Abstract
Purpose
Cryoballoon ablation (CBA) is an effective technique for pulmonary vein isolation (PVI). To date, there are no risk models to predict very late recurrence of atrial fibrillation (VLRAF) after CBA.
Methods
Retrospective analysis of a single-center database was performed. Inclusion criteria included PVI using CBA for atrial fibrillation (AF) without additional ablation targets, follow-up > 365 days, and no recurrent AF between 90 and 365 days after procedure. The primary endpoint was recurrent AF > 30 s > 12 months post-CBA. A risk model was created using clinical variables.
Results
Of 674 CBA performed from 2011 to 2016, 300 patients (200 male, 62.0 ± 9.9 years) met inclusion criteria. Of these, 159 (53.0%) patients had paroxysmal AF. Patients had an average of 9.5 ± 2.7 cryoballoon freezes, and no patients required additional radiofrequency ablation lesion sets. Over a follow-up of 995 ± 490 days, 77/300 (25.7%) patients exhibited VLRAF. Univariate and multivariate analyses demonstrated that Structural heart disease (1 point), Coronary artery disease (3 points), left Atrial diameter > 43 mm (1 point), Left bundle branch block (3 points), Early return of AF (4 points), and non-paroxysmal AF (3 points) were risk factors for VLRAF. Combining these variables into a risk model, SCALE-CryoAF, (min 0; max 15) predicted VLRAF with an area under the curve of 0.73.
Conclusion
SCALE-CryoAF is the first risk model to specifically predict first recurrence of AF beyond 1 year, VLRAF, after CBA. Model discrimination demonstrates that SCALE-CryoAF predicts VLRAF after CBA significantly better than other risk models for AF recurrence.
Keywords: Cryoballoon ablation, Atrial fibrillation, Risk score, Very late return of atrial fibrillation
1. Introduction
Cryoballoon ablation (CBA) has emerged as a viable alternative to radiofrequency ablation (RFA) to achieve pulmonary vein isolation (PVI) for patients with atrial fibrillation (AF) [1–3]. Multiple studies have found that CBA, when compared with RFA, produces similar short- and long-term freedom from AF, with an acceptable risk profile [2–5]. CBA has the advantage of requiring fewer ablation lesions per pulmonary vein and has been associated with shorter procedure times, decreased fluoroscopy times, and fewer cardiac perforations than RFA [2, 3, 5].
There is an increasing body of literature on risk factors for AF recurrence after CBA [4, 6–8]. However, to date, there are no risk scores to predict very late recurrence of AF > 12 months post-procedure (VLRAF) after CBA. While risk models for VLRAF have been isolated in a RFA population, due to the distinct nature of RFA and CBA, different factors may influence very late recurrence [9]. Determining which patients are at increased risk for VLRAF after CBA could assist in deciding the best candidates for consideration of additional lesion sets and optimal frequency of post-procedure monitoring for AF recurrence [9]. Additionally, a risk model to predict VLRAF after CBA may help physicians determine which patients may benefit from additional ablation techniques.
The present study creates a new risk model to specifically predict VLRAF after CBA and determines its utility relative to other risk models that have been developed to predict AF recurrence. This study also assesses the rate of VLRAF after CBA at a single center and investigates other risk factors for VLRAF after CBA.
2. Methods
2.1. Patients
Retrospective analysis of a prospectively maintained, single-center database of CBA cases was performed. This study included 674 patients who underwent CBA at Northwestern Memorial Hospital, Chicago, IL, between January 1, 2011, and December 31, 2016. Inclusion criteria included PVI using CBA without additional ablation targets for AF or atrial flutter, follow-up > 365 days, and no return of AF 90–365 days post-procedure (Fig. 1). The institutional review board at Northwestern University approved the study protocol.
Fig. 1.
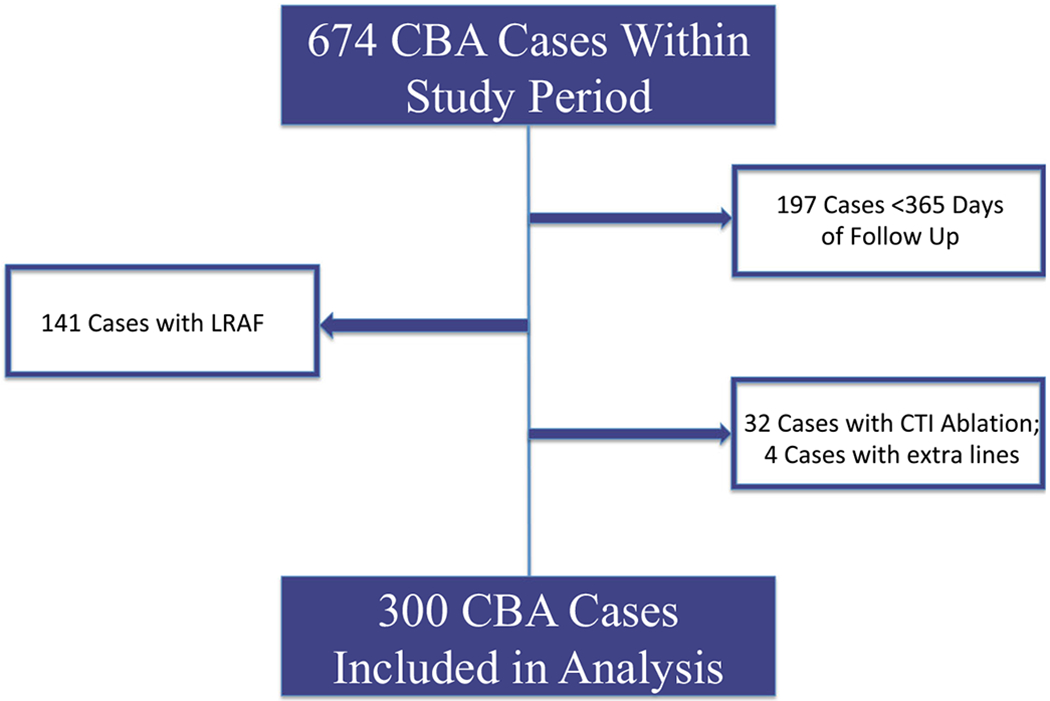
Patients included in analysis. CBA, cryoballoon ablation; LRAF, late return of atrial fibrillation (90–360 days post-ablation); CTI ablation, cavo-tricuspid isthmus ablation
2.2. Procedural details
