Abstract
Kinnow is a prevalent fruit crop of the mandarin group and belongs to the Rutaceae family. It is nutritionally rich in vitamin C, vitamin B, β-carotene, calcium, phosphorous and other health beneficing compounds. The fruit is grown commercially for fresh consumption and since the processing techniques are less prominent, a plentiful amount of harvested fruit goes for waxing and grading operations. To reduce the post-harvest losses, appropriate processing techniques need to be followed as considerable fruit waste is generated while processing. The foremost fruit wastes viz. peel and seeds are rich source of bioactive compounds and can be utilized for the extraction of aromatic compounds, essential oils and low-methoxyl pectin. Overall utilization of kinnow and its components through various technological interventions will not only enhance the profitability of processing industries but also assist in reducing the pollution load on the environment. The prevailing bitterness in kinnow juice has constrained its processing, value-addition, popularity and acceptability. Limited work has been done on kinnow processing leaving scarce relevant literature published on the post-harvest management. Efforts made by researchers worldwide, regarding the post-harvest application of kinnow and its by-products for product development, value addition and waste utilization is presented and discussed in this paper. This compiled information is envisioned to encourage the cottage food processing units in order to improvise the overall benefits along with achieving complete utilization of kinnow.
Keywords: Bitterness, By-product utilization, Citrus reticulate L., Kinnow, Post-harvest processing, Rutaceae, Value addition
Introduction
Citrus cultivation is a profitable venture and it contributes substantially in the economy of countries like USA, Brazil, Mexico, China, Iran, India, Spain and Greece. India is the 5th largest producer of citrus fruits worldwide, which includes the production of mandarins, grapefruit, lime, lemons, oranges, tangerines, etc. In the country, 10% of the total area among fruit crops has been occupied by citrus fruits and the production stands as 3rd following mango and banana. Production of the orange group including mandarin and kinnow in India was about 4.75 million tonnes obtained from 0.43 million hectares area (Mahawar et al. 2019). Citrus fruit group including lime/lemon, mandarin/sweet orange, kinnow are mainly grown with the objective of juice production and its processing. However, in that process, more than 40 million tons (mT) per annum of citrus wastes are produced by the industries indulged in juice processing which accounts for 50% of the original whole fruit mass (Sharma et al. 2017). These wastes viz. peels, seeds and pomace are considered to be a potential bio-resource material for several purposes. Processing of citrus wastes into products like pectin, flavonoids, fibers, animal feed, biofuel can not only reduce the quantum of the waste but also can elevate the prosperity of citrus industry (Mamma and Christakopoulos 2014).
Kinnow comes in ‘Mandarin’ group of citrus fruits and is grown on a wide scale in India and Pakistan. Kinnow was initially developed at the University of California Citrus Experiment Station in the year 1935 and was introduced in India during the early 1940s. It is a hybrid of two citrus cultivars namely ‘King’ (Citrus nobilis) and ‘Willow Leaf’ mandarin (Citrus deliciosa). In India, it is grown in parts of Punjab, Rajasthan, Himachal Pradesh, Haryana, Uttarakhand and Jammu & Kashmir with an overall production area of 0.33 million ha and production of 3.43 million tonnes (mT). The prevailing climatic conditions during winter helps for this ample production in these states which further helps in enhancing the sweetness index along with distinct taste. Punjab is India’s leading producer of kinnow with 29% of total national production (annual production of 1.1 mT from 0.048 mha area). Fazilka district of Punjab covers 55% area of cultivation and contributes 58% of total production.
Citrus fruits have a relatively longer post-harvest life in comparison with other tropical and subtropical fruits. Proper storage of citrus fruits for an extended period is very essential for their proper utilization in the glut season (Sonkar et al. 2008). Kinnow is highly cherished as fresh and in processed form as well due to its quality attributes, particularly its tangy taste. While at its peak production during winter, it is processed into juices by the industry and fruit vendors (Rafiq et al. 2018). The fruit is abundantly rich in vitamin C, vitamin B, β-carotene, calcium and phosphorous. Its startling colour, distinguishing flavour and nutritional content create an impulse for beverages preparation (Sogi and Singh 2001). Despite its commercial prominence and nutritional benefits, the availability of fruit during off-season is very limited due to its poor shelf life of 8–10 days.
The crucial post-harvest problem associated with kinnow is lack of proper marketing channels and poor post-harvest management practices. Unlike other citrus fruits, kinnow has poor keeping quality and cannot be transported in gunny bags. Simultaneous and cumulative production from various orchards often resulted in massive truckloads and upon sorting only, good quality fruit goes for fresh consumption leaving the low-grade fruits as a waste. Moreover, premature fruit drop and lack of on-farm processing facilities in the production catchments adds more to the existing fruit waste. The estimated pre-harvest drop of about 10–20% due to the decay and insect rearing ground also causes worries for farmers. With growing demand and consumption of kinnow, the magnitude of waste is proportionally escalating and generates ecological inconvenience. Discarding the fruit components without effective disposal is hazardous to the environment due to its unpleasant and unhygienic nature (Sharma et al. 2017).
Quantum of wastes resulted from processing (constituting 50% weight of fresh fruit) disturbs the total economy of the processes and hence demands urgent attention (Puri et al. 2011). Substantial amount of by-products comprising of peel (30–40%), pomace (juice sac residue), rag (membranes and cores), and seeds are expelled during citrus juice processing (Oberoi et al. 2011). It is a massive nutrient rich waste in the aggregated state. To counteract with the generation of this significant amount of waste, few indigenous technologies are operational in India for commercial production of kinnow juice and other value-added products. Through this review, we intended to provide a broad view of the available information regarding the scientific efforts made for value addition/processing of kinnow, likely ways to minimize the processing waste and future prospects of overall utilization of kinnow.
Physiology, maturity traits and physico-chemical properties
The shape of kinnow fruit is oblate with apex and base flattened, colour being deep orange-yellow. The fruit has two major components i.e. pericarp (peel) and endocarp, with the pericarp branching into epicarp (flavedo) and mesocarp (albedo). It is a mid-season variety, with few seeds adherent to 9–10 segments, smooth peel develops deep orange color on ripening, flavored juice (Ladaniya 2008). Different components of kinnow have been depicted in Fig. 1a. When the total soluble solids (TSS)/acid ratio reaches 12:1–14:1 and external colour turns orange, the fruit is considered to attain its full maturity stage.
Fig. 1.
a Pictorial representation of various components of Kinnow (Source: this study). b Pictorial representation of Kinnow orchard and harvesting using secateurs (Source: Anonymous 2019)
The peak harvesting season of kinnow in different states varies from November (Jammu & Kashmir), January (Himachal Pradesh), December–January (Haryana), January (Rajasthan) and January–February (Punjab), respectively (Anonymous 2018). The quality indices of kinnow for attaining optimal maturity are summarized below (Singh et al. 2005):
- Colour:
Golden yellow (peel), deep yellowish orange (pulp)
- TSS/acid ratio:
12:1–14:1
- Size:
5.0–9.7 cm
- Shape:
Moderate to oblate; both base and apex flattened or slightly depressed
- Appearance:
Very smooth, glossy and sometime faintly pitted
- Firmness:
Firm, not soft and easily peel able
The physical properties of any particular crop are of paramount importance in order to develop processing related machinery. Such physical properties were determined for a variety of citrus fruits by several researchers. The relevant information of kinnow in terms of physical, textural and chemical properties has been presented in Table 1.
Table 1.
