Abstract
Many biological processes are naturally regulated with spatiotemporal control. In order to perturb and investigate them, optochemical tools have been developed that convey similar spatiotemporal precision during study of the system. Pivotal to optochemical probes are photolabile protecting groups, so called caging groups, and recent developments have enabled new applications to cellular processes, including cell signaling. This review focusses on the advances made in the field of caging groups and their application in cell signaling through caged molecules such as neurotransmitters, lipids, secondary messengers, and proteins.
Introduction
Application of light as a non-invasive external trigger allows spatiotemporal control in dynamic systems to study dynamic biological processes [1–5]. It started when Engels and Hoffman first reported photoactivation of cyclic adenosine monophosphate (cAMP) [6] and adenosine triphosphate (ATP) [7], using 2-nitrobenzyl and (2-nitrophenyl)ethyl photolabile groups, respectively. The photolabile protecting groups, more commonly referred to as “caging groups”, have since been a cornerstone in optochemical biology. They have been used for the light-regulation of a variety of biomolecules, ranging from oligonucleotides [8,9], carbohydrates [10], proteins [2], and peptides [11], to small molecules (such as, cell signaling molecules [12], fluorophores [13], and chemical inducers of dimerization (CIDs) [14]), thus providing precise spatiotemporal control over biological processes in cells and animals. Over the last five years, there has been a surge toward improving the photophysical properties of caging groups, by shifting their absorption maxima towards the use of long-wavelength light for photoactivation, which reduces the potential for phototoxicity and enhances tissue penetration, as well as enabling decaging via multi-photon excitation. Several excellent review articles on caging groups exist, including a very comprehensive one by Klan et al. [15], and others focusing on two-photon applications [16–18]. This review summarizes most recent caging group developments (predominantly within the last five years), as well as recent applications of caging methodologies to the optical control of cell signaling. Complementary to caging groups, synthetic photoswitchable molecules [19,20], as well as natural photoswitchable proteins have been reviewed elsewhere and in this issue by Leippe and Frank. [21,22].
Advances in caging group development
Recent advances in caging group design have focused on optimizing several desirable properties including [15]: 1) red-shifted absorption maxima (λmax) towards far visible/NIR, 2) high molar extinction coefficient (ϵ) and quantum yield of decaging (φu) leading to higher decaging efficiency (ϵ x φu), 3) good aqueous solubility and stability, 4) non-toxic and low-absorbing photoreleased by-products, 5) large two-photon (2P) absorption (TPA) cross section (δa) which is used for quantifying the two-photon absorption of a chromophore, and 6) narrow absorption profile to enable multiplexing through orthogonal decaging experiments.
One challenge in caging group design is the difficulty in simultaneously optimizing both absorption maxima and quantum yield, where red-shifting the absorption by increasing conjugation sometimes leads to reduction in decaging efficiency. Additionally, introducing hydrophilic groups to achieve optimal solubility for in vivo applications often requires the presence of amine or hydroxy or alkyne handles on the caging group. The fine balance between background hydrolysis of caged compound and its rapid substrate release requires fine-tuning of pKa of both caging group and substrate. Rapid kinetics will allow investigation of fast cellular processes like neuronal signal transduction. Moreover, lack of background activity of the caged compound indicating high light to dark activity switching is desirable.
Coumarin-based caging groups
Coumarin-based caging groups have been applied towards a variety of studies in recent years due to ease of synthesis and rapid release of substrate. Recently, structural modifications have been made towards improving the photophysical properties like quantum yield and aqueous solubility. Efforts have built onto the 7-(diethylamino)-4-(hydroxymethyl)coumarin (DEACM) scaffold (Figure 1b) [23] to red-shift the absorption maximum. The developments can be broadly classified based on their electronic structure: Donor-π system-Acceptor (D-π-A) and Donor-π system-Donor (D-π-D). The D-π-A category exhibits push-pull effect where the chromophore is end-capped with an electron donor and an electron acceptor [24]. Substrates caged by coumarins are typically connected to the caging group through a carbonate, carbamate, phosphate, or carboxy moiety due to the requirement of low pKa in the leaving group [25]. Fournier et al. synthesized a series of such coumarin scaffolds where the structure bore an electron donating group (OMe/NEt2) at the 7-position and different electron withdrawing groups at 2/3 position/s aimed at extending the π-conjugation system [26]. Benzoic acid was utilized as the substrate to cage, and extensive investigation of the photophysical properties yielded three best candidates 1a-1c (Figure 1a), selected based on red-shifted absorption maxima and good quantum yield (Table 1) [26]. The caged tamoxifen analog 2 was employed to photoregulate the activity of an engineered transcription factor En2 in En2-ERT2 mRNA injected zebrafish embryos. Photoactivation of 2 upon 470 nm irradiation for 10 minutes led to observing 50 % of the expected phenotype, a reduction in size/ total absence of eyes [27].
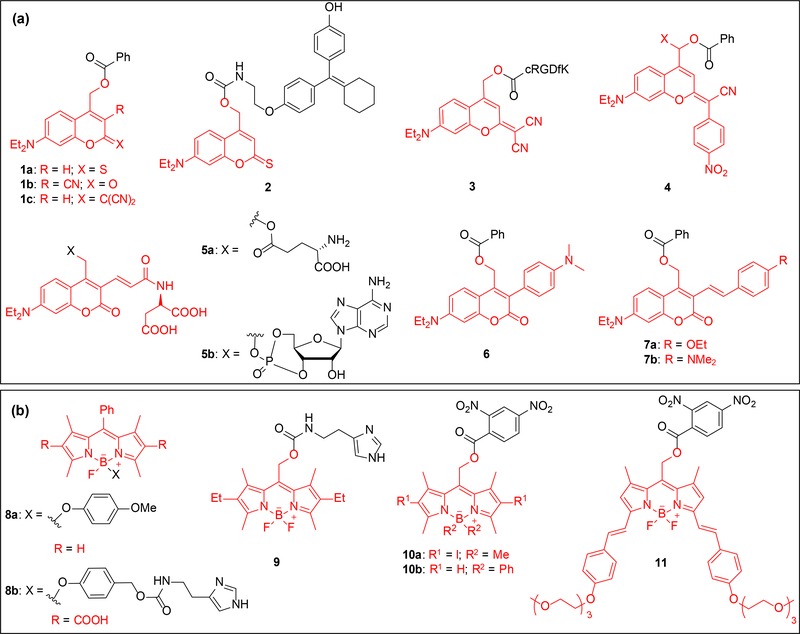
Figure 1.
Structures of coumarin and BODIPY caged substrates; caging groups are shown in red. (a) Structures include D-π-A type (1-5) and D-π-D (6-7) coumarin caged substrates. The structures include caged benzoic acids (1, 4, 6–7), a caged tamoxifen analogue (2), a caged cyclic RGDfK peptide (3), caged glutamic acid (5a) and caged cAMP (5b). (b) BODIPY caged molecules include 4-methoxyphenol (8a), caged histamine (8b, 9), and caged 2,4-dinitrobenzoic acid (10-11).
Table 1.
