Highlights
-
•
The application of electrocoagulation to remove the disadvantages of Soluble Algal Products.
-
•
Characterization of SAPs in a mixed microalgal culture.
-
•
Removing a major part of SAPs in terms of DOC, carbohydrate, and protein.
Keywords: Electrocoagulation, Microalgae, Mixed culture, Soluble Algal Products, Harvest
Abstract
The dewatering of algal culture requires coagulation of the algal cells. However, the coagulation in a continuous operation is slowed down through the excretion of Soluble Algal Products (SAPs). Electrocoagulation (EC), already utilized as a coagulation technique, has been investigated for its effects on SAPs characterizations. A mixed culture of Chlorella vulgaris, Scenedesmus Obliquus, Botryococcus braunii, Botryococcus sudeticus, and Afrocarpus falcatus was prepared and SAPs characteristics, including Specific Ultra Violet Absorbance (SUVA), Zeta potential, Molecular Weight (MW) fractionation, Dissolved Organic Carbon (DOC), protein and carbohydrate content, Excitation-Emission Matrix, and hydrophobicity using XAD resins, were measured and evaluated before and after electrocoagulation using mild steel and aluminum electrodes at 5 and 10 min. The results showed several improvements after EC. According to results, EC can render SAPs hydrophobicity up to 95 %, and the fluorescence peak results showed the complete removal of humic-like. Moreover, the SAPs were removed up to 21, 60, and 47 % for protein, carbohydrate and DOC, respectively. Results collectively showed that electrocoagulation might be able to mitigate the negative effects of growth on flocculation.
1. Introduction
The combination of wastewater treatment and microalgal cultivation has become a promising wastewater treatment strategy [1]. For a successful result, however, the biomass must be efficiently harvested from the cultivation broth. In addition, microalgae require 3726 kg fresh water to produce 1 kg biodiesel without recycling [2]. Although membrane bioreactors have been proved to be successful [3], the membrane fouling by algal, bacterial, and SAPs sources highly reduces the efficiency and membrane lifespan [4]. Moreover, the effect of SAPs is proved to be more dominant than algal and bacterial sources [5]. Furthermore, Discharging the medium into the pipe network, rivers, or lakes, will lead to bacterial growth, since SAPs serve as a great carbon source. Additionally, SAPs impact the taste and odor of fresh waters. On the other hand, keeping SAPs in the medium has shown to affect the microalgal growth [6]. Finally, studies have shown the links between the SAPs and production of precursors of disinfection by-products (DBPs) [[7], [8], [9]]. Thus, removal of SAPs effect seems to be critical for many operations.
SAPs include carbohydrates, protein, lipid, polysaccharides, amino acids, peptides, glycolic acid, organic phosphorus, enzymes, vitamins, hormonal substances, pigment, inhibitors and toxins [7,10]. Protein and carbohydrates dominate most of SAPs [11]. They originate from three sources: intracellular organic matter (IOMs) lysed from dead cells, desorbed surface-retained organic matters (SOMs) from cell surfaces, or secreted from living cells as extracellular organic matter (EOMs) [11]. Some SAPs like polyunsaturated aldehydes and precursors act as signal molecules and control the population density [12]. The presence of SAPs also largely affects the dosage of coagulants necessary for flocculation of microalgae in harvesting stage [[13], [14], [15], [16]]. SAPs with high molecular weight (MW) can aid coagulation whereas those of low MW can increase the negative surface charge [17].
Although treatment strategies, such as biological methods [18], coagulation [14], and ozonation [19] have been introduced, the application of electrocoagulation and its effects on the decrease of SAPs amount have not been fully investigated. In addition, this paper aims to study a mixed culture of microalgae which with further development in larger scale can simplify the operations in industry by removing the costs necessary for monoculture growth. Understanding the effects of the electrocoagulation on the characterization of SAPs in a mixed microalgal cultivation can facilitate the continuous algal dewatering and harvesting.
2. Materials and methods
Two sets of EC experiments were conducted independently to ensure the reproducibility of the results. Samples of each set underwent SAPs characterization tests. SAPs were characterized by composition through protein and carbohydrate tests, Excitation-Emission Matrix (EEM) by Fluorescence Spectrophotometer, hydrophobicity/hydrophilicity using XAD resins, Molecular Weight (MW) fractionation using fractionation membranes, dissolved organic carbon (DOC), specific ultraviolet absorbance (SUVA) using spectrophotometric measurements, and zeta potential (ZP) utilizing a Zetasizer before and after electrocoagulation and the results were compared accordingly. The details of each measurement have been provided below.
