Abstract
Background:
Treatment of those at clinical high-risk (CHR) for developing psychosis may lead to preventive strategies. However, attrition in trials may hamper efforts to detect effective changes and lead to bias. Our objective was to synthesize the relative attrition rates in clinical trials conducted in CHR for psychosis samples.
Method:
We searched the following electronic databases: MEDLINE, Embase, PsycINFO, CINAHL and EBM with no restrictions. Inclusion criteria was any treatment-based randomized controlled trial (RCT) conducted in CHR samples that reported attrition. Relative attrition rates were calculated using random-effects meta-analysis, stratified by time, and reported as odds ratios (ORs), proportions, and 95% confidence intervals (CIs).
Results:
Twenty-one RCTs met our inclusion criteria, including a total of 2,260 CHR participants. Attrition rates between all treatment types identified were not statistically different from control treatments at any time-point. When accessing overall trial attrition, the pooled attrition rate was 29.57% (95% CI=23.84–35.63%) with statistically significant heterogeneity (I2=88.70%; p<0.001). Furthermore, 11 trials had a subsequent follow-up after the intervention was conducted and the pooled attrition was 33.96% (95% CI=24.94–43.59%). When examining predictors of attrition, no statistically significant subgroup differences were observed in attrition rates.
Conclusions:
Almost one third of CHR participants will not complete participation in an RCT, however no predictors were found to be statistically significantly related to attrition. Methods to account for missing data and attrition are warranted in CHR trials to account for potential biases associated with high attrition rates.
Keywords: clinical high-risk, systematic review, meta-analysis, attrition, treatment
1. INTRODUCTION
Treatment of those at clinical high risk (CHR) of developing a psychotic disorder has the potential to lead to preventive strategies (Nelson et al, 2013). Although randomized controlled trials (RCT) are the gold standard for examining treatment efficacy and effectiveness, they still must overcome challenges such as treatment compliance, missing outcome data, and generalizability to real-world healthcare settings. These concerns are largely attributable to the completeness of study participation. While attrition is expected in any given research study, a significant amount of attrition can lead to bias, further complicating the statistical analysis and interpretation of the results (Molenberghs and Kenward, 2007).
Longitudinal studies conducted in youth at CHR of psychosis have found that attrition can range from 25% to 35% (Ruhrmann et al., 2010; Stowkowy et al, 2018). A recent longitudinal study indicated that there were no clinical, functional, or demographic characteristics that might help identify those who drop-out versus those who remain (Stowkowy et al, 2018). Treatment trials in CHR for psychosis samples have been increasing in recent years and are often longitudinal in nature. Since a primary concern of CHR trials is to determine the impact of a given treatment on symptoms, functioning, and transition to psychosis over time it may be important to know whether dropouts are related to increased symptoms at baseline, due to treatment effectiveness, or differences in trial designs.
To our knowledge, no meta-analysis has been conducted examining attrition in RCTs in CHR samples. However, previous reviews in serious mental illness have noted that placebo-based trials may be difficult to undertake as patients may feel that they are treated sub-optimally, experience deterioration in health, and therefore are unlikely to want to continue to participate in research (Hummer et al., 2003; Kemmler, Hummer, Widschwendter, & Fleischhacker, 2005; Roberts et al., 2002). Other reviews have examined differences in attrition between treatment and control groups, and indicated very similar rates of attrition between the two (Berlim, Van den Eynde, Tovar-Perdomo, & Daskalakis, 2014; Crutzen, Viechtbauer, Spigt, & Kotz, 2015). Exploring if there are specific reasons for differential attrition rates and determining if patterns of attrition exist in CHR trials may help researchers to optimally design research studies.
This systematic review provides an in-depth examination of attrition in RCTs in CHR samples. By investigating the relative attrition rates the evidence base will be enhanced which in turn may help researchers develop strategies to mitigate attrition in future trials. Therefore, the primary objective of this systematic review and meta-analysis was to summarize the relative attrition rates in all RCTs in CHR samples.
2. METHODS
A systematic literature review examining attrition rates in CHR for psychosis RCTs was conducted in accordance with the PRISMA guidelines and registered through PROSPERO a priori (CRD42018090329).
2.1. Search Strategy
A comprehensive search of the following databases was conducted: MEDLINE, CINAHL, EBM Reviews, Embase and PsycINFO. No date, language, or geographical restrictions were applied, and the search was conducted from database inception to June 28, 2017. The search was conducted in line with three previous reviews done by our research group (Devoe, Farris, Townes, & Addington, 2018a, 2018b; Devoe, Peterson, & Addington, 2018). In short, these previous reviews included different combinations of the following MeSH and keywords: “psychosis”, “schizophrenia”, “clinically high-risk”, “ultra high-risk”, “basic symptoms”, “treatment”, “experimental”, “trial”, “intervention”, and other related synonyms. This search was updated using SCOPUS up to and including any trials published prior to November 2018.
2.2. Inclusion Criteria
The inclusion criteria for this review were as follows: 1) study participants at CHR for psychosis (meeting criteria for at-risk mental state) or schizotypal disorders; 2) any intervention; 3) any control group; 4) follow-up of participants recording attrition and; 5) RCT study design. Case studies, observational interventions and abstracts not reporting attrition were excluded. Two authors, (MSF and DJD) screened first, titles/abstracts and second, full-text reviews (for publications meeting inclusion criteria) in duplication. A third reviewer (JA) resolved any conflicts.
2.3. Data Extraction
Data extraction was performed by one author (MSF) and verified by a second author (DJD). Information extracted from RCTs included: first author, year of publication, country, number of participants in total and for each trial group, type of intervention, type of control, treatment duration, CHR criterion, mean age, proportion of males, baseline attenuated psychotic symptom (APS) scores, baseline negative symptom scores, presence of a CONSORT flow diagram, reasons for attrition, methods for missing data applied, attrition rates at each follow-up time-point (for intervention and control arms separately and for all participants regardless of allocation), follow-up time point in months and if the primary outcome results were statistically significant or not. Attrition rates were extracted from the included trials at the end of the intervention and any further follow-ups if available. Overall trial attrition was derived by summing attrition rates from the intervention and control groups. This was an intention-to-treat meta-analysis that included all randomized individuals. Attrition was defined as individuals dropping out of the trial or lost to follow-up at any time-point, thus not completing the study. Transition to psychosis was not considered attrition.
The Cochrane risk-of-bias attrition domain was completed to assess differential attrition between treatment and control arms in the included RCTs.
