Abstract
Context
Serum uric acid has been linked to risk of type 2 diabetes (T2DM), but debate persists as to whether it plays a causal role. Indeed, it is unclear if changes in uric acid relate to the pathophysiologic determinants of T2DM (insulin resistance, beta-cell dysfunction), as would be expected if causal.
Objective
To evaluate the impact of changes in uric acid over 2 years on changes in insulin sensitivity, beta-cell function, and glycemia in women with and without recent gestational diabetes (GDM), a model of the early natural history of T2DM.
Design/Setting/Participants
At both 1 and 3 years postpartum, 299 women (96 with recent GDM) underwent uric acid measurement and oral glucose tolerance tests that enabled assessment of insulin sensitivity/resistance (Matsuda index, homeostasis model assessment of insulin resistance [HOMA-IR]), beta-cell function (insulin secretion-sensitivity index-2 [ISSI-2], insulinogenic index/HOMA-IR [IGI/HOMA-IR]), and glucose tolerance.
Results
Women with recent GDM had higher serum uric acid than their peers at both 1 year (281 ± 69 vs 262 ± 58 µmol/L, P = 0.01) and 3 years postpartum (271 ± 59 vs 256 ± 55 µmol/L, P = 0.03), coupled with lower insulin sensitivity, poorer beta-cell function, and greater glycemia (all P < 0.05). However, on fully adjusted analyses, neither uric acid at 1 year nor its change from 1 to 3 years was independently associated with any of the following metabolic outcomes at 3 years postpartum: Matsuda index, HOMA-IR, ISSI-2, IGI/HOMA-IR, fasting glucose, 2-hour glucose, or glucose intolerance.
Conclusion
Serum uric acid does not track with changes over time in insulin sensitivity, beta-cell function, or glycemia in women with recent GDM, providing evidence against causality in its association with diabetes.
Keywords: uric acid, insulin resistance, beta-cell dysfunction, type 2 diabetes, gestational diabetes
There is currently considerable interest in the impact of uric acid, a product of purine metabolism, on the development of diabetes and its complications (1,2). Notably, several epidemiological studies have demonstrated graded associations between baseline serum uric acid and subsequent type 2 diabetes (T2DM) (3–5). Meta-analyses have reported a 6% to 17% higher risk of T2DM for every 1 mg/dL increment in serum uric acid (6,7). However, whether uric acid plays a causal role in the development of T2DM remains a matter of contention (8,9). Indeed, uric acid is closely associated with other risk factors for T2DM, particularly adiposity, making it difficult to ascertain whether observed associations are truly independent of residual confounding (8–10). Moreover, 3 recent Mendelian randomization studies have demonstrated that uric acid-associated genetic loci can predict serum uric acid but do not predict diabetes, thereby arguing against a causal role (11–13). Thus, it remains unclear whether uric acid directly contributes to the pathophysiology of T2DM (8–10).
Another way to approach this unresolved question is to ask whether changes in uric acid relate to the pathophysiologic determinants of T2DM, namely beta-cell dysfunction and insulin resistance. In at-risk individuals, the development of T2DM is typically preceded by worsening beta-cell function in the setting of insulin resistance (14). Accordingly, if it were a causal mediator, one would anticipate that serum uric acid and changes therein should track with changes over time in beta-cell function or insulin resistance. However, while cross-sectional data has linked higher uric acid with lower insulin sensitivity (10), there is a relative paucity of human studies evaluating changes in uric acid in relation to these pathophysiologic processes. Reasons for this evidence gap include (1) the fact that most studies have measured uric acid at a single point in time only (ie, at baseline), (2) few have assessed beta-cell function and insulin sensitivity, and (3) even fewer have evaluated changes over time in these factors. Thus, recognizing these limitations, we sought to evaluate the longitudinal relationship over time between uric acid and concurrent changes in insulin sensitivity and beta-cell function in a population at risk for T2DM.
Women with a history of gestational diabetes mellitus (GDM) provide just such a population. Indeed, women with GDM have a 7-fold higher risk of developing T2DM, as compared to their peers (15). This risk is driven by progressive worsening of beta-cell function and insulin resistance over time (16). Moreover, this deterioration of beta-cell function and insulin sensitivity is apparent within the first 3 years after delivery (17,18). Accordingly, the early postpartum years in women with recent GDM provide a model of the early natural history of T2DM with which we can evaluate the impact of changes in uric acid on glucose homeostasis. Thus, our objective in this study was to characterize the relationship between changes in serum uric acid and concurrent changes in insulin sensitivity, beta-cell function, and glycemia between 1 and 3 years postpartum in women with and without recent GDM.
Methods
Study design and participants
The study population consisted of women participating in a prospective observational cohort study that is evaluating the relationship between glucose tolerance in pregnancy and metabolic function in the years after delivery. The protocol for this study has been described in detail previously (17,19). Briefly, women were first recruited at the time of antepartum GDM screening in late second and early third trimester. Participants underwent metabolic characterization at the time of recruitment in pregnancy and then repeated this assessment at 3 months and 1 year postpartum. At the latter visit, they were recruited into this ongoing observational cohort study for biannual serial metabolic characterization. This analysis reports on the association of serum uric acid levels with metabolic function in 299 women who have completed their 3-year postpartum visit. The study protocol has been approved by the Mount Sinai Hospital Research Ethics Board, and all participants provided written informed consent for their participation.
