Introduction
Glyphosate is the primary active ingredient in a number of publically available herbicides. Since the primary target of glyphosate is an enzyme found only in plants, mammalian toxicity of glyphosate has been assumed to be low.1 However, numerous case reports documenting toxic effects of glyphosate exposure in humans have been published.2,3 There has been a recent increase in media coverage as studies are ongoing regarding the potential effects of glyphosate.4 The primary cause of death in fatal cases of glyphosate exposure is not known, although several small studies indicate arrhythmias secondary to corrected QT (QTc) prolongation as the likely cause.2 Neurological effects, including confusion and coma, have also been documented.3
The following case report describes a young woman, exposed to high-concentrate glyphosate, who presented with syncope in the setting of electrocardiogram (ECG) findings of a left bundle branch block (LBBB), which evolved into a type I Brugada pattern.
Case report
A 30-year-old woman with past medical history of asthma presented after a witnessed syncopal episode, without prodrome, at home. Per her family, she was unconscious for at least 30 seconds and spontaneously awoke without requiring cardiopulmonary resuscitation. Family and patient denied any postsyncopal symptoms. The patient denied any history of prior syncopal events. She denied any use of illicit drugs or alcohol. She is a 1-pack-per-day smoker. Family history was positive for stroke in mother. There was no history of sudden cardiac death in her family. Further review of symptoms was positive for a rash on her hands and 10-pound weight gain over 3 months. Home medications consisted of albuterol inhaler and Valium 0.5 mg that the patient reported taking approximately “5 times per month.”
Upon arrival to the emergency room, the patient’s vital signs were as follows: temperature of 99.3°F, heart rate of 78 beats per minute, blood pressure of 79/52, respiratory rate of 20, SpO2 of 94%. The patient was given an intravenous bolus of 2 liters of lactated Ringer’s solution, and within 30 minutes her blood pressure had improved (to 130s/70s). Prior to laboratory results and after initial ECG, the patient was treated with 20 mg bicarbonate, 10 units of insulin with dextrose, and calcium gluconate because of presumed hyperkalemia. Comprehensive metabolic panel and complete blood count were both unremarkable, including no electrolyte abnormalities. Brain natriuretic peptide was within normal limits and troponin levels were negative, below the limit of detection for our laboratory. Urine toxicology was negative for all metabolites. Cardiac examination showed regular rate and rhythm without murmur. No S3 or S4 were noted. Jugular venous pressure was <5 cm at 45 degrees and no pedal edema was present. Pulmonary examination was unremarkable. The patient was fully alert and oriented, with no overt neurological deficits on examination.
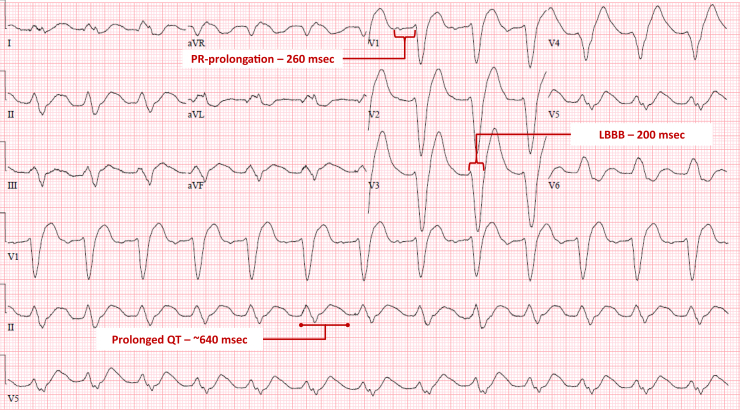
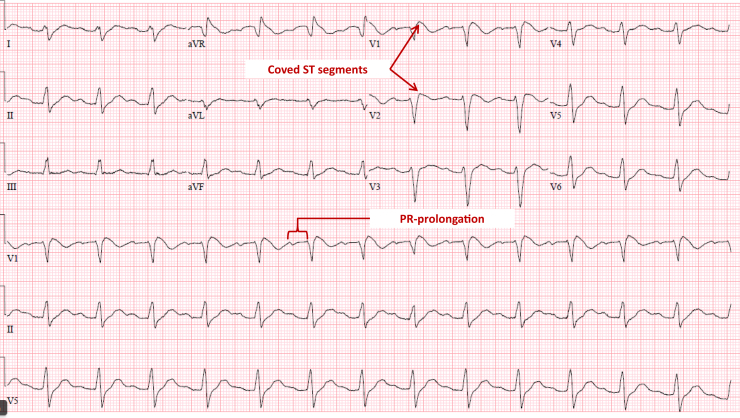
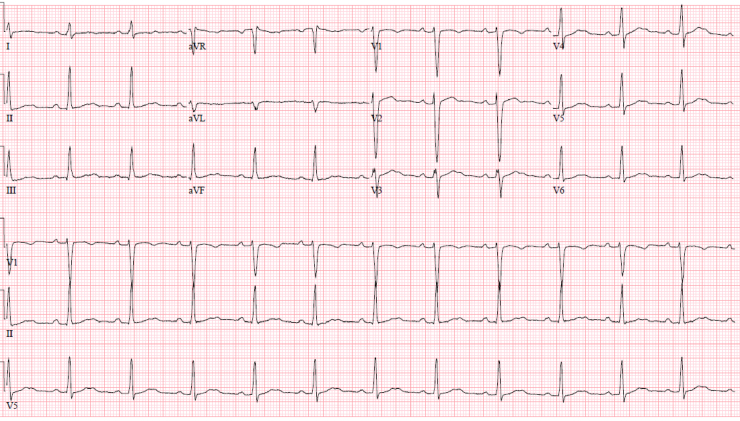
Baseline ECG from 10 years prior showed sinus tachycardia (Supplemental Figure). ECG on admission to this hospitalization, obtained approximately 4 hours post syncope, showed sinus rhythm at 75 beats per minute with first-degree atrioventricular (AV) block (PR 260 ms), LBBB (QRS 200 ms), and significantly prolonged QTC (670 ms) (Figure 1). An ECG done 8 hours after syncope showed sinus rhythm, prolonged PR interval, and coved ST segment in leads V1-V2, consistent with a Brugada type 1 pattern (Figure 2). ECG done on hospital day 2 demonstrated sinus rhythm with normal PR interval and narrow QRS (Figure 3).
