Abstract
It is an interesting topic to elucidate the interaction among plant proteins and bioactive lipid components. However, there is a shortage of understanding regarding the nature of the interaction between rice protein and conjugated linoleic acid (CLA). In this study, the intrinsic fluorescence intensity of rice glutelin (RG) was quenched upon increasing concentrations of CLA, indicating the occurrence of an interaction between them. Thermodynamic analysis showed that the RG-CLA binding process occurred spontaneously and hydrogen bonds were the primary driving force. Moreover, only one binding site was calculated between RG and CLA by the intrinsic fluorescence data. The surface hydrophobicity of RG was reduced with increasing CLA. Circular dichroism and synchronous fluorescence spectroscopy showed conformational and microenvironmental changes around the chromophores of RG. The α-helical content increased and β-sheet content declined after the binding reaction. The computational docking program displayed the target site in which CLA and amino acid residues of RG might be linked together. This study provides valuable insights into the nature of the interactions between plant proteins and fatty acids.
Keywords: Rice glutelin, Conjugated linoleic acid, Spectroscopy, Molecular docking
Introduction
Bioactive lipid components with beneficial health properties are found widely in dairy products (Benjamin and Spener 2009). Among the bioactive lipid components, conjugated linoleic acid (CLA) has attracted considerable attention for its health benefits, including its antidiabetic, antiatherogenic and anticarcinogenic properties (Belury 2002; Kim et al. 2016). However, the utilization of CLA in the food industry is restricted because it is unstable under natural conditions. The oxidation of CLA leads to the loss of biological activity and the occurrence of undesired compounds (Vélez et al. 2017). Incorporation of CLA into food protein-based materials may provide a simple way to keep CLA from degrading (Matalanis et al. 2011).
Proteins have previously been used as natural carriers of bioactive components due to their amphiphilic nature, ability to stabilize oil-in-water emulsions and film-forming abilities (Karaca et al. 2015; McClements et al. 2009). For example, β-lactoglobulin and its aggregates are the most studied systems for developing nanovehicles of docosahexaenoic acid (DHA) (Zimet and Livney 2009) and linoleic acid (Perez et al. 2014). Ovalbumin has also been demonstrated to have the ability to bind fatty acids such as linoleic acid and form complexes (Sponton et al. 2016). Complexes obtained by the interaction between protein and fatty acids can be applied in the food and pharmaceutical industries for the following reasons: (i) protection of fatty acids (polyunsaturated) from contact with pro-oxidant agents, such as oxygen, metals, light, and enzymes (Zimet and Livney 2009); (ii) improvement of bioavailability and water solubility of fatty acids allowing for their incorporation in more food systems (Ilyasoglu and El 2014); and (iii) some protein-fatty acid (oleic, linoleic) complexes have even presented antitumour activity (Fontana et al. 2013). Therefore, it is important to study the interactions between proteins and fatty acids.
Rice protein is a common plant protein that can interact with bioactive components, such as gallic acid, tea catechins, and pigments (Dai et al. 2017; Wang et al. 2016). Rice proteins are composed of albumin, globulin, glutelin, and prolamin based on solubility properties (Amagliani et al. 2017). Rice glutelin (RG) is the major storage protein in rice, which comprises two polypeptides with molecular weights of 30–40 kDa (α or acidic) and 19–23 kDa (β or basic). RG has been reported to have a high nutritive value, low cholesterol levels, and be hypoallergenic (Wang et al. 2016). For this reason, there is considerable interest in using RG as a functional ingredient in the food industry. In addition, it has been reported that RG can interact with bioactive compounds such as the procyanidin dimer (Dai et al. 2019) and gallic acid (Dai et al. 2017). Considering that CLA can also interact with some proteins (e.g., milk, egg proteins) (Le Maux et al. 2013; Sponton et al. 2016), and CLA can be consumed together with RG as dietary supplements for fat reduction in the fitness industry, it is important to investigate the interaction between RG and CLA, which has not yet been fully explored. Information derived from the results of the RG-CLA interaction could provide theoretical guidance to improve the performance of some existing nutritional products in the food industry.
The purpose of the present work was to study the molecular basis for the interaction between CLA and RG, as well as the effect of CLA on the conformation of RG.
Materials and methods
Materials
Cis-9, trans-11 conjugated linoleic acid (purity > 98%) and 1-anilinonaphthalene-8-sulfonic acid (ANS) were obtained from Sigma-Aldrich (Shanghai, China). Indica rice (Oryza sativa L.), “Gan Wan Xian 923” (No. 2000003) was purchased from the China Oil & Foodstuffs Corporation (Nanchang, Jiangxi, China). The Bradford protein assay kit was purchased from Beyotime Biotechnology Company (Shanghai, China). Ethanol (HPLC grade) was purchased from Aladdin (Shanghai, China). All other chemicals and reagents used were of analytical grade. Deionized water was purified using a Milli-Q water purification system (Millipore, MA, USA) and used throughout the experiments.
