Abstract
The aim of the study was to solve the mystery why sulfur-containing amino acids such as methionine can perform as an antioxidant during frying and hypothesized the antioxidative mechanisms. The results of this study revealed that sulfur-containing amino acids such as methionine failed to show DPPH· scavenging activity at room temperature but had valuable antioxidant activity based on OSI test at heated temperature. NMR analysis proved that methionine formed an intermediate molecule, 3-(methylthio)propylamine via decarboxylation during pyrolysis at heated temperature which was responsible for its antioxidant activity as shown by the OSI results. The mechanisms showed the proposed antioxidant behavior of methionine at heated temperature: (1) At heated temperature, 3-(methylthio)propylamine is generated by decarboxylation and (2) The antioxidant activity of 3-(methylthio)propylamine might be ascribed to the cooperation of amino group and the methylsulf-hydryl group in 3-methylthiopropylamine. From the frying study, methionine showed about 50% lower antioxidant capacity when compared TBHQ (tert-butylhydroquinone) based on OSI study, however, it has unexpected superior antioxidant activity under frying conditions that was on par with TBHQ. In summary, sulfur-containing amino acids with excellent antioxidant abilities might be useful for the food processing industry as antioxidant additives to extend shelf-life of food or food products.
Keywords: Methionine, Tert-butylhydroquinone, 3-(methylthio)propylamine, Antioxidant and Deep-fat frying
Introduction
Oxygen, widely found in the atmosphere, causes oxidative rancidity which is a major cause of food quality deterioration (Ahmed et al. 2016). Similarly, even though oxygen is essential for life, it is destructive to cells in the body known as oxidative stress (Lobo et al. 2010). As such, antioxidant systems are important in both food application and the human body in order to maintain good quality of food and protect the body respectively. The human body has naturally adapted over time and developed anti-oxidizing systems which includes enzymes (Luangwattananun et al. 2017), proteins (Serbanescu et al. 2017), small molecules (Rahajee et al. 2017) or even hormones (Kandemir et al. 2017) to counteract oxidation caused by various internal or external factors. Natural and synthetic antioxidants were also discovered or developed which are added to food products so as to protect food components prone to oxidation like fats or oil. Due to concerns about healthfulness of synthetic antioxidants and consumer preference for cleaner labels, new natural antioxidant systems are highly sought after.
Amino acids are known to be antioxidant in oils and fats and are also naturally present in the body as proteins or free forms (Huichun et al. 2003). Among the 20 common amino acids, some were identified to have the greatest total antioxidative capacity using micro-potassium permanganate titration and iodometric titration (Xu et al. 2017). These amino acids are: tryptophan and tyrosine (with electron-rich aromatic rings in side chains), cysteine and methionine (with sulfur atoms in side chains), histidine, lysine and arginine (with nitrogen atoms in side chains).
In the human body, methionine (as residues in proteins) can be readily oxidized to methionine sulfoxide by many reactive species which helps to protect other functionally essential residues from oxidative damage (Levine et al. 2000). Methionine and cysteine also accounted for about 40% to 80% of total antioxidant activity of human serum albumin by acting as a metal chelator and free radical scavenger respectively (Bourdon et al. 2005). In addition, oxidative stress induced by lead is reduced by sulphur-containing compounds such as methionine and cysteine in the body (Caylak et al. 2008). With the ubiquity of methionine sulfoxide reductases in virtually all forms of life, it also shows the important role methionine plays as a free radical scavenger (Elias et al. 2005).
In food applications, histidine was found to be a strong antioxidant in herring oil emulsion using oxygen consumption measurement method (Marcuse 1960). Methionine, threonine, lysine and histidine were also reported to slow the oxygen absorption rate in safflower oil-in-water emulsions (Riisom et al. 1980). Amino acid containing a thiol, thioether or extra anime group (such as arginine, cysteine, lysine, methionine and tryptophan) were found to have the strongest antioxidant activity under frying conditions (Hwang and Winkler-Moser 2017).
