Society of Australia and New Zealand, and Pediatric Academic Societies and American Academy of Pediatrics meetings 2004 ‐ 2008). The previously documented search strategy was expanded to include MeSH terms "[polygeline or albumins]" and text words "[starch or albumin or albumen or gelofusine or plasma]"
This review updates a previous version (Osborn 2004). For all versions, the standard search strategy of the Neonatal Review Group was used. The original review (Osborn 2001) included searches of the Oxford Database of Perinatal Trials. The January 2004 update included searches of CENTRAL (The Cochrane Library Issue 1, 2004), MEDLINE (1996 ‐ January 2004), EMBASE (1980 ‐ January 2004), previous reviews including cross references (all articles referenced), abstracts and conferences (Perinatal Society of Australia and New Zealand, and Pediatric Academic Societies and American Academy of Pediatrics meetings 1998 ‐ 2003). The search of MEDLINE included both MeSH searches using the following terms ("[colloids or plasma substitutes or sodium chloride or serum albumin or hypotension] and infant‐premature") and text searches using the following words ("[colloid or crystalloid or saline or volume or hypotension] and infant‐premature"). No language restriction was used.
Data collection and analysis
Criteria and methods used to assess the methodological quality of the included trials: Standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. The methodological quality of each trial was reviewed independently by the second review author. Particular emphasis was placed on allocation concealment, blinding, completeness of follow up and blinding of outcome assessment. Allocation concealment was ranked: Grade A: adequate; Grade B: uncertain; Grade C: clearly inadequate. Additional information was requested from authors of each trial to clarify methodology (see 'table of included studies' ‐ 'notes').
Methods used to collect data from the included trials: Each review author extracted data separately then compared and resolved differences. Additional data where required was requested from authors of trials (see 'table of included studies' ‐ 'notes').
Methods used to synthesise the data: Standard methods of Neonatal Review Group with use of relative risk and weighted mean difference where appropriate. The fixed effects model using RevMan 5.0 was used for meta‐analysis. Heterogeneity was explored using funnel plots and the chi2 statistic for heterogeneity, and quantified using the I2 statistic. Sensitivity analysis was performed on the basis of methodological quality.
Results
Description of studies
Eight studies were eligible for inclusion (see 'table of included studies'). An additional published report was found for one study (Lynch 2008). Data from a previously unpublished study was not provided by the author and is now listed as an excluded study (Wright 1995).
Infants: The largest study was the NNNI 1996 study with 776 enrolled infants. The other studies were all small with between 25 and 102 infants randomised. Infant numbers, weights and gestations are documented in the table "Characteristics of Included Studies'. Four studies enrolled very preterm infants on the basis of prematurity or low birth weight, not on the basis of haemodynamic compromise (Beverley 1985; Ekblad 1991; Gottuso 1976; NNNI 1996). Emery 1992 enrolled infants with a systolic BP < 40 mmHg. Infants with clinical volume overload were excluded. Infants in the study by Lundstrom 2000 were enrolled if the mean BP was in the normal range (29 ‐ 40 mmHg) and they had not received volume or inotrope in the preceding three hours. The authors state that this group were considered to be above the presumed normal upper limit for hypotension but from previous work may still be at risk of low left ventricular output and cerebral blood flow. Lynch 2008 enrolled hypotensive preterm infants before three days of age and with mean BP < 5th percentile. So 1997 enrolled mechanically ventilated preterm infants with hypotension (low mean BP according to birthweight strata).
Interventions: Beverley 1985 compared fresh frozen plasma 10 ml/kg on admission and repeated at 24 hours of age to no treatment. Ekblad 1991 compared fresh frozen plasma 10 ml/kg (given over three hours at < 5 hours of age, then daily for 3 days) to no treatment. Emery 1992 compared fresh frozen plasma to albumin 4.5% (15 ml/kg over three hours) given to infants on day one to four after birth. A control group given albumin 20% 5 ml/kg is not included in this review. Gottuso 1976 compared fresh frozen plasma 15 ml/kg to no treatment (supportive care only) in infants < 24 hours of age. Lundstrom 2000 compared albumin 20% 15 ml/kg to no treatment in infants with a mean postnatal age of 32 hours (range 5 ‐ 224). Lynch 2008 compared albumin 10 ml/kg with normal saline 10ml/kg infused over 20 minutes in infants < 3 days of age. Volume was repeated if hypotension persisted. The NNNI 1996 study compared infants < 2 hours of age randomised to either fresh frozen plasma 15 ml/kg, a gelatin‐based plasma substitute (Gelofusine) 20 ml/kg (given over 15 minutes and repeated after 24 hours) or to no treatment. So 1997 compared infants randomised to albumin 5% or normal saline 10 ml/kg over 30 minutes, with a maximum of three doses given if hypotension persisted.
Outcomes: the stated primary outcomes for the studies included P/IVH (Beverley 1985), water balance and renal function (Ekblad 1991), systolic BP one and four hours after infusion (Emery 1992), haemodynamic change (mean BP, left ventricular output and cerebral blood flow) (Lundstrom 2000), improvement in arterial BP to normal (> 10th percentile for mean BP for weight) at one hour after infusion (Lynch 2008), death before discharge or severe disability at two years (NNNI 1996) and successful treatment of hypotension (So 1997). The data for treatment failure in the studies by Lynch 2008 and So 1997 are the number of infants with persistent hypotension after volume therapy requiring inotropes. Gottuso 1976 did not state a primary outcome but measured mortality and blood gas data. Data from the NNNI 1996 study for grade 1 P/IVH, grade 2 ‐ 4 P/IVH and periventricular leucomalacia were available from a subgroup of units with routine scan facilities. Data from this study for grade 3 ‐ 4 P/IVH included late cystic parenchymal lesions. The data for severe neurodevelopmental disability included children who were blind, deaf, unable to walk, had a developmental quotient > 3 SD below mean or another severe disability. Hypotension in the NNNI 1996 study was defined as systolic BP < 35 mmHg. Treatment failure in this study was defined as the occurrence of hypotension on the first day after birth and was only available for a subgroup of infants born in 1990 and 1991.
In this update, the number of excluded reports has increased from 37 to 46 (see 'Characteristics of Excluded Studies'). These included 11 studies which had no control group (Alkalay 1999; Barr 1977; Bauer 1993; Belgaumkar 1998; Bignall 1989; Dimitriou 2001; Lambert 1998; Lay 1980; Nelle 1997; Pladys 1994; Pladys 1997; Sriram 1997). Three studies used insufficient volume to be eligible for inclusion, with less than 10 ml/kg volume given (Bland 1976; Greenough 1993; McMurray 1948). One of these also enrolled infants with a mean gestational age > 32 weeks (Bland 1976). One study enrolled term infants with suspected asphyxia (Gurkan 2000). Several studies treated infants predominately after the first 72 hours (Liet 2003; Liet 2006; McMurray 1948; Paul 2003; Upadhyay 2005). Four studies of red cell transfusion were in infants > 3 days old (Beeram 2003; Blank 1984; Meyer 1993; Nose 1996) and two studies (Paul 2002; Wong 2005) compared the effect of different red cell transfusion volumes in preterm infants with a low hematocrit. One study printed in abstract form only did not state volume of fresh frozen plasma given and data for analysis could not be extracted (Alexander 1976). Oca 2003 comparing volume expansion with albumin to normal saline enrolled hypotensive infants stratified by birth weight < 2500 g or > 2500 g. There were excess losses (23%) in the < 2500g strata to meet eligibility for inclusion in the review.
Risk of bias in included studies
See the table 'Characteristics of included studies'.
Seven studies reported adequate randomisation procedures and had adequate allocation concealment (Beverley 1985; Emery 1992; Gottuso 1976; Lundstrom 2000; Lynch 2008; NNNI 1996; So 1997). One study did not report the method of randomisation (Ekblad 1991). Lynch 2008 reported 'blinding' by sending the allocated solution from pharmacy in an opaque bag. No other study stated that the interventions were blinded in any way. Given the nature of the interventions, it is probable that caregivers were not blinded in any of the studies. Blinding measurement of outcomes was reported by Beverley 1985 (P/IVH), Lynch 2008 (all outcomes) and the NNNI 1996 study (neurodevelopmental assessment). Studies reporting no losses to follow‐up included Emery 1992; Gottuso 1976; Lundstrom 2000; NNNI 1996 study (neurodevelopmental assessment) and So 1997. Beverley 1985 reported seven (12.5%) losses. Data for P/IVH for the excluded infants is available and used in the analysis of mortality and P/IVH in this review. Ekblad 1991 reported outcomes for the same cohort of infants in two papers, with data reported on 38 of 40 infants in one paper (PDA) and 35 of 40 (renal function) in the other. Data for P/IVH and mortality for the excluded infants is available and used in the analysis of these outcomes in this review. The grade of P/IVH was not available for the excluded infants. The authors have been contacted to obtain this information. Data for PDA were not available for two infants. Lynch 2008 did not report post‐randomisation losses so losses are unclear. Lynch 2008 also reported PIVH for a subset of infants born < 1500g. Not all units in the NNNI 1996 study performed routine head ultrasound scans. Data for P/IVH were available for approximately 84% of 611 infants from centres with routine scan facilities. The NNNI 1996 study reported head ultrasound abnormalities in units with routine scanning facilities as intraventricular haemorrhage and periventricular abnormality in babies surviving at six weeks. This was then reclassified into subependymal haemorrhage, more severe haemorrhage, any cerebral abnormality, severe cerebral abnormality, and death or severe cerebral abnormality. Approximately 84% of surviving infants had data from both one and six weeks' ultrasounds. Only data from this subgroup of units with > 80% follow‐up and from surviving infants with both one and six weeks scans have been used in this review.
As the ascertainment of head ultrasound abnormality was different between the studies, data for P/IVH, grade 2 ‐ 4 P/IVH, grade 3 ‐ 4 P/IVH and periventricular leucomalacia are only combined in meta‐analysis in studies that report similar methods of ascertainment. Data are included where there is > 80% ascertainment of infants for the outcome.
Effects of interventions
VOLUME VS. NO TREATMENT IN VERY PRETERM INFANTS (Comparison 1):
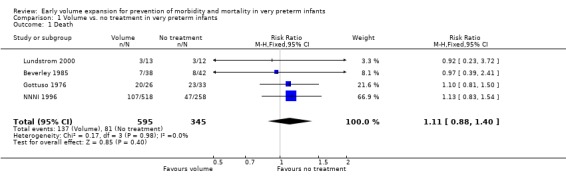
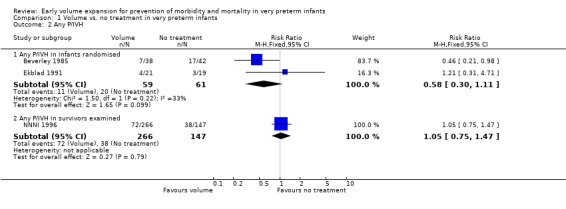
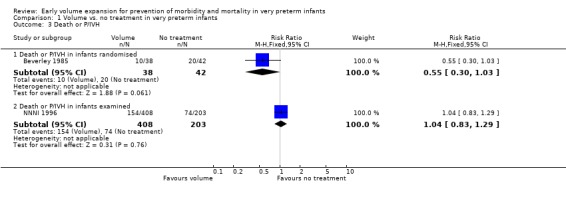
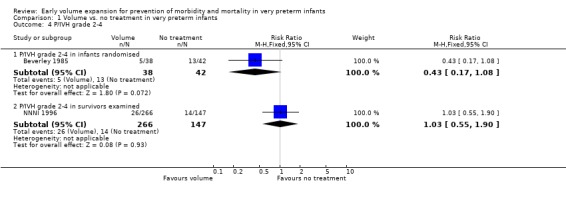
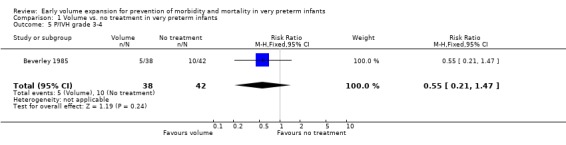
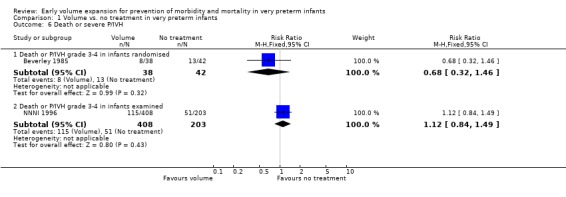
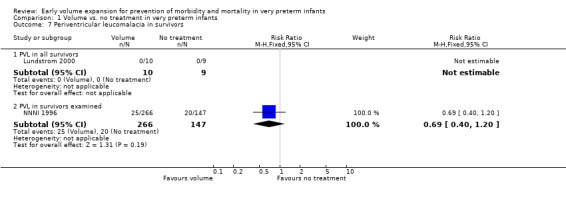
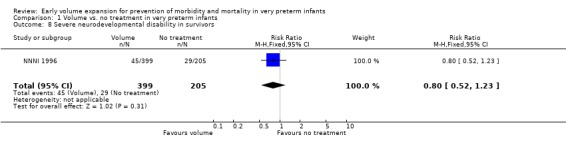
Primary outcomes: Five studies randomised infants to volume vs. no treatment in very preterm infants (Beverley 1985; Ekblad 1991; Gottuso 1976; Lundstrom 2000; NNNI 1996). Four studies with a total of 940 infants reported mortality (Beverley 1985; Gottuso 1976; Lundstrom 2000; NNNI 1996). None found a significant effect. Meta‐analysis showed the mortality was similar for infants who received volume compared to no treatment (typical relative risk 1.11, 95% confidence intervals 0.88, 1.40; typical risk difference 0.02, 95% CI ‐0.03, 0.08) (Outcome 1.1). Two of these studies (Beverley 1985; Ekblad 1991) reported P/IVH data on all infants randomised. Beverley 1985 found a significant reduction in P/IVH (RR 0.46, 95% CI 0.21, 0.98) whereas Ekblad 1991 found no significant difference (RR 1.21, 95% CI 0.31, 4.71). Meta‐analysis of these 2 studies showed no significant difference in P/IVH (typical RR 0.58, 95% CI 0.30, 1.11; typical RD ‐0.14, 95% CI ‐0.29, 0.01) (Outcome 1.2). Beverley 1985 found no significant difference in grade 2 ‐ 4 P/IVH (RR 0.43, 95% CI 0.17, 1.08) or grade 3‐4 P/IVH (RR 0.55, 95% CI 0.21, 1.47); the difference in rate of death or P/IVH was of borderline significance (RR 0.55, 95% CI 0.30, 1.03; RD ‐0.21, 95% CI ‐0.42, ‐0.01). There was no significant difference in death or grade 3 ‐ 4 P/IVH (RR 0.68, 95% 0.32, 1.46). For the 413 survivors who were examined at six weeks in centres with routine scan facilities in the NNNI 1996 study, no significant difference was found in P/IVH in survivors (RR 1.05, 95% CI 0.75, 1.47) or grade 2 ‐ 4 P/IVH in survivors (RR 1.03, 95% CI 0.55, 1.90). For the 611 infants randomised in these units, there was no difference in death or P/IVH (RR 1.04, 95% CI 0.83, 1.29) and death or grade 3‐4 P/IVH (RR 1.12, 95% CI 0.84, 1.49). Lundstrom 2000 reported no infants with periventricular leucomalacia in either group. In the 413 survivors examined, the NNNI 1996 study found no significant difference in periventricular leucomalacia (RR 0.69, 95% CI 0.40, 1.20). The NNNI 1996 study reported long‐term neurodevelopmental outcome in survivors. There was no significant difference in severe disability (RR 0.80, 95% CI 0.52, 1.23), cerebral palsy (RR 0.76, 95% CI 0.48, 1.20) and death or severe disability (RR 1.00, 95% CI 0.80, 1.24).