The decision to utilize the cryoballoon for PVI was at the discretion of treating physicians. Index CBA-PVI procedures were performed by six board-certified electrophysiologists with extensive ablation experience. Patients received conscious sedation or general anesthesia at the discretion of the treating physician. After obtaining access, trans-septal puncture across the interatrial septum was performed using an SL1 (Abbott, Chicago, IL) or Preface (Biosense Webster, New Brunswick, NJ) sheath and Bayliss RF needle (Bayliss, Burlington, MA). Intravenous heparin was given with an activated clotting time goal of > 300 s. The Arctic Front Advance (Medtronic Inc., Minneapolis, MN) cryoballoon catheter and lasso catheter were introduced into the left atrium using the Cryosheath (Medtronic Inc., Minneapolis, MN). 3D mapping was used at the discretion of the operator. A 28-mm second-generation cryoballoon was used in nearly all (> 97%) cases. CBA was performed at the ostium of each pulmonary vein, with pulmonary venogram obtained prior to each ablation in order to confirm appropriate location and balloon occlusion, and using entrance block as an endpoint. Lesion duration evolved over time from two 4-min freezes per vein to two 3-min freezes per vein, with some operators limiting veins to a single 3-min application if time to effect was < 30 s. Target temperatures were – 30 to −55°C for all patients, and esophageal monitoring was used for those patients receiving general anesthesia [5]. Entry and exit block were confirmed after PVI. During isolation of the right-sided PVs, a catheter was positioned in the superior vena cava to perform high-output pacing to monitor for phrenic nerve injury. Monitoring of compound motor action potential amplitude was added to the protocol early in the CBA experience [10]. No provocative pacing maneuvers were regularly performed after CBA to induce atrial arrhythmias. Cardioversion to sinus rhythm was performed if a patient remained in an atrial arrhythmia after CBA. If a patient developed early return of AF (ERAF) < 90 days post-CBA, the decision to perform a cardioversion or initiate new antiarrhythmic drugs was made by the treating physician.
2.3. Clinical follow-up
Antiarrhythmic medication was stopped after a 3-month post-procedure blanking period. Anticoagulants were continued based on CHA2DS2-VASc score. All patients had scheduled clinical follow-up every 6 months beginning 3 months after their ablation. AF recurrence was monitored by routine ECGs during office visits, external monitors, downloads from implanted devices, and readings from Kardia smartphone monitors (AliveCor, Mountain View, CA). Additional rhythm assessment was performed during symptoms suggestive of arrhythmia recurrence [5].
2.4. Definition of variables
Paroxysmal AF (pAF) was defined as AF that terminated without intervention within 7 days of onset. Persistent AF (PeAF) lasted > 7 days, or required a prior procedure (cardioversion or ablation) to terminate. Longstanding persistent AF (LPeAF) lasted continuously > 1 year [1]. Non-paroxysmal AF (NPAF) was defined as a composite of PeAF and LPeAF. Structural heart disease included cardiomyopathy (left ventricular ejection fraction (LVEF) < 50%), hypertrophic cardiomyopathy, and severe regurgitant or stenotic valvular disease as characterized by echocardiography. Coronary artery disease (CAD) was determined by prior coronary angiography, the need for prior percutaneous intervention or coronary artery bypass grafting, or ischemic heart disease with LVEF < 50%. Left atrial diameter (LAD) was determined either by transthoracic echocardiogram or cardiac magnetic resonance imaging, as available. LAD index was calculated by dividing LAD by body surface area (BSA). The determination of cutoffs to dichotomize continuous variables was based on prior work [9, 11–14].
2.5. Endpoints, data analysis, and creation of risk model
The primary endpoint for this study was VLRAF (i.e., no recurrent atrial arrhythmia 90–365 days post-CBA and having a first documented atrial arrhythmia > 365 days post-procedure without antiarrhythmic medications). Patient demographics, risk factors associated with VLRAF, and procedural complications were retrospectively analyzed from an electronic medical record, and data was entered into an IRB-approved database. Numerical results are reported as mean ± standard deviation, median (interquartile range), or number (%). Univariate statistical analyses were performed using chi-square or Fisher exact tests for categorical variables, and Student’s T tests for continuous variables, as appropriate. Multivariate analyses were performed on variables found to have a p < 0.1 according to univariate analysis to identify independent risk factors for VLRAF after CBA. A new model to predict VLRAF after CBA (SCALE-CryoAF) was created using the risk factors as determined by multivariate analysis. This model reflected the beta coefficients of the logistic regression relationships between the independent variables and VLRAF. Receiver operating characteristic curve (ROC) and area under the curve (AUC) analyses were performed using SPSS (IBM, Armonk, NY) and MedCalc (MedCalc Software, Ostend, Belgium) statistical software. Model discrimination was performed by comparing the AUC of SCALE-CryoAF to the AUCs of other risk scores for AF recurrence. p values < 0.05 were considered to be significant in this study.
