Abstract
The pathophysiology of schizophrenia includes altered neurotransmission, dysregulated intracellular signaling pathway activity, and abnormal dendritic morphology that contribute to deficits of synaptic plasticity in the disorder. These processes all require dynamic protein–protein interactions at cell membranes. Lipid modifications target proteins to membranes by increasing substrate hydrophobicity by the addition of a fatty acid or isoprenyl moiety, and recent evidence suggests that dysregulated posttranslational lipid modifications may play a role in multiple neuropsychiatric disorders, including schizophrenia. Consistent with these emerging findings, we have recently reported decreased protein S-palmitoylation in schizophrenia. Protein prenylation is a lipid modification that occurs upstream of S-palmitoylation on many protein substrates, facilitating membrane localization and activity of key intracellular signaling proteins. Accordingly, we hypothesized that, in addition to palmitoylation, protein prenylation may be abnormal in schizophrenia. To test this, we assayed protein expression of the five prenyltransferase subunits (FNTA, FNTB, PGGT1B, RABGGTA, and RABGGTB) in postmortem dorsolateral prefrontal cortex from patients with schizophrenia and paired comparison subjects (n = 13 pairs). We found decreased levels of FNTA (14%), PGGT1B (13%), and RABGGTB (8%) in schizophrenia. To determine whether upstream or downstream factors may be driving these changes, we also assayed protein expression of the isoprenoid synthases FDPS and GGPS1 and prenylation-dependent processing enzymes RCE and ICMT. We found these upstream and downstream enzymes to have normal protein expression. To rule out effects from chronic antipsychotic treatment, we assayed FNTA, PGGT1B, and RABGGTB in the cortex from rats treated long-term with haloperidol decanoate and found no change in the expression of these proteins. Given the role prenylation plays in localization of key signaling proteins found at the synapse, these data offer a potential mechanism underlying abnormal protein–protein interactions and protein localization in schizophrenia.
Subject terms: Molecular neuroscience, Schizophrenia, Long-term memory
Introduction
Altered neurotransmission is central to the pathophysiology of schizophrenia. Normal neurotransmission depends on regulation of receptor membrane localization and protein–protein interactions that regulate intracellular signaling activity1,2. Posttranslational modifications (PTMs), including lipid modification of proteins, have been shown to regulate neuronal functions and intracellular pathways by facilitating dynamic protein–protein interactions at membranes3–5. Altered posttranslational lipid modifications may mechanistically contribute to intracellular signaling abnormalities reported in schizophrenia6.
Posttranslational lipid modifications include the enzymatic addition of an isoprenyl group such as farnesyl or geranylgeranyl (collectively called prenylation), or a fatty acid moiety, such as a palmitoyl or myristoyl group. Dysregulated lipid modifications of proteins have been implicated in neuropsychiatric disorders, including Alzheimer’s disease7, Huntington’s disease8,9, and in a mouse model of schizophrenia10. Abnormal lipid modifications have been reported in schizophrenia in dorsolateral prefrontal cortex (DLPFC), including decreased protein S-palmitoylation11 and altered levels of a key N-myristoylated protein12. While S-palmitoylation, N-myristoylation, and prenylation pathways can act independently, in some cases combinations of these lipid modifications are necessary for efficient membrane targeting, protein–protein interactions, and conformational dynamics of essential intracellular signaling proteins, such as heterotrimeric G-proteins and small monomeric GTPases4,5,13–16. Abnormal G-protein signaling has been implicated in schizophrenia6,17–19, and heterotrimeric G-protein subunits have been shown to require modifications by each one of these lipid modifications20–24. Dysregulated prenylation could be a mechanism contributing to this illness via altered G-protein signaling.
Prenylation involves the addition of either farnesyl or geranylgeranyl isoprenoid group(s). Three prenylation enzymes are responsible for the thioether linkage of isoprenoid moieties—15-carbon farnesyl pyrophosphate (FPP) or 20-carbon geranylgeranyl pyrophosphate (GGPP)—to C-terminal cysteines: farnesyl transferase (FTase), geranylgeranyl transferase I (GGTase I), and geranylgeranyl transferase II (GGTase II)25,26. Each enzyme is comprised of an α and a β subunit. FTase and GGTase I have the same α subunit, FNTA, but have different β subunits: FNTB is the FTase β subunit, and PGGT1B the β subunit of GGTase I. GGTase II, which specifically geranylgeranylates Rab family proteins, is made of the RABGGTA and RABGGTB subunits26. The addition of isoprenyl moieties leads to increased protein hydrophobicity, which facilitates targeted localization to membranes, lateral movement within membranes, and substrate conformational changes that can influence dynamic protein–protein interactions15,16.
