Abstract
Purpose
To analyze the pathogen spectrum of isolated pathogens and antibiotic susceptibility trends of infectious endophthalmitis over 9 years from a large referral eye center in southern China.
Methods
Data from all inpatients who were clinically diagnosed with infectious endophthalmitis and underwent microbiological evaluation at the Zhongshan Ophthalmic Center from January 2010 to December 2018 were collected retrospectively and analyzed according to different clinical etiologies.
Results
A total of 816 cases were collected in the study. Open-globe injuries had caused 473 (57.97%) cases, 70 (8.58%) cases presented endophthalmitis after infectious keratitis, 156 (19.12%) cases were postoperative, and endogenous causes accounted for 117 (14.34%) cases. Among the 309 culture-positive cases, the predominant pathogen for both postoperative and posttraumatic endophthalmitis was gram-positive cocci (59.52% and 49.72%, respectively). Regarding keratitis-related endophthalmitis, the main pathogens were filamentous fungi (57.58%) and gram-negative bacilli (30.30%). The pathogens of endogenous endophthalmitis were almost evenly distributed among gram-positive cocci, gram-negative bacilli, and fungi. Eighty-five (10.42%) cases underwent evisceration/enucleation, including 42 cases secondary to keratitis-related endophthalmitis. The incidence of evisceration/enucleation was much higher in keratitis-related endophthalmitis than the total endophthalmitis population (χ2 =123.61, P<0.001). Overall bacteria showed high susceptibility to fluoroquinolones (75.36–100.00%). Gram-positive cocci showed much higher sensitivity to cephalosporins compared to gram-negative bacilli (85.11–92.59% vs 25.42–35.72%). For the five first-line antibiotics analyzed for time trend of susceptibility, four exhibited a significant decrease of susceptibility from 2010–2014 to 2015–2018.
Conclusion
Between 2010 and 2018, posttraumatic endophthalmitis was the most common form of the treated endophthalmitis in Zhongshan Ophthalmic Center. The causative pathogens varied according to different clinical settings. Even though the overall antibiotic susceptibilities were fairly high, we observed a substantial decrease of susceptibility for most first-line antibiotics.
Keywords: infectious endophthalmitis, pathogen spectrum, antibiotic resistance, time trend, evisceration/enucleation
Introduction
Infectious endophthalmitis is a common sight-threatening disease caused by intraocular microbial infection. Most endophthalmitis cases are exogenous, meaning the pathogens access the eye via open-globe trauma, intraocular operation, or sometimes, through corneal infection.1–3 Endogenous endophthalmitis is less common and occurs when intraocular pathogens gain access from the infected bloodstream.4 The urgency, severity, and prognosis of the endophthalmitis are variable, mostly depending on the causative pathogen.5 The spectrum of causative pathogens generally include gram-positive cocci, gram-negative bacilli and filamentous fungi. However, not only they can vary according to different clinical settings, they can also evolve over time and differ by region.6 To provide a regional reference for practitioners, it is necessary to update the epidemiology of causative endophthalmitis pathogens. As an infectious disease, antibiotic resistance is obviously a serious issue. The efficiency of first-line antibiotics has been seriously compromised ever since the rise of multiple drug resistance bacteria, especially so-called ESKAPE pathogens (E: Enterococcus faecium, S: Staphylococcus aureus, K: Klebsiella pneumoniae, A: Acinetobacter baumannii, P: Pseudomonas aeruginosa, E: Enterobacter spp.).7,8 To better guide empirical antibiotic treatment, antibiotic resistance trend is also an vital information. In light of the objective, we collected all the cases of infectious endophthalmitis treated at Zhongshan Ophthalmic Center from 2010 to 2018 to analyze the temporal trends of pathogen proportion and antibiotic susceptibilities.
Materials and Methods
The study was approved by the Ethics Committee of Zhongshan Ophthalmic Center and performed in compliance with the Declaration of Helsinki. All of the protocols and results interpretation were conducted according to the Clinical and Laboratory Standards Institute terms. The study was a retrospective review conducted among inpatients who were clinically diagnosed with infectious endophthalmitis at the Zhongshan Ophthalmic Center between January 2010 and December 2018. Cases with pathogen culture results were gathered and classified into four groups according to different clinical etiologies of infectious endophthalmitis: posttraumatic, microbial keratitis associated, endogenous, and postoperative infectious endophthalmitis. Medical charts including medical history, demographic data, laboratory results, and surgical records were collected and analyzed. As patient data were analyzed anonymously and maintained confidential, the patient consent was waived by the Ethics Committee of the Zhongshan Ophthalmic Center.
