Abstract
Background
Aging is associated with diminished testosterone (Te) secretion, which may be attributed to Leydig cell dysfunction, decreased pituitary stimulation, and altered Te feedback.
Objective
To study all regulatory nodes—gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH) and Leydig cell—in the same cohort of healthy men.
Study Design
This was a placebo-controlled, blinded, prospectively randomized cross-over study in 40 men, age range 19 to 73 years, and body mass index (BMI) range 20 to 34.3 kg/m2. A submaximal dose of the GnRH antagonist ganirelix was used to assess outflow of GnRH, by calculating the difference between LH output during the control arm and ganirelix arm. Ketoconazole (a steroidogenic inhibitor) was used to estimate feedback, by the difference in LH output during the ketoconazole and control arm. High-dose ganirelix and repeated LH infusions were used to measure testicular responsivity. Blood sampling was performed at 10-minute intervals.
Results
There were age-related, but not body composition–related decreases in estimated GnRH secretion, the feedback strength of Te on LH, and Leydig cell responsivity to LH, accompanied by changes in approximate entropy. Bioavailable Te levels were negatively related to both age and computed tomography (CT)–estimated abdominal visceral mass (AVF), without interaction between these variables. The LH response to a submaximal dose of GnRH was independent of age and AVF.
Conclusion
Advancing age is associated with (1) attenuated bioavailable Te secretion caused by diminished GnRH outflow and not by decreased GnRH responsivity of the gonadotrope, (2) diminished testicular responsivity to infused LH pulses, and (3) partial compensation by diminished Te feedback on central gonadotropic regulation.
Keywords: human, aging, feedback, feedforward, bioavailable testosterone, ganirelix
Circulating testosterone (Te) concentrations, especially bioavailable and free Te concentrations, decline with age (1–3). Meta-analysis and longitudinal studies have estimated an annual decrease in total, bioavailable, and free Te in healthy older men of 1% to 3% (4, 5). Relative depletion of androgen in the aging male may contribute to sarcopenia, osteopenia, diminished libido and potency, increased visceral adiposity, reduced aerobic capacity, and (possibly) impaired cognitive function (6, 7). The primary cause of the age-related decline in Te production has not been fully elucidated. Available studies point to possible defects in hypothalamic gonadotropin-releasing hormone (GnRH) feedforward (8), negative feedback of Te on the hypothalamus and pituitary (9, 10) and testicular responsiveness to luteinizing hormone (LH) (11, 12). However, the relative importance of the foregoing putative mechanisms as a function of age is unknown.
The hypothalamus-pituitary-gonadal (HPG) axis is a highly organized endocrine system, with multiple inputs to its hypothalamic components, i.e. the kisspeptin and GnRH neurons (13). Puberty and sexual maturity are driven by kisspeptin secreted by the KISS neurons in the arcuate nuclei. Inactivating mutations of kisspeptin and its receptor GRP54 are associated with central hypogonadism (14). Kisspeptin neurons project on hypothalamic GnRH neurons and stimulate the pulsatile secretion of GnRH (15). Hypothalamic GnRH is the principal, if not exclusive, feedforward signal that drives gonadotropin secretion by the human anterior pituitary gland, and the pulsatile LH feedforward signal stimulates testicular Leydig-cell steroidogenesis. Within this highly coordinated system, Te is a major negative-feedback signal that suppresses both GnRH and LH secretion. Conversely, Te withdrawal amplifies LH secretion. The decline in androgen availability with age is often associated with a decline in the growth hormone (GH)-insulin-like growth factor (IGF)-1 axis (16), possibly attributable in part to reduced hypothalamic output of growth hormone–releasing hormone (GHRH), and/or ghrelin (17).
Earlier studies developed a composite secretion-elimination model of joint feedback and feedforward coupling among GnRH, LH, and Te (18). This biomathematical construct predicts potential deterioration of GnRH drive to the pituitary gland, LH feedforward on the testis, and androgen feedback on GnRH and LH secretion in the aging male (19, 20). Given this background, the present study hypothesized that the age-related decline in androgen in healthy men is multifactorial, and caused by (1) central (hypothalamic-pituitary) impairment of GnRH and LH outflow, (2) diminished Leydig cell responsiveness to LH, and (3) possibly diminished Te feedback partly compensating for reduced central forward drive and Leydig cell responsiveness. Here, we explore presumptive hypothalamic, pituitary and testicular contributions to relative hypoandrogenemia in 40 healthy men, aged 19 to 73 years.
The study comprised 4 randomly ordered admissions to the Clinical Research Unit for each volunteer to conduct each of 4 experimental protocols to test distinct regulatory mechanisms: (1) central drive by GnRH, (2) pulsatile LH secretion, (3) feedback of Te on GnRH/LH secretion, and (4) the effect of exogenous LH pulses on Te secretion. Inasmuch as central neuronal release of GnRH is not determinable directly in the human, administration of a submaximally inhibitory dose of a selective GnRH-receptor antagonist (ganirelix) was used to probe endogenous GnRH outflow (21). To assess Te feedback, ketoconazole (KTCZ) was given to block steroidogenesis under concomitant glucocorticoid replacement, and hypothalamic-pituitary–regulated LH secretion was monitored. Leydig cell responsivity to LH pulses was addressed via subcutaneous (SC) injection of ganirelix to suppress endogenous LH secretion and intravenous (IV) infusion of successive pulses of recombinant human LH to drive Te secretion.
Unlike earlier publications, the present study explores multiple mechanisms in the same individual over a wide age and body mass index (BMI) span.
Methods
Subjects
Forty healthy, ambulatory community-dwelling men (mean age 47.8 years, range 19–73; Shapiro-Wilk test statistic 0.973; P = 0.457) participated in the Clinical Research Unit (CRU)-based studies. The mean BMI was 26.7, range 20–34.3 kg/m2; Shapiro-Wilk test statistic 0.966; P = 0.258. Screening endocrine data, measured in the Mayo Clinic Reference Laboratory, included (mean [range]): Te 540 ng/dL (265-1050), estradiol 26 pg/mL (14-50), LH 4.1 IU/L (3.5-12.5), follicle-stimulating hormone (FSH) 6.5 IU/L (1.8–16.3), sex hormone–binding globulin (SHBG) 33.2 nmol/L (16–86), prostate-specific antigen (PSA) 0.83 ng/mL (0.28–3.1), CT-determined AVF 157 cm2 (11–347), and total abdominal fat 306 cm2 (34–746). Volunteers were recruited by newspaper advertisements, local posters, the Clinical Trials Center web page and community (general and minority) bulletin boards.
Protocol
This was an investigator-initiated, FDA-approved prospectively randomized, double-blind placebo-controlled prospective cross-over study in adrenal-gonadal (cortisol and testosterone) and LH-downregulated men. Men who satisfied the inclusion and exclusion criteria (see below) and completed all 4 admissions underwent a single-slice CT of the abdomen as an exploratory measure to evaluate obesity (AVF). Data from prior single-slice CT scans were used if the CT scan was performed within the past year (from date of participant screening) and the individual had not had a greater than 6-lb (2.7 kg) weight change.
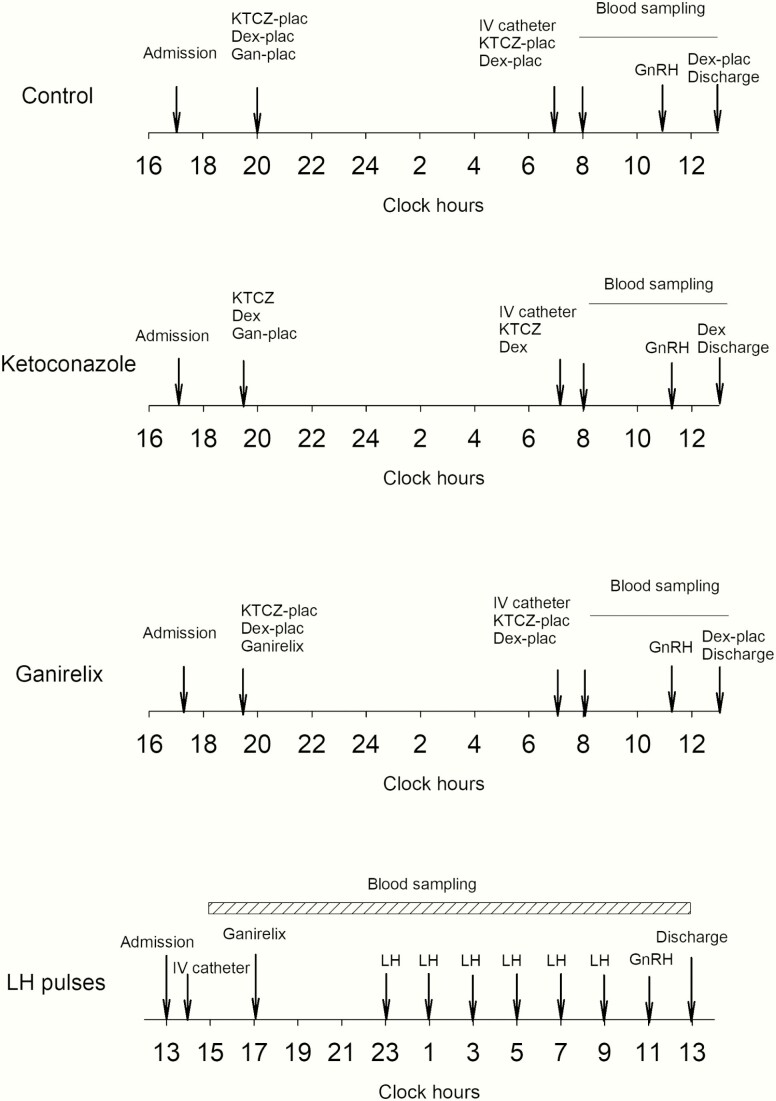
Participants were studied in the CRU on 4 occasions. Consecutive sessions were separated by at least 1 week and the order of the sessions was prospectively randomized. Scheduled sessions involved the administration of either (1) saline (placebo injection); (2) ganirelix alone; (3) KTCZ with dexamethasone; or (4) ganirelix with LH pulses. Administration of these 4 drugs tested, respectively: (1) control condition; (2) hypothalamic drive via endogenous GnRH by partial competitive ganirelix blockade of GnRH receptors; (3) Te’s feedback onto GnRH-LH responses to androgen deprivation; and (4) testicular function by response of Te to pulsatile LH drive. The schematic outline of the study is shown in Fig. 1. In 3 of the visits, participants were admitted to the CRU by 1730 hours to receive the interventional agent (below) at 2000 hours and stayed overnight in the CRU. An indwelling IV catheter was placed in a forearm vein the next morning by 0700 hours. Blood samples (1.5 mL) were withdrawn every 10 minutes from 0800 to 1300 hours. Serum LH and Te concentrations were measured in each sample by automated chemiluminescence assay. The specific inpatient interventions are: (1) saline control (placebo injection and placebo pills), (2) a single SC injection of 0.125 mg/m2 ganirelix and placebo pills, (3) placebo injection and 800 mg KTCZ and 0.75 mg dexamethasone administered orally, followed by 400 mg KTCZ at 0700 hours the following morning and 0.75 mg dexamethasone at the end of blood sampling. The second dose of KTCZ in the morning is required to ensure sustained androgen deprivation for the full duration of blood sampling. This intervention suppresses testicular and adrenal steroidogenesis overnight by > 85% in healthy young and older men (22). The third dose of dexamethasone at 1300 hours ensures adequate glucocorticoid replacement prior to discharge from the CRU. Importantly, the administered dose of dexamethasone does not interfere with LH secretion (23).
