Abstract
The distribution of chemical species and the mechanical modulation inside a single cell or tissue are of fundamental importance to characterize their physiological activity or their pathological conditions [1–4]. Here we analyse these properties by means of label free, non invasive, spectroscopic methods. In particular, we use a recently developed micro-spectrometer, which acquires simultaneously Raman and Brillouin spectra on the same point with subcellular resolution [5]. The techniques ability to analyse the chemical composition and the mechanical properties of single cells has been tested on NIH/3T3 murine fibroblast cells grown in adhesion on silicon substrates. Here we report the data acquired from fixed cells after their oncogenic transformation. Mechanical and chemical evolution is evident by direct inspection of raw data. Sharing our experimental records can be valuable for researchers interested in the analysis of single cells by Raman and Brillouin spectroscopy in order: i) to compare data acquired by different set-ups and ii) to correctly model the fitting functions.
Keywords: Biophotonics, Brillouin spectroscopy, Raman spectroscopy, Cell mechanics
Specifications Table
| Subject | Biophysics |
| Specific subject area | Optical Brillouin and Raman spectroscopy |
| Type of data | Raw and graph. The raw data are available in "Appendix A. Supplementary data" of the present article as zip file. |
| How data were acquired | Custom build microscope coupled with Brillouin and Raman spectrometers [6,7] |
| Data format | Raw |
| Parameters for data collection | The laser beam with λ = 532 nm and power on the sample P<3.5 mW is focalized on the single cells using UPLSAPO 60XW Olympus objective with NA1.2. In order to control the position on the cell, the Petri dish, thermalized at 37 °C, was inserted in a dedicated sample environment placed on a xyz translator stage (PI 611-3S Nanocube XYZ). Thanks to the piezoelectric control, it reaches a spatial resolution of 10 nm in a motion range of 100 μm for each axis. |
| Description of data collection | The Raman spectra were acquired by RM- Horiba iHR320 Triax using a 600 grooves mm−1 grating and an N2 cooled CCD detector (1024 × 256 pixels). The acquisition of Raman spectra up to 3000 cm−1 frequency shift is possible in this condition. At the same time, the High Contrast HC version of the Sandercock type tandem Fabry Perot (TFP-2) interferometer was used to acquire Brillouin spectra. |
| Data source location | IOM-CNR c/o Department of Physics and Geology University of Perugia Italy |
| Data accessibility | With the article |
Value of the Data
|
1. Data description
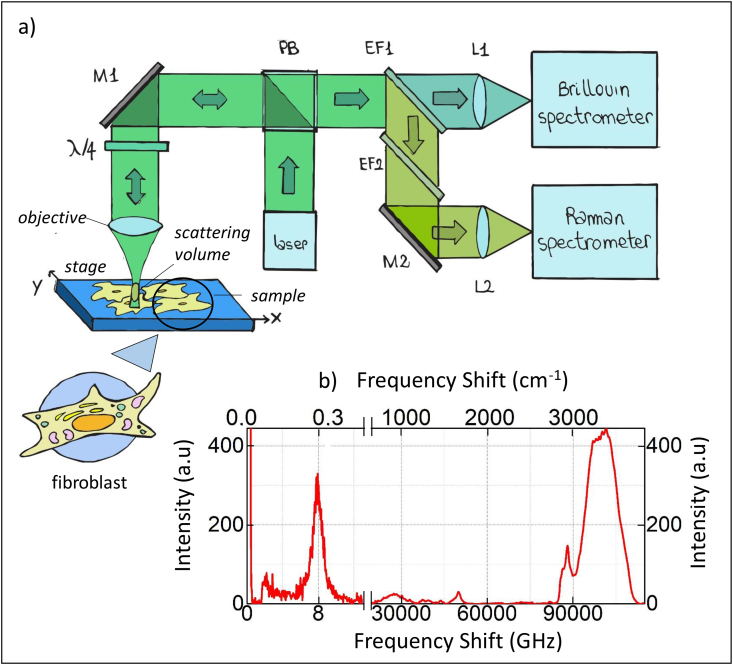
The growing importance to characterize mechanical properties, in addition to chemical ones, in biological framework is widely recognized in recent litterature [[1], [2], [3], [4]]. The data shared in the present paper were recorded investigating a single fixed cell using the novel experimental set-up recently assembled in our Lab able to simultaneously characterize chemical and mechanical properties of the investigated material [[5], [6], [7]]. The schematic picture of the optical system is reported in Fig. 1 a) (upper pannel). The Brillouin and Raman spectra simultaneously acquired probing the same position inside a single cell is reported in Fig. 1 b) (lower panel).
