Abstract
Introduction:
We investigated multi-domain baseline neurocognition of primary brain tumor patients prior to radiotherapy (RT), including clinical predictors of function and association between pre-RT and post-RT impairment on a prospective trial.
Methods:
A multi-domain neuropsychological battery (memory, executive functioning, language, attention, processing) was performed on 37 patients, pre-RT and 3-(n=21), 6-(n=22) and 12-(n=14) months post-RT. Impairment rate was the proportion of patients with standardized T-scores≤ 1.5 standard deviations below normative means. Per-patient impairment across all domains was calculated using a global deficit score (GDS; higher value indicates more impairment). Associations between baseline GDS and clinical variables were tested. Global GDS impairment rate at each time point was the fraction of patients with GDS scores> 0.5.
Results:
Statistically significant baseline neurocognitive impairments were identified on 4 memory (all p≤0.03) and 2 out of 3 (p=0.01, p=0.027) executive functioning tests. Per-patient baseline GDS was significantly associated with tumor volume (p=0.048), tumor type (p=0.043), seizure history (p=0.007), and use of anti-epileptics (p=0.009). The percentage of patients with the same impairment status at 3-, 6-, and 12-months as at baseline were 88%, 85%, and 85% respectively.
Conclusions:
Memory and executive functioning impairment were the most common cognitive deficits prior to RT. Patients with larger tumors, more aggressive histology, and use of anti-epileptics had higher baseline GDS values. GDS is a promising tool to encompass multi-domain neurocognitive function, and baseline GDS can identify those at risk of cognitive impairment.
Keywords: radiotherapy, neurocognitive function, primary brain tumors, global deficit score
Introduction
Post-treatment neurocognitive impairment is an unfortunate consequence of brain radiotherapy (RT) and is a critical outcome in brain tumor clinical trials[1]. As a result, there has been growing interest within the radiation oncology community to understand the brain physiology mediating these changes[2,3], including analyses of imaging biomarkers of microstructural changes that may underlie cognitive impairment[4,5]. Other investigations have looked into strategies to alter treatment in order to mitigate this adverse cognitive outcome[6–8]. As critical an outcome as neurocognitive functioning is, in the current clinical workflow for brain tumor RT, neither baseline nor follow-up formal neurocognitive evaluation are standard of care at most centers. While brain tumor patients do undergo standard neurological examination at baseline to detect gross deficits like sensory and motor deficiencies, or severe difficulties with expressive language, detecting subtle impairments in more complex cognitive functions requires formal neuropsychological testing. In order to better understand how and why neurocognitive changes occur in brain tumor patients receiving RT, it is critical to understand how cognitively impaired primary brain tumor patients are at the outset.
Baseline neuropsychological performance has shown to be a critical predictor of follow-up neurocognitive function for other interventions, such as systemic therapy[9]. In a cohort of 84 breast cancer patients, 35% exhibited cognitive impairment prior to systemic therapy initiation[9]. In a separate longitudinal study of breast cancer patients[10], the authors found that all patients who exhibited baseline impairment remained impaired at 12-months, while 48% of patients who were not impaired at baseline showed signs of impairment at follow-up after chemotherapy. Similar work in primary brain tumor patients before RT is lacking.
The goal of this study was to analyze and characterize baseline neurocognitive functioning of a cohort of primary brain tumor patients planned for fractionated RT, across multiple cognitive domains. Our questions included: What are the impairment rates across neurocognitive tests and domains prior to RT? Are there any significant associations between per-patient level of impairment and clinical characteristics? Are there any significant differences in per-patient impairment rates post-RT when compared to baseline? Detailed characterization of the baseline neurocognitive status of brain tumor patients undergoing RT (especially since many are post-operative) and how this status affects follow-up cognitive function may provide valuable insight to help guide intervention and neuro-protective strategies.
Material and Methods
Patient selection
Adult patients with primary brain tumors (n=37) were included on this IRB-approved longitudinal prospective clinical trial if: age≥18 years, Karnofsky performance status ≥70, estimated life expectancy >1 year, and ability to undergo neurocognitive testing in English. Clinical and tumor characteristics among the patient cohort at baseline are listed in Table 1. All patients received fractionated partial brain RT, and patients who had received prior RT were not eligible. Radiotherapy treatment characteristics are shown in Supplementary Table 1. None of the patients were receiving chemotherapy at the time of the baseline neurocognitive evaluation. The study was approved by the University of California San Diego and all patients provided written informed consent.
Table 1. Clinical and tumor characteristics at baseline.
