Abstract
Currently, Gram-negative bacteria have developed multidrug and broad-spectrum drug resistance, and the numbers of species and strains carrying mcr or blaNDM genes are increasing. In this study, mcr-1 and blaNDM distribution of 12,858 Gram-negative bacteria isolated from wildlife, patients, livestock, poultry and environment in 14 provinces of China from 2010 to 2019 and the antibiotics resistance in regard to polymyxins (polymyxin B and colistin) and carbapenems of positive strains were investigated. A total of 70 strains of 10 species carried the mcr-1 gene, positive rates of patients, livestock and poultry, and environmental strains were 0.62% (36/5,828), 4.07% (29/712), 5.43% (5/92), respectively. Six strains of 3 species carrying the blaNDM gene all came from patients 0.10% (6/5,828). Two new mcr-1 gene variants (GenBank: MK965883, MK965884) were identified, one of which contains premature stop codon. The drug susceptibility results showed that all mcr-1 carriers were sensitive to carbapenems, among which, 66 strains were resistant and 4 were sensitive to polymyxins. The strains with the blaNDM gene had different degrees of resistance to carbapenems and were sensitive to polymyxins. The findings that species carrying mcr-1 or blaNDM genes were limited and mostly normal flora of opportunistic or low pathogenic organisms indicated that transfer of mcr-1 and blaNDM genes between bacteria was relatively limited in China. The none detection among wildlife compared with other sources supports the speculation that the emergence of and increase in polymyxins and carbapenem-resistant strains was mainly related to the selective pressure of antibiotics.
Keywords: MCR, NDM, polymyxin, carbapenem, Gram-negative
Introduction
Bacterial resistance has been a global public health concern (Nolte, 2014; Alos, 2015). Currently, Gram-negative bacteria are developing multidrug resistance and broad-spectrum drug resistance, and the available antibiotics used in clinical treatment, agricultural and livestock production are gradually decreasing (Abdelraouf et al., 2017; Theuretzbacher, 2017). Polymyxins and carbapenems are among the antibiotics of last resort to treat Gram-negative bacteria infections. In 2009 and 2016, the superbug that carried the New Delhi metallo-beta-lactamase gene (blaNDM–1) (Yong et al., 2009; Kumarasamy et al., 2010) and the Escherichia. coli that carried the colistin resistance gene (mcr-1) (Liu et al., 2016) were identified. Until now, 9 subtypes of mcr (Liu et al., 2016; Xavier et al., 2016; AbuOun et al., 2017; Borowiak et al., 2017; Carattoli et al., 2017; Yin et al., 2017; Wang X. et al., 2018; Yang et al., 2018; Carroll et al., 2019) and 21 subtypes of blaNDM (Liu et al., 2018) have been published with papers. The discovery of these two types of resistance genes that can be horizontally transferred via plasmids has made researchers aware of the post-antibiotic era. Such plasmids usually carry other resistance genes, encoded for aminoglycosides and quinolones for instance (Carattoli, 2013; Rozwandowicz et al., 2018). The rapid horizontal spread of drug-resistant genes by plasmids is one of the reasons for the increasing number of multidrug resistant bacteria, however, the transfer range of bacteria species is unknown. Studies have confirmed that many countries and regions have isolated Gram-negative bacteria with mcr-1 gene (Fernandes et al., 2016; Hadjadj et al., 2017; Liu et al., 2017a), the blaNDM–1 gene (Wang et al., 2014; Michael et al., 2015; Zenati et al., 2016; Abderrahim et al., 2017; Madec et al., 2017; Riazzo et al., 2017; Yu et al., 2017), or both (Zheng et al., 2016; Liu et al., 2017b; Wang R. et al., 2018), from humans, the environment and animals. Whether isolates from antibiotics-free and depopulated areas, wildlife, carried mcr or blaNDM genes is undiscovered.
To explore the transfer range of polymyxins and carbapenems resistance genes, 12,858 Gram-negative isolates of ≥118 species were retrospectively collected. Considering limited genes can be screened for the 12,858 isolates, only the mcr-1 and blaNDM genes were chosen, for their wide dissemination around the world and across species (López et al., 2019). To confirm the effect of antibiotics on the emergence of resistant strains, strains isolated from wildlife, patients, livestock, poultry, and environment from past 10 years were screened. Strains positive with either of the gene were confirmed by open reading frame (ORF) sequencing, and were tested for antimicrobial susceptibility.
Materials and Methods
Bacteria Isolation and Identification
We retrospectively collected 12,858 Gram-negative bacteria that were isolated from wildlife (6,226/12,858), patients (5,828/12,858), livestock and poultry (712/12,858), and environment (92/12,858) in 14 provinces (Anhui, Beijing, Gansu, Guangxi, Guizhou, Hainan, Hunan, Jiangxi, Ningxia, Qinghai, Sichuan, Tianjin, Yunnan, Zhejiang) of China from 2010 to 2019. The study was approved by the ethics review committee of the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. Informed consent was obtained from participants. All strains were identified using VITEK II Compact system (bioMérieux, France) or API 20E strips (bioMérieux, France). The mcr-1 or blaNDM gene positive strains were identified again by VITEK II Compact system (bioMérieux, France), the results of which were consistent with the original ones.