Aqueous humor was aspirated from the anterior chamber through the limbus with a needle on a 1-mL syringe. Vitreous specimens were collected through the pars plana. Other specimens were optionally collected for microbial culture according to different clinical conditions. Corneal specimens were collected via scraping the base and edge of the corneal ulceration with a platinum spatula for microbial keratitis-associated endophthalmitis, blood specimens were drawn by peripheral venipuncture for some cases of endogenous endophthalmitis, and diseased intraocular tissue was removed for culture in case of evisceration or enucleation. The specimens were inoculated into bacterial and fungal media including blood agar, chocolate agar, brain heart infusion broth, thioglycolate (broth), Sabouraud agar, sheep blood agar, and potato glucose agar.
All bacterial isolates were identified using the automated system (VITEK 2 compact BioMérieux, Inc., Marcy-l’Étoile, France). Antibiotic susceptibility testing was performed, with minimum inhibitory concentration assay, for cephalosporins (cefazolin and cefuroxime sodium), fluoroquinolones (ofloxacin, levofloxacin, and moxifloxacin), aminoglycosides (tobramycin and gentamycin); penicillins (penicillin, methicillin, and oxacillin), and vancomycin. Due to the periodic suspension of some antibiotics, not every drug was tested for the same numbers of specimens. Fungi isolates were identified by experienced microbiological technicians according to the morphology and colony characteristics without testing for antifungal susceptibility.
Statistical Analysis
All analyses were performed with commercially available software (SPSS 17.0; SPSS Inc., Chicago, IL, USA). Culture-positive endophthalmitis was defined as positive pathogen detection from any specimen obtained from the same patient. For bacterial susceptibility, intermediate sensitivity was also considered sensitive. Differences between groups were compared with χ2 tests. To evaluate temporal trend in the distribution of pathogens detected, data were divided into two periods, 2010–2014 and 2015–2018. We performed χ2 tests to compare the distributions between these two periods. Post hoc multiple analyses were conducted to determine if the change for a certain organism was statistically significant. Two-tailed Student’s t-tests were used to determine statistical significance (P<0.05).
Results
A total of 816 cases that presented with infectious endophthalmitis were included in the study. The mean age was 38.54±21.62, ranged from1 month to 83 years. More than half (473, 57.97%) of cases were caused by open-globe injuries, 70 (8.58%) cases presented with endophthalmitis after infectious keratitis, 156 (19.12%) cases developed endophthalmitis after intraocular surgeries, and 117 (14.34%) cases were classified as endogenous endophthalmitis. As shown in Table 1, most patients were male (610 males, 206 females), mostly due to the male predominance of posttraumatic endophthalmitis.
Table 1.
Clinical Characteristics of Patients by Endophthalmitis Etiology in Zhongshan Ophthalmic Center
| Posttraumatic | Keratitis Related | Postoperative | Endogenous | Total | |
|---|---|---|---|---|---|
| Cases (n) | 473 (57.97%) | 70 (8.58%) | 156 (19.12%) | 117 (14.34%) | 816 (100.0%) |
| Sex (M/F) | 408/65 | 46/24 | 94/62 | 62/55 | 610/206 |
| Age (Mean±SD) | 31.81±16.99 | 46.74±26.86 | 59.46±17.68 | 32.83±21.19 | 38.54±21.62 |
| Culture-proven casesa | 177 (37.42%) | 33 (47.14%) | 42 (26.92%) | 57 (48.72%) | 309 (37.87%) |
| 2010–2014 | 61 | 3 | 15 | 28 | 107 |
| 2015–2018 | 116 | 33 | 27 | 29 | 202 |
| Cases underwent Evisceration/enucleation (n)b | 17 | 42c | 13 | 13 | 85 |
| Special pathogensd | Amoeba (1) | Parasites (5) | |||
| Notes | Silicone oil (2) |
Notes: aThe numbers in the parentheses represent the pathogen detection rates. bNumber of cases that ultimately underwent evisceration or enucleation. cThe incidence of evisceration/enucleation among keratitis-related endophthalmitis was higher than total population (χ2=123.61, P<0.001). dAmoeba was detected by corneal scraping. Parasites were diagnosed by pathological examination.