List of the photochemical properties of some of the new caging groups mentioned in the development section. λmax is the absorption maximum; ϵ is the molar extinction coefficient at the absorption maximum; Solvents used during photolysis: PBS (phosphate buffered saline pH=7.4), Tris is trisaminomethane buffer pH=7.5; φu is the quantum yield of decaging; δu = ϵ × φu is the decaging efficiency for single photon irradiation and is the product of the quantum yield and extinction coefficient; δu2P = δa × φu is two photon decaging action cross section for two photon irradiation where δa is the two photon absorption cross section (1 GM = 10−50 cm4 s photon−1).
| caged compound | λmax/nm | ϵ/mM−1cm−1 | solvent | φu/10−2 (λ/nm) | δu/M−1cm−1 | δu2P/GM (λ/nm) |
|---|---|---|---|---|---|---|
| 1a | 472 | 31 | 1:1 CH3CN:Tris | 0.12 (365) | 320 | - |
| 1c | 487 | 33 | 1:1 CH3CN:Tris | 0.07 (365) | 2 | - |
| 2 | 492 | 30 | 1:1 CH3CN:Tris | 0.24 (505) | 58 | - |
| 4b | 503 | 32 | MeOH | <0.001 (505) | 0.07 | - |
| 5a | 450 | 43 | PBS | 39 (450) | 16800 | 0.5 (900) |
| 6 | 407 | 29 | DMSO | 16 (405) | 4558 | 6 (760) |
| 7a | 430 | 30 | 9:1 MeOH:H2O | 45 (450) | 13500 | 26 (730) |
| 7b | 490 | 30 | 9:1 MeOH:H2O | 40 (450) | 12000 | - |
| 9 | 544 | 45 | 19:1 PBS:CH3CN | 0.01 (540) | 7 | - |
| 10a | 538 | 61 | MeOH | 28 (507) | 17056 | - |
| 11 | 661 | 65 | MeOH | 41 (532) | 3 | - |
| 12a | 668 | 40 | DMSO | - | - | - |
| 15a | 397 | 8 | PBS | 15 (405) | 1125 | 11 (800) |
| 16 | 400 | 11 | 19:1 PBS:DMSO | 22 (412) | 2500 | 20 (800) |
| 17a | 443 | 30 | MeOH | 1 (355) | 60 | |
| 19 | 440 | 66 | PBS | 23 (410) | - | 350 (810) |
| 20 | 362 | 19 | C6D6 | 30 (355) | 5631 | 120 (680) |
The Marchán group reported further improvement of green light activatable coumarin 1c and its application to a caged cRGDfK peptide 3 [28]. Presence of an α-methyl group speeds up decaging due to stabilization of the carbocation intermediate generated in the photolysis step [28]. Further red-shifted coumarin caged benzoic acids 4a and 4b (Figure 1a) were developed by replacing a cyano group in 1c with a nitrophenyl group [29]. However, the decaging quantum yield was significantly reduced (Table 1). The Ellis-Davies and Sabatini labs modified the DEACM scaffold with alkene and aspartate moieties yielding DEAC450 chromophore to shift the absorption maximum and enhance aqueous solubility, and applied it towards caging of glutamic acid (5a) [30]. Decaging of 5a with one-photon (473 nm) or two-photon (900 nm) irradiation at the spine heads on pyramidal neurons in isolated brain slices generated excitatory postsynaptic currents. Lack of response to 720 nm exposure enabled multiplexing of DEAC450 caging group with 4-carboxymethoxy-5,7-dinitroindoline (CDNI) and 4-methoxy-7-nitroindolinyl (MNI) scaffolds [15]. These results led to synthesis of caged cAMP 5b and its wavelength-selective decaging in presence of CDNI-caged γ-aminobutyric acid (GABA) [31].
D-π-D coumarin chromophores, generated through attachment of electron donating group at the 3-position – see 6 and 7, exhibit impressive two-photon properties (Table 1) [24,32,33]. Chitose et al. synthesized 6 which has a TPA cross section of 5.6 GM at 760 nm [33], 8-fold higher than the commonly used MNI chromophore [34], where GM (Goppert Mayer) is an unit of TPA cross section [35]. The Zhu group synthesized the coumarin chromophores 7a and 7b with electron rich styryl appendages at the 3-position [32]. This led to higher absorption maximum but more importantly produced an unconjugated by-product through intramolecular sequestration of the generated carbocation after photolysis. This prevents competitive absorption of the by-product at the absorption wavelength of the caged substrate.
Borondipyrromethene (BODIPY)-based caging groups
The BODIPY chromophore was serendipitously discovered as a caging group by the Urano group when they observed the release of an aryloxy group upon irradiation of 8a (Figure 1b) with 500 nm light [36]. An optimization study showed inverse correlation showed between fluorescence (φfl) and decaging quantum yields (φu), suggesting a photoinduced electron transfer (PeT)-based decaging process [15,25]. A major factor behind its excellent decaging efficiency is the large extinction coefficient (Table 1). Through a clever relay mechanism involving aryloxy decaging and subsequent 1,6-elimination and decarboxylation of a carbamate, the BODIPY group was applied to the caging of histamine (8b) [37].
The groups of Weinstain and Winter used a different connectivity, the meso-methylhydroxy position of BODIPY for caging [38,39]. Based on DFT calculations by the Klan group, BODIPY has a similar excited state structure as coumarins, xanthenes, and methine cyanines [40]. Decaging of 9 in HeLa cells using 500 nm light led to histamine-induced release of Ca2+ [38]. Systematic SAR studies on 32 meso-BODIPY scaffolds optimized their photophysical properties, leading to some candidates that showed exceptional promise in terms of absorption maxima and quantum yield (10a and 10b) [41]. The increased quantum yield for halogenated caged compounds provide evidence that decaging proceeds through a triplet state, as heavy atoms facilitate intersystem crossing (ISC) from the singlet to the triplet state [42]. The decaging efficiency which measures how efficiently a caged substrate is released and is the product of the extinction coefficient (ϵ) and the quantum yield (φu), surpassed 10,000 M−1 cm−1 for a few BODIPY chromophores. This can complicate handling of the caged compounds under ambient light. Winter recently reported a family of one-photon excitable BODIPY caging groups bearing styryl moieties that exhibit the most red-shifted absorption maxima for a BODIPY dye thus far (11) [43]. As a proof of principle, fluorescence of 11 was measured upon 635 nm light exposure and subsequent release of a quencher in HeLa cells [43].
Cyanine-based caging groups
Heptamethine cyanines commonly used for fluorescence applications [44] were employed by the Schnermann group as innovative near-infrared caging groups by converting the liability of cyanine photobleaching into a decaging strategy via localized photooxidation and cleavage of C-C double bonds upon irradiation with 690 nm light [45,46]. Caging and precise spatiotemporal release of 4-hydroxycyclofen 12a (Figure 2a) was demonstrated in MCF-7 cells [45]. The same chromophore was employed to cage combretastatin A4 as part of an antibody-drug conjugate (ADC) [47]. The antibody panitumumab ensured the localization of 13 to cells expressing EGFR while 690 nm irradiation controlled the release of the drug in a temporal fashion in a mouse xenograft model [47]. While the use of antibodies provides precise control over the delivery of the drug, the inherent fluorescence of cyanine enables tracking of the ADC and drug release can be monitored through decrease in fluorescence. The use of near-IR light further ensures good tissue penetration [48].
