Summary
Background
The estimated worldwide prevalence of non-alcoholic fatty liver disease (NAFLD) in adults is 25%; however, prevalence in young adults remains unclear. We aimed to identify the prevalence of steatosis and fibrosis in young adults in a sample of participants recruited through the Avon Longitudinal Study of Parents and Children (ALSPAC), based on transient elastography and controlled attenuation parameter (CAP) score.
Methods
In this population-based study, we invited active participants of the ALSPAC cohort to our Focus@24+ clinic at the University of Bristol (Bristol, UK) between June 5, 2015, and Oct 31, 2017, for assessment by transient elastography with FibroScan, to determine the prevalence of steatosis and fibrosis. FibroScan data were collected on histologically equivalent fibrosis stage (F0-F4) and steatosis grade (S0-S3); results with an IQR to median ratio of 30% or greater were excluded for median fibrosis results greater than 7·1 kPa, and CAP scores for steatosis were excluded if less than ten valid readings could be obtained. Results were collated with data on serology (including alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transferase) and exposures of interest: alcohol consumption (via the Alcohol Use Disorder Identification Test for Consumption [AUDIT-C] and the Diagnostic and Statistical Manual of Mental Disorders-5 criteria for alcohol use disorder), body-mass index (BMI), waist-to-height ratio, socioeconomic status (based on predefined ALSPAC markers), and sex. We used logistic regression models to calculate odds ratios (ORs) for the effect of exposures of interest on risk of steatosis and fibrosis, after dichotomising the prevalences of fibrosis and steatosis and adjusting for covariates (excessive alcohol intake [hazardous drinking, AUDIT-C score ≥5; or harmful drinking, evidence of alcohol use disorder], social class, smoking, and BMI).
Findings
10 018 active ALSPAC participants were invited to our Focus@24+ clinic, and 4021 attended (1507 men and 2514 women), with a mean age of 24·0 years (IQR 23·0–25·0). 3768 CAP scores were eligible for analysis. 780 (20·7% [95% CI 19·4–22·0]) participants had suspected steatosis (S1–S3; ≥248 dB/m), with 377 (10·0%) presenting with S3 (severe) steatosis (≥280 dB/m). A BMI in the overweight or obese range was positively associated with steatosis when adjusted for excessive alcohol consumption, social class, and smoking (overweight BMI: OR 5·17 [95% CI 4·11–6·50], p<0·0001; obese BMI: 27·27 [20·54–36·19], p<0·0001). 3600 participants had valid transient elastography results for fibrosis analysis. 96 participants (2·7% [95% CI 2·2–3·2]) had transient elastography values equivalent to suspected fibrosis (F2–F4; ≥7·9 kPa), nine of whom had values equivalent to F4 fibrosis (≥11·7 kPa). Individuals with alcohol use disorder and steatosis had an increased risk of fibrosis when adjusted for smoking and social class (4·02 [1·24–13·02]; p=0·02).
Interpretation
One in five young people had steatosis and one in 40 had fibrosis around the age of 24 years. The risk of fibrosis appears to be greatest in young adults who have harmful drinking patterns and steatosis. A holistic approach to the UK obesity epidemic and excessive drinking patterns is required to prevent an increasing health-care burden of adults with advanced liver disease in later life.
Funding
Medical Research Council UK, Alcohol Change UK, David Telling Charitable Trust.
Introduction
Non-alcoholic fatty liver disease (NAFLD) has an estimated worldwide prevalence in adults of approximately 25%.1 The disease is recognised as a spectrum of conditions, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), and ultimately cirrhosis. Worldwide, NASH prevalence in adults is estimated to be 6% in developed countries, with up to 40% of these individuals progressing to advanced fibrosis.1
This high prevalence has serious implications: almost a fifth of new hepatocellular carcinoma cases in developed countries are attributable to NAFLD.2 In the UK, NASH-related cirrhosis is among the most common indications for liver transplantation.3 An increased pool of steatotic donor livers has ramifications for recipients, with primary graft non-function and dysfunction increasingly associated with graft steatosis.4
The rise in the prevalence of NAFLD mirrors that of obesity and metabolic syndrome, and young adults with NAFLD represent the next major public health challenge for health services.5 Prospective cohort population studies in an adolescent setting (17–18 years) have estimated the prevalence of NAFLD to be between 2·5% and 12·8% in developed countries from liver ultrasound data.6, 7 Although liver biopsy is considered the gold standard for NAFLD assessment, it is unethical in large population studies, because of a risk of serious adverse events following the procedure.8
Research in context.
Evidence before this study
Non-alcoholic fatty liver disease (NAFLD) affects approximately a quarter of adults in developed countries, a substantial proportion of whom are at risk of developing liver failure and liver cancer, and an increased risk of death related to heart disease. One of the largest studies to analyse prevalence of NAFLD in young adults used data from the National Health and Nutrition Examination Survey. The estimated prevalence of suspected NAFLD in adults aged 18–35 years was 25%, under the criteria for NAFLD of an alanine aminotransferase concentration greater than 30 IU/L in men and greater than 19 IU/L in women, and a body-mass index greater than 25 kg/m2; however, these criteria are broad. To date, no study has used imaging to screen young adults and establish the true prevalence of NAFLD in this sparsely studied age group.
Added value of this study
This study is, to the best of our knowledge, the first to determine the prevalence of NAFLD in young adults with use of transient elastography, in an age group in which NAFLD burden is poorly characterised. The added value of our use of the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort is that, as the most phenotyped birth cohort worldwide, this group of young adults has already been assessed for NAFLD between the ages of 17 and 18 years, which acts as a comparator.
Implications of all the available evidence
We identified that around 20% of young adults in our cohort had steatosis of grade S1 or higher around the age of 24 years. One in 40 also had evidence of liver fibrosis, with participants at greatest risk of fibrosis being those with harmful drinking patterns and evidence of steatosis. Patients identified with steatosis and early fibrosis as young adults might present in earlier decades of life with advanced liver disease, placing increased strain on inpatient and transplant services. This outcome could have wide implications in the context of the national obesity epidemic in the UK. Increased public health interventions are required to tackle the obesity epidemic and excessive drinking in young adults, to attenuate their risk of NAFLD and alcohol-related liver disease, and therefore reduce the health-care burden of advanced liver disease.
To our knowledge, the largest study to analyse NAFLD prevalence in young adults used the US National Health and Nutrition Examination Survey. This study postulated a prevalence of 25% in people aged 18–35 years in the USA, under criteria for NAFLD of a body-mass index (BMI) greater than 25 kg/m2, and an alanine aminotransferase (ALT) concentration greater than 30 U/L in men and 19 U/L in women.9
Transient elastography, as a fast, simple, safe, and accurate technique, is considered the non-invasive standard for assessing liver fibrosis.10, 11, 12 To date, no population studies have looked into the prevalence of fibrosis and steatosis in young adults assessed by transient elastography.
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a general population, prospective birth cohort study of children born in the greater Bristol area of the UK, during 1991 and 1992. In this cohort, 1874 participants were assessed for NAFLD in their late teens (mean age 17·9 years), with a NAFLD prevalence of 2·5% identified by ultrasound.6 In the current study, we aimed to identify the prevalence of steatosis and fibrosis in young adults from the unselected, general population birth cohort of ALSPAC by use of transient elastography.
