Abstract
Introduction
A combined assessment of different parameters of cardiovascular (CV) risk and prognosis can be supportive and performed with cardiac magnetic resonance (CMR). Aortic stiffness, epicardial fat volume (EFV), left ventricular (LV) strain and fibrosis were evaluated within a single CMR examination and results were related to the presence of hypertension (HTN) and diabetes mellitus (DM).
Methods
20 healthy controls (57.2 ± 8.2 years(y); 26.2 ± 3.9 kg/m2), 31 hypertensive patients without DM (59.6 ± 6.7 y; 28.4 ± 4.7 kg/m2) and 12 hypertensive patients with DM (58.8 ± 9.9y; 30.7 ± 6.3 kg/m2) were examined at 1.5Tesla. Aortic stiffness was evaluated by calculation of aortic pulse wave velocity (PWV), EFV by a 3D-Dixon sequence. Longitudinal & circumferential systolic myocardial strain (LS; CS) were analyzed and T1-relaxation times (T1) were determined to detect myocardial fibrosis.
Results
EFV was highest in hypertensive patients with diabetes (78.4 ± 28.0 ml/m2) followed by only hypertensive patients (64.2 ± 27.3 ml/m2) and lowest in controls (50.3 ± 22.7 ml/m2; p < 0.05). PWV was higher in hypertensive patients with diabetes (9.8 ± 3.3 m/s) compared to only hypertensive patients (8.6 ± 1.7 m/s; p < 0.05) and to controls (8.1 ± 1.9 m/s; p < 0.05). LS&CS were worse in hypertensive patients with diabetes (LS:-20.9 ± 5.1% and CS: −24.4 ± 5.7%) compared to both only hypertensive patients (LS: −24.7 ± 4.6%; CS: −27.1 ± 5.0%; p < 0.05) and to controls (LS: −25.5 ± 3.8; CS: −28.3 ± 4.1%; p < 0.05). Both hypertensive groups with and without DM had higher T1́s (994.0 ± 43.2 ms; 991.6 ± 35.5 ms) than controls (964.6 ± 40.3 ms; p < 0.05).
Conclusion
CMR revealed increased aortic stiffness and EFV in hypertensive patients, which were even higher in the presence of DM. Also signs of LV myocardial fibrosis and a reduced strain were revealed. These parameters support the assessment of CV risk and prognosis. They can accurately be measured with CMR within a single examination when normally different techniques are needed.
1. Introduction
Cardiovascular (CV) disease is the number one cause of death. The assessment of CV risk is often performed by evaluation of traditional risk factors, which may not always be accurate [1], [2], [3], [4]. Up to date different additional parameters related to CV risk and prognosis have been introduced. A well known parameter is aortic pulse wave velocity (PWV). This is a measure of aortic stiffness and regarded as an important predictor of CV adverse events [5].
The amount of epicardial fat has also been related to the presence of CV risk factors, disease and events such as myocardial infarction. A metabolic and inflammatory role is discussed [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Parameters, such as the extent of myocardial fibrosis or parameters of left ventricular (LV) contractility, e.g. strain parameters, may be pathologic despite a normal ejection fraction and are both sensitive indicators for sub-clinical cardiac diseases, such as myocardial ischemia, hypertension, and heart failure [16], [17], [18], [19]. While normally different techniques are needed to assess these different parameters [5], [6], cardiac magnetic resonance (CMR) allows for accurate assessment of all within a single examination and without meaningful harm [20], [21], [22], [23]. PWV can accurately be measured using velocity-encoded MRI sequences. Dixon chemical shift imaging can measure epicardial fat volumetrically by 3-dimensional (3D) ECG-triggered sequences. Myocardial fibrosis can be assessed using myocardial T1-mapping techniques such as a modified Look-Locker inversion recovery (MOLLI) scheme and myocardial strain may be evaluated on cine datasets using dedicated softwares.
A combined measurement may support the evaluation of CV risk and prognosis.
The purpose of this CMR study was to evaluate aortic stiffness, epicardial fat volume (EFV), LV myocardial strain and fibrosis, and, to relate the results to the presence of hypertension (HTN) and diabetes mellitus (DM).