3. Results
3.1. Patient characteristics
There were 674 CBA performed during the study period. After exclusion of patients with return of AF 90–365 days post-CBA, < 365 days of follow-up, simultaneous ablations for atrial flutter, and additional radiofrequency ablation lines, 300 patients (200 male, 100 female, average age 62.0 ± 9.9 years) comprised the study group (Fig. 1). One hundred fifty-nine patients (53.0%) had pAF, 118 (39.3%) had PeAF, and 23 (7.7%) had LPeAF. The average duration of AF from initial diagnosis to CBA was 48.9 ± 60.9 months. For 50 patients (16.4%), this was their second ablation. Among those 50 patients, 7 patients (2.3%) had prior CBA and 43 patients (14.1%) had prior RFA. AF was the presenting rhythm in 79 (26.3%) patients, and 212 (70.1%) patients presented in sinus rhythm. Six patients (2%) presented in an atrial paced rhythm, and one patient each presented in a junctional rhythm, atrial flutter, and atrial-ventricular paced rhythm. Patients required an average of 9.5 ± 2.7 cryoballoon freezes per case. No patients required additional lesion sets beyond CBA of the PVs. Six patients had complications following the procedure. One patient each had hypotension requiring vasoactive medications, and a cerebral vascular accident. One patient had both phrenic nerve paralysis and a pericardial effusion not requiring drainage. There were two additional cases of phrenic nerve paralysis and one additional instance of pericardial effusion not requiring drainage. Among the 55 patients who had ERAF, 18 (32.7%) underwent cardioversion, 11 (20%) had initiation of a new antiarrhythmic drug, and 26 (47.3%) did not undergo any intervention. Over a mean follow-up of 33.2 ± 16.3 months, 77/300 (25.7%) patients exhibited VLRAF (Table 1). Of the 77 patients who developed VLRAF, the methods of determining procedure failure were as follows: ECG (57.1%), surface recorder (22.1%), Kardia (AliveCor, Mountain View, CA) smartphone monitor (9.1%), dual chamber pacemaker or dual chamber intra-cardiac defibrillator (5.2%), and implantable cardiac monitor (Reveal LINQ; Medtronic Inc., Minneapolis, MN) (6.5%).
Table 1.
Patient demographics and factors associated with VLRAF
| Variable | All patients (n = 300) | VLRAF (n = 77) | No VLRAF (n = 223) | p (VLRAF vs. no VLRAF) |
|---|---|---|---|---|
| Age | 62.0 ± 9.9 | 61.3 ± 10.4 | 62.3 ± 9.7 | 0.46 |
| Age > 65 | 127 (42.3%) | 34 (44.2%) | 93 (41.7%) | 0.71 |
| Male gender | 200 (66.6%) | 51 (66.2%) | 149 (66.8%) | 0.93 |
| Active smoker | 22 (7.3%) | 4 (5.2%) | 18 (8.1%) | 0.40 |
| Rhythm | ||||
| pAF | 159 (53.0%) | 28 (36.4%) | 131 (58.7%) | 0.001 |
| PeAF | 118 (39.3%) | 41 (53.2%) | 77 (34.5%) | 0.004 |
| LPeAF | 23 (7.7%) | 8 (10.4%) | 15 (6.7%) | 0.30 |
| Number of months of AF | 48.9 ± 60.9 | 55.7 ± 72.2 | 46.5 ± 56.6 | 0.27 |
| Structural heart disease | 40 (13.3%) | 15 (29.5%) | 25 (11.2%) | 0.06 |
| Pre-existing implantable defibrillator/Pacemaker | 20 (6.7%) | 5 (6.5%) | 15 (6.7%) | 0.94 |
| Left ventricular ejection fraction (%) | 56.8 ± 9.0 | 54.4 ± 11.4 | 57.7 ± 7.8 | 0.006 |
| Left ventricular ejection fraction < 55% | 113 (37.7%) | 36 (47.4%) | 77 (36.0%) | 0.080 |
| Left bundle branch block | 13 (4.4%) | 8 (10.4%) | 5 (2.3%) | 0.003 |
| Left atrium diameter (mm) | 39.2 ± 7.1 | 40.6 ± 7.5 | 38.8 ± 6.9 | 0.051 |
| LA diameter > 40 mm | 110 (37.9%) | 33 (44.6%) | 77 (35.6%) | 0.17 |
| LA diameter > 43 mm | 85 (29.3%) | 29 (39.2%) | 56 (25.9%) | 0.031 |
| LA diameter > 47 mm | 41 (14.1%) | 15 (20.3%) | 26 (12.0%) | 0.079 |
| Left atrium diameter index | 18.9 ± 3.6 | 19.4 ± 3.6 | 18.7 ± 3.6 | 0.14 |
| Left atrium diameter index > 23 | 30 (10.3%) | 12 (16.2%) | 18 (8.3%) | 0.14 |
| Body mass index | 29.4 ± 6.1 | 29.2 ± 5.3 | 29.5 ± 6.4 | 0.73 |
| Body surface area (m2) | 2.09 ± 0.28 | 2.11 ± 0.26 | 2.09 ± 0.28 | 0.61 |
