Abstract
Side effects of proton pump inhibitors (PPI) can be linked to the changes in the intestinal microbiome that occur during therapy, especially in long-term users. Therefore, the microbiome might also be a key player in the reduction of PPI side effects. We tested the effects of a three-month intervention with a multispecies synbiotic on intestinal inflammation, gut barrier function, microbiome composition, routine laboratory parameters and quality of life in patients with long-term PPI therapy. Thirty-six patients received a daily dose of a multispecies synbiotic for three months and were clinically observed without intervention for another three months. After intervention 17% of patients reached normal calprotectin levels; the overall reduction did not reach statistical significance (−18.8 ng/mg; 95%CI: −50.5; 12.9, p = 0.2). Elevated zonulin levels could be significantly reduced (−46.3 ng/mg; 95%CI: −71.4; −21.2; p < 0.001). The abundance of Stomatobaculum in the microbiome was reduced and Bacillus increased during the intervention. Furthermore, albumin, alkaline phosphatase and thrombocyte count were significantly increased and aspartate transaminase was significantly decreased during intervention. Gastrointestinal quality of life showed significant improvements. In conclusion, microbiome-related side effects of long-term PPI use can be substantially reduced by synbiotic intervention. Further studies are warranted to optimize dosage and duration of the intervention.
Subject terms: Microbiota, Nutrition, Quality of life
Introduction
In the last 30 years proton pump inhibitors (PPI) have established themselves as highly effective treatment of acid related diseases and became the most often prescribed class of drugs in gastroenterology1,2. Underneath their immaculate image of gastric protection, however, their side effect profile - although well described by high quality data - remained largely unaddressed3. Many of the side effects might be rooted in PPI-induced changes of the microbiome (i.e. dysbiosis): increased risk of enteric infections, bloating, flatulence, abdominal pain, stool frequency abnormalities, intestinal inflammation and vitamin B12 malabsorption4–12.
Long term PPI use can introduce ample alterations to the intestinal microbiome: Changes in composition, reduction of alpha diversity, small intestinal bacterial overgrowth and the manifestation of oral bacteria in more distal parts of the intestine have been observed13–19. A loss of alpha diversity and therefore a loss in pathogen-colonization resistance could account for the increased risk of enteric infections20,21. The change of composition, which includes the reduction of Clostridiales and the increase of Enterococcaceae and Streptococcaceae, and function of the microbiome might directly predispose for Clostridium difficile infections22. In children, persistent bowel symptoms during PPI use have been linked to small intestinal bacterial overgrowth23. The association with the microbiome could be a crucial factor for the amelioration of PPI side effects. Modulating the microbiome with probiotic bacteria might be able to reduce the burden of side effects during PPI therapies. The merit of this idea has been shown in a trial administering Lactobacillus reuteri together with PPI that could successfully reduce small intestinal bacterial overgrowth in children24. Probiotics also have additional effects of which PPI-treated patients could profit: they have been shown to reduce pathogen growth and drug-induced diarrhoea, ameliorate bowel symptoms, and improve gut barrier and liver function in previous reports25–35. Furthermore, the use of prebiotics (i.e. indigestible dietary fibre) or synbiotics (i.e. combination of probiotics and synbiotics) can support the resident microbiome and complement the effects of probiotic supplementation36. However, since PPI-specific data is still scarce, the routine use of probiotics during PPI therapy is not recommended yet1.
Therefore, we tested the hypothesis that the administration of probiotics reduces PPI side effects. We aimed to show the effects of a three-month intervention with a multispecies synbiotic on intestinal inflammation, gut barrier function, microbiome composition, routine laboratory parameters and gastrointestinal quality of life in patients with long-term PPI therapy.
Results
Patients
Fifty-seven patients with long-term PPI use were screened for the study; eight patients did not meet the inclusion/exclusion criteria (declined to participate: n = 5; PPI therapy to short: n = 2; active infection: n = 1). Forty-nine patients started the intervention and 36 finished it according to the protocol. Reasons for drop-out were withdrawn consent (n = 8), side-effects (gastric pain: n = 1; gastrointestinal discomfort: n = 2; constipation: n = 1) and liver transplantation (n = 1). See also Fig. 1. Patients were on average 63 years (95%CI: 59; 67) old, 47% were female and the average duration of PPI therapy was 63 months (95%CI: 44; 82). Reasons for PPI therapy were peptic ulcer/reflux disease (n = 21), polypharmacy (n = 8) and others (n = 7). Of the 36 analyzed patients, twelve had liver cirrhosis (details are given in Table S1). Patients stayed on their PPI regime throughout the study and did not make substantial changes to their diet. Patients’ characteristics are given in Table 1.
Figure 1.
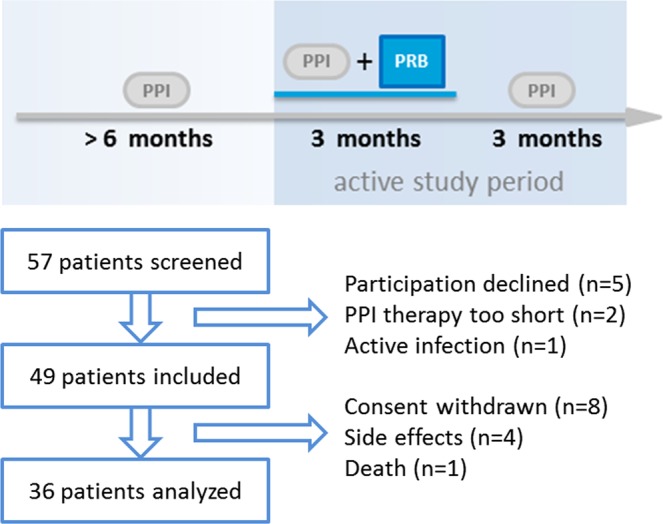
Enrolment scheme and time line.
Table 1.
