Abstract
In the present study, the antimicrobial and the insect repellent activity of 16 botanical extracts obtained by supercritical CO2 (SCF) extraction were evaluated. The present investigation was conducted as there is a necessity for exploration of natural botanical extracts that target both stored product insects and microbes. The antimicrobial activity was studied by disc diffusion and broth microdilution methods against ten microbial species, including Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis and Listeria monocytogenes), Gram-negative bacteria (Escherichia coli and Salmonella enterica), and fungi (Aspergillus flavus, Aspergillus paraciticus, Aspergillus ochraceous, Aspergillus niger and Penicillium verrucosum). Repellency assay was carried out by area preference method against three coleopteran insects (Tribolium castaneum, Rhyzopertha dominica and Sitophilus oryzae). Among all the extracts, thyme and ajwain were effective against all the tested bacteria with a minimum inhibition concentration (MIC) of 256–1024 µg/mL. Hop extract resulted in better antibacterial activity against all the tested Gram-positive bacteria with a MIC of 32–64 µg/mL. Oregano, thyme and ajwain extracts showed broad-spectrum antifungal activity against all the tested fungi with MIC of 128–1024 µg/mL. Most of the extracts exhibited class V (80.1–100%) repellency against T. castaneum. Extracts of hop, ajwain and thyme were found to have strong repellency against T. castaneum and R. dominica. Therefore, SCF extracts of ajwain and thyme can be explored further for the application of bio-extracts as a growth limiting factors in a microcosm where such consortia thrive.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04141-6) contains supplementary material, which is available to authorized users.
Keywords: Foodborne pathogens, Coleopteran insects, Super critical fluid extraction, Insect repellency, Microbial inhibition
Introduction
The food spoilage is a complex process, resultant of several physical, biological, chemical and environmental factors which cumulatively make the commodity unacceptable for human consumption. Spoilage of stored food grains is a major concern with almost 10 to 30% damage by stored product insects (Ferry et al. 2004; Bhavya et al. 2018). Adverse effects associated with stored-product insects can be classified broadly into two classes as either a direct or indirect effect on human food (Hubert et al. 2018). Direct effects by pests are through physical contamination of food by arthropod body parts/fragments and chemical contamination by carcinogenic or allergenic products related to their physiology. Complex indirect effects of stored product insects on food are through altering the humidity and temperature, in turn, creating favourable environmental conditions for the pathogenic bacteria and fungi. They also host/transmit microorganisms like Escherichia, Serratia, Streptococcus, Bacillus, and Klebsiella (Harein and De Las Casas 1968). Pathogenic fungi in stored product interact with the stored product pests resulting in mycotoxin contamination (Sinha and Sinha 1990; Hell et al. 2000). Toxic strains of Aspergillus flavus were isolated from insects like Sitophilus oryzae and Tribolium castaneum (Hell et al. 2000). Aspergillus species produce mycotoxins (secondary metabolites) like aflatoxin and ochratoxin, which are also associated with postharvest storage (Nidhina et al. 2017). Stored-product pests interact with symbiotic and parasitic bacteria by the transmission of bacteria (Channaiah et al. 2010) and hosting genes of antibiotic-resistance. Stored product insects serve as vectors for the human pathogens and antibiotic resistant strains (Larson et al. 2008). In the 21st century, foodborne diseases resulting from the ingestion of contaminated foods is a huge concern to public health.
Over the last few decades pest control measures involved the usage of toxic chemicals as insecticides and fumigants (Bomzan et al. 2018). Furthermore, public concern about food contamination with bactericidal and fungicidal residues has significantly increased, and the emerging industrialization of food manufacture catalyses the presence and spread of new or antibiotic-resistant pathogens (Tauxe et al. 2010). Considering all these factors, the development of new safe and biodegradable alternative strategy to combat the pest and microbial control is required. In this purview, the use of phytochemicals and plant extracts as insecticides, insect repellents, antiseptics and antimicrobial agents that are effective and economically feasible are of new interest to researchers from various fields. Apart from being used as pest control agents, these botanical extracts also have a wide range of application in culinary, human and veterinary medicine, cosmetic industry. Plants have an ability to synthesize a large pool of secondary metabolites capable of defending themselves against microbes, pests and herbivores. It is well established that plant extracts, and essential oils have a broad spectrum of antimicrobial activity against foodborne pathogens and spoilage microorganisms (Gyawali and Ibrahim 2014). Plants extracts/essential oils like basil, fennel, thyme, clove, cinnamon, oregano, mint, ajwain, and garlic have shown to have antimicrobial and insecticidal potential, which have advantages and disadvantages in maintaining human health (Pandey and Kumar 2013; Pandey et al. 2017; Messaoudi and Begaa 2018; Begaa and Messaoudi 2019). The plant extracts exhibiting antimicrobial property are mainly known by their bioactive molecules, such as phenolics, polyphenols, quinones, flavones, flavonoids, flavones, tannins, terpenoids, coumarins, alkaloids, lectins, polypeptides and other compounds (Cowan 1999). These bioactive compounds can be extracted by several extraction procedures. However, the toxicity of the solvents used in extraction techniques, the degradation of the compounds, and the selectivity of the process are key points that are to be considered during phytochemical extraction. In this context, supercritical fluid extraction (SCF) procedure indeed is a novel competitive alternative for the extraction of valuable bioactive compounds by which the final product obtained are without toxic residues, with no degradation of active components, and high purity (da Silva et al. 2016). There is no comprehensive study determining the efficacy of SCF extracts of spices and herbs on both microbial and stored product insects that are serious issues in safety and food spoilage. Hence, the objective of the present study was to determine the biological activity of SCF extracts like fennel, basil, lemon myrtle, oregano, sage, thyme, cinnamon, curcuma xanthorrhiza, licorice, garlic, ajwain, hop, clove, margosa, rosemary and schisandra against stored product pests and foodborne pathogens.
