Abstract
Background
The common GLA gene mutation p.F113L causes late-onset phenotype of Fabry disease (FD) with predominant cardiac manifestations. A founder effect of FD due to this mutation was found in the Portuguese region of Guimarães. Our study aims to deepen the knowledge on the natural history of this late-onset variant.
Methods
203 consecutive adult Fabry patients with p.F113L mutation (79 males; mean age 46 ± 18 years), from this region, were submitted at baseline to a predefined diagnostic protocol. The occurrence of FD manifestations was analyzed in each decade of age in both genders.
Results
In males, left ventricular hypertrophy (40.2%) and late gadolinium enhancement (21.4%) arose over 30 years; heart failure (HF) (21.9%), ventricular tachycardia (8.9%) and conduction disorders over 40 years; and bifascicular (13.1%) and complete atrioventricular blocks (5.9%) beyond 50 years of age. Cardiac manifestations occurred more commonly and 1–2 decades earlier in males; their frequency increased with age. Septum and posterior wall thickness, LV mass, QRS interval duration and pro-BNP levels increased with age in both genders. Mean survival free from HF (64 ± 1 vs. 76 ± 2 years) and pacemaker (71 ± 2 vs. 86 ± 1 years) was higher in females (p < .001). Albuminuria A2/A3 (33.7%), brain white matter lesions (50.3%) and sensorineural deafness (44.7%) arose before 30 years of age in both genders, increasing with age. Renal failure and stroke were rare. Lysosomal inclusions were demonstrated in podocytes of patients with proteinuria.
Conclusion
This study improves the knowledge on natural history of late-onset variants of FD, carrying major impact on clinical decisions and guidelines.
Keywords: Fabry disease, Late-onset, F113L, Natural history, Cardiac, Phenotype
1. Introduction
Fabry disease (FD) (OMIM 301500) is an X-linked lysosomal storage disorder caused by mutations in the GLA gene, leading to deficiency of the enzymatic activity of α-galactosidase A and subsequent lysosomal accumulation of globotriaosylceramide (GB3) and other related glycosphingolipids [1]. Multiorgan damage ensues, being death mainly driven by heart disease [2].
Mutations causing a virtually null enzymatic activity are associated to severe and early onset classical phenotypes, while mutations leading to a residual enzymatic activity are associated to attenuated and late-onset phenotypes. Classical phenotypes are characterized by early development, in childhood or adolescence, of acroparesthesias, neuropathic pain, hypohydrosis, heat, cold and exercise intolerance, cornea verticillata, angiokeratomas, gastrointestinal symptoms and proteinuria. In adulthood, patients also suffer from sensorineural deafness and cardiac, renal and cerebrovascular manifestations. In contrast, late-onset variants are characterized by the development of cardiac, renal and/or cerebrovascular manifestations in adulthood and the phenotype may be dominated by the involvement of an organ, such as the heart or the kidneys [1,3].
Despite being more prevalent [4], late-onset phenotypes were significantly underdiagnosed in the past [3]. Their increased recognition after the emergence of therapeutic options for FD raised the need to know their natural history, in order to reach evidence-based clinical decisions and accurately evaluate the efficacy of available therapies. Still, knowledge on their natural history has been hampered by the ethical issues related to the follow-up of untreated patients in a time when therapeutic options are available. Additionally, their slower disease course, inherently biased by older age and common comorbidities [[5], [6], [7]], further increases the difficulty of assessing the natural history of a disease already characterized by a high clinical heterogeneity even within the same genotype [5,6].
Current knowledge specifically on predominant cardiac phenotypes is frequently based on small cohorts [[8], [9], [10], [11], [12]] or registries [[12], [13], [14]] that are commonly biased by the overrepresentation of older, male, symptomatic and more severely affected patients. Female underrepresentation is a major issue in most previous studies [5,[8], [9], [10], [11], [12], [13], [14]], with a female/male ratio far below 2, ranging from 0.3 to 1.3. Comorbidities and concomitant medication are commonly not taken into account for the phenotype [8,[10], [11], [12], [13], [14], [15]]. Finally, the clinical description of cardiac predominant phenotypes is commonly focused on left ventricular hypertrophy (LVH) and evidence gaps exist on the natural history of other cardiac and extracardiac manifestations.
The GLA gene mutation c.337T>C (p.F113L) results in a misfolded enzyme that is unstable and consequently degraded at the neutral pH of the endoplasmic reticulum, which ultimately causes a late-onset phenotype of FD [6,16]. We have demonstrated since 2013 the existence of a founder effect of FD due to the p.F113L mutation in the Portuguese region of Guimarães, based on genealogy and haplotype analysis [6,17]. We have also reported its spread to many countries in the world due to migration in the last century and described the late-onset phenotype with predominant cardiac manifestations that was presented, at baseline, by a large cohort of patients with this mutation [6]. Aiming to deepen the knowledge on the natural history of late-onset FD, we herein provide a detailed description of the phenotype presented at baseline by a larger cohort of patients with the p.F113L mutation as well as the chronology of the clinical manifestations in both genders decade by decade, thereby obtaining an accurate overview of the natural history of this common late-onset FD variant.
2. Methods
2.1. Subjects
Between January 2008 and December 2018, we diagnosed, as previously described [6], 203 consecutive adult patients (≥18 years) with FD due to the p.F113 L mutation, from 34 different family pedigrees, in the Reference Center of Lysosomal Storage Disorders of Hospital Senhora da Oliveira – Guimarães. They shared a common ancestor nearly 400 years ago (Fig. S1) and lived all in the region of Guimarães, thereby also sharing environmental factors.
Fig. S1.
Family tree provided by the historians research, demonstrating the genealogical connection of 25 of 34 FD families with the p.F113L mutation to a common ancestor who was born in 1611 in the region of Guimarães. For simplification, only FD patients are depicted and respective spouses were removed from the pedigree.
2.2. Clinical evaluation of Fabry patients
At baseline, all patients were submitted to a predefined diagnostic protocol, including systematic multidisciplinary assessment (Cardiology, Internal Medicine, Neurology, Psychiatry, Ophthalmology, Otorhinolaryngology, Dermatology), blood and urine analysis with quantification of serum creatinine, albuminuria on 24 h-urine and albumin to creatinine ratio on random urine, estimation of glomerular filtration rate (eGFR) by the CKD-EPI formula, electrocardiogram, echocardiogram, cardiac MRI, 24 h-Holter, exercise stress test, brain MRI (by neuroradiologist), electromyography, audiometry and lung spirometry. Kidney biopsy was performed in Fabry patients presenting proteinuria ≥500 mg on 24 h-urine. The enzymatic activity of α-galactosidase A in plasma and leukocytes, the urinary GB3 and the plasma lyso-GB3 were measured as previously described [6].
2.3. Statistical analysis
Categorical variables were expressed as percentages and compared between groups of patients by the Chi-square test. Continuous variables were expressed as means and standard deviations and compared between groups of patients by the Student's t-test or Mann-Whitney test. The occurrence of key clinical manifestations of FD was evaluated by gender and age category (≤30; 31–40; 41–50; 51–60; 61–70; 71–80; >80 years). Polynomial regression (quadratic function) was used to best describe the relationship between age and LV mass, interventricular septum (IVS) and posterior wall (PW) thickness and QRS interval duration. An exponential model was used to best describe the relationship between age and pro-BNP levels. Kaplan-Meier curves were performed to estimate survival free from heart failure (HF) and pacemaker. Statistical significance was considered for p < .05.
2.4. Ethical issues
This research was approved by the hospital Ethics Committee and all patients provided signed informed consent.
3. Results
We assessed, at baseline, 203 consecutive adult patients with FD due to the p.F113L mutation, 79 males and 124 females (female/male ratio 1.6). Mean age at diagnosis was 46 ± 18 years and higher in males (49 ± 15 vs. 44 ± 19, p = .021). Thirty-four patients were index patients and the remaining were diagnosed through family screening (83.3%).