2.1. Microalgae medium and cultivation
A mixed culture containing Chlorella vulgaris, Scenedesmus Obliquus, Botryococcus braunii, Botryococcus sudeticus, and Afrocarpus falcatus was prepared and inoculated into a 4-liter cylindrical photobioreactor (PBR) fed with Bold’s Basal Medium (BBM) up to 3.5 liters. The PBR was illuminated using four 13 W 6700 K florescent lamps and aerated with 0.5 vvm of a mixed flow of air and CO2 (1.75 LPM air and its 5 % CO2 flow). In addition to the air flow, a magnet stirrer was utilized for further mixing. The final Dry Weight (DW) of the PBR was 2 mg.l−1. All analyses were conducted within 4 days of extraction and kept at 4 °C in the meantime.
2.2. Electrocoagulation cell
The EC cell consisted of a 250-mililiter beaker equipped with aluminum (Al) or mild steel (Fe) electrodes connected to a DC power supply. The sample volume was 200 milliliters, and EC lengths were 5 and 10 min. The sample naming was according to Table 1. Al5 and Al10 from now forth will refer to samples from aluminum electrodes with EC time of 5 and 10 min respectively, as well as Fe5 and Fe10 will determine electrocoagulation using mild steel electrodes for 5 and 10 min. Each sample was left to settle for 5 min before sampling. The current density for all experiments was 250 A.m−2, and the inter-electrode distance was 1 cm. The voltage was also monitored along all experiments. The cells were mixed using a magnet stirrer at a fixed speed for all samples. To obtain two samples of each Fe5, Fe10, Al5, and Al10, two sets of electrocoagulation experiments were run.
Table 1.
Samples’ Naming.
| Name | Description |
|---|---|
| Al5 | Sample with 5 minutes of electrocoagulation using aluminum electrodes |
| Al10 | Sample with 10 minutes of electrocoagulation using aluminum electrodes |
| Fe5 | Sample with 5 minutes of electrocoagulation using mild steel electrodes |
| Fe10 | Sample with 10 minutes of electrocoagulation using mild steel electrodes |
2.3. SAPs extraction method
SAPs were extracted by centrifuging the samples at 12857×g RCF for 15 min at the room temperature, and the subsequent filtering of the supernatant through a glass micro-fiber (0.7 mm Whatman GF/F glass micro-fiber) filter.
2.4. SAPs characterizations
2.4.1. Biochemical compositions
Proteins were measured through modified Lowry method [20] and carbohydrates were measured using the phenol-sulfuric method [21]. All analyses were carried out in triplicates and error bars were provided using standard deviation for the graphs.
2.4.2. Fluorescence spectroscopy
EEM values were obtained using a Cary Eclipse Fluorescence Spectrophotometer (Agilent, Netherlands) and a 4-mililiter length cuvette with 1 cm path. Emission spectra were scanned from 300 to 500 nm at 0.5 nm increments and excitation spectra were scanned from 250 to 400 nm with 5 nm increments [22]. The slits for excitation and emission were 5 nm. In addition, the deionized water blanks were used between every 4 analyses. It has to be noted that EEM values have been used to provide a more qualitative rather than a quantitative perspective to the investigation results [7].
2.4.3. XAD resin fractionation
A pair of XAD-7HP/XAD-4 columns was used to fractionate SAPs into hydrophobic and hydrophilic components [23]. A 250-ml SAPs sample was acidified to pH 2, and it was passed consecutively through the XAD-7HP and XAD-4 resins (5 ml resins in 10 mm glass tubes). The non-retained sample comprised the hydrophilic fraction (HPI). Each column was back-eluted with NaOH (0.1 M, 120 ml) such that the XAD- 7HP and XAD-4 resin back-effluent comprised the hydrophobic fraction (HPO) and transphilic fraction (TPI), respectively. The carbohydrate content of all fractions was measured as previously described.
2.4.4. MW fractionation
Vivaspin membrane (Sartorius, UK) was used to separate different sizes of biomolecules. Membrane sizes of 2, 5, 10, 30, 100, and 300 kDa were selected. The membranes were centrifuged at the speed and time given by the company manual. The supernatant of each size centrifugation was collected and tested for total carbohydrate and protein content.
2.4.5. DOC and SUVA
Dissolved Organic Carbon (DOC) was measured using an analyzer (Shimadzu TOC-5000A, Japan) by measuring the difference between total organic carbon and inorganic carbon. A UV/visible spectrophotometer was used at 254 nm to measure the absorbance at this wavelength. Next, the SUVA was calculated as UV254/DOC to reflect the concentration of the unsaturated compounds (including aromatic compounds) in the total dissolved organic carbon.
