Abstract
BACKGROUND
Morbidity from asthma is disproportionately higher among black patients than among white patients, and black patients constitute the minority of participants in trials informing treatment. Data indicate that patients with inadequately controlled asthma benefit more from addition of a long-acting beta-agonist (LABA) than from increased glucocorticoids; however, these data may not be informative for treatment in black patients.
METHODS
We conducted two prospective, randomized, double-blind trials: one involving children and the other involving adolescents and adults. In both trials, the patients had at least one grandparent who identified as black and had asthma that was inadequately controlled with low-dose inhaled glucocorticoids. We compared combinations of therapy, which included the addition of a LABA (salmeterol) to an inhaled glucocorticoid (fluticasone propionate), a step-up to double to quintuple the dose of fluticasone, or both. The treatments were compared with the use of a composite measure that evaluated asthma exacerbations, asthma-control days, and lung function; data were stratified according to genotypic African ancestry.
RESULTS
When quintupling the dose of fluticasone (to 250 μg twice a day) was compared with adding salmeterol (50 μg twice a day) and doubling the fluticasone (to 100 μg twice a day), a superior response occurred in 46% of the children with quintupling the fluticasone and in 46% of the children with doubling the fluticasone and adding salmeterol (P=0.99). In contrast, more adolescents and adults had a superior response to the addition of salmeterol than to an increase in the fluticasone (salmeterol–low-dose fluticasone vs. medium-dose fluticasone, 49% vs. 28% [P=0.003]; salmeterol–medium-dose fluticasone vs. high-dose fluticasone, 49% vs. 31% [P=0.02]). Neither the degree of African ancestry nor baseline biomarkers predicted a superior response to specific treatments. The increased dose of inhaled glucocorticoids was associated with a decrease in the ratio of urinary cortisol to creatinine in children younger than 8 years of age.
CONCLUSIONS
In contrast to black adolescents and adults, almost half the black children with poorly controlled asthma had a superior response to an increase in the dose of an inhaled glucocorticoid and almost half had a superior response to the addition of a LABA. (Funded by the National Heart, Lung, and Blood Institute; BARD ClinicalTrials.gov number, .)
Inhaled glucocorticoids are effective first-line therapies for asthma control, but when asthma remains poorly controlled, the recommended treatment is the addition of a long-acting β2-adrenergic receptor agonist (LABA).1-9 However, this recommendation is based on studies that included few patients who identified as black and does not account for differences in genetic ancestry.
Epidemiologic studies involving patients with asthma in the United States show a disproportionately greater burden of asthma (exacerbations, asthma-related urgent-care visits, hospitalizations, and deaths) in persons identified as “black” than in those identified as “white.”10-17 Although these disparities in asthma morbidity may be due to social, environmental, or cultural factors, such trends persist even after adjustment for contextual factors for which race or ethnic group may serve as surrogates.13-15,18 Studies show that black patients often have differential responses to medications for asthma, and they have more glucocorticoid resistance, less cellular sensitivity to glucocorticoids, and more eosinophilic inflammation during inhaled glucocorticoid treatment than do white patients.19,20 Furthermore, the response to pharmacotherapy for asthma can be affected by genetic variants that are distributed differentially among persons of diverse self-described races and ancestral backgrounds; these variants may contribute to differences between black patients and white patients with respect to the response to β2-agonists and inhaled glucocorticoids.21-27
Contrary to the findings in white patients,4-8 a subanalysis involving black patients in one study indicated that adding a LABA was not superior to increasing the dose of an inhaled glucocorticoid in persons who identify as black.8 In addition, a follow-up analysis showed that this finding occurred in black children with eczema.28 Furthermore, a retrospective study involving black adults suggested that the addition of LABAs may not confer the same benefit as an increased dose of inhaled glucocorticoids.29
We conducted two parallel Best African American Response to Asthma Drugs (BARD) trials to determine the preferred “step-up” strategy in children, adolescents, and adults who had at least one grandparent who identified as black. We further examined the extent to which biomarkers, patient characteristics, and ancestral informative genomic variation were predictive of a response to inhaled glucocorticoids or LABAs.
METHODS
OVERVIEW OF THE TRIALS
We conducted two prospective, randomized, double-blind, four-treatment, four-period, 56-week crossover trials. One trial involved children (5 to 11 years of age) with at least one grandparent who identified as black, and the other involved adolescents and adults who were 12 years of age or older and who had family backgrounds that were similar to those of the children. Patients in both trials had inadequately controlled asthma while receiving a low-dose inhaled glucocorticoid (fluticasone propionate at a dose of 50 μg twice daily in children and 100 μg twice daily in adolescents and adults) (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
In the trial involving children, we compared the efficacy of doubling the dose of an inhaled glucocorticoid (fluticasone propionate) to a dose of 100 μg, administered twice daily (the double-fluticasone group); doubling the dose of fluticasone to 100 μg and adding a LABA (salmeterol) at a dose of 50 μg (the salmeterol–double-fluticasone group); quintupling the dose of fluticasone to 250 μg (the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 μg and adding salmeterol at a dose of 50 μg (the salmeterol–quintuple-fluticasone group). Owing to the lack of a low-dose inhaled glucocorticoid–LABA combination (i.e., salmeterol–fluticasone propionate, both at a dose of 50 μg), we could not examine the effect of merely adding salmeterol to the baseline dose of inhaled glucocorticoid.
In the trial involving adolescents and adults, we compared the efficacy of adding twice-daily salmeterol at a dose of 50 μg to baseline twice-daily administration of fluticasone propionate at a dose of 100 μg (the salmeterol–fluticasone group); increasing the dose of fluticasone by a factor of 2.5 to 250 μg (the 2.5-fluticasone group); quintupling the dose of fluticasone to 500 μg (the quintuple-fluticasone group); or increasing the dose of fluticasone by a factor of 2.5 to 250 μg and adding salmeterol at a dose of 50 μg (the salmeterol–2.5-fluticasone group).
The trials were identical in design and step-up dosing strategies. However, the first step-up regimen was different in the two trials because of the above-described differences in available formulations for children.
OVERSIGHT OF THE TRIALS
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and approved by the AsthmaNet steering committee, an NHLBI-appointed protocol review committee, and a data and safety monitoring board. The authors vouch for the accuracy and completeness of the data, for the accuracy of the analyses, and for the fidelity of the trial to the protocol (available at NEJM.org). GlaxoSmithKline donated the medications for the trials but did not have any other role in the design of the trials, in the collection or interpretation of the data, or in the preparation of the manuscript.
PATIENTS
Patients of both sexes who were 5 years of age or older and who reported having or (in the case of children) were reported by a parent or guardian as having at least one black grandparent were recruited from nine AsthmaNet partnership sites. The patients had a baseline forced expiratory volume in 1 second (FEV1) of at least 40% of the predicted value after bronchodilator use (after four puffs of albuterol [90 μg per puff]) as well as a diagnosis of asthma confirmed by beta-agonist reversibility (an increase in the FEV1 of at least 12%), a methacholine provocation concentration causing a 20% decrease (PC20) in the FEV1 of 16 mg per milliliter or less, or an absolute difference in the percentage of the predicted FEV1 of at least 12 percentage points over two measurements documented within the previous year (Section 1 in the Supplementary Appendix).
