Abstract
The pig industry faces many animal welfare issues. Among these, biting behaviour has a high incidence. It is indicative of an existing problem in biters and is a source of physical damage and psychological stress for the victims. We categorize this behaviour into aggressive and non-aggressive biting, the latter often being directed towards the tail. This review focusses specifically on predisposing factors in early life, comprising the prenatal and postnatal periods up to weaning, for the expression of aggressive and non-aggressive biting later in life. The influence of personality and coping style has been examined in a few studies. It varies according to these studies and, thus, further evaluation is needed. Regarding the effect of environmental factors, the number of scientific papers is low (less than five papers for most factors). No clear influence of prenatal factors has been identified to date. Aggressive biting is reduced by undernutrition, cross-fostering and socialization before weaning. Non-aggressive biting is increased by undernutrition, social stress due to competition and cross-fostering. These latter three factors are highly dependent on litter size at birth. The use of familiar odours may contribute to reducing biting when pigs are moved from one environment to another by alleviating the level of stress associated with novelty. Even though the current environment in which pigs are expressing biting behaviours is of major importance, the pre-weaning environment should be optimized to reduce the likelihood of this problem.
Keywords: pre-weaning, swine, aggression, oral manipulation, tail biting
Implications
Biting behaviour in growing pigs impairs their welfare and leads to economic losses. We categorized this behaviour into aggressive and non-aggressive biting, the latter often being directed towards the tail. The environment in which pigs are expressing biting is of major importance, but predisposing factors acting in early life can also influence its expression. This review points out the detrimental influence of large litters on non-aggressive biting and the positive influence of social interactions between suckling piglets of different litters on aggressive biting later on. No clear conclusion emerged for other factors due to inconsistent results or paucity of information.
Introduction
Group-housed pigs in commercial production systems are susceptible to the performance of a variety of behaviours that contribute to reduced welfare. Most prominent are biting behaviours that directly result in more or less severe skin lesions, or in amputation of part of the tail or ears in post-weaning and fattening pigs. Indirectly, biting behaviour can result in injuries such as lameness due to slipping during fights (e.g. Anil et al., 2005; Maes et al., 2016) (sometimes lethal), infections due to wounds caused by biting (Schroëder-Petersen and Simonsen, 2001), immunosuppression (de Groot et al., 2001), reduced growth (e.g. Stookey and Gonyou, 1994) and, in some extreme cases, death (Sinisalo et al., 2012). Biting induces a reaction (retreat or attack) by the victim, except in severe cases when the wounded animal gradually gives up its resistance and its effort to flee (Sambraus, 1985) or when limitations imposed by the environment do not allow an effective escape by the recipient pig. Two types of biting can be identified (Simonsen, 1990) and will be referred to throughout the present text:
aggressive biting,
non-aggressive biting or oral manipulative biting.
Aggressive biting is common in the context of hierarchy formation and occurs mostly in the first hours after creating a new social group (Meese and Ewbank, 1973). It can also occur, to a lesser extent, in stable groups when animals compete for limited resources or when some pigs challenge the established hierarchical order (Meese and Ewbank, 1972 and 1973; Parois et al., 2017; Peden et al., 2018). Bites are targeted preferentially at the head and the shoulders (the front third of the body) but can also reach the flanks when delivered in a reverse parallel posture or the rump when delivered to retreating animals (McGlone, 1985; Fraser and Rushen, 1987; Turner et al., 2006).
Non-aggressive biting is largely unrelated to hierarchy formation and resource competition. It occurs mainly, though not exclusively, in barren environments where pigs are likely to be thwarted in their need to perform exploration, object play or foraging behaviours (EFSA, 2007). Non-aggressive biting is mainly targeted at the tail, but ears can also be the subject of biting (EFSA, 2007) as well as other parts of the body. These other parts of the body include flank biting (Petersen et al., 2008), leg biting (Beattie et al., 2000), penis biting in entire male pigs (Weiler et al., 2016), vulva biting in sows (Ladewig et al., 1984) or anus biting in fatteners (Blowey, 2003). Regarding tail or vulva biting, an aggressive motivation may result from competition for food or water in situations of limited access (Hansen et al., 1982; Van Putten and Vandeburgwal, 1990; Rizvi et al., 1998). Tail and ear lesions can also result from necrosis without other pigs’ intervention (Lechner et al., 2015), although this often leads to biting of the affected parts by other pigs once exudate and blood are present.
The important role of the immediate environment on the two types of biting behaviour is well recognized and has been reviewed (e.g. Schroëder-Petersen and Simonsen, 2001; Van de Weerd et al., 2005; EFSA, 2007). The influence of internal factors related to genotype or health is also recognized (for reviews see Moinard et al., 2003; Taylor et al., 2010; D’Eath et al., 2014; Valros and Heinonen, 2015). In addition to these factors, events affecting prenatal life as well as the early postnatal environment may also influence the later predisposition to both types of biting behaviours in pigs. The evidence for the existence of such early predisposing factors is evaluated in the present review, focussing on biting behaviours performed by young pigs after weaning and during the fattening period.
Taking into account that both types of biting involve at least one performer and one recipient, and that the reaction of the recipient is likely to influence the behaviour of the performer, we evaluate, whenever information is available, the effects of potential predisposing factors not only on the propensity of pigs to perform but also to receive such behaviours, as well as on the way pigs react to these behaviours. We consider various factors acting during the prenatal and early postnatal life and their effects during the post-weaning and fattening periods. Early postnatal life is defined as the whole period between birth and weaning. Before analysing the predisposing factors in detail, we firstly describe the main motivations of weaned or growing pigs to perform biting.
Motivations underlying biting behaviours and variability of expression between pigs
Motivations to bite
Aggressive biting and non-aggressive biting are, especially in practice, often discussed as if these are the same behaviours (Bracke et al., 2013; Benard et al., 2014). However, these should be considered differently because of differences in the underlying motivations, the part of the body that is concerned and the reaction of the recipient (Taylor et al., 2010). Indeed, the recipients of non-aggressive biting, such as tail biting, show no response or little reaction that consists mostly of avoidance (Taylor et al., 2010), whereas the recipients of aggressive biting often engage in reciprocal fighting (Turner et al., 2006).
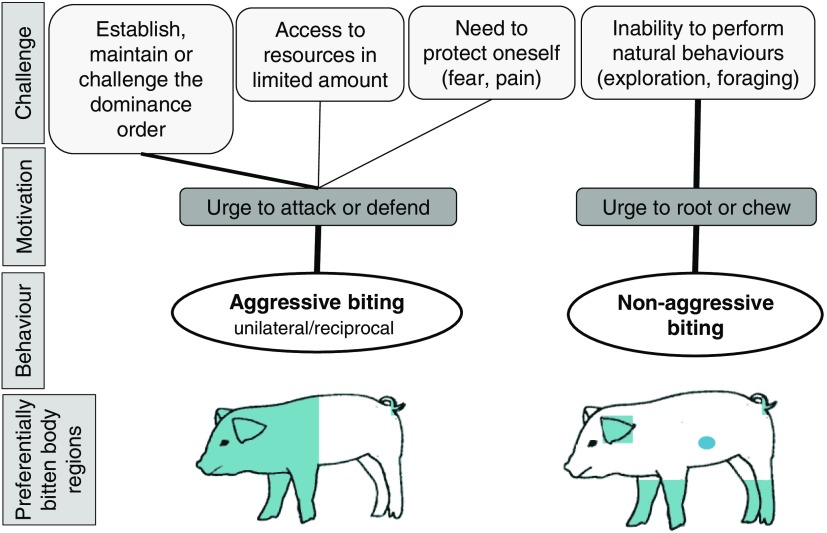
Aggressive biting occurs (1) during the formation of dominance relationships that dictate privileged access to potential resources and (2), subsequently, during the maintenance of these relationships when animals are competing for resources with limited access (Figure 1). The formation of dominance relationships occurs when unfamiliar pigs are mixed together to form new social groups, which is a common occurrence in commercial piggeries (Peden et al., 2018). The motivation to establish, defend or challenge a high dominance position, or to access resources, results in aggressive behaviour expressed through fighting and biting. In most cases, the target of biting is the front third of the body (Turner et al., 2006), but bites are often delivered to the rump of a retreating animal. In situations when animals try to access a feeder or a drinker, biting can be directed to the tail or the vulva as this is the most accessible part at that moment (Hansen et al., 1982; Van Putten and Vandeburgwal, 1990). Aggressive biting can also occur because of fear-induced and pain-induced aggression, as demonstrated in dogs (Jacobs et al., 2003).
Figure 1.
Targets of biting and main motivations of pigs to bite.
Non-aggressive biting largely results from the inability of pigs to express natural behaviour to root, chew and forage, as shown in numerous reviews (e.g. Schroëder-Petersen and Simonsen, 2001; Taylor et al., 2010; D’Eath et al., 2014; Valros and Heinonen, 2015). When this innate behaviour cannot be appropriately expressed, as is the case in most commercial conditions, this internal drive starts to be expressed in redirected behaviour. This urge to chew and root is redirected towards any available materials in the environment, including penmates. In field situations, aggressive and non-aggressive biting directed to the tail may sometimes be interrelated, since the presence of blood at the tail of one pig may attract other pigs (Fraser, 1987) that will develop non-aggressive tail biting.
Variability of expression of biting behaviours
During an episode of tail biting, some pigs in a pen perform (performers), receive (recipients), perform and receive (performers/recipients) or are not involved in tail biting (neutral) (Brunberg et al., 2011; Zonderland et al., 2011a). Regarding performers, there is substantial variation in the amount of tail biting and in other behaviours performed by these pigs. Some performers are considered as ‘fanatical’ biters, being hyperactive and going from one tail to another during an outbreak of biting, whereas other performers bite rather occasionally (Van de Weerd et al., 2005). A great variability also exists for the frequency of receipt of tail biting (Brunberg et al., 2011). Concerning aggressive biting, great variability is observed regarding the number of damaging interactions and the number of accumulated skin lesions during the 24 h after mixing unacquainted pigs (Turner et al., 2006). Part of this inter-individual variation within social groups can be explained by genetic factors, the personality or coping style of the animals and, in addition, by the influence of the prenatal and early postnatal environment.
Influence of personality and coping style on biting behaviours
Background
Personality is defined by a correlated set of individual behavioural and physiological traits that are consistent over lifetime and environmental contexts (Finkemeier et al., 2018). In humans, personality is described by five main dimensions. Among these, aggressiveness, exploration and boldness can be easily recognized and tested in farm animals. A coping style is defined by a coherent set of behavioural and physiological responses to an aversive stressor, with the responses being consistent over time (Koolhaas et al., 1999). Animals are classified as proactive (also referred to as ‘active’ or ‘high resisting’) when they have a strong fight/flight response, and reactive (also referred to as ‘passive’ or ‘low resisting’) when they have a low response. Personality and coping style are closely linked (Korte et al., 2005; Finkemeier et al., 2018). Indeed, proactive animals are considered to be more aggressive towards conspecifics, more exploratory, bold and active compared with reactive individuals. In pigs, coping style can be assessed through the backtest performed in suckling piglets (e.g. Bolhuis et al., 2005a). The classification into proactive and reactive is based on the number of escape attempts (i.e. bouts of struggling with at least the hind legs) that piglets display during the course of 60 s when they are gently placed on their back. ‘High resisting’ pigs perform more escape attempts. Backtest responses may change across multiple tests as shown by Zebunke et al. (2015). These authors performed four test repetitions between 1 and 4 weeks of age in 3555 piglets and concluded that the backtest rather indicates a coping disposition, which is modulated by environmental factors such as age and experience.