Characteristics of patients included in the analysis. (PPI: proton pump inhibitor).
| Analyzed patients (n = 36) | |
|---|---|
| Age (years) | 63 (59; 67) |
| Sex (f/m) | 17/19 (47/53%) |
| Duration of PPI (months) | 63 (44; 82) |
| PPI regime: | |
| Pantoprazole | 24 (67%) |
| −20 mg | 3 (8%) |
| −40 mg | 20(56%) |
| −60 mg | 1 (3%) |
| Esomeprazole | 7 (19%) |
| −20 mg | 2 (5%) |
| −40 mg | 5 (14%) |
| others | 5 (14%) |
| Reason for PPI: | |
| Peptic Ulcer/Reflux | 21 (58%) |
| Drug use/Polypharmacy | 8 (22%) |
| others | 7 (20%) |
| Cirrhosis (yes/no) | 12/24 (33/67%) |
Intestinal inflammation
Fecal calprotectin levels at baseline were elevated to 147.1 ng/mg (95%CI: 106.4; 187.8) and decreased on average by 18.8 ng/mg (95%CI: −50.5; 12.9, p = 0.2) during the intervention. Thirty patients (83%) had elevated calprotectin levels (i.e. >50 ng/mg) at baseline. Of these 30 patients, five (17%) showed normal calprotectin levels after the intervention, and therefore reached the primary endpoint. In total, 57% of patients with initially increased calprotectin levels showed a reduction of at least 10% after intervention. Details are given in Table 2.
Table 2.
Baseline values and changes in biomarkers for intestinal inflammation and permeability, liver function and Vitamin B12 levels throughout the study; data are given as mean (95% confidence interval), [aall patients, bselected patients with increased baseline values].
| Before Intervention | After Intervention | Follow up | p-value (Before vs. After) | p-value (Before Intervention vs. Follow-up) | |
|---|---|---|---|---|---|
| Calprotectin (ng/mg)a | 147.1 (106.4;187.8) | 128.3 (92.1; 164.5) | 142.9 (107.3; 178.4) | 0.192 | — |
| - when >50 ng/mg at baselineb | 168.7 (123.7; 213.7) | 142.6 (101.0; 184.2) | 161.5 (122.1; 200.9) | 0.090 | — |
| Zonulin (ng/mg)a | 60.9 (44.4; 77.5) | 51.2 (41.8; 60.6) | 51.9 (40.0; 63.8) | 0.385 | — |
| - when >50 ng/mg at baselineb | 100.1 (73.8; 126.4) | 53.8 (41.5; 66.0) | 48.7 (33.7; 63.8) | <0.001 | 0.007 |
| Lipopolysaccharide (EU/ml) | 1.9 (0.9; 2.9) | 1.6 (0.9; 2.3) | 2.3 (1.2; 3.4) | 0.428 | — |
| Lipopolysaccharide-binding protein (ng/ml) | 14.8 (12.3; 17.2) | 14.6 (12.8; 16.4) | 12.9 (10.9; 15.0) | 0.561 | — |
| sCD14 (µg/ml) | 2.3 (2.1; 2.6) | 2.5 (2.2; 2.8) | 2.1 (1.9; 2.2) | 0.177 | — |
| Albumin (g/dl) | 4.1 (4.0; 4.2) | 4.2 (4.1; 4.3) | 4.2 (4.1; 4.4) | 0.027 | 0.013 |
| Alanine aminotransferase (U/l) | 29.3 (24.2; 34.3) | 27.8 (23.4; 32.1) | 25.5 (22.4; 28.7) | 0.317 | — |
| Aspartate transaminase (U/l) | 32.0 (27.9; 36.1) | 29.1 (25.2; 33.1) | 28.1 (23.9; 32.3) | 0.016 | 0.005 |
| Alkaline Phosphatase (U/l) | 76.1 (67.0; 85.1) | 79.9 (70.9; 89.0) | 79.6 (69.5; 89.7) | 0.004 | 0.006 |
| Thrombocytes (G/l) | 196 (163; 229) | 207 (174; 240) | 216 (185; 247) | 0.010 | 0.138 |
| PZINR | 1.1 (1.0; 1.2) | 1.1 (1.0; 1.2) | 1.0 (0.9; 1.1) | 0.695 | — |
| Vitamin B12 (ng/l) | 406 (355; 457) | 416 (362; 469) | 445 (382; 508) | 0.784 | — |
| C-reactive proteins (mg/l) | 3.4 (2.0; 4.9) | 3.0 (2.1; 3.9) | 3.8 (2.2; 5.5) | 0.388 | — |
Since one third of the study cohort had liver cirrhosis as underlying disease, liver cirrhosis was analyzed as a potential confounder. Calprotectin levels were significantly higher in patients with cirrhosis as shown in Table S1, and the mean decrease after synbiotic intervention was larger in cirrhotic patients compared to liver healthy patients, although not statistically significant (−35.9 ng/mg (95%CI: −129.0; 57.2) vs. −10.2 ng/mg (95%CI: −32.9; 12.4), respectively, p = 0.07).
Gut permeability
Fecal zonulin levels were slightly increased at baseline (60.9 ng/mg, 95%CI: 44.4; 77.5) with 44% of patients showing increased levels (i.e. >50 ng/mg). Patients with zonulin levels > 50 ng/mg at baseline showed significant reduction of −46.3 ng/mg (95%CI: −71.4; −21.2; p < 0.001) during intervention and maintained low zonulin levels for three months after intervention had ended. When patients with elevated and normal zonulin levels were analyzed together, the reduction did not reach statistical significance (p = 0.4). There was no change in lipopolysaccharide, lipopolysaccharide-binding protein or sCD14 levels. For details see Table 2. Zonulin levels at baseline were similar in patients with and without liver cirrhosis and both groups showed comparable decrease during synbiotic intervention (−18.8 ng/mg (95%CI: −55.8; 18.25) vs. −5.2 ng/mg (95%CI: −24.5; 14.1), respectively, p = 0.5).
Gut microbiome
Patients showed microbiome changes consistent with the literature. Compared to a published control cohort, alpha diversity of patients before the intervention was significantly reduced (Chao1: p < 0.001). See also Fig. S1A. Beta diversity was significantly influenced by PPI use as assessed by redundancy analysis (RDA: Variance = 26.26, F = 1.7, p = 0.001) and shown in Fig. S1B. Since one third of the study cohort had liver cirrhosis as underlying disease, liver cirrhosis was analyzed as a potential confounder. Alpha diversity was not significantly different between patient groups, although liver healthy patients showed a somewhat higher Chao1 index as cirrhotic patients (p = 0.07). Beta diversity showed slight changes between the groups (RDA: Variance = 20.04, F = 1.3, p = 0.04). Details are given in Fig. S2. When underlying disease was used as a confounder in LEfSe analysis, PPI users still showed a significantly higher abundance of oral bacteria, such as Streptococcus, Lactobacillus, Veillonella and Rothia compared to controls (full list is provided in Fig. S2).
