Abstract
Background:
While several studies have evaluated predictors for atrial fibrillation (AF) recurrence following catheter ablation, there are limited data specific to cryoballoon ablation (CBA).
Methods:
We analyzed a prospective registry of patients at a single institution who underwent CBA. Recurrence of AF (RAF) was defined as recurrence of AF by 12-month follow-up, excluding the 3-month blanking period. Univariate analysis was performed to evaluate predictors of RAF. Receiver operating characteristic analysis was used to compare and evaluate the performance of various risk scores for discriminating risk of RAF.
Results:
There were 542 patients included in the analysis with mean age 61.3 ± 10.6 years, 67.9% male, and 51.6% paroxysmal AF (PAF). Overall, only left atrial diameter (LAD) > 40 mm and ERAF (early recurrence of AF within 0-3 month blanking period) were significant predictors of RAF. In the PAF specific subgroup, LAD > 40 mm, AF duration > 12 months, prior stroke or transient ischemic attack, ERAF, and having previously failed an antiarrhythmic drug were significant predictors of RAF. In persistent AF (PeAF) subgroup, obstructive sleep apnea (OSA) and ERAF were significant predictors of RAF. Out of clinical risk scores tested, BASEAF2 had the highest performance with area under the curve of 0.646 (95% confidence interval [0.548, 0.708]; P < .01).
Conclusions:
In this single-center retrospective study of CBA, we found only LAD > 40 mm and ERAF to be predictors of RAF. We identified OSA as a potential targetable risk factor in PeAF patients undergoing CBA. Out of risk scores tested for discriminating risk of RAF, BASEAF2 had the best performance.
Keywords: atrial fibrillation, cryoballoon ablation
1 |. INTRODUCTION
Cryoballoon ablation (CBA) is an effective and safe catheter-based treatment option in atrial fibrillation (AF) patients.1,2 However, recurrence rates of AF at 1 year following ablation have been reported to be as high as 40% after a 3-month blanking period.3 Several studies have used patients’ medical history and laboratory data to evaluate the predictors of AF and develop risk models for AF recurrence.4–10 Many of the existing risk scores were primarily established in the radiofrequency ablation population and did not include patients undergoing CBA8,10 or had a limited sample of these patients.7,9 Furthermore, most existing clinical risk scores have not been validated in an independent cohort.11
Given the increased adoption of CBA, it is important to identify the specific predictors of recurrence of AF following the use of this technology. Our study aimed to evaluate the predictors of recurrence of atrial fibrillation (RAF) between 3 and 12 months postprocedure in a large sample of patients at a single institution who underwent CBA.
2 |. METHODS
2.1 |. Patient population
We performed a retrospective analysis of a prospectively maintained, single-center database of patients who underwent CBA at our institution between January 2011 and December 2016. Patients who had concomitant cavotricuspid isthmus (CTI) ablation were excluded. Patients who had episodes of AF lasting longer than 7 days required antiarrhythmic drug (AAD) or electrical cardioversion for termination were defined as persistent (PeAF) and those whose episodes self-terminated within 7 days were defined as paroxysmal (PAF).12
Active smoking status and alcohol intake were dichotomously recorded based on chart review. Metabolic syndrome was defined as the patient having three of the following: (1) hypertension, (2) diabetes mellitus, (3) high-density lipoprotein (HDL) < 40 mg/dL for men or 50 mg/dL for women, and (4) triglycerides > 200 mg/dL. Structural heart disease included valvular disease and cardiomyopathy. Variant pulmonary vein anatomy, including the presence of a left common ostium, was identified on preprocedural cardiac magnetic resonance imaging (MRI). For patients who did not have a left atrial diameter (LAD) measurement on transthoracic echocardiogram (TTE), measurements were taken from cardiac MRI when available. Ejection fraction (EF) was collected on most recent TTE within 1 year of ablation. If no TTE was available, EF was taken from cardiac MRI if available or transesophageal echocardiogram (TEE) if cardiac MRI was not available. Intraoperative characteristics such as number of freezes, use of electrical or chemical cardioversion, and use of adenosine to unmask dormant conduction or isoproterenol to initiate non-PV triggers were also recorded.
2.2 |. Cryoballoon ablation procedure
The index CBA-pulmonary vein isolation (PVI) procedures were performed by six board-certified electrophysiologists with extensive ablation experience. Patients received conscious sedation or general anesthesia at the discretion of the treating physician. After obtaining access, transseptal puncture across the interatrial septum was performed using an SL1 or Preface sheath and Bayliss RF needle. Intravenous heparin was given with an activated clotting time goal of > 300 s. The Arctic Front cryoballoon catheter (Medtronic, Inc., Minneapolis, MN, USA) and Achieve mapping catheter (Medtronic, Inc.) were introduced into the left atrium using the Cryosheath. 3D Mapping was used at the discretion of the operator. The 28-mm second-generation cryoballoon was used in all cases. CBA was performed at the ostia of each pulmonary vein, with pulmonary venogram obtained prior to each ablation to confirm appropriate location and balloon occlusion of the ostia. Lesion duration evolved over time from two 4-min freezes per vein to two 3-min freezes per vein, with some operators limiting the veins to a single 3-min application if time to effect was <30 s. Target temperatures were −30°C to −55°C for all patients with esophageal monitoring used for those patients receiving general anesthesia. Entrance block was used to assess isolation of all pulmonary veins.
2.3 |. Postprocedural care
Oral anticoagulation was resumed within 6-24 h of the procedure in all patients. In general, antiarrhythmic medications were stopped after the blanking period.13
2.4 |. Follow-up/selection of RAF patients
Rhythm follow-up included, at minimum, a 3-week AF monitor at 3 months postprocedure, and 24- to 48-h Holter monitors thereafter at 6-month intervals up to 2 years, downloads from implanted devices, and readings from Kardia smartphone monitors (AliveCor, Mountain View, CA, USA) when available. All available data, including monthly rhythm downloads for patients, were analyzed. Additional monitoring was performed in response to patient symptoms. Surface electrocardiograms (ECGs) were obtained at each clinic visit. AF recurrence was defined as AF > 30 s on monitoring without a requirement for AAD. These designations are consistent with the HRS/EHRA/ECAS definition of recurrent AF.14 Date of first noted recurrence of AF as defined above was recorded and patients were categorized into two groups: (1) recurrent atrial fibrillation (RAF)-first recurrence after 3-month blanking period and before 12 months follow-up–and 2) No-RAF–no recurrence noted through 12 months postprocedure. Patients were also identified as having early recurrence of AF (ERAF) if they had AF recurrence during blanking period defined as 0 to 3 months postprocedure. The primary endpoint of this study was freedom from AF, atrial flutter, and atrial tachycardia between 3- and 12-months post-CBA.
2.5 |. Statistical analysis
Continuous variables were presented as mean value ± standard deviation, and categorical variables were presented as numbers (percentage). Comparison of continuous data between the PeAF versus PAF group and RAF versus No-RAF group were made by unpaired t-test, where categorical data were compared in both groups with chi-square test or Fisher’s exact tests as appropriate. APPLE (age > 65 years, PeAF, estimated glomerular filtration rate < 60, LAD ≥ 43 mm, EF < 50%), ALARMEc (Atrial fibrillation type, Left Atrium size, Renal insufficiency, Metabolic syndrome, cardiomyopathy), MB-LATER (Male, Bundle branch block, Left atrium ≥ 47 mm, Type of AF, and Early Recurrent AF), and BASE-AF2 (Body mass index ≥ 28 kg/m2, Atrial dilatation ≥40 mm, current Smoking, Early recurrence, duration of AF history, and PeAF), and CHA2DS2VASC scores were calculated for each patient.7–10 Univariate and multivariate logistic regression analyses were performed on both categorical and continuous variables to evaluate the association with RAF. Risk factors that were significant with P value < .05 in univariate analysis as predictors of RAF were entered into multivariate logistic regression analysis. Odds ratios (OR) and confidence intervals were calculated with a P-value < .05 indicating significance. Receiver operating characteristic (ROC) analysis was used to compare and evaluate the performance of various risk scores for discriminating risk of RAF. We did not create an arbitrary cutoff value for ROC curves similar to previous studies.7,8,10 All analyses were performed using SPSS software, version 20.0 (IBM Corp., Armonk, NY, USA).