Aggressive biting behaviour
The potential link between coping style and aggressive biting has been evaluated in several studies in pigs, with inconsistent results. Hessing et al. (1993) subjected suckling piglets to the backtest five times during the first 3 weeks of life. They also performed a social confrontation test at 1 week of age (mixing three animals from each of two litters together) in order to classify pigs as either aggressive or non-aggressive. Results showed that 75% of proactive pigs were aggressive, whereas 75% of reactive pigs were non-aggressive. Pigs that varied in their behavioural response during consecutive backtests (alternating between proactive and reactive; 21%) were equally distributed between aggressive and non-aggressive pigs. When social behaviour was tested in older animals, Bolhuis et al. (2005b) observed more aggressive behaviours (including biting) in proactive or ‘high resisting’ pigs, independent of the housing environment (enriched v. barren) applied before and after weaning. In the study of Melotti et al. (2011), the relationship between coping style and aggressiveness was more nuanced, with ‘high resisting’ pigs showing the same amount of aggression (head-knocks and/or bites) as ‘low resisting’ pigs, but being more persistent in their aggression. ‘High resisting’ pigs chased (bullied) other pigs more and fought more, independently of relative weight differences. In contrast, other studies found no relationship between the number of struggles in the backtest and aggressiveness indicators (number of attacks, bites, latency to attack) in a resident-intruder test (Forkman et al., 1995; D’Eath and Burn, 2002; Janczak et al., 2003). Moreover, aggressive traits measured in weaners, growers and gilts in their rearing pens during group mixing were poorly related to the number of escape attempts in the backtest (two repetitions performed at 12 and 19 days of age) with phenotypic correlations varying between −0.05 and 0.02 (Scheffler et al., 2016a). The discrepancy between test outcomes may be partly due to the variation in the manner of performing the backtest. Indeed, the findings showing a relationship between backtest response and aggression come from the same research group, where a great similarity in the procedures is expected. It can also be noted that, in all these experiments, the coping style was assessed early in the life of the pigs, and probably before they had time to develop their full personality when being confronted with a wide range of personal experiences. Therefore, increasing the time interval between the evaluation of coping style and aggressiveness may reduce the strength of the relationship between them.
The relationship between aggressiveness and personality traits measured in tests other than the backtest has less often been investigated. The response in the human approach test, commonly used to assess fear tendency (Finkemeier et al., 2018), was poorly correlated (Scheffler et al., 2016a) or not correlated (Janczak et al., 2003) with aggressiveness observed in rearing pens. Gilts classified as low or high responders after several behavioural tests (including restraint, handling, sudden human approach) did not differ in their number of attacks towards other gilts in a food competition test (Lawrence et al., 1991).
Non-aggressive biting behaviour
It has been reported that the total frequency of manipulatory behaviours towards penmates was lower from weaning until the end of the fattening period in ‘high resisting’ than in ‘low resisting’ pigs (in the backtest) that were kept in a barren environment (Bolhuis et al., 2005b; Bolhuis et al., 2006). This effect was not seen in an enriched environment, but it should be noted that the level of manipulatory behaviours was already very low in the ‘low resisting’ pigs. Specific tail manipulation behaviour was observed so rarely that the influence of the coping style could not be reliably tested. ‘Low resisting’ pigs showed more oral manipulation than ‘high resisting’ pigs when they experienced a change in the environment from enriched to barren housing (Melotti et al., 2011).
Piglets that showed a less fearful response pre-weaning in a novel environment test (‘Novel Box Test’) performed less tail biting later in life when housed in barren pens (Ursinus et al., 2014a). Chewing propensity at an early age has been tested as a personality trait that could predispose pigs for tail or ear biting later in life (Beattie et al., 2005). Indeed, chewing may refer to the exploration dimension of personality. Behaviour during a ‘Tail Chew Test’ (a salty rope and a plain one were presented to a piglet for 10 min) performed a couple of days before weaning (at 4 weeks of age) and 2 weeks later showed some stability over time. The behaviour directed towards these ropes was slightly positively correlated with ear biting observed between 4 and 7 weeks of age in the home pen, whereas tail biting was only positively correlated with results from the test at 6 weeks of age (Beattie et al., 2005).
Conclusion: personality and coping style
Currently, there is only limited evidence that personality and coping style, evaluated through behavioural tests before weaning, can predict aggressive or non-aggressive biting behaviour later in life. The response to the backtest has shown some relationship with both types of behaviours, with ‘low resisting’ pigs being more prone to non-aggressive biting (oral manipulation) and ‘high resisting’ ones more prone to show aggressive biting. This latter relationship was not observed in all studies being probably influenced by various factors. Consequently, the link between coping style, personality and biting behaviour would merit further investigation in different environments and at different ages. The various aspects of personality should also be considered, including boldness and exploration, and not solely the coping style.
Prenatal effects on biting behaviours
Foetal brain development is highly dependent upon adequate nutritional and endocrine support. Therefore, nutritional deficit or stress applied to the pregnant mother may have long-term consequences on cognitive and behavioural abilities of the offspring and hence on behavioural predisposition to bite.
Effects related to undernutrition of the foetus
Background
Undernutrition during prenatal life can be displayed by low birth weight. Low birth weight has a strong influence on growth rate during and after lactation, and hence on the liveweight at weaning or later at a given age. For example, Poore and Fowden (2003) found that low-birth-weight piglets had a lower growth rate until 3 months of age. These piglets also had higher adrenal-to-liveweight and adrenal cortex-to-medulla ratios and a greater cortisol response to ACTH stimulation at 3 months of age, even though differences were no longer detectable at 12 months of age.
There are many potential causes of undernutrition during prenatal life. Reduced nutrient supply can occur because of intra-uterine crowding and reduced placental area (Foxcroft and Town, 2004), undernutrition of the dam (Worobec et al., 1999; Bell and Ehrhardt, 2002) or maternal diseases that limit nutrient exchange to the foetuses (Gaccioli and Lager, 2016). In pigs, litter size is of particular interest, mainly because of the relationship with intra-uterine crowding (Foxcroft et al., 2006) and decreased average birth weight (Rutherford et al., 2013).
Aggressive biting behaviour
Taking into account the importance of liveweight at birth for growth rate (Douglas et al., 2013), an influence of nutrition during foetal life on biting behaviour is expected at least via the influence of liveweight on aggressiveness of pigs (cf. Postnatal undernutrition). However, in a competitive feeding test performed in gilts, the proportion of aggressive interactions initiated and the dominance ratio (ratio of the number of gilts she dominated to the number that dominated her) were not significantly predicted by size of litter at birth, body mass at birth or crown-rump length (which might be indicative of intra-uterine growth retardation) (Drickamer et al., 1999). Ruis et al. (2000) also found that pigs showing higher resistance in a backtest, which was moderately correlated to aggressiveness during a group-feeding competition test, were not heavier at birth.
Non-aggressive biting behaviour
In the study by Beattie et al. (2005), there was no significant difference in birth weight between pigs that expressed high or low levels of tail-chewing behaviour after weaning. Similarly, in the study by Ursinus et al. (2014b), birth weight did not differ between non-tail or ear biters, ‘medium’ tail or ear biters, and ‘high’ tail or ear biters, regardless of the environment (provided with a jute sack or not) and the stage of growth (post-weaning: 6 to 8 weeks of age; rearing: 11 weeks of age).
Conclusion: foetal undernutrition
The current balance of evidence fails to provide support for a role of prenatal nutrient deficiency in the ontogeny of later damaging behaviour.
Influence of non-nutritional sources of prenatal stress on biting behaviours
Background
Prenatal stress, that is, stress experienced while in the foetal environment, can result in long-term behavioural and biological changes of the offspring. This has been studied in various farm species, including pigs (reviewed by Kranendonk et al., 2008; Rutherford et al., 2012; Merlot et al., 2013). Prenatal stress can occur from a single or repeated stressor during gestation, such as malnutrition (discussed above), or from disease and social stress of the dam. In pigs, prenatal stress has been elicited through the dam by the administration of stress hormones (e.g. ACTH: Haussmann et al., 2000; hydrocortisone: Kranendonk et al., 2005), pain (e.g. Otten et al., 2001), rough handling (e.g. Lay et al., 2011) or social stress through group mixing during gestation (e.g. Couret et al., 2008; Rutherford et al., 2009). According to the stage of maturation of the foetus, the consequences of prenatal stress or of hormonal treatment may be different (reviewed by Kranendonk et al., 2008; Rutherford et al., 2012; Merlot et al., 2013).
When natural stressors are applied, they stimulate the hypothalamic–pituitary–adrenal axis of the dam (Brunton, 2013; Merlot et al., 2013). As a consequence, the nutrient supply to the foetuses can be modified and the transfer of cortisol to them can be increased. Both phenomena may alter the maturation of their neuroendocrine systems, with possible consequences after birth (Brunton, 2013; Merlot et al., 2013). In addition, undernutrition of the foetuses might influence biting behaviour through reduced birth weight per se (cf. Undernutrition of the foetus). Furthermore, the sympathetic nervous system (SNS) of the dam, and hence catecholamine release, is very likely to be stimulated with, again, possible consequences on the nutrient supply to the foetuses and long-term effects. When using hormonal treatment to mimic prenatal stress, the SNS component is not included.
Aggressive biting behaviour
During a social test (i.e. mixing with an unfamiliar pig for 60 min) performed at about 1.5 months of age, piglets born from sows treated with hydrocortisone during early (days 21 to 50), mid (days 51 to 80) or late (days 81 to 110) pregnancy performed the same number of aggressive encounters during the first 30 min as piglets born from control sows (Kranendonk et al., 2006). However, piglets born from sows treated during mid-pregnancy showed more aggressive encounters during the second 30 min of the test compared to piglets born from control sows or from sows treated during early or late gestation, suggesting a greater persistence of aggressive behaviour. In contrast, Lay et al. (2011) found no effect of sow stress treatment (ACTH administration or rough handling at days 42 to 77 of gestation) on the amount of offspring aggression during mixing. The influence of catecholamines was not specifically evaluated.
Non-aggressive biting behaviour
The hypothesis that prenatally stressed piglets will be better prepared for receiving stress (in the form of pain) and thus respond differently from control pigs has been challenged (Rutherford et al., 2009; Sandercock et al., 2011). Data show that prenatal stress due to social stress applied to the dam during mid-gestation increases the offsprings’ response to pain (Rutherford et al., 2009). Therefore, it can be hypothesized that prenatally stressed piglets are less susceptible to be recipients of biting due to an increased reaction to being bitten.
Offspring of sows that had received an ACTH challenge had significantly higher concentrations of plasma cortisol and healed slower after biopsy damage compared to control pigs (Haussmann et al., 2000). Therefore, they might be more prone to being bitten by other pigs due to the presence of persisting lesions.
Conclusion: prenatal stress
There are too few reports to reliably determine the influence of prenatal stress on aggressive behaviour. Prenatal stress might, however, have a favourable influence (pigs more responsive and hence probably more reactive to pain) on non-aggressive biting counterbalanced by a detrimental one (slower healing).
Postnatal effects on biting behaviours
An important part of the pig brain development takes place after birth and depends on nutritional and environmental inputs. Therefore, nutritional deficit or scarcity of sensory stimuli during that period may have long-term consequences on cognitive and behavioural abilities of pigs and hence on behavioural predisposition to bite.
Effects related to undernutrition
Background
Undernutrition of piglets during lactation can arise because of excessive competition at the udder in large litters (cf. Postnatal social stress), poor health and agalactia of the dam (Sauber et al., 1999; Pend et al., 2017) or poor health of the individual piglet itself. In the latter case, infection-induced cytokine production can reduce appetite and growth (Williams et al., 1997). Such undernutrition can be considered as a stressor with possible long-term consequences on the maturation of the neuroendocrine systems. In addition, it clearly influences the growth of pigs. Indeed, liveweight of growing pigs is greatly influenced by their liveweight at weaning, and hence milk intake and growth during lactation (Quiniou et al., 2002; Douglas et al., 2013).