There are several proposed mechanisms to explain antioxidant activity of amino acids in general. Amino acids can act as radical scavengers to quench singlet oxygen (Chloe and Min 2009) or chelate metals which could catalyse the breakdown of hydroperoxides into free radicals which causes oxidation (Decker et al. 2001). Amino acids can have synergistic interaction with tocopherols and other primary antioxidants to further improve antioxidant activity (Kamal–Eldin and Appelqvist 1996). Secondary compounds released from the interaction between amino acids and lipids could have antioxidant activity (Alaiz et al. 1995). With methionine commonly identified to have strong antioxidant activity in both living organisms and food applications, more research on how methionine exhibits such strong antioxidant activity would be done. However, specific mechanisms to explain antioxidant activity of methionine is still not known. Hence, there is a possibility that one of the mentioned mechanism could explain the antioxidant activity of methionine or there could be other mechanism which is still not discovered.
Therefore, the objective of this study was to solve the mystery why sulfur-containing amino acids such as methionine can perform as an antioxidant and hypothesize the antioxidative mechanisms. In addition, to demonstrate the effectiveness of methionine in the processes for food preparation involving high temperature. With this finding, it will be important to use these sulfur-containing amino acids in food processing industry as antioxidant additives to extend shelf-life of food or food products.
Materials and methods
Materials
DL-Methionine (min 99% purity) was purchased from Shijiazhuang Shixing Amino Acid Co., Ltd in China, 3-(methylthio)propylamine (min 97% purity) was purchased from Sigma-Aldrich (St Louis, Missouri, USA), Tertiary Butyl Hydroquinone (TBHQ, min 99% purity) and RBD palm oil w were obtained from Kemin Food technologies, Singapore. Antioxidant-free soybean oil and French fries used in the frying process were purchased from a local supermarket. All other chemicals were of analytical reagent AR grade.
2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay
The free-radical-scavenging activity of methionine was measured by 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH). Methionine (15 mg) was added to 10 ml of ethanol and the mixture was vortexed for 1 min before filtering through 0.2 μm PTFE syringe filter. A positive control using Tertiary Butyl Hydroquinone (TBHQ) was also prepared in a similar way. 2.95 ml of DPPH solution (0.740515 mN) in ethanol was added to 50 μl of filtered sample and positive control separately. A solvent blank was prepared by adding 3 ml of ethanol into a disposable cuvette while a reagent blank was prepared by adding 50 μl ethanol to 2.95 ml DPPH solution into a disposable cuvette. The absorbance values at 517 nm were measured by a UV–Visible spectrophotometer at every 15 s for 5 min. The percent inhibition was calculated by the following formula:
where A0 is the absorbance of the reagent blank, and A1 is the absorbance of the sample solution.
Oxidative stability index (OSI)
Oxidative stability index (OSI) were analysed following AOCS official method Cd 12b-92. Conductivity tubes were filled with 50 ml of deionized water and the probes were attached to the Oxidative Stability Instrument (Omnion OSI-24, ULTRA Scientific). Methionine and 3-(methylthio)propylamine were individually added into soybean oil at 100 ppm dosage. 5 g of each sample was weighed into a sample tube before covering it with a two-hole stopper containing a pasteur pipette and a glass tube. A silicon tube was placed between the compressed air outlet of the instrument and Pasteur pipette of the sample tube while another silicon tube was attached between the glass tube of the sample tube and the pasteur pipette of the conductivity tube. The sample tube was then placed into the thermo block at 100°C and the compressed air was turned on. The endpoint was automatically calculated when an induction point was reached. A similar procedure was done for methionine and 3-(methylthio)propylamine individually added into palm oil at 100 ppm dosage. The protection factor was calculated by the following formula:
where OSI0 is the average OSI of sample, and OSI1 is the average OSI of negative control.
Nuclear magnetic resonance (NMR) spectroscopy
3-(methylthio)propylamine (100 ppm) was prepared in palm oil. Methionine (100 ppm) was prepared in palm oil before heating at 100 °C and filtering through a 0.2 µm PTFE syringe filter. The samples were sent to Department of Chemistry, National University of Singapore for NMR analysis using Bruker 500 MHz NMR spectrometer. 50 µl of oil sample was diluted with 450 µl deuterated chloroform (CDCl3) before placing into the NMR spectrometer for analysis. A negative control (using palm oil) and positive control (using pure 3-(methylthio)propylamine) were prepared by diluting them in CDCl3 and deuterated water (D2O) respectively in a similar procedure before NMR analysis.