Given that prenylation occurs upstream of S-palmitoylation27, which is decreased in schizophrenia11, and facilitates the membrane targeting and resulting protein–protein interactions of key molecules associated with dynamic intracellular signaling4,5,28, we hypothesized that farnesylation and/or geranylgeranylation is also dysregulated in schizophrenia. Our own bioinformatic analysis of publically available datasets reflects a pattern of transcript expression differences for prenylation-associated enzymes and substrates in schizophrenia that further suggests dysregulation of this pathway. Accordingly, we assayed protein expression of the prenyltransferases subunits FNTA, FNTB, PGGT1B, RABGGTA, and RABGGTB in DLPFC from schizophrenia and matched comparison subjects. To further characterize the regulation of this modification, we also assayed protein expression of the upstream isoprenoid synthases farnesyl diphosphate synthase (FDPS) and geranylgeranyl pyrophosphate synthase (GGPS1) and the prenylation-dependent downstream processing enzymes Ras-converting enzyme (RCE) and isoprenylcysteine carboxyl methyltransferase (ICMT) in the same subjects. To identify the potential effects of antipsychotic treatment, we measured enzymes that were found altered in schizophrenia in the cortex from rats chronically treated with haloperidol decanoate.
Methods and materials
Human subjects
Samples of DLPFC (Brodmann area (BA) 9/46) from schizophrenia and matched comparison subjects (n = 13 pairs) were obtained from the from the Mount Sinai School of Medicine (MSSM) NIH Brain and Tissue Repository (Table 1) as previously described12,29. Neuropathological examination of all subjects was conducted, and each subject’s medical history was reviewed extensively; detailed information regarding assessment is available at http://icahn.mssm.edu/research/labs/neuropathology-and-brain-banking/neuropathology-evaluation. Subjects with previous drug or alcohol abuse, coma >6 h, suicide, or any evidence of neurodegenerative disease were excluded from the study. Next of kin consent was obtained for each subject. Subjects with schizophrenia all met Diagnostic and Statistical Manual of Mental Disorders, Third Edition-Revised criteria, diagnosed by at least two clinicians, with documented onset of psychosis prior to 40 years of age, and a minimum of 10 years hospitalization for the illness. Comparison subjects were similarly evaluated and free of any neurological or psychiatric conditions. Based on our previous protein postmortem studies, power analysis determined that this sample size was adequate to detect a moderate effect size ≥0.3 (α = 0.05, β = 0.2). We performed data analyses assuming equal variance as the subjects were matched pairs. Experimenters were blinded until data analyses.
Table 1.
Paired subject demographics.
| Pair | Subject | Sex/age | pH | PMI, h |
|---|---|---|---|---|
| 1 | Comparison | M/95 | 6.53 | 4.1 |
| Schizophrenia | M/97 | 6.50 | 9.3 | |
| 2 | Comparison | F/66 | 6.85 | 22.6 |
| Schizophrenia | F/62 | 6.74 | 23.7 | |
| 3 | Comparison | F/73 | 6.98 | 3.0 |
| Schizophrenia | F/70 | 6.51 | 13.2 | |
| 4 | Comparison | M/70 | 6.10 | 6.7 |
| Schizophrenia | M/70 | 6.35 | 7.2 | |
| 5 | Comparison | F/74 | 6.32 | 4.8 |
| Schizophrenia | F/75 | 6.49 | 21.5 | |
| 6 | Comparison | M/73 | 6.17 | 14.9 |
| Schizophrenia | M/73 | 6.50 | 7.9 | |
| 7 | Comparison | F/80 | 6.63 | 3.8 |
| Schizophrenia | F/81 | 6.67 | 15.1 | |
| 8 | Comparison | F/79 | 6.38 | 5.0 |
| Schizophrenia | F/77 | 6.01 | 26.1 | |
| 9 | Comparison | M/76 | 6.32 | 10.1 |
| Schizophrenia | M/80 | 6.37 | 9.7 | |
| 10 | Comparison | F/85 | 7.27 | 2.9 |
| Schizophrenia | F/84 | 6.80 | 15.4 | |
| 11 | Comparison | M/93 | 6.28 | 8.0 |
| Schizophrenia | M/92 | 6.67 | 21.9 | |
| 12 | Comparison | F/89 | 6.72 | 4.2 |
| Schizophrenia | F/89 | 6.20 | 17.7 | |
| 13 | Comparison | M/75 | 6.43 | 2.3 |
| Schizophrenia | M/78 | 6.64 | 9.6 | |
| Comparison (n = 13) | 79.1 ± 8.9 | 6.5 ± 0.3 | 7.1 ± 5.8 | |
| Schizophrenia (n = 13) | 79.1 ± 9.7 | 6.5 ± 0.2 | 15.3 ± 6.5 |
PMI postmortem interval, F female, M male
Antipsychotic-treated rats
Animal studies and procedures were performed in accordance to institutional guidelines and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Twenty male Sprague-Dawley rats (250 g) were housed in pairs during the 9-month course of the study. Haloperidol deconoate (28.5 mg/kg, n = 10) or vehicle (sesame oil, n = 10) was administered every 3 weeks via intramuscular injection for a total of 12 injections30,31. Animals were sacrificed by rapid decapitation, and the brains were harvested immediately. The right frontal cortex was dissected on wet ice, snap frozen in liquid nitrogen, and stored at −80 °C. Sample sizes were determined by previous studies, experimenters were blinded until data analyses, and sample groups were randomized.