There was no association between the enzymatic activity on plasma or leukocytes and the development of clinical manifestations in females.
3.1. Cardiac manifestations
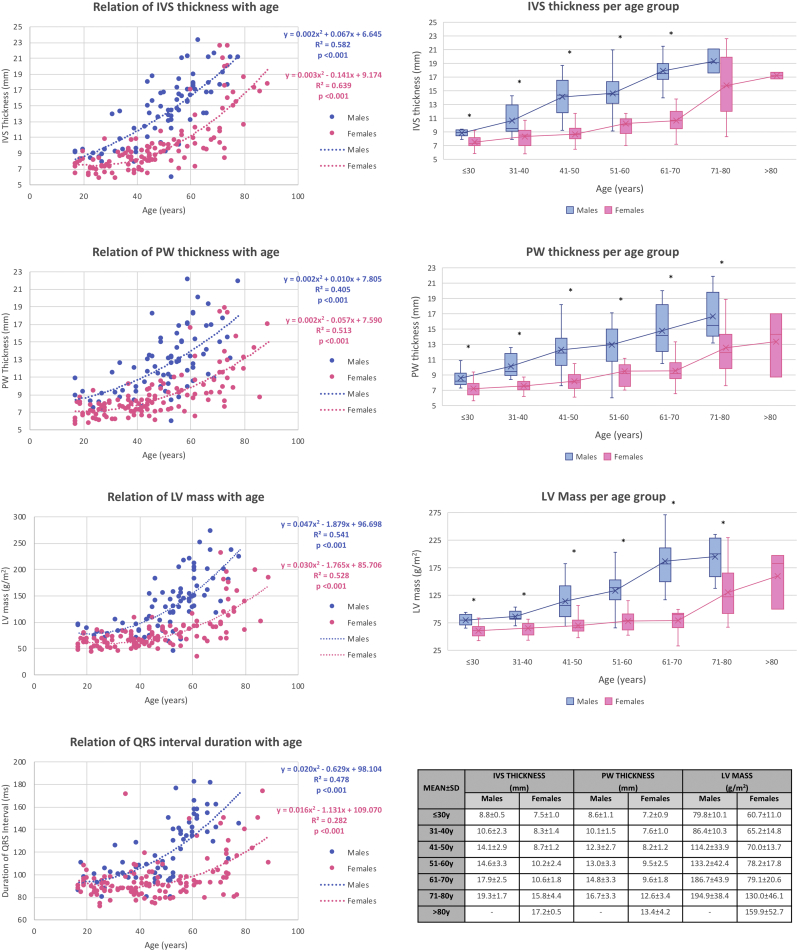
LVH (LV wall thickness > 12 mm) was found in 40.2% of the patients, more commonly in males (73.1% vs. 19.0%) (Table 1). In patients with LVH, mean LV mass was 147.5 ± 45.1 g/m2 and diastolic dysfunction was present in 67.1%. The frequency of LVH increased with age, arising in males over 30 years and females over 50 years and affecting all males beyond 60 years and all females beyond 80 years of age. Notably, 33.3% of males aged 31–40 years and 78.6% of males aged 41–50 years already presented LVH, while only 15.4% of females aged 51–60 years had LVH (Fig. 1). LV mass and IVS and PW thickness increased with age in both genders according to a quadratic model (Fig. 2). No correlation was found between these parameters and plasma lyso-GB3 in males.
Table 1.
Clinical phenotype of patients with late-onset FD due to the p.F113L mutation.
| Clinical manifestations | Fabry patients (n = 203) | Fabry males (n = 79) | Fabry females (n = 124) | p |
|---|---|---|---|---|
| Age at FD diagnosis (years) (mean ± SD) | 46 ± 18 | 49 ± 15 | 44 ± 19 | 0.021 |
| α-GAL A activity (mean ± SD) | ||||
| Plasma (nmol/h/mL) | 5.1 ± 5.1 | 0.4 ± 1.4 | 8.2 ± 4.2 | <0.001 |
| Leukocytes (nmol/h/mg) | 16.6 ± 17.4 | 2.0 ± 3.6 | 26.7 ± 15.9 | <0.001 |
| Disease biomarkers (mean ± SD) | ||||
| Urinary GB3 (μg/mmol creatinine) | 91.0 ± 152.1 | 181.7 ± 208.5 | 30.2 ± 23.1 | <0.001 |
| Plasma Lyso-GB3 (ng/mL) | - | 7.7 ± 3.0 | <2a | - |
| Cardiac manifestations | ||||
| LVH (%) | 80 (40.2%) | 57 (73.1%) | 23 (19.0%) | <0.001 |
| IVS thickness (mm) (mean ± SD) | 11.6 ± 4.4 | 14.3 ± 4.0 | 9.9 ± 3.6 | <0.001 |
| PW thickness (mm) (mean ± SD) | 10.3 ± 3.5 | 12.6 ± 3.5 | 8.9 ± 2.7 | <0.001 |
| LV mass (g/m2) (mean ± SD) | 100.3 ± 49.4 | 132.5 ± 51.8 | 79.6 ± 34.7 | <0.001 |
| LV ejection fraction (%) (mean ± SD) | 66.0 ± 7.0 | 65.9 ± 7.9 | 66.0 ± 6.3 | 0.710 |
| LV diastolic dysfunction (%) | 55 (27.8%) | 35 (44.9%) | 20 (16.7%) | <0.001 |
| Abnormal relaxation pattern (%) | 32 (58.2%) | 17 (48.6%) | 15 (75.0%) | |
| Pseudonormal pattern (%) | 22 (40.0%) | 17 (48.6%) | 5 (25.0%) | 0.145 |
| Restrictive pattern (%) | 1 (1.8%) | 1 (2.9%) | 0 (0.0%) | |
| LVH on cardiac MRI (%) | 60 (36.1%) | 44 (68.8%) | 16 (15.7%) | <0.001 |
| LV mass on cardiac MRI (g/m2) | 72.2 ± 29.2 | 93.8 ± 29.8 | 58.6 ± 18.8 | <0.001 |
| Late gadolinium enhancement (%) | 36 (21.4%) | 25 (39.1%) | 11 (10.6%) | <0.001 |
| Heart failure (%) | 44 (21.9%) | 26 (32.9%) | 18 (14.8%) | 0.002 |
| NYHA Class I (%) | 34 (77.3%) | 23 (88.5%) | 11 (61.1%) | |
| NYHA Class II (%) | 9 (20.5%) | 2 (7.7%) | 7 (38.9%) | 0.034 |
| NYHA Class III (%) | 1 (2.3%) | 1 (3.8%) | 0 (0.0%) | |
| NYHA Class IV (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Atrial fibrillation (%) | 9 (4.4%) | 6 (7.6%) | 3 (2.4%) | 0.081 |
| Atrial flutter (%) | 3 (1.5%) | 2 (2.5%) | 1 (0.8%) | 0.321 |
| Non-sustained ventricular tachycardia (%) | 18 (8.9%) | 11 (14.1%) | 7 (5.6%) | 0.040 |
| Atrioventricular block (%) | 30 (14.8%) | 16 (20.3%) | 14 (11.3%) | 0.079 |
| 1st degree (%) | 19 (10.5%) | 8 (12.3%) | 11 (9.5%) | 0.552 |
| 2nd degree (%) | 9 (4.5%) | 6 (7.6%) | 3 (2.4%) | 0.083 |
| 3rd degree (%) | 12 (5.9%) | 10 (12.7%) | 2 (1.6%) | 0.001 |
| Right bundle branch block (%) | 36 (19.6%) | 26 (38.8%) | 10 (8.5%) | <0.001 |
| Left anterior fascicular block (%) | 42 (23.0%) | 30 (45.5%) | 12 (10.3%) | <0.001 |
| Left bundle branch block (%) | 2 (1.1%) | 1 (1.6%) | 1 (0.9%) | 0.668 |
| Bifascicular block (%) | 26 (13.1%) | 19 (25.3%) | 7 (5.7%) | <0.001 |