Figure 1.
Schematic outline of the experimental protocols. The order of the 4 protocols was randomly assigned. In the overnight 10-minute blood sampling study, subjects received 1 mg ganirelix SC. LH pulses were given at 2-hour intervals, where 18.75 IU was infused over 6 minutes. A submaximal dose of GnRH (100 ng/kg) was administered IV at 1100 hours. In the other 3 arms, blood sampling was carried out every 10 minutes between 0800 and 1300 hours. The first dose of KTCZ was 800 mg, and the second dose 400 mg, together with 0.75 mg dexamethasone. A submaximal dose of ganirelix (0.125 mg/m2 body surface) was administered at 2000 hours. Both LH and Te were measured in all samples.
For the additional overnight admission, volunteers were admitted to the CRU at or before 1300 hours. An indwelling IV catheter was placed in a forearm vein by 1400 hours. Blood samples (1.5 mL) were withdrawn every 10 minutes for 22 hours from 1500 until 1300 hours the following day, for later assay of LH and Te concentrations. Ganirelix (1 mg SC) was given at 1700 hours to repress endogenous LH secretion. The subsequent stimulation protocol comprised pulsatile (6-minute bolus) IV infusions of recombinant human LH (18.75 IU, Serono) every 2 hours for 6 doses administered via infusion pump commencing at 2300 hours.
During all 4 sessions, a single additional, submaximally-effective GnRH stimulus (100 ng/kg IV bolus) was administered at 1100 hours to assess gonadotrope responsivity and corroborate the competitive nature of ganirelix’s inhibition of LH secretion (21).
Safety data
All volunteers completed a preliminary outpatient screening consisting of a complete history, physical examination, and screening biochemical measures of hepatic, renal, metabolic, and hematologic function (see Subjects). Volunteers 50 years and older underwent a screening electrocardiogram in the CRU. In keeping with standard medical practice, the PSA and a digital rectal examination (to assess the prostate gland) was performed in subjects over 40 years of age. A prostate exam was not required if one had been done in the past year and the PSA from the past year was within normal values.
Standardized supper (the evening of CRU admission), snacks (nondairy at 2000 and 0700 hours with oral KTCZ or placebo administration), as well as breakfast and lunch on day 2 were provided. Meals were caffeine-free throughout the inpatient study. Subjects were allowed to ambulate to the lavatory. Lights were off from 2300 until 0700 hours.
Investigational drugs
Ganirelix is a FDA-approved decapeptide analogue of GnRH, a single injection of which inhibits LH secretion rapidly (over 4–6 hours) and for up to 24 hours (24). Ganirelix was administered here under IND #66518. The drug has been administered daily in controlled ovulation-induction regimens to repress an endogenous LH surge (25, 26).
Ganirelix and recombinant human LH are used here together under a single protocol-specific FDA-approved investigator-initiated IND #58494. KTCZ and dexamethasone are FDA-approved drugs, here applied off-label under IND #63357. The protocol was approved by the Mayo Institutional Review Board. Witnessed written voluntary consent was obtained before study enrollment.
Criteria for exclusion
Exclusion criteria were recent (within 5 half-lives) use of psychotropic or neuroactive drugs; BMI below 19 or above 35 kg/m2; abnormal laboratory test results; drug or alcohol abuse; psychosis; depression, mania, or severe anxiety; acute or chronic organ-system disease; endocrinopathy; anabolic steroid use; nightshift work or recent (1 month) > 3 time-zones transmeridian travel; acute weight change (loss or gain of > 2 kg in 6 weeks); unwillingness to provide written informed consent; history or suspicion of prostatic disease (elevated PSA (> 4.0 ng/mL), indeterminate prostate nodule or mass, or obstructive uropathy; history of carcinoma (excluding localized basal cell carcinoma removed or surgically treated with no recurrence); history of thrombotic arterial disease (stroke, transient ischemic attacks, myocardial infarction, angina pectoris); or deep vein thrombophlebitis.
Analytical Methods
Assays
LH and Te were determined in duplicate and batch-wise in each subject. LH was measured using an automated 2-site monoclonal immunochemiluminescence assay with a sensitivity of 0.20 IU/L (first International Reference Preparation) and median inter- and intraassay coefficients of variation of 5.5% and 8.5%, respectively (DXL 800 automated immunoassay system, Beckman Instruments, Chaska, Minnesota) (27). Cross-reactivity with FSH, thyrotropin (thyroid-stimulating hormone), α-subunits, or free LH β-subunits is less than 0.1%. Total Te concentrations were assessed by liquid chromatography-tandem mass spectrometry (Agilent Technologies, Inc, Santa Clara, California). Sensitivity was 8 ng/dL, and median intra- and interassay coefficients of variation were 5.2% and 8.3%, respectively. Free and bioavailable hormone concentrations were calculated as described in the appendix of Takahashi et al (11). Estradiol was measured by liquid chromatography-tandem mass spectrometry (Agilent Technologies, Inc, Santa Clara, CA 95051). Interassay coefficients of variation for estradiol were 10.8% at 0.29 pg/mL and 5.1% at 32 pg/mL. SHBG was quantified by solid-phase chemiluminescent assay using the Siemens Immulite 2000 Automated Immunoassay System (Siemens Healthcare Diagnostics, Deerfield, Illinois (28). Interassay coefficients of variation for SHBG are 4.0% at 5.4 nmol/L and 5.9% at 74 nmol/L. Prolactin and FSH were measured by 2-site chemiluminescent sandwich immunoassays on a DXL 800 automated immunoassay system (Beckman Instruments). For prolactin, the interassay coefficients of variation were 3.7%, 2.1%, and 4.8% at 6.1, 16.4, and 34.5 µg/L; and for FSH the interassay coefficients of variation were 3.6%, 3.2%, and 4.7% at 6.5, 16.7, and 58.0 IU/L respectively (29, 30).
Calculations
The LH and bioavailable Te concentration time series (3 or 12 hours) were subjected to biexponential deconvolution analysis using independently determined estimates of 2-compartment LH and Te disappearance kinetics (31). The extended time series (2 hours) were used to estimate LH secretion before and after stimulation with GnRH. Primary analytical outcomes were (1) the difference in mean and median serum LH concentrations as well as in pulsatile and total LH secretion between the control and KTCZ arm, as a proxy for Te’s feedback control, and (2) the difference in pulsatile LH secretion between the ganirelix and control arm as a proxy for GnRH feedforward strength.
The bioavailable Te concentration responses to the exogenous LH pulses delivered at 2-hour intervals were used to estimate Leydig cell function via a Matlab program, which quantifies the unobserved dose-response function that couples paired hormone output (18, 19).
Approximate entropy (ApEn) was calculated on time-series. ApEn is a validated pattern-dependent regularity measure, which serves as a sensitive (> 90%) and specific (> 95%) discriminative metric of interactive complexity (number of connections) and strength (dose-response interface properties) in various interlinked (parameter-coupled) systems (31, 32). The statistic is calculated on a time series as a single, finite, positive, real number (between 0 and 2.3 in log base 10 data). Analogous to univariate ApEn, bivariate cross-ApEn is a scale- and model-independent 2-variable regularity statistic use to quantitate the relative pattern synchrony of coupled time series. Clinical experiments establish that changes in cross-ApEn reflect feedback and/or feedforward adaptations within an interlinked axis with high sensitivity and specificity (33).
Statistical assessment
Data are shown as mean and standard error of the mean. Comparisons among the 4 experimental arms and their possible interactions were performed with the 4 repeated measurements. Relations of LH and bioavailable Te with age and body composition (AVF) were assessed using linear regression analysis. Standardized linear regression coefficients (slopes, normalized to age- and AVF-SD-scores) were used to compare dependent variable effect sizes, such as relative effects of age and AVF on feedback and feedforward control. Statistical significance was set at P < 0.05. Calculations were performed with Systat version 13 (Systat Software, Inc, San Jose, CA) and with Matlab (MathWorks, Natick, Massachusetts).
