Abstract
Aim
The objective of this study was to assess the existing literature to determine if a relationship exists between hypothyroidism and periodontitis.
Methods
We used a modified approach to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses by searching five databases in addition to the gray literature. Keywords in the title and abstract fields, as well as subject headings for both periodontal disease and hypothyroidism, were used to search the existing literature for publications relevant to evaluation of the thyroid–periodontitis relationship.
Results
The authors screened 847 unique publications which, after applying inclusion and exclusion criteria, yielded 29 publications, which were further analyzed for relevance and applicability. Most of the included papers were cross‐sectional studies and retrospective chart reviews. Following critical analysis, four publications, including one abstract, were used to further assess the hypothyroid–periodontitis relationship.
Conclusions
There are very few high‐quality studies describing the potential association between hypothyroidism and periodontitis. In general, and among the included papers with the fewest confounding factors, a positive relationship between hypothyroidism and periodontitis was found. Further well‐controlled, prospective clinical and immunologic studies will be required to confirm that relationship.
Keywords: hypothyroidism, periodontitis, scoping review
1. INTRODUCTION
Relationships between periodontal diseases and a variety of systemic conditions have been described in the literature, including cardiovascular disease, cerebrovascular disease, respiratory disease, pregnancies resulting in preterm delivery or low birth weight infants, diabetes mellitus, rheumatoid arthritis, obesity, osteoporosis, and stress (R. Genco, Offenbacher, & Beck, 2002; R. J. Genco & Borgnakke, 2013). In addition, there is evidence that the severity and prevalence of periodontitis is greater in individuals with autoimmune diseases such as rheumatoid arthritis (Wolff et al., 2014) and inflammatory bowel diseases (Brito et al., 2008; Vavricka et al., 2013). The association between those conditions and periodontal disease might be due to common immunoinflammatory pathways in disease pathogenesis (Araujo, Melo, & Lima, 2015). It has been hypothesized that inflammatory mediators such as prostaglandin E2, interleukin‐1β, tumor necrosis factor‐α, and matrix metalloproteinases released locally as a consequence of periodontal disease lead to an alteration in bone hemostasis and might represent a risk factor for other systemic diseases such as rheumatoid arthritis (Araujo et al., 2015; Habashneh, Khader, Alhumouz, Jadallah, & Ajlouni, 2012). It is well established that the endocrine system can modulate the immune system in a bidirectional manner (Klein, 2006). Indeed, the relationship between diabetes mellitus and periodontitis offers a potential explanation for how acute and chronic inflammation, such as what occurs during periodontitis, might affect thyroid hormone production (R. J. Genco & Borgnakke, 2013).
Hypothyroidism is the most common hormone abnormality in humans and represents the most common thyroid disease. Hypothyroidism presents with a wide range of severity, from asymptomatic individuals to patients with multisystem failure (Roberts & Ladenson, 2004) due to decreased production of thyroxine (T4), triiodothyronine (T3), and calcitonin (Little, 2006), which causes decreased bone metabolism, maturation, and turnover and negatively affects bone homeostasis (Mosekilde & Melsen, 1978).
Consequently, the primary aim of this scoping review was to evaluate the quality and quantity of the existing literature describing the potential relationship between hypothyroidism and periodontitis. A secondary aim was to assess whether a relationship exists between hypothyroidism and periodontal disease severity.
2. MATERIALS AND METHODS
This review used the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses reporting guideline (Moher, Liberati, Tetzlaff, & Altman, 2010) and modified them to apply to this scoping review.
An initial electronic search was created by a health sciences librarian and completed on September 4, 2018, and supplemented on April 26, 2019, in the following databases: PubMed, Embase via http://Embase.com, CINAHL via EBSCOhost, Web of Science, and Evidence‐Based Medicine Reviews via Ovid, a database composed of seven other databases (including Cochrane Database of Systematic Reviews, Cochrane Register of Controlled Trials, ACP Journal Club, Health Technology Assessment, Database of Abstracts of Reviews of Effects, Cochrane Methodology Register, and National Health Service Economic Evaluation Database). The search included keywords for both hypothyroidism and periodontal disease and included subject terms from each database's thesaurus (such as Medical Subject Headings or MeSH) when possible, as well as keyword searching of title and abstract fields. No date or language restrictions were applied to the initial search. The exact search terminology used in PubMed can be found in Table 1. That search was applied to each database, with MeSH terms translated to reflect the controlled vocabulary of each individual database.
Table 1.
Search strategy for PubMed
| 1. “periodontal diseases”[MeSH Terms] |
| 2. periodont*[Title/Abstract] |
| 3. gingiv*[Title/Abstract] |
| 4. peri implantitis[Title/Abstract] |
| 5. alveolar bone[Title/Abstract] |
| 6. ((((“periodontal diseases”[MeSH Terms]) OR periodont*[Title/Abstract]) OR gingiv*[Title/Abstract]) OR peri implantitis[Title/Abstract]) OR alveolar bone[Title/Abstract] |
| 7. “thyroid diseases”[MeSH Terms] |
| 8. thyroid*[Title/Abstract] |
| 9. hypothyroid*[Title/Abstract] |
| 10. ((“thyroid diseases”[MeSH Terms]) OR thyroid*[Title/Abstract]) OR hypothyroid*[Title/Abstract] |
| 11. ((((((((((“periodontal diseases”[MeSH Terms]) OR periodont*[Title/Abstract]) R gingiv*[Title/Abstract]) OR peri implantitis[Title/Abstract]) OR alveolar bone[Title/Abstract])) AND (((“thyroid diseases”[MeSH Terms]) OR thyroid*[Title/Abstract]) OR hypothyroid*[Title/Abstract]) |
To assess the gray literature, the following resources were searched: Proquest Dissertations and Theses Global, http://clinicaltrials.gov, International Association for Dental Research (IADR) Abstract Archive (2001–present), American Thyroid Association (ATA) Meeting Abstracts (2011–present), and OpenGrey.eu. Proquest Dissertations and Theses Global allows for complex Boolean searching, which enabled the authors to use the same search that was used in the previously mentioned nongray databases. However, a search using the terms “periodontal disease” and “thyroid” or “periodontal disease” and “hypothyroidism” were used for searching the other gray literature resources. No date or language restrictions were used at this search stage with the exceptions of the IADR Abstract Archives and the ATA Meeting Abstracts, as the on‐line IADR Abstract Archives begins in the year of 2001, and the ATA Meeting Abstracts is freely available beginning only in 2011.
Our initial search on September 4, 2018, yielded 1,182 articles. When the search was updated on April 26, 2019, an additional 36 unique articles were identified. Three hundred seventy‐one articles were then excluded due to duplication. Collectively, both searches resulted in 847 articles that were subsequently screened by two reviewers (H. A. amd L. Y.) and resulted in the exclusion of 746 articles due to irrelevant title and/or abstract (i.e., not related to periodontitis and hypothyroidism). Our inclusion and exclusion criteria are summarized in Table 2.
