Abstract
Considering the increasing consumer demand for healthy food, the extract from broccoli by-products was studied. To this aim, in the first step, three extraction techniques were compared in terms of extraction efficiency. The best method was the pressurized liquid extraction. Then, the extract microencapsulation was optimized in terms of type of wall material (between Capsul and maltodextrins), concentration of wall material (10–20–30%, w/v), core/wall material ratio (1:2, 1:5, 1:10, 1:20) and inlet temperature (80, 100, 130, 150, 170 °C). The optimal conditions were found with 10% maltodextrins as wall material, core/wall material ratio 1/2 at 80 °C. Finally, the obtained microencapsulated extract was added at 5% (w/w) to fish burgers. Results demonstrated that total phenolic content, total flavonoids and antioxidant activity of enriched fish products were significantly higher than the control burgers, thus confirming that both process and cooking did not greatly affect the nutritional properties of extracted compounds.
Keywords: Broccoli by-products, PLE, SFE, UAE, Spray-drying process
Introduction
Nowadays, consumers are greatly careful to maintain healthy diet and lifestyle. In fact, according to several studies, demand for natural and health-promoting ingredients has increased. In this context, natural compounds from plants by-products can be considered valid alternative to synthetic substances (Djilas et al. 2009). Food by-products may be also used as food ingredients because of their properties as gelling and water binding agents (O’Shea et al. 2012). In addition, the use of by-products allows reducing not only the environmental impact but also the additional cost that the producer has to face for their disposal. Undoubtedly, fruit and vegetables are the main source of phytochemicals. In the current study, a particular attention has been paid to broccoli (Brassica oleracea var. italica) due to the recognized beneficial properties (Arnaiz et al. 2012). High contents of bioactive compounds such as glucosinates, isothiocyanates, phenolic compounds, vitamin A, C, E, K and several important minerals have been found in broccoli (Domínguez-Perles et al. 2010). During the manufacturing process of broccoli, high amount of waste, consisting of about 70% of plant, is generated (Arnaiz et al. 2012). To the best of our knowledge, a very few studies are related to the extraction or characterization of glucosinolate, or to the antioxidant activity of broccoli by-products (Arnáiz et al. 2016; Domínguez-Perles et al. 2010).
Conventional extraction technique, generally characterized by long extraction time, requiring high cost and high purity solvent, evaporation of the huge amount of solvent, low extraction selectivity and decomposition of thermolabile compounds, have been substituted by novel promising methods, more environmental friendly (Ares et al. 2015; Herrero et al. 2010). Among these green techniques, supercritical fluid extraction (SFE) is one of the most commonly employed methods to obtain bioactive compounds. As fluid, carbon dioxide (CO2) is widely used because it is cheap, environmental friendly and generally recognized as safe by FDA and EFSA (Herrero et al. 2006). The main disadvantage of CO2 is the low polarity, problem that can be overcome using modifiers (co-solvents), capable of hydrogen-bonding, dipole–dipole and others polarity interactions with analytes of interest (Maróstica Junior et al. 2010). Generally, ethanol is used as co-solvent considering good miscibility with CO2, non-toxicity and permissible use in the food and pharmaceutical industries. In addition to SFE, ultrasound assisted extraction (UAE) is another recent approach to obtain valuable compounds. According to Tao et al. (2014) in UAE is possible to use water as solvent, to shorten the extraction time, to reduce the organic solvent waste, to increase the extraction yield and to enhance the quality of extracts. This type of extraction uses sound mechanical waves with frequencies superior to 20 kHz and requires a liquid medium in which the waves are able to propagate up to the product, preserving the integrity of thermolabile, thermostable, hydro-soluble and liposoluble molecules. Specifically, cavitation forces, produced by the ultrasound application, lead a greater penetration of solvent into the food matrix and, consequently, an increase of the mass transfer rates of the bioactive compounds from food matrix into the extraction solvent (Gonzalez-Centeno et al. 2014). Under these conditions, the transfer of compounds to be extracted is facilitated and the extraction time is reduced (Marinelli et al. 2015). Another rapid and automated method is the pressurized liquid extraction (PLE), an emerging technique which uses liquid solvents at high temperature and pressure to enhance the extraction performance (Mustafa and Turner 2011).
The aim of the current study was to screen different extraction methods (SFE, UAE and PLE) to obtain a valuable extract from broccoli by-products. These techniques were compared in terms of extraction efficiency of the technique and in terms of total phenolic compounds (TPC), total flavonoid compounds (TFC) and antioxidant activity of the final extract. In addition, a spray-drying process of the extract was optimized to protect the bioactive compounds prior to the application to fish burgers. To this aim, microencapsulation conditions were optimized in terms of types of wall materials and their concentrations, core/wall ratio and inlet temperature. Although there are several studies on microencapsulation of bioactive compounds, most of them are focused on the optimization without studying any food applications. Therefore, the last aim of this study was to enrich fish burgers with 5% (w/w) of the broccoli by-product microencapsulated extract in order to enhance the healthy properties of fish.
Materials and methods
Raw materials and chemicals
Broccoli by-products (BRC) were supplied by a local industry located in Puglia (Farris, Foggia, Italy). The by-products were dried at 35 °C in a dryer (SG600, Namad, Rome, Italy) for 48 h. The dried broccoli samples were ground by a hammer mill (16/BV-Beccaria s.r.l., Cuneo, Italy) and then stored at 4 °C until further utilization.
