Abstract
Background
Cardiogenic shock (CS) due to takotsubo cardiomyopathy (TTC) is a life-threatening condition. Therapy is challenging because of the ambivalent effects of catecholamines. Catecholamines are required to stabilize blood pressure but might aggravate TTC. Cardiac assist devices could be a suitable solution for conserving catecholamines and the prevention of TTC perpetuation.
Case summary
We report the case of a male patient with refractory CS and severe respiratory insufficiency as a result of a reverse TTC, which involved both ventricles. Simultaneous circulatory support with an Impella CP® and veno-arterial extracorporeal membrane oxygenation was initiated for cardiopulmonary stabilization and catecholamine weaning. A giant, incidental pheochromocytoma was diagnosed as the cause of TTC. After drug treatment and resection of the tumour, biventricular function completely recovered within 7 weeks.
Discussion
A rare and challenging situation is the coincidence of a nor/epinephrine-secreting tumour, such as a pheochromocytoma, and severe CS complicating TTC. Although percutaneous left ventricular assist devices (pLVAD) are highly complicated and have shown conflicting results in terms of clinical efficacy for CS, its use may prevent the perpetuation of TTC due to reduced catecholamines requirement.
Keywords: Reverse takotsubo cardiomyopathy, Pheochromocytoma, pLVAD, ECMELLA, Case report
Learning points
The possibility of a pheochromocytoma should always be considered in the case of severe cardiogenic shock due to a takotsubo cardiomyopathy (TTC).
The implantation of a percutaneous left ventricular assist device reduces the need for catecholamines and prevents the deterioration of the TTC.
In the case of biventricular involvement of TTC, the combination of mechanical-circulatory support devices (ECMELLA) has to be taken into consideration.
Introduction
Takotsubo cardiomyopathy (TTC) is defined as transient regional systolic dysfunction despite a sufficient coronary oxygen supply.1 In most cases, TTC occurs in patients with severe emotional stress accompanied by a higher endogenous catecholamine level. However, catecholamine-secreting neuroendocrine tumours such as pheochromocytomas may also trigger TTC.2
Beside the classical form of TTC with apical ballooning, reverse forms are also described.3 Around 5% of all TTC patients develop cardiogenic shock (CS) with a high mortality rate.4 At the molecular level, the dysregulation of myocardial β2-adrenergic receptors, resulting in specific wall motion abnormalities with left ventricular apical hypokinesia and ballooning, is considered to be responsible.5
We report the case of a patient with severe CS and multiorgan failure due to reverse TTC caused by a giant incidental pheochromocytoma. Severely impaired biventricular function, in combination with an acute respiratory insufficiency, required combined mechanical circulatory support with veno-arterial extracorporeal membrane oxygenation (va-ECMO) and Impella CP®.
Timeline
| Time | Event |
|---|---|
| 0 day | Extirpation of a benign thyroglossal duct cyst |
| 0 day 10 h | Cardiogenic shock with severely impaired systolic left ventricular function |
| 0 day 14 h | Coronary angiography: no evidence of relevant coronary artery disease |
| 0 day 22 h | Admission to Heart Center Dresden (PaO2/FiO2 30 mmHg, 2.5 µg/kg/min norepinephrine |
| Transthoracic echocardiography: suspected for a reverse takotsubo cardiomyopathy because of akinesia of all basal and middle segments of the left ventricle with preserved kinetics of the apex (left ventricular ejection fraction, LVEF 15%) | |
| 0 day 24 h | Implantation of Impella CP® |
| 1 day 3 h | Implantation of veno-arterial extracorporeal membrane oxygenation (va-ECMO) due to deterioration of right ventricular function and persistent severe respiratory insufficiency |
| 1 day 20 h | Computed tomography scan detects a lesion of the adrenal gland suspicious of a pheochromocytoma |
| 6 days 10 h | End of intravenous norepinephrine administration |
| 7 days 0 h | Explanation of Impella CP® and va-ECMO due to improved biventricular function (LVEF 40%, TAPSE 19 mm) and respiratory situation (PaO2/FiO2 150 mmHg) |
| 8 days | Pheochromocytoma was confirmed by significantly increased plasma concentrations of metanephrine/normetanephrine |
| 9 days | Initiation of non-specific α adrenergic receptor inhibition with phenoxybenzamine |
| 28 days | Metaiodobenzylguanidine scintigraphy: no evidence for metastases of the pheochromocytoma or coincident paragangliomas |
| 33 days | Laparoscopic excision of the pheochromocytoma |
| 49 days | Discharge to rehabilitation with normalized biventricular function (LVEF 55%, TAPSE 25 mm) |
Case presentation
A 47-year-old man was referred to our centre for mechanical circulatory support for severe CS with multiorgan failure of unknown cause.