2. Materials and methods
The protocol for this retrospectively performed study was approved by the local ethic committee. All scans were performed on a 1.5 Tesla (T) MR system (Ingenia, Philips Healthcare, Best, The Netherlands) with a maximum gradient strength of 45mT/m and a maximum slew rate of 120mT/m/ms. A 32 channel torso coil with digital interface was used for signal reception. 20 healthy non-hypertensive controls without medications or history of CV disease and coronary artery disease (CAD) were included. They were assessed by detailed questionnaire, ruling out risk factors, previous medication or any history of CAD or hypertensive disease as ruled out by previous check-ups. 43 patients had a well-treated hypertension without CAD. Written informed consent was obtained from all study participants prior to CMR. Exclusion criteria included contraindications for CMR, valvular disorders, rhythm disorders and renal insufficiency.
2.1. MRI acquisition
Functional imaging: ECG-gated SSFP-cine images were obtained in breath-hold in the horizontal long axis, the vertical long axis, left ventricular outflow tract, and short axis for wall motion and functional analysis. Sequence parameters were as follows: field of view (FOV) = 350 × 350 mm2, slice thickness = 8 mm, pixel size = 1.7 × 1.7 mm2, reconstructed to 1 × 1 mm2, repetition time (TR) = 3.1 ms, echo time (TE) = 1.6 ms, flip angle (α) = 60°, parallel imaging factor (SENSE) = 2.5, number of cardiac phases reconstructed = 40.
Aortic stiffness, aortic PWV: ECG-gated SSFP-cine images of the aorta were acquired in a coronal and an oblique sagittal view, covering the aortic arch including the aorta ascendens (AA) and the proximal aorta descendens (AD). Based on these images, a 2D velocity-encoded MRI sequence was planned perpendicular through the AA and AD at the level of the bifurcation of the pulmonary artery with following parameters: FOV = 300 × 225 mm2, slice thickness = 8 mm, pixel size = 1.7 × 1.7 mm2, reconstructed to 1.2 × 1.2 mm2, TR = 6.5 ms, TE = 2.2 ms, α = 15°, number of heart phases = 130, velocity encoding was set to a maximum velocity of 1.5 m/s. Scan time was 2–3 min.
Dixon chemical shift imaging: A 3D transversal ECG-triggered and respiratory navigator gated magnetization prepared mDixon-sequence was acquired for assessment of EFV. Trigger delay was set to end-diastole and optimized by means of cine MRI data. The following sequence parameters were used: FOV = 350 × 302 × 180 mm3, voxel size = 1.5 × 1.5 × 3.0 mm3 (120 overcontiguous slices), reconstructed voxel size = 1.0 × 1.0 × 1.5 mm3, TR = 5.4 ms, TE1 / TE2 = 1.8 ms /4.0 ms; α = 20°, parallel imaging factor (SENSE) = 1.5 in both phase encoding directions, water fat shift = 0.16 pixel, arrhythmia rejection was applied, T2 preparation = 50 ms, acquisition window = 100–156 ms (selected based on cine MRI data). Net scan duration was 3–5 min. The average total scan duration time was about 7.5 min. In-phase (IP), opposed-phase (OP), water only (W), and fat only (F) images were reconstructed online at the scanner console [24].
Myocardial T1-mapping: For the assessment of T1 a 3(3)3(3)5 modified Look-Locker inversion recovery (MOLLI) scheme was used with the following parameters: FOV = 350 × 300 mm2, acquired voxel size = 2 × 2 × 10 mm3, reconstructed voxel size = 1.2 × 1.2 × 10 mm3, TR/TE = 2.2 / 1.0 ms, flip angle = 35°, parallel imaging with SENSE factor 2. End-diastolic short axis slices (basal, mid-ventricular, and apical) were obtained in breath hold [25]. T1-MOLLI maps were reconstructed online at the scanner console and mapping sequences were repeated in case of image artifacts.
2.2. Image analysis
Cardiac function analysis: Left ventricular end systolic and end diastolic volume (LVESV and LVEDV), left ventricular function (LVEF), interventricular septal diameter (IVSD) were determined offline using dedicated software (IntelliSpace Portal 7, Philips Healthcare).