RUN-IN PERIOD
Patients who were receiving an inhaled glucocorticoid or an inhaled glucocorticoid–LABA combination were included in the trials, except for those who had inadequately controlled asthma while they were receiving a high-dose inhaled glucocorticoid–LABA. The run-in period consisted of an open-label inhaled glucocorticoid (fluticasone propionate at a dose of 50 μg twice daily in children and fluticasone propionate at a dose of 100 μg twice daily in patients who were at least 12 years of age). Patients could undergo randomization if they were found to have inadequately controlled asthma within 2 to 10 weeks after they entered the run-in period (Section 2 in the Supplementary Appendix).
TREATMENT PERIODS
Patients who met the randomization criteria (Section 2 in the Supplementary Appendix) were randomly assigned to step-up treatment sequences in a four-way crossover design with add-on LABA, different strengths of increased doses of inhaled glucocorticoid, or an increased dose of inhaled glucocorticoid with LABA in dry-powder Diskus inhalers (Fig. S1 in the Supplementary Appendix). Each treatment period lasted 14 weeks. The initial 2 weeks of each period were considered to be a washout period for the previous treatment and a wash-in period for the new regimen. Data on asthma-control days during those 2 weeks were censored from the analyses.
PRIMARY OUTCOMES
The primary aims of these trials were to evaluate the superiority of different treatments and the effect of the proportion of African ancestry (as informed by ancestry informative markers as detailed below) on the composite clinical outcome. The primary clinical outcome of each trial was a hierarchical composite measure that sequentially evaluated asthma exacerbations (Section 3 in the Supplementary Appendix), asthma-control days, and the percentage of the predicted FEV1 at the end of the 14-week treatment regimens to determine a differential response.8 A treatment was deemed to be superior to another if there was a between-treatment differential response of at least one exacerbation, defined as worsening asthma events leading to treatment with systemic glucocorticoids or unscheduled health care utilization. If no exacerbation difference was identified, a differential of 31 annualized asthma-control days was evaluated, and if no differential in asthma-control days was identified, then an absolute difference of 5 percentage points in the percentage of the predicted FEV1 was evaluated. (The primary and prespecified comparisons are described in Section 7 in the Supplementary Appendix.)
Whole-blood DNA was genotyped with the use of Illumina Multi-Ethnic Global Array BeadChips and 117,053 informative single-nucleotide polymorphisms (linkage disequilibrium, r2≥0.1) selected for estimation of genetic ancestry. Genotype data were analyzed with 225 HapMap founders representing founders with ancestry from northern and western Europe (described in the Centre d’Etude du Polymorphisme Humain HapMap samples) and central west Africa (Yoruba) (http://ftp.ncbi.nlm.nih.gov/hapmap/) and 43 Native Americans to estimate the percentage of African, European, and Native American ancestry in each patient.30-32 Ancestry-based genetic analyses evaluated the association of the percentage of African ancestry with the primary composite outcome (Section 7 and Fig. S6 in the Supplementary Appendix).
SECONDARY OUTCOMES
Secondary outcomes included asthma-control days (according to the use of albuterol rescue, use of glucocorticoids, symptoms, unscheduled office visits, and peak flows that were <90% of the reference value determined during the run-in period for each patient) (Section 3 in the Supplementary Appendix); the FEV1, before or after bronchodilator use; and measures of asthma control. Asthma control was also assessed with the use of the Childhood Asthma Control Test (in which scores range from 0 [uncontrolled] to 27 [well controlled], with a minimally important difference of 2) and the Asthma Control Test (in which scores range from 5 [uncontrolled] to 25 [well controlled], with a minimally important difference of 3); higher values represent better asthma control in both instruments. Quality of life was assessed with the use of the Asthma Quality of Life Questionnaire (AQLQ) (in which scores range from 1 to 7 and higher scores represent less impairment, with a minimally important difference of 0.5).
EXPLORATORY OUTCOMES
The number and type of asthma exacerbations (i.e., visits to the emergency department and hospitalizations) were evaluated. Patient characteristics, including atopy, pulmonary function (e.g., the degree of bronchodilator reversibility and the degree of methacholine responsiveness), and selective biomarkers, (e.g., sputum eosinophils) were examined to evaluate the differential response to trial treatments.
SYSTEMIC EFFECTS
Overnight urine specimens were obtained for measurements of the ratio of cortisol to creatinine at baseline and after treatment intervals. Recent data from the AsthmaNet Step Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations trial33 showed a reduced linear growth rate associated with the use of high-dose glucocorticoids among children younger than 8 years of age, so we analyzed data stratified according to ages younger than 8 years and 8 years or older.
STATISTICAL ANALYSIS
In the primary analysis, the target sample size of 284 children and 291 adolescents and adults had 90% power with a two-sided alpha of 0.05 to identify an absolute difference of 20 percentage points in the percentage of patients with a superior response to one therapy over another for the composite asthma outcome, assuming a withdrawal rate of 20% among children and 35% among adults before complete trial data acquisition. This analysis modeled the probability of patients having a superior response to one specific treatment over another, defined according to the composite outcome. The composite outcome and its components were fit with the use of nonlinear mixed-effect models for each pair of treatment comparisons (Section 7 in the Supplementary Appendix).34,35
In the trial involving children (5 to 11 years of age), the primary prespecified comparison was between fluticasone propionate at a dose of 250 μg twice daily (quintuple fluticasone) and twice-daily fluticasone propionate at a dose of 100 μg plus salmeterol at a dose of 50 μg (salmeterol–double fluticasone). In the trial involving adolescents and adults, we were able to directly examine step-up regimens with a LABA as compared with increased doses of a glucocorticoid (at two doses), and we prespecified two primary comparisons: fluticasone propionate at a dose of 250 μg twice daily (2.5-fluticasone) versus twice-daily fluticasone at a dose of 100 μg plus salmeterol at a dose of 50 μg (salmeterol–fluticasone), and twice-daily fluticasone at a dose of 500 μg (quintuple fluticasone) versus twice-daily fluticasone at a dose of 250 μg plus salmeterol at a dose of 50 μg (salmeterol–2.5-fluticasone), without adjustment for multiple testing. (Section 7 in the Supplementary Appendix lists prespecified secondary comparisons of the other dose combinations.) The results of the trial involving children and those of the trial involving adults and adolescents were also compared.