On the previous day, the extirpation of a benign thyroglossal duct cyst was performed. During the operation, recurrent hypertensive episodes occurred. After extubation, the patient developed serious hypotension and pulmonary oedema. A transthoracic echocardiography (TTE) showed severely impaired left ventricular (LV) systolic function. A coronary angiogram showed no evidence of relevant coronary artery disease. A toxicological screening was negative. Medical history revealed only chronic polyarthritis and arterial hypertension.
At admission to our centre, the patient was invasively ventilated due to severe respiratory insufficiency (PaO2/FiO2 30 mmHg) and required massive doses of norepinephrine (2.5 µg/kg/min). Clinical observation showed a blood pressure of 75/49 mmHg, silent heart sounds without murmurs, arterial oxygen saturation of 86%, bilateral rales, and basal dullness at percussion. The patient’s heart rate was elevated at 180 b.p.m. due to sinus tachycardia. Due to pulseless electrical activity, resuscitation for approximately 5 min was necessary (1 mg epinephrine). A bedside TTE showed akinesia of all basal and middle segments of the left ventricle with preserved kinetics of the apex (left ventricular ejection fraction, LVEF 15%, Figure 1A and B, Supplementary material online, Video S1). Right ventricular (RV) function was preserved (TAPSE 17 mm). Decompensated myocardiopathy, similar to unknown dilatative cardiomyopathy, or florid myocarditis could be differential diagnoses; however, because of the typical LV kinetics, a reverse TTC was suspected. A percutaneous LV assist device (pLVAD), supplying circulatory support up to 3.5 L/min due to a micro-axial pump, was immediately implanted to unload the left ventricle, promote LV output, and reduce catecholamine levels. A LV endomyocardial biopsy revealed a moderate chronically active myocarditis with diffuse interstitial remodelling secondary to chronic polyarthritis. DNA testing, via the polymerase chain reaction, showed no evidence of acute or persistent myocardial infection.
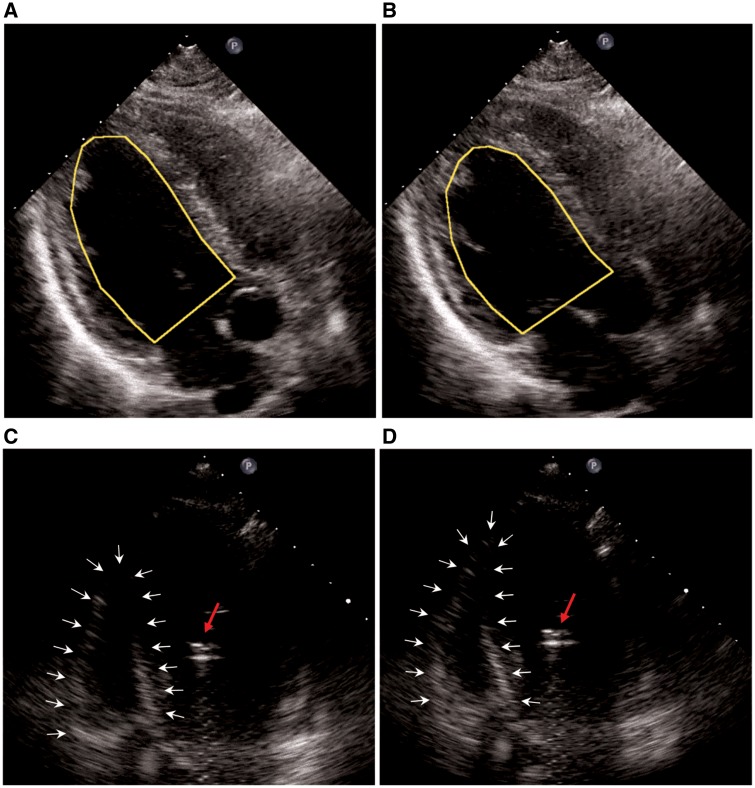
Figure 1.