Extraction of RG
RG was extracted according to the method reported by Dai et al. (2017). Rice samples were ground by high speed pulverizer (DFY-500, Wenling Linda Machinery Co., Zhejiang, China) and passed through an 80-mesh sieve to obtain the rice flour. Protein fractions were isolated from rice flour based on their solubility in specific solutions according to the Osborne procedure (Sodek and Wilson 1971). Albumin, globulin, prolamin, and glutelin fractions were sequentially extracted with deionized water, 50 mg mL−1 NaCl, 70% ethanol, and 0.1 mol L−1 NaOH, respectively. The glutelin fraction was isoelectrically precipitated at pH 4.8, recovered by centrifugation and dialyzed against distilled water to a neutral pH, followed by lyophilization. The protein content of RG was determined to be 94.2% with conversion factors of 5.95 using the Dumas combustion method (AOAC 992.23, 1990).
Preparation of the RG-CLA complex
Rice glutelin solution (0.65 mg mL−1) was prepared by dispersing the RG powder in phosphate-buffered saline (PBS, 0.01 mol L−1, pH 7.0). The concentration was measured with a protein assay kit (Xu et al. 2017). CLA solution (0.5 mmol L−1) was dissolved in ethanol at room temperature. The RG and CLA solutions were sterile filtered through 0.45 μm membrane filters (WondaDisc, Shimadzu-gl, Shanghai, China). The mixed RG-CLA system was prepared by adding the CLA solution successively into the RG solution with constant stirring.
UV absorption measurements
To consider the inner filter effect of CLA, the UV spectrum of CLA solution was monitored using a TU-1901 spectrometer (Persee, Beijing, China) according to Jia et al. (2017). In brief, 100 μL of 0.5 mmol L−1 CLA was dropped into 3 mL of stirring PBS solution, for a final concentration of CLA of 16.13 μmol L−1. The absorption spectrum of CLA was recorded from 200 to 400 nm. The PBS solution was used for background subtraction.
Fluorescence spectroscopy
Synchronous fluorescence spectroscopy (SFS) and the fluorescence emission spectra of RG and CLA complexes at 298, 304, and 310 K were detected by an F-7000 spectrofluorimeter (Hitachi, Tokyo, Japan) equipped with quartz cells (1.0 cm) and a thermostat bath according to the method described by Zeng et al. (2016). Briefly, the RG solution (3.0 mL, 0.65 mg mL−1) was titrated by successive additions of CLA solution (10 μL of a 0.5 mmol L−1 solution). The CLA maximum titration volume was 100 μL, regardless of the change in protein concentration (changed 3.23%). The emission spectra were excited at 280 nm and monitored from 300 to 500 nm (2.5 nm excitation and emission slit width). The spectral behaviours of the tyrosine and tryptophan residues of RG were observed (Δλ = 15 nm and Δλ = 60 nm, respectively). The appropriate blank corresponding to CLA was deducted to correct the background fluorescence intensity. The inner filter effect could be calculated with the following equation (Xu et al. 2015).
| 1 |
where Fobs and Fcorr are the observed and corrected fluorescence intensities, respectively; Aex and Aem represent the measured UV absorption values at the excitation and emission wavelength of RG with the addition of CLA, respectively; and dex and dem are the cuvette path lengths in the excitation and emission directions, respectively.
Surface hydrophobicity measurements
ANS-based measurements of surface hydrophobicity were performed on an F-7000 fluorescence spectrofluorimeter (Hitachi, Kyoto, Japan) using ANS as a probe. The excitation wavelength was set as 390 nm with slit widths of 5.0 nm and 2.5 nm for excitation and emission, respectively (Cao et al. 2013). ANS (16 μL, 8.0 mmol L−1) was injected into the RG solution (3.0 mL, 0.65 mg mL−1). Then, the RG solution containing ANS was titrated by successive additions of CLA solution (10 μL, 0.5 mmol L−1). The surface hydrophobicity was expressed as the relative ANS fluorescence intensity (Xu et al. 2015).
Circular dichroism (CD) spectra
The CD spectra of RG in the presence of CLA (4.95 and 9.80 μmol L−1) were monitored in the far-UV range (250–190 nm) under constant nitrogen flushing at room temperature (25 ± 1 °C) using a Bio-Logic MOS-450 CD spectrometer (SAS, Claix, France) (Xu et al. 2017). The CD spectra for all samples were corrected by subtracting the solvent background. The secondary structure contents of RG were estimated by the CD spectra using the online CONTIN program in DICHROWEB (http://dichroweb.cryst.bbk.ac.uk/html/home.shtml).