3 |. RESULTS
3.1 |. Patient characteristics
Of the 674 patients identified, 69 were excluded for having concomitant CTI ablation and 63 were excluded for having less than 3 months of follow-up data, leaving a study population of 542 patients. The study population had mean age 61.3 ±10.5 years, 67.2% male, 51.7% PAF (n = 280), and 48.3% PeAF (n = 262) (Table 1).
TABLE 1.
Baseline patient characteristics
| Variables | Overall n = 542 | PAF (n = 280) | PeAF (n = 262) | Chi square/T-value | P-value |
|---|---|---|---|---|---|
| Age (years) | 61.3 ± 10.5 | 60.8 ± 10.7 | 61.9 ± 10.3 | −1.22 | .22 |
| Male | 364 (67.2) | 173 (61.8) | 191 (72.9) | 7.58 | .01 |
| Months of AF | 55.3 ± 68.6 | 51.6 ± 74.4 | 59.4 ± 61.5 | −1.29 | .20 |
| Coronary artery disease | 86 (15.9) | 36 (12.9) | 50 (19.2) | 4.01 | .04 |
| Diabetes mellitus | 60 (11.1) | 31 (11.1) | 29 (11.1) | 0.00 | .99 |
| Hypertension | 251 (46.4) | 130 (46.4) | 121 (46.4) | 0.00 | .99 |
| BMI kg/m2 | 29.7 ± 6.3 | 29.1 ± 6.3 | 30.3 ± 6.2 | −2.28 | .02 |
| Metabolic syndrome (n = 391) | 137 (30.0) | 66 (27.7) | 71 (32.4) | 1.12 | .27 |
| Obstructive sleep apnea | 85 (15.8) | 36 (12.9) | 49 (18.8) | 3.57 | .06 |
| Prior stroke/TIA | 35 (6.5) | 20 (7.1) | 15 (5.7) | 0.46 | .50 |
| eGFR mL/min | 78.9 ± 18.5 | 79.3 ± 18.2 | 78.4 ± 18.8 | 0.54 | .59 |
| Serum creatinine (mg/dL) | 0.99 ± 0.2 | 0.98 ± 0.2 | 1.00 ± 0.2 | −1.55 | .12 |
| Current EtOH drinker | 276 (51.4) | 145 (51.8) | 131 (50.9) | 0.04 | .85 |
| Current smoker | 40 (7.5) | 15 (5.7) | 25 (10.1) | 3.53 | .06 |
| NYHA 3 or 4 | 11 (2.0) | 4 (1.4) | 7 (2.7) | 1.05 | .31 |
| Structural heart disease | 47 (8.7) | 28 (10.0) | 19 (7.3) | 1.29 | .26 |
| Variant PVA (n = 355) | 26 (6.8) | 18 (9.0) | 8 (4.4) | 3.04 | .08 |
| LVEF (%) | 56.2 ± 9.7 | 57.5 ± 8.2 | 54.8 ± 10.8 | 3.18 | .00 |
| Left atrium diameter (mm) | 39.4 ± 6.9 | 37.6 ± 6.4 | 41.3 ± 6.9 | −6.25 | .00 |
| Left atrium diameter index (mm/m2) | 18.8 ± 3.5 | 18.4 ± 3.4 | 19.3 ± 3.5 | −3.21 | .00 |
| QRS duration (ms) | 96.4 ± 20.9 | 95.3 ± 19.1 | 97.7 ± 22.6 | −1.36 | .120 |
| PR duration (ms) | 171.5 ± 35.2 | 167.9 ± 30.0 | 177.1 ± 41.7 | −2.47 | .01 |
| RBBB | 19 (3.5) | 9 (3.2) | 10 (3.9) | 0.15 | .70 |
| LBBB | 19 (3.5) | 9 (3.2) | 10 (3.9) | 0.15 | .70 |
| Days of follow-up | 1446 ± 14193.7 | 2024.1 ± 19739 | 530.6 ± 32.8 | 0.98 | .12 |
| EHRA score | 2.1 ± 0.2 | 2.1 (0.8) | 2.1 (0.7) | −0.06 | .71 |
Notes: Values are mean ± standard deviation or n (%).
Abbreviations: AF = atrial fibrillation; BMI = body mass index; EHRA = European Heart Rhythm Association; eGFR = estimated glomerular filtration rate; EtOH = ethanol; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association Functional Class; PAF = paroxysmal atrial fibrillation; PeAF = persistent atrial fibrillation; PVA = pulmonary vein anatomy; RBBB = right bundle branch block; TIA = transient ischemic attack; NYHA = New York Heart Association Functional Class.
Seventy-eight patients (13.8%) had undergone prior ablation for AF with 16 patients having previous CBA and 62 patients having previous RFA. The remaining 47 patients had prior ablation for atrial flutter and other supraventricular tachycardias. Of the 78 patients with prior AF ablations, all had PV to left atrium conduction recovery with an average of 3.3 PVs reconnected. The average number of reconnected PVs was 2.9 in patients with prior CBA (n = 16) and 3.5 in patients with prior RFA (n = 62). These findings likely represent a referral bias as patients with fewer reconnected PVs were likely to be treated with segmental PVI using RF at our institution. There were an average of 8.3 additional freezes delivered in patients with prior AF ablation (n = 78) with an average of 7.8 freezes in patients with prior CBA (n = 16) and 8.5 freezes in patients with prior RFA (n = 62). On average, there was an even distribution in the location of freezes between the four PVs (right superior PV, 2.2; right inferior PV, 2.1; left superior PV, 1.8; left inferior PV, 2.0).
Median follow up time was 21.5 months. Overall, 395/542 (72.9%) were free from AF at 12-month follow up. Of the total cohort, 17.3% of patients were on AAD (n = 94) at time of last follow-up. Patients who were on AAD at last follow-up were more likely to have had RAF with 29.9% (n = 44) on AAD at last follow-up in patients with RAF compared to 12.7% (n = 50) on AAD in those with No-RAF (OR 2.95, 95% confidence interval [CI; 1.86, 4.87], P < .01). In the former cases, AAD were used for the treatment of non-AF/atrial tachycardia arrhythmias.
Clinical, laboratory, and echocardiographic data were analyzed in patients categorized as PAF or PeAF (Table 1). Male gender and coronary artery disease were more prevalent in the PeAF group compared to PAF group. Furthermore, larger LAD with mean 41.3 ± 6.9 mm, larger LAD index with mean 19.3 ± 3.5 mm/m2, higher body mass index (BMI) with mean 30.3 ± 6.2 kg/m2, and lower EF with mean 54.8 ± 10.8% were found in the PeAF group.