Median values and interquartile ranges are listed for continuous variables (age, PTV volume, years of education) and breakdown by levels are listed for categorical variables (use of antiepileptic drugs, evidence of seizures, sex, use of steroids, surgery, tumor hemisphere, tumor location, and tumor type). All variables shown were used in building models to predict impairment at baseline.
| Characteristic | Patient Cohort, n = 37 [%] |
|---|---|
| Age [years] | |
| Median | 50 |
| Interquartile range | 40 – 59 |
| PTV volume [cm3] | |
| Median | 148 |
| Interquartile range | 51.8 – 331.8 |
| Years of formal education | |
| Median | 15 |
| Interquartile range | 12 – 18 |
| AED use at baseline: n [%] | |
| Yes | 21 [56.8%] |
| No | 16 [43.2%] |
| Seizures at baseline: n [%] | |
| Yes | 20 [54.1%] |
| No | 17 [45.9%] |
| Sex: n [%] | |
| Male | 21 [56.8%] |
| Female | 16 [43.2%] |
| Steroids: n [%] | |
| No | 19 [51.4%] |
| Yes | 18 [48.6%] |
| Surgery: n [%] | |
| Subtotal resection/biopsy | 25 [67.6] |
| Gross total resection | 6 [16.2%] |
| None | 6 [16.2%] |
| Tumor hemisphere: n [%] | |
| Left | 19 [51.4%] |
| Right | 11 [29.7%] |
| Other | 7 [18.9%] |
| Tumor location: n [%] | |
| Temporal | 10 [27.0%] |
| Frontal | 8 [21.6%] |
| Suprasellar | 6 [16.2%] |
| Cavernous sinus | 3 [8.1%] |
| Parietal | 3 [8.1%] |
| Cerebellar | 3 [8.1%] |
| Base of skull | 3 [8.1%] |
| Sphenoid wing | 1 [2.7%] |
| Tumor type: n [%] | |
| Glioblastoma | 12 [32.4%] |
| Meningioma | 9 [24.3%] |
| Anaplastic astrocytoma | 4 [10.8%] |
| Astrocytoma, grade 2 | 2 [5.4%] |
| Craniopharyngioma | 2 [5.4%] |
| Oligodendroglioma, grade 2 | 2 [5.4%] |
| Pituitary Adenoma | 2 [5.4%] |
| Ependymoma, grade 2 | 1 [2.7%] |
| Low grade chondrosarcoma | 1 [2.7%] |
| Pilocytic astrocytoma, grade 1 | 1 [2.7%] |
| Schwannoma | 1 [2.7%] |
Neurocognitive assessment
A formal comprehensive battery of 6 neurocognitive tests with 13 individual cognitive indices was performed on patients prior to, and at select time points after brain RT (3, 6, and 12 months), Table 2. These tests focused on the multiple cognitive domains most commonly affected in patients undergoing RT[11], including executive functioning, memory, language, attention and processing speed. Raw scores were converted to standardized T-scores, after adjusting for (where applicable) age, sex, ethnicity, and years of education. T-scores are standardized numerical values where the mean and standard deviation within the population are 50 and 10, respectively. Descriptions of the neurocognitive tests, along with additional references are presented in Supplementary Table 2.
Table 2. Baseline per-test impairment rate by neurocognitive test.
Per-test impairment rates are expressed as the percentage of patients with T-scores less than or equal to 35. A binomial test was used to evaluate whether the impairment rate was greater than that which could be observed in a normal population of healthy individuals (6.7%). Tests are sorted by impairment rate (largest to least).
| Test Name | Cognitive Domain | Impairment Rate [%, p-value] |
|---|---|---|
| HVLT-R Delayed recall | Memory | 27.0 [<0.001] |
| DKEFS VF Letter fluency | Executive functioning | 18.9 [0.01] |
| BVMT Delayed recall | Memory | 18.9 [0.01] |
| WCST Perseverative error | Executive functioning | 17.1 [0.027] |
| BVMT Total recall | Memory | 16.7 [0.03] |
| HVLT-R Total recall | Memory | 16.2 [0.034] |
| BNT Total score responses | Language | 13.9 [0.089] |
| WMS III Digit span | Attention | 12.1 [0.17] |
| DKEFS TMT Visual scanning | Attention | 11.4 [0.20] |
| DKEFS VF Category fluency | Language | 8.3 [0.44] |
| DKEFS TMT Letter sequencing | Processing speed | 8.3 [0.44] |
| DKEFS VF Switching accuracy | Executive functioning | 8.1 [0.45] |
| DKEFS TMT Number sequencing | Processing speed | 5.6 [0.70] |
HVLT-R: Hopkins Verbal Learning Test-Revised; DKEFS VF: Delis-Kaplan Executive Functioning System – Verbal Fluency; BVMT: Brief Visuospatial Memory Test; WCST: Wisconsin Cart Sorting Test; BNT: Boston Naming Test; WMS : Wechsler Memory Scale; DKEFS TMT : Delis-Kaplan Executive Functioning System – Trail Making Test.
