Abstract
Background
Immune cells express the vitamin (vit) D receptor, and vit D is a potent immune-modulator. A negative correlation between serum vit D levels and rheumatoid arthritis (RA) disease activity has been reported. Therefore, we aimed to investigate if the sufficient serum vit D level is helpful to control disease activity in RA patients treated with interleukin (IL)-6 receptor antibody tocilizumab.
Methods
RA patients taking tocilizumab were enrolled, and data were collected retrospectively. Disease activity scores (DAS) 28, serum vit D levels, modified Sharp scores of hand X-ray at the time of tocilizumab initiation, and follow-up data were analysed. Peripheral blood mononuclear cells were differentiated into T-helper (Th) 17 or osteoclasts in the presence of various concentrations of tocilizumab and/or 1,25(OH)2D. Th17 proportions were analysed by fluorescence-activated cell sorting. Supernatant cytokine levels were determined by enzyme-linked immunosorbent assay.
Results
Among 98 RA patients taking tocilizumab, 34 (34.7%) had sufficient serum 25(OH)D levels (≥ 30 ng/mL) when tocilizumab was initiated. At 24 weeks, vit D sufficient patients had greater DAS28 reduction (64.6% ± 15.5% vs. 52.7% ± 20.7%, P = 0.004), and lower disease activity (91.2% vs. 70.3%, P = 0.018) or remission (82.4% vs. 57.8%, P = 0.014). These differences in DAS28 reduction and the proportion of patients with remission persisted at 48 weeks. However, there was no significant difference in hand and wrist erosion progression. In vitro, tocilizumab and 1,25(OH)2D treatment synergistically suppressed IL-17 production and osteoclastogenesis.
Conclusion
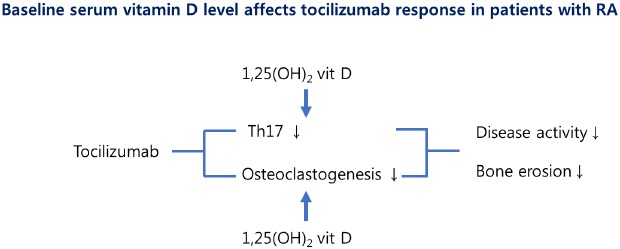
RA patients treated with IL-6 antibody show a better response when they have sufficient serum vit D. Tocilizumab and 1,25(OH)2D synergistically suppress IL-17 production and osteoclast differentiation in RA patients.
Keywords: Vitamin D, Rheumatoid Arthritis, Tocilizumab
Graphical Abstract
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that can cause cartilage and bone damage, as well as disability.1 Although the precise mechanism is not fully understood, aberrant immune reactions predominantly affecting synovial membranes in genetically susceptible subjects are known to play a central role.2 One of the current therapeutic strategies is targeting inflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-6.3 Vitamin (vit) D has been implicated in bone metabolism and calcium homeostasis.4 In addition, vit D receptor is expressed on immune cells,5 and the role of vit D in immunomodulation has been widely investigated. With regard to RA pathogenesis, vit D appears to regulate RA by suppressing pathogenic immune cells.6 For example, vit D suppresses the transcription of T-helper (Th) 1 proinflammatory cytokines, such as interferon (IFN)-γ, IL-17, and IL-21.5 It suppresses IL-17, IL-6, IL-1, and TNF-α production by Th1 cells. Vit D also inhibits Th1 and augments Th2 cell development,7 as well as decreases IFN-γ and IL-2 production by CD4+ T cells. Previous studies revealed that vit D levels are negatively associated with RA disease activity, and vit D regulates RA disease development.8,9 Moreover, 1,25(OH)2D3 suppresses Th17-mediated synovial inflammation, while anti-TNF-α treatment does not affect autocrine IL-17 production by Th17 derived from RA patients.10 The authors argued the suppressive function on IL-17 of vit D exerted an additional anti-inflammatory effect in TNF-α inhibitor-treated patients. Vit D also promotes the efficacy of CD28 co-stimulation blockade by abatacept.11 The study showed that the active metabolite of vit D (1,25[OH]2D3) suppressed the production of TNF-α, IFN-γ, and IL-17 by T cells when stimulated with anti-CD3. This was also the case with abatacept. These findings support the hypothesis that vit D can exert additive benefits with RA biologics treatment. Indeed, vit D supplementation in RA patients resulted in significantly decreased functional disability at month 6 in a recent clinical trial.12 In an open labelled randomized trial, 8 weeks of 1,25(OH)2D3 supplementation was associated with greater pain relief in early RA patients.13
Based on these previous observations, we aimed to investigate whether serum vit D level affects the therapeutic efficacy of IL-6 blockade in RA patients. We hypothesized that sufficient serum vit D level was associated with good treatment response and decreased bone erosion progression in RA patients on tocilizumab. We investigated if there was a difference in disease activity or bone erosion progression between groups with sufficient and insufficient serum 25(OH)D levels.