Table 2.
Aim 1: Inclusion and exclusion criteria
| Inclusion criteria |
| 1. Full‐text available. |
| 2. Both human and animal studies. |
| 3. All study designs. |
| 4. True periodontal outcome = any measure of periodontal status (bone loss, pocket depth, missing teeth, need for surgery, bleeding, suppuration, radiographic evidence of bone loss, including teeth or implants, or previous diagnosis of periodontal disease). |
| 5. Presence of hypothyroidism confirmed by laboratory testing (thyroid panel—TSH, free T4, free T3, or total T3) or medication. |
| Exclusion criteria |
| 1. Nonmammalian studies. |
| 2. Languages other than English. |
| 3. Hyperthyroidism or thyroid cancer. |
| 4. Wrong comparator = no comparison between periodontitis and hypothyroidism. |
| 5. Wrong intervention = the intervention was not for periodontitis diagnosis. |
| 6. Irrelevant outcome = the outcome wasn't related to periodontitis/hypothyroidism. |
| 7. Wrong patient population = no comparison of periodontitis and/or hypothyroidism. |
| 8. Journals of publication not cited in open access checklist for predatory publishers. |
One‐hundred one full‐text articles were further assessed for eligibility by the same two reviewers; any conflicts were resolved by a third reviewer (R. C.). A total of 72 articles were excluded for the following reasons: no full‐text available (three papers), not in English at the time of manual review (34 papers), not associated with a periodontal outcome or thyroid outcome (26 papers), related to hyperthyroidism or thyroid cancer but not hypothyroidism (two papers), incorrect/irrelevant comparator (i.e., no comparison between periodontitis and hypothyroidism; two papers), an incorrect or irrelevant intervention (i.e., an intervention not related to periodontal diagnosis; two papers), incorrect/irrelevant patient population (i.e., no comparison of periodontitis and or hypothyroidism; one paper), and if the journals of publication were cited in an open access checklist for predatory publishers (two papers). In addition, three papers were excluded due to duplication. As a result, a total of 28 full‐length papers and one abstract were included for review. The selection process used in our analysis is described in more detail in Figure 1.
Figure 1.
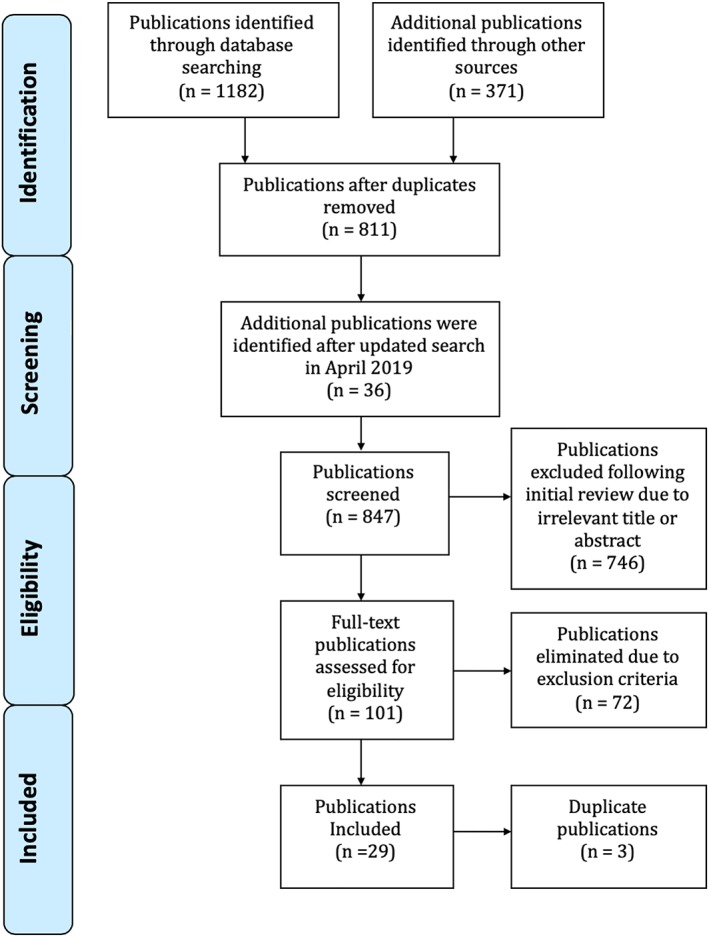
Publication selection methods
3. RESULTS
3.1. Aim 1
To evaluate the quality and quantity of the existing literature describing the potential relationship between hypothyroidism and periodontitis.
3.1.1. Outcome of search
After applying the inclusion, exclusion, and eligibility criteria noted above, a total of 29 publications, including one abstract, were considered for further analysis as shown in Table 3. We found that published papers describing the relationship between hypothyroid disease and periodontitis generally ranked poorly in the hierarchy of scientific evidence, and most were associated with significant methodological limitations. There were no published meta‐analyses or systematic reviews available that described the thyroid–periodontal disease relationship. Similarly, there were no randomized controlled human clinical trials and only one retrospective cohort study (that investigated dental implants). Only one intervention study and three case–control studies were available. There were 14 cross‐sectional studies (five of them are retrospective chart reviews): two review papers, three case reports, and four animal studies, in addition to one abstract.
Table 3.