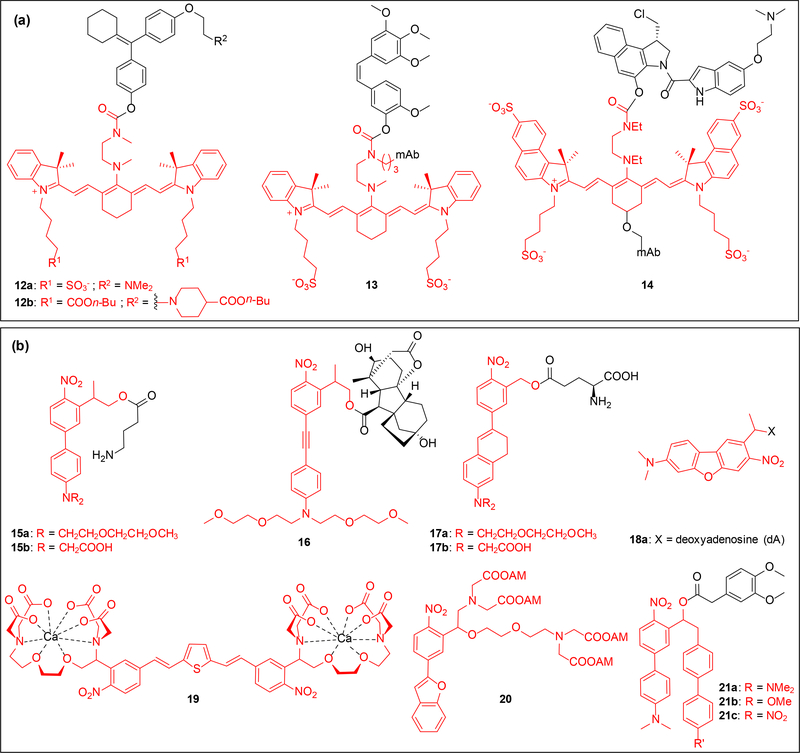
Figure 2.
Structures of heptamethine cyanine and new nitrobenzyl (NB) caged substrates; caging groups are shown in red. (a) Caged substrates include 4-hydroxycycofen (12), combretastatin A4 (13), and duocarmycin (14), the last two substrates were conjugated to the antibody panitumumab. (b) Structures include NPE-type (17-18 and 21) and NPP-type (15-16) caged molecules. Caged substrates include GABA (15), gibberellic acid (16), glutamic acid (17), deoxyadenosine (18), calcium chelators (19-20), and 3,4-dimethoxyphenylacetic acid (21);
Optimization of 12a via addition of lipophilic esters to both the caging group and substrate (12b, Figure 2a) enhances cellular uptake and localization [49]. Rational design of the cyanine structure led to development of a red-shifted and more hydrolytically stable dye [50]. Altering the heterocycle of the core and the linker domain yielded 14 which was used to spatiotemporally regulate release of duocarmycin from a cyanine-duocarmycin-panitumumab conjugate both in vitro and in vivo [50].
Ortho-nitrobenzyl (NB)-based caging groups
Despite being the most widely used caging group in biological applications, development of NB caging groups with more desirable properties has been challenging due to difficulties in optimizing the absorption and decaging properties simultaneously [51]. Increased conjugation has enhanced two-photon sensitivity but resulted in decrease in one-photon decaging efficiency in some cases [51–53]. Thus, recent efforts have focused on developing two photon sensitive chromophores.
Dialkylamino-biphenyl caging groups were introduced by Specht and Goeldner based on 2-(2-nitrophenyl) prop-1-yl (NPP) scaffold [54]. They synthesized caged GABA 15a and 15b (Figure 2b) which exhibited extraordinary TPA cross section of 11 GM at 800 nm and exhibited improved decaging efficiency over previously used coumarin caged GABA (0.37 GM at 800 nm) [53]. Irradiation of 15a or 15b with 800 nm light induced inhibitory postsynaptic GABAergic currents in rat cortical brain slices [54]. The dialkylamino group enables functionalization with PEG/2-carbonylmethyl moieties to improve aqueous solubility of the caged compound. Wombacher introduced a new caging group based on 15 where introduction of an alkyne led to a two-fold improvement in two-photon decaging efficiency by extending the π-conjugation, but there was no significant shift in the absorption maximum compared to 15. The caging group was installed on a plant-based CID, gibberellic acid (GA3) 16, to trigger protein dimerization and mitochondrial translocation of EGFP in COS-7 cells after 412 nm irradiation [55]. Supported by DFT calculations, Kobayashi developed a dialkyl-dihydronaphthalene and applied it to the caging of the glutamate derivatives 17a and 17b [56], which exhibited improved two-photon sensitivity compared to 15. Becker et al. recently reported a new red-shifted caged oligonucleotide 18, having excellent two-photon decaging properties at 840 nm but inert to one-photon irradiation [52]. Due to the exclusive response to two-photon photolysis, this caging group could be utilized in an orthogonal decaging approach with one-photon labile red-shifted caging groups.
Ellis-Davies and coworkers developed a bis-styrylthiophene (BIST) caging group with improved two-photon sensitivity and an absorption maximum at 440 nm [57]. The calcium chelator 19 generated by appending EGTA to the BIST caging group produced rapid “Ca2+ waves” in cardiac myocytes upon irradiation with both 405 nm and 810 nm [57]. This caging group can be multiplexed with other caged metal chelators to study the effect of controlled release of select ions in different signal transduction pathways [58]. Jakkampudi et al. synthesized the Ca2+ chelator 20 as a cell permeable acetoxymethyl (AM) ester which showed impressive two-photon properties (Table 1) [59]. Cultured neurons containing 20 were irradiated with 720 nm light leading to induction of calcium response as monitored through inhibitory post synaptic currents (IPSCs) at the dendritic edges [59]. Analysis of the photolysis products reveals that 19 and 20 selectively undergo release of the α-substituent, instead of fragmenting through an also conceivable β-elimination pathway [15]. Nakad et al. recently reported a series of optically activated reporter molecules 21a-c which upon decaging yields a fluorescent by-product [60], which can be used to monitor the amount of decaging.
Applications of caging groups in cell signaling studies
Some of the new, recently reported caging groups have found application in the context of cell signaling through caging of small molecule ligands [37,38], calcium ions [57,59], and secondary messengers [30,31,54]. Upon photoactivation, caged small molecule ligands bind to cell surface receptor and promote downstream signaling events through secondary messengers. Alternatively, caged secondary messengers, when photoreleased, activate intracellular targets to induce signal transduction and subsequently elicit a cellular response (Figure 3a).