| Active alcohol intake | 156 (52.0%) | 34 (44.7%) | 122 (54.7%) | 0.13 |
| Glomerular filtration rate (mL/min) | 78.7 ± 17.4 | 77.1 ± 17.1 | 79.3 ± 17.5 | 0.37 |
| Comorbid conditions | ||||
| Hypertension | 142 (47.5%) | 35 (45.5%) | 107 (48.2%) | 0.68 |
| Diabetes | 35 (11.7%) | 7 (9.1%) | 28 (12.6%) | 0.41 |
| Cerebrovascular accident | 19 (6.3%) | 2 (2.6%) | 17 (7.6%) | 0.12 |
| Coronary artery disease | 45 (15.0%) | 19 (24.7%) | 26 (11.7%) | 0.006 |
| Obstructive sleep apnea | 41 (13.7%) | 9 (11.7%) | 32 (14.3%) | 0.56 |
| Metabolic syndrome | 86 (34%) | 21 (30.4%) | 65 (35.4%) | 0.46 |
| Medications | ||||
| On ACE-i | 103 (34.6%) | 32 (41.6%) | 71 (32.1%) | 0.13 |
| On calcium channel blocker | 61 (20.4%) | 12 (15.6%) | 49 (22.1%) | 0.22 |
| On statin | 130 (43.6%) | 37 (48.1%) | 93 (42.1%) | 0.36 |
| On beta blocker | 171 (57.0%) | 45 (58.4%) | 126 (56.5%) | 0.77 |
| Prior Ablation | 50 (16.7%) | 13 (16.9%) | 37 (16.6%) | 0.95 |
| Procedural data | ||||
| 28 mm Arctic front balloon used | 292 (97.3%) | 76 (98.7%) | 216 (96.8%) | 1 |
| 23 mm Arctic Front Balloon Used | 8 (2.7%) | 1 (1.3%) | 7 (3.1%) | 0.39 |
| Left common Os | 18 (6.0%) | 5 (6.5%) | 13 (5.8%) | 0.83 |
| Total freezes (No.) | 9.5 ± 2.7 | 9.8 ± 2.7 | 9.4 ± 2.7 | 0.33 |
| Electrical cardioversion performed | 81 (27.0%) | 25 (32.5%) | 56 (25.1%) | 0.21 |
| Pharmacologic cardioversion performed | 8 (2.7%) | 3 (3.9%) | 5 (2.2%) | 0.44 |
| Spontaneous cardioversion observed | 32 (10.7%) | 5 (6.5%) | 27 (12.1%) | 0.17 |
| Adenosine administered | 50 (16.7%) | 15 (19.5%) | 35 (15.7%) | 0.44 |
| Isoproterenol administered | 29 (9.7%) | 4 (5.2%) | 25 (11.2%) | 0.12 |
| Procedure results | ||||
| Normal sinus rhythm following procedure | 298 (99.3%) | 77 (100%) | 221 (99.1%) | 0.40 |
| ERAF | 55 (18.3%) | 25 (32.5%) | 30 (13.5%) | <0.001 |
| VLRAF | 77 (25.7%) | x | x | x |
| Days of follow-up | 995 ± 490 | 1128 ± 494 | 949 ± 480 | 0.006 |
| Risk scores | ||||
| CHADS2 score | 0.81 ± 0.78 | 0.73 ± 0.77 | 0.83 ± 0.78 | 0.36 |
| CHA2DS2VASc score | 1.9 ± 1.3 | 1.87 ± 1.37 | 1.88 ± 1.24 | 0.95 |
| MB-LATER | 1.9 ± 1.4 | 2.50 ± 1.40 | 1.71 ± 1.29 | <0.001 |
| APPLE | 1.4 ± 1.1 | 1.73 ± 1.03 | 1.15 ± 1.01 | 0.004 |
| ALARMEc | 1.2 ± 1.0 | 1.44 ± 1.02 | 1.15 ± 1.01 | 0.06 |
| BASE-AF2 | 1.8 ± 1.3 | 2.17 ± 1.20 | 1.69 ± 1.25 | 0.02 |
| SCALE-CryoAF | 3.2 ± 2.9 | 5.03 ± 3.08 | 2.58 ± 3.08 | <0.001 |
pAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; LPeAF, longstanding persistent atrial fibrillation; NPAF, non-paroxysmal atrial fibrillation; AF, atrial fibrillation; LA, left atrium; BSA, body surface area; LA Diameter Index, left atrial diameter divided by body surface area; BMI, body mass index; CVA, cerebrovascular accident; TIA, transient ischemic attack; OSA, obstructive sleep apnea; CBA, cryoballoon ablation; AAD, antiarrhythmic drugs; ACE-i, angiotensin-converting enzyme inhibitor; CCB, calcium channel blocker; PV, pulmonary vein; ERAF, early return of atrial fibrillation (< 3 months post-procedure)
3.2. Factors associated with VLRAF
Of the clinical and imaging variables tested, univariate analysis revealed that having NPAF, LAD > 43 mm, left bundle branch block (LBBB), coronary artery disease (CAD), and ERAF < 90 days after the procedure were associated with VLRAF after CBA. Additional factors which nearly met statistical significance in univariate analysis included LVEF < 55%, and having structural heart disease (Table 1). There were no differences in rates of VLRAF between the patients who underwent intervention for ERAF and those who did not undergo intervention for ERAF (p = 1.0). There were also no differences in rates of VLRAF among patients who had prior ablations for AF and those undergoing their first ablation (p = 0.95).