Methods
Study design and population
We did a population-based study of young adults recruited through ALSPAC. ALSPAC is a prospective birth cohort study based at the University of Bristol (Bristol, UK) in southwest England.13, 14 The study website contains details of all available data through a fully searchable data dictionary and variable search tool. Briefly, ALSPAC recruited 15 454 pregnant women with expected delivery dates between April 1, 1991, and Dec 31, 1992, in the greater Bristol area.15 Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the National Research Ethics Service Committee South West—Frenchay (14/SW/1173 ALSPAC Focus@24+). Consent for use of biological samples in the current study was collected from offspring participants in accordance with the Human Tissue Act (2004). Informed consent for use of data collected with questionnaires and clinics was obtained from the offspring participants following the recommendations of the ALSPAC Ethics and Law Committee.
We invited active offspring participants of ALSPAC13 by letter and email to our Focus@24+ clinic at the University of Bristol between June 5, 2015, and Oct 31, 2017, for assessment by transient elastography (FibroScan 502 Touch; Echosens, Paris, France), with measurement of controlled attenuation parameter (CAP), as standardised non-invasive measures for assessment of fibrosis and quantification of steatosis in NAFLD.10, 16 Study data were collected and managed with REDCap electronic data capture tools hosted at the University of Bristol.17
Assessment of outcomes
All participants attending the Focus@24+ clinic were asked to fast for a minimum of 6 h, or overnight, before blood tests and subsequent transient elastography. Our serology analyses included liver function tests for ALT, aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT). Lipid profiles were also taken, of cholesterol, triglycerides, LDL, VLDL, and HDL. Serum concentrations of glucose and insulin were also used to calculate homoeostasis model assessment for insulin resistance (HOMA-IR) scores with the equation (fasting insulin [μU/mL] × fasting glucose [mmol/L])/22·5.18, 19
After phlebotomy, transient elastography was offered to all participants by trained field workers in the clinic. Ten valid readings were required to derive a CAP score and fibrosis result. The M probe was used initially unless the machine indicated use of the XL probe. In line with manufacturer instructions, cut-off values used for the M probe and XL probe were the same.20
Cut-off values for CAP score for different grades of steatosis (S0–S3) were derived from a meta-analysis on CAP technology: S0 was defined as a score of less than 248 dB/m (<10% steatosis); S1 as a score of 248 to less than 268 dB/m (10%–<33% steatosis [mild]); S2 as a score of 268 to less than 280 dB/m (33%–<66% steatosis [moderate]); and S3 as a score of 280 dB/m or more (≥66% steatosis [severe]).16 CAP scores of 248 dB/m or greater (≥S1) were considered as suspected steatosis. Participants' CAP scores were considered eligible for analysis if ten valid readings (100–400 dB/m) could be obtained.16
In the context that NAFLD is more common than alcohol-related liver disease in adolescents, and that NAFLD is present in more than 25% of the global adult population,1 when analysing the cohort as a whole, transient elastography cut-off values for NAFLD related to the METAVIR scoring system10 were used for fibrosis staging (F0–F4: F0–F1, <7·9 kPa; F2, 7·9 to <8·8 kPa; F3, 8·8 to <11·7 kPa; and F4, ≥11·7 kPa).10, 21 Participants with a transient elastography value of 7·9 kPa or greater (≥F2) were considered to have suspected fibrosis. If a participant's median fibrosis result was greater than 7·1 kPa, the IQR to median ratio had to be less than 30% to be considered valid.22
Assessment of exposures
Participants' alcohol consumption was assessed with questionnaires at private computer terminals. Questions were based on the Alcohol Use Disorder Identification Test for Consumption (AUDIT-C), and the Diagnostic and Statistical Manual of Mental Disorders-5 criteria for alcohol use disorder,23 from which we derived an AUDIT-C score and determined the presence of alcohol use disorder. Participants were then stratified according to a three-category ordinal variable, separating low-risk drinkers (AUDIT-C score <5 and absence of alcohol use disorder), hazardous drinkers (AUDIT-C score of ≥5 and absence of alcohol use disorder), and harmful drinkers (evidence of alcohol use disorder).
We calculated BMI and waist-to-height ratio as surrogate markers of adiposity.24 Participants with metabolic syndrome were identified according to the diagnostic criteria of the Adult Treatment Panel III/American Heart Institute/National Heart, Lung, and Blood Institute (ATPIII/AHA/NHLBI) scoring system, which involves elevated waist circumference, elevated triglycerides, low HDL, elevated blood pressure, and elevated fasting glucose.25 Participants were recorded as smokers if they had smoked in the 30 days before the clinic visit, including those who had used e-cigarettes. At the time mothers were recruited, the ALSPAC used three markers of socioeconomic status: parental social class, maternal education level achieved, and parental income.26, 27 All three variables were interrogated as exposures to identify whether social position was associated with our outcome measures.
We requested participants to specify all current medications in writing on attendance to the clinic, along with indication for treatment, and self-nominated text responses were logged.
Statistical analysis
Univariable linear regression models were used to examine differences across the ordinal categories of steatosis grade and fibrosis stage. Exposures of interest were BMI, waist-to-height ratio, alcohol consumption, socioeconomic status, and sex. When analysing fibrosis prevalence, we interrogated the exposure of steatosis, and combined harmful alcohol consumption (ie, alcohol use disorder) and steatosis. Likelihood ratio tests were used to assess the adequacy of a model in which change was restricted to be linear (ie, involving a single exposure and outcome of interest). Multivariable logistic regression was done after dichotomising the prevalences of fibrosis and steatosis, following which odds ratios (ORs) were calculated for exposures previously shown to be associated with the development of steatosis (BMI, waist-to-height ratio, alcohol consumption, maternal education [as a surrogate marker of socioeconomic status], and sex1, 5) and fibrosis (alcohol consumption, steatosis, and sex3, 21), while adjusting for covariates (excessive alcohol intake [hazardous and harmful drinking], social class, smoking, and BMI). Statistical analysis was done with Stata/MP (version 15.1). In line with the ALSPAC confidentiality policy, any analysed groups with less than five participants are expressed as n<5. This number can include zero.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
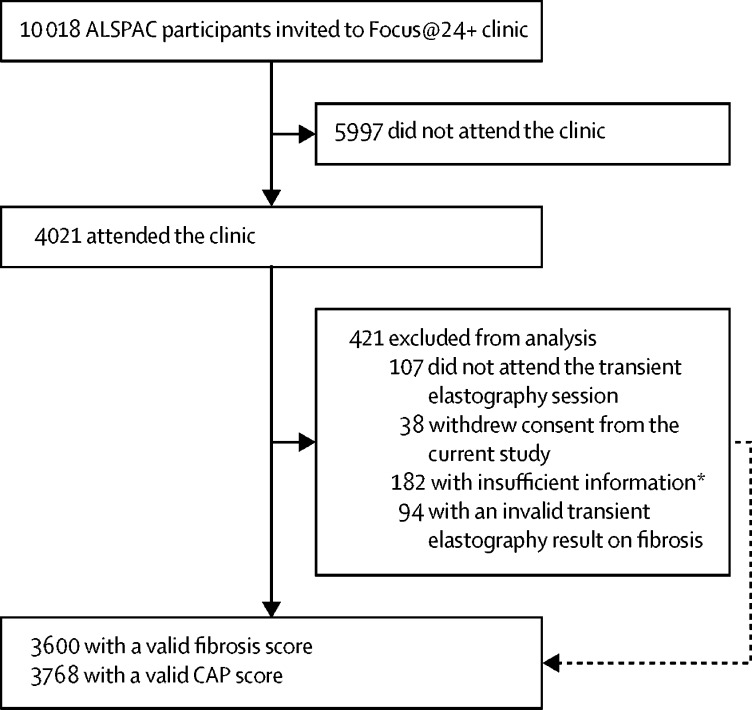
5436 original offspring participants of the ALSPAC have been lost to study attrition over the past three decades and did not have sufficient auxiliary information from which a response could be imputed.13 10 018 active ALSPAC participants were invited to our Focus@24+ clinic. Of those who were invited, 4021 (40·1%) attended (figure 1). Characteristics of participants who attended the clinic are shown in table 1. This population had a mean age of 24·0 years (IQR 23·0–25·0), with a range of 22–26 years, and comprised 1507 men and 2514 women. In total, 421 (10·5%) participants were excluded. Reasons for exclusion were not accepting the transient elastography session (n=107), withdrawal of consent from the current study (n=38), insufficient information for fibrosis or CAP measurement (n=182), or a transient elastography result with an IQR to median ratio of 30% or greater (n=94; figure 1).