Recruitment and antepartum assessment of glucose tolerance
As per standard clinical practice at our institution, pregnant women are screened for GDM between 24 and 28 weeks’ gestation by a 50-g glucose challenge test (GCT). If their GCT is abnormal (defined as plasma glucose ≥ 7.8 mmol/L at 1 hour after ingestion of 50-g glucose), then the women undergo a diagnostic oral glucose tolerance test (OGTT). In this study, women were recruited either before or after the screening GCT. Regardless of their GCT result, all study participants then underwent a 3-hour, 100-g OGTT for definitive ascertainment of their gestational glucose tolerance status. As previously described (17), the recruitment of women following an abnormal GCT serves to enrich the study population for those with GDM. GDM was defined by the National Diabetes Data Group criteria, which require at least 2 of the following on the OGTT: fasting blood glucose ≥ 5.8 mmol/L, 1-hour glucose ≥ 10.6 mmol/L, 2-hour glucose ≥ 9.2 mmol/L, or 3-hour glucose ≥ 8.1 mmol/L. Women diagnosed with GDM were referred to the specialized diabetes-in-pregnancy clinic at our center for antepartum glycemic management.
Metabolic characterization at 1 and 3 years postpartum
At both 1 year and 3 years postpartum, participants returned to the clinical investigation unit for metabolic characterization following an overnight fast. Each visit included a 2-hour, 75-g OGTT, where venous blood samples were drawn for measurement of glucose and specific insulin at fasting and at 30-, 60- and 120-minutes following ingestion of the glucose load. Specific insulin was measured with the Roche-Elecsys-1010 immunoassay analyzer and electrochemiluminescence immunoassay kit (Roche Diagnostics, Laval, Quebec). Glucose and serum uric acid (at fasting) were assessed by standard clinical biochemistry. On each OGTT, glucose tolerance status was defined as per Diabetes Canada Clinical Practice Guidelines (20). Prediabetes was defined as impaired fasting glucose, impaired glucose tolerance, or combined impaired fasting glucose and impaired glucose tolerance. At 3 years postpartum, estimated glomerular filtration rate (eGFR) was determined with the Modification of Diet in Renal Disease formula.
Insulin sensitivity was assessed with the Matsuda index, a measure of whole-body insulin sensitivity that has been validated against the hyperinsulinemic-euglycemic clamp (21). The homeostasis model assessment of insulin resistance (HOMA-IR) provided a secondary measure, primarily reflecting hepatic insulin resistance (22). Beta-cell function was assessed with the insulin secretion-sensitivity index-2 (ISSI-2), an established measure of beta-cell compensation on the OGTT that has been directly validated against the disposition index from the intravenous glucose tolerance test (23,24). A secondary measure of beta-cell function was provided by the insulinogenic index divided by HOMA-IR (IGI/HOMA-IR) (19).
Statistical analyses
All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). All tests were two-sided and performed at significance level P < 0.05. Continuous variables were tested for normality of distribution. Variables with normal distributions are presented as mean ± standard deviation, and those with skewed distributions are presented as median and interquartile range (25th–75th percentile). Characteristics of the women who had GDM and those who did not have GDM were compared at 1 year and 3 years postpartum by either one-way analysis of variance for continuous variables (if normally distributed) or the Kruskal-Wallis test for continuous variables (if skewed), and either the χ2 or Fisher exact test for categorical variables (Table 1). Mean adjusted levels of serum uric acid at 1 year postpartum and 3 years postpartum were compared between women with and without recent GDM after adjustment for age, ethnicity, family history of diabetes, current body mass index (BMI), duration of breastfeeding in the first year, and current glucose tolerance status (Fig. 1). Spearman correlation analyses were conducted to evaluate the respective associations of (1) baseline uric acid at 1 year postpartum and (2) baseline-adjusted change in uric acid between 1 and 3 years postpartum, with baseline-adjusted changes in metabolic factors (anthropometry, insulin sensitivity/resistance, beta-cell function, glycemia) between 1 and 3 years postpartum (Table 2). Multiple linear regression analyses were performed to determine whether uric acid at 1 year postpartum or the change in uric acid between 1 and 3 years postpartum was an independent predictor of the following metabolic outcomes at 3 years: (1) Matsuda index, (2) HOMA-IR, (3) ISSI-2, (4) insulinogenic index/HOMA-IR, (5) fasting glucose, and (6) 2-hour glucose. Each model included the following covariates: (1) diabetes risk factors (age, ethnicity, family history of diabetes, duration of breastfeeding, BMI at 1 year, change in BMI from 1 to 3 years); (2) the measure of the respective outcome variable at 1 year postpartum; and (3) both uric acid at 1 year and the change in uric acid from 1 to 3 years (Table 3). Inclusion of both baseline BMI and change in BMI was important to account for the known relationship between changes in weight and uric acid. Logistic regression was undertaken to determine whether baseline uric acid and/or the change in uric acid between 1 and 3 years was independently associated with the categorical outcome of prediabetes/diabetes at 3 years postpartum from the pool of potential predictors (age, ethnicity, family history of diabetes, duration of breastfeeding, BMI at 1 year, change in BMI from 1 to 3 years, uric acid at 1 year, change in uric acid from 1 to 3 years, and glucose tolerance status at 1 year)(Table 4). A backward selection model was constructed because when there are a moderate number of variables in the pool, backward selection may be preferable over forward selection insofar as the mean squared error values tend to be more nearly unbiased (because important predictors are retained at each step). A fully adjusted logistic regression model was also constructed as a sensitivity analysis.
