Abstract
The current study evaluated the key characters of aroma composition in diversified red wines (Cinsaut, Grenache, Cabernet Franc, Petit Verdot, Cabernet Sauvignon, Nielluccio, Tempranillo, Syrah, Merlot and Caladoc). Out of hundreds of volatile compounds 64 compounds were considered for study. Different groups consisting of fatty acids, volatile alcohols, aldehydes, esters, volatile phenols and terpenes were analysed using gas chromatography mass spectrometry coupled with thermal desorption (TD–GC–MS). Among all these diversified classes, alcohols were found as the most dominant group followed by esters and acids whereas aldehydes, phenols and terpenes were found to be minor compounds. Among the varieties, Nielluccio wine recorded highest concentration of total volatile compounds (191.53 mg/L) while, it was least in Caladoc wines (15.45 mg/L). The principal component analysis clearly differentiated Grenache wines based on their relationships between scores and their aroma composition followed by Nielluccio and Cinsuat wines. Out of sixty four compounds, only six aromatic compounds viz. butanediol, isoamyl actate, γ-Terpene, butanol, acetic acid and furfural have satisfying aroma descriptors with floral and fruity nuances and contribute to differentiate the Grenache wines from other varieties which have similar scores in PC1 analysis. The cluster analysis also suggested that the wines in the same group (Cinsaut, Tempranillo and Syrah), (Cabernet Franc, Cabernet Sauvignon, Caladoc and Merlot) and (Nielluccio and Petit Verdot) had similar aroma characterization. Grenache wines were well differentiated from the sub group formed by other red varieties.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04147-0) contains supplementary material, which is available to authorized users.
Keywords: Volatile compounds, Wines, Red wine varieties, Phenolics, TD–GC–MS
Introduction
Wine is one of the most complex alcoholic beverages produced by fermentation of grape juice. Wine quality is decided by grape variety, quality of grapes which are fermented, yeast strain and fermentation conditions. The presence of different flavour compounds in grape berry and the flavour produced during fermentation have own importance in attaining higher consumer preference. The wine flavour is composed of volatile compounds, which are especially responsible for the aroma. These characteristics are the results of complex interactions among different factors such as vineyard geography (Koundouras et al. 2006), grape variety (Armanino et al. 2008), yeast strain used for fermentation (Torrens et al. 2004), technical conditions of winemaking (Welke et al. 2014), etc. Presently, more than thousand aromatic compounds classified under higher alcohols, esters, organic acids, aldehydes, ethers, ketones and terpenes, volatile phenols etc. have been identified (Andujar-Ortiz et al. 2009). In addition to this, contribution of terpenols and C13-norisoprenoids to the character of wine is also reported (Vilanova et al. 2013). Concentration of aroma compounds in wines varies from few ng/L to mg/L (Cesnik et al. 2015).However, the influence of volatile compounds in wine aroma depends on their content, composition and the specific olfactory perception threshold of each compound (Welke et al. 2014). In other words, the overall volatome fingerprinting of the wine determine its aroma characteristics.
Volatile compositions in grapes and wines are studied by many workers (Vilanova et al. 2007). Volatile profiling have been performed in different varieties grown under various geographic regions, i.e. Cabernet Sauvignon from China, California and Brazil (Falcão et al. 2008; Tao et al. 2008), Sauvignon Blanc from New Zealand, France and Australia (Berna et al. 2009), Chardonnay from Croatia and China (Jiang and Zhang 2010), Tempranillo, Cabernet Sauvignon and Merlot from Spain (Ferreira et al. 2000). Effect of terroir and geographical conditions on wine compositions have been reflected in these studies. Well-known wine grape varieties are being grown under Indian conditions but as per available literature, no information on volatome finger printing is available.
Aroma compounds in wines are widely determined by using Gas chromatography (GC). Due to low level availability of aroma compounds in wines, suitable extraction/concentration step is needed. Liquid–liquid extraction (LLE) is primarily used for extracting volatiles from wine (Piñeiro et al. 2004). For volatile extraction, a technique based on Stir bar sorptive extraction has been developed where a SPE cartridge (SPE-td cartridge) is used in a thermal desorption system coupled with GC–MS. This technique is found suitable for screening of many volatile compounds in wines (Banerjee et al. 2015).
With rapid development of the wine industry in India, the quality of Indian wines is improving constantly and is getting accepted by Indian as well as foreign consumers. However, sensory data of Indian wines is still scarce, especially for wines of a specific denomination. The aim of the present study was to define the volatome profiling of red wine varieties grown under tropical climatic conditions of Maharashtra, India by using solid phase extraction followed by TD–GC–MS analysis.
Materials and Methods
Vineyard site
The research was carried out at the experimental farm of ICAR-National Research Centre for Grapes, Pune during the year 2013–2014. The vineyard is situated at mid-west Maharashtra state at an altitude of 559 m (18.32° N and 73.51° E) with tropical wet and dry climate and the average temperature is ranging between 25 and 35 °C during the peak period of season. Ten red wine grape varieties (Cinsaut, Grenache, Cabernet Franc, Petit Verdot, Cabernet Sauvignon, Nielluccio, Tempranillo, Syrah, Merlot and Caladoc) grafted onto 110-R rootstock were selected for the study. The vines were planted in North–South direction in black soil (EC 0.8 dS/m and pH 7.3) spaced at 2.66 m between the rows and 1.33 m between the vines. These vines were trained to mini Y trellis with cordons placed horizontally so as the shoots were positioned vertically.
Wine preparation
The grapes were harvested at 23°Brix corresponding to wines containing 11% ethanol (v/v). The grapes were processed following the traditional protocol of red wine making. Berries of ten different red wine varieties were destemmed and crushed on de-stemmer-cum-crusher and transferred to 20 L stainless steel containers. Potassium metabisulphite 5 mg/10 kg grape must was added to stop the activities of naturally available microorganisms. The grape musts were exposed to cold shock at 5 °C followed by pump. The must was incubated with commercial yeast strain EC1118 (Saccharomyces bayanus) in the form of dry active yeast (20 mg/L). The temperature for fermentation was maintained below 22 ± 2 °C with cold exchanger (Frozen water container) and the fermentation process was carried out for eleven days. After fermentation (reducing sugar < 2 g/L), the wines were separated from the skins and seeds. After required racking and separation of lees, 60 ppm SO2 was added and bottles were stored at 4 °C until analysis were carried out.
Chemicals and reagents
3-Octanol (> 97% purity), the internal standard (IS), was procured from Sigma Aldrich. Thomas Baker make sodium chloride (NaCl), sodium hydroxide (NaOH), glycerine, glucose and tartaric acid were used. Ethanol (99.9% purity) of SD Fine Chemicals Limited (Mumbai, India) was used and HPLC grade water was procured from Merck India Limited. SPE-tD cartridge (P/N C-SPTD10) used for volatile sorption was obtained from Markes International, Llantrisant, RCT, UK.