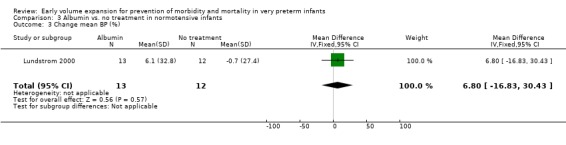
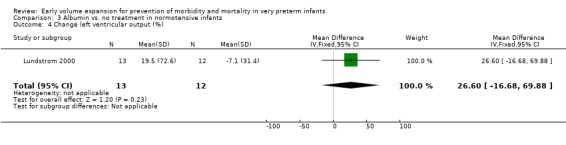
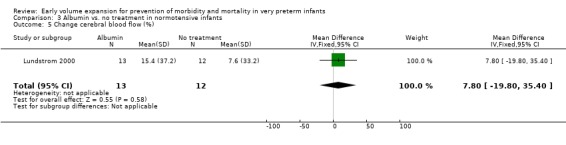
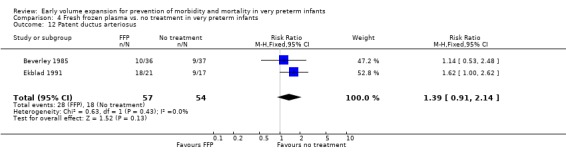
Secondary outcomes: The NNNI 1996 study found no significant difference in the rates of treatment failure for a subgroup of infants born 1990‐91 (hypotension: RR 0.55, 95% CI 0.24, 1.28) or in transient renal impairment (data not given in paper). Ekblad 1991 (2 infants lost from analysis) found an increase in PDA of borderline significance (RR 1.62, 95% CI 1.00, 2.62; RD 0.33. 95% CI 0.05, 0.61). Beverley 1985 found no difference in PDA (RR 1.14, 95% CI 0.53, 2.48). Meta‐analysis of the two studies found no significant difference in PDA (typical RR 1.39, 95% CI 0.91, 2.14) (Outcome 1.12). There was no significant difference in rates of pneumothorax, necrotising enterocolitis, or sepsis (Outcomes 1.13 ‐ 1.15). One study (Lundstrom 2000) found a non‐significant trend for volume to increase left ventricular output (mean difference 26.6 ml/kg/min, 95% CI ‐1.7, 69.9) and cerebral blood flow (MD 7.8 ml/kg/min, 95% CI ‐19.8, 35.4) compared to a control group, but to have little effect on mean BP (MD 6.8 mmHg, 95% CI ‐16.8, 30.4). No data were available for incidence of low blood flow, use of additional inotropes or retinopathy.
SUBGROUP ANALYSES ACCORDING TO TYPE OF VOLUME USED:
No studies were found that compared early red cell transfusion to a control group. No study reported any primary outcomes for the comparison of albumin and fresh frozen plasma.
COLLOID VS. CRYSTALLOID IN HYPOTENSIVE INFANTS (Comparison 2):
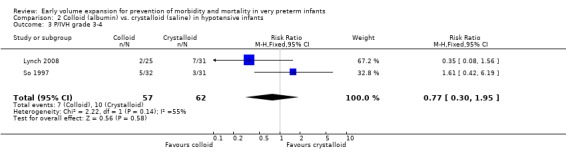
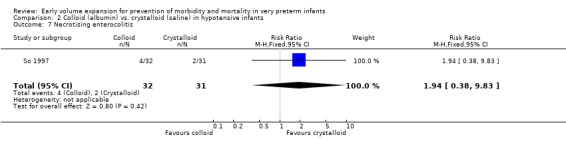
Primary outcomes: Two studies (So 1997; Lynch 2008) compared volume expansion using albumin 5% to normal saline 10ml/kg in hypotensive preterm infants. Meta‐analysis found no significant difference in mortality (typical RR 1.02; 95% CI 0.50, 2.06) (Outcome 2.1). Neither study reported a significant difference in PIVH or PIVH grade 3 ‐ 4, although the was substantial but not statistically significant heterogeneity in both analyses. Meta‐analysis found no significant difference in P/IVH (typical RR 0.95; 95% CI 0.56, 1.61) and grade 3 ‐ 4 P/IVH (typical RR 0.77; 95% CI 0.30, 1.95) (Outcomes 2.2, 2.3). No data were available for periventricular leucomalacia or long‐term disability.
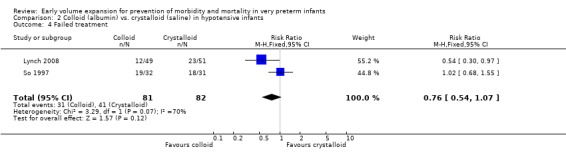
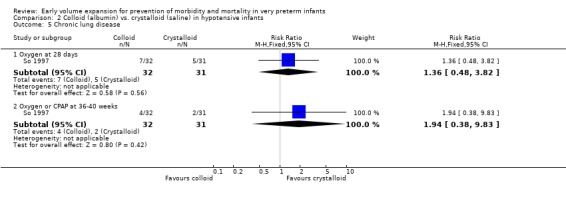
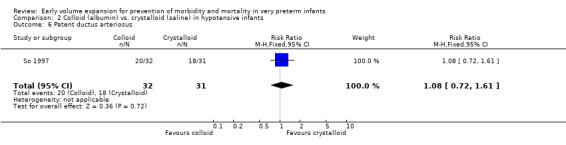
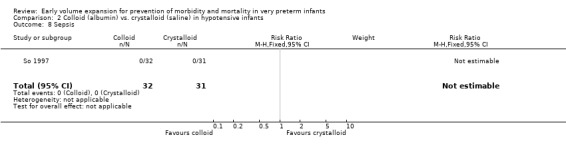
Secondary outcomes: One study (Lynch 2008), reported a significant reduction in treatment failure (persistent hypotension requiring inotropes (RR 0.54; 95% CI 0.30, 0.97) in infants receiving albumin compared to normal saline. So 1997 reported no significant difference in treatment failure (RR 1.02, 95% CI 0.68, 1.55). Meta‐analysis found no significant difference in treatment failure (two studies,163 infants; typical RR 0.76, 95% CI 0.54, 1.07) (Outcome 2.4). There was substantial (I2 70%) heterogeneity of borderline significance (chi2 p = 0.07). So 1997 reported no significant difference in chronic lung disease (oxygen requirements at 28 days or 36 weeks postmenstrual age), patent ductus arteriosus, necrotising enterocolitis and sepsis (Outcomes 2.5 ‐ 2.8). No data were available for retinopathy.
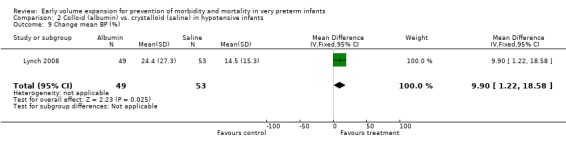
Other outcomes: Lynch 2008 reported (in abstract) a significantly greater increase in mean BP in infants receiving albumin compared to normal saline (MD 9.9%, 95% CI 1.22‐18.58). Lynch 2008 reported no significant difference in infants receiving a second bolus (albumin 61.2% vs. saline 64.7%), urine output (ml/kg/hr) for six hours after the first bolus (1.7 ± 2.2 vs. 1.8 ± 2) or cost of therapy per patient including pharmacy and nursing costs (albumin $US63.98 ± 8.03 vs. saline $US62.18 ± 8.58). So 1997 reported no significant difference in mean arterial BP (data presented in article in figure) but infants who received albumin received a significantly increased amount of extra volume for hypotension (mean 27.5 vs. 10.0 ml, p = 0.02) and had a significantly greater increase in weight in the first 48 hours (5.9 +\‐ 1.9% vs. 0.9 +\‐ 1.7%, p = 0.05).
ALBUMIN VS. SALINE:
See 'Colloid vs. crystalloid' above.
ALBUMIN VS. NO TREATMENT IN NORMOTENSIVE INFANTS (Comparison 3):
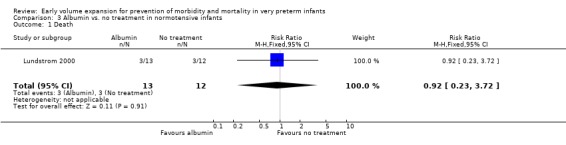
Primary outcomes: One study randomised 25 normotensive preterm infants to albumin 20% 15 ml/kg or no treatment (Lundstrom 2000) and found no significant difference in mortality (RR 0.92, 95% CI 0.23, 3.72) (Outcome 3.1) and no periventricular leucomalacia (Outcome 3.2) in either group. Secondary outcomes: Infants receiving albumin had a trend to increased left ventricular output and cerebral blood flow, with little change in mean BP (see above). No other outcome data are available.
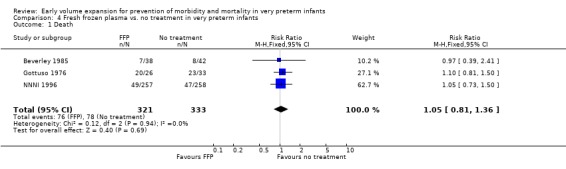
FRESH FROZEN PLASMA VS. NO TREATMENT IN VERY PRETERM INFANTS (Comparison 4):
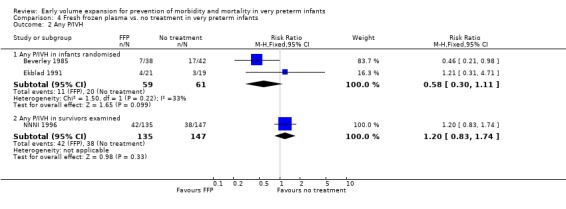
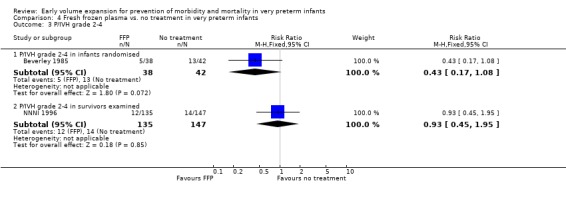
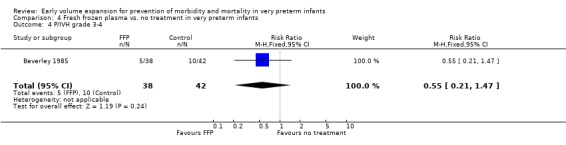
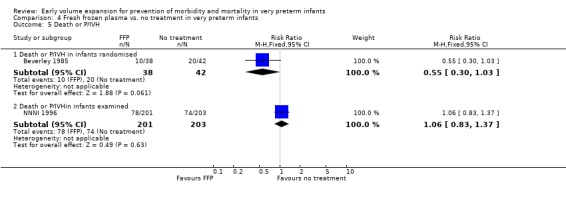
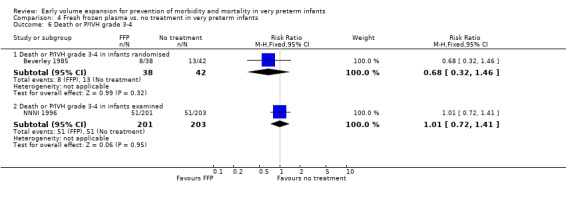
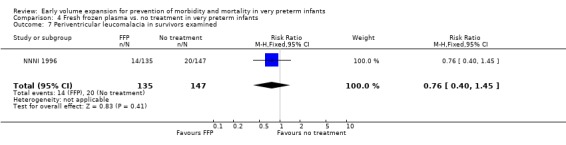
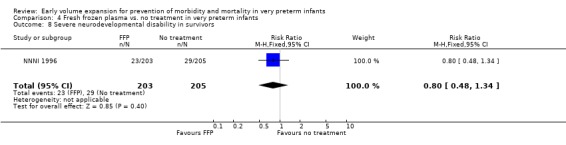
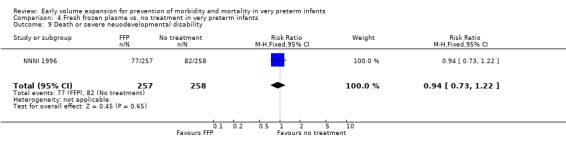
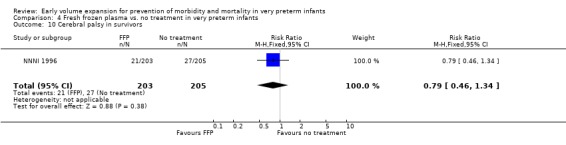
Primary outcomes: Four studies randomised infants to fresh frozen plasma or no treatment (Beverley 1985; Ekblad 1991; Gottuso 1976; NNNI 1996). Meta‐analysis of three studies reporting mortality data (Beverley 1985; Gottuso 1976; NNNI 1996) involving a total of 654 infants found no significant difference in mortality (typical RR 1.05, 95% CI 0.81, 1.36) (Outcome 4.1). Two studies (Beverley 1985; Ekblad 1991) reported data on all infants randomised. Beverley 1985 found a significant reduction in P/IVH (RR 0.46, 95% CI 0.21, 0.98) whereas Ekblad 1991 found no significant difference (RR 1.21, 95% CI 0.31, 4.71). Meta‐analysis of these 2 studies found a non‐significant trend to reduced P/IVH in infants receiving volume (typical RR 0.58, 95% CI 0.30, 1.11; typical RD ‐0.14, 95% CI ‐0.29, 0.01) (Outcome 4.2). One study, Beverley 1985 found a non‐significant trend to less P/IVH grade 3‐4 (RR 0.55, 95% CI 0.21, 1.47), reduced death or P/IVH of borderline significance (RR 0.55, 95% CI 0.30, 1.03; RD ‐0.21, 95% CI ‐0.42, ‐0.01) and no significant difference in death or grade 3‐4 P/IVH (RR 0.68, 95% CI 0.32, 1.46). From units with routine scan facilities, the NNNI 1996 study reported no difference in P/IVH in survivors examined (RR 1.20, 95% CI 0.83, 1.74), grade 2‐4 P/IVH in survivors examined (RR 0.93, 95% CI 0.45, 1.95), death or P/IVH (RR 1.06, 95% CI 0.83, 1.37), death or grade 3‐4 P/IVH (RR 1.01, 95% CI 0.72, 1.41) and periventricular leucomalacia in survivors (RR 0.76, 95% CI 0.40, 1.45). Rates of severe disability (RR 0.80, 95% CI 0.48, 1.34), death or severe disability (RR 0.94, 95% CI 0.73, 1.22) and cerebral palsy (RR 0.79, 95% CI 0.46, 1.34) were not significantly different.