Folin–Ciocalteu reagent, anhydrous sodium carbonate (Na2CO3), gallic acid monohydrate, sodium nitrite (NaNO2), aluminum chloride (AlCl3), sodium hydroxide (NaOH), quercetin, DPPH (2,2-Diphenyl-1-picrylhydrazyl), ABTS (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt), potassium persulfate (K2S2O8), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), absolute ethanol were supplied by Sigma-Aldrich (Milan, Italy). For the preparation of the phosphate buffered saline (PBS), the following salts were used: sodium phosphate dibasic hepta-hydrate (HNa2O4P*7H2O) and sodium phosphate monobasic mono-hydrate (H2NaO4P*H2O). These substances were purchased from Sigma-Aldrich (Milan, Italy). All the reagents were of analytical grade. Sapio (Monza, Italy) supplied CO2 with purity degree of 4.5 for SFE while N2 was provided by Air Liquide (Milan, Italy) with purity degree of 99.9%.
Supercritical fluid extraction (SFE)
The extraction process was performed according to the optimal conditions described by Arnáiz et al. (2016) using the supercritical fluid extractor Speed SFE-2 (Applied Separation, Allentown, USA). It was equipped with two pumps, one to push the supercritical CO2 throughout the system and the other one to add the organic co-solvent (ethanol). The system was set to the desired temperature (35 °C) and pressure (150 bar) with suitable switches, while the CO2 flow rate was regulated by means of a metering valve and set at 2 L/min. The collection condition was at room temperature and atmospheric pressure. According to the performed overall extraction curve (OEC), the whole process lasted 140 min, divided into 7 cycles. Each cycle consisted of 10 min of static phase, in order to maximize the contact of the supercritical solvent with the sample material and 10 min of dynamic phase, in which the 20% ethanol was pumped. Briefly, 33 g of finely ground by-products were put in a vessel (50 mL), placed in the extractor and allowed to equilibrate to the desired temperature. After reaching the set temperature, pressurization was initiated to make the extraction process. The obtained extract was collected in a separator vessel while CO2 was vented by a flow meter. The extract was placed overnight in a vacuum oven at 30 °C in order to remove ethanol. The solid residue was dissolved in 25 mL of absolute ethanol and centrifuged (5804R, Eppendorf, Milan, Italy) at 6000 rpm for 10 min before chemical analyses.
Ultrasound assisted extraction (UAE)
The extract was also obtained by means of UAE (USR-1500-50WL, Weal s.r.l., Milan, Italy), using only water as solvent, according to another study reported in the literature (Marinelli et al. 2015). The system consists of a reactor of 25 L capacity, in which the BRC flour was suspended in water at 1:10 (w/v) ratio (2.5 kg of by-products). The sample was ultrasonically treated for 60 min at acoustic frequency of 25 kHz and with ultrasonic power density of 50 W/L. A rise of 15 °C was observed (from 19 to 34 °C) within 60 min. The obtained extract was homogenized and a part of this placed overnight in a vacuum oven at 30 °C. The dry residue was dissolved in 25 mL of water and centrifuged at 6000 rpm for 10 min before analytical determinations.
Pressurized liquid extraction (PLE)
Pressurized liquid extraction was carried out with PLE equipment (LabService Analytica srl, Anzola Emilia, Bologna, Italy) according to the optimized method proposed by Ares et al. (2015) with slight modifications. The extraction cell was filled with 15 g of dry raw materials, mixed with inert matrix and glass beads to favor uniform distribution of the extraction solvent and maximize the extraction yield. The method was divided in more steps: (1) filling the cell with the extraction solvent (EtOH 70%) for 2.3 min in order to wet the sample; (2) increasing the pressure up to 1500 psi, (3) heating for 5 min up to a temperature of 60 °C; (4) static extraction (one extraction cycle, 5 min); (5) depressurization for 30 s; (6) washing the cell for 50 s; finally (7) purge the solvent from cell with N2 for 2 min. The extraction was performed in triplicate and between the replications a rinse was made to remove any residual. The obtained extract was dried in a vacuum oven at 30 °C overnight. Next, the solid residue was dissolved in 25 mL of ethanol and centrifuged for 10 min at 6000 rpm before chemical analyses.
Microencapsulation process of extract
Microencapsulation of the extract was carried out with a drying process using a mini Spray Dryer B-290 (BUCHI Labortechnik AG, Flawil, Switzerland), optimizing the following parameters: the type of wall material (Capsul, maltodextrins 14–16 DE), the concentration of wall material (10–20–30%), the core/wall material ratio (1:2, 1:5, 1:10, 1:20) and the temperature (80, 100, 130, 150, 170 °C). The optimization was based on the chemical characterization of the obtained powder (TPC, TFC, ABTS and DPPH assays). The resulting formulations were spray-dried at the following conditions: aspiration rate 100% and pump flow rate 25%. At the end of each drying session, the powders were collected, placed in closed vials and kept at room temperature in a dry and dark place until the analyses. For each considered parameter, the microencapsulation process was performed in triplicate.
Fish burgers preparation
Fresh sea bass fillets were obtained from a local seafood company, Minaba s.r.l. (Manfredonia, Foggia, Italy). In the laboratory the fish was cleaned and trimmed to remove bones and skin. The burgers with extract (BRC_FB) consisted of minced fish mixed with 5% w/w of microencapsulated extract and proper amount of whey proteins, parsley, potato flour and salt. The produced fish burgers were cooked in an electric convention oven (H2810, Hugin, Milan, Italy) at 180 °C for 15 min. Fish burgers without any extract were also prepared and used as reference samples (CTRL_FB). For each burger three replicates were made.