METHODS
Study population
Patients who visited Seoul St. Mary's Hospital, The Catholic University of Korea and initiated tocilizumab treatment from January 2013 onward were enrolled. Specifically, the first patient started tocilizumab on 2nd July 2013 and the last patient started tocilizumab on 23rd October 2014. All the patients were diagnosed with RA according to 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA14 with age over 19. Patients with concomitant severe illness that could affect the results of the study (e.g., parathyroid disease, malignancy) were excluded. Serum 25(OH)D3 level measured within 6 months before/after tocilizumab initiation was considered as the baseline level. As disease activity score (DAS) 28 was calculated every 6 months after initiation to meet reimbursement criteria; medical records were reviewed to obtain data.
Serum 25(OH)D level measurement
Serum level of 25(OH)D was derived from the laboratory result reported by department of laboratory medicine of Seoul St. Mary's Hospital. The level was determined by the automated machine (ADVIA Centaur® XPT; Siemens Aktiengesellschaft, Munich, Germany) using chemiluminescence immunoassay method.
Modified sharp score (mSHARP) of hands
mSHARP of the hands were measured as previously reported.15 Original mSHARP includes values for the hands and feet, we only used hand score because most patients did not have follow-up feet X-rays.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA) method followed by isolating CD4+ T cells using a Human CD4+T cell enrichment kit (Invitrogen, Carlsbad, CA, USA). The polarizing condition of T17 cells was as follows: CD3/CD28 T activator Dynabead (1:1 ratio with CD4+ T cell; Invitrogen), 10 µg/mL anti-IFN-γ antibody, 10 µg/mL anti-IL-4 antibody, 2 ng/mL recombinant transforming growth factor-β, 20 ng/mL recombinant IL-6 (R&D Systems, Minneapolis, MN, USA). Polarization to Th17 cells with various concentrations of tocilizumab and 1,25(OH)2D3 was performed for 3 days. After re-stimulation with 25 ng/mL phorbol 12-myristate 13-acetate and 250 ng/mL ionomycin with protein transport inhibitor Golgistop (BD Biosciences, San Jose, CA, USA), cells were stained with PerCP-Cy5.5-conjugated anti-CD4 antibody, phycoerythrin-conjugated anti-IL-17A antibody (BioLegend, San Diego, CA, USA). We prevented nonspecific antibody binding by pre-treatment with the human Fc gamma receptor binding inhibitor (Thermo Fisher Scientific, Waltham, MA, USA). Cells were first gated for singlets (FSC-H vs. FSC-A) then for lymphocytes (SSC-A vs. FSC-A) and live CD4+ T cells (fixable viability dye-e780; Invitrogen vs. CD4-PerCP-Cy5.5-A). Two independent experiments were performed with PBMCs from three individuals in each experiment.
Enzyme-linked immunosorbent assay
Culture supernatant from polarized cells was measured for IL-17A, TNF-α, and IL-6. Each duoset of the human IL-17A, TNF-α, and IL-6 development kit was purchased from R&D systems; we performed measurements according to the manufacturer's instructions.