Results
LH and Te pre-GnRH stimulation in the period from 0800 to 1100 hours
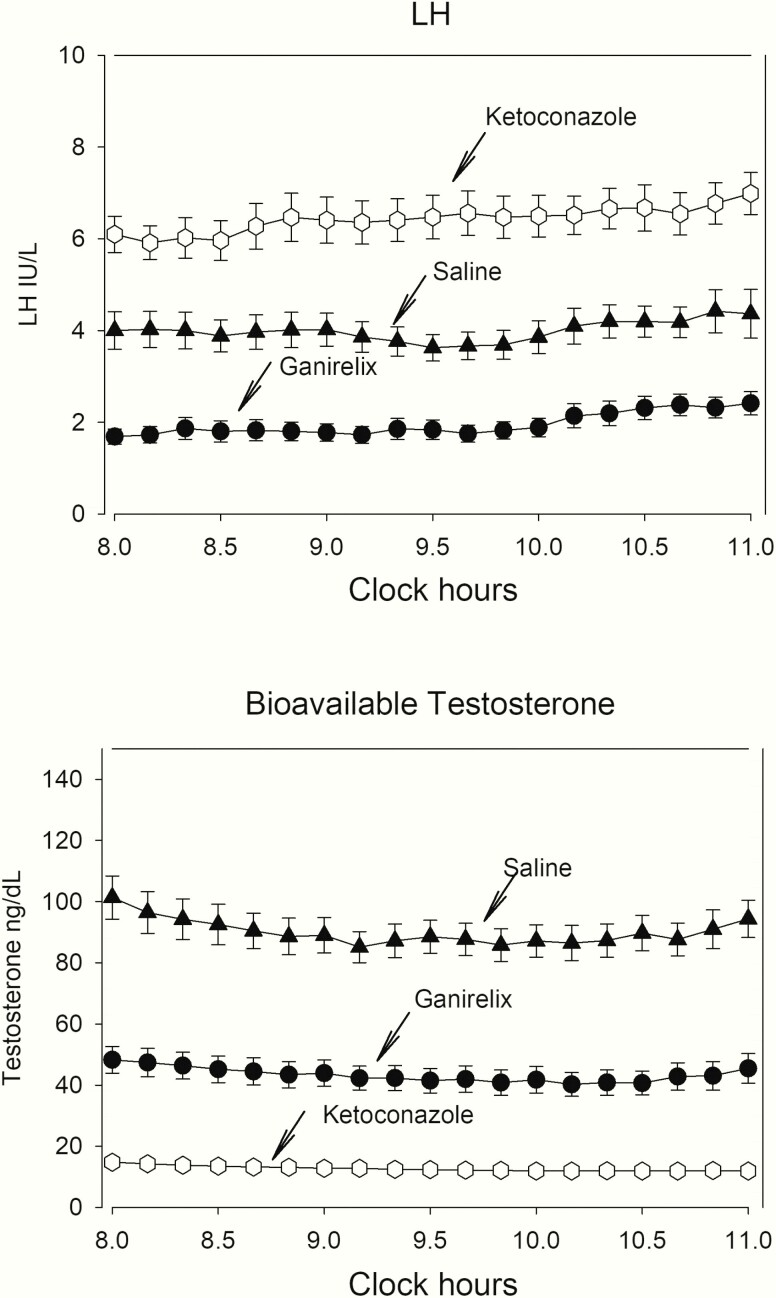
Serum LH concentrations during the 3-hour period on all 4 visits are depicted in the upper panel of Fig. 2. Mean and median LH concentrations were lower during submaximal GnRH-receptor blockade and increased during inhibition of Te synthesis by KTCZ, compared with the control (saline) visit. The statistical significance of these differences is shown in the upper rows of Table 1. The data inference of group differences was corroborated by the independent outcome of the deconvolution analysis of the LH profiles (lower rows of Table 1). Further analyses of the mean (median) LH levels of the 3-hour sampling period did not reveal significant regressions between the mean LH concentration on the one hand, and age, AVF, and total abdominal fat on the other (R values ranging between 0.030 and 0.252, with P values from 0.13 to 0.84).
Figure 2.
Serum LH and bioavailable Te concentration profiles in 40 men. Data are shown as mean and standard error of the mean.
Table 1.
Serum LH Concentrations and Secretion Parameters in 40 Healthy Men Sampled During 3 Hours at 10-Minute Intervals
| Serum concentrations | Control | Ganirelix | Ketoconazole | ANOVA |
|---|---|---|---|---|
| Mean 3-h LH (IU/L) | 3.99 ± 0.33 | 1.95 ± 0.19 | 6.42 ± 0.42 | <0.0001* |
| Median 3-h LH (IU/L) | 3.79 ± 0.32 | 1.81 ± 0.19 | 6.36 ± 0.42 | <0.0001* |
| Maximum LH (IU/L) | 6.02 ± 0.55 | 3.14 ± 0.30 | 8.32 ± 0.57 | <0.0001* |
| Minimum LH (IU/L) | 2.70 ± 0.25 | 1.26 ± 0.13 | 4.88 ± 0.31 | <0.0001* |
| Deconvolution | ||||
| Pulse frequency (#/3h) | 2.0 ± 0.1 | 1.8 ± 0.1 | 2.7 ± 0.2a | <0.0001 |
| Slow half-life (min) | 53 ± 4.0 | 63 ± 4.4 | 47 ± 2.3 | 0.007b |
| Mode (min) | 13.3 ± 1.1 | 14.1 ± 0.7 | 14.3 ± 0.8 | 0.47 |
| Basal secretion (IU/L/3h) | 15.7 ± 1.7 | 7.4 ± 1.0 | 26.0 ± 2.2 | <0.0001c |
| Pulsatile secretion (IU/L/3h) | 10.7 ± 1.3 | 5.1 ± 0.6 | 18.2 ± 2.3 | <0.0001c |
| Total secretion (IU/L/3h) | 26.4 ± 2.9 | 12.5 ± 1.4 | 44.3 ± 3.1 | <0.0001c |
| Mean pulse mass (IU/L) | 5.6 ± 0.5 | 3.0 ± 0.3 | 6.7 ± 0.7 | <0.0001c |
Data are shown as mean and SEM. The General Linear Model was used for the ANOVA of Repeat Measurements. Contrasts were evaluated with the Bonferroni correction.
Abbreviations: LH, luteinizing hormone.
Serum concentrations *Post hoc contrasts: Control vs Ganirelix and Ketoconazole: P < 0.0001, Ganirelix vs Ketoconazole: P < 0.0001. Deconvolution analysis: Contrasts were corrected with the Bonferroni procedure. aControl vs Ketoconazole P = 0.005, Ketoconazole vs Ganirelix P < 0.0001; bKetoconazole vs Ganirelix P = 0.007; cControl vs Ketoconazole and Ganirelix, and Ketoconazole vs Ganirelix P < 0.0001.
Serum bioavailable Te concentrations during the 3-hour pre-GnRH injection blood sampling period are depicted in the lower panel of Fig. 2. Statistical contrasts among mean (median, maximum, and minimum) concentrations are shown in Table 2 (upper rows). Highly significant differences were present between the 3 arms of the experiment, with lowest bioavailable Te levels during KTCZ treatment and with intermediate levels during ganirelix exposure. Table 2 also displays results of the multivariate ANOVA of mean 3-hour bioavailable Te concentrations and matching pulsatile Te secretion rates, except for the KTCZ-treated group, where deconvolution of the low, hardly-fluctuating concentrations was not possible. In the control arm, age was a significant (negative) predictor (R = −0.64; P < 0.0001; slope −1.51 ± 0.29) of deconvolution-derived secretion bioavailable Te secretion. This was true also in the ganirelix group (R = −0.46, P = 0.003; slope −2.22 ± 0.70). Body composition assessed by AVF was not a significant independent variable or interacting factor (Table 3, upper rows).
Table 2.
Serum Bioavailable Testosterone Concentrations and Deconvolution Analysis in 40 Healthy Men Sampled for 3 Hours at 10-Minute Intervals
| Serum bioavailable Te | Control | Ganirelix | Ketoconazole | ANOVA |
|---|---|---|---|---|
| Mean 3-h Te (ng/dL) | 89.7 ± 5.5 | 43.2 ± 4.1 | 12.7 ± 0.7 | <0.0001* |
| Median 3-h Te (ng/dL) | 88.5 ± 5.4 | 42.5 ± 4.1 | 12.5 ± 0.7 | <0.0001* |
| Maximum Te (ng/dL) | 111 ± 7.3 | 57 ± 5.1 | 14.9 ± 1.0 | <0.0001* |
| Minimum Te (ng/dL) | 74.5 ± 4.6 | 33.7 ± 3.2 | 11.6 ± 0.6 | <0.0001* |
| Deconvolution | ||||
| Pulse frequency (#/3h) | 1.8 ± 0.2 | 1.7 ± 0.2 | NA | 0.63 |
| Slow half-life (min) | 17.7 ± 1.0 | 26.8 ± 0.2 | NA | <0.0001 |
| Mode (min) | 18.7 ± 1.0 | 17.4 ± 0.9 | NA | 0.38 |
| Basal secretion (ng/dL/3h) | 920 ± 70 | 350 ± 60 | NA | <0.0001 |
| Pulsatile secretion (ng/dL/3h) | 120 ± 18 | 66 ± 11 | NA | <0.0001 |
| Total secretion (ng/dL/3h) | 1040 ± 78 | 426 ± 67 | NA | <0.0001 |
| Mean pulse mass (ng/dL) | 80 ± 12 | 39 ± 5 | NA | <0.0001 |
Data are shown as mean and SEM. The General Linear Model was used for the ANOVA of Repeat Measurements. Contrasts were evaluated with the Bonferroni correction.
Abbreviations: Te, testosterone.