RESULTS
BASELINE CHARACTERISTICS OF THE PATIENTS
Between January 2014 and March 2016, a total of 280 children (of 482 enrolled) and 294 adolescents and adults (of 536 enrolled) underwent randomization at nine centers (Figs. S2 and S3 and Table S1 in the Supplementary Appendix). The majority of children were male, and the majority of adolescents and adults were female (Table 1). As compared with the adolescents and adults, more children had a blood eosinophil count of at least 300 cells per cubic millimeter, and children had a higher percentage of the predicted FEV1, more courses of systemic glucocorticoids, and more unscheduled office visits and hospitalizations because of asthma in the previous year. (Complete baseline characteristics of the patients and of those who completed the trials as compared with those who discontinued the trials are provided in Table S1 in the Supplementary Appendix.)
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Children (5–11 Yr) (N = 280) |
Adolescents and Adults (≥12 Yr) (N = 294) |
|---|---|---|
| Demographic features | ||
| Age — yr | 8.5±1.8 | 37.3±16.1 |
| Male sex — no. (%) | 170 (60.7) | 95 (32.3) |
| Median percentage of African ancestry (IQR)† | 81.0 (73.4–85.6) | 82.1 (75.3–87.6) |
| Hispanic ethnic group — no. (%)‡ | 24 (8.6) | 9 (3.1) |
| Asthma history in previous 12 mo — no. (%) | ||
| One or more asthma episodes resulting in emergency care or unscheduled office visit | 208 (74.3) | 132 (44.9) |
| One or more overnight hospitalizations | 43 (15.4) | 14 (4.8) |
| One or more courses of systemic glucocorticoids | 172 (61.4) | 99 (33.7) |
| Medications used in previous 12 mo | ||
| Leukotriene-receptor antagonist or 5-lipoxygenase inhibitors — no. (%) | 107 (38.2) | 50 (17.0) |
| Oral glucocorticoids — no./total no. (%) | 170/277 (61.4) | 92/293 (31.4) |
| Inhaled or nebulized glucocorticoid monotherapy — no./total no. (%) | 246/279 (88.2) | 193/294 (65.6) |
| Inhaled glucocorticoid–LABA combination therapy — no./total no. (%) | 64/279 (22.9) | 139 /294 (47.3) |
| Clinical and spirometric features | ||
| Patients with ≥1 of 13 positive tests for allergens by ImmunoCAP assay — no./total no. (%) | 224/273 (82.1) | 244/287 (85.0) |
| Sputum eosinophil level ≥2% — no./total no. (%)§ | NA | 24/220 (10.9) |
| Median blood eosinophil absolute count — cells/mm3 (IQR)¶ | 340 (200–510) | 200 (100–300) |
| Median serum total IgE — IU/ml (IQR)‖ | 286.5 (92.0–693.5) | 174.0 (73.0–468.0) |
| FEV1 — % of predicted value** | 95.5±16.7 | 83.4±17.4 |
| Bronchodilator response (4 puffs) — % relative change†† | 13.79±14.48 | 12.47±12.43 |
| PC20 for methacholine — mg/ml‡‡ | ||
| Geometric mean | 1.32 | 1.71 |
| Coefficient of variation | 1.61 | 1.60 |
| Median score on Childhood Asthma Control Test or Asthma Control Test (IQR)§§ | 22 (19–24) | 19 (16–22) |
| Asthma-control days during 2 wk before randomization (%)¶¶ | 31.2±29.9 | 24.5±28.2 |
Plus–minus values are means ±SD. FEV1 denotes forced expiratory volume in 1 second, LABA long-acting beta-agonist, and NA not applicable.
Data were missing for 15 children and 10 adolescents and adults.
Race and ethnic group were reported by the patients or their parents or guardians.
Children younger than 12 years of age did not undergo sputum induction.
Data were missing for 5 children and 5 adolescents and adults.
Data were missing for 4 children and 4 adolescents and adults.
Data were missing for 5 children and 3 adolescents and adults.
Data were missing for 7 children.
PC20 denotes the provocative concentration of inhaled methacholine that results in a 20% reduction in the FEV1. Data were missing for 67 children and 44 adolescents and adults.
The score on the Childhood Asthma Control Test ranges from 0 to 27, with higher values representing better asthma control. The score on the Asthma Control Test ranges from 5 to 25, with higher values representing better asthma control. Data were missing for 1 patient in each group.
Patients who provided asthma-control information on fewer than 7 of the 14 days were excluded from this summary, and data were missing for 3 children and 5 adolescents and adults.
OUTCOMES IN CHILDREN
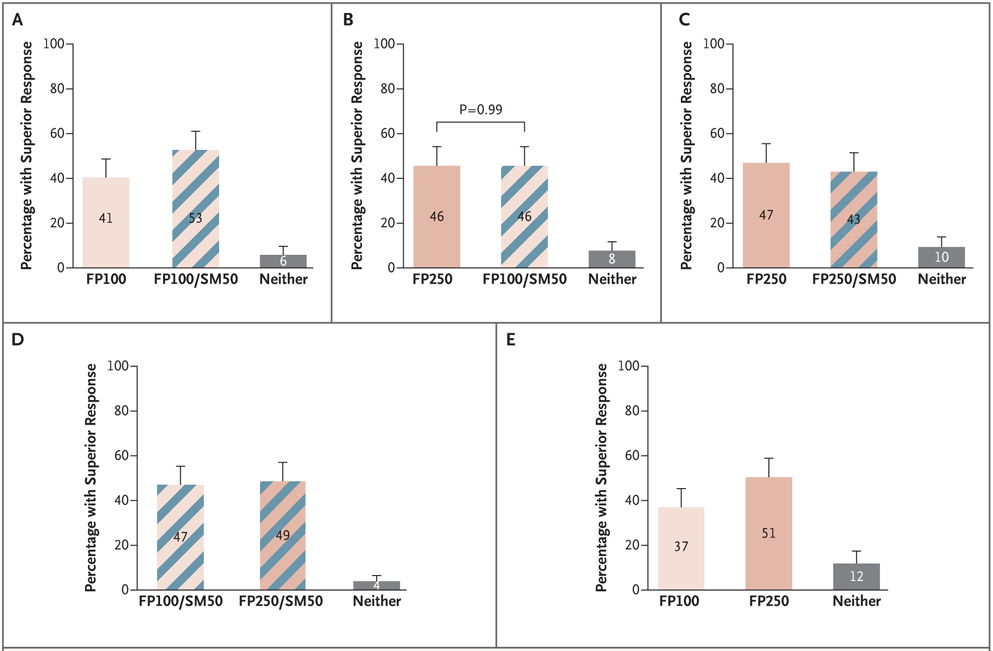
The majority of children had a differential outcome between treatments. The maximum percentage of patients who did not have a superior response in any paired intervention comparison was 12% (Fig. 1). There was no significant difference in the probability of a superior response when the dose of inhaled glucocorticoid was increased two steps to a quintupled dose of fluticasone propionate (250 μg) (46% superior) as compared with a two step-up strategy of adding a LABA (salmeterol) at a dose of 50 μg and increasing the dose of fluticasone to 100 μg (46% superior) (P=0.99) (Fig. 1B).