Frying trial and physio-chemical analysis of oil
The different treatments (100 ppm TBHQ, 100 ppm methionine and negative control) used for the frying experiment using soybean oil were prepared. The frying trial was conducted using a domestic deep-fat fryer with a 2-L-volume vessel (HD6159, Philips). For each deep-frying cycle, after heating the oil to and maintained constantly at 180 °C, French fries (100 g per batch) was added and deep fried for 3 min for one frying cycle. After every 10 frying cycles, oil top up of 100 ml from the respective treatments were added. Samples of frying oils (100 g) after every ten frying cycles were collected (0, 10, 20, 30, 40, 50, 60 and 70) and cooled to room temperature before storing at 4 °C prior to further analyses. Frying trials were conducted in duplicates. Peroxide value (PV), p-anisidine value (p-AV) and oxidative stability index (OSI) were analysed following AOCS official method Cd 8-53, Cd 18-90 and Cd 12b-92 respectively (AOCS 1990). %Total Polar Compounds (TPC) was measured using VITO 270 cooking oil tester. The sensor of VITO oil tester was submerged into the oil sample and moved gently for 30 s. This rapid method detects the dielectric constant of oil. This constant was converted to the content of total polar compounds (%) based on the formula set up by the manufacturer. All the analysis was carried out in duplicate.
Statistical analysis
Analysis of variance (One-way ANOVA) and Duncan’s multiple range tests were conducted using Statgraphics Plus version 5.0 software package at P < 0.05.
Results and discussion
2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay
The results for DPPH assay over 5 min and percent inhibition (%) are as shown in Table 1. Stable free radical, DPPH, is dark purple in colour (absorbed at 517 nm). With the presence of an antioxidant, DPPH free radical would be scavenged, resulting in the change of colour from purple to yellow (MacDonald-Wicks et al. 2006). Hence, the free radical scavenging activity could be observed by the reduction of absorbance value measured at 517 nm. From Table 1, there was no reduction in absorbance measured at 517 nm over 5 min for methionine sample which showed that there was no free radical scavenging activity seen for methionine. As shown in Table 1, the percent inhibition of the positive control (TBHQ) at 18.20% as compared to that of methionine at 0.24% also further indicated that methionine did not exhibit free radical scavenging activity when using DPPH analysis. Other study has also obtained similar negative results whereby methionine failed to show DPPH radical scavenging activity (Kim et al. 2018).
Table 1.
Absorbance of samples at 517 nm over 5 min and percent inhibition of TBHQ and methionine after 5 min
| Time (s) | Absorbance at 517 nm | |
|---|---|---|
| TBHQ | Methionine | |
| 0 | 0.815 | 0.833 |
| 15 | 0.803 | 0.833 |
| 30 | 0.798 | 0.832 |
| 45 | 0.788 | 0.833 |
| 60 | 0.782 | 0.832 |
| 75 | 0.772 | 0.833 |
| 90 | 0.765 | 0.834 |
| 105 | 0.756 | 0.834 |
| 120 | 0.749 | 0.833 |
| 135 | 0.742 | 0.832 |
| 150 | 0.737 | 0.833 |
| 165 | 0.729 | 0.833 |
| 180 | 0.723 | 0.832 |
| 195 | 0.717 | 0.833 |
| 210 | 0.712 | 0.833 |
| 225 | 0.707 | 0.833 |
| 240 | 0.702 | 0.834 |
| 255 | 0.697 | 0.833 |
| 270 | 0.692 | 0.833 |
| 285 | 0.688 | 0.834 |
| 300 | 0.683 | 0.833 |
| Percent inhibition (%) | 18.20 | 0.24 |
Oxidative stability index (OSI)
All oils and fats have a resistance to oxidation which depends on the degree of saturation, natural or added antioxidants, prooxidants or prior abuse. Oxidation is slow until this resistance is overcome, at this point oxidation accelerates and becomes very rapid. The length of time before this rapid acceleration of oxidation is the measure of the resistance to oxidation and is commonly referred to as Oxidative Stability Index (OSI). The oxidative stability instrument exposes the samples to heated temperature and constant flow of air to accelerate the development of oxidative rancidity. The results in Table 2 show the OSI for methionine treated soybean oil and palm oil. From Table 2, OSI for methionine treated soybean oil has higher OSI as compared to untreated soybean oil, showing that methionine helped to prolong the oil’s resistance to oxidation. Similarly, OSI for methionine treated palm oil was significantly (P < 0.05) higher as compared to that of untreated palm oil. Thus, the results showed that methionine has increased the oils’ resistance to oxidation, indicating that methionine has an antioxidant ability when added to soybean and palm oil under heated temperatures. This correlates to results of previous study which also show that methionine has antioxidant activity in soybean oil at frying temperatures (Hwang and Winkler-Moser 2017).