Table 1.
Comparison of Characteristics at 1 and 3 Years Postpartum Between Women Who Had GDM and Those Who Did Not Have GDM (Non-GDM)
| Non-GDM | GDM | ||
|---|---|---|---|
| At 1 Year Postpartum | (n = 203) | (n = 96) | P |
| Age (years) | 36 ± 4 | 36 ± 4 | 0.58 |
| Ethnicity: | 0.68 | ||
| White (%) | 72.9 | 68.8 | |
| Asian (%) | 10.3 | 13.5 | |
| Other (%) | 16.8 | 17.7 | |
| Family history of T2DM (%) | 57.6 | 64.6 | 0.25 |
| Months breastfeeding | 9 (4–12) | 10 (4–12) | 0.80 |
| Current smoking (%) | 4.5 | 2.1 | 0.51 |
| BMI (kg/m2) | 24.6 (21.9–29.1) | 25.4 (22.0–29.1) | 0.77 |
| Waist circumference (cm) | 87 ± 12 | 88 ± 13 | 0.31 |
| Matsuda index | 9.9 (5.5–14.2) | 8.0 (4.8–11.3) | 0.04 |
| HOMA-IR | 1.1 (0.7–1.8) | 1.2 (0.7–1.7) | 0.62 |
| ISSI-2 | 802 ± 317 | 672 ± 271 | 0.0009 |
| Insulinogenic index/HOMA-IR | 10.0 (6.1–16.4) | 7.4 (4.5–12.2) | 0.003 |
| OGTT: | |||
| Fasting glucose (mmol/L) | 4.7 ± 0.5 | 4.9 ± 0.5 | 0.005 |
| 2-hour glucose (mmol/L) | 6.0 ± 1.6 | 7.0 ± 1.9 | <0.0001 |
| Current glucose tolerance status: | 0.001 | ||
| Normal (%) | 86.5 | 70.3 | |
| Prediabetes/diabetes (%) | 13.5 | 29.7 | |
| Uric acid (µmol/L) | 262 ± 58 | 281 ± 69 | 0.01 |
| At 3 years postpartum | |||
| Current smoking (%) | 6.6 | 3.1 | 0.28 |
| BMI (kg/m2) | 25.9 ± 4.9 | 26.5 ± 6.0 | 0.34 |
| Waist circumference (cm) | 87 ± 12 | 89 ± 12 | 0.27 |
| Matsuda index | 8.4 (5.3–11.8) | 6.7 (4.2–10.1) | 0.01 |
| Serum creatinine (µmol/l) | 59.8 ± 9.5 | 57.8 ± 9.6 | 0.09 |
| eGFR (ml/min) | 108 ± 22 | 112 ± 22 | 0.09 |
| HOMA-IR | 1.2 (0.8–1.9) | 1.4 (0.8–2.2) | 0.12 |
| ISSI-2 | 860 ± 346 | 685 ± 374 | <0.0001 |
| Insulinogenic index/HOMA-IR | 11.2 (7.2–18.5) | 7.0 (4.7–12.1) | <0.0001 |
| OGTT: | |||
| Fasting glucose (mmol/L) | 4.6 ± 0.5 | 4.9 ± 0.6 | 0.0003 |
| 2-hour glucose (mmol/L) | 6.1 ± 1.7 | 7.3 ± 2.2 | <0.0001 |
| Current glucose tolerance status: | <0.0001 | ||
| Normal (%) | 85.7 | 64.6 | |
| Prediabetes/diabetes (%) | 14.3 | 35.4 | |
| A1c (%) | 5.5 ± 0.3 | 5.6 ± 0.4 | 0.03 |
| Uric acid (µmol/L) | 256 ± 55 | 271 ± 59 | 0.03 |
| Change in uric acid from 1 year (µmol/L) | –6.3 ± 40.8 | –8.6 ± 42.0 | 0.66 |
Continuous variables are shown as mean ± standard deviation (if normally distributed) or median followed by interquartile range in parentheses (if skewed).
Abbreviations: A1c, glycated hemoglobin; BMI, body mass index; eGFR, estimated glomerular filtration rate; GDM, gestational diabetes; HOMA-IR, homeostasis model assessment-insulin resistance; ISSI-2, insulin secretion-sensitivity index-2; OGTT, oral glucose tolerance test; T2DM, type 2 diabetes mellitus.