Screening and ORF Sequencing of mcr-1 or blaNDM Positive Strains
DNA templates were extracted by using TIANamp Bacteria DNA Kit. Positive controls were used for PCR. All 12,858 Gram-negative bacteria were screened for mcr-1 and blaNDM gene by screening primers (Table 1), and ORF of positive strains were further amplified, cloned and sequenced. The mcr-1 or blaNDM positive strains were confirmed only if ORF sequences were obtained.
TABLE 1.
The primers for screening and ORF amplification of the mcr-1 and blaNDM genes.
| Primer | Sequence (5′ → 3′) | Product size (bp) | Annealing temperature (°C) |
| mcr-1 | |||
| MCR-1_CLR5-F | CGGTCAGTCCGTTTGTTC | 309a | 54 |
| MCR-1_CLR5-R | CTTGGTCGGTCTGTAGGG | ||
| blaNDM | |||
| NDM-1_17U-F | CAGCACACTTCCTATCTC | 292a | 54 |
| NDM-1_17U-R | CCGCAACCATCCCCTCTT | ||
| mcr-1 | |||
| mcr-1 FL-F | AGAAGCACTGGGTGTAGAAT | 2189b | 54 |
| mcr-1 FL-R | GCCATGACAAGAGCGATA | ||
| mcr-1 | |||
| FR-mcr-FL-F | CATCAATCAGTGGAGCG | 2060b | 54 |
| FR-mcr-FL-R | CTCATCTCAGCAAGTAGG | ||
| mcr-1 | |||
| DR-mcr-FL-F | GCAGTATAATTGCCGTAA | 1841b | 50 |
| DR-mcr-FL-R | CTGACTGTGCTCAAGGGT | ||
| blaNDM | |||
| 21U-FL-F | TCGCATAAAACGCCTCTG | 1007b | 54 |
| 21U-FL-R | GAAACTGTCGCACCTCAT |
aScreening, bORF amplification.
Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations (MICs) of polymyxins (polymyxin B and colistin) and carbapenems of 70 strains with mcr-1 and 6 strains with blaNDM were determined by broth microdilution method in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (Clinical and Laboratory Standards Institute, 2019). Replication of sensitivity testing was conducted. Quality controls and breakpoints were in accordance with the European Committee on Antimicrobial Susceptibility Testing (2019) and the Clinical and Laboratory Standards Institute (2019) for polymyxins and carbapenems, respectively.
Results
Distribution of mcr-1 or blaNDM Positive Strains
A total of 70 strains with mcr-1 and 6 strains with blaNDM were confirmed. mcr-1 positive strains were isolated each year since 2012. The positive rate from 2012 to 2019 was 0.31% (2/649), 0.09% (1/1,169), 0.63% (7/1,105), 0.36% (9/2,520), 0.64% (34/5,334), 0.28% (4/1,420), 2.12% (9/424), and 8.16% (4/49), respectively. The isolation rates of blaNDM positive strains were 0.08% (2/2,520) in 2015, 0.06% (3/5,334) in 2016, and 0.24% (1/424) in 2018 (Table 2). Among the 70 strains with mcr-1, 36 were isolated from patients, with a positive rate of 0.62% (36/5,828) (Table 3). Thirty-five were isolated from diarrheal stool and 1 was from the sputum of an acute-pancreatitis patient. Twenty-nine strains were isolated from livestock and poultry specimens, with a positive rate of 4.07% (29/712), of which 24 isolates were from pig feces and 5 were from chicken feces. Five strains were isolated from environmental specimens, related to chicken slaughter, with a positive rate of 5.43% (5/92). Six blaNDM positive strains were isolated from stool specimens of diarrhea patients, with a positive rate of 0.10% (6/5,828). None of the strains isolated from wild animal specimens were mcr-1 or blaNDM positive, which accounted for 48.42% (6,226/12,858) of all screened strains. These strains were isolated from rodents besides marmots (66.48%, 4,139/6,226), marmots (19.03%, 1,185/6,226), birds (6.10%, 380/6,226), plateau pika (4.34%, 270/6,226) and bats (4.05%, 252/6,226).
TABLE 2.
The positive rate of strains with mcr-1 or blaNDM isolated in different years.
| No. mcr-1 positive strains (%) | No. blaNDM positive strain (%) | |
| 2010 | None | None |
| 2011 | None | None |
| 2012 | 2 (0.31) | None |
| 2013 | 1 (0.09) | None |
| 2014 | 7 (0.63) | None |
| 2015 | 9 (0.36) | 2 (0.08) |
| 2016 | 34 (0.64) | 3 (0.06) |
| 2017 | 4 (0.28) | None |
| 2018 | 9 (2.12) | 1 (0.24) |
| 2019 | 4 (8.16) | None |
| Total | 70 (0.54) | 6 (0.05) |
None: no strain positive for the mcr-1 or blaNDM gene.