Per-index rate of Impairment
The impairment rate at baseline for each cognitive index was determined as the percentage of patients with T-scores ≤ 35. A T-score of 35 is 1.5 standard deviations below the normative mean and has been commonly used as a threshold for neurocognitive impairment in brain tumor patients[12]. A one-sided binomial test was used to assess whether the impairment rate was greater than that expected in a normal population. In a normal distribution of T-scores (mean of 50, standard deviation of 10), 6.68% of the scores can be expected to be <=35.
Per-patient level of impairment
The per-patient level of impairment was measured in two ways: number of impaired indices (NII) and global deficit score (GDS). NII was calculated as the number of cognitive indices with T-scores≤35. GDS was calculated by transforming T-scores using a 5-point scale (Supplemental Figure 1) as implemented by Blackstone et al.[13] In previous studies, GDS values≥ 0.5 were associated with clinical ratings of overall impairments[14].
Association between GDS and patient characteristics
Clinical and tumor characteristics including age, gender, tumor type, tumor location, tumor hemisphere, evidence of seizures, use of antiepileptic drugs at baseline, use of steroids at baseline, years of education, and size of planning target volume (PTV) were individually tested for association with GDS scores. Nonparametric tests were used to avoid assumptions of normality. Spearman rank correlation coefficients were used for analyses of numeric variables. Kruskal-Wallis tests were used for categorical variables, with levels < 5 observations collapsed into an ‘other’ category. For variables with >2 categories and a significant Kruskal-Wallis test result, a post hoc pairwise Wilcoxon rank rest was used to determine which category pairs were different. In this case, p-values of multiple comparisons were adjusted to control the false discovery rate[15].
Trends in GDS over time
Cognitive indices were available for 37 patients at baseline, 21 patients at 3-months post-RT, 22 patients at 6-months post-RT, and 14 patients at 12-months post-RT. Post-RT GDS scores were determined as above. Wilcoxon signed-rank test was used to determine whether there was a statistically significant difference between post-RT GDS and baseline values. Impairment rates at each time point were determined by the proportion of patients with GDS values≥ 0.5. McNemar’s test was used to determine whether there was a statistically significant different in impairment rates between post-RT and baseline. Analyses in trends in GDS over time were then re-computed after stratifying the patients by their baseline impairment status using a GDS cutoff value of 0.5.
Results
Per-index impairment rates at baseline
Baseline impairment rates for the 13 cognitive indices used in this study are presented in Table 2. The 3 greatest impairment rates were observed in HVLT-R delayed recall (27%), DKEFS-VF letter fluency (18.9%), and BVMT delayed recall (18.9%) while the 3 lowest impairment rates were observed in DKEFS-TMT letter sequencing (8.3%), DKEFS-VF switching accuracy (8.1%), and DKEFS-TMT number sequencing (5.6%). Binomial tests indicated that 6 of the 13 indices had impairment rates statistically significantly (p<0.05) greater than that expected in a normal population of healthy individuals (Table 2). All 4 memory indices used in this study showed significant impairment rates.
Per-patient level of impairment at baseline: NII and GDS
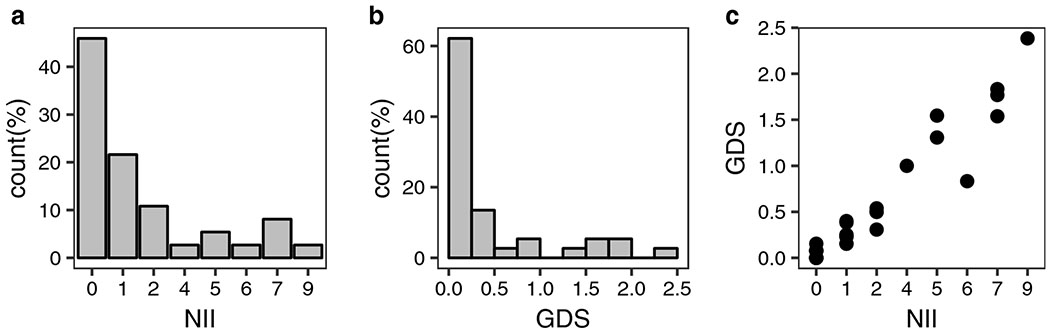
A summary of the baseline NII and GDS scores in the cohort is presented in Figure 1. About one-third (29.7%; 11 out of 37) of patients had GDS ≥0.5 (Figure 1b). The minimum NII value of these 11 patients was 2 indices. Spearman’s correlation coefficient test suggested a strong association between NII and GDS (rho=0.95, p<0.001; Figure 1c).