2.4.6. Zeta potential and charge density
Zeta potential was measured using a Zetasizer (Malvern, UK). The samples were kept at pH = 7 using phosphate buffer and the constant temperature of 25 °C.
3. Results and discussion
3.1. Molecular weight fractionation
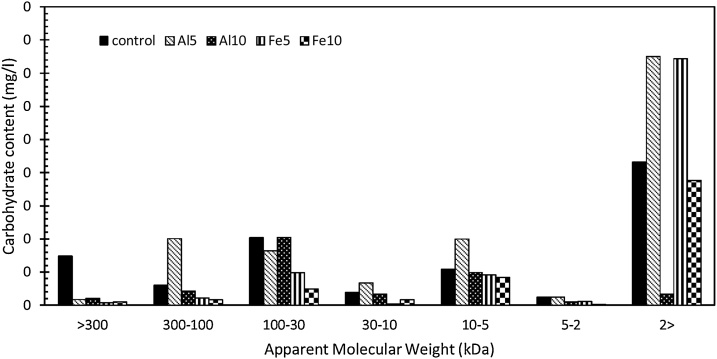
Electrocoagulation was conducted using Al and Fe electrodes and the SAPs characteristics were analyzed to investigate the effect of EC on SAPs. The results of total carbohydrates vs. MW fractionation for control and study samples after EC are depicted in the Fig. 1. Although MW of the SAPs varies significantly in different species, the molecules are mainly in the range of less than 3 kDa or more than 30 kDa [11]. The control sample included 40 % of total carbohydrate in SAPs MW above 30 kDa and 42 % in SAPs MW below 2 kDa. Similar results were also found in the literature [17]. After coagulation bigger carbohydrates are expected to be separated through settling or floatation. Thus, the percentage of carbohydrates bigger than 30 kDa is expected to fall while an increase in percentage of carbohydrates smaller than 3 kDa is predicted to be observed. Except for Al10, the total carbohydrates values for all the other samples fit to the predictions. The results for Al10 might be associated with over-dosage of electric charge leading to smaller EC efficiency. In addition, the increasing results of carbohydrate between 30 and 100 kDa for Al10 may also suggest the inefficiency of EC to coagulate these medium-sized particles resulting in their suspension in final medium. According to the results, the percentage of total carbohydrate containing molecules bigger than 30 kDa fell from 40 % in control sample to 26 %, 14 % and 8 % in Al5, Fe5, and Fe10, respectively. On the other hand, the percentage for carbohydrates smaller than 2 kDa rose from 42 % in the control sample to, respectively, 52 %, 76 %, and 72 % in the Al5, Fe5, and Fe10. It must be noted that the carbohydrate analysis shows that the total amount in control sample was 18.75 mg.l−1. This value fell into 15.41, 14.78, 18.03, and 13.15 mg.l−1. These values might suggest that a part of carbohydrates was separated, since the samples were taken from the solution and not the top layer separated by electrocoagulation.
Fig. 1.
MW distributions of SAPs in terms of carbohydrates content percentage for control sample, and the electrocoagulated samples of Al5, Al10, Fe5, and Fe10.
The carbohydrate separation is smaller than reported value by the chemical coagulation [14]. However, the total carbohydrates value was approximately 1 mg, which, considering the volume, is almost a quarter of the amount in this study.
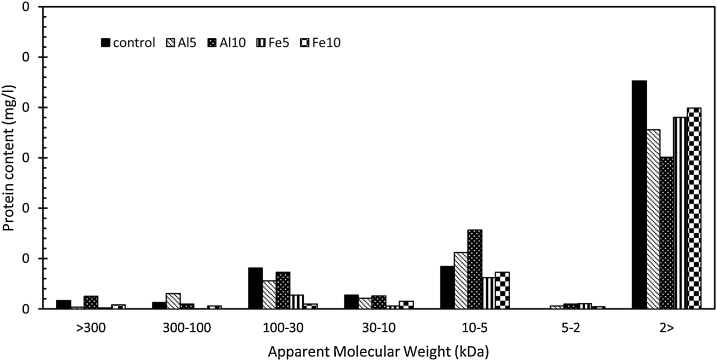
The protein content of the sample after EC was also measured and the results from MW fractionation are illustrated in Fig. 2. The control sample contained 16 % of its total protein at MW larger than 30 kDa. Also, 67 % of the SAPs contained oligopeptides, proteins smaller than 2 kDa. These results contradict with the results in previous works [4,17] where the major part of the proteins contained larger MW fractions. These results might be associated with the extraction technique [4]. Except for Al10, the protein size followed the same pattern as carbohydrates. The protein content tended towards the smaller MW fractions after EC. The proteins larger than 30 kDa consisted %13, 5, 5 of SAPs in Al5, Fe5, and Fe10, respectively.