Figure 1.
Participant flow chart
*182 with insufficient data for fibrosis measurement and 108 with insufficient data for CAP measurement; reasons included not achieving ten valid scan measurements or missing data.
Table 1.
Characteristics of ALSPAC participants who attended the Focus@24+ clinic
| All clinic attendees (n=4021) | ||
|---|---|---|
| Sex | ||
| Male | 1507 (37·5%) | |
| Female | 2514 (62·5%) | |
| Age, years | 24·0 (23·0–25·0) | |
| Parental social class*† | ||
| IV–V (partly skilled and unskilled occupation) | 111 (3·2%) | |
| III (non-manual and manual occupation) | 1100 (31·7%) | |
| II (managerial and technical occupation) | 1587 (45·7%) | |
| I (professional occupation) | 673 (19·4%) | |
| Parental income*† | ||
| Lowest 20% | 428 (13·0%) | |
| Second quintile | 571 (17·3%) | |
| Third quintile | 663 (20·1%) | |
| Fourth quintile | 769 (23·3%) | |
| Highest 20% | 869 (26·3%) | |
| Maternal education (highest level achieved)† | ||
| Lower than O-levels | 615 (17·0%) | |
| O-levels | 1223 (33·9%) | |
| Higher education | 1775 (49·1%) | |
| Smoker† | ||
| Yes | 1170 (30·2%) | |
| No | 2709 (69·8%) | |
| Alcohol consumption | ||
| Normal (AUDIT-C score <5) | 1813 (45·1%) | |
| Hazardous (AUDIT-C score ≥5) | 1697 (42·2%) | |
| Harmful (alcohol use disorder) | 511 (12·7%) | |
| BMI† | ||
| Underweight (<18·5 kg/m2) | 119 (3·0%) | |
| Normal weight (18·5 to <25 kg/m2) | 2323 (58·4%) | |
| Overweight (25 to <30 kg/m2) | 1002 (25·2%) | |
| Obese (≥30 kg/m2) | 533 (13·4%) | |
| Waist-to-height ratio† | ||
| Normal adiposity (<0·5) | 2856 (72·0%) | |
| Increased adiposity (≥0·5) | 1108 (28·0%) | |
Data are n (% of available data), median (IQR), or mean (SD). ALSPAC=Avon Longitudinal Study of Parents and Children. AUDIT-C=Alcohol Use Disorder Identification Test-C. BMI=body-mass index.
Missing data: parental social class, n=550; parental income, n=721; maternal education, n=408; smoking status, n=142; BMI, n=44; waist-to-height ratio, n=57.
In our check of current medications, no participants reported having viral hepatitis or to be taking nucleos(t)ide analogues or direct-acting antivirals. Autoimmune hepatitis requiring azathioprine, and overlap syndrome with autoimmune hepatitis and primary sclerosing cholangitis requiring prednisolone, mycophenolate mofetil, or ursodeoxycholic acid were reported in less than five participants each, but these individuals were not excluded on the basis of this being a general population study. 23 participants were taking insulin for type 1 diabetes. No participants were known to have type 2 diabetes.
Table 2 shows participant characteristics in relation to steatosis grade: 3768 had valid CAP scores (range 100–400 dB/m; mean 209·2 dB/m [SD 53·1]; 2341 women and 1427 men). 780 (20·7% [95% CI 19·4–22·0]) of the 3768 participants had S1 or greater steatosis; 377 (48·3%) of this group had S3 (severe) steatosis.
Table 2.
Serology and exposure factors according to steatosis grade (S0–S3)* in analysed participants (n=3768)
| S0 (n=2988; 79·3%) | S1 (n=281; 7·5%) | S2 (n=122; 3·2%) | S3 (n=377; 10·0%) | p value | ||
|---|---|---|---|---|---|---|
| Sex | .. | .. | .. | .. | <0·0001† | |
| Male (n=1427) | 1054 (73·8%) | 128 (9·0%) | 54 (3·8%) | 191 (13·4%) | .. | |
| Female (n=2341) | 1934 (82·6%) | 153 (6·5%) | 68 (2·9%) | 186 (7·9%) | .. | |
| Parental social class‡ | .. | .. | .. | .. | 0·75† | |
| IV–V (partly skilled and unskilled occupation; n=103) | 80 (77·7%) | 10 (9·7%) | 5 (4·9%) | 8 (7·8%) | .. | |
| III (non-manual and manual occupation; n=1020) | 789 (77·4%) | 77 (7·5%) | 37 (3·6%) | 117 (11·5%) | .. | |
| II (managerial and technical occupation; n=1492) | 1192 (79·9%) | 110 (7·4%) | 40 (2·7%) | 150 (10·1%) | .. | |
| I (professional occupation; n=641) | 524 (81·7%) | 49 (7·6%) | 22 (3·4%) | 46 (7·2%) | .. | |
| Smoker§ | .. | .. | .. | .. | 0·71† | |
| No (n=2528) | 2008 (79·4%) | 188 (7·4%) | 77 (3·0%) | 255 (10·1%) | .. | |
| Yes (n=1104) | 875 (79·3%) | 84 (7·6%) | 40 (3·6%) | 105 (9·5%) | .. | |
| Alcohol consumption | .. | .. | .. | .. | 0·26† | |
| Low risk (AUDIT-C score <5; n=1693) | 1317 (77·8%) | 130 (7·7%) | 66 (3·9%) | 180 (10·6%) | .. | |
| Hazardous (AUDIT-C score ≥5; n=1586) | 1281 (80·8%) | 110 (6·9%) | 44 (2·8%) | 151 (9·5%) | .. | |
| Harmful (alcohol use disorder; n=489) | 390 (79·8%) | 41 (8·4%) | 12 (2·5%) | 46 (9·4%) | .. | |
| BMI, kg/m2 | 22·9 (21·0–25·3) | 26·6 (24·1–29·9) | 27·7 (25·1–30·7) | 31·0 (27·2–35·2) | <0·0001¶ | |
| Obese participants (BMI ≥30 kg/m2) | 162 (5·4%) | 67 (24·0%) | 37 (30·3%) | 212 (56·2%) | <0·0001¶ | |
| Waist-to-height ratio | 0·45 (0·42–0·48) | 0·50 (0·47–0·55) | 0·51 (0·48–0·56) | 0·57 (0·52–0·63) | <0·0001¶ | |
| Homoeostasis model assessment for insulin resistance score (reference range <1·68) | 1·60 (1·13–2·23) | 2·31 (1·56–3·78) | 2·41 (1·54–3·88) | 3·60 (2·24–5·38) | <0·0001¶ | |
| Alanine aminotransferase, IU/L (reference range 10–35 IU/L) | 19·4 (14·8–26·8) | 21·4 (16·1–30·8) | 22·4 (17·4–34·8) | 30·5 (20·5–51·9) | <0·0001¶ | |
| Aspartate aminotransferase, IU/L (reference range 10–35 IU/L) | 23·9 (20·4–28·9) | 24·0 (20·1–28·2) | 24·9 (20·9–29·1) | 27·5 (22·7–35·7) | <0·0001¶ | |
| γ-Glutamyl transferase, IU/L (reference range <40 IU/L) | 15·0 (12·0–20·0) | 18·0 (13·0–26·0) | 18 (14·0–26·0) | 23·0 (16·0–38·0) | <0·0001¶ | |
| Cholesterol, mmol/L (reference range <5·2 mmol/L) | 4·4 (0·8) | 4·5 (0·8) | 4·6 (0·8) | 4·7 (0·9) | <0·0001¶ | |
| Triglycerides, mmol/L (reference range <1·7 mmol/L) | 0·8 (0·6–1·1) | 0·9 (0·6–1·2) | 1·1 (0·8–1·5) | 1·2 (0·8–1·8) | <0·0001¶ | |
| LDL, mmol/L | 2·4 (0·7) | 2·6 (0·7) | 2·7 (0·7) | 2·8 (0·9) | <0·0001¶ | |
| VLDL, mmol/L | 0·4 (0·3–0·5) | 0·4 (0·3–0·6) | 0·5 (0·4–0·7) | 0·5 (0·4–0·8) | <0·0001¶ | |
| HDL, mmol/L (reference range >1·45 mmol/L) | 1·6 (0·4) | 1·4 (0·4) | 1·3 (0·3) | 1·2 (0·3) | <0·0001¶ | |
| Metabolic syndrome (≥3 of 5 criteria‖; n=172) | 48 (27·9%) | 23 (13·4%) | 15 (8·7%) | 86 (50·0%) | <0·0001† | |
Data are n (% of available data), mean (SD; normally distributed variables), or median (IQR; non-normally distributed variables); numbers reflect available data and therefore do not always reflect the total population (data missing on 512 participants across all categories). AUDIT-C=Alcohol Use Disorder Identification Test-C. BMI=body-mass index.