Serum concentrations *Post-hoc contrasts: Control vs Ganirelix and Ketoconazole: P < 0.0001, Ganirelix vs Ketoconazole: P < 0.0001. Deconvolution analysis: Due to the very flat Te profile during effective Te inhibition, deconvolution analysis was impossible. Hence, comparisons between control and ganirelix was done with the Student t-test for paired data.
Table 3.
GLM Analysis of Age, AVF, and Their Interaction on Bioavailable Testosterone Concentrations and Secretion, and the Effect of GnRH Administration
| Age P value | AVF P value | Age*AVF P value | Overall ANOVA | |
|---|---|---|---|---|
| Mean 3h concentration Bio Te control | 0.003 | 0.227 | 0.519 | <0.0001 |
| Mean 3h concentration Bio Te ganirelix | 0.091 | 0.444 | 0.568 | 0.11 |
| Mean 3h concentration Bio Te KTCZ | 0.055 | 0.447 | 0.537 | 0.004 |
| Pulsatile Bio Te secretion control | 0.016 | 0.258 | 0.315 | 0.025 |
| Total Bio Te secretion control | 0.002 | 0.197 | 0.380 | <0.0001 |
| Pulsatile Bio Te secretion ganirelix | 0.029 | 0.361 | 0.468 | 0.028 |
| Total Bio Te secretion control ganirelix | 0.335 | 0.634 | 0.780 | 0.46 |
| Mean 2h Bio Te concentration control GnRH | <0.0001 | 0.049 | 0.180 | <0.0001 |
| Mean 2h Bio Te concentration ganirelix GnRH | <0.0001 | 0.012 | 0.046 | <0.0001 |
| Mean 2h Bio Te concentration KTCZ GnRH | 0.08 | 0.35 | 0.52 | 0.009 |
| AUC Bio Te control GnRH | <0.0001 | 0.05 | 0.183 | <0.0001 |
| AUC Bio Te ganirelix GnRH | <0.0001 | 0.01 | 0.043 | <0.0001 |
| AUC Bio Te KTCZ GnRH | 0.79 | 0.34 | 0.52 | 0.009 |
Mean levels were calculated from 18 samples collected at 10-min intervals between 0800 and 1100 h. Secretion was estimated by deconvolution. After injection of GnRH blood samples were withdrawn for 2 h. Abbreviations: AUC, area under the curve; Bio Te, bioavailable testosterone; KTCZ, ketoconazole.
Effects of GnRH administration on serum LH and Te concentrations
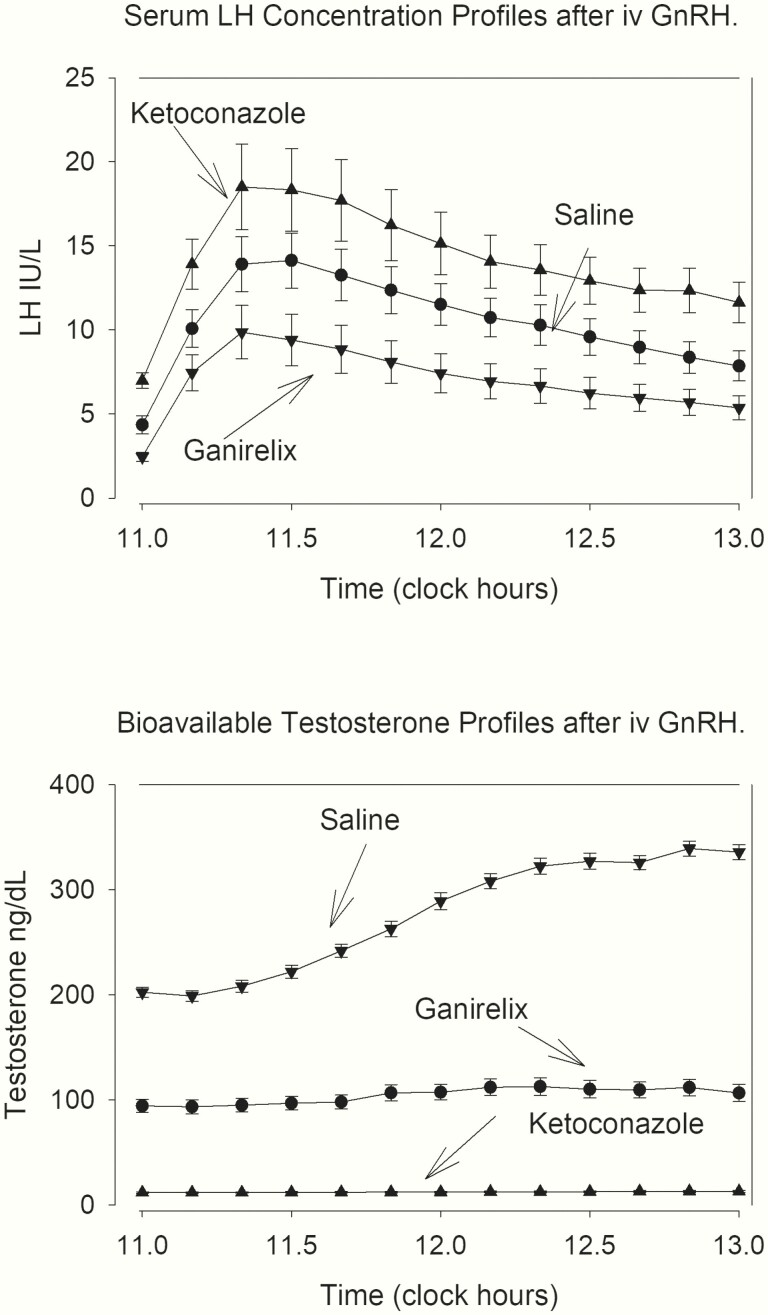
The serum LH and bioavailable Te concentration profiles after GnRH administration in the 3 arms are depicted in Fig. 3. The mean responses are shown in Table 4. There were significant differences between the 3 arms of the experiment, with low bioavailable Te levels in the KTCZ group associated with amplified LH response to GnRH. Importantly, the ganirelix arm still showed some LH increase, thus confirming the competitive nature of ganirelix. The mean LH response to GnRH injection in the ganirelix and KTCZ arm was not related to age, AVF, or the interaction. On the other hand, age was a strongly negative regressor and AVF a weakly negative predictor of 2-hour integrated bioavailable Te levels after GnRH injection in the control arm and after ganirelix administration. All 3 effects were lost during inhibition of Te secretion with KTCZ (Table 3). The interaction between the 2 variables of age and AVF was weak (Table 3).
Figure 3.
Serum LH and bioavailable Te concentration profiles after IV administration of GnRH (100 ng/kg). Note diminished LH responses in ganirelix-treated subjects, and the amplified LH release during KTCZ treatment.
Table 4.
Serum LH and Te Concentrations in 40 Healthy Men after GnRH Administration
| Serum concentrations | Control | Ganirelix | Ketoconazole | ANOVA |
|---|---|---|---|---|
| Mean 2-h LH (IU/L) | 10.14 ± 1.09 | 6.94 ± 1.00 | 13.77 ± 1.52 | <0.0001* |
| Maximum LH (IU/L) | 14.65 ± 1.62 | 9.95 ± 1.54 | 19.24 ± 2.50 | <0.0001* |
| Amplitude LH (IU/L) | 10.5 ± 1.3 | 7.5 ± 1.4 | 12.8 ± 2.3 | <0.0001** |
| AUC LH (IU/L) | 1358 ± 146 | 913 ± 132 | 1834 ± 204 | <0.0001* |
| Testosterone | ||||
| Mean 2-h Te (ng/dL) | 392 ± 19 | 276 ± 21 | 61 ± 3.5 | <0.0001*** |
| Maximum Te (ng/dL) | 458 ± 21 | 368 ± 24 | 68 ± 4.6 | <0.0001*** |
| Amplitude Te (ng/dL) | 122 ± 7.1 | 178 ± 14 | 11 ± 2.7 | <0.0001*** |
| AUC Te (ng/dL*103) | 4.72 ± 0.23 | 3.32 ± 0.25 | 0.73 ± 0.04 | <0.0001*** |
Data are shown as mean and standard error of the mean. The General Linear Model was used for the ANOVA of Repeat Measurements. LH Contrasts were evaluated with the Bonferroni correction.
Abbreviations: AUC, area under the curve; LH, luteinizing hormone.
Serum concentrations: *Post-hoc contrasts: Control versus Ganirelix and Ketoconazole, P < 0.0001, Ganirelix vs Ketoconazole, P < 0.0001.**Ganirelix versus control and ketoconazole, P < 0.0001. Te analysis: Contrasts were corrected with the Bonferroni procedure. ***Control vs Ganirelix and Ketoconazole, P < 0.0001; Ganirelix vs Ketoconazole, P < 0.0001.