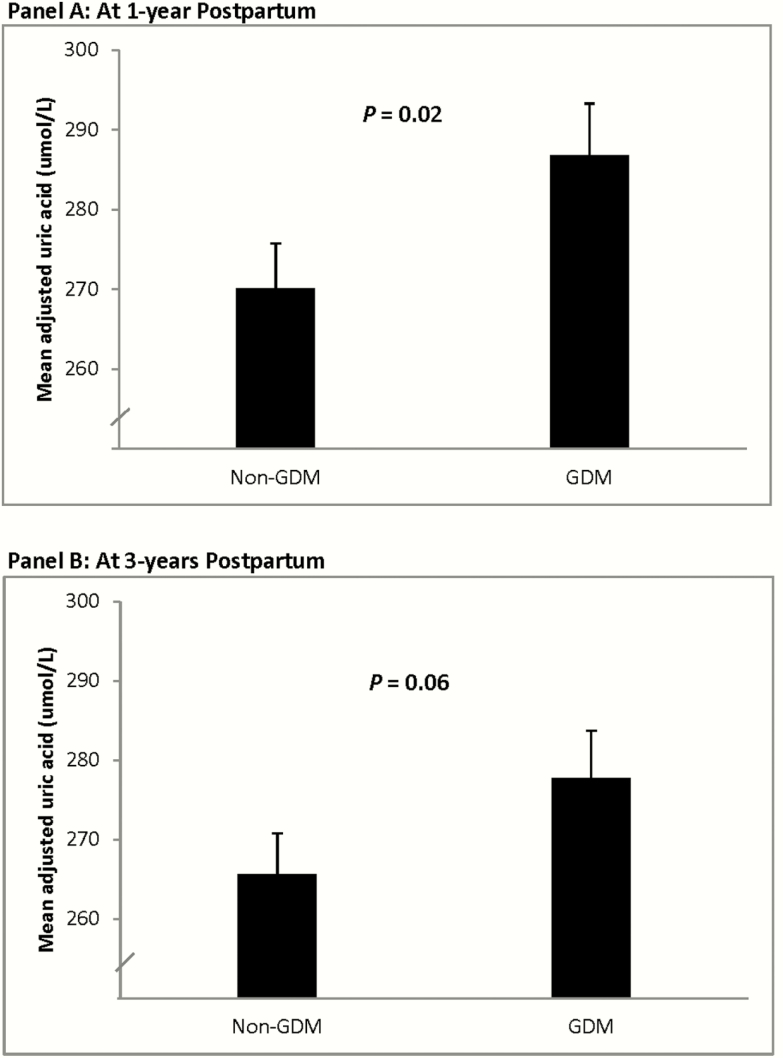
Figure 1.
Comparison of mean adjusted serum uric acid between women with and without recent GDM at (Panel A) 1 year postpartum and (Panel B) 3 years postpartum after adjustment for age, ethnicity, family history of diabetes, current BMI, duration of breastfeeding in first year, and current glucose tolerance. Abbreviation: GDM, gestational diabetes mellitus.
Table 2.
Spearman Correlations of (1) Uric Acid at 1 Year Postpartum and (2) Baseline-Adjusted Change in Uric Acid Between 1 and 3 Years Postpartum, With Baseline-Adjusted Changes in Metabolic Factors Between 1 and 3 Years Postpartum
| Baseline-Adjusted | ||||
| Change in Uric Acid | ||||
| Baseline-Adjusted Changes | Uric Acid at 1 Year | Between 1 and 3 Years | ||
| Between 1 and 3 years in: | r | P | r | P |
| BMI | 0.02 | 0.80 | 0.22 | 0.0003 |
| Waist | 0.14 | 0.02 | 0.17 | 0.005 |
| Matsuda index | –0.18 | 0.003 | –0.17 | 0.004 |
| HOMA-IR | 0.20 | 0.0008 | 0.07 | 0.27 |
| ISSI-2 | –0.15 | 0.01 | 0.02 | 0.78 |
| Insulinogenic index/HOMA-IR | –0.11 | 0.07 | –0.05 | 0.44 |
| Fasting glucose | 0.12 | 0.06 | –0.05 | 0.44 |
| 2-hour blood glucose | 0.13 | 0.02 | 0.06 | 0.32 |
Bold indicates P < 0.05.
Abbreviations: BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance; ISSI-2, insulin secretion-sensitivity index-2.
Table 3.
Serum Uric Acid at 1 Year Postpartum and Change in Uric Acid Between 1 and 3 Years Postpartum as Predictors of the Following Metabolic Outcomes at 3 Years Postpartum: (1) Matsuda Index, (2) HOMA-IR, (3) ISSI-2, (4) Insulinogenic Index/HOMA-IR, (5) Fasting Glucose, and (6) 2-Hour Glucose.
| Outcome at 3 Years | Predictor | Beta | T | P |
|---|---|---|---|---|
| Log Matsuda index | Uric acid at 1 year | –0.001031 | –1.82 | 0.07 |
| Change in uric acid from 1 to 3 years | –0.001190 | –1.57 | 0.12 | |
| Log HOMA-IR | Uric acid at 1 year | 0.000430 | 0.70 | 0.48 |
| Change in uric acid from 1 to 3 years | 0.000160 | 0.20 | 0.84 | |
| ISSI-2 | Uric acid at 1 year | –0.545933 | –1.37 | 0.17 |
| Change in uric acid from 1 to 3 years | –0.266129 | –0.50 | 0.62 | |
| Log IGI/HOMA-IR | Uric acid at 1 year | –0.002011 | –1.83 | 0.07 |
| Change in uric acid from 1 to 3 years | –0.000984 | –0.69 | 0.49 | |
| Fasting glucose | Uric acid at 1 year | –0.000433 | –0.76 | 0.45 |
| Change in uric acid from 1 to 3 years | –0.001083 | –1.43 | 0.15 | |
| 2-hour glucose | Uric acid at 1 year | 0.002975 | 1.45 | 0.15 |
| Change in uric acid from 1 to 3 years | 0.002460 | 0.90 | 0.37 |
Each model was adjusted for age, ethnicity, family history of diabetes, duration of breastfeeding, BMI at 1 year, change in BMI from 1 to 3 years, and the baseline measure of the respective outcome variable at 1 year postpartum.