Human in vitro osteoclastogenesis
PBMCs from healthy donor were isolated and cultured in 10% alpha minimum essential medium (Thermo Fisher Scientific) overnight. Non-adherent cells were removed, and attached cells were stimulated with 100 ng/mL macrophage colony-stimulating factor (M-CSF; R&D systems). After 3 days, osteoclast precursor cells were cultured further in the presence of 25 ng/mL M-CSF, 30 ng/mL receptor activator of nuclear factor kappa-B ligand (RANKL; R&D Systems), and various concentrations of tocilizumab and 1,25(OH)2D3 (Sigma, St. Louis, MO, USA) were treated for 9 days to generate osteoclasts. Tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells (MNCs) were regarded as osteoclasts. Two independent experiments were performed with PBMCs from three individuals in each experiment.
TRAP staining
A commercial TRAP kit (Sigma) was used according to the manufacturer's instructions; however, counterstaining with haematoxylin was omitted. TRAP-positive MNCs containing three or more nuclei were counted as osteoclasts.
Gene expression analysis using real-time polymerase chain reaction (PCR)
PCR amplification and analysis were performed on a Light Cycler 96 instrument (Roche, Mannheim, Germany). All reactions were performed using LightCycler SYBR Green I master (Roche), according to the manufacturer's instruction. The following primers were used: RANK sense 5′-ACCAGCATCAAAATCCCAAG-3′, antisense 5′-CCCCAAAGTATGTTGCATCC-3′; matrix metalloproteinase 9 (MMP9) sense 5′-TGGGGGGCAACTCGGC-3′, antisense 5′-GGAATGATCTAAGCCCAG-3′; cathepsin K sense 5′-TGAGGCTTCTCTTGGTGTCCATAC-3′, antisense 5′-AAAGGGTGTCATTACTGCGGG-3′; β-actin sense 5′-GGACTTCGAGCAAGAGATGG-3′, antisense 5′-TGTGTTGGGGTACAGGTCTTTG-3′. mRNA expression levels were normalized to that of β-actin.
Statistical analysis
We performed statistical analyses with GraphPad Prism (Version 8; GraphPad Inc., San Diego, CA, USA) and SPSS (version 24; IBM, Armonk, NY, USA). Continuous variables were analysed using Student's t-test or Mann-Whitney U test where appropriate. Three or more continuous variables were analysed with Kruskal-Wallis test or Wilcoxon signed rank test (with Bonferroni's correction for multiple comparison) where appropriate. Categorical variables were analysed using χ2 test. P < 0.05 was considered significant.
Ethics statement
This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital (KC14TISI0571). Informed consents were obtained from the subjects.
RESULTS
Baseline characteristics of the study population
A total of 98 RA patients were investigated. Baseline characteristics were obtained within 90 days of tocilizumab initiation. Table 1 summarizes the demographic and clinical characteristics of the patients at baseline. The mean age was 53.5 years, and 84 (85.7%) were women. The mean disease duration was 116.4 months. Among 98 patients, 34 (34.7%) had sufficient vit D levels (≥ 30 ng/mL) at tocilizumab initiation. Fifty patients were taking various kinds of vit D supplementation, but there was no significant difference in serum 25(OH)D level between vit D supplementation taking group and non-taking group (median [interquartile range, IQR], 26.1 [20.1–36.5] vs. 26.58 [16.6–29.6]; P = 0.393). There was no significant difference in age, gender, or disease activity between the vit D sufficient and insufficient groups.
Table 1. Baseline characteristics of the study subjects at tocilizumab initiation.