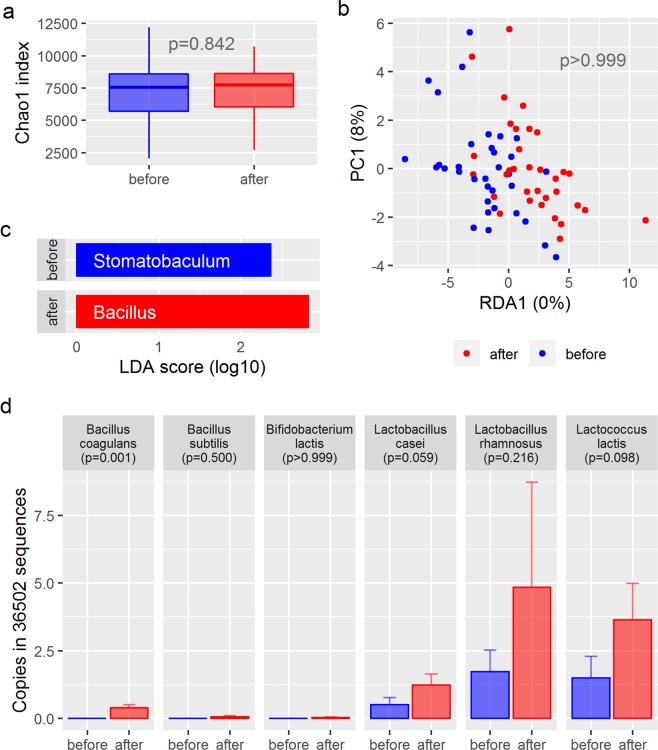
Microbiome composition was not substantially changed after synbiotic intervention. There was no change in alpha diversity as shown in Fig. 2a (Chao1: p = 0.8). Redundancy analysis showed no specific clustering of samples before and after intervention (p > 0.999, Fig. 2b) and no distinct networks could be identified for the two time points. LEfSe attributed the genus Stomatobaculum to samples before intervention and the genus Bacillus to samples after intervention (Fig. 2c). In addition, the family Bacillaceae and an operational taxonomic unit identified as Bacillus coagulans were significantly increased after intervention (p < 0.001 and p = 0.001, respectively). Bacillus coagulans was part of the synbiotic formulation used in this study. The other probiotic strains did not show a significant increase during intervention using 16S rRNA gene sequencing technology (Fig. 2d). No other changes in the microbiome composition could be observed (see Figs. S3 and S4).
Figure 2.
Changes in the microbiome of patients from baseline to end of intervention. (a) Chao1 index as a measurement for alpha diversity before and after intervention compared by paired t-test; (b) Redundancy analysis plot showing minimal changes in the overall beta-diversity of the microbiome during intervention. (c) LEfSe-Plot attributing the genus Stomatobaculum to pre-intervention samples and Bacillus to post-intervention samples. (d) Recovery rate of sequences matching the probiotic bacteria of the study product compared by Wilcoxon rank sum test. Bacteria not shown in this graph were not recovered.
Biochemical parameters
Albumin concentration increased significantly (+0.08 g/dl, 95%CI: 0.01; 0.15; p = 0.03) and aspartate transaminase (AST) concentration decreased significantly after treatment (−2.9U/l, 05%CI: −5.7; −0.1; p = 0.02). Alkaline phosphatase (AP) increased significantly, however within the normal range (+3.9 U/l, 95%CI: 1.2; 6.5; p = 0.004). Changes in albumin, AST and AP were sustained throughout the follow-up period. Thrombocyte count increased slightly after intervention (+11.3G/l, 95%CI: 3.3; 19.3; p = 0.01) but did not exceed the normal range in any case. Thrombocyte count at follow up was unchanged compared to baseline values. Vitamin B12 did not show any changes, however none of the patients had a vitamin B12 deficiency. None of the other biochemistry results were affected by synbiotic intervention. Details are given in Table 2.
AST and AP were significantly higher, and albumin and thrombocyte count were significantly lower in patients with cirrhosis as expected (see Table S1). Nevertheless, mean changes from baseline were comparable between patients with and without cirrhosis (Albumin: 0.05 g/dl (95%CI: −0.06; 0.2) vs. 0.1 g/dl (95%CI: 0.01; 0.2), respectively, p = 0.5; AST: −2.0U/l (95%CI: −9.8; 5.8) vs. −3.3 U/l (95%CI: −5.7; −0.9), respectively, p = 0.5; AP: 4.6 U/l (95%CI: −0.2; 9.4) vs. 3.5 U/l (95%CI: 0.06; 6.9), respectively, p = 0.6; thrombocyte count: 7.2G/l (95%CI: 0.5; 13.9) vs. 13.3G/l (95%CI: 1.6; 24.9), respectively, p = 0.4)
Quality of life
Gastrointestinal quality of life index (GIQLI) improved significantly during the intervention (total score: +8.9 points, 95%CI: 4.0; 13.8, p = 0.001). This was mainly facilitated by an improvement in quality of life related to symptoms and emotions which showed an increase of 4.5 points (95%CI: 1.8; 7.3, 0.002) and 1.2 points (95%CI: 0.3; 2.1, p = 0.01), respectively. While quality of life related to emotions returned to baseline values three months after the intervention had ended, quality of life related to gastrointestinal symptoms remained improved until the end of the follow-up period. The short-form (SF) 36 items and the remaining items of GIQLI remained unchanged throughout the study period. For details see Table 3.
Table 3.