Physical, textural and chemical properties of kinnow fruit (Source: this study)
| S. no. | Parameters | Value |
|---|---|---|
| Physical properties | ||
| 1. | Fruit weight (g) | 177.62 ± 21.10 |
| 2. | Fruit volume (ml) | 218.40 ± 33.70 |
| 3. | Major intercept (mm) | 60.00 ± 3.60 |
| 4. | Minor intercept (mm) | 74.00 ± 4.60 |
| 5. | Intermediate intercept (mm) | 73.01 ± 4.13 |
| 6. | Circumference (mm) | 242.12 ± 16.90 |
| 7. | Surface area (mm2) | 16,597.38 ± 1070.07 |
| 8. | Projected area based on length (cm2) | 56.44 ± 3.71 |
| 9. | Projected area based on width (cm2) | 43.48 ± 2.55 |
| 10. | Projected area based on height (cm2) | 43.70 ± 1.61 |
| 11. | Bulk density (g/ml) | 0.82 ± 0.09 |
| 12. | No. of vesicles | 10.60 ± 4.35 |
| 13. | Weight of flavedo (g) | 51.30 ± 21.45 |
| 14. | Weight of peeled fruit (g) | 124.20 ± 52.90 |
| 15. | Weight of pomace (g) | 44.40 ± 19.30 |
| 16. | Weight of juice (g) | 68.70 ± 29.60 |
| 17. | Thickness of peel (mm) | 4.01 ± 1.72 |
| 18. | No. of seeds | 19.40 ± 9.28 |
| 19. | Peel (%) | 29.00 ± 3.01 |
| 20. | Juice (%) | 38.00 ± 6.12 |
| 21. | Pomace (%) | 25.00 ± 4.01 |
| 22. | MC of peel (% d.b.) | 73.36 ± 30.12 |
| Textural properties of fruit | ||
| 23. | Bioyield point (kgf) | 14.16 ± 0.74 |
| 24. | Rupture (kgf | 13.73 ± 0.42 |
| 25. | Firmness (kgf) | 5.86 ± 0.29 |
| Properties of juice | ||
| 26. | TSS (°B) | 11.50 |
| 27. | Acidity (%) | 0.90 |
| 28. | TSS/acid ratio | 12:1 |
| 29. | Vitamin C (mg/100 ml juice) | 23.50 |
| 30. | Calcium (mg/100 ml) | 40.0 |
| 31. | Phosphorus (mg/100 ml) | 18.0 |
| 32. | Iron (mg/100 ml) | 0.40 |
| 33. | Turbidity (NTU) | 227.60 ± 9.30 |
Harvesting and post-harvest losses
Kinnow is generally harvested by secateurs/clipper (Fig. 1b), followed by dropping on the ground and collection in crates/bags which ultimately accounts for considerable post-harvest loss. Damaged fruits during harvest are neither suitable for marketing nor for consumption. Postharvest losses of kinnow were highest at farm level (32.4%) followed by picking (19.6%), packaging (3.5%), carrying (2.2%), loading and transportation (7.1%) (Ahmad et al. 2015). According to Singh et al. (2016), the combined harvest and post-harvest losses in kinnow are to the tune of 25–30%. However, this quantum of losses can be effectively minimized with appropriate processing of kinnow into different value-added products as described in the later part of this article.
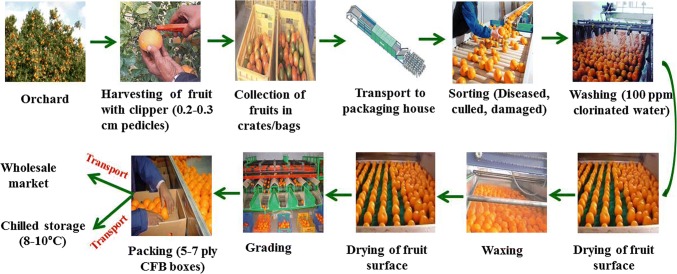
Packhouse operations
After harvesting, the general pack house operations involved in handling of kinnow includes (a) collection (b) pre-sorting (c) washing (d) grading (e) waxing (f) postharvest treatments (g) packaging. Packaging of the fruits is done manually in corrugated fiberboard CFB boxes with an approximate capacity of 10 kg each, however this can be changed as per the requirement. The series of operations has been depicted by the means of a schematic diagram (Fig. 2).
Fig. 2.
Schematic diagram of post-harvest pack house operations of Kinnow (Source: Bibwe et al. 2018)
Waxing and grading
Waxing is a very important unit operation followed on a wide scale for kinnow in the production catchments. As such, kinnow has a very short shelf life of 8–10 days at the ambient condition which can be extended to about 20 days if the fruit is properly waxed or wrapped. Waxing helps in reduction of moisture loss, provides the barrier for gas exchange, restore shiny appearance and provide support for preserving agents. For the said purpose of waxing, natural waxes like beeswax, carnauba wax, candelilla wax, wood rosin, and shellac can be used. Fungicides are usually added to the wax or wax emulsion. Waxing in combination with some chemicals viz. sesame oil, captafol, potassium permanganate, and mustard oil helped in improving the shelf life and reducing the physiological loss in weight (PLW) of kinnow (Sharma et al. 1991). Waxol™ is a commercial paraffin wax emulsion and its 12% concentration helped in the retention of highest quality kinnow for 35 days (Singh et al. 1973).
Particularly for kinnow, both unit operations i.e. waxing and grading are performed simultaneously for effective domestic and export marketing. This can be achieved either manually or with size/weight based graders. As per the grading standards suggested by Directorate of Marketing and Inspection, Government of India, kinnow can be graded into six grades based on fruit diameter viz, A: 60–64 mm, B: 65–69 mm, C: 70–72 mm, D: 72–74 mm, E: 75–79 mm and F: 80–85 mm, respectively (Dhatt and Mahajan 2007).
Packaging material and shelf life
Kinnow being one among the major citrus fruits in India, extensive studies have been carried out by researchers to examine the consequence of different chemical treatment, packaging material and storage conditions on its shelf life. Despite the research outcomes on packaging, efforts are still required in a direction to upgrade the existing packaging technique. It may be regarding the development of suitable coating material which can also be used for packaging in orchard itself. This will not only reduce the transportation cost and time associated to waxing/grading but will also provide direct benefit to the farmers. The promotion of suitable packaging technique with optimized storage conditions can enhance the seasonal availability in domestic/export market. The comprehensive information regarding the effect of packaging/coating on the shelf life of kinnow is tabulated in Table 2.
Table 2.
Packaging studies and treatments applied for shelf life extension of kinnow
| Packaging type/material | Treatment applied | Storage conditions | Shelf life (days) | References |
|---|---|---|---|---|
| 150-gauge polyethylene bags | 0.1% Bavistin solution | Evaporative cool chambers | 70 days | Thakur et al. (2002) |
| Ambient condition | 56 days | |||
| Cling film wrapped in combination with curing and coating | Neem oil (6%), til oil (4%), mustard oil (8%), wax (2.5%), carbendazim (1000 ppm) | Ambient storage | 84 days | Sonkar et al. (2009) |
| Cryovac heat shrinkable opti 200 (15 µ) and cling film (15 µ) | – | 18–20 °C, 80–85% RH | 20 days | Mahajan and Singh (2014) |
| – | Natural coating and synthetic coating | 5 ± 2 °C, 85–90% RH | 63 days | Ali et al. (2015) |
| – | Carboxy methyl cellulose and guar gum based coatings containing silver nanoparticles | 4 °C, 85–90% RH | 4 months | Shah et al. (2015) |
| 10 °C, 85–90% RH | 2 months |
Processing and value addition
Significant amount of pre-harvest losses, limited shelf life at ambient condition, poor postharvest management practices urged the farmers to explore various aspects of kinnow processing. Appropriate processing may help in minimizing the market glut and also extend its availability period. The desired characteristics for processing of kinnow include, fruit being slightly soft to firm, deep orange to red in colour, smooth-skinned with no deep grooves. From the processing perspective, the fruit is mainly processed in the form of juice related products like squash, nectar, ready to serve (RTS), fermented products, juice powder, etc. (Figure 3a). Such types of citrus beverages are possibly the most high-flying and unanimously recognized fruit drinks (Alam et al. 2019). Significant population around the globe prefers haze-less and sweet taste of the citrus juices. However, it is well-established fact that kinnow juice undergoes the process of delayed bitterness due to the existence and activation of bitter flavanone molecules during processing. This has significantly affected its consumer acceptability and processing on commercial scale. Therefore to overcome this hindrance, debittering of juice by various approaches as well as blending with suitable fruit/vegetable juices for making nutritive RTS beverages was considered as a convenient and economical alternative for effective utilization (Bhardwaj and Mukherjee 2011). Detailed narration about the prospects of juice bitterness and the efforts executed to reduce bitterness are briefly explained in the following section.
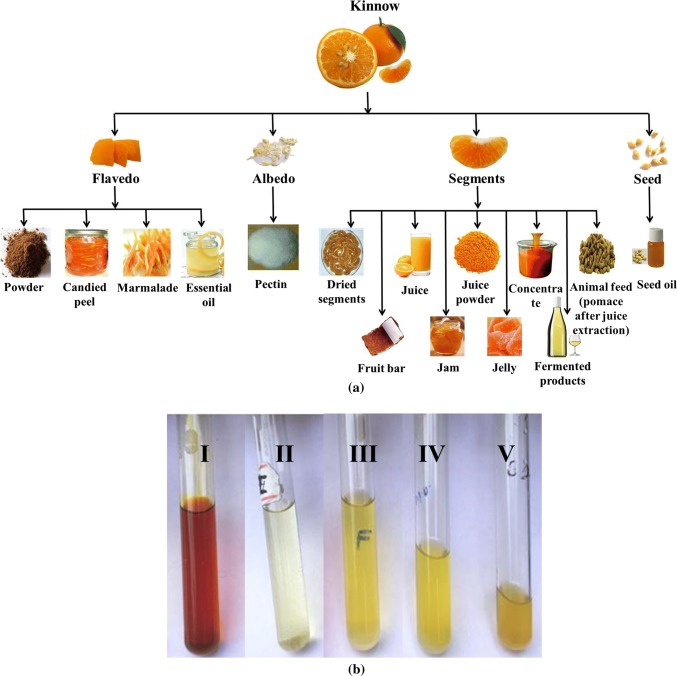
Fig. 3.