TABLE 3.
The positive rate of strains with mcr-1 or blaNDM isolated from different sources.
| No. strains | No. mcr-1 positive strains (%) | No. blaNDM postive strains (%) | |
| Wildlife | 6,226 | None | None |
| Livestock and poultry | 712 | 29 (4.07) | None |
| Environment | 92 | 5 (5.43) | None |
| Patients | 5,828 | 36 (0.62) | 6 (0.10) |
| Total | 12,858 | 70 (0.54) | 6 (0.05) |
None: no strain positive for the mcr-1 or blaNDM gene.
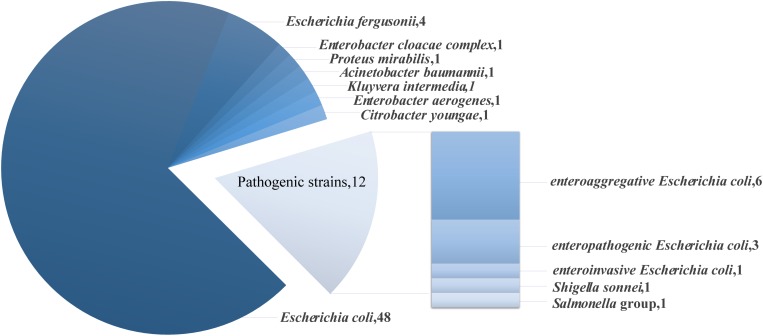
Of the mcr-1 positive strains, 83% were opportunistic or low pathogenic organisms, including 48 strains of Escherichia coli, 4 strains of Escherichia fergusonii, 1 strain of Enterobacter cloacae complex, 1 strain of Proteus mirabilis, 1 strain of Acinetobacter baumannii, 1 strain of Kluyvera intermedia, 1 strain of Enterobacter aerogenes and 1 strain of Citrobacter youngae (Figure 1). The remaining 17% of the strains were pathogenic strains, including 6 strains of enteroaggregative Escherichia coli (EAEC), 3 strains of enteropathogenic Escherichia coli (EPEC), 1 strain of enteroinvasive Escherichia coli (EIEC), 1 strain of Salmonella group and 1 strain of Shigella sonnei. Strains carrying the blaNDM gene that were low pathogenic or opportunistic organisms were 3 strains of Escherichia coli, 2 strains of Klebsiella pneumoniae ssp. pneumoniae and 1 strain of Klebsiella oxytoca.
FIGURE 1.
Species and number of mcr-1 positive strains.
Sequence Analysis of mcr-1 and blaNDM
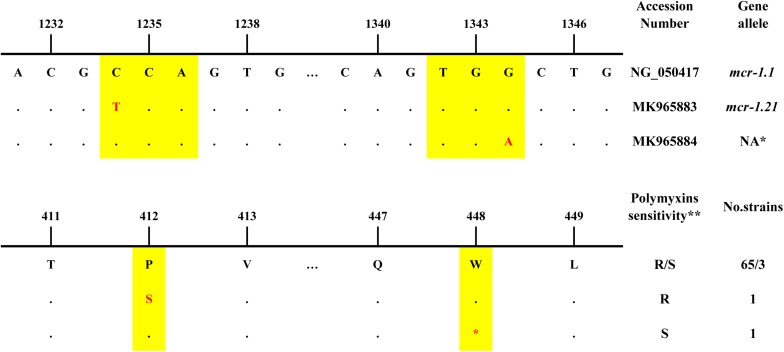
The sequence alignment of the ORF showed that 68 of 70 mcr-1 positive strains possessed an identical sequence compared to the reference sequence of the mcr-1.1 gene, 1626 bp (NCBI Reference Sequence: KP347127, region: 22413-24038) (Figure 2). Among these strains, 65 were resistant while 3 were sensitive to polymyxins. The additional 2 mcr-1 positive strains had a single base mutation compared to the reference sequence. One possessed mcr-1.21 (GenBank: MK965883), an Escherichia coli isolated from pig feces in Qinghai in 2016, which mutated at 1234 nt of reference sequence from C to T, resulting in a proline to serine change at the amino acid level. This strain shows resistance to polymyxins. The other strain possessed MK965884, an EAEC isolated from the stool of a diarrhea patient in Beijing in 2012. A base mutation site was located at 1344 nt of the reference sequence from G to A, resulting in a stop codon. The susceptibility results showed sensitivity to polymyxins.
FIGURE 2.
The nucleotide differences, amino acid differences and information of mcr-1 positive strains in this study *NA: not assigned by NCBI. **R: resistant, S:sensitive.
Of 6 blaNDM positive strains, 3 had identical sequences compared to the 813 bp reference sequence in the ORF of the blaNDM–1 gene (NCBI Reference Sequence: FN396876 REGION: 2420-3232), 2 were identical to the blaNDM–3 gene (NCBI Reference Sequence: JQ734687 REGION: 1-813), and 1 was identical to the 813 bp reference sequence in the ORF of the blaNDM–5 gene (NCBI Reference Sequence: JN104597 REGION: 115-927).
