Graphical abstract
Keywords: Aneurysm ruptured, Congenital, Bicuspid aortic valve, Valsalva sinus
Highlights
-
•
SOV-A is defined as abnormal dilation of the aortic root.
-
•
This is a rare entity, with an incidence between 0.14% and 3.5%.
-
•
SOV-A rupture is a complication generating fistulous trajectories between cavities.
-
•
The sign of “windsock” has been described for the ruptured SOV-A.
-
•
We present the case of a ruptured SOV-A in a patient with bicuspid aortic valve.
Introduction
Approximately 1 in 100 adults are born with congenital heart disease, which can be characterized, according to its complexity, as simple, moderate, or severe on the basis of the morbidity and mortality associated with each injury.1 Sinus of Valsalva aneurysm (SOV-A), although unusual, usually manifests when there is a complication such as rupture or endocarditis, and the overall prognosis is good with timely surgical correction. We present a case of ruptured SOV-A in a young patient with bicuspid aortic valve in which three-dimensional echocardiography was helpful for diagnosis and surgical planning.
Case Presentation
A 27-year-old male patient referred from another institution because of a 3-month history of worsening of functional class (New York Heart Association functional class III–IV), associated orthopnea, and paroxysmal nocturnal dyspnea that began after a common cold. Upon admission, physical examination showed blood pressure of 115/54 mm Hg, a heart rate of 93 beats/min, a respiratory rate of 19 breaths/min, oxygen saturation of 92% on room air, jugular venous distension (III/IV), palpable thrill in the anterior left chest region, palpable apex (between the fifth and sixth intercostal space with anterior axillary line), regular heart sounds, a continuous systolic/diastolic murmur (grade 3/6) at the right upper sternal border, and pansystolic murmur (grade 3/6) at the apex. He had hepatomegaly (the liver palpable 5 cm under the costal margin) and grade 2+ lower extremity pitting edema. Outside transthoracic echocardiography (TTE) revealed a subaortic ventricular septal defect. However, left heart catheterization and coronary angiography demonstrated the presence of an aortocameral fistula. On admission to our institution, chest radiography was performed, showing bilateral parahilar congestion and global cardiomegaly. Electrocardiography showed normal sinus rhythm with right-axis deviation. The patient was admitted for further testing and management of heart failure.
New TTE was performed, which showed a type 2 bicuspid aortic valve (BAV; fusion of the right coronary and noncoronary cusps) and the presence of turbulent continuous flow from the aortic root to the right atrium (Figure 1, Videos 1 and 2), with normal aortic root diameters. A nonstandard projection between the parasternal long axis and the right ventricular inflow tract showed aneurysmal dilation of the anterior sinus of Valsalva, with a mouth 10 mm in diameter and distal perforation (Videos 3-5, Figure 2). Spectral Doppler signal showed continuous flow between the aortic root and the right atrium (Figure 3, Video 6). Differentiation from a ventricular septal defect was obtained on anatomic analysis of the defect in the two-dimensional projections mentioned. The right ventricle was dilated, with systolic-diastolic flattening of the interventricular septum due to pressure and volume overload, tricuspid annular dilatation (26 mm/m2), and severe eccentric functional tricuspid regurgitation (Figure 4, Video 7) The left ventricle was severely dilated (end-diastolic volume 108 mL/m2), with borderline systolic dysfunction and a left ventricular ejection fraction of 51% calculated using the Simpson biplane method.
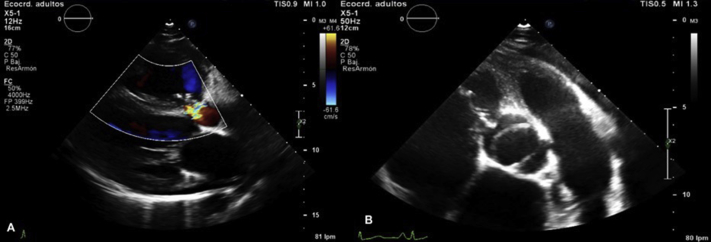
Figure 1.
TTE. (A) Parasternal long-axis view with color Doppler showing the presence of a turbulent systolic-diastolic flow from the aortic root to the right atrium. (B) Short-axis view showing BAV. Differentiation from a ventricular septal defect was obtained on anatomic analysis of the defect in the two-dimensional projections mentioned.
Figure 2.
TTE. (A, B) A nonstandard projection between the parasternal long-axis view and the right ventricular inflow tract with zoom, showing aneurysmal dilation of the anterior sinus of Valsalva with a mouth 10 mm in diameter and distal perforation.