Figure 1. Summary of per-patient level of impairment at baseline.
Summary statistics for NII (number of impaired indices) and GDS (global deficit scores) are displayed. Spearman’s rank correlation coefficient suggested a strong association between NII and GDS (r = 0.95, p<0.001). Figure 1c shows a scatter plot of the association between GDS and NII.
Association between baseline GDS and clinical characteristics
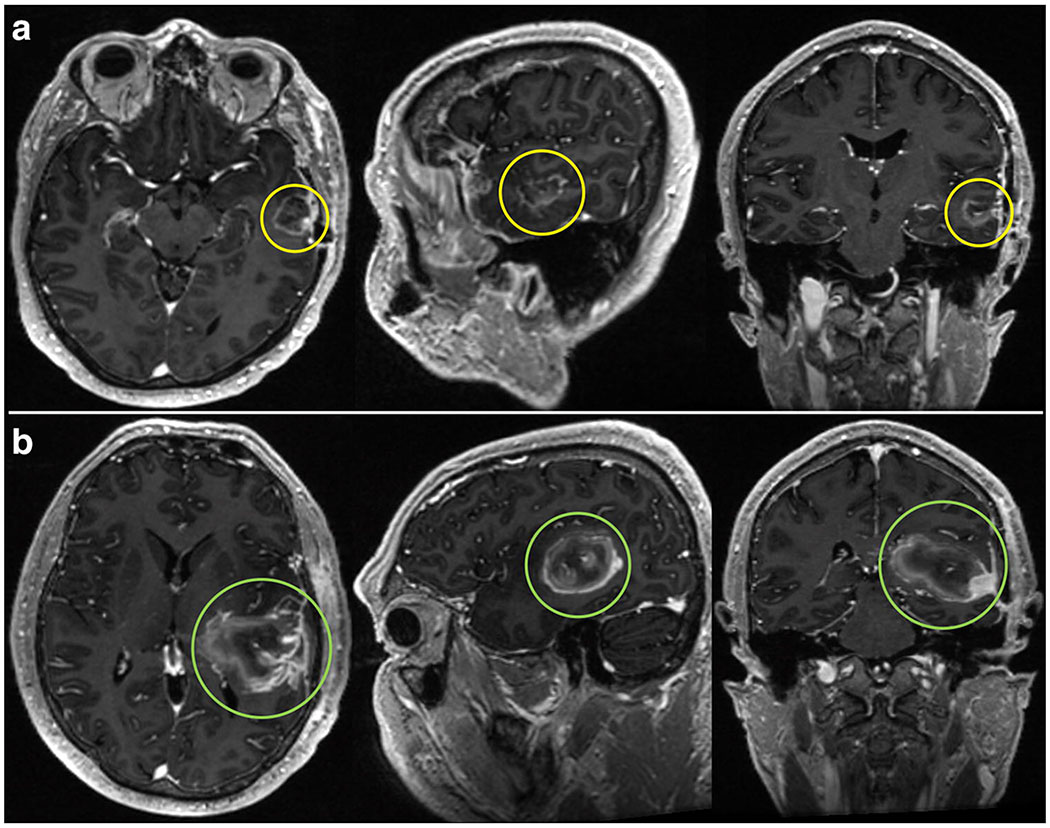
Spearman’s correlation coefficients showed a positive association between GDS and size of PTV (rho = 0.32, p = 0.048). Figure 2 demonstrates this: representative T1 post-contrast MRI showing tumor volume of a patient with GDS in the lower quartile (Figure 2A) and upper quartile (Figure 2B). Kruskal-Wallis tests showed significant associations between GDS and tumor type (X2 = 6.29, p = 0.043), tumor hemisphere (X2 =7.06, p = 0.029), seizures (X2 = 7.27, p = 0.0069), and use of antiepileptic drugs (X2 = 6.87, p = 0.0088). Post hoc analyses revealed a significant difference in GDS between meningioma (mean: 0.079) and GBM (mean: 0.71, p = 0.041) tumor types. No significant differences in tumor hemisphere were detected after adjusting for multiple comparisons. Supplementary Figure 2 shows plots of GDS against these clinical variables.