Materials and methods
Preparation and characterisation of supercritical extracts
All supercritical extracts used in this study were commercial products of Flavex Naturextrakte GmbH (Rehlingen, Germany) conforming to established product specifications.
Dry leaf materials (basil, lemon myrtle, oregano, sage and thyme) and hop cones were passed through a cutting mill and then pelletized. All other materials like fruits, buds, bark, root and seeds were first passed through a cutting mill (cinnamon, curcuma xanthorrhiza and licorice) or directly passed through a pin or turbo mill (ajwain, clove, margosa, rosemary and schisandra) to give a powder with particle size of 200–600 µm. Only garlic cloves were freshly mashed and after addition of 2% olive oil blended with kieselgur in a ratio of 2 + 3 to give a powder for extraction.
All powders were processed in a high-pressure extraction plant with 3 × 300 L extractor volume, typical extractor filling was 90 kg of powder and extraction time was 1.5 h on the average with CO2 mass flow of 600 kg/h according to a solvent ratio of 10 kg CO2/kg input material. The solvent amount was adjusted to obtain an extraction degree of NLT 90% of marker constituents based on the mass balance of feedstock, extract and residue. Typical extraction conditions for selective CO2 extracts containing mainly steam volatile mono- and sesquiterpenes (ajwain, basil, clove, fennel, lemon myrtle, oregano, thyme) were 90–120 bar and 40–60 °C. Total CO2 extracts which contain in addition non-volatile lipophilic constituents and oils (cinnamon, curcuma xanthorrhiza, garlic, hop, margosa and schisandra) were extracted at 250–350 bar and 50–60 °C. The antioxidative diterpene phenols of rosemary and sage as well as licorice phenols were extracted at 400–500 bar and 60 °C with a few percent of food grade ethanol as co-solvent.
All extracts apart from garlic, hop, licorice, rosemary and sage were composed by 100% of feedstock constituents; the others were standardised by addition of some vegetable oil–garlic with olive oil, licorice with MCT oil and hop, rosemary and sage with sunflower oil.
The composition of all supercritical CO2 extracts was analysed by HPLC–DAD and GC–MS. Authentic reference substances were used for identification and quantification as far as they were available. Supplementary Table 1 gives the extract composition of all product batches used for efficacy testing. Data were taken from the batch related certificates of analysis provided by the manufacturer.
Table 1.
Minimum inhibitory concentration of supercritical critical fluid extracts of botanicals against selected bacteria cultures
| Extract | S. aureus | L. monocytogenes | B. subtilis | E. coli | S. enterica |
|---|---|---|---|---|---|
| Minimum inhibitory concentration (µg/mL) | |||||
| Ajwain | 1024 | 512 | 256 | 512 | 512 |
| Oregano | 256 | 128 | 256 | ND | 256 |
| Lemon myrtle | > 2048 | 1024 | 512 | ND | ND |
| Cinnamon | 512 | 512 | 512 | ND | ND |
| Thyme | 512 | 256 | 256 | 256 | 512 |
| Licorice | 512 | ND | ND | ND | ND |
| Rosemary | 512 | 256 | ND | ND | ND |
| Sage | 512 | 512 | ND | ND | ND |
| Hop | 64 | 32 | 32 | ND | ND |
| Basil | ND | > 2048 | ND | ND | ND |
| Clove | ND | 1024 | ND | ND | ND |
ND Not determined
Bacterial, fungal strains and culture conditions
Bacillus subtilis (ATCC 6633), Escherichia coli (ATCC 11775), Listeria monocytogenes (ATCC 13932), Staphylococcus aureus (ATCC 12600) and Salmonella enterica subsp. enterica serovar Typhimurium (ATCC 25241) were obtained from Food Safety and Analytical Quality Control Laboratory at CSIR-CFTRI, Mysuru, India and stored in 20% glycerol at − 80 °C. Vials containing frozen stock cultures were thawed at room temperature and were reactivated before use in 10 ml of sterile brain heart infusion (BHI) broth at 37 ± 2 °C for 24 h under static conditions. The bacterial strains were grown and maintained in BHI agar slants at 37 ± 2 °C and 4 °C, respectively. The bacterial suspension in the BHI broth at an initial concentration of approximately 109 colony-forming units (CFU)/mL was used for further experiments.