| Variables | 25(OH)D ≥ 30 ng/mL (n = 34) | 25(OH)D < 30 ng/mL (n = 64) | P value | |
|---|---|---|---|---|
| Age, yr | 56.2 ± 12.0 | 52.1 ± 11.0 | 0.092 | |
| Gender, women | 30 (88.2) | 54 (84.4) | 0.765 | |
| Disease duration, mon | 143.7 ± 102.5 | 102.0 ± 82.2 | 0.045 | |
| Previous use of other biologics | 19 (55.9) | 33 (51.6) | 0.867 | |
| Methotrexate use | 29 (85.3) | 59 (92.2) | 0.309 | |
| Corticosteroid use | 28 (82.4) | 56 (87.5) | 0.550 | |
| 25(OH)D level, ng/mL | 37.8 ± 5.9 | 19.9 ± 6.0 | < 0.001 | |
| Rheumatoid factor, IU/mL | 146.6 ± 165.9 | 171.4 ± 284.4 | 0.641 | |
| Anti-CCP antibody, IU/mL | 176.8 ± 139.7 | 161.1 ± 138.6 | 0.770 | |
| ESR, mm/hr | 56.6 ± 26.4 | 57.1 ± 25.7 | 0.942 | |
| CRP, mg/dL | 2.3 ± 2.7 | 2.3 ± 2.5 | 0.976 | |
| DAS28 | 5.5 ± 0.8 | 5.4 ± 0.9 | 0.778 | |
| Year of TCZ start | ||||
| 2013 | 9 (26.5) | - | 1.000 | |
| 2014 | 12 (35.3) | - | 0.667 | |
| 2015 | 8 (23.5) | - | 1.000 | |
| 2016 | 5 (14.7) | - | 0.747 | |
| Cessation of TCZ during follow-up | 7 (20.6) | 12 (18.8) | 0.827 | |
| Reason for cessation | ||||
| Treatment failure | 2 | 6 | 0.633 | |
| Adverse event | 1 | 2 | 1.000 | |
| Clinical remission | 1 | 0 | 0.368 | |
| Consent withdrawal | 3 | 3 | 0.617 | |
| Follow-up loss | 0 | 1 | 1.000 | |
Data are presented as number (%) or mean ± standard deviation.
CCP = cyclic citrullinated peptide, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, TCZ = tocilizumab.
Patients with serum high 25(OH)D show better response to tocilizumab
To investigate the role of vit D in treatment response to tocilizumab, we compared disease activities of the two groups 24 and 48 weeks after tocilizumab initiation. Patients with sufficient serum vit D levels at baseline showed greater DAS28 reduction (64.6% ± 15.5% vs. 52.7% ± 20.7%; P = 0.004), and more patients achieved low disease activity (31 [91.2%] vs. 45 [70.3%]; P = 0.018) or remission (28 [82.4%] vs. 37 [57.8%]; P = 0.014) than patients with insufficient serum vit D level after 6 months of tocilizumab treatment (Table 2). The percentages of patients who achieved low disease activity were not different between the groups at week 48. However, the percent decrease in DAS28 and proportion of remission remained significantly higher in the vit D sufficient group.
Table 2. Clinical responses to tocilizumab at weeks 24 and 48.
| Efficacy measure | 25(OH)D ≥ 30 ng/mL (n = 34) | 25(OH)D < 30 ng/mL (n = 64) | P value | |
|---|---|---|---|---|
| At week 24 | ||||
| DAS28 reduction, % | 64.6 ± 15.5 | 52.7 ± 20.7 | 0.004 | |
| Low DAS28 (DAS28 ≤ 3.2) | 31 (91.2) | 45 (70.3) | 0.018 | |
| Remission (DAS28 ≤ 2.6) | 28 (82.4) | 37 (57.8) | 0.014 | |
| Clinically significant reduction ≥ 1.2 | 33 (97.1) | 59 (92.2) | 0.661 | |
| At week 48 | (n = 25) | (n = 50) | ||
| DAS28 reduction, % | 67.6 ± 13.9 | 59.8 ± 16.4 | 0.044 | |
| Low DAS28 (DAS28 ≤ 3.2) | 24 (96.0) | 43 (86.0) | 0.256 | |
| Remission (DAS28 ≤ 2.6) | 23 (92.0) | 35 (70.0) | 0.032 | |
| Clinically significant reduction ≥ 1.2 | 25 (100.0) | 49 (98.0) | 1.000 | |
Data are presented as number (%) or mean ± standard deviation.
DAS = disease activity score.
Radiologic progression is not affected by serum 25(OH)D level
Next, we investigated if the vit D level could affect radiographic progression. We compared baseline and follow-up X-rays from 80 patients: 27 vit D sufficient patients and 53 insufficient patients. The mean interval between hand X-rays was 14.7 months. The median (IQRs) value of differences in mSHARP hand score were 1 (0–8) and 1 (0–5.5) in the vit D sufficient and insufficient group, respectively (P = 0.979) (Table 3).