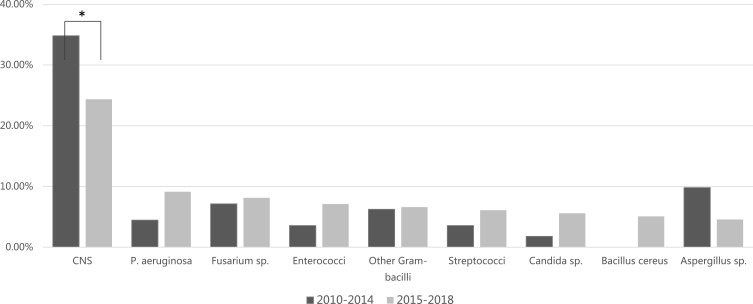
The distribution of 309 isolates is shown in Table 2. Overall, the predominant causative pathogens were gram-positive cocci (42.07–59.52%), except for keratitis-related endophthalmitis and endogenous endophthalmitis. Regarding keratitis-related endophthalmitis, the main causative pathogens were filamentous fungi (19/33 isolates, 57.58%) and gram-negative bacilli (10/33 isolates, 30.30%). For endogenous endophthalmitis, gram-negative bacilli counted for 33.33% (19/57 cases), while fungi counted for 38.56% (22/57 cases). Among the 57 endogenous endophthalmitis cases, 2 cases had mycotic dermatitis, 2 had sepsis and rest had unknown sources. Temporal trend in the distribution of pathogens detected was shown in Figure 1. There was a significant change in the percentage of the pathogens between these two periods (χ2=200.833, P=0.004). Post hoc analysis revealed that the percentage of coagulase-negative staphylococci (CNS) decreased significantly from 34.82% in 2010–2014 to 24.37% in 2015–2018 (P<0.001).
Table 2.
Genus Distribution of Pathogens Isolated from Endophthalmitis Patients by Clinical Setting
| Genus | Total | Posttraumatic | Keratitis Related | Postoperative | Endogenous |
|---|---|---|---|---|---|
| Gram-positive cocci | 130 (42.07%) | 88 (49.72%) | 2 (6.06%) | 25 (59.52%) | 15 (26.32%) |
| Staphylococcus epidermidis | 61 (19.74%) | 9 (5.08%) | 6 (14.29%) | 2 (3.51%) | |
| Other CNS | 26 (8.41%) | 54 (30.51%) | 1 (3.03%) | 7 (16.67%) | 8 (14.04%) |
| Enterococci | 18 (5.83%) | 6 (3.39%) | 10 (23.81%) | 2 (3.51%) | |
| Streptococci | 16 (5.18%) | 11 (6.21%) | 2 (4.76%) | 2 (3.51%) | |
| Staphylococcus aureus | 3 (0.97%) | 1 (1.75%) | |||
| Other Gram-positive cocci | 6 (1.94%) | 8 (4.52%) | 1 (3.03%) | ||
| Gram-negative bacilli | 83 (26.86%) | 44 (24.86%) | 10 (30.30%) | 10 (23.81%) | 19 (33.33%) |
| Pseudomonas aeruginosa | 23 (7.44%) | 3 (1.69%) | 9 (27.27%) | 4 (9.52%) | 7 (12.28%) |
| Serratia spp. | 20 (6.47%) | 4 (2.26%) | 2 (4.76%) | ||
| Monas spp. | 11 (3.56%) | 6 (3.39%) | 1 (2.38%) | 4 (7.02%) | |
| Enteric bacilli | 9 (2.91%) | 8 (4.52%) | 3 (7.14%) | 1 (1.75%) | |
| Other Gram-negative bacilli | 20 (6.47%) | 23 (12.99%) | 1 (3.03%) | 7 (12.28%) | |
| Gram-positive bacilli | 18 (5.83%) | 14 (7.91%) | 2 (6.06%) | 2 (4.76%) | |
| Bacillus cereus | 10 (3.24%) | 9 (5.08%) | 1 (3.03%) | ||
| Other Bacillus spp. | 4 (1.29%) | 4 (2.26%) | |||
| Propionibacterium acnes | 3 (0.97%) | 1 (3.03%) | 2 (4.76%) | ||
| Mycobacterium abscessus | 1 (0.32%) | 1 (0.56%) | |||
| Gram-negative vibrios | 2 (0.65%) | 2 (1.13%) | |||
| Vibrio vulnificus | 2 (0.65%) | 2 (1.13%) | |||
| Gram-negative cocci | 1 (0.32%) | 1 (1.75%) | |||
| Neisseria | 1 (0.32%) | 1 (1.75%) | |||
| Filamentous fungi | 60 (19.42%) | 26 (14.69%) | 19 (57.58%) | 3 (7.14%) | 12 (21.05%) |
| Fusarium spp. | 24 (7.77%) | 7 (3.95%) | 8 (24.24%) | 2 (4.76%) | 7 (12.28%) |
| Aspergillus spp. | 20 (6.47%) | 11 (6.21%) | 7 (21.21%) | 2 (3.51%) | |
| Mucor | 7 (2.27%) | 4 (2.26%) | 2 (6.06%) | 1 (1.75%) | |
| Sporothrix | 5 (1.62%) | 1 (0.56%) | |||
| Other filamentous fungi | 4 (1.29%) | 3 (1.69%) | 2 (6.06%) | 2 (4.76%) | 2 (3.51%) |
| Non-filamentous fungi | 15 (4.85%) | 3 (1.69%) | 2 (4.76%) | 10 (17.54%) | |
| Candida spp. | 13 (4.21%) | 3 (1.69%) | 2 (4.76%) | 8 (14.04%) | |
| Yeasts | 2 (0.65%) | 2 (3.51%) | |||
| Total isolated pathogens | 309 (100.0%) | 177 (100.0%) | 33 (100.0%) | 42 (100.0%) | 57 (100.0%) |
Abbreviation: CNS, coagulase-negative staphylococcus.