Figure 1. Percentage of Black Children (5 to 11 Years of Age) with Asthma Who Had a Superior Response to Specific Treatments, According to the Composite Outcome, at 14 Weeks.
Shown are the five prespecified comparisons of the percentages of patients with a superior response among those receiving twice-daily treatment with a low-dose inhaled glucocorticoid (fluticasone propionate) at a dose of 50 μg (FP100, the double-fluticasone group); a dose of fluticasone doubled to 100 μg with the addition of a LABA (salmeterol) at a dose of 50 μg (FP100/SM50, the salmeterol–double-fluticasone group); a dose of fluticasone quintupled to 250 μg (FP250, the quintuple-fluticasone group); or a dose of fluticasone quintupled to 250 μg with the addition of salmeterol at a dose of 50 μg (FP250/SM50, the salmeterol–quintuple-fluticasone group) with respect to the hierarchical composite outcome that incorporated asthma exacerbations, asthma-control days, and change in the forced expiratory volume in 1 second (FEV1). The numbers in each bar represent the percentage of patients who had a superior response to that specific treatment, as compared with the alternative treatment. Gray bars indicate the percentage of patients in whom one treatment was not superior to the other. The P value reflects a test of the coprimary null hypothesis that the probability of a superior response to each treatment would not differ. T bars indicate 95% confidence intervals.
In children, 53% of patients (95% confidence interval [CI], 45 to 61) in the salmeterol–double-fluticasone group had a superior response, as compared with 41% (95% CI, 33 to 49) in the double-fluticasone group (Fig. 1A), whereas 43% (95% CI, 35 to 52) in the salmeterol–quintuple-fluticasone group had a superior response, as compared with 47% (95% CI, 39 to 56) in the the quintuple-fluticasone group (Fig. 1C). A total of 51% of patients (95% CI, 42 to 59) had a superior response to a higher dose of inhaled glucocorticoid (in the quintuple-fluticasone group) as compared with 37% of patients (95% CI, 29 to 45) who had a superior response to a lower dose of inhaled glucocorticoid (in the double-fluticasone group) (Fig. 1E). (Differential responses to increasing the dose of inhaled glucocorticoid as compared with adding a LABA in the elements of the composite of FEV1, asthma-control days, or exacerbations are shown in Figures S4.1 through S4.3 in the Supplementary Appendix.)
OUTCOMES IN ADOLESCENTS AND ADULTS
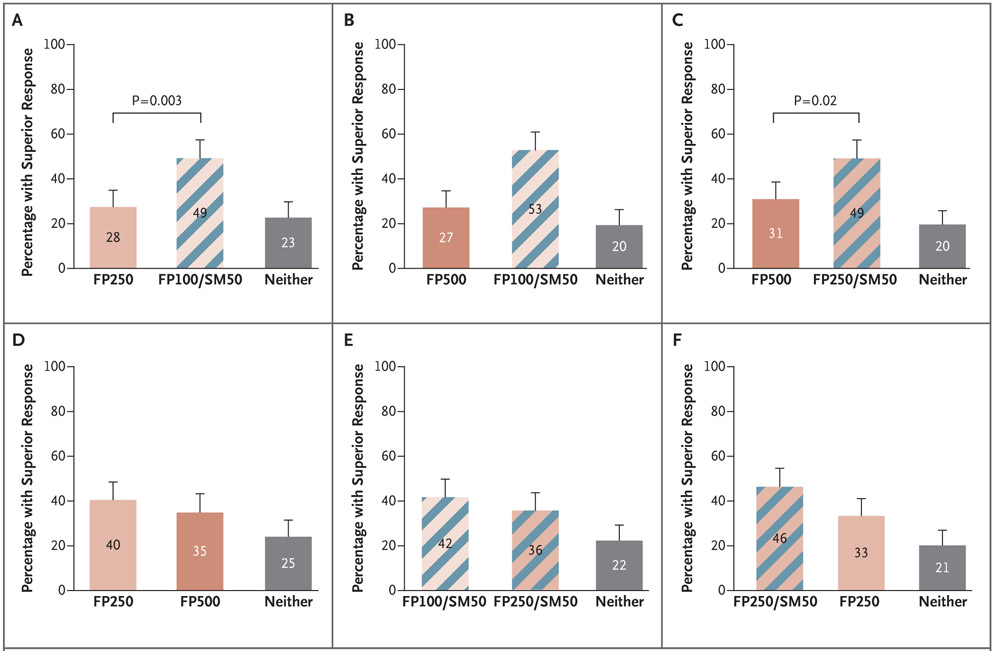
In all the comparisons in the trial involving adolescents and adults, 20 to 25% of the patients did not have a differential outcome between treatments (Fig. 2). More adolescents and adults had a superior response with the addition of a LABA than with either of the two step-up dose increases in inhaled glucocorticoids (49% in the salmeterol–fluticasone group vs. 28% in the 2.5-fluticasone group) (P=0.003) (Fig. 2A), and 49% in the salmeterol–2.5-fluticasone group versus 31% in the quintuple-fluticasone group (P = 0.02) (Fig. 2C). Differences in superior response rates were driven by differences in asthma-control days and FEV1 (Figs. S5.2A and S5.2B and S5.3A and S5.3B in the Supplementary Appendix). Exacerbations were infrequent and contributed only minimally to the composite outcome (Fig. S5.1.A and S5.1.B in the Supplementary Appendix). Increasing the dose of glucocorticoid by either a factor of 2.5 (fluticasone propionate at a dose of 250 μg) or a factor of 5 (fluticasone propionate at a dose of 500 μg) (Fig. 2D) or from 100 μg of fluticasone propionate to 250 μg of fluticasone propionate (accompanied by a LABA) (Fig. 2E) did not result in a significantly higher percentage of patients with a superior response.
Figure 2. Percentage of Black Adolescents and Adults with Asthma Who Had a Superior Response to Specific Treatments, According to the Composite Outcome, at 14 Weeks.
Shown are all the comparisons of the percentages of patients with a superior response among those receiving twice-daily treatment with fluticasone propionate at a dose of 100 μg plus salmeterol at a dose of 50 μg (FP100/SM50, the salmeterol–fluticasone group); a dose of fluticasone increased by a factor of 2.5 to 250 μg (FP250, the 2.5-fluticasone group); a dose of fluticasone quintupled to 500 μg (FP500, the quintuple-fluticasone group); or a dose of fluticasone increased by a factor of 2.5 to 250 μg with the addition of salmeterol at a dose of 50 μg (FP250/SM50, the salmeterol–2.5-fluticasone group) with respect to the hierarchical composite outcome that incorporated asthma exacerbations, asthma-control days, and the absolute change in the percentage of the predicted FEV1. The numbers in each bar represent the percentage of patients who had a superior response to that specific treatment, as compared with the alternative treatment. Gray bars indicate the percentage of patients in whom one treatment was not superior to the other. The P values reflect a test of the coprimary null hypotheses that the probability of a superior response to each treatment would not differ. T bars indicate 95% confidence intervals.
