Test your knowledge!
Take an interactive quiz related to this article: https://www.heartrhythmcasereports.com/content/quiz_archive
Introduction
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a rare inherited disease associated with progressive fibrofatty myocardial replacement predisposing to reentrant ventricular tachycardia (VT). Because of the high rate of VT recurrence despite pharmacological therapy and the improved procedural success rate thanks to the development of techniques and the combined endo-epicardial approach, catheter ablation takes an increasingly important place in the management of ventricular arrhythmia in ARVC/D. We will present the different aspects of how we approach VT ablation in patients with ARVC/D through 2 different clinical cases.
Case report 1
A 21-year-old athlete with ARVC/D was admitted to our institution for VT recurrence. He was implanted with a subcutaneous implantable cardioverter-defibrillator (S-ICD) 3 weeks earlier because of an episode of syncope with fast palpitations during exercise and documented spontaneous VT with left bundle branch block morphology and inferior axis during electrocardiographic monitoring (Figure 1). He had family history of ARVC/D with 2 affected uncles. Genetic testing was negative in the family for desmosomal gene mutations. Cardiac magnetic resonance imaging showed only mildly dilated right and left ventricles compatible with history of competitive sport (cycling). Shortly after S-ICD implantation, the patient experienced appropriate shocks because of VT recurrence despite beta-blocker therapy (nadolol 80 mg) and flecainide (200 mg).1 A combined endo-epicardial radiofrequency catheter ablation under general anesthesia was decided. The electroanatomic voltage mapping showed no endocardial substrate, whereas large areas of epicardial right ventricular (RV) scar with local abnormal ventricular activity (LAVA) and late potentials were observed (Figure 2). Programmed ventricular stimulation easily triggered the clinical VT. Activation mapping showed an epicardial VT circuit, dependent of a critical isthmus in the anterior epicardial RV outflow tract (Figure 2 and Supplemental Movie 1). We targeted the zone of the critical isthmus identified by activation mapping and all zones of endocardial and epicardial late potentials/LAVAs (40 W until loss of local capture). After 5 months follow-up, the patient was fine and no sustained VT reoccurred.
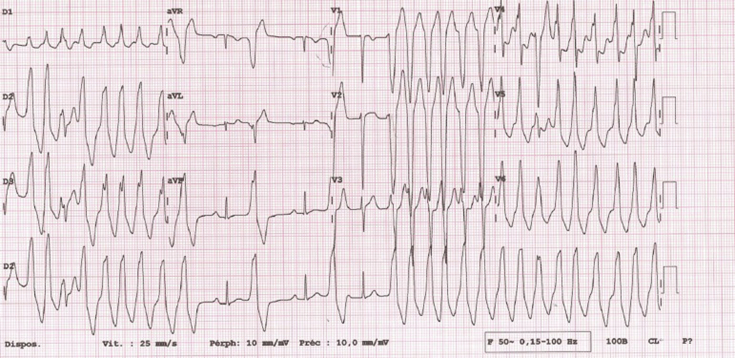
Figure 1.
Twelve-lead electrocardiogram of the clinical ventricular tachycardia.
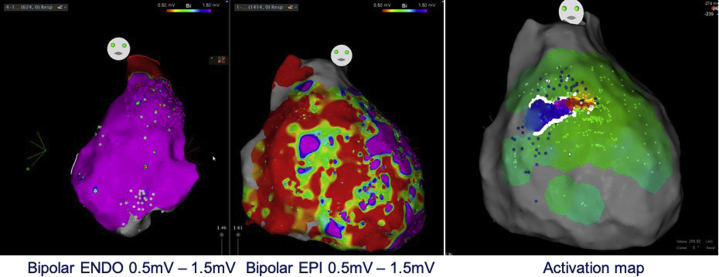
Figure 2.
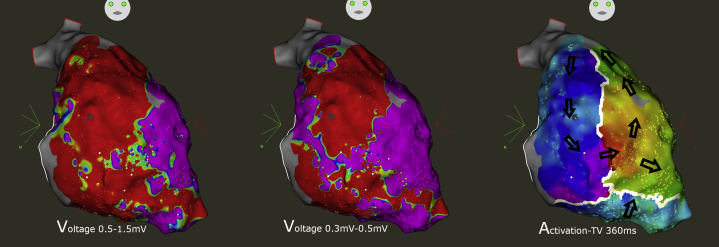
Endocardial and epicardial bipolar voltage map during sinus rhythm. No scar area was observed within the endocardium, contrasting with large scar areas within the right ventricular (RV) epicardium. The clinical ventricular tachycardia (VT) was induced and mapped, showing an epicardial circuit with a critical isthmus located at the anterior RV outflow tract. VT was successfully ablated at this site.