Fig. 1.
a) Schematic of the optical setup for simultaneous Brillouin-Raman micro spectroscopy. The optical components are labelled as: M (mirror), L (lent), EF (edge filter) and PB (polarized beam splitter) b) Brillouin and Raman spectrum simultaneously acquired inside the cell. For the Raman peaks assignment we refer to previous publications [5,14].
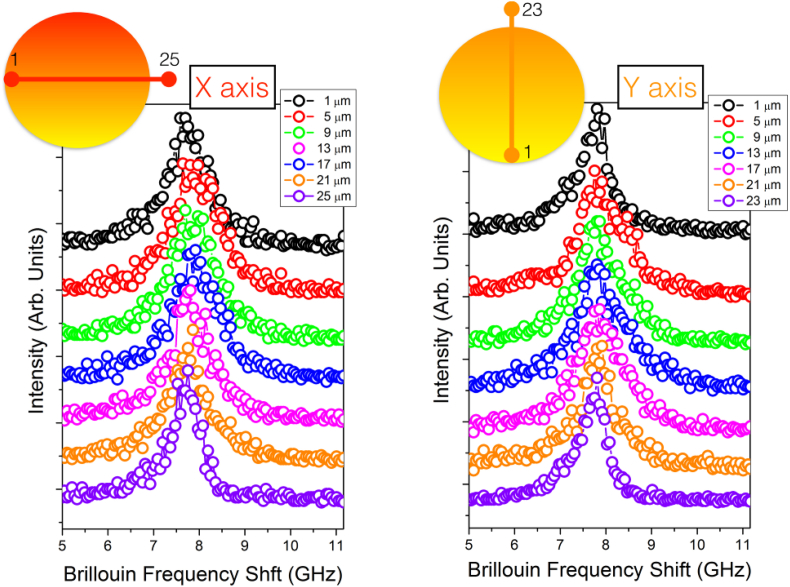
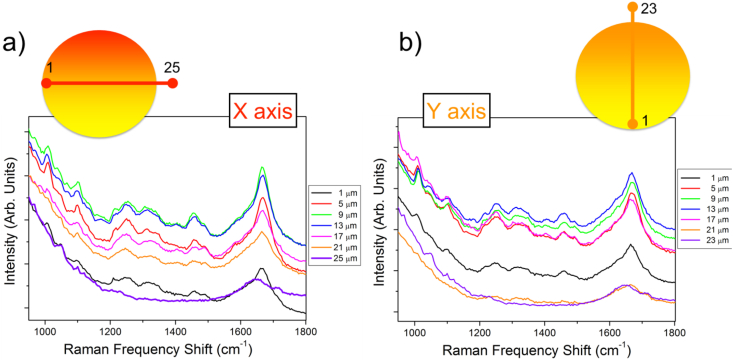
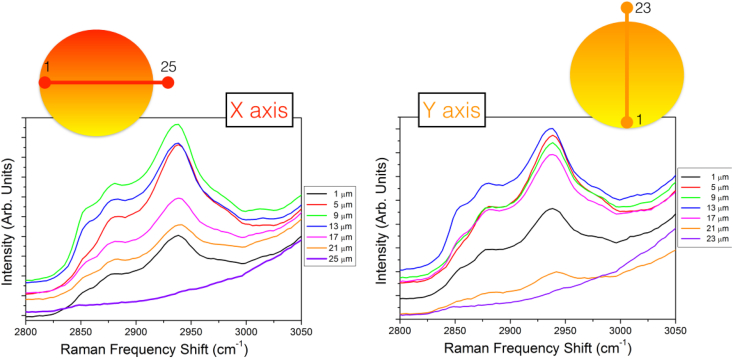
The whole dataset is archived in BRaM_Dataset.zip present in "Appendix A. Supplementary data" of the present article. Both Raman and Brillouin data are simultaneously acquired probing the same point inside the cell. They were collected as a function of the position in a fixed and transfected NIH/3T3 murine fibroblast (see below for the details) grown in adhesion on silicon substrates. In particular, the data were collected moving with a step of 2 μm crossing the cell from one side to the other, entering from the plasmatic membrane, through the cytoplasm into the nucleus, and exiting from the other side. A selection of the collected Brillouin spectra are reported in Fig. 2 and the low and high frequency region of the Raman spectra are reported in Fig. 3 and Fig. 4 respectively. The right and the left panel of the figures show the measurements along two perpendicular directions within the cell (x and y axis). Directly from the raw data, it is possible to appreciate modifications in the spectral shape. In fact, moving through different cellular points, modulation in the elastic properties is evidenced by the shift and broadening of the Brillouin peak related to the emergence of high frequency component (for the detailed analysis see Ref. [5]). Moreover, modifications in the relative concentration of the different chemical species are visible by changes in the relative intensity of the Raman peaks present in both high and low frequency range of the spectra.