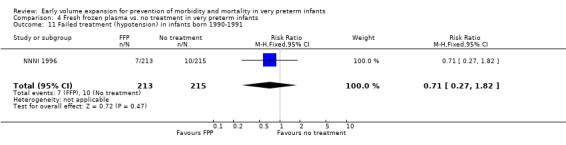
Secondary outcomes: The NNNI 1996 study found no significant difference in the rate of treatment failure in infants born 1990‐91 (hypotension: RR 0.71, 95% CI 0.27, 1.82). No data on the effect on blood flow were available. In the NNNI 1996 study, necrotising enterocolitis was significantly reduced (RR 0.22, 95% CI 0.06, 0.74) in infants receiving fresh frozen plasma, but sepsis significantly increased (RR 1.65, 95% CI 1.13, 2.40). Ekblad 1991 found an increase in PDA of borderline significance (RR 1.62, 95% CI 1.00, 2.62: RD 0.33, 95% CI 0.05, 0.61). Beverley 1985 found no difference in PDA (RR 1.14, 95% CI 0.53, 2.48). Meta‐analysis of these two studies found no significant difference in PDA (typical RR 1.39, 95% CI 0.91, 2.14) (Outcome 4.12). No significant difference was found in the rates pneumothorax (Outcome 4.13). The NNNI 1996 study found no significant difference in the rate of renal impairment (data not given in paper) and Ekblad 1991 found no significant difference in creatinine clearance and urinary fractional sodium excretion in the first 5 days after birth.
ALBUMIN VS. FRESH FROZEN PLASMA IN VERY PRETERM INFANTS:
One trial involving 20 infants in each group with a systolic BP < 40 mmHg compared fresh frozen plasma to albumin 4.5% 15 ml/kg (Emery 1992). No significant difference in change in mean BP was found. Both these groups had a significantly greater increase in mean BP than a control group who received albumin 20% 5 ml/kg. No difference in duration of ventilation was found. No other clinical data were available.
GELATIN‐BASED PLASMA SUBSTITUTE VS. NO TREATMENT IN VERY PRETERM INFANTS (Comparison 5):
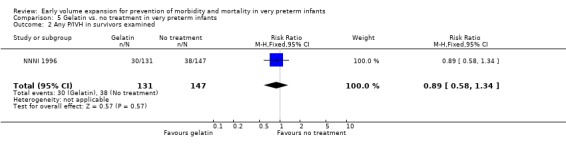
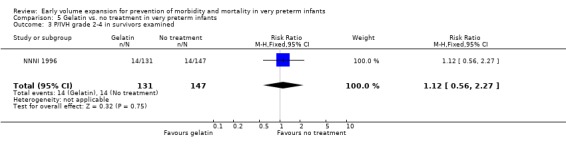
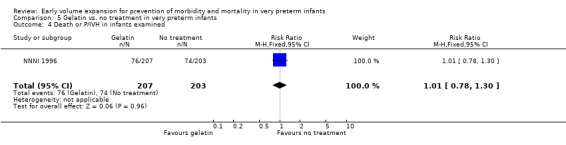
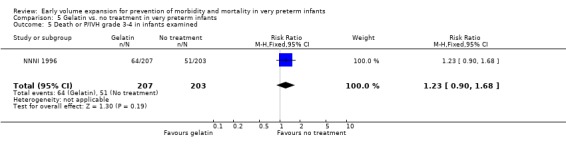
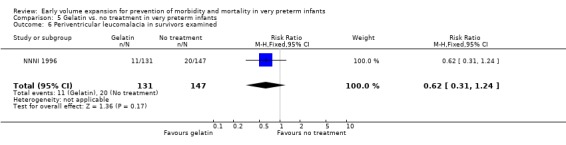
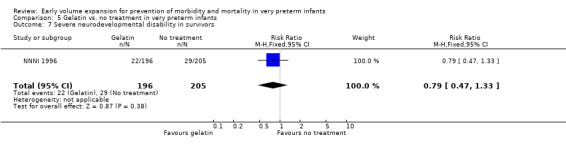
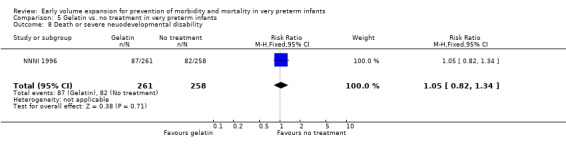
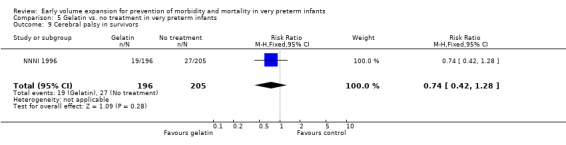
Primary outcomes: One study involving 519 infants compared a gelatin‐based plasma substitute 20 ml/kg with no treatment (NNNI 1996). No significant differences were found in mortality (RR 1.22, 95% CI 0.86, 1.72). In a subgroup of infants from centres with routine scanning facilities there was no significant difference found in P/IVH in survivors (RR 0.89, 95% CI 0.58, 1.34), grade 2‐4 P/IVH in survivors (RR 1.12, 95% CI 0.56, 2.27), death or P/IVH (RR 1.01, 95% CI 0.78, 1.30), death or grade 3‐4 P/IVH (RR 1.23, 95% CI 0.90, 1.68) or periventricular leucomalacia in survivors (RR 0.62, 95% CI 0.31, 1.24). Rates of severe disability in all survivors (RR 0.79, 95% CI 0.47, 1.33), death or severe disability (RR 1.05, 95% CI 0.82, 1.34) and cerebral palsy in all survivors (RR 0.74, 95% CI 0.42, 1.28) were not significantly different.
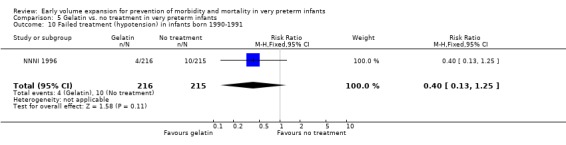
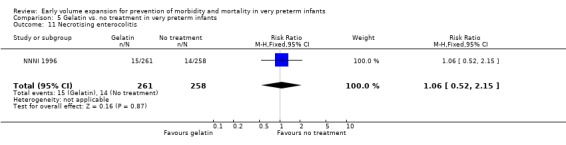
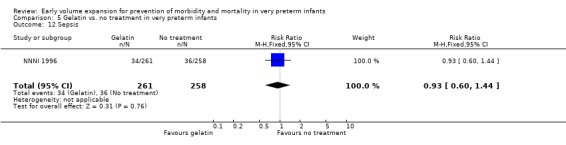
Secondary outcomes: The NNNI 1996 study found no significant differences in the rate of treatment failure in a subgroup of infants born 1990‐91 (hypotension: RR 0.40, 95% CI 0.13, 1.25), transient renal impairment, necrotising enterocolitis (RR 1.06, 95% CI 0.52, 2.15) and sepsis (RR 0.93, 95% CI 0.60, 1.44) . No data on the effect on blood flow, patent ductus arteriosus or retinopathy were available.
GELATIN‐BASED PLASMA SUBSTITUTE VS. FRESH FROZEN PLASMA IN VERY PRETERM INFANTS (Comparison 6):
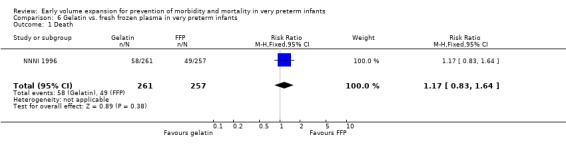
Primary outcomes: One study involving 519 infants compared a gelatin‐based plasma substitute with fresh frozen plasma 20 ml/kg (NNNI 1996). No significant difference was found in mortality (RR 1.17, 95% CI 0.83, 1.64). In a subgroup of infants born in centres with routine scanning facilities, the rates of P/IVH in survivors (RR 0.74, 95% CI 0.49, 1.10), grade 2‐4 P/IVH in survivors (RR 1.20, 95% CI 0.58, 2.50), death or P/IVH (RR 0.95, 95% CI 0.74, 1.21), death or grade 3‐4 P/IVH (RR 1.22, 95% CI 0.89, 1.67) and periventricular leucomalacia in survivors (RR 0.81, 95% CI 0.38, 1.72) were similar. Rates of severe disability in all survivors (RR 0.99, 95% CI 0.57, 1.72), death or severe disability (RR 1.11, 95% CI 0.86, 1.43) and cerebral palsy in all survivors (RR 0.94 95% CI 0.52, 1.69) were not significantly different.
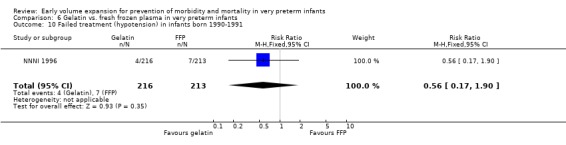
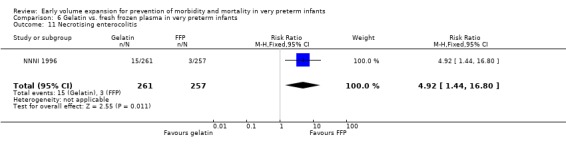
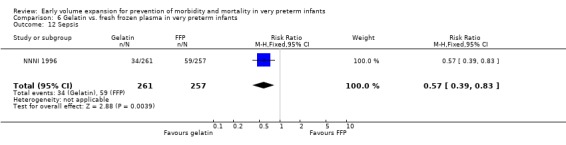
Secondary outcomes: The NNNI 1996 study found no significant difference in the rate of treatment failure in a subgroup of infants born 1990‐91 (hypotension: RR 0.56, 95% CI 0.17, 1.90), or for transient renal impairment. The rate of necrotising enterocolitis was significantly higher (RR 4.92, 95% CI 1.44, 16.80) and sepsis significantly lower (RR 0.57, 95% CI 0.39, 0.83) in infants who received the gelatin based plasma substitute. No data on the effect on blood flow or rates of patent ductus arteriosus and retinopathy were available.
BLOOD TRANSFUSION VS. NO TREATMENT VS. NO TREATMENT IN VERY PRETERM INFANTS:
No randomised study was found that compared early red cell transfusion to control.
OTHER SUBGROUP ANALYSES: 1) According to timing of treatment (data not in MetaView tables): Volume vs. no treatment: Early treatment (< 24 hours age): Four studies with gave volume expansion to infants within the first 24 hours after birth (Beverley 1985; Ekblad 1991; Gottuso 1976; NNNI 1996). Meta‐analysis of three studies with data on mortality for 915 infants (Beverley 1985; Gottuso 1976; NNNI 1996) found no significant difference (typical RR 1.11, 95% CI 0.88, 1.41). All other outcomes are as for the comparison 'volume vs. no treatment'.
Colloid (5% albumin) vs. crystalloid (normal saline): Early treatment (< 24 hours): One study (So 1997) enrolled infants with hypotension at < 2 hours of age and reported no significant difference in mortality (RR 1.36, 95% CI 0.48, 3.82), any P/IVH (RR 1.52, 95% CI 0.68, 3.42) and grade 3 ‐ 4 P/IVH (RR 1.61, 95% CI 0.42, 6.19). So 1997 reported no significant difference in treatment failure (RR 1.02, 95% CI 0.68, 1.55), rates of chronic lung disease (oxygen requirements at 28 days or 36 weeks postmenstrual age), patent ductus arteriosus, necrotising enterocolitis and sepsis. The other study (Lynch 2008) comparing albumin with normal saline enrolled hypotensive infants <3 days of age.