Tissue homogenization
Tissue samples from both human subjects and rats were homogenized in ice-cold homogenization buffer (5 mM Tris-HCl pH 7.5, 0.32 M sucrose) supplemented with protease and phosphatase inhibitor tablets (Complete Mini, EDTA-free and PhosSTOP; Roche Diagnostics, Mannheim, Germany), using A Power Gen 125 (ThermoFisher Scientific, Rockford, Illinois) homogenizer at speed setting 5 for 60 s. A BCA Protein Assay Kit (ThermoFisher Scientific) was used to determine protein concentration, and samples were stored at −80 °C.
Western blot analysis
Thawed homogenates were denatured at 70 °C for 10 min under reducing conditions and stored at −20 °C. Duplicate samples were loaded onto NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes using a BioRad Semi-Dry Transblotter (Hercules, CA). Membranes were incubated in Odyssey blocking buffer (LI-COR, Lincoln, NE) for 1 h at room temperature (RT) before being probed with the primary antibody diluted in LI-COR blocking buffer with 0.1% Tween-20, using the conditions indicated in Table 2. After incubation in primary antibody, membranes were washed in cold Tris-buffered saline + 0.05% Tween-20 (TBST) before being probed with IR-dye-labeled secondary antibody diluted in LI-COR blocking buffer + 0.1% Tween-20 for 1 h at RT. Finally, membranes were washed in cold TBST, then briefly rinsed in MilliQ water before being scanned with a LI-COR Odyssey imager. All antibodies were optimized for ideal conditions of each target protein within the linear range of detection for each assay and ensuring the primary antibody was present in excess (Table 2). Valosin-containing protein (VCP) has been shown to be unchanged in multiple regions of schizophrenia brain32,33 and was used as an intralane loading control for western blot normalization.
Table 2.
Antibodies used for western blot analyses.
| Target protein | Host species | Dilution | Incubation | Company | Catalog # |
|---|---|---|---|---|---|
| FNTA | Rabbit | 1:1000 | 16 h, 4 °C | Abcam | ab109738 |
| FNTB | Rabbit | 1:5000 | 16 h, 4 °C | Abcam | ab109625 |
| RABGGTA | Rabbit | 1:500 | 16 h, 4 °C | Abcam | ab118781 |
| RABGGTB | Mouse | 1:1000 | 16 h, 4 °C | Abnova | H00005876-M02 |
| PGGT1B | Mouse | 1:1000 | 16 h, 4 °C | Abnova | H00005229-M02 |
| FDPS | Rabbit | 1:1000 | 16 h, 4 °C | Abcam | ab153805 |
| GGPS1 | Rabbit | 1:1000 | 16 h, 4 °C | Abcam | ab167168 |
| RCE1 | Rabbit | 1:500 | 16 h, 4 °C | Abcam | ab62531 |
| CMT | Rabbit | 1:500 | 16 h, 4 °C | Abcam | ab80872 |
| VCP | Mouse | 1:25,000 | 1 h, RT | Abcam | ab11433 |
| VCP | Rabbit | 1:25,000 | 1 h, RT | Abcam | ab109240 |
Data analysis
Protein expression was determined using the LI-COR Odyssey 3.0 analytical software (Lincoln, NE). Intensity values were normalized to the intralane VCP intensity value after verifying that VCP was not changed between these schizophrenia and comparison subjects, consistent with previous reports32. Duplicate values were averaged for each subject. All dependent measures were tested for normal distribution with the D’Agostino–Pearson omnibus normality test. Normally distributed data were analyzed using two-tailed paired Student’s t tests, and Wilcoxon matched-pairs signed rank tests were used for non-normally distributed data using the GraphPad Prism software (GraphPad Software, La Jolla, CA). No dependent measures were found to be associated with age, pH, or postmortem interval using post hoc linear regression analyses. For all statistical tests, α = 0.05.
Bioinformatic analysis
We evaluated prenylation-associated targets for patterns of differential gene expression in schizophrenia using six publically available transcriptomic datasets generated from samples from the MSSM NIH Brain and Tissue Repository. These datasets include studies in gray matter homogenates from the middle temporal area (BA 21), temporopolar area (BA 38), anterior cingulate cortex (BA 32), and DLPFC (BA 46)34. Two datasets examined transcript levels in laser capture microdissected pyramidal neurons from superficial (lamina II–III) or deep (lamina V–VI) cortical layers of the DLPFC35. The data were processed and analyzed using various R packages for differential expression analysis such as edgeR, DESeq2, and limma36–38. The datasets are then aggregated and uniquely titled for processing. To visualize patterns of differential expression, a heatmap of log2 fold change values has been constructed to present harmonized data across all of the different datasets. Harmonization was achieved using empirical cumulative probabilities based on each dataset, and final harmonized values were presented as standardized values that range from −1 to 139. The unsupervised clustering and construction of heatmaps were done using the pheatmap R package40.