Aim 1: Summary of publications in initial search
| Author | Year of publication | Type of study | Association with hypothyroidism | Included/excluded |
|---|---|---|---|---|
| Alsaadi et al. | 2007 | Retrospective chart review (cross‐sectional) | No association between hypothyroidism and early implant failure | Excluded |
| Attard & Zarb | 2002 | Retrospective chart review (cross‐sectional) |
• No association between hypothyroidism and implant failure • Positive association between hypothyroidism and bone loss |
Included |
| Chatzopoulos et al. | 2016 | Cross‐sectional | No association between hypothyroidism and CPITN scores 3&4 | Excluded |
| Chrysanthakopoulos et al. | 2013 | Prospective cross‐sectional | Positive association between hypothyroidism and PD > 5 mm | Excluded |
| Chrysanthakopolulos et al. | 2016 | Prospective cross‐sectional | Positive association between hypothyroidism and PD > 5 mm | Excluded |
| Dalago et al. | 2017 | Cross‐sectional | No association between hypothyroidism and peri‐implantitis | Excluded |
| Molloy et al. | 2004 | Retrospective chart review (cross‐sectional) |
• Positive association between hypothyroidism and mild bone loss, but not severe • No association with tooth loss |
Excluded |
| Nesse et al. | 2010 | Cross‐sectional | No association between hypothyroidism and PD | Excluded |
| Rahangdale et al. | 2018 | Cross‐sectional | Positive association between hypothyroidism and PD, CAL | Included |
| Soomsawasdi (a) | 1965 | Cross‐sectional | Positive association between hypothyroidism and tooth mobility | Excluded |
| Soomsawasdi (b) | 1965 | Cross‐sectional | Positive association between hypothyroidism and missing teeth | Excluded |
| van Steenberghe et al. | 2002 | Cross‐sectional | No association between hypothyroidism and implant failure | Excluded |
| Xie & Ainmo | 1999 | Retrospective chart review (cross‐sectional) | No association between hypothyroidism and tooth loss | Excluded |
| Zeigler et al. | 2015 | Cross‐sectional | Positive association between high TSH levels and PD (5 mm and more) | Excluded |
| Babu et al. | 2016 | Case–control | Positive association between hypothyroidism and GI, PI (children) | Excluded |
| Beriashvili et al. | 2016 | Case–control | Positive association | Excluded |
| Saima et al. | 2016 | Case–control | Positive association between hypothyroidism and GI, PI (children) | Excluded |
| de Souza et al. | 2013 | Cohort study | No association between hypothyroidism and peri‐implantitis | Excluded |
| Bhankhar et al. | 2017 | Interventional | Positive association between hypothyroidism and CAL around 5 mm | Excluded |
| Patil et al. | 2011 | Review | Positive association | Excluded |
| Zahid et al. | 2011 | Review | Positive association | Excluded |
| Yerke et al. | 2019 | Abstract | Positive association | Included |
| Gupta et al. | 2014 | Case report | Unknown | Excluded |
| Patil et al. | 2012 | Case report | Positive association | Excluded |
| Yussif et al. | 2017 | Case report | Positive association | Excluded |
| De Toledo et al. | 1979 | Animal study—marmos model | Positive association | Excluded |
| Feitosa et al. | 2009 | Animal study—rat model | Positive association | Included |
| Pinto | 1974 | Animal study—rat model | Positive association | Excluded |
| Schneider | 1969 | Animal study—rat model | No association | Excluded |
Abbreviations: BOP, bleeding on probing; CAL, clinical attachment loss; CPITN, the community periodontal index of treatment needs; GI, gingival index; PD, pocket depth; TSH, thyroid‐stimulating hormone.
3.2. Aim 2
To assess the relationship between periodontal disease severity and hypothyroidism.
3.2.1. Relationship between hypothyroidism and periodontitis
All 29 publications noted in Table 3 were critically assessed for their ability to address a potential relationship between hypothyroidism and periodontitis. In general, papers were excluded if the patient populations consisted of only children or adolescents, because those groups are typically associated with a low prevalence of hypothyroid or periodontal diseases; if the study investigated primarily gingivitis or included patients having minimal probing depths rather than periodontitis; if there was no control group or baseline data available for data comparison; if potentially unreliable surrogate measurements of periodontitis were used (e.g., tooth loss and implant failure prior to abutment connection); if obsolete diagnostic methods for thyroid disease were used (e.g., measurement of the Achilles tendon reflex as a surrogate for hypothyroidism); reports on histological evaluation of inflammation without measurement of clinical periodontal parameters or bone levels; and studies that used patients with “thyroid disease” without distinguishing among subjects with hypothyroidism, hyperthyroidism, and thyroid cancer.
3.2.2. Papers excluded from analysis of hypothyroid–periodontitis relationships: Rationale and description
Two articles from Table 3 were review papers (Patil, Patil, & Gururaj, 2011; Zahid, Wang, & Cohen, 2011) that referred to primary articles also noted in that table. Consequently, those were excluded from the critical analysis, and the component papers were considered separately.
All the three case–control studies by Beriashvili, Nikolaishvili, Mantskava, Momtsemlidze, and Franchuk (2016), Saima, Tasneem, and Gowhar (2016), and Venkatesh Babu and Patel (2016) were excluded because their subject populations consisted of only children or adolescents. In addition, periodontal measurements in the excluded studies were based solely on the presence of gingival inflammation (gingivitis), and there were no measurements of pocket depth or bone levels. The study by Zeigler, Wondimu, Marcus, and Modéer (2015) also was excluded due to use of an adolescent patient population and a definition of pocket depths greater than 3 mm as “deep.” In addition, the objective of Zeigler et al. was to compare blood pressure to obesity, but the level of thyroid‐stimulating hormone (TSH) was used only as an adjusted confounder for that comparison. Consequently, TSH levels could not be directly assessed to measure the effect of hypothyroidism on periodontal disease.
The intervention study by Bhankhar, Hungund, Kambalyal, Singh, and Jain (2017) also was excluded. Although they found that alveolar bone loss was greater in hypothyroidism patients compared with the healthy controls, one of the limitations of this study was the absence of a control group consisting of normal thyroid function in patients with periodontitis. An additional weakness was that TSH levels were not measured posttreatment in the nonhypothyroidism group, so the posttreatment TSH levels in hypothyroidism group had no direct control comparison.
Two older papers by Soomsawasdi, Ringsdorf, and Cheraskin 1965a, 1965b) were excluded due to the use of a questionable (and currently abandoned) technique for the diagnosis of thyroid disease: the Burdick photomograph and electrocardiograph, which essentially is a measurement of a timed graphic curve of the Achilles tendon reflex for thyroid disease diagnosis. In addition, the authors acknowledged the considerable variability and uncertain appropriateness of their diagnostic methods, the subject population was limited (only females were included), and patient thyroid diagnoses were not subsequently confirmed by medical evaluation or laboratory testing.
The Xie and Ainamo (1999) study was excluded from analysis because their population consisted of only three groups of elderly patients (76, 81, and 86 years old) and hypothyroid disease was not separately reported. More specifically, patients with hypothyroidism, hyperthyroidism, and thyroid cancer were used for their analysis. Dental status was described as either edentulous or dentate (which was defined as having at least one tooth), which might not be appropriate for diagnosis of periodontitis, particularly in their elderly populations, and because no association between diabetes and tooth loss was found when this association has been established. Tooth loss also might be related to nonperiodontal etiologies such as trauma or caries. Finally, the authors also acknowledged that many patients with systemic diseases such as diabetes might not have survived to old age, which is an additional study limitation.
Chatzopoulos and Tsalikis (2016) were excluded because a community periodontal index of treatment needs score of 3 was used for diagnosis of periodontitis, which would include patients having any teeth with pocket depths greater than 3 mm. No additional periodontal criteria (e.g., bone levels and histology) were considered. Moreover, patient populations with hypothyroidism, hyperthyroidism, and thyroid cancer were pooled and used for analysis. In this study, the authors also did not find a relationship between diabetes and periodontitis, which also suggests that their inclusion criteria included a considerable number of patients having gingivitis or only slight periodontitis.