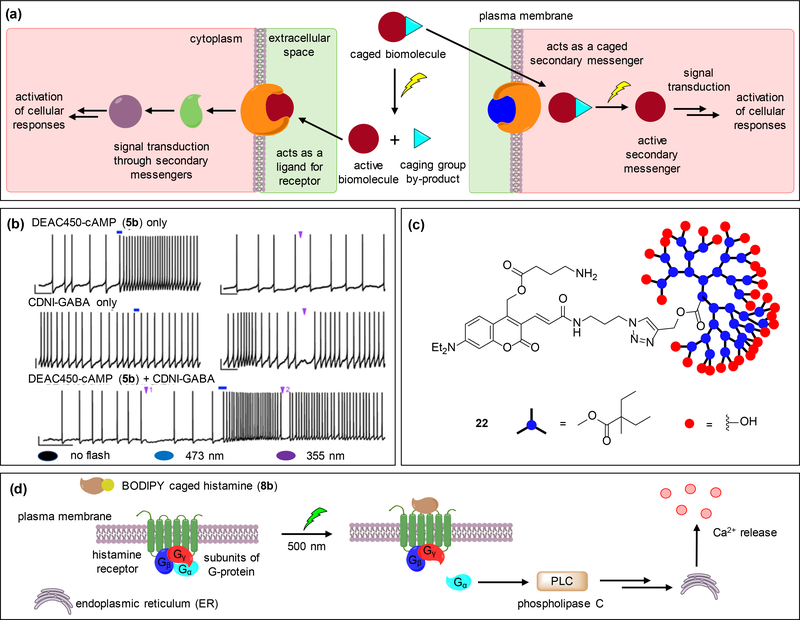
Figure 3.
Examples of applications of coumarin and BODIPY caging groups in cell-signaling. (a) The general approach includes a caged inactive biomolecule that is rendered functional upon light irradiation. The active biomolecule can act as a receptor ligand or a secondary messenger, both of which induce downstream signaling events and lead to a cellular response. (b) Whole cell current clamp recordings demonstrate the wavelength-selective, bi-directional modulation of neuronal firing in striatal cholinergic neurons through decaging of DEAC450-cAMP (5b) and CDNI-GABA within the same cell using one-photon irradiation. While decaging of 5b causes increase in the firing rates, decaging of CDNI-GABA transiently inhibits it. (c) Dendrimer-cloaked DEAC450-GABA (22) shields the substrate from interacting with the GABA-A receptors thereby reducing background activity. (d) Upon irradiation of BODIPY-caged histamine 8b, histamine binds to the H1 receptor and induces the G-protein mediated activation of phospholipase C, leading to calcium release. Adapted with permission from ref. [31], Copyright 2013 American Chemical Society.
Orthogonal decaging of neurotransmitters
The Ellis-Davies group synthesized DEAC-450 caged-cAMP (5b, Figure 1a) and used it in combination with CDNI-caged GABA. Using one-photon (473 nm and 365 nm) irradiation in a sequential fashion, they interrogated the bi-directional neuronal firing rates in single striatal cholinergic neurons [31]. Patch clamped single neurons from brain slices were incubated with 5b and CDNI-GABA, and whole-cell current clamp recordings were used to monitor spontaneous action potential firings. Irradiation with 473 nm enhanced the action potential firing rate while irradiation with 355 nm transiently inhibited it (Figure 3b). The wavelength orthogonality was demonstrated through induction of stimulatory and inhibitory response patterns by varying the order of the wavelength applied (Figure 3b). The orthogonality was extended to two-photon uncaging using DEAC450-Glu and CDNI-GABA, where irradiation with 900 nm along a basal dendrite elicited an action potential only to be reversed by the inhibitory currents induced through decaging of CDNI-GABA at 720 nm [12]. The neurotransmitter receptors being densely clustered, two-photon decaging offers a higher level of spatial control due to the irradiation of single spines and synapses. Additionally, orthogonally decaging excitatory and inhibitory compounds in one system allows precise study of the neurons in their natural environment where they are subjected to a complex mix of rapid excitatory and inhibitory stimuli to produce output signals.
Reduction in background activity of caged neurotransmitters
A major limitation of the caged compounds (Glu and GABA) is that they exhibit significant “GABA-A antagonism”, where the caged compound interacts with GABA-A receptors and dampens the spontaneous miniature inhibitory postsynaptic currents (mIPSCs) in patch clamped single neurons. This effect is pronounced for commonly used (MNI/CDNI) caged neurotransmitters employed at higher concentrations (1–12 mM), required for two-photon photolysis experiments, applied either through bath application or local perfusion [34,61]. To alleviate this issue, a “cloaked cage” strategy was developed where a dendrimer is attached to the caging group through “click” chemistry [62]. The bulky dendrimer envelopes the caged compound and prevents interaction with the receptors until photolyzed, similar to previous applications of PEG-modified caging groups blocking biological interactions until light-induced cleavage [63–65]. The application of the dendrimer-caged GABA 22 (Figure 3c) to patch-clamped prefrontal cortical neurons showed reduced binding affinity to receptors (IC50 = 0.9 mM) when compared to antagonists like bicuculline (IC50 = 0.5 μM) and caged GABA probes like DEAC-450-GABA (IC50 = 0.5 μM), as determined through blockade of electrically evoked inhibitory currents. Additionally, the higher two-photon cross-section of 22 enabled use of it at lower concentrations (66 μM) for two-photon decaging [30,34].
Decaging of small molecule ligands
The Urano group utilized 8b (Figure 1b) in HeLa cells to optically regulate histamine release as indicated by changes in intracellular Ca2+ concentration [37]. Irradiation of 8b with 500 nm light released histamine which targets membrane localized histamine H1 receptors to activate phospholipase-C (PLC) through the binding of Gα subunit of H1-GPCR. PLC further activates downstream targets like IP3 to eventually release calcium from the endoplasmic reticulum (Figure 3d). The di-carboxy group facilitates membrane localization of 8b near H1 receptors [66]. Treatment with pyrilamine, an H1 antagonist suppressed the Ca2+ response, indicating cells specifically responded to histamine produced through decaging of 8b [37].
Decaging of secondary messengers
In addition to applications of newly developed caging groups, the well-established nitrobenzyl and coumarin caging groups have found further use in recent biological applications, including control of cell signaling, due to their robust photophysical properties, ease of synthesis, and stability under physiological conditions [15]. The concentration gradients of distinct lipid species and their localization within the cell regulates signaling events [67]. Different potency due to subtle structural differences among lipids have prompted development of chemical tools to spatiotemporally investigate their function [67]. Wagner et al. reported a “click-cage” approach to synthesize organelle-specific DEACM caged lipid secondary messengers - arachidonic acid and sphingosine derivatives (Figure 4a) [68]. The click-cage is comprised of a DEACM caging group with a clickable alkyne handle enabling a modular design for attachment of established organelle targeting scaffolds [69]. Mitochondria, endoplasmic reticulum (ER), lysosome, and plasma membrane targeted sphingosine and arachidonic acid caged derivatives were synthesized and, along with corresponding organelle markers, applied to HeLa cells containing a fluorescent Ca2+ reporter. Localization of the caged compounds and markers was as expected (Figure 4a). Irradiation of defined regions in the cell with 385 nm light led to observation of vastly different signaling patterns in distinct cell organelles [68]. Robust calcium transients were observed in lysosomes, endoplasmic reticulum, and mitochondria but not at the plasma membrane through decaging of caged sphingosine derivatives, while stronger calcium responses were seen at the plasma membrane and mitochondria through decaging of caged arachidonic acid derivatives, compared to lysosomes. The observed results supports findings that TPC1, the main intracellular target of sphingosine is localized in lysosomes, while GPR40, a known target of arachidonic acid, is mainly localized at the plasma membrane [70,71]. This study shows that induction of lipid messengers at precise intracellular locations is critical for signaling outcome.