Fig. 2.
Sequence of Brillouin spectra probing different position inside the cell.
Fig. 3.
Sequence of selected Raman spectra probing the frequency region between 1000–1800 cm−1 acquired in different positions inside the cell.
Fig. 4.
Evolution of CH2 and CH3 stretching Raman band probing different positions inside the cell.
2. Experimental design, materials, and methods
The data were acquired using the Brillouin-Raman micro-spectroscopy set up extensively described elsewhere [5,6]. In brief, the laser light is focalized by a water immersion objective into the cell and the scattered light collected by the same objective is analysed in frequency by a HC-Tandem Fabry-Perot interferometer and by a single grating Raman spectrometer.
NIH/3T3 murine fibroblast cell line was purchased from American Type Culture Collection (ATCC). Cells were grown in Dulbecco Modified Eagle's Medium (DMEM) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 U/mL streptomycin and maintained at 37 °C in a 5% CO 2 humidified atmosphere. Cells were seeded in 6-well multiplates and transfected using Lipofectamine LTX with the expression vector pcDNA6/myc-His encoding the constitutively active mutant H-RasV12. The vector expressing the Ras mutant was previously described [15]. This mutation replaces the amino acid glycine with a valine, which makes the GTPase constitutively GTP bound. Transfected fibroblasts were selected using 4 μg/ml Blasticidin-S for 5 days. The expression of H-RasV12 was assessed by immunoblotting as previously shown [5]. Selected cells were trypsinized and seeded in silicon substrates sterilized with 100% ethanol washing and UV irradiation. Paraformaldehyde fixation was performed by incubating cells with 4% paraformaldehyde in PBS for 10 minutes at room temperature, then cells were washed twice with PBS. For the spectroscopic measurements, the cells were immersed in phosphate-buffered saline (PBS).