2) According to types of infants enrolled: a) Unselected preterm infants (not selected on the basis of cardiovascular compromise): All studies that compared volume to no treatment enrolled infants on the basis of gestation or birthweight. See comparison of volume vs. no treatment.
b) Infants with clinical evidence of cardiovascular compromise: Three studies enrolled infants with clinical evidence of cardiovascular compromise. Emery 1992 enrolled hypotensive infants (systolic BP < 40 mmHg) to fresh frozen plasma 15 ml/kg or albumin 4.5% 15 ml/kg. A control group received albumin 20% 5 ml/kg. Lynch 2008 enrolled hypotensive preterm infants (mean BP < 5th percentile according to Vermsold criteria) to 5% albumin vs. normal saline 10ml/kg. So 1997 enrolled hypotensive infants with a low mean BP (defined by weight strata) to albumin 10 ml/kg or isotonic saline 10 ml/kg. Only one study (Lynch 2008) reported a significant difference with a significant reduction in treatment failure in infants receiving albumin (RR 0.54; 95% CI 0.30, 0.97) and a significant increase in mean BP (WMD 9.90 mmHg, 9% CI 1.22, 18.58) compared to normal saline. No differences were found for any outcomes (see comparisons of 'fresh frozen plasma versus albumin' and 'colloid versus crystalloid'). No studies were found that compared volume to no treatment in infants with cardiovascular compromise.
c) Infants with low blood flow: No studies were found that compared volume to no treatment in infants with low blood flow.
HETEROGENEITY
No statistically significant heterogeneity was found for any analysis included in this review. Comparing volume to no treatment: One small study (Beverley 1985) found a reduced rate of P/IVH. The other studies (Ekblad 1991; NNNI 1996) and the overall meta‐analysis did not support a difference in P/IVH, high grade P/IVH or subsequent disability. Comparing albumin to normal saline: One study (Lynch 2008) reported a significant reduction in treatment failure and significantly greater increase in mean BP in hypotensive infants receiving albumin in the first 3 days. The other study (So 1997) reported no significant difference in treatment failure and no significant difference in % change in mean BP in infants enrolled <2 hours age.
SENSITIVITY ANALYSIS ACCORDING TO METHODOLOGICAL QUALITY (data not in MetaView tables)
The results of this review are not sensitive to excluding the study that did not state whether there was adequate allocation concealment (Ekblad 1991). Only one study stated that the interventions were blinded in any way (Lynch 2008) and this study compared 5% albumin with normal saline 10ml/kg in hypotensive infants in the first 3 days after birth. This study reported a significantly greater increase in mean BP in infants receiving albumin compared to normal saline (MD 9.9%, 95% CI 1.22, 18.58) but no significant difference in urine output (ml/kg/hr) for 6 hours after the 1st bolus (1.7 ± 2.2 vs. 1.8 ± 2) or cost of therapy per patient including pharmacy and nursing costs (albumin $US63.98 ± 8.03 vs. saline $US62.18 ± 8.58). Comparing volume with no treatment, one study with results for P/IVH had incomplete head ultrasound data at six weeks (NNNI 1996). Two studies had complete follow‐up of infants for P/IVH (Beverley 1985; Ekblad 1991) and found a non‐significant trend to reduced P/IVH (RR 0.6, 95% CI 0.3, 1.1; RD ‐0.14, 95% CI ‐0.29, 0.01) and grade 3 ‐ 4 P/IVH (RR 0.6, 95% CI 0.2, 1.5; RD ‐0.11, 95% CI ‐0.27, 0.06) in infants receiving volume. No significant difference for combined death or P/IVH (RR 0.95, 95% CI 0.74, 1.21) was found. The NNNI 1996 study had complete follow‐up for neurodevelopmental outcomes reporting no significant difference in severe disability (RR 0.80, 95% CI 0.52, 1.23), cerebral palsy (RR 0.76, 95% CI 0.48, 1.20) and death or severe disability (RR 1.00, 95% CI 0.80, 1.24).
Two studies were excluded from the analyses as insufficient volume (less than 10ml/kg) was given (Bland 1976; Greenough 1993) and the mean age of infants was > 32 weeks (Bland 1976). One study (Emery 1992) had data excluded from a subgroup of infants who received insufficient volume and did not meet the criteria for controls (given albumin 20% 5 ml/kg). These data do not meet inclusion criteria for this review. The excluded data from the study by Emery 1992 are given in 'results' in the comparison of 'fresh frozen plasma vs. albumin'. The inclusion of data from any of these comparisons has minimal impact on any of the estimates made in this review.
Discussion
This review examines evidence from randomised controlled trials for the use of early volume expansion in very preterm infants. In subgroup analysis, it looks for evidence for the use of different types of volume expansion and for the use of volume expansion in different types of infants. The analysis had a power of 80% to detect a 9% absolute difference in rates of combined death and severe disability between volume and control groups at the 5% significance level. Six of the 7 trials reported adequate randomisation procedures and allocation concealment. Only one reported efforts to blind caregivers with this study comparing use of albumin with normal saline in hypotensive infants (Lynch 2008). Only one trial provided data on long‐term neurodevelopment (NNNI 1996). This was the largest trial with no losses to follow‐up and blinded assessment of neurodevelopment at two years, including the Griffiths' Scales of Mental Development and clinical examination. This trial had incomplete ascertainment of head ultrasound findings in a subgroup of infants born in centres with routine scan facilities. The studies with complete ascertainment of head ultrasound abnormalities are much smaller (Beverley 1985; Ekblad 1991).
The studies comparing volume to no treatment did not enrol infants with evidence of cardiovascular compromise. Infants were predominantly enrolled on the basis of gestation or birthweight. However, by virtue of their gestation or birthweight, these infants were at risk of mortality, P/IVH and disability. Whether or not these infants had hypovolaemia was not measured. The studies that did enrol infants on the basis of cardiovascular compromise enrolled infants on the basis of low BP and compared different forms of volume expansion. They did not have a control group that received no treatment. Blood flow (left ventricular output and cerebral blood flow) was measured by only one study (Lundstrom 2000). This study enrolled infants with stable cardiovascular status and normal mean BP who had not received volume or inotropes in the preceding three hours. There is no data on blood flow in infants with cardiovascular compromise.
There is no evidence to support the routine use of volume expansion given to very preterm infants on the basis of gestational age or birthweight in the first days after birth. There is no evidence that routine early volume expansion decreases the incidence of cardiovascular compromise (hypotension) or mortality. Evidence from one study of a reduced rate of P/IVH is not supported by the overall meta‐analysis or any other study. No difference was seen in the rates of higher grade cerebral ischaemic lesions or subsequent disability. The observations from one study that infants who received fresh frozen plasma had a lower incidence of necrotising enterocolitis and higher incidence of sepsis should be treated with caution. The overall rate of mortality and disability were not different between infants who received fresh frozen plasma compared to no treatment in this study.
One study (Lynch 2008) reported a significant reduction in treatment failure (persistent hypotension) and a greater increase in mean BP in infants with hypotension treated with albumin compared to normal saline. Other clinical outcomes including mortality and P/IVH were not reported. The other study (So 1997) and the overall meta‐analysis do not support a reduction in rate of treatment failure or change in mean BP, and there was no significant difference in mortality, P/IVH or other clinical outcomes. Differences between the studies identified in subgroup analyses included the different timing of intervention (Lynch 2008 enrolled infants < 3 days age and So 1997 enrolled infants < 2 hours age) and the lack of blinding of treatment in one study (So 1997). One study (NNNI 1996) reported no significant difference in mortality, P/IVH or disability in infants who received a gelatin‐based plasma substitute compared to fresh frozen plasma. No data were available for the use of early blood transfusion. Data for the use of albumin compared to no treatment are only available from one small study with inadequate power to demonstrate any benefit and enrolling infants with a stable cardiovascular status.
Authors' conclusions
Implications for practice.
There is no evidence from randomised trials to support the routine use of early volume expansion in preterm infants without evidence of cardiovascular compromise. There is insufficient evidence to determine whether infants with evidence of cardiovascular compromise benefit from volume expansion. There is insufficient evidence to determine what type of volume expansion should be used in preterm infants (if at all) and for the use of early red cell transfusions. The significance of the finding of a significant increase in BP in hypotensive preterm infants in one trial comparing colloid (albumin) and crystalloid (saline) is unclear, but the overall meta‐analysis found no other clinical benefit in using albumin compared to saline.
Implications for research.
The question of whether volume expansion should be given before or in addition to inotropes in preterm infants with cardiovascular compromise has not been answered. Future trials of volume expansion should identify and enrol those infants with low cardiac output or organ perfusion. Further trials of albumin vs. saline are warranted in infants with cardiovascular compromise. In addition to important clinical outcomes (mortality, cerebral ischaemic lesions and neurodevelopment) future trials should measure changes in cardiac output and/or organ blood flow. Further research is required into methods of detecting hypovolaemia in preterm infants.
What's new
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Minor amendment ‐ Gaissmaier reference added. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 31 July 2008 | New search has been performed | This review is an update of the existing review "Early volume expansion for prevention of morbidity and mortality in very preterm infants", published in the Cochrane Database of Systematic Reviews, Issue 2, 2004 (Osborn 2004). Our updated literature search found a publication of study previously reported in abstract form only. Several studies did not meet eligibility criteria. Background updated and review updated to include outcomes from new publication. No changes in conclusions. |
| 20 April 2008 | Amended | Converted to new review format. |
| 6 February 2004 | New citation required but conclusions have not changed | This review is an update of the existing review "Early volume expansion for prevention of morbidity and mortality in very preterm infants", published in The Cochrane Library, Issue 3, 2001 (Osborn 2001). New information added includes identification of one new eligible study (Lynch 2002) comparing albumin and saline. Updated searches identified several studies that did not meet inclusion criteria. Data from one unpublished study (Wright 1995) have still not been obtainable from author. |
Data and analyses
Comparison 1. Volume vs. no treatment in very preterm infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.88, 1.40] |
| 2 Any P/IVH | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any P/IVH in infants randomised | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.11] |
| 2.2 Any P/IVH in survivors examined | 1 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.75, 1.47] |
| 3 Death or P/IVH | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Death or P/IVH in infants randomised | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.03] |
| 3.2 Death or P/IVH in infants examined | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.83, 1.29] |
| 4 P/IVH grade 2‐4 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 P/IVH grade 2‐4 in infants randomised | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.08] |
| 4.2 P/IVH grade 2‐4 in survivors examined | 1 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.55, 1.90] |
| 5 P/IVH grade 3‐4 | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.21, 1.47] |
| 6 Death or severe P/IVH | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Death or P/IVH grade 3‐4 in infants randomised | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.32, 1.46] |
| 6.2 Death or P/IVH grade 3‐4 in infants examined | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.84, 1.49] |
| 7 Periventricular leucomalacia in survivors | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 PVL in all survivors | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 PVL in survivors examined | 1 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.20] |
| 8 Severe neurodevelopmental disability in survivors | 1 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 9 Death or severe neuodevelopmental disability | 1 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.24] |
| 10 Cerebral palsy in survivors | 1 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.20] |
| 11 Failed treatment (hypotension) in infants born 1990‐1991 | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.28] |
| 12 Patent ductus arteriosus | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.91, 2.14] |
| 13 Pneumothorax | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.33, 1.92] |
| 14 Necrotising enterocolitis | 1 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.32, 1.27] |
| 15 Sepsis | 1 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.90, 1.83] |
| 16 Change mean BP (%) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 6.8 [‐16.83, 30.43] |
| 17 Change left ventricular output (%) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 26.6 [‐16.68, 69.88] |
| 18 Change cerebral blood flow (%) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 7.8 [‐19.80, 35.40] |
1.1. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 1 Death.
1.2. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 2 Any P/IVH.
1.3. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 3 Death or P/IVH.
1.4. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 4 P/IVH grade 2‐4.
1.5. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 5 P/IVH grade 3‐4.
1.6. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 6 Death or severe P/IVH.
1.7. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 7 Periventricular leucomalacia in survivors.
1.8. Analysis.
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 8 Severe neurodevelopmental disability in survivors.
1.9. Analysis.
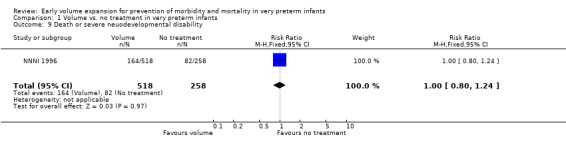
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 9 Death or severe neuodevelopmental disability.
1.10. Analysis.
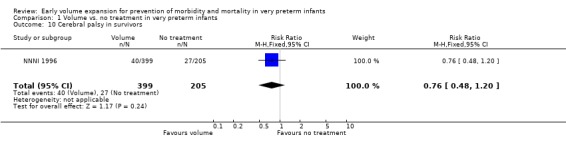
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 10 Cerebral palsy in survivors.
1.11. Analysis.
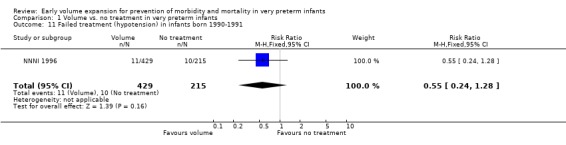
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 11 Failed treatment (hypotension) in infants born 1990‐1991.
1.12. Analysis.
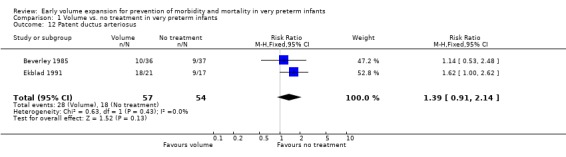
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 12 Patent ductus arteriosus.