Transthoracic echocardiography at admission (bedside, A and B diastolic & systolic). Akinesia of all basal and middle segments with preserved kinetics of the apex (left ventricular ejection fraction 15%). Transthoracic echocardiography post-Impella CP® (C and D diastolic & systolic). Right ventricular function deteriorated to a highly impaired degree after implantation of the Impella CP®, suggesting an right ventricular involvement of takotsubo cardiomyopathy.
Shortly after pLVAD implantation, RV function deteriorated (Figure 1C and D, Supplementary material online, Video S2). Furthermore, severe respiratory insufficiency persisted despite protective lung ventilation and antibiotic treatment (Figure 2A). Therefore, femoral veno-arterial extracorporeal membrane oxygenation (va-ECMO) was initiated, which unloaded the right ventricle and improved oxygenation as well as decarboxylation. To stabilize the patient, continuous venovenous hemodiafiltration and transfusion therapy were required.
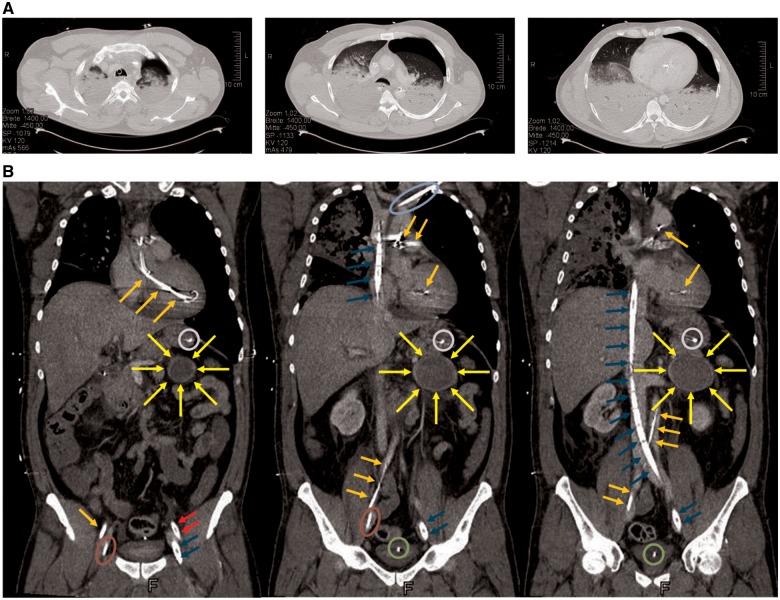
Figure 2.
Computed tomography of the thorax (A). Total dorsal consolidation of the lungs due to pulmonary oedema or secondary acute respiratory distress syndrome and a pneumothorax on the left side, which was caused by resuscitation. The pneumothorax was successfully evacuated by tube thoracostomy. Computed tomography of the thorax, abdomen, and pelvis (B). Imaging of a pheochromocytoma-suspected space-consuming lesion ( ) of the adrenal gland (diameter 6.7 cm) and the cannulization. Impella CP® optical (
) of the adrenal gland (diameter 6.7 cm) and the cannulization. Impella CP® optical ( ), veno-arterial extracorporeal membrane oxygenation arterial cannula (
), veno-arterial extracorporeal membrane oxygenation arterial cannula ( ), veno-arterial extracorporeal membrane oxygenation venous cannula (
), veno-arterial extracorporeal membrane oxygenation venous cannula ( ), Shaldon’s catheter (
), Shaldon’s catheter ( ), central venous catheter (
), central venous catheter ( ), urinary catheter (
), urinary catheter ( ), and feeding tube (
), and feeding tube ( ).
).