Antimicrobial Susceptibility Result
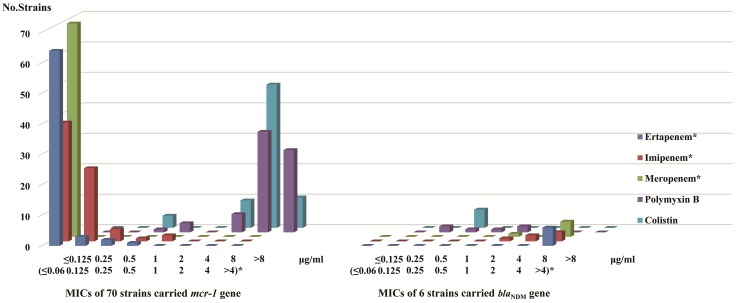
According to clinical breakpoint of carbapenem antibiotics, CLSI, all 70 strains carrying mcr-1 showed sensitivity to ertapenem, imipenem, and meropenem. Among the 6 strains with blaNDM, 5 were resistant to ertapenem (MIC > 4 μg/ml), imipenem (3 strains MIC > 4 μg/ml, 2 strains MIC = 4 μg/ml) and meropenem (MIC > 4 μg/ml), while the other strain was resistant to ertapenem (MIC > 4 μg/ml) but intermediate to imipenem (MIC = 2 μg/ml) and meropenem (MIC = 2 μg/ml). According to clinical breakpoint of EUCAST, among the 70 strains with the mcr-1 gene, 66 were resistant to polymyxins (polymyxin B: 6 strains MIC = 4 μg/ml, 33 strains MIC = 8 μg/ml, 27 strain MIC > 8 μg/ml. colistin: 9 strains MIC = 4 μg/ml, 47 strains MIC = 8 μg/ml, 10 strain MIC > 8 μg/ml) and the other four were sensitive to polymyxins (polymyxin B: 3 strains MIC = 1 μg/ml, 1 strain MIC = 0.5 μg/ml. colistin: 4 strains MIC = 0.5 μg/ml) (Table 4). Six strains with blaNDM were sensitive to polymyxins (Figure 3). The sensitivity data for all strains referred to Supplementary Table S1.
TABLE 4.
Strain information and MIC values of polymyxins sensitive strains.
| Bacteria | Host | Source | Year | mcr-1 accession number | Polymyxin B MICs (μg/ml) | Colistin MICs (μg/ml) | |
| QHXN2016-F75-2 | Escherichia coli | Pig | Feces | 2016 | NG_050417 | 1 | 0.5 |
| QHXN2016-F78-2 | Escherichia coli | Pig | Feces | 2016 | NG_050417 | 1 | 0.5 |
| GX1400471211 | Enterobacter aerogenes | Diarrhea patient | Stool | 2015 | NG_050417 | 1 | 0.5 |
| CY307223 | Escherichia coli | Diarrhea patient | Stool | 2012 | MK965884 | 0.5 | 0.5 |
FIGURE 3.
MICs distribution of carbapenem antibiotics and polymyxins in mcr-1 and blaNDM gene carriers MIC values (≤ 0.06 ∼>4 μg/ml)* in regard to ertapenem, imipenem, and meropenem. MIC values ≤0.125 ∼>8 μg/ml in regard to polymyxin B and colistin.
Discussion
The increase in Gram-negative bacterial strains carrying the mcr or blaNDM genes has led global health workers to reconsider infections and the treatment of bacteria infection (Kumarasamy et al., 2010). A total of 12,858 strains from ≥118 species of Gram-negative bacteria were screened, only to find 10 species carrying the mcr-1 gene and 3 species carrying blaNDM gene, indicating the species carrying these two genes and interspecific transfer of the two resistance genes were limited. The bacteria carrying these two resistance genes were mainly normal flora of opportunistic or low pathogenic organisms. Bacterial resistance is an ecological feature to maintain the lineage extension of the bacteria. The emergence of drug-resistant strains has a relationship with the widespread use of antibiotics (Liu et al., 2016). In fact, drug-resistant strains have existed for a long time (Haenni et al., 2016). Our study shows that the use of antibiotics may change the number of resistant strains, but it does not have an essential effect on the frequency of gene transfer. For example, metallo-β-lactamases itself is crucial in defining bacterial host specificity. The wider dissemination of NDM among different bacterial hosts, compared to VIM-2 and SPM-1, is mainly due to unique and singular features of this protein (López et al., 2019). In Gram-positive pathogens, the emergence of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA) were alarming (Centers for Disease Control and Prevention, 2002). However, as of May 2015, only 14 VRSA infections have been reported in patients from the United States (Walters et al., 2015).