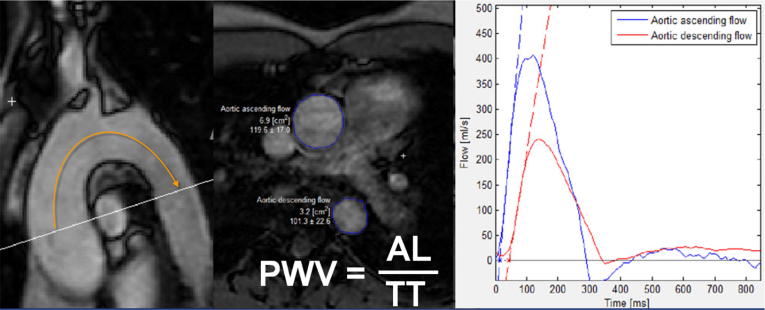
Aortic PWV: PWV quantification was performed using a tool implemented in the software Segment (Segment, version 1.9, R3918; http://segment.heiberg.se) [26]. First, the path length of the aortic arch (aortic length [AL]), i.e. the distance between the section through the AA and through the proximal AD, was measured between the center of the cross-sections of AA and proximal AD. The time interval between the arrival of the velocity waveform at the section AA and at the section of the proximal AD is called the transit time (TT) and was determined by contour-drawing in the aortic velocity maps. TT is measured as the time between the intercept of the two calculated tangents with the time axis. PWV was finally calculated by PWV = AL / TT [20] (Fig. 1). The post-processing time for the determination of aortic PWV was about 5 min.
Fig. 1.
PWV quantification using a tool implemented in the software Segment (Segment, version 1.9, R3918; http://segment.heiberg.se). A: path length of the aortic arch (aortic length = AL) which is the distance between the section through the Aorta ascendens (AA) and through the proximal Aorta descendens (AD) B: Region of interest (ROI) in the AA and in the AD in the aortic velocity maps C: Flow curves along with their respective calculated tangents. The transit time (TT) is the time between the two tangents. PWV = AL/TT.
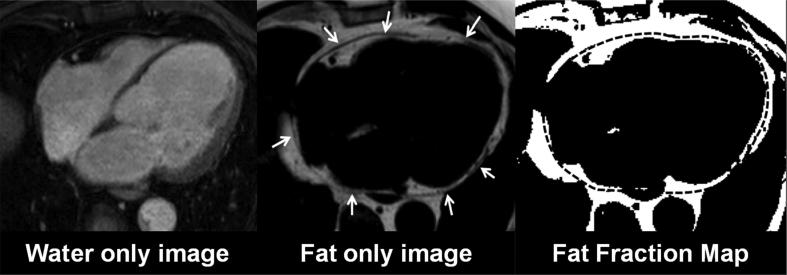
Epicardial fat volume: Dixon images were analyzed offline on a personal computer using dedicated software written in MATLAB (The MathWorks, Inc., Natick, MA) with an analysis time of about 7–10 min per subject. The epicardial fat volume was measured between the bifurcation of the pulmonary artery and the most inferior transversal slice of the myocardium [6]. A 3D region of interest (ROI) was defined by manually contouring the epicardial border in each slice. Fat-fraction maps were computed based on the fat- and water-only images with an appropriate noise threshold and correction for relaxation effects to identify voxels predominantly containing fat (Fig. 2). EFV was determined by multiplying the number of fat voxels inside the three-dimensional ROIs by the voxel size and normalized to the BSA [24].
Fig. 2.
Dixon images. A: Fat only image. B: Fat only Image with the epicardial outlines. C: Segmented fat voxels with the transferred region of interest.
Myocardial T1-mapping: T1 values were assessed using freely available software (Segment, version 1.9, R2783; http://segment.heiberg.se) and extracted from the relaxation maps using a segmental approach [27]. To exclude epicardial fat, blood pool and pericardial effusion from the analysis, endo- and epicardial borders were carefully contoured.
Myocardial strain analysis: LV strain analysis was performed by longitudinal strain measurements (LS) in 4-chamber long axis view cine datasets and circumferential strain measurements (CS) in short axis cine datasets using dedicated feature tracking software (2D Cardiac Performance Analysis MR, TomTec, Unterschleissheim, Germany) [21]. LV short axis circumferential strain was derived from a mid-ventricular short-axis slice. The LV endocardial borders were identified based on a manually drawn contour and then “tracked” automatically over the entire RR cycle from frame to frame. Peak segmental values were averaged resulting in a global longitudinal and circumferential LV peak systolic strain [21].
2.3. Statistical analysis
All statistical analyses were performed using SPSS, (IBM SPSS Statistics 22.0, Armonk, New York). Patients characteristics are presented as mean ± standard deviation or as absolute frequency. For comparison all subjects were grouped into controls, hypertensive patients without DM and hypertensive patients with DM. Continuous variables were tested for normal distribution. The independent 2‐sample Student t test or the Mann-Whitney-U test was used for comparison of continuous variables between different groups. The level of significance was set to 0.05.