Comparisons of clinical, laboratory, and echocardiographic data were also made between the RAF and No-RAF group (Table 2). There was no statistically significant difference in these parameters, with the exception of metabolic syndrome, which was more common in No-RAF group. In calculated risk scores, BASEAF2 and MB-LATER scores were higher in the RAF group compared to No-RAF.
TABLE 2.
Recurrence of atrial fibrillation versus no recurrence patient characteristics
| No-RAF (n = 395) | RAF (n = 147) | Chi square/T-score | P-value | |
|---|---|---|---|---|
| Age (years) | 61.3 ± 10.1 | 61.2 ± 11.7 | 0.16 | .88 |
| Male | 266 (67.3) | 98 (66.7) | 0.02 | .88 |
| Months of AF | 52.5 ± 69.4 | 63.3 ± 66.1 | −1.59 | .11 |
| PeAF | 187 (47.3) | 75 (51.0) | 0.58 | .45 |
| Prior AF ablation | 58 (14.7) | 20 (13.6) | 0.10 | .75 |
| Coronary artery disease | 61 (15.5) | 25 (17.0) | 0.19 | .67 |
| Diabetes mellitus | 44 (11.1) | 16 (11.0) | 0.00 | .95 |
| Hypertension | 183 (46.4) | 68 (46.3) | 0.00 | .97 |
| BMI kg/m2 | 29.6 ± 6.1 | 30.0 ± 6.6 | −0.80 | .54 |
| Metabolic syndrome (n = 457) | 109 (32.7) | 28 (22.6) | 4.43 | .04 |
| Obstructive sleep apnea | 58 (14.7) | 27 (18.8) | 1.31 | .25 |
| Prior stroke/TIA | 21 (5.3) | 14 (9.5) | 3.13 | .08 |
| eGFR mL/min | 78.6 ± 17.5 | 79.6 ± 20.8 | −0.56 | .58 |
| Serum creatinine (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.3 | −0.35 | .73 |
| Current smoker | 30 (8.0) | 10 (7.4) | 0.05 | .82 |
| Current EtOH drinker | 201 (51.1) | 75 (52.1) | 0.03 | .85 |
| Structural heart disease | 32 (8.1) | 15 (10.2) | 0.60 | .40 |
| NYHA 3 or 4 | 6 (1.5) | 5 (3.4) | 1.91 | .17 |
| Variant PVA (n = 355) | 22 (7.6) | 4 (4.3) | 1.17 | .28 |
| LVEF (%) | 56.4 ± 9.7 | 55.6 ± 9.6 | 0.87 | .39 |
| Left atrial diameter (mm) | 39.2 ± 6.9 | 40.0 ± 6.8 | −1.10 | .27 |
| Left atrial diameter index (mm/m2) | 18.8 ± 3.5 | 19.0 ± 3.4 | −0.58 | .54 |
| QRS duration (ms) | 97.1 ± 21.1 | 94.5 ± 20.2 | 1.26 | .21 |
| PR duration (ms) | 169.5 ± 32.2 | 177.3 ± 42.4 | −1.87 | .06 |
| RBBB | 17 (4.3) | 2 (1.4) | 2.76 | .10 |
| LBBB | 15 (3.8) | 4 (2.7) | 0.38 | .54 |
| EHRA score | 2.1 ±.74 | 2.2 ± 0.7 | −0.91 | .36 |
| Number of failed AAD | 1.2 ± 0.8 | 1.2 ± 0.7 | −0.11 | .91 |
| APPLE | 1.4 ± 1.1 | 1.6 ± 0.9 | −1.20 | .23 |
| ALARMEc | 1.2 ± 1.02 | 1.2 ± 1.0 | 0.60 | .55 |
| BASEAF2 | 1.8 ± 1.2 | 2.5 ± 1.2 | −4.80 | .00 |
| MB-LATER | 1.9 ± 1.3 | 2.2 ± 1.3 | −2.35 | .02 |
| CHA2DS2VASC | 1.8 ± 1.3 | 2.0 ± 1.4 | −1.83 | .07 |
Notes: Values are mean ± standard deviation or n (%).
Abbreviations: AAD = anti-arrhythmic drugs; AF = atrial fibrillation; BMI = body mass index; EHRA = European Heart Rhythm Association; EtoH = ethanol; eGFR = estimated glomerular filtration rate; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association Functional Class; PeAF = persistent atrial fibrillation; PVA = pulmonary vein anatomy; RBBB = right bundle branch block; RAF = recurrence atrial fibrillation; TIA = transient ischemic attack; NYHA = New York Heart Association Functional Class.
3.2 |. Predictors of AF
Results of univariate analysis are shown in Table 3. Of clinical, imaging, and intraprocedural variables tested, only LAD > 40 mm and ERAF were significant predictors of RAF in our population. A separate analysis of patients with prior AF ablation showed no predictors of RAF in this cohort.
TABLE 3.
Predictors of recurrence of atrial fibrillation
| Odds ratio | P-value | 95% Confidence interval | |
|---|---|---|---|
| Male | 0.97 | .88 | 0.65 to 1.45 |
| Age | 1.00 | .98 | 0.98 to 1.01 |
| Age > 65 years | 1.04 | .84 | 0.71 to 1.54 |
| PeAF | 1.16 | .45 | 0.79 to 1.70 |
| Months of AF | 1.00 | .11 | 1.00 to 1.01 |
| AF > 6 months | 1.34 | .32 | 0.75 to 2.39 |
| AF > 12 month | 1.48 | .10 | 0.93 to 2.40 |
| Prior AF ablation | 1.01 | .75 | 0.71 to 1.06 |
| ERAF | 3.57 | .00 | 2.34 to 5.46 |
| Coronary artery disease | 1.11 | .67 | 0.67 to 1.86 |
| Diabetes mellitus | 0.98 | .95 | 0.54 to 1.80 |
| Hypertension | 0.99 | .97 | 0.68 to 1.45 |
| BMI | 1.01 | .43 | 0.98 to 1.04 |
| BMI > 28 kg/m2 | 1.45 | .06 | 0.99 to 2.14 |
| Metabolic syndrome | 0.60 | .04 | 0.37 to 0.97 |
| eGFR | 1.00 | .58 | 0.99 to 1.01 |
| GFR < 60 mL/min | 1.30 | .38 | 0.73 to 2.23 |
| GFR < 68 mL/min | 1.16 | .50 | 0.75 to 1.80 |
| Serum creatinine | 1.16 | .73 | 0.50 to 2.74 |
| Obstructive sleep apnea | 1.34 | .25 | 0.81 to 2.21 |
| Prior stroke/TIA | 1.87 | .08 | 0.93 to 3.80 |
| Current EtoH drinker | 1.03 | .85 | 0.71 to 1.52 |
| Current smoker | 0.91 | .82 | 0.43 to 1.93 |
| Structural heart disease | 1.29 | .44 | 0.67 to 2.46 |
| NYHA 3 or 4 | 2.28 | .16 | 0.69 to 7.60 |
| LVEF | 1.00 | .40 | 0.97 to 1.01 |
| LVEF < 50% | 1.42 | .20 | 0.83 to 2.45 |
| Left atrial diameter | 1.01 | .27 | 0.99 to 1.05 |
| Left atrial diameter index (mm/m2) | 1.01 | .56 | 0.96 to 1.07 |
| LAD > 40 mm | 1.69 | .01 | 1.14 to 2.50 |
| QRS duration | 1.00 | .20 | 0.98 to 1.04 |
| QRS > 120 ms | 0.86 | .69 | 0.41 to 1.80 |
| PR duration | 1.01 | .04 | 1.00 to 1.01 |
| PR > 200 ms | 1.74 | .09 | 0.91 to 3.32 |
| RBBB | 0.31 | .10 | 0.07 to 1.34 |
| LBBB | 0.71 | .54 | 0.23 to 2.16 |
| Number of failed AAD | 1.01 | .91 | 0.79 to 1.30 |
| Failed AAD (y/n) | 1.59 | .11 | 0.90 to 2.81 |
| Variant PVA | 0.55 | .28 | 0.19 to 1.64 |
| Number of freezes | 1.03 | .44 | 0.96 to 1.10 |
| Electrical cardioversion | 1.34 | .16 | 0.89 to 2.00 |
| Chemical cardioversion | 1.96 | .25 | 0.61 to 6.26 |
| Adenosine given | 0.89 | .65 | 0.53 to 1.49 |
| Isoproterenol given | 1.14 | .61 | 0.49 to 2.67 |
| APPLE | 1.12 | .23 | 0.93 to 1.40 |
| ALARMEc | 0.94 | .55 | 0.78 to 1.16 |
| BASEAF2 score | 1.49 | .00 | 1.25 to 1.76 |
| BASEAF2 ≥ 3 | 3.00 | .00 | 1.97 to 4.57 |
| MB-LATER | 1.20 | .02 | 1.03 to 1.39 |
| MB-LATER≥ 2 | 1.51 | .04 | 1.01 to 2.27 |
| CHA2DS2VASC | 1.14 | .07 | 0.99 to 1.32 |
| EHRA score | 1.13 | .36 | 0.87 to 1.47 |
Abbreviations: AAD = anti-arrhythmic drugs; AF = atrial fibrillation; BMI = body mass index; EHRA = European Heart Rhythm Association; eGFR = estimated glomerular filtration rate; ERAF = early recurrence of atrial fibrillation; EtOH = ethanol; LAD = left atrial diameter; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association Functional Class;PeAF = persistent atrial fibrillation; PVA = pulmonary vein anatomy; RBBB = right bundle branch block; TIA = transient ischemic attack.