Computational docking simulations
Molecular docking was performed according to the LibDock algorithm via Discovery Studio 3.0 (Accelrys Inc., USA) as previously reported (Dai et al. 2017) with some modifications. The available crystal structure in the protein database (http://www.rcsb.org/pdb) was used as a homologous model of RG based on BLAST algorithms (Lindin et al. 2013). The sequence alignment between RG (GI number 20221) and pumpkin seed globulin (PDB ID: 2EVX) revealed the highest sequence identity of 46% (Dai et al. 2017; Xu et al. 2015). Since the identity of the template and target protein sequences was higher than 40%, the structure of the pumpkin seed globulin could be used to align the target sequences as a homologous model in the simulation (Taylor 1996). The CLA molecule was sketched in ChemBioOffice 2010 (PerkinElmer Inc., USA) with MMFF94 force field minimization. The homologous structure of the RG file was prepared by removing water. All hydrogen atoms were inserted by the CHARMm force field by “Prepare Protein” at pH 7.0. The RG structure was subsequently energy minimized by the Smart Minimizer algorithm. The CLA was treated with the “Prepare Ligands” module at pH 7.0. Finally, the optimal receptor-ligand interaction module was implemented with the CHARMm force field. RG cavities were applied to define the binding sites. Interaction energies of the identified RG and CLA interactions were calculated, and the simulation diagram was observed. The visual interaction between CLA and the rice protein was observed with the software Discovery Studio 2017 R2 (Accelrys Inc., USA).
Statistical analysis
All statistical analyses were conducted with SPSS version 25.0 for Windows (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) and Tukey’s test were used with a significance level of 5%.
Results and discussion
Fluorescence emission spectrum (FES)
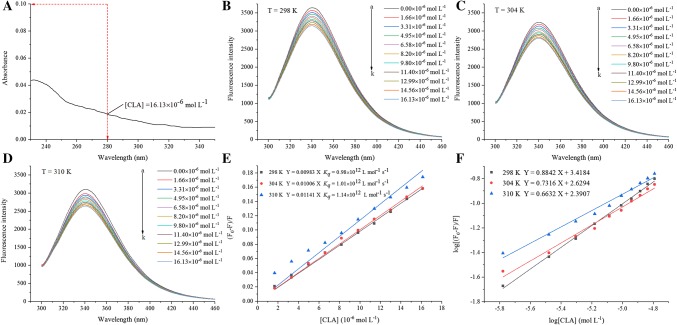
The structural changes and intermolecular interactions in the RG-CLA complex were detected by FES (Essemine et al. 2011). Fluorescence excitation of the RG-CLA complex was monitored at 280 nm, which is routinely used to estimate the changes in protein tertiary structure because the local environment of tryptophan and tyrosine groups are quite sensitive at this wavelength (Xu et al. 2015). The filter effect must be considered before fluorescent analysis since this would reduce the intensity of the excitation radiation when the total absorbance of the solution is higher than 0.1 aufs (Essemine et al. 2011). As shown in Fig. 1a, the absorbance at the excitation wavelength of 280 nm and emission wavelength of 340 nm for CLA its maximum concentration (16.13 μmol L−1) were 0.019 and 0.01 aufs, respectively, which were much lower than 0.1 aufs. Thus, the filter effect could be ignored (Van de Weert 2010). Figure 1b–d show the fluorescence spectra of RG with or without CLA at different temperatures. The fluorescence intensity of all the RG-CLA complexes decreased with increasing concentrations of CLA. RG exhibited a strong fluorescence emission band at approximately 340 nm. Moreover, there was no obvious shift in the maximum wavelength band in all samples. It can be concluded that CLA could quench the intrinsic fluorescence of RG by changing the microenvironment around the tyrosine and tryptophan residues (Boelens et al. 1985). The changes in the microenvironment may also change the surface hydrophobicity and conformation of RG, thus further influencing the binding and delivery ability of RG.
Fig. 1.
a Effect of the inner-filter effect on the fluorescence of CLA in a 1 × 1 cm cuvette; Effects of CLA on the fluorescence spectrum of RG at b 298 K, c 304 K, and d 310 K; e Stern–Volmer plots for the quenching of RG by CLA and the Kq of the RG-CLA complex in 298, 304, and 310 K; f Plots for the static quenching of RG by CLA (298, 304, and 310 K). CRG = 0.65 mg mL−1; CCLA = 0–16.13 μmol L−1
The quenching mechanism and binding constant
In addition to the inner filtration effect mentioned above, static and dynamic quenching are the most common types of luminescent quenching (Chen et al. 2014). The fluorescence spectra of RG with or without CLA at 298, 304, and 310 K are displayed in Fig. 1b–d, respectively. To determine the quenching type, the Stern–Volmer Eq. (2) was used to calculate the FES data at the three temperatures.
| 2 |
where [Q] is the CLA concentration; F0 and F represent the emission intensities of the RG-CLA complex before and after the addition of the quencher, respectively; Kq is the biomolecular quenching constant of RG; Ksv is the Stern–Volmer quenching constant, which is related to the stability of the complex; and τ0 is the unquenched lifetime of the biomolecule (τ0 = 10−8 s) (Xu et al. 2017).