Sample preparation
The volatile compounds were sorbed on a SPE-tD cartridge as per the below method (In-house generated).Wine sample (8 mL) of each variety was transferred to a 20 mL glass headspace vial and to it 3-octanol (150 µg/L) as internal standard (IS) and 2 g of NaCl were added. The SPE-tD cartridge and magnetic stirrer were inserted into the wine sample for sorption of volatile compounds. After crimping the vial, it was heated at 80 °C for 40 min followed by cooling for 20 min at room temperature on a magnetic stirrer. The SPE-tD cartridge with the volatile compounds was then removed from the headspace vial, wiped with tissue paper and transferred to an empty glass TD tube placed in a TD auto sampler attached to the GC–MS prior to analysis.
Volatile analysis by TD–GC/MS
The pre adsorbed SPE-tD cartridge with volatile compounds was inserted into the Thermal Desorption (TD) system (Series2 UNITY), which was equipped with a Series2 ULTRA auto sampler (100 sample capacity; Markes International, Llantrisant, RCT, UK). The SPE-tD cartridge was then heated at 180 °C for 20 min to desorb the volatiles. The volatiles released were passed from the auto sampler to the TD trap unit through a transfer line maintained at 200 °C. All the volatiles were trapped by a quartz cold trap maintained at − 20 °C using an electronic Peltier cooling system. The cold trap was then heated to 275 °C at 60 °C/s and maintained at that this temperature for 5 min. The desorbed vapours were transferred to the GC analytical column through a transfer line and analysed using GC–MS. An Agilent 7890A GC system was used in conjunction with an Agilent 5975C single quadrupole mass spectrometer (Santa Clara, CA, USA). The chromatographic separation was performed using a HP-INNOWAX capillary column. Helium was used as the carrier gas at a constant flow rate of 1.3 mL/min. The oven temperature was programmed from 40 °C (1 min hold) and ramped at 5 °C/min to 250 °C (24 min hold), resulting in a total run time of 67 min. Spectra were acquired in the mass range of 30–350 m/z. The volatile compounds were tentatively identified by comparing the full scan spectra to the in house targeted library using a minimum match factor of 90%. The target library was generated by copying spectra from the NIST11 Mass Spectral Library of known volatile compounds identified from the literature. Concentrations of the identified compounds were estimated against the IS 3-octanol (Tao et al. 2008).
Data processing analysis
The chromatogram of each wine sample contained large number of masses in the range of 30–350 m/z. The chromatogram was acquired using Agilent Chem Station (version MSD Chem Station E.02.02.1431) software and Agilent Mass Hunter software (version B.06.00) was used for targeted deconvolution for the positive masses. The software was set to compare the deconvoluted spectra against a target library comprising known wine volatiles. The integration parameters used for the confirmation of presence or absence of the compounds included retention time (retention window ± 0.2 min), ion ratio (minimum 50%) and minimum match factor as 90%.
Statistical analysis
The data was analysed using SAS version 9.3. Tukey’s test was used for the comparisons of varieties and the principal component analysis (PCA) and cluster analysis was performed by SAS enterprise guide 4.3.
Results and Discussion
Sixty four aromatic compounds quantified from studied wines were listed in Table 1, 2, 3, 4, and 5. Among the different aromatic compounds, alcohols were the most dominant group in all wines followed by esters and acids whereas aldehydes, phenols and terpenes were found at low concentration. During the period of study, total aroma composition was ranged from 191.53 mg/L for Nielluccio wine followed by 153.94 mg/L for Grenache and 124.12 mg/L for Petit Verdot wines, while it was least in Caladoc wine (15.45 mg/L). The highest concentration of volatile compounds in Nielluccio wine was represented by alcohols (61.56 mg/L), esters (65.56), terpenes (30.18 mg/L), acids (24.38 mg/L) and phenols (0.56 mg/L).Observed differences in the volatile composition of the wines in present study could be influenced by varietal effect. These results are quantitative rather than qualitative, which agrees with previous studies for volatile composition among the different varieties (Vilanova et al. 2007). Similarly, Song et al. (2013) reported that volatile composition of wine was entirely dependent on the variety employed.
Table 1.
Volatile acids in red wines (ppm)
| Varieties | Acetic acid | Dodecanoic acid | Hexanoic acid | n-Decanoic acid | Nonanoic acid | Octanoic Acid | Oxalic acid | Pentadecanoic acid | Propanoic acid | 2-methylpropanoic acid | Tetradecanoic acid | Undecanoic acid |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cinsaut | 3.05c | 0.48c | 0.32d | 0.39b | 0.08d | 1.04c | 0.29d | 0.42c | 0.06d | 0.39e | 0.98c | 0.42c |
| Grenache | 14.50a | 0.78b | 1.25b | 0.42a | 0.19a | 6.49a | 2.70a | 1.08a | 0.36a | 2.22b | 1.57a | 1.09a |
| Cabernet Franc | 0.28e | 0.09h | 0.12e | 0.07g | 0.02h | 0.19e | 0.01f | 0.06g | 0.01h | 0.10gf | 0.13i | 0.06g |
| Petit Verdot | 1.95d | 0.50c | 1.52a | 0.34c | 0.10c | 2.26b | 0.78c | 0.30d | 0.21c | 3.10a | 0.72d | 0.30d |
| Cabernet Sauvignon | 0.19e | 0.17g | 0.18e | 0.08f | 0.03g | 0.27b | 0.02f | 0.13f | 0.02g | 0.14gf | 0.28f | 0.13f |
| Nielluccio | 12.81b | 0.95a | 1.49a | 0.41a | 0.15b | 2.20ed | 1.21b | 0.70b | 0.33b | 2.02c | 1.42b | 0.69b |
| Tempranillo | 0.42e | 0.42d | 0.50c | 0.17e | 0.05e | 0.92b | 0.04f | 0.24e | 0.04f | 0.49d | 0.51e | 0.24e |
| Syrah | 2.30d | 0.37e | 0.18e | 0.17e | 0.03f | 0.31c | 0.21e | 0.22e | 0.05e | 0.43ed | 0.51e | 0.21e |
| Merlot | 0.25e | 0.20f | 0.26d | 0.25d | 0.05e | 0.41ed | 0.01f | 0.11f | 0.02g | 0.08g | 0.24g | 0.10f |
| Caladoc | 0.24e | 0.10h | 0.17e | 0.08f | 0.02gh | 0.37d | 0.01f | 0.02h | 0.01h | 0.18f | 0.18h | 0.04g |
| LSD 5% | 0.371 | 0.0258 | 0.069 | 0.0082 | 0.0065 | 0.1993 | 0.058 | 0.0402 | 0.0076 | 0.0961 | 0.0274 | 0.0343 |
Same character denotes no significance difference. However, the different characters (e.g. a, b, c) show significant differences
Table 2.