Chemical analyses
The evaluation of total phenols, flavonoids and antioxidant activity were performed on each extract, on the microencapsulated extract (50 mg dissolved in 20 mL of water) and on final fish burgers.
Determination of total phenol compounds
The extraction of poly-phenols was based on the method described by Biney and Beta (2014). Briefly, CTRL_FB and BRC_FB samples were dried at 30 °C and grounded. 1 g of each sample was mixed with 10 mL of acidified methanol (80% MeOH acidified with 1% HCl) and shaken at room temperature in darkness for 2 h at 300 rpm using orbital shaker (HS 260 BASIC, IKA, Staufen, Germany). Next, the samples were centrifuged at 5 °C for 15 min at 10,000 rpm (5804R, Eppendorf, Milan, Italy) and the supernatant was used for the analytical determinations. The extraction was carried out in triplicate. The total phenols were determined according to the Folin–Ciocalteu method described by Spinelli et al. (2016). In particular, 0.5 mL of each sample, diluted in a suitable way, and 2.5 mL of Folin–Ciocalteu reagent (diluted in water in a 1:10 ratio) were mixed and left to rest for 5 min. Then, an amount of 2 mL of Na2CO3 (4 g/100 mL) was added. The mixture was allowed to rest again for 2 h in darkness at room temperature. The absorbance was read at 740 nm using an UV–Vis spectrophotometer (UV1800, Shimadzu Italia s.r.l., Milan, Italy). Total phenolic content (TPC) was expressed as mg of gallic acid equivalents (GAEs) per gram of dry sample, according to a calibration curve (3.125–100 mg/L; R2 = 0.9989). For each sample, the analysis was carried out in triplicate.
Determination of total flavonoids
Flavonoids were quantified by aluminum trichloride method as described by Spinelli et al. (2016), using quercetin as standard. Briefly, 0.5 of mL of each sample was mixed with 2 mL of distilled H2O and 0.15 mL of 5% sodium nitrite (NaNO2) solution. After 6 min, 0.15 mL of a 10% aluminum chloride (AlCl3) solution was added and the mixture was allowed to stand for 6 min. Finally, 1 mL of 1 M NaOH and 1.2 mL of distilled water were added to the mixture. Only the fish burger extracts were filtered through a 0.45 µm syringe filter (Teknokroma Nylon 0.45 µm, Sant Cugat del Vallés, Barcelona, Spain). The solution was measured at 415 nm using the spectrophotometer. Total flavonoid content (TFC) was quantified by a calibration curve built in the range 6.25–400 mg/L (R2 = 0.9990) and expressed as mg of quercetin equivalents (QEs) per gram of dry sample. The analysis was carried out in triplicate for each sample.
Determination of antioxidant activity
The antioxidant activity of samples was assessed using two methods: ABTS and DPPH assays. The ABTS assay is based on the ability of antioxidants to interact with the radical cation ABTS·+ (2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) inhibiting its absorption at 734 nm. According to the study of Re et al. (1999) 7 mM ABTS stock water solution and 140 mM potassium persulfate were utilized. The ABTS radical cation (ABTS·+) was obtained by reacting ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before the analysis. The ABTS·+ solution was diluted with 5 mM phosphate buffered saline (PBS) at pH 7.4 to obtain an absorbance of 0.70 ± 0.02 at 734 nm. Then, 2.2 mL of ABTS·+ were added to 0.3 ml of each sample. The mixture was left to react for 6 min in the dark, at room temperature. The antioxidant activity was measured through the UV–Vis spectrophotometer at 734 nm. A calibration curve was made using Trolox standard in ethanol, at concentrations between 0.94 and 100 mg/L (R2 = 0.9995) and the antioxidant activity was expressed as mg Trolox equivalents (TEs) per gram of dry sample. All analyses were carried out in triplicate.
The DPPH assay (2,2-diphenyl-1-picrylhydrazyl) was based on the study of Mensor et al. (2001) with slight modifications. Briefly, 1.25 mL of each sample were mixed with 0.5 mL of 0.3 mM DPPH ethanol solution. The mixture was left to react for 30 min at room temperature and in the dark. Then, the absorbance was measured at 518 nm by means of the spectrophotometer. Ethanol (0.5 mL) plus sample (1.25 mL) was used as blank. DPPH solution (0.5 mL; 0.3 mM) plus ethanol (1.25 mL) was used as the control. The percentage antioxidant activity (AA) was calculated using the following equation:
The antioxidant activity was expressed as mg Trolox equivalents (TEs) per gram of dry sample using a calibration curve built between 1.56 and 100 mg/L (R2 = 0.9989). All samples were analyzed in triplicate.
Statistical analysis
All experimental data were subjected to one-way analysis of variance (ANOVA). To the aim, a Fisher’s test with the option of homogeneous groups (P < 0.05) was carried out to determinate significance differences among samples. STATISTICA 7.1 for Windows (StatSoft Italia s.r.l.) was used.