3. Results
A total of 63 subjects were enrolled in this study. 31 hypertensive patients had no diabetes mellitus (HTN; 14 men, mean age: 59.6 ± 6.7 years; BMI 28.4 ± 4.7 kg/m2) and 12 hypertensive patients had diabetes mellitus (HTN + DM; 7 men, mean age: 58.8 ± 9.9 years; BMI 30.7 ± 6.3 kg/m2). 20 persons served as non-hypertensive controls (11 men, mean age: 57.2 ± 8.2 years; BMI 26.2 ± 3.9 kg/m2).
There was no significant difference regarding age or gender between these groups. BMI was higher in HTN + DM patients compared to controls. Results were adjusted for BMI. EFV was highest in HTN + DM patients (78.4 ± 28.0 ml/m2) followed by HTN patients (64.2 ± 27.3 ml/m2) and lowest in controls (50.3 ± 22.7 ml/m2). PWV was also higher in hypertensive patients with DM (9.8 ± 3.3 m/s) compared to hypertensive patients without DM (8.6 ± 1.7 m/s; p < 0.05) and to controls (8.1 ± 1.9 m/s; p < 0.05).
There was no significant difference regarding PWV between controls and hypertensive patients without DM. Similarly LS and CS were worse in hypertensive patients with DM (LS: −20.9 ± 5.1% and CS: −24.4 ± 5.7%) compared to hypertensive patients without DM (LS: −24.7 ± 4.6% and CS: −27.1 ± 5.0%; p < 0.05) and to controls (LS: −25.5 ± 3.8% and CS: −28.3 ± 4.1%; p < 0.05). There was no significant difference regarding LS or CS between controls and hypertensive patients without DM. There was no differences regarding T1 between hypertensive patients with and without DM (994.0 ± 43.2 vs. 991.6 ± 35.5 ms), however, both had significantly higher T1 values as a sign of myocardial fibrosis compared to controls (964.6 ± 40.3 ms; p < 0.05).
The detailed characteristics of study participants and CMR-derived parameters are summarized in Table 1.
Table 1.
Results in dependency of the presence of hypertension resp. diabetes mellitus.
| Parameter | 1 Controls (N = 20) | 2 HTN (N = 31) | 3 HTN + DM (N = 12) | P 1 vs 2 | P 1 vs 3 | P 2 vs 3 |
|---|---|---|---|---|---|---|
| Men | 11 | 14 | 7 | 0.96 | 0.86 | 0.9 |
| Age (years) | 57.2 ± 8.2 | 59.6 ± 6.7 | 58.8 ± 9.9 | 0.34 | 0.62 | 0.8 |
| BMI [kg/m2] | 26.2 ± 3.9 | 28.4 ± 4.7 | 30.7 ± 6.3 | 0,12 | <0.05 | 0.2 |
| LVEF [%] | 64.8 ± 5.3 | 61.3 ± 6.6 | 63.0 ± 7.7 | 0.11 | 0.43 | 0.50 |
| LVEDVi [ml/m2] | 67.7 ± 12.9 | 70.7 ± 15.6 | 67.8 ± 12.0 | 0.59 | 0.98 | 0.60 |
| IVSD [mm] | 8.8 ± 1.6 | 10.3 ± 2.0 | 10.0 ± 2.1 | <0.05 | <0.05 | 0.7 |
| EFV [ml/m2] | 50.3 ± 22.7 | 64.2 ± 27.3 | 78.4 ± 28.0 | <0.05 | <0.05 | <0.05 |
| PWV (m/s) | 8.1 ± 1.9 | 8.6 ± 1.7 | 9.8 ± 3.3 | 0.14 | <0.05 | <0.05 |
| LS [%] | −25.5 ± 3.8 | −24.7 ± 4.6 | −20.9 ± 5.1 | 0.45 | <0.05 | <0.05 |
| CS [%] | −28.3 ± 4.1 | −27.1 ± 5.0 | −24.4 ± 5.7 | 0.24 | <0.05 | <0.05 |
| T1 [ms] | 964.6 ± 40.3 | 991.6 ± 35.5 | 994.0 ± 43.2 | <0.05 | <0.05 | 0.74 |
LVEF: left ventricular ejection fraction; LVEDVi: left ventricular end diastolic volume index; IVSD: interventricular septal diameter; EFV: epicardial fat volume; PWV: aortic pulse wave velocity; LS: longitudinal strain; CS: circumferential strain; T1: T1-relaxation times.