Aggressive biting behaviour
Liveweight at the time of mixing pigs into new groups is a major determinant of their aggressive behaviour, especially biting behaviour (e.g. Andersen et al., 2000; Desire et al., 2015; Scheffler et al., 2016b). Lighter animals demonstrate fewer aggressive behaviours. Even though lower liveweight at mixing may be related to a lower nutrient supply during lactation, it is far from being sufficient to demonstrate the role of early nutrition. To the best of our knowledge, only one study has reported the long-term influence of growth during lactation on aggressive biting (Drickamer et al., 1999). The results indicated that, in newly formed groups of 6- to 7-month-old gilts, the proportion of aggressive behaviours that each gilt initiated around feeding was positively correlated with her liveweight at 21 days of age. It was, however, not influenced by her liveweight at birth nor by her daily gain between birth and 21 days of age. This suggests that early-life nutrient supply, including both prenatal and lactational supply, may be important in competitive aggression.
Non-aggressive biting behaviour
It has anecdotally been reported that the pigs which perform injurious tail-biting behaviour are the smallest individuals within the group, or the so-called ‘runt’ pigs (Sambraus, 1985). When this has been investigated under experimental conditions, conflicting results have been obtained. Van de Weerd et al. (2005) reported that, while there was no difference in weight between pigs showing occasional tail biting and non-biting penmate controls, pigs showing persistent tail-biting behaviour were indeed significantly smaller individuals. These persistent biters, so-called ‘fanatical biters’, were described as being hyperactive pigs going from one tail to the other during a biting outbreak (Van de Weerd et al., 2005). In other studies where persistent tail-biting pigs have been identified, they have also tended to be lighter in weight compared with penmates (Zupan et al., 2012).
While smaller body size has therefore often been associated with tail-biting predisposition, it is less often documented when exactly – in pre or postnatal life – this reduced growth rate has occurred. In the study by Van de Weerd et al. (2005), ‘fanatical’ tail-biting pigs were lighter at the time of biting outbreaks but did not differ from other pigs (non-biters or sporadic biters) in their weight at birth or at weaning. This suggests a growth effect shortly prior to the appearance of injurious behaviour rather than an early-life effect. However, in the study by Beattie et al. (2005), although there was no significant difference in birth weight between pigs that expressed high or low tail-chewing behaviour after weaning, pigs that chewed most frequently showed significantly lower growth rates between birth and weaning (260 v. 285 g/day). This suggests an increased predisposition arising from nutrient deficiency during lactation. Zonderland et al. (2011b) also found tail-biting pigs in the post-weaning stage to have a significantly lower weaning weight compared with victims and a numerically lower weight (0.5 kg less) compared with control contemporaries. Further circumstantial evidence of a link between impaired early growth and tail biting comes from the observation of van Staaveren et al. (2017) of a negative correlation between average tail lesion score and weight at sale/transfer of a batch of weaners, and between the percentage of pigs with severe tail lesions in a herd and average daily gain in weaners. Several reasons may explain lower weight in biters. They may use up more energy due to their increased activity (e.g. Van de Weerd et al., 2005). They may have a reduced growth due to internal causes (e.g. health disorder as suggested by Valros and Heinonen, 2015) or may use tail biting as a strategy to displace heavier pigs from the feeder or the drinker when access is difficult (D’Eath et al., 2014).
Contrary to a negative relationship between growth and tail biting, Ursinus et al. (2014b), showed that liveweight at weaning and growth rate during lactation were higher in gilts classified as high tail biters compared with medium and non-tail biters during the first 4 weeks after weaning. However, these results were dependent on the rearing environment, since they were observed only when jute sacks were provided and hence when biting directed to congeners was mitigated. In addition, they were not consistent across ages, since the existing difference was not observed when animals were classified according to their behaviour 3 weeks later.
Conclusion: postnatal undernutrition
The influence of growth during lactation and weight at weaning on aggressive biting has been scarcely investigated. Available data suggest that reduced early nutrition decreases the occurrence of this behaviour. The current balance of evidence provides clear, though not unambiguous, evidence of a predisposing effect of undernutrition during lactation on subsequent manipulatory behaviour of weaned piglets. The tail-biting behaviour of growing or finishing pigs may be more related to subsequent growth rate immediately preceding onset of the problem.
Effects related to social stress due to competition for teats or other resources
Background
Colostrum and milk are essential to piglet survival as they provide nutrients necessary for thermoregulation and growth, as well as immunoglobulins and other cellular and humoral factors necessary for protection against diseases (Edwards, 2002; Salmon et al., 2009). During parturition and shortly after, colostrum is continuously available to the piglets, but thereafter milk can be consumed only during discrete ejections (De Passillé and Rushen, 1989; Fraser and Rushen, 1992). Disputes at the teats appear very early, in the first hours after birth of the first piglets (De Passillé and Rushen, 1989). These disputes enable winning piglets to gain access to a better functional teat during the brief period of time when milk is ejected (Fraser and Rushen, 1992). As a consequence, a stable ‘teat order’ emerges whereby piglets occupy the same teat at each suckling bout. A larger litter size is generally believed to increase disputes at the udder (Rutherford et al., 2013), but data from De Passillé and Rushen (1989) do not support this hypothesis for the first day of life when the teat order is being established. However, in fully established lactation, the occurrence of skin lesions in suckling piglets increases with litter size, suggesting a positive relationship between fighting and litter size (Norring et al., 2006). Increased competition in large litters is also associated with a more variable and lower growth rate on average (Ocepek et al., 2017). The current genetic selection for increasing litter size is likely to increase this competition (Ocepek et al., 2017). Another source of variation in the intensity of the competition to which piglets are subjected is their position at suckling. Piglets that use teats in the middle of the udder have potentially more competitors for the teats than those that use the anterior or posterior teats.
Aggressive biting behaviour
There is evidence that piglets that need to compete strongly for milk retain a heightened aggressiveness after weaning. Using a resident-intruder test at 18 to 19 days post-weaning, D’Eath and Lawrence (2004) found that piglets from larger litters were more aggressive after weaning. In contrast, Chaloupková et al. (2007) did not observe any influence of litter size on the frequency of agonistic behaviours in newly weaned and mixed piglets. Litter size in this study, however, was relatively small (10.8 in average compared to 12.5 in D’Eath and Lawrence, 2004) and may therefore have not resulted in much competition. Subsequently, Skok et al. (2014) showed that piglets that had sucked from middle teats were involved in more aggressive interactions with unfamiliar pigs post-weaning than those that had sucked from other parts of the udder. This effect did not seem to be related to liveweight at weaning, which was similar in piglets sucking anterior and middle teats.
Sibling competition is likely to occur in other contexts. As an example, piglets are born with little insulation and therefore face a major thermoregulatory challenge (Herpin et al., 2002). Securing access to a warm resting area is essential for survival and, as for other resources that affect fitness, competition should be expected where a warm area is too small. While there has been little work to quantify how much biting occurs to access a nest or creep area of fixed size, it most likely increases with litter size, and this early-life competition probably has similar effects on later behavioural development to that resulting from competition for access to teats.
Non-aggressive biting behavior
In the study by Ursinus et al. (2014b), females expressing a relatively high level of tail chewing and biting (both behaviours were registered in a single category) originated from larger litters (number of live-born piglets) compared with females with a relatively low level of tail chewing and biting. However, this result was dependent on the rearing environment, since it occurred only when pigs were housed in an environment enriched with jute sacks during lactation and after weaning. In a poor environment, high litter size was associated with a higher level of chewing directed to parts of the body other than the tail and ears.
Conclusion: postnatal competition
Taken together, these studies suggest that social competition experienced by piglets during lactation increases aggressive biting behaviour after weaning. In addition, non-aggressive biting behaviour may be increased in piglets originating from large litters. Taking into account the low number of studies, more data are needed to consolidate this conclusion.
Effects related to socialization of piglets by contact with piglets from other litters
Background
Under commercial conditions, pigs usually first encounter unfamiliar pigs at weaning at around 4 weeks of age, which is often accompanied by intense fighting and injuries from biting. Under natural conditions, young wild boar interact with piglets from other litters from the first week of life, without a high level of aggression or injurious bites (Gundlach, 1968). Piglets of domestic sows reared in a free-range environment also start interacting with non-familiar pigs within the first 12 days of life (Jensen and Redbo, 1987). As such, early-life socialization with unfamiliar animals is the norm in the wild ancestors of domestic pigs and, given the opportunity, domestic pigs revert to this practice.
In the wild, social groups usually comprise pigs that are related (Gabor et al., 1999). An early-life window of greater tolerance to unfamiliar conspecifics may be an adaptive response to the need of litters of wild pigs to integrate into this larger and related social group. Avoidance of damaging biting may promote individual fitness by reducing the energetic costs of fighting and the risk of attracting predators.
Domestic piglets in indoor housing seem to retain this willingness to engage in minimal aggression with unfamiliar litters pre-weaning. Indeed, even if the number of fights and skin lesions is increased by mixing litters (Wattanakul et al., 1997a and 1997b; Pedersen et al., 1998), pre-weaning socialization also stimulates play, resting together (Weary et al., 1999) and sharing of home pen areas (Weary et al., 2002). Therefore, a window of greater sociality is present in domestic pigs, and the indoor environment can be modified to allow voluntary integration of litters at a similar time as in the wild.
Aggressive biting behavior
Pre-weaning socialization reduces fighting when piglets are later mixed at weaning, although the mechanism of how it does so is not fully understood. Indeed, studies unanimously show evidence of a reduction in the frequency and/or duration of biting behaviour at post-weaning regrouping in pigs that have had the opportunity to socialize in early life (Weary et al., 1999; D’Eath, 2005; Kanaan et al., 2008; Kutzer et al., 2009; Salazar et al., 2018). While D’Eath (2005) reported that socialized pigs were quicker to attack a small, unfamiliar intruder introduced into the home pen, Wattanakul et al. (1997a) and Kanaan et al. (2008) found that socialized pigs took longer to attack a new pig. This discrepancy in attack latency is likely to result from the different social contexts in which aggressiveness was tested. The studies of Wattanakul et al. (1997a and 1997b) and Kanaan et al. (2008) involved mixing pigs in a novel environment with others of similar competitive ability, in contrast to that of D’Eath (2005) in which one pig had a clear competitive and residency advantage. Taken together, the evidence would suggest that socialized pigs take longer to enter into a fight, unless they have a home pen advantage and are faced with an inferior opponent, and are thus better able to efficiently establish dominance relationships. In addition, during a social encounter test performed a couple of days before or after weaning, piglets reared in a group farrowing system approached an unfamiliar piglet more quickly, stayed closer to it and were more active compared with piglets reared in individual farrowing pens (Hillmann et al., 2003). This was interpreted by the authors as a lesser but better adapted reaction to an unfamiliar pig. Finally, a recent work showed that socialized pigs solve dominance relationships sooner in a dyadic contest setting (Camerlink et al., 2019). It is assumed, but has never been tested, that the opportunity to engage in play fighting and other forms of social contact with unfamiliar animals pre-weaning allows a more rapid acquisition of mature social skills or cognitive ability.
Pre-weaning socialization can be achieved by allowing piglets, but not the sows, from adjacent farrowing pens to mix, or by using a multi-suckling system in which multiple sows and litters are allowed to integrate. It is possible that the benefit of socialization derives both from early-life contact with unfamiliar piglets but also from a more complex and larger physical environment. For example, Weary et al. (1999) allowed piglets to socialize between 11 days of age and weaning at 28 days, but the socialized piglets also had access to a communal area in which different enrichment objects were available. Similarly, Hillmann et al. (2003) offered more space per piglet and a more complex environment to socialized litters compared with un-socialized control ones. However, the work of Wattanakul et al. (1997a) and Kutzer et al. (2009) showed that removing the division between adjacent farrowing pens reduced post-weaning aggression and skin injuries, even though the floor space per piglet and level of enrichment of the environment remained the same. This indicates that socialization itself can reduce subsequent aggression independently of, even though potentially in addition to, environmental enrichment.