Fig. 2.
MW distributions of SAPs in terms of protein content percentage for control sample, and the electrocoagulated samples of Al5, Al10, Fe5, and Fe10.
The protein:carbohydrate ratio in the control sample was 1.66 mg mg−1. This ratio fell into 0.84 and 1.29 for Al5 and Fe5, respectively, but rose to 3.2 and 2.05 mg mg−1 for Al10 and Fe10, respectively. On one hand, the primary fall in protein: carbohydrate ratio in both Fe5 and Al5 may be associated with the fact that proteins are considerd as charge nuetralizers [24]. In other words, the surface charge on the proteins will interact with ions generated by electrocoagulation. The subsequent charge neutralization will lead to enhancment of coagulation, since particles can approach to form flocs. This procedure conclusively causes the seperation from the culture (the primary fall of protein). On the other hand, SAPs are dominated by hydrophobic proteins and hydrophylic carbohydrates [17], and the data obtained in this research (refer to XAD fractionation resluts) show that EC operation largely improves hydrophobicity leading to sepration of more carbohydrates by floatation. This conclusion is supported by the fact that the percentage of total carbohydrate value with MW larger than 30 kDa has fallen. Collectively, the big rise of protein:carbohydrate ratio in Fe10 and Al10 is more likely to be associated with the seperation of large hydrophilic carbohydrates compared to small proteins.
It must be noted that according to the reactions at the anode [25], with the same amount of mass, aluminium generates more ions, neutralizing more charges and leading to better results compared to steel. Furthermore, studies have shown that the affinity of protein to form complexes with organic matter is different with the affinity of protein with iron. [26]. Nevertheless, only the affinity at 60 kDa on two pure microalgae species has been investigated, and further investigattions are necessary to understand the impact.
3.2. Fluorescence spectroscopy
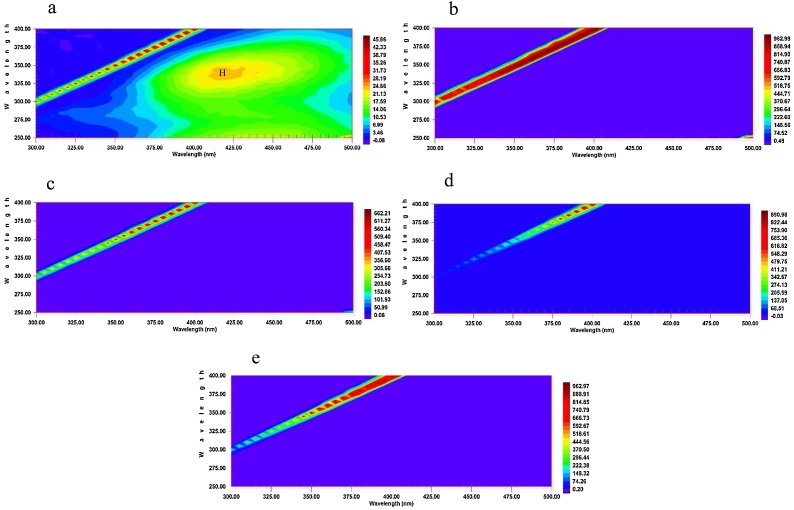
The results of fluorescence spectroscopy are illustrated in the Fig. 3. Qualitatively, humic-like substances, which comprise of an important part of SAPs, will show a peak at the Excitation value of 350 nm and an Emission range of 420−480 nm [27]. As it can be seen in Fig. 3.a, this peak, marked by the letter H, can be observed by a relatively moderate intensity. However, on all the other EEMs, the intensity has dramatically fallen. According to Fig. 3, in Al5, Al10, Fe5, and Fe10, the H peak intensity representing the humic-like substances has decreased. This weakened intensity might be interpreted as the separation of humic-like substances through EC application. Nevertheless, the remaining strips on all the florescence maps have also been present in other works on SAPs analysis [17].
Fig. 3.
