Abstract
Gut microbiota influences host behaviour and physiology, such as anxiety, stress, serotonergic and immune systems. These behavioural and physiological characteristics are related to feather pecking (FP), a damaging behaviour in chickens that reduces animal welfare and productivity. Moreover, high FP (HFP) and low FP (LFP) lines differed in microbiota composition. However, it is unknown whether microbiota can influence the development of FP. For the first time, we identified the effects of microbiota transplantation on FP, and behavioural and physiological characteristics related to FP. HFP and LFP chicks received sterile saline (control), HFP or LFP microbiota transplantation during the first two weeks post-hatch. Microbiota transplantation influenced behavioural responses of the HFP line during treatment and of the LFP line after treatment. In both lines, homologous microbiota transplantation (i.e., receiving microbiota from their line) resulted in more active behavioural responses. Furthermore, microbiota transplantation influenced immune characteristics (natural antibodies) in both lines and peripheral serotonin in the LFP line. However, limited effects on microbiota composition, stress response (corticosterone) and FP were noted. Thus, early-life microbiota transplantation had immediate and long-term effects on behavioural responses and long-term effects on immune characteristics and peripheral serotonin; however, the effects were dependent on host genotype. Since early-life microbiota transplantation influenced behavioural and physiological characteristics that are related to FP, it could thus influence the development of FP later in life.
Subject terms: Animal behaviour, Animal physiology, Immunology, Microbiology, Neuroscience
Introduction
Early-life is crucial for an animal’s behavioural and physiological development and early-life factors can have a profound impact on this development1. An important moment early in life is the rapid microbial colonization of the gut, leading to the establishment of the gut microbiota. The gut microbiota influences host behaviour and physiology2–5. Furthermore, altering microbiota composition, via for example anti- or probiotic treatment, affects anxiety, stress and activity6–8, as well as the serotonergic and immune systems in rodents9–12. Moreover, germ-free mice colonized with microbiota from another mouse strain exhibit behavioural profiles of the donor strain13. The gut microbiota seems to have similar effects in poultry, where altering microbiota composition affects fearfulness, memory, and serotonergic and immune systems14–16. Moreover, microbiota transplantation to germ-free quails has resulted in recipients adopting the fearful behaviour of donors early in life; however, this effect reversed later in life17. These findings suggest that the gut microbiota influences behavioural and physiological characteristics in poultry and could therefore influence a bird’s ability to cope with environmental and social challenges, such as those encountered in animal production systems.
Excessive damaging behaviours are indicative of an animal’s inability to cope with a restrictive environment and are frequently seen in production animals. Feather pecking (FP) in chickens is one such damaging behaviour, which involves hens pecking and pulling at feathers of conspecifics, thereby reducing animal welfare and productivity18. Feather pecking is multifactorial and has been linked to numerous behavioural characteristics, such as fearfulness, stress and activity, as well as physiological characteristics, such as serotonergic, dopaminergic and immune systems19–21. Since behavioural and physiological systems that are related to FP are also affected by the gut microbiota, microbiota might play a role in the development of FP. Indeed, lines selected for high FP (HFP) and low FP (LFP) differ in behavioural responses, stress response, activity, central serotonergic and dopaminergic activity, peripheral serotonin, innate and adaptive immune characteristics22–28. Moreover, the HFP and LFP lines differ in intestinal microbial metabolites and microbiota composition determined from caecal droppings and intestinal luminal content29–31. These findings point to a relationship between the gut microbiota and FP, however, it is unknown whether the gut microbiota influences the development of FP.
Therefore, this study aims to identify the effects of early-life microbiota transplantation on FP and behavioural and physiological characteristics related to FP in lines divergently selected for FP (HFP and LFP lines). We further identify the effects of microbiota transplantation on microbiota composition. We hypothesize that microbiota transplantation results in recipients adopting a similar behavioural profile as that seen in the donor line. For example, LFP birds receiving HFP microbiota show more FP and more active behavioural responses compared to LFP birds receiving LFP microbiota or control treatment.
Results
High and low feather pecking transplantation pools had distinct microbiota composition
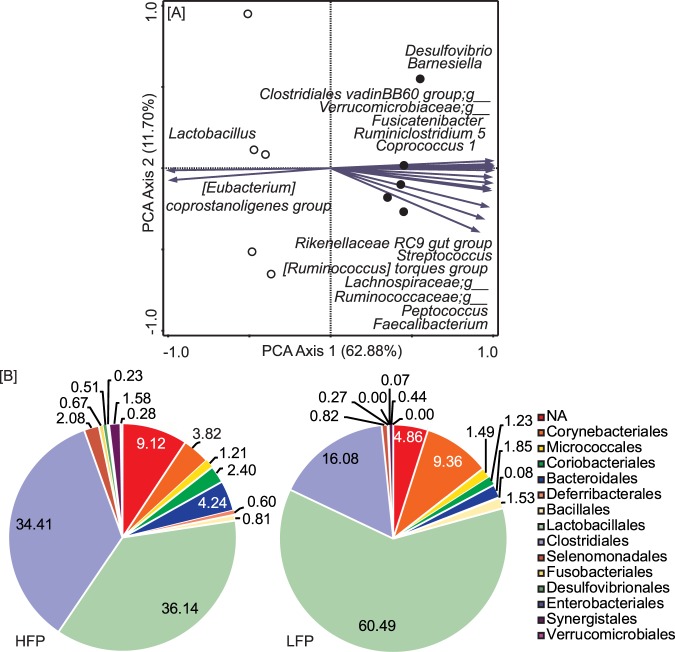
Gut microbiota was collected from adult chickens of the HFP and LFP lines that were shown to differ in microbiota composition31. Transplantation pools were made per line and could be distinguished from each other in terms of microbiota composition using a principal component analysis (PCA) (Fig. 1A). The orders of Clostridiales and Lactobacillales had the highest relative abundance in both pools. The HFP pool had a higher relative abundance of Clostridiales and the LFP pool had a higher relative abundance of Lactobacillales (Fig. 1B). The number of viable microorganisms in the pools was analysed using plate cultures, and both pools contained on average 4.75 × 106 viable aerobic colony forming units/mL and 5.1 × 106 viable anaerobic colony forming units/mL.
Figure 1.
(A) Biplot for principal component analysis (PCA) of transplantation pools’ microbiota composition. Samples are grouped by line: HFP (closed circles) and LFP (open circles) with 5 replicates per pool. Microbial groups for which the variation in relative abundance in the data is explained for at least 95% by the axes are represented as vectors. Groups that could not be assigned to a specific genus are classified by the family name appended with “;g_”. (B) Pie charts with average relative abundances of orders present in the HFP (left) and LFP (right) transplantation pools.
Early-life microbiota transplantation had limited effects on recipients’ microbiota composition
Newly hatched chicks received sterile saline (control), HFP or LFP microbiota transplantation within 6 h post-hatch to influence bacterial colonization32, and each day during the first 2 weeks post-hatch, a period when synapses are still being formed in the brain33. Microbiota was sampled from the luminal content of the ileum, caecum or colon at 5 days and 2 weeks of age to assess the effects of microbiota transplantation on recipients’ microbiota composition using 16S rRNA gene sequencing at the approximate genus-level. There was no overlap between microbiota composition of individual donors or the transplantation pools and that of recipients using a PCA (Sup. Fig. 1). Furthermore, multivariate redundancy analysis (RDA) showed a high overlap of line * treatment groups (Sup. Fig. 2), treatments (Sup. Fig. 3) and treatments within lines (Sup. Fig. 4–6). However, birds receiving the control treatment could be distinguished from birds receiving microbiota transplantation based on caecal microbial composition at 5 days and 2 weeks of age, where the control treatment explained 5.5% and 6.3%, respectively of the observed variation in microbiota composition (P = 0.032 and P = 0.004, respectively). Furthermore, within the HFP line, HFP birds receiving the control treatment could be distinguished from other groups in caecal microbial composition at 2 weeks of age, where the control treatment explained 11.2% of the observed variation in microbiota composition (P = 0.036; Fig. 2). This finding suggests the microbiota composition of HFP birds receiving the control treatment was distinct from that of HFP birds receiving HFP or LFP microbiota. To further analyse differences in microbiota composition, we determined whether line * treatment groups, treatments or treatments within lines differed in relative abundances of microbial groups. However, only a few microbial groups differed between line * treatment groups, treatments or treatments within lines, and most microbial groups that differed had on average low relative abundances (<1%) (Sup. Table 1). Overall, these results suggest limited effects of early-life microbiota transplantation on recipients’ microbiota composition.