Volatile alcohols in red wines (ppm)
| Varieties | 1-Heptanol | 1-Hexanol | 1-Hexanol, 2-ethyl- | 1-Octanol | 1-Pentanol, 3-methyl- | 2,3-Butanediol | 2-Butanol | 3-Hexen-1-ol, (Z)- | 6-Octen-1-ol, 3,7-dimethyl- [β-Citronellol] | 7-Octen-1-ol, 3,7-dimethyl-, (S)- [α-Citronellol] | Benzyl Alcohol | Benzyl alcohol,.alpha.-isobutyl-2,4,6-trimethyl- | n-Tridecan-1-ol | Phenylethyl Alcohol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cinsaut | 0.43b | 0.85d | 0.16de | 0.27d | 0.27d | 8.82c | 3.18d | 0.15c | 0.03de | 0.06d | 0.15e | 0.18b | 0.13d | 1.80d |
| Grenache | 0.22d | 4.94a | 1.91a | 0.26d | 1.44a | 7.83cd | 18.73a | 0.46a | 0.30a | 0.16b | 1.23a | 0.26a | 0.26c | 6.94c |
| Cabernet Franc | 0.02f | 0.15g | 0.05f | 0.11ef | 0.02f | 1.03ef | 0.60h | 0.00f | 0.00g | 0.01h | 0.03h | 0.04gf | 0.01gh | 0.42g |
| Petit Verdot | 0.16e | 2.65c | 0.69c | 0.78b | 0.26d | 26.64b | 9.12c | 0.35b | 0.12c | 0.15c | 0.50b | 0.13d | 0.35b | 7.51b |
| Cabernet Sauvignon | 0.03f | 0.40f | 0.08f | 0.15e | 0.05f | 2.45e | 0.96gh | 0.00f | 0.02f | 0.03f | 0.08g | 0.06e | 0.02gf | 0.81f |
| Nielluccio | 1.50a | 3.75b | 0.77b | 3.81a | 0.41b | 28.08a | 11.82b | 0.03e | 0.14b | 0.24a | 0.44c | 0.27a | 0.43a | 9.87a |
| Tempranillo | 0.03f | 0.84d | 0.15de | 0.24d | 0.18e | 7.17d | 2.13f | 0.15c | 0.02f | 0.00i | 0.06gh | 0.02h | 0.02gf | 0.97f |
| Syrah | 0.35c | 0.58e | 0.17d | 0.65e | 0.34c | 2.60e | 2.60e | 0.06d | 0.03de | 0.04e | 0.13ef | 0.16c | 0.07e | 1.33e |
| Merlot | 0.03f | 0.24g | 0.10ef | 0.02g | 0.15e | 1.89f | 1.25g | 0.00g | 0.03ef | 0.02g | 0.20d | 0.05ef | 0.03f | 1.11ef |
| Caladoc | 0.03f | 0.48ef | 0.07f | 0.06fg | 0.03f | 2.09e | 0.94gh | 0.06d | 0.04d | 0.06d | 0.09gh | 0.03gh | 0.00h | 0.94f |
| LSD 5% | 0.031 | 0.1445 | 0.068 | 0.0598 | 0.0431 | 1.0442 | 0.4185 | 0.0203 | 0.0088 | 0.0058 | 0.0385 | 0.0131 | 0.0112 | 0.2985 |
Same character denotes no significance difference. However, the different characters (e.g. a, b, c) show significant differences
Table 3.
Volatile aldehydes in red wines (ppm)
| Varieties | Acetaldehyde | Benzaldehyde | Butanol, 3-methyl- | Furfural | Nonanal |
|---|---|---|---|---|---|
| Cinsaut | 0.92b | 0.08d | 0.06b | 2.77c | 0.04 |
| Grenache | 1.28a | 0.33a | 0.03ef | 13.10a | 0.41 |
| Cabernet Franc | 0.16f | 0.02g | 0.17a | 0.24ef | 0.01 |
| Petit Verdot | 0.76c | 0.18c | 0.04 d | 6.07b | 0.22 |
| Cabernet Sauvignon | 0.15f | 0.04f | 0.03cd | 0.30ef | 0.00 |
| Nielluccio | 0.20e | 0.29b | 0.07b | 6.04b | 0.23 |
| Tempranillo | 0.24d | 0.06e | 0.05c | 0.40e | 0.04 |
| Syrah | 0.22de | 0.06e | 0.03de | 1.86d | 0.03 |
| Merlot | 0.16f | 0.05e | 0.03de | 0.29ef | 0.02 |
| Caladoc | 0.14f | 0.02f | 0.02f | 0.12f | 0.00 |
| LSD 5% | 0.028 | 0.0108 | 0.0067 | 0.215 | 0.0152 |
Same character denotes no significance difference. However, the different characters (e.g. a, b, c) show significant differences
Table 4.