Estimation of the feedforward strength of endogenous GnRH
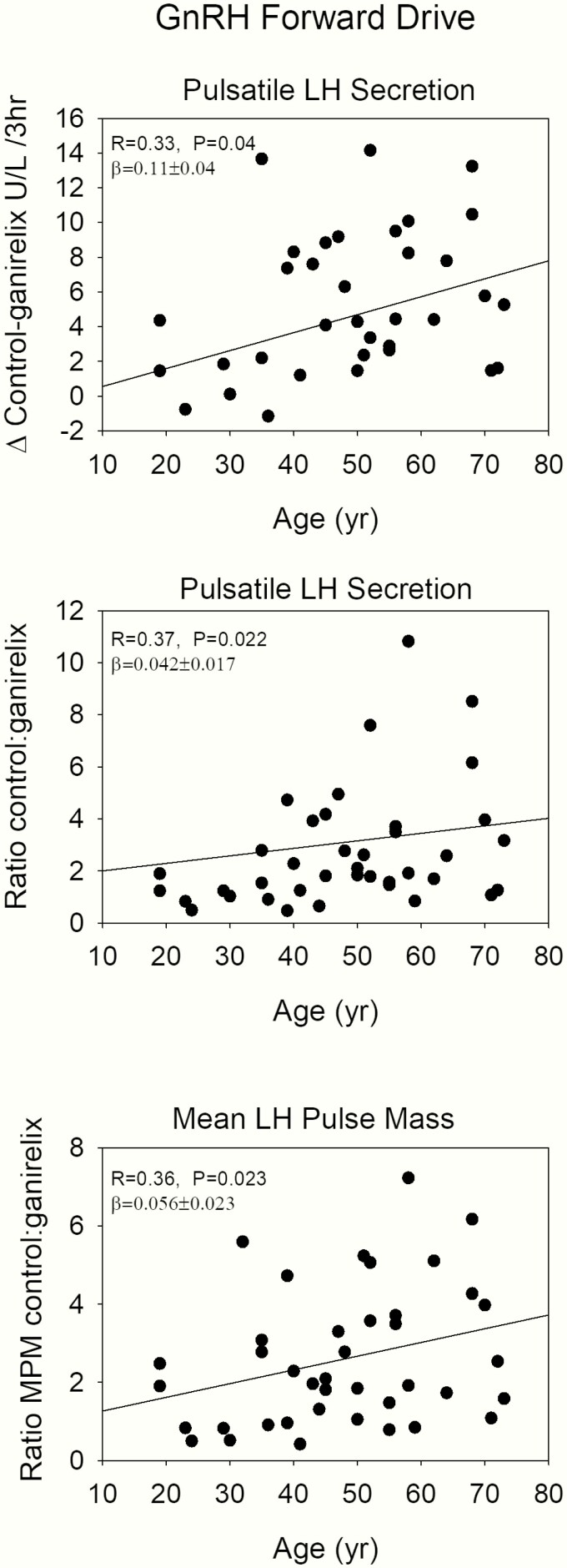
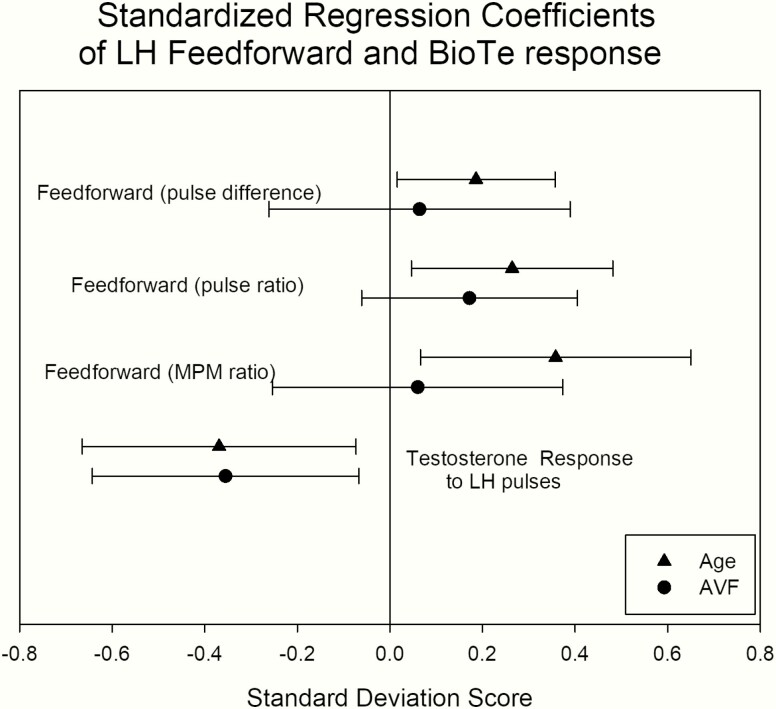
Endogenous GnRH regulates primarily LH pulse frequency and amplitude. Furthermore, if hypothalamic GnRH secretion were diminished, while GnRH action on gonadotropes were preserved (below) in aging individuals, inhibition of LH secretion by any fixed submaximally concentration of a competitive GnRH-receptor blocking agent would be more effective than in the case of strong GnRH signaling. Therefore, the difference in pulsatile LH secretion under the control condition and after ganirelix administration, estimated by deconvolution analysis, was used as a proxy for endogenous GnRH signaling as well as the ratio. Figure 4 presents this analysis, showing that algebraic differences between pulsatile LH secretion after saline (control) and after ganirelix administration were positively related to age. Analogously, the ratio of LH secretion under control conditions and after ganirelix administration was positively related to age, also indicating less GnRH outflow in older volunteers. These parameters were independent of AVF, as illustrated in Fig. 5, where standardized regression coefficients are plotted for the negative effects of age and AVF. This approach allows direct visual comparisons of slope effects (means ± 1.96 standard deviation) of age and AVF.
Figure 4.
Regression plots of parameters of inferred endogenous GnRH feedforward strength of endogenous GnRH on LH secretion versus age. With increasing age, GnRH forward strength diminishes. The upper panel shows the difference between pulsatile LH secretion during the control experiment and after ganirelix. The other panels give the ratios of pulsatile LH secretion and mean LH pulse mass.
Figure 5.
Standardized regression coefficients of feedback and feedforward signaling, calculated from deconvolution analysis and the endogenous dose-response relationship. The dots are the means of the slopes (triangles for age and circles for AVF) and bars represent ± 1.96 × SD. Error bars crossing the vertical zero line define nonsignificant slopes.
Estimation of the feedback strength of testosterone on LH secretion
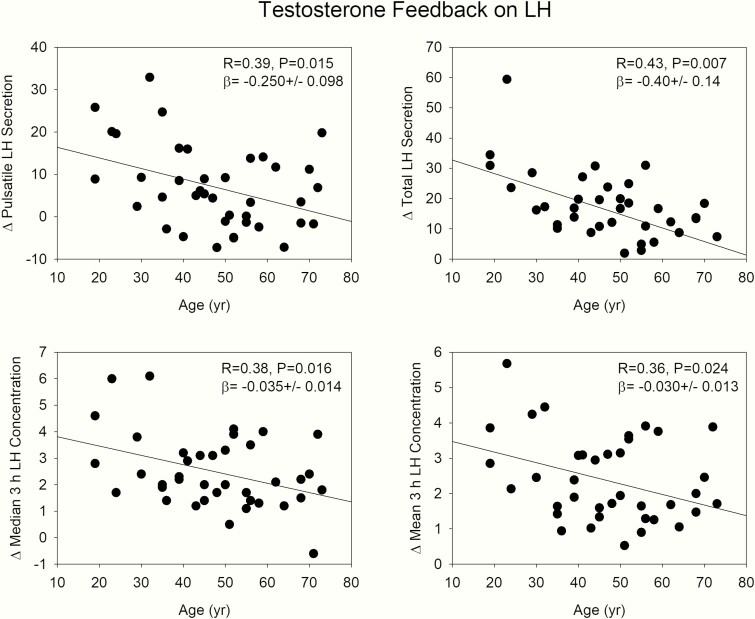
We used the LH difference, measured as (1) mean (median) serum levels and (2) pulsatile LH secretion during KTCZ treatment and the control arm as a proxy for feedback strength, wherein a high value would indicate strong feedback and conversely, a low number diminished feedback. Age was negatively related to all 4 of mean and median 3-hour concentration differences between the 2 arms, and the pulsatile LH and total LH secretion, as depicted in Fig. 6. This would be consistent with a possible age-related decrease in feedback strength. Body composition (AVF) was not related to the mean and median LH differences (R values from 0.21 to 0.29, with P values from 0.08 to 0.21) or pulsatile and total LH secretion (R values ranged from 0.16 to 0.28 and P values from 0.08 to 0.81). The standardized regression coefficients in Fig. 5 show this visually.
Figure 6.
Regression plots of Te feedback during KTCZ administration. Data are differences (mean, median, pulsatile, and total LH secretion) between KTCZ and control experiment. Diminished feedback leads to smaller numbers.