Predictors of RAF were further identified in PAF and PeAF subgroups. In PAF, LAD > 40 mm, prior stroke or transient ischemic attack (TIA), AF > 12 months, ERAF, and having previously failed an AAD were significant predictors of RAF (Table 4). In patients with PeAF, only obstructive sleep apnea and ERAF were significant predictors of RAF (Table 4).
TABLE 4.
Predictors of recurrence of atrial fibrillation in paroxysmal and persistent population
| Variable | PAF | PeAF | ||||
|---|---|---|---|---|---|---|
| Odds ratio | P-value | 95% Confidence interval | Odds Ratio | P-value | 95% Confidence interval | |
| Male | 0.70 | .21 | 0.41 to 1.21 | 1.39 | .31 | 0.74 to 2.59 |
| Age | 1.00 | .86 | 0.97 to 1.02 | 1.00 | .92 | 0.97 to 1.03 |
| Age > 65 years | 1.19 | .52 | 0.69 to 2.07 | 0.90 | .52 | 0.52 to 1.60 |
| Months of AF | 1.00 | .19 | 1.00 to 1.01 | 1.00 | .43 | 0.99 to 1.01 |
| AF > 6 months | 1.60 | .22 | 0.75 to 3.38 | 0.97 | .95 | 0.39 to 2.43 |
| AF > 12 month | 1.89 | .04 | 1.01 to 3.50 | 1.02 | .95 | 0.50 to 2.08 |
| ERAF | 4.55 | .00 | 2.45 to 8.43 | 2.83 | .00 | 1.57 to 5.10 |
| Coronary artery disease | 1.53 | .26 | 0.72 to 3.25 | 0.85 | .63 | 0.42 to 1.70 |
| Diabetes mellitus | 1.23 | .63 | 0.54 to 2.80 | 0.77 | .57 | 0.31 to 1.89 |
| Hypertension | 1.05 | .88 | 0.61 to 1.79 | 0.94 | .83 | 0.55 to 1.61 |
| BMI | 1.00 | .91 | 0.96 to 1.04 | 1.02 | .34 | 0.98 to 1.07 |
| BMI > 28 kg/m2 | 1.35 | .28 | 0.78 to 2.31 | 1.56 | .11 | 0.90 to 2.72 |
| Metabolic syndrome | 0.56 | .10 | 0.27 to 1.13 | 0.63 | .16 | 0.33 to 1.24 |
| eGFR | 1.00 | .68 | 0.98 to 1.01 | 1.01 | .23 | 1.00 to 1.02 |
| GFR < 60 mL/min | 2.11 | .05 | 0.99 to 4.51 | 0.72 | .48 | 0.30 to 1.77 |
| GFR < 68 mL/min | 1.40 | .29 | 0.75 to 2.62 | 0.95 | .88 | 0.52 to 1.75 |
| Serum creatine (mg/DL) | 1.49 | .53 | 0.43 to 5.18 | 0.88 | .84 | 0.27 to 2.93 |
| Obstructive sleep apnea | 0.67 | .38 | 0.28 to 1.61 | 2.06 | .03 | 1.07 to 3.93 |
| Prior stroke/TIA | 2.60 | .04 | 1.01 to 6.46 | 1.26 | .68 | 0.41 to 3.83 |
| Current EtoH intake | 1.05 | .85 | 0.61 to 1.80 | 1.02 | .93 | 0.59 to 1.76 |
| Current smoker | 0.74 | .65 | 0.20 to 2.71 | 0.99 | .97 | 0.39 to 2.46 |
| Structural heart disease | 1.12 | .72 | 0.49 to 2.80 | 1.50 | .41 | 0.57 to 3.97 |
| LVEF (%) | 0.98 | .20 | 0.95 to 1.01 | 1.00 | .96 | 0.98 to 1.03 |
| LVEF < 50% | 1.70 | .22 | 0.72 to 4.02 | 1.22 | .57 | 0.61 to 2.47 |
| Left atrial diameter (mm) | 1.02 | .35 | 0.98 to 1.07 | 1.01 | .70 | 0.97 to 1.05 |
| Left atrial diameter index (mm/m2) | 1.07 | .10 | 0.99 to 1.16 | 0.96 | .33 | 0.89 to 1.04 |
| Left atrial diameter > 40 mm | 2.12 | .01 | 1.18 to 2.81 | 1.37 | .26 | 0.79 to 2.39 |
| QRS duration | 0.99 | .36 | 0.98 to 1.01 | 0.99 | .34 | 0.98 to 1.01 |
| QRS > 120 ms | 0.89 | .84 | 0.28 to 2.82 | 0.81 | .67 | 0.31 to 2.13 |
| PR duration | 1.01 | .15 | 1.00 to 1.01 | 1.01 | .25 | 0.99 to 1.01 |
| PR > 200 ms | 2.16 | .09 | 0.88 to 5.31 | 1.00 | .29 | 0.99 to 1.01 |
| RBBB | 0.35 | .31 | 0.04 to 2.89 | 0.26 | .18 | 0.03 to 2.10 |
| LBBB | 0.36 | .31 | 0.04 to 2.89 | 1.05 | .94 | 0.27 to 4.20 |
| Number of failed AAD | 1.12 | .51 | 0.81 to 1.57 | 0.87 | .47 | 0.60 to 1.26 |
| Failed AAD (y/n) | 2.33 | .03 | 1.09 to 5.01 | 0.78 | .59 | 0.32 to 1.91 |
| Variant PVA | 0.74 | .68 | 0.20 to 2.68 | 0.36 | .32 | 0.04 to 2.99 |
| Number of freezes | 1.01 | .84 | 0.91 to 1.13 | 1.04 | .42 | 0.95 to 1.14 |
| Electrical cardioversion | 1.62 | .16 | 0.83 to 3.18 | 1.14 | .62 | 0.67 to 1.96 |
| Chemical cardioversion | 0.25 | .03 | 0.21 to 0.31 | 0.71 | .67 | 0.14 to 3.47 |
| Adenosine given | 1.61 | .18 | 0.83 to 3.2 | 0.41 | .02 | 0.18 to 0.98 |
| Isoprotenerol given | 1.49 | .61 | 0.36 to 6.10 | 0.95 | .92 | 0.33 to 2.76 |
| APPLE | 1.47 | .04 | 1.02 to 2.10 | 0.88 | .43 | 0.64 to 1.21 |
| ALARMEc | 0.90 | .58 | 0.61 to 1.32 | 0.75 | .14 | 0.51 to 1.10 |
| BASEAF2 score | 1.93 | .0 | 1.44 to 2.59 | 1.65 | .0 | 1.21 to 2.24 |
| BASEAF2 ≥ 3 | 5.19 | .0 | 2.41 to 11.14 | 4.11 | .0 | 1.96 to 8.65 |
| MB-LATER | 1.42 | .06 | 0.98 to 2.05 | 1.66 | .65 | 0.18 to 15.1 |
| MB-LATER≥ 2 | 2.02 | .03 | 1.05 to 3.89 | 2.02 | .03 | 1.05 to 3.89 |
| CHA2DS2VASC | 1.25 | .03 | 1.02 to 1.54 | 1.04 | .69 | 0.85 to 1.28 |
| EHRA score | 1.25 | .24 | 0.87 to 1.80 | 1.01 | .93 | 0.70 to 1.49 |
Abbreviations: AAD = anti-arrhythmic drugs; AF = atrial fibrillation; BMI = body mass index; EHRA = European Heart Rhythm Association; eGFR = estimated glomerular filtration rate; ERAF = early recurrence atrial fibrillation; EtOH = alcohol; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association Functional Class; PAF = paroxysmal atrial fibrillation; PeAF = persistent atrial fibrillation; PVA = pulmonary vein anatomy; RBBB = right bundle branch block; TIA = transient ischemic attack.