Figure 1.
Time trend of pathogens most frequently associated with infectious endophthalmitis in Zhongshan Ophthalmic Center. To evaluate the temporal trend in the distribution of pathogens, data were divided into two periods: 2010–2014 and 2015–2018. Chi-square analysis revealed a significant change in the percentage of pathogens between these two periods (χ2=200.833, P=0.004). Post hoc analysis revealed that the percentage of CNS decreased significantly from 34.82% in 2010–2014 to 24.37% in 2015–2018 (P<0.001). *P<0.01.
Abbreviations: CNS, coagulase-negative staphylococci; P, Pseudomonas.
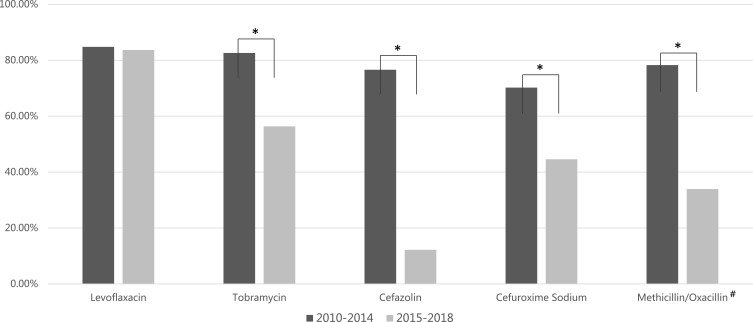
As presented in Table 3, bacteria showed generally high susceptibility to fluoroquinolones (75.36–100.00%). Gram-positive cocci showed much higher sensitivity to cephalosporins comparing to gram-negative bacilli (85.11–92.59% vs 25.42–35.72%). Regarding penicillins, overall sensitivity to penicillin was low (11.11–36.54%), and only half of CNS were sensitive to either methicillin or oxacillin. Fortunately, most gram-positive cocci were susceptible to vancomycin (97.72%). For the five antibiotics analyzed for susceptibility time trend, all except levofloxacin exhibited a significant decrease in susceptibility (Figure 2).
Table 3.
Overall Susceptibility Rates of Isolated Bacteria to Different Antibiotics in Zhongshan Ophthalmic Center
| Gram-Positive Cocci | CNS | Gram-Negative Bacilli | Gram-Positive Bacilli | |
|---|---|---|---|---|
| Levofloxacin | 78.70% (85/108) | 75.36% (52/69) | 89.39% (59/66) | 94.12% (16/17) |
| Ofloxacin | 95.24% (20/21) | 73.33% (11/15) | 100.0% (11/11) | 94.12% (16/17) |
| Moxifloxacin | 78.95% (45/57) | 80.39% (41/51) | / | / |
| Tobramycin | 49.51% (51/103) | 61.90% (39/63) | 77.61% (52/67) | 81.25% (13/16) |
| Gentamycin | 80.77% (42/52) | 80.39% (41/51) | 82.86% (29/35) | / |
| Cefazolin | 92.59% (25/27) | 100.0% (18/18) | 25.42% (15/59) | 100.0% (1/1) |
| Cefuroxime sodium | 85.11% (40/47) | 94.74% (18/19) | 35.71% (20/56) | 29.41% (5/17) |
| Vancomycin | 97.72% (59/61) | 98.04% (50/51) | / | / |
| Methicillin/oxacillin# | 55.26% (42/76) | 51.52% (34/66) | 52.00% (13/25) | / |
| Penicillin | 36.54% (19/52) | 11.11% (3/27) | 20.00% (2/10) | 17.65% (3/17) |
Notes: The numerator in the parentheses represents the number of isolates sensitive to the listed antibiotic, and the denominator represents the number of isolates that underwent susceptibility testing with the listed antibiotics. Gram-negative cocci and Gram-negative vibrion were omitted as the sample sizes were too small. #Bacteria isolates were sensitive to either methicillin or oxacillin.