Grading based on controlled attenuation parameter cut-off values in decibels per metre (dB/m): S0, <248 dB/m; S1, 248–<268 dB/m; S2, 268–<280 dB/m; and S3, ≥280 dB/m.16
Pearson's χ2 test.
Classes defined as in the ALSPAC cohort;13, 14 results on other socioeconomic markers not displayed because of a high proportion of missing data.
Defined as participants who had smoked ≤30 days before the clinic visit (including e-cigarette use).
Likelihood ratio test following univariable regression.
Metabolic syndrome criteria: (1) male waist circumference ≥102 cm or female waist circumference ≥88 cm; (2) triglyceride concent ration ≥1·7mmol/L; (3) HDL in men <1·93 mmol/L or in women <1·3mmol/L; (4) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg; and (5) fasting glucose ≥5·6 mmol/L.25
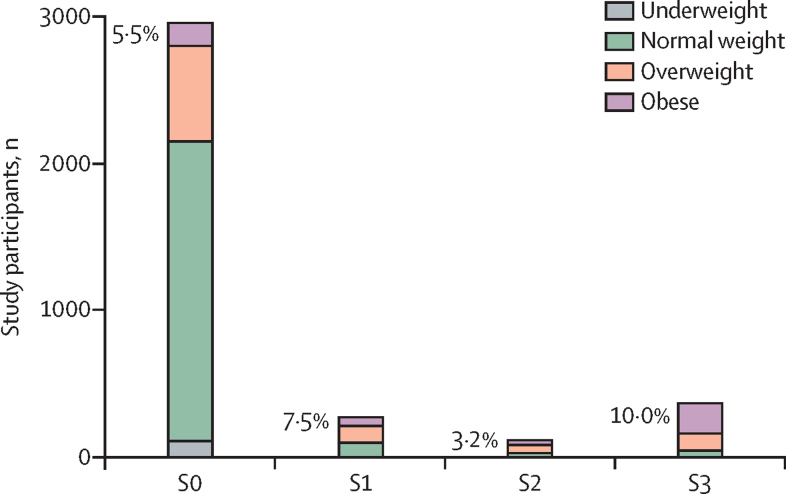
BMI was positively associated with increasing steatosis grade (p<0·0001), with the median BMI being in the overweight range (25 to <30 kg/m2) for S1 and S2 and the obese range (≥30 kg/m2) for S3 (table 2). As steatosis grade increased, the proportion of participants with obesity increased to 56·2% in participants with S3 grade steatosis (p<0·0001; table 2 and figure 2). Overall, a greater porportion of women had obesity compared with men (372 [15·0%] of 2479 vs 161 [10·7%] of 1498 with available data; not shown). Waist-to-height ratio was also positively associated with increasing steatosis grade (p<0·0001; table 2). Overall, a slightly greater proportion of men had increased adiposity (waist-to-height ratio ≥0·5) compared with women (428 [28·6%] of 1495 vs 680 [27·5%] of 2469 with available data; not shown). ALT, AST, and GGT were positively associated with increasing steatosis grade (all p<0·0001; table 2). Of participants with steatosis, 371 (60·2%) of 616 with available ALT data had an ALT concentration of less than 30 IU/L. In participants with valid data, 339 (59·9%) of 566 had steatosis without fibrosis and ALT less than 30 IU/L.
Figure 2.
Distribution of BMI categories across steatosis grade
Steatosis grade was derived from measurement of controlled attenuation parameter. Percentages represent obese participants at each grade.
HOMA-IR also increased with rising steatosis grade (p<0·0001; table 2). Similarly, cholesterol, triglyceride, LDL, and VLDL were positively associated with steatosis (all p<0·0001), whereas HDL showed a negative association (p<0·0001). Metabolic syndrome was positively associated with steatosis grade (p<0·0001), with 86 (50·0%) of 172 participants with metabolic syndrome having S3 steatosis.
No association was found between steatosis grade and parental social class (table 2), with similar findings for other markers of socioeconomic status (parental income and maternal education; not shown). Additionally, smoking and alcohol consumption had no association with steatosis grade (table 2).
On regression analyses, participants with a BMI in the obese range had a five times greater risk of steatosis than those with a BMI in the overweight range, following adjustment for excessive alcohol intake (ie, hazardous and harmful consumption), smoking, and social class (table 3). An increased risk of steatosis was also seen for adiposity with the surrogate marker of waist-to-height ratio, adjusted for the same covariates (table 3). Women had proportionally less steatosis than men, after adjusting for BMI, excessive alcohol intake, social class, and smoking (table 3). Maternal attainment of higher education appeared to be associated with a reduced risk of steatosis on unadjusted analysis, but after adjustment for BMI, excessive alcohol intake, and smoking, this relationship was lost (table 3).
Table 3.