a Pictorial representation of various components of kinnow along with their utilization (Source: this study). b Essential oil obtained from kinnow peel using different extraction methods (Source: Sharma 2016)
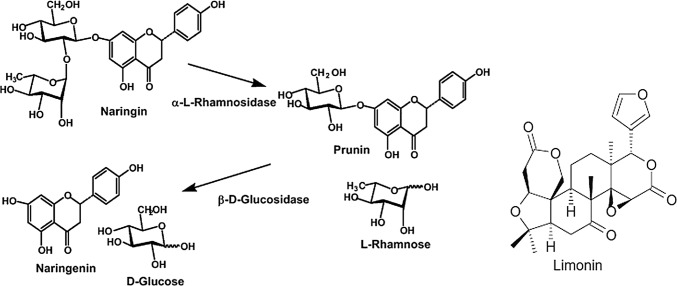
Debittering technology
The principal bitterness causing compounds (Naringin and Limonin) are present in peel and seed. Delayed bitterness is caused by the development of limonin. An enzyme (Limonoate-D-ring lactone hydrolase mainly present in seeds), catalyses the conversion of limonoate A-ring lactone (a non-bitter precursor) to bitter limonin in acidic condition of juice, resulting in delayed bitterness of juice after 3–4 h of extraction. The level of bitterness causing compounds varies in fruit components viz. peel and seeds. Premi et al. (1994) reported the highest Naringin content in the peel (0.422 mg/g), followed by the juice (0.230 mg/g) and seed (0.134 mg/g), whereas the limonin content was highest in the seed (9.50 mg/g), followed by the peel (4.69 mg/g) and juice (0.218 mg/g). Ilame and Singh (2018) adjudged the hollow fiber membrane with molecular weight 30 kDa best for improving the storage span of ultra-filtered kinnow juice to 60 days without any supplementation of preservative. This indicated that if seeds and peel (albedo + flavedo) are removed manually before juice extraction, bitterness level can be minimized due to a reduction in tissue disruption.
Sincere attempts have been made worldwide in order to reduce/remove the existing bitterness in kinnow juice. To reduce bitterness, a number of compounds have been identified which can mask the bitterness through their sweetening index e.g. naringin dihydrochalcone and neohesiperidin dihydrochalcone. Debittering can be achieved by absorption of the bitter substances on vinyl-dodecylbenzene resins. Singh et al. (2009) in their study allowed bittered kinnow juice to pass through layers of polymeric adsorbent resin and observed superior sensory characteristics throughout the storage. Naringin content in the juice can be controlled by immobilization of naringinase using 1% glutaraldehyde coated hen egg white and the bitterness can be reduced to 68% (Puri et al. 2011). Kaur (2017b) undertook a study for enzymatic debittering and aroma enhancement of kinnow juice using limonin dehydrogenase, naringinase and β-glucosidase enzymes. Maximum reduction in bitter component limonin 87.34%, naringin 58.41%, whereas an increase in glucose up to 4.38 µg/ml, acidity 30.13% and total sugar content 42.97 µg/ml were observed.
Pulp
Khalid et al. (2013) reported that kinnow pulp constitutes of juice (51–54%) with TSS (9–10.3°B), acidity (0.55–1.0%), ascorbic acid (41–53 mg/100 ml), total sugars (7.2–7.5%), total phenolic content (852–1059 ppm), antioxidant activity (65–73% DPPH inhibition), calcium (0.75 mg/100 ml) and iron (0.338 mg/100 ml), respectively. Aggarwal and Michael (2014) studied the properties of kinnow pulp and reported the corresponding values of moisture content (87.1%), TSS (10.8°B), acidity (0.69%), ascorbic acid (18.5 mg/100 ml), total sugars (4.4%), reducing sugars (2.9%), limonin (25 ppm), ash (0.46%), L value (lightness, 50.2), a value (redness and greenness, 16.0) and b value (yellowness and blueness, 38.4), respectively.
Juice
The juice content of kinnow fruit generally varies due to numerous factors such as variety, climate, cultural practices, etc. On a small scale, the juice is usually extracted by hand reaming where the fruits are halved in two parts and juice is extracted by reaming the halves on a suitable rosette. The rosette is usually conical in shape and has ribbed or grooved sides. This method of juice extraction is best, as it does not break the oil cells of the peel nor crush the seeds. On the contrary, mechanical juice extractors are also available for juice extraction where sometimes, the peel is removed manually and peeled kinnow is fed into the screw type juice extractor to extract the juice. Juice yield of around 40% by manual method and yield of around 60% can be obtained by mechanical juice extraction method as reported by various researchers (Sharma 2009). As per the reports of Bhardwaj and Mukherjee (2011), kinnow juice contains TSS (11.50°B), acidity (0.76%), ascorbic acid (21.15 mg/100 ml of juice), total sugars (7.50%), limonin (0.22 mg/ml of juice) and non-enzymatic browning (0.08). As already described, kinnow juice in pure form is not marketable and hence efforts were carried out employing kinnow as a substrate for the formulation of blended juice and other value-added products. This information is compiled and presented hereunder:
Blended juice
Blended juices prepared from the concentrates of two or more varieties are available in the market. Kinnow juice is blended with less acidic and less bitter citrus juice. During recent years, the concept of juice blending was trending and several reports are available in that similar line. The concise information has been compiled in Table 3.
Table 3.
Blending of kinnow juice with other juices and their properties
| Ratio of blended juice and storage conditions | TSS (°B) | Acidity (%) | AA (mg/100 g) | Total sugars (%) | Microbial population (cfu/ml juice) | Sensory qualities | Other quality parameters | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial | Yeast | Mould | (9 point hedonic scale) | L, TC, TP | ||||||
| Kinnow: aonla: ginger (92:5:3), 6 months, ambient condition (28 ± 4 °C) | 12.00 (14.50) | 0.80 (0.53) | 45.30 (38.95) | 7.44 (11.11) | 7.6 × 103 (4.0 × 103) | 3.4 × 103 (1.5 × 103) | 2.9 × 103 (2.1 × 103) | Fl: 7.50 (5.25), C: 8.20 (5.45), B:8.35 (5.83), OA: NR | L: 0.14 (0.30) | Bhardwaj and Mukherjee (2011) |
| Kinnow nectar 6 months, ambient condition | 15.00 (NR) | NR | 2.50 (0.41) | 10.87 (NR) | NR | NR | NR | Sensory qualities: NR | TC: 0.06 (0.02), TP: 6.67 (6.00) | Shubhra et al. (2014) |
| Kinnow nectar with blanched aloe juice (4%), 6 months, ambient condition | 15.00 (NR) | NR | 2.52 (0.50) | 10.91 (NR) | NR | NR | NR | Sensory qualities: NR | TC: 0.06 (0.03), TP: 6.85 (6.20) | |
| Kinnow nectar with unbalanced aloe juice (4%), 6 months, ambient condition | 15.00 (NR) | NR | 2.53 (0.52) | 10.93 (NR) | NR | NR | NR | Sensory qualities: NR |
TC: 0.07 (0.02) TP: 6.90 (6.24) |
|
| Carrot: kinnow (1:1) 3 months, refrigerated condition | 15.40 (16.30) | 0.46 (0.59) | 26.66 (26.54) | 21.75 (21.76) | NR | NR | NR | Fl: 8.40 (7.60), C: 8.60 (7.90), B: NR, OA: 8.50 (7.60) | NR | Ullah and Qazi (2015) |
| Kinnow: pomegranate: ginger: (89:10:1) + Lactobacillus acidophilus (10% of juice volume), 7 weeks, refrigerated condition (4 ± 1 °C) | 15.60 (14.30) | 0.76 (0.85) | 23.80 (20.40) | 6.52 (6.42) | ND (1.01) | ND (0.33) | NR | Fl: 8.60 (NR), C: 8.40 (NR), B: NR, OA: 8.30 (NR) | NR | Kaur (2017a) |
| Kinnow + aonla + aloe vera gel (5:70:25), 4 months, ambient condition | 50.03 (50.87) | 1.01 (1.86) | 71.87 (60.24) | 46.12 (49.43) | NR | NR | NR | OA: 8.33 (7.00) | NR | Balaji and Sikarwar (2018) |
Values in the parenthesis indicated after 6 month of storage at ambient storage
AA ascorbic acid (mg/100 g), Fl flavour, C colour, B bitterness, OA overall acceptability, L limonin (mg/ml juice), TC total carotenoids (mg/100 g), total phenols (mg/100 g), NR not reported
Fermented products
In addition to blended juices, efforts directed towards fermentation of kinnow juice/blended juice/peel/powder/paste are also reported in the literature. The process of fermentation was found to cause an increase in flavanone and carotenoid content while maintaining the levels of ascorbic acid. The fermented products contain health-related advantages and attempts made by researchers for their preparation are enlisted in Table 4.
Table 4.