A computed tomography scan of the chest, abdomen, and pelvis was performed 24 h later, which demonstrated an extensive dorsal consolidation of the lungs due to pulmonary oedema or secondary acute respiratory distress syndrome. Furthermore, a lesion of the adrenal gland (6.7 cm diameter) suspicious of a pheochromocytoma was detected (Figure 2B).
After 7 days, biventricular function (LVEF 40%, TAPSE 19 mm) and respiratory status (PaO2/FiO2 150 mmHg) improved, thus both assist devices were explanted. On the 6th day after admission, the patient no longer required norepinephrine. Therefore, the diagnosis of a pheochromocytoma was confirmed by significantly increased plasma concentrations of metanephrine/normetanephrine the following day. Non-specific α-adrenergic receptor inhibition with phenoxybenzamine (10 mg 1-1-1) was initiated, which markedly reduced the recurrence of hypertensive crises. Recurrent episodes of elevated blood pressure had been observed since admission with increasing frequency and severity until hemodynamic stabilization.
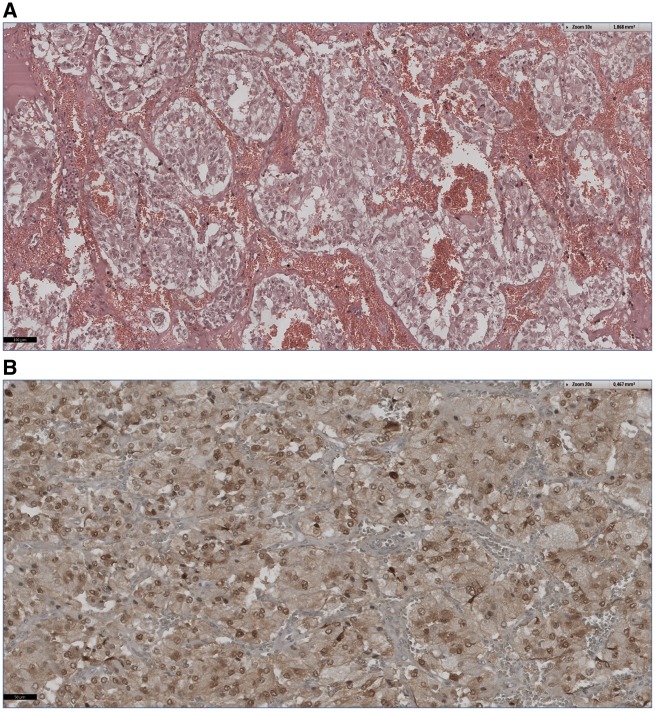
In the following days, weaning from the respirator and renal replacement therapy were successful. Metaiodobenzylguanidine scintigraphy showed no evidence of metastases of the pheochromocytoma or coincident paragangliomas. After signed informed consent was obtained, a laparoscopic excision was performed in the presence of intensive α-adrenergic receptor inhibition. Histological and immunohistochemical staining confirmed a well-differentiated neuroendocrine tumour of the adrenal gland without any evidence of malignancy (Figure 3).
Figure 3.
Typical histology and immunohistology of a pheochromocytoma. (A) Haematoxylin–eosin staining (10×) of large polygonal cells in an alveolar arrangement and pleomorphic cell cores. (B) Typical homogenous chromogranin A expression (20×).
After 49 days, the patient was discharged into follow-up care with normalized biventricular function (LVEF 55%, TAPSE 25 mm, E/E ′ <8, Supplementary material online, Video S3) and no neurological or cognitive deficit. Furthermore, TTE control confirmed a normal cardiac function three months after discharge.
Discussion
Therapy of severe CS due to TTC is difficult because of the ambivalent role of catecholamines. These adrenergic agents are needed for maintaining organ perfusion but lead to deterioration of the cardiomyopathy.6 A rare but challenging situation is the coincidence of a nor/epinephrine-secreting tumour, such as a pheochromocytoma or paraganglioma, as in the current case.