Figure 2. Tumor imaging by GDS quartile.
Representative T1 post-contrast MRI slices in axial, sagittal, and coronal planes showing tumor volume of a patient within the lower quartile of GDS values (Figure 2A) and a patient within the upper quartile of GDS values (Figure 2B).
Trends in GDS over time
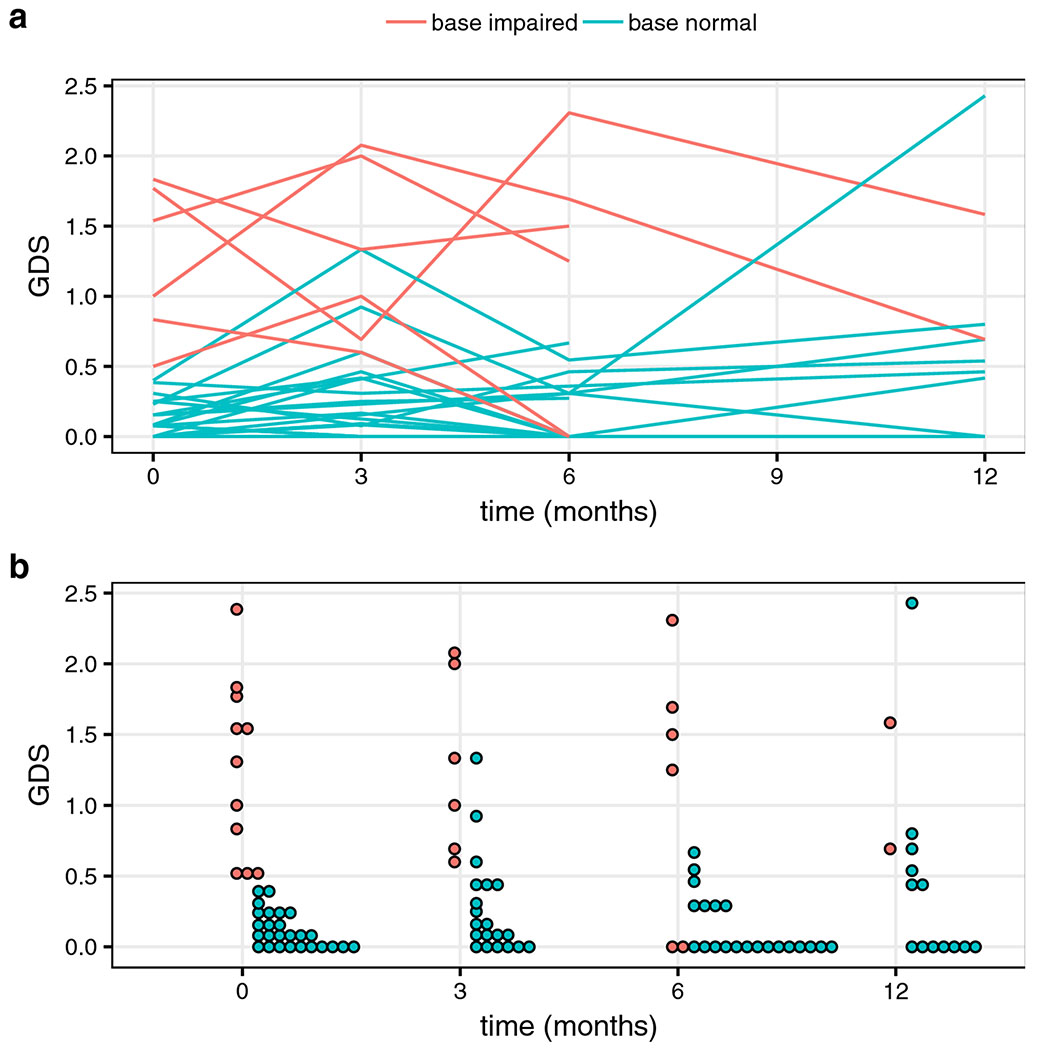
Mean GDS values at baseline, 3-, 6-, and 12-month time points were 0.45, 0.50, 0.36, and 0.50. Wilcoxon signed rank tests showed no significant difference in GDS when comparing each post-RT time point to baseline. Trends in GDS over time are plotted in Figure 3a and Figure 3b, color-coded by the baseline impairment status, as determined through GDS. The GDS impairment rates at baseline, 3-, 6-, and 12-months were 29.7 [95% CI: 15.8 – 47.0%], 34.6 [17.2 – 55.7%], 22.2 [8.6 – 42.2%], and 40% [16.3 – 67.7%] respectively. No significant differences in post-RT impairment rates compared to baseline were detected using McNemar’s test.