Fluorescence excitation–emission matrix (EEM) spectra for (a) control sample with a moderate-intensity peak for humic-like substances (with “H” mark); (b) Electrocoagulated sample with aluminum electrodes for 5 min showing no humic-like moderate-intensity peak(Al5) ; (c) Electrocoagulated sample with aluminum electrodes for 10 min showing no humic-like moderate-intensity peak (Al10); (d) Electrocoagulated sample with aluminum electrodes for 10 min showing no moderate-intensity humic-like peak (Fe5); (e) Electrocoagulated sample with aluminum electrodes for 10 min showing no moderate-intensity humic-like peak (Fe10). Z-axis = excitation (nm); X-axis = emission (nm); and Y-axis = intensity.
3.3. Zeta potential
To reach a sense of comparison and consistency, all the Zeta potential measurements were carried out for EC runs of 5 and 10 min. However, the Zeta potentials of the samples increased almost twice the -14.1 mV in control sample. This could be due to the charge overdose [28]. As a result, only in Zeta potential measurements, the experiments were executed for 60 s. Consequently, a sharp change of Zeta potential was obtained for both Al and Fe electrodes. The Zeta values for Al and Fe were -0.5 and -1.2 mV, respectively. The Zeta potential may be linked to the ionization of functional groups in the colloidal organics. Although the decrease in Zeta potential can vary in different species [14], the fact that the SAPs charge can be partially or completely neutralized may suggest the functionality of EC for the reduction of SAPs in the medium. The results can be comparable with chemical coagulation where a range of 90−5000 mg.meq−1 coagulant dose:charge equivalent was used to achieve similar neutralization [14]. Here again the aluminum worked better in neutralizing the charge, since for constant mass it can produce more ions.
3.4. DOC and SUVA
The carbohydrate:DOC ratio for the control sample was 0.49 mg. mg−1. Although it is suggested by other studies that carbohydrate:DOC ratio is consistent around 1 mg. mg−1 for different species [17], it has been shown that this ratio largely depends on the extraction method including temperature, pH, and centrifugation speed. The results in that study show that at higher centrifugation rates, such as this study, DOC values can be significantly larger than carbohydrate values leading to smaller carbohydrate:DOC ratio. In addition, centrifugation time can affect the DOC values much more than carbohydrate values [4]. According to the measurements carbohydrate:DOC ratios for all samples slightly changed. (Table 2).
Table 2.
A summary of sample characteristics after EC operations compared with control sample.
| Parameters | Control | Al5 | Al10 | Fe5 | Fe10 |
|---|---|---|---|---|---|
| SUVA (l m−1 mg −1) | 0.67 | 0.44 | 0.46 | 0.69 | 0.71 |
| Hydrophobicity (%) | 39.6 | 95.5 | 85.8 | 78.6 | 76.9 |
| Hydrophilicity (%) | 54.7 | 4.3 | 5.6 | 0.13 | 0.27 |
| Zeta potential (mV) | 14.1 | −0.5† | −1.2a | ||
| Carbohydrate: DOC (mg mg−1) | 0.49 | 0.44 | 0.32 | 0.5 | 0.41 |
| Protein: carbohydrate (mg mg−1) | 1.66 | 0.84 | 3.2 | 1.29 | 2.05 |
| Fluorescence peak | Humic-like substances | none | none | none | none |
| SAPs carbohydrates >30 kDa (%) | 40.64 | 25.82 | 56.19 | 13.73 | 8.27 |
| SAPs carbohydrates < 2 kDa (%) | 42.40 | 52.85 | 12.44 | 75.69 | 71.98 |
| SAPs proteins >30 kDa (%) | 16.44 | 13.23 | 17.15 | 5.11 | 5.13 |
| SAPs proteins < 2 kDa (%) | 66.92 | 63.21 | 50.86 | 81.06 | 74.69 |
Experiments were carried out in 60 s as explained in the content.
SUVA is an indicator for the presence of aromatic carbon compounds. The SUVA of the control sample was measured to be 0.67 l.m−1. mg−1 which matches with other works [17]. This value for Al5 and Al10 fell into 0.44 and 0.46 l.m−1. mg−1, respectively. However, the SUVA values for Fe5 and Fe10 increased to 0.69 and 0.71 l.m−1. mg−1, respectively. These results indicate that the aromatic carbon content in case of Al electrodes has fallen by 30 %. The increase in SUVA values for Fe electrodes, on the other hand, might have occurred as a result of interference of ferric (Fe+3) and ferrous (Fe+2) iron as previously discussed by researchers [29].