Fermented products prepared using kinnow and its components as a substrate
| Raw material | Inoculum | Fermentation period | Fermentation pH | Fermentation temperature | Ethanol quantity | TSS (°Brix) | pH | Acidity (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| Kinnow juice | Saccharomyces cerevisiae MTCC 180 (5%) | 10 days | 4.5 | 30 °C | 12.20% | 8.0 | 4.41 | 0.61 | Khandelwal et al. (2006) |
| Kinnow: cane juice blend (80:20) | Saccharomyces cerevisiae MTCC 180 (5%) | 8 days | 4.5 | 30 °C | 11.0% | 8.8 | 4.44 | 0.49 | Khandelwal et al. (2006) |
| Kinnow peel powder | Saccharomyces cerevisiae MTCC 180 7.5% (v/v) | 5 days | 5.4 | 29 °C | 11% | 26 | NR | NR | Panesar et al. (2009) |
| Pre-treated kinnow waste | Saccharomyces cerevisiae | 12 h | 5.6 | 37 °C | 42.8 g/l | NR | NR | NR | Oberoi et al. (2011) |
| Kinnow juice | Saccharomyces cerevisiae var. ellipsoideus | 10 days | NR | NR | 12.20% (v/v) | 7.99 | 3.74 | 0.86 | Joshi et al. (2012) |
| Kinnow juice and date paste | Saccharomyces cerevisiae MTCC-11815 | 8 days | 3.9 | NR | 9.4% | 0 | – | 0.78% | Dua and Kocher (2017) |
NR not reported
Dried segments
Sogi and Singh (2001) prepared candied segments by keeping the segments in 30°B syrup and cooked slowly to 80°B. After draining the syrup, segments were dried (55 °C for 5 h) and then coated with powdered sugar. Product was packed in polythene pouches and stored at ambient condition (16–20 °C) for 90 days. The reported quality parameters of the developed product were, TSS (83.50°B), acidity (0.65%), ascorbic acid (18.34 mg/100 ml of juice), total sugars (63.45%), reducing sugars (2.82%) with overall acceptability 7.73. Candied segments did not show any development of bitterness during the entire storage period. Alam et al. (2019) prepared candy from whole kinnow fruit using osmotic dehydration. The better configuration of higher water loss and lower solute gain was reported at osmotic process temperature (65 °C), sugar solution concentration (65–75°B), solution to fruit ratio (5:1) and immersion time (270 min), which corroborates the consistency of sugar solution concentration throughout the process.
Juice powder
Production of fruit juice powder assumed great dimensions in recent time due to its numerous advantages in reference to ease in handling, transportation and storage. Juice powder can be a suitable adjunct for confectionery, bakery and ice cream industries. Spray drying is the most widespread of methods for drying liquid foods. Juyal et al. (2015) analysed the kinnow juice powder recovery by spray drying technique and reported the recovery of 36.45% at a blend of kinnow juice and maltodextrin (60:40), 36.34% at 146.5 °C of inlet temperature and 36.69% at 26 °C of feed temperature, respectively. However, the powder recovery was 37.66% at 40:10:50 ratio of maltodextrin, sucrose and juice. During the study, the corresponding values of powder properties were moisture content (4.60–5.74%), dispersibility (86.07–87.24%), L (85.01–85.89), a (1.61–2.06), b (12.48–13.88), colour change (52.31–53.18), chroma (12.59–14.03) and hue angle (69.00–82.98), respectively.
Fruit bar
Kaur (2017b) prepared blended fruit bar from kinnow, grape and guava juice waste in varying proportions i.e. kinnow (100%), kinnow: grapes (50:50), kinnow: guava (50:50), kinnow: grapes: guava (33.3:33.3:33.3). Juice waste along with 20% sugar and 0.2% citric acid was concentrated by open pan concentration at 80 °C till 40°B. The resultant mix was poured on an aluminum tray in a thin layer (4–5 mm) and dried (50 °C for 12–18 h) till 17–18% moisture content. The fruit bar prepared from blend kinnow, grape and guava was found acceptable till 6 months of storage (14–32 °C). Average cost of the developed product was estimated as ₹ 60/kg signifying its economic feasibility from the perspective of manufacturers as well as consumers. The developed bar contained moisture content (17.63%), total solids (82.37°B), TSS (69.97°B), acidity (1.30%), pH (3.37), total sugars (64.95%), crude fiber (11.12%), ascorbic acid (275.20 mg/100 g), total phenolics (920 mg/100 g), total anthocyanins (4.60 mg/100 g), total carotenoids (0.68 mg/100 g) and antioxidant activity (190.47% DPPH/100 g), with 8.80 overall acceptability.
Concentrate
Concentration of liquid foods reduces the expenses involved in transportation, storage and handling operations. The process also ensures the utilization and availability of fruit juice throughout the year. The concentration of kinnow juice was done under vacuum (27–28 inch Hg) at 50–60 °C in a Bucchi type (glass apparatus) evaporator. The 72°B concentrate was packed in a glass container with 700 ppm SO2 and stored at − 18 °C without significant changes in physicochemical characteristics (total sugars and reducing sugars varied from 56.1 to 56.4% and 45.6–45.7%, respectively) along with no sign of fermentation or off odour (Thakur et al. 2000).
Khamrus and Pal (2002) used reverse osmosis for the concentration of kinnow juice and optimized the process conditions viz. operation pressure (40 bar) and temperature (40 °C). The resultant concentrated kinnow juice had 23°B TSS, 3.20 pH, 2.49% acidity, 17.25% total carbohydrates, 5.85% reducing sugars, 6.79% sucrose, 1.09 specific gravity, 6.21 cP viscosity and 9.23 brix-to-acid ratio, respectively. The process of concentration resulted in the recovery of 89.68% (acid components), 96.02% (total soluble solids), 95.50% (total carbohydrates), 94.32% (reducing sugars) and 98.22% (sucrose), respectively. Thakur et al. (2004) studied the effect of centrifugation (CF) and ultrafiltration (UF) on the concentration of kinnow juice by reverse osmosis (RO) as well as vacuum thermal evaporation (VTE). They emphasized that juice could be concentrated by RO up to 24–26°B and juice clarified with UF were concentrated to 26°B followed by CF clarified. The corresponding quality parameters for VTE treatment were TSS (43° B), acidity (2.33%), ascorbic acid content (56.48 mg/100 ml), reducing sugar (19.0%), total sugar (37.95%) and carotenoids content (1.79 mg/100 ml). The corresponding values for RO treatment were, 24°B, 1.7%, 25.90 mg/100 ml, 10.25%, 20.00%, 1.02 mg/100 ml, respectively. The statistical difference in sensorial characteristics of juice concentrate by different techniques was non-significant.
Jam
Sogi and Singh (2001) prepared jam from unpeeled and lye peeled kinnow segments. The jam was concentrated to 70°B, packed in airtight glass jars and stored under ambient condition (16–20 °C) for 105 days. From the sensorial data, bitterness didn’t occur in jam prepared from lye peeled segments till 105 days, whereas there was a sign of bitterness reported in jam prepared using unpeeled segments after 30 days, which gradually increased during storage. The prepared jam had TSS (70°B), acidity (1.34%), ascorbic acid (26.33 mg/100 ml of juice), total sugars (61.05%), flavour (8.33) and colour (8.13) with overall acceptability of 8.33.
By-product utilization
Since bitterness is the major reported hindrance in kinnow processing, for improving the overall utilization of kinnow, researchers have explored the option of by-product (peel and seeds) utilization. Similar to all other citrus fruits, in case of kinnow also, peel and pomace are the major by-products of processing industry and they collectively accounts for 55–60% of the fresh fruit. These by-products can be utilized for the recovery and purification of essential oils, production of pelletized dry peels as cattle feed and extraction of pectin (Fig. 4). The utilization of various by-products of kinnow is reported hereunder:
Fig. 4.