Figure 3.
TTE. (A) In the apical five-chamber view, the spectral continuous Doppler signal showed continuous flow between the aortic root and the right atrium. (B) Color Doppler (Video 15).
Figure 4.
TTE, apical four-chamber view, showing severe eccentric functional tricuspid regurgitation.
During his admission, the patient had a fever (39°C), for which transesophageal echocardiography was ordered to rule out infectious endocarditis; findings were negative, in agreement with blood cultures. Eventually fever was attributed to acute bronchitis.
Transesophageal echocardiography at the midesophagus showed the anterior SOV-A (saccular morphology with a 10-mm mouth, length 19 × 15 mm, with irregular edges) with a perforation at its distal end and a fistula between the aortic root and the right atrium. The aneurysm projected toward the tricuspid septal valve near the coaptation line (Videos 8-11), and this, in addition to tricuspid annular dilation, suggested the presence of severe tricuspid regurgitation. No images suggesting vegetations were identified in the BAV or any of the remaining valves. Neither aortic stenosis nor aortic regurgitation was visualized. Three-dimensional reconstruction of the aneurysm was performed (Video 12, Figure 5). The three-dimensional images were used as a complement to the two-dimensional images. The two-dimensional images, mainly TTE, were used for surgical planning; multiplanar reconstruction was not available.
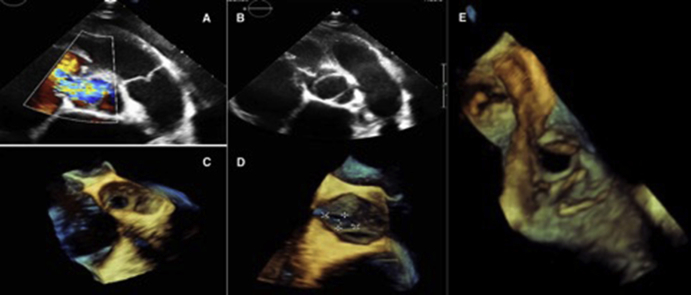
Figure 5.
TTE and transesophageal echocardiography. (A) TTE. In a parasternal short-axis (PSAX) view at the aortic level with color Doppler, the red arrow points to the ruptured SOV-A with a fistula between the aortic root and the right atrium. (B) TTE showing BAV in a PSAX view, with the ruptured SOV-A. (C–E) Three-dimensional transesophageal echocardiographic reconstruction from a live three-dimensional acquisition displayed from aortic (C, D) and right atrial (E) perspectives showing the communication between the two chambers.
The images of the cardiac catheterization previously done at an outside hospital were again reviewed, confirming the presence of an aortocameral fistula and dilatation of the right cavities (Figure 6).
Figure 6.
Left ventriculography. The contrast medium is moving from the aorta to the right atrium (white arrow).
The patient was taken to surgery, which confirmed the imaging findings in the aortic valve. The ruptured SOV-A was repaired with a double patch through the aorta and the right atrium. The tricuspid valve showed significant separation of its septal and anterior commissure with annular dilation. An anterior commissurorrhaphy was performed with bicuspidization of the tricuspid valve (Figures 7 and 8).
Figure 7.
Intraoperative findings: (A) Viewed from the right atrium, blood is passing through the ruptured SOV-A toward the right chamber (white arrow). (B, C) The site of the rupture of the SOV-A was exposed from the aortic view (white arrow).
Figure 8.
Intraoperative imager shows that ruptured SOV-A was repaired using a double Gore-Tex patch through the aorta and the right atrium.
Postoperative TTE showed resolution of the aneurysm, now replaced by increased echogenicity secondary to the patch without residual shunt. The tricuspid valve had no residual tricuspid regurgitation, with acceptable gradients. The average tricuspid gradient was 3 mm Hg at a heart rate of 75 beats/min (Figure 9, Videos 13-15).
Figure 9.
(A) TTE, parasternal long-axis view, showing resolution of the aneurysm without residual shunt. (B, C) Parasternal short-axis view at the level of the great vessels showing an increase echogenicity secondary to the patch without residual shunt and the tricuspid valve without residual tricuspid regurgitation.
The patient was discharged with no further complications on postoperative day 6.