Pigs are often regrouped several times after weaning and, at present, it is unknown whether the benefits of socialization are evident only during mixing at weaning or persist into later regrouping episodes. In pigs maintained in stable groups after weaning, a recent work has shown that socialized pigs had 19% fewer skin lesions from aggression compared with controls 4 weeks after weaning (Camerlink et al., 2018).
Non-aggressive biting behavior
In the study by Klein et al. (2016), piglets were allowed to socialize with piglets from three other litters starting at 10 days after parturition. Although tail biting occurred in all groups, a higher percentage of pigs from the early socialized groups had intact tails at day 100 of the fattening period, and their tails were significantly longer.
Conclusion: socialization
Altogether, these studies indicate that socializing piglets during lactation, by allowing them to interact with piglets from other litters, reduces aggressive biting at weaning and probably until some weeks after weaning. Even though more research is needed to substantiate the effect of socialization on non-aggressive biting, the first results are also in favour of a reduction of tail biting.
Effects related to cross-fostering
Background
Litter size has increased to such an extent that the number of live-born piglets often exceeds the number of functional teats. With the trend for more piglets than teats, management solutions such as cross-fostering and fostering to a nurse sow or supplementing with milk replacer have become standard practice in commercial pig husbandry (Baxter et al., 2013). If performed correctly, cross-fostering enhances survival prospects of piglets and can reduce the need for further management interventions. It is recommended to take place within the first 24 h after birth. As piglets get older, aggression after fostering is more intense and is associated with higher pre-weaning mortality (Straw et al., 1998). Piglets that are fostered may suffer from hunger and chilling during the process of acceptance, while all the piglets in the litter may suffer from social stress. Indeed, Heim et al. (2012) observed more fighting just after milk ejection in litters where half of the piglets were adopted, as well as in litters where all piglets were adopted, compared with litters with no adoption. Similarly, Robert and Martineau (2001) found more fighting in fostered litters compared with control litters, both during and between nursings. There are reports of long-term detrimental impacts of cross-fostering on survival, growth, behaviour, reproductive success and immunity (Baxter et al., 2013). Therefore, long-term effects of cross-fostering on aggressive and non-aggressive behaviours are expected.
Aggressive biting behavior
Compared with piglets originating from litters with no cross-fostering, piglets from litters with fostering at 6 days of age fought less immediately following weaning and social mixing performed around 18 days of age, as well as at 1 and 20 days later (Giroux et al., 2000). Being a resident or an intruder piglet at fostering did not change this effect. The occurrence of less fighting was accompanied by a tendency for fewer body lesions in the first week post-weaning, but not later on. The authors attributed the lower fighting frequency in litters with fostered piglets to prior experience of encountering unfamiliar piglets. It may have similar effects to the socialization performed later on during lactation (cf. Socialization of piglets). Similarly, Scheffler et al. (2016b) observed that pigs which had not been raised by their own dam showed fewer agonistic interactions and were less aggressive compared with non-cross-fostered animals when observed shortly after mixing at weaning or at transfer to the growing pens. More recently, Diaz et al. (2018) compared piglets originating from litters with no cross-fostering or from litters subjected to early (first week of life) or late (second and third weeks of life) cross-fostering. Pigs were inspected individually for the presence of body lesions during the post-weaning and fattening periods. Results did not show any difference in the presence of body lesions between treatments. This lack of difference may be due to the fact that lesions were determined several weeks after regrouping, whereas skin lesions commonly disappear in a couple of days, and to a binary scoring method unable to pick up differences in the severity and frequency of lesions.
Non-aggressive biting behavior
Moinard et al. (2003) found a higher incidence of tail biting in farms where cross-fostering was practised compared with farms with no cross-fostering. Since this was an epidemiological study, it cannot be elucidated whether fostering contributed directly to a later likelihood of tail-biting occurrence or whether this association was related to a common causal factor (e.g. herd size or litter size increasing the likelihood of fostering). In the study by Diaz et al. (2018), the presence of ear and tail lesions was not influenced by the occurrence of cross-fostering. However, pigs from fostered litters were more at risk of death and euthanasia, with severe tail lesions being one of the reasons for euthanasia. It suggests that cross-fostering promotes severe tail biting.
Conclusion: cross-fostering
Few studies have examined the influence of cross-fostering on aggressive and non-aggressive biting. Current evidence suggests that this practice may reduce aggressive behaviour and the amount of body lesions, particularly at regrouping, but with a decreasing influence over time. In contrast, it may increase non-aggressive biting after weaning. Taking into account the very low number of studies, more data are needed to consolidate this conclusion, especially regarding non-aggressive biting.
Effects related to age at weaning or artificial rearing
Background
In current intensive pig farms, weaning is abrupt and occurs usually between 3 and 5 weeks of age. This is much earlier than would be the case in natural conditions, where weaning is a very progressive process lasting for several weeks and ending at about 17 weeks of lactation (Jensen and Recen, 1989). Abrupt early weaning is highly stressful for the animals, as shown by the activation of the adrenal axis and changes in behaviour (Colson et al., 2006 and 2012). Alteration in behaviour is more profound when pigs are younger at weaning. Therefore, the behaviour of pigs during the post-weaning and fattening periods could differ according to the age at weaning. An extreme situation arises with ‘artificial rearing’ of piglets shortly after birth. This is performed when highly prolific sows have more piglets than teats and cross-fostering cannot be applied (Baxter et al., 2013). In this situation, piglets are usually allowed to suck colostrum from the dam and then transferred to a rearing pen, where they are provided with milk from a cup. This gives no opportunity to suckle, even though motivation to do so remains high (Noyes, 1976; Frei et al., 2018).
Aggressive biting behavior
Comparison of pigs weaned at about 10 or 30 days of age showed no difference between treatments in the occurrence of fighting behaviour, evaluated between 40 and 150 days of age (Hohenshell et al., 2000). Similarly, the frequency of aggressive behaviours measured at 42 days of age did not differ between pigs weaned at 7, 14 or 28 days of age (Worobec et al., 1999).
Non-aggressive biting behavior
Artificial rearing of piglets, separated from the sow between 3 and 6 days of age, resulted in high levels of belly nosing that lasted until at least 50 days of age (Hosp et al., 2014; Rzezniczek et al., 2015). Whether this very early separation from the dam results in a higher propensity for tail biting has not been evaluated. However, it is highly probable since significant correlations between tail-biting and belly-nosing behaviours have been described (Edwards, 2003; Brunberg et al., 2011).
Pigs weaned at 7 or 14 days of age showed a higher frequency of massaging penmates at 42 days of age than did pigs weaned at 28 days of age, but there was no effect on the occurrence of nosing-chewing penmates (Worobec et al., 1999). Comparing pigs weaned at around 10 and 30 days of age, Hohenshell et al. (2000) found a transient increase in manipulatory behaviours (nosing + biting + pushing + suckling part of another pig’s body) at 40 days of age, but no difference at 65, 102, 137 and 165 days. Furthermore, Algers (1984) found no difference between pigs weaned at 3 and 6 weeks of age in injuries caused by manipulation. Comparing pigs weaned at 4 and 6 weeks, Boe (1993) found a higher frequency of massaging and sucking penmates at the beginning of the fattening period in pigs weaned at the youngest age, but no increase in tail biting and tail lesions. Results indicated that the effect of the post-weaning environment had more influence than the age at weaning (Algers, 1984; Boe, 1993).
Conclusion: age at weaning or artificial rearing
Early weaning stimulates, at least transiently, massaging and/or chewing of penmates, with the risk of provoking damage if it is persistent. However, available data suggest that age at weaning has no clear influence on aggressive and non-aggressive biting in growing pigs.
Effects related to acute stress due to handling and routine practices
Piglets usually undergo a series of routine management practices within their first days of life, such as castration, tail docking, teeth clipping, ear tagging and medical treatments. These interventions certainly cause acute stress due to handling and/or pain (Prunier et al., 2005; Marchant-Forde et al., 2014), but it is not clear if such stressors may have a long-term effect on aggressive or non-aggressive biting. To the best of our knowledge, there are no data in the literature to support or refute such a hypothesis without difficulties of interpretation. For example, the influence of acute stress due to the surgery of tail docking is impossible to evaluate since it is confounded with the influence of shortening the tail, which itself reduces the likelihood of tail biting even if it does not fully eliminate it (EFSA, 2007). Similarly, the influence of acute stress due to surgical castration is confounded with the effect of the removal of testicular steroids that are known to have a great influence on behaviour.
Effects related to the housing environment
Background
The environment provided to piglets in most conventional farms is restrictive and does not fulfil their exploratory needs. This may result in behavioural and physiological disturbances, with potential long-term consequences on the ability of pigs to cope with their rearing conditions, as well as on their social skills and abilities to resolve social conflicts (de Jonge et al., 1996). The pre-weaning environment involves a number of aspects that act simultaneously on piglets, and so individual effects are usually difficult to isolate. Among these, restricted space and lack of enrichment material can be considered most important. Construction features such as crates may hinder vision and movement and thus proper communication between pigs, leading to increased agonistic behaviours (Lammers and Schouten, 1985). Other environmental aspects, such as continuous fan noise over certain thresholds (>85 dB), may also be important (Algers and Jensen, 1991).
Experimental data have shown that enriching the environment of growing pigs from birth until slaughter reduces the occurrence of penmate-directed manipulatory (nibbling, sucking or chewing ears, legs, feet or tails) and aggressive behaviours, as well as the occurrence of tail lesions at various ages (Beattie et al., 2000; Ursinus et al., 2014b). However, in these experiments, the effects of the early and current environments are confounded. Several experiments have been set up to evaluate the influence of the early environment per se on the behaviour of pigs observed during the subsequent post-weaning or fattening periods (Table 1).
Table 1.
Influence of the pre-weaning (preW) environment on the behaviour of pigs during the post-weaning (postW) or fattening periods. Positive effects are highlighted in light grey, negative effects in dark grey and lack of effects are not highlighted
| Reference | Housing during lactation | Age at weaning in days | Housing during the postW period | Housing during the fattening period | Effect of enrichment on tail- or ear-directed behaviours (nosing/chewing/biting) | Effect of enrichment on aggressive behaviours |
|---|---|---|---|---|---|---|
| Webster and Dawkins (2000) | Outdoors (arks with straw) v. indoors (concrete floor + straw, farrowing crate) | 21 to 28 | Straw-bedded, open-fronted pens with gale-breakers | Straw-bedded, open-fronted pens with gale-breakers | No effect at 1, 2 and 8 weeks postW | |
| Cox and Cooper (2001) | Outdoors (arks with straw) v. indoors (concrete floor + straw, farrowing crate) | 24 | Kennel with concrete floor + straw, outdoor area | NA1 | No effect during the 2 days postW | Less fighting behaviour during the 2 postW days |
| Van de Weerd et al. (2005) | Rooting box (chopped straw, hay shredded paper or compost in alternation) v. liquid dispenser v. straw bedding v. none | 28 | Rooting box v. dispenser v. straw bedding v. none between 28 and 56 days of age | Straw-bedded floor v. partly slatted floor with a plastic toy from 70 days of age | In straw-bedded pen: no effect of the preW and early postW environment on behaviour and tail lesions during fattening In partly slatted pen: higher level of manipulatory behaviours in pigs from liquid dispenser than from no enrichment but no effect on tail lesions during fattening |
NA1 |
| Chaloupková et al. (2007) | Enriched pen (straw, more space, no crate) v. enriched crate (straw, crate) v. Conventional (no straw carte) | 28 | Straw bedding | Slatted floor from 84 days of age | NA1 | No effect shortly postW Fewer agonistic interactions during a food competition test at 3 and 6 months of age in pigs from enriched pens compared to enriched and conventional crates |
| Vanheuke-lom et al. (2011) | Peat in a tray v. no peat | 28 | Peat in a tray v. no peat | Peat in a tray v. no peat | No effect during the postW and fattening periods regardless of postW environment | No effect during the postW and fattening periods regardless of postW environment |
| Statham et al. (2011) | Straw (1 kg twice a week) v. wood shavings (0.5 kg/day) | 25 | Straw-bedded floor | Straw-bedded floor | No effect during the postW and fattening periods on behaviour and tail-biting outbreaks | No effect during the postW and fattening periods |
| Telkänranta et al. (2014) | High (sisal ropes + a plastic ball + newspaper + wood shavings) v. low level (a plastic ball + wood shavings) of enrichment | 21 to 25 | Sisal ropes + a plastic ball + newspaper + wood shavings | NA1 | Lower prevalence of severe tail damage at 9 weeks of age in pigs from high enrichment pens but no effect on manipulation of piglets | NA1 |
| Martin et al. (2015) | Enriched (more space, no crate, more straw) v. conventional (less space, crate, few straw) | 27 | Deep straw bedding | NA1 | NA1 | No effect on the fighting behaviour but more lesions appeared at 3 days postW in pigs from enriched pens |
| Day et al. (2002) | Straw v. no straw | None v. minimal, v. substantial v. deep level of straw | No effect on tail biting | No effect on aggressive behaviours (biting excluded)More biting when fatteners are housed without straw | ||
| Bolhuis et al. (2006) | Straw v. no straw | Straw and no straw | No effect on manipulatory (belly nosing + manipulating ears, tail, other part of the body) behaviours in both current fattening environments | No effect on aggressive behaviours in both current fattening environments | ||
NA = no data available.