Volatile esters in red wines (ppm)
| Varieties | 1-Butanol, 3-methyl-, acetate [Isoamyl acetate] | 1-Butanol, 3-methyl-, propanoate [Isoamylpropanoate] | 2-Furancarboxylic acid, ethyl ester | 4-Decenoic acid, ethyl ester, (Z)- | Acetic acid, 2-phenylethyl ester [Phenethyl acetate] | Acetic acid, dimethoxy-, methyl ester | Benzeneacetic acid, ethyl ester [Ethyl phenacetate] | Butanedioic acid, diethyl ester [Diethyl succinate] | Butanedioic acid, hydroxy-, diethyl ester, (. ± .)- [Ethyl dl-malate] | Butanoic acid, 3-methyl-, ethyl ester [Ethyl isovalerate] | Decanoic acid, ethyl ester [Ethyl caprate] | Ethyl 9-hexadecenoate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cinsaut | 0.10c | 0.76b | 0.26c | 1.23c | 0.55c | 0.76c | 0.08c | 2.28d | 0.14c | 0.03e | 0.18c | 0.19a |
| Grenache | 0.70 | 0.36c | 0.43b | 7.01a | 0.72a | 1.35a | 0.30a | 9.64a | 0.44b | 0.15b | 0.36a | 0.09b |
| Cabernet Franc | 0.02d | 0.09e | 0.03f | 0.22f | 0.03e | 0.04f | 0.02f | 0.37g | 0.03e | 0.02f | 0.03f | 0.04e |
| Petit Verdot | 0.34bc | 0.15d | 0.44b | 2.70b | 0.26d | 0.82b | 0.13b | 5.61c | 0.24c | 0.10c | 0.25b | 0.04e |
| Cabernet Sauvignon | 0.16b | 0.26c | 0.05 | 0.35e | 0.07f | 0.10e | 0.04e | 0.85f | 0.07d | 0.02 | 0.04f | 0.06c |
| Nielluccio | 1.17a | 1.58a | 0.58a | 2.85b | 0.68b | 1.20a | 0.29a | 8.39b | 0.54a | 0.18a | 0.28b | 0.09b |
| Tempranillo | 0.01d | 0.23c | 0.06 | 1.07c | 0.03f | 0.08e | 0.04 | 1.37e | 0.07d | 0.03e | 0.06e | 0.06c |
| Syrah | 0.21bc | 0.81b | 0.15d | 0.38 | 0.09 | 0.22d | 0.07c | 1.60e | 0.12c | 0.03e | 0.10d | 0.10b |
| Merlot | 0.64b | 0.11d | 0.07e | 0.51d | 0.10e | 0.07e | 0.06d | 0.82f | 0.05d | 0.04d | 0.06e | 0.05d |
| Caladoc | 0.59b | 0.12d | 0.05e | 0.44d | 0.08e | 0.08e | 0.03 | 0.73f | 0.07d | 0.03e | 0.03f | 0.09b |
| LSD 5% | 0.02 | 0.0241 | 0.0158 | 0.0223 | 0.0281 | 0.0974 | 0.0049 | 0.0069 | 0.0064 | 0.0049 | 0.0408 | 0.0265 |
| Varieties | Ethyl hydrogen succinate | Hexadecanoic acid, ethyl ester [Ethyl palmitate] | Hexanoic acid, ethyl ester [Ethyl caproate] | Isoamyl lactate | Octanoic acid, 2-phenylethyl ester | Octanoic acid, ethyl ester [Ethyl caprylate] | Pentanoic acid, 4-oxo-, ethyl ester [Ethyl levulate] | Propanoic acid, 2-hydroxy-, ethyl ester [Ethyl lactate] | Propanoic acid, 2-methyl-, ethyl ester [Ethyl isobutyrate] | Tetradecanoic acid, ethyl ester [Ethyl myristate] | Tridecanoic acid, 3-hydroxy-, ethyl ester |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cinsaut | 0.89c | 1.03c | 0.58c | 5.01d | 1.30ab | 0.61d | 0.11d | 2.72b | 0.12c | 0.29c | 0.17b |
| Grenache | 0.90c | 1.54b | 1.06b | 1.65g | 1.64a | 2.12a | 0.93a | 8.58a | 0.57a | 0.58a | 0.28a |
| Cabernet Franc | 0.18 | 0.21fg | 0.13f | 1.43g | 0.08e | 0.07g | 0.02f | 0.54e | 0.04f | 0.05f | 0.01f |
| Petit Verdot | 0.96b | 0.57e | 0.33e | 17.21b | 0.61b | 0.84c | 0.36c | 2.80b | 0.49b | 0.33b | 0.12b |
| Cabernet Sauvignon | 0.47e | 0.26 | 0.22 | 2.14f | 0.13d | 0.10f | 0.03f | 0.48 | 0.03f | 0.07 | 0.03e |
| Nielluccio | 2.67a | 2.48a | 2.82a | 27.30a | 1.69a | 0.97b | 0.60b | 8.25a | 0.20c | 0.52a | 0.23a |
| Tempranillo | 0.25f | 0.31f | 0.47d | 6.38c | 0.08 | 0.32e | 0.02f | 0.10f | 0.09d | 0.11d | 0.08c |
| Syrah | 0.65d | 0.69d | 0.20f | 4.80e | 0.24c | 0.13f | 0.08e | 1.71c | 0.08e | 0.16d | 0.04d |
| Merlot | 0.40e | 0.58e | 0.00g | 2.87f | 0.27c | 0.12f | 0.03f | 0.69d | 0.06ef | 0.09e | 0.03e |
| Caladoc | 0.38e | 0.18g | 0.00g | 2.14f | 0.21c | 0.10f | 0.02f | 0.49e | 0.10cd | 0.06f | 0.02 |
| LSD 5% | 0.0245 | 0.2713 | 0.0299 | 0.0212 | 0.0084 | 0.0746 | 0.0049 | 0.0131 | 0.0088 | – | – |
Same character denotes no significance difference. However, the different characters (e.g. a, b, c) show significant differences
Table 5.
Volatile phenols and Volatile Terpenes in red wines (ppm)
| Varieties | Volatile phenols | Volatile Terpenes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2-Methoxy-4-vinylphenol | Phenol, 2-methoxy- | Phenol, 2-methyl- | Phenol, 4-ethyl- | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- [α-Terpinene] | 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- [gama-Terpene] | Cyclohexene, 1-methyl-4-(1-methylethylidene)- [Terpinolen] | D-Limonene | Naphthalene, 1,2,3,4-tetrahydro-1,1,6-trimethyl- [α-Ionene] | |
| Cinsaut | 0.20b | 0.14d | 0.03c | 0.14c | 0.76a | 5.02c | 0.57a | 0.64a | 1.26d |
| Grenache | 1.53a | 0.32a | 0.15a | 0.43a | 0.06c | 2.27e | 0.14b | 0.11b | 9.72a |
| Cabernet Franc | 0.07d | 0.04e | 0.00f | 0.02f | 0.03d | 1.10f | 0.00d | 0.02d | 0.33ef |
| Petit Verdot | 0.19b | 0.32a | 0.09b | 0.13d | 0.00e | 14.56b | 0.02cd | 0.02cd | 1.71c |
| Cabernet Sauvignon | 0.07d | 0.05e | 0.01e | 0.02f | 0.00e | 2.13e | 0.00d | 0.00e | 0.37ef |
| Nielluccio | 0.02e | 0.29b | 0.10b | 0.15b | 0.13b | 24.84a | 0.04c | 0.03c | 5.14b |
| Tempranillo | 0.12c | 0.04e | 0.01de | 0.03e | 0.00e | 3.31d | 0.01d | 0.00e | 1.17d |
| Syrah | 0.04de | 0.17c | 0.02d | 0.03e | 0.05c | 3.88d | 0.02cd | 0.02cd | 1.34d |
| Merlot | 0.06d | 0.13d | 0.01e | 0.03e | 0.01e | 2.58e | 0.01d | 0.00e | 0.57e |
| Caladoc | 0.02e | 0.04e | 0.01e | 0.01g | 0.00e | 2.42e | 0.00d | 0.00e | 0.25f |
| LSD 5% | 0.0316 | 0.0121 | 0.0076 | 0.0076 | 0.0161 | 0.65 | 0.0216 | 0.011 | 0.3115 |
Same character denotes no significance difference. However, the different characters (e.g. a, b, c) show significant differences
Based on their functional group and chemical structures, the aroma compositions of wines are classified as alcohols, volatile acids, aldehydes, esters, terpenes and volatile phenols. These are discussed in the following heads.