Discussion
SOV-A is a rare entity, with an incidence between 0.14% and 3.5% of patients taken to cardiac surgery in an Asian series and 0.09% in the general population.2 The incidence is 60% to 80% among men,3 and it is more frequent in the eastern hemisphere.4 SOV-A is defined as abnormal dilation of the aortic root that occurs because of thinning between the aortic annulus and the sinotubular junction,4 and it can be congenital (more frequent) or acquired. Congenital SOV-A occurs in the context of connective tissue diseases such as Marfan and Ehlers-Danlos syndrome; associations have been reported to other congenital heart diseases such as ventricular septal defect (supracrystal) in up to 31% and BAV in up to 9%, as in our patient. This particular case was related to the abnormal flow against the arterial wall secondary to the dysplastic valve, or as a result of the genetic defects responsible for the BAV.5 Acquired SOV-A is associated with aortic valve endocarditis, syphilis, tuberculosis, atherosclerosis, cystic medial necrosis of the middle, and chest trauma. It has also been associated with aortic and mitral regurgitation (in up to 44% and 9%, respectively).6
SOV-A originates most frequently from the right coronary sinus (78.2%), followed by the noncoronary sinus (20%) and the left coronary sinus (3%).
The clinical course of SOV-A without rupture is usually silent. However, a significant increase in its size compresses adjacent structures, manifesting on rare occasions as arrhythmias or blockages. SOV-A rupture is a frequent complication generating fistulous trajectories between two cavities, which typically manifests as acute or subacute heart failure depending on the size of the fistula and the speed of rupture; the receptor chamber will be the determinant of the clinical picture, presenting as right or left heart failure or even death if the rupture occurs in the pericardial space with cardiac tamponade.7
Right SOV-A rupture occurs more frequently in the right ventricle (81.4%) but also in the right atrium (18.6%), while those that originate in the noncoronary sinus are more likely to rupture in the right atrium (63%), and those in the left coronary sinus do so in the left-sided chambers.8
TTE is the first-line diagnostic study. The “windsock” sign has been described for ruptured SOV-A because of its elongated morphology with communication between two structures and perforation at its apex, as described in our case (Figure 2, Videos 5-8). Color and spectral continuous Doppler provides information about the rupture location and the continuous high-speed flow between the aortic root and the receiving chamber.7
Treatment for ruptured and unruptured SOV-A (with communication between the left and right chambers, severe aortic insufficiency, or accelerated growth with symptoms) is surgical, with three different repair approaches: through the aortic root by aortotomy, through the chamber receptor, or by combination of these two techniques, as in our patient. Choice of technique is determined by the size of the SOV-A, the chamber involved, and the associated cardiac pathology.9 The repair of the defect is made by primary closure for small aneurysms or with a patch for large aneurysms, with a survival rate described in the literature of about 90% at 15 years.10
Conclusion
SOV-A is an infrequent pathology and has a good prognosis with surgical management. Its clinical presentation depends on the speed and size when it ruptures and the receiving chamber when a fistula is established, manifesting in most cases as right-sided heart failure. TTE is the initial diagnostic modality, and other advanced imaging techniques are useful when there is doubt in the diagnosis or other associated heart diseases for surgical planning. The definitive treatment is surgical resection, with a good survival rate in experienced hands.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2019.11.001.
Supplementary Data
TTE. Parasternal long-axis view with color Doppler showing the presence of turbulent systolic-diastolic flow from the aortic root to the right atrium.
TTE. Short-axis view showing a BAV type 2 (fusion of the right coronary and noncoronary cusps).
TTE. A nonstandard projection between the PLAX view and the right ventricular inflow tract with zoom showing an aneurysmal dilation of the anterior sinus of Valsalva with a mouth of 10 mm in diameter and distal perforation.
3D TTE reconstruction from a live 3D acquisition, nonstandard projection between the PLAX view and the right ventricular inflow tract showing an aneurysmal dilation of the anterior sinus of Valsalva.
TTE. A nonstandard projection between the PLAX view and the right ventricular inflow tract with Zoom (with color Doppler) showing an aneurysmal dilation of the anterior sinus of Valsalva with a mouth of 10 mm in diameter and distal perforation.
TTE. Apical four-chamber view showing a severe eccentric functional tricuspid regurgitation.
TTE. Apical four-chamber view showing a severe eccentric functional tricuspid regurgitation.
A nonstandard TEE view at 75 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line.
A nonstandard TEE view at 75 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line (with color Doppler).
A nonstandard TEE view at 75 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line (zoom).
A nonstandard TEE view at 116 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line.
TTE. Parasternal short-axis view at the level of great vessels showing an increase echogenicity secondary to the patch without residual shunt and the tricuspid valve without residual tricuspid regurgitation.