| Pacemaker (%) | 13 (6.4%) | 10 (12.7%) | 3 (2.4%) | 0.004 |
| Implantable cardioverter-defibrillator (%) | 2 (1.0%) | 1 (1.3%) | 1 (0.8%) | 0.747 |
| Myocardial ischemic events (%) | 10 (4.9%) | 10 (12.7%) | 0 (0.0%) | <0.001 |
| Renal manifestations | ||||
| Albuminuria A2 (30-300mg/24 h) (%) | 48 (24.6%) | 28 (36.8%) | 20 (16.8%) | 0.002 |
| Albuminuria A3 (>300 mg/24 h) (%) | 18 (9.2%) | 12 (15.8%) | 6 (5.0%) | 0.011 |
| Albuminuria A2 or A3 (≥30 mg/24 h) (%) | 66 (33.7%) | 40 (52.6%) | 26 (21.7%) | <0.001 |
| Chronic kidney disease stages | ||||
| G1 (eGFR ≥90 mL/min/1.73m2) (%) | 139 (70.6%) | 45 (60.0%) | 94 (77.0%) | 0.011 |
| G2 (eGFR 60-89 mL/min/1.73m2) (%) | 45 (22.8%) | 23 (30.7%) | 22 (18.0%) | 0.040 |
| G3a (eGFR 45-59 mL/min/1.73m2) (%) | 10 (5.1%) | 6 (8.0%) | 4 (3.3%) | 0.143 |
| G3b (eGFR 30-44 mL/min/1.73m2) (%) | 1 (0.5%) | 0 (0.0%) | 1 (0.8%) | 0.432 |
| G4 (eGFR 15-29 mL/min/1.73m2) (%) | 1 (0.5%) | 1 (1.3%) | 0 (0.0%) | 0.201 |
| G5 (eGFR <15 mL/min/1.73m2) (%) | 1 (0.5%) | 0 (0.0%) | 1 (0.8%) | 0.432 |
| Chronic kidney disease stage ≥ G3 (%) | 13 (6.6%) | 7 (9.3%) | 6 (4.9%) | 0.225 |
| Neurological and neuropsychiatric manifestations | ||||
| Stroke (%) | 6 (3.0%) | 3 (3.8%) | 3 (2.4%) | 0.572 |
| Transient ischemic attack (%) | 1 (0.5%) | 0 (0.0%) | 1 (0.8%) | 0.424 |
| Brain white matter lesions (%) | 83 (50.3%) | 35 (53.8%) | 48 (48.0%) | 0.463 |
| Brain haemorrhage (%) | 2 (1.0%) | 2 (2.6%) | (0.0%) | 0.073 |
| Carpal tunnel syndrome (%) | 36 (19.7%) | 17 (23.9%) | 19 (17.0%) | 0.247 |
| Acroparesthesias (%) | 62 (30.7%) | 14 (17.9%) | 48 (38.7%) | 0.002 |
| Hypohidrosis (%) | 3 (1.5%) | 0 (0.0%) | 3 (2.4%) | 0.166 |
| Cold, heat or exercise intolerance (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Depression (%) | 41 (20.8%) | 12 (15.8%) | 29 (24.0%) | 0.169 |
| Anxiety (%) | 58 (29.3%) | 22 (28.9%) | 36 (29.5%) | 0.933 |
| Eye manifestations | ||||
| Cornea verticillata (%) | 19 (10.3%) | 11 (15.5%) | 8 (7.1%) | 0.068 |
| Cataracts (%) | 25 (13.5%) | 10 (14.3%) | 15 (13.0%) | 0.811 |
| Retinal vessel tortuosity (%) | 6 (3.3%) | 2 (2.9%) | 4 (3.5%) | 0.814 |
| Ear manifestations | ||||
| Sensorineural deafness (%) | 80 (44.7%) | 46 (62.2%) | 34 (32.4%) | <0.001 |
| Tinnitus (%) | 30 (16.3%) | 17 (23.0%) | 13 (11.8%) | 0.045 |
| Dermatological manifestations | ||||
| Angiokeratomas (%) | 4 (2.1%) | 0 (0.0%) | 4 (3.5%) | 0.106 |
| Pulmonary manifestations | ||||
| Lung obstructive disease (%) | 29 (17.5%) | 15 (22.7%) | 14 (14.0%) | 0.147 |
| Gastrointestinal manifestations (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Comorbidities | ||||
| Hypertension (%) | 66 (32.7%) | 30 (38.0%) | 36 (29.3%) | 0.198 |
| Diabetes mellitus (%) | 23 (11.4%) | 13 (16.5%) | 10 (8.1%) | 0.069 |
| Dyslipidemia (%) | 85 (42.1%) | 46 (58.2%) | 39 (31.7%) | <0.001 |
| Smoking (%) | 21 (10.4%) | 18 (22.8%) | 3 (2.4%) | <0.001 |
| Concomitant medications | ||||
| ACEI/ARB (%) | 79 (38.9%) | 41 (51.9%) | 38 (30.6%) | 0.002 |
p: Males vs. Females.
p < .05 are marked in bold.
Valid percentages are shown.
ACEI, angiotensin-converting-enzyme inhibitors; α-GAL A, α- galactosidase A; ARB, angiotensin II receptor blocker; e-GFR, estimated glomerular filtration rate; FD, Fabry disease; IVS, interventricular septum; LV, left ventricular; LVH, left ventricular hypertrophy; NYHA, New York Heart Association; PW, posterior wall.
Reference values: Enzymatic activity of α-galactosidase A on plasma 6–19 nmol/h/mL, and on leukocytes 36–80 nmol/h/mg; urinary GB3 0.87–13 μg/mmol creatinine; plasma Lyso-GB3 0–1.9 ng/mL
Below lower level of quantitation of plasma lyso-GB3 (2 ng/mL).
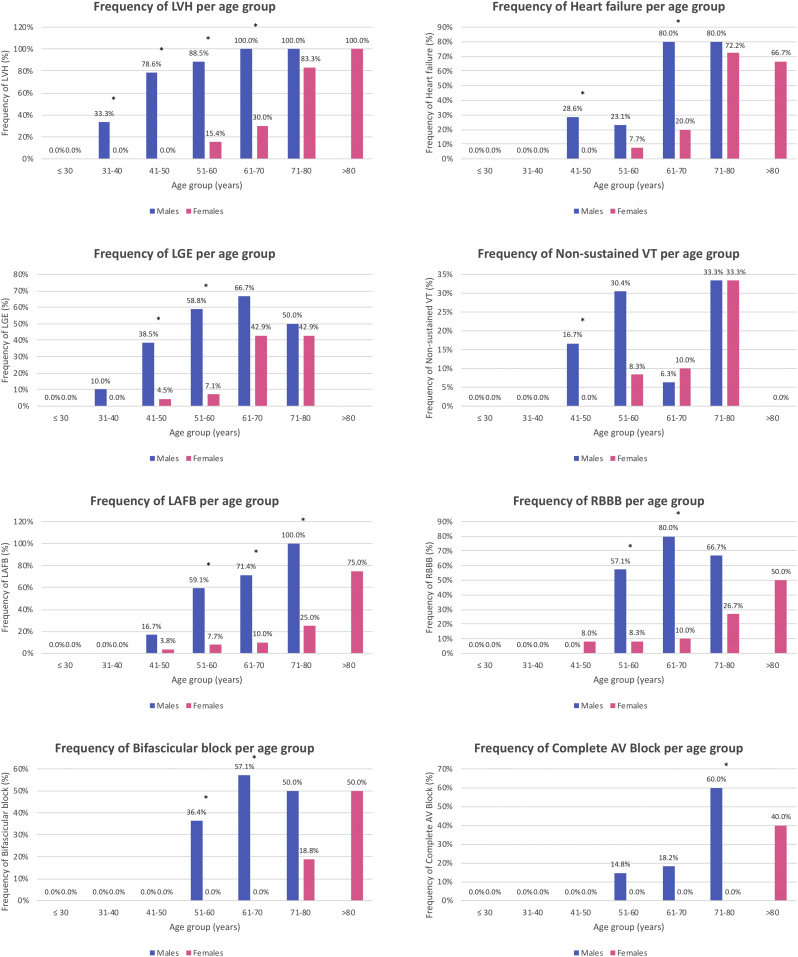
Fig. 1.