Abbreviations: BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin sensitivity/resistance; ISSI, insulin secretion-sensitivity index-2.
Table 4.
Backward Selection Logistic Regression Model of Prediabetes/Diabetes at 3 Years Postpartum.
| Variables in Final Model | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Glucose intolerance at 1 year | 8.88 | 4.24 – 18.59 | <0.0001 |
| Change in BMI from 1 to 3 years | 1.29 | 1.04 – 1.60 | 0.019 |
| BMI at 1 year | 1.08 | 1.01 – 1.15 | 0.028 |
| Age | 1.06 | 0.97 – 1.14 | 0.193 |
| Asian ethnicity | 2.02 | 0.76 – 5.40 | 0.160 |
| Non-white, non-Asian ethnicity | 1.44 | 0.59 – 3.52 | 0.429 |
| Family history of diabetes | 1.07 | 0.51 – 2.22 | 0.859 |
Covariates Available for Selection: Age, Ethnicity, Family History of Diabetes, Duration of Breastfeeding, BMI at 1 Year, Change in BMI From 1 to 3 Years, Uric Acid at 1 Year, Change in Uric Acid From 1 to 3 Years, and Glucose Tolerance Status at 1 Year. The following clinical risk factors for diabetes were forced into the model: age, ethnicity, family history of diabetes, and BMI at 1 year.
Abbreviations: BMI, body mass index; CI, confidence interval.
Results
Table 1 shows the demographic, clinical, and metabolic characteristics of the study population stratified into those who did not have GDM (n = 203) and those who did have GDM (n = 96). At 1 year postpartum, the 2 groups did not significantly differ in age, ethnicity, family history of T2DM, BMI, waist circumference, duration of breastfeeding, or current smoking status. As anticipated, women with recent GDM had lower whole-body insulin sensitivity (Matsuda index; P = 0.04), poorer beta-cell function (ISSI-2 P = 0.0009; IGI/HOMA-IR; P = 0.003), and greater glycemia (fasting glucose; P = 0.005, 2-hour glucose; P < 0.0001) than the non-GDM group. In addition, there was a higher prevalence of dysglycemia in the GDM group (29.7% vs 13.5%; P = 0.001), the vast majority of which was prediabetes. Of note, serum uric acid was also higher in the women with GDM compared to the non-GDM group at 1 year postpartum (281 ± 69 vs 262 ± 58 µmol/l; P = 0.01). These uric acid values were generally in the normal range of the laboratory assay (180–360 µmol/l).
At 3 years postpartum, metabolic differences between the groups persisted, with the GDM group displaying significantly lower insulin sensitivity (Matsuda index; P = 0.01), poorer beta-cell function (ISSI-2; P < 0.0001; IGI/HOMA-IR; P < 0.0001), greater glycemia (fasting glucose; P = 0.0003, 2-hour glucose; P < 0.0001), and higher prevalence of dysglycemia (35.4% vs 14.3%; P < 0.0001). Overall, serum uric acid increased between 1 and 3 years in 123 women. However, while serum uric acid remained higher in the GDM group (271 ± 59 vs 256 ± 55 µmol/l; P = 0.03), the change in uric acid concentration from 1 year to 3 years postpartum did not significantly differ between groups (P = 0.66).
We next sought to determine if the observed differences in uric acid levels between the groups were attributable to diabetes risk factors (age, ethnicity, family history of diabetes, BMI, breastfeeding). After adjustment for these risk factors and current glucose tolerance status, mean adjusted uric acid remained higher in the women with recent GDM than in the non-GDM group at 1 year postpartum (P = 0.02; Fig. 1A). Mean adjusted uric acid was also higher in the GDM group at 3 years postpartum, though at borderline significance (P = 0.06; Fig. 1B). With further adjustment for eGFR at 3 years, mean adjusted uric acid was higher in the GDM group than in the non-GDM group at 3 years (282 vs 268 µmol/l; P = 0.03)(data not shown).
Associations of uric acid with insulin sensitivity, beta-cell function, and glycemia
On Spearman correlation analysis (Table 2), uric acid at 1 year postpartum showed modest associations with baseline-adjusted changes between 1 and 3 years postpartum in waist circumference (r = 0.14; P = 0.02), HOMA-IR (r = 0.20; P = 0.0008) and 2-hour blood glucose (r = 0.13; P = 0.02), along with inverse associations with the Matsuda index (r = –0.18; P = 0.003) and ISSI-2 (r = –0.15; P = 0.01). To better elucidate if these may reflect direct associations, we next evaluated the correlations of the baseline-adjusted change in uric acid from 1 to 3 years with the concomitant baseline-adjusted changes in measures of adiposity, insulin sensitivity/resistance, beta-cell function, and glycemia. Baseline-adjusted change in uric acid showed modest associations with the concomitant baseline-adjusted changes in BMI (r = 0.22; P = 0.0003), waist circumference (r = 0.17; P = 0.005), and Matsuda index (r = –0.17; P = 0.004). There were no significant associations with changes in HOMA-IR, beta-cell function, or measures of glycemia.