Multivariate logistic regression analysis was also conducted to identify independent variables associated with RAF in both PAF and PeAF population (Table 5). Risk factors that were significant with P value < .05 in univariate analysis as predictors of RAF were entered into multivariate logistic regression analysis. For PAF, these variables were LAD > 40 mm, prior stroke or TIA, AF > 12 months, ERAF, and having previously failed AAD. For PeAF, these variables included OSA and ERAF. In multivariate logistic regression of PAF population, ERAF, AF > 12 months, and LAD > 40 mm were statistically significant predictors of RAF. In multivariate logistic regression of PeAF population, ERAF and OSA were statistically significant predictors of RAF.
TABLE 5.
Multivariate analysis
| Odds ratio | P-value | 95% confidence interval | |
|---|---|---|---|
| Paroxysmal AF | |||
| ERAF | 5.32 | .00 | 2.68 to 10.60 |
| LAD > 40 mm | 1.97 | .04 | 1.02 to 3.86 |
| Prior TIA/Stroke | 2.36 | .12 | 0.81 to 6.90 |
| AF > 12 months | 2.25 | .03 | 1.01 to 4.61 |
| Previously failed AAD | 1.42 | .42 | 0.60 to 3.35 |
| Persistent AF | |||
| Recurrence during blanking period | 3.95 | .00 | 2.10 to 7.42 |
| Obstructive sleep apnea | 2.42 | .01 | 1.20 to 4.85 |
Abbreviations: AAD = anti-arrhythmic drugs; AF = atrial fibrillation; ERAF = early recurrence of atrial fibrillation; TIA = transient ischemic attack.
3.3 |. Comparison of risk scores
BASEAF2 had the highest performance in discriminating risk of RAF with area under the curve (AUC) of 0.646, 95% CI [0.58, 0.71], and P < .01. MB-LATER was also statistically significant with AUC of 0.575, 95% CI [0.51, 0.64], and P < .05 in ROC analysis while APPLE and ALARMEc were not (Figure 1). In the PAF population, BASEAF2 had the largest AUC with 0.685, 95% CI [0.59, 0.78], and P < .01. No other risk score achieved statistical significance in the ROC analysis (Figure 2). In the PeAF population, BASEAF2 again had the highest performance with AUC of 0.608, 95% CI [0.52, 0.69], and P < .05 (Figure 3).
FIGURE 1.
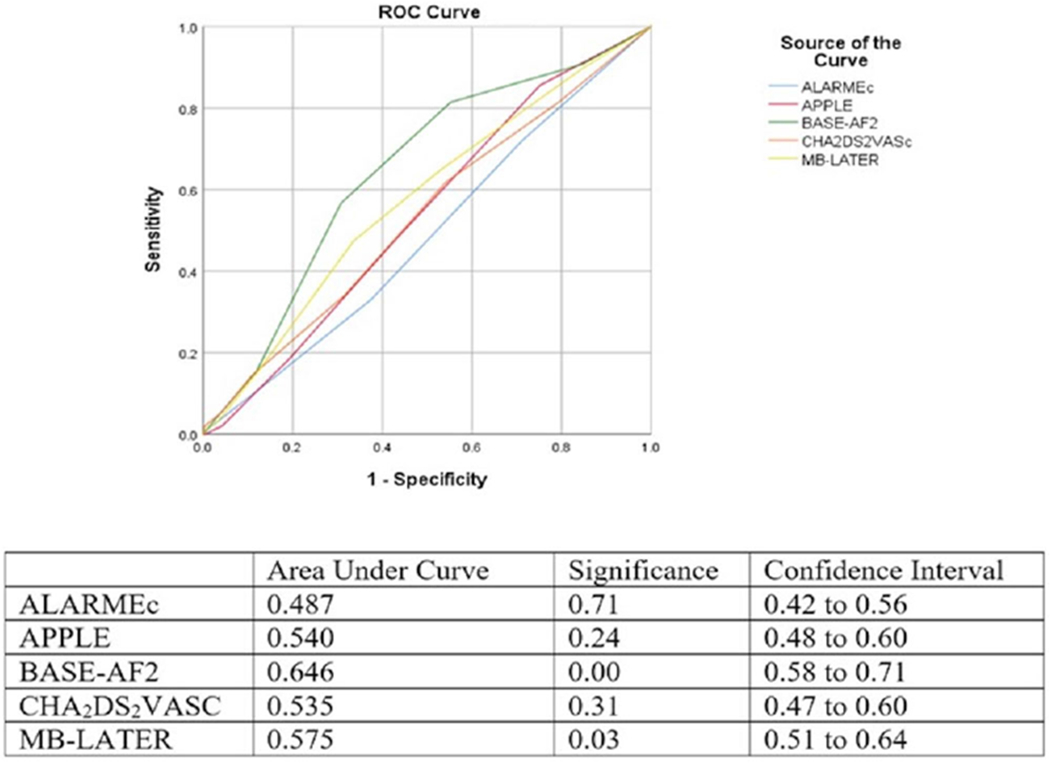
Receiver operating characteristic comparison of risk scores overall population [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
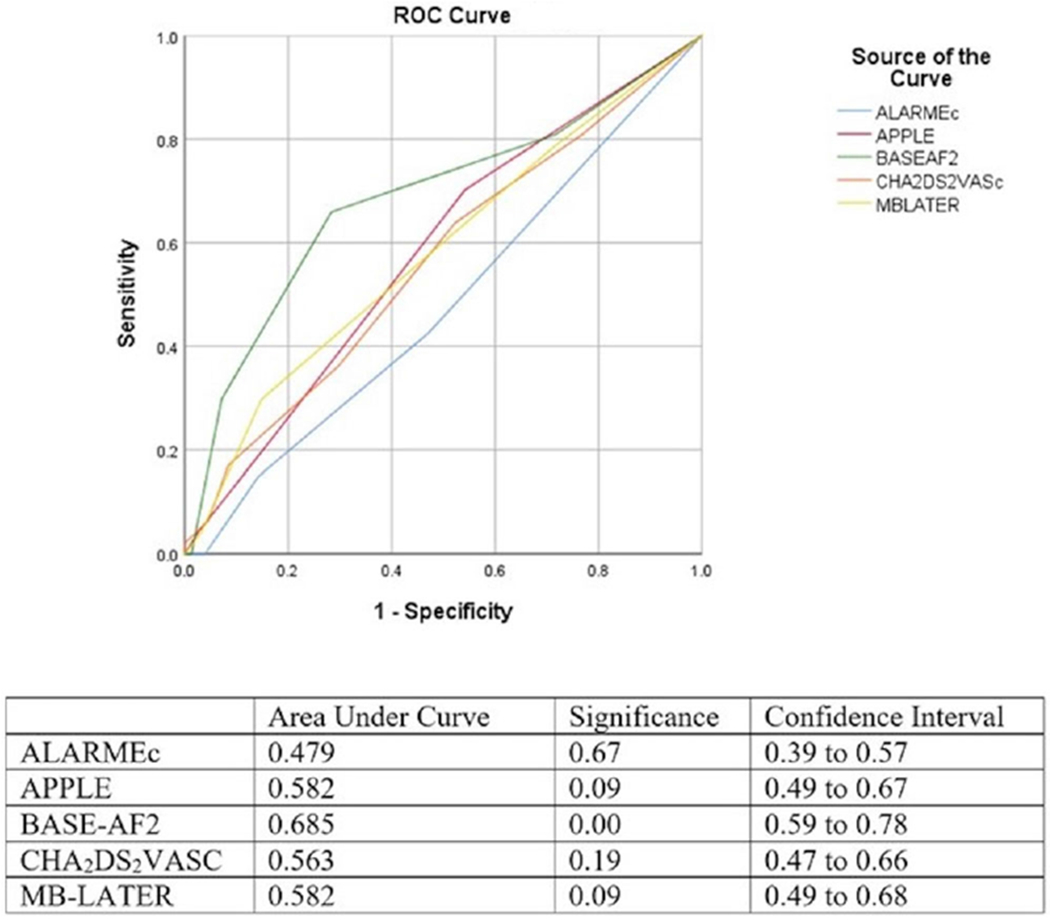
Receiver operating characteristic comparison of risk scores paroxysmal atrial fibrillation population [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
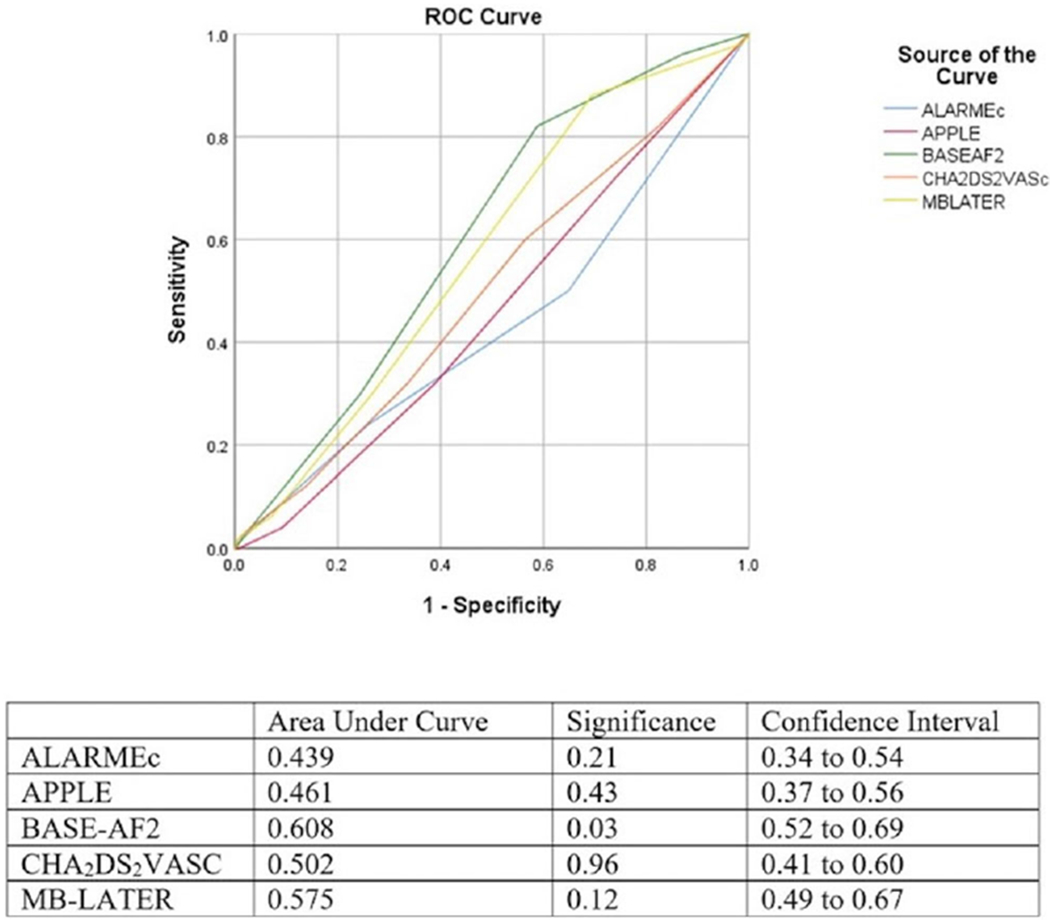
Receiver operating characteristic comparison of risk scores in persistent atrial fibrillation population [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
In this study, we analyzed the predictors of RAF in a large exclusively CBA population with close follow-up. The main findings of our study were that only LAD > 40 mm and ERAF were significant predictors of RAF and that BASEAF2 had the best performance of existing clinical risk scores in predicting RAF. Other studies have consistently shown a large LAD to be a predictor of recurrence following CBA.7,15,16 Similar to our data, other studies have also shown that recurrence of AF in blanking period is a predictor of later recurrence in both PAF and PeAF population.16–18 In contrast to exclusively CBA studies by Oto et al, Canpolat et al, and Akkaya et al, our study did not show smoking status, AF duration, PeAF, or BMI to be significant predictors of RAF.5,7,15 We also did not find additional components of PLAAF score, such as female gender and variant pulmonary vein anatomy, to be predictors of RAF.15 Our study did not find additional components of the ALARMEc risk score (mixed CBA and radiofrequency ablation population) including renal insufficiency, metabolic syndrome, and presence of cardiomyopathy to be significant predictors of RAF.9