Frequency of cardiac manifestations according to age category in male and female Fabry patients with the p.F113L mutation (LVH, heart failure, LGE, non-sustained VT, LAFB, RBBB, bifascicular block, complete AV block). * p < .05.
Fig. 2.
Left panel: Relationship of IVS and PW thickness, LV mass and QRS interval duration with age, depicted by a quadratic model; Right panel: IVS and PW thickness and LV mass per age category in male and female patients with FD due to the p.F113L mutation. The mean line is shown and means are marked on the boxplots with an X. * p < .05.
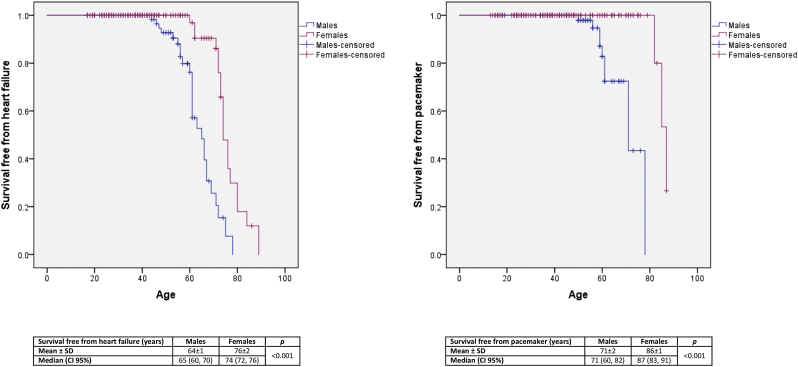
HF developed in 21.9%, more commonly in males (32.9% vs. 14.8%) (Table 1). In patients with LVH, it occurred in 55.0%, more commonly in females (78.3% vs. 45.6%, p = .008). HF arose in males over 40 years and females over 50 years. It is noteworthy that 28.6% of the males aged 41–50 years already presented HF. Its frequency increased with age, so that 80% of males over 60 years and nearly 70% of females over 70 years suffered from HF (Fig. 1). Most patients were in New York Heart Association (NYHA) class I or II (Table 1). pro-BNP levels increased with age according to an exponential model in males (R2 = 0.525; p < .001) and females (R2 = 0.464; p < .001). Mean survival free from HF was higher in females (64 ± 1 vs. 76 ± 2 years) (Fig. 3).
Fig. 3.
Kaplan-Meier curves of survival free from heart failure and survival free from pacemaker in male and female Fabry patients with the p.F113L mutation.
Cardiac MRI was performed in 166 patients (81.8%; 64 males and 102 females), and in 59 patients with LVH (73.8%; 44 males and 15 females). Sixty patients (one additional patient compared to echocardiography) fulfilled cardiac MRI criteria of LVH [18] (36.1%). LGE occurred in 4.8% of patients without LVH, without gender difference (5.3% vs. 4.7%, p = .918). In patients with LVH, LGE occurred in 52.5%, also without gender dominance (54.5% vs. 46.7%, p = .598). LGE arose in males over 30 years and females over 40 years and its frequency increased with age in both genders (Fig. 1). LV mass on MRI was a predictor of LGE (p < .001). LGE was associated with ST depression (p = .001) and negative T waves (p < .001) on the ECG, but still 17.8% of the patients without ST depression and 13.4% without negative T waves presented LGE.
24 h-Holter was performed in 185 patients (91.1%; 70 males and 115 females). Atrial fibrillation occurred in 4.4% (mean age at diagnosis: 70 ± 10 years). Non-sustained ventricular tachycardia (VT) occurred in 8.9%, mainly in males (14.1% vs. 5.6%) (Table 1), arising in males over 40 years and females over 50 years. It is relevant that 16.7% of the males aged 41–50 years already presented non-sustained VT. Its frequency increased with age, so that one third of the patients over 70 years had non-sustained VT (Fig. 1). Nevertheless, only one male and one female needed cardioverter-defibrillators for primary prevention of sudden cardiac death due to severe LVH with diffuse LGE and frequent non-sustained VT.
Left anterior fascicular block (LAFB) was found in 23.0% of the patients, right bundle branch block (RBBB) in 19.6%, bifascicular block (BB) in 13.1% and complete atrioventricular (AV) block in 5.9%, all more commonly in males (Table 1). LAFB developed in both genders since the age of 40 years and RBBB in females over 40 years and males over 50 years. BB occurred in males over 50 years, but only in females over 70 years. Likewise, complete AV block occurred in males since the age of 50 years, but only in females over 80 years. The frequency of all cardiac conduction disturbances increased with age in both genders, as well as the duration of the QRS interval (Fig. 1, Fig. 2). Notably, 59.1% of the males aged 51–60 years already presented LAFB, 57.1% of them had RBBB, 36.4% had BB and 14.8% had already suffered complete AV block and therefore implanted pacemaker (Fig. 1). Pacemaker implantation (6.4%) occurred two decades earlier in males [60 ± 7 (49–71) vs. 81 ± 5 (75–84), years] (Table 2), mainly in the emergency setting of a complete AV block. Kaplan-Meier curve of survival free from pacemaker is shown in Fig. 3.
Table 2.
Mean age at diagnosis of each clinical manifestation of FD in patients with the p.F113L mutation.
| Age at diagnosis (years) (Mean ± SD) (Min-Max) |
Fabry patients | Males | Females | p |
|---|---|---|---|---|
| LVH | 61 ± 12 (31–89) | 57 ± 10 (31–78) | 73 ± 8 (57–89) | <0.001 |
| LGE | 60 ± 9 (40–76) | 58 ± 8 (40–76) | 65 ± 8 (46–74) | 0.013 |
| Atrial fibrillation | 70 ± 10 (52–80) | 67 ± 11 (52–78) | 77 ± 3 (74–80) | 0.262 |
| Non-sustained VT | 62 ± 10 (45–77) | 57 ± 8 (45–77) | 70 ± 6 (60–77) | 0.003 |
| RBBB | 64 ± 9 (51–85) | 61 ± 6 (51–78) | 76 ± 9 (62–85) | 0.001 |
| LAFB | 63 ± 11 (42–89) | 60 ± 7 (47–78) | 72 ± 14 (42–89) | 0.003 |
| BB | 66 ± 9 (53–85) | 62 ± 6 (53–78) | 78 ± 6 (71–85) | <0.001 |
| Complete AV block | 63 ± 10 (49–83) | 60 ± 7 (49–71) | 79 ± 6 (75–83) | 0.036 |
| Pacemaker | 65 ± 11 (49–84) | 60 ± 7 (49–71) | 81 ± 5 (75–84) | 0.009 |
| Albuminuria A2 or A3 | 54 ± 14 (18–86) | 55 ± 12 (22–78) | 51 ± 18 (18–86) | 0.222 |
| CKD ≥ G3 | 70 ± 10 (50–86) | 66 ± 10 (50–78) | 75 ± 7 (66–86) | 0.202 |
| Brain WML | 53 ± 15 (18–89) | 53 ± 11 (27–75) | 52 ± 17 (18–89) | 0.912 |
| Stroke | 53 ± 12 (41–71) | 48 ± 5 (42–51) | 59 ± 16 (41–71) | 0.700 |
| Sensorineural deafness | 58 ± 13 (20–88) | 57 ± 12 (20–77) | 59 ± 15 (23–88) | 0.380 |
| Cornea verticillata | 60 ± 8 (43–72) | 59 ± 7 (46–68) | 63 ± 10 (43–72) | 0.238 |
p: Males vs. Females.
p < .05 are marked in bold.