Changes in gastrointestinal and health related quality of life throughout the study; data are given as mean (95% confidence interval). (GIQLI: gastrointestinal quality of life index; SF36: Short Form 36).
| Before Intervention | After Intervention | Follow up | p-value (Before vs. After) | p-value (Before Intervention vs. Follow-up) | |
|---|---|---|---|---|---|
| GIQLI | |||||
| Emotions | 14 (12; 15) | 15 (14; 17) | 14 (13; 16) | 0.010 | 0.719 |
| Medical treatment | 3 (3; 4) | 4 (3; 4) | 3 (3; 3) | 0.101 | — |
| Physical function | 16 (13; 18) | 17 (16; 19) | 16 (14; 18) | 0.089 | — |
| Social function | 12 (11; 13) | 13 (12; 14) | 11 (10; 12) | 0.060 | — |
| Symptoms | 54 (50; 57) | 58 (55; 61) | 55 (52 (59) | 0.002 | 0.028 |
| Total score | 98 (92; 105) | 107 (101; 113) | 100 (93; 107) | 0.001 | 0.159 |
| SF-36 | |||||
| General health | 58 (51; 66) | 57 (51; 64) | 52 (45; 59) | 0.600 | — |
| Social role | 80 (67; 92) | 85 (74; 96) | 76 (61; 91) | 0.507 | — |
| Physical role | 65 (51; 80) | 58 (43; 73) | 55 (38; 72) | 0.233 | — |
| Bodily pain | 60 (50; 71) | 67 (58; 76) | 53 (43; 64) | 0.124 | — |
| Physical functioning | 75 (67; 82) | 76 (69; 82) | 66 (59; 74) | 0.688 | — |
| Mental health | 70 (62; 77) | 73 (66; 79) | 69 (62; 75) | 0.263 | — |
| Social functioning | 79 (71; 88) | 85 (77; 92) | 73 (64; 83) | 0.101 | — |
| Vitality | 54 (46; 61) | 57 (51; 62) | 50 (43; 57) | 0.492 | — |
Cirrhosis did not affect GIQLI scores and their average improvements during synbiotic intervention were comparable between patients with and without cirrhosis (total score: 9.6 points (95%CI: −0.6; 19.7) vs. 8.6 points (95%CI: 2.6; 14.6), respectively, p = 0.6; symptoms: 4.8 points (95%CI: −1.5; 9.9)vs. 4.7 points (95%CI: 1.3; 8.1), respectively, p = 0.9; emotions: 0.5 points (95%CI: −1.1; 2.0) vs. 1.6 points (95%CI: 0.47; 2.7), respectively, p = 0.4).
Discussion
This study shows that a multispecies synbiotic containing twelve strains has the potential to improve PPI side effects after three months of intervention. We observed improvements in gut permeability, liver biochemistry parameters and quality of life; however intestinal inflammation and microbiome composition were unchanged.
Calprotectin levels were increased in the study patients, who were all free of any inflammatory bowel disease. This confirms previous reports that linked PPI use and PPI-associated changes in the microbiome to increased calprotectin levels, irrespective of underlying conditions11,12,37. Although some improvement in intestinal inflammation was observed, the primary endpoint that was defined as a normalization of elevated calprotectin levels was not reached. We were able to achieve a 10% reduction of calprotectin levels in 57% of the patients. It is yet unclear whether calprotectin needs to normalize in order to prevent PPI induced side effects or if a reduction will also provide clinical benefits. Since the average duration of PPI therapy was 63 months, a three-month synbiotic intervention during ongoing PPI treatment might also be too short to ameliorate inflammatory conditions.
On the other hand, zonulin levels were responsive to the intervention and, when elevated at baseline, showed significant reduction after three months and did not increase three months after the synbiotic intervention had stopped. The regulatory mechanism of probiotics on zonulin levels have been shown in trained men after a 14 week long intervention with a multispecies probiotic. Simultaneously, a decrease in TNFα and a reduction of exercise-induced protein oxidation was observed30. Also, perioperative interventions with multispecies probiotics in patients with colorectal cancer and/or colorectal liver metastases could reduce zonulin levels and reduce post-operative bacterial translocation, infectious events and duration of antibiotic therapy38,39. A probiotic intervention in cirrhosis failed to reduce zonulin levels after three and six months40, however, patients’ zonulin levels in that study were comparable to healthy controls. A recent report linked zonulin levels in cirrhosis to PPI-associated dysbiosis37.
Dysbiosis is well described in PPI users; the main features are a loss in diversity, oralization and small intestinal bacterial overgrowth13–15,18. Patients in our study exhibited typical changes in the composition of their microbiome compared to healthy controls without PPI use (details are given as Supplementary Information). Foremost, the increase in predominantly oral bacteria (i.e. oralization), including Streptococcus, Veillonella, Rothia, among others, is evident, as it has been described previously for PPI users15,37. Furthermore, the decrease of beneficial genera has been observed in our patients in accordance to the literature17,19. Unfortunately, changes in the microbiome composition due to the intervention were minimal after three months. Two genera were differentially abundant between the time points, Stomatobaculum and Bacillus. Stomatobaculum is an anaerobic bacterium from dental plaques41. The occurrence of this bacterium in stool can be a sign of oralization of the intestinal microbiome during PPI use. The abundance of Stomatobaculum was reduced during the intervention which might hint towards a potential role of probiotics in correcting PPI-induced dysbiosis (i.e. oralization), although Stomatobaculum is not a very prevalent oral taxon found in the intestinal microbiome of PPI users.
Bacillus increased during intervention, probably due to Bacillus coagulans that was ingested with the study product. Out of the twelve probiotic strains, only Bacillus coagulans was significantly increased. We were previously able to recover more strains in stool of patients with liver cirrhosis who used Ecologic Barrier/OmniBiotic Hetox (Winclove, Amsterdam, The Netherlands/Institut Allergosan Graz, Austria) - a product that shares 7 strains with the product under investigation in this study42. The reason for this difference is still elusive. The formulation and concentration of the study products differ between trials as well as the duration of intervention and the patient collective. Probiotic formulations have a wide range of bacterial content and evidence for dose dependent effects is still scarce43. Different formulations have different minimum concentrations to be effective; in irritable bowel syndrome, B. infantis 35624 could reduce symptoms at a daily dose of 10^8 CFU while higher dosages did not bring any additional benefit44. Other formulations on the other hand, do show dose dependent increases in efficacy45. The daily dose of bacteria in the study product with a total bacterial load of 8 × 109 was lower than other popular probiotics such as the original VSL#3 (Alphasigma, Covington, USA) or Ecologic Barrier (Winclove, Amsterdam, The Netherlands). These formulations contain 5 × 1011 and 1.5 × 1010 CFU per dose respectively and consist of eight/nine distinctive probiotic strains compared to twelve strains in the study product40,46. It is therefore possible that the concentration of each individual strain was too low to be detected by the sequencing effort in this study. Whether a higher dose of the study product could intensify the effects in patients with long-term PPI therapy, can only be clarified in additional clinical studies.