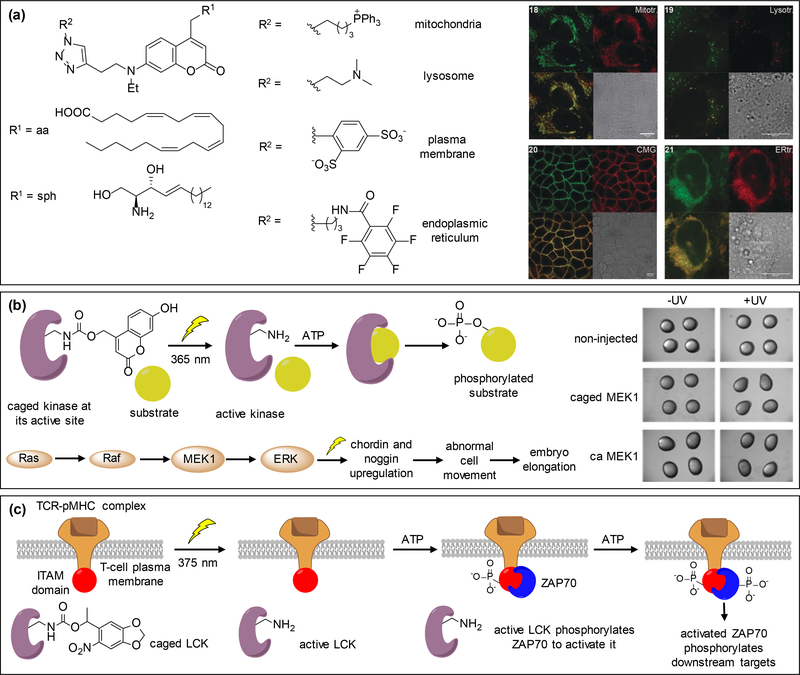
Figure 4.
Use of nitrobenzyl and coumarin caging groups in the control of cell signaling. (a) Organelle targeting DEACM-caged arachidonic acid and sphingosine derivatives are localized as expected: mitochondria (upper left), lysosome (upper right), plasma membrane (lower left), and endoplasmic reticulum (lower right). (b) A kinase is rendered inactive through caging of a catalytic site lysine with 7-hydroxycoumarin, blocking ATP binding until exposure to 365 nm irradiation. The active kinase then phosphorylates its downstream substrate. This was applied to MEK1 within the Ras/MAPK signaling pathway in developing zebrafish embryos. Hyperactivation of MEK1 induces embryo dorsalization leading to an elongation phenotype at 8 hours post fertilization. (c) LCK is rendered inactive through caging of its catalytic site until light irradiation with 375 nm activates the enzyme, which in turn activates ITAMs through phosphorylation. ZAP70 gets recruited by ITAMs and phosphorylated by LCK to activate downstream effector molecules and induce multiple signaling pathways. Adapted with permission from ref. [72] and [73], Copyright 2018, John Wiley and Sons and Copyright 2017, American Chemical Society, respectively.
Genetic code expansion using caged amino acids
Genetic code expansion, through expression of an engineered orthogonal tRNA/aminoacyl-tRNA synthetase pair, enables incorporation of unnatural amino acids into proteins in response to amber stop codons in cells and animals [74,75]. Among various chemically modified amino acids that were genetically encoded, caged analogs of tyrosine, cysteine, and lysine have found numerous applications in the control of protein function [2]. The Deiters lab utilized a genetically encoded 7-hydroxycoumarin lysine (HCK) replacing a critical lysine residue in the active site of MEK1 to extend applications of the optical control of MEK/ERK signaling from mammalian cells [76] to zebrafish embryos [72]. The caging group renders the MEK1 protein catalytically inactive by blocking the binding pocket of ATP until irradiation with 365 nm light makes the protein functional and allows phosphorylation of downstream targets (Figure 4b). Light activation of caged-MEK1 led to dorsalized embryos which was comparable to embryos expressing constitutively active MEK1 (caMEK1 in Figure 4b). The phenotypic results upon optical activation of caged-MEK1 at different time points revealed an essential time window (until 8 hours post-fertilization) for the MEK/ERK pathway to affect dorsal patterning. This critical information may be translated to treat human developmental defects due to MEK hyperactivation at an early developmental stage with pharmacological inhibitors. An earlier genetically encoded nitrobenzyl-caged lysine [77], was applied in triggering the TCR signaling pathway by James and Chin via optical regulation of lymphocyte tyrosine kinase (LCK) [73]. LCK was rendered inactive by caging the critical lysine residue K273 in the ATP binding pocket until 375 nm illumination led to phosphorylation of its downstream target ITAM. The kinase ZAP70 was then recruited and phosphorylated to initiate further downstream target phosphorylation and docking of binding proteins (Figure 4c). The kinetics of ZAP70 phosphorylation were measured by phospho-western blot analysis at different time points after decaging. Additionally, the authors revealed that LCK activity is mediated by autophosphorylation of its critical Y394 and a stimulatory role of the TCR coreceptor CD8 was observed through increased membrane recruitment of ZAP70 [73]. This work provides valuable mechanistic insights on the TCR signaling pathway and can be extended to other members of the SRC family of kinases.
Conclusions
Newer caging groups have improved several photophysical properties, especially shifting the absorbance maxima further into the visible/NIR region and enhancing the decaging efficiency. Increased π-conjugation has improved the decaging efficiency and has further sensitized nitrobenzyl and coumarin chromophores towards two-photon excitation which provides improved three-dimensional spatial control and opportunities for orthogonal decaging. The obtained wavelength orthogonality is an improvement over sequential activation studies which requires initial decaging of the red-shifted chromophore with longer wavelength, followed by activation of a second chromophore with shorter wavelength, typically UV light. Development of green light activatable BODIPY and near-IR activatable cyanine caging groups have further expanded the scope of one-photon irradiation, obviating the need to for a two-photon setup. The new caging groups have a broad substrate scope and have been applied to light-triggered release of alcohols, amines, thiols, carboxylates, and phosphates. The coumarin and BODIPY chromophores, however, generally require a carbonate or carbamate linkage, owing to the lower pKa requirement in the photolysis step compared to nitrobenzyl-based chromophores. In addition to choosing a caging group with red-shifted absorption maxima and good quantum yield for in vivo applications, fluorescent reporters with minimal spectral overlap with the caging group should be considered. The hydrophilicity/hydrophobicity of the caging group should be carefully considered based on solubility requirements and intracellular or membrane localization of the target. Moreover, decaging kinetics of caging groups should be considered for investigating fast cellular processes.