AV, atrioventricular; BB, bifascicular block; CKD, chronic kidney disease; LAFB, left anterior fascicular block; LGE, late gadolinium enhancement; LVH, left ventricular hypertrophy; RBBB, right bundle branch block; VT, ventricular tachycardia; WML, white matter lesions.
History of myocardial ischemic events was found exclusively in males (n = 10) (Table 1). Myocardial infarction occurred in eight and unstable angina in six males. All patients had significant stenoses/occlusions on coronary angiography, except one who had normal coronary arteries and suffered a type 2 myocardial infarction in the context of severe LVH. Six males underwent percutaneous coronary intervention and two underwent coronary artery bypass graft. Mean age at first myocardial ischemic event was 53 ± 10 years (42–76). All these patients presented cardiovascular risk factors: hypertension (60%), diabetes mellitus (10%), dyslipidemia (90%) and smoking (40%).
3.2. Extracardiac manifestations
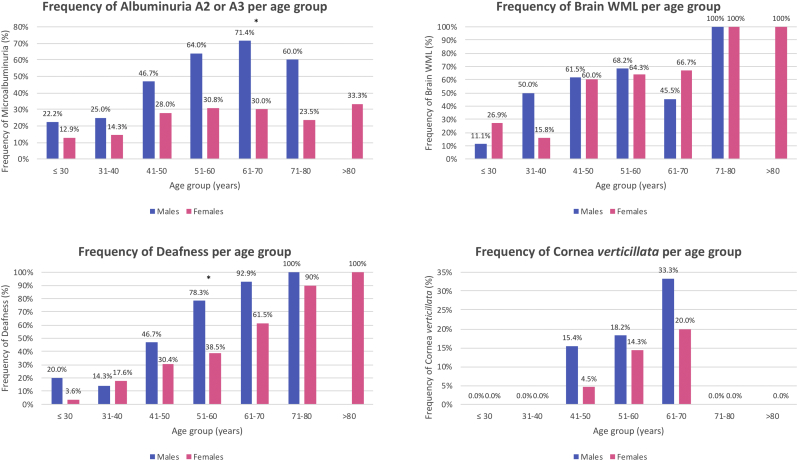
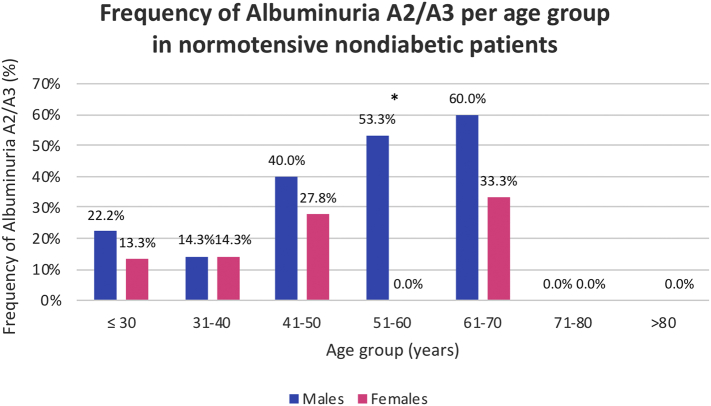
Albuminuria A2/A3 was found in 33.7%, mainly in males (52.6% vs. 21.7%) (Table 1). Of note, 23.1% of the patients with normoalbuminuria were under angiotensin-converting-enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB). Conversely, 51.5% of the patients with albuminuria A2/A3 had concomitant hypertension and 22.7% diabetes, although medically controlled. More importantly, albuminuria A2/A3 was found in 23.8% of the non-hypertensive non-diabetic patients, with a male predominance (39.1% vs. 15.5%, p = .002). Kidney biopsy showed lysosomal inclusions in podocytes of young p.F113 L patients with increased albuminuria (Fig. 4) and albuminuria A2/A3 was an early manifestation, affecting 22.2% of males and 12.9% of females before the age of 30 years. In males, its frequency increased progressively with age, reaching a peak of 71.4% in the 7th decade. In females, its frequency slowly increased until 40 years of age, when nearly one third of them had developed it (Fig. 5). A similar natural course was seen in the subset of patients without hypertension or diabetes (Fig. S2). Renal insufficiency (eGFR <60 mL/min/1.73m2) occurred rarely (6.6%), without gender dominance, and there were no cases of renal replacement therapy (RRT).
Fig. 4.
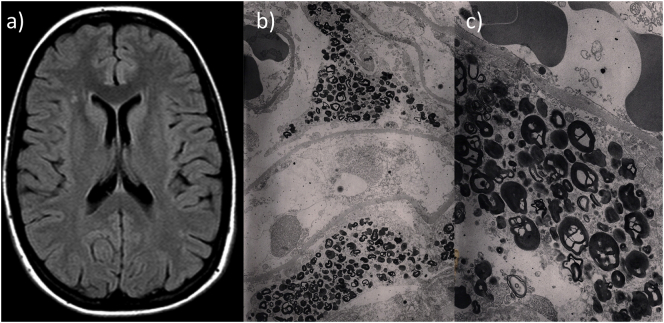
(a) Brain MRI showing WML lesions in a 29-year old female with FD due to the p.F113L mutation, in the absence of cardiovascular risk factors or other morbidities; (b, c) Electronic microscopy from a kidney biopsy showing lysosomal inclusions in renal podocytes in a 42-year old female with FD due to the p.F113L mutation, who presented proteinuria >1 g/24 h, in the absence of other morbidities.
Fig. 5.
Frequency of albuminuria A2/A3, brain WML, sensorineural deafness and cornea verticillata according to age category in male and female Fabry patients with the p.F113L mutation. * p < .05.
Fig. S2.
Frequency of albuminuria A2/A3 per age category in normotensive nondiabetic Fabry patients with the p.F113L mutation. * p < .05
Brain MRI was performed in 165 patients (81.3%; 65 males and 100 females). Brain white matter lesions (WML) occurred in 50.3%, without gender difference (53.8% vs. 48.0%) (Table 1). They were found in patients with cardiovascular risk factors, despite medically controlled, but also in 38.4% of the patients without these risk factors, without gender predominance. Brain WML were an early manifestation, affecting 11.1% of males and 26.9% of females under 30 years of age. Fig. 4 shows brain WML in a young female patient without comorbidities. Their frequency increased with age, being a universal finding in both genders over 70 years (Fig. 5). Stroke was rare and occurred in males (aged 42–51 years) and females (aged 41–71 years), all with concomitant cardiovascular risk factors and one with atrial fibrillation. Acroparesthesias occurred mainly in females and were predominantly mild (Table 1).
Sensorineural deafness was common (44.7%), particularly in males (62.2% vs.32.4%) (Table 1). It emerged early, affecting 20.0% of males and 3.6% of females under 30 years, and its frequency increased with age in both genders (Fig. 5). It occurred mainly at high frequencies and hearing aids were seldom indicated. Cornea verticillata was found in 10.3% of the patients. Notably, this is a milder form of cornea verticillata compared to the one that is typically observed in the classic patients. Angiokeratomas were rare (2.1%) and solitary.
4. Discussion
This study provides a detailed description of the natural history of the late-onset phenotype of FD due to one of the most common GLA gene mutations, the p.F113L mutation, thereby improving the knowledge on the natural history of nonclassical FD.
4.1. Cardiac manifestations
We have previously shown that, in this late-onset phenotype, cardiac manifestations carry the highest prognostic impact [6]. This study now shows that the first cardiac manifestations are LVH and LGE, which arise in males over 30 years and are followed by HF, non-sustained VT and cardiac conduction disorders, which arise in males over 40 years, culminating with the development of bifascicular block and complete AV block in males beyond the age of 50 years. Cardiac manifestations are more common and arise one to two decades earlier in males and their frequency increases with age in both genders. Likewise, the severity of LVH and cardiac blocks also increases with age, as demonstrated by the significant increase of LV mass, IVS and PW thickness and QRS interval with age.