Table 3. Comparison of radiologic progression between vitamin D sufficient and insufficient groups.
| Efficacy measure | 25(OH)D ≥ 30 ng/mL (n = 27) | 25(OH)D < 30 ng/mL (n = 53) | P value |
|---|---|---|---|
| Serum 25(OH)D level, ng/mL | 36.5 (32.4–42.1) | 21.6 (16.1–26.4) | < 0.001 |
| Interval from 1st to 2nd radiography, yr | 1.5 (1.2–2.2) | 1.5 (1.2–2.2) | 0.799 |
| 1st mSHARP, hand | 9 (0–63) | 9 (0–66.5) | 0.869 |
| 2nd mSHARP, hand | 17 (2–70) | 10 (1–73) | 0.846 |
| ΔmSHARP, hand | 1 (0–8) | 1 (0–5.5) | 0.979 |
Data are presented as median (interquartile ranges).
mSHARP = modified Sharp score.
1,25(OH)2D treatment suppressed IL-17 production synergistically with IL-6 blockade
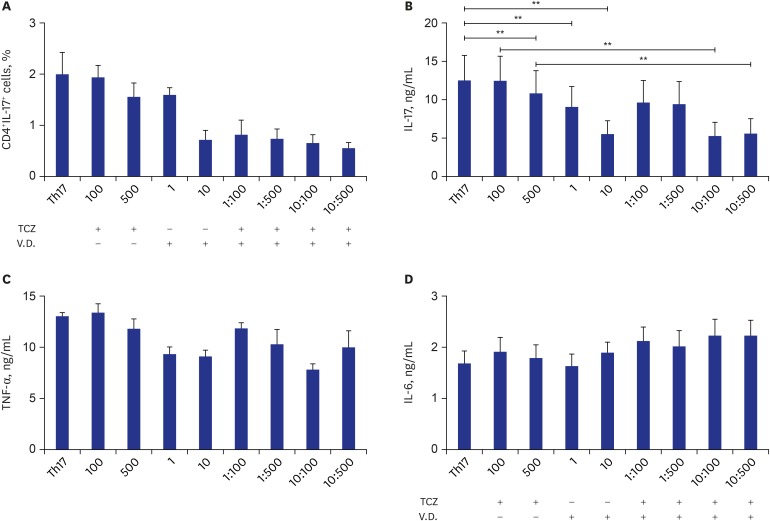
Th17 is a pathogenic cell in RA, so we assumed the reason of lower disease activity in the high vit D group was Th17 suppression by 1,25(OH)2D3 in synergy with IL-6 blockade. When CD4+ T cells obtained from healthy donors were differentiated into Th17 in the presence of various concentrations of tocilizumab and 1,25(OH)2D3, we observed that the CD4+IL-17+ population tended to be suppressed by a combination of tocilizumab and 1,25(OH)2D3, although it did not reach statistical significance (P = 0.063) (Fig. 1A). Interestingly, IL-17 concentration in the culture supernatant was suppressed by tocilizumab and 1,25(OH)2D3 treatment (Fig. 1B). We also observed a synergistic effect of tocilizumab and 1,25(OH)2D3 treatment. However, TNF-α (Fig. 1C) and IL-6 (Fig. 1D) levels were not affected by tocilizumab or 1,25(OH)2D3.
Fig. 1. Th17 differentiation in the presence or absence of tocilizumab and/or 1,25(OH)2D3. CD4+ T cells were isolated from peripheral blood mononuclear cells obtained from healthy donors (n = 3) and differentiated into Th17 in the presence or absence of various concentrations of tocilizumab and/or 1,25(OH)2D3. (A) CD4+IL17+ cell proportions were analysed by FACS. (B) Concentrations of IL-17, (C) TNF-α, and (D) IL-6.
Th = T-helper, IL = interleukin, FACS = fluorescence-activated cell sorting, TNF = tumour necrosis factor, TCZ = tocilizumab, V.D. = vitamin D.