In the context of cell signaling, coumarin caging groups have been used to cage neurotransmitters like GABA and glutamate, secondary messengers like cAMP and lipid molecules like DAG, arachidonic acid, and sphingosine. Wavelength-selective decaging studies using DEAC450-cAMP and CDNI-GABA have enabled mimicking the complex signaling dynamics occurring at synapses and wavelength orthogonality may further be used to study highly dynamic protein kinase/phosphatase signaling pathways and their crosstalk. The use of PEGs and dendrimers to alleviate background activity highlights the need for optimal sterics in the design of a caged compound. Induction of local concentration bursts through decaging of DEACM-caged sphingosine and arachidonic acid has been applied in conjunction with organelle targeting motifs to study lipid-mediated calcium signaling events at pre-defined intracellular locations with improved spatial resolution. The use of modular ‘click’ approach overcomes the limitation of synthesizing specific sets of caged compounds for each organelle where the caging group is directly attached to the targeting scaffold. While BODIPY-caged histamines have enabled investigation of the histamine-mediated calcium signaling pathway using green light, near-IR activatable cyanine dyes has so far been applied exclusively to targeted drug delivery. The cyanine dyes can be applied to orthogonal decaging experiments in combination with nitrobenzyl or coumarin caging groups. Caged amino acids have expanded the substrate scope of unnatural amino acid mutagenesis and provide blocking of protein function through caging of specific amino acid residues, which can be selected based on structural and/or mechanistic data. The loss of the caging group upon photolysis prevents potential perturbation of native protein function. 7-Hydroxycoumarin lysine was used to investigate the MAPK/ERK signaling pathway in zebrafish embryos and the study revealed an essential time window of MEK1 hyperactivation for potential therapeutic intervention in congenital diseases. Furthermore, a nitrobenzyl-caged lysine was used to investigate the TCR signaling pathway and quantify LCK catalytic activity in cells through direct activation of LCK thereby overcoming the limitation of steady-state measurements. Taken together, optochemical tools employing highly modular and synthetically readily accessible caging groups are highly tunable in their photophysical properties and have found applications in cell signaling, ranging from the optical control of metal ions and small molecule messengers to lipids and protein kinases. The developed probes have been applied in single cells, tissues, and whole animals, laying the foundation for a wide range of future studies.
Acknowledgements
We thank Nicholas Ankenbruck and Taylor Courtney for suggestions and the National Institutes of Health (R01GM112728 and R21HD085206) and the National Science Foundation (CBET-1603930) for funding.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.O’Banion CP, Lawrence DS: Optogenetics: A Primer for Chemists. Chembiochem 2018, 19:1201–1216. [DOI] [PubMed] [Google Scholar]
- 2.Courtney T, Deiters A: Recent advances in the optical control of protein function through genetic code expansion. Current Opinion in Chemical Biology 2018, 46:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ankenbruck N, Courtney T, Naro Y, Deiters A: Optochemical Control of Biological Processes in Cells and Animals. Angewandte Chemie International Edition 2017, 57:2768–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A: Light-Controlled Tools. Angewandte Chemie International Edition 2012, 51:8446–8476. [DOI] [PubMed] [Google Scholar]
- 5.Kowalik L, Chen JK: Illuminating developmental biology through photochemistry. Nature Chemical Biology 2017, 13:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engels J, Schlaeger EJ: Synthesis, structure, and reactivity of adenosine cyclic 3’,5’-phosphate-benzyltriesters. Journal of Medicinal Chemistry 1977, 20:907–911. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JH, Forbush B, Hoffman JF: Rapid photolytic release of adenosine 5’-triphosphate from a protected analog: utilization by the sodium:potassium pump of human red blood cell ghosts. Biochemistry 1978, 17:1929–1935. [DOI] [PubMed] [Google Scholar]
- 8.Ruble BK, Yeldell SB, Dmochowski IJ: Caged oligonucleotides for studying biological systems. Journal of Inorganic Biochemistry 2015, 150:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Deiters A: Optochemical control of deoxyoligonucleotide function via a nucleobase-caging approach. Accounts of chemical research 2014, 47:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bier C, Binder D, Drobietz D, Loeschcke A, Drepper T, Jaeger K-E, Pietruszka J: Photocaged Carbohydrates: Versatile Tools for Controlling Gene Expression by Light. Synthesis 2017, 49:42–52. [Google Scholar]
- 11.Lee H-M, Larson DR, Lawrence DS: Illuminating the Chemistry of Life: Design, Synthesis, and Applications of “Caged” and Related Photoresponsive Compounds. ACS Chemical Biology 2009, 4:409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amatrudo JM, Olson JP, Agarwal HK, Ellis-Davies GCR: Caged compounds for multichromic optical interrogation of neural systems. European Journal of Neuroscience 2014, 41:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W-h, Zheng G: Photoactivatable fluorophores and techniques for biological imaging applications. Photochemical & Photobiological Sciences 2012, 11:460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voß S, Klewer L, Wu Y-W: Chemically induced dimerization: reversible and spatiotemporal control of protein function in cells. Current Opinion in Chemical Biology 2015, 28:194–201. [DOI] [PubMed] [Google Scholar]
- 15.Klán P, Šolomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J: Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chemical Reviews 2013, 113:119–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe M, Chitose Y, Jakkampudi S, Thuy PTT, Lin Q, Van BT, Yamada A, Oyama R, Sasaki M, Katan C: Design and Synthesis of Two-Photon Responsive Chromophores for Near-Infrared Light-Induced Uncaging Reactions. Synthesis 2017, 49:3337–3346. [Google Scholar]
- 17.Piant S, Bolze F, Specht A: Two-photon uncaging, from neuroscience to materials. Optical Materials Express 2016, 6:1679–1691. [Google Scholar]
- 18.Bort G, Gallavardin T, Ogden D, Dalko PI: From one-photon to two-photon probes: “caged” compounds, actuators, and photoswitches. Angew Chem Int Ed Engl 2013, 52:4526–4537. [DOI] [PubMed] [Google Scholar]
- 19.Lerch MM, Hansen MJ, van Dam GM, Szymanski W, Feringa BL: Emerging Targets in Photopharmacology. Angew Chem Int Ed Engl 2016, 55:10978–10999. [DOI] [PubMed] [Google Scholar]
- 20.Szymański W, Beierle JM, Kistemaker HAV, Velema WA, Feringa BL: Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chemical Reviews 2013, 113:6114–6178. [DOI] [PubMed] [Google Scholar]
- 21.Leopold AV, Chernov KG, Verkhusha VV: Optogenetically controlled protein kinases for regulation of cellular signaling. Chemical Society Reviews 2018, 47:2454–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tischer D, Weiner OD: Illuminating cell signalling with optogenetic tools. Nature Reviews Molecular Cell Biology 2014, 15:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagen V, Bendig J, Frings S, Eckardt T, Helm S, Reuter D, Kaupp UB: Highly Efficient and Ultrafast Phototriggers for cAMP and cGMP by Using Long-Wavelength UV/Vis-Activation. Angewandte Chemie International Edition 2001, 40:1045–1048. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Xu W, Sheng P, Zhao G, Zhu D: Organic Donor–Acceptor Complexes as Novel Organic Semiconductors. Accounts of Chemical Research 2017, 50:1654–1662. [DOI] [PubMed] [Google Scholar]
- 25.Givens RS, Rubina M, Wirz J: Applications of p-hydroxyphenacyl (pHP) and coumarin-4-ylmethyl photoremovable protecting groups. Photochem Photobiol Sci 2012, 11:472–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fournier L, Aujard I, Le Saux T, Maurin S, Beaupierre S, Baudin J-B, Jullien L: Coumarinylmethyl Caging Groups with Redshifted Absorption. Chemistry – A European Journal 2013, 19:17494–17507. [DOI] [PubMed] [Google Scholar]
- 27.Fournier L, Gauron C, Xu L, Aujard I, Le Saux T, Gagey-Eilstein N, Maurin S, Dubruille S, Baudin JB, Bensimon D, et al. : A blue-absorbing photolabile protecting group for in vivo chromatically orthogonal photoactivation. ACS Chem Biol 2013, 8:1528–1536. [DOI] [PubMed] [Google Scholar]
- 28.Gandioso A, Cano M, Massaguer A, Marchán V: A Green Light-Triggerable RGD Peptide for Photocontrolled Targeted Drug Delivery: Synthesis and Photolysis Studies. The Journal of Organic Chemistry 2016, 81:11556–11564. [DOI] [PubMed] [Google Scholar]
- 29.Gandioso A, Contreras S, Melnyk I, Oliva J, Nonell S, Velasco D, Garcia-Amoros J, Marchan V: Development of Green/Red-Absorbing Chromophores Based on a Coumarin Scaffold That Are Useful as Caging Groups. J Org Chem 2017, 82:5398–5408. [DOI] [PubMed] [Google Scholar]
- 30.Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis-Davies GC: Optically selective two-photon uncaging of glutamate at 900 nm. J Am Chem Soc 2013, 135:5954–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson JP, Banghart MR, Sabatini BL, Ellis-Davies GC: Spectral evolution of a photochemical protecting group for orthogonal two-color uncaging with visible light. J Am Chem Soc 2013, 135:15948–15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Q, Yang L, Wang Z, Hua Y, Zhang D, Bao B, Bao C, Gong X, Zhu L: Coumarin Photocaging Groups Modified with an Electron-Rich Styryl Moiety at the 3-Position: Long-Wavelength Excitation, Rapid Photolysis, and Photobleaching. Angew Chem Int Ed Engl 2018, 57:3722–3726.•Development of π-extended coumarin dyes having styryl appendages at the 3-position led to red-shifted absorption maxima. The presence of styryl group stabilized the carbocation formed after photolysis, thereby improving the decaging efficiency and generating a non-absorbing photolysis by-product.