LVH prevalence on cardiac predominant phenotypes has ranged from 21-25% in the IVS4 phenotype [15,19] to 29–67% in the p.N215S phenotype [5,8,13], which might be explained by the GLA gene mutation, characteristics of the studied patients, but also different LVH definitions. There are several echocardiographic indices of LVH [20]. We have reported LVH in nearly 40% of p.F113L patients [6], based on the LVH definition of LV wall thickness > 12 mm, because this criterion carries a therapeutic impact, as it has been commonly used to establish the presence of LVH and mandate the initiation of specific therapy in Fabry patients [21].
Despite different LVH definitions, and similarly to our findings in the p.F113L phenotype, LVH has been described to arise as early as the third decade of life in patients with the p.N215S mutation [9,13] and after 30 years of age in patients with the IVS4 mutation [19]. Likewise, LVH prevalence increases with age in both genders in the IVS4 [19] and the p.N215S phenotypes [5]. However, IVS and PW thickness has been reported to increase progressively with age in males, remaining only mildly elevated in females with the p.N215S mutation [13]. In our study, LVH severity increased with age and severe LVH developed both in males and females with the p.F113L mutation.
Cardiac MRI is a valuable tool for the assessment of LVH and cardiac fibrosis [22]. However, most studies on cardiac predominant phenotypes do not provide cardiac MRI data [5,10,[13], [14], [15]]. In the p.N215S phenotype, two small studies have reported LGE, respectively, in 53% (8/15) [8] and 69% (18/26) [9], and as early as the third decade of life [9]. In a larger cohort of 100 IVS4 patients, Hsu et al. reported LVH based on cardiac MRI criteria in 28% and LGE in 38%. In patients without LVH, LGE was found in 38.1% of males and 16.7% of females, suggesting that cardiac fibrosis progressed in silence before the development of LVH. However, in patients with LVH, LGE was found in 61%, exclusively in males (77.3%), being surprisingly absent in females, but the characteristics of those six females were not reported [19]. Our study, based on a larger cohort of 166 patients who underwent cardiac MRI, found cardiac MRI criteria of LVH in 36.1% and an apparently lower prevalence of LGE in the overall cohort (21.4%), in patients with LVH (52.5%) and also in patients without LVH (5.3% of males and 4.6% of females), supporting the hypothesis of a lower frequency or later development of LGE in this late-onset phenotype [6]. Nevertheless, LGE was also an early manifestation in p.F113L phenotype, arising after the 30 years of age. Finally, although LGE was associated with ST depression and negative T waves on ECG, the finding of LGE in 17.8% of the patients without ST depression and in 13.4% without negative T waves argues against the hypothesis raised by Niemann et al. that their absence almost excludes LGE [23].
Most studies on the cardiac predominant phenotypes report on the prevalence of LVH, but data on the specific prevalence or natural course of HF, which is the actual clinical event associated with LVH, are very scarce. We have previously shown that HF occurs in nearly 20% of the p.F113L patients [6] and now we provide the first detailed data on its natural course.
We previously published for the first time the actual frequency of each cardiac rhythm and conduction disorder in a cardiac predominant phenotype [6]. Now, in this study, we provide the first detailed description of the chronology of each of the major cardiac rhythm and conduction disorders that occur and ultimately carry the highest impact on survival in cardiac predominant phenotypes.
In our study, all myocardial ischemic events, except one, were acute coronary syndromes in the setting of severe atherosclerotic coronary heart disease. The only myocardial ischemic event that could eventually be attributed to FD was a type 2 myocardial infarction that occurred in an elderly male with normal coronary arteries and a probable imbalance of myocardial oxygen supply and demand in the context of severe LVH. This finding, together with the typical absence of endothelial GB3 deposits in late-onset FD [24], suggests that myocardial ischemic events secondary to FD are rare in late-onset phenotypes. Some previous studies on late-onset FD reported myocardial ischemic events as Fabry cardiac events [3,13], but no information was given on coronary angiography findings or atherosclerotic risk of the patients. Moreover, percutaneous coronary intervention and coronary artery bypass graft have been considered cardiac events [3], despite the virtually null probability of representing manifestations of late-onset FD. Our study shows that we should cautiously attribute myocardial ischemic events to late-onset FD, because most of them are indeed acute coronary syndromes secondary to atherosclerotic coronary heart disease, which is a common comorbidity with advancing age.
4.2. Extracardiac manifestations
We have previously shown that, in this cardiac predominant phenotype, extracardiac involvement is common [6]. Now, we show that the earlier manifestations are extracardiac, with increased albuminuria, brain WML and sensorineural deafness arising before 30 years of age in both genders and increasing thereafter.
In the p.N215S phenotype, increased albuminuria has been reported, respectively, in 7.7% [9] and 50% of the patients [8] in two small studies and proteinuria in 32.4% of males and 6.4% of females in a larger study [5]. In the IVS4 phenotype, increased albuminuria has been described in 20% [15]. This wide range of results may be explained by different sample sizes and definitions of pathological albuminuria/proteinuria. Nevertheless, like in the p.F113L phenotype, microalbuminuria has been reported as an early manifestation, occurring before 40 years of age in the IVS4 phenotype [15] and as early as the second decade of life in the p.N215S phenotype [5]. Also similar to the p.F113L phenotype, its prevalence increased with age in both genders, but stabilized in middle-aged women with the p.N215S mutation [5]. Chronic kidney disease (CKD) ≥ G3 was reported in 21.6% of males and 2.4% of females with the p.N215S mutation and its prevalence increased with age. End-stage renal failure has been described in two young p.N215S males (25 and 38 years old) (2.4%) with marked persistent proteinuria [5]. In another study, 4% of p.N215S patients (males and females) developed CKD G5 with need for RRT before 65 years of age [13]. In the p.F113L phenotype, CKD ≥ G3 was only found in 9.3% of males and 4.9% of females, CKD G5 in 0.5% and no patients were under RRT.
This study also demonstrates the presence of lysosomal inclusions in renal podocytes of young patients presenting with increased albuminuria, without any known morbidities besides late-onset FD due to the p.F113L mutation, therefore supporting the hypothesis that increased albuminuria in these patients is indeed caused by FD. Likewise, lysosomal inclusions have also been found in renal podocytes of p.N215S patients [8,9].
Our study showed that brain WML are common manifestations (50.3%), which arise early before 30 years of age and increase progressively thereafter, becoming a universal finding in p.F113L males and females above 70 years of age. Small studies reported lower frequencies of brain WML in IVS4 patients (32%) [11,12] and p.N215S patients (21–32%) [8,9]. However, in a larger cohort of p.N215S patients, brain WML were found in 51.4% of males and 27.7% of females, appearing before 40 years of age, increasing progressively thereafter and affecting most elderly patients [5]. In the general population, brain WML have been associated with cerebrovascular risk factors and ageing [25,26]. A previous study reported the following age-related prevalences of brain WML in the general population: 3.5% (<40 years), 22% (41–50 years), 47% (51–60 years), 64% (61–70 years), 84% (71–80 years) and 95% (>81 years) [25]. Compared to the general population, it is striking in our FD cohort the higher prevalence of brain WML in young patients without other comorbidities, which also supports the notion that brain WML are caused by FD and not by ageing or other conditions. As late-onset phenotypes of FD are typically characterized by the absence of endothelial GB3 deposits [24], brain WML, particularly in young patients, are probably mainly caused by GB3 deposits in microglial cells and astrocytes and subsequent pro-inflammatory alterations [27]. Importantly, and in line with the absence of endothelial GB3 deposits, stroke remained a rare event, such as in the p.N215S [5,8,9,13], IVS4 [[10], [11], [12]] and overall late-onset phenotypes [3], occurring in patients with concomitant cardiovascular risk factors.