3.3. SCALE-CryoAF score
Based on the factors associated with VLRAF, a new risk model was created to predict VLRAF after CBA. Combining Structural heart disease (1 point), Coronary artery disease (3 points), left Atrial diameter > 43 mm (1 point), Left bundle branch block (3 points), Early return of AF (4 points), and non-paroxysmal AF (3 points) into a novel risk model, SCALE-CryoAF, (min 0; max 15) predicted VLRAF (VLRAF 5.03 ± 3.08; no VLRAF 2.58 ± 3.08; p < 0.001). Relative weights of parameters comprising the SCALE-CryoAF score were guided by beta coefficients from multivariate logistic regression analysis (Table 2). While LAD > 43 mm and structural heart disease did not exhibit statistical significance in logistic regression analysis, their inclusion in the risk model with a relative weight of 1 increased the AUC. These parameters were therefore included in the score. AF recurrence increased linearly with a higher SCALE-CryoAF score group (y = 21.3x – 14.5; r2 = 0.94) (Fig. 2). SCALE-CryoAF has an AUC of 0.73 (Fig. 3). This AUC demonstrates statistical significance when compared with the AUCs of MB-LATER, APPLE, ALARMEc, BASE-AF2, CHADS2, and CHA2DS2VASc (Table 3; Fig. 3).
Table 2.
Relative weights as determined by multivariate analysis
| Parameter | p value | Relative weight |
|---|---|---|
| Structural heart disease | 0.24 | 1 |
| Coronary artery disease | 0.008 | 3 |
| LAD > 43 mm | 0.49 | 1 |
| LBBB | 0.025 | 3 |
| ERAF | < 0.001 | 4 |
| NPAF | 0.024 | 3 |
LAD, left atrial diameter; LBBB, left bundle branch block; ERAF, early return of atrial fibrillation (< 3 months post-procedure); NPAF, non-paroxysmal atrial fibrillation
Fig. 2.
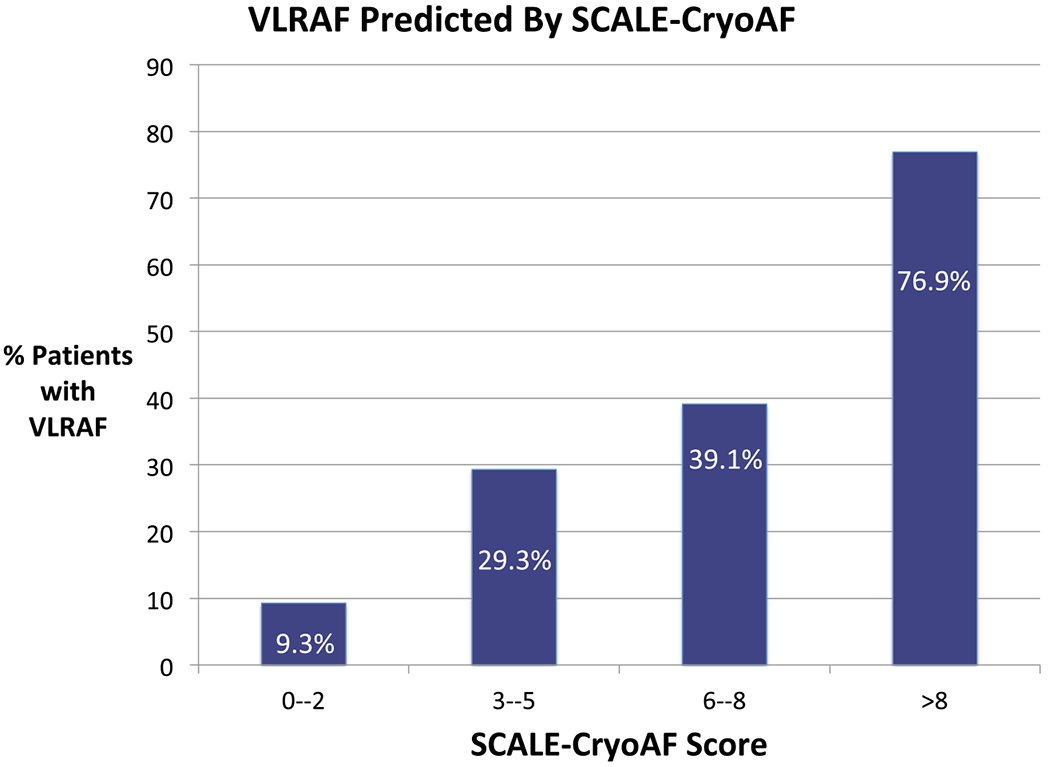
Percentage of patients with VLRAF as predicted by SCALE-CryoAF score. VLRAF, very late return of atrial fibrillation (> 360 days post-ablation)
Fig. 3.