4. Discussion
Atherosclerosis is the leading cause of death in the Western world [1]. However, there are possibilities to prevent CV events. Therefore it is important to assess individual CV risk early which is often performed by scoring systems [28], [29], [30]. Such calculated risk scores are not always accurate and sometimes may under- or overestimate individual CV risk [2], [3], [4]. Therefore the identification and exploration parameters related to CV risk and prognosis independent of the classical risk factors, and, which can easily be integrated into a routine imaging examination, is desirable. CMR is not widely used for the evaluation of parameter related to CV risk parameters and prognosis, though it has advantages. E.g., the 3D-Dixon technique measures, in contrast to echocardiography, the whole epicardial fat volume without ionizing radiation (as in computer tomography) [6], [24]. CMR can also determine PWV by flow measurements [20], [31] in particular aortic segments, whereas tonometric devices only measure a general arterial stiffness including different superficial locations such as between the carotid or brachial artery and the femoral artery. However, these vessels also involve muscular stiffening with a consecutively greater variance of PWV and also the real path length can only be estimated [5], [20], [32], [33]. The presence of myocardial fibrosis can be evaluated using T1 mapping techniques without the need for myocardial biopsy. LV strain analysis can detect subtle LV contractility disturbances even when LV ejection fraction may still be normal [16], [34], [35], [36], [37], [38], [39]. In the present study EFV was increased in patients with hypertension and even more increased in patients with hypertension and additional diabetes mellitus. PWV was increased and longitudinal and circumferential strain was worse in hypertensive patients with diabetes mellitus compared to hypertensive and non-hypertensive subjects, despite a preserved LV ejection fraction. Additionally signs of myocardial fibrosis were found in hypertensive patients (higher T1 values) compared to the non-hypertensive controls.
PWV is an important predictors of CV adverse events [5], [33]. A reduced aortic elasticity (higher aortic stiffness) leads to a higher aortic PWV and unfavorable effects on the heart and other organs. A pathologically increased PWV can eventually be detected before arterial hypertension is measured with conventional methods [5], [40]. Underlying mechanisms are an intima media hypertrophy, sclerosis and changes of the extracellular matrix with replacement of elastin by collagen. Metabolic and inflammatory mechanisms also play a role [5], [40], [41]. In hypertension an increased aortic stiffness occurs earlier in age as a result of structural changes due to aortic wall stretching and an accelerated development of atherosclerosis with consecutive arterial wall thickening [5], [33]. Diabetes mellitus may have an additive effect with dyslipidemia, disturbances in insulin sensitivity and endothelial function as possibly mechanisms, which may lead to vascular changes and vessel wall damage. An increased inflammatory burden may also play a role [42], [43]. A relationship between EFV and PWV has been described [44] and may be attributed to metabolic and inflammatory mechanisms [44], [45]. Normally, epicardial fat has beneficial effects through anti-atherogenic and anti-inflammatory adipokines [7], [46]. However, when it is pathologically increased it can unfavorably change because the hypertrophied epicardial fat becomes hypoxic and dysfunctional and also inflammatory factors invade it. Altogether such mechanisms contribute to vascular wall inflammation and atherogenesis [7], [46], [47]. Also an increased insulin resistance has been observed with an higher epicardial fat amounts and with increased visceral fat in general eventually contributing to the development of aortic stiffness [48], [49], [50], [51], [52], [53]. Local mechanisms may also play a role through the above explained, as epicardial fat has direct contact with the aorta as well as with the myocardium sharing the same blood supply [47], [54]. In concordance to previous studies hypertensive patients in general had higher T1-relaxation times than non-hypertensive controls - as a sign of myocardial fibrosis - and worse LV strain values with additional diabetes mellitus, despite a normal LV ejection fraction [16], [34], [35], [36], [38], [39]. Such changes occur physiologically as a result of aging, but are accelerated in the presence of CV risk factors such as hypertension or diabetes mellitus [23], [55] and may relate cardiac remodeling (e.g. increased mechanical stress, disturbed micro-vascular circulation, myocardial inflammation and oxidative stress). Eventually this may lead to an impaired regeneration of cardiomyocytes, to damaged myocardial fibers and to an increased interstitial collagen deposition. Also, the higher LV afterload and LV hypertrophy increases myocardial oxygen demand and impairs coronary artery perfusion which may worsen the situation and predisposing to myocardial dysfunction and fibrosis [35], [38], [56], [57], [58], [59]. The additional presence of a diabetes mellitus can augment these changes [19]. However, increased amounts of epicardial fat may also lead to myocardial fibrosis and contractility impairment through metabolically and inflammatory mechanisms such as cardiac steathosis and lipotoxicity [11], [14], [17].