1.13. Analysis.
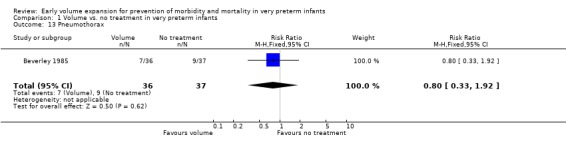
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 13 Pneumothorax.
1.14. Analysis.
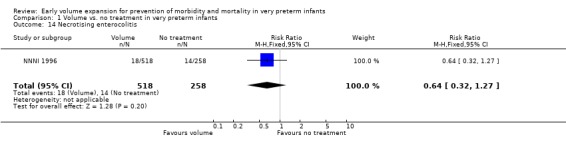
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 14 Necrotising enterocolitis.
1.15. Analysis.
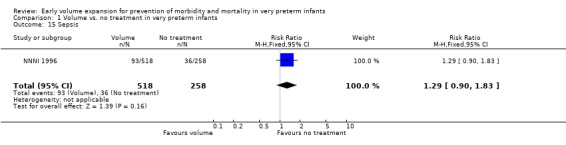
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 15 Sepsis.
1.16. Analysis.
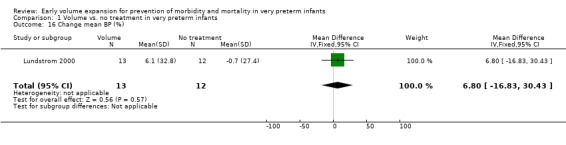
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 16 Change mean BP (%).
1.17. Analysis.
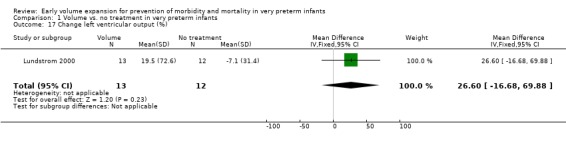
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 17 Change left ventricular output (%).
1.18. Analysis.
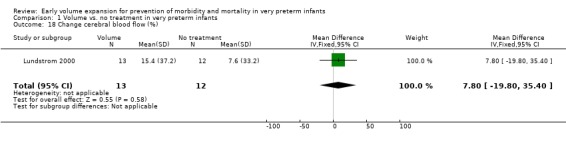
Comparison 1 Volume vs. no treatment in very preterm infants, Outcome 18 Change cerebral blood flow (%).
Comparison 2. Colloid (albumin) vs. crystalloid (saline) in hypotensive infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.50, 2.06] |
| 2 Any P/IVH | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.56, 1.61] |
| 3 P/IVH grade 3‐4 | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.30, 1.95] |
| 4 Failed treatment | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.07] |
| 5 Chronic lung disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oxygen at 28 days | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.48, 3.82] |
| 5.2 Oxygen or CPAP at 36‐40 weeks | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.38, 9.83] |
| 6 Patent ductus arteriosus | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.72, 1.61] |
| 7 Necrotising enterocolitis | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.38, 9.83] |
| 8 Sepsis | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change mean BP (%) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [1.22, 18.58] |
2.1. Analysis.
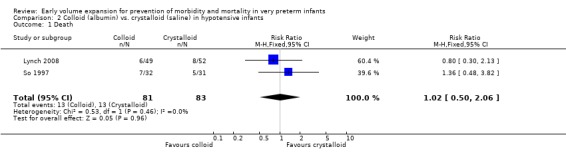
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 1 Death.
2.2. Analysis.
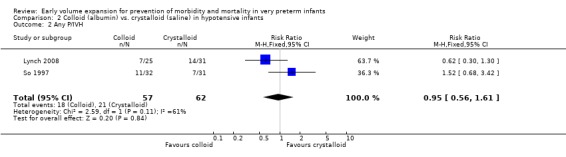
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 2 Any P/IVH.
2.3. Analysis.
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 3 P/IVH grade 3‐4.
2.4. Analysis.
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 4 Failed treatment.
2.5. Analysis.
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 5 Chronic lung disease.
2.6. Analysis.
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 6 Patent ductus arteriosus.
2.7. Analysis.
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 7 Necrotising enterocolitis.
2.8. Analysis.
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 8 Sepsis.
2.9. Analysis.
Comparison 2 Colloid (albumin) vs. crystalloid (saline) in hypotensive infants, Outcome 9 Change mean BP (%).
Comparison 3. Albumin vs. no treatment in normotensive infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.23, 3.72] |
| 2 Periventricular leucomalacia in survivors | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change mean BP (%) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 6.8 [‐16.83, 30.43] |
| 4 Change left ventricular output (%) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 26.6 [‐16.68, 69.88] |
| 5 Change cerebral blood flow (%) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 7.8 [‐19.80, 35.40] |
3.1. Analysis.
Comparison 3 Albumin vs. no treatment in normotensive infants, Outcome 1 Death.
3.2. Analysis.
Comparison 3 Albumin vs. no treatment in normotensive infants, Outcome 2 Periventricular leucomalacia in survivors.
3.3. Analysis.
Comparison 3 Albumin vs. no treatment in normotensive infants, Outcome 3 Change mean BP (%).
3.4. Analysis.
Comparison 3 Albumin vs. no treatment in normotensive infants, Outcome 4 Change left ventricular output (%).
3.5. Analysis.
Comparison 3 Albumin vs. no treatment in normotensive infants, Outcome 5 Change cerebral blood flow (%).
Comparison 4. Fresh frozen plasma vs. no treatment in very preterm infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.36] |
| 2 Any P/IVH | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any P/IVH in infants randomised | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.11] |
| 2.2 Any P/IVH in survivors examined | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.83, 1.74] |
| 3 P/IVH grade 2‐4 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 P/IVH grade 2‐4 in infants randomised | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.08] |
| 3.2 P/IVH grade 2‐4 in survivors examined | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 4 P/IVH grade 3‐4 | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.21, 1.47] |
| 5 Death or P/IVH | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Death or P/IVH in infants randomised | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.03] |
| 5.2 Death or P/IVHin infants examined | 1 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.37] |
| 6 Death or P/IVH grade 3‐4 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Death or P/IVH grade 3‐4 in infants randomised | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.32, 1.46] |
| 6.2 Death or P/IVH grade 3‐4 in infants examined | 1 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.72, 1.41] |
| 7 Periventricular leucomalacia in survivors examined | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.40, 1.45] |
| 8 Severe neurodevelopmental disability in survivors | 1 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.48, 1.34] |
| 9 Death or severe neuodevelopmental disability | 1 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.22] |
| 10 Cerebral palsy in survivors | 1 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.34] |
| 11 Failed treatment (hypotension) in infants born 1990‐1991 | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.82] |
| 12 Patent ductus arteriosus | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.91, 2.14] |
| 13 Pneumothorax | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.33, 1.92] |
| 14 Necrotising enterocolitis | 1 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.74] |
| 15 Sepsis | 1 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.13, 2.40] |
4.1. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 1 Death.
4.2. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 2 Any P/IVH.
4.3. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 3 P/IVH grade 2‐4.
4.4. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 4 P/IVH grade 3‐4.
4.5. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 5 Death or P/IVH.
4.6. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 6 Death or P/IVH grade 3‐4.
4.7. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 7 Periventricular leucomalacia in survivors examined.
4.8. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 8 Severe neurodevelopmental disability in survivors.
4.9. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 9 Death or severe neuodevelopmental disability.
4.10. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 10 Cerebral palsy in survivors.
4.11. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 11 Failed treatment (hypotension) in infants born 1990‐1991.
4.12. Analysis.
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 12 Patent ductus arteriosus.
4.13. Analysis.
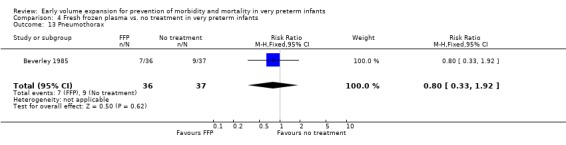
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 13 Pneumothorax.
4.14. Analysis.
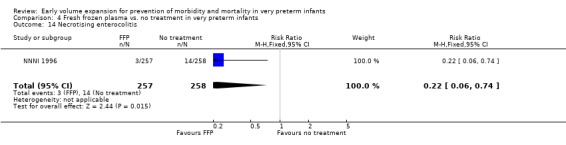
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 14 Necrotising enterocolitis.
4.15. Analysis.
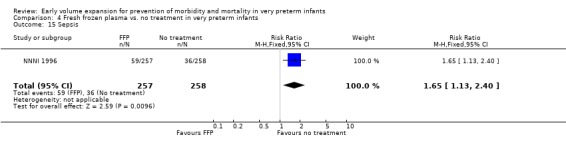
Comparison 4 Fresh frozen plasma vs. no treatment in very preterm infants, Outcome 15 Sepsis.
Comparison 5. Gelatin vs. no treatment in very preterm infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.86, 1.72] |
| 2 Any P/IVH in survivors examined | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.34] |
| 3 P/IVH grade 2‐4 in survivors examined | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.56, 2.27] |
| 4 Death or P/IVH in infants examined | 1 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.30] |
| 5 Death or P/IVH grade 3‐4 in infants examined | 1 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.90, 1.68] |
| 6 Periventricular leucomalacia in survivors examined | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.31, 1.24] |
| 7 Severe neurodevelopmental disability in survivors | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.33] |
| 8 Death or severe neuodevelopmental disability | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.34] |
| 9 Cerebral palsy in survivors | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.28] |
| 10 Failed treatment (hypotension) in infants born 1990‐1991 | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.13, 1.25] |
| 11 Necrotising enterocolitis | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.52, 2.15] |
| 12 Sepsis | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.60, 1.44] |
5.1. Analysis.
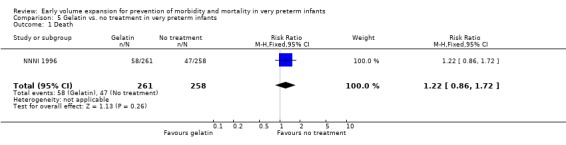
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 1 Death.
5.2. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 2 Any P/IVH in survivors examined.
5.3. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 3 P/IVH grade 2‐4 in survivors examined.
5.4. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 4 Death or P/IVH in infants examined.
5.5. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 5 Death or P/IVH grade 3‐4 in infants examined.
5.6. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 6 Periventricular leucomalacia in survivors examined.
5.7. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 7 Severe neurodevelopmental disability in survivors.
5.8. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 8 Death or severe neuodevelopmental disability.
5.9. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 9 Cerebral palsy in survivors.
5.10. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 10 Failed treatment (hypotension) in infants born 1990‐1991.
5.11. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 11 Necrotising enterocolitis.
5.12. Analysis.
Comparison 5 Gelatin vs. no treatment in very preterm infants, Outcome 12 Sepsis.
Comparison 6. Gelatin vs. fresh frozen plasma in very preterm infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.83, 1.64] |
| 2 Any P/IVH in survivors examined | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.10] |
| 3 P/IVH grade 2‐4 in survivors examined | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.58, 2.50] |
| 4 Death or P/IVH in infants examined | 1 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.21] |
| 5 Death or P/IVH grade 3‐4 in infants examined | 1 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.89, 1.67] |
| 6 Periventricular leucomalacia in survivors examined | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.38, 1.72] |
| 7 Severe neurodevelopmental disability in survivors | 1 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.57, 1.72] |
| 8 Death or severe neuodevelopmental disability | 1 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.86, 1.43] |
| 9 Cerebral palsy in survivors | 1 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.52, 1.69] |
| 10 Failed treatment (hypotension) in infants born 1990‐1991 | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.17, 1.90] |
| 11 Necrotising enterocolitis | 1 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.92 [1.44, 16.80] |
| 12 Sepsis | 1 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.83] |
6.1. Analysis.
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 1 Death.
6.2. Analysis.
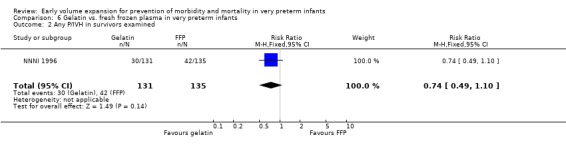
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 2 Any P/IVH in survivors examined.
6.3. Analysis.
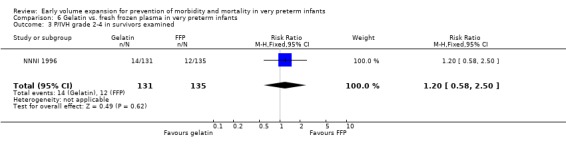
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 3 P/IVH grade 2‐4 in survivors examined.
6.4. Analysis.
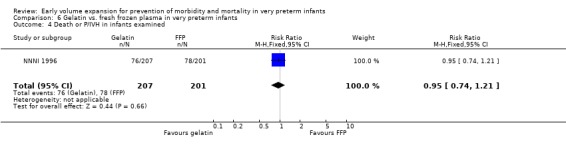
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 4 Death or P/IVH in infants examined.
6.5. Analysis.
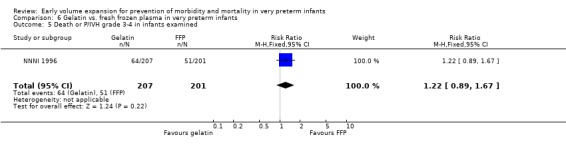
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 5 Death or P/IVH grade 3‐4 in infants examined.
6.6. Analysis.
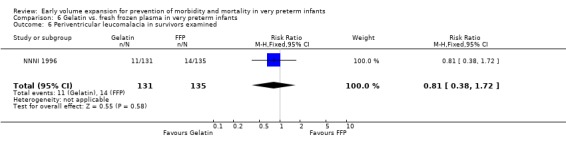
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 6 Periventricular leucomalacia in survivors examined.