2.4. Statistical Analysis
To compare attrition rates between intervention and control groups, odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. DerSimonian and Laird random-effects meta-analysis was used to pool these attrition ORs separately for all available treatments and follow-up time-points (DerSimonian and Laird, 1986). Based on these analyses, if attrition rates did not differ between intervention and control groups or different time-points, overall attrition rates were explored to determine if other factors influenced attrition. Cochran Q and I2 statistics were produced to examine heterogeneity; I2≥75% was deemed high heterogeneity (Higgins, Thompson, Deeks, & Altman, 2003).
To assess overall RCT attrition rates (combining intervention and control groups), the follow-up time-point closest to intervention completion for each RCT and proportions were calculated. A sensitivity analysis was completed on trials that had at least one follow-up assessment after intervention completion. The longest follow-up time-point was chosen for this sensitivity analysis to enable the inclusion of all available data. Random-effects proportion meta-analysis (metaprop) incorporating Freeman-Tukey double arcsine transformation (Freeman and Tukey, 1950) to stabilize the variances was used to pool the trial proportions. Further, univariate meta-regression was used to explore sources of differential attrition. Subgroup proportions of factors potentially associated with differential attrition were calculated and included: study location, type of intervention (medication or psychosocial treatment), number of sessions (for psychosocial treatment trials), type of control intervention (active or placebo/treatment as usual), duration of treatment intervention period (<24 or ≥24 weeks), treatment naïve (no, yes) RCT follow-up duration (<24 or ≥24 weeks), number of follow-up evaluations (1 or 2+), sample size (median <30 or ≥30), CHR criteria used in trial (Comprehensive assessment of the at-risk mental state [CAARMS], Early initial prodromal state [EIPS], international code of disease-10 [ICD-10], Positive and negative syndrome scale [PANSS] and Structured Interview of Psychosis-risk Syndromes [SIPS]), baseline attenuated psychotic symptom (APS) score and negative symptom score for intervention and control arms, statistically significant primary outcome results (no, yes), percent transition, reported a CONSORT diagram (no, yes), reported reasons for attrition (no, yes), Cochrane risk of bias – attrition domain (high, low), missing data methods (no, yes) and year of publication.
To assess publication bias we visually appraised a funnel plot for asymmetry and used Begg and Egger tests (Begg and Mazumdar, 1994; Egger, Smith, Schneider, & Minder, 1997). All data was analyzed using Stata (version13, StataCorp LP, College Station, Texas, United States) and results were deemed to be statistically significant with p<0.05.
3. RESULTS
3.1. Literature Search
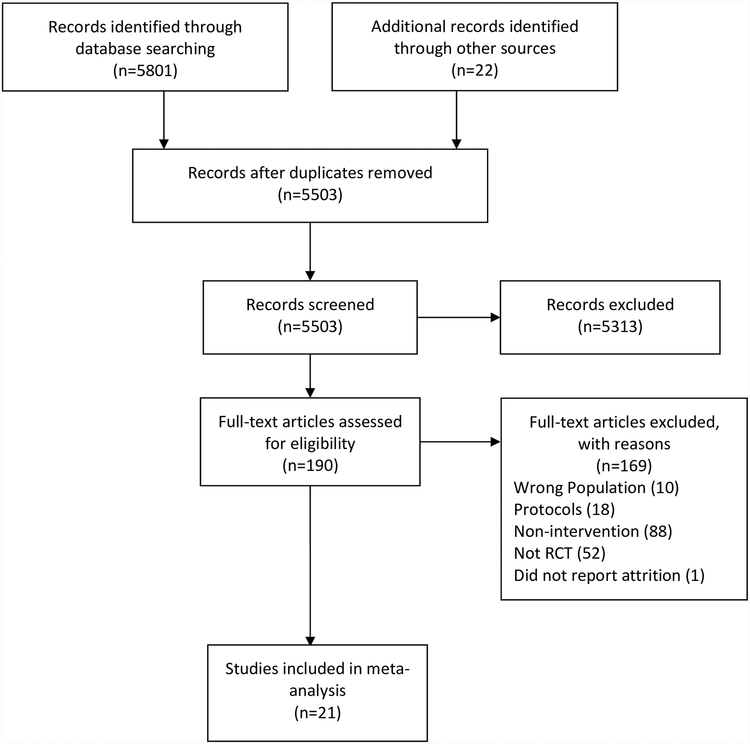
We identified 5,823 records in the initial database search and SCOPUS update (Figure 1). After removing duplicates, 5,503 studies remained, of which 190 were eligible for full-text screening. Twenty-one RCTs qualified for inclusion in this review (Addington et al., 2011; Albert et al., 2016; Amminger et al., 2010; Bechdolf et al., 2012; Cadenhead et al., 2017; Choi et al., 2016; Kantrowitz et al., 2015; Loewy et al., 2016; McGlashan et al., 2006; McGorry et al., 2017; McGorry et al., 2013; Miklowitz et al., 2014; Morrison et al., 2012; Morrison et al., 2004; Nordentoft et al., 2006; Piskulic, Barbato, Liu, & Addington, 2015; Piskulic, Romanowska, & Addington, 2018; Ruhrmann et al., 2007; Stain et al., 2016; van der Gaag et al., 2012; Woods et al., 2017).
Figure 1.
Flowchart of systematic literature review to identify included studies
3.2. Trial Characteristics
Characteristics of included RCTs (n=21) are provided in Table 1. In total, there were 2,260 CHR participants with a mean age of 20.0 years and 55% male. Cognitive behavioural therapy (CBT) was the most common treatment (n=5), followed by cognitive remediation therapy (CRT) (n=4), interpersonal therapies (IPT) (n=3), omega-3 (n=3), amisulpride (n=1), family therapy (n=1), N-Methyl-D-Aspartic acid receptor (NMDAR) regulators (n=1), olanzapine (n=1), risperidone plus CBT (n=1) and ziprasidone (n=1). Thirteen trials reported reasons for attrition, while 12 RCTs reported methods for handling missing data. Intervention group attrition ranged from 7–63%, while control group attrition rates ranged from 3–54%, respectively.
Table 1.