The total detection rate of this study was lower than other studies, mainly because 48.42% (6,226/12,858) of the strains were isolated from wildlife, while neither mcr-1 nor blaNDM genes was positive. Among the wildlife, marmots, plateau pika and bats live away from humans in this study. Rodents besides marmot (such as Rattus flavipectus and Rattus norvegicus) and birds mainly live between human surroundings and lands away from humans. The existence of antibiotic selective pressure away from humans is minimal, where no strain was found carrying mcr-1 or blaNDM gene. Among other sources, the highest detection rate of mcr-1 was found in strains from the environment, which related to chicken slaughter (5.43%, 5/92), followed by livestock and poultry (4.07%, 29/712). Colistin has been used as a feed additive for livestock and poultry for growth promotion and disease prevention, especially for the treatment of gastrointestinal infections in livestock and poultry (Catry et al., 2015), which may cause widespread resistance of strains in livestock and poultry, further resulting in pollution of the environment, food, water and so on. The mcr-1 gene was detected in bacteria isolated from imported chickens in Denmark (Hasman et al., 2015), food (Kuo et al., 2016; Yang et al., 2019) and wastewater (Zhao and Zong, 2016; Zhao et al., 2017) in China. In patients, the strains carrying the mcr-1 gene (0.62%, 36/5,828) or blaNDM gene (0.10%, 6/5,828) are possibly related to the use of polymyxins or carbapenems in hospitals and in-hospital transmission of drug-resistant strains. Therefore, it is possible that the increase in these two kinds of resistant strains is mainly due to the use of polymyxins or carbapenems antibiotics.
Different antibiotics usage may lead to various isolates selection. No resistant strains in this study co-harbored mcr-1 and blaNDM–1 gene, while in other studies from China, such strains were seen (Zheng et al., 2016; Liu et al., 2017b; Wang R. et al., 2018). For instances, in patients, different antibiotics treatment were adopted according to different illness and severity. For livestock and poultry, antibiotics usage may also varied with breeding scales and farms. In addition to mcr-1 and blaNDM–1 gene, other genotype combination were also reported from China (Kong et al., 2017; Liu et al., 2017b; Chavda et al., 2018; Wang Q. et al., 2018; Wang R. et al., 2018). The mcr and blaNDM genes co-harbored isolates from China are often E. coli., and E. cloacae, etc., occasionally. Further restrictions should be made on antibiotics usage for relevant occasions.
Compared with the ORF of the mcr-1.1 gene, it was found that two strains have variants. One variant mcr-1.21 (GenBank: MK965883), changed from C to T at locus 1234 nt, which changed the codon from proline to serine but did not affect the polymyxin-resistant phenotype of the strain. The other variant (GenBank: MK965884) changed from G to A at locus 1344 nt, resulting in a codon change from tryptophan to stop codon. Strain of this variant was sensitive to polymyxins. It was speculated that the translation of this mcr-1 gene was terminated when containing premature stop codon and the functional mcr-1 protein was not completely expressed, it truncated MCR family phosphoethanolamine – lipid A transferase. These two strains were isolated from pig and diarrhea patients, respectively, implying that mutations may be in response to antibiotic selection and certain environmental stresses. Some researchers have also detected variants of the mcr-1 gene from bacteria isolated in environment or healthy individuals (Lu et al., 2017). In addition, three strains with the mcr-1 gene were found to be sensitive to polymyxins, suggesting that the gene does not play a role in certain strains (Quan et al., 2017), which may be related to the metabolism of the strains. Another possible mechanism is the inhibition of mcr-1 gene expression or the inactivation of phosphoethanolamine transferase it encodes (Liassine et al., 2016). In addition, multiple primers were designed to amplify the ORF of mcr-1 positive strains, indirectly reflecting that the flanking regions of the ORF in this study may be variable. Variations did existed in the upstream of the ORF, compared with reference plasmid (KP347127): sequences amplified by the primer mcr-1 FL showed T to C mutation at 22377nt. As to sequences amplified by primer FR-mcr-FL, shortly after the forward primer (KP347127:22091-22107) was a homologous fragments of ≥256 bp, which should be located at 20897-21152 of KP347127, prior to the location of forward primer.
Many genes are responsible and important for polymyxins or carbapenems resistance, e.g., blaOXA (Evans and Amyes, 2014) and blaKPC (Pitout et al., 2015). To focus on both mobile and widespread genes, only mcr-1 and blaNDM genes were screened for 12,858 strains, which is on the other hand, the limitation of the study. There may exist other important resistance mechanisms or genes in our strains. We would further resolve it using strains in this study and isolated afterward. The none detection of wildlife isolates from depopulated areas, in sharp contrast with positive findings of isolates from antibiotics-using areas, emphasized the importance of antibiotics management. On the other hand, the majority of strains collected from wildlife and patients is also the limitation of the study and should be considered when making conclusion.
The mcr-1 and blaNDM genes, mainly encoded by plasmids (Kumarasamy et al., 2010; Liu et al., 2016), have caused harm to livestock, poultry, humans and the environment, and therefore active measures should be taken against the bacteria. Our study have demonstrated that the transfer of mcr-1 or blaNDM genes between bacteria may be limited in China, however, the emergence of and increase in polymyxins and carbapenem-resistant strains was mainly related to the selective pressure of antibiotics. When using polymyxins and carbapenems antibiotics for disease prevention and control in the clinical setting, poultry, livestock and environment, strict management of usage is essential (Walsh and Wu, 2016; Al-Tawfiq et al., 2017), to prevent further intra- and interspecies dissemination of resistance genes and to control the spread of a broad-spectrum drug resistant or multidrug resistant bacteria.