6.7. Analysis.
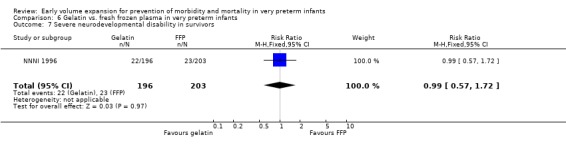
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 7 Severe neurodevelopmental disability in survivors.
6.8. Analysis.
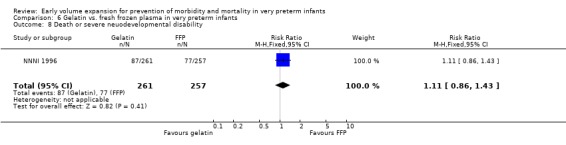
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 8 Death or severe neuodevelopmental disability.
6.9. Analysis.
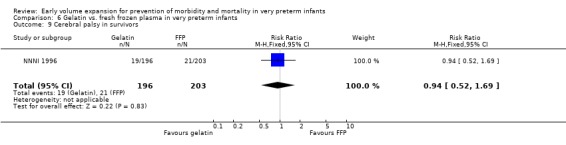
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 9 Cerebral palsy in survivors.
6.10. Analysis.
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 10 Failed treatment (hypotension) in infants born 1990‐1991.
6.11. Analysis.
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 11 Necrotising enterocolitis.
6.12. Analysis.
Comparison 6 Gelatin vs. fresh frozen plasma in very preterm infants, Outcome 12 Sepsis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beverley 1985.
| Methods | Adequate randomisation: yes, sealed envelopes. Allocation concealment: yes. Blinding of intervention: no. Blinding of measurement: yes. Losses to follow up: yes, 7 (9%) infants excluded from study due to death (1), intraventricular hemorrhage on admission (1) and treatment of hypotension or coagulopathy with fresh frozen plasma (5 controls). Data available for mortality and intraventricular haemorrhage in excluded infants. | |
| Participants | Infants <1500g or < 32 weeks. Mean gestation: Treatment group: 29.4 weeks (sd 2.4); Control: 28.8 (sd 2.1). Mean birthweight: Treatment group: 1246g (sd 400); Control: 1216g (sd 320). | |
| Interventions | Intervention (n = 38): fresh frozen plasma 10 ml/kg on admission and 24 hours of age. Control (n = 42): no treatment. | |
| Outcomes | Stated primary outcome: P/IVH. Other outcomes: mortality, respiratory distress syndrome, ventilation, pCO2 > 7kPa, pH < 7.15, mean maximal peak inspiratory pressure, mean maximal inspired oxygen, pneumothorax, patent ductus arteriosus, coagulation studies and platelet counts. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ekblad 1991.
| Methods | Adequate randomisation: method not stated. Allocation concealment: unclear. Blinding of intervention: no. Blinding of measurement: no. Losses to follow‐up: yes. Outcomes reported for 38 of 40 infants in one paper (PDA) and 35 of 40 (renal function) in the other. Data for P/IVH and mortality for the excluded infants is available and used in the analysis of these outcomes. | |
| Participants | Appropriate for gestational age preterm infants stratified by gestation: < 30 weeks (n = 19), 30‐34, weeks (n = 19). < 5 hours of age. Mean gestation: 27.8 (sd 1.7); Control: 27.6 (sd 1.6). Mean birthweight: Treatment: 1375g. Control: 1448g. | |
| Interventions | Intervention (n = 21): Fresh frozen plasma 10 ml/kg over 2 hours, daily for 3 days, no additional sodium. Control (n = 19): no treatment, sodium added to fluids. | |
| Outcomes | Stated primary outcome: water balance and extracellular volume (bromide space), renal function. Other outcomes: ventilatory assistance, respiratory distress syndrome, patent ductus arteriosus, P/IVH. | |
| Notes | Data for grade of P/IVH in excluded infants requested from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Emery 1992.
| Methods | Adequate randomisation: yes, sealed envelope. Allocation concealment: yes. Blinding of intervention: no. Blinding of measurement: no. Losses to follow‐up: none. | |
| Participants | Preterm infants day 1‐4 (23‐35 weeks, (552‐1954g), hypotension with systolic BP < 40 mmHg. Exclusions: clinical fluid overload. Median gestation: Group 1: 26 weeks (range 23‐34); Group 2: 27 weeks (range 24‐25). Median birthweight: Group 1: 958g (566‐1880). Group 2: 844g (690‐1954). | |
| Interventions | Intervention: Group 1 (n = 20) fresh frozen plasma 15 ml/kg over 3 hours. Group 3 (n = 20) 4.5% albumin 15 ml/kg over 3 hours. | |
| Outcomes | Stated primary outcome: systolic BP one and 4 hours after infusions. Other outcomes: none. | |
| Notes | No data available for inclusion in meta‐analysis. Median change BP available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gottuso 1976.
| Methods | Two centres. Adequate randomisation: yes, used sealed envelopes. Allocation concealment: yes. Blinding of intervention: no. Blinding of measurement: no. Losses to follow‐up: none. | |
| Participants | Stratified into 3 groups: Group A; birth weight 700‐1000g, < 24 hours age. Group B: 1001‐2000g, severe respiratory distress syndrome, < 24 hours age. Group C: > 1000g, any age, partial thromboplastin time > 60 secs, and acidosis or hypoxia in 60% inspired oxygen. | |
| Interventions | Intervention: Supportive care and fresh frozen plasma 15 ml/kg (n = 26). Control: supportive care only (n = 33). | |
| Outcomes | Stated primary outcome: none stated. Other outcomes: mortality, pH, pCO2, FiO2/PaO2, intracranial and pulmonary haemorrhage. | |
| Notes | Infants randomised to exchange transfusion excluded from review. Group A and B infants received treatment at mean age 7.4 to 12 hours. Group C median age 20 hours. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lundstrom 2000.
| Methods | Adequate randomisation: yes, sealed envelopes. Allocation concealment: yes. Blinding of intervention: no. Blinding of measurement: not stated. Losses to follow‐up: none. | |
| Participants | Preterm infants < 33 weeks (median 28, range 25‐32), arterial line, mean arterial BP 29 to 40 mmHg, normal blood glucose, no volume or inotrope support within preceding 3 hours. Mean postnatal age = 31.8 hrs (range 5‐224). Mean gestation: Intervention: 27.9 weeks; Control: 28.7 weeks Mean birthweight: Intervention: 1134g; Control: 1238g. | |
| Interventions | Intervention (n = 13): albumin 20% 15 ml/kg. Control (n = 12): no treatment. | |
| Outcomes | Stated primary outcome: mean arterial BP, left ventricular output, global cerebral blood flow. Other outcomes: mortality, P/IVH, periventricular leucomalacia not available according to treatment groups. | |
| Notes | Data from publication used. Clinical data for mortality obtained from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lynch 2008.
| Methods | Single centre. Adequate randomisation: yes, performed by a pharmacist using computer code generated after obtaining consent. Allocation concealment: yes. Blinding of intervention: yes, fluid was sent to the NICU in syringe concealed by an opaque wrapper. Blinding of measurement: yes. Losses to follow‐up: unclear. Abstract reported 53 infants in normal saline group, publication 52 infants. | |
| Participants | Infants admitted to NICU <24 hours age, ≦ 3 days age, mean arterial pressure <5th percentile by Versmold criteria for >10 minutes and parental permission. Infants mean gestational age (weeks) albumin group: 30.8±4.4; normal saline: 30.1±4.1. Mean birth weight (g): albumin: 1617±838; normal saline: 1528±830. | |
| Interventions | 5% albumin 10ml/kg infused over 20 minutes (n = 49). Normal saline 10ml/kg infused over 20 minutes (n = 53). Volume repeated if failure of response. Treatment success defined as resolution of hypotension after 2 boluses ‐ infants received dopamine. | |
| Outcomes | Primary outcomes: improvement in arterial BP to normal (greater than the 10th percentile for mean BP for weight) at 1 hour after infusion. Other outcomes: urine output, use of second bolus of volume, use of pressure support, average cost of treatment, average amount of nursing time required for preparation of drug. Treatment failure defined as need for pressor support for persisting hypotension (mean BP<5th percentile). Reported PIVH for infants <1500g only. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
NNNI 1996.
| Methods | Multicentre. Adequate randomisation: yes, central telephone randomisation using minimization. Allocation concealment: yes. Blinding of intervention: no. Blinding of measurement: yes. Losses to follow‐up: none for death and disability, incomplete head ultrasound data (in units with routine scanning ‐ 84% for 6 weeks survivors). | |
| Participants | Infants < 32 weeks, < 2 hours old and clinician uncertain as to whether to use volume, no indications or contraindications to any of the interventions. Median gestation: 29 weeks (range 27‐31) Median birthweight (interquartile range): Group 1: 1265g (981‐1543); Group 2: 1254g (965‐1535); Control: 1240g (980‐1510). | |
| Interventions | Intervention: Group 1 ( n = 257). Fresh frozen plasma 20 ml/kg over 15 mins, 10 ml/kg 24hrs later. Group 2 (n = 261). Gelatin‐based plasma substitute (Gelofusine) 20 ml/kg over 15 mins, 10 ml/kg 24hrs later. Control (n = 258): Maintenance fluids 60‐120 ml/kg/day. Other management according to local physician. | |
| Outcomes | Stated primary outcome: death before discharge or severe disability at 2 years. Other outcomes: P/IVH on 1 and 6 week ultrasound. | |
| Notes | 77% of eligible infants enrolled. P/IVH data taken from units with routine scanning only. Data for reclassified P/IVH and periventricular leucomalacia requested from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
So 1997.
| Methods | Adequate randomisation: yes, computer used. Allocation concealment: yes. Blinding of intervention: no. Blinding of measurement: no. Losses to follow‐up: none. | |
| Participants | Infants < 34 weeks, birthweight < 2000g, mechanically ventilated for RDS, hypotension (MAP < 25, 30, 35 mmHg at < 1kg, 1‐1.49 kg, 1.5‐1.99 kg respectively) at < 2 hours age, no previous fluids or inotropes. Exclusions: infants of mothers given antihypertensives within 24 hours of birth, severe congenital anomalies, cyanotic heart disease or left ventricular outflow anomalies Mean gestation: Intervention group: 28.1 weeks (sd 4.0); Control: 28.5 weeks (sd 2.8). Mean birthweight: Intervention: 1123g (sd 458); Control: 1163g (sd 367). | |
| Interventions | Intervention (n = 32): 5% albumin 10 ml/kg over 30 mins up to 3 doses. Control (n = 31): isotonic saline 10 ml/kg over 30 mins up to 3 doses. | |
| Outcomes | Stated primary outcome: treatment failure (persistent hypotension requiring dopamine). Other outcomes: need for extra volume, weight gain, urine output, serum sodium, patent ductus (echo confirmed), P/IVH (ultrasound at 2 and 6 days and 3 weeks), necrotising enterocolitis, chronic lung disease (oxygen at 28 days and 36 weeks post conception), and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 1976 | Fresh frozen plasma versus no treatment. Printed in abstract form only. Volume of fresh frozen plasma not stated. No clinical data could be extracted for inclusion in review. |
| Alkalay 1999 | No control group. |
| Andrew 1993 | Randomised trial of platelet transfusion in thrombocytopenic newborns. |
| Barr 1977 | No control group. |
| Bauer 1993 | No control group. |
| Beeram 2003 | Randomised trial of two different volumes of packed red cell transfusions in haemodynamically stable very low birth weight infants. |
| Belgaumkar 1998 | Retrospective cohort of 10 ml/kg 4.5% albumin infusion in 26 ventilated normotensive neonates with metabolic acidosis. |
| Bell 1980 | Randomised trial of higher and lower fluid intakes. |
| Bignall 1989 | No control group. |
| Bland 1976 | Insufficient volume to meet inclusion criteria. Mean age of infants > 32 weeks. Randomised preterm infants < 37 weeks with serum protein < 4.6 g% to receive glucose versus albumin 25% 8 ml/kg within 2 hours of birth. |
| Blank 1984 | Randomized to red cell transfusion to maintain Hb > 10 g/L or control group. Intervention predominantly late. |
| Chan 1976 | Randomised to albumin 1g/kg versus no treatment prior to exchange transfusion. |
| Costarino 1992 | Randomised trial of higher and low sodium intake. |
| Delivoria 1976 | Used alternation to assign infants to exchange transfusion or control. |
| Dimitriou 2001 | No control group. Observed effect of albumin infusion on BP, pH and core‐peripheral temperature difference. |
| Dixon 1999 | Randomised trial of 10ml/kg 4.5% albumin versus 4.2% sodium bicarbonate in acidotic newborn infants. Gestations not reported. Infants with hypotension or evidence of acute blood loss excluded. |
| Ekblad 1987 | Randomised to high and low sodium intake. |
| Greenough 1993 | Given inadequate volume: 20% albumin 5 ml/kg given at maintenance fluid rate instead of maintenance fluids. Placebo was maintenance fluids. |
| Gurkan 2000 | Randomised trial of 20% albumin 0.5g/kg in term newborns with perinatal asphyxia. |
| Hosono 2001 | Randomised trial of albumin infusion in term infants with non‐haemolytic hyperbilirubinaemia. |
| Kanarek 1991 | Randomised after 48‐72 hours of age to addition of albumin to total parenteral nutrition. |
| Kavvadia 2000 | Trial of fluid restriction. |
| Lambert 1998 | No control group. |
| Lay 1980 | No control group. |
| Leake 1976 | Infants 3 to 16 days age. Infants randomly assigned to different infusion rates of maintenance fluids. |
| Liet 2003 | Neonates 690–4030g, gestational age 26–40 weeks without cardiac or renal failure or major hemostasis abnormalities and requiring a peripherally inserted central catheter for parenteral nutrition. Median (1st/3rd quartiles) age enrolment HES group: day 3 (3, 6); saline: day 5 (4, 6). |
| Liet 2006 | Hypotensive preterm infants (mean gestational age of 29 +/‐3 week) randomly allocated to 10ml/kg 5% albumin versus isotonic saline versus hydroxyethyl starch (HES). Infants age at randomisation (days): albumin: 2+/‐1; saline: 6 +/‐10; HES: 5 +/‐ 6. |
| McMurray 1948 | Insufficient volume: used 3ml 25g/dl albumin injected per pound bodyweight 1–2 times weekly versus no albumin. Given after day 3. Treatment allocation by alternation with process not strictly adhered to. |
| Meyer 1993 | Randomised to packed red cell transfusion to maintain hematocrit > 0.35 or control. Mean time of transfusion 28 days |
| Nelle 1997 | Enrolled preterm infants with anaemia. Studied effect of blood transfusion. No control group. |
| Nose 1996 | Infants < 4 days old excluded. Randomised study of 2 different infusion rates of red cell transfusion. |
| Oca 2003 | Enrolled 50 newborn infants with hypotension in first 24 hours. Stratified randomisation of infants <2500g and >=2500. Excluded because of excessive post randomisation losses (9/39 =23%) for infants <2500g. |
| Papile 1978 | Observational study of effects of sodium bicarbonate in acidotic infants. |
| Paul 2002 | Comparison of 10 versus 20 ml/kg red blood cells in infants <1500g. Mean postnatal age 23‐26 days. |
| Paul 2003 | Enrolled infants aged 1–38 months, allocated randomly to 20 ml/kg HES 70/0.5 or Ringer's lactate during the first hour of urological surgery. |
| Pladys 1994 | No control group. |
| Pladys 1997 | No control group. |
| Sinclair 1968 | Infants randomised to varying oxygen, alkali (rapid infusion of sodium bicarbonate) and ventilation strategies. |
| Sriram 1997 | No control group. |
| Stoddart 1996 | Enrolled neonates undergoing major surgery. They were excluded if the body weight was less than 2kg. |
| Upadhyay 2005 | Enrolled infants with septic shock 1 month to 12 years age. |
| Warburton 1983 | Randomised to different volumes of maintenance fluids. |
| Weisman 1992 | Randomised infants with early onset sepsis to 10ml/kg intravenous immunoglobulin or albumin. |
| Wirth 1979 | Control group received bicarbonate. Crossover study of bicarbonate and albumin. Gestational ages of infants not stated. Printed in abstract form only. |
| Wong 2005 | Infants <1500g with HCT <0.30 randomised to 2 different red cell transfusion volumes. |
| Wright 1995 | Unable to obtain data from author. |
Contributions of authors
DO wrote the review update. Both authors performed the updated literature search, assessed eligibility, critically appraised articles and performed data extraction independently.