COMPARISON OF THE TWO TRIALS
In adolescents and adults, the addition of a LABA was more likely to produce superior responses than increasing the dose of an inhaled glucocorticoid. In contrast, children had a response to stepped increases in the dose of inhaled glucocorticoid (Fig. 3A, and Figs. S4 and S5 in the Supplementary Appendix).
Figure 3. Comparison of the Primary Composite Outcome in the Trial involving Adolescents and Adults and the Trial involving Children.
Shown are the prespecified comparisons of the percentages of patients with a superior response. Each panel shows a comparison of a similar step-up in therapy for children and for adolescents and adults. Shown are responses at 14 weeks in adolescents and adults and in children who at baseline had poorly controlled asthma while receiving twice-daily treatment with a low-dose inhaled glucocorticoid (fluticasone propionate) (50 μg in children and 100 μg in adolescents and adults). In children, the step-up trial treatments included doubling the dose of fluticasone to 100 μg (FP100, the double-fluticasone group); doubling the dose of fluticasone to 100 μg and adding salmeterol at a dose of 50 μg (FP100/SM50, the salmeterol–double-fluticasone group); quintupling the dose of fluticasone to 250 μg (FP250, the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 μg and adding salmeterol at a dose of 50 μg (FP250/SM50, the salmeterol–quintuple-fluticasone group). In adolescents and adults, the step-up interventions included adding salmeterol to the baseline dose of fluticasone (FP100/SM50, the fluticasone–salmeterol group), increasing the dose of fluticasone by a factor of 2.5 (FP250, the 2.5-fluticasone group), increasing the dose of fluticasone by a factor of 2.5 and adding salmeterol (FP250/SM50, the salmeterol–2.5-fluticasone group), or quintupling the dose of fluticasone (FP 500). A superior response was determined with respect to the hierarchical composite outcome that incorporated asthma exacerbations, asthma-control days, and the change in the FEV1. The numbers in each bar represent the percentage of patients who had a superior response to that specific treatment as compared with the alternative treatment. Gray bars indicate the percentage of patients in whom one treatment was not superior to the other. The two groups of patients (patients from the trial involving children and those from the trial involving adolescents and adults) were compared to identify interactions between the two groups and the composite superiority outcome. T bars indicate 95% confidence intervals.
SECONDARY OUTCOMES
The results of prespecified secondary outcomes and analyses are provided in Figures S4 and S5 and Table S8 in the Supplementary Appendix. Consistent with the trend seen in the evaluation of the composite outcome, more children had reduced asthma exacerbations with a higher dose of inhaled glucocorticoid (7%; 95% CI, 2 to 13) than with a lower-dose of inhaled glucocorticoid (2%; 95% CI, 0 to 5). Higher-dose inhaled glucocorticoids also produced greater changes in the percentage of the predicted FEV1 before or after bronchodilator use (2.3%; 95% CI, 0.7 to 4.0) than a lower-dose inhaled glucocorticoid (1.6%; 95% CI, 1 to 3.0).
Adolescents and adults had more asthma-control days with the addition of a LABA than with an increase in the dose of inhaled glucocorticoid (a 14-day-per-year difference [95% CI, 1 to 26] in the salmeterol–fluticasone group vs. the 2.5-fluticasone group and a 14-day-per-year difference [95% CI, 3 to 25] in the salmeterol–2.5-fluticasone group vs. the quintuple-fluticasone group) (Table S8 in the Supplementary Appendix). There was also an absolute difference in the percentage of the predicted FEV1 before bronchodilator use (the salmeterol–fluticasone group vs. the 2.5-fluticasone group, 1.2 percentage points [95% CI, 0.2 to 2.3]; and the salmeterol–2.5-fluticasone group vs. the quintuple-fluticasone group, 0.9 percentage points [95% CI, −0.1 to 1.9]) (Table S8 in the Supplementary Appendix). However, the addition of a LABA did not differentially affect asthma exacerbations, the FEV1 after bronchodilator use, the results of the Asthma Control Test, or the results of the AQLQ.
GENETIC AFRICAN ANCESTRY AND THERAPEUTIC OUTCOMES
Among patients in both age groups, there was a broad distribution in the percentage of African ancestry, ranging from 2 to 100%, with a mean of 81.0% in children and 82.1% in adolescents and adults (Table 1, and Fig. S6 in the Supplementary Appendix); this distribution was comparable to that in black populations in previous studies.36 There were no significant interactions between the percentage of African ancestry and the primary composite outcome or any of the individual outcomes (Tables S2 and S3 in the Supplementary Appendix). We were unable to identify a cutoff for the percentage of African ancestry that was predictive of therapeutic response (Tables S4 through S7 in the Supplementary Appendix), and we did not find significant, consistent, meaningful associations between African ancestry and the treatment response when the extremes of ancestry were compared (Figs. S7.1 and S7.2 in the Supplementary Appendix).
For the primary composite outcome in both trials, none of the prespecified biomarkers or patient characteristics identified a group of patients who were more likely to have a response to the addition of one therapy than to another.
SAFETY
In the trial involving children, the highest dose of inhaled glucocorticoid (250 μg twice daily) was associated with a decrease in the ratio of urinary cortisol to creatinine in those who were younger than 8 years of age (Table S9 in the Supplementary Appendix), whereas no such effects were seen in adolescents and adults. There were no other significant differences among the treatment groups in either trial with respect to respiratory tract infections or pneumonia.
DISCUSSION
Studies involving children and adults with asthma have been conducted primarily in white populations; these studies have shown that when escalating asthma therapy, the addition of a LABA is more likely to produce a superior response than an increase in the dose of an inhaled glucocorticoid.5-8 These data have influenced guidelines regarding escalation of therapy when patients present with asthma that is not well controlled; however, black patients with asthma have not been included in the clinical trials on which the guidelines were based.10,11,13-16
In the current trials, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma (46%) had improved asthma outcomes when the dose of inhaled glucocorticoid was increased rather than with the addition of a LABA. Furthermore, we discovered that in contrast to both black adults and white adults and white children, black children had a response to stepped increases in the dose of inhaled glucocorticoid. Our results are all the more striking in that in our parallel trial we confirmed that adolescents and adults who had at least one grandparent who identified as black had responses similar to those reported in white adults — that is, the addition of a LABA in adults was more likely to lead to superior responses in a larger group of patients than an increase in the dose of an inhaled glucocorticoid. These findings suggest that data cannot be extrapolated from clinical trials involving mixed populations to specific subgroups, including those of different ages and races.
We found that larger percentages of children than had been previously reported in mixed populations had a response to increasing doses of glucocorticoids than to the addition of a LABA.8 Although a recent trial involving predominantly white children with mild-to-moderate persistent asthma showed that quintupling the dose of inhaled glucocorticoids for 7 days at the early signs of loss of asthma control was not better in preventing exacerbations than maintaining the use of low-dose inhaled glucocorticoids,33 that trial involved short-term administration of higher-dose inhaled glucocorticoids in patients with acute deterioration of asthma and thus is not comparable to our trials examining the effects of long-term treatment.