A Stern–Volmer plot with linear correlation coefficient (R2 > 0.99) was found in Fig. 1e. The good linear relationship at different temperatures generally represents only one quenching mechanism (dynamic or static) occurred (Jia et al. 2017). The Ksv (Ksv = Kqτ0) values [0.98 × 104 (298 K), 1.01 × 104 (304 K), and 1.14 × 104 L mol−1 (310 K)] tended to increase as the temperature increased. In addition, the binding constants (Kq) were 0.98 × 1012, 1.01 × 1012, and 1.14 × 1012 L mol−1 s−1 for the samples at 298, 304, and 310 K, respectively. These values were higher than the maximum diffusion collision quenching constant (2.0 × 1010 L mol−1 s−1), indicating that the quenching type was a typical static quenching (Shi et al. 2017).
For static quenching, the association constant (Ka) of the RG-CLA complex and the number of binding sites per protein (n) could be obtained from the following double logarithmic Eq. (3):
| 3 |
The high linear correlation coefficient (R2 > 0.99) at the different temperatures in Fig. 1f demonstrated that the assumptions underlying the derivation of Eq. (3) were valid. The values of n at the experimental temperatures were approximately equal to 1, which indicated the existence of one independent class of binding site between CLA and RG. The experimental results are summarized in Table 1. The Ka values [2.62 × 103 (298 K), 0.43 × 103 (304 K), and 0.25 × 103 L mol−1 (310 K)] of the RG-CLA complexes decreased with increasing temperature. The calculated Ka values of the RG-CLA complexes were close to the values of the RG-Gallic acid complexes (Ka = 3.6 × 103 L mol−1) (Dai et al. 2017) and higher than that of milk malvidin-3-O-glucoside complexed with α-casein (Ka = 0.51 × 103 L mol−1) and β-casein (Ka = 0.46 × 103 L mol−1) (He et al. 2016), which indicated that CLA had a moderate affinity for RG. The binding affinity is an important parameter for proteins to deliver bioactive compounds. In addition, the binding level between delivering the protein and bioactive compounds is critical and directly correlates with the in vivo efficacy of the bioactive compounds (Xiao et al. 2009). Based on the results of Ka, it was speculated that RG could successfully bind with CLA, which might lead to facilitated absorption and greatly increase the bioavailability of CLA (Cao et al. 2013).
Table 1.
Quenching constants KSV, binding constants Ka, and relative thermodynamic parameters for the interaction of CLA with RG at different temperatures
| T (K) | KSV (104 L mol−1) | Ra | Ka (103 L mol−1) | n | Rb | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K−1) |
|---|---|---|---|---|---|---|---|---|
| 298 | 0.98 | 0.9991 | 2.62 | 0.88 | 0.997 | − 19.01 | ||
| 304 | 1.01 | 0.9991 | 0.43 | 0.73 | 0.9835 | − 16.33 | − 151.97 | − 446.19 |
| 310 | 1.14 | 0.9901 | 0.25 | 0.66 | 0.9944 | − 13.65 |
Ra is the correlation coefficient for the KSV values. Rb is the correlation coefficient for the Ka values
Thermodynamic parameters
The basic thermodynamic parameters were calculated to explore the primary forces contributing to the stability of RG-CLA complex. The dominant binding forces that may take place between small molecules and proteins are hydrophobic interactions, hydrogen bonding and electrostatic forces (Chen et al. 2014). When ΔS < 0 and ΔH < 0, the dominating forces are hydrogen bonds and van der Waals forces, whereas if ΔS > 0 and ΔH > 0, hydrophobic forces control the interaction; when ΔS > 0 and ΔH < 0, this means that electrostatic forces are the primary factor (Ross and Subramanian 1981). The van’t Hoff Eqs. (4, 5) could be applied to determine the changes of enthalpy (ΔH), entropy (ΔS) and Gibbs free energy (ΔG).
| 4 |
| 5 |
where R is the ideal gas constant (8.314 J mol−1 K−1) and T is the temperature in Kelvin. Ka was derived from the preceding Eq. (3) at several different temperatures. The ΔS and ΔH values can be calculated from the intercept and slope of the linear plot of lnKa versus T−1. Equation (5) was used to determine the Gibbs free energy change (ΔG).
The negative ΔG demonstrated that the binding of RG-CLA occurred spontaneously (Table 1). The ΔS and ΔH values for the association of CLA and RG were − 446.19 kJ mol−1 K−1 and − 151.97 kJ mol−1, respectively. Both ΔS and ΔH values were less than 0, which indicated that hydrogen bonds and van der Waals forces played main roles in the binding of RG and CLA, and enthalpy drove the binding interaction. The result was similar to the previous study of Zeng et al. (2016), who demonstrated that the primary driving force for the binding of apigenin and α-glucosidase was hydrogen bonding.
Surface hydrophobicity
ANS was utilized as a fluorescent probe for detecting hydrophobic sites and exploring the conformational changes of RG in the RG-CLA complex. The surface hydrophobicity of RG decreased gradually with increasing concentrations of CLA from 0 to 16.13 μmol L−1 (Fig. 2). The decrease in surface hydrophobicity of the RG-CLA complex might be related to changes in the polarity of the solution environment (exposure of hydrophobic amino acids). Jia et al. (2017) reported that the combination of β-lactoglobulin-polyphenols would induce the exposure of hydrophobic regions, which in turn would reduce the surface hydrophobicity of β-lactoglobulin.