Discussion
Few data are available on pharmacological antiarrhythmic therapy in ARVC/D. In the first publication by Wichter and colleagues,2 sotalol (320–480 mg/day) demonstrated a large benefit over other antiarrhythmic drugs to prevent VT.2 However, data from the North American ARVC/D registry did not support these results and concluded that only amiodarone therapy was associated with reduction of VT burden.3 Unpublished data from our group with 130 patients treated with flecainide therapy in association with beta-blockers showed a significant reduction of ventricular arrhythmia burden with good tolerance. In the present case, VT reoccurred under this association and amiodarone may be more effective. However, the chronic use of amiodarone is associated with high risk of complications and would therefore be avoided in this young patient.
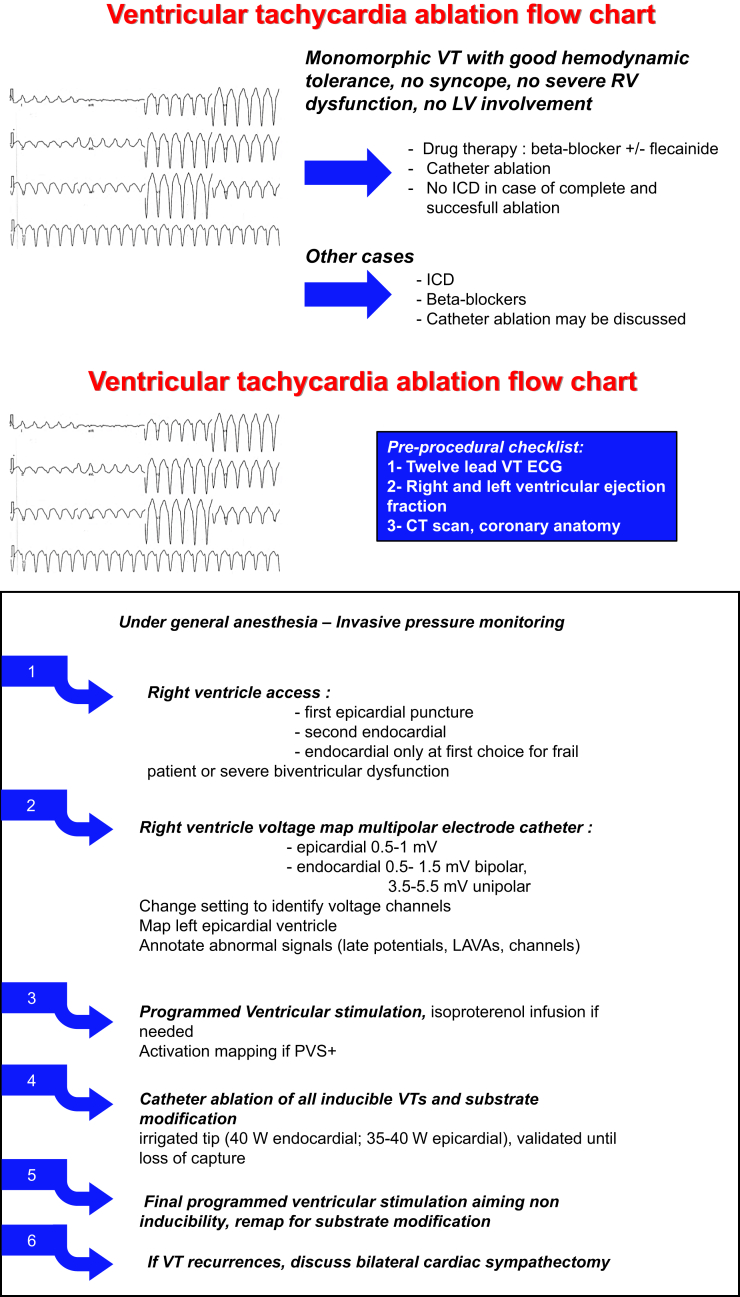
ICD is indicated in secondary prevention in ARVC/D patients. However, there is some evidence that patients with well-tolerated monomorphic VT can be managed by ablation only.4 In our center, we propose this strategy only in patients with no other risk factors for sudden death, especially in absence of severe RV dysfunction, left ventricular (LV) involvement, or syncope (Figure 3). In these cases, it is important to make sure that ablation is complete and successful. Patients should be maintained on beta-blocker therapy and close follow-up should be done. S-ICD is a promising therapy in young patients at high risk of transvenous ICD-related complications5,6 and was proposed to this patient to that end. However, the use of S-ICD in ARVC/D patients remains debatable as monomorphic VT, accessible to antitachycardia pacing (ATP) therapy, is much more frequent than polymorphic VT or ventricular fibrillation. Data from the North American registry that analyzed ICD therapy in 104 ARVC/D patients showed that 97% of ventricular arrhythmia were monomorphic VT that were successfully terminated by ATP in 91%.7 In addition, there are some concerns about undersensing issues, mostly owing to the frequent microvoltage observed in these patients. However, transvenous ICDs are associated with considerable morbidity related to device damage and inappropriate therapies in ARVC/D patients, up to 4.4% and 3.7% per year, respectively.5 In this setting, catheter ablation is a preferable option to lower the VT burden and avoid appropriate shocks. Catheter ablation is the treatment of choice for recurrent VT despite antiarrhythmic therapy in ARVC/D patients, with a class IIa recommendation for this indication in the international task force consensus statement on ARVC/D management.8 Catheter ablation has been shown to decrease ICD therapy burden and to allow a good VT control without need for amiodarone therapy in most ARVC/D patients.9,10 Recent data showed that bilateral cardiac sympathetic denervation was effective in 5 of 8 ARVC/D patients with refractory VT despite catheter ablation as a last-resort therapy.11 Because of limited data on this technique in ARVC/D and procedure-related complications, bilateral cardiac sympathetic denervation should be considered for refractory VTs only after failure of catheter ablation.