Data Availability Statement
The datasets generated for this study can be found in the https://www.ncbi.nlm.nih.gov/pathogens/isolates#/refgene/gene_family:(blaNDM), https://www.ncbi.nlm.nih.gov/pathogens/isolates#/refgene/gene_family:(mcr-1), MK965884.
Author Contributions
XW and HJ contributed to the conception and design of the work. RF, CL, SQ, JL, MX, and DL performed the experiments. RF, CL, and RD drafted the manuscript. RF and RD performed the analysis and interpretation of the data. XW supervised the work. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank American Journal Experts for their critical editing and helpful comments regarding our manuscript (Sub ID S86HSZSZC).
Footnotes
Funding. This work was supported by the National Sci-Tech Key Project (2018ZX10713-003-002 and 2018ZX10713-001-002).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00121/full#supplementary-material
References
- Abdelraouf K., Linder K. E., Nailor M. D., Nicolau D. P. (2017). Predicting and preventing antimicrobial resistance utilizing pharmacodynamics: part II Gram-negative bacteria. Expert. Opin. Drug Metab. Toxicol. 13 705–714. 10.1080/17425255.2017.1329417 [DOI] [PubMed] [Google Scholar]
- Abderrahim A., Djahmi N., Pujol C., Nedjai S., Bentakouk M. C., Kirane-Gacemi D., et al. (2017). First Case of NDM-1-Producing Klebsiella pneumoniae in annaba university hospital. Algeria. Microb. Drug Resist. 23 895–900. 10.1089/mdr.2016.0213 [DOI] [PubMed] [Google Scholar]
- AbuOun M., Stubberfield E. J., Duggett N. A., Kirchner M., Dormer L., Nunez-Garcia J., et al. (2017). mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in great britain from 2014 to 2015. J. Antimicrob. Chemother. 72 2745–2749. 10.1093/jac/dkx286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alos J. I. (2015). Antibiotic resistance: a global crisis. Enferm. Infecc. Microbiol. Clin. 33 692–699. [DOI] [PubMed] [Google Scholar]
- Al-Tawfiq J. A., Laxminarayan R., Mendelson M. (2017). How should we respond to the emergence of plasmid-mediated colistin resistance in humans and animals? Int. J. Infect. Dis. 54 77–84. 10.1016/j.ijid.2016.11.415 [DOI] [PubMed] [Google Scholar]
- Borowiak M., Fischer J., Hammerl J. A., Hendriksen R. S., Szabo I., Malorny B. (2017). Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 72 3317–3324. 10.1093/jac/dkx327 [DOI] [PubMed] [Google Scholar]
- Carattoli A. (2013). Plasmids and the spread of resistance. Int. J. Med. Microbiol. 303 298–304. 10.1016/j.ijmm.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Carattoli A., Villa L., Feudi C., Curcio L., Orsini S., Luppi A., et al. (2017). Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro. Surveill. 22:30589. 10.2807/1560-7917.ES.2017.22.31.30589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll L. M., Gaballa A., Guldimann C., Sullivan G., Henderson L. O., Wiedmann M. (2019). Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype typhimurium isolate. mBio 10:e00853-19.. 10.1128/mBio.00853-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catry B., Cavaleri M., Baptiste K., Grave K., Grein K., Holm A., et al. (2015). Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents 46 297–306. 10.1016/j.ijantimicag.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, (2002). Staphylococcus aureus resistant to vancomycin–United States, 2002. Morb. Mortal. Wkly. Rep. 51 565–567. [PubMed] [Google Scholar]
- Chavda B., Lv J., Hou M., Chavda K. D., Kreiswirth B. N., Feng Y., et al. (2018). Coidentification of mcr-4.3 and blaNDM-1 in a Clinical Enterobacter cloacae Isolate from China. Antimicrob. Agents Chemother. 62:e00649-18. 10.1128/AAC.00649-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2019). Performance Standards for Antimicrobial Susceptibility Testing. Avliable at: http://www.clsi.org (accessed January, 2019). [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (2019). Breakpoint tables for interpretation of MICs and zone Diameters. Avaliable at: http://www.eucast.org (accessed January 1, 2019). [Google Scholar]
- Evans B. A., Amyes S. G. (2014). OXA beta-lactamases. Clin. Microbiol. Rev. 27 241–263. 10.1128/CMR.00117-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M. R., Moura Q., Sartori L., Silva K. C., Cunha M. P., Esposito F., et al. (2016). Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Euro. Surveill. 21:30214. 10.2807/1560-7917.ES.2016.21.17.30214 [DOI] [PubMed] [Google Scholar]