Results and discussion
Identification of the most efficient extraction technique
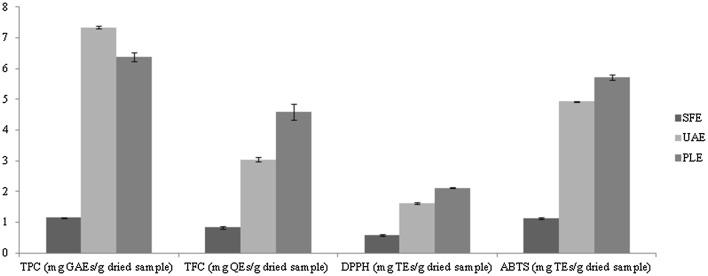
In order to identify an extraction method able to give an extract with high concentration of bioactive compounds, three techniques were compared. To the aim, total phenolic compounds (TPC), total flavonoid compounds (TFC) and antioxidant activity (DPPH and ABTS) of the extracts were measured. As can be seen in Fig. 1, the highest total phenolic content was obtained by UAE (7.33 ± 0.04 mg GAEs/g dw), followed by PLE (6.37 ± 0.15 mg GAEs/g dw) and SFE (1.15 ± 0.01 mg GAEs/g dw). This result is in accordance with other data reported in the literature. Nayak et al. (2015) also compared different non-conventional extraction techniques to recover bioactive compounds from Citrus sinensis peels and observed that UAE gave higher amount of total phenols compared to PLE. Also Liazid et al. (2010) noted higher yield of polyphenols by UAE than PLE. This behavior could be attributed to the cavitation phenomena generated by the ultrasonic waves (Gonzalez-Centeno et al. 2014). The PLE differed slightly from UAE, while SFE showed a much lower yield than the other two methods. This finding is most probably due to the fact that SFE is a more selective technique; its efficiency depends on solubility of compounds in the supercritical fluid and depends on the density of the fluid (Cheah et al. 2010). Furthermore, even if supercritical fluids have similar characteristics to liquids, these fluids are less able to break the bonds between matrix and analyte.
Fig. 1.
Total phenols, total flavonoids and antioxidant activity (DPPH and ABTS assays) of broccoli extract obtained by different extraction techniques (SFE, UAE and PLE). TPC total phenolic content, TFC total flavonoid content, DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2′-azino-bis(3-ethylben- zothiazoline-6-sulfonic acid), GAEs gallic acid equivalents, QEs quercetin equivalents, TEs Trolox equivalents
As far as the other parameters are concerned (TFC, DPPH and ABTS), the PLE technique represented the best extraction method. Specifically, high content of flavonoids (4.59 ± 0.27 mg QEs/g dw) and antioxidant activity (2.11 ± 0.02 and 5.72 ± 0.08 mg TEs/g dw, for DPPH and ABTS, respectively) were recorded. The high content of total flavonoids could be linked to the greater sensibility of these substances to the extraction time. Long time could cause their degradation. In fact, the PLE was shorter than the other two extraction techniques. The extraction process took about 20 min, 1 h and 140 min for PLE, UAE and SFE, respectively. This result was also confirmed by Casazza et al. (2010), who also compared several non-conventional extraction methods (UAE, MAE and HPTE) versus classic solid–liquid extraction in terms of extraction yield and antioxidant power of the extract and observed that prolonging the time over 30 min the yield of total flavonoids decreased. Furthermore, the PLE gave a better extract from the qualitative point of view, since it contained greater antioxidant activity compared to SFE and UAE extracts. It is known that the antioxidant activity depends more significantly from the quality of the phenolic compounds than from the quantity (Otero-Pareja et al. 2015). According to the obtained data, we can conclude that the PLE represented the most efficient extraction technique to recover bioactive compounds from broccoli by-products. It showed only a little difference in TPC compared to UAE, but according to Nayak et al. (2015) it could be due to interactions between phenolic and non-phenolic compounds during the extraction.
Extract microencapsulation
As Jafari et al. (2008) stated, the successful of the microencapsulation process of nutraceutical extracts relies on achieving the maximum retention of the core within the powdered material. Hence, for the optimization of the extract microencapsulation, different parameters were considered: wall materials, their concentrations, core/wall ratio and inlet temperature, making a total of 28 experiments. As Table 1 and Fig. 2 show, the microencapsulated extract was chemically characterized in terms of total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity by means DPPH and ABTS assays. The inlet temperature was fixed at 170 °C and the effects of the concentration of Capsul and maltodextrins on the bioactive compounds were investigated. As can be seen in Table 1, the properties of selected wall materials statistically affected the encapsulation efficiency of samples. Specifically, it was observed low retention of bioactive compounds by increasing the concentration of both wall materials from 10 to 30% (w/v), probably due to the increase in total solid content and consequently high viscosity of the infeed emulsion. Some authors also noted that there is an optimal level of total solid concentration (Jafari et al. 2008; Reineccius and Bangs 1985), so, if more wall material was added, the solution become over-saturated and therefore, the un-dissolved wall material does not have any protective effect, thus reducing the core compounds retention. Furthermore, increasing the solid amount, the emulsion viscosity increased. This finding improved the retention up to an optimal level, beyond which the retention decreased due to the long spray-drying process and the slow droplet formation (Jafari et al. 2008; Rosenberg et al. 1990). A more viscous emulsion generated larger drops. The larger the particle size, the lower the retention, because the formation time of droplets membrane was longer and increased the loss of bioactive substances. Another reason for the decrease of core compound retention with increasing the wall material concentration was the presence of agglomerations and cakes in the powder, as also noted by Sansone et al. (2011). These authors identified the cause of this behavior in the delay of a semi-permeable layer development. In agreement with results also recorded by Sansone et al. (2011), the less concentration of wall material (10%) was found the most appropriate to microencapsulate the extract. Maltodextrins and Capsul are encapsulating agents widely used in the food industry; however, even though Capsul has good retention, stability and emulsifying properties (Aburto et al. 1998), in Table 1 it can be observed that the preservation of bioactive compounds was significantly greater by using maltodextrins. According to Bakowska-Barczak and Kolodziejczyk (2011), maltodextrins are produced from the acid hydrolysis of corn starch, and are characterized by high water solubility and low viscosity. Most likely, thanks to these structural chemical differences, maltodextrins appeared to be a wall material that offers greater bioactive compounds protection than the Capsul. In previous studies it has been also confirmed the microencapsulation efficiency of maltodextrins. Sansone et al. (2011) for example studied the capacity of maltodextrin/pectin matrix to spray-dry polyphenol-rich extracts, and Silva et al. (2013) demonstrated the feasibility to microencapsulate jaboticaba extracts with 30% maltodextrins. As reported in the study of Wu et al. (2014) it was shown that maltodextrins could provide a good sulforaphane retention after spray-drying. Hence, maltodextrins have been found more suitable for microencapsulating our extract.