Furthermore, we observed a slight but significant increase in albumin levels and a decrease in the levels of the liver enzyme AST. The positive effects of probiotics on AST in non-alcoholic fatty liver disease are well documented47,48. Moreover, probiotic interventions in patients with and without different liver diseases were also attested a significant reduction of AST49. Albumin is an indicator for liver function and for the absence of inflammation. Besides liver function, albumin also reflects the nutritional status of a patient. However, nutritional habits stayed unchanged throughout the study and the patients were all well-nourished, excluding nutrition as a possible reason for the increase in albumin in this study. Alkaline phosphatase also increased significantly during the intervention and higher levels were sustained during the follow-up period. Only 3/36 measurements were outside the normal range at the end of intervention, all 3 were within 1.5x of the upper limit of normal and gamma-glutamyl-transferase did not increase. This excludes cholestatic liver damage as possible source for the increase in AP. Human AP in serum is a mixture of several isoenzymes with different functions and AP levels can also be influenced by age, diet and various diseases50. Serum AP outside the normal range has been associated with higher mortality51,52. However, other studies show that AP can also exert beneficial effects. In animal models of necrotizing enterocolitis, intestinal AP administration could reduce systemic inflammation53 and protect gut barrier function in neonatal rats54. Moreover, in-vitro studies have indicated intestinal AP in the up-regulation of tight junction proteins55. Recombinant AP is under investigation to prevent sepsis-associated acute kidney injury in humans56, however, the phase II study failed to show a clinical benefit57. In addition to mammalian isoenzymes, several probiotic strains in the study product have shown substantial AP activity, which might contribute to the detoxification of lipopolysaccharide58. Whether these enzymes translocate through the intestinal barrier and contribute to the total serum AP activity is not clear. A more comprehensive study of AP isoenzymes is warranted to answer this question. Taken together the changes in AST and albumin speak to the hepatoprotective effect of probiotics that has been indicated in previous reports40,59,60.
Quality of life is an important outcome assessor and reflects the impact of health care interventions on the patients’ everyday life and well-being61,62. The improvement in quality of life in the present study was based on a reduction of bowel symptoms and negative emotions. This reflects previously shown positive effects of probiotics on bowel symptoms in irritable bowel syndrome and functional bowel disorder63–65. Bacillus coagulans as a single-strain synbiotic formulation has been shown to reduce abdominal pain and diarrhea in irritable bowel syndrome patients66. Bacillus coagulans was also the most prominent probiotic strain in the presented study, which sparks interest in the modes of action of this probiotic bacterium. The microbiome can influence quality of life and features of depression, and the potential to improve sad mood by modulating the microbiome with probiotics has been demonstrated before67,68. This is in accordance to the improved quality of life in regards to emotions that has been observed in the presented study. There were no changes in quality of life measured with the SF-36, although both questionnaires deal with patients’ emotions. A previous report by Shi et al. found that even though both instruments show validity on their own and there are clear correlation between the outcomes of the two instruments, GIQLI showed superior responsiveness in regards to emotional well-being in patients with cholecystectomy69. It, therefore, seems to be more suitable for studying the impact of interventions in gastrointestinal diseases. Regardless of the questionnaire, quality of life is a subjective measure and the effects observed in this study need to be validated in a blinded, controlled trial.
To our knowledge this is the first study analyzing the effect of a synbiotic intervention on intestinal inflammation, gut permeability, microbiome composition, liver function and quality of life in long term PPI users. So far only one small study addressed the effect of probiotics in children receiving a 12 week treatment with PPI for gastroesophageal reflux. In this study no microbiome sequencing data were presented, but small intestinal bacterial overgrowth (measured with a hydrogen breath test) could be reduced significantly24.
The foremost limitation of this study is the design as open labelled, uncontrolled trial. To our knowledge comparable trials in adults to address PPI-induced dysbiosis are missing. Therefore, a pilot trial was necessary on which decisions about endpoint and sample size for a full-sized randomized control trial can be based. A full-sized randomized control trial without pilot data would not have been economically feasible. This trial was supposed to detect potential targets for synbiotic interventions in patients with long-term PPI use, in order to inform upcoming trials by us and others. The primary and many of the secondary endpoints are hard endpoints likely not influenced by the lack of a placebo control; however, the chosen trial design limits the interpretation of the more subjective measures, such as quality of life. The dropout rate of 27% was higher than expected (20%). Adverse effects related to abdominal discomfort accounted for one third of drop-outs. Since we performed the study as an open-label pilot trial, we are not able to discuss the relation of the product to the side effects. In a previous study using a multispecies probiotic containing 9 strains (7 of which are also present in the study product of this study) a much lower dropout rate (2%) and no side effects were observed in the treated group; in the placebo group the drop-out rate was 23%40. Generally, there is little information about the side-effect profile of individual strains, therefore a meaningful discussion about adverse effects of specific probiotics in this and other trials is not yet possible and comprehensive studies are warranted.
In conclusion: Synbiotic interventions have the potential to improve side effects of long-term PPI therapy in cirrhotic and liver healthy patients. Future studies are necessary to validate the here presented benefits and may find valuable therapeutic targets in gut permeability, liver function parameters and quality of life.
Methods
Between September 2017 and March 2018 an open-label pilot trial was conducted at the University Hospital Graz/Austria (Division of Gastroenterology and Hepatology). Patients from the department’s outpatient clinic were included in the study if they were at least 18 years old, gave written informed consent und had a history of continuous PPI use for at least 6 months. Exclusion criteria were active infections at time of inclusion, antibiotic therapy including prophylactic therapy within 14 days of inclusion, inflammatory bowel disease, consumption of pre-, pro- or synbiotics other than the study product, and concomitant diseases or circumstances that suggests that the patient is not eligible. A daily dose of 4 g of a multispecies probiotic (Institute Allergosan, Graz, Austria/Winclove Probiotics, Amsterdam, The Netherlands) was administered to all patients for three months followed by a three-month observation period without intervention. The study product contained 12 strains (Bacillus coagulans W183, Bacillus subtilis W201, Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Bifidobacterium lactis W51, Lactobacillus acidophilus W37, Lactobacillus acidophilus W22, Lactobacillus casei W56, Lactobacillus rhamnosus W71, Lactobacillus salivarius W24, Lactococcus lactis W19, Propionibacterium freudenreichii W200) in a total concentration of 2 × 109 cfu/g. The probiotic strains were selected based on their capacity to inhibit pathogens (Salmonella, E. coli and C. difficile), improve intestinal barrier function and the ability to produce vitamins. The bacteria were embedded in a prebiotic matrix of corn starch, maltodextrin, fructo-oligosaccharide P6, inulin P2 and vegetable protein. Patients dissolved the powder in 125 ml of water and drank the suspension after a 10 minute activation period.