The study by Molloy, Wolff, Lopez‐Guzman, and Hodges (2004) found that thyroid disease was more prevalent among patients with mild bone loss, but not with tooth loss. In their analysis, the authors did not differentiate between hyperthyroidism, hypothyroidism, or thyroid cancer, which may have affected the results. In addition, it is arguable whether measurement of tooth loss is a sufficiently sensitive diagnostic indicator for periodontitis and was therefore excluded from critical analysis.
In the two publications by Chrysanthakopoulos and Chrysanthakopoulos (2013, 2016), a positive association was found between periodontal disease and hypothyroidism. They defined periodontitis by the presence of pocket depths equal or greater than 5 mm and excluded patients that had been recently treated for periodontal disease, to obtain patients with “clinically established periodontitis.” However, those studies also pooled patients with thyroid disease to include hypothyroidism, hyperthyroidism, and thyroid cancer in one category. The authors further reported that as many as one third of their patient pool was reported to have hyperthyroidism. The authors also did not specifically indicate if the endocrine results had been adjusted for diabetes or smoking, and therefore, both studies were excluded.
Nesse et al. (2010) evaluated periodontitis and hypothyroidism in control patients, periodontitis patients in dental clinics, and periodontitis patients in periodontal clinics. The results were adjusted for age, gender, and smoking. However, periodontitis was defined as having pocket depths greater than 3 mm and, although full mouth probing depths were recorded for patients with community periodontal index of treatment needs scores 3 or more, clinical periodontal data were unavailable in 28% of patients, smoking data were missing from 50% of one of the clinic sites, and smoking status was significantly different between the groups. An additional limitation of this study was that patients with periodontitis were significantly older than the control group and more likely to be female, which also might have affected the study outcome.
All of the three case reports by Patil and Giri (2012), Gupta, Goel, Solanki, and Gupta (2014), and Yussif, El‐Mahdi, and Wagih (2017) were excluded because a periodontal diagnosis was not obtained in the Gupta et al. report, there were no periodontal measurements, and the patient's chief complaint was the presence of retained deciduous teeth. The other two case reports (Patil & Giri, 2012; Yussif et al., 2017) were excluded in our analysis, as case reports do not rank highly in the hierarchy of scientific evidence. In both of those cases, there was a periodontal diagnosis based on clinical periodontal measurements such as pocket depth, bleeding on probing, tooth mobility, and radiographic bone loss. In both cases, resolution of periodontal disease was observed following diagnosis and treatment of hypothyroid disease concurrent with periodontal therapy. However, the absence of a control group and the study design remains the major limitation of those papers.
Five papers were related to dental implants and thyroid disease. Of those, four were excluded: van Steenberghe, Jacobs, Desnyder, Maffei, and Quirynen (2002) and Alsaadi, Quirynen, Komarek, and van Steenberghe (2007) were not further considered because they evaluated the relationship between thyroid disease and early implant failure, which included the time period from fixture placement up to—but before—abutment connection. Consequently, implant failure during that period most likely represents a lack of osseointegration and might not be an appropriate indicator of chronic periodontal disease. de Souza et al. (2013) were excluded because measurements were nonstandardized, uncorrected for distortion, and not adjusted using proportionality or computer‐based methods for differences in angulation, which may affect outcomes. de Souza et al., as well as Dalago, Schuldt Filho, Rodrigues, Renvert, and Bianchini (2017), did not find a relationship between peri‐implantitis and smoking, or with peri‐implantitis and diabetes. There also was no distinction among hypothyroidism, hyperthyroidism, and other thyroid disease in either study, because patients with any or all of those conditions were pooled and considered to have “thyroid disease.” Additionally, de Souza et al. also acknowledged that their small sample size might have influenced their results, and only 7.3% of patients in the study by Dalago et al. had a diagnosis consistent with peri‐implantitis.
Our review also identified a number of animal studies relevant to the search terms. Three of those papers were excluded for several reasons. The 1969 rat study by Schneider was not further considered because experimental periodontal disease was not induced in this model, which evaluated “naturally occurring periodontal disease.” In addition, only gingival inflammation was assessed, as well as the presence of “degenerative periodontosis.” There was no control group, and no measurements of bone loss were recorded. Similarly, the rat study by Pinto (1974) and the De Toledo, Bozzo, Do Nascimento, and Sallum (1979) study in marmosets were based on descriptive histology that qualitatively assessed inflammation in the periodontal ligament, without any defined or direct measurements of periodontal disease such as bone loss, pocket depth, or attachment loss. Moreover, no control groups were presented in either of those studies.
3.2.3. Papers included in the analysis of hypothyroid–periodontitis relationships: Rationale and description
Of the 29 publications obtained from the results of the primary search, 25 were excluded as described above and four remained for further descriptive analysis to address Aim 2. The four remaining publications are listed in Table 4 and are described in more detail as follows:
Table 4.
Aim 2: [Appendix 1] Summary of the publications included for analysis of the periodontitis–hypothyroidism relationship
| Author | Year of publication | Type of study | Association between hypothyroidism and periodontitis | Periodontal diagnosis as defined in the paper | Hypothyroid disease as defined in the paper |
|---|---|---|---|---|---|
| Attard & Zarb | 2002 | Retrospective chart review (cross‐sectional) |
• No association between hypothyroidism and implant failure • Positive association between hypothyroidism and bone loss (compare with control group) |
Radiographic bone loss around implant |
• Diagnosed with primary hypothyroidism • On thyroid replacement medication |
| Feitosa et al. | 2009 | Animal study (rat model) | Positive association in hypothyroidism rats and bone loss in ligature‐induced periodontitis model | Bone resorption |
• Hypo‐thyroidism induced by the ingestion of a solution containing propylthiouracil (Propilracil, 100 mg) • Taking antithyroid drug that blocks thyroid hormone synthesis |
| Rahangdale et al. | 2018 | Cross‐sectional | Positive association between hypothyroidism and PD, CAL | Pocket depth, clinical attachment loss, and bone loss |
• Diagnosed with primary hypothyroidism On thyroid replacement medication |
| Yerke et al. | 2019 | Abstract | Positive association with deep pocket ≥5 or ≥6 mm | Pocket depth | Patient medical history for use of prescription medication(s) for thyroid hormone supplementation |
Rahangdale and Galgali (2018), in a comparative cross‐sectional study involving 102 human subjects, found significantly greater mean pocket depths and more clinical attachment loss among patients with hypothyroidism, compared with patients without thyroid disease. Groups were statistically adjusted for age, and plaque scores for both groups were similar. The authors concluded that patients taking thyroxine replacement medications were more likely to have periodontal disease than euthyroid control subjects.