Table 1.
Chemical characterization of broccoli by-products powder obtained at different core/wall ratio and concentration of wall material
| TPC (mg GAEs/g dw) | TFC (mg QEs/g dw) | DPPH (mg TEs/g dw) | ABTS (mg TEs/g dw) | |
|---|---|---|---|---|
| 1:20 | ||||
| Capsul 10% | 2.10 ± 0.09c | 0a | 1.37 ± 0.04c | 4.99 ± 0.07f |
| MD 10% | 3.50 ± 0.07e | 2.69 ± 0.40c | 4.86 ± 0.06f | 4.23 ± 0.03e |
| Capsul 20% | 1.29 ± 0.05b | 0a | 1.07 ± 0.02b | 3.60 ± 0.10d |
| MD 20% | 2.51 ± 0.11d | 1.42 ± 0.31b | 4.50 ± 0.06e | 2.32 ± 0.03b |
| Capsul 30% | 0.86 ± 0.12a | 0a | 0.90 ± 0.04a | 2.99 ± 0.08c |
| MD 30% | 1.97 ± 0.10c | 0.54 ± 0.23a | 4.36 ± 0.04d | 1.67 ± 0.03a |
| 1:10 | ||||
| Capsul 10% | 4.56 ± 0.17e | 1.15 ± 0.23a | 2.13 ± 0.03a | 6.82 ± 0.15e |
| MD 10% | 5.93 ± 0.25f | 2.89 ± 0.20d | 5.26 ± 0.06f | 7.01 ± 0.07e |
| Capsul 20% | 2.54 ± 0.05b | 1.21 ± 0.12a | 2.51 ± 0.01b | 5.34 ± 0.09d |
| MD 20% | 3.35 ± 0.02d | 2.42 ± 0.23c | 4.82 ± 0.06e | 4.11 ± 0.11b |
| Capsul 30% | 1.81 ± 0.07a | 1.01 ± 0.12a | 2.26 ± 0.03c | 4.42 ± 0.11c |
| MD 30% | 2.90 ± 0.09c | 1.55 ± 0.51b | 4.59 ± 0.05d | 3.08 ± 0.11a |
| 1:5 | ||||
| Capsul 10% | 7.92 ± 0.10d | 4.23 ± 0.23c | 3.04 ± 0.04c | 9.76 ± 0.19d |
| MD 10% | 9.31 ± 0.22e | 5.71 ± 0.00d | 5.62 ± 0.06f | 10.50 ± 0.15e |
| Capsul 20% | 4.62 ± 0.04b | 3.16 ± 0.31b | 2.92 ± 0.01b | 7.91 ± 0.02c |
| MD 20% | 5.37 ± 0.07c | 3.49 ± 0.00b | 5.03 ± 0.02e | 6.67 ± 0.17b |
| Capsul 30% | 3.21 ± 0.02a | 2.09 ± 0.20a | 2.70 ± 0.02a | 6.45 ± 0.09b |
| MD 30% | 4.47 ± 0.02b | 1.68 ± 0.35a | 4.87 ± 0.03d | 5.10 ± 0.14a |
| 1:2 | ||||
| Capsul 10% | 13.97 ± 0.11e | 12.48 ± 0.31e | 5.66 ± 0.01e | 13.39 ± 0.08d |
| MD 10% | 16.04 ± 0.14f | 12.14 ± 0.20e | 5.68 ± 0.09e | 15.23 ± 0.20e |
| Capsul 20% | 8.53 ± 0.16c | 6.51 ± 0.35c | 6.54 ± 0.01b | 11.73 ± 0.14c |
| MD 20% | 10.40 ± 0.15d | 7.72 ± 0.20d | 5.56 ± 0.00d | 11.60 ± 0.12c |
| Capsul 30% | 6.10 ± 0.09a | 4.50 ± 0.40b | 3.25 ± 0.01a | 9.79 ± 0.06b |
| MD 30% | 7.33 ± 0.15b | 3.56 ± 0.12a | 5.30 ± 0.03c | 8.84 ± 0.19a |
MD maltodextrins 14–16 DE, TPC total phenolic content, TFC total flavonoid content, DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2′-azino-bis(3-ethylben- zothiazoline-6-sulfonic acid), GAEs gallic acid equivalents, QEs quercetin equivalents, TEs Trolox equivalents. a–fData in columns with different superscript are significantly different (P < 0.05)
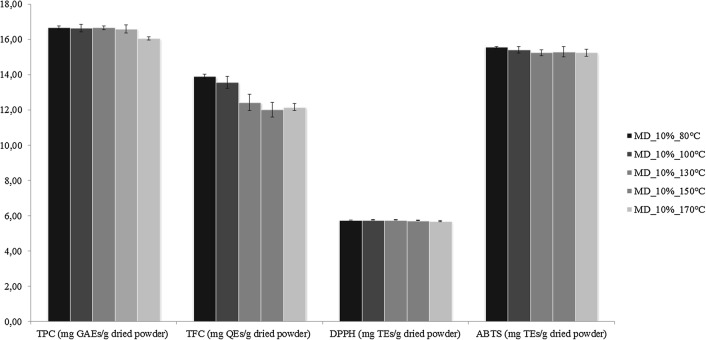
Fig. 2.