Alcohols
Alcohols represented the largest group in terms of concentration of aromatic compounds identified indifferent red wines. These compounds are produced mainly during fermentation of sugar and yeast metabolism. Variations in composition of volatile alcohols were obtained in this study. It was maximum in Nielluccio wine (61.56 mg/L) followed by Petit Verdot (49.41 mg/L) while the least was observed in Cabernet Franc wine (2.49 mg/L). The principal compound of alcohols is 2, 3-Butanediol and its highest concentration was estimated in the wine made from Nielluccio grape variety. The presence of these compounds at higher concentration in these wines may be due to anabolic pathway from glucose or catabolic pathway from their corresponding amino acids (valine, leucine, phenylalanine) during fermentation (Perestrelo et al. 2006). The varieties showed varied concentration of aromatic compounds. The highest concentration of 1-Hexanol (4.94 mg/L), 1-Hexanol, 2-ethyl (1.91 mg/L), 1-Pentanol, 3-methyl (1.44 mg/L), 2-Butanol (18.73 mg/L), 3-Hexen-1-ol, (Z)- (0.46 mg/L), dimethyl- [β-Citronellol] (0.30 mg/L), Benzyl Alcohol (1.23 mg/L), Benzyl alcohol, α-Isobutyl-2,4,6-trimethyl (0.26 mg/L) were recorded in wine made from Grenache variety. However, 1-Heptanol (1.50 mg/L), 1-Octanol (3.81 mg/L), 2,3-Butanediol (28.08 mg/L), 7-Octen-1-ol, 3,7-dimethyl-, (S)- [α-Citronellol] (0.24 mg/L), Benzyl alcohol, α-Isobutyl-2,4,6-trimethyl (0.27a), n-Tridecan-1-ol (0.43 mg/L), Phenylethyl Alcohol (9.87 mg/L) were present at higher level in Nielluccio wine. The wine made from Cabernet Franc recorded least concentration of 1-Heptanol (0.02 mg/L), 1-Hexanol (0.15 mg/L), 1-Hexanol, 2-ethyl (0.05 mg/L), 1-Pentanol, 3-methyl (0.02 mg/L), 2-Butanol (0.60 mg/L), 3-Hexen-1-ol, (Z) (ND), 3-Octanol (0.21 mg/L), 6-Octen-1-ol, 3,7-dimethyl- [β-Citronellol] (ND), 7-Octen-1-ol, 3,7-dimethyl-, (S)- [α-Citronellol] (0.01 mg/L), Benzyl alcohol (0.03 mg/L), α-Isobutyl-2,4,6-trimethyl (0.04 mg/L), n-Tridecan-1-ol (0.01 mg/L), and Phenylethyl alcohol (0.42 mg/L). These variations can be explained by the conditions of fermentation, which are associated to higher temperatures, since low temperatures favours the formation of fruity esters in red wines (Clarke and Bakke 2004). Elevated fermentation temperatures, the encouragement of an aerobic environment, excessive turbidity within the must, chaptalisation, and fermenting within pressure tanks all encourage the production of higher alcohols. An increased pH will also result in larger amounts of fusel alcohols (Zoecklein et al. 2015).
Acids
Different types of acids considered to be class of compounds to contribute to the aroma of wine. The production of acids depends on the composition of must and fermentation conditions. The higher concentration of volatile acids was recorded in Grenache wine (32.65 mg/L) followed by wine obtained from Nielluccio variety (24.38 mg/L). The wine prepared from Cabernet Franc showed lower concentration of volatile acids (1.14 mg/L). Approximately 40% of total aroma composition of wine is contributed by volatile acids. It is mainly comprised by acetic acid (35.99 mg/L), Octanoic acid (14.46 mg/L) and Hexanoic acid (5.99 mg/L). Higher concentrations of some acids are described as the aroma of rancid, cheesy and vinegar like aromas, but they are usually present at low concentration in healthy wine (Louw et al. 2010). Acids at concentration of 4 to 10 mg/L impart mild and pleasant aroma to wine; however, beyond the level of 20 mg/L, wines affected negatively (Shinohara 1985). These acids might have a positive impact on aroma of the wines examined in the current study since their levels were recorded below 20 mg/L. It has been reported that acids are mainly produced during fermentation (Schreier and Jennings 1979). In the present study, the wines made from Cinsaut, Cabernet Franc, Cabernet Sauvignon, Tempranillo, Syrah, Merlot and Caladoc contained volatile acids less than 10.00 mg/L. Wines made from Grenache and Nielluccio grape varieties registered with 32.65 and 24.38 mg/L volatile acids. Results indicated that wines made from major varieties except Grenache and Nielluccio, produced with lower concentrations of volatile acids which related to mild and pleasant aromas.
Aldehydes
Significant differences were recorded for combinations of different aldehydes in studied wine (Table 3). The wine made from Grenache contained higher aldehydes (15.15 mg/L) which was comprised by Furfural (13.10 mg/L), Acetaldehyde (1.28 mg/L), Nonanal (0.41 mg/L) and Benzaldehyde (0.33 mg/L). The wine made from Petit Verdot has total aldehyde composition of 7.27 mg/L which was contributed by Furfural (6.07 mg/L), Acetaldehyde (0.76 mg/L), Nonanal (0.22 mg/L) and Benzaldehyde (0.18 mg/L). However, minimum aldehyde content (0.30 mg/L) was recorded in Caladoc wine in the form of Acetaldehyde (0.14 mg/L), Furfural (0.12 mg/L) and Benzaldehyde (0.02 mg/L). The concentration of Furfural was dominant across the varietal wines except Caladoc. It was ranged from 13.10 mg/L in Grenache to 0.12 mg/L in Caladoc. The variable results obtained in this study might be due to the factors like, fermentation temperature and amount of SO2 present in must are responsible for formation of carbonyl compounds during vinification (Nykänen 1986).
Furfural compound has a sweet, caramel or butterscotch aroma but the concentration of furfural in studied wines was less than threshold limit i.e. 20 mg/L (Chatonnet et al. 1993). In the present study, the higher concentration of benzaldehyde was obtained for Grenache wines directly related to the concentration of benzyl alcohol which corroborated by higher concentration of this compounds. At low levels, it may be responsible for a pleasant fruity aroma but at high concentration it possesses a pungent irritating odor (Miyake and Shibamoto 1993). While, all analyzed wines have low concentration of benzaldehyde than threshold level.