Fig. 2.
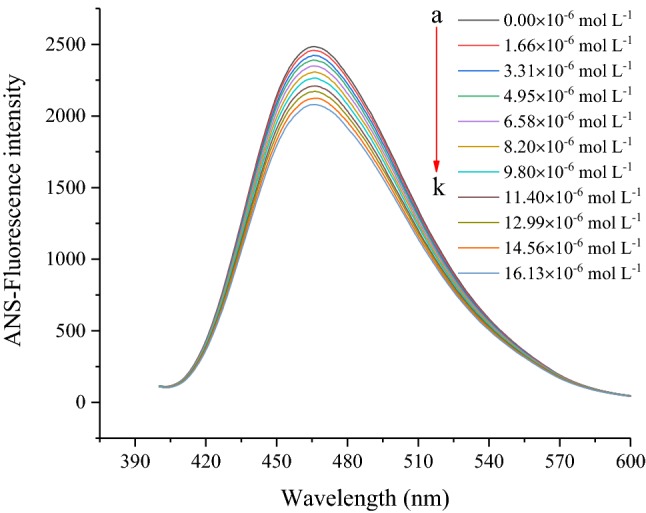
Changes in ANS fluorescence intensity of RG at different concentrations of CLA. CRG = 0.65 mg mL−1; CCLA = 0–16.13 μmol L−1
Synchronous fluorescence spectroscopy
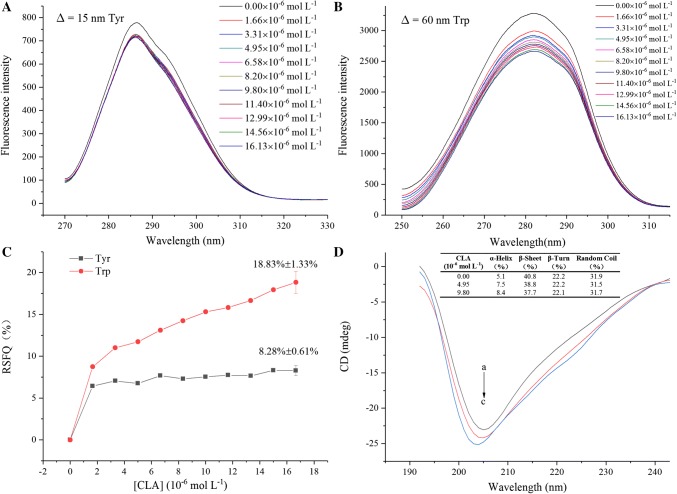
SFS was utilized to further explore the effects of CLA on the structure of RG. This approach could provide evidence of the speculated changes in the polarity of the microenvironment around the fluorophores (Xu et al. 2015). The fluorescent spectra curves of the RG solutions containing different CLA concentrations are displayed in Fig. 3a, b. The maximum emission wavelengths of the tryptophan and tyrosine residues were maintained at approximately 283 and 287 nm, respectively. The fluorescence of RG was mainly attributed to tryptophan since the fluorescence intensity of tryptophan was much greater than that of tyrosine. The fluorescence intensity of tyrosine and tryptophan decreased sharply after the addition of CLA. The fluorescence intensity of tryptophan decreased by 18.83 ± 1.33%, which was significantly (p < 0.05) higher than the decrease in the tyrosine intensity (8.28 ± 0.61%) when the concentration of CLA was 16.13 μmol L−1. This suggested that tryptophan contributed to the interaction process more than tyrosine, and that tryptophan might be closer to the binding sites.
Fig. 3.
Synchronous fluorescence spectra of RG with different concentrations of CLA at a ∆λ = 15 nm and b ∆λ = 60 nm. CCLA = 0–16.13 μmol L−1; c Comparative evaluation of the CLA effect on the ratios of synchronous fluorescence quenching (RSFQ) of RG with CCLA = 0–16.13 μmol L−1; d CD spectra of RG in the presence of CLA at 0, 4.95 and 9.80 μmol L−1. CRG = 0.65 mg mL−1
Circular dichroism characteristics
CD spectroscopy was used to investigate the secondary structural changes of RG. The changes in the far-UV CD spectra of RG with and without CLA are displayed in Fig. 3d. The values of negative ellipticity increased with increasing CLA concentration, reflecting partial changes in the RG secondary structure. The untreated RG sample contained approximately 5.1% α-helix, 40.8% β-sheet, 22.2% β-turn, and 31.9% random coil. When the concentration of CLA rose to 9.80 μmol L−1, the contents of α-helix significantly (p < 0.05) increased to 8.4% with a reduction in β-turn contents (to 37.7%), while the β-sheet (22.1%) and random coil (31.7%) showed no significant (p > 0.05) changes. According to Xu et al. (2015), an increase in α-helix proportion may be related to ligand molecules that are embedded in the hydrophobic regions of proteins, which is in agreement with the data of the hydrophobicity analysed in the previous sections. It can be concluded that the insertion of CLA facilitated the unfolding of polypeptide chains, the collapsing of hydrogen bonding networks, and resulted in the rearrangement of these hydrogen bonds leading to conformational changes in RG, as suggested by Paramonov et al. (2006).