Characteristics of included RCTs (n=21)
| Author, year | Country | Intervention | Control | Treatment Duration (weeks) | CHR criterion | CHR Patients | Missing Data Methods | Reported Reasons for Attrition | End of Treatment Attrition % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age M±SD | Male N (%) | Inter-vention | Control | ||||||||
| Amisulpride | ||||||||||||
| Ruhrmann, 2007 | Germany | Amisulpride: Mean dose 188.7 mg/day + NFI | NFI | 12 | EIPS | 124 | 25.6±6.3 | 70 (57) | LOCF | Yes | 29% | 46% |
| Cognitive Behavioral Therapy (CBT) | ||||||||||||
| Addington, 2011 | Canada | CBT | Supportive therapy | 24 | SIPS | 51 | CBT: 20.8±4.5 Supportive: 21.1±3.7 |
36 (71) | GLM | Yes | 30% | 33% |
| Van der Gaag, 2012 | Nether-lands | CBT + TAU | TAU | 24 | CAARMS | 196 | CBT: 22.7±5.6 TAU: 22.6±5.4 |
97 (49) | LOCF, MI | Yes | 14% | 13% |
| Morrison, 2004 | United Kingdom | CBT | Monitoring | 24 | PANSS | 58 | 22±4.5 | 40 (69) | NR | Yes | 30% | 30% |
| Morrison, 2012 | United Kingdom | CBT + monitoring | Monitoring | 24 | CAARMS | 288 | 20.7±4.3 | 180 (63) | GLM | Yes | 33% | 31% |
| Stain, 2016 | Australia, New Zealand | CBT + TAU | NDRL | 24 | CAARMS | 57 | CBT: 16.2±2.7 NDRL: 16.5±3.2 |
23 (40) | NR | No | 43% | 37% |
| Cognitive Remediation Therapy (CRT) | ||||||||||||
| Choi, 2016 | USA | CR | Tablet games | 8 | SIPS | 62 | CR: 18.2±3.8 Control: 18.5±3.7 |
30 (49) | NR | No | 10% | 3% |
| Loewy, 2016 | USA | CR | Computer games | 8 | SIPS | 83 | CR: 17.8±3.1 Control: 18.7±4.6 |
42 (51) | GLM | No | 38% | 48% |
| Piskulic, 2015 | Canada | CR | Computer games | 12 | SIPS | 32 | CR: 19.7±5.7 Control: 17.5±3.5 |
21 (66) | GLM | Yes | 52% | 40% |
| Piskulic, 2017 | Canada | CR + Motivational Interviewing | CR | 10 | SIPS | 12 | 19.5±NR | 5 (42) | NR | No | 29% | 20% |
| Family Therapy | ||||||||||||
| Miklowitz, 2014 | Canada, USA | FFT | Enhanced care | 24 | SIPS | 129 | 17.4±4.1 | 74 (57) | NR | No | 17% | 25% |
| Interpersonal therapies (IPT) | ||||||||||||
| Albert, 2016 | Denmark | IPT | TAU | 104 | ICD-10 | 83 | 26.6±4.4 | 38 (46) | MI | Yes | 23% | 34% |
| Bechdolf, 2012 | Germany | IPT | Supportive counselling | 52 | EIPS | 128 | IPT: 25.2±5.4 Supportive: 26.8±6.2 |
81 (63) | NR | Yes | 19% | 12% |
| Nordentoft, 2006 | Denmark | IPT | TAU | 104 | ICD-10 | 79 | 24.9±4.9 | 53 (67) | NR | No | 14% | 22% |
| N-Methyl-D-Aspartic acid receptor (NMDAR) | ||||||||||||
| Kantrowitz, 2016 | USA | D-serine: 60 mg/kg | Placebo | 16 | SIPS | 35 | D-serine: 20±4.9 placebo: 19±3.5 |
23 (65) | GLM | Yes | 55% | 54% |
| Olanzapine | ||||||||||||
| McGlashan, 2006 | Canada, USA | Olanzapine: 5–15 mg/day | Placebo | 52 | SIPS | 60 | Olanzapine:18.2±5.5 Placebo: 17.2±4.0 |
39 (65) | LOCF | Yes | 55% | 35% |
| Omega-3 | ||||||||||||
| Amminger, 2010 | Austria | Omega-3 PUFA: 1.2 g/day | Placebo | 12 | PANSS | 81 | Omega: 16.8±2.4 Placebo: 16.0±1.7 |
27 (33) | LOCF | Yes | 7% | 5% |
| Cadenhead, 2017* | Canada, USA | Omega-3:740 mg EPA, 400 mg DHA/day | Placebo | 24 | SIPS | 127 | 18.8±NR | 71 (56) | GLM | No | 45% | 39% |
| McGorry, 2017 | Multi-national | Omega-3 ω−3 PUFA: 1.4 g/day + CBCM | Placebo + CBCM | 24 | CAARMS | 304 | Omega: 19.4±4.8 Placebo: 18.9±4.3 |
139 (46) | GLM | Yes | 25% | 26% |
| Risperidone + CBT | ||||||||||||
| McGorry, 2013 | Australia | Risperidone: 0.5–2 mg/day + CBT or CBT + placebo | Supportive therapy + placebo | 52 | CAARMS | 115 | 18.1±3.0 | 45 (39) | NR | Yes | Risperidone + CBT: 30%; CBT + placebo 25% | Supportive therapy + placebo: 21% |
| Ziprasidone | ||||||||||||
| Woods, 2017* | USA | Ziprasidone: 20–160 mg/d | Placebo | 24 | SIPS | 51 | 22.3±4.2 | 32(64) | NR | No | 63% | 52% |
Abbreviations: CAARMS=Comprehensive assessment of at-risk mental states; CBCM=Cognitive-behavioral case management; CBT=cognitive behavioral therapy; CRT=cognitive remediation therapy; DHA=docosahexaenoic acid; EIPS=Early initial prodromal state; EPA=eicosapentaenoic acid; FFT=Family focused therapy; GLM=generalized linear model; ICD=International code of disease; IPT=Interpersonal therapies; MI=multiple imputation; NDRL=Non Directive Reflective Listening; NFI=needs focused intervention; NR=not reported; PANSS=Positive and negative syndrome scale; PUFA =polyunsaturated fatty acid; RCT=Randomized controlled trial; SIPS=Structured Interview of Psychosis-risk Syndromes; TAU=treatment as usual; USA=United States of America
Abstract only.
3.3. Primary Attrition Results Comparing Intervention and Control Groups
The pooled odds of attrition between CBT and control groups at 6-, 12- and 24-months were not statistically significantly different (6-months: OR=1.06, 95% CI=0.76–1.49; n=5; 12-months: OR=1.02, 95% CI=0.74–1.42, n=5; 24-months: OR=0.87, 95% CI=0.62–1.22, n=3) nor was there statistically significant heterogeneity between time-points (p=0.99). Other treatment versus control comparisons, while limited to a sample size of only 2–3 trials, were not statistically significantly different between groups or follow-up time-points (Table 2). Although, omega-3 odds of attrition at 12-months were 1.53 times higher than controls with a trend towards significant heterogeneity (p=0.07).
Table 2.