Table 2.
OSI results for methionine treated palm oil and soybean oil
| Sample | Soybean oil | Palm oil | ||
|---|---|---|---|---|
| Average OSI (h) | Protection factor | Average OSI (h) | Protection factor | |
| Negative control | 9.23a | 1.00 | 13.08a | 1.00 |
| Methionine | 9.65a | 1.05 | 14.50b | 1.11 |
| 3-(methylthio)propylamine | 11.53b | 1.25 | 14.40b | 1.10 |
a,bMeans within a column (between samples) with different letters are significantly different (P < 0.05)
As such, the investigation of the mechanisms why methionine exhibit antioxidant activity at heated temperature was carried out. Since the DPPH assay at room temperature showed that methionine failed to show DPPH radical scavenging activity, as such this mechanism to explain antioxidant ability of methionine is no longer valid. Similarly, there was no metallic ions or tocopherols added during OSI analysis. Thus, the mechanisms of chelating metal and synergistic interaction with tocopherols or other antioxidants are also eliminated. Therefore, a hypothesis is proposed that methionine may possess a different antioxidant pathway depending on the chemical forms under frying temperature. Possible degradation pathways of methionine at heated temperature include the formation of 3-(methylthio)propylamine by decarboxylation during pyrolysis and methional by Strecker degradation (Kamal–Eldin and Appelqvist 1996). 3-(methylthio)propylamine hydrochloride was found to have antioxidant activity on the autoxidation of sodium linolenate (Nagano et al. 2014). In addition, since Strecker degradation is less likely to occur during OSI analysis as soybean and palm oil were used (which contained little carbohydrates), the hypothesis that 3-(methylthio)propylamine is responsible for antioxidant activity in methionine was formed.
Hence, OSI analysis for 3-(methylthio)propylamine was done in soybean and palm oil to validate this hypothesis and the result was also shown in Table 2. From Table 2, the protection factor for both methionine and 3-(methythio)propylamine at the same dosage of 100 ppm were similar at 1.05 and 1.08 for soybean oil and 1.11 and 1.10 for palm oil respectively. This further showed that 3-(methylthio)propylamine is the potential molecule formed during pyrolysis of methionine at heated temperatures which is responsible for methionine’s antioxidant ability. From here, more studies need to be conducted to validate this hypothesis such as using NMR analysis.
Nuclear magnetic resonance (NMR) spectroscopy
In order to prove that 3-(methylthio)propylamine was formed from methionine at heated temperature, NMR spectroscopy was used. NMR spectroscopy is one analysis method for structure determination of unknown compound present. Table 3 shows the peak signals captured during NMR analysis for pure 3-(methylthio)propylamine and extra peak signals (not found in negative control) for both 3-(methylthio)propylamine in palm oil and methionine heated in palm oil (at 100 °C). Figure 1a shows the overall spectrum of negative control, 3-(methylthio)propylamine in palm oil and methionine heated in palm oil. Figure 1b–d show a clearer spectrum for signal 1 and 2, signal 2 and 3, signal 4 and 5 respectively. Figure 1e shows a partial spectrum with signal 2, 3, 4 and 5 for pure 3-(methylthio)propylamine, negative control, 3-(methylthio)propylamine in palm oil and heated methionine in palm oil. Figure 1f shows the hydrogen atoms in 3-(methylthio)propylamine corresponding to each signal.
Table 3.