Influence of exogenous LH pulses on Te secretion
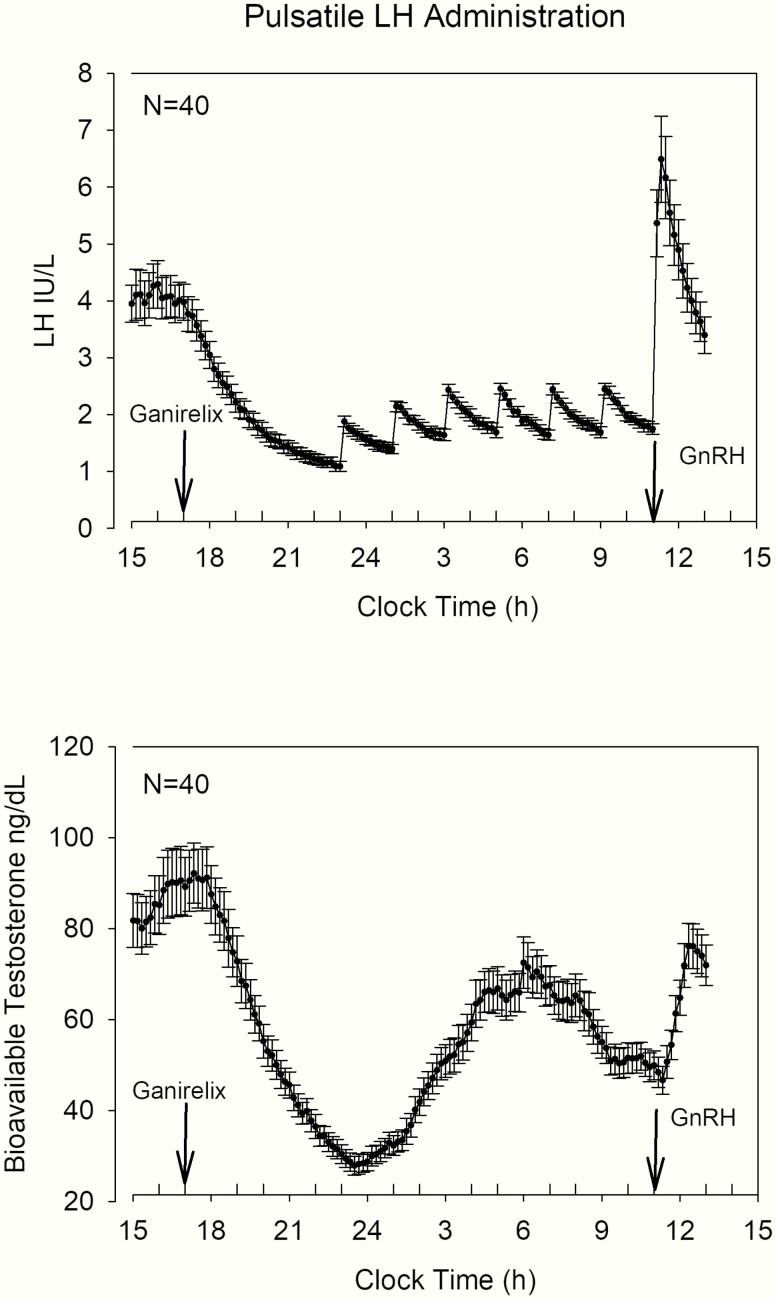
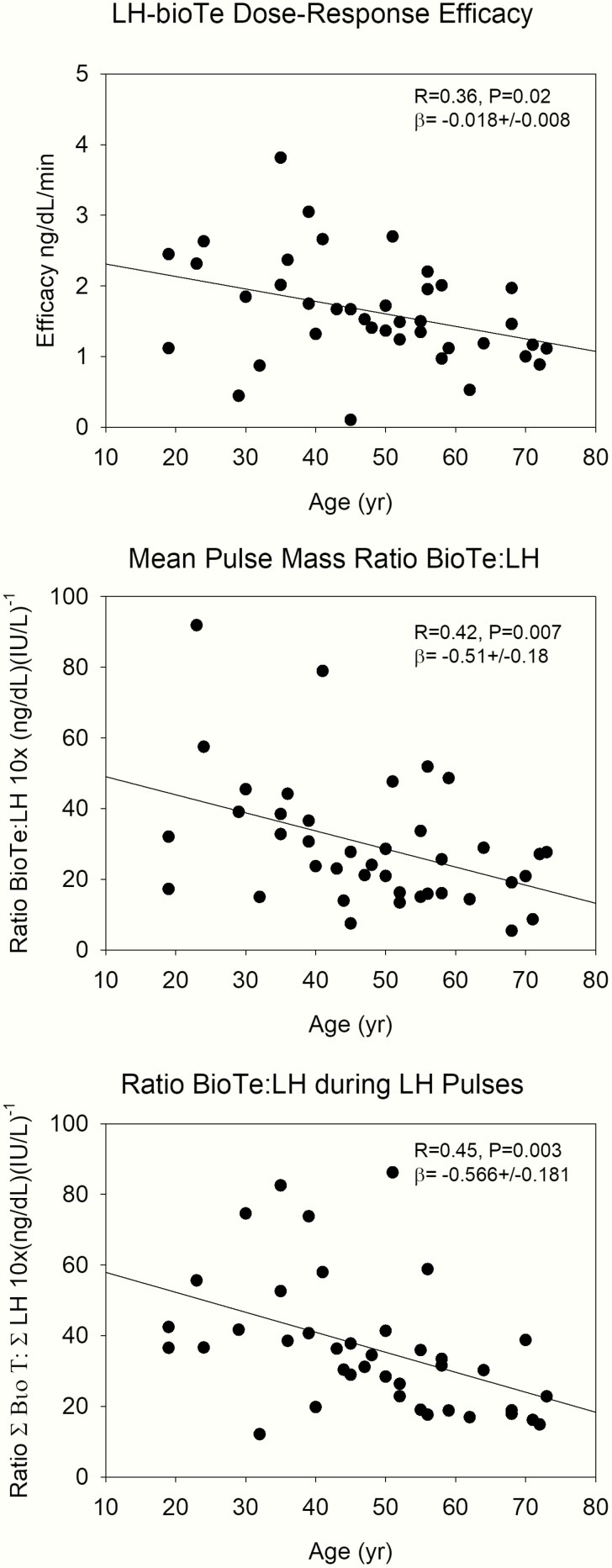
After the administration of ganirelix, endogenous LH and bioavailable Te concentrations decreased acutely, as shown in Fig. 7. Here, data were analyzed in 2-hour bins to match 2-hour infused LH pulses. ANOVA for bioavailable Te and LH yielded overall P < 0.0001, contrasts for LH were P < 0.0001 for time bins 2, 3, and 4 versus time bin 1, while for bioavailable Te, time bins 3 and 4 were significant versus time bin 1. At 2300 hours when the IV LH pulses were started, there were synchronized exogenous LH pulses and endogenous bioavailable Te increases until 0600 hours. The combination of ganirelix and exogenous LH pulses was designed to explore the relationship between LH and bioavailable Te with respect to age and body composition. Thus, the time period in which the LH pulses were administered was analyzed separately from the effect of GnRH injection. Here, we used 3 different approaches. First, we applied dose-response construction (20, 34), comprising logistic regressions between LH concentration pulses and the corresponding bioavailable Te secretion rates (see Methods section). Efficacy (maximal bioavailable Te secretion rate) was negatively related to age (see Fig. 8, upper panel), but LH-Te slope (sensitivity) and LH EC50 (concentration at which half maximal effect occurs) were not. Furthermore, body composition (AVF) was not related to LH-Te dose-response parameters. Second, the ratio between mean bioavailable Te and LH pulse mass (as estimated by deconvolution analysis in this time window) was used as a surrogate for testis responsivity. This measure was also negatively related to age (see Fig. 8, middle panel), and independent of AVF. Third, a model-free approach on the LH and bioavailable Te profiles was applied. The ratios of the areas under the curve for successive 2-hour bins were used as a potential proxy for testicular sensitivity. The ratio of bioavailable Te/LH areas showed a significantly negative relation to age, consistent with less responsiveness of the testis under a fixed LH drive (see Fig. 8, lower panel). This was not present for AVF.
Figure 7.
Serum concentration plots of the overnight blood 10-minute sampling study. After ganirelix administration (labeled arrow) LH and bioavailable Te levels decreased. Pulsatile IV infusions of LH resulted in a reproducible serum LH pattern. The timing of the GnRH injection is shown by the labeled arrow.
Figure 8.
Regression of Leydig cell responsivity to LH pulses on age, estimated by 3 different methods. The upper panel shows analytical reconstruction of the efficacy of the endogenous LH-Te dose-response relation. The middle panel gives the ratio of mean LH and bioavailable Te pulse mass calculated by deconvolution analysis, and the lower panel the ratio of the integrated areas, calculated with the trapezoidal method.
Effect of the GnRH injection
Both LH and bioavailable Te concentrations increased acutely after the administration of GnRH (Fig. 7). The LH concentration response was not related to age and/or body composition, whereas integrated bioavailable Te concentrations were negatively related to age (R = −0.61; P = 0.0001, and regression slope −10.1 ± 2.1) but not to AVF. Comparable results were found for mean concentrations and LH and bioavailable Te concentration peaks and amplitudes (data not shown).
Approximate entropy and cross-approximate entropy
The results of these analyses are listed in Table 5. During KTCZ treatment the decreased feedback of Te depletion increased LH ApEn, reflected in diminished regularity of LH secretion. On the other hand, diminished GnRH feedforward by ganirelix increased LH regularity (lower ApEn). Shutting down Te secretion via KTCZ or ganirelix was accompanied by more regular Te secretion. During the control experiment (with placebo treatment) neither LH ApEn, nor bioavailable Te ApEn were related to age or body composition. The coupling strength of LH and Te was explored with cross-ApEn. Both in the feedforward and feedback direction coupling became stronger (cross-ApEn lower) during KTCZ and ganirelix treatment. Age, but not AVF, was positively related to cross-ApEn in the ganirelix-treated group (denoting loss of LH-Te synchrony), but not in the other arms (forward direction: R = 0.511, P = 0.001; feedback direction R = 0.345, P = 0.003). During the pulsatile LH administration, LH-ApEn was not related to age, but bioavailable Te-ApEn increased with age (R = 0.49, P = 0.001, slope 0.01 ± 0.003), consistent with abnormal testis response patterns. A borderline significant positive relation was present for bioavailable Te and AVF (R = 0.39, P = 0.015).
Table 5.
Approximate and Cross-approximate Entropy of LH and Bioavailable Testosterone
| Control | KTCZ | Ganirelix | ANOVA | |
|---|---|---|---|---|
| LH | 1.073 ± 0.032 | 1.150 ± 0.040a | 0.982 ± 0.049 | 0.017 |
| BioTe | 1.273 ± 0.038b | 0.751 ± 0.081 | 1.096 ± 0.047 c | <0.0001 |
| X-ApEn LH→BioTe | 1.287 ± 0.043 d | 0.680 ± 0.067 | 1.185 ± 0.069 d | <0.0001 |
| X-ApEn BioTe→LH | 1.319 ± 0.045 | 1.443 ± 0.122 e | 1.030 ± 0.063f | 0.001 |
Data are shown as mean ± SEM. Statistical comparisons were done with ANOVA for repeated measurements. Abbreviations: BioTe: bioavailable testosterone, X-ApEn: cross approximate entropy. Contrasts: aKTCZ vs control, P = 0.025; bcontrol vs KTCZ, P < 0.0001; cganirelix vs KTCZ, P = 0.004; dControl and ganirelix vs KTCZ, P < 0.0001; eganirelix vs KTCZ, P = 0.012, fcontrol vs ganirelix, P = 0.004.