Chemical characterization of broccoli by-products powder obtained at different inlet- temperatures. TPC total phenolic content, TFC total flavonoid content, DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2′-azino-bis(3-ethylben- zothiazoline-6-sulfonic acid), GAEs gallic acid equivalents, QEs quercetin equivalents, TEs Trolox equivalents
Microencapsulation efficiency was not only affected by the type of wall material but also by the core/wall ratio, term commonly used to indicate the concentration of core material and that has great effect on the properties of final powder (Hogan et al. 2001). In general, there is an optimal and specific core-to-wall ratio, although in most of the previous studies core/wall ratio of 1:4 was adopted. As shown in Table 1, results indicate that increasing core/wall ratio from 1:20 to 1:2, TPC, TFC, DPPH and ABTS increased. Most probably, the reason may be associated with the instability of the prepared mixtures when the core/wall ratio decreased (Shu et al. 2006). Furthermore, this trend was confirmed by the findings of Goula and Adamopoulos (2011), who, developing a new technique for lycopene microencapsulation by spray-drying, noted that increasing core/wall from 1/19 to 1/3, the encapsulation efficiency increased. Therefore, the most advantageous core-to-wall ratio for microencapsulating broccoli by-products extracts was identified in 1/2. Finally, keeping core/wall ratio of 1/2 and using 10% maltodextrins as wall material, the effect of different inlet temperatures was also assessed. As can be seen in Fig. 2, when the inlet temperature increased from 80 to 170 °C, DPPH (5.68–5.75 mg TEs/g powder) and ABTS (15.23–15.55 mg TEs/g powder) remained almost unchanged. On the contrary, TPC (16.04–16.66 mg GAEs/g powder) and especially TFC (12.01–13.89 mg QEs/g powder) were more affected by the temperature. Several authors have argued that with high air inlet temperature, premature release and degradation of the encapsulated ingredients can occur (Cai and Corke 2000; Goula and Adamopoulos 2011; Silva et al. 2013; Tonon et al. 2011; Wu et al. 2014). Specifically, Zakarian and King (1982) reported that elevated temperature caused the formation of cracks in the membrane, inducing an excessive evaporation of pigments. Contrarily, lower temperatures produced powders with agglomerations that reduce the exposition to oxygen, defending the pigment against the oxidation (Quek et al. 2007). Based on the results obtained, 80 °C represented the most suitable inlet temperature for microcapsules production. Another experimental evidence recorded with microencapsulation is how these TPC, TFC, DPPH parameters are going to increase after microencapsulation as compared to broccoli extract. A very few information can be found in the scientific literature to justify this trend (Arana-Sánchez et al., 2010); however, an interesting explanation was suggested by Böger et al. (2018) who microencapsulated the extract from grape seed oil by spray drying. These authors also observed an increase of phenolic compounds and antioxidant activity when maltodextrins were adopted in the microencapsulation. They suggested that the unexpected increment recorded could be attributed to compositional changes during the microencapsulation process. The same authors also suggest that with high probability Maillard reaction products with antioxidant properties could take place during the thermal processing that occur in the spray drying.
In conclusion, the optimal conditions for the microencapsulation of broccoli by-product extract were established as follows: concentration of maltodextrins at 10%, ratio of core to wall material of 1/2 and inlet temperature of 80 °C. Applying these experimental conditions, the highest values of TPC (16.66 ± 0.11 mg GAEs/g powder), TFC (13.89 ± 0.12 mg QEs/g powder), DPPH (5.75 ± 0.01 mg TEs/g powder) and ABTS (15.55 ± 0.05 mg TEs/g powder) were found (Fig. 2).
Fish burgers enriched with microencapsulated extract
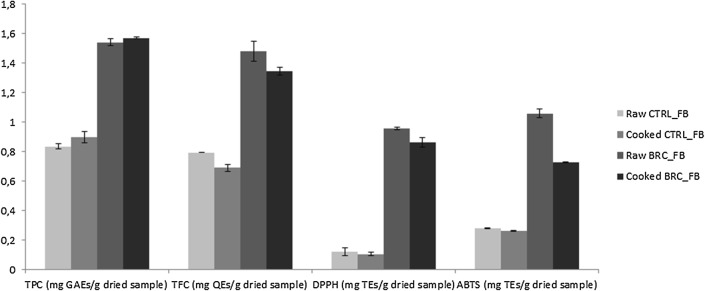
Figure 3 shows the total phenolic content, total flavonoid content and antioxidant activity of raw and cooked fish-burgers with and without extract. As expected, the incorporation of 5% microencapsulated extract significantly enhanced the concentration of bioactive compounds and the antioxidant activity when compared to the control (P < 0.05). After cooking the TFC and the antioxidant activity of the fortified samples were slightly reduced (raw BRC_FB presented 1.48 ± 0.07 mg QEs g−1, 0.96 ± 0.01 mg TEs g−1 for DPPH and 1.06 ± 0.03 mg TEs for ABTS while cooked BRC_FB 1.35 ± 0.03 mg QEs g−1, 0.86 ± 0.03 TEs g−1 for DPPH and 0.73 ± 0.00 TEs g−1 for ABTS) but no significant variations (P > 0.05) were found in terms of TPC concentrations in both raw and cooked samples (1.54 mg GAEs g−1 in the raw burger and 1.57 mg GAEs g−1 in the cooked sample), thus confirming the potential application of broccoli by-product as valid food ingredient.