Figure 3.
Our workflow for ventricular tachycardia management and catheter ablation in arrhythmogenic right ventricular cardiomyopathy/dysplasia.
If catheter ablation is the treatment of choice for drug-refractory VT in ARVC/D, ablation can be challenging. Endocardial-only ablation is associated with a high rate of VT recurrence in ARVC/D.12 Fibro-fatty replacement leads to areas of slow conduction and constitutes the main substrate for reentrant VT in ARVC/D. It usually progresses from the epicardium to the endocardium during the evolution of the disease.13,14 Polin and colleagues15 described the utility of unipolar endocardial mapping to identify epicardial scar. Using the value of 5.5 mV as a cut-off value, unipolar mapping identified low bipolar epicardial signal (0.5–1 mV)15 (Figure 4). From what is known from the natural history of the disease, VT substrate is likely to be mainly epicardial at early stages, especially in young patients with no RV dysfunction. The usual electrocardiographic criteria to predict epicardial origin of VT are not reliable in ARVC/D, as they have been developed for LV VTs.16 Philips and colleagues evaluated the endocardial scar surface at 5.5 cm2 vs 46.8 cm2 at the epicardial side.17 Several recent studies have demonstrated the superiority of combined endo-epicardial access to improve success rates of the procedure in ARVC/D.18 Combined endo-epicardial access at first step is now the routine approach in our daily practice. This strategy is supported by the 2015 expert consensus statement that proposed “a combined endocardial/epicardial VT ablation approach as an initial ablation strategy, provided that the operator and electrophysiologic laboratory are experienced performing epicardial VT ablation in patients with ARVC/D” (class IIa recommendation).8 Our procedure workflow is detailed in Figure 3. Because of concerns on the risk of right ventricle puncture during epicardial access (4.5%–17%),19 we usually begin the procedure with the epicardial puncture and mapping. This allows potential bleeding to stop before heparin infusion, which is needed for endocardial catheter placement. We use an anterior approach to prevent liver puncture, in particular in this population at risk of RV failure and liver enlargement. Preprocedural imaging for ARVC/D ablation is very useful and mandatory when available, allowing localizing scar tissue before the procedure and to merge the RV morphology with electroanatomical mapping to ensure that the whole RV is mapped. This is particularly important for some areas difficult to reach through endocardial access, such as the RV free wall and the apex. It also allows localizing coronary arteries before epicardial ablation.
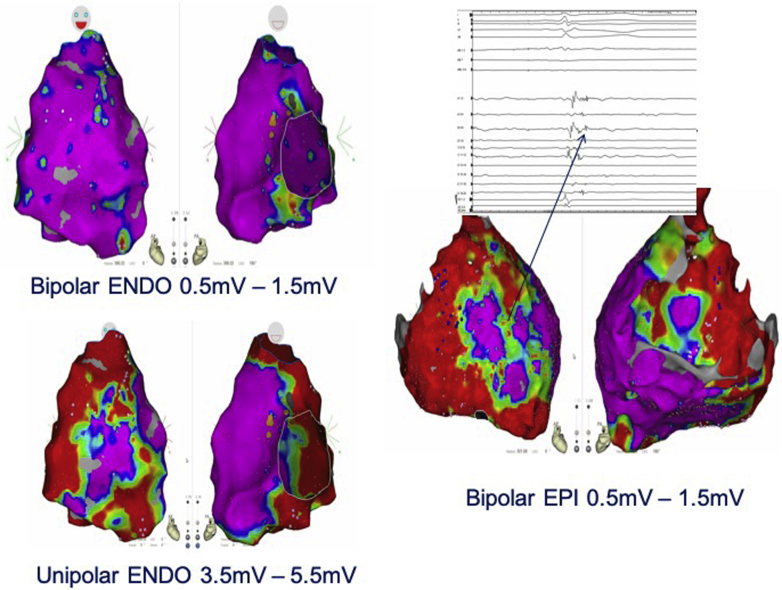
Figure 4.