TTE. Parasternal long-axis view showing the resolution of the aneurysm without residual shunt.
Parasternal short-axis view at the level of the great vessels showing an increase echogenicity secondary to the patch without residual shunt.
Parasternal short-axis view at the level of the great vessels showing the tricuspid valve without residual tricuspid regurgitation.
References
- 1.Otto C.M. 5th ed. Elsevier; Philadelphia: 2017. The Practice of Clinical Echocardiography; pp. 894–895. [Google Scholar]
- 2.Ring W.S. Congenital Heart Surgery Nomenclature and Database Project: aortic aneurysm, sinus of Valsalva aneurysm, and aortic dissection. Ann Thorac Surg. 2000;69:S147–S163. doi: 10.1016/s0003-4975(99)01242-4. [DOI] [PubMed] [Google Scholar]
- 3.Ott D.A. Aneurysm of the sinus of Valsalva. Pediatr Card Surg. 2006;9:165–176. doi: 10.1053/j.pcsu.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Feldman D.N., Roman M.J. Aneurysms of the sinuses of Valsalva. Cardiology. 2006;106:73–81. doi: 10.1159/000092635. [DOI] [PubMed] [Google Scholar]
- 5.Carità P., Dendramis G., Novo G., Clara E., Neglia L., Mignano A. Bicuspid aortic valve and unruptured sinus of Valsalva aneurysm, a rare association. Int J Cardiol. 2016;202:103–105. doi: 10.1016/j.ijcard.2015.08.189. [DOI] [PubMed] [Google Scholar]
- 6.Moustafa S., Mookadam F., Cooper L., Carolina N. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol. 2007;99:1159–1164. doi: 10.1016/j.amjcard.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Mcgregor P., Temtanakitpaisan Y., Hiltbolt A., Aragam J.R. A spectrum of sinus of Valsalva aneurysm—from the young to the old. Echocardiography. 2017;34:1524–1530. doi: 10.1111/echo.13655. [DOI] [PubMed] [Google Scholar]
- 8.Sarıkaya S., Adademir T., Elibol A., Büyükbayrak F., Onk A., Kirali K. Surgery for ruptured sinus of Valsalva aneurysm: 25-year experience with 55 patients. Eur J Cardio-Thoracic Surg. 2013:591–596. doi: 10.1093/ejcts/ezs450. [DOI] [PubMed] [Google Scholar]
- 9.Weinreich M., Yu P., Trost B. Sinus of Valsalva aneurysms: review of the literature and an update on management. Clin Cardiol. 2015;38:185–189. doi: 10.1002/clc.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan F., Huo Q., Qiao J., Murat V. Surgery for sinus of Valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16:361–365. doi: 10.1177/021849230801600504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE. Parasternal long-axis view with color Doppler showing the presence of turbulent systolic-diastolic flow from the aortic root to the right atrium.
TTE. Short-axis view showing a BAV type 2 (fusion of the right coronary and noncoronary cusps).
TTE. A nonstandard projection between the PLAX view and the right ventricular inflow tract with zoom showing an aneurysmal dilation of the anterior sinus of Valsalva with a mouth of 10 mm in diameter and distal perforation.
3D TTE reconstruction from a live 3D acquisition, nonstandard projection between the PLAX view and the right ventricular inflow tract showing an aneurysmal dilation of the anterior sinus of Valsalva.
TTE. A nonstandard projection between the PLAX view and the right ventricular inflow tract with Zoom (with color Doppler) showing an aneurysmal dilation of the anterior sinus of Valsalva with a mouth of 10 mm in diameter and distal perforation.
TTE. Apical four-chamber view showing a severe eccentric functional tricuspid regurgitation.
TTE. Apical four-chamber view showing a severe eccentric functional tricuspid regurgitation.
A nonstandard TEE view at 75 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line.
A nonstandard TEE view at 75 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line (with color Doppler).
A nonstandard TEE view at 75 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line (zoom).
A nonstandard TEE view at 116 showing the SOV-A projected toward the tricuspid septal valve near the coaptation line.
TTE. Parasternal short-axis view at the level of great vessels showing an increase echogenicity secondary to the patch without residual shunt and the tricuspid valve without residual tricuspid regurgitation.
TTE. Parasternal long-axis view showing the resolution of the aneurysm without residual shunt.
Parasternal short-axis view at the level of the great vessels showing an increase echogenicity secondary to the patch without residual shunt.
Parasternal short-axis view at the level of the great vessels showing the tricuspid valve without residual tricuspid regurgitation.