Intervention versus control attrition rates at the end of the trial intervention
| Treatment | No. of trials | Pooled attrition OR (95% CI) | Time-point specific heterogeneity | Overall heterogeneity P-value |
|---|---|---|---|---|
| CBT | ||||
| 6-months | 5 | 1.06 (0.76–1.49) | 0.0%; p=0.99 | 0.99 |
| 12-months | 5 | 1.02 (0.74–1.42) | 0.0%; p=0.77 | |
| 24-months | 3 | 0.87 (0.62–1.22) | 0.0%; p=0.98 | |
| CRT | ||||
| 2-months | 3 | 0.85 (0.39–4.89) | 0.0%; p=0.38 | - |
| Interpersonal therapies | ||||
| 12-months | 2 | 1.06 (0.38–2.98) | 42.6%; p=0.19 | 0.51 |
| 24-months | 3 | 0.77 (0.46–1.29) | 0.0%; p=0.63 | |
| Omega-3 | ||||
| 6-months | 3 | 1.07 (0.71–1.61) | 0.0%; p=0.75 | 0.35 |
| 12-months | 3 | 1.53 (0.68–3.43) | 0.0%; p=0.07 | |
Abbreviations: CBT=cognitive behavioural therapy; CI=confidence interval; CRT=cognitive remediation therapy; NMDAR=N-Methyl-D-Aspartic acid receptor; OR=odds ratio
3.4. Overall Trial Attrition
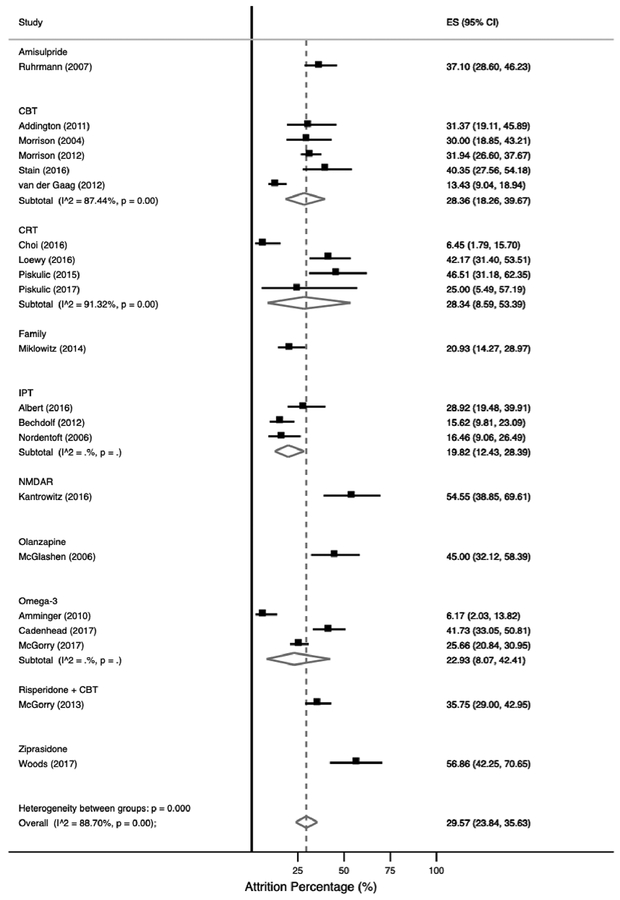
Figure 2 presents the overall pooled trial attrition for each individual trial. The pooled overall trial attrition was 29.57% (95% CI=23.84–35.63%) and there was evidence of high between trial heterogeneity (88.70%; p<0.001). The lowest relative attrition rate reported was 6.17% (95% CI=2.03–13.82) (Amminger, et al., 2010); while the highest relative attrition was 56.86% (95% CI=42.25–70.65%), respectively (Woods, et al., 2017). The majority of omega-3 trials and IPT trials had attrition below the pooled estimate (19.82%, 95% CI=12.43–28.39%, n=3; 22.93%, 95% CI=8.07–42.41%, n=3) compared to interventions such as ziprasidone (56.86%, 95% CI=42.25–70.65%), n=1) olanzapine (45.00%, 95% CI=32.12–58.39%, n=1) and NMDAR (52.27%, 95% CI=36.69–67.54%, n=1), respectively. Further, a sensitivity analysis examining 11 trials with a subsequent follow-up after the treatment intervention was conducted and the pooled attrition was 33.96 (95% CI=24.94–43.59%) (Addington, et al., 2011; Albert, et al., 2016; Amminger, et al., 2010; Bechdolf, et al., 2012; McGlashan, et al., 2006; McGorry, et al., 2017; Morrison, et al., 2012; Nordentoft, et al., 2006; Piskulic, et al., 2015; Stain, et al., 2016; van der Gaag, et al., 2012).
Figure 2. Pooled overall study attrition at the end of treatment intervention in included RCTs.
Abbreviations: CBT=cognitive behavioural therapy; CI=confidence interval; CRT=cognitive remediation; ES=effect estimate; IPT=interpersonal therapies; NMDAR= N-Methyl-D-Aspartic acid receptor.
3.5. Predictors of Differential Attrition
To understand how different trial factors contributed to heterogeneity in the pooled attrition rate among the included trials, subgroup and meta-regression analyses were performed and presented in Table 3. Medication versus psychosocial treatment type, baseline APS and negative symptoms in the intervention and control arms, and percent transitioned were not statistically significant in subgroup or meta-regression analyses. On another note, RCTs resulting in statistically significant primary results (i.e., transition to psychosis) (24.20%, 95% CI=15.90–33.57%) versus trials with null findings (35.80%, 95% CI=30.31–41.48%) was trending towards a statistically significant difference (p=0.06). Additionally, treatment naïve samples had a 10% lower pooled attrition rate (24.40%, 95% CI=17.54–31.98%, n=9) than samples which were not treatment naïve (34.06%, 95% CI=25.29–43.40%, n=12), albeit not statistically significant (p=0.11). Further, attrition was 8–22% higher in trials that used SIPS criteria to characterize CHR (36.14%, 95% CI=25.62–47.35%; n=10) relative to other CHR criteria including CAARMS, EIPS, ICD-10 and PANSS; although not statistically significantly different (p=0.22).
Table 3.