Implantation of a pLVAD has several advantages. First, it provides hemodynamic stabilization and sufficient organ perfusion. Second, it can reduce catecholamine dependency and consequently prevent TTC perpetuation. Therefore, the pLVAD, which uses a micro-axial pump, is the most suitable device because of its ability to unload the left ventricle without increasing afterload, contrarily to va-ECMO.7 The current case is a rare incidence of pheochromocytoma-induced TTC with uncontrolled endogenous catecholamine secretion. Data regarding the treatment of TTC with biventricular involvement are rare. Additional wall motion abnormalities of the right ventricle are apparent in 25% of TTC patients and are associated with a more severe impairment of the LV systolic function,8 which may be caused by extensively increased catecholamine levels, like in cases of incidental pheochromocytomas. In the current case, the endogenous release of catecholamines was confirmed through elevated plasma levels of normetanephrine and metanephrine. The measurement was performed more than 24 h after stopping intravenous norepinephrine administration. Therefore, interference with the vasopressor, which has a very short biological half-life of 2–2.5 min, was excluded.9
The combination of a pLVAD and va-ECMO is known to induce synergistic haemodynamic effects, such as venting of the left ventricle through the micro-axial pump, as va-ECMO increases LV afterload.10 Furthermore, oxygenation and decarboxylation can be improved in severe respiratory insufficiency through extracorporeal membrane oxygenation. In the current case, the combined use of both assist devices enabled protective lung ventilation with a sufficient oxygen supply and the weaning of catecholamines, leading to the recovery of biventricular function.
The endomyocardial biopsy showed a chronic active myocarditis with diffuse interstitial remodelling. Its occurrence was attributed to myocardial involvement of the chronic polyarthritis.
In summary, pheochromocytoma or paraganglioma should always be considered in the case of severe CS due to TTC. The implantation of a pLVAD might reduce the need for catecholamines and prevent the deterioration of TTC. In the case of biventricular involvement of TTC and additional severe respiratory insufficiency, the combination of mechanical-circulatory support devices (ECMELLA) must be taken into consideration.
Lead author biography

Dr Johannes Mierke is a resident physician currently training at the Herzzentrum Dresden, University Clinic, Germany. He received the Doctor of Medicine degree in the field of vascular remodeling from Technische Universität Dresden in 2017. Afterwards, he became research fellow of Faculty of Medicine Carl Gustav Carus (TU Dresden). He is participated in the Dresden Impella Registry. His research interests are the acute cardiovascular care of the cardiogenic shock and the vascular remodeling.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 2. Salathe M, Weiss P, Ritz R.. Rapid reversal of heart failure in a patient with phaeochromocytoma and catecholamine-induced cardiomyopathy who was treated with captopril. Br Heart J 1992;68:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF.. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 4. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E.. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–1529. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe M, Izumo M, Akashi YJ.. Novel understanding of takotsubo syndrome. Int Heart J 2018;59:250–255. [DOI] [PubMed] [Google Scholar]
- 6. Coupez E, Eschalier R, Pereira B, Pierrard R, Souteyrand G, Clerfond G, Citron B, Lusson J-R, Mansencal N, Motreff P.. A single pathophysiological pathway in Takotsubo cardiomyopathy: Catecholaminergic stress. Arch Cardiovasc Dis 2014;107:245–252. [DOI] [PubMed] [Google Scholar]
- 7. Werdan K, Gielen S, Ebelt H, Hochman JS.. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156–167. [DOI] [PubMed] [Google Scholar]
- 8. Haghi D, Athanasiadis A, Papavassiliu T, Suselbeck T, Fluechter S, Mahrholdt H, Borggrefe M, Sechtem U.. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J 2006;27:2433–2439. [DOI] [PubMed] [Google Scholar]
- 9. Beloeil H, Mazoit J-X, Benhamou D, Duranteau J.. Norepinephrine kinetics and dynamics in septic shock and trauma patients. Br J Anaesth 2005;95:782–788. [DOI] [PubMed] [Google Scholar]
- 10. Fiedler AG, Dalia A, Axtell AL, Ortoleva J, Thomas SM, Roy N, Villavicencio MA, D’Alessandro DA, Cudemus G.. Impella placement guided by echocardiography can be used as a strategy to unload the left ventricle during peripheral venoarterial extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 2018;32:2585–2591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.