Sources of support
Internal sources
RPA Newborn Care, Royal Prince Alfred Hospital, Sydney, Australia.
External sources
NSW Centre for Perinatal Health Services Research, University of Sydney, Australia.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Beverley 1985 {published data only}
- Beverley DW, Pitts‐Tucker TJ, Congdon PJ, Arthur RJ, Tate G. Prevention of intraventricular haemorrhage by fresh frozen plasma. Archives of Disease in Childhood 1985;60:710‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekblad 1991 {published data only}
- Ekblad H, Kero P, Korvenranta H. Renal function in preterm infants during the first five days of life: influence of maturation and early colloid treatment. Biology of the Neonate 1992;61:308‐17. [DOI] [PubMed] [Google Scholar]
- Ekblad H, Kero P, Shaffer SG, Korvenranta H. Extracellular volume in preterm infants: influence of gestational age and colloids. Early Human Development 1991;27:1‐7. [DOI] [PubMed] [Google Scholar]
Emery 1992 {published data only}
- Emery EF, Greenough A, Gamsu HR. Randomised controlled trial of colloid infusions in hypotensive preterm infants. Archives of Disease in Childhood 1992;67:1185‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottuso 1976 {published data only}
- Gottuso MA, Williams ML, Oski FA. The role of exchange transfusion in the management of low‐birth‐weight infants with and without severe respiratory distress syndrome. Journal of Pediatrics 1976;89:279‐85. [DOI] [PubMed] [Google Scholar]
Lundstrom 2000 {published data only}
- Lundstrom K, Pryds O, Greisen G. The haemodynamic effects of dopamine and volume expansion in sick preterm infants. Early Human Development 2000;57:157‐3. [DOI] [PubMed] [Google Scholar]
- Lundstrom KE. A randomized, controlled study of the influence of dopamine and volume expansion on CBF, LVO and MABP in hypotensive, preterm, infants. Proceedings of 14th European Congress of Perinatal Medicine, Helsinki, Finland. 1994:500.
Lynch 2008 {unpublished data only}
- Lynch SK, Mullett MD, Graeber JE, Polak MJ. A comparison of albumin‐bolus therapy versus normal saline‐bolus therapy for hypotension in neonates. Journal of Perinatology 2008;28:29‐33. [DOI] [PubMed] [Google Scholar]
- Lynch SK, Stone CS, Graeber J, Polak MJ. Colloid vs. cystalloid therapy for hypotension in neonates. Pediatric Research. 2002; Vol. 51:384A.
- Mullett MD, Lynch SK, Graeber JE, Stone C, Polak MJ. Cost comparison of albumin (ALB) versus cormal saline (NS) therapy for hypotension in neonates. Pediatric Research. 2002; Vol. 51:370A.
NNNI 1996 {published data only}
- Fooks J, Fritz S, Tin W, Yudkin P, Johnson A, Elbourne D, Hey E. A comparison of two methods of follow‐up in a trial of prophylactic volume expansion in preterm babies. Paediatric and Perinatal Epidemiology 1998;12:199‐216. [DOI] [PubMed] [Google Scholar]
- Fooks J, Mutch L, Yudkin P, Johnson A, Elbourne D. Comparing two methods of follow up in a multicentre randomised trial. Archives of Disease in Childhood 1997;76:369‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Northern Neonatal Nursing Initiative [NNNI] Trial Group. A randomized trial comparing the effect of prophylactic intravenous fresh frozen plasma, gelatin or glucose on early mortality and morbidity in preterm babies. European Journal of Pediatrics 1996;155:580‐8. [DOI] [PubMed] [Google Scholar]
- The Northern Neonatal Nursing Initiative [NNNI] Trial Group. Randomized trial of prophylactic early fresh‐frozen plasma or gelatin or glucose in preterm babies: outcome at 2 years. Lancet 1996;348:229‐32. [PubMed] [Google Scholar]
So 1997 {published data only}
- So KW, Fok TF, Ng PC, Wong WW, Cheung KL. Isotonic saline vs 5% albumin as volume expander. Pediatric Research. 1996; Vol. 39:246A.
- So KW, Fok TF, Ng PC, Wong WW, Cheung KL. Randomised controlled trial of colloid or crystalloid in hypotensive preterm infants. Archives of Disease in Childhood 1997;76:F43‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 1976 {published data only}
- Alexander G, Visveshwara N, Bauer CR. Prophylactic exchange transfusion in the very low birth weight infant. Pediatric Research 1976;10:420. [Google Scholar]
Alkalay 1999 {published data only}
- Alkalay AL, Galvis S, Ferry DA, Sola A. Packed red blood cell transfusions do not modify cardiac function of premature infants with "very low hematocrits". Pediatric Research 1999;45:261A. [Google Scholar]
Andrew 1993 {published data only}
- Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A, Watts J, Saigal S, Milner R, Wang E. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. Journal of Pediatrics 1993;123:285‐91. [DOI] [PubMed] [Google Scholar]
Barr 1977 {published data only}
- Barr PA, Bailey PE, Sumners J, Cassady G. Relation between arterial blood pressure and blood volume and effect of infused albumin in sick preterm infants. Pediatrics 1977;60:282‐9. [PubMed] [Google Scholar]
Bauer 1993 {published data only}
- Bauer K, Linderkamp O, Versmold HT. Short‐term effects of blood transfusion on blood volume and resting peripheral blood flow in preterm infants. Acta Paediatrica 1993;82:1029‐33. [DOI] [PubMed] [Google Scholar]
Beeram 2003 {unpublished data only}
- Beeram MR, Ginther S, Olvera R, Loughran C. Safety and efficacy of high volume (20cc/kg) vs low volume (15cc/kg) packed red blood cell transfusions (PRBCTs) in very low birthweight infants (VLBW): double blinded randomized study. Pediatric Research 2003;53:A2063. [Google Scholar]
Belgaumkar 1998 {published data only}
- Belgaumkar A, Greenough A, Kavvadia V, Dimitriou G. Metabolic acidosis: Response to albumin infusion. European Journal of Pediatrics 1998;157:520‐1. [DOI] [PubMed] [Google Scholar]
Bell 1980 {published data only}
- Bell EF, Warburton D, Stonestreet BS, Oh W. High‐volume fluid intake predisposes premature infants to necrotising enterocolitis. Lancet 1979;2 (8133):90. [DOI] [PubMed] [Google Scholar]
- Bell EF. Warburton D, Stonestreet BS, Oh W. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. NEJM 1980;302:598‐604. [DOI] [PubMed] [Google Scholar]
Bignall 1989 {published data only}
- Bignall S, Bailey PC, Bass CA, Cramb R, Rivers RP, Wadsworth J. The cardiovascular and oncotic effects of albumin infusion in premature infants. Early Human Development 1989;20:191‐201. [DOI] [PubMed] [Google Scholar]
Bland 1976 {published data only}
- Bland RD, Clarke TL, Harden LB. Rapid infusion of sodium bicarbonate and albumin into high‐risk premature infants soon after birth: a controlled, prospective trial. American Journal of Obstetrics and Gynecology 1976;124:363‐7. [DOI] [PubMed] [Google Scholar]
Blank 1984 {published data only}
- aBlank JP, Sheagren TG, Vajaria J, Mangurten HH, Benawra RS, Puppala BL. The role of RBC transfusion in the premature infant. Am J Dis Child 1984;138:831‐3. [DOI] [PubMed] [Google Scholar]
Chan 1976 {published data only}
- Chan G, Schiff D. Variance in albumin loading in exchange transfusions. American Journal of Perinatology 1976;88:609‐13. [DOI] [PubMed] [Google Scholar]
Costarino 1992 {published data only}
- Costarino AT Jr, Gruskay JA, Corcoran L, Polin RA, Baumgart S. Sodium restriction versus daily maintenance replacement in very low birth weight premature neonates: a randomized, blind therapeutic trial. Journal of Pediatrics 1992;120:88‐106. [DOI] [PubMed] [Google Scholar]
Delivoria 1976 {published data only}
- Delivoria‐Papadopoulos M, Miller LD, Forster RE, Oski FA. The role of exchange transfusion in the management of low‐birth‐weight infants with and without severe respiratory distress syndrome. Journal of Pediatrics 1976;89:273‐8. [DOI] [PubMed] [Google Scholar]
Dimitriou 2001 {published data only}
- Dimitriou G, Greenough A, Mantagos J, Skinner S. Metabolic acidosis, core‐peripheral temperature difference and blood pressure response to albumin infusion in hypotensive, very premature infants. Journal of Perinatal Medicine 2001;29:442‐5. [DOI] [PubMed] [Google Scholar]
Dixon 1999 {published data only}
- Dixon H, Hawkins K, Stephenson T. Comparison of albumin versus bicarbonate treatment for neonatal metabolic acidosis. European Journal of Pediatrics 1998;158:414‐5. [DOI] [PubMed] [Google Scholar]
Ekblad 1987 {published data only}
- Ekblad H, Kero P, Takala J, Korvenranta H, Valimaki I. Water, sodium and acid‐base balance in premature infants: therapeutical aspects. Acta Paediatrica Scandinavica 1987;76:47‐53. [DOI] [PubMed] [Google Scholar]
Greenough 1993 {published data only}
- Greenough A, Emery E, Hird MF, Gamsu HR. Randomised controlled trial of albumin infusion in ill preterm infants. European Journal of Pediatrics 1993;152:157‐9. [DOI] [PubMed] [Google Scholar]
Gurkan 2000 {published data only}
- Gurkan F, Haspolat K, Yaramis A, Ece A. Beneficial effect of human albumin on neonatal cerebral edema. American Journal of Therapeutics 2000;8:253‐4. [DOI] [PubMed] [Google Scholar]
Hosono 2001 {published data only}
- Hosono S, Ohno T, Kimoto H, Nagoshi R, Shimizu M, Nozawa M. Effects of albumin infusion therapy on total and unbound bilirubin values in term infants with intensive phototherapy. Pediatrics International 2001;43:8‐11. [DOI] [PubMed] [Google Scholar]
Kanarek 1991 {published data only}
- Kanarek KS, Adamson M, Williams PR, Blair C. Concurrent administration of albumin with total parenteral nutrition (TPN) in ill newborn infants. Pediatric Research. 1991; Vol. 29:1771.