Figure 2.
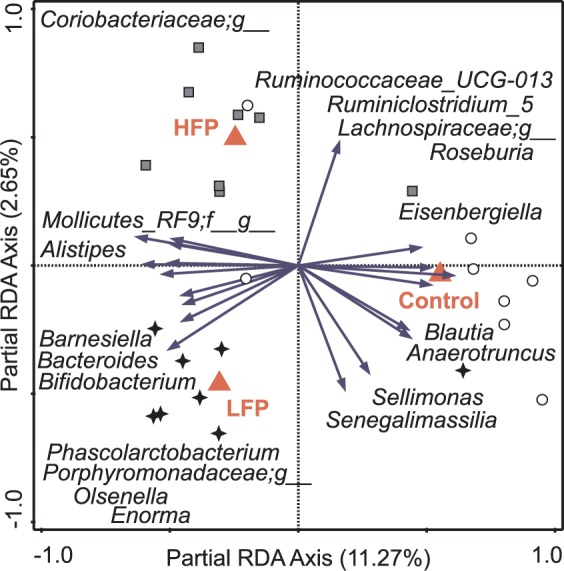
Triplot for partial redundancy analysis (RDA) of caecum microbiota composition of the high feather pecking (HFP) line at 2 weeks of age. Microbial groups for which the variation in relative abundance in the data is explained for at least 20% by the axes are represented as vectors. Nominal environmental treatment variables are represented by red triangles. Samples are grouped by treatment: HFP microbiota (grey squares), control (white circles) and low feather pecking (LFP) microbiota (black stars). Groups that could not be assigned to a specific genus are classified by the family name appended with “;g_”.
Early-life microbiota transplantation influenced behavioural responses
Several behavioural tests were performed to assess fearfulness and the stress response34,35. During treatment, birds were tested in a novel object test at 3 days of age and a novel environment test at 1 week of age. After treatment, birds were tested in a second novel object test at 5 weeks of age, a tonic immobility test at 9 weeks of age, an open field test at 13 weeks of age and a manual restraint test at 15 weeks of age. Fearfulness and the stress response were measured given that anxiety-like behaviour and stress were influenced by gut microbiota in rodents2,5, and fearfulness and stress sensitivity are related to the development of FP19. Here, findings with P-values < 0.1 were reported concerning the effects of line * treatment interactions, treatment and treatment within lines, but a complete overview is given in Sup. Table 2.
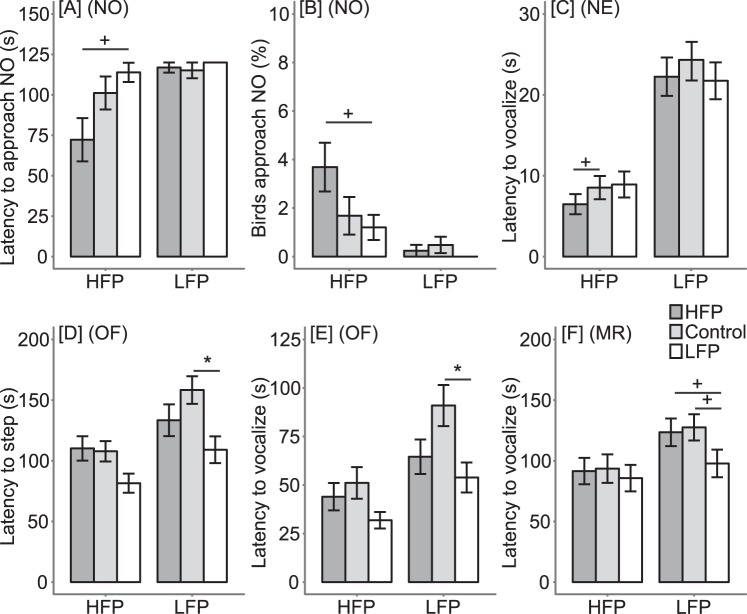
During treatment, significant line * treatment interactions were found for latency to approach the novel object (χ2 = 16.32, df = 5, P = 0.006), the percentage of birds approaching the novel object (χ2 = 22.69, df = 5, P < 0.001) (Sup. Fig. 7) and flight attempts during the novel environment test (F2,41 = 3.27, P = 0.048) (Sup. Fig. 8). However, no treatment effects were found on behavioural responses to the novel object or novel environment. We further analysed the effect of treatment within lines because we were interested in whether treatments within a line differed from each other. Interestingly, we found tendencies for treatment effects within the HFP line, where it tended to affect the latency to approach the novel object (χ2 = 5.71, df = 2, P = 0.058; Fig. 3A), percentage of birds approaching the novel object (χ2 = 5.17, df = 2, P = 0.075; Fig. 3B) and the latency to vocalize in the novel environment test (F2,20 = 2.63, P = 0.097; Fig. 3C). HFP chicks receiving HFP microbiota tended to approach the novel object sooner (P = 0.064) and more birds tended to approach it (P = 0.091) compared to HFP chicks receiving LFP microbiota. Furthermore, HFP chicks receiving HFP microbiota tended to vocalize sooner compared to HFP chicks receiving the control treatment (P = 0.091). These results suggest that during treatment, HFP chicks receiving HFP microbiota showed more active behavioural responses compared to HFP chicks receiving LFP microbiota or the control treatment.
Figure 3.
(A) Mean latency (±SE) for three birds to approach the novel object (NO) and (B) mean percentage (±SE) of birds approaching the NO at 3 days of age; (C) mean latency to vocalize (±SE) in the novel environment (NE) test at 1 week of age; (D) mean latency to step (±SE) and (E) mean latency to vocalize (±SE) in the open field (OF) test at 13 weeks of age; (F) mean latency to vocalize (±SE) in the manual restraint (MR) test at 15 weeks of age for the high (HFP) and low feather pecking (LFP) lines treated with HFP microbiota, control treatment or LFP microbiota. +denotes tendencies (P < 0.1) and *denotes significant differences (P < 0.05) between treatments within lines.
After treatment, significant line * treatment interactions were found on latency to approach the novel object (χ2 = 20.38, df = 5, P = 0.001) and percentage of birds approaching the novel object (χ2 = 19.35, df = 5, P = 0.002) (Sup. Fig. 7). Furthermore, treatment effects were found for the number of inductions needed to reach tonic immobility (F2,43 = 3.39, P = 0.043) and latency to step (F2,43 = 7.42, P = 0.002) and vocalize (F2,43 = 5.66, P = 0.007) in the open field test. Birds receiving LFP microbiota needed fewer inductions to reach tonic immobility compared to birds receiving HFP microbiota (P = 0.043) (Sup. Fig. 9A). In the open field test, birds receiving LFP microbiota stepped and vocalized sooner compared to birds receiving the control treatment (P = 0.001 and P = 0.005, respectively) and tended to step sooner compared to birds receiving HFP microbiota (P = 0.059) (Sup. Fig. 9B,C). To explore these treatment effects in more detail, we analysed treatment effects within lines. Interestingly, we found treatment effects within the LFP line, where it affected latency to step (F2,20 = 5.77, P = 0.011; Fig. 3D) and vocalize (F2,20 = 5.13, P = 0.016; Fig. 3E) in the open field test and latency to vocalize during restraint (F2,20 = 3.79, P = 0.04; Fig. 3F). In the open field test, LFP birds receiving LFP microbiota stepped (P = 0.008) and vocalized (P = 0.013) sooner compared to LFP birds receiving the control treatment. In the manual restraint test, LFP birds receiving LFP microbiota tended to vocalize sooner compared to LFP birds receiving HFP microbiota or the control treatment (P = 0.096 and P = 0.051, respectively). These results suggest that after treatment LFP birds receiving LFP microbiota showed more active behavioural responses compared to LFP birds receiving HFP microbiota or the control treatment.