Figure 1.
Initial electrocardiogram on presentation. Four hours after syncope. LBBB = left bundle branch block.
Figure 2.
Electrocardiogram 8 hours after syncope.
Figure 3.
Electrocardiogram on day 2 of hospitalization.
During the admission, the patient did not experience any significant cardiac or respiratory symptoms. In addition to the fluid resuscitation initiated in the emergency department (2 liters), the patient received an additional 2 liters of normal saline during hospital day 1. However, throughout the remainder of the admission, the patient spontaneously diuresed 8.5 liters and was net negative approximately 2.5 liters at time of discharge. No diuretics were administered. In addition to electrolyte imbalances (which were excluded in the emergency room), our differential for her syncope and ECG changes included medication adverse effects, other drug toxicity, or a genetic sodium channel disorder. Throughout the hospitalization, 3 separate rounding teams inquired about suicidal intentions, initiation of new medications, and illicit drug use. The patient consistently denied the above on each occasion. Exposure to herbicides or pesticides was discussed on day 1, which the patient initially denied. On hospital day 2, after mentioning specific brands of pesticides and herbicides, she admitted that she had been using Roundup in her yard. She reported using Roundup super concentrate (50% glyphosate concentrate) the prior evening. She had been using this product regularly while doing yard work for several weeks, without any protective gloves. On several occasions, including the day prior, she had spilled a small amount of the solution on her hands. The patient consistently reported this exposure was accidental, denying any intentional ingestion. She further denied any depressive symptoms, suicidal ideation, or prior history of self-harm.
Imaging studies, including chest radiograph and noncontrast computed tomography head and abdomen, were unremarkable. A noncontrast computed tomography chest showed mild pulmonary edema. Telemetry monitoring did not identify any further arrhythmias. Further workup with transthoracic echocardiogram showed normal ejection fraction and paradoxical septal wall motion. Cardiac magnetic resonance imaging was also unremarkable, without evidence of late gadolinium enhancement or myocardial inflammation. She was discharged on hospital day 3 with scheduled follow-up. An ambulatory patch monitor, worn for 14 days, subsequently showed sinus rhythm with narrow QRS and no arrhythmias.
Discussion
This patient presented with syncope that was concerning for a cardiogenic etiology in the setting of an LBBB that progressed to a Brugada type 1 pattern and then normalized within 24 hours. There were no clear causes or exposures other than concentrated glyphosate, whose cardiovascular side effects are poorly understood. However, case reports have previously shown QTc prolongation, intraventricular conduction delays, and first-degree AV block associated with glyphosate exposure.2 Interestingly, on our patient’s initial ECGs (Figures 1 and 2), she had transient PR prolongation. There have also been at least 3 cases in which patients exposed to glyphosate developed ventricular tachycardia and subsequent death.5 Primary skin exposure usually leads to rash and does not lead to significant systemic absorption. The primary form of exposure leading to toxicity is ingestion. In our patient, she had been using 50% glyphosate often for several weeks. It is possible that repeated skin exposure may have allowed for systemic accumulation of toxic doses of glyphosate.