Example of 23-year-old man carrying a PKP2 mutation with exercise-induced ventricular tachycardia from right ventricular outflow tract. The endocardial bipolar voltage map (0.5–1.5 mV) during sinus rhythm showed no significant area of scar, contrary to the unipolar voltage map (3.5–5.5 mV), which predicted areas of scar observed in the epicardium.
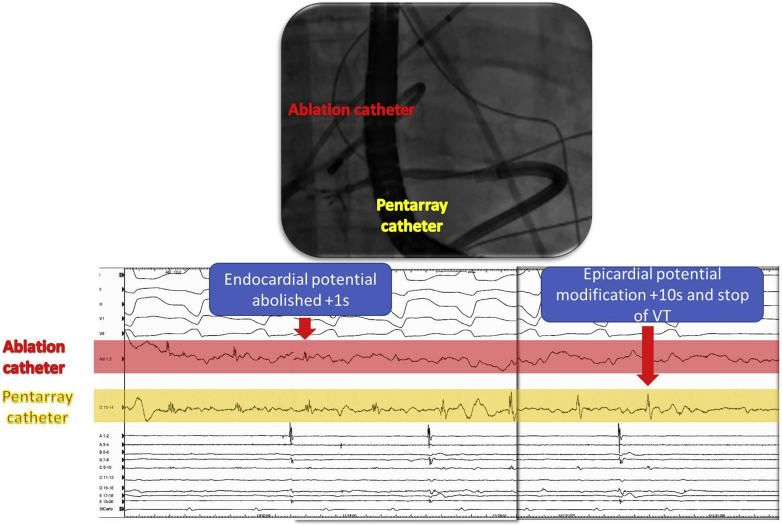
When facilities for epicardial ablation are not available, endocardial-only ablation may be an alternative option. Komatsu and colleagues20 demonstrated the ability to eliminate endocardial and epicardial LAVA potential with endocardial-only ablation, as shown in these examples (Figure 5 and Supplemental Movie 2). However, this approach abolished epicardial LAVA in only 40% of ARVC/D patients. Endocardial-only ablation could, however, be effective and safe in patients with acute hemodynamic decompensation (AHD) or with very advanced disease, where endocardial substrate may be more important.
Figure 5.
Example of an ablation of epicardial local electrogram by endocardial ablation. The endocardial ablation led to the modification of the local epicardial electrogram recorded by the multielectrode catheter placed in front of the ablation catheter. Ventricular tachycardia was stopped from endocardium at this site.
General anesthesia is a debatable strategy for VT ablation because of concerns about the risk of AHD during the procedure and the risk of VT noninducibility. However, epicardial access is painful and endo-epicardial catheter ablations are usually long procedures. In the study by Santangeli and colleagues,21 general anesthesia was associated with a higher risk of AHD during VT catheter ablation. However, in our experience, VTs are usually well tolerated under general anesthesia in ARVC/D patients if LV ejection fraction is preserved, even in case of RV dysfunction. Data from Nof and colleagues22 suggested that general anesthesia did not promote noninducibility of VT. For these reasons, general anesthesia remains, in our opinion, the best option for these procedures, especially in young patients.
Clinical VT must be aimed at as the primary target. Clinical VT induction and activation mapping allows determining the critical isthmus of the tachycardia. As an alternative, if the VT is noninducible or nontolerated, the critical isthmus can be determined by pace mapping23 (Figure 6). However, the arrhythmogenic VT substrate is often complex and extended; thus clinical VT-only ablation can be insufficient to achieve good long-term results. The ablation of all inducible VTs should be the rule. This is a classical endpoint for VT ablation. In our experience, the mean number of morphologies induced in ARVC/D patients is 2.3 (ranging from 1 to 8). In the recent study from Santangeli and colleagues,4 the procedural endpoint was noninducibility of any sustained VT from at least 2 RV sites. This approach was associated with low VT recurrence. In patients with nonischemic cardiomyopathy, noninducibility at the end of the procedure was associated with lower VT recurrence and mortality.24
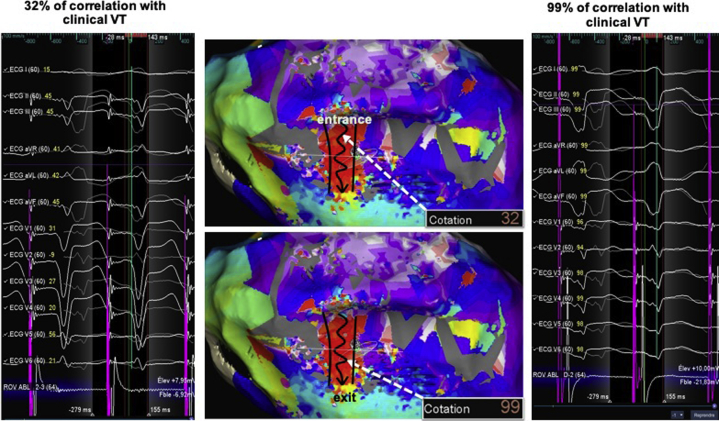
Figure 6.