**P < 0.01.
1,25(OH)2D3 treatment suppressed osteoclast differentiation synergistically with IL-6 blockade
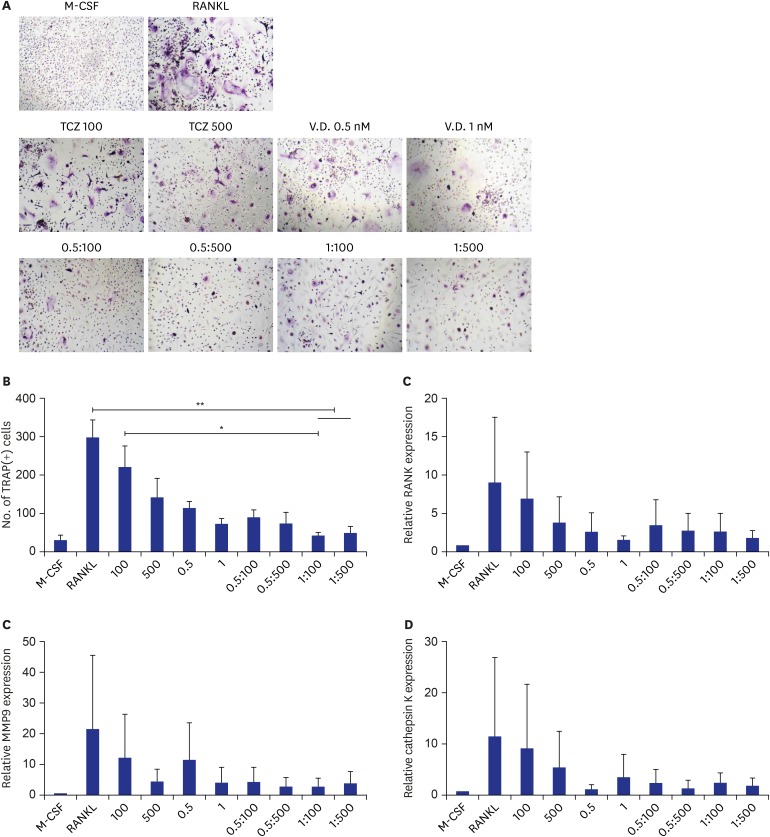
Although serum vit D difference did not affect radiographic progression represented by mSHARP hand score in our study population, we observed that tocilizumab and 1,25(OH)2D3 dose dependently suppressed osteoclastogenesis in vitro, shown by reduced number of TRAP-positive osteoclasts (Fig. 2A and B). When 1,25(OH)2D3 was co-applied with tocilizumab, there was an additive synergistic effect in osteoclastogenesis suppression compared to tocilizumab-only treated cells. We also investigated the expression of osteoclast-related molecules such as RANK (Fig. 2C), MMP9 (Fig. 2D), and cathepsin K (Fig. 2E). Vit D tended to additively suppress osteoclastogenic markers when added to tocilizumab, but the difference was not significant.
Fig. 2. Osteoclastogenesis in the presence or absence of tocilizumab and/or 1,25(OH)2D3. Peripheral blood mononuclear cells obtained from healthy donors (n = 3) were differentiated into osteoclasts with RANKL and M-CSF treatment in presence or absence of various concentrations of tocilizumab and/or 1,25(OH)2D3. (A) Representative H&E stain, (B) number of TRAP-positive giant cells, relative mRNA expressions of (C) RANK, (D) MMP9, and (E) cathepsin K.
RANKL = receptor activator of nuclear factor kappa-B ligand, M-CSF = macrophage colony-stimulating factor, H&E = hematoxylin and eosin, TRAP = tartrate-resistant acid phosphatase, RANK = receptor activator of nuclear factor kappa-B, MMP9 = matrix metalloproteinase 9, TCZ = tocilizumab, V.D. = vitamin D.
*P < 0.05; **P < 0.01.
DISCUSSION
Our results indicate that serum vit D levels affect the treatment response of RA patients on tocilizumab. To the best of our knowledge, this is the first study to investigate the role of vit D in IL-6 blockade strategy for RA treatment.