- Hadjadj L., Riziki T., Zhu Y., Li J., Diene S., Rolain J. (2017). Study of mcr-1 gene-mediated colistin resistance in Enterobacteriaceae isolated from humans and animals in different countries. Genes 8:394. 10.3390/genes8120394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M., Poirel L., Kieffer N., Chatre P., Saras E., Metayer V., et al. (2016). Co-occurrence of extended spectrum beta lactamase and MCR-1 encoding genes on plasmids. Lancet Infect. Dis. 16 281–282. 10.1016/s1473-3099(16)00007-4 [DOI] [PubMed] [Google Scholar]
- Hasman H., Hammerum A. M., Hansen F., Hendriksen R. S., Olesen B., Agersø Y., et al. (2015). Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Eurosurveillance 20 1–5. 10.2807/1560-7917.ES.2015.20.49.30085 [DOI] [PubMed] [Google Scholar]
- Kong L. H., Lei C. W., Ma S. Z., Jiang W., Liu B. H., Wang Y. X., et al. (2017). Various Sequence Types of Escherichia coli Isolates coharboring blaNDM-5 and mcr-1 genes from a commercial swine farm in China. Antimicrob. Agents Chemother. 61:e02167-16.. 10.1128/AAC.02167-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy K. K., Toleman M. A., Walsh T. R., Bagaria J., Butt F., Balakrishnan R., et al. (2010). Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10 597–602. 10.1016/S1473-3099(10)70143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Huang W. C., Wang H. Y., Shiau Y. R., Cheng M. F., Lauderdale T. L. (2016). Colistin resistance gene mcr-1 in Escherichia coli isolates from humans and retail meats, Taiwan. J. Antimicrob. Chemother. 71 2327–2329. [DOI] [PubMed] [Google Scholar]
- Liassine N., Assouvie L., Descombes M.-C., Dénervaud Tendon V., Kieffer N., Poirel L., et al. (2016). Very low prevalence of MCR-1/MCR-2 plasmid-mediated colistin resistance in urinary tract Enterobacteriaceae in Switzerland. Int. J. f Infect. Dis. 51 4–5. 10.1016/j.ijid.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Liu L., Feng Y., Mcnally A., Zong Z. (2018). blaNDM-21, a new variant of blaNDM in an Escherichia coli clinical isolate carrying blaCTX-M-55 and rmtB. J. Antimicrob. Chemother. 73 2336–2339. 10.1093/jac/dky226 [DOI] [PubMed] [Google Scholar]
- Liu B.-T., Song F.-J., Zou M., Hao Z.-H., Shan H. (2017a). Emergence of colistin resistance gene mcr-1 in Cronobacter sakazakii producing NDM-9 and in Escherichia coli from the same animal. Antimicrob. Agents Chemother. 61:e01444-16.. 10.1128/AAC.01444-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.-T., Song F.-J., Zou M., Zhang Q.-D., Shan H. (2017b). High incidence of Escherichia coli strains coharboring mcr-1 and blaNDM from chickens. Antimicrob. Agents Chemother. 61:e02347-16.. 10.1128/AAC.02347-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Y., Wang Y., Walsh T. R., Yi L. X., Zhang R., Spencer J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16 161–168. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- López C., Ayala J. A., Bonomo R. A., González L. J., Vila A. J. (2019). Protein determinants of dissemination and host specificity of metallo-β-lactamases. Nat. Commun. 10:3617. 10.1038/s41467-019-11615-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Hu Y., Luo M., Zhou H., Wang X., Du Y., et al. (2017). MCR-1.6, a New MCR Variant Carried by an IncP Plasmid in a Colistin-Resistant Salmonella enterica Serovar Typhimurium Isolate from a Healthy Individual. Antimicrob. Agents Chemother. 61:e02632-16.. 10.1128/AAC.02632-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec J. Y., Haenni M., Nordmann P., Poirel L. (2017). Extended-spectrum beta-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: a threat for humans? Clin. Microbiol. Infect. 23 826–833. 10.1016/j.cmi.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Michael G. B., Freitag C., Wendlandt S., Eidam C., Fessler A. T., Lopes G. V., et al. (2015). Emerging issues in antimicrobial resistance of bacteria from food-producing animals. Future Microbiol. 10 427–443. 10.2217/fmb.14.93 [DOI] [PubMed] [Google Scholar]
- Nolte O. (2014). Antimicrobial resistance in the 21st century: a multifaceted challenge. Protein Pept. Lett. 21 330–335. 10.2174/09298665113206660106 [DOI] [PubMed] [Google Scholar]
- Pitout J. D., Nordmann P., Poirel L. (2015). Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob. Agents Chemother. 59 5873–5884. 10.1128/AAC.01019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J., Li X., Chen Y., Jiang Y., Zhou Z., Zhang H., et al. (2017). Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect. Dis. 17 400–410. 10.1016/S1473-3099(16)30528-X [DOI] [PubMed] [Google Scholar]