Esters
Ethyl ester group of aroma compounds is found most important in deciding wine aromas. In general, fruity and floral aromas in wines are dependent on ester compounds. Reaction of acetyl-CoA with higher alcohols degrade amino acids or carbohydrates and form acetate and esters (Perestrelo et al. 2006). During grape fermentation process fatty acid ethyl esters are produced enzymatically. In the present investigation, significant differences were noted in composition of esters (Table 4). The concentration was varied in wines Nielluccio (65.56 mg/L) to Cabernet Franc (3.70 mg/L). Among the different esters, Isoamyl lactate was found at highest concentration of (70.93 mg/L) followed by Butanedioic acid, diethyl ester [Diethyl succinate] (31.66 mg/L) while, least concentration of Butanoic acid, 3-methyl-, ethyl ester [Ethyl isovalerate] (0.63 mg/L) was found in our study. Although their concentration differed in red wines varieties, Isoamyl lactate, dimethyl succinate, ethyl lactate, 4- Decennoic acid and ethyl ester were the major esters found in the aroma compounds (Zhang et al. 2010). Hexanoic acid, ethyl ester [Ethyl caproate] were absent in Merlot and Caladoc wines. These esters contributed to odours of forest and berry fruits as previously reported (Ferreira et al. 2000). It was mentioned that the esters contribute stronger aromas and also found that high temperature during fermentation process favours the formation of more ethyl esters (Ferreira et al. 2000). The results for Ethyl esters found in our study might be due to formation of corresponding amino acids by Strecker degradation or from some amino acid derivatives of branched chain fatty acids (Ferreira et al. 2000) which are important contributors to the fruit and sweet aroma of wine (Aznar et al. 2001). Similarly, Meilgaard (1975) and Li et al. (2008) reported these compounds as important contributors to fruity character of red wines (Li et al. 2008; Meilgaard 1975). However, aromatic esters investigated including Acetic acid, 2-Phenylethyl ester [Phenethyl acetate], Benzeneacetic acid, ethyl ester [Ethyl phenacetate] have been considered as important flavour contributors to wine aroma and they give the aroma of floral, cherry, stone-fruit and dry plum (Fang and Qian 2006). Some other esters were also quantified, although they had low concentration in red wine varieties with the exception of diethyl succinate whose concentration was highest. Higher contents of diethyl succinate in red wines could results from skin contact treatment (Selli et al. 2006) and could be due to malolactic fermentation, which more frequently take place in red wines (Gil et al. 2006).
Phenols
Volatile phenols bearing a vinyl radical and an ethyl radical are abundant in red wines (Vilanova et al. 2007). The compositions of volatile phenol varied significantly in different red wines are presented in Table 5. Highest volatile phenols were observed in Grenache (2.43 mg/L) wine followed by Petit Verdot (0.73 mg/L) while least concentration was noted in Caladoc wine (0.08 mg/L). Among the volatile phenols, 2-Methoxy-4-vinylphenol was available with highest concentration i.e. 2.32 mg/L followed by Phenol, 2-methoxy (1.54 mg/L); while Phenol, 2-methyl (0.43 mg/L) was found with lowest concentration. Song et al. (2013) stated that the formation of 2-Methoxy-4-vinylphenol was related to non-oxidative decarboxylation of some phenolic acids (p-coumaric and ferulic acid) and is catalysed by cinnamate decarboxylases produced by S. cerevisiae during alcohol fermentation. Lower concentration of this compound in the present investigation was due to the inhibitory action of some tannic substances on action of cinnamate decarboxylases (Chatonnet et al. 1993; Clarke and Bakke 2004). This compound has a pleasant spicy aroma at low and moderate concentration as recorded in this study.
Terpenes
Terpenes are secondary plant constituents; its biosynthesis begins with acetyl coenzyme A (Acetyl CoA). Terpenes are not changed by the yeast metabolism during fermentation (Mateo and Jiménez 2000). Grape derived terpenoids are responsible for fruity and flowery notes in wines and contribute significantly to varietal aroma character (Ebeler 2001). In the present study, five terpenes (3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- [α-Terpinene], 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- [gamma-Terpene], Cyclohexene, 1-methyl-4-(1-methylethylidene)- [Terpinolen], D-Limonene, Naphthalene, 1,2,3,4-tetrahydro-1,1,6-trimethyl- [α-Ionene],) were detected in the wines made from different varieties. However, the concentrations of these terpenes were lower in comparison with other aromatic compounds. The quantity of gamma-Terpenes (1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl))- (62.11 mg/L)was higher and followed by α-Ionene (Naphthalene, 1,2,3,4-tetrahydro-1,1,6-trimethyl-) (21.86 mg/L) while, Terpinolen (Cyclohexene, 1-methyl-4-(1-methylethylidene)) was recorded with least concentration (0.81 mg/L). Among the terpenes, the highest concentration of D-Limonene was found in Cinsaut (0.64 mg/L) followed by Grenache (0.11 mg/L), while it was minimum (0.02 mg/L) in Cabernet Franc, Petit Verdot, Nielluccio and Syrah (Table 5). It was absent in Cabernet Sauvignon, Tempranillo, Merlot and Caladoc varieties. Results obtained in this study confirm the findings of Zhang et al. (2007) who reported terpenyl compounds could serve as potential indicators to distinguish wine derived from Chardonnay from Cabernet Sauvignon and Cabernet Gernischt. In our study, Nielluccio wine showed highest concentration of 1, 4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)-[gamma-Terpene] (24.84 mg/L) followed by Petit Verdot (14.56 mg/L). Cabernet Sauvignon wine showed only gamma-terpinene (2.13 mg/L)] and α-Ionene (0.37 mg/L). The differences observed in present study could be attributed to viticulture and oenological practices. Alpha terpinol showed highest value (0.76 mg/L) for Cinsuat wine; however, it was absent in Petit Verdot, Cabernet Sauvignon and Tempranillo wines. Low concentrations of these compounds were found in Merlot (0.01 mg/L), Cabernet Franc (0.03 mg/L), Syrah (0.05 mg/L), Grenache (0.06 mg/L) and Nielluccio (0.13 mg/L) wines. These compounds have often been considered important contributors to the aroma of wine. Higher levels of these compounds are responsible for rose aroma (Ribéreau Gayon et al. 2006). Terpenes contribute for overall flavour of grapes and wines and also act as markers for each variety also (Oliveira et al. 2006).
Principal component analysis
The principal component analysis (PCA) was performed on wines volatile composition and presented in Fig. S1 (supplementary). The PCA provides a visual representation of the relationship between the wines based on the volatile composition. Two PCs are selected by their value, accounted for 94.66% of the variance explaining 86.22% for PC 1 and 8.44% for PC 2. The wines are grouped in accordance to their aroma composition by their PC1 and PC2 scores. Grenache wines being differentiated by their higher score in PC1 have positive score for PC1 and PC2 followed by Nielluccio and Cinsaut wine have positive score for PC1 and PC2 while, Caladoc, Merlot, Cabernet Sauvignon and Petit Verdot have negative score for PC2 and their values were near to 0 in PC2. Grenache wines could be clearly differentiated according to their relationships between scores and aroma composition was followed by Nielluccio and Cinsuat wines in present study.