Aggressive biting behavior
Webster and Dawkins (2000) studied how piglets raised indoors or outdoors before weaning differed in their behaviour after weaning into a pen with concrete floor covered by straw. Compared to the indoor environment, the outdoor environment offered piglets more space, more rooting material and opportunities for social interactions with other litters, and so many factors were confounded. The authors did not observe any difference between indoor and outdoor pigs concerning the fighting behaviour observed just after weaning, as well as at 1, 2 and 8 weeks after weaning. Using the same experimental model, Cox and Cooper (2001) focussed more on the period after weaning with a more detailed ethogram. Indoor piglets showed more fighting behaviours during the first 2 days after weaning. Working only with indoor pigs, Chaloupková et al. (2007) tested the influence of the pre-weaning environment on agonistic behaviours on the day after weaning at 4 weeks of age, and on the behaviour during resource competition tests at 3 and 6 months of age. Treatments were conventional crates (slatted floor, no straw), enriched farrowing crates (straw-bedded pen, 10% additional area) and enriched farrowing pens (straw-bedded pen, 60% additional area, no crate). Pigs were housed in straw-bedded pens after weaning and in slatted pens thereafter, when food competition tests were performed. No effect of the pre-weaning environment was detected on agonistic behaviours immediately after weaning. However, pigs from the enriched farrowing pens showed fewer agonistic interactions during feed competition tests at 3 and 6 months of age compared with pigs from the two other environments. In a factorial design, Vanheukelom et al. (2011) evaluated the influence of providing peat during lactation to pigs which either subsequently did or did not have access to peat later on in life. They found no influence of the pre-weaning environment on fighting behaviour during the post-weaning and fattening periods, whereas the presence of peat in the current environment reduced fighting during the post-weaning period. Similarly, Statham et al. (2011) did not find an effect of adding straw on the floor of farrowing pens (1 kg twice a week) on agonistic behaviour of pigs during the post-weaning and finishing periods. These pigs were housed after weaning on a solid concrete floor with straw added at regular intervals. Martin et al. (2015) found that housing piglets during lactation in an enriched environment (280% more space plus fresh long-stemmed straw), in comparison to a conventional one, increased the appearance of skin lesions between weaning and 3 days later. However, this did not influence the latency to first fight after weaning nor the occurrence of fighting behaviours during the post-weaning period (28 to 56 days of age).
Prolonging the same level of enrichment before and after weaning (experience with straw v. no straw), Day et al. (2002) compared the influence of the early environment in fattening pigs housed with four levels of straw provision (none, minimal, substantial and deep). The early environment had no influence on aggressive behaviours excluding biting. However, there was a significant interaction between the early and current environments for biting any part of another pig except the tail, presumably reflecting aggressive biting. Indeed, biting behaviour was influenced by the early environment only when pigs had no access to straw during the fattening period: an increase was shown in pigs having had an early experience with straw. Similarly, Bolhuis et al. (2006) prolonged the enrichment with straw during lactation to the post-weaning period. From 70 days of age, pigs were exposed either to straw or not in a two factorial design. These did not find any significant effect of the early environment on aggressive behaviour.
Whether the influence of a poor pre-weaning environment on piglet behaviour is age-dependent remains to be evaluated. It is known that piglets stay close to the sow during the first 4 days of life (Kirkden et al., 2013). Therefore, it may be expected that they are relatively unaware of their environment beyond the maternal presence and that the consequences of a barren environment are minor. From day 4 of age until weaning, piglets’ environmental needs may change as they grow. According to Lewis et al. (2006), piglets would not interact with shredded paper or natural fibre ropes before 10 days of age, although this may not apply to all types of enrichment materials. The same authors found that, when piglets were offered shredded paper or natural fibre ropes in the farrowing crate, enrichment characteristics were already relevant between 14 and 26 days of age, since piglets offered shredded paper and ropes spent substantial time interacting with the enrichment but were much more interested in paper than ropes.
Other forms of enrichment related to olfactory, taste and auditory senses are also possible but have not been investigated for their influence on aggressive biting, except with the aim of familiarizing pigs with their environment between phases of rearing. For example, a pleasant odorant molecule (isoamyl acetate = banana scent) was topically applied on the skin of the sows’ mammary glands during lactation and on the feeders after weaning (Fuentes et al., 2012). Piglets in contact with the scent during lactation had fewer agonistic interactions, including biting after weaning. Since the banana scent is unlikely to inhibit aggressive behaviours, the effect is probably linked to a decrease in stress due to novelty of the environment at weaning. In another attempt, feed supplemented with an anisic flavour using transanethol was given to sows during gestation and/or lactation, and piglets received a feed with the same flavour in addition to a standard feed during the 2 weeks after weaning (Oostindjer et al., 2010). Exposure to the flavour during gestation and/or lactation had no effect on the amount of aggressive behaviour in the hours after weaning. However, latency to fight after weaning increased in pigs exposed to the flavour during gestation but not during lactation.
Non-aggressive biting behavior
Comparing commercial farms with a history of tail biting during the previous 6 months with farms with no tail biting, Moinard et al. (2003) suggested a link between the degree of enrichment in farrowing accommodation and tail biting. They found that renewing straw daily in the farrowing pen was more frequent in farms with no tail biting. However, this effect may have been confounded with a more frequent use of straw during the later stages of pig production. Cox and Cooper (2001) did not find differences between indoor and outdoor pre-weaning environments on tail-biting levels performed by piglets during the first 2 days after weaning. Van de Weerd et al. (2005) compared different enrichment materials (rooting box with chopped straw, hay, shredded paper or compost in alternation, liquid dispenser, straw bedding or none) provided for 4 weeks either during lactation or during the immediate post-weaning period. From 10 weeks of age until slaughter at around 90 kg liveweight, pigs were reared on partly slatted floors with a minimum legal amount of enrichment or on straw bedding. The early environment had no influence on tail biting observed during fattening, in contrast to effects of the current environment. Some effects of the post-weaning environment were observed on behaviour during fattening, but only in the poor environment, with pigs having the liquid dispenser showing more manipulatory behaviours than those with no enrichment. Similarly, Statham et al. (2011) did not find an effect of adding straw on the floor of farrowing pens in pigs subsequently reared on solid concrete floors with straw. Outbreaks of tail biting occurred at the same level in both experimental groups, and frequencies of tail biting and chewing behaviours observed at 7, 11, 15 and 19 weeks of age were also similar. Similarly, Vanheukelom et al. (2011) did not show an influence of enriching the pre-weaning environment with peat on manipulatory behaviours (chewing and non-violent biting any part of a congener) during the post-weaning and fattening periods, whereas peat during the post-weaning period reduced manipulatory behaviours. Telkänranta et al. (2014) compared two levels of enrichment during lactation (sisal ropes + a plastic ball + newspaper + wood shavings v. a plastic ball + wood shavings) in pigs reared in an identically enriched environment after weaning (sisal ropes + a plastic chewing toy + wood shavings). They observed a lower prevalence of severe tail damage at 9 weeks of age in pigs from the richer lactational environment (10% v. 32%), even though the frequency of penmate manipulation, defined as touching any part of the body, was not influenced by the lactational environment.
Confounding the influence of enrichment before and after weaning (experience with straw v. no straw), Day et al. (2002) did not show any significant influence of the early environment on tail biting expressed by fattening pigs, regardless of the level of enrichment in the current environment, whereas the presence of straw in the current fattening environment reduced tail biting. Similarly, Bolhuis et al. (2006) did not find any influence of early experience with straw on manipulatory behaviours (belly nosing + manipulating ears, tail, other part of the body) in fatteners, regardless of the current environment, whereas the presence of straw in the current environment reduced these behaviours.
The benefit of familiarity with the environment between phases of rearing the pigs was also investigated using a special flavour as a continuous stimulus in the environment (Oostindjer et al., 2011). Piglets were exposed to an anisic flavour for 2 weeks following weaning, after being exposed to a control or to the same anisic flavour during prenatal life and the lactational period via their mother’s feed. Manipulatory behaviour (nibbling, sucking or chewing body parts of penmates) was reduced in piglets exposed to the anisic in their early life. Fewer vocalizations and shorter latency to eat were also observed, suggesting that these pigs were less stressed by weaning.
Conclusion: environmental enrichment
Enriching the environment during lactation has shown diverse effects on both aggressive and non-aggressive biting depending on the study. Some studies do not show any significant effect, whereas others indicate a promising positive reduction in biting or, on the contrary, an increase. This suggests that the nature of the enrichment during the pre-weaning period, as well as the housing after weaning are of great importance to determine the effects. For example, it is likely that pre-weaning-enriched conditions may be detrimental for piglets, at least regarding aggressive biting, if pigs are deprived of enrichment after weaning. Another promising way to reduce harmful behaviours after weaning would be to ensure some familiarity with the environment when animals are moved between houses using, for example, continuous exposure to a familiar pleasant scent.
Overall conclusion
Literature on the early-life factors predisposing to biting is variable according to the type of factor, and results are not always consistent. Regarding non-aggressive biting, its relatively low frequency and unpredictable nature make it difficult to analyse and may explain, at least in part, a lack of the influence of some treatments and/or inconstancy between some studies.
The influence of personality has been poorly examined. Most studies used the response to a backtest to assess coping style, which only reflects one part of personality. Moreover, this assessment is often performed at a very young age, probably well before personality is fully established. Therefore, there is a large scope for new investigations evaluating how personality and its development influence biting. For most of the environmental factors having a potential influence on future biting behaviours, the number of scientific papers is low (less than five) and sometimes there are no data (Figure 2). Only the influence of early socialization on aggressive biting, poor nutrition on non-aggressive biting, and poor environment on both types of biting have been more fully investigated. Sometimes the conclusions differ between studies, suggesting that the influence of one factor may depend on other factors or on the age when effects were observed. This is, for example, the case for the influence of poor housing on both types of biting. Overall, the conditions of the current environment during the post-weaning or fattening periods are probably of greater importance, and may mask or interact with those existing before weaning.