Primary endpoint was the normalization of calprotectin levels in stool; this was also the basis for the sample size calculation. Secondary endpoints were gut permeability (assessed by faecal zonulin levels, serum levels lipopolysaccharide, lipopolysaccharide-binding protein and sCD14), microbiome composition, gastrointestinal and health-related quality of life [assessed by gastrointestinal quality of life index (GIQLI) and Short Form (SF)-36 questionnaires], vitamin B12 levels and routine laboratory parameters. Dietary habits were tracked by an extensive, validated food frequency questionnaire70.
The study protocol was approved by the Research Ethics Board of the Medical University of Graz (29-552 ex 16/17) and was registered prior to the inclusion of the first patient at clinicaltrials.gov (NCT03220802, 18.07.2017). All study procedures were performed according to the Declaration of Helsinki and Good Clinical Practice. For more detailed information, the study protocol is provided as Supplementary Information.
Laboratory assessments
Commercially available ELISA were used to assess calprotectin and zonulin in stool (Immundiagnostik, Bensheim, Germany), as well as LBP and sCD14 in plasma (Hycult Biotechnology, Uden, Netherlands and R&D Systems, Abbington, UK, respectively). Lipopolysaccharide was measured with a cell-based detection system (Invitrogen, Toulouse, France) using an adapted protocol40. Reference values for normal calprotectin levels (<50 ng/mg) were provided by the manufacturer. Reference values for low zonulin levels (<50 ng/mg) were established in our laboratory as recommended by the manufacturer with samples from healthy volunteers with normal calprotectin levels.
Microbiome analysis
Stool samples were collected by the patients in sterile collection tubes either on the same day or the evening before the study visit. Samples were kept on 4 °C until arrival at the hospital and then frozen immediately at −80 °C. DNA was isolated with the MagNA Pure LC DNA Isolation Kit III (Bacteria, Fungi) (Roche, Mannheim, Germany) according to manufacturer’s instructions. Hypervariable region V1-V2 was amplified (primers: 27F-AGAGTTTGATCCTGGCTCAG; R357-CTGCTGCCTYCCGTA) and sequenced using Illumina Miseq technology (Illumina, Eindhoven, Netherlands) as described before71. Paired end reads were then joined by fastq-join tool. Primers were removed by cutadapt 1.6 and USEARCH 6.172 was used for reference based chimera detection. Open reference operational taxonomic unit picking was done with SILVA v132 as reference database. When necessary, sequences were blasted in the NCBI database for further classification73. Clustering was performed by UCLUST74 with a 97% sequence similarity threshold. Fasttree was used to generate a phylogenetic tree. After pre-processing, an average of 68 014 (range from 36 502 to 89 457) reads per sample was available. Data was normalized by rarefaction (sampling depth: 36 502 reads). Rarefied data was used for alpha diversity assessment with Chao1 index and beta diversity assessment with redundancy analysis with one or two explanatory variables. LDA effect size (LEfSe) was used to find differentially abundant taxa on family, genus and OTU level. An LDA score (log10) of 2 or higher was regarded as significant. Network analysis was done based on Spearman correlation where only positive correlations were taken into account. Data analysis was performed with QIIME1.9.175 implemented on a local Galaxy instance (https://galaxy.medunigraz.at/), redundancy analysis, network analysis and linear discriminant analysis effect size (LEfSe) were done in the web-based software Calypso 8.84 (http://cgenome.net/wiki/index.php/Calypso)76.
In order to establish that patients included in the study show PPI-associated dysbiosis, an already published cohort71 of 19 healthy controls without PPI intake was used to compare the composition of the microbiome between the groups. A brief description of the control cohort is given in Table S2.
Statistical analysis
All patients who finished the study per protocol were included in the analysis. Count data is presented as absolute numbers and percentages, continuous data as means and 95% confidence intervals. Differences between values before and after intervention/follow up were evaluated with Wilcoxon signed-rank tests. SPSS 23 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis and the R packages “ggplot2” and “ggpubr” for visualization77–79.
Data availability
Sequencing data is made publicly available at the NCBI Sequence Read Archive under the accession number PRJNA532853. All other data is shared upon reasonable request sent to the corresponding author.
Supplementary information
Acknowledgements
We want to thank Ingeborg Klymiuk at the Center for Medical Research in Graz for her help with the 16S rRNA gene sequencing. The study was funded by MEFOgraz (Vereinigung Forschungsförderung Meduni Graz). The study product was produced by Wincolve BV (Amsterdam, the Netherlands) and provided free of charge by Institut Allergosan (Graz, Austria). The funding institution was not involved in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Author contributions
A.H. analyzed the data, interpreted the results, drafted the manuscript and obtained funding, B.L. contributed to the design of the study, N.F., M.S., I.K., F.R., A.B. acquired data, V.S. designed the study, interpreted the results, drafted the manuscript and obtained funding. All authors read and approved the final version of the manuscript.
Competing interests
A.H. received travel grants from Winclove Probiotics, outside the submitted work; V.S. received personal fees from Institut Allergosan, personal fees from Winclove Probiotics, grants from Fresenius Kabi Austria and personal fees from Ferring, outside the submitted work; B.L., N.F., M.S., I.K., F.R., A.B. have nothing to disclose. All authors have read the journal’s policy on conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59550-x.