Exposures associated with the presence of steatosis in analysed participants (n=3768)
| Participants with steatosis | Unadjusted OR (95% CI) | p value | Adjusted for | Adjusted OR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| BMI | ||||||
| Normal | 171/2206 (7·8%) | Reference | .. | Excessive alcohol intake†, social class, smoking | Reference | .. |
| Overweight | 285/936 (30·4%) | 5·34 (4·33–6·60) | <0·0001 | .. | 5·17 (4·11–6·50) | <0·0001 |
| Obese | 316/478 (66·1%) | 23·8 (18·61–30·47) | <0·0001 | .. | 27·27 (20·54–36·19) | <0·0001 |
| Waist-to-height ratio* | ||||||
| Normal adiposity (<0·5) | 254/2718 (9·3%) | Reference | .. | Excessive alcohol intake†, social class, smoking | Reference | |
| Increased adiposity (≥0·5) | 517/1007 (51·3%) | 10·24 (8·56–12·24) | <0·0001 | .. | 10·73 (8·79–13·10) | <0·0001 |
| Alcohol consumption† | ||||||
| Low risk | 376/1693 (22·2%) | Reference | .. | Social class, smoking | Reference | .. |
| Hazardous (AUDIT-C score ≥5) | 305/1586 (19·2%) | 0·83 (0·70–0·99) | 0·04 | .. | 0·87 (0·71–1·05) | 0·14 |
| Harmful (alcohol use disorder) | 99/489 (20·2%) | 0·89 (0·69–1·14) | 0·35 | .. | 0·92 (0·69–1·22) | 0·57 |
| Maternal education | ||||||
| Primary level | 149/573 (26·0%) | Reference | .. | BMI, excessive alcohol intake†, smoking | Reference | .. |
| Secondary level | 257/1147 (22·4%) | 0·82 (0·65–1·04) | 0·10 | .. | 1·03 (0·77–1·37) | 0·85 |
| Higher education | 298/1673 (17·8%) | 0·62 (0·49–0·77) | <0·0001 | .. | 1·06 (0·81–1·40) | 0·66 |
| Sex | ||||||
| Male | 373/1427 (26·1%) | Reference | .. | BMI, excessive alcohol intake†, social class, smoking | Reference | .. |
| Female | 407/2341 (17·4%) | 0·59 (0·51–0·70) | <0·0001 | .. | 0·46 (0·37–0·57) | <0·0001 |
OR=odds ratio. BMI=body-mass index. AUDIT-C=Alcohol Use Disorder Identification Test-C.
Threshold for normal and increased adiposity as defined previously.24
According to AUDIT-C score and the Diagnostic and Statistical Manual of Mental Disorders-5 criteria for alcohol use disorder;23 excessive intake defined as hazardous or harmful consumption.
On evaluation of alcohol-related steatosis, 489 (13·0%) of 3768 participants showed evidence of harmful alcohol consumption (ie, alcohol use disorder), 99 (20·2%) of whom had steatosis. On unadjusted analysis, no evidence was found of an increased risk of steatosis with harmful alcohol intake (table 3).
3600 participants had valid transient elastography results (range 1·3–44·5 kPa; mean 4·7 kPa [SD 1·5]; table 4). 96 (2·7% [95% CI 2·2–3·2]) of the participants had transient elastography values equivalent to METAVIR F2–F4 fibrosis (42 with F2 fibrosis [≥7·9 kPa], 45 with F3 fibrosis [≥8·8 kPa], and nine with F4 fibrosis [≥11·7 kPa]). CAP score was positively associated with increasing fibrosis stage (p<0·0001; table 4), with the nine participants with suspected F4 fibrosis having a mean CAP score of 283·4 dB/m, equating to S3 steatosis. Similar positive associations with fibrosis stage were seen with BMI (p<0·0001) and waist-to-height ratio (p=0·009); although the median BMI in the suspected F4 group was only borderline overweight (table 4). No association was found between metabolic syndrome and fibrosis stage in our cohort (p=0·20).
Table 4.
Serology and exposure factors according to fibrosis stage (F0–F4)* in analysed participants (n=3600)
| F0–F1 (n=3504; 97·3%) | F2 (n=42; 1·2%) | F3 (n=45; 1·3%) | F4 (n=9; 0·3%) | p value | ||
|---|---|---|---|---|---|---|
| Sex | .. | .. | .. | .. | 0·002† | |
| Male | 1337 | 27 | 22 | <5 | .. | |
| Female | 2167 | 15 | 23 | <5 | .. | |
| Parental social class‡ | .. | .. | .. | .. | 0·22† | |
| IV-V (partly and unskilled occupation) | 99 | <5 | <5 | <5 | .. | |
| III (non-manual and manual occupation) | 961 | 9 | 9 | <5 | .. | |
| II (managerial and technical occupation) | 1401 | 16 | 19 | <5 | .. | |
| I (professional occupation) | 613 | <5 | 9 | <5 | .. | |
| Smoker§ | .. | .. | .. | .. | 0·12† | |
| No | 2359 | 30 | 25 | 9 | .. | |
| Yes | 1018 | 10 | 16 | <5 | .. | |
| Alcohol consumption | .. | .. | .. | .. | 0·092† | |
| Low risk (AUDIT-C score <5) | 1573 | 17 | 16 | <5 | .. | |
| Hazardous (AUDIT-C score ≥5) | 1481 | 13 | 22 | <5 | .. | |
| Harmful (alcohol use disorder) | 450 | 12 | 7 | <5 | .. | |
| Controlled attenuation parameter score (dB/m) | 208·5 (52·2) | 219·8 (57·2) | 221·1 (57·5) | 283·4 (96·1) | <0·0001¶ | |
| BMI, kg/m2 | 23·6 (21·5–26·7) | 23·6 (21·9–26·5) | 25·3 (22·2–27·9) | 25·5 (22·9–40·9) | <0·0001¶ | |
| Obese participants (BMI ≥30 kg/m2) | 407 | 5 | 9 | <5 | <0·0001 | |
| Waist-to-height ratio | 0·46 (0·43–0·50) | 0·45 (0·41–0·50) | 0·46 (0·43–0·51) | 0·48 (0·44–0·69) | 0·009¶ | |
| Homoeostasis model assessment for insulin resistance score (reference range <1·68) | 1·7 (1·2–2·5) | 1·6 (1·1–2·5) | 1·7 (1·3–3·1) | 1·3 (0·7–5·9) | 0·16¶ | |
| Alanine aminotransferase, IU/L (reference range 10–35 IU/L) | 20·4 (15·3–29·0) | 21·3 (17·7–35·6) | 27·6 (17·9–42·2) | 33·1 (29·2–45·3) | <0·0001¶ | |
| Aspartate aminotransferase, IU/L (reference range 10–35 IU/L) | 24·1 (20·6–29·1) | 26·2 (21·2–30·5) | 26·2 (21·5–36·4) | 37·7 (27·4–43·4) | <0·0001¶ | |
| γ-Glutamyl transferase, IU/L (reference range <40 IU/L) | 16·0 (12·0–22·0) | 22·0 (17·0–32·0) | 19·0 (15·0–31·0) | 24·5 (20·0–40·0) | <0·0001¶ | |
| Cholesterol, mmol/L (reference range <5·2 mmol/L) | 4·4 (0·8) | 4·4 (1·0) | 4·3 (1·1) | 4·6 (0·8) | 0·47¶ | |
| Triglycerides, mmol/L (reference range <1·7 mmol/L) | 0·8 (0·6–1·1) | 0·9 (0·6–1·1) | 0·8 (0·7–1·0) | 0·7 (0·6–1·3) | 0·36¶ | |
| LDL, mmol/L | 2·4 (0·8) | 2·4 (1·1) | 2·3 (0·9) | 2·8 (0·9) | 0·51¶ | |
| VLDL, mmol/L | 0·4 (0·3–0·5) | 0·4 (0·3–0·5) | 0·4 (0·3–0·4) | 0·3 (0·3–0·6) | 0·25¶ | |
| HDL, mmol/L (reference range >1·45 mmol/L) | 1·6 (0·4) | 1·5 (0·5) | 1·6 (0·4) | 1·4 (0·4) | 0·94¶ | |
| Metabolic syndrome (≥3 of 5 criteria‖; n=162) | 153 | <5 | <5 | <5 | 0·20† | |
Data are n, mean (SD; normally distributed variables), or median (IQR; non-normally distributed variables); numbers reflect available data and therefore do not always reflect the total population (data missing on 526 participants across all categories). Any analysed groups with less than five participants are expressed as n<5 in line with the ALSPAC confidentiality policy (percentages not provided for the same reason). AUDIT-C=Alcohol Use Disorder Identification Test-C. BMI=body-mass index.