3.5. XAD resin fractionation
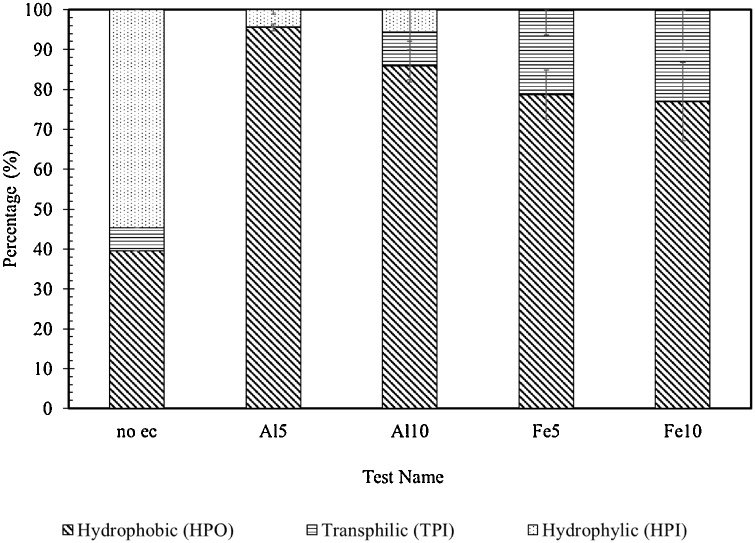
As it can be seen from Fig. 4, the algal culture in this study is highly hydrophilic (54 %). Similar results were obtained in the literature [4,17]. This high amount of hydrophilic content usually includes polysaccharides and hydroxyl acids [30]. In membranes, fouling is largely caused through hydrophilic compounds [8]. After EC, the hydrophilicity decreased into 4 and 5 percent for Al5 and Al10 samples, respectively, and almost zero in both Fe samples. The hydrophobicity is the primary force for the hydrophobic articles and air bubbles [31]. In addition, cross-linking of proteins coagulation which is sequent to denaturation of the proteins usually involves polyfunctional agents including metal ions in solution [32]. Given the fact that floatation by bubbles is considered one of the major mechanisms of electrocoagulation in separating colloids, the increase in hydrophobicity will, therefore, enhance the efficiency of coagulation. As the results show, EC itself can increase the hydrophobicity. In other works in literature, hydrophobicity was improved by adding collectors and raising the dosage [33]. The main measure of hydrophobic compounds comprises of large molecules (>10 kDa). On the other hand, hydrophilic compounds largely contain smaller molecules (<1 kDa) [34]. Consequently, by raising the amount of hydrophobic molecules in the algal culture, the subsequent coagulation will be greatly improved. Furthermore, hydrophilic compounds mainly include neutral polysaccharides and acidic sugars [30]. Given the carbohydrate:DOC ratio after EC and the fact that both DOC and total carbohydrate values have decreased independently, the data suggest the separation of more hydrophilic compounds.
Fig. 4.
Percentage of total carbohydrates in the SAP present in the HPO, TPI and HPI fractions for control sample (without EC) and other samples undergone EC experiments.
A summary of characterization of SAPs in all samples has been collected in Table 2.
3.6. SAP removal
According to one study, algal organic matter was removed at an efficiency between 46 and 71 % [35] while in another study this efficiency ranged between 18 and 50 % [36]. Here, in this study SAPs, in terms of DOC was removed by 35, 40, 42, and 47 % for Al5, Al10, Fe5, and Fe10, respectively. In terms of protein content only, results show that SAPs were reduced by 5, 20, 8, and 21 % for the same samples. And finally as for carbohydrate perspective, EC separated the SAPs by 41, 60, 39, and 56 %. These results are quite comparable with the abovementioned studies. It must be noted that the efficiency of the SAPs removal is largely dependent on factors like pH, and MW fractionation of the content. pH, for instance, determines which complexation would occur and therefore changes can affect the separation [37]. In other words, the SAPs separation can be improved by parametric optimization of EC using pH, current density, inter-electrode gap, time-length, etc. While the “optimization” of SAPs removal may be the subject of a separate study, in this paper the “possibility” of this removal using EC was mostly focused. On the other hand, the results here show that a large portion of SAPs contains low MW molecules. These small molecules cause inefficient flocculation, since the SAPs must cross-link metal compounds to form flocs [14]. As a result, even in case of smaller efficiencies of removal, an increase in the demand of coagulants will be most likely to result in higher removal efficiencies. It must be remarked that with proper preparations like sufficient dosage of coagulants, and optimum pH, metal-organic complexes can be removed rapidly using the process of sweep flocculation [38]. Thus, a separate study is required to investigate the optimization of EC for SAPs removal.