- Riazzo C., Lopez-Cerero L., Rojo-Martin M. D., Hoyos-Mallecot Y., Fernandez-Cuenca F., Martin-Ruiz J. L., et al. (2017). First report of NDM-1-producing clinical isolate of Leclercia adecarboxylata in Spain. Diagn. Microbiol. Infect. Dis. 88 268–270. 10.1016/j.diagmicrobio.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Rozwandowicz M., Brouwer M. S. M., Fischer J., Wagenaar J. A., Gonzalez-Zorn B., Guerra B., et al. (2018). Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 73 1121–1137. 10.1093/jac/dkx488 [DOI] [PubMed] [Google Scholar]
- Theuretzbacher U. (2017). Global antimicrobial resistance in Gram-negative pathogens and clinical need. Curr. Opin. Microbiol. 39 106–112. 10.1016/j.mib.2017.10.028 [DOI] [PubMed] [Google Scholar]
- Walsh T. R., Wu Y. (2016). China bans colistin as a feed additive for animals. Lancet Infect. Dis. 16 1102–1103. 10.1016/s1473-3099(16)30329-2 [DOI] [PubMed] [Google Scholar]
- Walters M. S., Eggers P., Albrecht V., Travis T., Lonsway D., Hovan G., et al. (2015). Vancomycin-resistant staphylococcus aureus - delaware, 2015. Morb. Mortal. Wkly. Rep. 64:1056. 10.15585/mmwr.mm6437a6 [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang P., Zhao D., Jiang Y., Zhao F., Wang Y., et al. (2018). Emergence of tigecycline resistance in Escherichia coli co-producing MCR-1 and NDM-5 during tigecycline salvage treatment. Infect. Drug Resist. 11 2241–2248. 10.2147/IDR.S179618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Liu Y., Zhang Q., Jin L., Wang Q., Zhang Y., et al. (2018). The prevalence of colistin resistance in Escherichia coli and Klebsiella pneumoniae isolated from food animals in China: coexistence of mcr-1 and blaNDM with low fitness cost. Int. J. Antimicrob,. Agents 51 739–744. 10.1016/j.ijantimicag.2018.01.023 [DOI] [PubMed] [Google Scholar]
- Wang X., Wang Y., Zhou Y., Li J., Yin W., Wang S. (2018). Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microbes Infec. 7:122. 10.1038/s41426-018-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang Z., Hao Q., Wu J., Xiao J., Jing H. (2014). Complete genome sequence of Acinetobacter baumannii ZW85-1. Genome Announc. 2:e01083-13. 10.1128/genomeA.01083-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier B. B., Lammens C., Ruhal R., Kumar-Singh S., Butaye P., Goossens H., et al. (2016). Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro. Surveill. 21:30280. 10.2807/1560-7917.ES.2016.21.27.30280 [DOI] [PubMed] [Google Scholar]
- Yang F., Shen C., Zheng X., Liu Y., El-Sayed Ahmed M. A. E., Zhao Z., et al. (2019). Plasmid-mediated colistin resistance gene mcr-1 in Escherichia coli and Klebsiella pneumoniae isolated from market retail fruits in Guangzhou, China. Infect. Drug Resist. 12 385–389. 10.2147/IDR.S194635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Q., Li Y. X., Lei C. W., Zhang A. Y., Wang H. N. (2018). Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 73 1791–1795. 10.1093/jac/dky111 [DOI] [PubMed] [Google Scholar]
- Yin W., Li H., Shen Y., Liu Z., Wang S., Shen Z., et al. (2017). Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio 8:e00543-17.. 10.1128/mBio.00543-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong D., Toleman M. A., Giske C. G., Cho H. S., Sundman K., Lee K., et al. (2009). Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53 5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Wang Y., Chen Z., Zhu X., Tian L., Li L., et al. (2017). Outbreak of nosocomial NDM-1-producing Klebsiella pneumoniae ST1419 in a neonatal unit. J. Glob. Antimicrob. Resist. 8 135–139. 10.1016/j.jgar.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Zenati K., Touati A., Bakour S., Sahli F., Rolain J. M. (2016). Characterization of NDM-1- and OXA-23-producing Acinetobacter baumannii isolates from inanimate surfaces in a hospital environment in Algeria. J. Hosp. Infect. 92 19–26. 10.1016/j.jhin.2015.09.020 [DOI] [PubMed] [Google Scholar]
- Zhao F., Zong Z. (2016). Kluyvera ascorbata strain from hospital sewage carrying the mcr-1 colistin resistance gene. Antimicrobial. Agents Chem. 60 7498–7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Feng Y., Lü X., Mcnally A., Zong Z. (2017). IncP plasmid carrying colistin resistance gene mcr-1 in Klebsiella pneumoniae from hospital sewage. Antimicrobial. AgentsChem. 61:e02229-16.. 10.1128/AAC.02229-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Dong H., Xu H., Lv J., Zhang J., Jiang X., et al. (2016). Coexistence of MCR-1 and NDM-1 in Clinical Escherichia coli Isolates. Clin. Infect. Dis. 63 1393–1395. 10.1093/cid/ciw553 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in the https://www.ncbi.nlm.nih.gov/pathogens/isolates#/refgene/gene_family:(blaNDM), https://www.ncbi.nlm.nih.gov/pathogens/isolates#/refgene/gene_family:(mcr-1), MK965884.