Discussion
This clinical study estimated age- and body fat-related changes in GnRH outflow, GnRH effect, Te feedback and Te secretion in response to exogenous LH pulses in 40 healthy men over a range of age and visceral abdominal fat. Age, but not body fat, was associated with diminished GnRH outflow, normal GnRH effect, decreased testicular responsivity to infused LH pulses and diminished Te feedback. The empirical outcomes directly support earlier theoretical predictions based upon a biomathematical model (35).
Pulsatile LH secretion by the pituitary gland is regulated by hypothalamic kisspeptin- and GnRH-secreting neurons, as inferred in humans, isolated rat hypothalamic explants, and hypophyseal-portal blood of the ewe (18, 36, 37). Thereby, Leydig cells are exposed to pulsatile LH, which promotes Te synthesis and pulsatile Te secretion (38). This schema is supported by loss-of-function mutations of the human genes for kisspeptin and kisspeptin receptor (GPR54) (39, 40).
The present study used an indirect approach to estimate the endogenous GnRH signal, namely, the difference in LH pulse amplitude during saline and ganirelix treatment. This analysis predicted decreased output of GnRH-stimulated LH secretion with age. In contrast, prior GnRH dose-response studies revealed comparable GnRH efficacy (maximal effect) with increased GnRH sensitivity (steeper GnRH dose-LH response curve) in older and young men. Moreover, pulsatile IV infusions of GnRH for 2 weeks yielded comparable 24-hour pulsatile bioactive and immunoreactive LH secretion in older and young individuals (41, 42). Investigations in the aged male brown Norway rat have disclosed decreases in hypothalamic GnRH mRNA and peptide content with unchanged LH responses to LH (43).
Sex steroids have a restraining effect on gonadotropin secretion. The present analyses demonstrate that, compared with young men, older men manifest less amplification of LH secretion during experimental Te deprivation. A second approach, which used exogenous Te to suppress LH secretion, inferred either increased or decreased feedback in older individuals (44–46). A third approach using intensive (2.5-minute interval) blood sampling and cross-entropy analysis of Te and LH secretion estimated relative feedback failure in older men (10). This result is comparable to that of a fourth approach, based upon a dynamic biomathematical feedback model in men ages 18 to 65 years studied under graded transdermal Te feedback (47).
The sites of impaired Te feedback (pituitary and/or hypothalamus) and the mechanisms involved (direct, after 5α-reduction or after aromatization of Te) are not inferable from the present analyses. However, available data show that kisspeptin but not GnRH neurons contain estrogen receptor α, allowing estrogen negative feedback thereby (48). This pathway could explain why Te infusions in orchidectomized Rhesus monkeys downregulate hypothalamic KiSS-1 gene expression (49). In clinical investigations of Te feedback, both estrogen receptor and androgen receptor are partly involved, since flutamide, bicalutamide, tamoxifen, and anastrozole all augment LH pulse frequency (a property conferred by GnRH pulses) and reduce LH secretory regularity (a property conferred by both GnRH and LH secretion) (50, 51).
Pharmacologic human chorionic gonadotropin injections do not stimulate maximal (young adult) Te secretion in aging men (5, 52). The present investigation employed nearly physiological IV pulses of human recombinant LH infused under ganirelix suppression of endogenous LH to document attenuated Leydig-cell Te secretion in older men, as illustrated in earlier paradigms (11, 53). Moreover, further analysis suggested lower LH efficacy (maximal Te response) and unchanged sensitivity (slope of the LH-Te secretion curve) and potency (EC50) in older men, as inferred independently from 24-hour LH and Te secretion profiles analyzed analogously (19). In relation to presumptive loss of LH efficacy, the number of Leydig cells in older men is reported as decreased or normal (54, 55), while their ultrastructure and biochemistry are abnormal in humans (56) and in a rat model (57).
Prior studies of exogenous GnRH’s stimulation of LH secretion describe either diminished or increased pituitary responses in older men (58, 59). In the current study, based upon a total of 160 GnRH challenges, no age-related decline in LH secretion was detectable. This outcome agrees with that of a direct GnRH dose-response study conducted previously in which men were maintained at experimentally fixed Te concentrations (58). Therefore, the decrease in pulsatile LH secretion in older individuals should reflect diminished GnRH outflow and not blunted pituitary responsiveness.
LH secretion did not correlate with BMI over the wide range examined in this study. Given that the highest BMI was 34.3 kg/m2, our finding agrees with a review in which only 2 out of 9 studies of LH secretion observed an inverse correlation with BMI, when BMI exceeded 40 kg/m2 (60, 61).Unlike LH, in the case of Te, 18 of 20 studies report a negative relation between BMI and total Te, and 10 of 14 also do so for free Te (61). In our study, whereas CT-estimated intraabdominal fat was a strong negative covariate of bioavailable Te concentrations, it did not explain the effects of age on LH or Te secretion.
As a secondary outcome, unihormonal LH ApEn (pattern irregularity) increased with age under experimental Te deprivation, as observed for pituitary secretion in other endocrine axes under corresponding feedback withdrawal (17, 62-64). Moreover, bihormonal LH-Te joint synchrony also deteriorated with age in humans (59). The basic defect responsible for these pattern-sensitive changes is unknown. Importantly, all subjects were evaluated under the same sampling conditions of exercise, sleep and food intake in order to prevent confounding effects on the HPG axis (65).
Several caveats apply. Although hypothesis-driven, this study’s cross-sectional design limits causal conclusions. Thus, further studies are needed with long-term follow-up in larger cohorts of men, including even older volunteers.
In summary, this study discloses age-associated decreases in estimated GnRH secretion, pulsatile LH feedforward on Te secretion, and Te feedback on the GnRH-LH unit, with preserved GnRH action in healthy men.
Acknowledgments
Financial Support: Supported in part via R01 AG019695, R01 AG029362, R01 AG031763 and P30 DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD). The project described was supported by UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS) and 60NANB10D005Z from the National Institute of Standards and Technology.
Glossary
Abbreviations
- ApEn
approximate entropy
- AVF
abdominal visceral mass
- BMI
body mass index
- CT
computed tomography
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GH
growth hormone
- GHRH
growth hormone–releasing hormone
- HPG axis
hypothalamic-pituitary-gonad axis
- IGF-1
insulin-like growth factor 1
- IV
intravenous
- LH
luteinizing hormone
- PSA
prostate-specific antigen
- SHBG
sex hormone–binding globulin
- SC
subcutaneous
- Te
testosterone
Additional Information
Disclosure Summary: The authors have nothing to disclose.
References
- 1. Araujo AB, Mohr BA, McKinlay JB. Changes in sexual function in middle-aged and older men: longitudinal data from the Massachusetts Male Aging Study. J Am Geriatr Soc. 2004;52(9):1502–1509. [DOI] [PubMed] [Google Scholar]
- 2. Svartberg J, Midtby M, Bønaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromsø Study. Eur J Endocrinol. 2003;149(2):145–152. [DOI] [PubMed] [Google Scholar]
- 3. Liu PY, Veldhuis JD. The hypothalamo-pituitary unit, testis, and male accessory organs. In: Yen and Jaffe’s Reproductive Endocrinology. Amsterdam: Elsevier; 2018; 285–300. [Google Scholar]
- 4. Gray A, Berlin JA, McKinlay JB, Longcope C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol. 1991;44(7):671–684. [DOI] [PubMed] [Google Scholar]
- 5. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. [DOI] [PubMed] [Google Scholar]
- 6. van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85(9):3276–3282. [DOI] [PubMed] [Google Scholar]
- 7. Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol. 1997;146(8):609–617. [DOI] [PubMed] [Google Scholar]
- 8. Veldhuis JD, Zwart A, Mulligan T, Iranmanesh A. Muting of androgen negative feedback unveils impoverished gonadotropin-releasing hormone/luteinizing hormone secretory reactivity in healthy older men. J Clin Endocrinol Metab. 2001;86(2):529–535. [DOI] [PubMed] [Google Scholar]
- 9. Mulligan T, Iranmanesh A, Johnson ML, Straume M, Veldhuis JD. Aging alters feed-forward and feedback linkages between LH and testosterone in healthy men. Am J Physiol. 1997;273(4):R1407–R1413. [DOI] [PubMed] [Google Scholar]
- 10. Veldhuis JD, Iranmanesh A, Keenan DM. Erosion of endogenous testosterone-driven negative feedback on pulsatile luteinizing hormone secretion in healthy aging men. J Clin Endocrinol Metab. 2004;89(11):5753–5761. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab. 2007;92(9):3626–3632. [DOI] [PubMed] [Google Scholar]
- 12. Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Aging in healthy men impairs recombinant human luteinizing hormone (LH)-stimulated testosterone secretion monitored under a two-day intravenous pulsatile LH clamp. J Clin Endocrinol Metab. 2005;90(10):5544–5550. [DOI] [PubMed] [Google Scholar]
- 13. Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147(3):1154–1158. [DOI] [PubMed] [Google Scholar]
- 14. Wahab F, Quinton R, Seminara SB. The kisspeptin signaling pathway and its role in human isolated GnRH deficiency. Mol Cell Endocrinol. 2011;346(1-2):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harter CJL, Kavanagh GS, Smith JT. The role of kisspeptin neurons in reproduction and metabolism. J Endocrinol. 2018;238(3):R173–R183. [DOI] [PubMed] [Google Scholar]
- 16. Roelfsema F, Veldhuis JD. Growth Hormone Dynamics in Healthy Adults Are Related to Age and Sex and Strongly Dependent on Body Mass Index. Neuroendocrinology. 2016;103(3-4):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J Clin Endocrinol Metab. 2004;89(5):2290–2300. [DOI] [PubMed] [Google Scholar]
- 18. Keenan DM, Veldhuis JD. Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1755–R1771. [DOI] [PubMed] [Google Scholar]
- 19. Keenan DM, Veldhuis JD. Stochastic model of admixed basal and pulsatile hormone secretion as modulated by a deterministic oscillator. Am J Physiol. 1997;273(3 Pt 2):R1182–R1192. [DOI] [PubMed] [Google Scholar]
- 20. Keenan DM, Veldhuis JD. A biomathematical model of time-delayed feedback in the human male hypothalamic-pituitary-Leydig cell axis. Am J Physiol. 1998;275(1):E157–E176. [DOI] [PubMed] [Google Scholar]
- 21. Liu PY, Pincus SM, Takahashi PY, et al. Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade. Am J Physiol Endocrinol Metab. 2006;290(1):E34–E41. [DOI] [PubMed] [Google Scholar]
- 22. Zwart AD, Iranmanesh A, Veldhuis JD. Disparate serum free testosterone concentrations and degrees of hypothalamo-pituitary-luteinizing hormone suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab. 1997;82(7):2062–2069. [DOI] [PubMed] [Google Scholar]
- 23. Veldhuis JD, Lizarralde G, Iranmanesh A. Divergent effects of short term glucocorticoid excess on the gonadotropic and somatotropic axes in normal men. J Clin Endocrinol Metab. 1992;74(1):96–102. [DOI] [PubMed] [Google Scholar]
- 24. Rabinovici J, Rothman P, Monroe SE, Nerenberg C, Jaffe RB. Endocrine effects and pharmacokinetic characteristics of a potent new gonadotropin-releasing hormone antagonist (Ganirelix) with minimal histamine-releasing properties: studies in postmenopausal women. J Clin Endocrinol Metab. 1992;75(5):1220–1225. [DOI] [PubMed] [Google Scholar]
- 25. Nelson LR, Fujimoto VY, Jaffe RB, Monroe SE. Suppression of follicular phase pituitary-gonadal function by a potent new gonadotropin-releasing hormone antagonist with reduced histamine-releasing properties (ganirelix). Fertil Steril. 1995;63(5):963–969. [DOI] [PubMed] [Google Scholar]
- 26. de Jong D, Macklon NS, Mannaerts BM, Coelingh Bennink HJ, Fauser BC. High dose gonadotrophin-releasing hormone antagonist (ganirelix) may prevent ovarian hyperstimulation syndrome caused by ovarian stimulation for in-vitro fertilization. Hum Reprod. 1998;13(3):573–575. [DOI] [PubMed] [Google Scholar]
- 27.RRID:AB_2750984 2019 https://scicrunch.org/resolver/AB_2750984.