Fig. 3.
Chemical characterization (TPC, TFC, DPPH and ABTS) of raw and cooked fish-burgers with and without broccoli powder at 5%. TPC total phenolic content, TFC total flavonoid content, DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2′-azino-bis(3-ethylben- zothiazoline-6-sulfonic acid), GAEs gallic acid equivalents, QEs quercetin equivalents, TEs Trolox equivalents, CTRL_FB control fish-burger, BRC_FB fish-burger enriched with broccoli powder
Conclusion
Three extraction methods (SFE, UAE, PLE) were compared in terms of extraction efficiency. Among them, PLE was found as best technique because gave the greatest content of bioactive compounds (6.37 ± 0.15 mg GAE/g dw and 4.59 ± 0.27 mg QE/g dw) and the highest antioxidant activity (2.11 ± 0.02 and 5.72 ± 0.08 mg TEs/g dw, for DPPH and ABTS, respectively) of the relative extract. In the second step, the spray-drying of the extract was optimized. For the investigated working conditions, the powder with high concentration of phenols, flavonoids and antioxidant activity was obtained using maltodextrins at 10% as wall material and keeping core/wall ratio of 1/2 at 80 °C as inlet temperature. Finally, the obtained powder was used as food ingredient and added at 5% (w/w) in fish burgers to increase the nutritional value. The obtained data showed that, even if cooking slight affected the concentration of bioactive compounds and the antioxidant activity, relevant differences were recorded between control and fortified fish burgers. Therefore, broccoli by-products extracts can be considered promising source to design new foods with interesting healthy properties.
Compliance with ethical standards
Conflict of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aburto LC, Tavares DQ, Martucci ET. Microencapsulação de óleo essencial de laranja. Food Sci Technol (Campinas) 1998;18:45–48. doi: 10.1590/S0101-20611998000100010. [DOI] [Google Scholar]
- Arana-Sánchez A, Estarrón-Espinosa M, Obledo-Vázquez EN, Padilla-Camberos E, Silva-Vázquez R, Lugo-Cervantes E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated in β-cyclodextrin. Lett Appl Microbiol. 2010;50:585–590. doi: 10.1111/j.1472-765X.2010.02837. [DOI] [PubMed] [Google Scholar]
- Ares AM, Bernal J, Nozal MJ, Turner C, Plaza M. Fast determination of intact glucosinolates in broccoli leaf by pressurized liquid extraction and ultra high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res Int. 2015;76:498–505. doi: 10.1016/j.foodres.2015.06.037. [DOI] [PubMed] [Google Scholar]
- Arnaiz E, Bernal J, Martin MT, Nozal MJ, Bernal JL, Toribio L. Supercritical fluid extraction of free amino acids from broccoli leaves. J Chromatogr A. 2012;1250:49–53. doi: 10.1016/j.chroma.2012.04.066. [DOI] [PubMed] [Google Scholar]
- Arnáiz E, Bernal J, Martín MT, Diego JC, Bernal JL, Recio LT. Optimisation of the supercritical fluid extraction of antioxidants from broccoli leaves. Food Anal Methods. 2016;9:2174–2181. doi: 10.1007/s12161-016-0399-4. [DOI] [Google Scholar]
- Bakowska-Barczak AM, Kolodziejczyk PP. Black currant polyphenols: their storage stability and microencapsulation. Ind Crops Prod. 2011;34:1301–1309. doi: 10.1016/j.indcrop.2010.10.002. [DOI] [Google Scholar]
- Biney K, Beta T. Phenolic profile and carbohydrate digestibility of durum spaghetti enriched with buckwheat flour and bran. LWT Food Sci Technol. 2014;57:569–579. doi: 10.1016/j.lwt.2014.02.033. [DOI] [Google Scholar]
- Böger BR, Geogetti SR, Koruzawa LE. Microencapsulation of grape seed oil by spray drying. Food Sci Technol. 2018;38:263–270. doi: 10.1590/fst.04417. [DOI] [Google Scholar]
- Cai YZ, Corke H. Production and properties of spray-dried Amaranthus Betacyanin pigments. J Food Sci. 2000;65:1248–1252. doi: 10.1111/j.1365-2621.2000.tb10273.x. [DOI] [Google Scholar]
- Casazza AA, Aliakbarian B, Mantegna S, Cravotto G, Perego P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J Food Eng. 2010;100:50–55. doi: 10.1016/j.jfoodeng.2010.03.026. [DOI] [Google Scholar]
- Cheah ELC, Heng PWS, Chan LW. Optimization of supercritical fluid extraction and pressurized liquid extraction of active principles from Magnolia officinalis using the Taguchi design. Sep Purif Technol. 2010;71:293–301. doi: 10.1016/j.seppur.2009.12.009. [DOI] [Google Scholar]
- Djilas S, Čanadanović-Brunet J, Ćetković G. By-products of fruits processing as a source of phytochemicals. Chem Ind Chem Eng Q. 2009;15:191–202. doi: 10.2298/CICEQ0904191D. [DOI] [Google Scholar]
- Domínguez-Perles R, Martínez-Ballesta MC, Carvajal M, García-Viguera C, Moreno DA. Broccoli-derived by-products—a promising source of bioactive. J Food Sci. 2010;75:C383–C392. doi: 10.1111/j.1750-3841.2010.01606.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Centeno MR, Knoerzer K, Sabarez H, Simal S, Rossello C, Femenia A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—a response surface approach. Ultrason Sonochem. 2014;21:2176–2184. doi: 10.1016/j.ultsonch.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Goula AM, Adamopoulos KG (2011) Optimization of lycopene microencapsulation by spray drying. In: Paper presented at the ICEF 11-international congress on engineering and food, Athens, Greece