Abbreviation: CNS, coagulase-negative staphylococci.
Figure 2.
Time trend of overall antibiotic susceptibility rate from 2010 to 2018 in Zhongshan Ophthalmic Center. Some antibiotics were omitted as the numbers of isolates that underwent susceptibility testing were not comparable between periods. The overall susceptibility rate for tobramycin decreased significantly from 82.61% in 2010–2014 to 56.34% in 2015–2018 (P=0.001). Similarly, cefazolin decreased from 76.60% to 12.20% (P<0.000), cefuroxime sodium from 70.21% to 44.59% (P=0.006), and methicillin/oxacillin from 78.26% to 33.93% (P<0.000).
Notes: *P<0.01. #Bacteria isolates were sensitive to either methicillin or oxacillin.
Among the 816 cases, 85 (10.42%) underwent evisceration/enucleation. Forty-two of these cases were secondary to keratitis-related endophthalmitis, and these accounted for almost half of the cases of keratitis-related endophthalmitis (47.19%, 42/89). The incidence of evisceration/enucleation was much higher for keratitis-related endophthalmitis compared to the total endophthalmitis population (χ2=123.61, P<0.001). Detailed causative pathogens detected are shown in Table 4. All pathogens detected in those cases with evisceration/enucleation were either gram-negative bacilli or fungi, while Pseudomonas aeruginosa was the most commonly detected pathogen.
Table 4.
Pathogen Spectrum of Endophthalmitis Patients Who Underwent Evisceration/Enucleation
| Evisceration (n) | Enucleation (n) | |
|---|---|---|
| Orbital cellulitis/panophthalmitis | 10 | 2 |
| No orbital cellulitis/panophthalmitis | 68 | 5 |
| Pathogen distribution |
P. aeruginosa (10) Aspergillus spp. (5) Other Gram- bacilli (3) Other filamentous fungi (2) Fusarium spp. (2) Bacillus cereus (2) Monas spp. (1) Enteric bacilli (1) Candida spp. (1) Mucor (1) Sporothrix (1) Pathogen unknown (49) |
Enteric bacilli (1) Pathogen unknown (6) |
| Total (n) | 78 | 7 |
Abbreviation: P, Pseudomonas.
Discussion
More than half of infectious endophthalmitis cases in the present study were due to open-globe trauma. Unsurprisingly, most posttraumatic infectious endophthalmitis patients were males. The result was consistent with our previous work, as well as the study by Bhoomibunchoo et al that reported that posttraumatic endophthalmitis constituted 43.1% of infectious endophthalmitis cases in northeast Thailand.9,10 In contrast, postoperative infection is the leading cause for infectious endophthalmitis in developed countries (38.4–74.3%), where industrial and agricultural injuries are much less common.11–14
Causative Pathogen Spectrum by Clinical Setting
Gram-positive cocci were the most commonly detected pathogens in posttraumatic and postoperative endophthalmitis, which is similar to other reports in multiple countries.15–19 Notably, gram-positive cocci usually only become pathogenic following injury or operation when the ocular defense homeostasis is altered. It is worth mentioning that the ratio of CNS, which account for the majority of gram-positive cocci, decreased over time. The trend was consistent with our previous study that also showed that the proportion of CNS recovered from infectious keratitis cases decreased from 2010 to 2018.10 A similar study from India also reported that percentage of bacteria had decreased over from 1991 to 2015.6 As majority of the microflora in the conjunctival sac were bacteria, the decreasing in bacterial infection might be explained by hygiene improvement over the last couple of decades in developing countries.