We found evidence of adrenal axis suppression in young children (<8 years of age) (Table S9 in the Supplementary Appendix) at the highest dose of inhaled glucocorticoid we tested (fluticasone propionate at a dose of 250 μg). Our trial was not long enough to assess effects on growth. However, the findings with regard to the adrenal axis at high doses of inhaled glucocorticoids are of potential concern, although it is not clear how our findings would extrapolate to other formulations of inhaled glucocorticoids.
African ancestry, as determined by patterns of genetic markers, has been associated with asthma-related phenotypes including low lung function and exacerbations.36-38 However, we did not find that African ancestry was associated with differential responses in adolescents and adults or with differential responses in children (Figs. S7.1 and S7.2 in the Supplementary Appendix). Nevertheless, the absence of a global ancestral effect does not exclude potential effects of either asthma severity loci or pharmacogenetic loci differentially inherited among persons across varying ancestral backgrounds.21-27 We were also not able to detect phenotypic or biomarker characteristics that were associated with a differential response to a specific therapy. A larger trial might have the power to determine which phenotypic or specific pharmacogenetic variant panels could have the power to detect such differences.
In conclusion, our prospective, randomized BARD trials comparing several strategies of treatment escalation for asthma in children and in adolescents and adults who had at least one grandparent who identified as black showed that outcomes differed in children and adults, and the results in these children differed from those previously reported in studies involving white children. In contrast to black adults and white persons of all ages, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma had a superior response to an increased dose of an inhaled glucocorticoid over the addition of a LABA. A larger, more simplified trial should be undertaken to determine the best treatment approach for black children with poorly controlled asthma despite the use of standard doses of an inhaled glucocorticoid.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute.
Dr. Wechsler reports receiving grant support and consulting fees from AstraZeneca, Novartis, Sanofi, and GlaxoSmithKline, consulting fees from Regeneron Pharmaceuticals, Mylan, Genentech, Restorbio, Equillium, Boston Scientific, Genzyme, Gala Therapeutics, and Pulmatrix, fees for serving on a data and safety monitoring board from Sentien Biotechnologies, grant support, consulting fees, advisory board fees, and donated drugs from Teva Pharmaceuticals, consulting fees and donated drugs from Boehringer Ingelheim, and donated drugs from Merck; Dr. Szefler, receiving consulting fees, paid to his institution, and donated drugs from Boehringer Ingelheim, receiving fees for manuscript preparation and advisory board fees, paid to his institution, from Genentech, fees for attending meetings, paid to his institution from and serving as manager of a grant for GlaxoSmithKline, consulting fees, paid to his institution, from Aerocrine, AstraZeneca, Daiichi Sankyo, Roche, Propeller Health, Sanofi, and Regeneron Pharmaceuticals, advisory board fees, paid to his institution, and donated drugs from Teva Pharmaceuticals, and donated drugs from Merck; Dr. Pongracic, receiving donated drugs from Boehringer Ingelheim, Merck, Teva Pharmaceuticals, and GlaxoSmithKline; Drs. Chinchilli and Lima, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Ms. Kunselman, receiving donated drugs from Boehringer Ingelheim and Teva Pharmaceuticals, and receiving donated drugs from and owning stock in Merck; Dr. Mauger, receiving grant support and donated drugs from GlaxoSmithKline, Genentech, Vifor Pharma, Boehringer Ingelheim, and Teva Pharmaceuticals, grant support from Sanofi and AstraZeneca, fees for serving on a data and safety monitoring board from Novartis, and donated drugs from Merck; Dr. Bleecker, receiving consulting fees and donated drugs from Boehringer Ingelheim, donated drugs from Merck and Teva Pharmaceuticals, and consulting fees from AstraZeneca, MedImmune, GlaxoSmithKline, Novartis, and Sanofi–Regeneron, and participating in trials as an employee of Wake Forest School of Medicine and the University of Arizona for AstraZeneca, MedImmune, Boehringer Ingelheim, Cephalon–Teva Pharmaceuticals, Genentech, GlaxoSmithKline, Johnson & Johnson (Janssen), Novartis, and Sanofi–Regeneron; Dr. Bacharier, receiving consulting fees and lecture fees from Aerocrine, GlaxoSmithKline, Genentech–Novartis, and AstraZeneca, advisory board fees and donated drugs from Merck, fees for serving on a data safety monitoring board from DBV Technologies, consulting fees, lecture fees, and donated drugs from Teva Pharmaceuticals and Boehringer Ingelheim, honoraria from WebMD–Medscape, advisory board fees and lecture fees from Sanofi–Regeneron, advisory board fees and consulting fees from Vectura, and advisory board fees from Circassia; Dr. Beigelman, Ms. Benson, and Dr. Blake, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Cabana, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, and consulting fees from Genentech and Novartis; Dr. Cardet, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Castro, receiving grant support, lecture fees, and donated drugs from Boehringer Ingelheim, donated drugs from Merck, consulting fees, lecture fees, and donated drugs from Teva Pharmaceuticals, consulting fees and lecture fees from Boston Scientific and Genentech, consulting fees from Nuvaira, Aviragen, 4D Pharma, VIDA Diagnostics, Mallinckrodt Pharmaceuticals, Theravance, Therabron, and Vectura, grant support, consulting fees, and lecture fees from Sanofi-Aventis, grant support and lecture fees from AstraZeneca and GlaxoSmithKline, grant support from Chiesi and Novartis, and lecture fees from Regeneron Pharmaceuticals; Dr. Chmiel, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, consulting fees from Albumedix, Catabasis, Paka Pulmonary Pharmaceuticals, Patara Pharma, and pH Pharma, and grant support form Vertex; Dr. Covar, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, and grant support from GlaxoSmithKline; Dr. Denlinger, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, grant support and consulting fees from AstraZeneca, and consulting fees from Sanofi–Regeneron; Dr. DiMango, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Gentile, receiving donated drugs from Boehringer Ingelheim and Teva Pharmaceuticals, grant support and donated drugs from Merck, grant support from GlaxoSmithKline, grant support, consulting fees, and lecture fees from Stallergenes Greer, and fees for serving on a data and safety monitoring board from AstraZeneca; Dr. Holguin, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Jackson, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, grant support from GlaxoSmithKline, advisory board fees from Novartis, Sanofi–Regeneron, and AstraZeneca, fees for serving on a steering committee from Vifor Pharma, consulting fees from Commense, and fees for serving on a data and safety monitoring board from Pfizer; Dr. Kraft, receiving grant support from Chiesi and Sanofi; Drs. LaForce and Lang, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Lemanske, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, and