Computational docking simulations
Protein-ligand docking (LibDock) is a high-throughput docking algorithm that positions catalyst-generated ligand conformations in the protein active site based on polar interaction sites (hotspots) (Singh et al. 2017). It was used to extrapolate the specific binding sites of CLA in RG and examined the validity of the previously obtained experimental data. According to previous reports (Dai et al. 2017; Xu et al. 2015), the RG model was constructed using the template of the pumpkin seed globulin structure based on a homology modelling approach. The results of the docking study showed 10 poses. The best pose from the docking was selected according to the LibDock score. The higher the LibDock score, the greater the probability of ligand-protein binding. The pose with the highest LibDock score (115.949) in Table 2 represented the most stable conformation, which was selected for analysis regarding the interaction between RG and CLA. The 3D docking model (Fig. 4a) and schematic 2D diagram (Fig. 4b) showed clearly that CLA embedded itself into a cavity on the RG surface and interacted with some amino acid residues. As shown in Fig. 4b, the potential interaction sites between RG and CLA involved Leu 29, Asp 56, Glu 57, Cys 60, Arg 164, Asp 54, Pro 172, Thr 163, Gln 53, Gly 62, Asn 64, Gln 59, Cys 27, Arg 28, Ala 26, and Phe 58. Owing to the many hydroxyl groups in CLA, hydrogen bonds occurred between CLA and the amino acid residues Arg 188 and Val 63. The distances of these two hydrogen bonds (5.37 Å 4.86 Å, respectively) are displayed in Fig. 4b. In addition, the main interaction forces were van der Waals forces and hydrogen bonds, which agreed with the preceding thermodynamic results. This information may enable RG to serve as a transport protein of fatty acids, protecting fatty acids from degradation and improving their bioavailability in future nutritional products.
Table 2.
Parameters of the LibDock protocol docking results
| Pose number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| LibDock score | 115.95 | 93.65 | 85.13 | 82.81 | 81.76 | 76.23 | 75.87 | 70.43 | 61.73 | 59.85 |
Fig. 4.
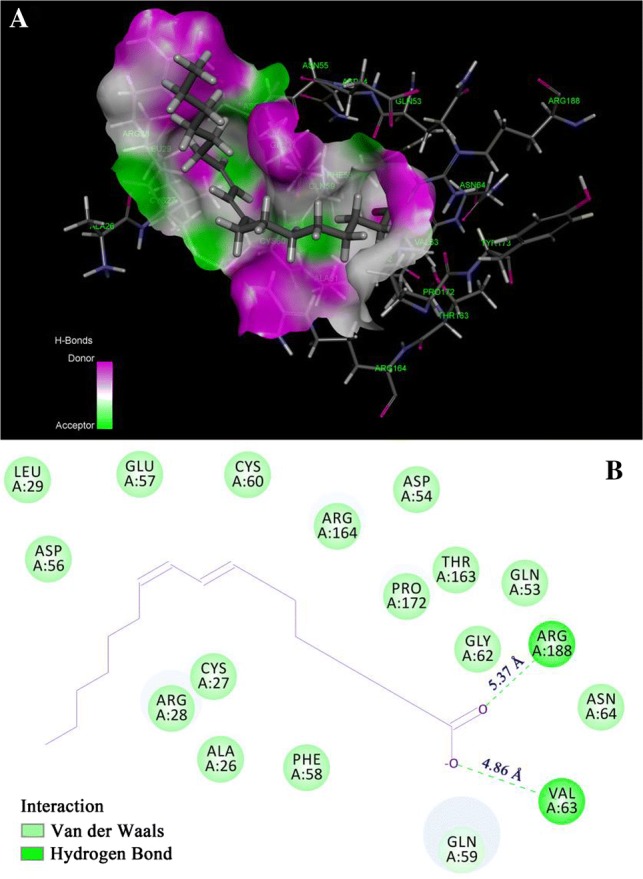
a The hydrogen bond surface of the protein receptor interacting with CLA; b 2D schematic interaction diagram between CLA and RG. The colour of the amino acid residue is drawn according to the interaction. The blue and green colours represent the donors and acceptors of hydrogen bonds, respectively (colour figure online)
Conclusion
This research demonstrated that RG interacted with CLA and formed a stabilized RG-CLA complex. Typical static fluorescence quenching was induced by the RG-CLA interaction. Hydrogen bonds played a key role in the binding process. The CD results indicated that the RG-CLA complex led to secondary structural changes in RG. Computational docking simulations visually showed that CLA was embedded into a cavity on the RG surface. These results might be useful for understanding the essence of interactions between bioactive lipid components and plant proteins. Therefore, this study proposes a new strategy to enhance the performance of nutritional products for food retailers and manufacturers.