Results
Prenyltransferase subunits are abnormally expressed in schizophrenia
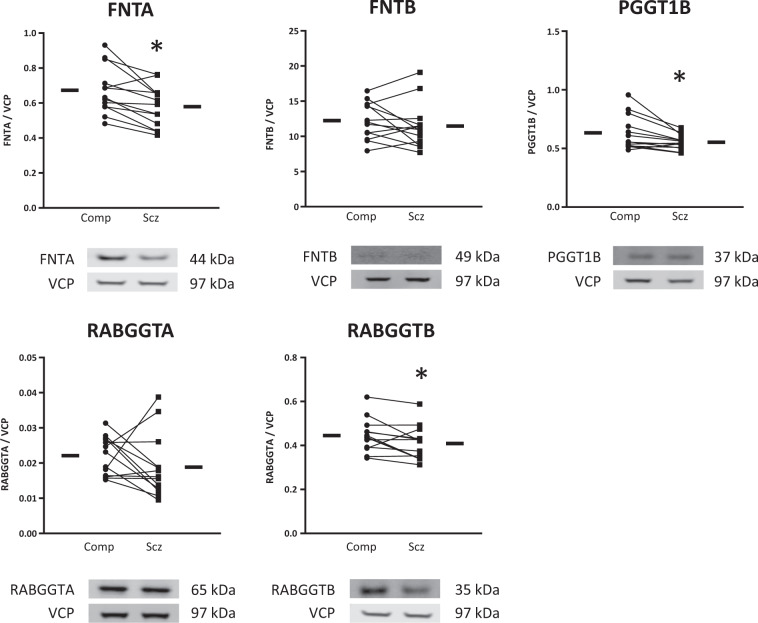
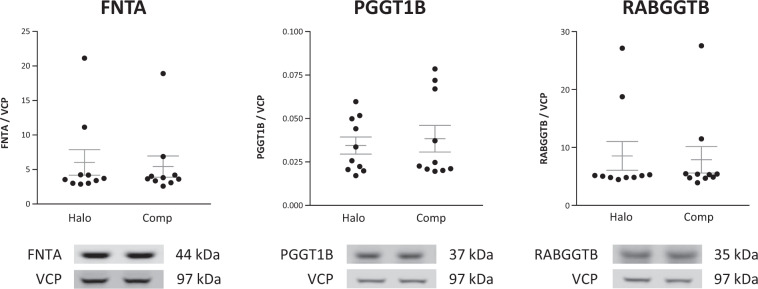
We found that each prenyltransferase enzyme had decreased expression of either or both of its respective α and β subunits in schizophrenia relative to comparison subjects. The FTase and GGTase I α subunit, FNTA, was decreased 14% (t(12) = 3.74, p = 0.003). The GGTase I β subunit PGGT1B was decreased 13% (W = −77, p = 0.004) and the Rab protein-specific GGTase II β subunit RABGGTB was decreased 8% (t(12) = 2.29, p = 0.04) in schizophrenia (Fig. 1). We also assayed FNTA, PGGT1B, and RABGGTB in rats chronically treated with haloperidol decanoate and found that haloperidol treatment did not affect the expression of these proteins in these rats (Fig. 2).
Fig. 1. Decreased prenyltransferase subunit expression in schizophrenia.
Western blots were used to assay protein expression in DLPFC of prenyltransferase subunits in schizophrenia (Scz) and matched, comparison subjects (Comp). FNTA, PGGT1B, and RABGGTB were decreased in schizophrenia. Data are expressed for each subject as the ratio of the signal intensity for protein of interest divided by the intensity of intralane VCP. Center lines are means. *p < 0.05.
Fig. 2. Chronic antipsychotic treatment does not alter the expression of FNTA, PGGT1B, and RABGGTB in rats.
Western blotting techniques were used to assay the protein expression of FNTA, PGGT1B, and RABGGTB in frontal cortex from adult male rats treated with haloperidol decanoate (28.5 mg/kg every 3 weeks for 9 months; Halo) or vehicle (Comp). Data are expressed as a ratio of signal intensity for each target protein to intensity for VCP. Chronic haloperidol treatment did not change the expression of these prenyltransferase subunits in rat cortex. Center lines are means ± S.E.M.
Expression of upstream isoprenoid synthases and downstream prenylation-dependent enzymes are normal in schizophrenia
To determine whether upstream lipid donor synthesis of FPP or GGPP was altered in schizophrenia, we assayed the synthases for these lipid pyrophosphate molecules, FDPS and GPPS1. No differences in the expression of FDPS or GPPS1 were identified in schizophrenia (Table 3). To determine whether enzymes downstream of prenyl attachment that are involved in the secondary processing of prenylproteins were altered, we measured the protein expression of RCE and ICMT in schizophrenia and found normal expression of both (Table 3).
Table 3.