lecture fees from Thermo Fisher Scientific; Dr. Long, receiving donated drugs from Teva Pharmaceuticals; Dr. Lugogo, receiving grant support, advisory board fees, and donated drugs from GlaxoSmithKline, grant support, consulting fees, and advisory board fees from AstraZeneca, consulting fees, advisory board fees, and donated drugs from Teva Pharmaceuticals, grant support from Genentech, grant support and advisory board fees from Sanofi–Regeneron, and donated drugs from Merck and Boehringer Ingelheim; Dr. Martinez, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, grant support from Johnson & Johnson, and consulting fees from Copeval and Commense; Dr. Meyers, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Moore, receiving grant support and donated drugs from Boehringer Ingelheim, donated drugs from Merck and Teva Pharmaceuticals, grant support and advisory board fees from AstraZeneca, GlaxoSmithKline, and Sanofi–Regeneron, and grant support from Novartis, Cumberland Pharmaceuticals, and Gossamer Bio; Drs. Moy, Naureckas, and Olin, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Peters, receiving advisory board fees from AstraZeneca, GlaxoSmithKline, Mylan, Teva Pharmaceuticals, Sanofi–Regeneron, and Theravance, fees for serving as clinical trial adjudicator from Quintiles, fees for serving on a data and safety monitoring board from Genentech, fees for serving as chair of a data and safety monitoring board from Novartis, and honoraria from PRIME; Drs. Phipatanakul, Que, Raissy, and Robison, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Ross, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, and grant support, paid to her institution, from AstraZeneca; Dr. Sheehan, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Smith, receiving donated drugs from Boehringer Ingelheim and Teva Pharmaceuticals, and fees for serving on a data and safety monitoring board and donated drugs from Merck; Dr. Solway, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, advisory board fees from PulmOne Advanced Medical Devices, advisory board fees, honoraria, and travel support from Regeneron–Sanofi–Genzyme, holding patents #6,090,618, #6,114,311, #6,284,743, #6,291,211, #6,297,221, #6,331,527, and #7,169,764 on a smoothmuscle gene promoter (SM22 alpha), holding pending patent PCT/US2014/032186 on a method for determining respiratory physiological parameters, holding pending patent 62/872,980 on remodilins for airway remodeling and organ fibrosis, and holding pending patent 62/828,122 on remodilins to prevent or treat cancer metastasis, glaucoma, and hypoxia; Drs. Sorkness and Sullivan-Vedder, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals; Dr. Wenzel, receiving donated drugs from Boehringer Ingelheim, Merck, and Teva Pharmaceuticals, grant support and consulting fees from AstraZeneca and Sanofi, and consulting fees from Pieris Pharmaceuticals; Dr. White, receiving advisory board fees and donated drugs from Boehringer Ingelheim, and donated drugs from Merck and Teva Pharmaceuticals; and Dr. Israel, receiving grant support and consulting fees from AstraZeneca, Novartis, and Genentech, consulting fees from Regeneron Pharmaceuticals, Bird Rock Bio, Nuvelution Pharmaceuticals, Vitaeris, Sanofi Genzyme, Entrinsic Health Solutions, Pneuma Respiratory, 4D Pharma, Sienna Biopharmaceuticals, and Equillium, grant support, consulting fees, and donated drugs from Merck, Teva Pharmaceutical Industries, and GlaxoSmithKline, serving as a consultant for Vorso, receiving grant support and donated drugs from Vifor Pharma, Boehringer Ingelheim, and Teva Pharmaceuticals, grant support from Sanofi and AstraZeneca, and donated drugs from Circassia. No other potential conflict of interest relevant to this article was reported.
We thank Elizabeth Juniper, M.S.C.P., for granting permission to use asthma-control and quality-of-life questionnaires, Michelle Freemer, M.D., and Robert Smith, of the National Heart, Lung, and Blood Institute, for their support of these trials, and William Busse, M.D., for chairing the AsthmaNet steering committee.
Footnotes
A complete list of the investigators in the NHLBI AsthmaNet is provided in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
Michael E. Wechsler, National Jewish Health, Denver, University of Colorado School of Medicine, Aurora, Colorado
Stanley J. Szefler, University of Colorado School of Medicine, Children’s Hospital Colorado, Aurora, Colorado
Victor E. Ortega, Wake Forest School of Medicine, Winston-Salem, North Carolina
Jacqueline A. Pongracic, Ann and Robert H. Lurie Children’s Hospital of Chicago
Vernon Chinchilli, Penn State University, Pennsylvania
John J. Lima, Nemours Children’s Health System, Jacksonville, Florida
Jerry A. Krishnan, University of Illinois at Chicago, Chicago
Susan J. Kunselman, Penn State University, Pennsylvania
David Mauger, Penn State University, Pennsylvania
Eugene R. Bleecker, University of Arizona Health Sciences, Tucson
Leonard B. Bacharier, Washington University School of Medicine, St. Louis
Avraham Beigelman, Washington University School of Medicine, St. Louis
Mindy Benson, University of California, San Francisco (UCSF), San Francisco, UCSF Benioff Children’s Hospital, Oakland, California
Kathryn V. Blake, Nemours Children’s Health System, Jacksonville, Florida
Michael D. Cabana, University of California, San Francisco (UCSF), San Francisco, California
Juan-Carlos Cardet, University of South Florida Morsani College of Medicine, Tampa, Florida, Brigham and Women’s Hospital and Harvard Medical School, Boston
Mario Castro, Washington University School of Medicine, St. Louis
James F. Chmiel, University Hospitals Rainbow Babies and Children’s Hospital, Case Western Reserve University School of Medicine, Cleveland
Ronina Covar, National Jewish Health, Denver, University of Colorado School of Medicine, Aurora, Colorado
Loren Denlinger, University of Wisconsin–Madison, Madison, Wisconsin
Emily DiMango, Columbia University Irving Medical Center, New York
Anne M. Fitzpatrick, Emory University, Atlanta
Deborah Gentile, Hershey, and Allegheny General Hospital, Pittsburgh, Pennsylvania
Nicole Grossman, Brigham and Women’s Hospital and Harvard Medical School, Boston
Fernando Holguin, University of Colorado School of Medicine, Aurora, Colorado
Daniel J. Jackson, University of Wisconsin–Madison, Madison, Wisconsin
Harsha Kumar, University of Illinois at Chicago, Chicago
Monica Kraft, University of Arizona Health Sciences, Tucson
Craig F. LaForce, North Carolina Clinical Research, Raleigh, North Carolina
Jason Lang, Nemours Children’s Health System, Jacksonville, Florida
Stephen C. Lazarus, University of California, San Francisco (UCSF), San Francisco, California
Robert F. Lemanske, Jr, University of Wisconsin–Madison, Madison, Wisconsin
Dayna Long, University of California, San Francisco (UCSF), San Francisco, UCSF Benioff Children’s Hospital, Oakland, California
Njira Lugogo, Duke University Medical Center, Durham, North Carolina
Fernando Martinez, University of Arizona Health Sciences, Tucson
Deborah A. Meyers, University of Arizona Health Sciences, Tucson
Wendy C. Moore, Wake Forest School of Medicine, Winston-Salem, North Carolina
James Moy, Rush University Medical Center, Chicago
Edward Naureckas, University of Chicago, Chicago