Pace mapping to identify and validate clinical ventricular tachycardia (VT) isthmus. Example of an epicardial VT ablation with the Precision system (Abbott, St. Paul, MN) and HD Grid multipolar catheter in an arrhythmogenic right ventricular cardiomyopathy/dysplasia patient. This is a very nice example of bad to good pace map transition within the critical isthmus, which was identified by activation mapping, from 32% correlation from the channel entrance to a quasi-perfect 99% correlation from the channel exit.
Substrate modification is an alternative to activation/entrainment mapping and has been associated with good procedural success in different cardiomyopathies.25 Several strategies had been described to overcome the noninducibility or the poor hemodynamic tolerance of clinical VT; most were elaborated from ischemic cardiomyopathy.26 Scar dechanneling, which aims to ablate conduction channel entrances, was the only substrate modification strategy investigated specifically in ARVC/D and has been shown to be effective with good long-term outcomes.14 Dechanneling endo-epicardially allows dealing with complex substrate. It is our routine practice to perform at the first step epicardial and endocardial voltage maps with annotation of all pathologic signals (LAVAs, late potentials, voltage channels) (Figure 7 and Supplemental Movie 3). As the second step, we aim to induce and map the VT with activation/entrainment mapping or using pace mapping for poorly tolerated VTs. In the third step, we complete ablation with substrate modification aiming to ablate all abnormal signals, especially late potentials. For entrainment and substrate mapping, we always use a multipolar mapping catheter to achieve high-density multielectrode mapping, which has been shown to be superior to conventional point-by-point mapping. Multielectrode mapping is particularly useful in ARVC/D with such a complex substrate and enables to create faster activation mapping during ongoing VT. We always use an irrigated catheter. The classical setting is 35–40 W and effective lesions are validated by local loss of capture and/or drop in impedance of 10 ohms and/or disappearance of the local potential. Contact force catheter may be an additional value aiming at 9g epicardially and 8g endocardially.27 Epicardial fat, the hallmark of ARVC/D, may protect the surviving myocardial fibers from radiofrequency lesion. To ensure effective lesions, we usually use high power (35–40 W) for epicardial ablation and add endocardial applications in front (“sandwich-like” ablation).
Figure 7.
Examples of late potentials recorded within the critical isthmus of the ventricular tachycardia in 2 different arrhythmogenic right ventricular cardiomyopathy/dysplasia patients. Top: Recording with HD grid multipolar catheter (Abbott, St. Paul, MN). Bottom: Recording with PentaRay multipolar catheter (Biosense Webster, Irvine, CA).
Case report 2
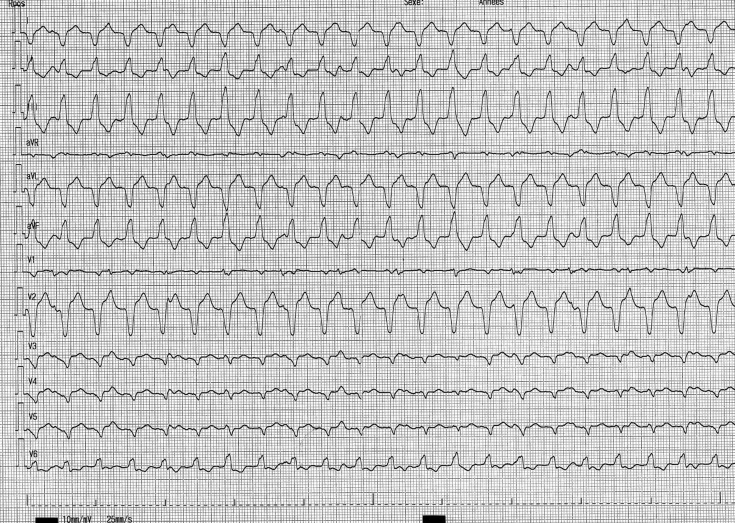
A 36-year-old patient with ARVC/D diagnosed since 2008 was admitted in 2018 for electrical storm in the setting of amiodarone-induced hyperthyroidism. He had been implanted with a transvenous ICD in 2012 because of advanced disease with biventricular dysfunction. He experienced several ATP therapies (about 50) for well-tolerated monomorphic sustained VT (Figure 8). He displayed severe biventricular dysfunction with LV ejection fraction at 35% and RV ejection fraction at 18%. He was first managed medically with bisoprolol titrated to 10 mg/day and, after endocrinologist approval, reintroduction of amiodarone. As VT reoccurred despite pharmacologic therapy, plasmapheresis was decided to lower circulating thyroid hormone levels. Despite normalized T3 level, the patient experienced several episodes of VT.