References
- 1.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson S, Langstrom G, Rikner L, Carlsson R, Naesdal J. Omeprazole and H2-receptor antagonists in the acute treatment of duodenal ulcer, gastric ulcer and reflux oesophagitis: a meta-analysis. European journal of gastroenterology & hepatology. 1995;7:467–475. [PubMed] [Google Scholar]
- 3.Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nature reviews. Gastroenterology & hepatology. 2017;14:697–710. doi: 10.1038/nrgastro.2017.117. [DOI] [PubMed] [Google Scholar]
- 4.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. The American journal of gastroenterology. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 5.Leonard Jennifer, Marshall John K., Moayyedi Paul. Systematic Review of the Risk of Enteric Infection in Patients Taking Acid Suppression. The American Journal of Gastroenterology. 2007;102(9):2047–2056. doi: 10.1111/j.1572-0241.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 6.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Alimentary pharmacology & therapeutics. 2011;34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 7.Compare D, et al. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. European journal of clinical investigation. 2011;41:380–386. doi: 10.1111/j.1365-2362.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmulson MJ, Frati-Munari AC. Bowel symptoms in patients that receive proton pump inhibitors. Results of a multicenter survey in Mexico. Revista de gastroenterologia de Mexico. 2019;84:44–51. doi: 10.1016/j.rgmx.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Marcuard SP, Albernaz L, Khazanie PG. Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12) Annals of internal medicine. 1994;120:211–215. doi: 10.7326/0003-4819-120-3-199402010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Therapeutic advances in drug safety. 2013;4:125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen M. Proton pump inhibitors may cause elevation in faecal calprotectin levels. The British journal of general practice: the journal of the Royal College of General Practitioners. 2016;66:350. doi: 10.3399/bjgp16X685813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poullis, A., Foster, R., Mendall, M. A., Shreeve, D. & Wiener, K. Proton pump inhibitors are associated with elevation of faecal calprotectin and may affect specificity. European journal of gastroenterology & hepatology15, 573–574, author reply 574 (2003). [DOI] [PubMed]
- 13.Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42. doi: 10.1186/2049-2618-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. Journal of gastroenterology. 2018;53:27–36. doi: 10.1007/s00535-017-1371-9. [DOI] [PubMed] [Google Scholar]
- 15.Imhann F, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clooney AG, et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Alimentary pharmacology & therapeutics. 2016;43:974–984. doi: 10.1111/apt.13568. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol. 2014;307:G951–957. doi: 10.1152/ajpgi.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda A, et al. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clinical and translational gastroenterology. 2015;6:e89. doi: 10.1038/ctg.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson MA, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, McLean JS, Guo L, Lux R, Shi W. The social structure of microbial community involved in colonization resistance. The ISME Journal. 2014;8:564–574. doi: 10.1038/ismej.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case TJ. Invasion resistance arises in strongly interacting species-rich model competition communities. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9610–9614. doi: 10.1073/pnas.87.24.9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedberg DE, et al. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015;149:883–885.e889. doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieczkowska A, Landowski P, Zagozdzon P, Kaminska B, Lifschitz C. Small Bowel Bacterial Overgrowth Associated with Persistence of Abdominal Symptoms in Children Treated with a Proton Pump Inhibitor. The Journal of pediatrics. 2015;166:1310–1312 e1311. doi: 10.1016/j.jpeds.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. Is It Useful to Administer Probiotics Together With Proton Pump Inhibitors in Children With Gastroesophageal Reflux? Journal of neurogastroenterology and motility. 2018;24:51–57. doi: 10.5056/jnm17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson ML, et al. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Applied and environmental microbiology. 1993;59:15–20. doi: 10.1128/AEM.59.1.15-20.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lidbeck A, Gustafsson JA, Nord CE. Impact of Lactobacillus acidophilus supplements on the human oropharyngeal and intestinal microflora. Scandinavian journal of infectious diseases. 1987;19:531–537. doi: 10.3109/00365548709032419. [DOI] [PubMed] [Google Scholar]
- 27.Blaabjerg Sara, Artzi Daniel, Aabenhus Rune. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis. Antibiotics. 2017;6(4):21. doi: 10.3390/antibiotics6040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattani R, Palda VA, Hwang SW, Shah PS. Probiotics for the prevention of antibiotic-associated diarrhea and Clostridium difficile infection among hospitalized patients: systematic review and meta-analysis. Open medicine: a peer-reviewed, independent, open-access journal. 2013;7:e56–67. [PMC free article] [PubMed] [Google Scholar]
- 29.Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World journal of gastroenterology. 2015;21:3072–3084. doi: 10.3748/wjg.v21.i10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamprecht M, et al. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. Journal of the International Society of Sports Nutrition. 2012;9:45. doi: 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World journal of hepatology. 2015;7:425–442. doi: 10.4254/wjh.v7.i3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 33.Lata J, et al. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. European journal of gastroenterology & hepatology. 2007;19:1111–1113. doi: 10.1097/MEG.0b013e3282efa40e. [DOI] [PubMed] [Google Scholar]
- 34.Dhiman RK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–1337.e1323. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Kirpich IA, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol (Fayetteville, N.Y.) 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krumbeck JA, Maldonado-Gomez MX, Ramer-Tait AE, Hutkins RW. Prebiotics and synbiotics: dietary strategies for improving gut health. Current opinion in gastroenterology. 2016;32:110–119. doi: 10.1097/mog.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 37.Horvath A, et al. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Scientific reports. 2019;9:12000. doi: 10.1038/s41598-019-48352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC gastroenterology. 2015;15:34. doi: 10.1186/s12876-015-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu ZH, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. The American journal of clinical nutrition. 2013;97:117–126. doi: 10.3945/ajcn.112.040949. [DOI] [PubMed] [Google Scholar]
- 40.Horvath A., Leber B., Schmerboeck B., Tawdrous M., Zettel G., Hartl A., Madl T., Stryeck S., Fuchs D., Lemesch S., Douschan P., Krones E., Spindelboeck W., Durchschein F., Rainer F., Zollner G., Stauber R. E., Fickert P., Stiegler P., Stadlbauer V. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Alimentary Pharmacology & Therapeutics. 2016;44(9):926–935. doi: 10.1111/apt.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sizova MV, et al. Stomatobaculum longum gen. nov., sp. nov., an obligately anaerobic bacterium from the human oral cavity. International journal of systematic and evolutionary microbiology. 2013;63:1450–1456. doi: 10.1099/ijs.0.042812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath A, et al. Changes in the intestinal microbiome during probiotic intervention in cirrhosis. Journal of Hepatology. 2019;70:e625–e854. doi: 10.1016/S0618-8278(19)31290-3. [DOI] [Google Scholar]