In summary, these results indicate that behavioural responses were influenced by early-life microbiota transplantation, where effects were found during treatment in the HFP line and after treatment in the LFP line. Furthermore, in both lines, birds receiving homologous microbiota transplantation (i.e., receiving microbiota from their line) showed more active behavioural responses.
Early-life microbiota transplantation influenced natural antibodies and peripheral serotonin, but not corticosterone
Several physiological characteristics were measured after the treatment period. Natural antibody titres (NAb, an antibody that binds antigen without prior intentional exposure to that antigen36) were measured at 5, 10 and 15 weeks of age as NAb’s play an essential role in both innate and adaptive immunity37–39 and were therefore used as a general immune characteristic. At 15 weeks of age, corticosterone level after manual restraint was used as an indicator of the physiological stress response23, and whole blood serotonin level was used as an indicator of central serotonin levels40. These physiological characteristics were measured given that the immune, stress and serotonergic systems are influenced by gut microbiota in rodents3–5, and these systems are related to FP19–21. Here, findings with P-values < 0.1 were reported concerning the effects of line * treatment interactions, treatment and treatment within lines, but a complete overview is given in Sup. Table 3.
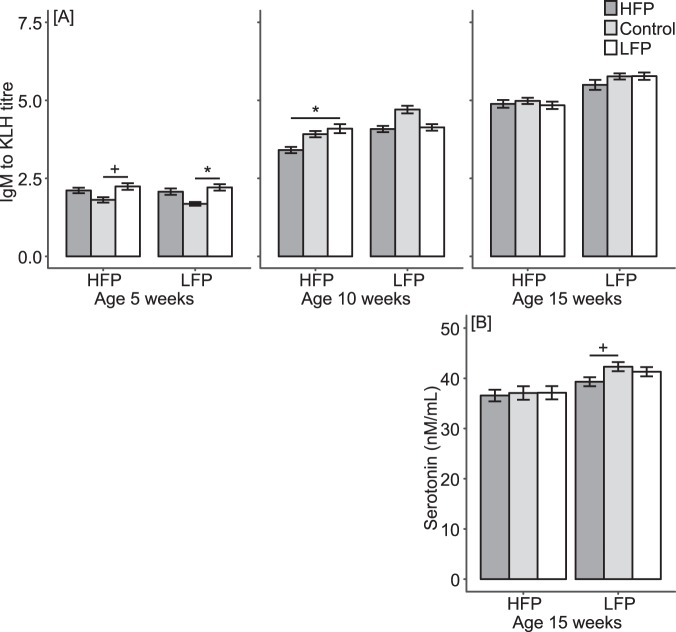
No effects of line * treatment interactions were found on any of the physiological characteristics. However, treatment effects were found on IgM NAb titres at 5 (F2,43 = 7.87, P = 0.001) and 10 weeks of age (F2,43 = 7.94, P = 0.031). Birds receiving the control treatment had lower IgM titres compared to birds receiving HFP (P = 0.018) or LFP microbiota (P = 0.001) at 5 weeks of age. However, at 10 weeks of age, they had higher IgM titres compared to birds receiving HFP microbiota (P = 0.026) (Sup. Fig. 10). To explore these treatment effects in more detail we analysed treatment effects within lines. In the HFP line, treatment tended to influence IgM NAb titres at 5 weeks of age (F2,20 = 3.18, P = 0.063) and significantly influenced IgM NAb titres at 10 weeks of age (F2,20 = 4.03, P = 0.034) (Fig. 4A). HFP birds receiving LFP microbiota tended to have higher IgM titres compared to HFP birds receiving the control treatment at 5 weeks of age (P = 0.064) and further had higher IgM titres compared to HFP birds receiving HFP microbiota at 10 weeks of age (P = 0.031). In the LFP line, we found a treatment effect on IgM NAb titres at 5 weeks of age (F2,20 = 4.41, P = 0.026) (Fig. 4A). LFP birds receiving LFP microbiota had higher IgM titres compared to LFP birds receiving the control treatment at 5 weeks of age (P = 0.025). Furthermore, treatment tended to influence peripheral serotonin within the LFP line (F2,20 = 2.62, P = 0.098; Fig. 4B), where LFP birds receiving HFP microbiota tended to have lower serotonin levels compared to LFP birds receiving the control treatment (P = 0.084).
Figure 4.
(A) Mean IgM natural antibody titres to keyhole limpet haemocyanin (KLH) (±SE) at 5, 10 and 15 weeks of age and (B) mean serotonin level (±SE) at 15 weeks of age for the high (HFP) and low feather pecking (LFP) lines treated with HFP microbiota, control treatment or LFP microbiota. +denotes tendencies (P < 0.1) and *denotes significant differences (P < 0.05) between treatments within lines.
Overall, these results suggest that early-life microbiota transplantation influenced IgM NAb in both lines and tended to influence peripheral serotonin in the LFP line after the treatment period. However, early-life microbiota transplantation did not influence IgG NAb or corticosterone.
Early-life microbiota transplantation had limited effects on feather pecking
Feather pecking was observed between 0–1, 2–3, 4–5, 9–10 and 14–15 weeks of age at the pen level and was categorized into gentle FP (subdivided into exploratory and stereotyped FP) and severe FP. Here, gentle FP typically does not result in damage, and severe FP is the problematic behaviour in terms of damage to the recipient in animal production systems19. During and after the treatment period, significant line * treatment interactions were found for stereotyped FP and severe FP but not exploratory FP (for a complete overview see Sup. Table 4 and Sup. Fig. 11A–C). After treatment, a tendency for a treatment effect on exploratory FP was found for weeks 2–3 (F2,43 = 2.34, P = 0.083), where birds receiving HFP microbiota tended to show less exploratory FP compared to birds receiving LFP microbiota (P = 0.085). We explored treatment effects in more detail by analysing treatment effects within lines. In the LFP line, a tendency for a treatment effect was found for exploratory FP in weeks 4–5 (χ2 = 5.16, df = 2, P = 0.076). LFP birds receiving HFP microbiota tended to show more exploratory FP compared to LFP birds receiving the control treatment (P = 0.09). In summary, these results indicate that FP up to 15 weeks of age was not influenced by early-life microbiota transplantation.
Discussion
This study aimed to identify effects of early-life microbiota transplantation on feather pecking (FP), and behavioural and physiological characteristics related to FP in high FP (HFP) and low FP (LFP) selection lines. We hypothesized that microbiota transplantation would result in recipients adopting a similar behavioural profile as that seen in the donor line. To summarize, behavioural responses were influenced by early-life microbiota transplantation, where the effects of homologous transplantation (i.e., receiving microbiota from their line) were seen during treatment in the HFP line and after treatment in the LFP line. Concerning physiological characteristics, we found effects on IgM natural antibodies in both lines and a tendency on peripheral serotonin in the LFP line, but not on IgG natural antibodies or corticosterone. Furthermore, early-life microbiota transplantation had limited effects on microbiota composition and FP.
Microbiota composition of recipients
Early-life microbiota transplantation had limited effects on microbiota composition of recipients. A potential explanation for this could be that transplantation pools consisted of adult microbiota. Adult microbiota might not be able to colonize and remain within the gut of newly hatched chicks since the gut microbiota is still developing and undergoes a rapid succession until it is completely developed and stable around seven weeks of age32,41. Furthermore, we sampled luminal content instead of mucosal scrapings. Mucosa-associated microbiota composition might be more involved in communication with the host given its proximity42 and differs from luminal microbiota composition43,44. Mucosa-associated microbiota composition might have been altered by our microbiota transplantation, but the FP selection lines did not differ in mucosa-associated microbiota composition in our previous study31. Previous studies in rodents and quails found effects of microbiota transplantation on microbiota composition of recipients13,17,45–47. However, these studies used pseudo-germ-free (i.e., received antibiotic treatment before transplantation) or germ-free animals and most identified microbiota composition from faecal samples. Using (pseudo-) germ-free animals allows for stronger effects of microbiota transplantation on microbiota composition as these animals are sterile or depleted of microbiota and are often housed in a sterile environment. However, such animal models are rather extreme, making it difficult to translate findings to ‘normally’ occurring situations. Furthermore, identifying microbiota composition from different gut sections (i.e., ileum, caecum or colon) is crucial, as microbiota composition of faecal samples is variable because it originates from different gut sections48, which differ in microbiota composition43,49,50. Thus, differences found in previous studies might be due to faecal sampling.