To evaluate the above described parameters MR-sequences were acquired that can easily be integrated into the routine workflow of a CMR study and with a reasonable additional scan time. This is a clear advantage of CMR, because all measurements can be performed in a single examination, in contrast to other approaches which necessitate the application of different modalities [5], [6], [20]. CMR therefore should be used to support the assessment of CV risk and prognosis.
The low number of subjects examined in this study is a limitation. Therefore, the results cannot be generalized and further studies with higher numbers of patients in these different groups are needed. The observational and explorative study design did not allow for the determination of the exact causality or pathogenesis of the described findings. A further limitation is that hypertensive patients used anti-hypertensive medication. These are known to reduce PWV, which may explain the lack of difference between patients with only hypertension and non-hypertensive controls [5], [60]. Another limitation is that the patients of the study were somewhat obese, although the differences did not reach a statistical significance. However, as obesity may also independently induce a cardiomyopathie, this may have been of influence. Further studies in non-obese hypertensive and diabetic patients are warranted. Furthermore there is a lack of data concerning duration of hypertension or diabetes, medical treatment of hypertension and diabetes and the presence of other cardiovascular risk factors like hypercholesterolemia. This may have an impact over results interpretation.
5. Conclusion
CMR evaluation revealed an increased aortic stiffness and epicardial fat volume in hypertensive patients. Both parameters were even higher in the presence of an additional diabetes mellitus. Hypertensive patients also had signs of LV myocardial fibrosis and a reduced strain, in spite of a normal LV ejection fraction. CMR can determine these different parameters of CV risk and prognosis within a single examination, when normally different techniques are needed. It can therefore be used to support the assessment of CV risk and prognosis.
References
- 1.Yusuf S., Hawken S., Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P., LaBree L., Azen S.P. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 3.Hense H.W., Schulte H., Lowel H. Framingham risk function overestimates risk of coronary heart disease in men and women from Germany–results from the MONICA Augsburg and the PROCAM cohorts. Eur. Heart J. 2003;24:937–945. doi: 10.1016/s0195-668x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P., Smith S.C., Jr., Grundy S.M. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001;104:1863–1867. doi: 10.1161/hc4201.097189. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S., Cockcroft J., Van Bortel L. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 6.Dey D., Nakazato R., Li D. Epicardial and thoracic fat - Noninvasive measurement and clinical implications. Cardiovasc. Diagn. Ther. 2012;2:85–93. doi: 10.3978/j.issn.2223-3652.2012.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iacobellis G., Bianco A.C. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011;22:450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazurek T., Zhang L., Zalewski A. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 9.Iacobellis G., Pistilli D., Gucciardo M. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Tanami Y., Jinzaki M., Kishi S. Lack of association between epicardial fat volume and extent of coronary artery calcification, severity of coronary artery disease, or presence of myocardial perfusion abnormalities in a diverse, symptomatic patient population: results from the CORE320 multicenter study. Circ. Cardiovasc. Imaging. 2015;8:e002676. doi: 10.1161/CIRCIMAGING.114.002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyman K., Graner M., Pentikainen M.O. Cardiac steatosis and left ventricular function in men with metabolic syndrome. J. Cardiovasc. Magn. Reson. 2013;15:103. doi: 10.1186/1532-429X-15-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahabadi A.A., Berg M.H., Lehmann N. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 13.Mahabadi A.A., Lehmann N., Kalsch H. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovascular Imaging. 2014;7:909–916. doi: 10.1016/j.jcmg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Ngo D.T., Gokce N. Epicardial adipose tissue: a benign consequence of obesity? Circ. Cardiovasc. Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.115.003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homsi R., Sprinkart A.M., Gieseke J. 3D-Dixon cardiac magnetic resonance detects an increased epicardial fat volume in hypertensive men with myocardial infarction. Eur. J. Radiol. 