Staging based on transient elastography cut-off values: F0–F1, <7·9 kPa; F2, 7·9 to <8·8 kPa; F3, 8·8 to <11·7 kPa; and F4, ≥11·7 kPa.
Pearson's χ2 test.
Classes defined as in the ALSPAC cohort;13, 14 results on other socioeconomic markers not displayed because of a high proportion of missing data.
Defined as participants who had smoked ≤30 days before the clinic visit (including e-cigarette use).
Likelihood ratio test following univariable regression.
Metabolic syndrome criteria: (1) male waist circumference ≥102 cm or female waist circumference ≥88 cm; (2) triglyceride concentration ≥1·7mmol/L; (3) HDL in men <1·93 mmol or in women <1·3mmol/L; (4) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg; and (5) fasting glucose ≥5·6 mmol/L.25
On multivariable regression, female sex was associated with a significant reduction in the risk of fibrosis, after adjusting for BMI, excessive alcohol intake, smoking, and social class (table 5). Harmful alcohol consumption had some association with increased risk of fibrosis when adjusted for CAP, BMI, smoking, and social class (table 5). After adjusting for excessive alcohol intake, BMI, smoking, and social class, no association was found between increasing steatosis grade and fibrosis (table 5). No interaction was identified between steatosis and alcohol in the context of fibrosis (interaction parameter 1·70 [95% CI 0·56–5·16]; p=0·35). However, concurrent alcohol use disorder and steatosis increased the risk of fibrosis (after adjustment for smoking and social class), compared with steatosis or alcohol use disorder alone (table 5).
Table 5.
Exposures associated with the presence of fibrosis in analysed participants (n=3600)
| Participants with fibrosis | Unadjusted OR (95% CI) | p value | Adjusted for | Adjusted OR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Alcohol consumption* | ||||||
| Low risk | 37/1610 (2·3%) | Reference | .. | CAP, BMI, smoking, social class | Reference | .. |
| Hazardous | 39/1520 (2·6%) | 1·12 (0·71–1·77) | 0·63 | .. | 1·38 (0·79–2·41) | 0·26 |
| Harmful (alcohol use disorder) | 20/470 (4·3%) | 1·89 (1·09–3·29) | 0·02 | .. | 2·11 (1·02–4·37) | 0·04 |
| Steatosis | ||||||
| S0 | 69/2795 (2·5%) | Reference | .. | Excessive alcohol intake*, BMI, smoking, social class | Reference | .. |
| S1 | 6/264 (2·3%) | 0·92 (0·40–2·14) | 0·84 | .. | 0·60 (0·21–1·73) | 0·34 |
| S2 | 3/109 (2·8%) | 1·12 (0·35–3·61) | 0·85 | .. | 1·19 (0·35–4·01) | 0·78 |
| S3 | 16/343 (4·7%) | 1·93 (1·11–3·37) | 0·02 | .. | 0·83 (0·35–1·96) | 0·67 |
| Alcohol use disorder and steatosis | ||||||
| No alcohol use disorder or steatosis | 27/1231 (2·2%) | Reference | .. | Smoking, social class | Reference | 0·13 |
| Steatosis, no alcohol use disorder | 10/346 (2·9%) | 1·33 (0·64–2·77) | 0·45 | .. | 1·56 (0·63–3·84) | 0·33 |
| Alcohol use disorder, no steatosis | 13/368 (3·5%) | 1·63 (0·83–3·20) | 0·15 | .. | 2·05 (0·86–4·91) | 0·11 |
| Alcohol use disorder and steatosis | 7/94 (7·4%) | 3·59 (1·52–8·47) | 0·004 | .. | 4·02 (1·24–13·02) | 0·02 |
| Sex | ||||||
| Male | 54/1391 (3·9%) | Reference | .. | BMI, excessive alcohol intake*, smoking, social class | Reference | .. |
| Female | 42/2209 (1·9%) | 0·48 (0·32–0·72) | <0·0001 | .. | 0·51 (0·31–0·84) | 0·008 |
OR=odds ratio. CAP=controlled attenuation parameter.
According to AUDIT-C score and the Diagnostic and Statistical Manual of Mental Disorders-5 criteria for alcohol use disorder;23 excessive intake defined as hazardous or harmful consumption.
ALT, AST, and GGT appeared to be positively associated with increasing fibrosis stage (all p<0·0001; table 4). However, in our multivariable regression models with ALT, AST, and GGT as the dependent variables, and fibrosis stage as the independent variable, with adjustment for CAP score, no such relationships were identified (ALT, p=0·16; AST, p=0·27; and GGT, p=0·46). Thus, the changes in liver function tests with increasing fibrosis stage on univariable regression were probably dependent on CAP in the context of suspected NAFLD. No associations were found between fibrosis stage and lipid profile, HOMA-IR, smoking status, or social class (table 4).
94 participants had transient elastography values with an elevated IQR to median ratio (≥30%). These participants had higher CAP scores than the group with IQR to median ratios within the valid range, and higher BMI and adiposity (appendix).
Discussion
In our general population cohort sampled from the ALSPAC cohort, around one in five young adults had steatosis, and one in 40 had liver fibrosis. Our estimate of NAFLD is lower than that in the previous largest attempt to analyse prevalence in young adults from National Health and Nutrition Examination Survey data,9 which found a prevalence of 25% in people aged 18–35 years, albeit with a different assessment modality. In our study, BMI and adiposity were independent predictors of steatosis despite adjusting for excessive alcohol consumption. To the best of our knowledge, the prevalence of steatosis detected with CAP measurement in our birth cohort is one of the first attempts to assess prevalence of NAFLD in young adults in the UK.
Although 96 participants were identified with suspected F2–F4 fibrosis, these cases cannot be solely attributed to NAFLD. No associations were found between steatosis and metabolic syndrome, harmful alcohol use (ie, alcohol use disorder), or fibrosis; however, participants with steatosis and alcohol use disorder had a four times greater risk of developing fibrosis, when adjusted for confounders. These patients could represent participants with both alcohol-related and non-alcoholic fatty liver disease.
In a similar general population study by Petta and colleagues in Palermo, Italy,28 890 adults (mean age 53 years) were assessed for NAFLD with transient elastography and CAP, with similar cut-off values for fibrosis stage and steatosis grade. They identified 428 (48·1%) of their population to have NAFLD, 27·4% of whom had type 2 diabetes or impaired fasting glucose. Of the 890 participants, 28 (3·1%) had NAFLD and evidence of advanced fibrosis (≥F3). By comparison, only 1·5% of our cohort had F3–F4 fibrosis. Although no ALSPAC participants had known type 2 diabetes, HOMA-IR in participants with steatosis (≥S1) in Petta and colleagues' study was similar to that in our S3 group (3·2 vs 3·6).28
Our study had limitations. Transient elastography is an extensively validated modality with good diagnostic ability to detect fibrosis equivalent to stage F2 and higher.10, 20 However, this ability is lost when attempting to differentiate between F0 and F1 fibrosis. As a result, this population study could under-report the number of participants with fibrosis as we cannot comment on those with early F1 fibrosis.10
Although we estimated the prevalence of suspected NAFLD to be 20·7%, when evaluating associations with transient elastography values equivalent to fibrosis stage in multivariable regression, no association was found with CAP score alone. Ultimately, the staging cut-off values based on transient elastography and cause of fibrosis are validated in patients with histologically proven advanced liver disease, and caution should be applied in interpreting transient elastography results indicative of fibrosis in young adults in a general population. In this context, the proportion of people with advanced fibrosis in our cohort could be an overestimation. Full liver screens looking for other causes of liver disease, such as viral hepatitis, autoimmune hepatitis, and primary biliary cholangitis, were not done in our participants. However, the prevalence of viral hepatitis in southwest England is low, with hepatitis C prevalence in the greater Bristol area estimated at 0·31%, according to the Operational Delivery Network profile tool of Public Health England. In England, of the 571 positive hepatitis C antibody or ribonucleic acid tests in 2018–19, less than 5% were in 15–24 year olds.29 Additionally, the proportion of positive laboratory reports for acute or chronic hepatitis B is 6·8 per 100 000 of the population in the southwest region.30 Furthermore, in our large population study, the gold standard method of liver biopsy was not ethically viable. Therefore, the definitive cause of fibrosis was only speculated to be NAFLD in participants with steatosis in the absence of harmful alcohol consumption.