Univariate meta-regression examining overall study attrition (n=21)
| Predictors of attrition | No. trials | Pooled ES (95% CI) | P heterogeneity (within group) | Univariate meta-regression P-value |
|---|---|---|---|---|
| Trial location | ||||
| Australia | 2 | 36.74% (30.82–42.86%) | - | 0.22 |
| Europe | 8 | 21.55% (14.22–29.88%) | 89.0% | |
| Multinational | 1 | 25.66% (20.84–30.95%) | - | |
| North America | 10 | 36.14% (25.62–47.35%) | 87.5% | |
| Type of treatment | ||||
| Medication | 8 | 36.10% (25.91–46.95%) | 91.0% | 0.11 |
| Psychosocial treatment | 13 | 25.52% (19.15–32.43%) | 85.1% | |
| Number of sessions (for psychosocial treatment trials) | 9 | - | - | 0.95 |
| Type of control | ||||
| Active | 7 | 23.67% (15.01–33.52%) | 86.4% | 0.34 |
| Placebo/treatment as usual | 14 | 32.58% (25.09–40.53%) | 90.0% | |
| Duration of treatment | ||||
| <24 weeks | 7 | 28.93% (14.13–46.33%) | 92.6% | 0.86 |
| ≥24 weeks | 14 | 29.79% (23.97–35.94%) | 86.4% | |
| Treatment naïve | ||||
| No | 12 | 34.06% (25.29–43.40%) | 87.3% | 0.11 |
| Yes | 9 | 24.40% (17.54–31.98%) | 88.9% | |
| Follow-up duration | ||||
| <24 weeks | 14 | 30.42% (23.34–37.98%) | 87.4% | 0.51 |
| ≥24 weeks | 7 | 28.08% (18.23–39.09%) | 91.1% | |
| Number of follow-ups | ||||
| 1 | 8 | 34.48% (27.43–41.89%) | 73.7% | 0.22 |
| 2+ | 13 | 26.85% (19.42–34.97%) | 91.0% | |
| CHR criteria | ||||
| CAARMS | 5 | 28.37% (19.99–37.57%) | 94.9% | 0.22 |
| EIPS | 2 | 25.45% (20.23–31.05%) | - | |
| ICD-10 | 2 | 22.54% (16.36–29.37%) | - | |
| PANSS | 2 | 14.57% (9.11–20.99%) | - | |
| SIPS | 10 | 36.14% (25.62–47.35%) | 87.8% | |
| Baseline APS score | 21 | - | - | 0.82 |
| Baseline negative score | 21 | - | - | 0.62 |
| Statistically significant primary outcome results | ||||
| No | 10 | 35.80% (30.31–41.48%) | 70.2% | 0.06 |
| Yes | 11 | 24.20% (15.90–33.57%) | 91.0% | |
| Percent transition | 17 | - | - | 0.19 |
| Reported a CONSORT diagram | ||||
| No | 3 | 32.44% (7.82–63.78%) | - | 0.48 |
| Yes | 18 | 29.02% (23.42–34.94%) | 86.6% | |
| Reported reasons for attrition | ||||
| No | 8 | 30.01% (18.64–42.70%) | 89.8% | 0.71 |
| Yes | 13 | 29.27% (22.63–36.36%) | 88.7% | |
| Cochrane risk of bias – attrition | ||||
| High | 10 | 32.44% (23.48–42.08%) | 87.1% | 0.56 |
| Low | 11 | 27.28% (19.69–35.58%) | 90.5% | |
| Missing data methods | ||||
| No | 9 | 26.09% (18.44–34.53%) | 88.7% | 0.35 |
| Yes | 12 | 32.45% (24.01–41.48%) | 89.5% | |
| Year of publication | 21 | - | - | 0.70 |
Abbreviations: APS=attenuated psychotic symptoms; CAARMS=Comprehensive assessment of the at-risk mental state; CI=confidence interval; EIPS=Early initial prodromal state; ES=effect estimate; ICD=international code of disease; PANSS=Positive and negative syndrome scale; SIPS=Structured Interview of Psychosis-risk Syndromes.
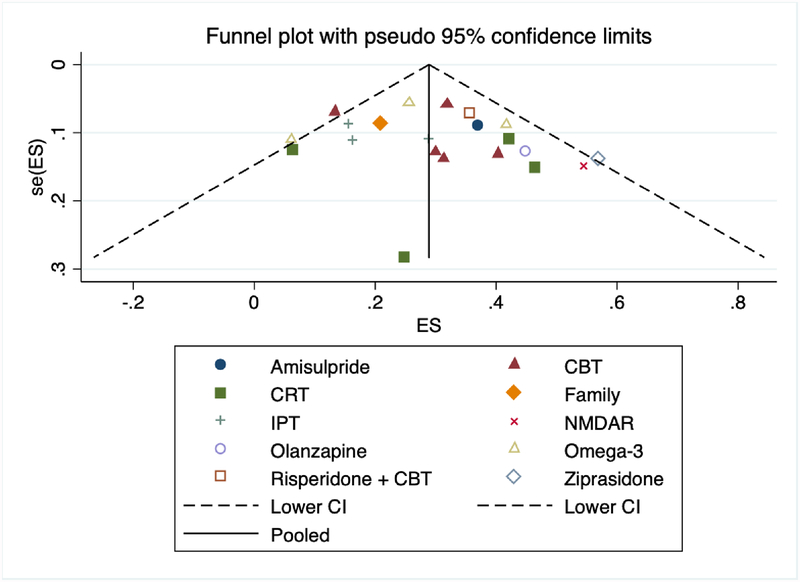
3.6. Publication Bias
A visual representation of funnel plots revealed a fairly even distribution of effect estimates (Figure 3). Further, both the Begg and Egger tests supported no publication bias (p=0.23) and no small-study effects (p=0.16).
Figure 3. Publication bias funnel plot.
Abbreviations: CBT=cognitive behavioral therapy; CI=confidence interval; CRT=cognitive remediation; ES=effect estimate; IPT=interpersonal therapies; NMDAR=N-Methyl-D-Aspartic acid receptor; SE=standard error.
3.7. Risk of Bias Assessment
A majority of the RCTs had a low risk-of-bias for the attrition domain (n=11), while 10 RCTs had high risk-of-bias.
4. DISCUSSION
This systematic literature review and meta-analysis found that the odds of attrition in CHR for psychosis RCTs between intervention and control groups were not statistically significantly different. This lack of a difference was consistent when stratified by treatment type and follow-up time-point. However, overall the end of trial attrition rate in RCTs included in this review reached a pooled estimate of almost 30%, increasing to 34% at later follow-ups. Consequently, almost one third of CHR participants are dropping out and/or are lost to follow-up. Further, while the pooled attrition estimate amongst the included trials was deemed to have high heterogeneity, when examining different clinical and trial characteristics to explain the heterogeneity, none of the subgroups were statistically significantly different from one another.