Structures of limonin and naringin and their precursors (Source: Puri et al. 2011)
Peel utilization
During processing of kinnow into juice, about 30–34% of fruit peel is obtained as the primary waste component (Rafiq et al. 2018). Exploration of potential benefits of peel has been an active area of research worldwide with the aim to minimize environmental hazards. Kinnow peel is reported to have total solids (22.45%), TSS (12.50°B), ascorbic acid (41.57 mg/100 g), acidity (1.38%), total sugar (6.23%), reducing sugar (5.99%), ash (0.67%), carotenoids (13.65 mg/100 g), β-carotene (7.43 mg/100 g), pectin (1.85%) and fat (0.77%) as documented by Aggarwal and Sandhu (2003). As per the reports of Tumbas et al. (2010), mandarin peel extract (MPE) contains hesperidin (80.9 mg/g extract) and narirutin (15.3 mg/g extract). MPE showed prominent free radical scavenging activity towards DPPH (ECDPPH·50 = 0.179 mg/ml) and hydroxyl radicals (EC·OH50 = 0.415 mg/ml). Babbar et al. (2011) reported antioxidant activity (51.7 mg trolox equivalent/g-dw) and total phenol (17.5 mg Gallic acid equivalent/g-dw) in kinnow peel making it suitable for food and pharmaceutical industries. Kinnow peel is reportedly a rich source of ascorbic acid (47.52 mg/100 g), pectin (18.56%), naringin (358 µg/g) and limonin (60.75 µg/g) as documented by Sidhu et al. (2016). Being the potential source of phenolic compounds with functional, antioxidant and antimicrobial properties, peel was utilized for extraction of polyphenol compounds and preparation of value-added products as discussed hereunder:
Puri et al. (2011) extracted naringin from kinnow peel waste and used infrared spectroscopy for its molecular characterization. An effort to develop ice cream using frozen un-blanched and blanched kinnow peel was executed by Mann et al. (2013) and they reported that incorporation of frozen kinnow peel (5% of blanched and 3% of unbleached peel) was helpful in improving the appearance, flavour and overall acceptability. Panesar (2014) used kinnow peel waste with Monascus purpureus MTCC 369 (Microbial Type Culture Collection and Gene Bank 369) at submerged fermentation conditions to attain red pigment. A mixture of kinnow peel powder and pea pod supplemented with 0.1% magnesium sulphate incubated (35 °C for 9 days) with pH (6.5) yielded maximum pigment production. Another encouraging study by Safdar et al. (2017), where kinnow peel was used for extraction of eleven phenolic compounds including five phenolic acids and six flavonoids (gallic acid, chlorogenic acid, ferulic acid, coumaric acid, caffeic acid, catechins, epicatechins, hesperidin, naringenin, quercetin, kaempferol) using maceration and ultrasound-assisted extraction technique.
Drying of kinnow fruit and peel
Drying is one of the oldest and highly utilized techniques to increase the shelf-life of food products. In that similar line, Sharma (2016) investigated that time required for drying of kinnow peel by mechanical (60 °C) and solar (35 °C) drying was 30.5 h and 8 h, respectively to reach final moisture content 7%. Mahawar et al. (2017) illustrated the effect of drying temperature and drying technique using selected drying models (Page, Modified Page, Henderson and Pabis, Logarithmic). Among the models, page model satisfactorily described the drying curves with a high correlation coefficient (R2 ≥ 0.99) for all drying conditions. Alam et al. (2019) investigated the mass transfer kinetics of osmotically dehydrated kinnow in sugar syrup. The better configuration of higher water loss and lower solute gain was obtained at osmotic process temperature (65 °C), sugar syrup (65–75°B), solution to fruit ratio (5:1) and immersion time (270 min), which confirmed the uniformity of sugar syrup concentration. Rafiq et al. (2019) compared the effect of drying techniques (tray, vacuum and freeze) on chemical composition, color and antioxidant activity of kinnow peel. The retention of polyphenolic characteristics and color attributes was highest in freeze drying followed by vacuum drying substantiated their promising incorporation in food products.
Pectin extraction from peel
Singh and Dhillon (2007) suggested that pectin as a gellying agent can be used for preparation of jam, jelly, ketchup and ice cream. In addition, it has applications as a fat replacement and as a thickening agent. In a study conducted by the same authors, kinnow peel was utilized for the extraction of pectin using a combination of sodium and potassium salts. During the study, 14.80% (w/w) pectin yield was obtained from steam blanched and sun dried powdered samples. In another attempt to extract pectin, Sharma et al. (2013) acquired pectin yield of 16.1% from dried kinnow peel and 6.2% in pomace, respectively at extraction conditions of temperature (60 °C), pH (1.75) and extraction time (70 min) using water acidified with HNO3.
Yousuf (2019) utilized kinnow peel for extraction of pectin and bioactive compounds using the sequential microwave assisted solvent extraction for comprehensive valorization. Optimum extraction of bioactive compounds were obtained at microwave power (480.173 W), microwave time (3.127 min), solvent volume (103.458 ml) and particle size (0.543 mm), whereas, for pectin extraction, the optimum parameters were microwave power (476.58 W), microwave time (2.59 min), solvent volume (155.41 ml) and pH (1.05). Pectin yield (27.58%), equivalent weight (767.83 mg), methoxyl content (7.23%), anhydrouronic acid content (60.42%) and degree of esterification (64.679%) were recorded. Study also revealed that use of citrus peel extract (200 – 1000 ppm) could increase the shelf life of flaxseed oil up to 60 days and extracted pectin could be used for the jam preparation.
Peel powder and candied peel
In a promising study by Sharma (2016), the quality parameters of the powder obtained by drying of kinnow peel using mechanical and solar drying techniques were compared. The powder obtained from mechanical drying had better swelling index (23.78 ml water/g DM), water retention capacity (7.21 g water/g DM), solubility (37.33%), oil retention capacity (2.29 g oil/g DM), total phenols (0.510 g gallic acid/100 g DM), flavanoids content (18.80 mg reutin/100 g DM), total antioxidant capacity (84.39 g ascorbic acid/100 g DM), respectively. The corresponding values for solar drying was 21.94 ml water/g DM, 5.76 g water/g DM, 36.8%, 2.14 g oil/g DM, 0.488 g gallic acid/100 g DM, 14.15 mg reutin/100 g DM, 80.97 g ascorbic acid/100 g DM, respectively.
Aggarwal and Michael (2014) attempted to use kinnow peel for candy preparation using different proportions of sucrose and fructose. They found that the candy prepared with 100% fructose as well as 25:75 (sucrose: fructose) were best in view of organoleptic characteristics. The candied peel contains TSS (70°B), acidity (0.18%), ascorbic acid (11.7 mg/100 g), total sugars (42%) and juice limonin content (0.41 mg/ml) with overall acceptability (8.4). Sidhu et al. (2016) prepared kinnow peel candy and peel powder using osmotic dehydration and packed in four packaging materials i.e. high-density polyethylene (HDPE), low-density polyethylene (LDPE), laminate bag and glass jar. The overall acceptability of candy packed in HDPE bag and peel powder packed in the laminate bag was superior after 60 days of storage under ambient (37–44 °C, 56% RH) and refrigerated conditions (4–6 °C, 95% RH).
Essential oil
Essential oils extracted from citrus wastes are beneficial owing to their strong antimicrobial, antioxidant, and anti-inflammatory properties. They have a number of potential applications, such as ingredients in food additives, preservatives against spoilage, pharmaceuticals and cosmeceuticals (Sharma et al. 2017).
Kamal et al. (2011) extracted essential oil from fresh, ambient and oven-dried kinnow peels by the hydro-distillation method. The maximum amount of oil was obtained from oven-dried (0.50%) followed by ambient dried (0.48%) and fresh (0.30%) peel samples. Using GC and GC/MS, a total of 16–27 chemical constituents were identified in the peel essential oils. The limonin content was in the range of 64.1–71.1%. Ahmed et al. (2016) reported that essential oil extracted from kinnow peel by cold pressing method followed by centrifugation (15,000 rpm at 28–30 °C for 45 min) contains 42.73% of aldehyde content, 2.21 of acid number and 23.70 of ester number. Sharma (2016) extracted kinnow oil from peel using different extraction methods namely (1) physical method involves shredding/peeling, mechanical pressing, filtration and centrifugation, (2) physical method and hydrodistillation, (3) fresh peel by hydrodistillation, (4) mechanical dried (60 °C) peel by hydrodistillation, (5) solar-dried (60 °C) peel by hydrodistillation method. The essential oil yield (%) and extraction time (min) from selected methods were 0.179 and 25, 0.291 and 122, 0.365 and 285, 0.910 and 91, 0.617 and 115, respectively. Specific gravity, refractive index, acid value, free fatty acid, saponification value, ester, % glycerin of oil from above methods ranged from 0.803 to 0.856, 1.352–1.486, 3.46–5.02 mg KOH/g, 1.74–2.53%, 181.73–186.90 mg KOH/g, 176.71–182.97 mg KOH/g, 9.66–10.11%, respectively. The oil obtained from all five extraction methods was insoluble in water (Fig. 3b). Goyal and Kaushal (2018) reported the yield of essential oil obtained from kinnow peels was 0.3 ml/100 g dried peel powder. Similarly, Dwivedi et al. (2018) in their study recovered kinnow oil (0.04%) containing 96% of d-limonin by directly processing of kinnow waste using hydro-distillation. The compound D-limonin can be effectively utilized as a renewable feedstock for producing a diverse range of Bronsted acidic ionic liquids.