2016 doi: 10.1016/j.ejrad.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Donekal S., Venkatesh B.A., Liu Y.C. Interstitial fibrosis, left ventricular remodeling, and myocardial mechanical behavior in a population-based multiethnic cohort: the Multi-Ethnic Study of Atherosclerosis (MESA) study. Circ. Cardiovasc. Imaging. 2014;7:292–302. doi: 10.1161/CIRCIMAGING.113.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crendal E., Dutheil F., Naughton G. Increased myocardial dysfunction, dyssynchrony, and epicardial fat across the lifespan in healthy males. BMC Cardiovasc. Disord. 2014;14:95. doi: 10.1186/1471-2261-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y.W., Su C.T., Sung J.M. Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction. Clin. J. Am. Soc. Nephrol. 2013;8:1564–1574. doi: 10.2215/CJN.10671012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitt B., Zannad F. The detection of myocardial fibrosis: an opportunity to reduce cardiovascular risk in patients with diabetes mellitus? Circ. Cardiovasc. Imaging. 2012;5:9–11. doi: 10.1161/CIRCIMAGING.111.971143. [DOI] [PubMed] [Google Scholar]
- 20.Wentland A.L., Grist T.M., Wieben O. Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc. Diagn. Ther. 2014;4:193–206. doi: 10.3978/j.issn.2223-3652.2014.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hor K.N., Gottliebson W.M., Carson C. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3:144–151. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Homsi R., Meier-Schroers M., Gieseke J. 3D-Dixon MRI based volumetry of peri- and epicardial fat. Int. J. Cardiovasc. Imaging. 2016;32:291–299. doi: 10.1007/s10554-015-0778-8. [DOI] [PubMed] [Google Scholar]
- 23.Liu C.Y., Liu Y.C., Wu C. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homsi R., Meier-Schroers M., Gieseke J. 3D-Dixon MRI based volumetry of peri- and epicardial fat. Int. J. Cardiovasc. Imaging. 2015 doi: 10.1007/s10554-015-0778-8. [DOI] [PubMed] [Google Scholar]
- 25.Messroghli D.R., Radjenovic A., Kozerke S. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn. Reson. Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 26.Dorniak K., Hellmann M., Rawicz-Zegrzda D. A novel tool for phase contrast MR-derived pulse wave velocity measurement - validation against applanation tonometry and phantom studies. J. Cardiovasc. Magn. Reson. 2015 Feb;17(Suppl 1):P40. [Google Scholar]
- 27.Cerqueira M.D., Weissman N.J., Dilsizian V. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int. J. Cardiovasc. Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 28.Jousilahti P., Laatikainen T., Peltonen M. Primary prevention and risk factor reduction in coronary heart disease mortality among working aged men and women in eastern Finland over 40 years: population based observational study. BMJ. 2016;352:i721. doi: 10.1136/bmj.i721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assmann G., Schulte H., Cullen P. Assessing risk of myocardial infarction and stroke: new data from the Prospective Cardiovascular Munster (PROCAM) study. Eur. J. Clin. Invest. 2007;37:925–932. doi: 10.1111/j.1365-2362.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- 30.Assmann G., Schulte H., Cullen P. New and classical risk factors–the Munster heart study (PROCAM) Eur. J. Med. Res. 1997;2:237–242. [PubMed] [Google Scholar]
- 31.Homsi R., Thomas D., Gieseke J. Epicardial fat volume and aortic stiffness in healthy individuals: a quantitative cardiac magnetic resonance study. Rofo. 2016;188:853–858. doi: 10.1055/s-0042-110098. [DOI] [PubMed] [Google Scholar]
- 32.Liu C.S., Li C.I., Shih C.M. Arterial stiffness measured as pulse wave velocity is highly correlated with coronary atherosclerosis in asymptomatic patients. J. Atheroscler. Thromb. 2011;18:652–658. doi: 10.5551/jat.7021. [DOI] [PubMed] [Google Scholar]
- 33.Baulmann J., Homsi R., Uen S. Pulse wave velocity is increased in patients with transient myocardial ischemia. J. Hypertens. 2006;24:2085–2090. doi: 10.1097/01.hjh.0000244959.92856.7e. [DOI] [PubMed] [Google Scholar]
- 34.Diez J., Lopez B., Gonzalez A. Clinical aspects of hypertensive myocardial fibrosis. Curr. Opin. Cardiol. 2001;16:328–335. doi: 10.1097/00001573-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Rosen B.D., Saad M.F., Shea S. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2006;47:1150–1158. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 36.Diez J., Querejeta R., Lopez B. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 37.Bull S., White S.K., Piechnik S.K. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi C.J., Wu C.O., Tee M. The association between cardiovascular risk and cardiovascular magnetic resonance measures of fibrosis: the Multi-Ethnic Study of Atherosclerosis (MESA) J. Cardiovasc. Magn. Reson. 2015;17:15. doi: 10.1186/s12968-015-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambale Venkatesh B., Volpe G.J., Donekal S. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the Multi-Ethnic Study of Atherosclerosis study. Hypertension. 2014;64:508–515. doi: 10.1161/HYPERTENSIONAHA.114.03697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Rourke M. Mechanical principles in arterial disease. Hypertension. 1995;26:2–9. doi: 10.1161/01.hyp.26.1.2. [DOI] [PubMed] [Google Scholar]