Attrition rates between any treatment and control group comparisons were not different amongst any of the treatment types identified or at different follow-up time-points. However, meta-analyses examining individuals with severe mental illness diagnoses (e.g., schizophrenia) found there to be substantial differences, which were ultimately attributed to placebo-based control regimens (Kemmler, et al., 2005; Stroup, 2006). A potential reason for this difference between our review and other reviews citing higher attrition rates in placebo-based versus active control group trials is that CHR for psychosis individuals are putatively prodromal and are not experiencing full-blown psychotic symptoms. Whereas individuals with serious mental illness, may be actively experiencing deterioration and increased severity of symptoms. Therefore, these individuals may be more reluctant to continue to participate in research when not experiencing improvements in care. Another reason for this difference may be that almost all placebo-based trials in our review had a needs-based intervention of some kind within the control arm, thus not a true placebo control arm.
When identifying attrition by combining all participants in the included RCTs to present overall attrition rates, we report a pooled attrition rate of almost 30% and individual trial arm attrition rates ranging from 3–63%. Our review was comparable to other published reviews of youth and young adults. For example, in two reviews (Rice et al., 2014; Valimaki, Anttila, Anttila, & Lahti, 2017) both of which focused on web-based and social networking interventions for adolescent depression, attrition rates ranging from 0–61% were reported. Further, a review specifically examining treatment dropout in child and adolescent outpatient mental health care interventions reported a mean dropout of 28.4% (range was 16–50%) (de Haan, Boon, de Jong, Hoeve, & Vermeiren, 2013). Although, this review did not focus on a specific disorder, dropouts were reported to be lower in effectiveness trials, potentially due to the strict inclusion criteria employed in such trials. Furthermore, Haan et al., provided evidence that lower perceived relevance of the treatment led to more dropouts. As a result, participants may be less motivated to complete the treatment. In contrast, CHR intervention trials to date are not typically designed with treatment specific needs-based selection criteria nor are the treatments tailored to the individual’s main mental health concerns (e.g., primary outcomes are usually transition to psychosis, while individuals may be more concerned with anxiety or social functioning) (Addington, Devoe, & Santesteban-Echarri, 2019), and therefore, CHR trials may be more vulnerable to attrition.
Other reviews not focused on youth or young adults also reported similar attrition rates. For example, a meta-analysis evaluating antipsychotic drug trials observed an overall pooled attrition rate of 33% (Wahlbeck, Tuunainen, Ahokas, & Leucht, 2001). A review examining CBT interventions amongst a range of mental health disorders presented a pooled attrition rate of 26% during treatment, only 4% lower than the attrition rates reported in this review (Fernandez, Salem, Swift, & Ramtahal, 2015). However, another review focusing on generalized anxiety disorder reported a pooled attrition rate of 17% (Gersh et al., 2017). Altogether in systematic reviews examining attrition in serious mental illness individuals, and particularly for the CHR for psychosis literature, attrition rates exceed 20%, and therefore treatment effects may be biased.
Our meta-analysis did not find any statistically significant factors related to differential attrition. However, a trend towards a significant difference was observed between trials reporting statistically significant primary outcome results versus those without significant outcome results. Trials with null primary outcome findings reported a higher pooled attrition rate. One explanation may be due to the nocebo effect, that is negative expectations of an intervention that cause the individual to experience negative outcomes. It may have been the case that participants in the trials with null primary outcome results were not experiencing changes in symptoms, or their expected benefits of the intervention, which substantially could lead to negative experiences like worsening of symptoms, functioning or other outcome measures. As a result, they were more likely to discontinue trial participation. This would in turn affect the significance of the trial results and attrition rates. A further explanation is that the trials with null primary outcome results had a numerically higher average length of treatment relative to trials with statistically significant primary outcome results, ultimately giving participants more opportunity to drop out of the trial with a longer intervention period. However, since this finding was not observed to be statistically significant, these explanations should be interpreted with caution.
Several trials discussed attrition and half compared completers to non-completers. All five CBT trials discussed attrition (Addington, et al., 2011; Morrison, et al., 2012; Morrison, et al., 2004; Stain, et al., 2016; van der Gaag, et al., 2012), Stain et al., reported high attrition rates aligned with previous literature, Addington et al., suggested that several individuals may have left the study because they made some improvement, or the study was too time consuming. Morrison et al. 2004, discussed the highly mobile nature of the CHR population to explain high attrition. In van der Gaag et al., several individuals were lost due to time consuming travel; and when examining differences between in participants, only age was significant in relation to attrition rates.
Of the CRT trials, three of the four trials reported high attrition rates (>25%) (Loewy, et al., 2016; Piskulic, et al., 2015; Piskulic, et al., 2018), largely due to dis-interest in the training programs. Choi et al., learned from the previous CRT trials and specifically designed their training program to promote sustained engagement through a neurofeedback mechanism and using game learning contexts that might engage young people; thus reducing the attrition rates drastically (Choi, et al., 2016). Interestingly, Nordentoft et al., reporting on an IPT trial, reported that attrition was statistically significantly more frequent among individuals who used cannabis at least monthly at trial entry compared to those who reported no use or less frequent use (Nordentoft, et al., 2006). Whereas, Albert et al., another IPT trial, did not observe the same attrition trend (Albert, et al., 2016). Altogether, lack of engagement and/or cannabis use could also potentially be reasons for attrition in other studies but was neither reported or recorded.
In the antipsychotic trials, attrition was generally higher in the antipsychotic groups potentially due to side effects (e.g., weight gain in the olanzapine trial) (McGlashan, et al., 2006; McGorry, et al., 2013). However, in Ruhrmann et al. 2007, early attrition in the control group (needs focused intervention) was notably higher than the amisulpride treatment arm, reporting that psychosocial treatment alone may not meet individual’s mental health needs, relative to combination interventions such as CBT plus risperidone (Ruhrmann, et al., 2007). With the diversity of interventions and the limited number of interventions in the CHR context, consistent trends and patterns explaining reasons for attrition are sparse.
This study has a number of strengths, first, to the best of our knowledge, this is the first systematic review and meta-analysis to examine the intervention, control and trial attrition rates in RCTs involving CHR for psychosis samples. In addition, we followed a rigorous process and utilized several online databases to ensure a comprehensive search of the literature was conducted. There are, however, several limitations. First, attrition rates varied greatly among the included trials. The variation in attrition rates represent the potential for very low attrition rates as achieved by some trials in this review, while others with higher attrition (i.e., >20%) may have a high risk of bias. When examining different trial characteristics that may have been attributable to this variation, we did not find any statistically significant trends. The subgroup and meta-regression analyses may have been underpowered and thus, these results are possibly inconclusive at this point in time. However, similar to a recent publication (Stowkowy et al., 2018) exploring attrition in a large CHR for psychosis prospective cohort study, no trends were observed, therefore, firm conclusions about predictors of attrition cannot be drawn from our results nor can they be ruled out. This literature is still in its infancy as the earliest RCT included in this review was published in 2004 (Morrison, et al., 2004) and publications in this area are becoming more common as early intervention is gaining attention and importance for combating mental health issues.