Acknowledgements
The authors thank the National Natural Science Foundation of China (31460394, 31760441), and the Support Program for Outstanding Youth Talents in Jiangxi Province (20171BCB-23026).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amagliani L, O’ Regan J, Kelly AL, O’ Mahony JA. The composition, extraction, functionality and applications of rice proteins: a review. Trends Food Sci Technol. 2017;64:1–12. doi: 10.1016/j.tifs.2017.01.008. [DOI] [Google Scholar]
- AOAC Official Methods of Analysis (1990) AOAC official method 992.23 crude protein in cereal grains and oilseeds. In Cereal foods, Chapter 32, p 27
- Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- Benjamin S, Spener F. Conjugated linoleic acids as functional food: an insight into their health benefits. Nutr Metab. 2009;6:36. doi: 10.1186/1743-7075-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens R, Scheek R, Dijkstra K, Kaptein R. Sequential assignment of imino-and amino-proton resonances in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy-application to a lac operator fragment. J Magn Reson. 1985;62:378–386. [Google Scholar]
- Cao H, Jing X, Wu D, Shi Y. Methylation of genistein and kaempferol improves their affinities for proteins. Int J Food Sci Nutr. 2013;64(4):437–443. doi: 10.3109/09637486.2012.759186. [DOI] [PubMed] [Google Scholar]
- Chen H, Jin Y, Ding X, Wu F, Bashari M, Chen F, Cui ZW, Xu XM. Improved the emulsion stability of phosvitin from hen egg yolk against different pH by the covalent attachment with dextran. Food Hydrocoll. 2014;39:104–112. doi: 10.1016/j.foodhyd.2013.12.031. [DOI] [Google Scholar]
- Dai T, Yan X, Li Q, Li T, Liu C, McClements DJ, Chen J. Characterization of binding interaction between rice glutelin and gallic acid: multi-spectroscopic analyses and computational docking simulation. Food Res Int. 2017;102:274–281. doi: 10.1016/j.foodres.2017.09.020. [DOI] [PubMed] [Google Scholar]
- Dai T, Chen J, McClements DJ, Hu P, Ye X, Liu C, Li T. Protein-polyphenol interactions enhance the antioxidant capacity of phenolics: analysis of rice glutelin-procyanidin dimer interactions. Food Funct. 2019;10:765–774. doi: 10.1039/C8FO02246A. [DOI] [PubMed] [Google Scholar]
- Essemine J, Hasni I, Carpentier R, Thomas T, Tajmir-Riahi H. Binding of biogenic and synthetic polyamines to β-lactoglobulin. Int J Biol Macromol. 2011;49:201–209. doi: 10.1016/j.ijbiomac.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Fontana A, Spolaore B, de Laureto PP. The biological activities of protein/oleic acid complexes reside in the fatty acid. BBA-Proteins Proteom. 2013;1834(6):1125–1143. doi: 10.1016/j.bbapap.2013.02.041. [DOI] [PubMed] [Google Scholar]
- He Z, Xu M, Zeng M, Fang Q, Chen J. Interactions of milk α- and β-casein with malvidin-3-O-glucoside and their effects on the stability of grape skin anthocyanin extracts. Food Chem. 2016;199:314–322. doi: 10.1016/j.foodchem.2015.12.035. [DOI] [PubMed] [Google Scholar]
- Ilyasoglu H, El SN. Nanoencapsulation of EPA/DHA with sodium caseinate-gum arabic complex and its usage in the enrichment of fruit juice. LWT-Food Sci Technol. 2014;56(2):461–468. doi: 10.1016/j.lwt.2013.12.002. [DOI] [Google Scholar]
- Jia J, Gao X, Hao M, Tang L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017;228:143–151. doi: 10.1016/j.foodchem.2017.01.131. [DOI] [PubMed] [Google Scholar]
- Karaca AC, Low N, Nickerson M. Potential use of plant proteins in the microencapsulation of lipophilic materials in foods. Trends Food Sci Technol. 2015;42:5–12. doi: 10.1016/j.tifs.2014.11.002. [DOI] [Google Scholar]
- Kim JH, Kim Y, Kim YJ, Park Y. Conjugated linoleic acid: potential health benefits as a functional food ingredient. Annu Rev Food Sci Technol. 2016;7:221–244. doi: 10.1146/annurev-food-041715-033028. [DOI] [PubMed] [Google Scholar]
- Le Maux S, Bouhallab S, Giblin L, Brodkorb A, Croguennec T. Complexes between linoleate and native or aggregated β-lactoglobulin: interaction parameters and in vitro cytotoxic effect. Food Chem. 2013;141:2305–2313. doi: 10.1016/j.foodchem.2013.05.031. [DOI] [PubMed] [Google Scholar]