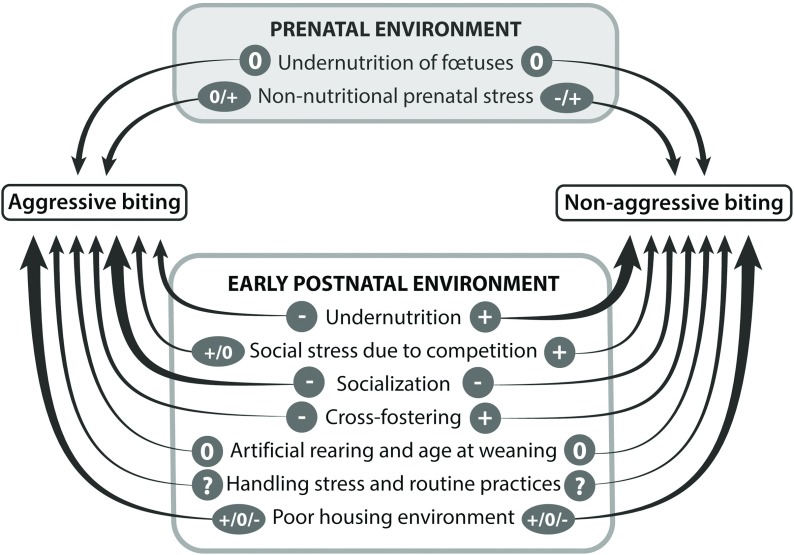
Figure 2.
Summary of the effects of prenatal and pre-weaning environments of pigs on the occurrence of their biting behaviours later in life. When at least five studies are available, the arrows are drawn with a thick line. Signs above the arrows indicate that there is at least one study showing that the considered factor increases (+), has no effect (0) or decreases (−) the occurrence of biting. A question mark indicates that there is no information due to a lack of published studies.
Regarding aggressive biting, undernutrition, cross-fostering and socialization early in life reduces its later occurrence. The practical consequence is that any means to allow piglets from different litters to interact from the second week of age should be encouraged. Regarding non-aggressive biting, undernutrition, social stress due to competition and cross-fostering stimulate its occurrence later in life. These three factors are highly dependent on litter size at birth. Therefore, the full consequences of large litters at birth should be evaluated in terms of health, welfare and performance over the whole life of pigs in order to make a more comprehensive assessment of the advantages and drawbacks of a high litter size. Regarding both types of biting, the use of familiar odours may contribute to their reduction when pigs are moved from one stage of production to another, by alleviating the level of stress associated with novelty of the environment. Therefore, this is a promising method of improvement that needs more research for validation and implementation. Finally, it should be remembered that these early environmental factors are likely to interact with genetic predisposing factors.
Acknowledgements
This review paper is based upon work from COST Action CA15134 – Synergy for preventing damaging behaviour in group-housed pigs and chickens (GroupHouseNet), supported by COST (European Cooperation in Science and Technology; www.cost.eu). The text represents the authors’ views and does not necessarily represent a position of the Commission, which will not be liable for the use made of such information.
A. Prunier 0000-0003-3070-6613
Declaration of interest
There is no conflict of interest involved with this paper.
Ethics statement
This paper is a review of published information. No new ethical approval was required.
Software and data repository resources
No new data were generated in this paper.
References
- Algers B 1984. Animal health in fladeck rearing of weaned piglets. Zentralblatt Fur Veterinarmedizin Reihe a-Journal of Veterinary Medicine Series a-Animal Physiology Pathology and Clinical Veterinary Medicine 31, 1–13. [DOI] [PubMed] [Google Scholar]
- Algers B and Jensen P 1991. Teat stimulation and milk producion during early lactation in sows: effect of continuous noise. Canadian Journal of Animal Science 71, 51–60. [Google Scholar]
- Andersen IL, Andenæs H, Bøe KE, Jensen P and Bakken M 2000. The effects of weight asymmetry and resource distribution on aggression in groups of unacquainted pigs. Applied Animal Behaviour Science 68, 107–120. [DOI] [PubMed] [Google Scholar]
- Anil L, Anil SS, Deen J, Baidoo SK and Wheaton JE 2005. Evaluation of well-being, productivity, and longevity of pregnant sows housed in groups in pens with an electronic sow feeder or separately in gestation stalls. American Journal of Veterinary Research 66, 1630–1638. [DOI] [PubMed] [Google Scholar]
- Baxter EM, Rutherford KMD, D’Eath RB, Arnott G, Turner SP, Sandoe P, Moustsen VA, Thorup F, Edwards SA and Lawrence AB 2013. The welfare implications of large litter size in the domestic pig II: management factors. Animal Welfare 22, 219–238. [Google Scholar]
- Beattie VE, Breuer K, O’Connell NE, Sneddon IA, Mercer JT, Rance KA, Sutcliffe MEM and Edwards SA 2005. Factors identifying pigs predisposed to tail biting. Animal Science 80, 307–312. [Google Scholar]
- Beattie VE, O’Connell NE and Moss BW 2000. Influence of environmental enrichment on the behaviour, performance and meat quality of domestic pigs. Livestock Production Science 65, 71–79. [Google Scholar]
- Bell AW and Ehrhardt RA 2002. Regulation of placental nutrient transport and implications for fetal growth. Nutrition Research Reviews 15, 211–230. [DOI] [PubMed] [Google Scholar]
- Benard M, Schuitmaker TJ and Buning TD 2014. Scientists and Dutch pig farmers in dialogue about tail biting: unravelling the mechanism of multi-stakeholder Learning. Journal of Agricultural & Environmental Ethics 27, 431–452. [Google Scholar]
- Blowey RW 2003. Anal biting in pigs. Veterinary Record 152, 667–667. [PubMed] [Google Scholar]
- Boe K 1993. The effect of age at weaning and post-weaning environment on the behavior of pigs. Acta Agriculturae Scandinavica Section a-Animal Science 43, 173–180. [Google Scholar]
- Bolhuis JE, Schouten WGP, Schrama JW and Wiegant VM 2005. a. Individual coping characteristics, aggressiveness and fighting strategies in pigs. Animal Behaviour 69, 1085–1091. [Google Scholar]
- Bolhuis JE, Schouten WGP, Schrama JW and Wiegant VM 2005. b. Behavioural development of pigs with different coping characteristics in barren and substrate-enriched housing conditions. Applied Animal Behaviour Science 93, 213–228. [Google Scholar]
- Bolhuis JE, Schouten WGP, Schrama JW and Wiegant VM 2006. Effects of rearing and housing environment on behaviour and performance of pigs with different coping characteristics. Applied Animal Behaviour Science 101, 68–85. [Google Scholar]
- Bracke MBM, Lauwere CCD, Wind SMM and Zonerland JJ 2013. Attitudes of Dutch pig farmers towards tail biting and tail docking. Journal of Agricultural & Environmental Ethics 26, 847–868. [Google Scholar]
- Brunberg E, Wallenbeck A and Keeling LJ 2011. Tail biting in fattening pigs: associations between frequency of tail biting and other abnormal behaviours. Applied Animal Behaviour Science 133, 18–25. [Google Scholar]
- Brunton PJ 2013. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction 146, R175–R189. [DOI] [PubMed] [Google Scholar]
- Camerlink I, Farish M, D’Eath R, Arnott G, & Turner SP 2018. Long term benefits on social behaviour after early life socialization of piglets. Animals, 8, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerlink I, Turner SP, Farish M, & Arnott G 2019. Advantages of social skills for contest resolution. Royal Society Open Science, 6, 181456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloupková H, Illmann G, Bartos L and Spinka M 2007. The effect of preweaning housing on the play and agonistic behaviour of domestic pigs. Applied Animal Behaviour Science 103, 25–34. [Google Scholar]
- Colson V, Martin E, Orgeur P and Prunier A 2012. Influence of housing and social changes on growth, behaviour and cortisol in piglets at weaning. Physiology and Behavior 107, 59–64. [DOI] [PubMed] [Google Scholar]
- Colson V, Orgeur P, Foury A and Mormede P 2006. Consequences of weaning piglets at 21 and 28 days on growth, behaviour and hormonal responses. Applied Animal Behaviour Science 98, 70–88. [Google Scholar]
- Couret D, Otten W, Puppe B, Prunier A and Merlot E 2008. Behavioural, endocrine and immune responses to repeated social stress in pregnant gilts. Animal 3, 118–127. [DOI] [PubMed] [Google Scholar]
- Cox LN and Cooper JJ 2001. Observations on the pre- and post-weaning behaviour of piglets reared in commercial indoor and outdoor environments. Animal Science 72, 75–86. [Google Scholar]
- D’Eath RB 2005. Socialising piglets before weaning improves social hierarchy formation when pigs are mixed post-weaning. Applied Animal Behaviour Science 93, 199–211. [Google Scholar]
- D’Eath RB, Arnott G, Turner SP, Jensen T, Lahrmann HP, Busch ME, Niemi JK, Lawrence AB and Sandoe P 2014. Injurious tail biting in pigs: how can it be controlled in existing systems without tail docking? Animal 8, 1479–1497. [DOI] [PubMed] [Google Scholar]
- D’Eath RB and Burn CC 2002. Individual differences in behaviour: a test of ‘coping style’ does not predict resident-intruder aggressiveness in pigs. Behaviour 139, 1175–1194. [Google Scholar]
- D’Eath RB and Lawrence AB 2004. Early life predictors of the development of aggressive behaviour in the domestic pig. Animal Behaviour 67, 501–509. [Google Scholar]
- Day JEL, Burfoot A, Docking CM, Whittaker X, Spoolder HAM and Edwards SA 2002. The effects of prior experience of straw and the level of straw provision on the behaviour of growing pigs. Applied Animal Behaviour Science 76, 189–202. [Google Scholar]
- de Groot J, Ruis MAW, Scholten JW, Koolhaas JM and Boersma WJA 2001. Long-term effects of social stress on antiviral immunity in pigs. Physiology & Behavior 73, 145–158. [DOI] [PubMed] [Google Scholar]
- de Jonge FH, Bokkers EAM, Schouten WGP and Helmond FA 1996. Rearing piglets in a poor environment: developmental aspects of social stress in pigs. Physiology & Behavior 60, 389–396. [DOI] [PubMed] [Google Scholar]
- De Passillé AMB and Rushen J 1989. Suckling and teat disputes by neonatal piglets. Applied Animal Behaviour Science 22, 23–38. [Google Scholar]
- Desire S, Turner SP, D’Eath RB, Doeschl-Wilson AB, Lewis CRG and Roehe R 2015. Analysis of the phenotypic link between behavioural traits at mixing and increased long-term social stability in group-housed pigs. Applied Animal Behaviour Science 166, 52–62. [Google Scholar]
- Diaz JAC, Manzanilla EG, Diana A and Boyle LA 2018. Cross-fostering implications for pig mortality, welfare and performance. Frontiers in Veterinary Science 5, 1–10. doi: 10.3389/fvets.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SL, Edwards SA, Sutcliffe E, Knap PW and Kyriazakis I 2013. Identification of risk factors associated with poor lifetime growth performance in pigs. Journal of Animal Science 91, 4123–4132. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Arthur RD and Rosenthal TL 1999. Predictors of social dominance and aggression in gilts. Applied Animal Behaviour Science 63, 121–129. [Google Scholar]
- Edwards S 2003. Environmental and genetic influences on the development of adverse behaviour in pigs. Final report for DEFRA project AW0126. Retrieved on 1 March 2019 from http://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&ProjectID=8900&FromSearch=Y&Status=3&Publisher=1&SearchText=pig.