Attard and Zarb (2002) could not demonstrate an association between hypothyroidism and implant failure, but there was a relationship between peri‐implant radiographic bone loss in patients with hypothyroidism, compared with normal controls. The lack of association with implant failure might be related to the relatively large amount of bone loss required for implant failure. The strengths of this study included patient matching for age, gender, site, and prosthetics between the hypothyroid and control groups, the use of only hypothyroid patients, and use of standardized radiographs. Limitations included small sample size (56 patients), the retrospective nature of their study, and use of self‐reported hypothyroidism in lieu of a formal diagnosis.
One well‐designed animal study by Feitosa et al. (2009) was included in our analysis. The authors used an experimental periodontitis ligature model in a rat model system. Three groups were included in the study: control, hypothyroidism, and hyperthyroidism, and thyroid status was confirmed using laboratory blood testing for TSH. Periodontal bone levels were measured via histomorphometric analysis. They found that an increase in periodontitis‐related bone loss was associated with hypothyroidism (but not with hyperthyroidism).
One abstract by Yerke, Levine, and Cohen (2019) was included in our analysis. That study evaluated the association between hypothyroidism and severity of periodontitis among 538 patients with moderate to severe periodontitis. The extent of periodontal disease was evaluated by measuring the percentage of teeth with probing depths greater than or equal to 5 or 6 mm, and hypothyroidism was assessed through review of patient medical histories for use of prescription thyroid hormone supplementation in combination with a diagnosis of hypothyroidism. They found that patients with hypothyroidism had significantly more teeth with deeper periodontal probing depths, compared with patients without thyroid disease. In that study, all patients were examined by single examiner, but the retrospective natural of that analysis was a limitation because the dosage and duration of thyroid hormone supplementation was not determined.
4. DISCUSSION
Our results indicate that there are very few high‐quality studies describing the potential association between hypothyroidism and periodontitis. It appears that most of the available literature consists of retrospective cross‐sectional studies, case reports, and retrospective chart review analyses. Our results are similar to the two review papers (Patil et al., 2011; Zahid et al., 2011) that were identified in our scoping review for Aim 1, both of which found that, although there is evidence that a potential relationship might exist between hypothyroidism and periodontitis, existing literature lacks adequate randomized controlled clinical trials and observational studies to support this claim. Nevertheless, our review suggests that a positive relationship between hypothyroidism and periodontitis most likely exists and that this relationship might be more apparent among subjects with severe periodontitis, compared with patients with mild periodontal disease.
In the critical analysis section of our review, we identified three full‐length papers and one abstract that demonstrated a positive relationship between those two conditions. All the excluded publications were compromised due to lack of defined periodontal measurements, as well as the use of relatively unreliable methods for diagnosis of hypothyroid disease. Consequently, it was not possible to perform a systematic review or meta‐analysis of the periodontitis–thyroid disease relationship. Therefore, a scoping review was performed to assess the quality of the existing literature.
The lack of relevant papers might be due to the effects of thyroid hormone replacement therapy on hypothyroidism, which might mask the effect of untreated hypothyroid disease on periodontal disease, particularly in patients with mild to moderate periodontitis. Indeed, many of the papers considered for review used a variety of definitions for periodontitis, and there was no consistent classification of disease. Due to this inconsistency in disease classification among papers, comparison of results from individual publications was challenging. Furthermore, no high‐level evidence was found upon our search, as no meta‐analysis, systematic reviews, or randomized clinical trials were found. As a result, our analysis was focused on a limited number of cross‐sectional studies and case reports, which have a lower level of evidence due to the retrospective nature of the study design, limited sample size, and a higher potential of bias. Indeed, in the review of periodontal manifestations of systemic diseases from the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions (Albandar, Susin, & Hughes, 2018), an established relationship between hypothyroidism and periodontitis was not described. This omission most likely was due to the lack of relevant literature supporting such an association.
One limitation of our study is that only papers written in English were included in our review, and other studies reporting results in languages other than English might be available.
With those limitations, we believe that there is evidence suggesting that a relationship between hypothyroidism and periodontitis likely exists, and we speculate that the relationship may be more apparent in individuals with more severe periodontitis. Perhaps the strongest support for that conclusion comes from the results of Feitosa et al. (2009), where an experimental model of ligature‐induced periodontitis was evaluated in rats with and without thyroid disease, Rahangdale and Galgali (2018), who found a higher pocket depth and clinical attachment loss in hypothyroid patients compared with healthy patients, and by Yerke et al. (2019), who demonstrated a relationship between those conditions in patients with moderate to advanced periodontitis, as well as by Attard and Zarb (2002), who described an association between hypothyroidism and peri‐implantitis.
Hypothyroid patients appear to have a decreased bone turnover rate secondary to slower resorption during bone remodeling (Mosekilde & Melsen, 1978; Mosekilde, Melsen, Bagger, Myhre‐Jensen, & Schwartz Sorensen, 1977) that results in an increase in bone mass (Eriksen, Mosekilde, & Melsen, 1986). Because thyroid hormone receptors are present in human bone (Abu, Bord, Horner, Chatterjee, & Compston, 1997), it has been proposed that thyroid hormone might act directly on bone cells via specific nuclear receptors or indirectly via increasing the secretion of growth hormone and insulin‐like growth factor (Abu et al., 1997). Patients with hypothyroidism tend to exhibit higher than normal bone density, whereas subjects with hyperthyroidism, characterized by lower levels of TSH, appear to experience bone loss and have a higher fracture incidence (Lakatos, 2003; Mosekilde, Eriksen, & Charles, 1990). Animal models of hypothyroidism also have demonstrated alterations in bone metabolism (Britto, Fenton, Holloway, & Nicholson, 1994; Monfoulet et al., 2011), possibly through a mechanism by which thyroid hormone has direct or indirect effects on osteoblasts and osteoclasts.
Other drugs can affect thyroid function in addition to medications, such as levothyroxine and liothyronine, which are primarily used to treat hypothyroidism. Tyrosine kinase inhibitors used in cancer treatment have the potential to modify thyroid hormone metabolism or directly induce hypothyroidism (Barbesino, 2010; Desai et al., 2006; Mannavola et al., 2007). Immunomodulating agents such as alemtuzumab, interferon, and other cytokines also can affect thyroid function, although the mechanisms have not been fully elucidated (Coles et al., 1999; Eskes & Wiersinga, 2009; Investigators, 2008; Rao, Kremenevskaja, Resch, & Brabant, 2005; Schou, Amdisen, Jensen, & Olsen, 1968; Tran, Malcolm Reeves, Gibson, & Attia, 2009). Other medications known to have thyroid effects include lithium, amiodarone, and oral estrogens (Eskes & Wiersinga, 2009; Rao et al., 2005; Schou et al., 1968; Tahboub & Arafah, 2009).