There are multiple potential reasons for the lack of significant predictors of RAF in our study. A primary consideration is that the predictors for RAF after CBA may be different from predictors after radiofrequency ablation. For example, CBA in comparison to radiofrequency ablation may provide a more continuous circumferential ablation lesion and has a lower risk of post-procedural atrial flutters and atrial tachycardias.2,19 In addition, the definition of recurrence as a dichotomous variable may obscure differences in AF burden. This reduction of AF burden is clinically significant as multiple studies have suggested a direct correlation between AF burden and increased morbidity and mortality in terms of stroke and heart failure.20–23 Despite this, AF is still most often viewed as a dichotomous variable, one that is either present or absent. Guidelines for anti-coagulation support this perspective, and studies such as ours, BASEAF2, APPLE, ALARMEc, MB-LATER, and PLAAF all use a 30-s threshold to define AF recurrence.7–10,24 Looking at RAF through this strict dichotomous prism instead of considering AF burden may limit identification of consistent predictors and be of less clinical utility. Finally, our time point of recurrence at 3-12 months was different from the PLAAF study that evaluated recurrence at 36 months.15
When looking specifically at PAF population, our study showed LAD > 40 mm, previous stroke/TIA, AF > 12 months, and previously failed AAD to be predictors of RAF. The association between stroke/TIA and RAF may relate to the presence of atrial myopathy that may consequently lead to failure of PVI.25 Length of time greater than 12 months between first documentation of AF and ablation may be indicative of greater cumulative AF burden leading to LA remodeling and increased vulnerability to AF induction and maintenance: “AF begets AF.”26 Patients who failed AADs have been shown to have higher rates of AF recurrence than those undergoing ablation as first line therapy, which is consistent with our findings.27
As PeAF has been shown in previous studies to be associated with significantly greater recurrence and overall lower success rates following ablation, it is interesting to note the lack of significant predictors–aside from obstructive sleep apnea (OSA)–in that subgroup in our study.6,7,28 Several studies have established a clear association between OSA and RAF following catheter ablation.29,30 However, what is of significant debate is the extent to which the severity of OSA determines RAF and the possibly bidirectional relationship between OSA and AF.29,31,32 Potential mechanistic links include sympathetic hyperactivity, cardiac remodeling due to changes in intrathoracic pressure, and hypoxemia.33 The association between OSA and RAF has been established in CBA population, although this was not isolated to a PeAF subset as in our study.34 There is an overall lack of modifiable predictors of RAF in PeAF population undergoing CBA and may underscore the poorly understood nature of the complex arrhythmogenic substrate that drives recurrence in patients with PeAF.35 A recent study of PeAF patients undergoing CBA by Akkaya et al showed that an enlarged LA area and AF duration > 2 years were nonmodifiable predictors.36 OSA was not evaluated in this study and has not been evaluated in other studies looking at PeAF patients undergoing CBA.37 Given the lack of modifiable risk factors in PeAF patients to prevent recurrence, the identification in our study of OSA as a potentially targetable risk factor is intriguing. Previous studies–although not exclusively in CBA population–have demonstrated both the underdiagnosis of OSA in AF patients and the efficacy of treating OSA in preventing AF recurrence.38–41 Further investigation into the efficacy of screening for undiagnosed OSA or more aggressively treating OSA prior to CBA in PeAF population is necessary.
4.1 |. Comparison of existing risk scores