Signals obtained from NMR spectroscopy
| Signal | Type of signal | Peak signals (ppm) | ||
|---|---|---|---|---|
| 3-(methylthio)propylamine | 3-(methylthio)propylamine in palm oila | Heated methionine in palm oila | ||
| 1 | Triplet | 2.7779 | 3.3682 | 3.3617 |
| 2.7642 | 3.3551 | 3.3492 | ||
| 2.7504 | 3.3424 | 3.3363 | ||
| 2 | Triplet | 2.5309 | 2.6160 | 2.6201 |
| 2.5167 | 2.6020 | 2.5968 | ||
| 2.5020 | 2.5880 | 2.5757 | ||
| 3 | Singlet | 2.0690 | 2.0881 | 2.0895 |
| 4 | Quintet | 1.7382 | 1.8293 | 1.8333 |
| 1.7245 | 1.8155 | 1.8197 | ||
| 1.7105 | 1.8016 | 1.8060 | ||
| 1.6963 | 1.7877 | 1.7916 | ||
| 1.6821 | 1.7739 | 1.7787 | ||
| 5 | Singlet | 1.4485 | 1.5962 | 1.5979 |
aThese are peak signals identified to be found in these samples but not in negative control (palm oil)
Fig. 1.
a Overall NMR spectrum and b partial NMR spectrum for negative control, 3-(methylthio)propylamine in palm oil and heated methionine in palm oil showing signal 1 and 2, c signal 2 and 3, d signal 4 and 5, e partial NMR spectrum for 3-(methylthio)propylamine, negative control, 3-(methylthio)propylamine in palm oil and heated methionine in palm oil showing signal 2, 3, 4 and 5 and f Chemical structure of 3-(methylthio)propylamine
Fig. 2.
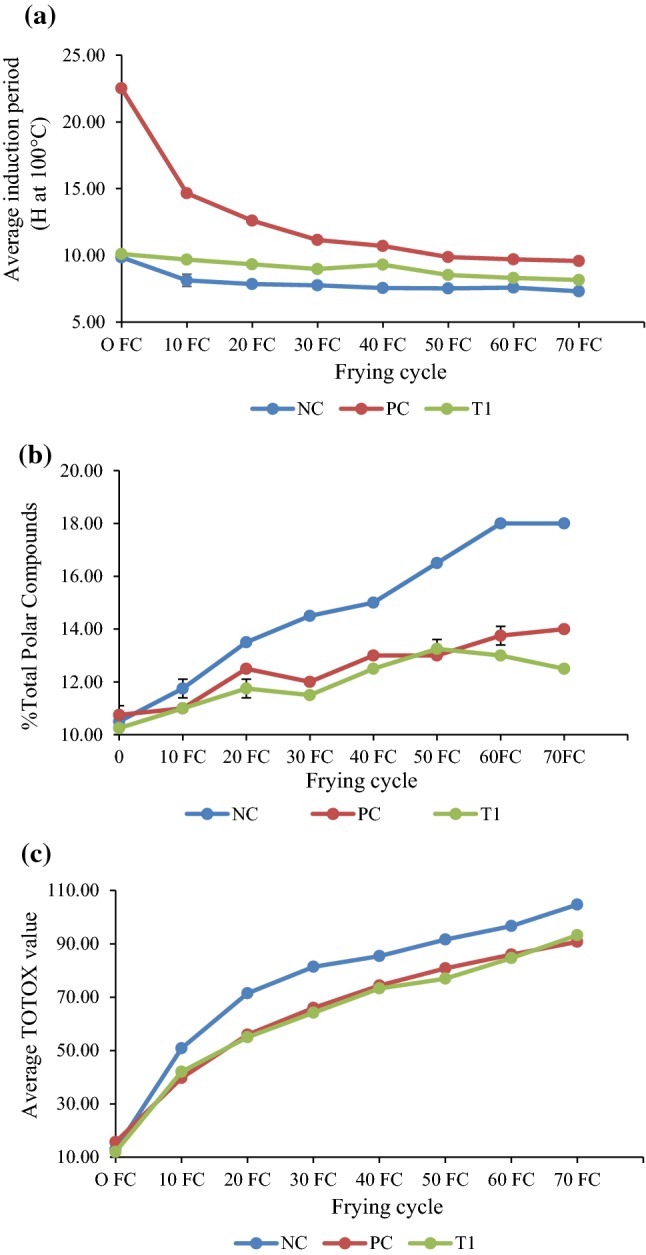
Changes in (a) induction period, b average %TPC and c average TOTOX value of soybean oil during frying
From Table 3, it is shown that there were 5 signals captured by NMR spectroscopy for pure 3-(methylthio)propylamine compound diluted in D2O. The hydrogen atoms responsible for each of the corresponding signals produced in the spectral data were also determined and identified as shown in Fig. 1f. Briefly, the hydrogen atoms in the methyl group (C1) formed a singlet signal at 2.0690 ppm in D2O solvent. The hydrogen atoms from C2, C3 and C4 formed triplet, triplet and quintet signals at 2.5167 ppm, 1.7105 ppm and 2.7642 ppm respectively. The hydrogen atoms in the amine group formed a singlet at 1.4485 ppm.