Prenylation-associated enzyme expression levels in schizophrenia and comparison subjects.
| Comparison | Schizophrenia | Test statistic | p value | |
|---|---|---|---|---|
| Prenylsynthases | ||||
| FDPS | 0.649 ± 0.067 | 0.641 ± 0.067 | t(12) = 0.35 | 0.73 |
| GGPS1 | 0.194 ± 0.066 | 0.189 ± 0.059 | t(12) = 0.36 | 0.72 |
| Prenyltransferase subunits | ||||
| FNTA | 0.673 ± 0.136 | 0.580 ± 0.117 | t(12) = 3.74 | 0.003* |
| FNTB | 12.24 ± 2.618 | 11.47 ± 3.235 | W = −25 | 0.41 |
| PGGT1B | 0.633 ± 0.146 | 0.553 ± 0.063 | W = −77 | 0.004* |
| RABGGTA | 0.022 ± 0.006 | 0.019 ± 0.009 | t(12) = 1.14 | 0.28 |
| RABGGTB | 0.445 ± 0.076 | 0.409 ± 0.078 | t(12) = 2.29 | 0.04* |
| Prenylcysteine-processing enzymes | ||||
| RCE | 0.127 ± 0.038 | 0.119 ± 0.033 | W = −11 | 0.74 |
| ICMT | 0.197 ± 0.061 | 0.188 ± 0.055 | t(12) = 0.68 | 0.51 |
Comparison and schizophrenia values are reported as means ± S.D.
*p ≤ 0.05
Transcript levels of prenylation-associated enzymes and prenylated substrates are altered in schizophrenia
Bioinformatic analysis of transcriptomic datasets generated from samples from the MSSM NIH Brain and Tissue Repository revealed that genes associated with prenylation demonstrate altered patterns of gene expression in schizophrenia. Genes encoding for upstream prenyl synthases, prenyltransferase subunits, prenylcysteine-processing enzymes, and some GTPases (substrates of prenylation) exhibit differential expression relative to comparison subjects in one or more of the datasets evaluated (Supplementary Fig. S1, Supplementary Table S1).
Discussion
Neurotransmission, synaptic plasticity, dendritic dynamics, and protein subcellular localization have all been reported to be abnormal in schizophrenia. Prenylation is a cytosolic PTM that enables many GTPases associated with these processes to correctly localize for signaling transduction16,19,21,26,41–44. GTPases have been shown to require combinations of lipid modifications including S-palmitoylation, N-myristoylation, and prenylation, which facilitate membrane-dependent GTPase activity20–24. Given that a deficit in protein S-palmitoylation has been reported in schizophrenia45, we hypothesized that abnormal prenylation may also contribute to altered G-protein signaling pathways implicated in the illness6,17–19. We found protein expression of FNTA, PGGT1B, and RABGGTB prenyltransferase subunits decreased in schizophrenia DLPFC relative to paired comparison subjects, changes not likely due to chronic antipsychotic treatment. Bioinformatic assessments identified patterns of differential gene expression of prenylation-associated enzymes and substrates in schizophrenia. For individual genes, the direction and magnitude of differences appears to vary by brain region and cortical layer; however, identification of prenylation-associated differences across multiple datasets suggests that this functional pathway is involved in this illness. Together, these data are consistent with our previous findings of abnormal lipid modifications in schizophrenia, including abnormal S-palmitoylation and decreased expression of an N-myristoylated protein in schizophrenia DLPFC11,12.
Given that FNTA, PGGT1B, and RABGGTB were decreased, each prenyltransferase enzyme αβ complex then has at least one abnormally expressed subunit, and GGTase I has reduced expression of both its α and β subunits. Previous reports demonstrated transcript-level upregulation of two of these subunits, FNTA and RABGGTB, in schizophrenia superior temporal gyrus (BA 22)46 and prefrontal cortex (BAs 9 and 10)47, respectively. These changes reported for transcript expression are in the opposite direction of the protein expression changes identified in the current study, which might suggest that upstream or downstream regulatory molecules may also be altered in schizophrenia, or may reflect cellular compensation.
Since upstream or downstream factors could be driving the changes in prenyltransferase expression, we also assayed protein expression of the isoprenoid synthases, FDPS and GGPS1, and prenylprotein-processing enzymes, RCE and ICMT. FDPS and GGPS1 catalyze the production of the key intermediates in the mevalonate pathway that are the lipid donors for farnesylation and geranylgeranylation, FPP and GGPP, which are attached to proteins by their respective prenyltransferase(s)26. Following cytosolic prenylation, many prenylproteins are targeted to the endoplasmic reticulum, where they are subject to additional processing steps before they can ultimately be trafficked to the correct membrane destination25. For substrates of FTase and GGTase I that contain a C-terminal “CAAX” amino acid sequence (where C is cysteine, A is any aliphatic amino acid, and X is any amino acid), RCE cleaves the AAX residues from the prenyl-cysteine, and ICMT subsequently catalyzes the methylation of the prenyl-cysteine25. These upstream and downstream enzymes, however, were not found to be differently expressed in schizophrenia DLPFC. Together, these data suggest that isoprenoid lipid donor synthesis and prenyl-cysteine modifications that occur following prenylation are normal in schizophrenia, but the actual attachment of prenyl groups to protein substrates could be impaired.