Our findings also have applicability to clinical practice. Specifically, periodontists should consider thyroid disease, and endocrinologists should consider the presence of periodontitis, during patient evaluation because early diagnosis and intervention for both conditions is likely to mutually improve patient outcomes. Ideally, dentists should be able to recognize local and systemic symptoms that may indicate undiagnosed thyroid diseases, or signs of medications that affect thyroid function, and recommend appropriate patient referrals when necessary. Similarly, physicians should consider clinical history or signs of periodontal diseases during medical evaluations.
From the authors' knowledge, this study is the first scoping review to evaluate the available literature regarding the association between periodontitis and hypothyroidism. Our study also identifies gaps and opportunities for future research in this area by conducting a well‐designed randomized clinical trial that include comprehensive assessment of immune status. A better understanding of the potential effect of thyroid hormone dysfunction in the outcome and severity of periodontitis might be important for the decrease in morbidity of both diseases.
5. CONCLUSIONS
There are very few high‐quality studies describing the potential association between periodontitis and hypothyroidism, with most of the available literature consisting of retrospective cross‐sectional studies and chart reviews. In general, a positive association between periodontitis and hypothyroidism was found. Further well‐controlled, prospective clinical and immunologic studies will be required to confirm that relationship, to measure the strength of any association with disease severity, to establish causality, and to establish the role of either disease in the pathogenesis of the other.
CONFLICT OF INTEREST
None of the authors have any financial or commercial associations or conflicts of interest.
CLINICAL RELEVANCE
SCIENTIFIC RATIONALE FOR THE STUDY
The potential association between periodontitis and a variety of immunologic and inflammatory conditions provides a foundation for a possible relationship between hypothyroidism and periodontitis.
PRINCIPAL FINDINGS
There are very few high‐quality studies describing the potential association between hypothyroidism and periodontitis. Nevertheless, analysis of the most relevant clinical and experimental studies supported the existence of a positive relationship between hypothyroidism and periodontitis.
PRACTICAL IMPLICATIONS
Periodontists should consider thyroid disease, and endocrinologists should consider the presence of periodontitis, during patient evaluation because early diagnosis and intervention for both conditions is likely to mutually improve patient outcomes.
ACKNOWLEDGEMENTS
This study was supported in part by the William M. Feagans Endowed Chair Research Fund (SUNYAB) and the Department of Periodontics and Endodontics, University at Buffalo, School of Dental Medicine, State University of New York.
Aldulaijan HA, Cohen RE, Stellrecht EM, Levine MJ, Yerke LM. Relationship between hypothyroidism and periodontitis: A scoping review. Clin Exp Dent Res. 2020;6:147–157. 10.1002/cre2.247
REFERENCES
- Abu, E. O. , Bord, S. , Horner, A. , Chatterjee, V. K. , & Compston, J. E. (1997). The expression of thyroid hormone receptors in human bone. Bone, 21(2), 137–142. 10.1016/S8756-3282(97)00097-5 [DOI] [PubMed] [Google Scholar]
- Albandar, J. M. , Susin, C. , & Hughes, F. J. (2018). Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. Journal of Periodontology, 89(Suppl 1), S183–s203. 10.1002/jper.16-0480 [DOI] [PubMed] [Google Scholar]
- Alsaadi, G. , Quirynen, M. , Komarek, A. , & van Steenberghe, D. (2007). Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. Journal of Clinical Periodontology, 34(7), 610–617. 10.1111/j.1600-051X.2007.01077.x [DOI] [PubMed] [Google Scholar]
- Araujo, V. M. , Melo, I. M. , & Lima, V. (2015). Relationship between periodontitis and rheumatoid arthritis: Review of the literature. Mediators of Inflammation, 2015, 259074–259015. 10.1155/2015/259074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard, N. J. , & Zarb, G. A. (2002). A study of dental implants in medically treated hypothyroid patients. Clinical Implant Dentistry and Related Research, 4(4), 220–231. 10.1111/j.1708-8208.2002.tb00174.x [DOI] [PubMed] [Google Scholar]
- Barbesino, G. (2010). Drugs affecting thyroid function. Thyroid, 20(7), 763–770. 10.1089/thy.2010.1635 [DOI] [PubMed] [Google Scholar]
- Beriashvili, S. , Nikolaishvili, M. , Mantskava, M. , Momtsemlidze, N. , & Franchuk, K. (2016). Changes in tooth hard tissue minerali‐zation and blood rheology in healthy adolescents and those with thyroid dysfunction. Georgian Medical News, 260(Issue), 28–34. [PubMed] [Google Scholar]
- Bhankhar, R. R. , Hungund, S. , Kambalyal, P. , Singh, V. , & Jain, K. (2017). Effect of nonsurgical periodontal therapy on thyroid stimulating hormone in hypothyroid patients with periodontal diseases. Indian Journal of Dental Research, 28(1), 16–21. 10.4103/ijdr.IJDR_174_16 [DOI] [PubMed] [Google Scholar]
- Brito, F. , de Barros, F. C. , Zaltman, C. , Carvalho, A. T. , Carneiro, A. J. , Fischer, R. G. , … Figueredo, C. M. (2008). Prevalence of periodontitis and DMFT index in patients with Crohn's disease and ulcerative colitis. Journal of Clinical Periodontology, 35(6), 555–560. 10.1111/j.1600-051X.2008.01231.x [DOI] [PubMed] [Google Scholar]
- Britto, J. M. , Fenton, A. J. , Holloway, W. R. , & Nicholson, G. C. (1994). Osteoblasts mediate thyroid hormone stimulation of osteoclastic bone resorption. Endocrinology, 134(1), 169–176. 10.1210/endo.134.1.8275930 [DOI] [PubMed] [Google Scholar]
- Chatzopoulos, G. S. , & Tsalikis, L. (2016). Periodontal treatment needs and systemic diseases in an older population in Greece. Journal of Clinical and Experimental Dentistry, 8(1), e32–e37. 10.4317/jced.52763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysanthakopoulos, N. A. , & Chrysanthakopoulos, P. A. (2013). Examination of the relation between periodontal disease indices in patients with systemic diseases. Acta Stomatologica Croatica, 47(3), 217–232. 10.15644/asc47/3/3 [DOI] [Google Scholar]