4. Conclusion
In the present research, the Soluble Algal Products (SAPs) were characterized after an electrocoagulation operation. Based on the results obtained, electrocoagulation positively affects the SAPs. In other words, those SAPs features which decrease the efficiency of coagulation overtime were effectively removed or mitigated. As a result, in case of continuous electrocoagulation for algal harvesting and dewatering, the effect of SAPs which remain in the culture after each cycle of operation can be moderated. Moreover, for similar reasons if other technologies are aimed to be used for separating algal biomass, electrocoagulation seems quite promising as a pretreatment unit. However, a further study on the parameters of electrocoagulation seems necessary to optimize the work of such target.
Author statement
This manuscript is a joint work by all of the authors. P Rafiee is responsible for writing the manuscript, conducting the experiments and analyzing the data. S Ebrahimi and YW Tong jointly helped to conceptualize the idea and develop the methodology. M Hosseini provided guidance in data analysis.
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00433.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Zhu L., Wang Z., Shu Q., Takala J., Hiltunen E., Feng P. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013;47:4294. doi: 10.1016/j.watres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Yang J., Xu M., Zhang X., Hu Q., Sommerfeld M., Chen Y. Life-cycle analysis on biodiesel production from microalgae: water footprint and nutrients balance. Bioresour. Technol. 2011;102:159. doi: 10.1016/j.biortech.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Rossignol N., Vandanjon L., Jaouen P., Quemeneur F. Membrane technology for the continuous separation microalgae/culture medium: compared performances of cross-flow microfiltration and ultrafiltration. Aquac. Eng. 1999;20:191. [Google Scholar]
- 4.Chu H., Yu H., Tan X., Zhang Y., Zhou X., Yang L. Extraction procedure optimization and the characteristics of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) from Chlorella pyrenoidosa. Colloids Surf. B Biointerfaces. 2015;125:238. doi: 10.1016/j.colsurfb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Babel S., Takizawa S., Ozaki H. Factors affecting seasonal variation of membrane filtration resistance caused by Chlorella algae. Water Res. 2002;36:1193. doi: 10.1016/s0043-1354(01)00333-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T.-Y., Yu Y., Wu Y.-H., Hu H.-Y. Inhibitory effects of soluble algae products (SAP) released by Scenedesmus sp. LX1 on its growth and lipid production. Bioresour. Technol. 2013;146:643. doi: 10.1016/j.biortech.2013.07.142. [DOI] [PubMed] [Google Scholar]
- 7.Her N., Amy G., Park H.-R., Song M. Characterizing algogenic organic matter (AOM) and evaluating associated NF membrane fouling. Water Res. 2004;38:1427. doi: 10.1016/j.watres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Gao N., Deng Y., Yao J., Zhang K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012;46:1233. doi: 10.1016/j.watres.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Tabatabai S.A.A., Schippers J.C., Kennedy M.D. Effect of coagulation on fouling potential and removal of algal organic matter in ultrafiltration pretreatment to seawater reverse osmosis. Water Res. 2014;59:283. doi: 10.1016/j.watres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Biddanda B., Benner R. Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnol. Oceanogr. 1997;42:506. [Google Scholar]
- 11.Zhuang L.-L., Wu Y.-H., Espinosa V.M.D., Zhang T.-Y., Dao G.-H., Hu H.-Y. Soluble Algal Products (SAPs) in large scale cultivation of microalgae for biomass/bioenergy production: a review. Renew. Sustain. Energy Rev. 2016;59:141. [Google Scholar]
- 12.Leflaive J., Ten‐Hage L. Chemical interactions in diatoms: role of polyunsaturated aldehydes and precursors. New Phytol. 2009;184:794. doi: 10.1111/j.1469-8137.2009.03033.x. [DOI] [PubMed] [Google Scholar]
- 13.Garzon-Sanabria A.J., Ramirez-Caballero S.S., Moss F.E., Nikolov Z.L. Effect of algogenic organic matter (AOM) and sodium chloride on Nannochloropsis salina flocculation efficiency. Bioresour. Technol. 2013;143:231. doi: 10.1016/j.biortech.2013.05.125. [DOI] [PubMed] [Google Scholar]
- 14.Henderson R.K., Parsons S.A., Jefferson B. The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae. Water Res. 2010;44:3617. doi: 10.1016/j.watres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Vandamme D., Foubert I., Fraeye I., Muylaert K. Influence of organic matter generated by Chlorella vulgaris on five different modes of flocculation. Bioresour. Technol. 2012;124:508. doi: 10.1016/j.biortech.2012.08.121. [DOI] [PubMed] [Google Scholar]