Several existing risk scores estimate RAF following catheter-based ablation, including APPLE, ALARMEc, BASEAF2, and MB-LATER.7–10 However, the CBA specific data are limited in these risk scores with no CBA data in APPLE and MB-LATER and only 140 CBA patients in ALARMEc and 246 in BASEAF2.7–10 Our sample size was also significantly larger than the recently published PLAAF score (n = 440), which evaluated predictors of 3-year outcome after CBA. We were not able to calculate PLAAF score in patients as we did not assess left atrial area in all patients. While our study did not independently find the same significant predictors that comprise the BASEAF2 score, BASEAF2 clearly had the best performance in the overall population in our study as well as the PAF and PeAF subgroups. Interestingly, despite using exclusively Very Late Recurrence of Atrial Fibrillation (VLRAF) patients in the creation of their risk score, MB-LATER performed better than APPLE or ALARMEc, neither of which reached statistical significance.10 These results indicate the superiority of BASEAF2 to other scores and validate this score in discriminating risk of RAF in an exclusively CBA population. This validation has clinical importance as the BASEAF2 score can help clinicians in assessing which patients would likely benefit from CBA alone as opposed to consideration of alternative treatment options that may include lesion sets beyond PVI.
4.2 |. Strengths/limitations
This study reports predictors of recurrence in one of the largest CBA populations in the literature to date.7,42 Furthermore, this study analyzed key variables that previous studies did not. For example, the presence of OSA was not analyzed in BASEAF2, ALARMEc, APPLE, or MB-LATER despite strong evidence of an association between RAF and OSA.7–10,43,44 Similarly, the aforementioned studies did not look at New York Heart Association functional classification and only MB-LATER evaluated the number of previously failed AAD. Other factors that we analyzed that were not consistently evaluated in previous studies included the presence of variant pulmonary vein anatomy, stroke/TIA, QRS duration on electrocardiogram, glomerular filtration rate, and metabolic syndrome.11 When metabolic syndrome was evaluated, there was not a clear definition as seen in ALARMEc.9 This study was limited by its retrospective nature. Given retrospective nature, there was no standardized protocol for OSA screening or regular sleep monitoring. There was variability in ablation protocol including time of freeze and use of time to isolation that may impact ablation outcome. However, previous studies have shown the equivalent efficacy of single 3-min freeze versus double 4-min freeze and time to isolation strategy versus fixed ablation strategy.45–47 In patients with redo ablations, the high rate of vein reconnection per patient is likely due to selection bias as patients with one or two veins reconnected would have received RF and not been included in our study. Many patients did not have continuous rhythm monitoring post-CBA and reliance on scheduled clinic ECGs, short-term Holter monitors, and patient symptoms may underestimate episodes of AF recurrence. While AAD were usually stopped after 3 months, this was not universally true in all patients potentially impacting AF recurrence. Furthermore, although the study population was a heterogeneous group, there may be bias related to the fact that these patients were treated at a single high-volume referral center. We defined AF recurrence dichotomously similar to previous studies and did not evaluate AF burden. Future studies should evaluate AF recurrence in terms of AF burden given clinical significance.
5 |. CONCLUSION
In this single-center retrospective study of CBA, we found only LAD > 40 mm and recurrence during blanking period to be a predictor of RAF. In the PeAF population, we identified obstructive sleep apnea as a potential targetable risk factor to prevent RAF. Out of all risk scores tested in discriminating risk of recurrence, BASEAF2 had the best overall performance and in the PAF and PeAF subgroups. Whether patients at higher risk of recurrence benefit from additional lesion sets outside of cryoballoon PVI requires further study.
Acknowledgments
DISCLOSURES/CONFLICTS OF INTEREST
Bradley P. Knight- receives honoraria for consulting and speaking for Medtronic Inc. Editor of PACE Journal. Rod S. Passman- receives research support, consulting fees and speaker fees from Medtronic; royalties from UpTo Date; and research support from Pfizer and Kardia. Northwestern University receives fellowship support from Medtronic, Inc.