Figure 3. Trends in GDS over time.
GDS values are plotted for ‘base impaired and ‘base normal’ patients, color-coded as ‘base impaired’ if their baseline GDS values were greater than or equal to 0.5, and ‘base normal’ if otherwise. Mean GDS values at baseline (0 months), 3-, 6-, and 12-months were 0.45, 0.50, 0.36, and 0.50. Figure 3a uses lines to group values belonging to the same patient. Figure 3b uses a dot plot to track ‘base impaired’ and ‘base normal’ across time.
In patients with mean GDS values less than 0.5 at baseline (‘base-normal’), mean GDS values at baseline, 3-, 6-, and 12-month time points were 0.11, 0.27, 0.14, and 0.41, respectively. In patients with GDS values greater than 0.5 at baseline (‘base-impaired’), mean GDS values at baseline, 3-, 6- and 12-month time points were 1.25, 1.28, 1.13, and 1.14 respectively. A statistically significant difference (p = 0.009) was detected in “base normal” GDS scores at 3-months post-RT compared to baseline. Using a GDS cutoff value of 0.5 to classify patients at each time point, the percentages of patients with the same classification as that at baseline were 88%, 85%, and 85% at the 3-, 6-, and 12-month time points respectively.
Discussion
We present the first prospective study to fully characterize and quantify baseline neurocognitive functioning of brain tumor patients prior to RT, including impairment rates across multiple cognitive domains and the value of baseline neurocognitive impairment as a predictor of post-RT neurocognitive function. We introduce the composite global deficit score (GDS)[13], which considers both the number and severity of deficits in neurocognitive performance within the entire test battery across multiple domains, to better understand neurocognitive performance. Baseline per-test impairment rates ranged between 5.6 and 27%, with the largest rate observed on the HVLT-R delayed recall memory test. Several clinical variables, including size of PTV, tumor type, use of anti-epileptic drugs, and history of seizures, were significantly associated with baseline GDS. The majority of patients were classified with the same impairment status post-RT as they were at baseline.
Among the entire multi-domain battery, significant baseline impairment was observed in memory and executive functioning. Deficits in memory and executive functioning have long been considered sequelae of RT[16]. Much of the focus in this field has been on memory impairment as a result of RT, leading to clinical trials investigating the impact of hippocampal avoidance[17] to help mitigate these effects. Our work suggests that almost one-third of patients about to undergo radiotherapy already have memory impairment. Executive functioning concerns abilities including multi-tasking, cognitive flexibility, and planning, and is critical to occupational status and quality of life[5]. Our recently published work highlights the association between early imaging biomarkers of radiation-associated damage to the dorsal cingulum and post-RT executive functioning, after accounting for baseline functioning[5].
Patients with larger PTV volumes (Figure 2b), glioblastoma tumor types (compared to meningioma), history of seizures and use of anti-epileptic drugs were more likely to have greater levels of baseline impairment as measured by GDS. These results are consistent with literature on pre-operative and epilepsy patients[12,18],and suggest that in order to better understand the impact of treatment effects, it is imperative to formally measure baseline (pre-RT) functioning, especially in patients with larger and more aggressive tumors and those with seizures. Cross-sectional studies using post-RT measurements of neurocognitive functioning without controlling for baseline may be at-risk of misrepresenting the impact of radiation therapy on neurocognition. Future larger longitudinal studies may be able to document a patient’s journey along the spectrum of cognitive impairment after RT, answering such questions as: Were patients who were initially without signs of impairment getting worse? Is RT worsening pre-existing impairments that are measurable before therapy began? These investigations may go a long way to helping identify those patients who are at the highest risk of experiencing cognitive impairment, and perhaps those who would benefit the most from cognitive interventional therapies[19].