According to the Fig. S2 (supplementary), PC1 and PC2 contributed 94.66% of the total variance and differentiated by means of their corresponding vector over each axis. This pattern shows their contribution to the respective component. In this way, six aromatic compounds numbered 18 (Butanediol), 48 (Isoamyllactate), 61 (gamma-Terpene), 19 (Butanol), 1 (Acetic acid) and 31 (Furfural) have positive coefficient and most contributing compounds to PC1 and PC2. Butanediol, Isoamyl lactate, gamma-Terpenes were most important compounds contributing to PC1 with positive coefficient. Only two No. 39 (Ethyl phenacetate) and No. 44 (Ethy-l-9-hexadecenoate) compounds showed negative coefficient for PC1. These compounds contributing to PC1 have agreeable aroma descriptors with flora and fruity nuances and contribute to differentiate the Grenache wines from other varieties which have similar scores for PC1. The compounds number as (1)Acetic acid, (31) furfural, (34) Isoamyl propanoate, (40) Diethyl succinate, (36) Decenoic acid ethyl esters, (27) phenyl ethyl alcohol, (52) ethyl lactate, (64) alpha Ionen, (49) Octanoic acid phenyl ethyl esters with fruity, green and honey as more pleasant aroma descriptors and most influencing factors for PC2.
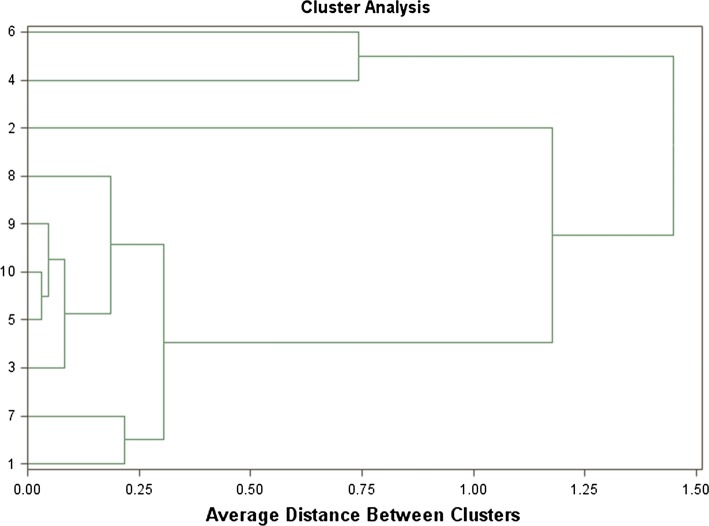
Cluster analysis
Cluster analysis was performed to elucidate aroma characteristics of different red wine varieties (Fig. 1). The dendrogram present two different groups among the varieties analysed for the aromatic compounds. First group comprising the most aromatic varieties such as Nielluccio and Petit Verdot and second is fairly homogenized group which include Cinsaut, Tempranillo and Syrah varieties. However, another more heterogeneous group in which a subgroup was constituted by varieties such as Cabernet Franc, Cabernet Sauvignon, Caladoc and Merlot. Grenache wine was well differentiated from the sub group formed by the other red varieties. This group appears to be completely separate from the second group of the wines analysed. The results of cluster analysis suggested that the wines in the same group had similar aroma characterization and also confirm the results with principal component analysis (Fig. 1).
Fig. 1.
Dendrogram obtained from the cluster analysis of the aromatic compounds of red wine varieties namely Cinsaut (1) Grenache (2), Cabernet Franc (3), Petit Verdot (4), Cabernet Sauvignon (5), Nielluccio (6), Tempranillo (7), Syrah (8), Merlot (9) and Caladoc (10)
Conclusion
This study provides better knowledge of the volatile composition of wines (Cinsaut, Grenache, Cabernet Franc, Petit Verdot, Cabernet Sauvignon, Nielluccio, Tempranillo, Syrah, Merlot and Caladoc) grown in India. The results recorded for the wine varieties can be characterized and grouped by their volatile compositions. Nielluccio wines showed highest concentrations of volatile compounds followed by Grenache and Petit Verdot while, volatile compounds was least in Caladoc wines. PCA showed similarities for the volatile composition of Cabernet Franc, Cabernet Sauvignon, Caladoc and Merlot. However, fairly homogenized group was obtained for Cinsaut, Tempranillo and Syrah. The wines made from Grenache, Nielluccio and Petit Verdot showed higher values for volatile composition and found more aromatic wines prepared from grapes grown in Indian conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The authors are thankful to the Director General of Agriculture, Food Processing and Territorial Policies of the Ministry of Agriculture and Fisheries, Government of France for providing the planting material to carry out research work on evaluation of wine varieties under Pune conditions. The Director, ICAR-NRC Grapes, Pune also deserves for sincere thanks for providing the guidance and required facilities for carrying out the research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andujar-Ortiz I, Moreno-Arribas MV, Martín-Álvarez PJ, Pozo-Bayón MA. Analytical performance of three commonly used extraction methods for the gas chromatography–mass spectrometry analysis of wine volatile compounds. J Chromatogr A. 2009;1216(43):7351–7357. doi: 10.1016/j.chroma.2009.08.055. [DOI] [PubMed] [Google Scholar]
- Armanino C, Casolino MC, Casale M, Forina M. Modelling aroma of three Italian red wines by headspace–mass spectrometry and potential functions. Anal Chim Acta. 2008;614(2):134–142. doi: 10.1016/j.aca.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Aznar M, López R, Cacho JF, Ferreira V. Identification and quantification of impact odorants of aged red wines from rioja. GC—olfactometry, quantitative GC–MS, and odor evaluation of HPLC fractions. J Agric Food Chem. 2001;49(6):2924–2929. doi: 10.1021/jf001372u. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Kambale N, Uttare S, Kandaswamy C. Volatile profiling in wine using gas chromatography mass spectrometry with thermal desorption. Hyderabad: Agilent; 2015. [Google Scholar]
- Berna AZ, Trowell S, Clifford D, Cynkar W, Cozzolino D. Geographical origin of Sauvignon blanc wines predicted by mass spectrometry and metal oxide based electronic nose. Anal Chim Acta. 2009;648(2):146–152. doi: 10.1016/j.aca.2009.06.056. [DOI] [PubMed] [Google Scholar]
- Cesnik HB, Bavcar D, Lisjak K. Volatile profile of wine Teran PTP. Acta Agric Slov. 2015;105:5–14. [Google Scholar]
- Chatonnet P, Duborudieu D, Boidron JN, Lavigne L. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J Sci Food Agric. 1993;62(2):191–202. [Google Scholar]
- Clarke RJ, Bakke J. Wine: flavour chemistry. Food Chem. 2004;95(4):683. [Google Scholar]