Although limited effects of early-life microbiota transplantation on microbiota composition were found, microbiota transplantation did affect behavioural responses and natural antibodies and tended to affect peripheral serotonin. Early-life microbiota transplantation potentially influenced brain, immune and serotonergic system functioning. Microbiota transplantation was given during the first two weeks post hatch when both the brain and immune system are still developing33,51,52. There is extensive evidence that gut microbiota affects brain, central serotonergic and immune system functioning3,4,53, and it seems to influence peripheral serotonergic system functioning as well. For example, germ-free mice had lower mRNA levels of tryptophan hydroxylase (enzyme for serotonin synthesis) and higher mRNA levels of the serotonin transporter in intestinal cells54. However, it should be noted that we cannot exclude that other factors in the transplantation pools might have contributed to altering behavioural responses, natural antibodies and peripheral serotonin, for example, viruses or fungi55,56. We suggest that microbiota transplantation influenced brain, serotonergic and immune system functioning, which (in)directly resulted in differences in behavioural responses, natural antibodies and serotonin.
Are recipients adopting the behavioural profile of donors?
Previously, HFP birds showed more active responses compared to LFP birds in the behavioural tests that were performed in the present study24,25,27 and other behavioural tests57. Thus, during the treatment period, behavioural responses seem to be adopted from donors in the HFP line as HFP chicks receiving HFP microbiota tended to show more active responses (i.e., approached novel object sooner and more birds approached it, vocalized sooner) compared to HFP chicks receiving LFP microbiota or the control treatment. In contrast, after the treatment period behavioural responses were not adopted from donors in the LFP line as LFP birds receiving LFP microbiota showed more active responses (i.e., stepped and vocalized sooner) compared to LFP birds receiving HFP microbiota or the control treatment. Previous studies in rodents show that behavioural profiles of donors are adopted by recipients via microbiota transplantation13,45,47,58. However, similar to our findings, a recent study using early-life microbiota transplantation in quails showed that birds adopted the behaviour of donors early in life, but that this effect was reversed later in life17. Thus, early-life microbiota transplantation can affect behavioural responses in poultry, and behavioural profiles can be adopted. However, these effects are dependent on age and genotype.
Although behavioural responses were influenced by early-life microbiota transplantation, FP was not, and recipients did not adopt FP behaviour of donors. An explanation for this finding might be that FP usually increases from the egg-laying period onwards (approximately 20 weeks of age)59,60, and we observed FP till 15 weeks of age. It should further be noted that effects on FP might be missed as FP was observed for a limited amount of time, and some variation in severe FP was observed between pens, which is probably caused by severe FP only being performed by few individuals59. Further research is needed to identify the potential effects of early-life microbiota transplantation on FP at adult age.
Homologous microbiota transplantation
Interestingly, in both the HFP and LFP lines, homologous microbiota transplantation (i.e., receiving microbiota from their line) resulted in birds showing more active responses, which suggests reduced fearfulness given that silence and inactivity are related to high fearfulness34,61. Therefore, homologous transplantation could be a potential approach to lower fearfulness in chickens. Many studies show that FP is related to high fearfulness62–64, indicating that receiving homologous transplantation might reduce the risk of birds developing FP. However, it should be noted that no treatment effects within lines were found on severe FP or tonic immobility duration, the measure for innate fearfulness in chickens34. Furthermore, we previously showed that HFP birds had shorter tonic immobility duration compared to LFP birds25, suggesting that FP is related to low fearfulness in the FP selection lines. However, another study reported no difference in tonic immobility duration between the FP selection lines65. Thus, homologous transplantation could be used to reduce fearfulness in poultry, for example by providing faeces from mother hens to chicks, thereby potentially reducing the development of FP.
Microbiota transplantation might be seen as a type of vertical transmission, where microbiota is transferred from mother hens to chicks. Vertical transmission might play an important role in initiating a host-specific gut microbiota, which could improve the host’s immune system and brain development. For example, germ-free mice colonized with human microbiota showed impaired immune system development compared to mice colonized with mouse microbiota66, suggesting that host-specific microbiota is required for immune system maturation. Although this result is noted from a comparison of inter vs. intra species microbiota transplantation, it could still point to improved immune system development through homologous transplantation. Moreover, germ-free mice receiving homologous transplantation had higher brain-derived neurotrophic factor (BDNF) levels in the hippocampus but not in the amygdala compared to germ-free mice receiving microbiota from another strain13. This finding suggests that homologous transplantation might improve brain development, since BDNF is involved in neuronal differentiation, synapse formation and plasticity67. Thus, receiving homologous transplantation could improve the immune system and brain development, potentially altering natural antibodies and behavioural responses. Furthermore, fearfulness was shown to decrease with age in chickens68,69, suggesting that homologous transplantation might accelerate behavioural development. Further research is needed to identify whether homologous transplantation improves immune system and brain development in poultry.
Effects of microbiota transplantation depend on the recipient’s genotype
During treatment, microbiota transplantation tended to influence behavioural responses in the HFP line. However, after treatment, it influenced behavioural responses in the LFP line. A potential explanation for this could be that the HFP line seems to have a more responsive immune system, which reacts more strongly to the environment26–28. A more responsive immune system might result in HFP birds responding more strongly to microbiota transplantation with the synthesis and release of pro-inflammatory cytokines70. Peripherally produced pro-inflammatory cytokines can act on the brain71, where they alter serotonergic and dopaminergic neurotransmission72, which have been indicated to play a role in the development of FP20. However, we did not identify pro-inflammatory cytokine levels or brain neurotransmission in the present study. Yet, direct-fed microbials were shown to alter intestinal mRNA levels of pro-inflammatory cytokines73, and probiotic treatment altered serotonergic and dopaminergic neurotransmission16 in broilers, indicating that microbiota could influence cytokine levels, and serotonergic and dopaminergic neurotransmission in poultry. Thus, further research is needed to identify the effects of microbiota transplantation on cytokine levels and brain neurotransmission in poultry. Furthermore, it should be noted that after the treatment period, both lines responded similarly to microbiota transplantation concerning IgM natural antibodies, and no differences were found for IgG natural antibodies. In addition, behavioural responses of HFP birds tended to be influenced by receiving HFP microbiota compared to LFP microbiota or the control treatment, indicating that receiving any type of adult microbiota composition was not sufficient to alter behavioural responses in the HFP line. It is possible that HFP microbiota had specific effects on other immune characteristics; however, this hypothesis needs further investigation.
After treatment, microbiota transplantation influenced behavioural responses in the LFP line. These effects do not seem to be explained by differences in peripheral serotonin. LFP birds receiving HFP microbiota tended to have lower peripheral serotonin levels compared to LFP birds receiving the control treatment, while behavioural differences were observed between LFP birds receiving LFP microbiota and LFP birds receiving HFP microbiota or the control treatment. Moreover, it should be noted that serotonin cannot cross the blood-brain barrier74; thus, caution is needed when using peripheral serotonin levels as an indicator for central serotonin levels. Nevertheless, it is interesting that LFP birds receiving HFP microbiota tended to have lower peripheral serotonin, as we previously found that HFP birds had lower peripheral serotonin compared to LFP birds27. This finding might point to an increased risk for developing FP in LFP birds receiving HFP microbiota as low peripheral serotonin levels are related to high FP40,64,75. However, the potential pathway through which microbiota transplantation influences behavioural responses in LFP birds remains unclear.