The suspected NAFLD prevalence of 20·7% is a substantial increase from a sample of the same cohort 6 years earlier (mean age 17·9 years), when prevalence was estimated at 2·5%.6 This previous analysis used ultrasound to assess steatosis, and did not include mild (S1) steatosis in its definition of NAFLD. Ultrasound has been superseded in clinical practice by transient elastography and CAP measurement for assessment of fibrosis and quantification of steatosis. Therefore, the prevalence of NAFLD could have been under-reported previously in this cohort. A similar population-based cohort study in Australia that used ultrasound assessment reported a prevalence of 12·8% for NAFLD.7 Ultrasound has greater than 90% sensitivity to detect steatosis of 30% or more, which corresponds to high S1 and low S2 steatosis.31 13·2% of our participants had S2–S3 steatosis, and therefore a substantial increase in the prevalence of NAFLD in the ALSPAC birth cohort seems to have occurred between 18 to 24 years, despite differences in methods used for detection.
This study did not collect data on grams of alcohol consumed by participants. To compensate for these missing data, we risk-stratified our participants using AUDIT-C scores and evidence of alcohol use disorder into the categories of hazardous and harmful drinking. As a result, the true number of participants with excessive alcohol consumption might be under-reported. Similarly, we did not have data on physical activity to comment on its effect as a predictor of steatosis and fibrosis. However, physical activity relates closely to our key exposures of obesity and adiposity, both of which were shown to be positively associated with steatosis around the age of 24 years.
The ALSPAC birth cohort might not be truly representative of the wider NAFLD prevalence in the UK. The demographic profile of the catchment area population in southwest England, and the differential attrition, has created an over-representation of affluent groups and an under-representation of ethnic minorities.13 Although our study found no association between social class and increased steatosis and fibrosis, we acknowledge that participants that continue to attend our clinics tend to have higher-level educational attainment, introducing a selection bias.13, 14 Furthermore, the southwest region of England has the third lowest adult prevalence of obesity in the UK, at 23%.32 Therefore, this study might underestimate the prevalence of NAFLD in young adults for the whole of the UK, and particularly for regions such as northeast England, where the prevalence of adult obesity is 30%.32
We found obesity to be the strongest factor associated with steatosis. Correspondingly, overweight and obese BMI and increased adiposity were the most powerful predictors of steatosis despite adjustments for excessive alcohol consumption, social class, and smoking. These findings reflect previous work that identified a dose-response relationship between BMI and steatosis outcomes.33
The prevalence of NAFLD appeared to be greater in young men than in young women, a shift from our earlier assessment at 17 years, in which prevalence was similar across the sexes.6 Interestingly, this prevalence in young men was despite proportionally more female participants having obesity than male participants (15·0% vs 10·7%), which contrasts with national data from Cancer Research UK, showing that the prevalence of adult obesity is higher in men than women. Part of this discrepancy could be due to a slightly greater proportion of men in our cohort having increased adiposity compared with women, based on waist-to-height ratio (28·6% vs 27·5%). These findings are consistent with another large epidemiological study of electronic health records, which identified male sex as an independent risk factor for NASH and NAFLD.34
Strong evidence exists that alcohol-related liver disease is linked to socioeconomic deprivation, and similarly, that deprivation is linked to obesity in the paediatric and adolescent settings.3, 35, 36 We found no association between the severity of steatosis or fibrosis and socioeconomic status. Furthermore, no association was identified between smoking and suspected NAFLD, despite smoking being a well recognised risk factor for the development of NAFLD.37 However, most studies looking at the association of smoking with NAFLD have been in middle-aged adults (aged 40–60 years), and the effects of smoking might have been too premature to be apparent in the young adults of our birth cohort.
2·7% of our cohort had evidence of F2-equivalent or greater fibrosis. Steatosis combined with alcohol use disorder had the highest risk of fibrosis compared with alcohol use disorder or steatosis alone. This result is reflective of the complex relationship between NAFLD and alcohol. Moderate alcohol consumption (<20 g/day) has been purported to be protective against steatohepatitis in studies of patients with NAFLD.38, 39 However, chronic alcohol consumption in the context of NAFLD has been associated with increased risk of hepatocellular carcinoma.40, 41 In 2019, a cohort study of UK and US patients with alcohol-related hepatitis that short-term mortality was two times greater in patients with obesity.42 Ultimately in our study, we did not identify any protective effect of alcohol consumption on steatosis, and at least seven participants had suspected fibrosis in the setting of alcohol-related liver disease with steatosis.
Overall our findings have serious public health implications for the UK. Half of our participants with steatosis had S3 steatosis, which theoretically has the greatest association with the risk of NASH, compared with lower grades of steatosis. Progression to fibrosis ranges between 33% and 40% in the literature once NASH is established.43, 44 Evidence exists for steatosis or non-alcoholic fatty liver progressing to fibrosis, with concurrent diabetes a strong risk factor.45 We are not aware of any patients with confirmed type 2 diabetes in our cohort of young adults, but the mean HOMA-IRs for all the steatosis groups were indicative of insulin resistance (≥1·68). Although no association was seen between HOMA-IR and increasing fibrosis stage in participants with suspected NAFLD, HOMA-IR was associated with increasing CAP score, supporting the concept that insulin resistance is a major facilitator of steatosis in the context of NAFLD.46
In conclusion, this study provides evidence that the obesity epidemic is affecting the future health of young adults in the UK, by increasing their risk of NASH-related cirrhosis, hepatocellular carcinoma, and complications of metabolic syndrome. Crucially, these outcomes can be avoided with stringent public health measures, starting with increased awareness of NAFLD among the general population. We identified participants in our cohort with NAFLD-related fibrosis, but the strongest association with increased severity of fibrosis was found in participants with harmful alcohol consumption and hepatic steatosis.
Acknowledgments
Acknowledgments
We are extremely grateful to all the families who took part in this study, to the midwives for their help in recruiting participants, and to the whole Avon Longitudinal Study of Parents and Children team, including the interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. We are also thankful to Professor Nick Sheron at the Institute of Hepatology, Foundation for Liver Research, Kings College London (London, UK), who contributed to the original study design for the Focus@24+ clinic. This publication is the work of the authors, who serve as guarantors for the contents of this paper. The UK Medical Research Council (MRC) and Wellcome Trust (102215/2/13/2) and the University of Bristol (Bristol, UK) provide core support for the Avon Longitudinal Study of Parents and Children. This work was undertaken with the support of the MRC and Alcohol Research UK (MR/L022206/1), and the David Telling Charitable Trust. We also acknowledge support from The Centre for the Development and Evaluation of Complex Interventions for Public Health Improvement, a UK Clinical Research Collaboration (UKCRC) Public Health Research Centre of Excellence (joint funding [MR/KO232331/1] from the British Heart Foundation, Cancer Research UK, the UK Economic and Social Research Council, the MRC, the Welsh Government, and the Wellcome Trust, under the UKCRC); the UK National Institute for Health Research (NIHR) School of Public Health Research, the NIHR Health Protection Research Unit in Evaluation, and the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust (Bristol, UK); and the University of Bristol.