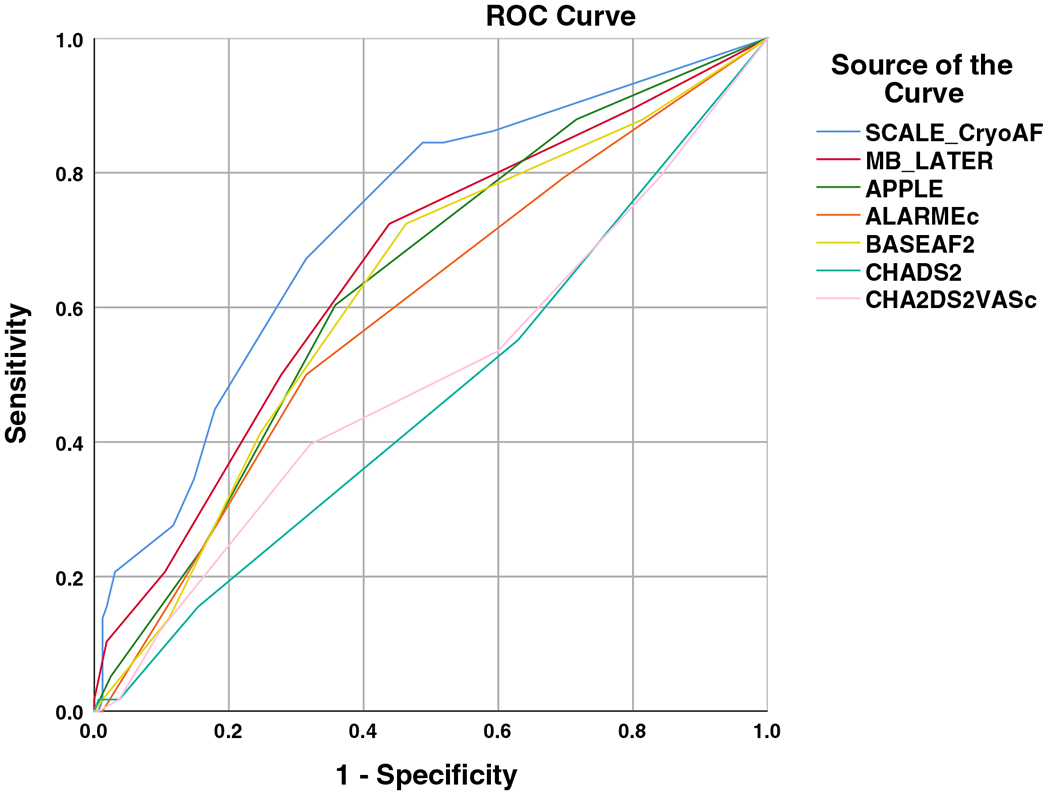
Comparison of ROC curves
Table 3.
Comparison of risk models
| Score | AUC (95% CI) | p (compared with SCALE-CryoAF) |
|---|---|---|
| SCALE-CryoAF | 0.73 (0.67–0.80) | – |
| MB-LATER | 0.65 (0.58–0.73) | 0.0066 |
| BASE-AF2 | 0.62 (0.54–0.69) | 0.0004 |
| APPLE | 0.62 (0.54–0.69) | 0.0030 |
| ALARMEc | 0.58 (0.50–0.67) | 0.0002 |
| CHADS2 | 0.47 (0.39–0.54) | 0.0005 |
| CHA2DS2VASc | 0.50 (0.42–0.58) | 0.0001 |
4. Discussion
CBA is increasing in popularity as an alternative to RFA, but prior risk prediction models for VLRAF have been designed in the RFA population. This study of 300 CBA cases is the first to create a risk model for VLRAF specifically after CBA.
While multiple studies have assessed long-term success rates of CBA, none has specifically studied VLRAF. The BASE-AF2 score, comprised of body mass index (BMI) > 28, active smoking, NPAF, duration of AF > 6 years, LAD > 40 mm, and ERAF, successfully predicted AF recurrence after CBA at extended follow-up of 20 months. Similarly, the PLAAF score that includes NPAF, left atrial area, abnormal pulmonary vein (PV) anatomy, months of AF, and gender also predicted AF recurrence after CBA at 3 years of follow-up [11]. While BASE-AF2 and PLAAF evaluate risk factors for AF recurrence at an extended period after CBA, these scores do not specifically assess VLRAF [7, 11, 15]. This is an important distinction, as a significant percentage of patients who do not develop recurrence of AF after 12 months will demonstrate recurrence at extended follow-up [9]. While factors such as BMI, gender, abnormal PV anatomy, and duration of AF may influence recurrence of AF < 12 months after CBA as shown in BASE-AF2 and PLAAF, our study did not find these variables significant in specifically predicting VLRAF.
The MB-LATER score was recently shown to predict VLRAF after RFA [9]. Risk factors for VLRAF after RFA in this study included male gender, bundle branch block, LAD > 47 mm, type of AF (pAF, PeAF, LPeAF), and ERAF [9]. While LAD, type of AF and ERAF were also included in the SCALE-CryoAF score, our study found that these variables did not fully account for VLRAF after CBA. This discrepancy in risk factors influencing VLRAF shows that there may be specific characteristics unique to CBA that influence recurrence of AF > 12 months post-procedure.
Factors that have been shown to impact AF recurrence regardless of ablation technique or time to follow-up include type of AF (pAF vs. NPAF), enlarged LAD, ERAF, and reduced ejection fraction [7, 9, 11–14, 16–19]. The consistency in these risk factors between studies suggests that these are AF-specific characteristics that impact general arrhythmia recurrence. Other factors included in risk models are likely specific to the time period in which recurrence is evaluated, and the ablation technique used.