Conclusion
This is the first study to investigate effects of early-life microbiota transplantation on FP, and behavioural and physiological characteristics related to FP. In conclusion, early-life microbiota transplantation influenced behavioural responses that are related to FP. Effects were seen during treatment in the HFP line and after treatment in the LFP line. In both lines, homologous microbiota transplantation resulted in more active behavioural responses, indicating lower fearfulness. Early-life microbiota transplantation further influenced physiological characteristics after treatment, including immune characteristics (natural antibodies) in both lines and peripheral serotonin in the LFP line, but had limited effects on microbiota composition, the physiological stress response (corticosterone) and FP. Thus, early-life microbiota transplantation had immediate and long-term effects on behavioural responses and long-term effects on immune characteristics and peripheral serotonin. However, the effects were genotype dependent. Since microbiota transplantation influenced behavioural and physiological characteristics that are related to FP, it could thereby influence the development of FP. However, microbiota transplantation did not influence FP up to adolescent age and more research is needed to identify whether it could influence FP later in life.
Material and Methods
Animals and housing
White Leghorn birds from the 19th generation of lines selected for high (HFP) and low feather pecking (LFP) were used (see Kjaer et al.76 for the selection procedure). A total of 576 birds (HFP = 288 and LFP = 288) were hatched from two batches of eggs with 3 weeks between batches. Eggs were incubated at an average eggshell temperature of 37.8 °C and an average relative humidity of 55.7%. Eggs were not disinfected, and lines were distributed randomly within the incubator. Eggs were placed in hatching baskets on embryonic day 18, and cardboard was placed on the bottom to prevent cross-contamination. From embryonic day 19, we collected hatched chicks (dry and wet chicks) every 6 h. There was no light in the incubator, and eggshells were removed to limit chicks from pecking at the environment or eggshells through which they could obtain bacteria. Chicks received a neck tag with a unique number and their first treatment and were weighed. Chicks were then placed in separate hatching baskets according to line * treatment group (line [HFP or LFP] * treatment [HFP microbiota, control or LFP microbiota]) with 6 experimental groups in total, which were distributed randomly within the incubator. On embryonic day 21, chicks were sexed and placed in pens according to experimental group with an approximate 50/50 male/female distribution. Non-beak-trimmed birds were used and were housed in groups of 12 birds per pen. At 5 days, 2 weeks and 10 weeks of age, group size was reduced for microbiota sampling (n = 10–11 birds per pen, n = 7–10 birds per pen and n = 6–9 birds per pen, respectively). Batches had the same housing conditions and experimental setup with 4 pens for each experimental group and 24 pens in total, with an overall total of 8 pens for each experimental group and 48 pens for both batches. During hatching and for the first 5 weeks of age, extra hygienic measures were taken to prevent cross-contamination. Gloves were worn when handling birds and switched between treatments. Cover shoes were used when entering a pen and switched between pens.
At all times, water and feed were provided ad libitum. Birds received a commercial rearing diet 1 from hatching until 8 weeks of age and a commercial rearing diet 2 from 8 until 16 weeks of age for laying hens (Agruniek Rijnvallei Voer B.V.). Each floor pen (h: 2 m, l: 1 m, w: 2 m) had wood shavings on the floor, two perches installed 45 cm above the floor and visual barriers of 1.5 m high to prevent birds in adjacent pens from seeing each other. Post hatch, the temperature was maintained at approximately 33 °C and gradually lowered to 24 °C at 4 weeks of age. The light regime was 23 L:1D post-hatch and was gradually reduced to 8 L:16D at 4 weeks of age. Light intensity for each pen ranged between 45 and 81 LUX (average 62.6 LUX) as measured with a Voltcraft MS-1300 light meter (Conrad Electric Benelux). The study was originally designed to end at 30 weeks of age. However, due to unforeseen practical issues that would have seriously influenced our experimental results and were beyond our control, we had to postpone the start of the experiment and were subsequently unable to maintain birds for longer than 15 weeks of age. The experiment was approved by the Central Authority for Scientific Procedures on Animals according to Dutch law (no: AVD104002015150-1).
Treatment
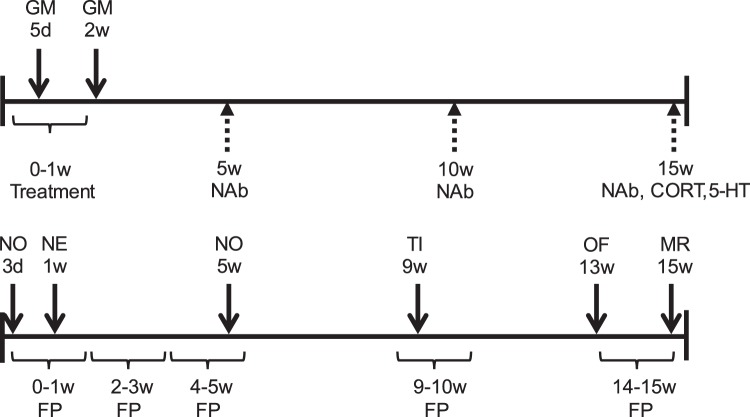
Microbiota transplantation consisted of a mixed pool of luminal content of the ileum, caeca and colon from either HFP or LFP adult birds collected during a previous experiment31. Pooled samples were stored at −80 °C until use. The number of viable aerobic and anaerobic microorganisms in pooled samples was determined using plate cultures with a blood agar medium. Plates were incubated overnight under aerobic or anaerobic conditions at 37 °C, and colonies were counted. Before treatment, pooled samples were defrosted in a 37 °C water bath for 5 min and then centrifuged at 5250 × g for 10 min. The microbial pellet was re-suspended in sterile saline (0.9% NaCl, half of the original volume was added). The control treatment consisted of sterile saline. Treatments were kept on ice in between processing steps and during administration. The first treatment was given within 6 h post-hatch. Thereafter, chicks received treatments daily during the first two weeks post-hatch. Each treatment consisted of 100 µL of the treatment solution administered orally using a pipette (see Fig. 5 for the timeline of the experiment).
Figure 5.
Timeline of the experiment. The upper line indicates physiological measures: microbiota sampling (above the line) and microbiota transplantation treatment and blood sampling (below the line) performed at specific ages in days (d) or weeks (w). GM = gut microbiota, NAb = natural antibodies, CORT = corticosterone and 5-HT = serotonin. The lower line indicates behavioural measures: behavioural tests (above the line) and feather pecking observations (below the line). FP = feather pecking observations, NO = novel object test, NE = novel environment test, TI = tonic immobility test, OF = open field test and MR = manual restraint test.
Microbiota sampling
At 5 days and 2 weeks of age, 8 birds of each experimental group (1 per pen) were randomly selected and sacrificed for the collection of gut microbiota. We collected luminal content from a ±2 cm midsection of the ileum (between Meckel’s diverticulum and the ileo-caeca-colic junction), one of the caeca and the colon. Samples were stored in cryovials at −80 °C until further analysis.
Microbiota analysis
Microbiota composition of transplantation pools (HFP 5 replicates and LFP 5 replicates) and luminal content (n = 8 for each experimental group, gut section and age, total of n = 288) was determined via total DNA extraction, PCR amplification and sequencing as described previously31. PCR amplification was performed with primers directed to the V5-V6 region of the bacterial 16S rRNA gene, namely, BSF784F (5′-RGGATTAGATACCC) and 1064 R (5′-CGACRRCCATGCANCACCT). Data were processed using NG-Tax, an in-house bioinformatics pipeline, as described by Ramiro-Garcia et al.77, which resulted in a minimum of 29324 reads and a maximum of 607793 reads per sample.
Behavioural observations and tests
Feather pecking (FP) behaviour was observed between 0–1, 2–3, 4–5, 9–10 and 14–15 weeks of age. Birds were further subjected to five behavioural tests: novel object, novel environment, open field, tonic immobility and manual restraint. The order of testing was randomized at an individual level, except for FP observations and novel object tests, which were randomized at the pen level because we observed FP and behavioural responses to the novel object at pen level. The experimenters were blinded to the lines and treatments.
Feather pecking observations
Each pen was observed for 30 min, either in the morning (8:30 h-12:30 h) or in the afternoon (12:30 h-16:30 h) after a 2.5-min habituation period. FP was divided into gentle feather pecks (subdivided into exploratory FP and bouts of stereotyped FP) and severe FP as adapted from Newberry et al.59. Reliability between the two observers (inter-observer agreement) was high for all FP behaviours (Pearson correlations: exploratory FP (0.92), stereotyped FP (0.85) and severe FP (0.97)).