- 28.RRID:AB_2750986 2019 https://scicrunch.org/resolver/AB_2750986.
- 29.RRID:AB_2750985 2019 https://scicrunch.org/resolver/AB_2750985.
- 30.RRID:AB_2750983 2019 https://scicrunch.org/resolver/AB_2750983.
- 31. Liu PY, Keenan DM, Kok P, Padmanabhan V, O’Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297(2):E538–E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88(6):2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci U S A. 1996;93(24):14100–14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci U S A. 2001;98(7):4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keenan DM, Takahashi PY, Liu PY, et al. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology. 2006;147(6):2817–2828. [DOI] [PubMed] [Google Scholar]
- 36. Caraty A, Lomet D, Sébert ME, Guillaume D, Beltramo M, Evans NP. Gonadotrophin-releasing hormone release into the hypophyseal portal blood of the ewe mirrors both pulsatile and continuous intravenous infusion of kisspeptin: an insight into kisspeptin’s mechanism of action. J Neuroendocrinol. 2013;25(6):537–546. [DOI] [PubMed] [Google Scholar]
- 37. Clarke IJ. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology. 1993;133(4):1624–1632. [DOI] [PubMed] [Google Scholar]
- 38. Foresta C, Bordon P, Rossato M, Mioni R, Veldhuis JD. Specific linkages among luteinizing hormone, follicle-stimulating hormone, and testosterone release in the peripheral blood and human spermatic vein: evidence for both positive (feed-forward) and negative (feedback) within-axis regulation. J Clin Endocrinol Metab. 1997;82(9):3040–3046. [DOI] [PubMed] [Google Scholar]
- 39. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 40. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol. 1999;141(3):257–266. [DOI] [PubMed] [Google Scholar]
- 42. Veldhuis JD, Iranmanesh A, Mulligan T. Age and testosterone feedback jointly control the dose-dependent actions of gonadotropin-releasing hormone in healthy men. J Clin Endocrinol Metab. 2005;90(1):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl. 2000;21(1):72–84. [PubMed] [Google Scholar]
- 44. Deslypere JP, Kaufman JM, Vermeulen T, Vogelaers D, Vandalem JL, Vermeulen A. Influence of age on pulsatile luteinizing hormone release and responsiveness of the gonadotrophs to sex hormone feedback in men. J Clin Endocrinol Metab. 1987;64(1):68–73. [DOI] [PubMed] [Google Scholar]
- 45. Muta K, Kato K, Akamine Y, Ibayashi H. Age-related changes in the feedback regulation of gonadotrophin secretion by sex steroids in men. Acta Endocrinol (Copenh). 1981;96(2):154–162. [DOI] [PubMed] [Google Scholar]
- 46. Winters SJ, Atkinson L. Serum LH concentrations in hypogonadal men during transdermal testosterone replacement through scrotal skin: further evidence that ageing enhances testosterone negative feedback. The Testoderm Study Group. Clin Endocrinol (Oxf). 1997;47(3):317–322. [DOI] [PubMed] [Google Scholar]
- 47. Liu PY, Takahashi PY, Roebuck PD, Veldhuis JD. Age or factors associated with aging attenuate testosterone’s concentration-dependent enhancement of the regularity of luteinizing hormone secretion in healthy men. J Clin Endocrinol Metab. 2006;91(10):4077–4084. [DOI] [PubMed] [Google Scholar]
- 48. Javed Z, Qamar U, Sathyapalan T. The role of kisspeptin signalling in the hypothalamic-pituitary-gonadal axis–current perspective. Endokrynol Pol. 2015;66(6):534–547. [DOI] [PubMed] [Google Scholar]
- 49. Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2007;19(6):432–438. [DOI] [PubMed] [Google Scholar]
- 50. Schnorr JA, Bray MJ, Veldhuis JD. Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous gonadotropin-releasing hormone-stimulated luteinizing hormone and follicle-stimulating hormone secretion in young men. J Clin Endocrinol Metab. 2001;86(6):2600–2606. [DOI] [PubMed] [Google Scholar]
- 51. Veldhuis JD, Iranmanesh A. Short-term aromatase-enzyme blockade unmasks impaired feedback adaptations in luteinizing hormone and testosterone secretion in older men. J Clin Endocrinol Metab. 2005;90(1):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Winters SJ, Sherins RJ, Troen P. The gonadotropin-suppressive activity of androgen is increased in elderly men. Metabolism. 1984;33(11):1052–1059. [DOI] [PubMed] [Google Scholar]
- 53. Veldhuis JD, Veldhuis NJ, Keenan DM, Iranmanesh A. Age diminishes the testicular steroidogenic response to repeated intravenous pulses of recombinant human LH during acute GnRH-receptor blockade in healthy men. Am J Physiol Endocrinol Metab. 2005;288(4):E775–E781. [DOI] [PubMed] [Google Scholar]
- 54. Neaves WB, Johnson L, Porter JC, Parker CR Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59(4):756–763. [DOI] [PubMed] [Google Scholar]
- 55. Petersen PM, Seierøe K, Pakkenberg B. The total number of Leydig and Sertoli cells in the testes of men across various age groups - a stereological study. J Anat. 2015;226(2):175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paniagua R, Nistal M, Sáez FJ, Fraile B. Ultrastructure of the aging human testis. J Electron Microsc Tech. 1991;19(2):241–260. [DOI] [PubMed] [Google Scholar]
- 57. Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143(5):1637–1642. [DOI] [PubMed] [Google Scholar]
- 58. Iranmanesh A, Mulligan T, Veldhuis JD. Age in men does not determine gonadotropin-releasing hormone’s dose-dependent stimulation of luteinizing hormone secretion under an exogenous testosterone clamp. J Clin Endocrinol Metab. 2010;95(6): 2877–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu PY, Pincus SM, Keenan DM, Roelfsema F, Veldhuis JD. Analysis of bidirectional pattern synchrony of concentration-secretion pairs: implementation in the human testicular and adrenal axes. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R440–R446. [DOI] [PubMed] [Google Scholar]
- 60. Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79(4):997–1000. [DOI] [PubMed] [Google Scholar]
- 61. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293–311. [DOI] [PubMed] [Google Scholar]
- 62. Lado-Abeal J, Hickox JR, Cheung TL, Veldhuis JD, Hardy DM, Norman RL. Neuroendocrine consequences of fasting in adult male macaques: effects of recombinant rhesus macaque leptin infusion. Neuroendocrinology. 2000;71(3):196–208. [DOI] [PubMed] [Google Scholar]
- 63. Roelfsema F, Pereira AM, Adriaanse R, et al. Thyrotropin secretion in mild and severe primary hypothyroidism is distinguished by amplified burst mass and Basal secretion with increased spikiness and approximate entropy. J Clin Endocrinol Metab. 2010;95(2):928–934. [DOI] [PubMed] [Google Scholar]
- 64. Roelfsema F, Aoun P, Takahashi PY, Erickson D, Yang R, Veldhuis JD. Regulation of pulsatile and entropic ACTH secretion under fixed exogenous secretagogue clamps. J Clin Endocrinol Metab. 2017;102(7):2611–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iranmanesh A, Lawson D, Veldhuis JD. Glucose ingestion acutely lowers pulsatile LH and basal testosterone secretion in men. Am J Physiol Endocrinol Metab. 2012;302(6):E724–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]