- 33.Chitose Y, Abe M, Furukawa K, Lin JY, Lin TC, Katan C: Design and Synthesis of a Caged Carboxylic Acid with a Donor-pi-Donor Coumarin Structure: One-photon and Two-photon Uncaging Reactions Using Visible and Near-Infrared Lights. Org Lett 2017, 19:2622–2625. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaki M, Hayama T, Kasai H, Ellis-Davies GCR: Two-photon uncaging of gamma-aminobutyric acid in intact brain tissue. Nat Chem Biol 2010, 6:255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svoboda K, Yasuda R: Principles of Two-Photon Excitation Microscopy and Its Applications to Neuroscience. Neuron 2006, 50:823–839. [DOI] [PubMed] [Google Scholar]
- 36.Sano Y, Watanabe W, Matsunaga S: Chromophore-assisted laser inactivation--towards a spatiotemporal-functional analysis of proteins, and the ablation of chromatin, organelle and cell function. J Cell Sci 2014, 127:1621–1629. [DOI] [PubMed] [Google Scholar]
- 37.Umeda N, Takahashi H, Kamiya M, Ueno T, Komatsu T, Terai T, Hanaoka K, Nagano T, Urano Y: Boron dipyrromethene as a fluorescent caging group for single-photon uncaging with long-wavelength visible light. ACS Chem Biol 2014, 9:2242–2246. [DOI] [PubMed] [Google Scholar]
- 38.Rubinstein N, Liu P, Miller EW, Weinstain R: meso-Methylhydroxy BODIPY: a scaffold for photolabile protecting groups. Chem Commun (Camb) 2015, 51:6369–6372. [DOI] [PubMed] [Google Scholar]
- 39.Goswami PP, Syed A, Beck CL, Albright TR, Mahoney KM, Unash R, Smith EA, Winter AH: BODIPY-derived photoremovable protecting groups unmasked with green light. J Am Chem Soc 2015, 137:3783–3786. [DOI] [PubMed] [Google Scholar]
- 40.Solomek T, Wirz J, Klan P: Searching for Improved Photoreleasing Abilities of Organic Molecules. Acc Chem Res 2015, 48:3064–3072. [DOI] [PubMed] [Google Scholar]
- 41.Slanina T, Shrestha P, Palao E, Kand D, Peterson JA, Dutton AS, Rubinstein N, Weinstain R, Winter AH, Klan P: In Search of the Perfect Photocage: Structure-Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J Am Chem Soc 2017, 139:15168–15175.• Systemic investigation of meso-methyl BODIPY scaffolds led to new caging groups with high extinction coefficients and high decaging efficiences. Rational design including heavy atom substitutions of the core and boron methylation led to greatly improved BODIPY chromophores.
- 42.Zhang X-F: BODIPY photosensitizers based on PET and heavy atom effect: A comparative study on the efficient formation of excited triplet state and singlet oxygen in BODIPY dimers and monomers. Journal of Photochemistry and Photobiology A: Chemistry 2018, 355:431–443. [Google Scholar]
- 43.Peterson JA, Wijesooriya C, Gehrmann EJ, Mahoney KM, Goswami PP, Albright TR, Syed A, Dutton AS, Smith EA, Winter AH: Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J Am Chem Soc 2018, 140:7343–7346. [DOI] [PubMed] [Google Scholar]
- 44.Sun W, Guo S, Hu C, Fan J, Peng X: Recent Development of Chemosensors Based on Cyanine Platforms. Chemical Reviews 2016, 116:7768–7817. [DOI] [PubMed] [Google Scholar]
- 45.Gorka AP, Nani RR, Zhu J, Mackem S, Schnermann MJ: A near-IR uncaging strategy based on cyanine photochemistry. J Am Chem Soc 2014, 136:14153–14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorka AP, Schnermann MJ: Harnessing cyanine photooxidation: from slowing photobleaching to near-IR uncaging. Current Opinion in Chemical Biology 2016, 33:117–125.• First report describing the use of near-IR light to spatiotemporally release a drug from an antibody-drug conjugate in cells and mice. The inherent fluorescence of the cyanine was used as a location tracker and the irradiation-induced loss of emission served as a marker for drug release.