The factors included in SCALE-CryoAF were all independent predictors of VLRAF in our population. In multivariate analysis, presence of LBBB had the highest relative weight. Multiple prior studies have shown that decreased ventricular synchrony from LBBB or chronic right ventricular pacing leads to increased left atrial size and decreased left atrial contractility [20–22]. Both of these factors have been shown to significantly increase the probability of AF recurrence after ablation, and LBBB may represent progression of disease responsible for AF [7, 9, 12–14, 16–19, 23, 24]. However, due to the low absolute number of patients who had LBBB, it was given a lower relative weight in the risk model. In multivariate analysis, structural heart disease and LAD > 43 mm did not demonstrate statistical significance. However, when these two factors were removed from the score, the AUC decreased. This suggests that the independent significance of these scores is significant enough to contribute to the ultimate risk model. Enlarged LAD has been included in most risk models for AF recurrence and has been widely regarded as a factor that increases AF burden [7, 9, 12–14, 16–19, 23]. While LAD > 47 mm trended towards significance as a predictor of VLRAF, it did not meet the threshold for inclusion into the risk model. This is likely secondary to the small overall number of patients with this clinical characteristic. Structural heart disease has been considered to be a root cause of AF and risk factor for recurrence after treatment [25]. Therefore, the significant contribution of structural heart disease to our risk model is not surprising.
While a number of the factors included in SCALE-CryoAF have been shown to predict recurrence of AF after RFA, this is the first study to show that they predict VLRAF after CBA. Additionally, the factors may carry different weights in RFA and CBA populations, likely due to the differences in ablation technique. The study population from which the SCALE-CryoAF score was derived includes 50 patients in whom CBA was their second ablation. Having a prior ablation was not a risk factor for VLRAF in this study; however, this study may be underpowered to detect a significant effect of repeat ablation on VLRAF. By including patients with prior ablations, the SCALE-CryoAF score has applicability to all patients undergoing CBA, not just patients undergoing their index ablation.
Knowledge of factors associated with VLRAF after CBA has a number of distinct benefits. Five of the six clinical variables included in SCALE-CryoAF (structural heart disease, CAD, LAD > 43 mm, LBBB, NPAF) are known prior to CBA. If a patient has the majority of these clinical variables, additional lesion sets and substrate modification techniques may be warranted along with CBA to reduce the patient’s chances of developing VLRAF. The SCALE-CryoAF score may be used to guide decision making about post-ablation screening in patients at higher-than-average risk for VLRAF after CBA. Patients with a high SCALE-CryoAF score may require more intensive post-operative monitoring should anticoagulation be stopped as a result of shared decision making. While patients with a low score may only require monitoring every 6 months [26], patients with a high score may benefit from more rigorous arrhythmia detection strategies such as implantable loop recorders, smartphone-based monitors, or extended-wear external monitors.
The AUC of SCALE-CryoAF indicates that the score successfully predicts VLRAF after CBA [27, 28]. SCALE-CryoAF produced an AUC that was significantly greater than all other risk scores tested. We were unable to compare SCALE-CryoAF with PLAAF as we do not routinely measure LA area on pre-ablation imaging [11]. While showing significance in our single-center patient population, SCALE-CryoAF requires validation in an external patient cohort. This study was also limited by its retrospective nature. Similar to other studies, a longer follow-up time was associated with a higher rate of return of AF in our population and duration of follow-up was an independent risk factor for VLRAF in our study [9]. Finally, a criticism of prior studies that have included ERAF in risk models is that a clinician cannot use the model prior to an ablation to determine if a patient has a high chance of having recurrent AF [7–9]. As ERAF is only one of six clinical variables included in SCALE-CryoAF, if patients already have a high-risk score before CBA, they may warrant additional lesion sets to prevent VLRAF. Future studies should prospectively validate the SCALE-CryoAF score. As LBBB was the strongest predictor of VLRAF in our population, future studies may also investigate the effect of cardiac resynchronization among patients with LBBB on development of VLRAF after CBA.
5. Conclusion
SCALE-CryoAF, comprised of Structural heart disease (1), Coronary artery disease (3), left Atrial diameter > 43 mm (1), Left bundle branch block (3), Early return of AF (4), and non-paroxysmal AF (3), is the first risk score to specifically predict VLRAF after CBA. This risk model may help guide pre-operative planning and post-operative management after CBA.
Acknowledgments
Conflict of interest Bradley P. Knight receives honoraria for consulting and speaking for Medtronic Inc. Rod S. Passman receives research support, consulting fees, and speaker fees from Medtronic; royalties from UpToDate; and research support from Pfizer and Kardia. Northwestern University receives fellowship support from Medtronic, Inc.