- Chrysanthakopoulos, N. A. , & Chrysanthakopoulos, P. A. (2016). Association between indices of clinically‐defined periodontitis and self‐reported history of systemic medical conditions. Journal of Investigative and Clinical Dentistry, 7(1), 27–36. 10.1111/jicd.12119 [DOI] [PubMed] [Google Scholar]
- Coles, A. J. , Wing, M. , Smith, S. , Coraddu, F. , Greer, S. , Taylor, C. , … Waldmann, H. (1999). Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. The Lancet, 354(9191), 1691–1695. 10.1016/S0140-6736(99)02429-0 [DOI] [PubMed] [Google Scholar]
- Dalago, H. R. , Schuldt Filho, G. , Rodrigues, M. A. , Renvert, S. , & Bianchini, M. A. (2017). Risk indicators for peri‐implantitis. A cross‐sectional study with 916 implants. Clinical Oral Implants Research, 28(2), 144–150. 10.1111/clr.12772 [DOI] [PubMed] [Google Scholar]
- De Toledo, S. , Bozzo, L. , Do Nascimento, A. , & Sallum, A. W. (1979). Changes of the thyroid gland and periodontal tissues of marmosets related to experimental hypothyroidism. Revista Brasileira de Pesquisas Médicas e Biológicas, 12(2‐3), 141–146. [PubMed] [Google Scholar]
- Desai, J. , Yassa, L. , Marqusee, E. , George, S. , Frates, M. C. , Chen, M. H. , … Demetri, G. D. (2006). Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Annals of Internal Medicine, 145(9), 660–664. 10.7326/0003-4819-145-9-200611070-00008 [DOI] [PubMed] [Google Scholar]
- Eriksen, E. F. , Mosekilde, L. , & Melsen, F. (1986). Kinetics of trabecular bone resorption and formation in hypothyroidism: Evidence for a positive balance per remodeling cycle. Bone, 7(2), 101–108. 10.1016/8756-3282(86)90681-2 [DOI] [PubMed] [Google Scholar]
- Eskes, S. A. , & Wiersinga, W. M. (2009). Amiodarone and thyroid. Best Practice & Research Clinical Endocrinology & Metabolism, 23(6), 735–751. 10.1016/j.beem.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Feitosa, D. S. , Marques, M. R. , Casati, M. Z. , Sallum, E. A. , Nociti, F. H. Jr. , & de Toledo, S. (2009). The influence of thyroid hormones on periodontitis‐related bone loss and tooth‐supporting alveolar bone: A histological study in rats. Journal of Periodontal Research, 44(4), 472–478. 10.1111/j.1600-0765.2008.01144.x [DOI] [PubMed] [Google Scholar]
- Genco, R. , Offenbacher, S. , & Beck, J. (2002). Periodontal disease and cardiovascular disease: Epidemiology and possible mechanisms. Journal of the American Dental Association (1939), 133(Suppl), 14s–22s. 10.14219/jada.archive.2002.0375 [DOI] [PubMed] [Google Scholar]
- Genco, R. J. , & Borgnakke, W. S. (2013). Risk factors for periodontal disease. Periodontology 2000, 62(1), 59–94. 10.1111/j.1600-0757.2012.00457.x [DOI] [PubMed] [Google Scholar]
- Gupta, R. , Goel, K. , Solanki, J. , & Gupta, S. (2014). Oral manifestations of hypothyroidism: A case report. Journal of Clinical and Diagnostic Research, 8(5), 20–22. 10.7860/JCDR/2014/8905.4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashneh, R. A. , Khader, Y. S. , Alhumouz, M. K. , Jadallah, K. , & Ajlouni, Y. (2012). The association between inflammatory bowel disease and periodontitis among Jordanians: A case‐control study. Journal of Periodontal Research, 47(3), 293–298. 10.1111/j.1600-0765.2011.01431.x [DOI] [PubMed] [Google Scholar]
- Investigators, C. T (2008). Alemtuzumab vs. interferon beta‐1a in early multiple sclerosis. New England Journal of Medicine, 359(17), 1786–1801. [DOI] [PubMed] [Google Scholar]
- Klein, J. R. (2006). The immune system as a regulator of thyroid hormone activity. Experimental Biology and Medicine (Maywood, N.J.), 231(3), 229–236. 10.1177/153537020623100301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos, P. (2003). Thyroid hormones: Beneficial or deleterious for bone? Calcified Tissue International, 73(3), 205–209. 10.1007/s00223-002-0027-8 [DOI] [PubMed] [Google Scholar]
- Little, J. W. (2006). Thyroid disorders. Part II: Hypothyroidism and thyroiditis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 102(2), 148–153. 10.1016/j.tripleo.2005.05.070 [DOI] [PubMed] [Google Scholar]
- Mannavola, D. , Coco, P. , Vannucchi, G. , Bertuelli, R. , Carletto, M. , Casali, P. G. , … Fugazzola, L. (2007). A novel tyrosine‐kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. The Journal of Clinical Endocrinology & Metabolism, 92(9), 3531–3534. 10.1210/jc.2007-0586 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2010). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. International Journal of Surgery, 8(5), 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Molloy, J. , Wolff, L. F. , Lopez‐Guzman, A. , & Hodges, J. S. (2004). The association of periodontal disease parameters with systemic medical conditions and tobacco use. Journal of Clinical Periodontology, 31(8), 625–632. 10.1111/j.1600-051X.2004.00539.x [DOI] [PubMed] [Google Scholar]
- Monfoulet, L. E. , Rabier, B. , Dacquin, R. , Anginot, A. , Photsavang, J. , Jurdic, P. , … Chassande, O. (2011). Thyroid hormone receptor beta mediates thyroid hormone effects on bone remodeling and bone mass. Journal of Bone and Mineral Research, 26(9), 2036–2044. 10.1002/jbmr.432 [DOI] [PubMed] [Google Scholar]
- Mosekilde, L. , Eriksen, E. F. , & Charles, P. (1990). Effects of thyroid hormones on bone and mineral metabolism. Endocrinology and Metabolism Clinics of North America, 19(1), 35–63. 10.1016/S0889-8529(18)30338-4 [DOI] [PubMed] [Google Scholar]
- Mosekilde, L. , & Melsen, F. (1978). Morphometric and dynamic studies of bone changes in hypothyroidism. Acta Pathologica et Microbiologica Scandinavica. Section A, 86(1), 56–62. [DOI] [PubMed] [Google Scholar]
- Mosekilde, L. , Melsen, F. , Bagger, J. P. , Myhre‐Jensen, O. , & Schwartz Sorensen, N. (1977). Bone changes in hyperthyroidism: Interrelationships between bone morphometry, thyroid function and calcium‐phosphorus metabolism. Acta Endocrinologica, 85(3), 515–525. 10.1530/acta.0.0850515 [DOI] [PubMed] [Google Scholar]