- 16.Vandamme D., Muylaert K., Fraeye I., Foubert I. Floc characteristics of Chlorella vulgaris: influence of flocculation mode and presence of organic matter. Bioresour. Technol. 2014;151:383. [Google Scholar]
- 17.Henderson R.K., Baker A., Parsons S.A., Jefferson B. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms. Water Res. 2008;42:3435. doi: 10.1016/j.watres.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Liu D., Lu L., Zhao Z., Xu Y., Cui F. Degradation of algal organic matter using microbial fuel cells and its association with trihalomethane precursor removal. Bioresour. Technol. 2012;116:80. doi: 10.1016/j.biortech.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Paralkar A., Edzwald J.K. Effect of ozone on EOM and coagulation. Am. Water Works Assoc. J. 1996;88:143. [Google Scholar]
- 20.Fr B., Griebe T., Nielsen P. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 1995;43:755. doi: 10.1007/BF00164784. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X., Bishop P.L., Kinkle B.K. Comparison of extraction methods for quantifying extracellular polymers in biofilms. Water Sci. Technol. 1999;39:211. [Google Scholar]
- 22.Baker A. Fluorescence properties of some farm wastes: implications for water quality monitoring. Water Res. 2002;36:189. doi: 10.1016/s0043-1354(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 23.Malcolm R.L., MacCarthy P. Quantitative evaluation of XAD-8 and XAD-4 resins used in tandem for removing organic solutes from water. Environ. Int. 1992;18:597. [Google Scholar]
- 24.Liao B., Allen D., Droppo I., Leppard G., Liss S. Surface properties of sludge and their role in bioflocculation and settleability. Water Res. 2001;35:339. doi: 10.1016/s0043-1354(00)00277-3. [DOI] [PubMed] [Google Scholar]
- 25.Mollah M.Y.A., Schennach R., Parga J.R., Cocke D.L. Electrocoagulation (EC)—science and applications. J. Hazard. Mater. 2001;84:29. doi: 10.1016/s0304-3894(01)00176-5. [DOI] [PubMed] [Google Scholar]
- 26.Pivokonsky M., Kloucek O., Pivokonska L. Evaluation of the production, composition and aluminum and iron complexation of algogenic organic matter. Water Res. 2006;40:3045. doi: 10.1016/j.watres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Coble P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996;51:325. [Google Scholar]
- 28.López-Maldonado E., Oropeza-Guzman M., Jurado-Baizaval J., Ochoa-Terán A. Coagulation–flocculation mechanisms in wastewater treatment plants through zeta potential measurements. J. Hazard. Mater. 2014;279:1. doi: 10.1016/j.jhazmat.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Weishaar J.L., Aiken G.R., Bergamaschi B.A., Fram M.S., Fujii R., Mopper K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003;37:4702. doi: 10.1021/es030360x. [DOI] [PubMed] [Google Scholar]
- 30.Edzwald J. Coagulation in drinking water treatment: particles, organics and coagulants. Water Sci. Technol. 1993;27:21. [Google Scholar]
- 31.Ducker W.A., Xu Z., Israelachvili J.N. Measurements of hydrophobic and DLVO forces in bubble-surface interactions in aqueous solutions. Langmuir. 1994;10:3279. [Google Scholar]
- 32.Boye J., Ma C., Harwalkar V. Thermal denaturation and coagulation of proteins. Food Sci. Technol. 1997:25. New York-Marcel Dekker. [Google Scholar]
- 33.Garg S., Li Y., Wang L., Schenk P.M. Flotation of marine microalgae: effect of algal hydrophobicity. Bioresour. Technol. 2012;121:471. doi: 10.1016/j.biortech.2012.06.111. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y., Hu H.-Y., Li X., Wu Y.-H., Zhang X., Jia S.-L. Accumulation characteristics of soluble algal products (SAP) by a freshwater microalga Scenedesmus sp. LX1 during batch cultivation for biofuel production. Bioresour. Technol. 2012;110:184. doi: 10.1016/j.biortech.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Vandamme D., Foubert I., Muylaert K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013;31:233. doi: 10.1016/j.tibtech.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Widrig D.L., Gray K.A., McAuliffe K.S. Removal of algal-derived organic material by preozonation and coagulation: monitoring changes in organic quality by pyrolysis-GC-MS. Water Res. 1996;30:2621. [Google Scholar]
- 37.Moreno-Casillas H.A., Cocke D.L., Gomes J.A., Morkovsky P., Parga J., Peterson E. Electrocoagulation mechanism for COD removal. Sep. Purif. Technol. 2007;56:204. [Google Scholar]
- 38.Duan J., Gregory J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003;100:475. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.