Footnotes
Compliance with ethical standards
The institutional review board at Northwestern University approved the study protocol.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016;37(38):2858–65. 10.1093/eurheartj/ehw285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16): 1713–23. 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Vogt J, Heintze J, Gutleben KJ, Muntean B, Horstkotte D, Nolker G. Long-term outcomes after cryoballoon pulmonary vein isolation: results from a prospective study in 605 patients. J Am Coll Cardiol. 2013;61(16):1707–12. 10.1016/j.jacc.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Wasserlauf J, Pelchovitz DJ, Rhyner J, Verma N, Bohn M, Li Z, et al. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2015;38(4): 483–9. 10.1111/pace.12582. [DOI] [PubMed] [Google Scholar]
- 6.Bitter T, Nolker G, Vogt J, Prinz C, Horstkotte D, Oldenburg O. Predictors of recurrence in patients undergoing cryoballoon ablation for treatment of atrial fibrillation: the independent role of sleep-disordered breathing. J Cardiovasc Electrophysiol. 2012;23(1):18–25. 10.1111/j.1540-8167.2011.02148.x. [DOI] [PubMed] [Google Scholar]
- 7.Canpolat U, Aytemir K, Yorgun H, Sahiner L, Kaya EB, Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish Cryoablation Registry. Int J Cardiol. 2013;169(3):201–6. 10.1016/j.ijcard.2013.08.097. [DOI] [PubMed] [Google Scholar]
- 8.Oto A, Aytemir K, Canpolat U, Karakulak U, Evranos B, Sahiner L, et al. Pulmonary vein isolation with the cryoballoon technique in atrial fibrillation treatment: single centre experience. Turk Kardiyol Dern Ars. 2013;41(4):299–309. 10.5543/tkda.2013.37096. [DOI] [PubMed] [Google Scholar]
- 9.Mujovic N, Marinkovic M, Markovic N, Shantsila A, Lip GY, Potpara TS. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: the MB-LATER clinical score. Sci Rep. 2017;7:40828 10.1038/srep40828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi F, Dubuc M, Guerra PG, Delisle S, Romeo P, Landry E, et al. Diaphragmatic electromyography during cryoballoon ablation: a novel concept in the prevention of phrenic nerve palsy. Heart Rhythm. 2011;8(6):885–91. 10.1016/j.hrthm.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Akkaya E, Berkowitsch A, Greiss H, Hamm CW, Sperzel J, Neumann T, et al. PLAAF score as a novel predictor of long-term outcome after second-generation cryoballoon pulmonary vein isolation. Europace. 2017;20:f436–43. 10.1093/europace/eux295. [DOI] [PubMed] [Google Scholar]
- 12.Kornej J, Hindricks G, Arya A, Sommer P, Husser D, Bollmann A. The APPLE score - a novel score for the prediction of rhythm outcomes after repeat catheter ablation of atrial fibrillation. PLoS One. 2017;12(1):e0169933 10.1371/journal.pone.0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P, et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015;104(10):871–6. 10.1007/s00392-015-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojcik M, Berkowitsch A, Zaltsberg S, Hamm CW, Pitschner HF, Kuniss M, et al. Score associated with the outcome after multiple ablation procedures in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2014;37(6):682–90. 10.1111/pace.12356. [DOI] [PubMed] [Google Scholar]
- 15.Aryana A, Bowers MR, O’Neill PG. Outcomes of cryoballoon ablation of atrial fibrillation: a comprehensive review. J Atr Fibrillation. 2015;8(2):1231 10.4022/jafib.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojcik M, Berkowitsch A, Greiss H, Zaltsberg S, Pajitnev D, Deubner N, et al. Repeated catheter ablation of atrial fibrillation: how to predict outcome? Circ J. 2013;77(9):2271–9. [DOI] [PubMed] [Google Scholar]
- 17.McCready JW, Smedley T, Lambiase PD, Ahsan SY, Segal OR, Rowland E, et al. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace. 2011;13(3):355–61. 10.1093/europace/euq434. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs V, May HT, Bair TL, Crandall BG, Cutler M, Day JD, et al. The impact of risk score (CHADS2 versus CHA2DS2-VASc) on long-term outcomes after atrial fibrillation ablation. Heart Rhythm. 2015;12(4):681–6. 10.1016/j.hrthm.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Arya A, Hindricks G, Sommer P, Huo Y, Bollmann A, Gaspar T, et al. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace. 2010;12(2): 173–80. 10.1093/europace/eup331. [DOI] [PubMed] [Google Scholar]
- 20.Pastore G, Zanon F, Baracca E, Aggio S, Corbucci G, Boaretto G, et al. The risk of atrial fibrillation during right ventricular pacing. Europace. 2016;18(3):353–8. 10.1093/europace/euv268. [DOI] [PubMed] [Google Scholar]
- 21.Xie JM, Fang F, Zhang Q, Chan JY, Yip GW, Sanderson JE, et al. Left atrial remodeling and reduced atrial pump function after chronic right ventricular apical pacing in patients with preserved ejection fraction. Int J Cardiol. 2012;157(3):364–9. 10.1016/j.ijcard.2010.12.075. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357(10):1000–8. 10.1056/NEJMoa071880. [DOI] [PubMed] [Google Scholar]
- 23.Montserrat S, Gabrielli L, Bijnens B, Borras R, Berruezo A, Poyatos S, et al. Left atrial deformation predicts success of first and second percutaneous atrial fibrillation ablation. Heart Rhythm. 2015;12(1):11–8. 10.1016/j.hrthm.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 24.Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S. Left atrial strain: a useful index in atrial fibrillation. Int J Cardiol. 2016;220:208–13. 10.1016/j.ijcard.2016.06.197. [DOI] [PubMed] [Google Scholar]
- 25.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9): 1453–68. 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 26.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9(6):335–79. 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 27.Hanley JM,B The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 28.Fawcett T An introduction to ROC analysis. Pattern Recogn Lett. 2006;27:861–74. [Google Scholar]