To our knowledge, this is the first study describing the natural course of sensorineural deafness in a cardiac predominant phenotype of FD. Our study shows for the first time that this common manifestation (44.7%) arises before 30 years of age in males and females with the p.F113L mutation and increases with age, virtually affecting all the elderly individuals.
4.3. Strengths and limitations
This study represents one of the largest cohorts of adult patients with late-onset FD due to the same GLA gene mutation, which provides a unique opportunity to perform a gender and age-stratified analysis and obtain a reliable overview of the natural history of this late-onset variant. Most patients were diagnosed through family screening, which allowed the diagnosis of patients from both genders, all age categories and disease status, capturing the full spectrum of the disease. The female/male ratio was 1.6, closer to the expected ratio in an X-linked disease, considerably reducing the bias related to female underrepresentation of previous studies [5,[8], [9], [10], [11], [12], [13], [14]]. We analyzed baseline data at the time of FD diagnosis, so the disease status of the patients reflects the natural course of the disease until that moment, without the influence of FD specific therapy. We also evaluated concomitant morbidities and medications and accounted for their influence on the phenotype. Patients were systematically submitted to a predefined multidisciplinary diagnostic protocol, which assured a high level of completeness and detail of the data on all organ system manifestations, overcoming common bias of retrospective studies or registries. Finally, p.F113L is one of the most common GLA gene mutations causing late-onset FD, so these natural history data will have an impact on the clinical decisions of many patients.
However, the natural history presented in this study does not represent a longitudinal follow-up of these patients, rather it was built based on the baseline data presented by untreated adult patients distributed through the full range of age categories. The longitudinal follow-up of untreated patients in a time where therapies are available raises ethical issues and, given the attenuated and slow course of the disease, a long time of follow-up would be needed to capture its natural history. Nevertheless, considering the large size of our cohort, the fact that it includes all the patients diagnosed with FD within these 34 families and the fact that most of them were diagnosed through family screening and not because of medical attention driven by symptoms, it is reasonable to expect that the data within each age category is a reliable representation of the clinical heterogeneity of the disease at each timeframe of its natural course.
4.4. Future directions
Our study demonstrates a high clinical heterogeneity in both males and females with this late-onset phenotype, with some patients developing an earlier and more severe phenotype than others. Studies specifically addressing the efficacy of therapies on late-onset phenotypes are urgently needed to ascertain if all patients benefit from therapy. If that would not be the case, the holy grail would be, within the high clinical heterogeneity of late-onset phenotypes, to correctly select the patients with higher risk of disease progression and faster disease course who could benefit or benefit the most from therapy.
Moreover, the best timing to start therapy in FD is not clear, particularly in classic females and late-onset phenotypes (males and females). Current guidelines recommend to start therapy in these subsets of patients as soon as there is evidence of target organ damage [28]. Some studies have already shown that early treatment is better than late treatment [29,30]. However, how early should the treatment be started is not yet defined, although from a theoretical point view it would make sense to start treatment before target organ damage occurs.
Therefore it is essential to deepen the study on the natural history of FD, find the determinants of phenotype and determine the predictors of disease progression in both genders, so we could practice Precision Medicine for each individual patient, based on factors much beyond gender, age or GLA gene mutation, and accurately determine, for each individual, if he is a good candidate for therapy, the best therapy and the best timing to start it in order to avoid target organ damage.
5. Conclusion
Our study on the natural history of the p.F113L phenotype found many similarities to the natural history of other predominant cardiac phenotypes, suggesting a general common natural course to these FD variants, despite the different GLA gene mutations. Nevertheless, this study comes to fill many evidence gaps on the natural history of these FD phenotypes, namely on HF, cardiac rhythm and conduction disturbances, ischemic myocardial events and sensorineural deafness. It also provides cardiac and brain MRI data on a large cohort of patients with late-onset FD. Finally, the demonstration of lysosomal inclusions on renal podocytes of young p.F113L patients with proteinuria as well as of brain WML in young patients without morbidities besides late-onset FD due to the p.F113L mutation supports the hypothesis that these clinical manifestations are caused by FD pathology. In summary, our study improves the knowledge on the natural history of late-onset FD, which is essential to make evidence-based clinical decisions and guidelines and to accurately evaluate the clinical efficacy of FD therapies.
The following are the supplementary data related to this article.
Funding
No funding sources.
Disclosures
Olga Azevedo and Gabriel Miltenberger-Miltenyi have received educational and/or research grants from Shire Human Genetic Therapies, Amicus and Sanofi Genzyme. Miguel Gago has received educational grants from Shire Human Genetic Therapies and Sanofi Genzyme. Maria José Guimarães has received educational and research grants from Sanofi Genzyme. The remaining authors have no disclosures.
Author statement
Olga Azevedo - Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing original draft, review & editing.
Miguel F Gago - Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; review & editing of manuscript.
Gabriel Miltenberger-Miltenyi – Data analysis; Investigation; review & editing of manuscript.
Ana Raquel Robles - Data analysis; Investigation; review & editing of manuscript.
Maria Antónia Costa - Data analysis; Investigation; review & editing of manuscript.
Olga Pereira - Data analysis; Investigation; review & editing of manuscript.
Ana Teresa Vide - Data analysis; Investigation; review & editing of manuscript.
Gonçalo Castelo Branco - Data analysis; Investigation; review & editing of manuscript.
Sónia Simões - Data analysis; Investigation; review & editing of manuscript.
Maria José Guimarães - Data analysis; Investigation; review & editing of manuscript.
Ana Salgado - Data analysis; Investigation; review & editing of manuscript.
Nuno Sousa – Supervision; review & editing of manuscript.
Damião Cunha - Supervision; review & editing of manuscript.
Acknowledgements
The authors thank to all the health professionals of the Reference Center on Lysosomal Storage Disorders of Hospital Senhora da Oliveira, Guimarães, Portugal, for their important contribution to the diagnosis, treatment and follow-up of FD patients; to Dr. Pedro Pereira, for the selection of the figures of electronic microscopy of the kidney biopsy; to the historians Dr. Rui Faria, Dr. Alice Martins and Dr. Fátima Dias, for their continuous collaboration in the genealogical research of Fabry families with the founder mutation p.F113L; and to the patients and families.