Aspergillus ochraceous (MTCC 1810), Aspergillus flavus (MTCC 277), Aspergillus niger (MTCC 281), Penicillium verrucosum (MTCC 1758), and Aspergillus parasiticus (MTCC 2796) were procured from Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India. Fungi used in the experiment were cultured on potato dextrose agar (PDA) slants and incubated at 25 ± 2 °C and were maintained at 4 °C until use. The fungal spore suspension prepared in 0.9% saline solution containing approx. 106 CFU/mL was used in the studies.
Rearing of stored product insects
Rhyzopertha dominica (lesser grain borer), Tribolium castaneum (red flour beetle) and Sitophilus oryzae (rice weevil) being maintained at Department of Food Protection and Infestation Control, CSIR-CFTRI, Mysuru, India were cultured on conditioned whole wheat (moisture content of 11.5–12.5%), and maintained at 28 ± 2 °C and 70 ± 5% RH and a 16:8 light: dark photoperiod. Adult insects of 1–2 weeks age were used in all the experiments. Cultures were maintained in the laboratory without exposure to any insecticide. All experiments were carried out under the same environmental conditions.
In vitro antibacterial activity by disc diffusion method
Antibacterial activity of sixteen supercritical extracts against five pathogenic bacteria (three Gram-positive and two Gram-negative) was investigated by the agar disk diffusion method as described by Rohinishree and Negi (2016). Briefly, the BHI agar medium was seeded with 0.1% of inoculum (107 log CFU/mL) and poured onto the plates. After solidification, the sterile filter discs (6 mm diameter, Hi-Media, Mumbai) were placed, and 10 μL of the extract was added on the disc and incubated at 37 ± 2 °C for 24 h. The plates containing sterile discs without the addition of the extracts served as negative control. The sensitivity of the bacteria to the supercritical extracts was determined by measuring the size of the inhibitory zones (including the diameter of the disk) around the discs on the agar surface, and values < 10 mm were considered as not active (i.e., negative) against tested bacteria. Extracts with the inhibition zone of > 15 mm were tested for their minimum inhibition concentration (MIC) against the selected bacteria.
In vitro antifungal activity by disc diffusion method
Antifungal activity of ten supercritical extracts against five fungi namely A. ochraceous, A. flavus, A. niger, P. verrucosum and A. parasiticus, were investigated by the agar disk diffusion method as mentioned by Chandrasekaran and Venkatesalu (2004). The PDA agar medium was seeded with a spore suspension of approx. 106 CFU/mL by spread plate method. The sterile filter discs (6 mm diameter, Hi-Media, Mumbai) were placed, and 10 μL of the extract was added on the disc and incubated at 25 ± 2 °C for 3–5 days. The in vitro antifungal activity was determined by measuring the zones of growth inhibition including the diameter of the discs. The inhibition zones with a diameter of < 10 mm are considered as not active (i.e., negative) and extracts with inhibition zones of > 20 mm were tested for their minimum inhibition concentration (MIC) against the selected fungi.
Determination of minimum inhibition concentration (MIC) against selected foodborne pathogens
Supercritical extracts exhibiting an inhibition zone ≥ 15 mm in diameter was screened to determine the MIC by standard two-fold microbroth dilution methodology given by Goyal and Kaushik (2011) with slight modifications. Bacterial suspensions were prepared as mentioned earlier and diluted with BHI broth to obtain a final inoculum of 107 CFU/mL in the assay for all the bacteria (based on optical density values at 630 nm previously calibrated against viable counts). A stock solution of each SCF extract was serially diluted in 96 well microtiter plate with BHI broth to obtain a concentration ranging from 4 to 2048 µg/mL of dimethyl sulfoxide (DMSO). Wells containing BHI broth, inoculated broth and DMSO treated broth served as media, negative and diluent controls. A standardized inoculum of each bacterial strain was added (5 µL) to each well and microtiter plates were then incubated at 37 ± 2 °C for 24 h. Following incubation, MIC was calculated as the lowest concentration of the extract inhibiting the growth of bacterial strain by measuring optical density at 630 nm using Varioskan flash reader (Thermo scientific, Bengaluru, India). The MIC of the samples was determined from the absorbance by comparing to the controls. The MIC results are means of four replicates from two separate experiments.
Determination of minimum inhibition concentration against the test fungi
The MIC for all the selected SCF extracts was performed by the broth microdilution method (Gomez-Lopez et al. 2005). The fungal spore suspension was prepared into sterile 0.9% saline solution as stated earlier. The suspension was adjusted to yield final inoculum of 105 CFU/mL by diluting into RPMI-1640 media (with l-glutamine, without NaHCO3 and supplemented with 2% glucose and 0.165 M MOPS (3-(N-morpholino) propanesulfonic acid), pH 7). Fungal spore suspensions were prepared as mentioned earlier and diluted with RPMI media to obtain a final inoculum of 105 CFU/mL in the assay for all the fungi (based on haemocytometer counts). A stock solution of each SCF extract was serially diluted in 96 well microtiter plate with RPMI media to obtain a concentration ranging from 4 to 2048 µg/mL of DMSO. Wells containing RPMI media, inoculated broth and DMSO treated broth served as media, negative and diluent controls. The plates were incubated at 25 ± 2 °C for 48 to 72 h. Absorbance was measured at 492 nm using Varioskan flash reader (Thermo Scientific) at 48 and 72 h. Further, visual observation of hyphal growth of the fungi was also examined. The MIC of the samples was determined from the absorbance by comparing to the controls. The MIC results are means of four replicates from two separate experiments.