Infectious keratitis-associated endophthalmitis cases were mainly caused by fungi (57.58%) and gram-negative bacilli (30.30%) in the present study, which is much different from the pathogen spectrum for posttraumatic and postoperative endophthalmitis. Infectious keratitis-associated endophthalmitis might be the most sight-devastating type of endophthalmitis etiologies.14,20 About half of these cases required evisceration/enucleation, consistent with previous studies that reported evisceration/enucleation rates of 57.9% and 62.2% among patients with endophthalmitis that progressed from infectious keratitis.21,22 Dave et al studied an evisceration population of 791 cases, and reported that Pneumococci (17.53%), Aspergillus (14.95%), and Pseudomonas (12.11%) were the three most common pathogens.23 One study showed that gram-positive cocci (mainly Streptococcus spp.) also played an important role in the occurrence of evisceration/enucleation, other than gram-negative bacilli and fungi.24 Interestingly, all the pathogens recovered from evisceration/enucleation cases in the present study were either gram-negative bacilli or fungi, regardless of the etiology. However, we only isolated 30 pathogens, which might be the explanation for the discrepancy. Nonetheless, it is highly possible that endophthalmitis that progressed from infectious keratitis would ultimately require evisceration/enucleation, especially if it was due to gram-negative bacilli or fungi infection. It is worth mentioning that endoscopic vitrectomy might be helpful in reducing the evisceration rates in cases with concurrent keratitis, practitioners can consider attempting the endoscopy to avoid the predicament in the future.25
The causative pathogens of endogenous endophthalmitis in the present study were almost evenly distributed among gram-positive organisms, gram-negative organisms, and fungi. It was well known that causative pathogens of endogenous endophthalmitis vary according to primary systemic conditions.4,26,27 Previous reports described various causative organisms showing dominance in different regions. Specifically, gram-negative organisms (mainly Klebsiella pneumoniae) accounted for 44–48% of causative organisms in studies from Southeast Asia; Connell and colleagues reported that 65% of endogenous endophthalmitis cases were caused by fungi among 41 culture-proven cases in Australia.28–31 Unfortunately, there was insufficient relevant information to proceed with a comparison with the current study. This could be explored in future investigations.
Profile and Time Trends of Antibiotic Susceptibilities
Since the treatment of endophthalmitis usually starts with empiric antibiotic treatment, it is essential to have regional susceptibility profiles of first-line antibiotics. In the present study, both quinolones and cephalosporins had fairly high susceptibility rates regarding gram-positive cocci. Gram-negative bacilli were particularly susceptible to either quinolones or aminoglycosides. Yet, the rise of antibiotic resistance is no doubt a serious challenge in worldwide clinical practice.32–35 The result of the present study also emphasizes this issue. Among the five antibiotics analyzed for time trends from 2010–2014 to 2015–2018, four showed substantial decreases over time. Furthermore, the trend corroborated the result of our previous investigation that was conducted to evaluate the causative pathogens isolated from infectious keratitis at the same institution over the same time period (2010–2018).10 Notably, that study showed that more than half of the antibiotics also exhibited trends of reduced susceptibility. It is reasonable to presume that altered antibiotic susceptibilities in ocular infection are more likely correlated to antibiotic susceptibilities of microbial infection in the same region, rather than restricted to the ophthalmic field. Recent studies from Guangzhou reported continuously increasing trends of multidrug-resistance bacteria and antibiotic-resistant gram-positive bacteria.36,37 The danger of antibiotic resistance cannot be overemphasized, and the current results further demonstrate the urgent need to monitor and control antibiotic resistance.
The current study has some limitations. First, there is a potential risk of sampling bias since it was a hospital-based study. Second, detailed clinical characteristics and visual outcomes were not presented. Information regarding antibiotic susceptibilities of some first-line antibiotics, including ceftazidime, was missing due to periodic suspension.38 Nonetheless, to the best of our knowledge, this study is the first report to provide a general overview of endophthalmitis with such a large number of culture-proven pathogens at the largest referral ophthalmic center in southern China.
Conclusion
Between 2010 and 2018, posttraumatic endophthalmitis was the most common type of endophthalmitis treated in Zhongshan Ophthalmic Center. Gram-positive cocci were the most commonly detected pathogens in posttraumatic and postoperative endophthalmitis. Keratitis-associated endophthalmitis was mainly caused by fungi. Even though the overall antibiotic susceptibilities were fairly high, there was a substantial decrease of susceptibility for most first-line antibiotics.
Funding Statement
This study was supported by grants from the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (30306020240020130 and 3030902113030), and grants from the National Natural Science Foundation of China (81974135, 81400381, and 81900851).