Patients with sufficient vit D levels showed better treatment responses 24 and 48 weeks after tocilizumab initiation. A previous study showed that when CCR6+ memory Th cells were co-cultured with RA synovial fibroblasts, 1, 25(OH)2D3 suppressed T cell production of IL-17A, IFN-γ, and IL-6.16 Likewise, we confirmed that vit D suppresses IL-17 production in addition to IL-6 blockade in vitro. In this way, vit D sufficiency seems to explain our observation of improved treatment response to tocilizumab at weeks 24 and 48. Beside this direct inhibition on inflammatory cytokines, DAS28 difference may have resulted from difference in pain perception. DAS28 is calculated with the number of tender/swollen joints, the level of acute phase reactant, and patient global assessment scales from 0–100. Therefore, subjective pain perception can exert a profound effect on DAS28. Gendelman et al.17 reported that supplement of 4,000 IU vit D lead to faster decline of pain visual analogue scale scores than placebo in patients with musculoskeletal pain.
Tocilizumab suppressed disease activity represented by DAS28 and prevented bone erosion at 2 years more efficiently than the methotrexate-treated group of early RA patients.18 Decreased inflammation may indirectly contribute to reduced bone erosion; however, a direct suppressive mechanism was recently reported. Wu et al.19 found that IL-6 enhanced osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. This enhancing role of IL-6 requires the presence of soluble IL-6R.20 Consistent with this, we observed a direct suppressive effect of tocilizumab (antibody to IL-6R) on RANKL-/M-CSF-induced osteoclastogenesis in vitro.
The effect of 1,25(OH)2D3 on osteoclasts has been controversial. Gu et al.21 reported that 48 hours of 1,25(OH)2D3 treatment enhanced RANKL- and M-CSF-induced osteoclast differentiation from RAW264.7 cells. Also, osteoclast number and resorption activity are increased when human CD14+ monocytes are differentiated into osteoclasts in the presence of 1,25(OH)2D3. Conversely, vit D treatment suppresses osteoclast resorptive capacity by modifying their surface adhesion and migration properties.22 In the current study, we observed decreased numbers of TRAP(+) cells with active vit D treatment, and the combination of tocilizumab and 1,25(OH)2D showed a superior suppressive effect compared to tocilizumab treatment alone. Despite the promising effect observed in vitro, there was no difference in hand erosion progression represented by mSHARP hand score. This might be due to the variable and short follow-up periods, underscoring the need for long-term observational studies.
There are several limitations of our study. First, it was a retrospective design with a relatively small sample size, and the follow-up intervals of X-rays varied Especially, even though we tried to include as many patients as possible, the number of enrolled patients was not calculated on the basis of statistical estimation. Therefore, it is unclear if the negative results in mSHARP are appropriately powered. Second, we could only obtain serial X-rays of the hands; radiographic progression was not assessed in feet, so we could not calculate the complete mSHARP score. Third, serum 25(OH)D was only measured at baseline, so vit D level variation during the follow-up period was not considered in the analysis. Moreover, serum vit D level is influenced by the degree of the individual's sun exposure. Therefore, variation in insolation according to the season should be taken into account considering that the measurement of vit D level was performed throughout the year.
In conclusion, patients on tocilizumab are more likely to achieve remission or low disease activity when they maintain sufficient serum vit D levels. This seems to be due to the suppressive effects of tocilizumab and vit D on Th17 development and osteoclastogenesis. Considering its direct suppressive effect on osteoclastogenesis in synergy with IL-6 blockade, future prospective studies may also reveal a suppressive effect on radiographic progression.
Footnotes
Funding: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C1062).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung SM, Park S.
- Data curation: Kim H, Jung SM.
- Formal analysis: Kim H, Lee J,1 Jung SM, Lee J,2 Cho ML.
- Investigation: Kim H, Baek SY, Hong SM, Kwok SK.
- Methodology: Baek SY, Hong SM.
- Project administration: Lee J1.
- Supervision: Cho ML, Kwok SK.
- Writing - original draft: Lee J2.
- Writing - review & editing: Cho ML, Kwok SK.