- 47.Nani RR, Gorka AP, Nagaya T, Kobayashi H, Schnermann MJ: Near-IR Light-Mediated Cleavage of Antibody-Drug Conjugates Using Cyanine Photocages. Angew Chem Int Ed Engl 2015, 54:13635–13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henderson TA, Morries LD: Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatric disease and treatment 2015, 11:2191–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorka AP, Yamamoto T, Zhu J, Schnermann MJ: Cyanine Photocages Enable Spatial Control of Inducible Cre-Mediated Recombination. Chembiochem 2018, 19:1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nani RR, Gorka AP, Nagaya T, Yamamoto T, Ivanic J, Kobayashi H, Schnermann MJ: In Vivo Activation of Duocarmycin-Antibody Conjugates by Near-Infrared Light. ACS Cent Sci 2017, 3:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aujard I, Benbrahim C, Gouget M, Ruel O, Baudin JB, Neveu P, Jullien L: o-nitrobenzyl photolabile protecting groups with red-shifted absorption: syntheses and uncaging cross-sections for one- and two-photon excitation. Chemistry 2006, 12:6865–6879. [DOI] [PubMed] [Google Scholar]
- 52.Becker Y, Unger E, Fichte MAH, Gacek DA, Dreuw A, Wachtveitl J, Walla PJ, Heckel A: A red-shifted two-photon-only caging group for three-dimensional photorelease. Chem Sci 2018, 9:2797–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gug S, Bolze F, Specht A, Bourgogne C, Goeldner M, Nicoud JF: Molecular engineering of photoremovable protecting groups for two-photon uncaging. Angew Chem Int Ed Engl 2008, 47:9525–9529. [DOI] [PubMed] [Google Scholar]
- 54.Donato L, Mourot A, Davenport CM, Herbivo C, Warther D, Leonard J, Bolze F, Nicoud JF, Kramer RH, Goeldner M, et al. : Water-soluble, donor-acceptor biphenyl derivatives in the 2-(o-nitrophenyl)propyl series: highly efficient two-photon uncaging of the neurotransmitter gamma-aminobutyric acid at lambda = 800 nm. Angew Chem Int Ed Engl 2012, 51:1840–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schelkle KM, Griesbaum T, Ollech D, Becht S, Buckup T, Hamburger M, Wombacher R: Light-Induced Protein Dimerization by One- and Two-Photon Activation of Gibberellic Acid Derivatives in Living Cells. 2015, 54:2825–2829. [DOI] [PubMed] [Google Scholar]
- 56.Boinapally S, Huang B, Abe M, Katan C, Noguchi J, Watanabe S, Kasai H, Xue B, Kobayashi T: Caged glutamates with pi-extended 1,2-dihydronaphthalene chromophore: design, synthesis, two-photon absorption property, and photochemical reactivity. J Org Chem 2014, 79:7822–7830. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal HK, Janicek R, Chi SH, Perry JW, Niggli E, Ellis-Davies GC: Calcium Uncaging with Visible Light. J Am Chem Soc 2016, 138:3687–3693.• One of few examples of a blue light absorbing nitrobenzyl chromophore with high two-photon sensitivity used in biological applications. A calcium chelator was generated using the BIST chromophore and activation was demonstrated in cardiac myocytes upon one-photon and two-photon irradiation.
- 58.Maret W: Crosstalk of the group IIa and IIb metals calcium and zinc in cellular signaling. Proceedings of the National Academy of Sciences 2001, 98:12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakkampudi S, Abe M, Komori N, Takagi R, Furukawa K, Katan C, Sawada W, Takahashi N, Kasai H: Design and Synthesis of a 4-Nitrobromobenzene Derivative Bearing an Ethylene Glycol Tetraacetic Acid Unit for a New Generation of Caged Calcium Compounds with Two-Photon Absorption Properties in the Near-IR Region and Their Application in Vivo. ACS Omega 2016, 1:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abou Nakad E, Bolze F, Specht A: o-Nitrobenzyl photoremovable groups with fluorescence uncaging reporting properties. Org Biomol Chem 2018, 16:6115–6122. [DOI] [PubMed] [Google Scholar]
- 61.Amatrudo JM, Olson JP, Lur G, Chiu CQ, Higley MJ, Ellis-Davies GC: Wavelength-selective one- and two-photon uncaging of GABA. ACS Chem Neurosci 2014, 5:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richers MT, Amatrudo JM, Olson JP, Ellis-Davies GC: Cloaked Caged Compounds: Chemical Probes for Two-Photon Optoneurobiology. Angew Chem Int Ed Engl 2017, 56:193–197.••Introduced a “cloaking” technology by appending a large neutral dendrimer to a coumarin caged GABA. This overcame the problem of background GABA-A receptor antagonism exhibited by the caged molecules at concentrations required for two-photon photolysis in brain tissue. This is another interesting application of polymer attachment through a caging group and subsequent light-triggered release from the biologically active molecules.
- 63.Ts KK-S, Leung K-K, Liu H-W, Lo KK-W: Photoactivatable cytotoxic agents derived from mitochondria-targeting luminescent iridium(iii) poly(ethylene glycol) complexes modified with a nitrobenzyl linkage. Chemical Communications 2016, 52:4557–4560. [DOI] [PubMed] [Google Scholar]
- 64.Govan JM, McIver AL, Deiters A: Stabilization and photochemical regulation of antisense agents through PEGylation. Bioconjug Chem 2011, 22:2136–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georgianna WE, Lusic H, McIver AL, Deiters A: Photocleavable polyethylene glycol for the light-regulation of protein function. Bioconjug Chem 2010, 21:1404–1407. [DOI] [PubMed] [Google Scholar]
- 66.Parsons ME, Ganellin CR: Histamine and its receptors. British journal of pharmacology 2006, 147 Suppl 1:S127–S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laguerre A, Schultz C: Novel lipid tools and probes for biological investigations. Curr Opin Cell Biol 2018, 53:97–104. [DOI] [PubMed] [Google Scholar]
- 68.Wagner N, Stephan M, Hoglinger D, Nadler A: A Click Cage: Organelle-Specific Uncaging of Lipid Messengers. Angew Chem Int Ed Engl 2018, 57:13339–13343.••Localized decaging of arachidonic acid and sphingosine through attachment of organelle-targeting moieties to coumarin-caged compounds in a “click-cage” approach allows for cellular signaling perturbation with improved spatial precision and provides insights into differential signaling patterns at different cellular locations.
- 69.Xu W, Zeng Z, Jiang J-H, Chang Y-T, Yuan L: Discerning the Chemistry in Individual Organelles with Small-Molecule Fluorescent Probes. Angewandte Chemie International Edition 2016, 55:13658–13699. [DOI] [PubMed] [Google Scholar]
- 70.Hoglinger D, Haberkant P, Aguilera-Romero A, Riezman H, Porter FD, Platt FM, Galione A, Schultz C: Intracellular sphingosine releases calcium from lysosomes. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, et al. : Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422:173. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Hemphill J, Samanta S, Tsang M, Deiters A: Genetic Code Expansion in Zebrafish Embryos and Its Application to Optical Control of Cell Signaling. J Am Chem Soc 2017, 139:9100–9103.••The first example of optical control of photocaged amino acids in animals and extended the substrate scope for unnatural amino acid mutagenesis in zebrafish embryos. Light-activation of genetically incorporated photocaged lysine enabled mapping of the temporal effects of MEK1 hyperactivation on zebrafish embryo development, providing a potential time window for pharmacological intervention in the corresponding human developmental diseases.
- 73.Liaunardy-Jopeace A, Murton BL, Mahesh M, Chin JW, James JR: Encoding optical control in LCK kinase to quantitatively investigate its activity in live cells. Nat Struct Mol Biol 2017, 24:1155–1163.••Optical control of the kinase LCK through genetic incorporation of a nitrobenzyl-caged lysine enabled quantification of phosphorylation kinetics in live cells – an approach which is broadly applicable to other kinases. It also provided insights into the mechanistic role of LCK and its interaction with other regulatory proteins in the TCR signaling pathway.
- 74.Chin JW: Expanding and reprogramming the genetic code. Nature 2017, 550:53. [DOI] [PubMed] [Google Scholar]
- 75.Brown W, Liu J, Deiters A: Genetic Code Expansion in Animals. ACS Chemical Biology 2018, 13:2375–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gautier A, Deiters A, Chin JW: Light-activated kinases enable temporal dissection of signaling networks in living cells. J Am Chem Soc 2011, 133:2124–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gautier A, Nguyen DP, Lusic H, An W, Deiters A, Chin JW: Genetically Encoded Photocontrol of Protein Localization in Mammalian Cells. Journal of the American Chemical Society 2010, 132:4086–4088. [DOI] [PubMed] [Google Scholar]