Figure 8.
Twelve-lead electrocardiogram of the clinical monomorphic ventricular tachycardia.
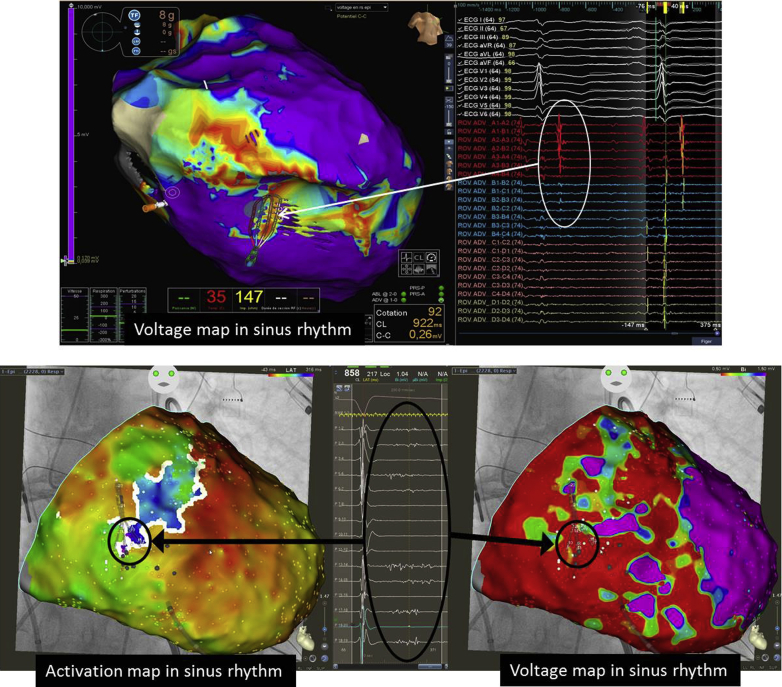
Because of concerns about the hemodynamic tolerance of epicardial ablation under general anesthesia (the PAAINESD score of this patient would be 15 if general anesthesia were used for ablation), we chose to perform endocardial-only ablation under sedation to avoid AHD. The clinical monomorphic VT was easily induced and mapped with good tolerance. The VT reentrant circuit was located in the endocardium with a critical isthmus within the RV free wall (Figure 9). VT was successfully ablated at this site with no further recurrence.
Figure 9.
Activation and endocardial bipolar voltage mapping from an arrhythmogenic right ventricular cardiomyopathy/dysplasia patient with electrical storm. Ventricular tachycardia circuit was dependent of a critical isthmus located in the endocardial right ventricular free wall. Voltage mapping by modifying the voltage scale (left and middle) allowed identifying the low voltage channel between 2 areas of dense scar.
The patient underwent thyroidectomy after control of VT and normalization of thyroid hormone levels with no complication. After 10 months follow-up, no sustained VT reoccurred. The patient is currently listed for heart transplantation because of persistent symptomatic right heart failure.
Discussion
Electrical storm is associated with a high morbidity and mortality, especially in patients with LV dysfunction. Patients with unstable VT or repetitive ventricular fibrillation may benefit from mechanical hemodynamic support. The PAAINESD score, based on baseline patient characteristics, has been proposed to predict acute decompensation during VT ablation procedures and patients who may benefit from prophylactic mechanical support.28 The French national consensus expert statement recommends that ablation of complex arrhythmia such as epicardial VT or in the setting of electrical storm should be performed in an expert center with cardiac surgery and emergency hemodynamic support implantation facilities.29 Catheter ablation is the treatment of choice for refractory VT in the setting of electrical storms. Recent data from our group showed good efficacy of urgent VT ablation in ARVC/D patients experiencing electrical storms.30 In frail patients, as in this case, we choose to perform endocardial-only ablation under sedation to avoid AHD.
If we fail to ablate the VT isthmus endocardially, we first try to use half-saline dilution for catheter cooling. The next step would be to perform under conscious sedation epicardial puncture, as there is strong evidence that epicardial puncture can be performed safely after heparin reversal or continuous anticoagulation in expert centers.31
Neuromodulation techniques demonstrate a growing interest for VT control in the setting of electrical storm since the first encouraging report by Nademanee and colleagues.32 As stated above, there are some recent encouraging data in ARVC/D.11 Surgical sympathectomy using thoracoscopy is the gold standard but is sometimes difficult in frail patients, as in this case. Echocardiography-guided percutaneous sympathectomy is a useful alternative for patients with hemodynamic instability.33 In the present case, sympathectomy would definitely be considered as an option in case of ablation failure.
Acknowledgments
The authros thank Mr Simon (Biosense Webster, Irvine, CA) for map editing.
Footnotes
Funding: The authors report no funding.