Determination of insect repellency of SCF-CO2 extracts by contact assay with area preference method
Half filter paper discs (Whatman No. 1, diameter 9 cm) were prepared, and extracts were added to each half-disc, which were air-dried for 10 min. Each treated half-disc was then attached lengthwise, edge-to-edge, to a control half-disc with adhesive tape and placed in a Petri dish (diameter 9 cm); the inner surface of the petri dish was smeared with liquid Teflon (insect anti-slip) to prevent insects escaping. The orientation of the seam was changed in the replicates to avoid the effects of any external directional stimulus affecting the distribution of the test insects. Twenty adult insects were released in the middle of each filter-paper circle. Each concentration was tested 4 times, and insects that settled on each half of the filter paper disc were counted at hourly intervals for 5 h. The equation of percentage repellency (PR) for a given treatment time is given as follows (1):
| 1 |
where Nc, the number of insects in the untreated (control); and Nt, treated areas, respectively. The averages were then assessed to the different class using the scale described by Jilani and Su (1983). PR > 0.01 to < 0.1 = class 0; 0.1 to 20 = class I; 20.1 to 40 = class II; 40.1 to 60 = class III; 60.1to 80 = class IV; 80.1 to 100 = class V.
Statistical analysis
Values are expressed as mean ± standard error mean of three independent experiments done in triplicates. Statistical analysis was performed using SPSS 20 version (IBM SPSS Statistics 20), and significance between the groups was determined by one-way analysis of variance (ANOVA) with Tukey’s post t test. Cluster analysis was performed to group cases according to their similarity of the response. Based on the repellent activity, the hierarchical cluster analysis was performed with 48 data points corresponding to average PR values of the extracts. The Cluster Analysis was performed using SPSS 20 version with Hierarchical Cluster Between-groups linkage method and Squared Euclidean distance as a measurement. Generalized linear models (GLM) was fitted to assess the effects of extracts and concentrations on the repellency activity against insects. Value of p < 0.05 was considered as the significant difference between the groups.
Results and discussions
In vitro antimicrobial activity by disc diffusion method
Disc diffusion assay was performed as an initial screening process for selecting the extracts to determine the MIC values. The diameter of inhibition zones (mm) of 16 extracts against five different bacteria was presented in supplementary Table 2. Most of the extracts exhibited an adequate inhibitory effect on L. monocytogenes, where ajwain and oregano showed 63 mm inhibition. Cinnamon, rosemary and Hop showed a positive impact on Gram-positive bacteria tested (S. aureus, B. subtilis and L. monocytogenes). In the present study, SCF extract of rosemary inhibited S. aureus with an inhibition zone of 19 mm but did not inhibit E. coli. In contrary, essential oil of rosemary was found to inhibit both E. coli and S. aureus with an inhibition zone of 19 mm at 1:4 dilution (Hosni et al. 2013). Santoyo et al. (2005) reported an inhibition zone of 18, 17 and 29 for E. coli, B. subtilis and S. aureus, respectively, when treated with 200 µL of SCF rosemary extract. In the present investigation, ajwain, thyme and oregano SCF extracts showed antibacterial effect on all the tested bacteria. The essential oil of thyme was found to inhibit both E. coli and S. aureus with an inhibition zone of 15 and 13 mm at 1:32 dilution, respectively (Hosni et al. 2013). While, Karakaya et al. (2011) reported that SCF extract of oregano didn’t show any inhibition zone against E. coli, S. aureus and L. monocytogenes. The difference in the results may be due to the variation in the bacterial strains used, method of extraction, concentration and difference in composition of the extract. However, our observations are in tune with Santoyo et al. (2006), who have also recorded an inhibition zone of 19, 20, 21 and 18 mm against E. coli, B. subtilis, S. aureus and A. niger, respectively, with SCF oregano extract. This inhibitory effect may be attributed to the major compound present in the oregano extract, carvacrol at 40% (Santoyo et al. 2006), while it is 50% in SCF extract used in the present study.
Table 2.
Minimum inhibitory concentration of supercritical critical fluid extracts of botanicals against selected fungal cultures
| Extract | A. niger | A. ochraceous | P. verrucosum | A. flavus | A. parasiticus |
|---|---|---|---|---|---|
| Minimum inhibitory concentration (µg/mL) | |||||
| Ajwain | 512 | 512 | 256 | 1024 | 1024 |
| Oregano | 512 | 512 | 128 | 512 | 256 |
| Lemon myrtle | > 2048 | 2048 | 2048 | ND | ND |
| Cinnamon | 128 | 64 | 64 | 128 | ND |
| Thyme | 512 | 256 | 256 | 512 | 256 |
| Clove | 1024 | 1024 | 512 | 2048 | 512 |
| Garlic | > 2048 | ND | > 2048 | ND | > 2048 |
ND Not determined
In the present study, inhibition zone was also determined against A. ochraceous, A. flavus, A. niger, P. verrucosum, and A. parasiticus using 10 supercritical extracts (Supplementary Table 3). Oregano, ajwain and thyme extracts were effective against all tested fungi. Curcuma extract had no inhibitory effect on any of the tested fungi. Basil and lemon myrtle extracts showed inhibition zone (> 12 mm) against all the tested fungi except for A. parasiticus. The essential oil (10 µL) of ajwain seeds showed 9 mm of inhibition zone against A. niger (Shokri et al. 2016), which was contrary to our results (> 80 mm inhibition) with SCF extract, which may be due to different extraction procedure. In hydrodistillation, essential oil may suffer chemical alteration if the compounds are sensitive (Sovilj et al. 2011). Moreover, recovery of essential oil is better in SCF compared to hydrodistillation (Khajeh et al. 2004).