There were several notable trends in impairment over time. Impairment rates prior to RT (29%) in this study are similar to those reported by Wefel[9] prior to chemotherapy (35%) in breast cancer patients. However, the largest percentage of patients who could be classified as “newly impaired” at the follow-up time points (i.e. previously unimpaired at baseline) was 15.4% at 12 months post-RT compared to roughly 48% in the longitudinal study of breast cancer patients undergoing chemotherapy[10]. Thus it appears in our study that a minority of patients started out unimpaired only to become impaired as a consequence of brain RT. Further investigation is needed in to what factors make certain individuals at higher risk of becoming newly impaired post-RT. Apart from a handful of patients at 6-months, all the patients whose GDS values were 0 at the follow-up time points had GDS values of 0 at baseline. This suggests that patients who were experiencing some form of cognitive impairment prior to RT were not likely to have resolution of this impairment in the year following treatment.
When considering the entire cohort, the rate of GDS impairment and mean GDS values did not reliably change from baseline to post-RT time points. However, when stratified by baseline impairment status, we detected a significant increase in the mean GDS value of ‘base normal’ patients at 3-months post-RT, potentially reflecting some acute to subacute and potentially reversible neurocognitive side effects of RT[1]. Given that our data show the relatively stable nature of GDS in this “baseline normal” group of patients, a baseline GDS cutoff value of 0.5 could potentially be used to stratify patients as ‘normal’ and ‘impaired’ with the notion that the vast majority of patients will maintain this cognitive impairment status in the year following RT. This may allow physicians to determine those brain tumor patients who may benefit the most from neurocognitive interventions[19].
We have validated the use of GDS to encapsulate the overall cognitive functioning of brain tumor patients across multiple neurocognitive test scores and domains, which is highly valuable in clinical and research applications when a number of different cognitive domains are of interest. GDS was highly correlated with the number of indices with T-scores less than 35. There are several other strategies for incorporating the scores of multiple neurocognitive tests into a single outcome metric. The Alliance NCCTG N0574[20] trial used cognitive progression, defined as a greater than one standard deviation decline in any of 6 cognitive tests. The NRG clinical trial[21] (NRG-BN005) comparing proton beam or intensity-modulated RT uses a Clinical Trial Battery Composite score[11], calculated as the mean z-score from a selection of neurocognitive tests: HVLT-R, Controlled Word Association, and Trail Making Tests. There is no clearstandardized approach to measure neurocognitive change in this patient population, but our results suggest that composite outcome measures may quantify similar functioning deficits.
This study has several limitations. The sample size is relatively small, yet this study is prospective and longitudinal with close-follow up, and comparable in size to other similar single institution prospective cohort studies[22,23] of cognitive function in primary brain tumor patients. The neurocognitive assessments are robust and performed by the same expert neuropsychology team. As such, these cognitive batteries take up to 2-3 hours to perform at each time point, which is why these data are so valuable but also why there are few studies of this nature with large sample sizes. Future work towards shorter but equally robust testing batteries is warranted. In addition, we used cognitive tests with alternate forms, to avoid patient “learning” of the tests. Similar to other trials[20], follow-up neurocognitive test scores were not corrected for practice effects[24]. However, this was mitigated by the use of a composite measure of neurocognitive function (GDS) as an outcome instead of focusing on individual test scores. Several patients in the cohort did not complete all the follow up assessments to date, which limits the scope and generalizability of trends in follow-up rates of impairment reported in this study. No statistically significant changes in impairment rate from baseline to follow-up were detected; however a future study with a larger sample size may be able to detect clinically significant changes in GDS within this time period. Finally, the study cohort represented a variety of tumor types, location, and sizes, which are representative of the primary brain tumor population that present for adjuvant brain RT. This heterogeneity allowed us to investigate several associations between clinical variables and baseline GDS, yet the sample size was not large enough to explore potential confounder effects between predictors in a multivariable approach. This is most certainly the goal in future studies.
We plan to use the results of this analysis in future investigations to stratify patients into 2 groups based on their baseline GDS impairment status and so assess whether patients will experience different vulnerability to radiation dose effects based on their level of cognitive functioning prior to therapy. Furthermore, it is possible that clinically significant changes in impairment can be detected with more nuanced analysis of the cognitive data. GDS is not able to detect patients whose scores decreased, but remained above a value of 40. These high functioning patients present a particular challenge in research and clinical practice as they do not meet criteria for impairment, but they may experience significant functional decline and reduced quality of life.
In conclusion, the majority of brain tumor patients exhibited some form of neurocognitive impairment, particularly in memory and executive functioning, when tested prior to actually receiving RT. The accurate measurement of baseline neurocognitive functioning might help identify patients who are at risk of cognitive impairment and those who may need intervention. Furthermore, studies such as these can help inform the design of clinical trials with cognitive endpoints.