Many other possible etiologies of ECG abnormalities were considered in this patient. The ECG abnormalities on presentation (Figure 1) can be observed in severe hyperkalemia as well as tricyclic antidepressant overdose.6, 7, 8 However, the patient’s potassium level, which was drawn prior to any treatments given, was normal at 4.8 mmol/L (lab reference range 3.5–5.3 mmol/L) at time of admission and remained normal during the hospitalization. Magnesium levels were also within normal limits. Phosphorus levels were not checked, although this has been implicated previously as a cause of arrhythmia.9 The patient did not have a history of any psychiatric conditions and vehemently denied any pill ingestion, illicit drug use, or intentions of harming herself. This was of particular concern, as similar cases of young patients with abnormal ECG findings have been reported in the setting of suicide attempts with toxic ingestions.10 The “coved” ST elevations noted in Figure 2 suggest sodium channel dysfunction and mimic a Brugada pattern. Therefore, we also considered a channelopathy/primary arrhythmia syndrome in our differential diagnosis. In the absence of any prior similar presentations, we feel that the aforementioned abnormal ECG findings in this otherwise healthy young woman are best attributable to a cardiotoxic effect of repeated exposure to highly concentrated glyphosate. Specifically, we hypothesize that sodium channel blockade from this compound created an ECG pattern similar to what might be seen with an overdose of a sodium channel blocking medication such as flecainide or a tricyclic antidepressant. In retrospect, treatment with hypertonic saline may have produced an even more rapid electrocardiographic recovery.11 The 4 L of normal saline that the patient received on admission may have contributed to the rapid normalization of her ECG.
Conclusions
Glyphosate exposure has been implicated as the potential cause of diffuse electrophysiological depolarization and repolarization conduction abnormalities, including prolonged QTc, intraventricular block, and AV conduction delay. These changes can lead to the development of life-threatening arrhythmias.
Key Teaching Points.
-
•
Glyphosate, a common active ingredient in publicly available herbicides, may be more harmful to humans than previously thought.
-
•
Glyphosate may have cardiotoxic effects mirroring acute sodium channel blocker overdose.
-
•
A complete history including potential toxic exposures is essential in the evaluation of cardiogenic syncope.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.hrcr.2019.10.014
Appendix A. Supplementary data
Baseline electrocardiogram from 10 years prior showing sinus tachycardia.
References
- 1.Steinrucken H.C., Amrhein N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun. 1980;94:1207–1212. doi: 10.1016/0006-291x(80)90547-1. [DOI] [PubMed] [Google Scholar]
- 2.Gress S., Lemoine S., Séralini G.E., Puddu P.E. Glyphosate-based herbicides potently affect cardiovascular system in mammals: review of the literature. Cardiovasc Toxicol. 2015;15:117–126. doi: 10.1007/s12012-014-9282-y. [DOI] [PubMed] [Google Scholar]
- 3.Talbot A.R., Shiaw M.H., Huang J.S. Acute poisoning with a glyphosate-surfactant herbicide ('Roundup’): a review of 93 cases. Hum Exp Toxicol. 1991;10:1–8. doi: 10.1177/096032719101000101. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan M. Roundup’s Risks Could Go Well Beyond Cancer. https://www.bloomberg.com/opinion/articles/2019-06-04/roundup-cancer-risk-is-only-one-danger-to-humans-animals
- 5.Kim Y.H., Lee J.H., Hong C.K. Heart rate-corrected QT interval predicts mortality in glyphosate-surfactant herbicide-poisoned patients. Am J Emerg Med. 2014;32:203–207. doi: 10.1016/j.ajem.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Reingardienė D., Vilčinskaitė J., Bilskienė D. Brugada-like electrocardiographic patterns induced by hyperkalemia. Medicina (Kaunas) 2013;49:148–153. [PubMed] [Google Scholar]
- 7.Littmann L., Gibbs M.A. Electrocardiographic manifestations of severe hyperkalemia. J Electrocardiol. 2018;51:814–817. doi: 10.1016/j.jelectrocard.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Monteban-Kooistra W.E., van den Berg M.P., Tulleken J.E., JJM Ligtenberg, Meertens J.H.J.M., Zijlstra J.G. Brugada electrocardiographic pattern elicited by cyclic antidepressants overdose. Intensive Care Med. 2006;32:281–285. doi: 10.1007/s00134-005-0007-3. [DOI] [PubMed] [Google Scholar]
- 9.Jacob R., Patel R.S., Fuentes F. Less phosphorus, more problems: hypophosphatemia induced polymorphic ventricular tachycardia in a young male. Int J Clin Cardiol. 2018;5:112. [Google Scholar]
- 10.Noti F., Asatryan B., Seiler J. Unexplained cardiac arrest in an apparently healthy young woman what is the underlying substrate of the arrhythmia? Circulation. 2018;137:1863–1866. doi: 10.1161/CIRCULATIONAHA.118.034238. [DOI] [PubMed] [Google Scholar]
- 11.Di Grande A., Giuffrida C., Narbone G. Management of sodium-channel blocker poisoning: the role of hypertonic sodium salts. Eur Rev Med Pharmacol Sci. 2010;14:25–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline electrocardiogram from 10 years prior showing sinus tachycardia.