J. Tod Olin, National Jewish Health, Denver, University of Colorado School of Medicine, Aurora, Colorado
Stephen P. Peters, Wake Forest School of Medicine, Winston-Salem, North Carolina
Wanda Phipatanakul, Boston Children’s Hospital, Boston
Loretta Que, Duke University Medical Center, Durham, North Carolina
Hengameh Raissy, University of New Mexico, Albuquerque
Rachel G. Robison, Ann and Robert H. Lurie Children’s Hospital of Chicago
Kristie Ross, University Hospitals Rainbow Babies and Children’s Hospital, Case Western Reserve University School of Medicine, Cleveland
William Sheehan, Boston Children’s Hospital, Boston
Lewis J. Smith, Northwestern University Feinberg School of Medicine, Chicago
Julian Solway, University of Chicago, Chicago
Christine A. Sorkness, University of Wisconsin–Madison, Madison, Wisconsin
Lisa Sullivan-Vedder, Aurora Sinai Medical Center, Milwaukee, Wisconsin
Sally Wenzel, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
Steven White, University of Chicago, Chicago
Elliot Israel, Brigham and Women’s Hospital and Harvard Medical School, Boston
REFERENCES
- 1.Boulet LP, Turcotte H, Prince P, et al. Benefits of low-dose inhaled fluticasone on airway response and inflammation in mild asthma. Respir Med 2009;103:1554–63. [DOI] [PubMed] [Google Scholar]
- 2.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000;343:332–6. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003;361:1071–6. [DOI] [PubMed] [Google Scholar]
- 4.Adams NP, Bestall JC, Jones P, Lasserson TJ, Griffiths B, Cates CJ. Fluticasone at different doses for chronic asthma in adults and children. Cochrane Database Syst Rev 2008;4:CD003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med 2010;363:1715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauwels RA, Löfdahl C-G, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 1997;337:1405–11. [DOI] [PubMed] [Google Scholar]
- 7.O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164:1392–7. [DOI] [PubMed] [Google Scholar]
- 8.Lemanske RF Jr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 2010; 362:975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinbami L The state of childhood asthma, United States, 1980–2005. Adv Data 2006;381:1–24. [PubMed] [Google Scholar]
- 11.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3 2012;35:1–58. [PubMed] [Google Scholar]
- 12.Homa DM, Mannino DM, Lara M. Asthma mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990–1995. Am J Respir Crit Care Med 2000;161:504–9. [DOI] [PubMed] [Google Scholar]
- 13.Adams RJ, Smith BJ, Ruffin RE. Factors associated with hospital admissions and repeat emergency department visits for adults with asthma. Thorax 2000;55: 566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisner MD, Katz PP, Yelin EH, Shiboski SC, Blanc PD. Risk factors for hospitalization among adults with asthma: the influence of sociodemographic factors and asthma severity. Respir Res 2001;2: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griswold SK, Nordstrom CR, Clark S, Gaeta TJ, Price ML, Camargo CA Jr. Asthma exacerbations in North American adults: who are the “frequent fliers” in the emergency department? Chest 2005; 127:1579–86. [DOI] [PubMed] [Google Scholar]
- 16.El-Ekiaby A, Brianas L, Skowronski ME, et al. Impact of race on the severity of acute episodes of asthma and adrenergic responsiveness. Am J Respir Crit Care Med 2006;174:508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: rethinking the inner-city asthma epidemic. J Allergy Clin Immunol 2015;135:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper RS, Nadkarni GN, Ogedegbe G. Race, ancestry, and reporting in medical journals. JAMA 2018;320:1531–2. [DOI] [PubMed] [Google Scholar]
- 19.Nyenhuis SM, Krishnan JA, Berry A, et al. Race is associated with differences in airway inflammation in patients with asthma. J Allergy Clin Immunol 2017; 140(1):257–65.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federico MJ, Covar RA, Brown EE, Leung DY, Spahn JD. Racial differences in T-lymphocyte response to glucocorticoids. Chest 2005;127:571–8. [DOI] [PubMed] [Google Scholar]
- 21.Israel E, Lasky-Su J, Markezich A, et al. Genome-wide association study of short-acting β2-agonists: a novel genome-wide significant locus on chromosome 2 near ASB3. Am J Respir Crit Care Med 2015;191:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tantisira KG, Lasky-Su J, Harada M, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med 2011; 365:1173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himes BE, Jiang X, Hu R, et al. Genome-wide association analysis in asthma subjects identifies SPATS2L as a novel bronchodilator response gene. PLoS Genet 2012;8(7):e1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israel E, Chinchilli VM, Ford JG, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004;364:1505–12. [DOI] [PubMed] [Google Scholar]
- 25.Ortega VE, Meyers DA. Pharmacogenetics: implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol 2014;133:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortega VE, Hawkins GA, Moore WC, et al. Effect of rare variants in ADRB2 on risk of severe exacerbations and symptom control during longacting β agonist treatment in a multiethnic asthma population: a genetic study. Lancet Respir Med 2014; 2:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drake KA, Torgerson DG, Gignoux CR, et al. A genome-wide association study of bronchodilator response in Latinos implicates rare variants. J Allergy Clin Immunol 2014;133:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malka J, Mauger DT, Covar R, et al. Eczema and race as combined determinants for differential response to step-up asthma therapy. J Allergy Clin Immunol 2014;134:483–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsler ME, Castro M, Lehman E, et al. Impact of race on asthma treatment failures in the Asthma Clinical Research Network. Am J Respir Crit Care Med 2011; 184:1247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao X, Bigham AW, Mei R, et al. A genomewide admixture mapping panel for Hispanic/Latino populations. Am J Hum Genet 2007;80:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res 2009;19:1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The International HapMap Consortium. The International HapMap Project. Nature 2003;426:789–96. [DOI] [PubMed] [Google Scholar]
- 33.Jackson DJ, Bacharier LB, Mauger DT, et al. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med 2018;378:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidian M, Giltinan DM. Nonlinear models for repeated measurement data. New York: Chapman & Hall, 1995. [Google Scholar]
- 35.Vonesh EF, Chinchilli VM. Linear and nonlinear models for the analysis of repeated measurements. New York: Marcel Dekker, 1997. [Google Scholar]
- 36.Kumar R, Seibold MA, Aldrich MC, et al. Genetic ancestry in lung-function predictions. N Engl J Med 2010;363:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rumpel JA, Ahmedani BK, Peterson EL, et al. Genetic ancestry and its association with asthma exacerbations among African American subjects with asthma. J Allergy Clin Immunol 2012;130:1302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pino-Yanes M, Thakur N, Gignoux CR, et al. Genetic ancestry influences asthma susceptibility and lung function among Latinos. J Allergy Clin Immunol 2015;135:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.