Kinnow seeds
Kinnow fruit contains average 20–25 seeds which can be used for limonin extraction. The available scientific reports revealed that the composition of kinnow seeds was examined by different researchers in order to further understand its appropriate utilization. Anwar et al. (2008) reported that kinnow seed contains 31.15% oil content, 9.56% protein content, 6.50% fiber content and 5.60% ash content, respectively. Babbar et al. (2011) reported that kinnow seeds have antioxidant activity (20.50 mg trolox equivalent/g-dw), total phenol (3.68 mg Gallic acid equivalent/g-dw), respectively. Liu et al. (2012) in their study extracted limonin using alkaline solution at the optimized extraction conditions of pH (11), temperature (70 °C), alkaline solution/seeds ratio 20:1 (v/w) and ultrasonic power (800 W for 30 min). The yield of 7.5 mg/g limonin/citrus seeds of 98% purity was obtained. Juhaimi et al. (2016) studied the detailed proximate composition of kinnow seed and reported the parameters viz. moisture (4.07 ± 0.87%), ash (4.51 ± 0.81%), crude oil (28.39 ± 3.21%), crude protein (15.17 ± 0.98%) and crude fiber (16.38 ± 1.78%). In addition, macro elements (mg/kg dry matter) viz. Ca (7619 ± 306), Mg (1186 ± 9), K (10,334 ± 37), P (3119 ± 33), S (1132 ± 5), and microelement (mg/kg dry matter) viz. B (12.91), Cr (0.396), cu (9.30), Fe (40.50), Mn (6.08), Mo (0.318), Ni (0.51), Zn (14.67) were recorded. Sugar content (g/kg) was also estimated including, sugar glucose (5.30 ± 0.39), fructose (5.83 ± 0.44), rafinose (4.86 ± 0.36), staxioz (5.59 ± 0.40), saccharose (5.29 ± 0.40) and galactose (3.59 ± 0.29), respectively.
Seed oil
Juhaimi et al. (2016) reported that kinnow seeds are considered to be a potential oil source due to their fatty acid composition and important tocopherol. Moreover, due to high protein, mineral and fiber contents, it might be used for edible applications as well as the production of potential value-added products. It consists of 98.6% of fatty acid which includes pammitic acid (21.9%), stearic acid (4.0%), oleic acid (21.3%), cis-vaccenic acid (2.0%), linoleic acid (43.7%), linolenic acid (5.0%), arachidic acid (0.4%), elcosenoic acid (0.1%), behenic acid. (0.2%) and 13.7 mg/kg of tocopherl content which includes α-Tocopherol (7.1 mg/kg), γ-Tocopherol (6.6 mg/kg).
Anwar et al. (2008) reported that extracted kinnow seed oil exhibited an iodine value of 104.80 (g of I/100 g of oil), refractive index (40 °C), 1.465, density (25 °C) 0.927 mg/ml, saponification value 186 mg of KOH/g of oil, unsaponifiable matter 0.48%, acid value 1.30 mg KOH/g of oil, color (red units) 2.50, colour (yellow units) 20.00. The oil revealed good oxidative stability as indicated by the determinations of specific extinctions at 232 and 270 nm (2.64 and 0.81, respectively), p-anisidine value (3.15) and peroxide value (2.40 mequiv/kg of oil).
Animal feed
Devatkal et al. (2010) replaced synthetic anti-oxidants in meat products using kinnow rind powder extract as they are rich in phenolic compounds having free radical scavenging activity. In another interesting study, retention of the color and oxidative stability of the raw ground goat meat using 2% kinnow peel powder with 2% salt was reported as the auto-oxidation and salt-induced lipid oxidation was minimized (Devatkal and Naveena 2010).
Kour et al. (2016) evaluated the effect of kinnow mandarin waste (KMW) inclusion in the ration on feed intake and nutrient utilization in goats. It was found that, 40% KMW inclusion widened the Ca: P ratio due to its high calcium content suggesting that maintaining the ratio needs careful consideration while feeding. There was no adverse effect of KMW inclusion in dietary ration on the general health of the goats as indicated by blood biochemical parameter.
FAO (2017) reported that kinnow waste (KW) can be used fresh or after sun drying as a component of total mixed ration (TMR). The KW contains approximately 20% dry matter (DM) and 12% crude protein on DM basis. Fresh KW and wheat straw were mixed in 80:20 ratio (on fresh basis) and ensiled for 42 days. The recommended level of KW-wheat straw silage in TMR was @ 25% on DM basis. The sundried, ground KW can completely replace cereal grains on crude protein basis, in the concentrate mixture and can be fed to animals after mixing with berseem (Trifolium alexandrium) hay in 50:50 ratio on DM basis. Peels contain approximately 7% limonin on DM basis and was incorporated in commercial broiler ration at @ 1% which improved the performance of broilers considerably.
Chaudhary et al. (2017) formulated paddy straw based feed blocks containing KMW and other ingredients in a manual densification machine. The feed block was tested on adult goats and it was concluded that it can be utilized without affecting the nutrient intake and digestibility. Further, due to the poor binding and durability of blocks, the formulation was not recommended to be densified in manual block making machine.
Toxic, regulatory status and identification of processed products
Sharma et al. (2017) emphasized that maintaining quality aspects of processed products prepared from citrus waste is a significant barrier in the food processing industry including the issues related to shelf life and food safety. Furthermore, some phytochemicals, such as D-limonin having an adverse effect on the processing methods must be excluded by efficient quality control systems. Approximately 89% of the initial D-limonin was removed during pre-treatment for producing ethanol (Oberoi et al. 2011). D-limonin has applications in the manufacturing of food and medicines as a flavoring agent. In addition, the chemical industry, cosmetics firms and domestic household products also employ D-limonin. Therefore, its effective removal prior to further use is necessary to avoid any complications (Mamma and Christakopoulos 2014). Peel oil obtained from citrus fruits contains D-limonin and its occupational exposure may lead to skin allergy. The wine prepared from fermented peel may contain impurities. Kinnow seeds are an important source of health beneficial nutrients, however excessive consumption may lead to kidney damage. The pulp and juice (except bitterness and microbial contamination) don’t have toxicity-related issues.
The rules and regulations for the manufacturing and sale of processed food items are different in different countries. In India, FSSAI (Food Safety Standard Authority of India) is the nodal agency to undertake the registration and licensing of processed food from various industries/companies/entrepreneurs. FSSAI has prescribed the limits of adulterants and other preservatives with their proper labeling which is mandatory to follow by each and every manufacturer. The entry of kinnow based products into the local market and for export purpose is regulated by internal and external food safety agencies.
Citrus fruits are composed of complex combinations of aroma/volatile compounds which help in their identification. It consists of mono‐ and sesquiterpenes which accumulate in specialized oil glands in the flavedo and oil bodies in the juice sacs. The estimation of such compounds needs sophisticated instruments like gas–liquid chromatography/high-performance liquid chromatography.
Miscellaneous use
Apart from the above-mentioned applications, kinnow fruit and its by-products are having the potential to be utilized for volatile oils, flavouring compounds, reformed fruit fragments, fatty acids, enzymes, biogas, citric acid, microbial biomass, vinegar, etc. (Singh and Dhillon 2007). Researchers have also reported the utilization of kinnow waste for the production of single-cell protein, multiple enzymes, bio-ethanol and as animal feed. Oberoi et al. (2010) emphasized that dried kinnow pulp supplemented with wheat bran (4:1) resulted in the highest filter paper cellulase activity (13.4 IU/gds). Singla et al. (2019) developed dietary fiber enriched vermicelli from wheat flour supplemented with debittered kinnow by-products (pulp residue and pomace) and found that incorporation of 15% debittered kinnow pulp residue and pomace in vermicelli was better in terms of quality characteristics of extruded products.
Economic analysis
Grover et al. (2013) conducted an economic analysis of emerging marketing channel (EMC) and traditional marketing channel (TMC) for the marketing of kinnow in Punjab region. Their study revealed that, although kinnow growers who sold their fruits through EMC had to incur higher marketing costs, yet the net price received by them was about 20% higher than TMC. Further, TMC’s share in the price paid by the consumers under TMC was only 33.70% as compared to that of emerging marketing channel share of 55 percent. Hence, this resulted in higher benefit–cost ratio (BCR) in EMC as compared to that in TMC for the enterprises.
Kaur and Singla (2016) studied the economic viability of kinnow orchards in Fazilka district of Punjab and reported BCR (2.04), net present value (₹ 3, 02, 289.78) and internal rate of return (40%) at 10% of discount, respectively indicating it as a profitable venture.
Bibwe et al. (2018) reported that, approximately ₹ 1,10,89,000/- as fixed capital and ₹ 1,80,12,645/- as working capital is needed for the establishment of 5 tonnes/h capacity kinnow waxing and grading plant. The breakeven point was 86.40% with an assumption that the effective utilization of plant capacity will be approximately 60, 70 80 and 100% in 1st, 2nd, 3rd and 4th year, respectively of the establishment.
Nawaz et al. (2018) conducted a study in Sargodha, Toba Tek Singh (TTS) and Vehari districts of Punjab (Pakistan) to assess the economic analysis of kinnow during on year and off-year. During off-year, fruit grade prize was observed to be higher as compared to on-year prices. Similarly, more income from orchards was reduced in Vehari and TTS than Sargodha during off-year.