- Ebeler SE. Analytical chemistry: unlocking the secrets of wine flavor. Food Rev Int. 2001;17:45–64. [Google Scholar]
- Falcão LD, de Revel G, Rosier JP, Bordignon-Luiz MT. Aroma impact components of Brazilian Cabernet Sauvignon wines using detection frequency analysis (GC–olfactometry) Food Chem. 2008;107(1):497–505. [Google Scholar]
- Fang Y, Qian MC. Quantification of selected aroma-active compounds in Pinot Noir wines from different grape maturities. J Agric Food Chem. 2006;54(22):8567–8573. doi: 10.1021/jf061396m. [DOI] [PubMed] [Google Scholar]
- Ferreira V, López R, Cacho JF. Quantitative determination of the odorants of young red wines from different grape varieties. J Sci Food Agric. 2000;80(11):1659–1667. [Google Scholar]
- Gil M, Cabellos JM, Arroyo T, Prodanov M. Characterization of the volatile fraction of young wines from the denomination of origin “Vinos de Madrid” (Spain) Anal Chim Acta. 2006;563(1):145–153. [Google Scholar]
- Jiang B, Zhang Z. Volatile compounds of young wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay varieties grown in the Loess Plateau Region of China. Molecules. 2010;15(12):9184. doi: 10.3390/molecules15129184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundouras S, Marinos V, Gkoulioti A, Kotseridis Y, van Leeuwen C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated Cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J Agric Food Chem. 2006;54(14):5077–5086. doi: 10.1021/jf0605446. [DOI] [PubMed] [Google Scholar]
- Li H, Tao YS, Wang H, Zhang L. Impact odorants of Chardonnay dry white wine from Changli county (China) Eur Food Res Technol. 2008;227:287–292. [Google Scholar]
- Louw L, Tredoux A, Van Rensburg P, Kidd MNT, Nieuwoudt H. Fermentation-derived aroma compounds in varietal young wines from South Africa. S Afr J Enol Vitic. 2010;31:213–225. [Google Scholar]
- Mateo JJ, Jiménez M. Monoterpenes in grape juice and wines. J Chromatogr A. 2000;881(1):557–567. doi: 10.1016/s0021-9673(99)01342-4. [DOI] [PubMed] [Google Scholar]
- Meilgaard MC. Flavour chemistry of beer. Part II: flavour and threshold of 239 aroma volatiles. MBAA Technol Q Master Brew Assoc. 1975;12:151–168. [Google Scholar]
- Miyake T, Shibamoto T. Quantitative analysis of acetaldehyde in foods and beverages. J Agric Food Chem. 1993;41(11):1968–1970. [Google Scholar]
- Nykänen L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am J Enol Vitic. 1986;37(1):84–96. [Google Scholar]
- Oliveira JM, Faria M, Sá F, Barros F, Araújo IM. C6-alcohols as varietal markers for assessment of wine origin. Anal Chim Acta. 2006;563:300–309. [Google Scholar]
- Perestrelo R, Fernandes A, Albuquerque FF, Marques JC, Câmara JS. Analytical characterization of the aroma of Tinta Negra Mole red wine: identification of the main odorants compounds. Anal Chim Acta. 2006;563(1):154–164. [Google Scholar]
- Piñeiro Z, Palma M, Barroso CG. Determination of terpenoids in wines by solid phase extraction and gas chromatography. Anal Chim Acta. 2004;513(1):209–214. [Google Scholar]
- Ribéreau Gayon P, Dubourdieu D, Doneche B, Lonvaud A. Handbook of enology. Volume 1. The microbiology of wine and vinifications. Chichester: Wiley; 2006. [Google Scholar]
- Schreier P, Jennings WG. Flavor composition of wines: a review. Crit Rev Food Sci Nutr. 1979;12(1):59–111. doi: 10.1080/10408397909527273. [DOI] [PubMed] [Google Scholar]
- Selli S, Canbas A, Cabaroglu T, Erten H, Günata Z. Aroma components of cv. Muscat of Bornova wines and influence of skin contact treatment. Food Chem. 2006;94(3):319–326. [Google Scholar]
- Shinohara T. Gas chromatographic analysis of volatile fatty acids in wines. Agric Biol Chem. 1985;49(7):2211–2212. [Google Scholar]
- Song JQ, Li H, Liang YY, Tao YS, Mi Q, Qian MC. Characterization of volatile components of red and sparkling wines from a new wine grape cultivar ‘Meili’ (Vitis vinifera L.) Vitis. 2013;52(1):41–48. [Google Scholar]
- Tao Y, Li H, Wang H, Zhang L. Volatile compounds of young Cabernet Sauvignon red wine from Changli County (China) J Food Compost Anal. 2008;21(8):689–694. [Google Scholar]
- Torrens J, Riu-Aumatell M, López-Tamames E, Buxaderas S. Volatile compounds of red and white wines by headspace-solid-phase microextraction using different fibers. J Chromatogr Sci. 2004;42(6):310–316. doi: 10.1093/chromsci/42.6.310. [DOI] [PubMed] [Google Scholar]
- Vilanova M, Zamuz S, Vilariño F, Sieiro C. Effect of terroir on the volatiles of Vitis vinifera cv. Albariño. J Sci Food Agric. 2007;87(7):1252–1256. [Google Scholar]
- Vilanova M, Genisheva Z, Grana M, Oliveira JM. Determination of odorants in varietal wines from International grape cultivars (Vitis vinifera) grown in NW Spain. S Afr Enol Vitic. 2013;34(2):212–222. [Google Scholar]
- Welke JE, Zanus M, Lazzarotto M, Alcaraz Zini C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res Int. 2014;59:85–99. [Google Scholar]
- Zhang M, Xu Q, Duan C, Qu W, Wu Y. Comparative study of aromatic compounds in young red wines from Cabernet Sauvignon, Cabernet Franc, and Cabernet Gernischet varieties in China. J Food Sci. 2007;72(5):C248–C252. doi: 10.1111/j.1750-3841.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li L, Gao N, Wang D, Gao Q, Jiang S. Feature extraction and selection from volatile compounds for analytical classification of Chinese red wines from different varieties. Anal Chim Acta. 2010;662(2):137–142. doi: 10.1016/j.aca.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Zoecklein B, Fugelsang K, Gump B, Nury F. Wine analysis and production. New York City: Kluwer Academic/Plenum Publishers; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.