Lee J,1 Lee Jaeseon; Lee J,2 Lee Jennifer.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–2348. doi: 10.1016/S0140-6736(17)31491-5. [DOI] [PubMed] [Google Scholar]
- 4.Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):E1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13(1):21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 6.Wen HY, Luo J, Li XF, Wei DD, Liu Y. 1,25-Dihydroxyvitamin D3 modulates T cell differentiation and impacts on the production of cytokines from Chinese Han patients with early rheumatoid arthritis. Immunol Res. 2019;67(1):48–57. doi: 10.1007/s12026-018-9033-4. [DOI] [PubMed] [Google Scholar]
- 7.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 8.Bellan M, Sainaghi PP, Pirisi M. Role of vitamin D in rheumatoid arthritis. Adv Exp Med Biol. 2017;996:155–168. doi: 10.1007/978-3-319-56017-5_13. [DOI] [PubMed] [Google Scholar]
- 9.Lee YH, Bae SC. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: a meta-analysis. Clin Exp Rheumatol. 2016;34(5):827–833. [PubMed] [Google Scholar]
- 10.van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Cornelissen F, van Leeuwen JP, et al. TNF blockade requires 1,25(OH)2D3 to control human Th17-mediated synovial inflammation. Ann Rheum Dis. 2012;71(4):606–612. doi: 10.1136/annrheumdis-2011-200424. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DH, Jeffery LE, Soskic B, Briggs Z, Hou TZ, Raza K, et al. 1,25(OH)2D3 promotes the efficacy of CD28 costimulation blockade by abatacept. J Immunol. 2015;195(6):2657–2665. doi: 10.4049/jimmunol.1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soubrier M, Lambert C, Combe B, Gaudin P, Thomas T, Sibilia J, et al. A randomised, double-blind, placebo-controlled study assessing the efficacy of high doses of vitamin D on functional disability in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(6):1056–1060. [PubMed] [Google Scholar]
- 13.Mukherjee D, Lahiry S, Thakur S, Chakraborty DS. Effect of 1,25 dihydroxy vitamin D3 supplementation on pain relief in early rheumatoid arthritis. J Family Med Prim Care. 2019;8(2):517–522. doi: 10.4103/jfmpc.jfmpc_446_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde DM. Plain X-rays in rheumatoid arthritis: overview of scoring methods, their reliability and applicability. Baillieres Clin Rheumatol. 1996;10(3):435–453. doi: 10.1016/s0950-3579(96)80043-4. [DOI] [PubMed] [Google Scholar]
- 16.Dankers W, González-Leal C, Davelaar N, Asmawidjaja PS, Mus AM, Hazes JM, et al. 1,25(OH)2D3 and dexamethasone additively suppress synovial fibroblast activation by CCR6+ T helper memory cells and enhance the effect of tumor necrosis factor alpha blockade. Arthritis Res Ther. 2018;20(1):212. doi: 10.1186/s13075-018-1706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gendelman O, Itzhaki D, Makarov S, Bennun M, Amital H. A randomized double-blind placebo-controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Lupus. 2015;24(4-5):483–489. doi: 10.1177/0961203314558676. [DOI] [PubMed] [Google Scholar]
- 18.Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert-Roth A, Blanco R, et al. Tocilizumab combination therapy or monotherapy or methotrexate monotherapy in methotrexate-naive patients with early rheumatoid arthritis: 2-year clinical and radiographic results from the randomised, placebo-controlled FUNCTION trial. Ann Rheum Dis. 2017;76(7):1279–1284. doi: 10.1136/annrheumdis-2016-210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q, Zhou X, Huang D, Ji Y, Kang F. IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell Physiol Biochem. 2017;41(4):1360–1369. doi: 10.1159/000465455. [DOI] [PubMed] [Google Scholar]
- 20.Feng W, Liu H, Luo T, Liu D, Du J, Sun J, et al. Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-κB, ERK and JNK signaling pathways. Sci Rep. 2017;7:41411. doi: 10.1038/srep41411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J, Tong XS, Chen GH, Wang D, Chen Y, Yuan Y, et al. Effects of 1α,25-(OH)2D3 on the formation and activity of osteoclasts in RAW264.7 cells. J Steroid Biochem Mol Biol. 2015;152:25–33. doi: 10.1016/j.jsbmb.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Kogawa M, Findlay DM, Anderson PH, Atkins GJ. Modulation of osteoclastic migration by metabolism of 25OH-vitamin D3. J Steroid Biochem Mol Biol. 2013;136:59–61. doi: 10.1016/j.jsbmb.2012.09.008. [DOI] [PubMed] [Google Scholar]