Determination of minimum inhibition concentration against the test bacteria and fungi
Those extracts with an inhibition zone of above 15 mm were selected for determining the MIC against the foodborne pathogenic bacteria (Table 1). Hop extract was found to have least MIC of 32 µg/mL against L. monocytogenes and B. subtilis; 64 µg/mL against S. aureus. Even, Rój et al. (2015) reported a lower MIC value (20 µg/mL) for SCF extract of hop against L. monocytogenes and S. aureus and a higher MIC value of > 2560 µg/mL against E. coli. In the present study, sage, rosemary, licorice, thyme, and cinnamon showed 512 µg/mL of MIC against S. aureus. In contrast, with rosemary leaf SCF extract, Genena et al. (2008) have shown a MIC (µg/mL) of 1000 and a low MIC of 250 against E. coli and S. aureus, respectively. SCF extracts of oregano and thyme showed MIC of 200 µg/mL against L. monocytogenes (Gutierrez et al. 2009). Ivanovic et al. (2013) reported that a MIC (µg/mL) of 640 and 20 of clove bud and oregano SCF extract is required to inhibit the growth of S. aureus. While, SCF extract of Greek oregano was found to have MIC of 320 and 640 µg/mL against S. aureus and E. coli, respectively (Stamenic et al. 2014). Contrary to these reports, in our experiments, we found that a MIC of 256 µg/ml of oregano SCF extract is required to inhibit the growth of S. aureus. Lemon myrtle and cinnamon showed 512 µg/mL MIC of against B. subtilis. Ajwain and thyme showed 512 µg/ml of MIC, whereas, oregano showed 256 µg/mL of MIC against S. enterica. Ajwain showed MIC of 256 µg/mL against E. coli. SCF extracts of thyme and rosemary exhibited MIC of ≤ 40 and 640 µg/mL against B. subtilis, 640 and 2560 µg/mL against E. coli, respectively (Ivanovic et al. 2012). We observed inhibition with 256 µg/mL concentration against both B. subtilis and E. coli with SCF extract of thyme.
Cinnamon was found to be effective against all the tested fungi by MIC determination (Table 2). The MIC of 64 µg/mL for cinnamon was obtained against P. verrucosum and A. ochraceous, whereas, it was 128 µg/mL for A. flavus and A. niger. Cinnamon showed 128 µg/mL of MIC, whereas, oregano, ajwain and clove showed 512 µg/mL of MIC against A. niger. The essential oil of Trachyspermum ammi showed MIC of 1500 µg/mL against A. niger (Shokri et al. 2016). In our study, ajwain and thyme showed 256 µg/mL of MIC, whereas, clove showed 512 µg/mL of MIC against P. verrucosum. Thyme and oregano showed 256 µg/mL of MIC, whereas, clove showed 512 µg/mL of MIC against A. parasiticus.
Repellent activity of supercritical extracts against three stored products insects
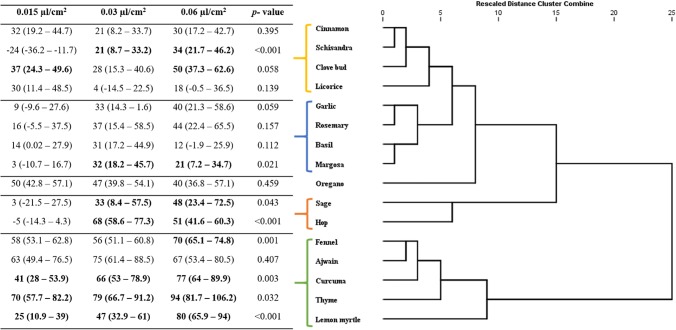
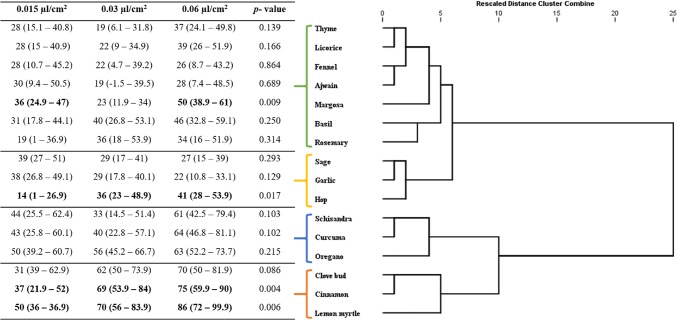
Supercritical fluid extracts were found to have a good insect repellent activity against all the three test insects used in the experiment, i.e., R. dominica, T. castaneum, S. oryzae (Figs. 1, 2, 3). Repellent effect of extracts significantly differed among insects (GLM, p < 0.05) and were positively associated with high concentration of extracts. In the present study, extracts exhibiting PR values > 40% were considered to be strong repellents. Hierarchical cluster analysis yielded different responses classes based on the similarity of behavioral response of the insect towards the extracts (Figs. 1, 2, 3). The PR values for almost all the tested extracts were higher than 80% at the test concentration of 0.06 µL/cm2. At least 6 extracts did not exhibit a significant repellency even at the maximum test concentration against R. dominica and S. oryzae. Licorice, rosemary and garlic extracts did not cause any repellency against both R. dominica and S. oryzae. Extracts of margosa, basil, hop, schisandra, curcuma and clove bud, oregano, sage, hop were found to be repellent at least at one of the test concentration against S. oryzae and R. dominica, respectively. Fennel, ajwain, curcuma, thyme, lemon myrtle extracts had significant repellence at all the test concentration against R. dominica (Fig. 2). Whereas, S. oryzae was repelled by oregano, clove, cinnamon and lemon myrtle extracts (Fig. 3).