Finally, we performed multiple linear regression analyses to determine if uric acid at 1 year and/or its change from 1 to 3 years postpartum was independently associated with metabolic outcomes at 3 years after adjustment for diabetes risk factors (age, ethnicity, family history of diabetes, BMI at 1 year, breastfeeding), the change in BMI, and the baseline measure at 1 year of the respective metabolic outcome (Table 3). These models revealed that neither uric acid at 1 year nor the change in uric acid from 1 to 3 years was a significant independent predictor of any of the following outcomes at 3 years postpartum: (1) Matsuda index; (2) HOMA-IR; (3) ISSI-2; (4) IGI/HOMA-IR; (5) fasting glucose; or (6) 2-hour glucose. These findings were unchanged with further adjustment of each model for eGFR at 3 years (data not shown). We also confirmed that neither uric acid at 1 year nor the change in uric acid from 1 to 3 years was a significant independent predictor of glycated hemoglobin at 3 years after adjusting for the same variables as in Table 3 except for baseline glycated hemoglobin (data not shown). Moreover, on backward selection logistic regression analysis (Table 4), neither uric acid measure emerged as a significant independent predictor of prediabetes/diabetes at 3 years postpartum (ie, neither was retained in the model). These findings were unchanged when we constructed the fully adjusted logistic regression model as a sensitivity analysis, with neither baseline uric acid (OR = 1.01; 95% confidence interval [CI], 1.00–1.01; P = 0.15) nor the change in uric acid (OR = 1.01; 95% CI, 1.00–1.02; P = 0.16) emerging as significant predictors (data not shown).
Finally, to test the robustness of these findings, we performed a series of sensitivity analyses in which the multiple linear regression models in Table 3 were repeated under various restrictions. First, we confirmed that neither uric acid at 1 year nor the change in uric acid between 1 and 3 years was a significant independent predictor of metabolic outcomes at 3 years (Matsuda index, HOMA-IR, ISSI-2, IGI/HOMA-IR, fasting glucose, or 2-hour glucose) when restricted to only the women in whom uric acid increased between 1 and 3 years (Table 5). Second, to eliminate any confounding effect of GDM, we confirmed that neither uric acid measure was a significant independent predictor of these outcomes when restricted to only the women (n = 203) who did not have GDM (Table 6). Third, to address the possibility of reverse causality, we confirmed that these findings were unchanged when the models were restricted to only those women who maintained normal glucose tolerance at 3 years (data not shown).
Table 5.
Serum uric acid at 1 Year Postpartum and Change in Uric Acid Between 1 and 3 Years Postpartum as Predictors of the Indicated Metabolic Outcomes at 3 Years Postpartum in Women in Whom Uric Acid Increased Between 1 and 3 Years (n = 123).
| Outcome at 3 Years | Predictor | Beta | T | P |
|---|---|---|---|---|
| Log Matsuda index | Uric acid at 1 year | –0.001492 | –1.79 | 0.08 |
| Change in uric acid from 1 to 3 years | 0.003319 | 1.62 | 0.11 | |
| Log HOMA-IR | Uric acid at 1 year | 0.000716 | 0.77 | 0.44 |
| Change in uric acid from 1 to 3 years | –0.003485 | –1.55 | 0.12 | |
| ISSI-2 | Uric acid at 1 year | –0.500765 | –0.93 | 0.35 |
| Change in uric acid from 1 to 3 years | –1.370566 | –1.03 | 0.30 | |
| Log IGI/HOMA-IR | Uric acid at 1year | –0.001200 | –0.81 | 0.42 |
| Change in uric acid from 1 to 3 years | –0.005490 | –1.57 | 0.12 | |
| Fasting glucose | Uric acid at 1 year | –0.000446 | –0.49 | 0.62 |
| Change in uric acid from 1 to 3 years | –0.001579 | –0.73 | 0.47 | |
| 2-hour glucose | Uric acid at 1 year | 0.005240 | 1.64 | 0.10 |
| Change in uric acid from 1 to 3 years | –0.000211 | –0.03 | 0.98 |
Each model was adjusted for age, ethnicity, family history of diabetes, duration of breastfeeding, BMI at 1 year, change in BMI from 1 to 3 years, and the baseline measure of the respective outcome variable at 1 year postpartum.
Abbreviations: BMI, body mass index; IGI, insulinogenic index; HOMA-IR, homeostasis model assessment-insulin resistance; ISSI-2, insulin secretion-sensitivity index-2.
Table 6.
Serum Uric Acid at 1 Year Postpartum and Change in Uric Acid Between 1 and 3 Years Postpartum as Predictors of the Indicated Metabolic Outcomes at 3 Years Postpartum in Women Who Did Not Have GDM (n = 203).
| Outcome at 3 Years | Predictor | Beta | T | P |
|---|---|---|---|---|
| Log Matsuda index | Uric acid at 1 year | –0.001039 | –1.46 | 0.15 |
| Change in uric acid from 1 to 3 years | –0.001084 | –1.18 | 0.24 | |
| Log HOMA-IR | Uric acid at 1 year | 0.000775 | 1.00 | 0.32 |
| Change in uric acid from 1 to 3 years | 0.000679 | 0.69 | 0.49 | |
| ISSI-2 | Uric acid at 1 year | –0.512043 | –1.02 | 0.31 |
| Change in uric acid from 1 to 3 years | –0.422132 | –0.65 | 0.52 | |
| Log IGI/HOMA-IR | Uric acid at 1 year | –0.001535 | –1.14 | 0.26 |
| Change in uric acid from 1 to 3 years | –0.001920 | –1.13 | 0.26 | |
| Fasting glucose | Uric acid at 1 year | –0.000556 | –0.82 | 0.42 |
| Change in uric acid from 1 to 3 years | –0.000195 | –0.22 | 0.82 | |
| 2-hour glucose | Uric acid at 1 year | 0.001070 | 0.43 | 0.67 |
| Change in uric acid from 1 to 3 years | 0.001168 | 0.37 | 0.71 |
Each model was adjusted for age, ethnicity, family history of diabetes, duration of breastfeeding, BMI at 1 year, change in BMI from 1 to 3 years, and the baseline measure of the respective outcome variable at 1 year postpartum.