3-(methylthio)propylamine was spiked into palm oil and the NMR spectroscopy was done using CDCl3 as solvent. The peak signals in this sample were compared to that in the negative control and it was found that 4 extra peak signals which were seen in this sample and not in negative control were peak signals due to the added 3-(methylthio)propylamine into palm oil. The 4 extra signals (2 triplets, 1 singlets and 1 quintet) captured by NMR spectroscopy corresponded to all the hydrogen atoms of 3-(methylthio)propylamine except for the hydrogen atoms in the amine group. These signals also have a chemical shift downfield the spectral data (when compared to the signals obtained from pure 3-(methylthio)propylamine) due to the different solvents used for these two samples and the interactions between 3-(methylthio)propylamine and palm oil. As a result, the peak signal corresponding to hydrogen atoms from amine group was shifted downfield into one of the peak signal formed by palm oil at 1.59 ppm. The interaction between 3-(methylthio)propylamine and palm oil also resulted in other unknown signals formed.
With this, methionine was spiked into palm oil and heated at 100 °C to mimic the OSI conditions before NMR analysis. However, due to low solubility of methionine in oil, the methionine could not be fully dissolved into the oil. Thus, the sample was filtered before NMR analysis to ensure no excess solid methionine particles were present which would greatly affect NMR signals. Similarly, the results for this sample showed 4 extra peak signals at the exact same positions of the spectra data and same peak patterns to the results obtained from 3-(methythio)propylamine in palm oil. Similarly, due to the chemical shift downfield, the peak signal corresponding to hydrogen atoms from amine group of 3-(methylthio)propylamine formed from heated methionine was shifted downfield into one of the peak signal formed by palm oil at 1.59 ppm. Hence, this shows that 3-(methylthio)propylamine was indeed formed from methionine at heated temperature resulting in similar NMR results. The signals obtained in this sample were much smaller in intensity as compared to that for 3-(methylthio)propylamine in palm oil sample because the NMR analysis required a high concentration of methionine spiked in palm oil which was much above the maximum solubility concentration of methionine in palm oil. Thus, methionine was not fully dissolved into palm oil and excess solid methionine was filtered resulting in a lower concentration of 3-(methylthio)propylamine formed from lesser dissolved methionine during heating. Other unknown signals were also resulted in the interaction between heated methionine and palm oil. Some of these unknown signals were slightly different from those found in 3-(methylthio)proylamine in palm oil sample because the interactions between the 3-(methylthio)propylamine and palm oil may be different due to the differences between the pure and converted form (from methionine).
With this NMR analysis, the hypothesis was proven to be correct that 3-(methylthio)propylamine was formed from methionine at heated temperature and thus is responsible for the antioxidant activity in methionine as shown in OSI results. The mechanisms showed the proposed antioxidant behavior of methionine at heated temperature:
At heated temperature, 3-(methylthio)propylamine is generated by decarboxylation

The antioxidant activity of 3-(methylthio)propylamine might be ascribed to the cooperation of amino group and the methylsulf-hydryl group in 3-methylthiopropylamine:

Frying trial and physio-chemical analysis of oil
Methionine was dosed into soybean oil for a frying trial to determine its efficacy in improving frying performance in terms of induction period (Oxidative Stability Index, OSI), %total polar compound (TPC) and TOTOX value (using peroxide and p-anisidine value).
Induction period is a direct evidence for changes in oxidative resistance. Figure 2a shows that both positive control (TBHQ) and methionine treated oil have significantly (P < 0.05) higher induction periods as compared to untreated control from 10th to 50th frying cycles, suggesting that both positive control (TBHQ) and methionine demonstrated relatively strong antioxidant activity even though TBHQ still performed slightly better.