Many prenylproteins belong to the superfamily of G-proteins, which play key roles in synaptic regulation. Heterotrimeric G-protein α, β, and γ subunits require lipid modifications for membrane targeting, protein interaction, and activity. Gα subunits can be N-myristoylated and/or S-palmitoylated14,22,48, and Gγ subunits are prenylated16. Prenylation is also required for some proteins involved with the regulation of Ca2+ signaling, spinogenesis, and synaptogenesis49,50, pathways which have been implicated in schizophrenia18,43,51,52.
Many members of the Ras protein superfamily of small GTPases, which include Ras, Rho, Rab, Rap, Arf, Ran, and Rheb subfamilies, are also prenylated4. The Rab and Rho protein subfamilies regulate membrane trafficking and cytoskeletal dynamics, as well as spatiotemporal aspects of vesicular recycling within the cell53. These proteins require prenylation for correct interactions with their effector proteins at cell membranes54,55. Ras subfamily GTPases are involved in signal transduction and the regulation of gene expression56 and recent studies suggest that Ras family protein signaling plays a role in memory formation57. Given that pharmacological inhibition of Ras farnesylation prevents signal transduction to downstream targets in cell culture58, decreased expression of functional FTases could inhibit the interaction of Ras proteins with their effectors and contribute to altered intracellular signaling and working memory in schizophrenia. Age-related downregulation of PGGT1B in mice is associated with decreased membrane-associated Rho-GTPases involved in synaptic plasticity. Inhibition of GGTase I in vitro leads to decreased protein levels of synaptophysin and GAP-4359, and these proteins are also decreased in schizophrenia brain60–62. Studies using statins, which inhibit the activity of the mevalonate pathway that produces FPP and GGPP, and prenylation-specific inhibitors in neuronal cultures, have reported decreased dendritic aborization63,64 and increased axonal growth65,66. These reports emphasize that morphological abnormalities can arise from altered prenylation and suggest one potential mechanism contributing to dendritic spine abnormalities in schizophrenia brain43,67.
Often the isoprenyl PTM by itself is not sufficient to maintain a strong membrane association, and many prenylated proteins require a second signal for membrane localization. This second signal is typically the addition of a palmitoyl group or the presence of a polybasic domain4,68. We have previously reported a deficit of protein S-palmitoylation in schizophrenia11, and it is likely that the subset of proteins which require both prenylation and S-palmitoylation are more susceptible to dysfunction if both of these pathways are impaired. Some members of the Ras subfamily are known to be both prenylated and S-palmitoylated, with prenylation occurring upstream of S-palmitoylation4,27,28. Our prior report of decreased S-palmitoylation identified a widespread decrease of S-palmitoylation across many proteins, including Ras, but did not identify alterations in the expression levels of enzymes that attach or cleave palmitoyl groups that would explain the reduction11. Our current finding of reduced prenyltransferase expression suggests that defective prenylation of Ras could potentially prevent the appropriate S-palmitoylation of these molecules, consistent with the findings of our previous study. If this is indeed the case, altered Ras lipidation might impair correct intracellular localization and/or signaling of these small GTPases. Deficits in DLPFC function, circuitry, and working memory have been repeatedly implicated in schizophrenia69,70. Considering that Ras family signaling contributes to memory formation57, abnormal lipid modifications in this brain region may contribute to memory-associated deficits in the disorder.
Postmortem brain studies in schizophrenia inherently have limitations. We evaluated gene expression differences and measured protein expression levels in aged subjects, and these data may not generalize to different age groups or earlier stages of the illness. Our protein study is also restricted to the DLPFC, thus additional brain regions will need to be examined to determine whether these findings are widespread or brain region specific. Furthermore, given that some enzymes measured in this study demonstrate cell-type-specific patterns of expression, prenylation pathway alterations identified here may preferentially impact a distinct subpopulation of cells and cell-type-specific protein measures will be necessary to investigate this possibility. Another major limitation of schizophrenia postmortem studies is that chronic antipsychotic treatment may affect the expression of some proteins independent of the disorder. To rule out the potential effects of long-term antipsychotic use, we assayed these proteins in frontal cortex of rats chronically treated with haloperidol decanoate. We did not find any effects of long-term haloperidol treatment on protein levels of FNTA, PGGT1B, or RABGGTB, which suggests that reduced prenyltransferase subunit expression identified in schizophrenia is likely due to the illness and not a result of chronic antipsychotic treatment. However, it is important to note that the haloperidol studies were not performed in an animal model of schizophrenia, and potential interactions between disease pathophysiology and long-term antipsychotic use that may influence protein prenylation cannot be completely ruled out.