Novel object test
At 3 days and 5 weeks of age, the response to a novel object (NO) was tested at the pen level. At 3 days of age (n = 48), the NO was a wooden block (h: 8 cm, l: 5 cm, w- 2.5 cm) wrapped with coloured tape (green, bright pink, light pink and yellow)64. At 5 weeks of age (n = 48), the NO test was repeated with a plastic stick (l: 50 cm, d: 3.5 cm) wrapped with coloured tape (red, white, green, black, and yellow)78. The test started 10 sec after one experimenter had placed the NO on the floor in the centre of the home pen. The latency for three birds to approach the NO at a distance of <25 cm and the number of birds that were within <25 cm of the NO were recorded by another experimenter every 10 sec for the 2-min test duration. Two experimenters tested all pens at 3 days and 5 weeks of age.
Novel environment test
At 1 week of age, the response to a novel environment64 was tested for a duration of 1 min (n = 520). All birds from a pen were taken and placed in a cardboard box in front of the home pen. The average time difference between the first and last bird to be tested was 9 min. Birds were then individually taken to one of three test locations, where birds were placed inside a white bucket (h: 57 cm, l: 32 cm, w: 22 cm) at the start of the test. The latency to vocalize, the number of vocalizations and flight attempts were recorded. Together, three experimenters tested all birds where each experimenter tested approximately one-third of the birds.
Tonic immobility test
At 9 weeks of age, birds were tested in a tonic immobility (TI) test79 for a maximum duration of 5 min (n = 458). Half of the birds in a pen were taken and transported in a cardboard box to a room near the testing rooms. The average time difference between the first and last bird to be tested was 8 min. Birds were individually taken to one of two test rooms, where they were placed in a supine position in a metal cradle with their head suspended from the side of the cradle. Each bird was restrained for 10 sec and when the bird remained in this position after release, TI duration was recorded until the bird returned to an upright position. If this occurred within 10 sec after release TI was induced again, with a maximum of three attempts at inducing TI. Together, two experimenters tested all birds, and each experimenter tested approximately half of the birds.
Open field test
At 13 weeks of age, birds were tested in an open field (OF) test80 for a duration of 5 min (n = 409). Birds were individually taken and transported to the test room in a cardboard box. The OF was a square wooden enclosure (h: 1.22 m, l: 1.15 m, w: 1 m) with a video camera positioned above it. A bird was placed in the centre of the OF, and the test started when the lights were switched on. One experimenter recorded the latency to vocalize and the number of vocalizations. A second experimenter recorded the latency to step and the number of steps from a monitor in an adjacent room. Three experimenters tested all birds, and each experimenter tested approximately one-third of the birds for vocalizations or steps.
Manual restraint test
At 15 weeks of age, birds were tested in a manual restraint (MR) test81 for a duration of 5 min (n = 409). Birds were individually taken and transported to one of two test rooms in a cardboard box. Birds were placed on their right side on a table. The right hand of the experimenter covered the bird’s back, and the left hand gently stretched the bird’s legs. The latencies to vocalize and struggle and the number of vocalizations and struggles were recorded. Together, four experimenters tested all birds, and each experimenter tested approximately one-fourth of the birds. Fifteen min after the start of MR, blood samples were drawn from the wing vein for assessment of the peak plasma corticosterone level82.
Blood collection and analyses
Blood was collected from all birds at 5, 10 and 15 weeks of age. Blood was taken from the wing vein using a heparinized syringe and kept on ice after blood sampling. Blood samples for corticosterone and natural antibodies were centrifuged at 5250 × g for 10 min at room temperature, and the obtained plasma was stored at −20 °C until further analysis. Whole blood samples (1 mL) for the determination of serotonin were stored at −20 °C until further analysis27.
Plasma IgM and IgG natural antibody titres
Samples from all weeks were used for the determination of IgM and IgG natural antibody titres against keyhole limpet haemocyanin via an indirect enzyme-linked immunosorbent assay as described previously83 with the following modifications. Serial dilutions of plasma were made in four steps starting with a 1:40,000 dilution in phosphate buffer saline containing 0.05% Tween 20 and 1% horse plasma (100 µL in each well). Peroxidase-conjugated goat-anti-chicken IgM (A30-102P, Bethyl; dilution 1:20,000) or goat-anti-chicken IgG (A30-104P, Bethyl; dilution 1:20,000) was used as secondary antibody (100 µL in each well).
Plasma corticosterone
Samples from week 15 were used for the determination of plasma corticosterone concentrations via a radioimmunoassay kit (MP Biomedicals) as described previously84.
Whole blood serotonin
Samples from week 15 were used for the determination of whole blood serotonin concentration (nmol/mL) via a fluorescence assay as described previously81. The centrifugation steps were performed at 931 × g, and fluorescence was determined in a Perkin-Elmer 2000 Fluorescence spectrophotometer at 295 and 540 nm.
Statistical analysis
A power analysis was performed for the main variable FP. Using an alpha of 0.05 and a power of 0.8, we calculated that with a mean of 10 FP bouts and a standard deviation of 5 we needed a sample size of 8 pens per experimental group (pen is the experimental unit as individuals within one pen can influence each other’s behaviour).
To plot the microbial composition of the transplantation pools, principal component analysis was used as implemented in CANOCO 5 software package (Biometris). The relative contribution of 259 genus-level phylogenetic groups identified by 16S rRNA gene sequencing was used as response variables. To relate changes in microbial composition to explanatory variables, redundancy analysis was used as implemented in the CANOCO 5 software package. Line * treatment interaction was introduced as a nominal variable, and we further tested treatment and treatment effects within lines separately. The relative contribution of 259 genus-level phylogenetic groups identified by 16S rRNA gene sequencing was used as response variables. Analyses were performed for each age (5 days and 2 weeks of age) and gut section (ileum, caecum or colon) separately. The Monte Carlo Permutation test (number of permutations 499) was applied to test for significance of the effect of line * treatment, treatment or treatment within lines on microbiota composition. Batch and sex were included as covariates. P-values were corrected using a Benjamini Hochberg correction31.
SAS Software version 9.4 was used for statistical analysis (SAS Institute). Linear mixed models for line * treatment effects were tested for each age separately and consisted of fixed effects of line * treatment, line, treatment, sex and batch and the random effect of pen within line and treatment. Linear mixed models for treatment effects within lines consisted of fixed effects of treatment, sex and batch and the random effect of pen within treatment. Linear mixed models for FP behaviours and behavioural responses to NO did not include the fixed effect of sex or a random effect as they were tested at the pen level. Test time (morning 8:00 h–12:30 h or afternoon 12:30 h–18:00 h) was added as a fixed effect for the TI, OF and MR tests. Experimenter was added as a fixed effect for the NE, TI, OF and MR tests. The model residuals were visually examined for normality. Variables were square-root transformed (i.e., latency to vocalize and frequency of vocalizations in the NE test; TI duration; latency to vocalize and step, step and vocalization frequency in the OF test; latency to struggle and vocalize in the MR test; corticosterone and serotonin) to obtain normality of model residuals. Generalized linear mixed models with a binary distribution were used to test the effects of line*treatment, line and treatment or treatment effects within lines for flight attempts in the NE test, the number of inductions needed to reach TI, and struggle and vocalization frequency in the MR test. A backward regression procedure was used when fixed effects (i.e., line * treatment, test time or experimenter) had P > 0.1. Post hoc pairwise comparisons were corrected by Tukey–Kramer adjustment. Kruskal Wallis tests were used to analyse line * treatment, treatment and treatment effects within lines for individual microbial groups, stereotyped FP, severe FP and behavioural responses to the NO test, and post hoc comparisons were made with the Dwass, Steel, Critchlow-Fligner method. All data are presented as (untransformed) mean ± standard error (SE)25,27.
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplementary information
Acknowledgements
We would like to thank I. van den Anker-Hensen, J. Arts, L. Nieuwe Weme and in particular G. de Vries Reilingh for their assistance during the experiment. We would further like to thank R. Koopmanschap and G. de Vries Reilingh for their assistance during blood analyses and S. Aalvink and M. van Gaal for their assistance during microbiota analyses. R. Baris, C. Deemter, C. Duivenvoorden, V. Lefoul, C. Nauta, A. Roelofs and Y. van de Weetering (MSc students, Wageningen University) are gratefully acknowledged for their help during the experiment. We thank the staff of experimental farm “CARUS” for their excellent animal care. This study is partly funded by the project “WIAS Graduate Programme” (no: 022.004.005), which is financially supported by the Netherlands Organization for Scientific Research (NWO).