- Herrero M, Cifuentes A, Ibánez E. Sub- and supercritical fluid extraction offunctional ingredients from different natural sources: plants, food-by-products, algae and microalgae—a review. Food Chem. 2006;98:136–148. doi: 10.1016/j.foodchem.2005.05.058. [DOI] [Google Scholar]
- Herrero M, Mendiola JA, Cifuentes A, Ibáñez E. Supercritical fluid extraction: recent advances and applications. J Chromatogr A. 2010;1217:2495–2511. doi: 10.1016/j.chroma.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Hogan SA, McNamee BF, O’Riordan ED, O’Sullivan M. Microencapsulating properties of sodium caseinate. J Agric Food Chem. 2001;49:1934–1938. doi: 10.1021/jf000276q. [DOI] [PubMed] [Google Scholar]
- Jafari SM, Assadpoor E, He Y, Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Dry Technol. 2008;26:816–835. doi: 10.1080/07373930802135972. [DOI] [Google Scholar]
- Liazid A, et al. Evaluation of various extraction techniques for obtaining bioactive extracts from pine seeds. Food Bioprod Process. 2010;88:247–252. doi: 10.1016/j.fbp.2009.11.004. [DOI] [Google Scholar]
- Marinelli V, Padalino L, Nardiello D, Del Nobile MA, Conte A. New approach to enrich pasta with polyphenols from grape marc. J Chem. 2015;2015:8. doi: 10.1155/2015/734578. [DOI] [Google Scholar]
- Maróstica Junior MR, Leite AV, Romanelli V, Dragano N. Supercritical fluid extraction and stabilization of phenolic compounds from natural sources—review (supercritical extraction and stabilization of phenolic compounds) Open Chem Eng J. 2010;4:51–60. [Google Scholar]
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG. Screening of brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res PTR. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Mustafa A, Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Nayak B, Dahmoune F, Moussi K, Remini H, Dairi S, Aoun O, Khodir M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015;187:507–516. doi: 10.1016/j.foodchem.2015.04.081. [DOI] [PubMed] [Google Scholar]
- O’Shea N, Arendt EK, Gallagher E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov Food Sci Emerg Technol. 2012;16:1–10. doi: 10.1016/j.ifset.2012.06.002. [DOI] [Google Scholar]
- Otero-Pareja MJ, Casas L, Fernandez-Ponce MT, Mantell C, Martinez de la Ossa EJ. Green extraction of antioxidants from different varieties of red grape pomace. Molecules (Basel, Switzerland) 2015;20:9686–9702. doi: 10.3390/molecules20069686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek SY, Chok NK, Swedlund P. The physicochemical properties of spray-dried watermelon powders. Chem Eng Process. 2007;46:386–392. doi: 10.1016/j.cep.2006.06.020. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reineccius GA, Bangs WE. Spray drying of food flavors. III. Optimum infeed concentrations for the retention of artificial flavors. Perfum Flavorist. 1985;9(2):27–29. [Google Scholar]
- Rosenberg M, Kopelman IJ, Talmon Y. Factors affecting retention in spray-drying microencapsulation of volatile materials. J Agric Food Chem. 1990;38:1288–1294. doi: 10.1021/jf00095a030. [DOI] [Google Scholar]
- Sansone F, Mencherini T, Picerno P, d’Amore M, Aquino RP, Lauro MR. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J Food Eng. 2011;105:468–476. doi: 10.1016/j.jfoodeng.2011.03.004. [DOI] [Google Scholar]
- Shu B, Yu W, Zhao Y, Liu X. Study on microencapsulation of lycopene by spray-drying. J Food Eng. 2006;76:664–669. doi: 10.1016/j.jfoodeng.2005.05.062. [DOI] [Google Scholar]
- Silva PI, Stringheta PC, Teófilo RF, de Oliveira IRN. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J Food Eng. 2013;117:538–544. doi: 10.1016/j.jfoodeng.2012.08.039. [DOI] [Google Scholar]
- Spinelli S, Conte A, Lecce L, Padalino L, Del Nobile MA. Supercritical carbon dioxide extraction of brewer’s spent grain. J Supercrit Fluids. 2016;107:69–74. doi: 10.1016/j.supflu.2015.08.017. [DOI] [Google Scholar]
- Tao Y, Zhang Z, Sun DW. Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc: influence of acoustic energy density and temperature. Ultrason Sonochem. 2014;21:1461–1469. doi: 10.1016/j.ultsonch.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Tonon RV, Freitas SS, Hubinger MD. Spray drying of açai (Euterpe Oleraceae mart.) juice: effect of inlet air temperature and type of carrier agent. J Food Process Preserv. 2011;35:691–700. doi: 10.1111/j.1745-4549.2011.00518.x. [DOI] [Google Scholar]
- Wu Y, Zou L, Mao J, Huang J, Liu S. Stability and encapsulation efficiency of sulforaphane microencapsulated by spray drying. Carbohyd Polym. 2014;102:497–503. doi: 10.1016/j.carbpol.2013.11.057. [DOI] [PubMed] [Google Scholar]
- Zakarian JA, King CJ. Volatiles loss in the nozzle zone during spray drying of emulsions. Ind Eng Chem Process Des Dev. 1982;21:107–113. doi: 10.1021/i200016a019. [DOI] [Google Scholar]