Only seven RCTs in this review used advanced methods, such as multiple imputation to account for missing data. Future research initiatives should consider including these statistical methods to mitigate risk of bias (Leon et al., 2006). Further, while there were various unique intervention comparisons found in this review, in some cases, only one trial represented each treatment comparison. Since CHR individuals are difficult to recruit, attrition rates are high and clinical outcomes, such as transition to psychosis, are uncommon, we suggest that future trials factor in a 30% attrition rate. This may impact resources and budget allocated to future interventions, however, it will better ensure the statistical power to detect statistically significant associations where present. In addition, due to the random nature of attrition in CHR samples, as there were no statistically significant differences in attrition amongst the included trials, improvement in attrition rates may be difficult to achieve. Finally, while approximately half of the RCTs listed reasons for attrition and/or compared completers versus non-completers, some insights as to why attrition rates are high were surfaced. Future interventions specifically tailoring interventions to improve participant engagement as shown in Choi et al. 2016, are promising.
In conclusion, almost one third of CHR participants, on average, will not complete a trial. This represents a significant concern for internal and external validity of these RCTs results. The lack of understanding of CHR attrition rates interferes with improving participant engagement and ultimately, needs to be addressed in future trials. Further, understanding the motivations for CHR individuals to continue to participate in research is important moving forward in this field. Finally, maximizing engagement throughout the trial and subsequently attempting to account for missing data using statistical methods will help to improve the confidence in trial results.
Acknowledgements:
This work was supported by NIH grant RO1MH105178 awarded to Dr. Jean Addington and by the Alberta Innovates Graduate Studentship awarded to Dan Devoe.
Data sharing:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
5. REFERENCES
- Addington J, Devoe D, & Santesteban-Echarri O (2019). Multidisciplinary Treatment for Individuals at Clinical High Risk of Developing Psychosis. Current Treatment Options in Psychiatry, 6(1), pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Epstein I, Liu L, French P, Boydell K, & Zipursky R (2011). A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia Research, 125(1), pp. 54–61. [DOI] [PubMed] [Google Scholar]
- Albert N, Glenthøj L, Melau M, Jensen H, Hjorthøj C, & Nordentoft M (2016). Course of illness in a sample of patients diagnosed with a schizotypal disorder and treated in a specialized early intervention setting. Findings from the 3.5 year follow-up of the OPUS II study. Schizophrenia Research, 182, pp. 24–30. doi: 10.1016/j.schres.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Amminger G, Schafer M, Papageorgiou K, Klier C, Cotton S, Harrigan S, … Berger G (2010). Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of General Psychiatry, 67(2), pp. 146–154. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Wagner M, Ruhrmann S, Harrigan S, Putzfeld V, Pukrop R, … Klosterkotter J (2012). Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry, 200(1), pp. 22–29. doi: 10.1192/bjp.bp.109.066357 [DOI] [PubMed] [Google Scholar]
- Begg C, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, pp. 1088–1101. [PubMed] [Google Scholar]
- Berlim M, Van den Eynde F, Tovar-Perdomo S, & Daskalakis Z (2014). Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychological medicine, 44(2), pp. 225–239. [DOI] [PubMed] [Google Scholar]
- Cadenhead K, Addington J, Cannon T, Cornblatt B, Mathalon D, McGlashan T, … Woods S (2017). 23. Omega-3 Fatty Acid Versus Placebo in a Clinical High-Risk Sample From the North American Prodrome Longitudinal Studies (NAPLS) Consortium. Schizophrenia Bulletin, 43(suppl_1), pp. S16–S16. doi: 10.1093/schbul/sbx021.042 [DOI] [Google Scholar]
- Choi J, Corcoran C, Fiszdon J, Stevens M, Javitt D, Deasy M, … Pearlson G (2016). Pupillometer-Based Neurofeedback Cognitive Training to Improve Processing Speed and Social Functioning in Individuals at Clinical High Risk for Psychosis. Psychiatric Rehabilitation Journal, p No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutzen R, Viechtbauer W, Spigt M, & Kotz D (2015). Differential attrition in health behaviour change trials: a systematic review and meta-analysis. Psychology & health, 30(1), pp. 122–134. [DOI] [PubMed] [Google Scholar]
- de Haan AM, Boon AE, de Jong JT, Hoeve M, & Vermeiren RR (2013). A meta-analytic review on treatment dropout in child and adolescent outpatient mental health care. Clin Psychol Rev, 33(5), pp. 698–711. doi: 10.1016/j.cpr.2013.04.005 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, & Laird N (1986). Meta-analysis in clinical trials. Controlled clinical trials, 7(3), pp. 177–188. [DOI] [PubMed] [Google Scholar]
- Devoe DJ, Farris MS, Townes P, & Addington J (2018a). Attenuated psychotic symptom interventions in youth at risk of psychosis: A systematic review and meta-analysis. Early Interv Psychiatry doi: 10.1111/eip.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe DJ, Farris MS, Townes P, & Addington J (2018b). Interventions and social functioning in youth at risk of psychosis: A systematic review and meta-analysis. Early Interv Psychiatry doi: 10.1111/eip.12689 [DOI] [PubMed] [Google Scholar]
- Devoe DJ, Peterson A, & Addington J (2018). Negative Symptom Interventions in Youth at Risk of Psychosis: A Systematic Review and Network Meta-analysis. Schizophr Bull, 44(4), pp. 807–823. doi: 10.1093/schbul/sbx139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith G, Schneider M, & Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj, 315(7109), pp. 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Salem D, Swift J, & Ramtahal N (2015). Meta-analysis of dropout from cognitive behavioral therapy: Magnitude, timing, and moderators. Journal of Consulting and Clinical Psychology, 83(6), p 1108. [DOI] [PubMed] [Google Scholar]
- Freeman M, & Tukey J (1950). Transformations related to the angular and the square root. The Annals of Mathematical Statistics, pp. 607–611. [Google Scholar]
- Gersh E, Hallford D, Rice S, Kazantzis N, Gersh H, Gersh B, & McCarty C (2017). Systematic review and meta-analysis of dropout rates in individual psychotherapy for generalized anxiety disorder. Journal of anxiety disorders [DOI] [PubMed] [Google Scholar]