Supplementary Material
Acknowledgments
Funding support
This work was funded, in part, by: National Institute of Health (NIH) grants #1KL2TR001444 (JAH-G), #UL1TR000100 (JAH-G), R01CA238783-01 (JAH-G), TL1TR001443 (KRT, DM), R01NS065838 (C.R.M.); American Cancer Society Pilot Award ACS-IRG 70-002 (JAH-G); American Cancer Society Research Scholar Grant RSG-15-229-01-CCE (C.R.M.).
Conflicts of Interest
Drs. Seibert, Dr. Hattangadi-Gluth, and Dr. Karunamuni report grant funding from Varian Medical Systems, unrelated to the present study.
References
- 1.Makale MT, McDonald CR, Hattangadi-Gluth JA, et al. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 2017;13:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene-Schloesser D, Robbins ME, Peiffer AM, et al. Radiation-induced brain injury: A review. Front Oncol 2012;2:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene-Schloesser DM, Moore E, Robbins ME. Molecular pathways: Radiation-induced cognitive impairment. Clin Cancer Res 2013;19:2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacob J, Durand T, Feuvret L, et al. Cognitive impairment and morphological changes after radiation therapy in brain tumors: A review. Radiother Oncol 2018;128:221–228. [DOI] [PubMed] [Google Scholar]
- 5.Tringale KR, Nguyen T, Bahrami N, et al. Identifying early diffusion imaging biomarkers of regional white matter injury as indicators of executive function decline following brain radiotherapy: A prospective clinical trial in primary brain tumor patients. Radiother Oncol 2019;132:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol 2010;97:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karunamuni RA, Moore KL, Seibert TM, et al. Radiation sparing of cerebral cortex in brain tumor patients using quantitative neuroimaging. Radiother Oncol 2016;118:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murzin VL, Woods K, Moiseenko V, et al. 4Π Plan Optimization for Cortical-Sparing Brain Radiotherapy. Radiother Oncol 2018;127:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wefel JS, Lenzi R, Theriault R, et al. “Chemobrain” in breast carcinoma? A prologue. Cancer 2004;101:466–475. [DOI] [PubMed] [Google Scholar]
- 10.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and Late Onset Cognitive Dysfunction Associated With Chemotherapy in Women With Breast Cancer. Cancer 2010;116:3348–3356. [DOI] [PubMed] [Google Scholar]
- 11.Noll KR, Bradshaw ME, Weinberg JS, et al. Relationships between neurocognitive functioning, mood, and quality of life in patients with temporal lobe glioma. Psychooncology 2018;26:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noll KR, Sullaway C, Ziu M, et al. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol 2014;17:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackstone K, Moore DJ, Franklin DR, et al. Defining Neurocognitive Impairment in HIV: Deficit Scores versus Clinical Ratings. Clin Neuropsychol; 26 Epub ahead of print 2012. DOI: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichenberg A, Harvey PD, Bowie CR, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull 2009;35:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Society RS. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing Author (s): Yoav Benjamini and Yosef Hochberg Source: Journal of the Royal Statistical Society. Series B (Methodological), Vol. 57, No. 1 Published by:. 2018;57:289–300. [Google Scholar]
- 16.Makale MT, McDonald CR, Hattangadi-Gluth JA, et al. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 2016;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gondi V, Pugh SL, Tome WA, et al. Preservation of Memory With Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment During Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J Clin Oncol 2014;32:3810–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology 2002;58:S21–S26. [DOI] [PubMed] [Google Scholar]
- 19.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol 2006;7:517–523. [DOI] [PubMed] [Google Scholar]
- 20.Brown PD, Asher AL, Ballman K V, et al. NCCTG N0574 (Alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. J Clin Oncol 2015;33:LBA4–LBA4. [Google Scholar]
- 21.Proton Beam or Intensity-Modulated Radiation Therapy in Preserving Brain Function in Patients With IDH Mutant Grade II or III Glioma - Full Text View - ClinicalTrials.gov CinicalTrials.gov Available from: https://clinicaltrials.gov/ct2/show/NCT03180502. Accessed June 27, 2018.
- 22.Chapman CH, Zhu T, Nazem-zadeh M, et al. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother Oncol 2016;120:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma TM, Grimm J, McIntyre R, et al. A prospective evaluation of hippocampal radiation dose volume effects and memory deficits following cranial irradiation. Radiother Oncol 2017;125:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg TE, Harvey PD, Wesnes KA, et al. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2015;1:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.