Epilogue
Kinnow is amongst the major citrus crop and the availability of fresh fruit is very seasonal and localized. However, during its peak time of production a substantial amount of waste is generated. Hence, for its efficient utilization and to augment its availability during off-season, its appropriate processing and value addition in required not only for its preservation and increased storage life but also as ready reconstitution for consumption. From the perspective of post-harvest utilization, several waxing and grading plants are established in the growing belt, but the number of juice processing industries/pilot plant is scanty. Various forms of value-added products are available in the market but the commercialization of such products hasn’t taken place and the full-fledged large scale processing is still underway. Economical and suitable process protocol/technology for debittering needs to be standardized and validated to enhance the potential of the juice industry. In addition, popularization of kinnow juice among masses demands the development of suitable process protocol/technology which can be effortlessly adopted either at small/medium/large scale for the processing of this nutritious fruit. There also exists enormous scope for the overall utilization of kinnow at an industrial scale which appears to be a profitable venture. The quantum of waste generated during the peak season can’t be managed by the production of a few value-added products and it won’t be economically feasible. Hence, the production of some more alternative products should be explored.
Funding
Funding was provided by Indian Council of Agricultural Research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aggarwal P, Michael M. Effect of replacing sucrose with fructose on the physico-chemical sensory characteristics of kinnow candy. Czech J Food Sci. 2014;32:158–163. doi: 10.17221/221/2013-CJFS. [DOI] [Google Scholar]
- Aggarwal P, Sandhu KS. Effect of harvesting time on physico-chemical properties of juice components of Kinnow. J Food Sci Technol. 2003;40:666–668. [Google Scholar]
- Ahmad UI, Ying L, Mushtaq K, Bashir MK. An econometric estimation of post-harvest losses of Kinnow in Pakistan. Int J Econ Commer Manag. 2015;3(5):773–783. [Google Scholar]
- Ahmed MM, Rehman S, Qureshi TM, Nadeem M, Asghar M. Variability in peel composition and quality evaluation of peel oils of citrus varieties. J Agric Res. 2016;54(4):747–756. [Google Scholar]
- Alam MS, Kaur M, Ramya HG. Mass transfer kinetics for osmotic dehydration of kinnow fruit in sugar solution. Proc Natl Acad Sci India Sect B Biol Sci. 2019;89(1):361–370. doi: 10.1007/s40011-017-0951-z. [DOI] [Google Scholar]
- Ali MA, Zulfiqar A, Arif AM, Khan AR, Iqbal Z, Khan MA. Effect of natural and synthetic fruit coatings on the postharvest quality of kinnow mandarins. Agric Eng Int CIGR J. 2015;17(1):197–206. [Google Scholar]
- Anonymous (2018) Value chain strategy of kinnow in Fazilka district of Punjab. Report by NHRDF, Ministry of Agriculture and Farmers Welfare; Department of Agriculture, Cooperation & Farmers Welfare
- Anonymous (2019) https://en.wikipedia.org/wiki/Kinnow. Accessed on 18 July 2019
- Anwar F, Naseer R, Bhanger MI, Ashraf S, Talpur FN, Aladedunye FA. Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. J Am Oil Chem Soc. 2008;85:321–330. doi: 10.1007/s11746-008-1204-3. [DOI] [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Balaji V, Sikarwar PS. Development and evaluation of physico chemical properties of kinnow-aonla-aloe vera blended squash. Int J Curr Microbiol Appl Sci. 2018;7(4):113–122. doi: 10.20546/ijcmas.2018.704.013. [DOI] [Google Scholar]
- Bhardwaj RL, Mukherjee S. Effects of fruit juice blending ratios on kinnow juice preservation at ambient storage condition. Afric J Food Sci. 2011;5(5):281–286. [Google Scholar]
- Bibwe B, Mahawar MK, Kumar R, Saharan V, Gupta RK, Vishwakarma RK (2018) Status of Kinnow waxing and grading plants in Punjab and Rajasthan, Technical Bulletin, ICAR-CIPHET, Ludhiana, pp 1–35
- Chaudhary S, Rastogi A, Sharma RK, Raghuwanshi P, Khan N. Formulation of kinnow mandarin (Citrus Nobilis Lour × Citrus Deliciosa Tenora) waste and paddy straw based complete feed blocks and its utilization by goats. Indian J Anim Res. 2017;51(1):105–110. [Google Scholar]
- Devatkal SK, Naveena BM. Effect of salt, kinnow and pomegranate fruit by-product powders on color and oxidative stability of raw ground goat meat during refrigerated storage. Meat Sci. 2010;85:306–311. doi: 10.1016/j.meatsci.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85:155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Dhatt AS, Mahajan BVC (2007) Horticulture, post-harvest technology, harvesting, handling and storage of horticultural crops. Punjab Horticultural Postharvest Technology Centre, Punjab Agricultural University Campus, pp 1–30
- Dua K, Kocher GS. Fermentative processing of kinnow juice and extraction of limonin from kinnow waste. Curr Trends Biomed Eng Biosci. 2017;4(3):555637. [Google Scholar]
- Dwivedi P, Singh M, Sehra N, Pandey N, Sangwan RS, Mishra BB. Processing of wet Kinnow mandarin (Citrus reticulata) fruit waste into novel Brønsted acidic ionic liquids and their application in hydrolysis of sucrose. Bioresour Technol. 2018;250:621–624. doi: 10.1016/j.biortech.2017.11.100. [DOI] [PubMed] [Google Scholar]
- FAO (2017). https://www.feedipedia.org/node/23511. Accessed on 19 July 2019
- Goyal L, Kaushal S. Evaluation of chemical composition and antioxidant potential of essential oil from citrus reticulata fruit peels. Adv Res. 2018;15(2):1–9. doi: 10.9734/AIR/2018/41981. [DOI] [Google Scholar]
- Grover DK, Singh J, Singh JM, Kumar S. An economic analysis of marketing of kinnow in Punjab: emerging vis-a-vis traditional marketing channels. Agric Update. 2013;8(3):484–491. [Google Scholar]
- Ilame SA, Singh SV. Physico-chemical properties of ultrafiltered kinnow (mandarin) fruit juice. J Food Sci Technol. 2018;55(6):2189–2196. doi: 10.1007/s13197-018-3136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi VK, Kumar V, Kumar A. Physico-chemical and sensory evaluation of wines from different citrus fruits of Himachal Pradesh. Int J Food Ferment Technol. 2012;2(2):145–148. [Google Scholar]
- Juhaimi FAL, Matthaus B, Ozcan MM, Ghafoor K. The physico-chemical properties of some citrus seeds and seed oils. Zeitschrift für Naturforschung C. 2016;71(3–4):79–85. doi: 10.1515/znc-2016-0004. [DOI] [PubMed] [Google Scholar]
- Juyal D, Singh A, Kumar M. Production of powder from kinnow juice using spray drying technology. Agric Res J. 2015;52(1):37–41. [Google Scholar]
- Kamal GM, Anwar F, Hussain AI, Sarri N, Ashraf MY. Yield and chemical composition of citrus essential oils as affected by drying pretreatment of peels. Int Food Res J. 2011;18(4):1275–1282. [Google Scholar]
- Kaur B (2017a) Probiotication of blended kinnow juice at pilot scale. M.Sc Thesis submitted to Punjab Agricultural University, Ludhiana
- Kaur M (2017b) Enzymatic debittering and aroma enhancement of kinnow juice. M.Sc Thesis submitted to Punjab Agricultural University, Ludhiana
- Kaur M (2017c) Development of high fibre and antioxidant rich fruit bars from fruit juice waste. M.Sc Thesis submitted to Punjab Agricultural University, Ludhiana
- Kaur M, Singla N. An economic analysis of kinnow cultivation and marketing in Fazilka district of Punjab. Ind J Econ Dev. 2016;12(4):711–718. doi: 10.5958/2322-0430.2016.00195.5. [DOI] [Google Scholar]
- Khalid MS, Malik AU, Khalid S, Hafez O, Amin M (2013) Kinnow mandarin: the premier citrus of Pakistan, Agriculture Information Bank. http://www.agrinfobank.wordpress.com/2013/05/13/kinnow-mandarin-the-premier-citrus-of-pakistan/. Accessed 26 Aug 2019
- Khamrus K, Pal D. Application of reverse osmosis for concentration of Kinnow mandarin juice. J Food Sci Technol. 2002;39:310–312. [Google Scholar]
- Khandelwal P, Kumar V, Das N, Tyagi MS. Development of a process for preparation of pure & blended kinnow wine without debittering kinnow mandarin juice. Int J Food Saf. 2006;8:24–29. [Google Scholar]