Abbreviations: BMI, body mass index; GDM, gestational diabetes mellitus; HOMA-IR, homeostasis model assessment-insulin resistance; IGI, insulinogenic index; ISSI-2, insulin secretion-sensitivity index-2.
Discussion
In this study, we show that serum uric acid is higher in women with recent GDM than in their peers at both 1 year and 3 years postpartum, a window of time during which this patient population is undergoing the early pathophysiologic changes that lead to T2DM. Notably, in this setting, neither uric acid at 1 year nor its change from 1 to 3 years was independently associated with concurrent changes in insulin sensitivity, beta-cell function, or glycemia. Furthermore, neither uric acid measure was a significant predictor of prediabetes/diabetes at 3 years postpartum. Taken together, these findings suggest that uric acid does not track with the early pathophysiologic determinants of T2DM and thus provide evidence against causality in its association with diabetes.
Previous cross-sectional studies have yielded conflicting findings on uric acid concentrations in women with a history of GDM, with some reporting higher levels (25,26) and others reporting no differences compared with women who did not have GDM (27,28). While demonstrating that women with GDM have higher uric acid than their peers at 2 points in time, this study offered the opportunity to address the broader question of the relationship between uric acid and the pathophysiologic processes that lead to T2DM. Indeed, we have previously demonstrated that recent GDM predicts distinct trajectories of declining insulin sensitivity, worsening beta-cell function, and rising glycemia (fasting and 2-hour blood glucose) between 1 and 3 years postpartum in this study population (17). Moreover, in this setting and study population, hepatic markers (such as fetuin A and alanine aminotransferase/aspartate aminotransferase ratio) have been shown to predict changes in insulin sensitivity, beta-cell function, and glycemia (19). Accordingly, we sought to evaluate uric acid with the same model.
With this approach, we found that there was no association between changes in uric acid and changes in insulin sensitivity, beta-cell function, and glycemia between 1 and 3 years postpartum. While residual confounding is always a consideration with observational data, it should be recognized that its potential impact is problematic for inferring causality from a positive association. In contrast, the complete absence of associations between uric acid and changes in insulin sensitivity, beta-cell function, and glycemia during a window in which these factors are known to be changing argues against a causal role.
The current study thus adds to a growing body of evidence suggesting that higher serum uric acid does not directly contribute to the development of diabetes. First, 3 Mendelian randomization studies (which theoretically obviate the potential for residual confounding) have found that genes that predict uric acid levels do not predict risk of diabetes (11–13). Second, a recent study that applied the counterfactual framework in causal mediation analysis found that the association between uric acid and insulin resistance is mediated by adiposity (10). Third, a randomized controlled trial in 74 men found that lowering serum uric acid with allopurinol did not alleviate fructose-induced insulin resistance (29). Against this background, the current study adds an additional line of evidence in the case against uric acid as a factor contributing to the early pathophysiology of T2DM.
These data hold implications for future studies. First, they suggest that therapy that specifically targets the lowering of serum uric acid is unlikely to prevent the development of T2DM in at-risk individuals. Second, while arguing against serum uric acid, these data do not necessarily preclude any involvement of the xanthine oxidase pathway. Indeed, in a recent prospective cohort study, serum xanthine oxidase activity, but not uric acid concentration, was associated with the risk of incident T2DM (30). Similarly, this study cannot rule out the possibility that intracellular uric acid (ie, not reflected in serum levels) may relate to glucose metabolism (8).
A limitation of this study is the use of OGTT-based surrogate indices rather than clamp studies in the assessment of insulin sensitivity and beta-cell function. However, owing to their invasive nature and time requirements, it would be difficult to complete 2 clamp studies 2 years apart in 299 new mothers. Moreover, the Matsuda index, HOMA-IR, ISSI-2, and insulinogenic index/HOMA-IR are validated measures that have been widely used in previous studies (17,19,21-24) and the serial OGTTs on which they were determined also enabled assessment of glucose tolerance status. It is also possible that this sample size did not provide sufficient power to detect associations of uric acid with glucose homeostasis. However, these data showed no evidence of association. In contrast, with this same study population and methodology, the independent associations of hepatic markers with changes in insulin sensitivity, beta-cell function, and glycemia were readily apparent (19).
In conclusion, serum uric acid did not track with changes over time in insulin sensitivity, beta-cell function, or glycemia in women with recent GDM. Moreover, neither baseline uric acid nor its change from 1 to 3 years postpartum was an independent predictor of prediabetes/diabetes at 3 years. In demonstrating that serum uric acid does not track with the early pathophysiologic determinants of T2DM, this study provides a new line of evidence against causality in the ongoing debate on the association of uric acid with diabetes.