- Higgins J, Thompson S, Deeks J, & Altman D (2003). Measuring inconsistency in meta-analyses. BMJ: British Medical Journal, 327(7414), p 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer M, Holzmeister R, Kemmler G, Eder U, Hofer A, Kurzthaler I, … Fleischhacker W (2003). Attitudes of patients with schizophrenia toward placebo-controlled clinical trials. The Journal of clinical psychiatry, 64(3), pp. 277–281. [DOI] [PubMed] [Google Scholar]
- Kantrowitz J, Woods S, Petkova E, Cornblatt B, Corcoran C, Chen H, … Javitt D (2015). D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: A pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. The Lancet Psychiatry, 2(5), pp. 403–412. [DOI] [PubMed] [Google Scholar]
- Kemmler G, Hummer M, Widschwendter C, & Fleischhacker W (2005). Dropout rates in placebo-controlled and active-control clinical trials of antipsychotic drugs: a meta-analysis. Archives of General Psychiatry, 62(12), pp. 1305–1312. [DOI] [PubMed] [Google Scholar]
- Leon A, Mallinckrodt C, Chuang-Stein C, Archibald D, Archer G, & Chartier K (2006). Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry, 59(11), pp. 1001–1005. [DOI] [PubMed] [Google Scholar]
- Loewy R, Fisher M, Schlosser D, Biagianti B, Stuart B, Mathalon D, & Vinogradov S (2016). Intensive Auditory Cognitive Training Improves Verbal Memory in Adolescents and Young Adults at Clinical High Risk for Psychosis. 42(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Zipursky R, Perkins D, Addington J, Miller T, Woods S, … Breier A (2006). Randomized, Double-Blind Trial of Olanzapine Versus Placebo in Patients Prodromally Symptomatic for Psychosis. The American Journal of Psychiatry, 163(5), pp. 790–799. [DOI] [PubMed] [Google Scholar]
- McGorry P, Nelson B, Markulev C, Yuen H, Schafer M, Mossaheb N, … Amminger G (2017). Effect of omega-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: The NEURAPRO randomized clinical trial. 74(1), pp. 19–27. [DOI] [PubMed] [Google Scholar]
- McGorry P, Nelson B, Phillips L, Yuen H, Francey S, Thampi A, … Yung A (2013). Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. The Journal of clinical psychiatry, 74(4), pp. 349–356. [DOI] [PubMed] [Google Scholar]
- Miklowitz D, O’Brien M, Schlosser D, Addington J, Candan K, Marshall C, … Cannon T (2014). Family-focused treatment for adolescents and young adults at high risk for psychosis: results of a randomized trial. Journal of the american academy of child and adolescent psychiatry, 53(8), pp. 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs G, & Kenward M (2007). Missing data in clinical studies: John Wiley & Sons. [Google Scholar]
- Morrison A, French P, Stewart S, Birchwood M, Fowler D, Gumley A, … Dunn G (2012). Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. 344, p 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A, French P, Walford L, Lewis S, Kilcommons A, Green J, … Bentall R (2004). Cognitive therapy for the prevention of psychosis in people at ultra-high risk: Randomised controlled trial. The British Journal of Psychiatry, 185(4), pp. 291–297. [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Thorup A, Petersen L, Ohlenschlaeger J, Melau M, Christensen T, … Jeppesen P (2006). Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the OPUS trial. A randomized clinical trial of integrated treatment and standard treatment. Schizophrenia Research, 83(1), pp. 29–40. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Barbato M, Liu L, & Addington J (2015). Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. 225(1–2), pp. 93–98. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Romanowska S, & Addington J (2018). Pilot study of cognitive remediation and motivational interviewing in youth at risk of serious mental illness. Early Interv Psychiatry, 12(6), pp. 1193–1197. doi: 10.1111/eip.12520 [DOI] [PubMed] [Google Scholar]
- Rice SM, Goodall J, Hetrick SE, Parker AG, Gilbertson T, Amminger GP, … Alvarez-Jimenez M, (2014). Online and social networking interventions for the treatment of depression in young people: a systematic review. J Med Internet Res, 16(9), p e206. doi: 10.2196/jmir.3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L, Warner T, Brody J, Roberts B, Lauriello J, & Lyketsos C (2002). Patient and psychiatrist ratings of hypothetical schizophrenia research protocols: assessment of harm potential and factors influencing participation decisions. American Journal of Psychiatry, 159(4), pp. 573–584. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Bechdolf A, Kuhn KU, Wagner M, Schultze-Lutter F, Janssen B, … Klosterkotter J (2007). Acute effects of treatment for prodromal symptoms for people putatively in a late initial prodromal state of psychosis. British Journal of Psychiatry, 191(SUPPL. 51), pp. s88–s95. [DOI] [PubMed] [Google Scholar]
- Stain H, Bucci S, Baker A, Carr V, Emsley R, Halpin S, … Startup M (2016). A randomised controlled trial of cognitive behaviour therapy versus non-directive reflective listening for young people at ultra high risk of developing psychosis: The detection and evaluation of psychological therapy (DEPTh) trial. Schizophrenia Research, 176(2–3), pp. 212–219. [DOI] [PubMed] [Google Scholar]
- Stowkowy J, Liu L, Cadenhead KS, Tsuang MT, Cannon TD, Cornblatt BA, … Addington J (2018). Exploration of clinical high-risk dropouts. Schizophr Res, 195, pp. 579–580. doi: 10.1016/j.schres.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup T (2006). higher rates of attrition in antipsychotic treatment arms of placebo controlled trials than in trials with active comparators. Evidence-based mental health, 9(3), p 70. [DOI] [PubMed] [Google Scholar]
- Valimaki M, Anttila K, Anttila M, & Lahti M (2017). Web-Based Interventions Supporting Adolescents and Young People With Depressive Symptoms: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth, 5(12), p e180. doi: 10.2196/mhealth.8624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag M, Nieman DH, Rietdijk J, Dragt S, Ising HK, Klaassen RM, … Linszen DH (2012). Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: a randomized controlled clinical trial. Schizophr Bull, 38(6), pp. 1180–1188. doi: 10.1093/schbul/sbs105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlbeck K, Tuunainen A, Ahokas A, & Leucht S (2001). Dropout rates in randomised antipsychotic drug trials. Psychopharmacology, 155(3), pp. 230–233. [DOI] [PubMed] [Google Scholar]
- Woods S, Saksa J, Compton M, Daley M, Rajarethinam R, Graham K, … McGlashan T (2017). 112. Effects of Ziprasidone Versus Placebo in Patients at Clinical High Risk for Psychosis. Schizophrenia Bulletin, 43(Suppl 1), pp. S58–S58. doi: 10.1093/schbul/sbx021.150 [DOI] [Google Scholar]