Conflicts of interest: Dr Waintraub received consulting fees from Boston, Microport, and Medtronic. Dr Gandjbakhch received consulting fees from Boston, Microport, Medtronic, and Abbott.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2019.09.006.
Appendix. Supplementary data
Activation map showing an epicardial circuit with a critical isthmus located at the anterior right ventricular outflow tract. Clinical ventricular tachycardia was successfully ablated at this site.
Example of an ablation of epicardial local electrogram by endocardial ablation in an arrhythmogenic right ventricular cardiomyopathy/dysplasiapatient carrying a PKP2 mutation. In this other case, the endocardial ablation led to the disappearance of the local epicardial late potentials recorded by the Pentaray multipolar catheter (Biosense) placed in front of the endocardial ablation catheter.
Example of late potentials recorded in an arrhythmogenic right ventricular cardiomyopathy/dysplasia patient carrying a PKP2 mutation. This movie shows the epicardial activation map during sinus rhythm showing an area of very late potentials recorded with Pentaray multipolar catheter (Biosense). Ventricular tachycardia was ablated successfully at this site.
References
- 1.Ermakov S., Gerstenfeld E.P., Svetlichnaya Y., Scheinman M.M. Use of flecainide in combination antiarrhythmic therapy in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2017;14:564–569. doi: 10.1016/j.hrthm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Wichter T., Paul T.M., Eckardt L. Arrhythmogenic right ventricular cardiomyopathy. Antiarrhythmic drugs, catheter ablation, or ICD? Herz. 2005;30:91–101. doi: 10.1007/s00059-005-2677-6. [DOI] [PubMed] [Google Scholar]
- 3.Marcus G.M., Glidden D.V., Polonsky B. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol. 2009;54:609–615. doi: 10.1016/j.jacc.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santangeli P., Tung R., Xue Y. Outcomes of catheter ablation in arrhythmogenic right ventricular cardiomyopathy without background implantable cardioverter defibrillator therapy: a multicenter international ventricular tachycardia registry. JACC Clin Electrophysiol. 2019;5:55–65. doi: 10.1016/j.jacep.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Schinkel A.F.L. Implantable cardioverter defibrillators in arrhythmogenic right ventricular dysplasia/cardiomyopathy: patient outcomes, incidence of appropriate and inappropriate interventions, and complications. Circ Arrhythm Electrophysiol. 2013;6:562–568. doi: 10.1161/CIRCEP.113.000392. [DOI] [PubMed] [Google Scholar]
- 6.Orgeron G.M., Bhonsale A., Migliore F. Subcutaneous implantable cardioverter-defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia: a transatlantic experience. J Am Heart Assoc. 2018;7:e008782. doi: 10.1161/JAHA.118.008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Link M.S., Laidlaw D., Polonsky B. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol. 2014;64:119. doi: 10.1016/j.jacc.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrado D., Wichter T., Link M.S. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015;132:441–453. doi: 10.1161/CIRCULATIONAHA.115.017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero J., Grushko M., Briceño D.F., Natale A., Di Biase L. Radiofrequency ablation in arrhythmogenic right ventricular cardiomyopathy (ARVC) Curr Cardiol Rep. 2017;19:82. doi: 10.1007/s11886-017-0893-3. [DOI] [PubMed] [Google Scholar]
- 10.Santangeli P., Zado E.S., Supple G.E. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:1413–1421. doi: 10.1161/CIRCEP.115.003562. [DOI] [PubMed] [Google Scholar]
- 11.Assis F.R., Krishnan A., Zhou X. Cardiac sympathectomy for refractory ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2019;16:1003–1010. doi: 10.1016/j.hrthm.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Philips B., Madhavan S., James C. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:499–505. doi: 10.1161/CIRCEP.111.968677. [DOI] [PubMed] [Google Scholar]
- 13.Basso C., Thiene G., Corrado D., Angelini A., Nava A., Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 14.Berruezo A., Acosta J., Fernández-Armenta J. Safety, long-term outcomes and predictors of recurrence after first-line combined endoepicardial ventricular tachycardia substrate ablation in arrhythmogenic cardiomyopathy. Impact of arrhythmic substrate distribution pattern. A prospective multicentre study. Europace. 2017;19:607–616. doi: 10.1093/europace/euw212. [DOI] [PubMed] [Google Scholar]