- Kour R, Rastogi A, Sharma RK, Singh M. Evaluation of kinnow mandarin fruit waste in rations of goats. Indian J Anim Nutr. 2016;33(4):416–420. doi: 10.5958/2231-6744.2016.00074.8. [DOI] [Google Scholar]
- Ladaniya MS. Citrus fruit: biology, technology and evaluation. San Diego: Elsevier Academic Press; 2008. [Google Scholar]
- Liu C, Liu J, Rong Y, Liang N, Rong L. Aqueous extraction of limonin from Citrus reticulate Blanco. Czech J Food Sci. 2012;4:364–368. doi: 10.17221/108/2011-CJFS. [DOI] [Google Scholar]
- Mahajan BVC, Singh R. Effect of packaging films on shelf life and quality of kinnow fruits packed in consumer packages. Int J Farm Sci. 2014;4(1):92–98. [Google Scholar]
- Mahawar MK, Jalgaonkar K, Bibwe B, Ghodki B, Bhushan B. Mathematical modelling and drying kinetics of kinnow and sweet lime peels. Int J Chem Stud. 2017;5(6):885–888. [Google Scholar]
- Mahawar MK, Bibwe B, Jalgaonkar K, Ghodki BM. Mass modeling of kinnow mandarin based on some physical attributes. J Food Process Eng. 2019 doi: 10.1111/jfpe.13079. [DOI] [Google Scholar]
- Mamma D, Christakopoulos P. Biotransformation of citrus by-products into value added products. Waste Biomass Valor. 2014;5(4):529–549. doi: 10.1007/s12649-013-9250-y. [DOI] [Google Scholar]
- Mann S, Minhas KS, Aggarwal P. Development of phytochemical rich ice cream incorporating kinnow peel. Glob J Sci Front Res. 2013;13(4):1–3. [Google Scholar]
- Nawaz R, Abbasi NA, Hafiz IA, Khalid A, Ahmad T. Economic analysis of citrus (Kinnow mandarin) during on-year and off-year in the Punjab province, Pakistan. J Hortic. 2018;5(4):1–6. doi: 10.4172/2376-0354.1000250. [DOI] [Google Scholar]
- Oberoi HS, Chavan Y, Bansal S, Dhillon GS. Production of cellulases through solid state fermentation using kinnow pulp as a major substrate. Food Bioprocess Technol. 2010;3(4):528–536. doi: 10.1007/s11947-008-0092-8. [DOI] [Google Scholar]
- Oberoi HS, Vadlani PV, Nanjundaswamy A, Bansal S, Singh S, Kaur S, Babbar N. Enhanced ethanol production from Kinnow mandarin (Citrus reticulata) waste via a statistically optimized simultaneous saccharification and fermentation process. Bioresour Technol. 2011;102:1593–1601. doi: 10.1016/j.biortech.2010.08.111. [DOI] [PubMed] [Google Scholar]
- Panesar R. Bioutilization of kinnow waste for the production of biopigments using submerged fermentation. Int J Food Nutr Sci. 2014;3(1):9–13. [Google Scholar]
- Panesar PS, Panesar R, Singh B. Application of response surface methodology in the optimization of process parameters for the production of Kinnow wine. Nat Prod Radiance. 2009;8(4):366–373. [Google Scholar]
- Premi BR, Lal BB, Joshi VK. Distribution pattern of bittering principle in Kinnow fruits. J Food Sci Technol. 1994;31:140–141. [Google Scholar]
- Puri M, Kaur A, Schwarz WH, Singh S, Kennedy JF. Molecular characterization and enzymatic hydrolysis of naringin extracted from kinnow peel waste. Int J Bio Macromol. 2011;48:58–62. doi: 10.1016/j.ijbiomac.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Rafiq S, Kaul R, Sofi SA, Bashir N, Nazir F, Ahmad Nayik G. Citrus peel as a source of functional ingredient: a review. J Saudi Soc Agric Sci. 2018;17(4):351–358. [Google Scholar]
- Rafiq S, Singh B, Gat Y. Effect of different drying techniques on chemical composition, color and antioxidant properties of kinnow (Citrus reticulata) peel. J Food Sci Technol. 2019;56(5):2458–2466. doi: 10.1007/s13197-019-03722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar MN, Kausar T, Jabbar S, Mumtaz A, Ahad K, Saddozai AA. Extraction and quantification of polyphenols from kinnow (Citrus eticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal. 2017;25:488–500. doi: 10.1016/j.jfda.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SWA, Jahangir M, Qaiser M. Storage stability of Kinnow fruit (Citrus reticulata) as affected by CMC and guar gum-based silver nanoparticles based coating. Molecules. 2015;20:22645–22661. doi: 10.3390/molecules201219870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AD. Postharvest technology of Kinnow. Jodhpur: Scientific Publishers; 2009. pp. 1–115. [Google Scholar]
- Sharma P (2016) Development of oil extraction method from kinnow peel. M.Tech. Thesis, Department of Processing and Food Engineering, Punjab Agricultural University, Ludhiana, Punjab, India
- Sharma RK, Sandooja JK, Singhrot RS. A note on enhances shelf life of kinnow by some chemicals. Haryana J Hort Sci. 1991;20(3–4):216–217. [Google Scholar]
- Sharma H, Bhatia S, Alam MS. Studies on pectin extraction from kinnow peel and pomace. J Res Punjab Agric Univ. 2013;50(3 & 4):128–130. [Google Scholar]
- Sharma P, Chand T, Sharma SR. Evaluation of drying kinetics and physico-chemical characteristics of dried kinnow peel. Agric Res J. 2017;54(4):545–550. doi: 10.5958/2395-146X.2017.00104.1. [DOI] [Google Scholar]
- Shubhra B, Swati K, Singh RP, Savita S. Studies on aloe juice supplemented kinnow nectar. Res J Agric For Sci. 2014;2(8):14–20. [Google Scholar]
- Sidhu N, Arora M, Alam MS. Biochemical, microbial stability and sensory evaluation of osmotically dehydrated kinnow peel candy and peel powder. Int J Sci Res. 2016;5(9):1428–1436. [Google Scholar]
- Singh M, Dhillon SS. Extraction of pectin from kinnow peels. Int J Environ Stud. 2007;64(3):287. doi: 10.1080/00207230500241488. [DOI] [Google Scholar]
- Singh BP, Gupta AK, Chundawat BS. Effect of various treatments on storage of Kinnow fruits. Punjab Hortic J. 1973;13(3–4):161–164. [Google Scholar]
- Singh D, Jain RK, Agarwal M, Kumar A, Singh DB (2005) Bulk handling of kinnow mandarin. Technical bulletin, ICAR-CIPHET Abohar
- Singh SV, Jain RK, Gupta AK. Changes in quality of debittered kinnow juice during storage. J Food Sci Technol. 2009;46(6):598–600. [Google Scholar]
- Singh SV, Jain RK, Gupta AK. Adsorptive reduction of naringin from kinnow mandarin juice with non-ionic macroporpus adsorbent resin. Ind Chem Eng. 2016;58(2):136–156. [Google Scholar]
- Singla G, Krishania M, Sandhu PP, Sangwan RS, Panesar PS. Value addition of kinnow industry byproducts for the preparation of fiber enriched extruded products. J Food Sci Technol. 2019;56(3):1575–1582. doi: 10.1007/s13197-019-03670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogi DS, Singh S. Studies on bitterness development in Kinnow juice ready-to-serve beverage, squash, jam and candy. J Food Sci Technol. 2001;38:433–438. [Google Scholar]
- Sonkar RK, Sarnaik DA, Dikshit SN, Saroj PL, Huchche AD. Post-harvest management of citrus fruits: a review. J Food Sci Technol. 2008;45(3):199–208. [Google Scholar]
- Sonkar RK, Sarnaik DA, Dikhshit SN, Saxena RR. Individually stretch cling film wrapped kinnow mandarin under ambient storage. Ind J Hortic. 2009;66:22–27. [Google Scholar]
- Thakur NK, Kaushal Lal BB, Joshi VK. Effect of different conditions of storage on physio-chemical and microbial qualities of debittered kinnow juice concentrate. J Food Sci Technol. 2000;37(4):415–418. [Google Scholar]
- Thakur KS, Kaushal BBL, Sharma RM. Effect of different post-harvest treatments and storage conditions on the fruit quality of Kinnow. J Food Sci Technol. 2002;39(6):609–618. [Google Scholar]
- Thakur V, Manjunath SS, Gupta DKD. Comparative evaluation of clarification and concentration technique for kinnow fruit juice. J Food Sci Technol. 2004;41(4):382–385. [Google Scholar]
- Tumbas VT, Cetkovic GS, Djilas SM. Antioxidant activity of mandarin (Citrus reticulate L.) peel. Acta period technol. 2010;41:195–203. doi: 10.2298/APT1041195T. [DOI] [Google Scholar]
- Ullah N, Qazi IM. Preservation of ready to serve blended carrot and kinnow (mandarin) drink by ginger extract. J Food Process Technol. 2015;6(4):1–6. [Google Scholar]
- Yousuf (2019) Comprehensive valorization of citrus (Kinnow Mandarin) peel through sequential microwave assisted solvent extraction of bioactive compounds and pectin for their application in food. PhD Thesis submitted to G.B. Pant University of Agriculture and Technology, Pantnagar (Uttarakhand)