References
- 1.Germain D.P. Fabry disease. Orphan. J. Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta A., Clarke J.T., Giugliani R., Elliott P., Linhart A., Beck M., Sunder-Plassmann G., FOS Investigators Natural course of Fabry disease: changing pattern of causes of death in FOS - Fabry outcome survey. J. Med. Genet. 2009;46:548–552. doi: 10.1136/jmg.2008.065904. [DOI] [PubMed] [Google Scholar]
- 3.Arends M., Wanner C., Hughes D., Mehta A., Oder D., Watkinson O.T., Elliott P.M., Linthorst G.E., Wijburg F.A., Biegstraaten M., Hollak C.E. Characterization of classical and nonclassical Fabry disease: a multicenter study. J. Am. Soc. Nephrol. 2017;28:1631–1641. doi: 10.1681/ASN.2016090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spada M., Pagliardini S., Yasuda M., Tukel T., Thiagarajan G., Sakuraba H. High incidence of later-onset Fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavalle L., Thomas A.S., Beaton B., Ebrahim H., Reed M., Ramaswami U. Phenotype and biochemical heterogeneity in late onset Fabry disease defined by N215S mutation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azevedo O., Gal A., Faria R. Founder effect of Fabry disease due to p.F113L mutation: clinical profile of a late-onset phenotype. Mol. Genet. Metab. 2019 doi: 10.1016/j.ymgme.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Kleinert J., Dehout F., Schwarting A., Lorenzo A.G., Ricci R., Kampmann C. Prevalence of uncontrolled hypertension in patients with Fabry disease. Am. J. Hypertens. 2006;19:782–787. doi: 10.1016/j.amjhyper.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Alharbi F.J., Baig S., Auray-Blais C., Boutin M., Ward D.G., Wheeldon N., Steed R., Dawson C., Hughes D., Geberhiwot T. Globotriaosylsphingosine (Lyso-Gb3) as a biomarker for cardiac variant (N215S) Fabry disease. J. Inherit. Metab. Dis. 2018;41:239–247. doi: 10.1007/s10545-017-0127-2. [DOI] [PubMed] [Google Scholar]
- 9.Oder D., Liu D., Hu K., Üçeyler N., Salinger T., Müntze J., Lorenz K., Kandolf R., Gröne H.J., Sommer C., Ertl G., Wanner C., Nordbeck P. α-Galactosidase A genotype N215S induces a specific cardiac variant of fabry disease. Circ. Cardiovasc. Genet. 2017;10 doi: 10.1161/CIRCGENETICS.116.001691. pii: e001691. [DOI] [PubMed] [Google Scholar]
- 10.Lin H.Y., Liu H.C., Huang Y.H., Liao H.C., Hsu T.R., Shen C.I., Li S.T., Li C.F., Lee L.H., Lee P.C., Huang C.K., Chiang C.C., Lin C.Y., Lin S.P., Niu D.M. Effects of enzyme replacement therapy for cardiac-type Fabry patients with a Chinese hotspot late-onset Fabry mutation (IVS4+919G>A) BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.J., Hung S.C., Hsu T.R., Ko S.C., Chui-Mei T., Huang C.C., Niu D.M., Lin C.P. Brain MR imaging findings of cardiac-type Fabry disease with an IVS4+919G>A mutation. AJNR Am. J. Neuroradiol. 2016;37:1044–1049. doi: 10.3174/ajnr.A4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H.J., Hsu T.R., Hung S.C., Yu W.C., Chu T.H., Yang C.F., Bizjajeva S., Tiu C.M., Niu D.M. A comparison of central nervous system involvement in patients with classical Fabry disease or the later-onset subtype with the IVS4+919G>a mutation. BMC Neurol. 2017;17 doi: 10.1186/s12883-017-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germain D.P., Brand E., Burlina A., Cecchi F., Garman S.C., Kempf J., Laney D.A., Linhart A., Maródi L., Nicholls K., Ortiz A., Pieruzzi F., Shankar S.P., Waldek S., Wanner C., Jovanovic A. Phenotypic characteristics of the p.Asn215Ser (p.N215S) GLA mutation in male and female patients with Fabry disease: a multicenter Fabry registry study. Mol. Genet. Genom. Med. 2018 doi: 10.1002/mgg3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H.C., Perrin A., Hsu T.R., Yang C.F., Lin H.Y., Yu W.C., Niu D.M. Age at first cardiac symptoms in Fabry disease: association with a Chinese hotspot Fabry mutation (IVS4+919G>A), classical Fabry mutations, and sex in a Taiwanese population from the Fabry outcome survey (FOS) JIMD Rep. 2015;22:107–113. doi: 10.1007/8904_2015_418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin H.Y., Huang C.H., Yu H.C., Chong K.W., Hsu J.H., Lee P.C., Cheng K.H., Chiang C.C., Ho H.J., Lin S.P., Chen S.J., Lin P.K., Niu D.M. Enzyme assay and clinical assessment in subjects with a Chinese hotspot late-onset Fabry mutation (IVS4+919G→A) J. Inherit. Metab. Dis. 2010;33:619–624. doi: 10.1007/s10545-010-9166-7. [DOI] [PubMed] [Google Scholar]
- 16.Ishii S., Chang H., Kawasaki K., Yasuda K., Wu H., Garman S.C. Mutant α-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem. J. 2007;406:285–295. doi: 10.1042/BJ20070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azevedo O., Gal A., Rodrigues D. Miocardiopatia Hipertrófica secundária a doença de Fabry: Evidência de um efeito fundador na região de Guimarães [Abstract] Rev. Port. Cardiol. 2013;32:28. (Espec Congr) [Google Scholar]
- 18.Hudsmith L.E., Petersen S.E., Francis J.M., Robson M.D., Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2005;7(5):775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 19.Hsu T.R., Hung S.C., Chang F.P., Yu W.C., Sung S.H., Hsu C.L. Later onset Fabry disease, cardiac damage progress in silence: experience with a highly prevalent mutation. J. Am. Coll. Cardiol. 2016;68:2554–2563. doi: 10.1016/j.jacc.2016.09.943. [DOI] [PubMed] [Google Scholar]
- 20.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 21.Biegstraaten M., Arngrímsson R., Barbey F., Boks L., Cecchi F., Deegan P.B., Feldt-Rasmussen U., Geberhiwot T., Germain D.P., Hendriksz C., Hughes D.A., Kantola I., Karabul N., Lavery C., Linthorst G.E., Mehta A., van de Mheen E., Oliveira J.P., Parini R., Ramaswami U., Rudnicki M., Serra A., Sommer C., Sunder-Plassmann G., Svarstad E., Sweeb A., Terryn W., Tylki-Szymanska A., Tøndel C., Vujkovac B., Weidemann F., Wijburg F.A., Woolfson P., Hollak C.E. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphan. J. Rare Dis. 2015;10 doi: 10.1186/s13023-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry R., Shah R., Saiedi M., Patil S., Ganesan A., Linhart A., Selvanayagam J.B. The role of cardiac imaging in the diagnosis and management of Anderson-Fabry disease. JACC Cardiovasc. Imaging. 2019 Jul;12(7 Pt 1):1230–1242. doi: 10.1016/j.jcmg.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Niemann M., Hartmann T., Namdar M., Breunig F., Beer M., Machann W., Herrmann S., Ertl G., Wanner C., Weidemann F. Cross-sectional baseline analysis of electrocardiography in a large cohort of patients with untreated Fabry disease. J. Inherit. Metab. Dis. 2013;36:873–879. doi: 10.1007/s10545-012-9540-8. [DOI] [PubMed] [Google Scholar]
- 24.von Scheidt W., Eng C.M., Fitzmaurice T.F., Erdmann E., Hübner G., Olsen E.G. An atypical variant of Fabry’s disease with manifestations confined to the myocardium. N. Engl. J. Med. 1991;324:395–399. doi: 10.1056/NEJM199102073240607. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang F.-J., Chen Y., He W.-B., Cai Z.-Y. Prevalence of white matter hyperintensities increases with age. Neural Regen. Res. 2018;13:2141–2146. doi: 10.4103/1673-5374.241465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao D., Cooper L., Cai J., Toole J., Bryan N., Burke G., Shahar E., Nieto J., Mosley T., Heiss G. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC study. Neuroepidemiology. 1997;16:149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 27.Rozenfeld P., Feriozzi S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol. Genet. Metab. 2017;122:19–27. doi: 10.1016/j.ymgme.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz A., Germain D.P., Desnick R.J., Politei J., Mauer M., Burlina A., Eng C., Hopkin R.J., Laney D., Linhart A., Waldek S., Wallace E., Weidemann F., Wilcox W.R. Fabry disease revisited: management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018;123:416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Arends M., Wijburg F.A., Wanner C., Vaz F.M., van Kuilenburg A.B.P., Hughes D.A., Biegstraaten M., Mehta A., Hollak C.E.M., Langeveld M. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol. Genet. Metab. 2017;121:157–161. doi: 10.1016/j.ymgme.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Zamorano J., Serra V., Pérez de Isla L., Feltes G., Calli A., Barbado F.J., Torras J., Hernandez S., Herrera J., Herrero J.A., Pintos G. Usefulness of tissue Doppler on early detection of cardiac disease in Fabry patients and potential role of enzyme replacement therapy (ERT) for avoiding progression of disease. Eur. J. Echocardiogr. 2011;12:671–677. doi: 10.1093/ejechocard/jer109. [DOI] [PubMed] [Google Scholar]