- 15.Polin G.M., Haqqani H., Tzou W. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 16.Bazan V., Bala R., Garcia F.C. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006;3:1132–1139. doi: 10.1016/j.hrthm.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Philips B., te Riele A.S.J.M., Sawant A. Outcomes and ventricular tachycardia recurrence characteristics after epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm. 2015;12:716–725. doi: 10.1016/j.hrthm.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Romero J., Cerrud-Rodriguez R.C., Biase L.D. Combined endocardial-epicardial versus endocardial catheter ablation alone for ventricular tachycardia in structural heart disease: a systematic review and meta-analysis. JACC Clin Electrophysiol. 2019;5:13–24. doi: 10.1016/j.jacep.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Lim H.S., Sacher F., Cochet H. Safety and prevention of complications during percutaneous epicardial access for the ablation of cardiac arrhythmias. Heart Rhythm. 2014;11:1658–1665. doi: 10.1016/j.hrthm.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu Y., Daly M., Sacher F. Endocardial ablation to eliminate epicardial arrhythmia substrate in scar-related ventricular tachycardia. J Am Coll Cardiol. 2014;63:1416–1426. doi: 10.1016/j.jacc.2013.10.087. [DOI] [PubMed] [Google Scholar]
- 21.Santangeli P., Muser D., Zado E.S. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia. Circ Arrhythm Electrophysiol. https://www.ahajournals.org/doi/abs/10.1161/CIRCEP.114.002155 Available at: [DOI] [PubMed]
- 22.Nof E., Reichlin T., Enriquez A.D. Impact of general anesthesia on initiation and stability of VT during catheter ablation. Heart Rhythm. 2015;12:2213–2220. doi: 10.1016/j.hrthm.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 23.de Chillou C., Sellal J.-M., Magnin-Poull I. Pace mapping to localize the critical isthmus of ventricular tachycardia. Card Electrophysiol Clin. 2017;9:71–80. doi: 10.1016/j.ccep.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Hu J., Zeng S., Zhou Q. Can ventricular tachycardia non-inducibility after ablation predict reduced ventricular tachycardia recurrence and mortality in patients with non-ischemic cardiomyopathy? A meta-analysis of twenty-four observational studies. Int J Cardiol. 2016;222:689–695. doi: 10.1016/j.ijcard.2016.07.200. [DOI] [PubMed] [Google Scholar]
- 25.Jaïs P., Maury P., Khairy P. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 26.Sadek M.M., Schaller R.D., Supple G.E. Ventricular tachycardia ablation – the right approach for the right patient. Arrhythm Electrophysiol Rev. 2014;3:161–167. doi: 10.15420/aer.2014.3.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno H., Vergara P., Maccabelli G. Contact force monitoring for cardiac mapping in patients with ventricular tachycardia. J Cardiovasc Electrophysiol. 2013;24:519–524. doi: 10.1111/jce.12080. [DOI] [PubMed] [Google Scholar]
- 28.Muser D., Castro S.A., Liang J.J., Santangeli P. Identifying risk and management of acute haemodynamic decompensation during catheter ablation of ventricular tachycardia. Arrhythm Electrophysiol Rev. 2018;7:282–287. doi: 10.15420/aer.2018.36.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maury P., Defaye P., Klug D. Position paper concerning the competence, performance and environment required in the practice of complex ablation procedures. Arch Cardiovasc Dis. 2019;112:67–73. doi: 10.1016/j.acvd.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Laredo M, Da Silva LO, Extramiana F, et al. Catheter ablation of electrical storm in patients with arrhythmogenic right ventricular cardiomyopathy [published online ahead of print July 5, 2019]. Heart Rhythm. doi: 10.1016/j.hrthm.2019.06.022. [DOI] [PubMed]
- 31.Nakamura T., Davogustto G.E., Schaeffer B. Complications and anticoagulation strategies for percutaneous epicardial ablation procedures. Circ Arrhythm Electrophysiol. 2018;11:e006714. doi: 10.1161/CIRCEP.118.006714. [DOI] [PubMed] [Google Scholar]
- 32.Nademanee K., Taylor R., Bailey W.E., Rieders D.E., Kosar E.M. Treating electrical storm: sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation. 2000;102:742–747. doi: 10.1161/01.cir.102.7.742. [DOI] [PubMed] [Google Scholar]
- 33.Prabhu M.A., Prasad S.B.V., Abhilash S.P., Thajudeen A., R B.K., Namboodiri N. Left sympathetic cardiac denervation in managing electrical storm: acute outcome and long term follow up. J Interv Card Electrophysiol. 2016;47:285–292. doi: 10.1007/s10840-016-0153-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activation map showing an epicardial circuit with a critical isthmus located at the anterior right ventricular outflow tract. Clinical ventricular tachycardia was successfully ablated at this site.
Example of an ablation of epicardial local electrogram by endocardial ablation in an arrhythmogenic right ventricular cardiomyopathy/dysplasiapatient carrying a PKP2 mutation. In this other case, the endocardial ablation led to the disappearance of the local epicardial late potentials recorded by the Pentaray multipolar catheter (Biosense) placed in front of the endocardial ablation catheter.
Example of late potentials recorded in an arrhythmogenic right ventricular cardiomyopathy/dysplasia patient carrying a PKP2 mutation. This movie shows the epicardial activation map during sinus rhythm showing an area of very late potentials recorded with Pentaray multipolar catheter (Biosense). Ventricular tachycardia was ablated successfully at this site.