Contributors
KWMA analysed data and wrote the manuscript. GSF helped with data interpretation, advised on statistical analysis, and reviewed the manuscript. GH collected and interpreted data. AJP and FHG provided clinical input for data interpretation and reviewed the manuscript. JH advised on statistical analysis, reviewed the manuscript, and supervised KWMA. MH advised on statistical analysis, reviewed the manuscript, and supervised KWMA and the project.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317–324. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 3.Williams R, Alexander G, Armstrong I. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;391:1097–1107. doi: 10.1016/S0140-6736(17)32866-0. [DOI] [PubMed] [Google Scholar]
- 4.de Graaf EL, Kench J, Dilworth P. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the donor risk index. J Gastroenterol Hepatol. 2012;27:540–546. doi: 10.1111/j.1440-1746.2011.06844.x. [DOI] [PubMed] [Google Scholar]
- 5.Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology. 2017;65:2100–2109. doi: 10.1002/hep.29068. [DOI] [PubMed] [Google Scholar]
- 6.Lawlor DA, Callaway M, Macdonald-Wallis C. Nonalcoholic fatty liver disease, liver fibrosis, and cardiometabolic risk factors in adolescence: a cross-sectional study of 1874 general population adolescents. J Clin Endocrinol Metab. 2014;99:E410–E417. doi: 10.1210/jc.2013-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayonrinde OT, Olynyk JK, Marsh JA. Childhood adiposity trajectories and risk of nonalcoholic fatty liver disease in adolescents. J Gastroenterol Hepatol. 2015;30:163–171. doi: 10.1111/jgh.12666. [DOI] [PubMed] [Google Scholar]
- 8.Seeff LB, Everson GT, Morgan TR. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877–883. doi: 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mrad RA, Merjaneh N, Mubarak G, Lopez R, Zein NN, Alkhouri N. The increasing burden of nonalcoholic fatty liver disease among young adults in the United States: a growing epidemic. Hepatology. 2016;64:1386–1387. doi: 10.1002/hep.28555. [DOI] [PubMed] [Google Scholar]
- 10.Wong VW, Vergniol J, Wong GL. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 11.Foucher J, Chanteloup E, Vergniol J. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo S, Buonocore M, Del Poggio A. Head-to-head comparison of transient elastography (TE), real-time tissue elastography (RTE), and acoustic radiation force impulse (ARFI) imaging in the diagnosis of liver fibrosis. J Gastroenterol. 2012;47:461–469. doi: 10.1007/s00535-011-0509-4. [DOI] [PubMed] [Google Scholar]
- 13.Boyd A, Golding J, Macleod J. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser A, Macdonald-Wallis C, Tilling K. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northstone K, Lewcock M, Groom A. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res. 2019;4:51. doi: 10.12688/wellcomeopenres.15132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlas T, Petroff D, Sasso M. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarafidis PA, Lasaridis AN, Nilsson PM. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens. 2007;21:709–716. doi: 10.1038/sj.jhh.1002201. [DOI] [PubMed] [Google Scholar]
- 19.Shashaj B, Luciano R, Contoli B. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016;53:251–260. doi: 10.1007/s00592-015-0782-4. [DOI] [PubMed] [Google Scholar]
- 20.Eddowes PJ, Sasso M, Allison M. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 22.Lucidarme DFJ, Le Bail B, Costera L. The ratio interquartile range/median value of liver stiffness measurement is a key factor of accuracy of transient elastography (FibroScan (R)) for the diagnosis of liver fibrosis. Hepatology. 2007;46:318A. [Google Scholar]
- 23.American Psychiatric Association . 5th edn. American Psychiatric Association Publishing; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 24.Brambilla P, Bedogni G, Heo M, Pietrobelli A. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. Int J Obes. 2013;37:943–946. doi: 10.1038/ijo.2013.32. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Daniels SR. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 26.Melotti R, Heron J, Hickman M, Macleod J, Araya R, Lewis G. Adolescent alcohol and tobacco use and early socioeconomic position: the ALSPAC birth cohort. Pediatrics. 2011;127:e948–e955. doi: 10.1542/peds.2009-3450. [DOI] [PubMed] [Google Scholar]
- 27.Gregg P, Propper C, Washbrook E. Centre for Market and Public Organisation; Bristol: 2008. Understanding the relationship between parental income and multiple child outcomes: a decomposition analysis. Centre for Market and Public Organization working paper 08/193. [Google Scholar]
- 28.Petta S, Di Marco V, Pipitone RM. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: genetic and metabolic risk factors in a general population. Liver Int. 2018;38:2060–2068. doi: 10.1111/liv.13743. [DOI] [PubMed] [Google Scholar]
- 29.Public Health England Hepatitis C in England 2019. Working to eliminate hepatitis C as a major public health threat. April, 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/798270/HCV_in-England_2019.pdf
- 30.Public Health England Hepatitis B in the south east. 2016 data. March, 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/801176/South_east_hepatitis_B_2016.pdf
- 31.Palmentieri B, de Sio I, La Mura V. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–489. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 32.NHS Digital Statistics on obesity, physical activity and diet. England: 2018. April 4, 2018. https://files.digital.nhs.uk/publication/0/0/obes-phys-acti-diet-eng-2018-rep.pdf
- 33.Fan R, Wang J, Du J. Association between body mass index and fatty liver risk: a dose-response analysis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loomis AK, Kabadi S, Preiss D. Body mass index and risk of nonalcoholic fatty liver disease: two electronic health record prospective studies. J Clin Endocrinol Metab. 2016;101:945–952. doi: 10.1210/jc.2015-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nau C, Schwartz BS, Bandeen-Roche K. Community socioeconomic deprivation and obesity trajectories in children using electronic health records. Obesity (Silver Spring) 2015;23:207–212. doi: 10.1002/oby.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinra S, Nelder RP, Lewendon GJ. Deprivation and childhood obesity: a cross sectional study of 20 973 children in Plymouth, United Kingdom. J Epidemiol Community Health. 2000;54:456–460. doi: 10.1136/jech.54.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:753–759. doi: 10.1016/j.jhep.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn W, Sanyal AJ, Brunt EM. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2012;57:384–391. doi: 10.1016/j.jhep.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajifathalian K, Torabi Sagvand B, McCullough AJ. Effect of alcohol consumption on survival in nonalcoholic fatty liver disease: a national prospective cohort study. Hepatology. 2019;70:511–521. doi: 10.1002/hep.30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahli A, Hellerbrand C. Alcohol and obesity: a dangerous association for fatty liver disease. Dig Dis. 2016;34(suppl 1):32–39. doi: 10.1159/000447279. [DOI] [PubMed] [Google Scholar]
- 41.Kechagias S, Blomdahl J, Ekstedt M. Alcohol consumption in non-alcoholic fatty liver disease—harmful or beneficial? Hepatobiliary Surg Nutr. 2019;8:311–313. doi: 10.21037/hbsn.2019.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker R, Kim SJ, Im GY. Obesity in acute alcoholic hepatitis increases morbidity and mortality. EBioMedicine. 2019;45:511–518. doi: 10.1016/j.ebiom.2019.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labenz C, Huber Y, Kalliga E. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther. 2018;48:1109–1116. doi: 10.1111/apt.14976. [DOI] [PubMed] [Google Scholar]
- 46.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.