Total polar content is one of the key quality parameter to judge the quality of cooking oil or frying oil. The polar compounds are results of oxidation of fat or oil during deep-fat frying. Figure 2b shows that untreated oil has the highest % TPC throughout the frying process, suggesting that both positive control (TBHQ) and methionine demonstrated antioxidant activity. Methionine treated oil also generally has significantly (P < 0.05) lower %TPC compared to TBHQ treated oil at the same inclusion rate of 100 ppm from 20th to 70th frying cycles (except at 50th frying cycle), indicating that methionine performed significantly (P < 0.05) better than TBHQ in improving frying performance.
Peroxide value (PV) is a measure of the amount of peroxides formed in the fats and oils throughout the oxidation process which are unstable intermediates that decompose into various carbonyls and other secondary oxidation byproducts readily whereas p-anisidine value (AV) measures aldehydes which are less easily destroyed under heated conditions. As such, it would be more appropriate to compare the quality of the oxidized oil using the TOTOX value (defined as 2 × PV + AV) which is a parameter regularly used to determine oil quality (Nayak et al. 2016; Moigradean et al. 2012; Decker et al. 2010) with combined PV and AV results using a mathematical function. Figure 2c shows that untreated oil has the highest TOTOX value while methionine and TBHQ treated oil were generally on par in terms of TOTOX value throughout the frying process, indicating that methionine has similar antioxidant activity level as TBHQ in improving frying performance.
Overall, methionine was shown to exhibit antioxidant activity in frying application and its performance was comparable with TBHQ based on the various physio-chemical analysis methods as shown in Table 4.
Table 4.
Comparison of antioxidant activity in frying application between methionine and TBHQ
| Physio-chemical analysis method | Methionine performance as compared to TBHQ |
|---|---|
| Induction period | Slightly lower |
| Total polar compound | Slightly higher |
| TOTOX value | On-par |
Conclusion
The results of this study revealed that sulfur-containing amino acids such as methionine failed to show DPPH· scavenging activity at room temperature but had valuable antioxidant activity based on OSI test at heated temperature. NMR analysis proved that methionine formed an intermediate molecule, 3-(methylthio)propylamine via decarboxylation during pyrolysis at heated temperature which was responsible for its antioxidant activity as shown by the OSI results.
The mechanisms showed the proposed antioxidant behavior of methionine at heated temperature: (1) At heated temperature, 3-(methylthio)propylamine is generated by decarboxylation and (2) The antioxidant activity of 3-(methylthio)propylamine might be ascribed to the cooperation of amino group and the methylsulf-hydryl group in 3-methylthiopropylamine.
From the frying study, methionine showed about 50% lower antioxidant capacity when compared TBHQ (tert-butylhydroquinone) based on OSI study, however, it has unexpected superior antioxidant activity under frying conditions that was on par with TBHQ.
In summary, sulfur-containing amino acids with excellent antioxidant abilities might be useful for the food processing industry as antioxidant additives to extend shelf-life of food or food products.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed M, Pickova J, Ahmad T, Liaquat M, Farid A, Jahangir M. Oxidation of lipids in foods. Sarhad J Agric. 2016;32(3):230–238. doi: 10.17582/journal.sja/2016.32.3.230.238. [DOI] [Google Scholar]
- Alaiz M, Zamora R, Hidalgo FJ. Antioxidative activity of €-2-octenal/amino acids reaction products. J Agric Food Chem. 1995;43:795–800. doi: 10.1021/jf00051a044. [DOI] [Google Scholar]
- AOCS . Official methods and recommended practices. 5. Champaign: American Oil Chemists’ Society Press; 1990. [Google Scholar]
- Bourdon E, Loreau N, Lagrost L, Blache D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic Res. 2005;39:15–20. doi: 10.1080/10715760400024935. [DOI] [PubMed] [Google Scholar]