Abbreviation
CNS, coagulase-negative staphylococcus.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19(3):227–234. doi: 10.1111/1469-0691.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relhan N, Forster RK, Flynn HW Jr. Endophthalmitis: then and now. Am J Ophthalmol. 2018;187:xx–xxvii. doi: 10.1016/j.ajo.2017.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safneck JR. Endophthalmitis: A review of recent trends. Saudi J Ophthalmol. 2012;26(2):181–189. doi: 10.1016/j.sjopt.2012.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham ET, Flynn HW, Relhan N, Zierhut M. Endogenous Endophthalmitis. Ocul Immunol Inflamm. 2018;26(4):491–495. doi: 10.1080/09273948.2018.1466561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30(3):597–613. doi: 10.1128/CMR.00113-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph J, Sontam B, Guda SJM, et al. Trends in microbiological spectrum of endophthalmitis at a single tertiary care ophthalmic hospital in India: a review of 25 years. Eye (Lond). 2019;33(7):1090–1095. doi: 10.1038/s41433-019-0380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajdacs M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi: 10.3390/molecules24050892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajdacs M, Albericio F. Antibiotic resistance: from the bench to patients. Antibiotics (Basel). 2019;8(3):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhoomibunchoo C, Ratanapakorn T, Sinawat S, Sanguansak T, Moontawee K, Yospaiboon Y. Infectious endophthalmitis: review of 420 cases. Clin Ophthalmol. 2013;7:247–252. doi: 10.2147/OPTH.S39934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L, Duan F, Yang Y, Lou B, Liang L, Lin X. Nine-year analysis of isolated pathogens and antibiotic susceptibilities of microbial keratitis from a large referral eye center in southern China. Infect Drug Resist. 2019;12:1295–1302. doi: 10.2147/IDR.S206831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karacal H, Kymes SM, Apte RS. Retrospective analysis of etiopathogenesis of all cases of endophthalmitis at a large tertiary referral center. Int Ophthalmol. 2007;27(4):251–259. doi: 10.1007/s10792-007-9068-3 [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Orlans HO, Hornby SJ, Bowler IC. Microbiology and visual outcomes of culture-positive bacterial endophthalmitis in Oxford, UK. Graefes Arch Clin Exp Ophthalmol. 2014;252(11):1825–1830. doi: 10.1007/s00417-014-2658-7 [DOI] [PubMed] [Google Scholar]
- 13.Moloney TP, Park J. Microbiological isolates and antibiotic sensitivities in culture-proven endophthalmitis: a 15-year review. Br J Ophthalmol. 2014;98(11):1492–1497. doi: 10.1136/bjophthalmol-2014-305030 [DOI] [PubMed] [Google Scholar]
- 14.Zapp D, Loos D, Feucht N, et al. Microbial keratitis-induced endophthalmitis: incidence, symptoms, therapy, visual prognosis and outcomes. BMC Ophthalmol. 2018;18(1):112. doi: 10.1186/s12886-018-0777-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed Y, Schimel AM, Pathengay A, Colyer MH, Flynn HW Jr. Endophthalmitis following open-globe injuries. Eye (Lond). 2012;26(2):212–217. doi: 10.1038/eye.2011.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhagat N, Nagori S, Zarbin M. Post-traumatic Infectious Endophthalmitis. Surv Ophthalmol. 2011;56(3):214–251. doi: 10.1016/j.survophthal.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 17.Gokce G, Sobaci G, Ozgonul C. Post-traumatic endophthalmitis: a mini-review. Semin Ophthalmol. 2015;30(5–6):470–474. doi: 10.3109/08820538.2013.877939 [DOI] [PubMed] [Google Scholar]
- 18.Slean GR, Shorstein NH, Liu L, Paschal JF, Winthrop KL, Herrinton LJ. Pathogens and antibiotic sensitivities in endophthalmitis. Clin Exp Ophthalmol. 2017;45(5):481–488. doi: 10.1111/ceo.2017.45.issue-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodjikian L, Salvanet-Bouccara A, Grillon S, et al. Postcataract acute endophthalmitis in France: national prospective survey. J Cataract Refract Surg. 2009;35(1):89–97. doi: 10.1016/j.jcrs.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 20.Henry CR, Flynn HW Jr, Miller D, Forster RK, Alfonso EC. Infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012;119(12):2443–2449. doi: 10.1016/j.ophtha.2012.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malihi M, Li X, Patel S, et al. Infectious keratitis-associated endophthalmitis: a 14-Year Study. Retina. 2017;37(4):662–666. doi: 10.1097/IAE.0000000000001204 [DOI] [PubMed] [Google Scholar]