- Edwards SA 2002. Perinatal mortality in the pig: environmental or physiological solutions? Livestock Production Science 78, 3–12. [Google Scholar]
- EFSA 2007. Scientific report on the risks associated with tail biting in pigs and possible means to reduce the need for tail docking considering the different housing and husbandry systems. EFSA Journal 611, 1–100. [Google Scholar]
- Finkemeier MA, Langbein J and Puppe B 2018. Personality research in mammalian farm animals: concepts, measures, and relationship to welfare. Frontiers in Veterinary Science 5, 1–15. doi: 10.3389/fvets.2018.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkman B, Furuhaug IL and Jensen P 1995. Personality, coping paterns, and agression in piglets. Applied Animal Behaviour Science 45, 31–42. [Google Scholar]
- Foxcroft GR, Dixon WT, Novak S, Putman CT, Town SC and Vinsky MDA 2006. The biological basis for prenatal programming of postnatal performance in pigs. Journal of Animal Science 84 (suppl. E), E105–E112. [DOI] [PubMed] [Google Scholar]
- Foxcroft GR and Town SC 2004. Prenatal programming of postnatal performance – the unseen cause of variance. Advances in Pork Production, 15, 269–279. [Google Scholar]
- Fraser D 1987. Attraction to blood as a factor in tail-biting by pigs. Applied Animal Behaviour Science 17, 61–68. [Google Scholar]
- Fraser D and Rushen J 1987. Aggressive behavior. Veterinary Clinics of North America-Food Animal Practice 3, 285–305. [DOI] [PubMed] [Google Scholar]
- Fraser D and Rushen J 1992. Colostrum intake by newborn piglets. Canadian Journal of Animal Science 72, 1–13. [Google Scholar]
- Frei D, Wurbel H, Wechsler B, Gygax L, Burla JB and Weber R 2018. Can body nosing in artificially reared piglets be reduced by sucking and massaging dummies? Applied Animal Behaviour Science 202, 20–27. [Google Scholar]
- Fuentes M, Otal J, Hevia ML, Quiles A and Fuentes FC 2012. Effect of olfactory stimulation during suckling on agonistic behavior in weaned pigs. Journal of Swine Health and Production 20, 25–33. [Google Scholar]
- Gabor TM, Hellgren EC, Van den Bussche RA and Silvy NJ 1999. Demography, sociospatial behaviour and genetics of feral pigs (Sus scrofa) in a semi-arid environment. Journal of Zoology 247, 311–322. [Google Scholar]
- Gaccioli F and Lager S 2016. Placental nutrient transport and intrauterine growth restriction. Frontiers in Physiology 7, 1–8. doi: 10.3389/fphys.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux S, Robert S and Martineau GP 2000. The effects of cross-fostering on growth rate and post-weaning behavior of segregated early-weaned piglets. Canadian Journal of Animal Science 80, 533–538. [DOI] [PubMed] [Google Scholar]
- Gundlach H 1968. Maternal care, pre- and postnatal, behavioral ontogeny, and circadian activity of the European wild boar (Sus scrofa L). Zeitschrift fur Tierpsychologie 25, 955–995. [PubMed] [Google Scholar]
- Hansen LL, Hagelso AM and Madsen A 1982. Behavioral results and perforamnce of bacon pigs fed ad-libitum from one or several feeders. Applied Animal Ethology 8, 307–333. [Google Scholar]
- Haussmann MF, Carroll JA, Weesner GD, Daniels MJ, Matteri RL and Lay DC 2000. Administration of ACTH to restrained, pregnant sows alters their pigs hypothalamic-pituitary-adrenal (HPA) axis. Journal of Animal Science 78, 2399–2411. [DOI] [PubMed] [Google Scholar]
- Heim G, Mellagi APG, Bierhals T, Souza LPD, Fries HCCD, Piuco P, Seidel E, Bernardi ML, Wentz I and Bortolozzo FP 2012. Effects of cross-fostering within 24 h after birth on pre-weaning behaviour, growth performance and survival rate of biological and adopted piglets. Livestock Science 150, 121–127. [Google Scholar]
- Herpin P, Damon M and Le Dividich J 2002. Development of thermoregulation and neonatal survival in pigs. Livestock Production Science 78, 25–45. [Google Scholar]
- Hessing MJC, Hagelso AM, Vanbeek JAM, Wiepkema PR, Schouten WGP and Krukow R 1993. Individual behavioral characteristics in pigs. Applied Animal Behaviour Science 37, 285–295. [Google Scholar]
- Hillmann E, von Hollen F, Bunger B, Todt D and Schrader L 2003. Farrowing conditions affect the reactions of piglets towards novel environment and social confrontation at weaning. Applied Animal Behaviour Science 81, 99–109. [Google Scholar]
- Hohenshell LM, Cunnick JE, Ford SP, Kattesh HG, Zimmerman DR, Wilson ME, Matteri RL, Carroll JA and Lay DC 2000. Few differences found between early- and late-weaned pigs raised in the same environment. Journal of Animal Science 78, 38–49. [DOI] [PubMed] [Google Scholar]
- Hosp H, Rzezniczek M, Weber R and Hillmann E 2014. Effects of artificial rearing on the behaviour of piglets after regrouping. In Internationalen Arbeitstagung Angewandte Ethologie bei Nutztieren der Deutschen Veterinärmedizinischen Gesellschaft e.V. (DVG) (ed. Erhard M, Pollmann U, Reiter K and Waiblinger S). Kuratorium für Technik und Bauwesen in der Landwirtschaft, Freiburg/Breisgau, Germany.
- Jacobs C, De Keuster T and Simoens P 2003. Assessing the pathological extent of aggressive behaviour in dogs – A review of the literature. Veterinary Quarterly 25, 53–60. [DOI] [PubMed] [Google Scholar]
- Janczak AM, Pedersen LJ and Bakken M 2003. Aggression, fearfulness and coping styles in female pigs. Applied Animal Behaviour Science 81, 13–28. [Google Scholar]
- Jensen P and Recen B 1989. When to wean – observations from free-ranging domestic pigs. Applied Animal Behaviour Science 23, 49–60. [Google Scholar]
- Jensen P and Redbo I 1987. Behavior during nest leaving in free-ranging domestic pigs. Applied Animal Behaviour Science 18, 355–362. [Google Scholar]
- Kanaan VT, Pajor EA, Lay DC Jr., Richert BT and Garner JP 2008. A note on the effects of co-mingling piglet litters on pre-weaning growth, injuries and responses to behavioural tests. Applied Animal Behaviour Science 110, 386–391. [Google Scholar]
- Kirkden RD, Broom DM and Andersen IL 2013. Piglet mortality: management solutions. Journal of Animal Science 91, 3361–3389. [DOI] [PubMed] [Google Scholar]
- Klein S, Patzkewitschl D, Reese S and Erhard M 2016. Effects of socializing piglets in lactation on behaviour, including tail-biting, in growing and finishing pigs. Tieraerztliche Praxis Ausgabe Grosstiere Nutztiere 44, 141–150. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW and Blokhuis HJ 1999. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews 23, 925–935. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC and McEwen BS 2005. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews 29, 3–38. [DOI] [PubMed] [Google Scholar]
- Kranendonk G, Hopster H, Fillerup M, Ekkel ED, Mulder EJH and Taveme MAM 2006. Cortisol administration to pregnant sows affects novelty-induced locomotion, aggressive behaviour, and blunts gender differences in their offspring. Hormones and Behavior 49, 663–672. [DOI] [PubMed] [Google Scholar]
- Kranendonk G, Hopster H, van Eerdenburg F, van Reenen K, Fillerup M, de Groot J, Korte M and Taverne M 2005. Evaluation of oral administration of cortisol as a model for prenatal stress in pregnant sows. American Journal of Veterinary Research 66, 780–790. [DOI] [PubMed] [Google Scholar]
- Kranendonk G, Mulder EJH, Parvizi N and Taverne MAM 2008. Prenatal stress in pigs: experimental approaches and field observations. Experimental and Clinical Endocrinology & Diabetes 116, 413–422. [DOI] [PubMed] [Google Scholar]
- Kutzer T, Buenger B, Kjaer JB and Schrader L 2009. Effects of early contact between non-littermate piglets and of the complexity of farrowing conditions on social behaviour and weight gain. Applied Animal Behaviour Science 121, 16–24. [Google Scholar]
- Ladewig J, Kloeppel P and Kallweit E 1984. A case of reverses cannibalism: the piglets damaging the sow. Annales de la Recherche Vétérinaire 15, 275–277. [PubMed] [Google Scholar]
- Lammers GJ and Schouten WGP 1985. Effect of pen size on the development of agonistic behavior in piglets. Netherlands Journal of Agricultural Science 33, 305–307. [Google Scholar]
- Lawrence AB, Terlouw EMC and Illius AW 1991. Individual differences in behavioral responses of pigs exposed to non-social and social challenges. Applied Animal Behaviour Science 30, 73–86. [Google Scholar]
- Lay DC Jr., Kattesh HG, Cunnick JE, Daniels MJ, Kranendonk G, McMunn KA, Toscano MJ and Roberts MP 2011. Effect of prenatal stress on subsequent response to mixing stress and a lipopolysaccharide challenge in pigs. Journal of Animal Science 89, 1787–1794. [DOI] [PubMed] [Google Scholar]
- Lechner M, Langbein F and Reiner G 2015. Necrosis and cannibalism – an overview. Tieraerztliche Umschau 70, 505–514. [Google Scholar]
- Lewis E, Boyle LA, O’Doherty JV, Lynch PB and Brophy P 2006. The effect of providing shredded paper or ropes to piglets in farrowing crates on their behaviour and health and the behaviour and health of their dams. Applied Animal Behaviour Science 96, 1–17. [Google Scholar]
- Maes D, Pluym L and Peltoniemi O 2016. Impact of group housing of pregnant sows on health. Porcine Health Management 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant-Forde JN, Lay DC Jr., McMunn KA, Cheng HW, Pajor EA and Marchant-Forde RM 2014. Postnatal piglet husbandry practices and well-being: the effects of alternative techniques delivered in combination. Journal of Animal Science 92, 1150–1160. [DOI] [PubMed] [Google Scholar]
- Martin JE, Ison SH and Baxter EM 2015. The influence of neonatal environment on piglet play behaviour and post-weaning social and cognitive development. Applied Animal Behaviour Science 163, 69–79. [Google Scholar]
- McGlone JJ 1985. A quantitative ethogram of aggressive and submissive behaviours in recently regrouped pigs. Journal of Animal Science 61, 559–565. [PubMed] [Google Scholar]
- Meese GB and Ewbank R 1972. Note on instability of dominace hierarchy and variations in level of aggression withing groups of fattening pigs. Animal Production 14, 359–362. [Google Scholar]
- Meese GB and Ewbank R 1973. Establisment and nature of dominance hierarchy in domesticated pigs. Animal Behaviour 21, 326–334. [Google Scholar]
- Melotti L, Oostindjer M, Bolhuis JE, Held S and Mendl M 2011. Coping personality type and environmental enrichment affect aggression at weaning in pigs. Applied Animal Behaviour Science 133, 144–153. [Google Scholar]
- Merlot E, Quesnel H and Prunier A 2013. Prenatal stress, immunity and neonatal health in farm animal species. Animal 12, 2016–2025. [DOI] [PubMed] [Google Scholar]
- Moinard C, Mendl M, Nicol CJ and Green LE 2003. A case control study of on-farm risk factors for tail biting in pigs. Applied Animal Behaviour Science 81, 333–355. [Google Scholar]
- Norring M, Valros A, Munksgaard L, Puumala M, Kaustell KO and Saloniemi H 2006. The development of skin, claw and teat lesions in sows and piglets in farrowing crates with two concrete flooring materials. Acta Agriculturae Scandinavica Section a-Animal Science 56, 148–154. [Google Scholar]
- Noyes L 1976. A behavioural comparison of gnotobiotic with normal neonate pigs, indicating stress in the former. Applied Animal Ethology 2, 113–121. [Google Scholar]
- Ocepek M, Newberry RC and Andersen IL 2017. Trade-offs between litter size and offspring fitness in domestic pigs subjected to different genetic selection pressures. Applied Animal Behaviour Science 193, 7–14. [Google Scholar]