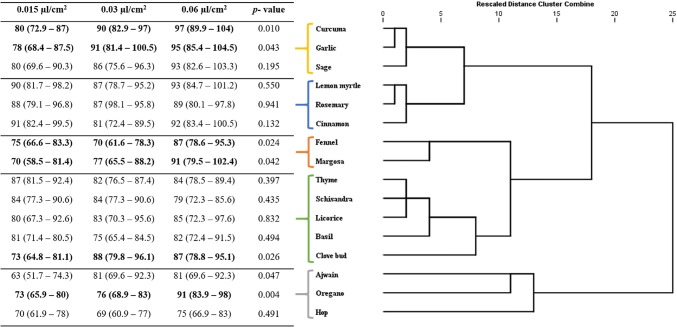
Fig. 1.
Response of T. castaneum adults to the supercritical critical fluid extracts at 3 concentrations (0.015, 0.03, 0.06 µL/cm2): dendrogram determined by hierarchical cluster analysis. Values in bold lettering were significantly different with the Post Hoc Bonferroni correction method. P value of the GLM of the interaction concentration-product (dose-dependency) on the repellency
Fig. 2.
Response of R. dominica adults to the supercritical critical fluid extracts of plant botanicals at 3 concentrations (0.015, 0.03, 0.06 µL/cm2): dendrogram determined by hierarchical cluster analysis. Values in bold lettering were significantly different with the Post Hoc Bonferroni correction method. P-value of the GLM of the interaction concentration-product (dose-dependency) on the repellency
Fig. 3.
Response of S. oryzae adults to the supercritical critical fluid extracts of plant botanicals at 3 concentrations (0.015, 0.03, 0.06 µL/cm2): dendrogram determined by hierarchical cluster analysis. Values in bold lettering were significantly different with the Post Hoc Bonferroni correction method. P value of the GLM of the interaction concentration-product (dose-dependency) on the repellency
While testing the repellency activity, 11 out of 16 extracts showed Class-V repellency against T. castaneum. Hop, curcuma, thyme, ajwain and clove, cinnamon, lemon myrtle exhibited class IV repellency against R. dominica and S. oryzae, respectively (Fig. 4). Lemon myrtle and oregano showed good repellency against all the three tested coleopteran insects.
Fig. 4.
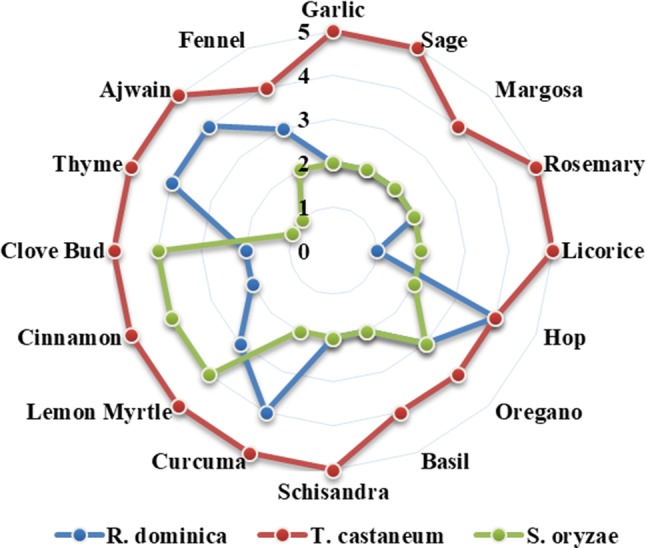
Repellency response of T. castaneum, R. dominica and S. oryzae adults to the supercritical critical fluid extracts of plant botanicals. (PR values > 0.01 to < 0.1 = class 0; 0.1 to 20 = class 1; 20.1 to 40 = class 2; 40.1 to 60 = class 3; 60.1 to 80 = class 4; 80.1 to 100 = class 5)
Conclusion
To summarize, hop extract resulted in better antibacterial activity against all the tested Gram-positive bacteria with a MIC of 32-64 µg/mL and oregano, thyme and ajwain extracts indicated broad-spectrum antifungal activity against all the tested fungi with MIC in the range of 128-1024 µg/mL. Though most of the extracts exhibited class V repellency against T. castaneum, maximum repellency observed against S. oryzae, and R. dominica was limited to class IV. Extracts of hop, ajwain and thyme were found to have strong repellency against T. castaneum and R. dominica. Ajwain and thyme SCF extracts showed both insect repellent and antimicrobial activity, which can be attributed to its high thymol content (> 50%). Since, natural compounds are always considered as safer food preservatives over synthetic chemicals, these SCF extracts have a potential scope in food grain storage application. Efficacy of these SCF extracts as fumigants and antimicrobials in food system can be explored in future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Director, CSIR-Central Food Technological Research Institute, Mysuru for the support and facilities provided for this study. This research was financially supported by Department of Biotechnology, Government of India (BT/IN/Finnish/06/AP/2013).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Begaa S, Messaoudi M. Toxicological aspect of some selected medicinal plant samples collected from Djelfa, Algeria Region. Biol Trace Elem Res. 2019;187(1):301–306. doi: 10.1007/s12011-018-1365-3. [DOI] [PubMed] [Google Scholar]
- Bhavya ML, Chandu AGS, Devi SS. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind Crops Prod. 2018;126:434–439. doi: 10.1016/j.indcrop.2018.10.043. [DOI] [Google Scholar]