- 22.O’Neill EC, Yeoh J, Fabinyi DC, et al. Risk factors, microbial profiles and prognosis of microbial keratitis-associated endophthalmitis in high-risk eyes. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1457–1462. doi: 10.1007/s00417-014-2732-1 [DOI] [PubMed] [Google Scholar]
- 23.Dave TV, Dave VP, Sharma S, et al. Infectious endophthalmitis leading to evisceration: spectrum of bacterial and fungal pathogens and antibacterial susceptibility profile. J Ophthalmic Inflamm Infect. 2019;9(1):9. doi: 10.1186/s12348-019-0174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuriyan AE, Weiss KD, Flynn HW Jr, et al. Endophthalmitis caused by streptococcal species: clinical settings, microbiology, management, and outcomes. Am J Ophthalmol. 2014;157(4):774–780 e771. doi: 10.1016/j.ajo.2013.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dave VP, Pappuru RR, Tyagi M, Pathengay A, Das T. Endoscopic vitrectomy in endophthalmitis: initial experience of 33 cases at a tertiary eye care center. Clin Ophthalmol. 2019;13:243–251. doi: 10.2147/OPTH.S185716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maitray A, Rishi E, Rishi P, et al. Endogenous endophthalmitis in children and adolescents: case series and literature review. Indian J Ophthalmol. 2019;67(6):795–800. doi: 10.4103/ijo.IJO_710_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modjtahedi BS, Finn AP, Barb SM, et al. Characteristics and outcomes of endogenous endophthalmitis: eight-year experience at a tertiary care center. Ophthalmol Retina. 2019;3(1):61–72. doi: 10.1016/j.oret.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 28.Cho H, Shin YU, Siegel NH, et al. Endogenous endophthalmitis in the American and Korean population: an 8-year retrospective study. Ocul Immunol Inflamm. 2018;26(4):496–503. doi: 10.1080/09273948.2016.1195000 [DOI] [PubMed] [Google Scholar]
- 29.Silpa-Archa S, Ponwong A, Preble JM, Foster CS. Culture-positive endogenous endophthalmitis: an eleven-year retrospective study in the central region of Thailand. Ocul Immunol Inflamm. 2018;26(4):533–542. doi: 10.1080/09273948.2017.1355469 [DOI] [PubMed] [Google Scholar]
- 30.Sharma S, Padhi TR, Basu S, Kar S, Roy A, Das T. Endophthalmitis patients seen in a tertiary eye care centre in Odisha: a clinico-microbiological analysis. Indian J Med Res. 2014;139(1):91–98. [PMC free article] [PubMed] [Google Scholar]
- 31.Connell PP, O’Neill EC, Fabinyi D, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye (Lond). 2011;25(1):66–72. doi: 10.1038/eye.2010.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grzybowski A, Brona P, Kim SJ. Microbial flora and resistance in ophthalmology: a review. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):851–862. doi: 10.1007/s00417-017-3608-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao M, Ying GG, Singer AC, Zhu YG. Review of antibiotic resistance in China and its environment. Environ Int. 2018;110:160–172. doi: 10.1016/j.envint.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 34.Alos JI. Antibiotic resistance: a global crisis. Enferm Infecc Microbiol Clin. 2015;33(10):692–699. doi: 10.1016/j.eimc.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 35.Roope LSJ, Smith RD, Pouwels KB, et al. The challenge of antimicrobial resistance: what economics can contribute. Science. 2019;364(6435). doi: 10.1126/science.aau4679 [DOI] [PubMed] [Google Scholar]
- 36.Gao K, Guan X, Zeng L, et al. An increasing trend of neonatal invasive multidrug-resistant group B streptococcus infections in southern China, 2011-2017. Infect Drug Resist. 2018;11:2561–2569. doi: 10.2147/IDR.S178717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z, Xie J, Peters BM, et al. Longitudinal surveillance on antibiogram of important Gram-positive pathogens in Southern China, 2001 to 2015. Microb Pathog. 2017;103:80–86. doi: 10.1016/j.micpath.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 38.Dave VP, Pathengay A, Nishant K, et al. Clinical presentations, risk factors and outcomes of ceftazidime-resistant Gram-negative endophthalmitis. Clin Exp Ophthalmol. 2017;45(3):254–260. doi: 10.1111/ceo.2017.45.issue-3 [DOI] [PubMed] [Google Scholar]