- Kanarek KS, Williams PR, Blair C. Concurrent administration of albumin with total parenteral nutrition in sick newborn infants. J Parenter Enteral Nutr 1992;16:49‐53. [DOI] [PubMed] [Google Scholar]
Kavvadia 2000 {published data only}
- Greenough A, Cheeseman P, Kavvadia V, Dimitriou G, Morton M. Colloid infusion in the perinatal period and abnormal neurodevelopmental outcome in very low birth weight infants. European Journal of Pediatrics 2000;161:319‐23. [DOI] [PubMed] [Google Scholar]
- Greenough A, Cheeseman P, Kavvadia V, Dimitriou G, Morton M. Colloid infusion in the perinatal period and abnormal neurodevelopmental outcome in very low birth weight infants. European Journal of Pediatrics 2002;161:319‐23. [DOI] [PubMed] [Google Scholar]
- Kavvadia V, Greenough A, Dimitriou G, Hooper R. Randomised trial of fluid restriction in ventilated very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;83:F91‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert 1998 {published data only}
- Lambert HJ, Baylis PH, Coulthard MG. Central‐peripheral temperature difference, blood pressure, and arginine vasopressin in preterm neonates undergoing volume expansion. Archives of Disease in Childhood 1998;78:F43‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lay 1980 {published data only}
- Lay KS, Bancalari E, Malkus H, Baker R, Strauss J. Acute effects of albumin infusion on blood volume and renal function in premature infants with respiratory distress syndrome. Journal of Pediatrics 1980;97:619‐23. [DOI] [PubMed] [Google Scholar]
Leake 1976 {published data only}
- Leake RD, Zakauddin S, Trygstad CW, Fu P, Oh W. The effects of large volume intravenous fluid infusion on neonatal renal function. Journal of Pediatrics 1976;89:968‐72. [DOI] [PubMed] [Google Scholar]
Liet 2003 {published data only}
- Liet JM, Bellouin AS, Boscher C, Lejus C, Roze JC. Plasma volume expansion by medium molecular weight hydroxyethyl starch in neonates: A pilot study. Pediatric Critical Care Medicine 2003;4:305‐7. [DOI] [PubMed] [Google Scholar]
Liet 2006 {published data only}
- Liet JM, Kuster A, Denizot S, Caillaux‐Varin G, Gras‐Leguen C, Roze JC. Effects of hydroxyethyl starch on cardiac output in hypotensive neonates: A comparison with isotonic saline and 5% albumin. Acta Paediatrica 2006;95:555‐60. [DOI] [PubMed] [Google Scholar]
McMurray 1948 {published data only}
- McMurray L‐G, Roe JH, Sweet LK. Plasma protein studies on normal newborn and premature infants. I. Plasma protein values for normal full term and normal premature infants; II. Use of concentrated normal human serum albumin in treatment of premature infants. Am J Dis Child 1948;75:265‐78. [PubMed] [Google Scholar]
Meyer 1993 {published data only}
- Meyer J, Sive A, Jacobs P. Empiric red cell transfusion in asymptomatic preterm infants. Acta Paediatr 1993;82:30‐4. [DOI] [PubMed] [Google Scholar]
Nelle 1997 {published data only}
- Nelle M, Hoecker C, Linderkamp O. Effects of red cell transfusion on pulmonary blood flow and right ventricular systolic time intervals in neonates. European Journal of Pediatrics 1997;156:553‐6. [DOI] [PubMed] [Google Scholar]
Nose 1996 {published data only}
- Nose Y, Tamai H, Shimada S, Funato M. Haemodynamic effects of differing blood transfusion rates in infants less than 1500 g. Journal of Paediatrics and Child Health 1996;32:177‐82. [DOI] [PubMed] [Google Scholar]
Oca 2003 {published data only}
- Oca MJ, Nelson M, Donn SM. Randomized trial of normal saline (NS) versus 5% albumin (ALB) for the treatment of neonatal hypotension. Pediatric Research 1999;45:215A. [DOI] [PubMed] [Google Scholar]
- Oca MJ, Nelson M, Donn SM. Randomized trial of normal saline versus 5% albumin for the treatment of neonatal hypotension. Journal of Perinatology 2003;23:473‐6. [DOI] [PubMed] [Google Scholar]
Papile 1978 {published data only}
- Papile LA, Burstein J, Burstein R, Koffler H, Koops B. Relationship of intravenous sodium bicarbonate infusions and cerebral intraventricular hemorrhage. Journal of Pediatrics 1978;93:834‐6. [DOI] [PubMed] [Google Scholar]
Paul 2002 {published data only}
- Paul DA, Leef KH, Locke RG, Stefano JL. Transfusion volume in infants with very low birth weight: a randomized trial of 10 versus 20 ml/kg. Journal of Pediatric Hematology/Oncology 2002;24:43‐6. [DOI] [PubMed] [Google Scholar]
Paul 2003 {published data only}
- Paul M, Dueck M, Joachim Herrmann H, Holzki J. A randomized, controlled study of fluid management in infants and toddlers during surgery: Hydroxyethyl starch 6% (hes 70/0.5) vs lactated ringer's solution. Paediatric Anaesthesia 2003;13:603‐8. [DOI] [PubMed] [Google Scholar]
Pladys 1994 {published data only}
- Pladys P, Betremieux P, Lefrancois C, Schleich JM, Gourmelon N, Marec B. Doppler echocardiography in the evaluation of volume expansion effects in newborn infants. Arch Pediatr 1994;1:470‐6. [PubMed] [Google Scholar]
Pladys 1997 {published data only}
- Pladys P, Wodey E, Betremieux P, Beuchee A, Ecoffey C. Effects of volume expansion on cardiac output in the preterm infant. Acta Paediatrica 1997;86:1241‐5. [DOI] [PubMed] [Google Scholar]
Sinclair 1968 {published data only}
- Sinclair JC, Engel K, Silverman WA. Early correction of hypoxemia and acidemia in infants of low birth weight: a controlled trial of oxygen breathing, rapid alkali infusion, and assisted ventilation. Pediatrics 1968;42:565‐89. [PubMed] [Google Scholar]
Sriram 1997 {published data only}
- Sriram S, Gizowski K, Ramirez J, Agarwala B, Ruschhaupt D, Meadow W. Hemodynamic consequences of volume infusions in the NICU: What's cardiac output got to do with it?. Pediatric Research 1997;41:179A. [Google Scholar]
Stoddart 1996 {published data only}
- Stoddart PA, Rich P, Sury MR. A comparison of 4.5% human albumin solution and Haemaccel in neonates undergoing major surgery. Paediatric Anaesthesia 1996;6:103‐6. [DOI] [PubMed] [Google Scholar]
Upadhyay 2005 {published data only}
- Upadhyay M, Singhi S, Murlidharan J, Kaur N, Majumdar S. Randomized evaluation of fluid resuscitation with crystalloid (saline) and colloid (polymer from degraded gelatin in saline) in pediatric septic shock. Indian Pediatrics 2005;42:223‐31. [PubMed] [Google Scholar]
Warburton 1983 {published data only}
- Warburton D, Bell EF, Stonestreet BS, Oh W. Echocardiographic effects of high and low volumes of maintenance fluid administration in low‐birth‐weight infants. Dev Parmacol Ther 1983;6:45‐54. [DOI] [PubMed] [Google Scholar]
Weisman 1992 {published data only}
- Weisman LE, Stoll BJ, Kueser TJ, Rubio TT. Frank CG, Heiman HS, Subramanian KN, Hankins CT. Anthony BF, Cruess DF, et al. Intravenous immune globulin therapy for early‐onset sepsis in premature neonates. Journal of Pediatrics 1992;121:434‐43. [DOI] [PubMed] [Google Scholar]
Wirth 1979 {published data only}
- Wirth FH, Squires P. The treatment of metabolic acidosis (MA) in newborn infants with respiratory distress (RD). A random prospective trial alternating albumin and NaHCO3 infusions in the same infant. Pediatric Research 1979;13:484. [Google Scholar]
- Wirth FH, Squires P. The treatment of metabolic acidosis in newborn infants with respiratory distress. A random prospective trial alternating albumin and NaHCO3 infusions in the same infant. Clinical Research 1979;27:824A. [Google Scholar]
Wong 2005 {published data only}
- Wong W, Fok TF, Lee CH, Ng PC, So KW, Ou Y, Cheung KL. Randomised controlled trial: Comparison of colloid or crystalloid for partial exchange transfusion for treatment of neonatal polycythaemia. Archives of Disease in Childhood Fetal & Neonatal Edition 1997;77:F115‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1995 {published data only}
- Wright IMR, Levene MI, Arthur RA, Martinez D. A randomised controlled trial of fresh frozen plasma and volume expansion in very low birthweight and sick preterm infants. British Paediatric Association Annual Meeting. 1995; Vol. 67:52A.
Additional references
Alderson 2004
- The Albumin Reviewers (Alderson P, Bunn F, Lefebvre C, Li Wan Po A, Li L, Roberts I, Schierhout G). Human albumin solution for resuscitation and volume expansion in critically ill patients (Cochrane Review). Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001208.pub2] [DOI] [PubMed] [Google Scholar]
Bauer 1993
- Bauer K, Linderkamp O, Versmold HT. Systolic blood pressure and blood volume in preterm infants. Archives of Disease in Childhood 1993;69:521‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bignall 1989
- Bignall S, Bailey PC, Bass CA, Cramb R, Rivers RP, Wadsworth J. The cardiovascular and oncotic effects of albumin infusion in premature infants. Early Human Development 1989;20:191‐201. [DOI] [PubMed] [Google Scholar]
Bourchier 1997
- Bourchier D, Weston PJ. Randomised trial of dopamine compared with hydrocortisone for the treatment of hypotensive very low birthweight infants. Archives of Disease in Childhood 1997;76:F174‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaissmaier 1999
- Gaissmaier RE, Pohlandt F. Single‐dose dexamethasone treatment of hypotension in preterm infants. Journal of Pediatrics 1999;134:701‐5. [DOI] [PubMed] [Google Scholar]
Goldberg 1980
- Goldberg RN, Chung D, Goldman SL, Bancalari E. The association of rapid volume expansion and intraventricular hemorrhage in the preterm infant. Journal of Pediatrics 1980;96:1060‐3. [DOI] [PubMed] [Google Scholar]
Goldstein 1995
- Goldstein RF, Thompson RJ Jr, Oehler JM, Brazy JE. Influence of acidosis, hypoxemia, and hypotension on neurodevelopmental outcome in very low birth weight infants. Pediatrics 1995;95:238‐43. [PubMed] [Google Scholar]
Greenough 1993a
- Greenough A, Emery EF. Randomized trial comparing dopamine and dobutamine in preterm infants. European Journal of Pediatrics 1993;152:925‐7. [DOI] [PubMed] [Google Scholar]
Hentschel 1995
- Hentschel R, Hensel D, Brune T, Rabe H, Jorch G. Impact on blood pressure and intestinal perfusion of dobutamine or dopamine in hypotensive preterm infants. Biology of the Neonate 1995;68:318‐24. [DOI] [PubMed] [Google Scholar]
Hunt 2004
- Hunt RW, Evans N, Rieger I, Kluckow M. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. Journal of Pediatrics 2004;145:588‐92. [DOI] [PubMed] [Google Scholar]
Klarr 1994
- Klarr JM, Faix RG, Pryce CJ, Bhatt‐Mehta V. Randomized, blind trial of dopamine versus dobutamine for treatment of hypotension in preterm infants with respiratory distress syndrome. Journal of Pediatrics 1994;125:117‐22. [DOI] [PubMed] [Google Scholar]
Kluckow 1996
- Kluckow M, Evans N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. Journal of Pediatrics 1996;129:506‐12. [DOI] [PubMed] [Google Scholar]
Kluckow 2000
- Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage. Archives of Disease in Childhood 2000;82:F188‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorenz 1998
- Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med 1998;152:426‐35. [DOI] [PubMed] [Google Scholar]
Low 1993
- Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The ssociation between preterm newborn hypotension and hypoxemia and outcome during the first year. Acta Paediatrica 1993;82:433‐7. [DOI] [PubMed] [Google Scholar]
Meek 1999
- Meek JH, Tyszckuk L, Elwell CE, Wyatt S. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Archives of Disease in Childhood 1999;81:F15‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miall‐Allen 1987
- Miall‐Allen VM, Vries LS, Whitelaw AG. Mean arterial blood pressure and neonatal cerebral lesions. Archives of Disease in Childhood 1987;62:1068‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miletin 2008
- Miletin J, Dempsey EM. Low superior vena cava flow on day one and adverse outcome in the very low birth weight infant. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(5):F368‐71. [DOI] [PubMed] [Google Scholar]
Osborn 2002
- Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. Journal of Pediatrics 2002;140:183‐91. [DOI] [PubMed] [Google Scholar]
Osborn 2003
- Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 2003;112:33‐9. [DOI] [PubMed] [Google Scholar]
Osborn 2004a
- Osborn DA, Evans N, Kluckow M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central‐peripheral temperature difference. Archives of Disease in Childhood Fetal & Neonatal Edition 2004;89:F168‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2007
- Osborn DA, Evans N, Kluckow M, Bowen JR, Rieger I. Low superior vena cava flow and effect of inotropes on neurodevelopment to 3 years in preterm infants. Pediatrics 2007;120:372‐80. [DOI] [PubMed] [Google Scholar]
Osborn 2007a
- Osborn DA, Paradisis M, Evans N. The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellicer 2005
- Pellicer A, Valverde E, Elorza MD, Madero R, Gaya F, Quero J, Cabanas F. Cardiovascular support for low birth weight infants and cerebral hemodynamics: A randomized, blinded, clinical trial. Pediatrics 2005;115:1501‐12. [DOI] [PubMed] [Google Scholar]
Roze 1993
- Roze JC, Tohier C, Maingueneau C, Lefevre M, Mouzard A. Response to dobutamine and dopamine in the hypotensive very preterm infant. Archives of Disease in Childhood 1993;69:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subhedar 2003
- Subhedar NV, Shaw NJ. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001242.] [DOI] [PubMed] [Google Scholar]
Valverde 2006
- Valverde E, Pellicer A, Madero R, Elorza D, Quero J, Cabanas F. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: Analysis of systemic effects and neonatal clinical outcomes. Pediatrics 2006;117:e1213‐22. [DOI] [PubMed] [Google Scholar]
Van Marter 1990
- Marter LJ, Leviton A, Allred EN, Pagano M, Kuban KC. Hydration during the first days of life and the risk of bronchopulmonary dysplasia in low birth weight infants. Journal of Pediatrics 1990;116:945‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Osborn 2001
- Osborn DA, Evans N. Early volume expansion for prevention of morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002055] [DOI] [PubMed] [Google Scholar]
Osborn 2004
- Osborn DA, Evans N. Early volume expansion for prevention of morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002055.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