- Bomzan DP, Bhavya ML, Chandu AGS, Manivannan S, Lavanya G, Ramasamy K, Pasha A. Potential of pyrethroid-synergised pyrethrum on stored product insects and implications for use as prophylactic sprays. J Food Sci Technol. 2018;55(6):2270–2278. doi: 10.1007/s13197-018-3144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol. 2004;91(1):105–108. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Channaiah LH, Subramanyam B, McKinney LJ, Zurek L. Stored-product insects carry antibiotic-resistant and potentially virulent enterococci. FEMS Microbiol Ecol. 2010;74(2):464–471. doi: 10.1111/j.1574-6941.2010.00949.x. [DOI] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva RP, Rocha-Santos TA, Duarte AC. Supercritical fluid extraction of bioactive compounds. Trends Analyt. Chem. 2016;76:40–51. doi: 10.1016/j.trac.2015.11.013. [DOI] [Google Scholar]
- Ferry N, Edwards MG, Gatehouse JA, Gatehouse AM. Plant–insect interactions: molecular approaches to insect resistance. Curr Opin Biotechnol. 2004;15(2):155–161. doi: 10.1016/j.copbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Genena AK, Hense H, Smânia Junior A, Souza SMD. Rosemary (Rosmarinus officinalis): a study of the composition, antioxidant and antimicrobial activities of extracts obtained with supercritical carbon dioxide. Food Sci. Technol. (Campinas) 2008;28(2):463–469. doi: 10.1590/S0101-20612008000200030. [DOI] [Google Scholar]
- Gomez-Lopez A, Aberkane A, Petrikkou E, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M. Analysis of the influence of Tween concentration, inoculum size, assay medium, and reading time on susceptibility testing of Aspergillus spp. J Clin Microbiol. 2005;43(3):1251–1255. doi: 10.1128/JCM.43.3.1251-1255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P, Kaushik P. In vitro evaluation of antibacterial activity of various crude leaf extracts of Indian sacred plant, Ocimum sanctum L. Br Microbiol Res J. 2011;1(3):70. doi: 10.9734/BMRJ/2011/312. [DOI] [Google Scholar]
- Gutierrez J, Barry-Ryan C, Bourke P. Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009;26(2):142–150. doi: 10.1016/j.fm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- Harein PK, De Las Casas E. Bacteria from granary weevils collected from laboratory colonies and field infestations. J Econ Entomol. 1968;61(6):1719–1720. doi: 10.1093/jee/61.6.1719. [DOI] [Google Scholar]
- Hell K, Cardwell KF, Setamou M, Schulthess F. Influence of insect infestation on aflatoxin contamination of stored maize in four agroecological regions in Benin. Afr. Entomol. 2000;8(2):169–177. [Google Scholar]
- Hosni K, Hassen I, Chaâbane H, Jemli M, Dallali S, Sebei H, Casabianca H. Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): impact on yield, chemical composition and antimicrobial activity. Ind Crops Prod. 2013;47:291–299. doi: 10.1016/j.indcrop.2013.03.023. [DOI] [Google Scholar]
- Hubert J, Stejskal V, Athanassiou CG, Throne JE. Health hazards associated with arthropod infestation of stored products. Ann Rev Entomol. 2018;63:553–573. doi: 10.1146/annurev-ento-020117-043218. [DOI] [PubMed] [Google Scholar]
- Ivanovic J, Misic D, Zizovic I, Ristic M. In vitro control of multiplication of some food-associated bacteria by thyme, rosemary and sage isolates. Food Control. 2012;25(1):110–116. doi: 10.1016/j.foodcont.2011.10.019. [DOI] [Google Scholar]
- Ivanovic J, Dimitrijevic-Brankovic S, Misic D, Ristic M, Zizovic I. Evaluation and improvement of antioxidant and antibacterial activities of supercritical extracts from clove buds. J Funct Foods. 2013;5(1):416–423. doi: 10.1016/j.jff.2012.11.014. [DOI] [Google Scholar]
- Jilani G, Su HC. Laboratory studies on several plant materials as insect repellants for protection of cereal grains. J Econ Entomol. 1983;76(1):154–157. doi: 10.1093/jee/76.1.154. [DOI] [Google Scholar]