In summary, we found decreased protein expression of the prenyltransferase subunits FNTA, PGGT1B, and RABGGTB in schizophrenia DLPFC as well as evidence from a bioinformatic analysis of differential gene expression of prenylation-associated genes across multiple brain regions and cortical layers. These data are consistent with other evidence of abnormal lipid modifications in schizophrenia11,12 and suggest a potential mechanism for our report of reduced Ras S-palmitoylation in the face of normal palmitoyltransferase expression. Because of its importance in G-protein signaling and small GTPase activity, abnormal prenylation is also a potential mechanism underlying altered intracellular signaling, dendritic dynamics, and subcellular protein localization previously reported in schizophrenia. Decreased protein expression of these prenyltransferase subunits could contribute to many facets of the pathophysiology of schizophrenia by its important role in multiple cell biological processes.
Supplementary information
Acknowledgements
The authors gratefully acknowledge Dr. Rosalinda Roberts and the Alabama Brain Collection for providing materials used to optimize conditions used in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-019-0610-7).
References
- 1.Hamm HE. The many faces of G protein signaling. J. Biol. Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 3.Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2006;2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- 4.Resh MD. Covalent lipid modifications of proteins. Curr. Biol. 2013;23:R431–R435. doi: 10.1016/j.cub.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 6.Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol. Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- 7.Hottman DA, Li L. Protein prenylation and synaptic plasticity: implications for Alzheimer’s disease. Mol. Neurobiol. 2014;50:177–185. doi: 10.1007/s12035-013-8627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanai A, et al. Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function. Nat. Neurosci. 2006;9:824–831. doi: 10.1038/nn1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singaraja RR, et al. Altered palmitoylation and neuropathological deficits in mice lacking HIP14. Hum. Mol. Genet. 2011;20:3899–3909. doi: 10.1093/hmg/ddr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukai J, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat. Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinner AL, Tucholski J, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Decreased protein S-palmitoylation in dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res. 2016;177:78–87. doi: 10.1016/j.schres.2016.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinner AL, Haroutunian V, Meador-Woodruff JH. Alterations of the myristoylated, alanine-rich C kinase substrate (MARCKS) in prefrontal cortex in schizophrenia. Schizophr. Res. 2014;154:36–41. doi: 10.1016/j.schres.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buss JE, Mumby SM, Casey PJ, Gilman AG, Sefton BM. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc. Natl Acad. Sci. USA. 1987;84:7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–1652. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 15.Escriba, P. V., Wedegaertner, P. B., Goni F. M. & Vogler O. Lipid-protein interactions in GPCR-associated signaling. Biochim. Biophys. Acta1768, 836–852 (2007). [DOI] [PubMed]
- 16.Higgins JB, Casey PJ. The role of prenylation in G-protein assembly and function. Cell. Signal. 1996;8:433–437. doi: 10.1016/s0898-6568(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 17.Bychkov ER, Ahmed MR, Gurevich VV, Benovic JL, Gurevich EV. Reduced expression of G protein-coupled receptor kinases in schizophrenia but not in schizoaffective disorder. Neurobiol. Dis. 2011;44:248–258. doi: 10.1016/j.nbd.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catapano L. A., Manji H. K. G protein-coupled receptors in major psychiatric disorders. Biochim. Biophys. Acta1768, 976–993 (2007). [DOI] [PMC free article] [PubMed]
- 19.Datta, D., Arion, D., Corradi, J. P. & Lewis, D. A. Altered expression of CDC42 signaling pathway components in cortical layer 3 pyramidal cells in schizophrenia. Biol. Psychiatry78, 775–785 (2015). [DOI] [PMC free article] [PubMed]
- 20.Casey PJ. Lipid modifications of G proteins. Curr. Opin. cell Biol. 1994;6:219–225. doi: 10.1016/0955-0674(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 21.Casey PJ, Moomaw JF, Zhang FL, Higgins YB, Thissen JA. Prenylation and G protein signaling. Recent Prog. Horm. Res. 1994;49:215–238. doi: 10.1016/b978-0-12-571149-4.50015-5. [DOI] [PubMed] [Google Scholar]
- 22.Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J. Biol. Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- 23.Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem. Sci. 1995;20:181–187.. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- 24.McCallum JF, et al. The role of palmitoylation of the guanine nucleotide binding protein G11 alpha in defining interaction with the plasma membrane. Biochem. J. 1995;310(Pt 3):1021–1027. doi: 10.1042/bj3101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 26.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 27.Rocks O, et al. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 2010;141:458–471. doi: 10.1016/j.cell.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Resh MD. Targeting protein lipidation in disease. Trends Mol. Med. 2012;18:206–214. doi: 10.1016/j.molmed.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powchik P, et al. Postmortem studies in schizophrenia. Schizophr. Bull. 1998;24:325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- 30.Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr. Res. 2005;75:303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Kashihara K, et al. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biol. Psychiatry. 1986;21:650–656. doi: 10.1016/0006-3223(86)90126-5. [DOI] [PubMed] [Google Scholar]
- 32.Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J. Neural Transm. 2009;116:487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stan AD, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roussos P, Katsel P, Davis KL, Siever LJ, Haroutunian V. A system-level transcriptomic analysis of schizophrenia using postmortem brain tissue samples. Arch. Gen. Psychiatry. 2012;69:1205–1213. doi: 10.1001/archgenpsychiatry.2012.704. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, C. R. et al. Neuron-specific deficits of bioenergetic processes in the dorsolateral prefrontal cortex in schizophrenia. Mol. Psychiatry24, 1319–1328 (2018). [DOI] [PMC free article] [PubMed]
- 36.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouillard, A. D. et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford)2016, baw100 (2016). [DOI] [PMC free article] [PubMed]
- 40.Kolde, R. pheatmap: pretty heatmaps. R package version 1.0.10, https://www.rdocumentation.org/packages/pheatmap/versions/1.0.10 (2018).