Author contributions
J.v.d.E., A.L., B.R., H.S., M.N. and B.K. designed the study. J.v.d.E., A.L. and B.R. planned the experiments. J.K. provided the selection lines. J.v.d.E., A.L. and B.R. collected the data. H.d.V. performed the microbiota analysis. J.v.d.E. performed the data analysis. J.v.d.E. wrote the first draft. J.v.d.E., A.L., B.R., H.d.V., J.K., H.S., M.N. and B.K. reviewed and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59125-w.
References
- 1.Pryce CR, Rüedi-Bettschen D, Dettling AC, Feldon J. Early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci. Biobehav. Rev. 2005;29:23–50. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 3.Sommer F, Bäckhed F. The gut microbiota - masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 4.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Scik. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceylani, T., Jakubowska-Doğru, E., Gurbanov, R., Teker, H. T. & Gozen, A. G. The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age. Heliyon, 4 (2018). [DOI] [PMC free article] [PubMed]
- 8.Hoban AE, et al. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience. 2016;339:463–477. doi: 10.1016/j.neuroscience.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Yousefi B, et al. Probiotics importance and their immunomodulatory properties. J. Cell. Physiol. 2019;234:8008–8018. doi: 10.1002/jcp.27559. [DOI] [PubMed] [Google Scholar]
- 10.Fröhlich EE, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain. Behav. Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y-N, et al. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J. Gastroenterol. 2018;24:338–350. doi: 10.3748/wjg.v24.i3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge X, et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017;15:1–9. doi: 10.1186/s12967-016-1105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bercik P, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609.e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Parois S, Calandreau L, Kraimi N, Gabriel I, Leterrier C. The influence of a probiotic supplementation on memory in quail suggests a role of gut microbiota on cognitive abilities in birds. Behav. Brain Res. 2017;331:47–53. doi: 10.1016/j.bbr.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Cox CM, Dalloul RA. Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef. Microbes. 2015;6:45–52. doi: 10.3920/BM2014.0062. [DOI] [PubMed] [Google Scholar]
- 16.Yan FF, Wang WC, Cheng HW. Bacillus subtilis based probiotic improved bone mass and altered brain serotoninergic and dopaminergic systems in broiler chickens. J. Funct. Foods. 2018;49:501–509. doi: 10.1016/j.jff.2018.09.017. [DOI] [Google Scholar]
- 17.Kraimi N, et al. Effects of gut microbiota transfer on emotional reactivity in Japanese quails (Coturnix japonica) J. Exp. Biol. 2019;222:1–9. doi: 10.1242/jeb.202879. [DOI] [PubMed] [Google Scholar]
- 18.Brunberg EI, et al. Omnivores going astray: A review and new synthesis of abnormal behavior in pigs and laying hens. Front. Vet. Sci. 2016;3:57. doi: 10.3389/fvets.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodenburg TB, et al. The prevention and control of feather pecking in laying hens: identifying the underlying principles. Worlds. Poult. Sci. J. 2013;69:361–374. doi: 10.1017/S0043933913000354. [DOI] [Google Scholar]
- 20.de Haas EN, van der Eijk JAJ. Where in the serotonergic system does it go wrong? Unravelling the route by which the serotonergic system affects feather pecking in chickens. Neurosci. Biobehav. Rev. 2018;95:170–188. doi: 10.1016/j.neubiorev.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Parmentier HK, Rodenburg TB, de Vries Reilingh G, Beerda B, Kemp B. Does enhancement of specific immune responses predispose laying hens for feather pecking? Poult. Sci. 2009;88:536–542. doi: 10.3382/ps.2008-00424. [DOI] [PubMed] [Google Scholar]
- 22.Kjaer JB. Feather pecking in domestic fowl is genetically related to locomotor activity levels: Implications for a hyperactivity disorder model of feather pecking. Behav. Genet. 2009;39:564–570. doi: 10.1007/s10519-009-9280-1. [DOI] [PubMed] [Google Scholar]
- 23.Kjaer JB, Guémené D. Adrenal reactivity in lines of domestic fowl selected on feather pecking behavior. Physiol. Behav. 2009;96:370–373. doi: 10.1016/j.physbeh.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Kops MS, et al. Brain monoamine levels and behaviour of young and adult chickens genetically selected on feather pecking. Behav. Brain Res. 2017;327:11–20. doi: 10.1016/j.bbr.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 25.van der Eijk JAJ, Lammers A, Li P, Kjaer JB, Rodenburg TB. Feather pecking genotype and phenotype affect behavioural responses of laying hens. Appl. Anim. Behav. Sci. 2018;205:141–150. doi: 10.1016/j.applanim.2018.05.027. [DOI] [Google Scholar]
- 26.Buitenhuis AJ, Kjaer JB, Labouriau R, Juul-Madsen HR. Altered circulating levels of serotonin and immunological changes in laying hens divergently selected for feather pecking behavior. Poult. Sci. 2006;85:1722–8. doi: 10.1093/ps/85.10.1722. [DOI] [PubMed] [Google Scholar]
- 27.van der Eijk JAJ, Lammers A, Kjaer JB, Rodenburg TB. Stress response, peripheral serotonin and natural antibodies in feather pecking genotypes and phenotypes and their relation with coping style. Physiol. Behav. 2019;199:1–10. doi: 10.1016/j.physbeh.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 28.van der Eijk JAJ, et al. Chicken lines divergently selected on feather pecking differ in immune characteristics. Physiol. Behav. 2019;212:112680. doi: 10.1016/j.physbeh.2019.112680. [DOI] [PubMed] [Google Scholar]
- 29.Meyer B, Zentek J, Harlander-Matauschek A. Differences in intestinal microbial metabolites in laying hens with high and low levels of repetitive feather-pecking behavior. Physiol. Behav. 2013;110–111:96–101. doi: 10.1016/j.physbeh.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Birkl P, et al. Differences in cecal microbiome of selected high and low feather-pecking laying hens. Poult. Sci. 2018;97:3009–3014. doi: 10.3382/ps/pey167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Eijk Jerine A.J., de Vries Hugo, Kjaer Joergen B., Naguib Marc, Kemp Bas, Smidt Hauke, Rodenburg T. Bas, Lammers Aart. Differences in gut microbiota composition of laying hen lines divergently selected on feather pecking. Poultry Science. 2019;98(12):7009–7021. doi: 10.3382/ps/pez336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballou AL, et al. Development of the chick microbiome: How early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:1–12. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson R, et al. Biochemical, behavioural and electrophysiological investigations of brain maturation in chickens. Brain Res. Bull. 2008;76:217–223. doi: 10.1016/j.brainresbull.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Forkman B, Boissy A, Meunier-Salaün M-C, Canali E, Jones RB. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007;92:340–374. doi: 10.1016/j.physbeh.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Korte SM, Beuving G, Ruesink W, Blokhuis HJ. Plasma catecholamine and corticosterone levels during manual restraint in chickens form a high and low feather pecking line of laying hens. Physiol. Behav. 1997;62:437–441. doi: 10.1016/S0031-9384(97)00149-2. [DOI] [PubMed] [Google Scholar]
- 36.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: A key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 37.Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol. Today. 2000;21:624–630. doi: 10.1016/S0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 38.Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol. 2009;30:43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J. Immunol. 2015;194:13–20. doi: 10.4049/jimmunol.1400844. [DOI] [PubMed] [Google Scholar]
- 40.Uitdehaag KA, et al. Effects of genetic origin and social environment on behavioral response to manual restraint and monoamine functioning in laying hens. Poult. Sci. 2011;90:1629–36. doi: 10.3382/ps.2010-01292. [DOI] [PubMed] [Google Scholar]
- 41.Hill RA, Welsh TH, Poulos SP, Gabler NK, Connor EE. Growth and development symposium: Intestinal development and growth. J. Anim. Sci. 2011;89:833–834. doi: 10.2527/jas.2010-3764. [DOI] [PubMed] [Google Scholar]
- 42.Ouwerkerk JP, de Vos WM, Belzer C. Glycobiome: Bacteria and mucus at the epithelial interface. Best Pract. Res. Clin. Gastroenterol. 2013;27:25–38. doi: 10.1016/j.bpg.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Awad WA, et al. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 2016;6:1–17. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen KN, Henriksen M, Bisgaard M, Nielsen OL, Christensen H. Investigation of chicken intestinal bacterial communities by 16S rRNA targeted fluorescence in situ hybridization. Antonie Van Leeuwenhoek. 2008;94:423–437. doi: 10.1007/s10482-008-9260-0. [DOI] [PubMed] [Google Scholar]
- 45.Yang, C. et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry, 9 (2019). [DOI] [PMC free article] [PubMed]
- 46.Zheng P, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 47.Kelly JR, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Sekelja M, et al. Abrupt temporal fluctuations in the chicken fecal microbiota are explained by its gastrointestinal origin. Appl. Environ. Microbiol. 2012;78:2941–2948. doi: 10.1128/AEM.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu, S. et al. Bacterial Community Mapping of the Mouse Gastrointestinal Tract. PLoS One, 8 (2013). [DOI] [PMC free article] [PubMed]
- 50.Lu J, et al. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fellah, J. S., Jaffredo, T., Nagy, N. & Dunon, D. Development of the avian immune system. In Avian Immunology 45–63 (Elsevier, 2014).