Acknowledgments
Financial Support: This study was supported by operating grants from the Canadian Institutes of Health Research (CIHR) (MOP-84206 and PJT-156286) and Diabetes Canada (CDA-OG-3-15-4924-RR). B.Z. is the Stephen and Suzie Pustil Diabetes Research Scientist at Mount Sinai Hospital. R.R. holds the Boehringer Ingelheim Chair in Beta-cell Preservation, Function, and Regeneration at Mount Sinai Hospital, and his research program is supported by the Sun Life Financial Program to Prevent Diabetes in Women.
Author Contributions: R.R., A.J.H., P.W.C., and B.Z. designed and implemented the study. R.R. and C.Y. contributed to the analysis plan and interpretation of the data. C.Y. performed the statistical analyses. A.V. wrote the first draft. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript. R.R. is guarantor, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. The protocol is available from R.R. on request.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- GCT
glucose challenge test
- GDM
gestational diabetes mellitus
- HOMA-IR
homeostasis model assessment of insulin resistance
- IGI
insulinogenic index
- ISSI-2
insulin secretion-sensitivity index-2
- OGTT
oral glucose tolerance test
- T2DM
type 2 diabetes mellitus
Additional Information
Disclosure Summary: The authors have no conflicts of interest to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21(6):1291–1298. [DOI] [PubMed] [Google Scholar]
- 3. Kramer CK, von Mühlen D, Jassal SK, Barrett-Connor E. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose: the Rancho Bernardo Study. Diabetes Care. 2009;32(7):1272–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Juraschek SP, McAdams-Demarco M, Miller ER, et al. Temporal relationship between uric acid concentration and risk of diabetes in a community-based study population. Am J Epidemiol. 2014;179(6):684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shani M, Vinker S, Dinour D, et al. High normal uric acid levels are associated with an increased risk of diabetes in lean, normoglycemic healthy women. J Clin Endocrinol Metab. 2016;101(10):3772–3778. [DOI] [PubMed] [Google Scholar]
- 6. Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lv Q, Meng XF, He FF, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. Plos One. 2013;8(2):e56864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson RJ, Merriman T, Lanaspa MA. Causal or noncausal relationship of uric acid with diabetes. Diabetes. 2015;64(8): 2720–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism. 2006;55(10):1293–1301. [DOI] [PubMed] [Google Scholar]
- 10. Mazidi M, Katsiki N, Mikhailidis DP, Banach M. The link between insulin resistance parameters and serum uric acid is mediated by adiposity. Atherosclerosis. 2018;270:180–186. [DOI] [PubMed] [Google Scholar]
- 11. Sluijs I, Holmes MV, van der Schouw YT, et al. ; InterAct Consortium A mendelian randomization study of circulating uric acid and type 2 diabetes. Diabetes. 2015;64(8):3028–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfister R, Barnes D, Luben R, et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54(10):2561–2569. [DOI] [PubMed] [Google Scholar]
- 13. Keenan T, Zhao W, Rasheed A, et al. Causal assessment of serum urate levels in cardiometabolic diseases through a mendelian randomization study. J Am Coll Cardiol. 2016;67(4):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. [DOI] [PubMed] [Google Scholar]
- 16. Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010;59(10):2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kramer CK, Swaminathan B, Hanley AJ, et al. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of β-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care. 2014;37(12):3262–3269. [DOI] [PubMed] [Google Scholar]
- 18. Xiang AH, Takayanagi M, Black MH, et al. Longitudinal changes in insulin sensitivity and beta cell function between women with and without a history of gestational diabetes mellitus. Diabetologia. 2013;56(12):2753–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinnaduwage L, Ye C, Hanley AJ, et al. Changes over time in hepatic markers predict changes in insulin sensitivity, β-cell function, and glycemia. J Clin Endocrinol Metab. 2018;103(7):2651–2659. [DOI] [PubMed] [Google Scholar]
- 20. Diabetes Canada Clinical Practice Guidelines Expert Committee. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10–S15. [DOI] [PubMed] [Google Scholar]
- 21. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 23. Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring). 2008;16(8):1901–1907. [DOI] [PubMed] [Google Scholar]
- 24. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26(12):1198–1203. [DOI] [PubMed] [Google Scholar]
- 25. Molęda P, Fronczyk A, Safranow K, Majkowska L. Is uric acid a missing link between previous gestational diabetes mellitus and the development of type 2 diabetes at a later time of life? Plos One. 2016;11(5):e0154921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leng J, Wang L, Wang J, et al. Uric acid and diabetes risk among Chinese women with a history of gestational diabetes mellitus. Diabetes Res Clin Pract. 2017;134:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim C, Cheng YJ, Beckles GL. Cardiovascular disease risk profiles in women with histories of gestational diabetes but without current diabetes. Obstet Gynecol. 2008;112(4):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zajdenverg L, Rodacki M, Faria JP, Pires ML, Oliveira JE, Halfoun VL. Precocious markers of cardiovascular risk and vascular damage in apparently healthy women with previous gestational diabetes. Diabetol Metab Syndr. 2014;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). 2010;34(3):454–461. [DOI] [PubMed] [Google Scholar]
- 30. Li X, Meng X, Gao X, et al. Elevated serum xanthine oxidase activity is associated with the development of type 2 diabetes: a prospective cohort study. Diabetes Care. 2018;41(4):884–890. [DOI] [PubMed] [Google Scholar]