Acknowledgments
SC acknowledges the support from PAT (Autonomous Province of Trento) (GP/PAT/2012) ‘Grandi Progetti 2012’ Project ‘MaDEleNA’. MM acknowledges the European Commission under the EU Horizon 2020 Programme Grant Agreement No: 644852, PROTEUS. SC acknowledges financial support from Consiglio Nazionale delle Ricerche-Istituto Officina dei Materiali.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2020.105223.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Prevedel R., Diz-Muñoz A., Ruocco G., Antonacci G. Brillouin microscopy - a revolutionary tool for mechanobiology? Nat. Methods. 2019;16:969–977. doi: 10.1038/s41592-019-0543-3. [DOI] [PubMed] [Google Scholar]
- 2.Caponi S., Canale C., Cavalleri O., Vassalli M. Characterization tools for mechanical probing of biomimetic materials. In: Kumar C., editor. Nanotechnology Characterization Tools for Tissue Engineering and Medical Therapy. Springer, Berlin, Heidelberg; 2019. pp. 69–111. [Google Scholar]
- 3.Palombo F., Fioretto D. Brillouin light scattering: applications in biomedical sciences. Chem. Rev. 2019;119:7833–7847. doi: 10.1021/acs.chemrev.9b00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margueritat J., Virgone-Carlotta A., Monnier S., Delanoë-Ayari H., Mertani H.C., Berthelot A., Martinet Q., Dagany X., Rivière C., Rieu J.P., Dehoux T. High-frequency mechanical properties of tumors measured by Brillouin light scattering. Phys. Rev. Lett. 2019;122:018101. doi: 10.1103/PhysRevLett.122.018101. [DOI] [PubMed] [Google Scholar]
- 5.Mattana S., Mattarelli M., Urbanelli L., Sagini K., Emiliani C., Serra M.D., Fioretto D., Caponi S. Non-contact mechanical and chemical analysis of single living cells by microspectroscopic techniques. Light Sci. Appl. 2018;7:17139. doi: 10.1038/lsa.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarponi F., Mattana S., Corezzi S., Caponi S., Comez L., Sassi P., Morresi A., Paolantoni M., Urbanelli L., Emiliani C., Roscini L., Corte L., Cardinali G., Palombo F., Sandercock J.R., Fioretto D. High-performance versatile setup for simultaneous Brillouin-Raman microspectroscopy. Phys. Rev. X. 2017;7:031015. [Google Scholar]
- 7.Mercatelli R., Mattana S., Capozzoli L., Ratto F., Rossi F., Pini R., Fioretto D., Pavone F.S., Caponi S., Cicchi R. Morpho-mechanics of human collagen superstructures revealed by all-optical correlative micro-spectroscopies. Commun. Biol. 2019;2:117. doi: 10.1038/s42003-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarcelli G., Polacheck W.J., Nia H.T., Patel K., Grodzinsky A.J., Kamm R.D., Yun S.H. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat. Methods. 2015;12:1132–1134. doi: 10.1038/nmeth.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsayad K., Werner S., Gallemí M., Kong J., Sánchez Guajardo E.R., Zhang L., Jaillais Y., Greb T., Belkhadir Y. Mapping the subcellular mechanical properties of live cells in tissues with fluorescence emission-Brillouin imaging. Sci. Signal. 2016;9:rs5. doi: 10.1126/scisignal.aaf6326. [DOI] [PubMed] [Google Scholar]
- 10.Bevilacqua C., Sánchez-Iranzo H., Richter D., Diz-Muñoz A., Prevedel R. Imaging mechanical properties of sub-micron ECM in live zebrafish using Brillouin microscopy. Biomed. Optic Express. 2019;10:1420. doi: 10.1364/BOE.10.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonacci G., de Turris V., Rosa A., Ruocco G. Background-deflection Brillouin microscopy reveals altered biomechanics of intracellular stress granules by ALS protein FUS. Commun. Biol. 2018;1:139. doi: 10.1038/s42003-018-0148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonacci G., Braakman S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci. Rep. 2016;6:37217. doi: 10.1038/srep37217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caponi S., Fioretto D., Mattarelli M. On the actual spatial resolution of Brillouin imaging. Optics Lett. 2020 doi: 10.1364/OL.385072. [DOI] [PubMed] [Google Scholar]
- 14.Caponi S., Liguori L., Giugliarelli A., Mattarelli M., Morresi A., Sassi P., Urbanelli L., Musio C. Raman micro-spectroscopy: a powerful tool for the monitoring of dynamic supramolecular changes in living cells. Biophys. Chem. 2013;182:58–63. doi: 10.1016/j.bpc.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Armeni T., Ercolani L., Urbanelli L., Magini A., Magherini F., Pugnaloni A., Piva F., Modesti A., Emiliani C., Principato G. Cellular redox imbalance and changes of protein S-glutathionylation patterns are associated with senescence induced by oncogenic H-Ras. PLoS One. 2012;7:e52151. doi: 10.1371/journal.pone.0052151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.