- 52.Cebra JJ. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 1999;69:1046–1051. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 53.Burokas A, et al. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 54.Sjögren K, et al. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enaud R, et al. The mycobiome: A neglected component in the microbiota-gut-brain axis. Microorganisms. 2018;6:22. doi: 10.3390/microorganisms6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cadwell K. The virome in host health and disease. Immunity. 2015;42:805–813. doi: 10.1016/j.immuni.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Haas EN, Nielsen BL, Buitenhuis AJ, Rodenburg TB. Selection on feather pecking affects response to novelty and foraging behaviour in laying hens. Appl. Anim. Behav. Sci. 2010;124:90–96. doi: 10.1016/j.applanim.2010.02.009. [DOI] [Google Scholar]
- 58.Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: Experimental evidence and clinical implications. Curr. Opin. Microbiol. 2013;16:240–245. doi: 10.1016/j.mib.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Newberry RC, Keeling LJ, Estevez I, Bilcik B. Behaviour when young as a predictor of severe feather pecking in adult laying hens: The redirected foraging hypothesis revisited. Appl. Anim. Behav. Sci. 2007;107:262–274. doi: 10.1016/j.applanim.2006.10.010. [DOI] [Google Scholar]
- 60.Bright A. Time course of plumage damage in commercial layers. Vet. Rec. 2009;164:334–335. doi: 10.1136/vr.164.11.334. [DOI] [PubMed] [Google Scholar]
- 61.Jones RB. Fear and adaptability in poultry: insights, implications and imperatives. Poult. Sci. J. 1996;52:131–174. [Google Scholar]
- 62.Uitdehaag KA, Komen H, Rodenburg TB, Kemp B, van Arendonk J. The novel object test as predictor of feather damage in cage-housed Rhode Island Red and White Leghorn laying hens. Appl. Anim. Behav. Sci. 2008;109:292–305. doi: 10.1016/j.applanim.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Rodenburg TB, et al. Genetic and phenotypic correlations between feather pecking and open-field response in laying hens at two different ages. Behav. Genet. 2004;34:407–415. doi: 10.1023/B:BEGE.0000023646.46940.2d. [DOI] [PubMed] [Google Scholar]
- 64.de Haas EN, et al. Predicting feather damage in laying hens during the laying period. Is it the past or is it the present? Appl. Anim. Behav. Sci. 2014;160:75–85. doi: 10.1016/j.applanim.2014.08.009. [DOI] [Google Scholar]
- 65.Rodenburg TB, de Haas EN, Nielsen BL, Buitenhuis AJ. Fearfulness and feather damage in laying hens divergently selected for high and low feather pecking. Appl. Anim. Behav. Sci. 2010;128:91–96. doi: 10.1016/j.applanim.2010.09.017. [DOI] [Google Scholar]
- 66.Chung H, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vicario-Abejón C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat. Rev. Neurosci. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- 68.Albentosa MJ, Kjaer JB, Nicol CJ. Strain and age differences in behaviour, fear response and pecking tendency in laying hens. Br. Poult. Sci. 2003;44:333–344. doi: 10.1080/00071660310001598085. [DOI] [PubMed] [Google Scholar]
- 69.Hocking P.M., Channing C.E., Waddington D., Jones R.B. Age-related changes in fear, sociality and pecking behaviours in two strains of laying hen. British Poultry Science. 2001;42(4):414–423. doi: 10.1080/00071660120070686. [DOI] [PubMed] [Google Scholar]
- 70.Kaiser Pete, Stäheli Peter. Avian Immunology. 2014. Avian Cytokines and Chemokines; pp. 189–204. [Google Scholar]
- 71.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee KW, Kim DK, Lillehoj HS, Jang SI, Lee SH. Immune modulation by Bacillus subtilis-based direct-fed microbials in commercial broiler chickens. Anim. Feed Sci. Technol. 2015;200:76–85. doi: 10.1016/j.anifeedsci.2014.12.006. [DOI] [Google Scholar]
- 74.Pietraszek MH, et al. Relationship between serotonergic measures in periphery and the brain of mouse. Life Sci. 1992;51:75–82. doi: 10.1016/0024-3205(92)90221-A. [DOI] [PubMed] [Google Scholar]
- 75.de Haas EN, Kemp B, Bolhuis JE, Groothuis T, Rodenburg TB. Fear, stress, and feather pecking in commercial white and brown laying hen parent-stock flocks and their relationships with production parameters. Poult. Sci. 2013;92:2259–2269. doi: 10.3382/ps.2012-02996. [DOI] [PubMed] [Google Scholar]
- 76.Kjaer JB, Sørensen P, Su G. Divergent selection on feather pecking behaviour in laying hens (Gallus gallus domesticus) Appl. Anim. Behav. Sci. 2001;71:229–239. doi: 10.1016/S0168-1591(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 77.Ramiro-Garcia J, et al. NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000Research. 2016;5:1791. doi: 10.12688/f1000research.9227.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welfare Quality®. Welfare Quality®assessment protocol for poultry (broilers, laying hens). (2009).
- 79.Jones RB, Faure JM. Sex and strain comparisons of tonic immobility (‘Righting time’) in the domestic fowl and the effects of various methods of induction. Behav. Processes. 1981;6:47–55. doi: 10.1016/0376-6357(81)90015-2. [DOI] [PubMed] [Google Scholar]
- 80.Rodenburg TB, Uitdehaag KA, Ellen ED, Komen J. The effects of selection on low mortality and brooding by a mother hen on open-field response, feather pecking and cannibalism in laying hens. Anim. Welf. 2009;18:427–432. [Google Scholar]
- 81.Bolhuis JE, et al. Effects of genetic group selection against mortality on behavior and peripheral serotonin in domestic laying hens with trimmed and intact beaks. Physiol. Behav. 2009;97:470–475. doi: 10.1016/j.physbeh.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Fraisse F, Cockrem JF. Corticosterone and fear behaviour in white and brown caged laying hens. Br. Poult. Sci. 2006;47:110–119. doi: 10.1080/00071660600610534. [DOI] [PubMed] [Google Scholar]
- 83.Berghof TVL, et al. Genetic and non-genetic inheritance of natural antibodies binding keyhole limpet hemocyanin in a purebred layer chicken line. PLoS One. 2015;10:e0131088. doi: 10.1371/journal.pone.0131088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buyse J, et al. Effect of corticosterone on circulating concentrations of corticosterone, prolactin, thyroid hormones and somatomedin C and on fattening in broilers selected for high or low fat content. J. Endocrinol. 1987;112:229–237. doi: 10.1677/joe.0.1120229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.