- 41.McEniery C.M., Yasmin Hall IR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J. Am. Coll. Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 42.Lyons T.J., Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl. Res.: J. Lab. Clin. Med,. 2012;159:303–312. doi: 10.1016/j.trsl.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saely C.H., Rein P., Vonbank A. Type 2 diabetes and the progression of visualized atherosclerosis to clinical cardiovascular events. Int. J. Cardiol. 2013;167:776–780. doi: 10.1016/j.ijcard.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 44.Kim B.J., Kim B.S., Kang J.H. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int. J. Cardiol. 2013;167:2234–2238. doi: 10.1016/j.ijcard.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Park H.E., Choi S.Y., Kim H.S. Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J. Atheroscler. Thromb. 2012;19:570–576. doi: 10.5551/jat.12484. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgibbons T.P., Czech M.P. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J. Am. Heart Assoc. 2014;3:e000582. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talman A.H., Psaltis P.J., Cameron J.D. Epicardial adipose tissue: far more than a fat depot. Cardiovasc. Diagn. Ther. 2014;4:416–429. doi: 10.3978/j.issn.2223-3652.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi K.M., Lee K.W., Seo J.A. Relationship between brachial-ankle pulse wave velocity and cardiovascular risk factors of the metabolic syndrome. Diabet. Res. Clin. Pract. 2004;66:57–61. doi: 10.1016/j.diabres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi N., Suzuki K., Tatara K. Clustered features of the metabolic syndrome and the risk for increased aortic pulse wave velocity in middle-aged Japanese men. Angiology. 2003;54:551–559. doi: 10.1177/000331970305400504. [DOI] [PubMed] [Google Scholar]
- 50.Wildman R.P., Mackey R.H., Bostom A. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42:468–473. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira I., Snijder M.B., Twisk J.W. Central fat mass versus peripheral fat and lean mass: opposite (adverse versus favorable) associations with arterial stiffness? The Amsterdam growth and health longitudinal study. J. Clin. Endocrinol. Metab. 2004;89:2632–2639. doi: 10.1210/jc.2003-031619. [DOI] [PubMed] [Google Scholar]
- 52.Begum N., Song Y., Rienzie J. Vascular smooth muscle cell growth and insulin regulation of mitogen-activated protein kinase in hypertension. Am. J. Physiol. 1998;275:C42–C49. doi: 10.1152/ajpcell.1998.275.1.C42. [DOI] [PubMed] [Google Scholar]
- 53.Ulrich P., Cerami A. Protein glycation, diabetes, and aging. Recent Prog. Horm. Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Rosito G.A., Massaro J.M., Hoffmann U. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 55.Anversa P., Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 56.Brilla C.G., Janicki J.S., Weber K.T. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ. Res. 1991;69:107–115. doi: 10.1161/01.res.69.1.107. [DOI] [PubMed] [Google Scholar]
- 57.Shimoni S., Gendelman G., Ayzenberg O. Differential effects of coronary artery stenosis on myocardial function: the value of myocardial strain analysis for the detection of coronary artery disease. J. Am. Soc. Echocardiogr. 2011;24:748–757. doi: 10.1016/j.echo.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Liang H.Y., Cauduro S., Pellikka P. Usefulness of two-dimensional speckle strain for evaluation of left ventricular diastolic deformation in patients with coronary artery disease. Am. J. Cardiol. 2006;98:1581–1586. doi: 10.1016/j.amjcard.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 59.Nucifora G., Schuijf J.D., Delgado V. Incremental value of subclinical left ventricular systolic dysfunction for the identification of patients with obstructive coronary artery disease. Am. Heart J. 2010;159:148–157. doi: 10.1016/j.ahj.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 60.Dudenbostel T., Glasser S.P. Effects of antihypertensive drugs on arterial stiffness. Cardiol. Rev. 2012;20:259–263. doi: 10.1097/CRD.0b013e31825d0a44. [DOI] [PMC free article] [PubMed] [Google Scholar]