- Caylak E, Aytekin M, Halifeoglu I. Antioxidant effects of methionine, a-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp Toxicol Pathol. 2008;60(4–5):289–294. doi: 10.1016/j.etp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Chloe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Compr Rev Food Sci Food Saf. 2009;8:345–358. doi: 10.1111/j.1541-4337.2009.00085.x. [DOI] [Google Scholar]
- Decker EA, Ivanov V, Zhu B-Z, Frei B. Inhibition of low-density lipoprotein oxidation by carnosine and histidine. J Agric Food Chem. 2001;49:511–516. doi: 10.1021/jf0010533. [DOI] [PubMed] [Google Scholar]
- Decker EA, Elias RJ, McClements JD. Oxidation in foods and beverages and antioxidant applications: management in different industry sectors. Amsterdam: Elsevier; 2010. p. 201. [Google Scholar]
- Elias RJ, McClements DJ, Decker EA. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase beta-lactoglobulin in oil-in-water emulsions. J Agric Food Chem. 2005;53(26):10248–10253. doi: 10.1021/jf0521698. [DOI] [PubMed] [Google Scholar]
- Huichun W, Huaming C, Chyuanyuan S. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Hwang HS, Winkler-Moser JK. Antioxidant activity of amino acids in soybean oil at frying temperature: structural effects and synergism with tocopherols. Food Chem. 2017;221:1168–1177. doi: 10.1016/j.foodchem.2016.11.042. [DOI] [PubMed] [Google Scholar]
- Kamal–Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- Kandemir YB, Aydin C, Gorgisen G. The effects of melatonin on oxidative stress and prevention of primordial follicle loss via activation of mTOR pathway in the rat ovary. Mol Cell Biol. 2017;63:100–106. doi: 10.14715/cmb/2017.63.2.16. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Jang H-J, Cho W-Y, Yeon S-J, Lee C-H. In vitro antioxidant actions of sulfur-containing amino acids. Arab J Chem. 2018;20:141–148. [Google Scholar]
- Levine RL, Moskovitz J, Stadtman ER. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life. 2000;50(4–5):301–307. doi: 10.1080/15216540051081056. [DOI] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luangwattananun P, Eiamphungporn W, Songtawee N, Bulow L, Isarankura N, Ayudhya CIN, Prachayasittikul V, Yainoy S. Improving enzymatic activities and thermostability of a tri-functional enzyme with SOD, catalase and cell-permeable activities. J Biotechnol. 2017;247:50–59. doi: 10.1016/j.jbiotec.2017.03.001. [DOI] [PubMed] [Google Scholar]
- MacDonald-Wicks LK, Wood LG, Garg ML. Methodology for the determination of biological antioxidant capacity in vitro: a review. J Sci Food Agric. 2006;86:2046–2056. doi: 10.1002/jsfa.2603. [DOI] [Google Scholar]
- Marcuse Antioxidative effect of amino acids. Nature. 1960;186:886–887. doi: 10.1038/186886a0. [DOI] [PubMed] [Google Scholar]
- Moigradean D, Poiana M-A, Gogoasa I. J Agroaliment Proc Technol. 2012;18(4):272–276. [Google Scholar]
- Nagano Y, Samejima H, Kinoshita S. Antioxidant Activity of 3-Methylthiopropylamine Hydrochloride. Agric Biol Chem. 2014;32(7):846–850. doi: 10.1080/00021369.1968.10859150. [DOI] [Google Scholar]
- Nayak RK, Dash U, Rayaguru K. Quality assessment of mustard oil in deep fat frying. Asian J Dairy Food Res. 2016;35(2):168–171. [Google Scholar]
- Rahaiee S, Hashemi M, Shojaosadati SA, Moini S, Razavi SH. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: antioxidant activities, bioavailability and anticancer properties. Int J Biol Macromol. 2017;9:401–408. doi: 10.1016/j.ijbiomac.2017.02.095. [DOI] [PubMed] [Google Scholar]
- Riisom T, Sims RJ, Fioriti JA. Effect of amino acids on the autoxidation of safflower oil in emulsion. J Am Oil Chem Soc. 1980;57:354–359. doi: 10.1007/BF02662057. [DOI] [Google Scholar]
- Serbanescu GL, Gruia MI, Bara M, Anghel RM. The evaluation of the oxidative stress for patients receiving neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Med Life. 2017;10:99–103. [PMC free article] [PubMed] [Google Scholar]
- Xu N, Chen G, Liu H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules. 2017;22:2066. doi: 10.3390/molecules22122066. [DOI] [PMC free article] [PubMed] [Google Scholar]