- Karakaya S, El SN, Karagözlü N, Şahin S. Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanum vulgare ssp. hirtum) by using different extraction methods. J Med Food. 2011;14(6):645–652. doi: 10.1089/jmf.2010.0098. [DOI] [PubMed] [Google Scholar]
- Khajeh M, Yamini Y, Sefidkon F, Bahramifar N. Comparison of essential oil composition of Carum copticum obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem. 2004;86(4):587–591. doi: 10.1016/j.foodchem.2003.09.041. [DOI] [Google Scholar]
- Larson Z, Subramanyam B, Zurek L, Herrman T. Diversity and antibiotic resistance of enterococci associated with stored-product insects collected from feed mills. J Stored Prod Res. 2008;44(2):198–203. doi: 10.1016/j.jspr.2007.08.007. [DOI] [Google Scholar]
- Messaoudi M, Begaa S. Application of INAA technique for analysis of essential trace and toxic elements in medicinal seeds of Carum carvi L. & Foeniculum vulgare Mill. used in Algeria. J Appl Res Med Aromat Plants. 2018;9:39–45. [Google Scholar]
- Nidhina N, Bhavya ML, Bhaskar N, Muthukumar SP, Murthy PS. Aflatoxin production by Aspergillus flavus in rumen liquor and its implications. Food Control. 2017;71:26–31. doi: 10.1016/j.foodcont.2016.05.051. [DOI] [Google Scholar]
- Pandey AK, Kumar S. Perspective on plant products as antimicrobial agents: a review. Pharmacologia. 2013;4(7):469–480. doi: 10.5567/pharmacologia.2013.469.480. [DOI] [Google Scholar]
- Pandey AK, Kumar P, Singh P, Tripathi NN, Bajpai VK. Essential oils: sources of antimicrobials and food preservatives. Front Microbiol. 2017;7:2161. doi: 10.3389/fmicb.2016.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohinishree YS, Negi PS. Effect of licorice extract on cell viability, biofilm formation and exotoxin production by Staphylococcus aureus. J Food Sci Technol. 2016;53(2):1092–1100. doi: 10.1007/s13197-015-2131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rój E, Tadić VM, Mišić D, Žižović I, Arsić I, Dobrzyńska-Inger A, Kostrzewa D. Supercritical carbon dioxide hops extracts with antimicrobial properties. Open Chem. 2015;13(1):1157–1171. doi: 10.1515/chem-2015-0131. [DOI] [Google Scholar]
- Santoyo S, Cavero S, Jaime L, Ibanez E, Senorans FJ, Reglero G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J Food Prot. 2005;68(4):790–795. doi: 10.4315/0362-028X-68.4.790. [DOI] [PubMed] [Google Scholar]
- Santoyo S, Cavero S, Jaime L, Ibanez E, Senorans FJ, Reglero G. Supercritical carbon dioxide extraction of compounds with antimicrobial activity from Origanum vulgare L.: determination of optimal extraction parameters. J Food Prot. 2006;69(2):369–375. doi: 10.4315/0362-028X-69.2.369. [DOI] [PubMed] [Google Scholar]
- Shokri H, Sharifzadeh A, Khosravi AR. Antifungal activity of the Trachyspermum ammi essential oil on some of the most common fungal pathogens in animals. Iran J Vet Med. 2016;10(3):173–180. [Google Scholar]
- Sinha AK, Sinha KK. Insect pests, Aspergillus flavus and aflatoxin contamination in stored wheat: a survey at North Bihar (India) J Stored Prod Res. 1990;26(4):223–226. doi: 10.1016/0022-474X(90)90026-O. [DOI] [Google Scholar]
- Sovilj MN, Nikolovski BG, Spasojević MĐ. Critical review of supercritical fluid extraction of selected spice plant materials. Maced J Chem Chem Eng. 2011;30(2):197–220. doi: 10.20450/mjcce.2011.35. [DOI] [Google Scholar]
- Stamenic M, Vulic J, Djilas S, Misic D, Tadic V, Petrovic S, Zizovic I. Free-radical scavenging activity and antibacterial impact of Greek oregano isolates obtained by SFE. Food Chem. 2014;165:307–315. doi: 10.1016/j.foodchem.2014.05.091. [DOI] [PubMed] [Google Scholar]
- Tauxe RV, Doyle MP, Kuchenmüller T, Schlundt J, Stein CE. Evolving public health approaches to the global challenge of foodborne infections. Int J Food Microbiol. 2010;139:S16–S28. doi: 10.1016/j.ijfoodmicro.2009.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.