REFERENCES
- 1.Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: First results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. [DOI] [PubMed] [Google Scholar]
- 2.Kuck K-H, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Eng J Med. 2016;374:2235–2245. [DOI] [PubMed] [Google Scholar]
- 3.Curnis A, Salghetti F, Cerini M, et al. Efficacy of second-generation cryoballoon ablation in paroxysmal and persistent atrial fibrillation patients. J Cardiovasc Med. 2017;18:655–662. [DOI] [PubMed] [Google Scholar]
- 4.Arya A, Hindricks G, Sommer P, et al. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace. 2009;12:173–180. [DOI] [PubMed] [Google Scholar]
- 5.Oto A, Aytemir K, Canpolat U, et al. Pulmonary vein isolation with the cryoballoon technique in atrial fibrillation treatment: Single centre experience. Turk Kardiyol Dern Ars. 2013;41:299–309. [DOI] [PubMed] [Google Scholar]
- 6.D’Ascenzo F, Corleto A, Biondi-Zoccai G, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: A meta-analysis. Int J Cardiol. 2013;167:1984–1989. [DOI] [PubMed] [Google Scholar]
- 7.Canpolat U, Aytemir K, Yorgun H, Sahiner L, Kaya EB, Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: Promising results from the Turkish Cryoablation Registry. Int J Cardiol. 2013;169:201–206. [DOI] [PubMed] [Google Scholar]
- 8.Kornej J, Hindricks G, Shoemaker MB, et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015;104:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojcik M, Berkowitsch A, Greiss H, et al. Repeated catheter ablation of atrial fibrillation: How to predict outcome. Circ J. 2013;77:2271–2279. [DOI] [PubMed] [Google Scholar]
- 10.Mujović N, Marinković M, Marković N, Shantsila A, Lip GY, Potpara TS. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: The MB-LATER clinical score. Sci Rep. 2017;7:40828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng H, Bai Y, Shantsila A, Fauchier L, Potpara TS, Lip GYH. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: A systematic review. Clin Res Cardiol. 2017;106:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Members ATF, Camm AJ, Lip GY, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: An update of the 2010 ESC guidelines for the management of atrial fibrillation developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 13.Wasserlauf J, Pelchovitz DJ, Rhyner J, et al. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2015; 38:483–489. [DOI] [PubMed] [Google Scholar]
- 14.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9:335–379. [DOI] [PubMed] [Google Scholar]
- 15.Akkaya E, Berkowitsch A, Greiss H, et al. PLAAF score as a novel predictor of long-term outcome after second-generation cryoballoon pulmonary vein isolation. Europace. 2018;20:f436–f443. [DOI] [PubMed] [Google Scholar]
- 16.Gerede DM, Candemir B, Vurgun VK, et al. Prediction of recurrence after cryoballoon ablation therapy in patients with paroxysmal atrial fibrillation.Anatol J Cardiol. 2015;16:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade JG, Khairy P, Macle L, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: Insights from the multicenter sustained treatment of paroxysmal atrial fibrillation (STOP AF) trial. Circ Arrhythm Electrophysiol. 2014;7:69–75. [DOI] [PubMed] [Google Scholar]
- 18.Ciconte G, Baltogiannis G, de Asmundis C, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: A comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace. 2015;17: 559–565. [DOI] [PubMed] [Google Scholar]
- 19.AkerstrÖm F, Bastani H, Insulander P, Schwieler J, Arias MA, Jensen-Urstad M. Comparison of regular atrial tachycardia incidence after circumferential radiofrequency versus cryoballoon pulmonary vein isolation in real-life practice. J Cardiovasc Electrophysiol. 2014;25:948–952. [DOI] [PubMed] [Google Scholar]
- 20.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Eng J Med. 2018; 378:417–427. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Reynolds K, Yang J, et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: The KP-RHYTHM study. JAMA Cardiol. 2018;3: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LY, Chung MK, Allen LA, et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: A scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 24.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 25.Goldberger JJ, Arora R, Green D, et al. Evaluating the atrial myopathy underlying atrial fibrillation: Identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nattel S, Harada M. Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–2345. [DOI] [PubMed] [Google Scholar]
- 27.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Prior antiarrhythmic drug use and the outcome of atrial fibrillation ablation. Europace. 2012;14:646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: A systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–1216. [DOI] [PubMed] [Google Scholar]
- 29.Dewire J, Calkins H. Impact of obstructive sleep apnea on outcomes of catheter ablation of atrial fibrillation. J Atr Fibrillation. 2013;5:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. [DOI] [PubMed] [Google Scholar]
- 31.Tang RB, Dong JZ, Liu XP, et al. Obstructive sleep apnoea risk profile and the risk of recurrence of atrial fibrillation after catheter ablation. Europace. 2009;11:100–105. [DOI] [PubMed] [Google Scholar]
- 32.Chilukuri K, Dalal D, Gadrey S, et al. A prospective study evaluating the role of obesity and obstructive sleep apnea for outcomes after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:521–525. [DOI] [PubMed] [Google Scholar]
- 33.Latina JM, Estes NAM 3rd, Garlitski AC. The relationship between obstructive sleep apnea and atrial fibrillation: A complex interplay. Pulm Med. 2013;2013:621736–621736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitter T, Nolker G, Vogt J, Prinz C, Horstkotte D, Oldenburg O. Predictors of recurrence in patients undergoing cryoballoon ablation for treatment of atrial fibrillation: The independent role of sleep-disordered breathing. J Cardiovasc Electrophysiol. 2012; 23:18–25. [DOI] [PubMed] [Google Scholar]
- 35.Fiala M. Catheter ablation for persistent and long-standing persistent atrial fibrillation. J Atr Fibrillation. 2016;9:1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akkaya E, Berkowitsch A, Zaltsberg S, et al. Second-generation cryoballoon ablation for treatment of persistent atrial fibrillation: Three-year outcome and predictors of recurrence after a single procedure. J Cardiovasc Electrophysiol. 2018;29:38–45. [DOI] [PubMed] [Google Scholar]
- 37.Koektuerk B, Yorgun H, Hengeoez O, et al. Cryoballoon ablation for pulmonary vein isolation in patients with persistent atrial fibrillation: One-year outcome using second generation cryoballoon. Circ Arrhythm Electrophysiol. 2015;8:1073–1079. [DOI] [PubMed] [Google Scholar]
- 38.Abumuamar AM, Dorian P, Newman D, Shapiro CM. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin Cardiol. 2018;41:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: Clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331–337. [DOI] [PubMed] [Google Scholar]
- 40.Shukla A, Aizer A, Holmes D, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: A meta-analysis. JACC Clin Electrophysiol. 2015;1:41–51. [DOI] [PubMed] [Google Scholar]
- 41.Tung P, Anter E. Atrial fibrillation and sleep apnea: Considerations for a dual epidemic. J Atr Fibrillation. 2016;8:1283–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oto A, Aytemir K, Canpolat U, et al. [Pulmonary vein isolation with the cryoballoon technique in atrial fibrillation treatment: Single centre experience]. Turk Kardiyol Dern Ars. 2013;41:299–309. [DOI] [PubMed] [Google Scholar]
- 43.Shukla A, Aizer A, Holmes D, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: A meta-analysis. JACC Clin Electrophysiol. 2015;1:41–51. [DOI] [PubMed] [Google Scholar]
- 44.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. [DOI] [PubMed] [Google Scholar]
- 45.Ciconte G, De Asmundis C, Sieira J, et al. Single 3-minute freeze for second-generation cryoballoon ablation: One-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015;12:673–680. [DOI] [PubMed] [Google Scholar]
- 46.Pott A, Kraft C, Stephan T, Petscher K, Rottbauer W, Dahme T. Time-to-isolation guided titration of freeze duration in 3rd generation short-tip cryoballoon pulmonary vein isolation - Comparable clinical outcome and shorter procedure duration. Int J Cardiol. 2018;255:80–84. [DOI] [PubMed] [Google Scholar]
- 47.Su W, Aryana A, Passman R, et al. Cryoballoon best practices II: Practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm. 2018;15:1348–1355. [DOI] [PubMed] [Google Scholar]