- 41.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller TM, Remedies CE, Haroutunian V, Meador-Woodruff JH. Abnormal subcellular localization of GABAA receptor subunits in schizophrenia brain. Transl. Psychiatry. 2015;5:e612. doi: 10.1038/tp.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ide M, Lewis DA. Altered cortical CDC42 signaling pathways in schizophrenia: implications for dendritic spine deficits. Biol. Psychiatry. 2010;68:25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Pinner, A. L., Tucholski, J., Haroutunian, V., McCullumsmith, R. E. & Meador-Woodruff, J. H. Decreased protein S-palmitoylation in dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res.177, 78–87 (2016). [DOI] [PMC free article] [PubMed]
- 46.Bowden NA, Scott RJ, Tooney PA. Altered gene expression in the superior temporal gyrus in schizophrenia. BMC Genomics. 2008;9:199. doi: 10.1186/1471-2164-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maycox PR, et al. Analysis of gene expression in two large schizophrenia cohorts identifies multiple changes associated with nerve terminal function. Mol. Psychiatry. 2009;14:1083–1094. doi: 10.1038/mp.2009.18. [DOI] [PubMed] [Google Scholar]
- 48.Wedegaertner PB. Lipid modifications and membrane targeting of G alpha. Biol. Signals Recept. 1998;7:125–135. doi: 10.1159/000014538. [DOI] [PubMed] [Google Scholar]
- 49.Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nat. Rev. Mol. Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- 51.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res. Brain Res. Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 52.Datta D, Arion D, Corradi JP, Lewis DA. Altered expression of CDC42 signaling pathway components in cortical layer 3 pyramidal cells in schizophrenia. Biol. Psychiatry. 2015;78:775–785. doi: 10.1016/j.biopsych.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem. Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- 54.Seabra MC. Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal. 1998;10:167–172. doi: 10.1016/s0898-6568(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 55.Nishimura A, Linder ME. Identification of a novel prenyl and palmitoyl modification at the CaaX motif of Cdc42 that regulates RhoGDI binding. Mol. Cell. Biol. 2013;33:1417–1429. doi: 10.1128/MCB.01398-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hancock JF. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003;4:373–384.. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 57.Ye X, Carew TJ. Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron. 2010;68:340–361. doi: 10.1016/j.neuron.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox AD, et al. The CAAX peptidomimetic compound B581 specifically blocks farnesylated, but not geranylgeranylated or myristylated, oncogenic ras signaling and transformation. J. Biol. Chem. 1994;269:19203–19206. [PubMed] [Google Scholar]
- 59.Afshordel S, Wood WG, Igbavboa U, Muller WE, Eckert GP. Impaired geranylgeranyltransferase-I regulation reduces membrane-associated Rho protein levels in aged mouse brain. J. Neurochem. 2014;129:732–742. doi: 10.1111/jnc.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian SY, Wang JF, Bezchlibnyk YB, Young LT. Immunoreactivity of 43 kDa growth-associated protein is decreased in post mortem hippocampus of bipolar disorder and schizophrenia. Neurosci. Lett. 2007;411:123–127. doi: 10.1016/j.neulet.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 61.Fung SJ, Sivagnanasundaram S, Weickert CS. Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol. Psychiatry. 2011;69:71–79. doi: 10.1016/j.biopsych.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch. Gen. Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- 63.Kim WY, et al. Statins decrease dendritic arborization in rat sympathetic neurons by blocking RhoA activation. J. Neurochem. 2009;108:1057–1071. doi: 10.1111/j.1471-4159.2008.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulz JG, et al. HMG-CoA reductase inhibition causes neurite loss by interfering with geranylgeranylpyrophosphate synthesis. J. Neurochem. 2004;89:24–32. doi: 10.1046/j.1471-4159.2003.02305.x. [DOI] [PubMed] [Google Scholar]
- 65.Li H, et al. Protein prenylation constitutes an endogenous brake on axonal growth. Cell Rep. 2016;16:545–558. doi: 10.1016/j.celrep.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Pooler AM, Xi SC, Wurtman RJ. The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor pravastatin enhances neurite outgrowth in hippocampal neurons. J. Neurochem. 2006;97:716–723. doi: 10.1111/j.1471-4159.2006.03763.x. [DOI] [PubMed] [Google Scholar]
- 67.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 69.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 70.Potkin SG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr. Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.