- 43.Sanders Mary Ellen. Probiotics: Definition, Sources, Selection, and Uses. Clinical Infectious Diseases. 2008;46(s2):S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 44.Whorwell PJ, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. The American journal of gastroenterology. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 45.Szulińska Monika, Łoniewski Igor, van Hemert Saskia, Sobieska Magdalena, Bogdański Paweł. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients. 2018;10(6):773. doi: 10.3390/nu10060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venturi A, et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Alimentary pharmacology & therapeutics. 1999;13:1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 47.Ma YY, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World journal of gastroenterology. 2013;19:6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A. SL, D. VR, Manohar T, A. AL. Role of Probiotics in the Treatment of Nonalcoholic Fatty Liver Disease: A Meta-analysis. Euroasian journal of hepato-gastroenterology. 2017;7:130–137. doi: 10.5005/jp-journals-10018-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalesi S, et al. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis. European journal of nutrition. 2018;57:2037–2053. doi: 10.1007/s00394-017-1568-y. [DOI] [PubMed] [Google Scholar]
- 50.Le DMH, Millan JL. Structural evidence of functional divergence in human alkaline phosphatases. The Journal of biological chemistry. 2002;277:49808–49814. doi: 10.1074/jbc.M207394200. [DOI] [PubMed] [Google Scholar]
- 51.Regidor DL, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. Journal of the American Society of Nephrology: JASN. 2008;19:2193–2203. doi: 10.1681/asn.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gleissenthall GV, et al. Tryptophan metabolism in post-withdrawal alcohol-dependent patients. Alcohol and alcoholism. 2014;49:251–255. doi: 10.1093/alcalc/agu011. [DOI] [PubMed] [Google Scholar]
- 53.Riggle KM, et al. Intestinal alkaline phosphatase prevents the systemic inflammatory response associated with necrotizing enterocolitis. The Journal of surgical research. 2013;180:21–26. doi: 10.1016/j.jss.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rentea RM, et al. Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. Journal of pediatric surgery. 2012;47:1135–1142. doi: 10.1016/j.jpedsurg.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 55.Liu W, et al. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. Journal of the American College of Surgeons. 2016;222:1009–1017. doi: 10.1016/j.jamcollsurg.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters E, Masereeuw R, Pickkers P. The potential of alkaline phosphatase as a treatment for sepsis-associated acute kidney injury. Nephron. Clinical practice. 2014;127:144–148. doi: 10.1159/000363256. [DOI] [PubMed] [Google Scholar]
- 57.Pickkers P, et al. Effect of Human Recombinant Alkaline Phosphatase on 7-Day Creatinine Clearance in Patients With Sepsis-Associated Acute Kidney Injury: A Randomized Clinical Trial. Jama. 2018;320:1998–2009. doi: 10.1001/jama.2018.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemert Saskia Van, Ormel Geline. Influence of the Multispecies Probiotic Ecologic<sup>®</sup> BARRIER on Parameters of Intestinal Barrier Function. Food and Nutrition Sciences. 2014;05(18):1739–1745. doi: 10.4236/fns.2014.518187. [DOI] [Google Scholar]
- 59.Shukla S, Shukla A, Mehboob S, Guha S. Meta-analysis: the effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Alimentary pharmacology & therapeutics. 2011;33:662–671. doi: 10.1111/j.1365-2036.2010.04574.x. [DOI] [PubMed] [Google Scholar]
- 60.Stadlbauer V, et al. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J. Hepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Sousa KH, Williamson A. Symptom status and health-related quality of life: clinical relevance. Journal of advanced nursing. 2003;42:571–577. doi: 10.1046/j.1365-2648.2003.02660.x. [DOI] [PubMed] [Google Scholar]
- 62.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. Journal of the American Society of Nephrology: JASN. 2001;12:2797–2806. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 63.Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. American family physician. 2017;96:170–178. [PubMed] [Google Scholar]
- 64.Hungin APS, Mitchell CR, Whorwell P, Mulligan C, Cole O. Systematic review: probiotics in the management of lower gastrointestinal symptoms - an updated evidence-based international consensus. 2018;47:1054–1070. doi: 10.1111/apt.14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ringel-Kulka T, et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. Journal of clinical gastroenterology. 2011;45:518–525. doi: 10.1097/MCG.0b013e31820ca4d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogha M, Esfahani MZ, Zargarzadeh AH. The efficacy of a synbiotic containing Bacillus Coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterology and hepatology from bed to bench. 2014;7:156–163. [PMC free article] [PubMed] [Google Scholar]
- 67.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behavior, and Immunity. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Valles-Colomer Mireia, Falony Gwen, Darzi Youssef, Tigchelaar Ettje F., Wang Jun, Tito Raul Y., Schiweck Carmen, Kurilshikov Alexander, Joossens Marie, Wijmenga Cisca, Claes Stephan, Van Oudenhove Lukas, Zhernakova Alexandra, Vieira-Silva Sara, Raes Jeroen. The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology. 2019;4(4):623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 69.Shi H-Y, et al. Responsiveness and Minimal Clinically Important Differences after Cholecystectomy: GIQLI Versus SF-36. Journal of Gastrointestinal Surgery. 2008;12:1275. doi: 10.1007/s11605-008-0526-7. [DOI] [PubMed] [Google Scholar]
- 70.Haftenberger M, et al. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutrition journal. 2010;9:36. doi: 10.1186/1475-2891-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stadlbauer V, et al. Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Scientific reports. 2017;7:15601. doi: 10.1038/s41598-017-15650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics (Oxford, England) 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coordinators NR. Database resources of the National Center for Biotechnology Information. Nucleic Acids Research. 2016;44:D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics (Oxford, England) 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 75.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zakrzewski M, et al. Calypso: a user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics (Oxford, England) 2017;33:782–783. doi: 10.1093/bioinformatics/btw725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots, https://cran.r-project.org/package=ggpubr (2018).
- 78.Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag New York, 2009).
- 79.R_Core_Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria., https://www.R-project.org/ (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data is made publicly available at the NCBI Sequence Read Archive under the accession number PRJNA532853. All other data is shared upon reasonable request sent to the corresponding author.