- Lindin I, Wuxiuer Y, Kufareva I, Abagyan R, Moens U, Sylte I, Ravna AW. Homology modeling and ligand docking of Mitogen-activated protein kinase-activated protein kinase 5 (MK5) Theor Biol Med Model. 2013;10:56. doi: 10.1186/1742-4682-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalanis A, Jones OG, McClements DJ. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011;25:1865–1880. doi: 10.1016/j.foodhyd.2011.04.014. [DOI] [Google Scholar]
- McClements DJ, Decker EA, Park Y, Weiss J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit Rev Food Sci Nutr. 2009;49:577–606. doi: 10.1080/10408390902841529. [DOI] [PubMed] [Google Scholar]
- Paramonov SE, Jun HW, Hartgerink JD. Self-assembly of peptide-amphiphile nanofibers: the roles of hydrogen bonding and amphiphilic packing. J Am Chem Soc. 2006;128(22):7291–7298. doi: 10.1021/ja060573x. [DOI] [PubMed] [Google Scholar]
- Perez AA, Andermatten RB, Rubiolo AC, Santiago LG. β-Lactoglobulin heat-induced aggregates as carriers of polyunsaturated fatty acids. Food Chem. 2014;158:66–72. doi: 10.1016/j.foodchem.2014.02.073. [DOI] [PubMed] [Google Scholar]
- Ross PD, Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochem. 1981;20(11):3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Shi M, Huang LY, Nie N, Ye JH, Zheng XQ, Lu JL, Liang YR. Binding of tea catechins to rice bran protein isolate: interaction and protective effect during in vitro digestion. Food Res Int. 2017;93:1–7. doi: 10.1016/j.foodres.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Singh S, Awasthi M, Pandey VP, Dwivedi UN. Lipoxygenase directed anti-inflammatory and anti-cancerous secondary metabolites: ADMET-based screening, molecular docking and dynamics simulation. J Biomol Struct Dyn. 2017;35:657–668. doi: 10.1080/07391102.2016.1159985. [DOI] [PubMed] [Google Scholar]
- Sodek L, Wilson CM. Amino acid compositions of proteins isolated from normal, opaque-2, and floury-2 corn endosperms by a modified Osborne procedure. J Agr Food Chem. 1971;19(6):1144–1150. doi: 10.1021/jf60178a011. [DOI] [Google Scholar]
- Sponton OE, Perez AA, Carrara CR, Santiago LG. Complexes between ovalbumin nanoparticles and linoleic acid: stoichiometric, kinetic and thermodynamic aspects. Food Chem. 2016;211:819–826. doi: 10.1016/j.foodchem.2016.05.137. [DOI] [PubMed] [Google Scholar]
- Taylor WR. Multiple protein sequence alignment: algorithms and gap insertion. Method Enzymol. 1996;266(1):343–367. doi: 10.1016/S0076-6879(96)66022-4. [DOI] [PubMed] [Google Scholar]
- Van de Weert M. Fluorescence quenching to study protein-ligand binding: common errors. J Fluoresc. 2010;20:625–629. doi: 10.1007/s10895-009-0572-x. [DOI] [PubMed] [Google Scholar]
- Vélez MA, Perotti MC, Zanel P, Hynes ER, Gennaro AM. Soy PC liposomes as CLA carriers for food applications: preparation and physicochemical characterization. J Food Eng. 2017;212:174–180. doi: 10.1016/j.jfoodeng.2017.06.001. [DOI] [Google Scholar]
- Wang L, Xu Y, Zhou S, Qian H, Zhang H, Qi X, Fan M. Interaction between Vaccinium bracteatum Thunb. leaf pigment and rice proteins. Food Chem. 2016;194:272–278. doi: 10.1016/j.foodchem.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Xiao J, Cao H, Wang Y, Zhao J, Wei X. Glycosylation of dietary flavonoids decreases the affinities for plasma protein. J Agr Food Chem. 2009;57(15):6642–6648. doi: 10.1021/jf901456u. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu W, Zhong J, Luo L, Liu C, Luo S, Chen L. Binding interaction between rice glutelin and amylose: hydrophobic interaction and conformational changes. Int J Biol Macromol. 2015;81:942–950. doi: 10.1016/j.ijbiomac.2015.09.041. [DOI] [PubMed] [Google Scholar]
- Xu X, Luo L, Liu C, Zhang Z, Mcclements DJ. Influence of electrostatic interactions on behavior of mixed rice glutelin and alginate systems: pH and ionic strength effects. Food Hydrocoll. 2017;63:301–308. doi: 10.1016/j.foodhyd.2016.09.005. [DOI] [Google Scholar]
- Zeng L, Zhang G, Lin S, Gong D. Inhibitory mechanism of apigenin on α-Glucosidase and synergy analysis of flavonoids. J Agr Food Chem. 2016;64:6939–6949. doi: 10.1021/acs.jafc.6b02314. [DOI] [PubMed] [Google Scholar]
- Zimet P, Livney YD. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2009;23(4):1120–1126. doi: 10.1016/j.foodhyd.2008.10.008. [DOI] [Google Scholar]