- Oostindjer M, Bolhuis JE, Simon K, van den Brand H and Kemp B 2011. Perinatal flavour learning and adaptation to being weaned: all the pig needs is smell. PLOS ONE 6, 1–7. doi: 10.1371/journal.pone.0025318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostindjer M, Bolhuis JE, van den Brand H, Roura E and Kemp B 2010. Prenatal flavor exposure affects growth, health and behavior of newly weaned piglets. Physiology & Behavior 99, 579–586. [DOI] [PubMed] [Google Scholar]
- Otten W, Kanitz E, Tuchscherer M and Nurnberg G 2001. Effects of prenatal restraint stress on hypothalamic-pituitary-adrenocortical and sympatho-adrenomedullary axis in neonatal pigs. Animal Science 73, 279–287. [Google Scholar]
- Parois S, Larzul C and Prunier A 2017. Associations between the dominance status and sexual development, skin lesions or feeding behaviour of intact male pigs. Applied Animal Behaviour Science 187, 15–22. [Google Scholar]
- Peden RSE, Turner SP, Boyle LA and Camerlink I 2018. The translation of animal welfare research into practice: the case of mixing aggression between pigs. Applied Animal Behaviour Science 204, 1–9. [Google Scholar]
- Pedersen LJ, Studnitz M, Jensen KH and Giersing AM 1998. Suckling behaviour of piglets in relation to accessibility to the sow and the presence of foreign litters. Applied Animal Behaviour Science 58, 267–279. [Google Scholar]
- Pend W, Jenny B, Torgerson P, Spring P, Kuemmerlen D and Sidler X 2017. Effect of herd health management on the prevalence of Postpartum Dysgalaktie Syndrome (PPDS) and the treatment incidence. Schweizer Archiv Fur Tierheilkunde 159, 109–116. [DOI] [PubMed] [Google Scholar]
- Petersen HH, Nielsen EO, Hassing AG, Ersboll AK and Nielsen JP 2008. Prevalence of clinical signs of disease in Danish finisher pigs. Veterinary Record 162, 377–382. [DOI] [PubMed] [Google Scholar]
- Poore KR and Fowden AL 2003. The effect of birth weight on hypothalamo-pituitary-adrenal axis function in juvenile and adult pigs. Journal of Physiology-London 547, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier A, Mounier AM and Hay M 2005. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. Journal of Animal Science 83, 216–222. [DOI] [PubMed] [Google Scholar]
- Quiniou N, Dagorn J and Gaudré D 2002. Variation of piglets’ birth weight and consequences on subsequent performance. Livestock Production Science 78, 63–70. [Google Scholar]
- Rizvi S, Nicol CJ and Green LE 1998. Risk factors for vulva biting in breeding sows in south-west England. Veterinary Record 143, 654–658. [PubMed] [Google Scholar]
- Robert S and Martineau GP 2001. Effects of repeated cross-fosterings on preweaning behavior and growth performance of piglets and on maternal behavior of sows. Journal of Animal Science 79, 88–93. [DOI] [PubMed] [Google Scholar]
- Ruis MAW, Brake JHAT, Burgwal JAVD, Jong ICD, Blokhuis HJ and Koolhaas JM 2000. Personalities in female domesticated pigs: behavioural and physiological indications. Applied Animal Behaviour Science 66, 31–47. [Google Scholar]
- Rutherford KMD, Baxter EM, D’Eath RB, Turner SP, Arnott G, Roehe R, Ask B, Sandoe P, Moustsen VA, Thorup F, Edwards SA, Berg P and Lawrence AB 2013. The welfare implications of large litter size in the domestic pig I: biological factors. Animal Welfare 22, 199–218. [Google Scholar]
- Rutherford KMD, Donald RD, Arnottt G, Rooke JA, Dixon L, Mehers JJM, Turnbull J and Lawrence AB 2012. Farm animal welfare: assessing risks attributable to the prenatal environment. Animal Welfare 21, 419–429. [Google Scholar]
- Rutherford KMD, Robson SK, Donald RD, Jarvis S, Sandercock DA, Scott EM, Nolan AM and Lawrence AB 2009. Pre-natal stress amplifies the immediate behavioural responses to acute pain in piglets. Biology Letters 5, 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzezniczek M, Gygax L, Wechsler B and Weber R 2015. Comparison of the behaviour of piglets raised in an artificial rearing system or reared by the sow. Applied Animal Behaviour Science 165, 57–65. [Google Scholar]
- Salazar LC, Ko HL, Yang CH, Llonch L, Manteca X, Camerlink I and Llonch P 2018. Early socialisation as a strategy to increase piglets’ social skills in intensive farming conditions. Applied Animal Behaviour Science 206, 25–31. [Google Scholar]
- Salmon H, Berri M, Gerdts V and Meurens F 2009. Humoral and cellular factors of maternal immunity in swine. Developmental and Comparative Immunology 33, 384–393. [DOI] [PubMed] [Google Scholar]
- Sambraus HH 1985. Mouth-based anomalous syndromes In World animal science, A5, ethology of farm animals. A comprehensive study of the behavioural features of common farm animals (ed. Fraser AF), pp. 391–422. Elsevier, Amsterdam, The Nertherlands. [Google Scholar]
- Sandercock DA, Gibson IF, Rutherford KMD, Donald RD, Lawrence AB, Brash HM, Scott EM and Nolan AM 2011. The impact of prenatal stress on basal nociception and evoked responses to tail-docking and inflammatory challenge in juvenile pigs. Physiology & Behavior 104, 728–737. [DOI] [PubMed] [Google Scholar]
- Sauber TE, Stahly TS and Nonnecke BJ 1999. Effect of level of chronic immune system activation on the lactational performance of sows. Journal of Animal Science 77, 1985–1993. [DOI] [PubMed] [Google Scholar]
- Scheffler K, Stamer E, Traulsen I and Krieter J 2016. a. Relationship between behavioural tests and agonistic interactions at different age levels in pigs. Applied Animal Behaviour Science 177, 19–24. [Google Scholar]
- Scheffler K, Stamer E, Traulsen I and Krieter J 2016. b. Estimation of genetic parameters for agonistic behaviour of pigs at different ages. Journal of Agricultural Science 154, 732–741. [Google Scholar]
- Schroëder-Petersen D and Simonsen H 2001. Tail biting in pigs. Veterinary Journal 162, 196–210. [DOI] [PubMed] [Google Scholar]
- Simonsen HB 1990. Behaviour and distribution of fattening pigs in the multi-activity pen. Applied Animal Behaviour Science 27, 311–324. [Google Scholar]
- Sinisalo A, Niemi JK, Heinonen M and Valros A 2012. Tail biting and production performance in fattening pigs. Livestock Science 143, 220–225. [Google Scholar]
- Skok J, Prevolnik M, Urek T, Mesarec N and Skorjanc D 2014. Behavioural patterns established during suckling reappear when piglets are forced to form a new dominance hierarchy. Applied Animal Behaviour Science 161, 42–50. [Google Scholar]
- Statham P, Green L and Mendl M 2011. A longitudinal study of the effects of providing straw at different stages of life on tail-biting and other behaviour in commercially housed pigs. Applied Animal Behaviour Science 134, 100–108. [Google Scholar]
- Stookey JM and Gonyou HW 1994. The effects of regrouping on behavioral and production parameters in finishing swine. Journal of Animal Science 72, 2804–2811. [DOI] [PubMed] [Google Scholar]
- Straw BE, Dewey CE and Burgi EJ 1998. Patterns of crossfostering and piglet mortality on commercial US and Canadian swine farms. Preventive Veterinary Medicine 33, 83–89. [DOI] [PubMed] [Google Scholar]
- Taylor NR, Main DCJ, Mendl M and Edwards SA 2010. Tail-biting a new perspective. Veterinary Journal 186, 137–147. [DOI] [PubMed] [Google Scholar]
- Telkänranta H, Swan K, Hirvonen H and Valros A 2014. Chewable materials before weaning reduce tail biting in growing pigs. Applied Animal Behaviour Science 157, 14–22. [Google Scholar]
- Turner SP, Farnworth MJ, White IMS, Brotherstone S, Mendl M, Knap P, Penny P and Lawrence AB 2006. The accumulation of skin lesions and their use as a predictor of individual aggressiveness in pigs. Applied Animal Behaviour Science 96, 245–259. [Google Scholar]
- Ursinus WW, Reenen CGV, Reimert I and Bolhuis JE 2014. a. Tail biting in pigs: blood serotonin and fearfulness as pieces of the puzzle? PLOS ONE 9, e107040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursinus WW, Wijnen HJ, Bartels AC, Dijvesteijn N, van Reenen CG and Bolhuis JE 2014. b. Damaging biting behaviors in intensively kept rearing gilts: the effect of jute sacks and relations with production characteristics. Journal of Animal Science 92, 5193–5202. [DOI] [PubMed] [Google Scholar]
- Valros A and Heinonen M 2015. Save the pig tail. Porcine Health Management 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Weerd HA, Docking CM, Day JEL and Edwards SA 2005. The development of harmful social behaviour in pigs with intact tails and different enrichment backgrounds in two housing systems. Animal Science 80, 289–298. [Google Scholar]
- Van Putten G and Vandeburgwal JA 1990. Vulva biting in group-housed sows: preliminary report. Applied Animal Behaviour Science 26, 181–186. [Google Scholar]
- van Staaveren N, Teixeira DL, Hanlon A and Boyle LA 2017. Pig carcass tail lesions: the influence of record keeping through an advisory service and the relationship with farm performance parameters. Animal 11, 140–146. [DOI] [PubMed] [Google Scholar]
- Vanheukelom V, Driessen B, Maenhout D and Geers R 2011. Peat as environmental enrichment for piglets: the effect on behaviour, skin lesions and production results. Applied Animal Behaviour Science 134, 42–47. [Google Scholar]
- Wattanakul W, Sinclair AG, Stewart AH, Edwards SA and English PR 1997. b. Performance and behaviour of lactating sows and piglets in crate and multisuckling systems: a study involving European White and Manor Meishan genotypes. Animal Science 64, 339–349. [Google Scholar]
- Wattanakul W, Stewart AH, Edwards SA and English PR 1997. a. Effects of grouping piglets and changing sow location on suckling behaviour and performance. Applied Animal Behaviour Science 55, 21–35. [Google Scholar]
- Weary DM, Pajor EA, Bonenfant M, Fraser D and Kramer DL 2002. Alternative housing for sows and litters Part 4. Effects of sow-controlled housing combined with a communal piglet area on pre- and post-weaning behaviour and performance. Applied Animal Behaviour Science 76, 279–290. [Google Scholar]
- Weary DM, Pajor EA, Bonenfant M, Ross SK, Fraser D and Kramer DL 1999. Alternative housing for sows and litters: 2. Effects of a communal piglet area on pre- and post-weaning behaviour and performance. Applied Animal Behaviour Science 65, 123–135. [Google Scholar]
- Webster S and Dawkins M 2000. The post-weaning behaviour of indoor-bred and outdoor-bred pigs. Animal Science 71, 265–271. [Google Scholar]
- Weiler U, Isernhagen M, Stefanski V, Ritzmann M, Kress K, Hein C and Zols S 2016. Penile Injuries in Wild and Domestic Pigs. Animals, 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NH, Stahly TS and Zimmerman DR 1997. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. Journal of Animal Science 75, 2481–2496. [DOI] [PubMed] [Google Scholar]
- Worobec EK, Duncan IJH and Widowski TM 1999. The effects of weaning at 7, 14 and 28 days on piglet behaviour. Applied Animal Behaviour Science 62, 173–182. [Google Scholar]
- Zebunke M, Repsilber D, Nuernberg G, Wittenburg D and Puppe B 2015. The backtest in pigs revisited – an analysis of intra-situational behaviour. Applied Animal Behaviour Science 169, 17–25. [Google Scholar]
- Zonderland JJ, Kemp B, Bracke MBM, den Hartog LA and Spoolder HAM 2011. a. Individual piglets’ contribution to the development of tail biting. Animal 5, 601–607. [DOI] [PubMed] [Google Scholar]
- Zonderland JJ, Schepers F, Bracke MBM, den Hartog LA, Kemp B and Spoolder HAM 2011. b. Characteristics of biter and victim piglets apparent before a tail-biting outbreak. Animal 5, 767–775. [DOI] [PubMed] [Google Scholar]
- Zupan M, Janczak AM, Framstad T and Zanella AJ 2012. The effect of biting tails and having tails bitten in pigs. Physiology & Behavior 106, 638–644. [DOI] [PubMed] [Google Scholar]