- Nesse, W. , Dijkstra, P. U. , Abbas, F. , Spijkervet, F. K. , Stijger, A. , Tromp, J. A. , … Vissink, A. (2010). Increased prevalence of cardiovascular and autoimmune diseases in periodontitis patients: A cross‐sectional study. Journal of Periodontology, 81(11), 1622–1628. 10.1902/jop.2010.100058 [DOI] [PubMed] [Google Scholar]
- Patil, B. S. , & Giri, G. R. (2012). A clinical case report of Hashimoto's thyroiditis and its impact on the treatment of chronic periodontitis. Nigerian Journal of Clinical Practice, 15(1), 112–114. 10.4103/1119-3077.94113 [DOI] [PubMed] [Google Scholar]
- Patil, B. S. , Patil, S. , & Gururaj, T. R. (2011). Probable autoimmune causal relationship between periodontitis and Hashimotos thyroidits: A systemic review. Nigerian Journal of Clinical Practice, 14(3), 253–261. 10.4103/1119-3077.86763 [DOI] [PubMed] [Google Scholar]
- Pinto, A. C. G. (1974). Effect of hypothyroidism obtained experimentally in periodontium of rat. Journal of Periodontology, 45(4), 217–221. 10.1902/jop.1974.45.4.217 [DOI] [PubMed] [Google Scholar]
- Rahangdale, S. I. , & Galgali, S. R. (2018). Periodontal status of hypothyroid patients on thyroxine replacement therapy: A comparative cross‐sectional study. Journal of Indian Society of Periodontology, 22(6), 535–540. 10.4103/jisp.jisp_316_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, A. , Kremenevskaja, N. , Resch, J. , & Brabant, G. (2005). Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/β‐catenin signalling. European Journal of Endocrinology, 153(6), 929–938. 10.1530/eje.1.02038 [DOI] [PubMed] [Google Scholar]
- Roberts, C. G. , & Ladenson, P. W. (2004). Hypothyroidism. Lancet, 363(9411), 793–803. 10.1016/s0140-6736(04)15696-1 [DOI] [PubMed] [Google Scholar]
- Saima, S. , Tasneem, S. A. , & Gowhar, O. (2016). Oral health status of children suffering from thyroid disorders. Annals of Dental Specialty, 4(2), 25–28. [Google Scholar]
- Schneider, L. C. (1969). Periodontal disease in hypothyroid adult rats. Archives of Oral Biology, 14(10), 1169–1175. 10.1016/0003-9969(69)90156-3 [DOI] [PubMed] [Google Scholar]
- Schou, M. , Amdisen, A. , Jensen, S. E. , & Olsen, T. (1968). Occurrence of goitre during lithium treatment. British Medical Journal, 3(5620), 710–713. 10.1136/bmj.3.5620.710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomsawasdi, P. , Ringsdorf, W. M. Jr. , & Cheraskin, E. (1965a). Photomotogrammetry and oral health. I. A study of euthyroidism and clinical tooth mobility. Periodontics, 3, 60–65. [PubMed] [Google Scholar]
- Soomsawasdi, P. , Ringsdorf, W. M. Jr. , & Cheraskin, E. (1965b). Photomotogrammetry and oral health. Ii. A study of euthyroidism and tooth loss. Journal of Periodontology, 36, 115–120. 10.1902/jop.1965.36.2.115 [DOI] [PubMed] [Google Scholar]
- de Souza, J. G. , Neto, A. R. , Filho, G. S. , Dalago, H. R. , de Souza Junior, J. M. , & Bianchini, M. A. (2013). Impact of local and systemic factors on additional peri‐implant bone loss. Quintessence International, 44(5), 415–424. 10.3290/j.qi.a29152 [DOI] [PubMed] [Google Scholar]
- van Steenberghe, D. , Jacobs, R. , Desnyder, M. , Maffei, G. , & Quirynen, M. (2002). The relative impact of local and endogenous patient‐related factors on implant failure up to the abutment stage. Clinical Oral Implants Research, 13(6), 617–622. 10.1034/j.1600-0501.2002.130607.x [DOI] [PubMed] [Google Scholar]
- Tahboub, R. , & Arafah, B. M. (2009). Sex steroids and the thyroid. Best Practice & Research Clinical Endocrinology & Metabolism, 23(6), 769–780. 10.1016/j.beem.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Tran, H. A. , Malcolm Reeves, G. E. , Gibson, R. , & Attia, J. R. (2009). Development of thyroid diseases in the treatment of chronic hepatitis C with α‐interferon may be a good prognosticator in achieving a sustained virological response: A meta‐analysis. Journal of Gastroenterology and Hepatology, 24(7), 1163–1168. 10.1111/j.1440-1746.2009.05874.x [DOI] [PubMed] [Google Scholar]
- Vavricka, S. R. , Manser, C. N. , Hediger, S. , Vogelin, M. , Scharl, M. , Biedermann, L. , … Frei, P. (2013). Periodontitis and gingivitis in inflammatory bowel disease: A case‐control study. Inflammatory Bowel Diseases, 19(13), 2768–2777. 10.1097/01.MIB.0000438356.84263.3b [DOI] [PubMed] [Google Scholar]
- Venkatesh Babu, N. S. , & Patel, P. B. (2016). Oral health status of children suffering from thyroid disorders. Journal of the Indian Society of Pedodontics and Preventive Dentistry, 34(2), 139–144. 10.4103/0970-4388.180443 [DOI] [PubMed] [Google Scholar]
- Wolff, B. , Berger, T. , Frese, C. , Max, R. , Blank, N. , Lorenz, H. M. , & Wolff, D. (2014). Oral status in patients with early rheumatoid arthritis: A prospective, case‐control study. Rheumatology (Oxford), 53(3), 526–531. 10.1093/rheumatology/ket362 [DOI] [PubMed] [Google Scholar]
- Xie, Q. F. , & Ainamo, A. (1999). Association of edentulousness with systemic factors in elderly people living at home. Community Dentistry and Oral Epidemiology, 27(3), 202–209. 10.1111/j.1600-0528.1999.tb02011.x [DOI] [PubMed] [Google Scholar]
- Yerke, L. M. , Levine, M. J. , & Cohen, R. E. (2019). Potential relationship between hypothyroidism and periodontal disease severity. Journal of the Endocrine Society, 3(Suppl. 1). 10.1210/js.2019-mon-616 [DOI] [Google Scholar]
- Yussif, N. M. , El‐Mahdi, F. M. , & Wagih, R. (2017). Hypothyrodism as a risk factor of periodontitis and its relation with vitamin D deficiency: Mini‐review of literature and a case report. Clinical Cases in Mineral and Bone Metabolism, 14(3), 312–316. 10.11138/ccmbm/2017.14.3.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid, T. M. , Wang, B. Y. , & Cohen, R. E. (2011). The effects of thyroid hormone abnormalities on periodontal disease status. Journal of the International Academy of Periodontology, 13(3), 80–85. [PubMed] [Google Scholar]
- Zeigler, C. C. , Wondimu, B. , Marcus, C. , & Modéer, T. (2015). Pathological periodontal pockets are associated with raised diastolic blood pressure in obese adolescents. BMC Oral Health, 15(1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]


